Dear Editor,
Here we report two patients with gastrointestinal (GI) metastases from breast cancer (BC) and provide a brief summary of the characteristics and management of this rare clinical entity (the main studies are summarized in Table 1).
Table 1.
Main studies of breast cancer in patients with gastrointestinal metastasis
| Author | No. of patients | Age (yr) | Location | Histology of primary site | Receptors of primary site | Treatment of primary site |
|---|---|---|---|---|---|---|
| Liu (2012) | 12 | 59 | L | Medullary | NR | Surgery, CT |
| Amin (2016) | 1 | 46 | L | DIC | HR+ | Surgery, CT, HT, RT |
| Lai (2016) | 1 | 54 | L | LIC | HR+ | Surgery, CT, HT, RT |
| Nakayama (2015) | 1 | 42 | L | LIC | HR+ | Surgery |
| Koike (2014) | 3 | 42,54, 54 | L (2), Both (2) | LIC (2), DIC (1) | HR+ (2); HR− (1) | Surgery (3), CT (2) |
| Reiman (2001) | 1 | 64 | R | LIC | HR+ | Surgery, CT, HT, RT |
| Pera (2001) | 1 | 45 | L | LIC | HR+ | Surgery, RT |
| Hara (2010) | 1 | 74 | L | DIC | HR− | Surgery, RT |
| Ciulla (2008) | 1 | 70 | L | LIC | HR+ | Surgery, HT, |
| Pectasides (2016) | 8 | 59.5* | NR | LIC (6), DIC (2) | HR+ (8) | Surgery (8), CT (7), HT (2) |
| Fernandess (2016) | 4 | 61.5* | NR | LIC (2), DIC (2) | HR+ | NR |
| Jones (2007) | 2 | 51,61 | L (1), R (1) | LIC | HR+ (2) | Surgery, RT; Surgery, CT, HT |
| Kudo (2005) | 1 | 59 | NR | NR | NR | Surgery |
| Dumoulin (2009) | 1 | 60 | NR | LIC | HR+ | Surgery, CT, RT |
| Almubaraka (2011) | 35 | 62* | NR | LIC (34), DIC (1) | HR+ (19), NR (16) | Surgery (29); NR (6) |
| Okido (2011) | 1 | 48 | R | LIC | HR+ | Surgery, CT, HT, RT |
| Andriola (2012) | 1 | 63 | L | Mixed | NR | Surgery, CT, |
| Michalopoulos (2004) | 2 | 55,57 | L | LIC (1), DIC (1) | NR | Surgery, CT |
| Zhou (2012) | 1 | 54 | R | DIC | HR+ | Surgery, CT, HT |
| Cho (2011) | 1 | 46 | Both | DIC | HR+ | Surgery, HT, RT |
| Gizzi (2015) | 1 | 72 | L | LIC | HR+ | Surgery, CT, HT, RT |
| Ali (2016) | 1 | 61 | L | DIC | HR+ | Surgery, CT, HT, RT |
| Maekawa (2012) | 1 | 52 | R | DIC | HR+ | Surgery, CT, HT, RT |
| Matsuda (2012) | 1 | 62 | L | LIC | NR | Surgery, CT, RT |
| Uygun (2006) | 1 | 43 | R | Mixed | HR+ | Surgery, CT, HT, RT |
| Mostafa (2002) | 1 | 56 | NR | DIC | HR+ | Surgery, HT, RT |
| Nikoli (2012) | 1 | 70 | L | DIC | HR+ | Surgery, CT, HT, RT |
| Feng (2009) | 1 | 49 | R | DIC | NR | Surgery, CT |
| Mistrangelo (2011) | 1 | 80 | Both | LIC | NR | Surgery |
| Malhotra (2009) | 1 | 71 | NR | LIC | NR | NR |
| Buka (2016) | 1 | 58 | R | LIC | HR+ | CT, HT, RT |
| Guzmán (2017) | 1 | 58 | Both | LIC | HR+ | Surgery, CT, HT, RT |
, median age/months; −, gastric metastasis appeared before primary BC; yr, years old; mo, months; GI, gastrointestinal; NR, not reported; HR, hormone receptor; HER2, human epithelial receptor 2; L, left; R, right; DIC, ductal infiltrating carcinoma; LIC, lobular infiltrating carcinoma; CT, chemotherapy; RT, radiotherapy; HT, hormonal therapy.
Case 1
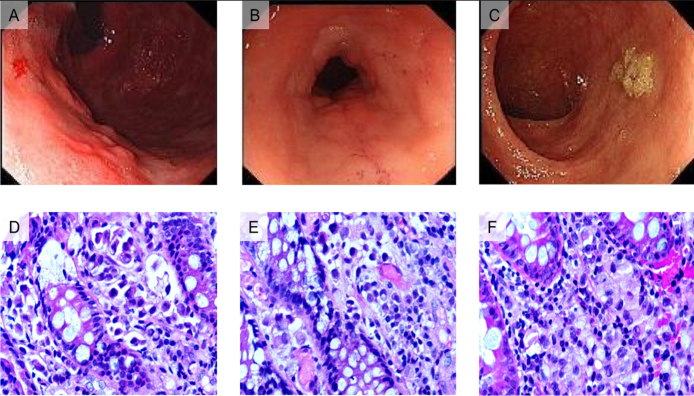
A 42-year-old woman presented to our hospital with a complaint of a palpable mass in the left breast for 2 months in February 2014. Biopsies of the primary tumor and left axillary lymph nodes were obtained, and the pathological results showed invasive lobular cancer and lymph node metastases. Based on the Union for International Cancer Control Tumor Node Metastasis classification, she was diagnosed with BC (cT4bN1Mx). Immunohistochemical (IHC) examinations revealed that estrogen receptor (ER) and progesterone receptor (PgR) were both positive, whereas human epidermal growth receptor 2 (HER2) was negative. After six cycles of preoperative chemotherapy (a combination of docetaxel, epirubicin, and cyclophosphamide, TEC), the primary tumor and the axillary lymph nodes shrank obviously, and she underwent a left modified radical mastectomy. Postoperatively, tamoxifen was given. In June 2016, she complained of upper abdominal discomfort. Abdomen computerized tomography (CT) revealed increased thickness of the greater omentum and multiple mesenteric lymph node metastases. Gastroscopy showed a local protuberant lesion on the body of the stomach and obvious thickness of mucosa of gastroesophageal angle (Figure 1A, 1D). The histological examinations and IHC staining of both biopsies obtained from previously described lesions indicated BC metastases. Two months later, although the symptom of feeling full eased, a colon stenosis was identified by colonoscopy (Figure 1B, 1E). In October 2017, she was diagnosed with bladder metastasis. IHC results of these biopsies were consistent with those of the primary BC. Since June 2016, she was treated with docetaxel and capecitabine for six cycles with stable disease, second- line gemcitabine and cisplatin for six cycles with stable disease, and third-line paclitaxel and carboplatin for two cycles with progressive disease. She died in February 2018 due to cancer progression.
Figure 1.
Endoscopic views and pathological staining of gastric and colon specimens from two patients.
A) The gastroscopic view of the first patient found a local protuberant lesion on the body of the stomach and obvious thickening of the mucosa of the gastroesophageal angle. B) A colon stenosis was identified by colonoscopy in the first patient. C) The colonoscopic features of the second patient showed a flat bulging. D) Numerous signet ring cells were observed in the hematoxylin and eosin (HE) staining of the gastric specimen obtained from the first patient. E) HE staining of the colon specimen showing strands of invasive cells in the mucosa. F) HE staining of the colon specimen obtained from the second patient showing strands of invasive cells in the mucosa.
Case 2
In 2013, a 58-year-old woman underwent a right BC modified radical mastectomy. Pathological examination revealed infiltrated ductal cancer with ipsilateral nodal involvement, which was ER positive, PgR positive, and HER2 negative. She received adjuvant chemotherapy and radiotherapy. In April 2017, she returned to our hospital with complaints of a persistent stomach burning sensation, sour regurgitation, a change in bowel habits and weight loss. Enhanced abdominal CT revealed a marked increase in the thickness of the sigmoid colon wall, and serum tumor biomarker tests showed elevated levels of carcinoembryonic antigen (CEA), CA19-9, and CA125. Positron emission tomography-computed tomography showed metastases in sigmoid colon and lymph nodes surrounded. IHC findings of the colonoscopy samples indicated that the tumor cells were positive for cytokeratin (CK) 7 and gross cystic disease fluid protein-15 (GCDFP-15) and negative for ER, PgR, and CK20. She was diagnosed with sigmoid colon metastasis from breast cancer based on historical examinations and IHC findings (Figure 1C, 1F). Because of her poor performance status, she did not receive any oncotherapy and died soon after hospital discharge.
The incidence of GI metastasis from BC has been reported to range from 0.07% to 18%, while 8%–35% of patients with BC were found to have GI metastasis in the autopsy series (1,2). Most of the GI metastases were metachronous and occurred 0–300 months after the primary BC surgery (1).
The lack of specific and serious symptoms of GI metastases often leads to delayed diagnosis and treatment. Most patients with BC with suspected GI metastasis could be confirmed by endoscopic biopsies and IHC analysis, which are currently the only reliable methods to distinguish the metastatic GI tumor from the primary GI tumor (1,3). Metastatic BC is usually positive for ER, PgR, CK7, GCDFP-15, and CEA and negative for CK20 (2). Nearly half of the patients’ hormone receptors (HR) and HER2 status can change during chemotherapy, which makes it essential to obtain IHC results of GI metastasis. The alterations of such receptors may help to determine appropriate treatments and prognosis (4,5). In the second case, a transition in HR status was detected when comparing the IHC results of the primary site with those of the metastatic GI site (from HR positive to HR negative). Concordant HER2 status is significantly related to longer overall survival (OS), independent of whether the patients are treated with trastuzumab (5). Thus, monitoring the treatment of metastatic BC involves a series of assessments, and the need for clinicians to integrate different forms of information to make a determination of the effectiveness of the treatment and its toxicity.
Most patients with BC with GI metastasis receive systemic treatment, and clinicians must take the presenting symptoms, age, performance status, HR status and previous systemic treatments into consideration (1). Patients with metastatic BC usually develop many localized problems that may benefit from local irradiation, surgery, or regional chemotherapy. Patients with BC who respond to a hormonal therapy maneuver, with either shrinkage of the tumor or long-term disease stabilization, should receive another hormonal therapy upon disease progression (5). As seen in the first case, the woman became resistant to hormonal therapy and chemotherapy was restarted.
The median OS after the diagnosis of GI metastasis from BC may range from 3 to 41 months, which is clearly shorter than that of patients with BC with other metastases (1). The survival rate for HR matching cases is higher than that for nonmatching cases. These changes in subtypes are important because each subtype has a different prognosis and unambiguous treatment response to hormonal therapy and chemotherapy (5).
In conclusion, the occurrence of GI metastasis from BC is a rare but possible event. Endoscopic examination, re-biopsy, and IHC staining of the suspected GI metastasis are essential for accurate diagnosis and determining the appropriate treatment, the main component of which is systemic chemotherapy in most cases. Patients with BC with confirmed GI metastasis are associated with poor survival. Furthermore, alterations in HR/HER2 status may be a risk factor for OS.
Footnotes
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - C.H.Z., X.F.F., Y.Y.; Design - C.H.Z., X.F.F.; Supervision - X.F.F., Y.Y.; Materials - L.Z.Z., J.L.T., D.L.; Data Collection and/or Processing - L.Z.Z., J.L.T., D.L.; Analysis and/or Interpretation - D.L.; Literature Search - L.Z.Z., J.LT.; Writing Manuscript - C.H.Z., X.F.F, D.L.; Critical Review - Y.Y.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.McLemore EC, Pockaj BA, Reynolds C, et al. Breast cancer: presentation and intervention in women with gastrointestinal metastasis and carcinomatosis. Ann Surg Oncol. 2005;12:886–9. doi: 10.1245/ASO.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 2.Koike K, Kitahara K, Higaki M, et al. Clinicopathological features of gastric metastasis from breast cancer in three cases. Breast Cancer. 2014;21:629–34. doi: 10.1007/s12282-011-0284-3. [DOI] [PubMed] [Google Scholar]
- 3.Taal BG, Peterse H, Boot H. Clinical presentation, endoscopic features, and treatment of gastric metastases from breast carcinoma. Cancer. 2000;89:2214–21. [PubMed] [Google Scholar]
- 4.Carlos Villa Guzmán J, Espinosa J, Cervera R, Delgado M, Patón R, Cordero García JM. Gastric and colon metastasis from breast cancer: case report, review of the literature, and possible underlying mechanisms. Breast Cancer. 2017;9:1–7. doi: 10.2147/BCTT.S79506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liedtke C, Broglio K, Moulder S, et al. Prognostic impact of discordance between triple-receptor measurements in primary and recurrent breast cancer. Ann Oncol. 2009;20:1953–8. doi: 10.1093/annonc/mdp263. [DOI] [PMC free article] [PubMed] [Google Scholar]