Abstract
Rationale: Less invasive, nonsurgical approaches are needed to treat severe emphysema.
Objectives: To evaluate the effectiveness and safety of the Spiration Valve System (SVS) versus optimal medical management.
Methods: In this multicenter, open-label, randomized, controlled trial, subjects aged 40 years or older with severe, heterogeneous emphysema were randomized 2:1 to SVS with medical management (treatment) or medical management alone (control).
Measurements and Main Results: The primary efficacy outcome was the difference in mean FEV1 from baseline to 6 months. Secondary effectiveness outcomes included: difference in FEV1 responder rates, target lobe volume reduction, hyperinflation, health status, dyspnea, and exercise capacity. The primary safety outcome was the incidence of composite thoracic serious adverse events. All analyses were conducted by determining the 95% Bayesian credible intervals (BCIs) for the difference between treatment and control arms. Between October 2013 and May 2017, 172 participants (53.5% male; mean age, 67.4 yr) were randomized to treatment (n = 113) or control (n = 59). Mean FEV1 showed statistically significant improvements between the treatment and control groups—between-group difference at 6 and 12 months, respectively, of 0.101 L (95% BCI, 0.060–0.141) and 0.099 L (95% BCI, 0.048–0.151). At 6 months, the treatment group had statistically significant improvements in all secondary endpoints except 6-minute-walk distance. Composite thoracic serious adverse event incidence through 6 months was greater in the treatment group (31.0% vs. 11.9%), primarily due to a 12.4% incidence of serious pneumothorax.
Conclusions: In patients with severe heterogeneous emphysema, the SVS shows significant improvement in multiple efficacy outcomes, with an acceptable safety profile.
Clinical trial registered with www.clinicaltrials.gov (NCT01812447).
Keywords: chronic obstructive pulmonary disease, FEV, quality of life
At a Glance Commentary
Scientific Knowledge on the Subject
Although no medical therapy provides relief from the progressive disability of severe emphysema, improved lung function and survival has been seen with lung volume reduction surgery. However, eligibility for lung volume reduction surgery is contingent upon the patient’s overall health status and pattern of emphysema and is only offered at a limited number of centers. Thus, there is substantial need for less invasive treatment options for severe emphysema.
What This Study Adds to the Field
The Spiration Valve System (SVS) consists of a one-way valve that blocks inspired airflow to distal portions of the lung affected by disease. Treatment of severe heterogeneous emphysema with the SVS in medically optimized participants achieved significant improvements in FEV1, hyperinflation, target lobe volume, dyspnea, and quality of life measures compared with optimal medical management alone. SVS offers clinically relevant benefits to severely ill patients with emphysema. The current study refined objective methods for using quantitative computed tomography as a tool to assess target lobe emphysema characteristics and determine eligibility for bronchoscopic lung volume reduction therapy.
Chronic obstructive pulmonary disease (COPD) affects an estimated 16 million U.S. residents (1) and is the fourth leading cause of death in the United States (2). Emphysema alone affects 4.7 million U.S. residents and is associated with progressive physical activity limitations, dyspnea, and reduced quality of life (QoL) (3, 4).
Pharmacologic COPD treatments have limited benefit (5). Inhaled therapies reduce annual decline in FEV1 more than placebo; however, observed declines have not been clinically relevant. Other guideline-recommended treatments include pulmonary rehabilitation (PR) and continuous oxygen therapy (6), but no medical therapy provides relief from the progressive disability of severe emphysema (5).
The National Emphysema Treatment Trial showed that lung volume reduction surgery (LVRS) improved survival compared with medical treatment in participants with upper-lobe emphysema and low exercise capacity, and also improved health status, dyspnea, exercise capacity, and lung function (7). Although effective, most qualifying individuals (80%) are ineligible for LVRS, primarily due to the potential morbidity associated with surgery and the pattern of emphysema and severity of lung function (8, 9). Thus, there is a substantial need for less invasive treatment options for severe emphysema.
The Spiration Valve System (SVS; formerly known as the intrabronchial valve) consists of a one-way valve that blocks inspired airflow using a flexible umbrella design. This allows for bronchoscopic placement in selected airway regions and limits airflow to distal portions of the lung affected by emphysema. The SVS has been evaluated in prior clinical studies using a bilateral, partial occlusion treatment methodology (10, 11), which proved ineffective. However, Eberhardt and colleagues (12) along with other subsequent studies (13, 14) showed that unilobar total occlusion may provide similar physiologic and clinical benefits to LVRS, including reduced hyperinflation, leading to improved lung function and clinical status, in a minimally invasive and potentially reversible manner (15).
Previous studies using endobronchial valves to treat patients with hyperinflated emphysema have reported that absence of collateral ventilation is pivotal in achieving lobar atelectasis, the overall treatment goal of this therapy (13, 16, 17). Collateral ventilation can be assessed using a balloon-tipped catheter placed bronchoscopically to measure flow and pressure distally in the targeted lobe (18). Alternatively, structural integrity of the fissure(s) adjacent to the targeted lobe can be assessed by quantitative high-resolution computed tomography (HRCT), which also acts as a marker for collateral ventilation and aids in patient and lobe selection (19). The EMPROVE trial represents the largest multicenter study using HRCT analysis of fissure integrity for patient selection and targeted lobar treatment.
The results of the current research have been published in the form of two abstracts presented at the American Thoracic Society (20) and the European Respiratory Society (21) meetings in 2018.
Methods
The EMPROVE study was a prospective, open-label, randomized, controlled, multicenter trial to assess the safety and efficacy of the SVS procedure in participants with severe heterogeneous emphysema.
Participant Population
Up to 220 participants were to be randomized from 41 investigational sites (Appendix E2 in the online supplement), with the potential for the study to be stopped early for success or futility. Institutional review boards at each site approved the study, and all participants provided written informed consent (Appendix E1). Eligible participants were 40 years of age or older, met American Thoracic Society/European Respiratory Society Guidelines criteria for management of stable COPD, and were able to perform a 6-minute-walk test (6MWT) ≥140 m. Disease severity was assessed by HRCT. Participants were required to have ≥40% emphysema destruction in the target lobe (assessed at −920 Hounsfield units) and a ≥10% disease emphysema severity difference with the ipsilateral lobe. The target and ipsilateral lobes were required to be separated by an intact fissure, estimated visually to be ≥90% complete with no segmental vessels crossing between adjacent lobes (as assessed by the CT corelab; MedQIA). Eligible participants had severe dyspnea (Modified Medical Research Council scale [mMRC] ≥2); severe obstructive disease FEV1 ≤45% of predicted, after bronchodilators; and hyperinflation defined as TLC ≥100% and residual volume (RV) ≥150% of predicted. Participants agreed to attend required follow-up visits and maintain consistent nutrition and exercise habits during the study period (Appendix E3).
All subjects who had not completed a PR program in the prior 2 years were screened to determine if they should complete a PR program before entering the trial (Appendix E4). Baseline testing included pulmonary function, CT, and QoL assessments (Appendix E5). Randomization occurred within the electronic data capture system at a preprocedure visit (2:1 randomization to treatment or control group). Patients in both the treatment and control groups received optimal medical management throughout the study; the treatment group additionally received bronchoscopic SVS placement.
Procedure
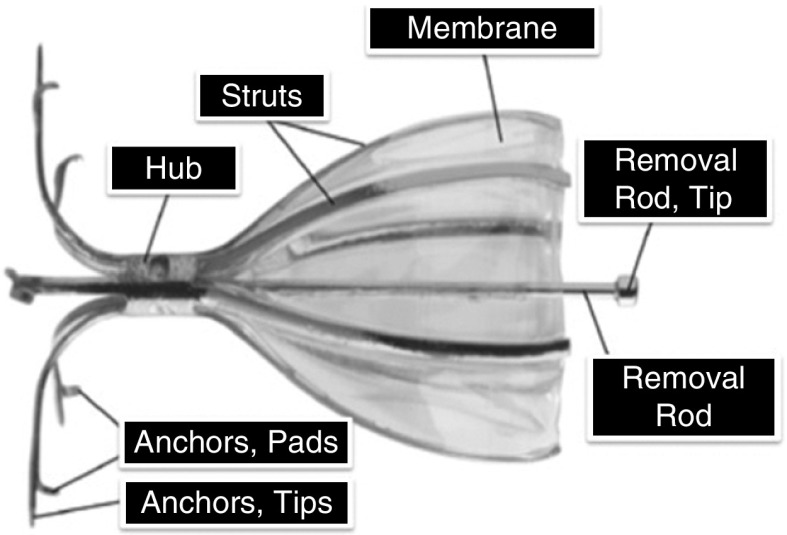
The SVS valve is designed for placement in selected regions of bronchial airways using a flexible bronchoscope, deployment catheter, and accompanying loader. The valve has a flexible umbrella that blocks inspired airflow to distal portions of lungs affected by disease, while allowing air and mucus to clear proximally from treated airways. Valves are removable using a flexible bronchoscope and forceps, if necessary. The valve comprises a frame made of a super-elastic, biocompatible alloy (Nitinol) and a polyurethane membrane (Figure 1). The membrane is held against the airway mucosa by six flexible struts, which expand and contract with airway movement during breathing. The valve is secured in position with five anchors and tips that gently penetrate the airway wall to a controlled depth.
Figure 1.
Key components of the Spiration Valve.
An airway sizing system and calibrated balloon was used to determine the appropriate valve width size (5, 6, 7, and 9 mm [the 9-mm valve was introduced after the initial 29 subjects had been randomized in the study]) to treat target lobe airways ranging from 4.75 to 8.75 mm. The treatment algorithm called for the complete occlusion of one lobe; this was achieved by using one or more SVS valves to occlude all segments (i.e., lobar, segmental, and/or subsegmental airways). HRCT imaging and, if necessary, lung perfusion was used to select treatment lobes. Either upper or lower lobes could be targeted for treatment; the right middle lobe was not treated in this study. When two lobes both met criteria for emphysema and heterogeneity, the lobe with the lowest perfusion was treated. To limit subsequent adverse events, physicians were asked to follow a checklist to limit procedure duration. Treated patients remained in the hospital for at least 1 day. The total duration of post-procedural hospitalization was at the discretion of the local investigator and within the norms of clinical practice at the local center.
Outcome Measures
Follow-up and outcome assessments were scheduled for 2 weeks, 1, 3, and 6 months, and annually through 2 or 5 years for the control and treatment groups, respectively. The primary effectiveness endpoint was mean change in FEV1 post-bronchodilator from baseline to 6 months between treatment and control groups; 12-month results are also reported. Secondary effectiveness endpoints were FEV1 difference between responders, defined as a 15% or greater improvement; target lobe volume (TLV) reduction, only assessed in the SVS treatment group, measured by quantitative CT (QCT); hyperinflation, measured by the ratio of RV to TLC; health status and QoL, measured by St. George’s Respiratory Questionnaire (SGRQ); dyspnea, measured by mMRC; and exercise capacity, measured by 6MWT. HRCT, plethysmography, and exercise assessments only occurred between baseline and 6 months; therefore, TLV, hyperinflation, and 6MWT data were not assessed at 12 months.
The primary safety endpoint was the incidence of prespecified composite thoracic serious adverse events (SAEs; Appendix E6) through 6 months; secondary safety endpoints were the rate of each category of thoracic SAEs and thoracic SAE rate per patient-year.
Statistical Analysis
Analyses of SVS effectiveness and durability were conducted at 6 and 12 months, respectively. Using a Bayesian adaptive design (22, 23), two interim analyses of sample size adequacy were conducted when 100 and 160 participants were enrolled, at which time the predictive probability of eventual success was calculated. Based on these analyses, enrollment could be stopped early for futility or probable eventual success, whereas follow-up continued until the last subject reached 6 months. The maximum possible sample size was 220 (Appendix E7). Subjects with missing data were included in the analysis via Bayesian multiple imputation. The primary effectiveness objective (superiority of SVS based on FEV1 change from baseline to 6 mo) was considered statistically significant if the posterior probability (PP) exceeded 0.982, a prespecified threshold value chosen to control type I error rate (under simulation) of 0.025 or less.
Primary and secondary safety analyses were conducted by determining the 95% Bayesian credible intervals (BCIs) for the difference and ratio of composite SAE probabilities, as well as each individual thoracic SAE category, in the treatment and control groups (Appendix E8). Secondary effectiveness endpoints were computed as the difference between treatment and control groups at 6 and 12 months compared with baseline. Statistical analysis was conducted in the R statistical language (version 3.4.0; R Foundation for Statistical Computing).
Results
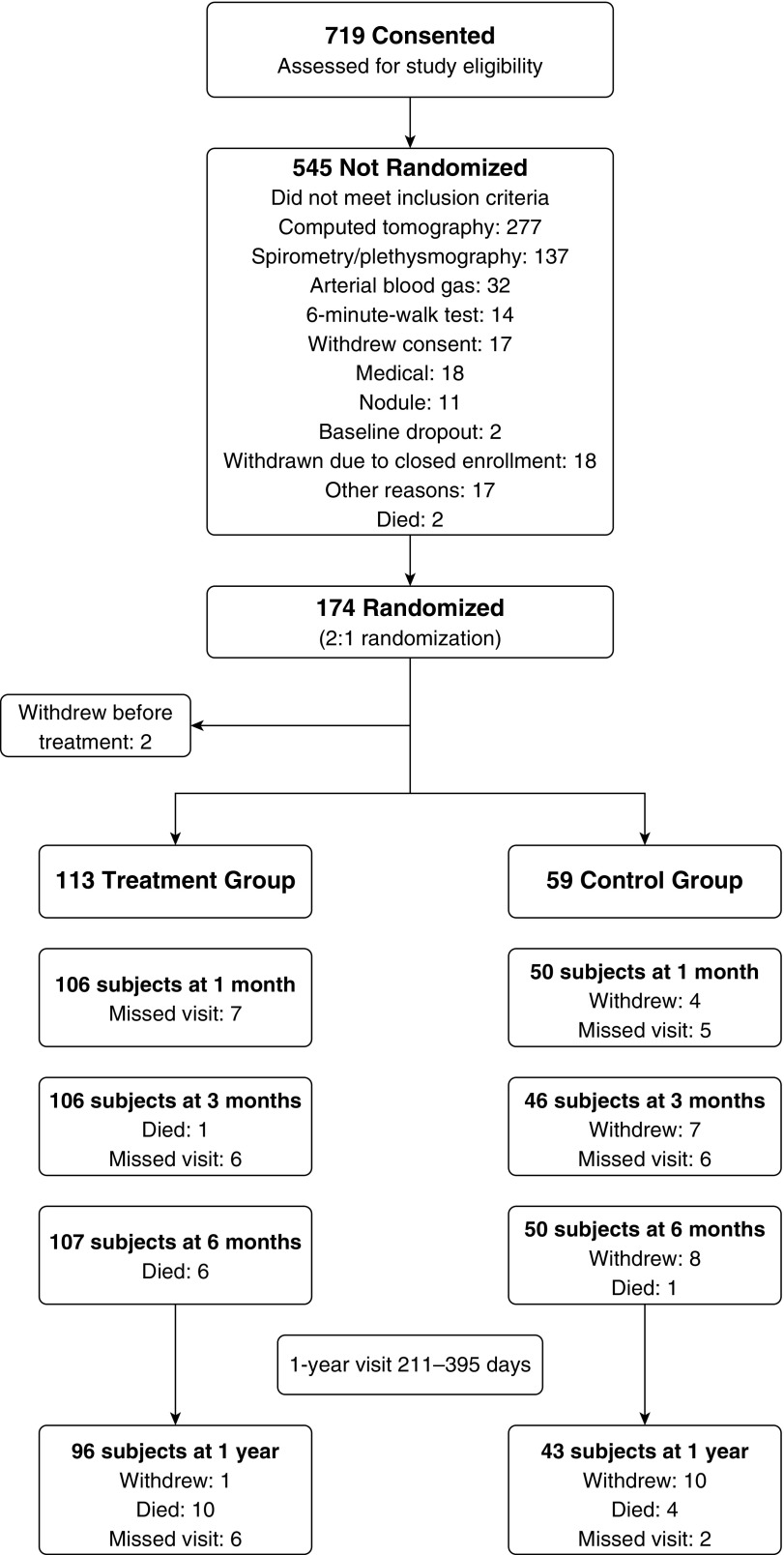
The trial was conducted from October 8, 2013 to May 3, 2017 at 41 clinical sites (Appendix E1) with 172 participants ultimately randomized to treatment (n = 113, 65.7%) and control (n = 59, 34.3%) groups at 31 clinical sites (Figure 2). Enrollment was stopped when the predictive probability of success with the existing cohort was greater than 0.999. By 6 months in the treatment group, 6 subjects had died and 107 had an evaluable visit. By 12 months, 96 subjects had evaluable visits. In the control group (n = 59), 8 participants withdrew and 1 died, leaving 50 evaluable subjects at 6 months. By 12 months, 43 subjects had an evaluable visit.
Figure 2.
Study subject disposition flow chart.
Treatment and control group participants had similar baseline characteristics. Demographic data, use of pulmonary medications and supplemental oxygen, medical history, lung function, arterial blood gas results, exercise tolerance, SGRQ, mMRC dyspnea scores, and HRCT characteristics were all comparable (Table 1 and Appendix E9, Table E2). The only demographic difference was sex; the control group had approximately 15% more males.
Table 1.
Subject Demographics and Baseline Characteristics
| Treatment Group (n = 113) | Control Group (n = 59) | ||||
|---|---|---|---|---|---|
| Characteristics | n | Mean ± SD or n (%) | n | Mean ± SD or n (%) | Difference (T − C; 95% BCI) |
| Sex, M | 113 | 54 (47.8) | 59 | 38 (64.4) | −30.9% to −0.8% |
| Age, yr | 113 | 66.7 ± 6.6 | 59 | 68.1 ± 6.4 | −3.4 to 0.7 |
| BMI, kg/m2 | 113 | 25.3 ± 4.3 | 59 | 24.6 ± 5.2 | −0.8 to 2.3 |
| FEV1, L | 113 | 0.825 ± 0.264 | 59 | 0.792 ± 0.260 | −0.051 to 0.116 |
| FEV1% predicted | 113 | 30.8 ± 8.1 | 59 | 28.5 ± 8.5 | −0.4 to 5.0 |
| FVC, L | 113 | 2.492 ± 0.754 | 59 | 2.633 ± 0.757 | −0.384 to 0.101 |
| FVC% predicted | 113 | 70.2 ± 16.5 | 59 | 70.5 ± 16.7 | −5.6 to 5.0 |
| TLC, L | 113 | 7.215 ± 1.530 | 59 | 7.649 ± 1.431 | −0.904 to 0.035 |
| TLC% predicted | 113 | 126.5 ± 14.5 | 59 | 128.2 ± 17.0 | −6.9 to 3.5 |
| RV, L | 113 | 4.573 ± 1.253 | 59 | 4.848 ± 1.199 | −0.665 to 0.115 |
| RV% predicted | 113 | 207.5 ± 45.0 | 59 | 213.4 ± 49.3 | −21.3 to 9.4 |
| RV/TLC ratio | 113 | 0.632 ± 0.080 | 59 | 0.632 ± 0.086 | −0.028 to 0.026 |
| Prescribed O2 | 113 | 59 | |||
| Proportion | 51 (45.1) | 27 (45.8) | −15.7 to 14.9 | ||
| L/min | 1.18 ± 1.43 | 1.16 ± 1.47 | −0.45 to 0.49 | ||
| Po2, mm Hg | 112 | 67.9 ± 10.2 | 59 | 68.0 ± 11.6 | −3.6 to 3.5 |
| Pco2, mm Hg | 112 | 40.2 ± 5.7 | 59 | 40.9 ± 6.0 | −2.7 to 1.1 |
| Pulmonary rehabilitation | 113 | 59 | |||
| Before enrollment | 113 (100) | 59 (100) | −11.8 to 13.4 | ||
| During follow-up period | 39 (34.5) | 18 (30.5) | |||
| 6MWT, m | 113 | 303.5 ± 84.6 | 59 | 306.9 ± 104.2 | −34.8 to 28.0 |
| Dyspnea, mMRC | 113 | 2.7 ± 0.7 | 59 | 2.7 ± 0.6 | −0.2 to 0.2 |
| COPD assessment test | 113 | 21.8 ± 6.8 | 59 | 20.0 ± 6.3 | −0.3 to 3.9 |
| SGRQ total | 113 | 57.2 ± 14.8 | 59 | 54.6 ± 13.6 | −1.9 to 7.1 |
| TLV, L | 113 | 1.843 ± 0.602 | 59 | 1.820 ± 0.456 | −0.140 to 0.187 |
| Target lobe | 113 | 59 | |||
| Left lower | 27 (23.9) | 9 (15.3) | −4.2% to 19.5% | ||
| Left upper | 66 (58.4) | 37 (62.7) | −17.8% to 12.0% | ||
| Right lower | 7 (6.2) | 7 (11.9) | −15.9% to 2.8% | ||
| Right upper | 13 (11.5) | 6 (10.2) | −9.4% to 10.1% | ||
| Emphysema severity, % | 113 | 63.6 ± 10.1 | 59 | 61.6 ± 11.6 | −1.6 to 5.5 |
| Emphysema heterogeneity, % | 113 | 25.3 ± 12.0 | 59 | 23.3 ± 11.6 | −1.8 to 5.8 |
Definition of abbreviations: 6MWT = 6-minute-walk test; BCI = Bayesian credible interval; BMI = body mass index; C = control; COPD = chronic obstructive pulmonary disease; mMRC = Modified Medical Research Council; O2 = oxygen; RV = residual volume; SGRQ = St. George’s Respiratory Questionnaire; T = treatment; TLV = target lobe volume.
Mean procedure duration, defined as the time between bronchoscope insertion and removal, was 24.3 minutes (range, 9–73 min). The mean and median duration of hospitalization was 3.83 days and 1 day, respectively (Appendix E10, Table E3). Target lobes, defined by preprocedural imaging, were primarily on the left side (82.3%), with 58.4% being the left upper lobe (Appendix E10, Table E4). QCT was used for target lobe selection in 97.4% of cases. In the remaining three cases, where two potential target lobes were identified by QCT, perfusion scan results were used, and final determination of the target lobe was by the CT corelab. A total of 476 valves were placed in 113 treatment group participants (mean number per participant, 3.83 ± 1.48; Appendix E10, Table E5).
Efficacy Outcomes
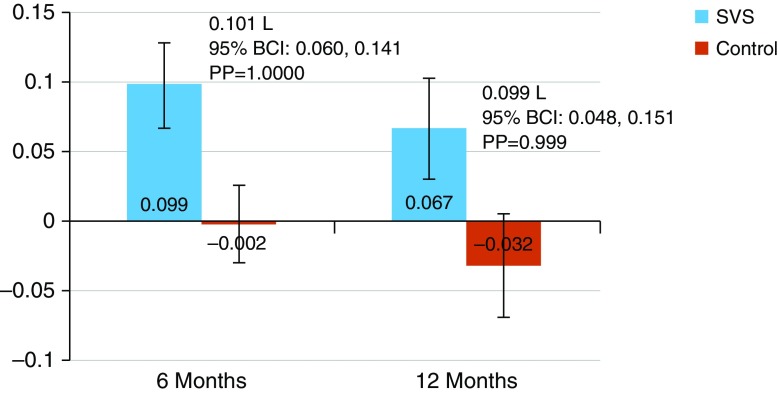
The SVS treatment group had significant FEV1 improvements (Figure 3). At 6 months, the treatment group improved by 0.099 L on average from baseline (95% BCI, 0.069–0.128), whereas the control group changed by −0.002 L (95% BCI, −0.030 to 0.026), for a between-group difference of 0.101 L (95% BCI, 0.060–0.141). At 12 months, the treatment group improved by 0.067 L on average (95% BCI, 0.031 to 0.103), whereas the control group decreased by −0.032 L (95% BCI, −0.069 to 0.005), for a between-group difference of 0.099 L (95% BCI, 0.048–0.151). (Appendix E11, Table E6).
Figure 3.
Change in FEV1 at 6 and 12 months; data are shown as mean and 95% Bayesian credible interval (BCI). PP = posterior probability; SVS = Spiration Valve System.
Secondary effectiveness outcomes were also improved in the SVS-treated group. At 6 and 12 months, the between-group difference in FEV1 responder rates (improvement, ≥15%) was estimated at 25.7% (95% BCI, 12.5%–37.5%; 0.9998 PP) and 30.4% (95% BCI, 16.8%–42.5%; 0.9999 PP), respectively, in favor of SVS (Table 2 and Appendix E11, Tables E7–E9).
Table 2.
Responder Rates for All Effectiveness Outcomes
| Outcome Measure Responder Rates | Treatment Group [n/N (%)] | Control Group [n/N (%)] |
|---|---|---|
| FEV1 (≥15% improvement) | ||
| 6 mo | 39/106 (36.8) | 5/50 (10.0) |
| 12 mo | 32/86 (37.2) | 2/39 (5.1) |
| TLV (≥350 ml reduction) | ||
| 6 mo | 76/102 (74.5) | NA |
| RV (≥310 ml reduction) | ||
| 6 mo | 53/105 (50.5) | 16/50 (32.0) |
| SGRQ (≥4 point reduction) | ||
| 6 mo | 57/105 (54.3) | 9/50 (18.0) |
| 12 mo | 48/95 (50.5) | 9/41 (22.0) |
| mMRC (≥1 point reduction) | ||
| 6 mo | 57/107 (53.3) | 9/50 (18.0) |
| 12 mo | 46/94 (48.9) | 3/41 (7.3) |
| 6MWT (≥25 m improvement) | ||
| 6 mo | 33/102 (32.4) | 11/48 (22.9) |
Definition of abbreviations: 6MWT = 6-minute-walk test; mMRC = Modified Medical Research Council; NA = not applicable; RV = residual volume; SGRQ = St. George’s Respiratory Questionnaire; TLV = target lobe volume.
At 6 months, treatment group participants had a significant reduction in TLV as measured by QCT (−0.974 L [95% BCI, −1.119 to −0.829]), with a 1.0000 PP for mean change less than 0 (Table 3 and Appendix E11, Table E10). Using a 350-ml reduction in TLV as a threshold, 75% of the SVS-treated group achieved a clinically meaningful improvement, with 40% of the entire treatment cohort achieving complete atelectasis of the target lobe.
Table 3.
Secondary Effectiveness Outcomes
| Outcome Measure Described as Change from Baseline | Treatment Group [Mean ± SD (N)] | Control Group [Mean ± SD (N)] | Difference between Groups (95% BCI) | Posterior Probability of Superiority |
|---|---|---|---|---|
| TLV, L | ||||
| 6 mo | −0.974 ± 0.74 (102) | NA | −0.974 (−1.12 to −0.83)* | 1.0000 |
| RV, L | ||||
| 6 mo | −0.402 ± 0.85 (105) | −0.042 ± 0.58 (50) | −0.361 (−0.59 to −0.13) | 0.9990 |
| RV/TLC | ||||
| 6 mo | −0.035 ± 0.08 (105) | 0.005 ± 0.04 (50) | −0.039 (−0.06 to −0.02) | 1.0000 |
| SGRQ | ||||
| 6 mo | −8.1 ± 17.1 (105) | 4.8 ± 10.6 (50) | −13.0 (−17.4 to −8.5) | 1.0000 |
| 12 mo | −5.8 ± 16.8 (95) | 3.7 ± 10.9 (41) | −9.5 (−14.4 to −4.7) | 1.0000 |
| mMRC | ||||
| 6 mo | −0.6 ± 1.0 (107) | −0.0 ± 0.6 (50) | −0.6 (−0.9 to −0.3) | 1.0000 |
| 12 mo | −0.6 ± 1.1 (94) | 0.2 ± 0.6 (41) | −0.9 (−1.2 to −0.6) | 1.0000 |
| 6MWT, m | ||||
| 6 mo | −4.4 ± 76.7 (102) | −11.3 ± 51.4 (48) | 6.9 (−14.2 to 28.2) | 0.7438 |
Definition of abbreviations: 6MWT = 6-minute-walk test; BCI = Bayesian credible interval; mMRC = modified Medical Research Council; NA = not applicable; RV = residual volume; SGRQ = St. George’s Respiratory Questionnaire; TLV = target lobe volume.
Prespecified hierarchy of testing: TLV, hyperinflation (RV/TLC), SGRQ, dyspnea (mMRC), 6MWT, all at 6 months.
Compared with baseline.
The SVS treatment group also had significantly greater mean RV/TLC improvement. The between-group difference at 6 months was −0.039 (95% BCI, −0.058 to −0.020; 1.0000 PP) in favor of SVS (Table 3 and Appendix E11, Table E11a).
There was significantly greater mean improvement in SGRQ (health status) for SVS treatment versus control groups at 6 months, with a between-group difference of −13.0 points (95% BCI, −17.4 to −8.5; 1.0000 PP). Results at 12 months were −9.5 points (95% BCI, −14.4 to −4.7; 1.0000 PP) (Table 3 and Appendix E11, Table E12).
Dyspnea, as measured by mMRC, was significantly improved with SVS treatment, with a between-group difference of −0.6 (95% BCI, −0.9 to −0.3; 1.0000 PP) at 6 months and −0.9 (95% BCI, −1.2 to −0.6; 1.0000 PP) at 12 months (Table 3 and Appendix E11, Table E13).
Although not a secondary endpoint of the study, the COPD assessment test scores were improved by 4.3 points at 6 months and 5.3 points at 12 months in the treatment group compared with the control group, and were statistically significant at both time points (Appendix E11, Table E16).
Change in exercise capacity, measured by 6MWT, was not statistically significant at 6 months, with a between-group difference of 6.9 m (95% BCI, −14.2 to 28.2; 0.7438 PP) (Table 3 and Appendix E11, Table E14).
Table 2 provides responder rates for all secondary efficacy outcomes.
Safety Outcomes
Short term (0–6 mo)
At 6 months, the incidence of composite thoracic SAEs was 31.0% in the treatment group and 11.9% in the control group for a statistically significant between-group difference of 19.1% (95% BCI, 5.9–29.7). The higher treatment group incidence was primarily due to a 12.4% (95% BCI, 4.6–18.6) increased incidence of serious pneumothorax (Appendix E12, Tables E17 and E18), which was statistically significant. Over this time, 32 monitored events of pneumothorax were reported, with 18 protocol-defined (Appendix E6) serious incidents in 16 (14.2%) of 113 treatment group participants, and 14 nonserious pneumothorax events in 13 (11.5%) treatment group participants. The majority (66%) of these pneumothorax events occurred within 3 days of the procedure, within the average hospital stay duration (Appendix E12, Figure E1). Of the 16 subjects with serious pneumothorax events, 11 (69%) had one or more valves removed per the defined pneumothorax management protocol (Appendix E5). Five (5) of these subjects had valves reimplanted upon cessation of the pneumothorax, and this subset showed a TLV reduction of −834.0 ml compared with only −19.2 ml in those that did not have valves replaced. There were no other statistically significant between-group differences in thoracic SAEs by category.
There were six (5.3%) deaths in the treatment group and one (1.7%) death in the control group (Appendix E12, Table E19). This difference between groups was not statistically significant. Only one death (occurring at Day 95 after SVS procedure) was adjudicated by the study clinical events committee as possibly related to the device due to pneumothorax in the contralateral untreated lobe, which did not resolve before death (Table E20).
There were no statistically significant between-group differences for non-horacic SAEs, with 11.5% and 3.4% nonthoracic SAEs in the treatment and control groups, respectively (Appendix E12, Table E19).
Long term (6–12 mo)
Between 6 and 12 months, the incidence of composite thoracic SAEs was 21.4% in the treatment group versus 10.6% in the control group (Appendix E12, Table E17), with a between-group difference of 10.7% (95% BCI, −3.0 to 21.2), which was not statistically significant. There were no statistically significant between-group differences in thoracic SAEs by category. There were 3 nonserious events of pneumothorax in 2 of 113 (1.7%) treatment subjects, and no additional serious pneumothorax events (Appendix E12, Figure E1). Three SAEs were adjudicated as device related (one case of infection, one of pneumonia, and one death). There were four (3.9%) deaths in the treatment group (one of which was device related) and three (6.4%) in the control group (Appendix E12, Tables E17 and S19; death details in Table E21). There were no unanticipated device-related SAEs or migration, erosion, or expectoration reported through 12 months of follow-up.
There were no statistically significant between-group differences for nonthoracic SAEs, with rates of 12.6% and 12.8% in the treatment and control groups, respectively (Appendix E12, Table E19).
Discussion
The EMPROVE trial evaluated the safety and efficacy of the SVS compared with optimal medical management in patients with severe heterogeneous emphysema. Although prior SVS trials using bilateral, partial occlusion of the target lobe did not show consistent improvement (10, 11), the results of the EMPROVE trial, with single-lobe, total lobar occlusion, shows marked benefits. At 6 months, the primary outcome and a majority of secondary outcome measures were improved in the SVS treatment group compared with the control group. There was a significant between-group increase in mean FEV1 from baseline (0.101 L) and a 25.7% between-group difference in FEV1 responder rates (defined as improvement of ≥15%). These results persisted at 12 months. The SVS treatment group also saw significant reductions in TLV, hyperinflation, and dyspnea. Improved health status and QoL was observed as an 8.1-point mean reduction in the SGRQ, which exceeds the 4-point minimum score change defined as clinically relevant (24). These efficacy results are very comparable to other randomized clinical trials using one-way valves in a unilateral lobar treatment paradigm (13, 14, 16, 25).
Although the SVS treatment group performed better on the 6MWT than the control group (between-group difference, 6.9 m), this difference was not statistically significant. In contrast, patients who underwent endobronchial valve treatment in the recent LIBERATE (Lung Function Improvement after Bronchoscopic Lung Volume Reduction with Pulmonx Endobronchial Valves Used in Treatment of Emphysema) trial performed significantly better on 6MWT than control subjects. This improvement is not surprising, as LIBERATE patients were required to maintain a supervised PR program throughout study follow-up (25), and PR has been shown to improve exercise capacity in patients with COPD (26). The EMPROVE study was designed with the understanding that only ∼40% of patients with COPD actually adhere to a PR program due to problems with access and prohibitive cost (27). As such, EMPROVE subjects were required to have been in a PR program in the 2 years before study enrollment (Appendix E4), but were not mandated to maintain a supervised PR program throughout the study follow-up, with only 34.5% and 32.7% of EMPROVE treatment and control subjects, respectively, maintaining a PR regimen through the 12-month follow-up. Thus, the difference between the two trials highlights the importance of additional exercise training by way of PR in translating improved lung function into enhanced exercise performance.
Mean procedure time in EMPROVE (24 min) was also shorter than that observed in the LIBERATE trial (34 min) (25). This is relevant because shorter procedure times have been associated with fewer procedure-related complications (11). In the EMPROVE study, post-SVS treatment risks were generally minor and tended to diminish over time. The primary safety outcome, incidence of composite thoracic SAEs, was greater in the treatment than in the control group (31.0% and 11.9%, respectively). However, pneumothorax was the only individual SAEs with significantly higher treatment group incidence, similar to comparable studies (13, 25). Early-onset pneumothorax in the treatment group likely resulted from lung conformation changes due to acute reduction in lung volume by valve therapy, triggering rapid ipsilateral nontargeted lobe expansion, a recognized indicator of successful target lobe occlusion (28). There was no statistically significant difference in mortality between the study groups at any time point. The 5.3% mortality rate in the treatment group is similar to the 3.1–5.0% documented in other randomized valve trials (11, 25, 29, 30, 31), and lower than the 7.9–12% documented in randomized LVRS trials (7). There were no unanticipated device-related SAEs.
The results of the EMPROVE trial also demonstrate that using HRCT analysis for fissure integrity ≥90% is a useful method to select patients for lack of collateral ventilation that are most likely to achieve targeted lobe atelectasis and improved clinical outcomes. The procedural time for SVS performance was less than other trials using physiological assessment for collateral ventilation, and avoids added procedural costs (32, 33). Moreover, in a broader clinical context, HRCT quantitative assessment of fissure integrity may be easier to implement.
Strengths and Limitations
Strengths of the EMPROVE trial include its use of an adaptive sample size, thus shortening overall enrollment time, and planned long-term follow-up: 5 and 2 years for the treatment and control groups, respectively. A key study limitation was the lack of TLV and hyperinflation assessments at 12 months, which would have provided mechanistic data to support improvements in functional and QoL parameters. In addition, the EMPROVE study, and other recent multicenter, randomized controlled trials, did not blind either subjects or assessors (16, 25, 34). Although this may introduce bias to the QoL assessments and the 6MWT, it is unlikely that measures such as lung function, TLV, and hyperinflation would be affected by this approach.
Conclusions
Treatment of severe heterogeneous emphysema with the SVS in medically optimized participants selected for fissure integrity ≥90% by quantitative HRCT achieved significant improvements in FEV1, hyperinflation, TLV, dyspnea, and QoL measures compared with optimal medical management alone. The SVS offers clinically relevant benefits for severely ill patients with emphysema and, although there are risks with the therapy, they are primarily manageable and tend to diminish over time.
The results of the EMPROVE trial and other randomized trials of valve therapy have led to the inclusion of endobronchial valve therapy as an important component of the clinical therapy recommendations for the underserved patient population with severe emphysema (35, 36).
Acknowledgments
Acknowledgment
The authors thank the EMPROVE trial steering committee, comprised of Douglas Wood, M.D. (Chair), Robert Wise, M.D., Felix Herth, M.D., Christopher Cooper, M.D., Paul Jones, M.D., Atul Mehta, M.D., Steve Springmeyer, M.D., Daniel Sterman, M.D., and Greg Sessler; Nawzer Mehta, Ph.D., and Douglas Sheffield, Ph.D., for overall study management; Michelle Tobin, Susan Anton, Tom Matthews, Gerald Guidry, and Eriika Etshokin for investigational center oversight and data monitoring; Lauri DeVore for procedure support and patient recruitment; Jacki Campbell, Victoria Simonnet, and Amanda Whitson for safety reporting and monitoring; Bill Sirokman and David Himes for data management; Andy Mugglin, Ph.D., for performing all statistical analyses; and Caitlin Rothermel, M.P.H., for medical writing and editorial assistance. Additional contributions: safety oversight of the study was provided by a Data and Safety Monitoring Board, comprised of John Beamis, M.D. (Chair), Greg Campbell, Ph.D. (statistician), Frank Detterbeck, M.D., Barry Make, M.D., and Jonathon Truwit, M.D. The Clinical Events Committee adjudicated adverse events and was comprised of Matthew Brenner, M.D. (Chair), Richard Helmers, M.D., Eric Vallières, M.D., and Roger Yusen, M.D. The study Medical Monitors were Robert Kruklitis, M.D., and Daniel Nader, M.D.
Footnotes
Supported by Spiration Inc. d.b.a. Olympus Respiratory America funding of the EMPROVE trial. Spiration Inc. d.b.a. Olympus Respiratory America helped with trial design and review and clarification of the methods of the manuscript.
Author Contributions: G.J.C. is the principal investigator of the study and collaborated on design of the study, advised on medical issues during the conduct of the study, actively recruited and treated patients in the study, participated in acquisition of data, analysis and interpretation of the data, and development of the manuscript. K.H., D.R.L., and R.C. are investigators in the study and actively recruited and treated patients in the study, participated in acquisition of data, helped with the interpretation of the data, and provided revisions to the manuscript. A.D., K.V., D.K.H., A.M., M.Z., S.B.B., R.C.H., A.W., K.C., M.J.R., P.R.B., M.A.-H., J.M.M., R.K., A.S., C.M.K., C.R.L., M.F.R., W.B.A., P.V.K., G.X.M., D.W.J., M.G.G., A.A.M., C.A.H., C.R., R.A.M., A.H.C., S.S.M., R.S.W., B.C., G.E.H., and S.M. are investigators in the study and actively recruited and treated patients in the study, participated in acquisition of data, and provided revisions to the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201902-0383OC on July 31, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: for the EMPROVE Study Group
References
- 1.Wheaton AG, Cunningham TJ, Ford ES, Croft JB Centers for Disease Control and Prevention (CDC) Employment and activity limitations among adults with chronic obstructive pulmonary disease: United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64:289–295. [PMC free article] [PubMed] [Google Scholar]
- 2.U.S. Department of Health and Human Services, National Institutes of Health and National Heart, Lung, and Blood Institute. COPD national action plan. Washington, D.C.: U.S. Department of Health and Human Services; 2017 [accessed 2018 Jul 6]. Available from: https://www.nhlbi.nih.gov/sites/default/files/media/docs/COPD National Action Plan 508_0.pdf.
- 3.van Agteren JE, Hnin K, Grosser D, Carson KV, Smith BJ. Bronchoscopic lung volume reduction procedures for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2017;2:CD012158. doi: 10.1002/14651858.CD012158.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaplan RM, Ries AL. Health-related quality of life in emphysema. Proc Am Thorac Soc. 2008;5:561–566. doi: 10.1513/pats.200706-080ET. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vogelmeier CF, Criner GJ, Martínez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Arch Bronconeumol. 2017;53:128–149. doi: 10.1016/j.arbres.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Qaseem A, Wilt TJ, Weinberger SE, Hanania NA, Criner G, van der Molen T, et al. American College of Physicians; American College of Chest Physicians; American Thoracic Society; European Respiratory Society. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179–191. doi: 10.7326/0003-4819-155-3-201108020-00008. [DOI] [PubMed] [Google Scholar]
- 7.Fishman A, Martinez F, Naunheim K, Piantadosi S, Wise R, Ries A, et al. National Emphysema Treatment Trial Research Group. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003;348:2059–2073. doi: 10.1056/NEJMoa030287. [DOI] [PubMed] [Google Scholar]
- 8.Cooper JD, Patterson GA, Sundaresan RS, Trulock EP, Yusen RD, Pohl MS, et al. Results of 150 consecutive bilateral lung volume reduction procedures in patients with severe emphysema. J Thorac Cardiovasc Surg. 1996;112:1319–1329. doi: 10.1016/S0022-5223(96)70147-2. [Discussion, pp. 1329–1330.] [DOI] [PubMed] [Google Scholar]
- 9.McKenna RJ, Jr, Brenner M, Fischel RJ, Singh N, Yoong B, Gelb AF, et al. Patient selection criteria for lung volume reduction surgery. J Thorac Cardiovasc Surg. 1997;114:957–964. doi: 10.1016/S0022-5223(97)70010-2. [Discussion, pp. 964–967.] [DOI] [PubMed] [Google Scholar]
- 10.Ninane V, Geltner C, Bezzi M, Foccoli P, Gottlieb J, Welte T, et al. Multicentre European study for the treatment of advanced emphysema with bronchial valves. Eur Respir J. 2012;39:1319–1325. doi: 10.1183/09031936.00019711. [DOI] [PubMed] [Google Scholar]
- 11.Elstad MR, Mehta AC, Nader D, Rai N, Mularski RA, Sterman DH, et al. Bronchial valve treatment of emphysema: procedure and device safety results from a double-blind randomized trial [abstract] Am J Respir Crit Care Med. 2012;185:A1112. [Google Scholar]
- 12.Eberhardt R, Gompelmann D, Schuhmann M, Reinhardt H, Ernst A, Heussel CP, et al. Complete unilateral vs partial bilateral endoscopic lung volume reduction in patients with bilateral lung emphysema. Chest. 2012;142:900–908. doi: 10.1378/chest.11-2886. [DOI] [PubMed] [Google Scholar]
- 13.Klooster K, ten Hacken NHT, Hartman JE, Kerstjens HAM, van Rikxoort EM, Slebos D-J. Endobronchial valves for emphysema without interlobar collateral ventilation. N Engl J Med. 2015;373:2325–2335. doi: 10.1056/NEJMoa1507807. [DOI] [PubMed] [Google Scholar]
- 14.Davey C, Zoumot Z, Jordan S, McNulty WH, Carr DH, Hind MD, et al. Bronchoscopic lung volume reduction with endobronchial valves for patients with heterogeneous emphysema and intact interlobar fissures (the BeLieVeR-HIFi study): a randomised controlled trial. Lancet. 2015;386:1066–1073. doi: 10.1016/S0140-6736(15)60001-0. [DOI] [PubMed] [Google Scholar]
- 15.Hopkinson NS. Endobronchial valves as a treatment for emphysema: moving out of the shadow of lung volume reduction surgery. Am J Respir Crit Care Med. 2016;194:1039–1040. doi: 10.1164/rccm.201609-1808ED. [DOI] [PubMed] [Google Scholar]
- 16.Kemp SV, Slebos DJ, Kirk A, Kornaszewska M, Carron K, Ek L, et al. TRANSFORM Study Team *. A multicenter randomized controlled trial of Zephyr endobronchial valve treatment in heterogeneous emphysema (TRANSFORM) Am J Respir Crit Care Med. 2017;196:1535–1543. doi: 10.1164/rccm.201707-1327OC. [DOI] [PubMed] [Google Scholar]
- 17.Liberator C, Shenoy K, Marchetti N, Criner G. The role of lobe selection on FEV1 response in endobronchial valve therapy. COPD. 2016;13:477–482. doi: 10.3109/15412555.2015.1115007. [DOI] [PubMed] [Google Scholar]
- 18.Gompelmann D, Eberhardt R, Michaud G, Ernst A, Herth FJ. Predicting atelectasis by assessment of collateral ventilation prior to endobronchial lung volume reduction: a feasibility study. Respiration. 2010;80:419–425. doi: 10.1159/000319441. [DOI] [PubMed] [Google Scholar]
- 19.Li S, Wang G, Wang C, Gao X, Jin F, Yang H, et al. The REACH trial: a randomized controlled trial assessing the safety and effectiveness of the Spiration® Valve System in the treatment of severe emphysema. Respiration. 2019;97:416–427. doi: 10.1159/000494327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Criner GJ, Delage A, Voelker KG for the EMPROVE Trial Investigator Group. The EMPROVE trial: a randomized, controlled multicenter clinical study to evaluate the safety and effectiveness of the Spiration® Valve System for single lobe treatment of severe emphysema [abstract] Am J Respir Crit Care Med. 2018;197:A7753. [Google Scholar]
- 21.Criner GJ, Delage A, Voelker K. Endobronchial valves for severe emphysema: 12-month results of the EMPROVE trial. Eur Respir J. 2018;52:OA4928. [Google Scholar]
- 22.Broglio KR, Connor JT, Berry SM. Not too big, not too small: a Goldilocks approach to sample size selection. J Biopharm Stat. 2014;24:685–705. doi: 10.1080/10543406.2014.888569. [DOI] [PubMed] [Google Scholar]
- 23.Saville BR, Connor JT, Ayers GD, Alvarez J. The utility of Bayesian predictive probabilities for interim monitoring of clinical trials. Clin Trials. 2014;11:485–493. doi: 10.1177/1740774514531352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation: the St. George’s Respiratory Questionnaire. Am Rev Respir Dis. 1992;145:1321–1327. doi: 10.1164/ajrccm/145.6.1321. [DOI] [PubMed] [Google Scholar]
- 25.Criner GJ, Sue R, Wright S, Dransfield M, Rivas-Perez H, Wiese T, et al. LIBERATE Study Group. A multicenter randomized controlled trial of Zephyr endobronchial valve treatment in heterogeneous emphysema (LIBERATE) Am J Respir Crit Care Med. 2018;198:1151–1164. doi: 10.1164/rccm.201803-0590OC. [DOI] [PubMed] [Google Scholar]
- 26.Celli BR. Pulmonary rehabilitation in patients with COPD. Am J Respir Crit Care Med. 1995;152:861–864. doi: 10.1164/ajrccm.152.3.7663796. [DOI] [PubMed] [Google Scholar]
- 27.Keating A, Lee A, Holland AE. What prevents people with chronic obstructive pulmonary disease from attending pulmonary rehabilitation? A systematic review. Chron Respir Dis. 2011;8:89–99. doi: 10.1177/1479972310393756. [DOI] [PubMed] [Google Scholar]
- 28.Valipour A, Slebos DJ, de Oliveira HG, Eberhardt R, Freitag L, Criner GJ, et al. Expert statement: pneumothorax associated with endoscopic valve therapy for emphysema: potential mechanisms, treatment algorithm, and case examples. Respiration. 2014;87:513–521. doi: 10.1159/000360642. [DOI] [PubMed] [Google Scholar]
- 29.Herth FJ, Noppen M, Valipour A, Leroy S, Vergnon JM, Ficker JH, et al. International VENT Study Group. Efficacy predictors of lung volume reduction with Zephyr valves in a European cohort. Eur Respir J. 2012;39:1334–1342. doi: 10.1183/09031936.00161611. [DOI] [PubMed] [Google Scholar]
- 30.Criner GJ, Cordova FC, Furukawa S, Kuzma AM, Travaline JM, Leyenson V, et al. Prospective randomized trial comparing bilateral lung volume reduction surgery to pulmonary rehabilitation in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160:2018–2027. doi: 10.1164/ajrccm.160.6.9902117. [DOI] [PubMed] [Google Scholar]
- 31.Hillerdal G, Löfdahl CG, Ström K, Skoogh BE, Jorfeldt L, Nilsson F, et al. Comparison of lung volume reduction surgery and physical training on health status and physiologic outcomes: a randomized controlled clinical trial. Chest. 2005;128:3489–3499. doi: 10.1378/chest.128.5.3489. [DOI] [PubMed] [Google Scholar]
- 32.Herth FJ, Eberhardt R, Gompelmann D, Ficker JH, Wagner M, Ek L, et al. Radiological and clinical outcomes of using Chartis™ to plan endobronchial valve treatment. Eur Respir J. 2013;41:302–308. doi: 10.1183/09031936.00015312. [DOI] [PubMed] [Google Scholar]
- 33.Schuhmann M, Raffy P, Yin Y, Gompelmann D, Oguz I, Eberhardt R, et al. Computed tomography predictors of response to endobronchial valve lung reduction treatment: comparison with Chartis. Am J Respir Crit Care Med. 2015;191:767–774. doi: 10.1164/rccm.201407-1205OC. [DOI] [PubMed] [Google Scholar]
- 34.Valipour A, Slebos DJ, Herth F, Darwiche K, Wagner M, Ficker JH, et al. IMPACT Study Team. Endobronchial valve therapy in patients with homogeneous emphysema: results from the IMPACT study. Am J Respir Crit Care Med. 2016;194:1073–1082. doi: 10.1164/rccm.201607-1383OC. [DOI] [PubMed] [Google Scholar]
- 35.Deslee G Time to Translate Randomized Controlled Trial Results into Routine Clinical Practice. Endobronchial lung volume reduction in severe emphysema. Am J Respir Crit Care Med. 2018;198:1110–1112. doi: 10.1164/rccm.201805-0983ED. [DOI] [PubMed] [Google Scholar]
- 36.Herth FJF, Slebos DJ, Criner GJ, Valipour A, Sciurba F, Shah PL. Endoscopic lung volume reduction: an expert panel recommendation: update 2019. Respiration. 2019;97:548–557. doi: 10.1159/000496122. [DOI] [PubMed] [Google Scholar]