The CFTR (cystic fibrosis transmembrane conductance regulator) protein regulates airway mucus viscosity and surface fluid pH (1). Defective CFTR leads to severe chronic airway infection and inflammation that destroys the structural support of bronchi. Correction of the basic defect by highly effective modulators of CFTR function is fueling the hope of a cure or effective control for people with cystic fibrosis (CF) (2). However, structural damage to CF airways is likely to persist even in those individuals whose CFTR function is restored. Furthermore, inflammatory mediators such as oxidants and proteases can suppress CFTR function (3, 4). If all individuals are to fully benefit from CFTR modulator therapies, we will need a better understanding of chronic inflammatory pathways associated with CF lung disease.
Chronic lung disease remains the leading cause of morbidity and mortality in CF (5). The cardinal feature of CF lung disease is the presence of bronchiectasis: floppy cystic airways bearing abundant purulent secretions. The appearance and severity of bronchiectasis is strongly associated with the abundance of airway secretion neutrophil elastase (6). One of the key cytokines driving neutrophilic inflammation in the CF lung is IL-1β (7).
The regulation of IL-1β synthesis and its activation involves a complex interplay of cellular danger sensors, metabolic reprogramming, and post-transcriptional protein processing (8). The study published in this issue of the Journal by McElvaney and colleagues (pp. 1381–1391) documents these interactions in CF lung and peripheral blood neutrophils and provides key information pointing to potential new antiinflammatory strategies (9). These immunometabolic changes in neutrophils are independent of CFTR function.
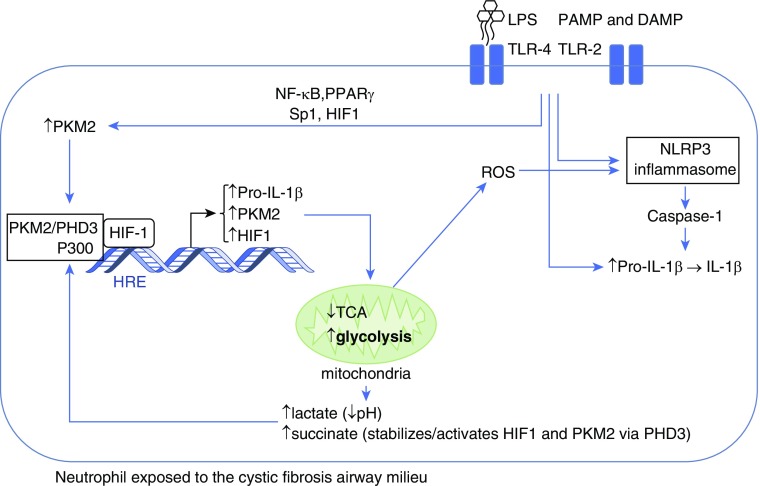
Neutrophil immunometabolism is altered by the CF airway environmental signals known as damage-associated molecular patterns and pathogen-associated molecular patterns, particularly bacterial LPS (Figure 1). Once engaged by LPS, the neutrophil increases transcription of the key metabolic protein, PKM2 (pyruvate kinase M2 isoform). PKM2 undergoes post-translational modification, forming a transcriptional complex with PHD3 (prolyl hydroxylase-3), P300, and HIF1 (hypoxia inducible factor-1) to induce several glycolytic proteins (10). Neutrophil metabolism of glucose is thus reprogrammed toward glycolysis (known as the Warburg effect), rather than oxidative phosphorylation through the Krebs cycle (tricarboxylic acid cycle and citric acid cycle). The HIF1 complex binds to the HRE (HIF1-responsive element) and increases neutrophil PKM2, HIF1, and pro–IL-1β synthesis. Increased glycolysis was confirmed in the CF neutrophil cytosol by high PKM2, lactate, and succinate as well as low pH.
Figure 1.
Neutrophil immunometabolism in cystic fibrosis. On exposure to LPS in the cystic fibrosis airway, the neutrophil increases its cytoplasmic levels of the M2 isoform of PKM2 (pyruvate kinase) and pro–IL-1β. Simultaneously, the NLRP3 inflammasome is activated through several mechanisms. PKM2 in association with PHD3 (prolyl hydroxylase-3) is stabilized and in conjunction with the coactivator P300 facilitates HIF1 (hypoxia inducible factor-1)–dependent transcription that further increases the synthesis of pro–IL-1β, PKM2, and HIF1, while reprogramming mitochondria to deviate glucose from the tricarboxylic acid cycle (TCA) to the glycolytic pathway. Increased glycolysis results in production of lactate, acidification of the cytoplasm, and accumulation of succinate, which helps stabilize the HIF1 complex. Mitochondria release reactive oxygen species (ROS) that further activate the NLRP3 inflammasome, which converts pro–IL-1β to active IL-1β through caspase-1–mediated cleavage. DAMP = damage-associated molecular patterns; NF-κB = nuclear factor-κB; PAMP = pathogen-associated molecular patterns; PPARγ = peroxisome proliferator-activated receptor-γ; TLR = toll-like receptors.
The current study reveals that the amount of circulating LPS in CF blood is sufficient to increase neutrophil PKM2 and pro–IL-1β but not to activate pro–IL-1β. In contrast, the amount of LPS in the CF lung is sufficient to assemble the NLRP3 inflammasome and initiate caspase-1–dependent cleavage and activation of pro–IL-1β. Interestingly, the levels of IL-1β in CF airway secretions strongly correlate with neutrophil burden and patient outcomes. The addition of an NLRP3 inflammasome diarylsulfonylurea-containing inhibitor (MCC950), blocked the LPS-dependent IL-1β production both in vitro and in animal models of LPS exposure and Pseudomonas aeruginosa airway infection.
The NLRP3 inflammasome is a protein complex comprising an intracellular sensor (Nod-1–like receptor), procaspase-1, and the inflammasome adaptor protein ASC, an activating adaptor for procaspase-1 (11). Oligomerization of the NLRP3 inflammasome into a functional complex activating IL-1β occurs in the presence of mitochondria-derived reactive oxygen species, phagolysosomal destabilization, or bacterial cytotoxins that induce potassium efflux (12). Although the exact mechanism(s) by which the NLRP3 inflammasome is assembled remains unknown, the importance of this complex and its effects on IL-1β activation in CF lung disease is strongly supported by the results of this study as well as the work of previous investigators (13).
Inhibition of the IL-1β and/or the NLRP3 inflammasome represents an attractive goal in the quest to find new efficient strategies to control CF inflammation and possibly improve pathogen clearance. Several molecules capable of preventing the metabolic reprogramming toward glycolysis exist, some of which could be quickly amenable to clinical trials (14). The current study reveals that blocking glycolysis with 2-deoxyglucose decreases neutrophil PKM2, a key factor that may be linked to increased NLRP3 inflammasome activity. Whether inhibition of glycolysis alone will be sufficient to control CF neutrophilic lung inflammation remains unknown. Alternatively, anakinra, an inhibitor of the IL-1β receptor in use for many years in the treatment of rheumatoid arthritis, has a favorable safety profile and has shown benefit in murine models of CF lung disease (13). The antiinflammatory effects of MCC950 in this study further suggest that the NLRP3 inflammasome and its resultant release of IL-1β represent key targets worthy of further investigation.
The novelty of linking inflammation with metabolic reprogramming in the neutrophil certainly provides an exciting advancement in our knowledge of CF airway inflammation. However, further work is needed before translation of these findings into clinical studies. The current study includes a short-term intervention of NLRP3 suppression in murine models of LPS exposure and lung infection. The degree to which the bacterial pathogen lung burden was suppressed was modest and was measured at an early time point. Lung disease in CF is a chronic lifelong condition. There is reason to believe that inhibition of glycolytic pathways or of IL-1β over several years could be associated with significant adverse events. The chronic use of IL-1β in rheumatoid arthritis can be associated with a mildly increased risk of serious infection (15), a fact that may give pause to clinicians treating patients who already are at risk of life-threatening infectious complications. Effective inhibition of glycolytic pathways could also have unwanted effects on tissues known for their high glucose uptake and metabolism, such as the heart.
In summary, McElvaney and colleagues have clearly demonstrated that the neutrophil undergoes marked changes in immunometabolism during its encounter with the CF lung environment and that these changes have profound effects on the cell’s capacity to enhance IL-1β–dependent inflammation, a cytokine strongly correlated with parameters of poor patient outcome (9). The study highlights the importance of neutrophil metabolic reprogramming characterized by the Warburg effect and NLRP3 inflammasome assembly, both of which represent potential areas of novel therapeutic strategies for CF lung disease.
Footnotes
A.M.C. is a member of the Fonds de Recherche du Québec–Santé (FRQS)-funded Centre de Recherche Clinique du Centre Hosptalier Universitaire de Sherbrooke (CHUS) and was supported by a grant from the Canadian Institutes of Health Research.
Originally Published in Press as DOI: 10.1164/rccm.201908-1558ED on September 5, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Stoltz DA, Meyerholz DK, Welsh MJ. Origins of cystic fibrosis lung disease. N Engl J Med. 2015;372:1574–1575. doi: 10.1056/NEJMc1502191. [DOI] [PubMed] [Google Scholar]
- 2.Gentzsch M, Mall MA. Ion channel modulators in cystic fibrosis. Chest. 2018;154:383–393. doi: 10.1016/j.chest.2018.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cantin AM, Bilodeau G, Ouellet C, Liao J, Hanrahan JW. Oxidant stress suppresses CFTR expression. Am J Physiol Cell Physiol. 2006;290:C262–C270. doi: 10.1152/ajpcell.00070.2005. [DOI] [PubMed] [Google Scholar]
- 4.Le Gars M, Descamps D, Roussel D, Saussereau E, Guillot L, Ruffin M, et al. Neutrophil elastase degrades cystic fibrosis transmembrane conductance regulator via calpains and disables channel function in vitro and in vivo. Am J Respir Crit Care Med. 2013;187:170–179. doi: 10.1164/rccm.201205-0875OC. [DOI] [PubMed] [Google Scholar]
- 5.Corriveau S, Sykes J, Stephenson AL. Cystic fibrosis survival: the changing epidemiology. Curr Opin Pulm Med. 2018;24:574–578. doi: 10.1097/MCP.0000000000000520. [DOI] [PubMed] [Google Scholar]
- 6.Sly PD, Gangell CL, Chen L, Ware RS, Ranganathan S, Mott LS, et al. AREST CF Investigators. Risk factors for bronchiectasis in children with cystic fibrosis. N Engl J Med. 2013;368:1963–1970. doi: 10.1056/NEJMoa1301725. [DOI] [PubMed] [Google Scholar]
- 7.Montgomery ST, Dittrich AS, Garratt LW, Turkovic L, Frey DL, Stick SM, et al. Interleukin-1 is associated with inflammation and structural lung disease in young children with cystic fibrosis. J Cyst Fibros. 2018;17:715–722. doi: 10.1016/j.jcf.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Wen H, Ting JP, O’Neill LA. A role for the NLRP3 inflammasome in metabolic diseases: did Warburg miss inflammation? Nat Immunol. 2012;13:352–357. doi: 10.1038/ni.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McElvaney OJ, Zaslona Z, Becker-Flegler K, Palsson-McDermott EM, Boland F, Gunaratnam C, et al. Specific inhibition of the NLRP3 inflammasome as an antiinflammatory strategy in cystic fibrosis. Am J Respir Crit Care Med. 2019;200:1381–1391. doi: 10.1164/rccm.201905-1013OC. [DOI] [PubMed] [Google Scholar]
- 10.Prakasam G, Iqbal MA, Bamezai RNK, Mazurek S. Posttranslational modifications of pyruvate kinase M2: tweaks that benefit cancer. Front Oncol. 2018;8:22. doi: 10.3389/fonc.2018.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henao-Mejia J, Elinav E, Strowig T, Flavell RA. Inflammasomes: far beyond inflammation. Nat Immunol. 2012;13:321–324. doi: 10.1038/ni.2257. [DOI] [PubMed] [Google Scholar]
- 12.Rathinam VA, Vanaja SK, Fitzgerald KA. Regulation of inflammasome signaling. Nat Immunol. 2012;13:333–342. doi: 10.1038/ni.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iannitti RG, Napolioni V, Oikonomou V, De Luca A, Galosi C, Pariano M, et al. IL-1 receptor antagonist ameliorates inflammasome-dependent inflammation in murine and human cystic fibrosis. Nat Commun. 2016;7:10791. doi: 10.1038/ncomms10791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corcoran SE, O’Neill LA. HIF1α and metabolic reprogramming in inflammation. J Clin Invest. 2016;126:3699–3707. doi: 10.1172/JCI84431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salliot C, Dougados M, Gossec L. Risk of serious infections during rituximab, abatacept and anakinra treatments for rheumatoid arthritis: meta-analyses of randomised placebo-controlled trials. Ann Rheum Dis. 2009;68:25–32. doi: 10.1136/ard.2007.083188. [DOI] [PMC free article] [PubMed] [Google Scholar]