To the Editor:
Subjects who fall into the same Global Initiative for Obstructive Lung Disease (GOLD) category of chronic obstructive pulmonary disease (COPD) severity are remarkably heterogeneous, and this diversity is often difficult to handle from a therapeutic standpoint (1). Computed tomography (CT) has been instrumental in identifying COPD subphenotypes, such as airway disease (AD) and parenchymal destruction (emphysema), the relative contribution of which varies from patient to patient. Importantly, emphysema is detected by CT scan in 20% of the smokers who do not meet the spirometric criteria of COPD (2). Recent studies have highlighted some major differences between emphysema and AD, such that they are now believed to be two specific endotypes (3) that can overlap with each other, and not manifestations of the same disease.
Cigarette smoke, the major risk factor for COPD in developed countries, causes pulmonary inflammation that persists long after smoking cessation, suggesting self-perpetuating adaptive immune responses similar to those that occur in autoimmune diseases. Increases in the number and size of B cell–rich lymphoid follicles (LFs) have been shown in patients in severe stages of COPD (4), and increased B-cell products (autoantibodies) have been observed in the blood and lungs of patients with COPD (5, 6). Oligoclonal rearrangement of the immunoglobulin genes has been observed in B cells isolated from COPD LFs, suggesting that a specific antigenic stimulation drives B-cell proliferation. Consistently, we have shown that in the COPD lung, there is an overexpression of BAFF (B-cell activation factor of the TNF family), which is a key regulator of B-cell homeostasis in several autoimmune diseases (7) and is involved in the growth of LFs in COPD. However, a network analysis of lung transcriptomics showed that a prominent B-cell molecular signature characterized emphysema preferentially but was absent in AD independently of the degree of airflow limitation (8). In the current study, we investigated the correlation between B-cell responses in lung tissue from patients with COPD and healthy smokers, and the extent of emphysema versus airflow limitation.
Methods
We collected formalin-fixed paraffin-embedded lung sections from 52 subjects undergoing lung volume reduction surgery or transplant for treatment of severe emphysema, or lung resection for a solitary peripheral nodule (the lung tissue studied was at least 10 cm away from the nodule). The subjects were classified as 1) active or former smokers with GOLD stages 1–2 or GOLD stages 3–4 COPD, or 2) healthy smokers without COPD (SC; see Table 1). None of the subjects had evidence of respiratory tract infection at the time of the surgery. The lung sections were immunostained for 1) 1:200 murine anti-CD20 (B-cell marker) and 1:100 rat anti-BAFF; 2) 1:50 rat anti CD45R (hematopoietic origin cell marker expressed on B cells), 1:100 rabbit anti-CD138 (plasma cell marker), and 1:50 murine anti-CD10 (immature B-cell and follicle center B-cell [centrocyte] marker); 3) 1:50 rat anti-CD45R, 1:50 rabbit anti-IgD, and 1:50 murine anti-CD24 (naive B cells); and 4) 1:50 rat anti-CD45R, 1:50 murine anti-IgG, and 1:50 rabbit anti-CD27 (memory B cells). All of the antibodies were obtained from Abcam. Appropriate isotype-matched, nonimmune control antibodies were used for each staining. For each sample, at least 20 randomly selected, nonconsecutive, high-magnification fields were evaluated using a Leica epifluorescence microscope. The numbers of parenchymal, vascular, and bronchial LFs (defined as aggregates containing more than 40 contiguous mononuclear cells that demonstrated the characteristic topographical arrangement of B cells) (7), BAFF+ B cells, BAFF+ alveolar type I and type II cells, CD138+, CD10+, CD24+, IgD+, IgG+, and CD27+ B cells were counted and normalized by alveolar tissue area using MetaMorph software. The analysis of the CT scans was performed by two independent experts in chest CT scans. The Chest Imaging Platform software (https://chestimagingplatform.org) was used to quantify emphysema as the percentage ratio of low-attenuation areas below a threshold of −950 Hounsfield units (%LAA950) (9).
Table 1.
Selected Demographics, Comorbidities, and Medication Use of the Study Participants
| Selected Demographics | n | GOLD 1–2 | GOLD 3–4 | SC | All Subjects | P Value |
|---|---|---|---|---|---|---|
| Total number of participants | 23 | 18 | 11 | 52 | ||
| Sex, % female | 52 | 35% | 56% | 18% | 49% | 0.122 |
| Age | 52 | 63.45 (10.73) | 60.17 (5.01) | 63.73 (11.98) | 62.35 (9.40) | 0.479 |
| Percentage of current smokers | 52 | 30% | 11% | 27% | 23% | 0.273 |
| Pack-years | 49 | 52.36 (42.63) | 51.35 (20.72) | 54.00 (33.80) | 52.34 (33.75) | 0.982 |
| FEV1% predicted | 52 | 76.95 (18.51) | 29.89 (11.11) | 91.27 (15.93) | 63.43 (29.88) | <0.001 |
| FEV1/FVC | 50 | 60.95 (8.02) | 43.47 (14.25) | 78.40 (7.72) | 58.45 (16.50) | <0.001 |
| DlCO% | 34 | 67.29 (23.35) | 42.83 (22.41) | 68.29 (16.77) | 58.61 (24.37) | 0.014 |
| Kco% | 34 | 79.00 (26.27) | 50.08 (33.10) | 87.43 (26.44) | 70.27 (32.23) | 0.016 |
| Comorbidities | ||||||
| Hypertension | 52 | 36% | 28% | 42% | 35% | 0.757 |
| Gastroesophageal reflux disease | 52 | 14% | 6% | 8% | 10% | 0.837 |
| Hyperlipidemia | 52 | 9% | 11% | 25% | 13% | 0.449 |
| Diabetes mellitus | 52 | 18% | 6% | 0% | 10% | 0.272 |
| Lung adenocarcinoma | 52 | 41% | 6% | 50% | 31% | 0.009 |
| Squamous cell lung cancer | 53 | 36% | 0% | 25% | 21% | 0.009 |
| Medications | ||||||
| LABA/LAMA/SABA | 52 | 32% | 100% | 17% | 52% | <0.001 |
| Inhaled corticosteroids | 52 | 18% | 78% | 25% | 40% | <0.001 |
| Statins | 52 | 36% | 17% | 50% | 33% | 0.158 |
| Protonic pump inhibitors | 52 | 27% | 22% | 42% | 29% | 0.542 |
| ACE inhibitors/angiotensin receptor blockers | 52 | 32% | 17% | 25% | 25% | 0.560 |
| Calcium antagonist | 52 | 23% | 11% | 25% | 19% | 0.607 |
| Diuretics | 52 | 14% | 22% | 17% | 17% | 0.893 |
| Oral corticosteroids | 52 | 5% | 22% | 8% | 12% | 0.248 |
| β blockers | 52 | 5% | 11% | 25% | 12% | 0.208 |
Definition of abbreviations: ACE = angiotensin-converting enzyme; GOLD = Global Initiative for Obstructive Lung Disease; LABA = long-acting β-agonist; LAMA = long-acting muscarinic antagonist; SABA = short-acting β-agonist; SC = smokers without chronic obstructive pulmonary disease.
The values are expressed as mean (SD). P values for difference across the three groups represent one-way ANOVA for continuous measures and Fisher’s exact test for categorical measures.
Statistical analysis
Associations between BAFF+ B cells, BAFF+ alveolar cells, DlCO, Kco and CD10+, CD24+, CD27+, CD138+, IgD+, and IgG+ B cells were tested with Spearman’s rank correlation tests. To determine whether %LAA950 and FEV1% predicted (FEV1%pred) were independently associated with the B-cell–related parameters measured, multivariate linear regression models were used that included, among other covariates, either %LAA950 or FEV1%pred as the independent predictor. For cellular parameters that showed a significant association with both %LAA950 and FEV1%pred, mutually adjusted models that included both predictors were assessed. The models included all subjects from both COPD groups (stages 1–2 and 3–4) as well as smoking control subjects.
The independent relationship between selected cellular parameters and emphysema and FEV1 was displayed and tested with Spearman’s correlation after the participants were stratified into groups according to GOLD stage (GOLD 1–2 and GOLD 3–4) and emphysema level (above or below the median %LAA950), respectively. Smoking control subjects were kept as a separate group in these graphs.
Results
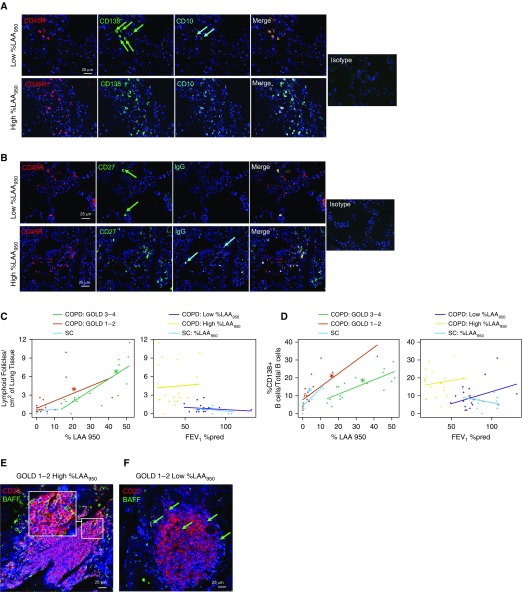
As expected, %LAA950 and FEV1%pred correlated inversely with each other (r = −0.766; P < 0.001), and they were both associated with the number of LFs, BAFF+ B cells and alveolar cells, CD10+ B cells and plasma cells (Figure 1A), and memory and IgG+ B cells (Figure 1B) when tested separately in multivariate linear regression models (Table 2, left columns). However, when they were mutually adjusted in the same regression models (Table 2, right columns), only %LAA950, and not FEV1%pred, remained significantly and strongly associated with all of these cellular parameters. We did not find any significant association between the numbers of IgD+ and CD24+ cells and %LAA950 and FEV1%pred (data not shown). From the analysis of consecutive tissue sections, we observed that, interestingly, in subjects with the highest %LAA950 values, most of the CD138+ B cells tended to cluster together and were also CD10+. Some of these cells were also expressing either CD27 or IgG, or both. In contrast, in the subjects with low %LAA950 values, only a minority of CD138+ B cells were also positive for CD10.
Figure 1.
Increases in B cell–adaptive immune responses are associated with the extent of emphysema and not with airflow limitation. (A) Triple immunofluorescence staining for CD45R (B-cell marker), CD138 (plasma-cell marker), CD10 (immature B-cell and follicle center B-cell [centrocyte] marker), and the merge panel. The green arrows indicate CD138+ B cells, and cyan arrows indicate CD10+ B cells. (B) Triple immunofluorescence staining for CD45R, CD27 (memory B cells), IgGs, and the merge panel. The green arrows indicate CD27+ B cells, and the cyan arrows indicate IgG+ B cells. Isotype control merge figures are shown on the right of both A and B. (C and D) Stratified graphs are presented for the association of low-attenuation areas below a threshold of −950 Hounsfield units (%LAA950) and FEV1% predicted (FEV1%pred) with (C) the number of lymphoid follicles (LFs)/cm2 of lung tissue and (D) the %CD138+ B cells/total B cells. For each graph, the relationship of the parameter of interest with %LAA950 within different Global Initiative for Obstructive Lung Disease (GOLD) stages (1–2 vs. 3–4) is shown in the left panel, and the relationship with FEV1%pred within different levels of emphysema is shown in the right panel. SC = smokers without chronic obstructive pulmonary disease (COPD). (E and F) Double-immunofluorescence pictures of formalin-fixed paraffin-embedded lung sections from 1) a patient with GOLD 1–2 COPD and severe emphysema (E), showing robust BAFF (B-cell activation factor of the TNF family) staining in most of the alveolar cells, LF B cells, and parenchymal B cells; and 2) a patient with GOLD 1–2 COPD and low emphysema (F), showing fewer BAFF+ alveolar cells, B cells within the LF, and parenchymal B cells. In E, the inset shows a detail of an LF, with the great majority of B cells expressing BAFF. The green arrows indicate BAFF+ B cells and alveolar cells.
Table 2.
Adjusted Coefficients for the Associations of Emphysema (%LAA950) and FEV1% Predicted with Cellular Parameters of B-Cell Activation in Separate (Left Columns) and Mutually Adjusted (Right Columns) Linear Regression Models
| Separate Models: %LAA950 or FEV1%pred As Predictor |
Mutually Adjusted Models: Both %LAA950 and FEV1%pred As Predictors |
||||||
|---|---|---|---|---|---|---|---|
| Dependent Variable | Predictor | Adjusted Coefficient* | 95% CI | Adjusted P Value | Adjusted Coefficient* | 95% CI | Adjusted P Value |
| No. of lymphoid follicles/cm2 of lung tissue, log† | %LAA950 | 0.023 | 0.014, 0.032 | <0.001 | 0.021 | 0.010, 0.032 | <0.001 |
| FEV1%pred | −0.009 | −0.014, −0.003 | 0.004 | −0.002 | −0.008, 0.004 | 0.505 | |
| No. of BAFF+ B cells/cm2 of alveolar tissue, log† | %LAA950 | 0.018 | 0.010, 0.026 | <0.001 | 0.016 | 0.006, 0.026 | 0.003 |
| FEV1%pred | −0.007 | −0.012, −0.002 | 0.005 | −0.002 | −0.007, 0.004 | 0.517 | |
| No. of BAFF+ alveolar cells/cm2 of alveolar tissue, log† | %LAA950 | 0.010 | 0.005, 0.016 | 0.001 | 0.008 | 0.001, 0.016 | 0.031 |
| FEV1%pred | −0.005 | −0.008, −0.001 | 0.007 | −0.002 | −0.006, 0.002 | 0.321 | |
| Percentage of CD10+ B cells/total B cells | %LAA950 | 0.326 | 0.236, 0.415 | <0.001 | 0.338 | 0.228, 0.449 | <0.001 |
| FEV1%pred | −0.093 | −0.159, −0.027 | 0.007 | 0.012 | −0.048, 0.072 | 0.683 | |
| Percentage of CD27+ B cells/total B cells | %LAA950 | 0.278 | 0.093, 0.464 | 0.004 | 0.239 | 0.010, 0.467 | 0.041 |
| FEV1%pred | −0.112 | −0.217, −0.007 | 0.038 | −0.037 | −0.161, 0.086 | 0.546 | |
| Percentage of CD138+ B cells/total B cells | %LAA950 | 0.377 | 0.238, 0.516 | <0.001 | n/a | n/a | n/a |
| FEV1%pred | −0.049 | −0.144, 0.047 | 0.311 | n/a | n/a | n/a | |
| Percentage of IgG+ B cells/total B cells | %LAA950 | 0.152 | 0.080, 0.224 | <0.001 | n/a | n/a | n/a |
| FEV1%pred | −0.020 | −0.065, 0.026 | 0.395 | n/a | n/a | n/a | |
Definition of abbreviations: BAFF = B-cell activation factor of the TNF family; CI = confidence interval; FEV1%pred = FEV1% predicted; n/a = not applicable; %LAA950 = low-attenuation areas below a threshold of −950 Hounsfield units.
Models included all patients with chronic obstructive pulmonary disease (without stratification by Global Initiative for Obstructive Lung Disease stage) and smoking control subjects.
Also adjusted for sex, age, smoking status, and presence of lung cancer. Pack-years were excluded from the models owing to missing data for three participants. Results were confirmed in a sensitivity analysis after further adjustment for pack-years.
Dependent variables were first log-transformed in base 10 to achieve normalization. Participants with no lymphoid follicles were transformed to the base 10 log of 0.1.
Consistent with these results, as shown in Figure 1C, levels of %LAA950 correlated significantly with the number of LFs both among subjects in GOLD stages 1–2 and among those in GOLD stages 3–4 (left panel). However, after stratification by emphysema levels, FEV1%pred did not correlate with the number of LFs among subjects with low or high emphysema (right panel). Similarly, %LAA950 was found to be associated with the percentage of plasma cells in each COPD group as well as among SC (Figure 1D, left panel), whereas no association was found between FEV1%pred and the percentage of plasma cells in either of the emphysema groups or among SC (Figure 1D, right panel). In line with these results, %LAA950, but not FEV1%pred, was also shown to be significantly associated with the other B-cell subpopulations studied when stratified into the same groups (data not shown). As expected, LFs in lungs from subjects with high %LAA950 were very rich in BAFF (Figure 1E), in contrast to the subjects with low %LAA950, where low pulmonary LF BAFF levels were observed (Figure 1F). The numbers of BAFF+ B cells and alveolar cells were highly correlated with the numbers of LFs (r = 0.7 and 0.6, respectively), CD10+ B cells (r = 0.6 and 0.7, respectively), plasma cells (r = 0.4 and 0.6, respectively), memory B cells (r = 0.4 and 0.5, respectively), and IgG+ B cells (r = 0.3 and 0.5, respectively). The DlCO and Kco values were also strongly correlated with the numbers of LFs (r =−0.5), BAFF+ B cells (r =−0.6), and BAFF+ parenchymal cells (r = −0.5), and with CD10+ B cells (r =−0.5). In addition, Kco was also correlated with the number of plasma cells and memory B cells (r =−0.4).
Discussion
These data are in line with previous findings that the presence of emphysema, and not the degree of airflow limitation, is correlated with a specific lung endotype dominated by B-cell responses (8). We now extend these findings to all COPD GOLD stages and SC, showing that an upregulation of the B-cell immune compartment in lung tissue is directly linked to %LAA950 and not to FEV1%pred. Our results support the hypothesis that an overactivation of the B-cell compartment, characterized by increases in naive, memory, and antibody-producing B cells and expression of BAFF by B cells and alveolar cells, is abundant in the emphysematous lung, either as a consequence or as a concurrent cause of the ongoing emphysematous process (10). Importantly, the cellular readouts of activation of the B-cell compartment were also significantly directly associated with the extent of emphysema in the smokers without airflow limitation. This suggests that increases in B cell–adaptive immune responses are present before lung function starts to decline. We should acknowledge that the association between B cells and emphysema in our cross-sectional study does not provide proof of a causal association (cause–effect), and could be due to chance, bias, confounding, and/or reverse causation (effect–cause), the effects of which need to be explored in future studies analyzing broader cohorts of subjects.
These observations may open new therapeutic paths for patients with COPD, as the complexity of B-cell maturation presents opportunities for therapeutic interventions. Currently, there is a lack of disease-modifying therapies for COPD, mainly because available therapies target patients with COPD as a whole and cluster them simply according to their airflow limitation. We believe that further characterization of a B-cell endotype associated with emphysema could 1) shift the notion that patients with COPD, even within the same GOLD stage, are pathobiologically similar and thus require similar clinical management; and 2) define the clinical phenotype (likely emphysema) that could benefit from therapies targeting B cells or B-cell products (e.g., BAFF), leading to earlier and more personalized therapeutic interventions that may greatly alleviate the burden of COPD.
Footnotes
Supported by funds from the Asthma and Airway Disease Research Center (University of Arizona), Flight Attendants Medical Research Institute grant YFAC141004, a Parker B. Francis Foundation Fellowship, and grant PI16/01149 from the Spanish Government.
Author Contributions: F.P. conceived the project and designed the experiments. J.-L.S., B.B., M.K., F.D.M., G.B., J.P.d.-T., R.S.J.E., S.G., and F.P. conducted experiments and/or contributed to data analysis and interpretation. All authors contributed to the writing and editing of the manuscript.
Originally Published in Press as DOI: 10.1164/rccm.201903-0632LE on July 26, 2019
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Soriano JB, Polverino F, Cosio BG. What is early COPD and why is it important? Eur Respir J. 2018;52:1801448. doi: 10.1183/13993003.01448-2018. [DOI] [PubMed] [Google Scholar]
- 2.Woodruff PG, Barr RG, Bleecker E, Christenson SA, Couper D, Curtis JL, et al. SPIROMICS Research Group. Clinical significance of symptoms in smokers with preserved pulmonary function. N Engl J Med. 2016;374:1811–1821. doi: 10.1056/NEJMoa1505971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petersen H, Vazquez Guillamet R, Meek P, Sood A, Tesfaigzi Y. Early endotyping: a chance for intervention in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2018;59:13–17. doi: 10.1165/rcmb.2018-0002PS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 5.Núñez B, Sauleda J, Antó JM, Julià MR, Orozco M, Monsó E, et al. PAC-COPD Investigators. Anti-tissue antibodies are related to lung function in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011;183:1025–1031. doi: 10.1164/rccm.201001-0029OC. [DOI] [PubMed] [Google Scholar]
- 6.Lee SH, Goswami S, Grudo A, Song LZ, Bandi V, Goodnight-White S, et al. Antielastin autoimmunity in tobacco smoking-induced emphysema. Nat Med. 2007;13:567–569. doi: 10.1038/nm1583. [DOI] [PubMed] [Google Scholar]
- 7.Polverino F, Cosio BG, Pons J, Laucho-Contreras M, Tejera P, Iglesias A, et al. B cell-activating factor: an orchestrator of lymphoid follicles in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;192:695–705. doi: 10.1164/rccm.201501-0107OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faner R, Cruz T, Casserras T, López-Giraldo A, Noell G, Coca I, et al. Network analysis of lung transcriptomics reveals a distinct B-cell signature in emphysema. Am J Respir Crit Care Med. 2016;193:1242–1253. doi: 10.1164/rccm.201507-1311OC. [DOI] [PubMed] [Google Scholar]
- 9.Müller NL, Staples CA, Miller RR, Abboud RT. “Density mask”: an objective method to quantitate emphysema using computed tomography. Chest. 1988;94:782–787. doi: 10.1378/chest.94.4.782. [DOI] [PubMed] [Google Scholar]
- 10.Cosio MG, Saetta M, Agusti A. Immunologic aspects of chronic obstructive pulmonary disease. N Engl J Med. 2009;360:2445–2454. doi: 10.1056/NEJMra0804752. [DOI] [PubMed] [Google Scholar]