Abstract
Rationale: Studies of the immune responses at the site of respiratory syncytial virus (RSV) infection are sparse despite nearly five decades of research into understanding RSV disease.
Objectives: To investigate the role of mucosal innate immune responses to RSV and respiratory viral load in infants hospitalized with the natural disease.
Methods: Cytokines, viral load, and type 2 innate lymphoid cell (ILC2) levels in nasal aspirates, collected within 24 hours of enrollment, from infants hospitalized with RSV infection were quantified.
Measurements and Main Results: RSV severity in infants was categorized based on admission to the general ward (moderate) or the pediatric ICU (severe). Evaluable subjects included 30 patients with severe and 63 patients with moderate disease (median age, 74 d; range, 9–297 d). ILC2s were found in the nasal aspirates of patients with severe disease (0.051% of total respiratory CD45+ cells) to a significantly greater extent than in patients with moderate disease (0.018%, P = 0.004). Levels of IL-4, IL-13, IL-33, and IL-1β were significantly higher in nasal aspirates of patients with severe disease compared with those of patients with moderate disease. Factors associated with disease severity were gestational age (odds ratio, 0.49; 95% confidence interval, 0.29–0.82; P = 0.007) and IL-4 (odds ratio, 9.67; 95% confidence interval, 2.45–38.15; P = 0.001).
Conclusions: This study shows, for the first time, that elevated levels of ILC2s is associated with infant RSV severity. The findings highlight the dominance of type-2 responses to RSV infection in infants and suggest an important role of ILC2 in shaping the immune response early during RSV infection.
Keywords: type 2 innate lymphoid cell, infant, respiratory syncytial virus, severity, immune response
At a Glance Commentary
Scientific Knowledge on the Subject
The severity of respiratory syncytial virus (RSV) infection in human infants has been related to type 2–biased immune responses. Although there is a great body of animal studies providing mechanistic insights into the role of peripheral innate lymphoid type 2 cells (ILC2s) in mediating type 2 responses in different lung diseases, direct evidence of the involvement of respiratory ILC2 in infant RSV severity is lacking.
What This Study Adds to the Field
This study, for the first time, reveals the association of elevated levels of respiratory ILC2 and their associated cytokines with RSV severity in infants during the early phase of infection at the site of initial infection using nasal aspirates. Thus, these findings provide not only a new insight about the local innate immune responses, but also a translation from animal models to human patients, and they suggest the important role of ILC2s in shaping the immune response in the early phase of RSV infection.
Respiratory syncytial virus (RSV) is the leading cause of acute lower respiratory tract infection and severe bronchiolitis in infants globally (1). Age at initial RSV infection is a major predictor of disease severity (2–4). However, the reason that infants are more susceptible to severe RSV infection and severe disease remains unclear. Manifestations of severe RSV infection include respiratory insufficiency and severe apnea that requires admission to the pediatric ICU (PICU) and mechanical ventilator support. Although a licensed immunoprophylactic for RSV (palivizumab) exists and effectively reduces RSV hospitalization rates (5), there is no vaccine or specific antiviral treatments approved for use in infants. Currently, the mainstay for the treatment of severe RSV disease is supportive care.
In humans, both viral load (3, 4) and T-helper cell type 2 (Th2)-biased responses, characterized by exacerbated IL-4 and IL-13 secretion and low IFN-γ, have been associated with RSV pathogenesis (6). Recent studies have suggested the critical role of type 2 innate lymphoid cells (ILC2s) in shaping and promoting Th2 responses in respiratory and pulmonary compartments in humans (7–9). ILC2s, together with ILC1s and ILC3s, are characterized as rare innate immune cells with lymphoid morphology, but phenotypically distinct from T, B, and myeloid cells (10). Despite their scarcity, ILC2s are the primary ILC population in the lung and rapidly secrete large amounts of type 2 cytokines upon airway inflammation (7). Using a novel neonatal mouse model, we previously demonstrated that the rapid increase in pulmonary ILC2s, which is driven by robust induction of IL-33, coincided with Th2-biased responses after RSV neonatal infection (11). These studies implied the key role of ILC2 as the early responders to RSV infection and demonstrated their central role in orchestrating the subsequent adaptive immune response. Although IL-33, a known inducer of ILC2s (12), has been detected at higher levels in infants with RSV bronchiolitis compared with healthy control infants (11, 13), no data exist on respiratory ILC2 in RSV-infected infants. In the current study, we describe the early infant immune response to RSV infection and viral load at the site of initial infection. Our studies were performed on nasal aspirates isolated from infants infected with RSV and hospitalized at Le Bonheur Children’s Hospital (Memphis, TN). We hypothesized that elevated levels of ILC2 and their associated cytokines (IL-13 and IL-4) and inducing cytokine (IL-33) are positively correlated with RSV severity in infants early during infection.
Methods
For a detailed description, see the online supplement.
Study Participants
The study was approved by the Institutional Review Board of the University of Tennessee Health Sciences Center. The selection criteria are described in Table E1 in the online supplement. RSV infection was confirmed in the research laboratory using RSV-PCR, as previously described (14). We compared moderately (admitted to the general ward) and severely (admitted to PICU) RSV-infected infants admitted to Le Bonheur Children’s Hospital from 2013 to 2016.
Nasal Aspirate Collection and Quantification of Viral Load and Cytokine Levels
Nasal aspirates were collected within 24 hours of enrollment. Supernatants from nasal aspirates were centrifuged at 1,000 × g for 3 minutes, and 250-μl aliquots were stored at −80°C until use. The cell pellet was washed twice by gently mixing with 5 ml of RPMI-1640 and then filtered through a 40-μm cell strainer before being centrifuged at 3,000 × g for 3 minutes. Pelleted cells were suspended in Recovery Cell Culture Freezing Medium (ThermoFisher) at 3 × 106 cell/ml, and 1-ml aliquots were stored at −150°C until use. The viral load and cytokine levels in the nasal aspirate were quantified with qRT-PCR and multiplex assay (Meso Scale Discovery), respectively.
Flow Cytometry Analysis
Frozen cells were thawed using CTL antiaggregate wash solution (ImmunoSpot) once in RPMI-1640 (CTL buffer). Briefly, the vial of cells was quickly thawed in a 37°C bead bath until the ice chunk was released from the wall of the tube. The cells were then added to 9 ml of 37°C CTL buffer. The thawed cells were subsequently washed twice with 10 ml of warm CTL buffer with centrifugation at 500 × g for 5 min at room temperature. The resulting cells were suspended in 250 μl of warm CTL buffer. Approximately 105–106 nasal aspirate cells were sequentially stained with human Fc blocking (CD16/CD32) and a panel of monoclonal antibodies at 4°C for 30 minutes. The stained cells were then fixed before flow cytometric analysis with a BD FACSCanto II flow cytometer and subsequently FlowJo v.10. ILC2s were identified as CD45+Lin−CD127+CD161+CRTH2+c-kit− with gating based on fluorescence minus one controls. The antibody panel and gating strategy are described in Table E4 and Figure E1, respectively.
Statistical Analysis
The data are described using descriptive statistics. Comparisons were performed using a two-tailed Mann-Whitney U test for continuous variables and chi-square or Fisher’s exact test for qualitative and categorical variables. We examined the relationship of all variables with the outcome of disease severity and computed unadjusted odds ratios (ORs) with 95% confidence interval (CI) using bivariate and multivariable logistic regression. Analyses were performed using SAS v9.3 (SAS Institute Inc.).
Results
Characteristics of Infants Enrolled with RSV Infection
Subjects included 30 severely (PICU) and 63 moderately (non-PICU) infected infants with RSV hospitalized from 2013 to 2016. Subjects had a median age of 70.5 (patients with severe disease) and 78 (patients with moderate disease) days, respectively (Table 1). Most infants with severe RSV were mechanically ventilated (86.7%) and hospitalized for a longer period compared with infants with moderate RSV (Table 1). This supports the classification of disease severity based on PICU status.
Table 1.
Demographic and Clinical Characteristics of Enrolled Infants with Respiratory Syncytial Virus
| Characteristics | Severe (PICU) (n = 30) | Moderate (non-PICU) (n = 63) |
|---|---|---|
| Age at enrollment | ||
| Age, median (range), d | 70.5 (11–297) | 78 (9–288) |
| Age ≤3 mo old, n (%) | 20 (66.7) | 37 (58.7) |
| Age >3 mo old, n (%) | 10 (33.3) | 26 (41.3) |
| Gestational age, median (range), wk | 36 (32–40) | 38.3 (30–41) |
| Preterm, n (%) | ||
| No | 13 (43.3) | 44 (69.8) |
| Yes | 17 (56.7) | 19 (30.2) |
| Birthweight, median (range), lb* | 5.5 (3.9–8.7) | 6.7 (1.3–9.5) |
| Enrollment weight, median (range), lb | 10.7 (5.0–19.4) | 12 (5.9–24.7) |
| Sex, n (%) | ||
| Male | 17 (56.7) | 41 (65.1) |
| Female | 13 (43.3) | 22 (34.9) |
| Race, n (%) | ||
| White | 16 (53.3) | 23 (36.5) |
| African American | 11 (36.7) | 39 (61.9) |
| Others (Asian + Hispanic) | 3 (10) | 1 (1.6) |
| History of being in NICU, n (%) | ||
| No | 14 (46.7) | 45 (71.4) |
| Yes | 16 (53.3) | 18 (28.6) |
| Time in NICU, median (range), d | 14 (7–30) | 14 (8 - 30) |
| Breastfeeding, n (%)* | ||
| No | 12 (41.4) | 30 (47.6) |
| Yes | 17 (58.6) | 33 (52.4) |
| Sick contact, n (%) | ||
| No | 11 (36.7) | 19 (30.2) |
| Yes | 19 (63.3) | 44 (69.8) |
| History of wheezing, n (%) | ||
| No | 21 (70) | 51 (81) |
| Yes | 9 (30) | 12 (19) |
| Exposure to tobacco, n (%) | ||
| No | 23 (76.7) | 55 (87.3) |
| Yes | 7 (23.3) | 8 (12.7) |
| Day care, n (%) | ||
| No | 25 (83.3) | 51 (80.9) |
| Yes | 5 (16.7) | 12 (19.1) |
| Parental smoking, n (%) | ||
| No | 23 (76.7) | 46 (73) |
| Yes | 7 (23.3) | 17 (27) |
| History of family asthma, n (%) | ||
| No | 17 (56.7) | 24 (38.1) |
| Yes | 13 (43.3) | 39 (61.9) |
| Fever, n (%) | ||
| No | 16 (53.3) | 30 (47.6) |
| Yes | 14 (46.7) | 33 (52.4) |
| Duration of symptoms before collecting of nasal aspirates, median (range), d | 3.5 (1–7) | 4 (2–12) |
| Oxygen supplementation, n (%)* | ||
| No | 0 (0) | 15 (24.2) |
| Yes | 30 (100) | 47 (75.8) |
| LOS, median (range), d | 15 (4–103) | 4 (1–25) |
| DOS, median (range), d | 3.5 (1–7) | 4 (2–12) |
| Mechanical ventilation, n (%) | ||
| No | 4 (13.3) | 63 (100) |
| Yes | 26 (86.7) | 0 (0) |
| RSV subtype | ||
| A | 22 (73.3) | 37 (58.7) |
| B | 8 (26.7) | 26 (41.3) |
Definition of abbreviations: DOS = duration of oxygen supplement; LOS = length of stay; PICU = pediatric ICU; NICU = neonatal ICU; RSV = respiratory syncytial virus.
Preterm indicates gestational age <37 weeks; breastfeeding was self-reported by parents; sick contact indicates if the patient recently had contact with sick individuals before this illness; history of wheezing indicates if the patient ever wheezed before this illness; exposure to tobacco smoke indicates if the patient has ever been exposed to tobacco smoke before this illness; day care indicates if there are more than than five children at the day care; parental smoking was self-reported by parents; history of family asthma indicates if there is anyone in the family that has asthma, including mother, father, brothers, or sisters; and fever indicates, with this illness, if the patient had run a fever before being admitted to the hospital.
Missing one data point.
The median gestational ages (GAs) were 36 and 38.3 weeks (Table 1) for severe and moderate groups, respectively. In addition, 56.7% of infants with severe RSV had GA <37 weeks (preterm infants); this is higher than 30.2% of infants with moderate RSV. Admission to the neonatal ICU (NICU) at birth also differed with severity with 53.3% of infants with severe RSV and only 28.6% of infants with moderate RSV having been admitted to the NICU. Infants in the two study groups were not equally distributed according to race. White race constitutes 53.3% of total severe cases compared with 36.5% of total moderate cases (Table 1). There is also no significant difference between the median days of duration of symptoms before collection of samples of patients with severe disease (3.5 d) and that of patients with moderate disease (4 d) (Table 1).
Global Respiratory Cytokine Profiles Are Associated with RSV Disease Severity at the Early Phase of the Disease
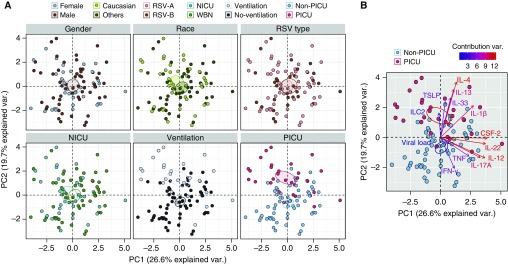
To explore the association between immune responses and disease severity, we conducted principal component (PC) analysis (PCA) on 13 continuous immune-related variables (11 cytokines, ILC2s, and viral load). Then, PCA maps were colored by sex, race, and RSV subtype, past NICU stay, mechanical ventilation, and PICU admission. Figure 1 illustrates our PCA analysis with immune-related variables, with the first and second PCs (PC1 and PC2) accounting for 26.6% and 19.7%, respectively, of all the variance of immune mediators. Thus, in contrast to other demographic variables (sex, race, RSV type, and NICU status), PICU and mechanical ventilation status form discrete clusters with limited overlap (Figure 1A). Interestingly, IL-1β, IL-13, IL-4, IL-33, and ILC2s of infants with severe RSV grouped together and situated opposite to IFN-γ, TNF (tumor necrosis factor), IL-12, IL-17A, and IL-22 of infants with moderate RSV group (Figure 1B) implying that analyzed immune mediators were associated with disease severity and independent of sex, race and RSV type.
Figure 1.
Immune mediators define disease severity. Principal component (PC) analysis (PCA) of 13 continuous immune-related variables (including viral load) from 93 subjects was used to identify the first PCs (PC1 and PC2), which together explain 46.3% of the variation in the data. (A) PCA maps (or scores plots), which show the scores on each PC for each infant. Then, PCA maps were colored by sex, race, respiratory syncytial virus (RSV) type, neonatal ICU (NICU) status, and mechanical ventilation and pediatric ICU (PICU) status (from left to right). Individual points represent individual infants. Infants that are close together have similar profiles of immune mediators (including viral load). The ellipses (with 95% confidence interval) depict a separation between two different populations. (B) PCA biplot (or loading plot) shows the immune mediators, which load on the respective PCs. The vectors represent individual immune mediators. Type II cytokines covary, as evidenced by clustering within the same quadrants of the PCA biplot. The color of vectors represents the loading of the contribution of the individual immune mediators. WBN = well-baby nursery.
Type 2 Immunity Is Associated with Severity of RSV Disease in Infants
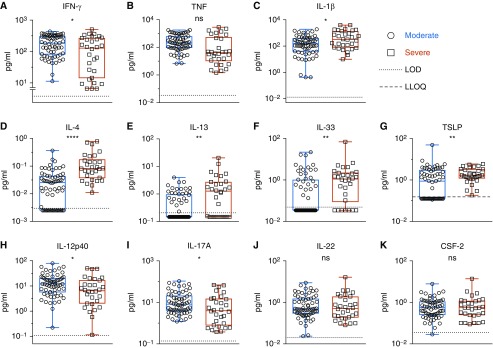
Indeed, the moderate group displayed more elevated levels of IFN-γ, IL-12p40, and IL-17A compared with infants with severe RSV (Figures 2A, 2H, and 2I). Conversely, the level of respiratory type 2 cytokines (IL-4 and IL-13) and IL-33 were significantly higher in the severe group related to the moderate group (Figures 2D–2F). It suggests that, although infants with severe RSV displayed a predominance of type 2 cytokines in response to RSV infection, type 1 and type 3 cytokines were preferentially expressed in patients with moderate RSV.
Figure 2.
Infants with severe respiratory syncytial virus disease express higher levels of type 2 cytokines. Cytokine expression in nasal aspirates, collected within 24 hours of enrollment, was compared between patients with severe (n = 30) and moderate disease (n = 63) and are represented as box-and-whisker plots. Whiskers represent maximum and minimum values; boxes represent 25th–75th percentiles. The median is represented by the middle line. Significance was determined using the Mann-Whitney U test. *P ≤ 0.05, **P ≤ 0.01, and ****P ≤ 0.0001. LLOQ = lower limit of quantification; LOD = limit of detection; ns = nonsignificant.
The Frequency of ILC2, but Not Viral Load, Is Significantly Associated with RSV Severity
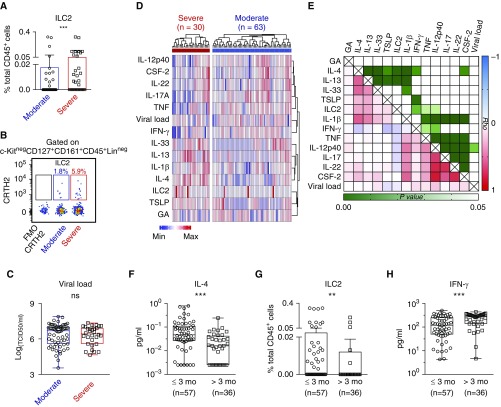
Intriguingly, the frequency of ILC2, a central mediator of type 2 immunity, was significantly greater in infants with severe versus moderate RSV (Figures 3A and 3B). Similar observations were made by comparing the absolute numbers of ILC2 and total cell counts in nasal aspirates between infants with moderate RSV and those with severe RSV (Figure E1C). Examining viral load from nasal aspirates obtained within 24 hours of enrollment, we found that viral load was not significantly different between moderate and infants with severe RSV (Figure 3C). Similarly, there was no noticeable association between viral load with age at enrollment, sex, race, prematurity, or NICU status (Figure E2).
Figure 3.
The age-related frequency of nasal aspirate innate lymphoid type 2 cells (ILC2s) and type 2 immune mediators were associated with respiratory syncytial virus (RSV) disease severity. (A–C) The frequency of ILC2s (A), and the dot plot (B), showing CRTH2+ILC2s gated on c-Kit− CD127+CD161+CD45+Lin− populations, and viral load in nasal aspirates from infants with severe versus moderate RSV (C). (D) A heatmap with hierarchical clustering, illustrating cytokine expression of individual infants with RSV. (E) The heat map indicates the Spearman rank correlation coefficient (Rho) (varying from positive to negative correlation; red–white–blue) and P ≤ 0.05 (light green to dark green cells) and P > 0.05 (white cells). (F–H) Expression of IL-4 (F), ILC2 (G), and IFN-γ (H) in RSV-infected infants ≤3 months or >3 months of age. GA = gestational age; ns = not significant. **P ≤ 0.01 and ***P ≤ 0.001.
Levels of Type 1 and Type 2 Immune Mediators Are Age Dependent
We next examined the relationship between immune/viral mediators and demographic characteristics of RSV-infected infants. Younger-GA infants tended to have higher levels of IL-4, IL-13, and ILC2 and clustered together. This clustering was distinct from the IFN-γ, IL-12, and IL-17A cluster (Figure 3D). However, there was no statistically significant correlation between GA and any cytokines, ILC2s, or viral load (Figure 3E). In addition, patients with higher IL-4 levels tended to have high levels of IL-13, IL33, and IL-1β, and relatively high ILC2 frequency and lower IFN-γ (Figures 3D and 3E). Of note, most type 2 cytokines exhibited a positive correlation with other type 2 cytokines, as well as with ILC2. The levels of IL-4 were negatively correlated with that of IFN-γ (Figure 3E). Similarly, there is a highly positive correlation among type 3 cytokines (Figure 3E).
Intriguingly, levels of IL-4 and ILC2 in nasal aspirates were remarkably lower in RSV-infected infants over 3 months compared with infants 3 months of age or younger (Figures 3F and 3G). Conversely, IFN-γ levels were significantly higher in RSV-infected infants over 3 months compared with infants 3 months of age or younger (Figure 3H). The 3-month cutoff of age at enrollment was selected based on the age distribution of our cohort (Figure E3A). Similar to IL-4, the levels of IL-13 and IL-33 were also significantly higher in the younger RSV-infected group (Figures E3B and E3C). Of note, the majority of patients with undetectable IL-13 and IL-33 had the moderate disease and was significantly older at enrollment (Figure E3D). There was no significant association between GA and IL-13/IL-33 level (Figure E3D).
Elevated Levels of Respiratory IL-4 and Young GA Are Significant Risk Factors for Severe RSV Disease
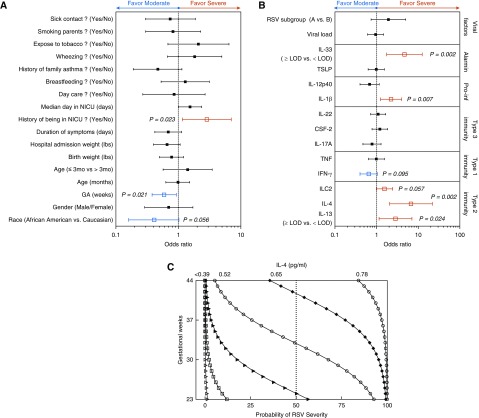
The relationship of individual variables with the outcome of disease severity was examined using bivariate logistic regression (Figure 4). The risk of RSV severity was increased in infants with younger GA (OR, 0.59; 95% CI, 0.38–0.93; P = 0.021; Figure 4A and Table E5A). Furthermore, infants admitted to NICU were at higher risk for severe disease relative to well-born babies (OR, 2.86; 95% CI, 1.16–7.04; P = 0.023; Figure 4A and Table E5A). Intriguingly, infants with higher levels of respiratory IL-4 and IL-1β were at increased risk of severe RSV disease (IL-4: OR, 6.71; 95% CI, 2.06–21.89; P = 0.002; IL-1β: OR, 2.23; 95% CI, 1.24–4.00; P = 0.007; Figure 4B and Table E5A). The risk of severe disease also significantly increased in infants with detectable levels of IL-13 or IL-33 (Figure 4B and Table E5A) and infants with higher frequencies of ILC2, but in a marginally significant manner (OR, 1.55; 95% CI, 0.986–2.44; P = 0.057) (Figure 4B and Table E5A). However, only IL-4 and GA were retained as potential risk factors in the final multivariable logistic regression model (IL-4: OR, 9.67; 95% CI, 2.45–38.16; P = 0.001; GA: OR, 0.49; 95% CI, 0.29–0.82; P = 0.007; Table E5B). Figure 4C illustrates the combined influence of IL-4 and GA on infant RSV severity in the final multivariable logistic regression. The effect of IL-4 was greater at younger GA and progressively reduced to negligible levels, at <0.39 pg/ml. Thus, for a 37-week GA patient, the probability of developing severe RSV increases 24.4% (from 3.9% to 28.3%) as the level of IL-4 in nasal aspirate increases from 0.39 pg/ml to 0.52 pg/ml. However, for a patient with younger GA (30 wk), the probability of severe RSV increases 50.6% (from 19.4% to 70%) as the level of IL-4 increases from 0.39 pg/ml to 0.52 pg/ml. Therefore, younger GA and higher IL-4 levels result in the highest probability for severe RSV. The final multivariable results are exploratory, and all analyses should be examined further and validated in similar cohorts.
Figure 4.
Younger gestational age (GA) positively correlates with higher levels of IL-4 in response to respiratory syncytial virus (RSV) infection and more severe disease. (A and B) Plots of risk factors for the development of severe RSV, using bivariate logistic regression of (A) background variables and (B) immune/host-related variables, with pediatric ICU admission status as the dependent variable. The vertical line represents an odds ratio of 1. Odds ratio with 95% confidence interval; P ≤ 0.05. (C) The combined influence of nasal aspirate levels of IL-4 and GA on the severity of RSV disease in infants was assessed using multivariable logistic regression. The logarithm of the odds Pr/(1 − Pr), where Pr is the probability of the disease severity, depends on a vector of predictors (IL-4 and GA), is logit(Pr) = log[Pr/(1 − Pr)] = β0 + βIL4 × IL4 + βGA × GA, where β is a vector of regression coefficients. In the final logistic regression model, β0 = −0.62, βIL4 (0.13 pg/ml) = 2.269, and βGA (2.76 wk) = −0.709. Duration of symptoms refers to duration of symptoms before sampling. ILC2 = innate lymphoid type 2 cell; LOD = limited of detection; NICU = neonatal ICU; Pro-inf = proinflammatory cytokines.
Discussion
Our study confirms that preterm birth is a major risk factor for severe RSV disease, including the requirement of mechanical ventilation and longer length of stay (LOS) (15–18). The younger the GA, the greater the risk of severe disease (Figures 4A and 4C and Table E5). After discharge from the NICU, preterm infants are more often rehospitalized with lower respiratory tract infections, presumably due to reduced immunoglobulin concentrations and immature immune systems. GA is positively correlated with the level of maternal immunoglobulins (18–20), and the expression of CD14 and MD-2 on leukocytes (21), which plays an important role in RSV fusion protein–mediated Toll-like receptor 4 activation (22). Interestingly, the impairment of CD14 and MD-2 in preterm infants was corrected by treatment of IFN-γ (21), suggesting a correlation between GA and the expression of IFN-γ. However, there was no correlation between GA and IFN-γ in nasal aspirates in our current cohort (Figure 3E).
Our data reveal that elevated levels of IL-4, IL-13, IL1-β, and IL-33 upon hospitalization with RSV disease are significant risk factors for severity of disease (Figure 4B and Table E5), whereas significantly higher levels of IFN-γ (Figure 2) dominate in infants with moderate disease. These results support previous studies describing the sustainable association of elevated IL-4/IFN-γ ratios, IL-1β, and IL-33 levels in either respiratory secretions or sera (an indication of type 2 bias) with acute RSV bronchiolitis in infants (13, 23–29). In agreement with our data, a recent report also revealed that IFN-γ levels in nasal aspirates are lower in children with severe RSV bronchiolitis (30). Interestingly, this same study did not report a change in IL-4 or IL-13 levels between children with severe or moderate RSV bronchiolitis in contrast to our current findings and that of other studies (25, 26). There are several differences between these studies that may account for this discrepancy, including patient demographics, cohort size, and sampling methods.
Furthermore, others have observed that during the acute phase of RSV disease, circulating CD4+ T cells from symptomatic ambulatory infants expressed a remarkably greater level of IFN-γ compared with those from hospitalized infants (31), suggesting that type 1-skewed responses are protective against severe disease. Similarly, circulating CD8+ T cells expressed a significantly greater level of IFN-γ in hospitalized infants with moderate versus severe RSV disease (32). However, the adaptive immune responses in the peripheral blood compartment may not accurately reflect the local immune responses, especially during the early phase of the infection. It is also evident that acute RSV infection induces a relative predominance of Th2 cytokines in nasal washes compared with those in plasma from infected children (33). Having previously demonstrated the association of the increase in the number of lung ILC2s with enhanced IL-13 expression in neonatal mouse models of RSV (11), we, therefore, sought to determine the ILC2 compartments of the local innate immune responses using nasal aspirates collecting within 24 hours of enrollment. Our study thus reveals the higher frequency and absolute number of ILC2s in nasal aspirates from infants with severe, but not moderate, RSV disease (Figures 3A and 3B and Figure E5C), and it furthermore demonstrates that elevated frequencies of ILC2s increased the risk for severe RSV (Figure 4B and Table E5A; P = 0.057). Others have observed significantly higher GATA3/T-bet (essential transcript factors for ILC2/Th2 and Th1, respectively) mRNA ratio in nasal aspirates from hospitalized infants with severe RSV compared with moderate RSV (26), suggesting the association of ILC2 and Th2 polarization with illness severity. The important role of ILC2 in mediating type 2 immunity has also been shown in different lung diseases (34, 35) and neonatal mouse models of RSV (11). Although we have no direct evidence that ILC2s are the source of IL-4/IL-13, the timing (i.e., early in the infection) and recent studies demonstrate the ability of ILC2s to produce these cytokines and to promote Th2 polarization via either direct cellular contact (36) or IL-4/IL-13 signaling (35, 37, 38). Our findings demonstrate that ILC2 frequencies are highly correlated with IL-4 and IL-13 in same nasal aspirates (Figures 3D and 3E) and that these correlate with disease severity in human infants (Figure 4B and Table E5A), highlighting the role of ILC2 and type 2 cytokines in RSV human disease.
Others have shown that the expression of these type 2 cytokines is age differentiated in infants infected with RSV. Specifically, IL-4 levels in nasopharyngeal secretions are remarkably lower in RSV-infected infants over 3 months of age versus infants 3 months of age or younger (39). These findings corroborate our current observation. Although nasal aspirate levels of IL-4 and ILC2 were inversely associated with age at enrollment, IFN-γ levels were significantly greater in RSV-infected infants over 3 months of age than in infants 3 months of age or younger (Figures 3F–3H and Table E6). It implies that younger age at infection is a risk factor for RSV severity and a potential confounder in immune responses to RSV infection. However, in our current cohort, age at enrollment did not show a statistically significant association with disease severity (Figure 4A and Table E5A), and any significant effects on the association between immune responses and disease severity (Table E7). Of note, all patients in our current cohort are <1 year old, with 61.2% 3 months old or younger (Figure E3A). The positively skewed age distribution may explain why age at enrollment was not a significant risk factor for disease severity in our study.
Understanding the association of viral load and RSV severity is also essential for developing antiviral therapies, vaccine design, and validation of therapeutics/vaccines. However, the relationship between RSV severity and viral load is unclear. RSV viral load has been found to be directly proportional to LOS, respiratory failure, and ICU requirements (4, 40–42). Nevertheless, others have failed to identify a correlation between viral load in nasopharyngeal lavage (43) or nasal aspirates with either LOS or duration of oxygen supplement or severe bronchiolitis in either infants (44) or children (45). Our single-time-point data upon hospital admission for RSV disease also showed no significant difference in nasal aspirate viral load between infants with severe and moderate disease (Figure 3C). Like other studies (46, 47), we found that viral load in the nasal aspirate did not vary by sex, race, age at enrollment, and prematurity (Figure E2). Differences in sample size, the time point of sampling, cohort demographics, and the target viral genes for viral load analysis may explain the inconsistency of viral load data in the literature. Together with findings from these previous studies, our data, however, highlight the complexity of RSV pathogenesis, which is presumably determined by the interaction between immune responses and viral replication.
Overall, our current study examined viral load and immunological mediators from nasal aspirates collected within 24 hours of enrollment, and hence represents the interaction between viral replication and immune response at a more relevant infected site and at an earlier phase of infection relative to other studies (23, 25, 48). Using nasal aspirate collection, which is more accessible and less invasive than peripheral blood collection, we demonstrate a feasible method for characterizing cellular and humoral components of mucosa innate immune responses. Thus, our findings reveal the significant association of elevated levels of IL-4, IL-13, IL-33, and ILC2s in nasal aspirates with severe RSV disease, and a strong correlation between the frequencies of ILC2s and their associated cytokines (IL-4 and IL-13) and inducing cytokine (IL-33) in nasal aspirates from RSV-infected infants. Although IL-33/ILC2 axis–mediated type-2–biased responses have been shown in neonatal experimental models, these represent the first data on IL-33 and ILC2 responses in RSV-infected infants at the site of infection. Therefore, our data provide a translation from our mouse model (11) to human patients and suggest the important role of ILC2s in shaping the immune response in the early phase of infection. We hypothesized that levels of ILC2s and their associated cytokines at the early phase of infection determine the balance of Th1/2–adaptive immune responses at the latter phase of infection in infants with RSV. Our future studies with longitudinally collected nasal aspirates will delineate this possibility.
Our current studies have certain limitations. Due to the difficulty of finding cells in nasal aspirates from noninfected infants, we did not use uninfected infants as the control population. In addition, the bioactivity of IL-33 has been elegantly demonstrated to be highly susceptible to oxidation (49). Unfortunately, commercial reagents to specifically detect nonoxidized forms of IL-33 in human specimens were not available at the time of our studies, and thus precluded us from examining these forms of IL-33. However, the enhanced levels of IL-33 coinciding with the elevated ILC2 numbers/frequencies in nasal aspirates from infants with severe RSV disease strongly suggest the association of IL-33/ILC2 with RSV severity. Considering that more patients with severe disease tend to have higher levels of extracellular mediators, our cytokine data from nasal aspirates are not normalized for sample contents (e.g., by total protein) and are presented as per sample volume. This is consistent with how viral load is also quantified. Another limitation of our study was that we did not examine IL-5, another ILC2-associated cytokine associated with induction of eosinophil responses early in infection. These limitations can be addressed in further cohorts.
Acknowledgments
Acknowledgment
The authors are grateful to the patients and their parents/guardians who participated in this research and made it possible.
Footnotes
Supported by NIH (National Institute of Allergy and Infectious Diseases) grant AI090059.
Author Contributions: L.D.V. designed and executed data collection, performed data analyses, and drafted the manuscript; D.Y. helped design the study; D.S. and R.T. performed data collection; T.L.J. performed statistical analyses; J.D.V. and his laboratory enrolled study subjects, collected clinical data and nasal aspirates, and edited the manuscript; S.A.C. conceptualized and designed the study and drafted the manuscript; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201812-2366OC on June 25, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Paes B. Respiratory syncytial virus in otherwise healthy prematurely born infants: a forgotten majority. Am J Perinatol. 2018;35:541–544. doi: 10.1055/s-0038-1637762. [DOI] [PubMed] [Google Scholar]
- 2.Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360:588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El Saleeby CM, Bush AJ, Harrison LM, Aitken JA, Devincenzo JP. Respiratory syncytial virus load, viral dynamics, and disease severity in previously healthy naturally infected children. J Infect Dis. 2011;204:996–1002. doi: 10.1093/infdis/jir494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeVincenzo JP, El Saleeby CM, Bush AJ. Respiratory syncytial virus load predicts disease severity in previously healthy infants. J Infect Dis. 2005;191:1861–1868. doi: 10.1093/infdis/jir494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olchanski N, Hansen RN, Pope E, D’Cruz B, Fergie J, Goldstein M, et al. Palivizumab prophylaxis for respiratory syncytial virus: examining the evidence around value. Open Forum Infect Dis. 2018;5:ofy031. doi: 10.1093/ofid/ofy031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russell CD, Unger SA, Walton M, Schwarze J. The human immune response to respiratory syncytial virus infection. Clin Microbiol Rev. 2017;30:481–502. doi: 10.1128/CMR.00090-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mjösberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, et al. Human IL-25– and IL-33–responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12:1055–1062. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- 8.Dhariwal J, Cameron A, Trujillo-Torralbo M-B, Del Rosario A, Bakhsoliani E, Paulsen M, et al. MRC-GSK Strategic Alliance Consortium. Mucosal type 2 innate lymphoid cells are a key component of the allergic response to aeroallergens. Am J Respir Crit Care Med. 2017;195:1586–1596. doi: 10.1164/rccm.201609-1846OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poposki JA, Klingler AI, Tan BK, Soroosh P, Banie H, Lewis G, et al. Group 2 innate lymphoid cells are elevated and activated in chronic rhinosinusitis with nasal polyps. Immun Inflamm Dis. 2017;5:233–243. doi: 10.1002/iid3.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TKA, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saravia J, You D, Shrestha B, Jaligama S, Siefker D, Lee GI, et al. Respiratory syncytial virus disease is mediated by age-variable IL-33. PLoS Pathog. 2015;11:e1005217. doi: 10.1371/journal.ppat.1005217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saluzzo S, Gorki A-D, Rana BMJ, Martins R, Scanlon S, Starkl P, et al. First-breath-induced type 2 pathways shape the lung immune environment. Cell Reports. 2017;18:1893–1905. doi: 10.1016/j.celrep.2017.01.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.García-García ML, Calvo C, Moreira A, Cañas JA, Pozo F, Sastre B, et al. Thymic stromal lymphopoietin, IL-33, and periostin in hospitalized infants with viral bronchiolitis. Medicine (Baltimore) 2017;96:e6787. doi: 10.1097/MD.0000000000006787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perkins SM, Webb DL, Torrance SA, El Saleeby C, Harrison LM, Aitken JA, et al. Comparison of a real-time reverse transcriptase PCR assay and a culture technique for quantitative assessment of viral load in children naturally infected with respiratory syncytial virus. J Clin Microbiol. 2005;43:2356–2362. doi: 10.1128/JCM.43.5.2356-2362.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Resch B, Gusenleitner W, Müller W. The impact of respiratory syncytial virus infection: a prospective study in hospitalized infants younger than 2 years. Infection. 2002;30:193–197. doi: 10.1007/s15010-002-2122-1. [DOI] [PubMed] [Google Scholar]
- 16.Horn SD, Smout RJ. Effect of prematurity on respiratory syncytial virus hospital resource use and outcomes. J Pediatr. 2003;143(Suppl):S133–S141. doi: 10.1067/s0022-3476(03)00509-2. [DOI] [PubMed] [Google Scholar]
- 17.Gunville CF, Sontag MK, Stratton KA, Ranade DJ, Abman SH, Mourani PM. Scope and impact of early and late preterm infants admitted to the PICU with respiratory illness. J Pediatr. 2010;157:209–214, e1. doi: 10.1016/j.jpeds.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLaurin KK, Farr AM, Wade SW, Diakun DR, Stewart DL. Respiratory syncytial virus hospitalization outcomes and costs of full-term and preterm infants. J Perinatol. 2016;36:990–996. doi: 10.1038/jp.2016.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ballow M, Cates KL, Rowe JC, Goetz C, Desbonnet C. Development of the immune system in very low birth weight (less than 1500 g) premature infants: concentrations of plasma immunoglobulins and patterns of infections. Pediatr Res. 1986;20:899–904. doi: 10.1203/00006450-198609000-00019. [DOI] [PubMed] [Google Scholar]
- 20.Conway SP, Dear PR, Smith I. Immunoglobulin profile of the preterm baby. Arch Dis Child. 1985;60:208–212. doi: 10.1136/adc.60.3.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tissières P, Ochoda A, Dunn-Siegrist I, Drifte G, Morales M, Pfister R, et al. Innate immune deficiency of extremely premature neonates can be reversed by interferon-γ. PLoS One. 2012;7:e32863. doi: 10.1371/journal.pone.0032863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rallabhandi P, Phillips RL, Boukhvalova MS, Pletneva LM, Shirey KA, Gioannini TL, et al. Respiratory syncytial virus fusion protein–induced Toll-like receptor 4 (TLR4) signaling is inhibited by the TLR4 antagonists Rhodobacter sphaeroides lipopolysaccharide and eritoran (E5564) and requires direct interaction with MD-2. MBio. 2012;3:e00218–12. doi: 10.1128/mBio.00218-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Román M, Calhoun WJ, Hinton KL, Avendaño LF, Simon V, Escobar AM, et al. Respiratory syncytial virus infection in infants is associated with predominant Th-2–like response. Am J Respir Crit Care Med. 1997;156:190–195. doi: 10.1164/ajrccm.156.1.9611050. [DOI] [PubMed] [Google Scholar]
- 24.Legg JP, Hussain IR, Warner JA, Johnston SL, Warner JO. Type 1 and type 2 cytokine imbalance in acute respiratory syncytial virus bronchiolitis. Am J Respir Crit Care Med. 2003;168:633–639. doi: 10.1164/rccm.200210-1148OC. [DOI] [PubMed] [Google Scholar]
- 25.Hassan MA, Eldin AM, Ahmed MM. T-helper2/T-helper1 imbalance in respiratory syncytial virus bronchiolitis in relation to disease severity and outcome. Egypt J Immunol. 2008;15:153–160. [PubMed] [Google Scholar]
- 26.Caballero MTSM, Serra ME, Acosta PL, Marzec J, Gibbons L, Salim M, et al. TLR4 genotype and environmental LPS mediate RSV bronchiolitis through Th2 polarization. J Clin Invest. 2015;125:571–582. doi: 10.1172/JCI75183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bendelja K, Gagro A, Bace A, Lokar-Kolbas R, Krsulovic-Hresic V, Drazenovic V, et al. Predominant type-2 response in infants with respiratory syncytial virus (RSV) infection demonstrated by cytokine flow cytometry. Clin Exp Immunol. 2000;121:332–338. doi: 10.1046/j.1365-2249.2000.01297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Díaz PV, Valdivia G, Gaggero AA, Bono MR, Zepeda G, Rivas M, et al. Pro-inflammatory cytokines in nasopharyngeal aspirate from hospitalized children with respiratory syncytial virus infection with or without rhinovirus bronchiolitis, and use of the cytokines as predictors of illness severity. Medicine (Baltimore) 2015;94:e1512. doi: 10.1097/MD.0000000000001512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bertrand P, Lay MK, Piedimonte G, Brockmann PE, Palavecino CE, Hernández J, et al. Elevated IL-3 and IL-12p40 levels in the lower airway of infants with RSV-induced bronchiolitis correlate with recurrent wheezing. Cytokine. 2015;76:417–423. doi: 10.1016/j.cyto.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 30.Thwaites RS, Coates M, Ito K, Ghazaly M, Feather C, Abdulla F, et al. Reduced nasal viral load and IFN responses in infants with respiratory syncytial virus bronchiolitis and respiratory failure. Am J Respir Crit Care Med. 2018;198:1074–1084. doi: 10.1164/rccm.201712-2567OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pinto RA, Arredondo SM, Bono MR, Gaggero AA, Díaz PV. T helper 1/T helper 2 cytokine imbalance in respiratory syncytial virus infection is associated with increased endogenous plasma cortisol. Pediatrics. 2006;117:e878–e886. doi: 10.1542/peds.2005-2119. [DOI] [PubMed] [Google Scholar]
- 32.Chen ZM, Mao JH, Du LZ, Tang YM. Association of cytokine responses with disease severity in infants with respiratory syncytial virus infection. Acta Paediatr. 2002;91:914–922. doi: 10.1080/080352502760272588. [DOI] [PubMed] [Google Scholar]
- 33.Bermejo-Martin JF, Garcia-Arevalo MC, De Lejarazu RO, Ardura J, Eiros JM, Alonso A, et al. Predominance of Th2 cytokines, CXC chemokines and innate immunity mediators at the mucosal level during severe respiratory syncytial virus infection in children. Eur Cytokine Netw. 2007;18:162–167. doi: 10.1684/ecn.2007.0096. [DOI] [PubMed] [Google Scholar]
- 34.Gold MJ, Antignano F, Halim TY, Hirota JA, Blanchet MR, Zaph C, et al. Group 2 innate lymphoid cells facilitate sensitization to local, but not systemic, TH2-inducing allergen exposures. J Allergy Clin Immunol. 2014;133:1142–1148. doi: 10.1016/j.jaci.2014.02.033. [DOI] [PubMed] [Google Scholar]
- 35.Pelly VS, Kannan Y, Coomes SM, Entwistle LJ, Rückerl D, Seddon B, et al. IL-4–producing ILC2s are required for the differentiation of TH2 cells following Heligmosomoides polygyrus infection. Mucosal Immunol. 2016;9:1407–1417. doi: 10.1038/mi.2016.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mirchandani AS, Besnard AG, Yip E, Scott C, Bain CC, Cerovic V, et al. Type 2 innate lymphoid cells drive CD4+ Th2 cell responses. J Immunol. 2014;192:2442–2448. doi: 10.4049/jimmunol.1300974. [DOI] [PubMed] [Google Scholar]
- 37.Noval Rivas M, Burton OT, Oettgen HC, Chatila T. IL-4 production by group 2 innate lymphoid cells promotes food allergy by blocking regulatory T-cell function. J Allergy Clin Immunol. 2016;138:801–811, e9. doi: 10.1016/j.jaci.2016.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jia Y, Fang X, Zhu X, Bai C, Zhu L, Jin M, et al. IL-13+ type 2 innate lymphoid cells correlate with asthma control status and treatment response. Am J Respir Cell Mol Biol. 2016;55:675–683. doi: 10.1165/rcmb.2016-0099OC. [DOI] [PubMed] [Google Scholar]
- 39.Kristjansson S, Bjarnarson SP, Wennergren G, Palsdottir AH, Arnadottir T, Haraldsson A, et al. Respiratory syncytial virus and other respiratory viruses during the first 3 months of life promote a local TH2-like response. J Allergy Clin Immunol. 2005;116:805–811. doi: 10.1016/j.jaci.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 40.Fodha I, Vabret A, Ghedira L, Seboui H, Chouchane S, Dewar J, et al. Respiratory syncytial virus infections in hospitalized infants: association between viral load, virus subgroup, and disease severity. J Med Virol. 2007;79:1951–1958. doi: 10.1002/jmv.21026. [DOI] [PubMed] [Google Scholar]
- 41.Zhou L, Xiao Q, Zhao Y, Huang A, Ren L, Liu E. The impact of viral dynamics on the clinical severity of infants with respiratory syncytial virus bronchiolitis. J Med Virol. 2015;87:1276–1284. doi: 10.1002/jmv.24111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hasegawa K, Jartti T, Mansbach JM, Laham FR, Jewell AM, Espinola JA, et al. Respiratory syncytial virus genomic load and disease severity among children hospitalized with bronchiolitis: multicenter cohort studies in the United States and Finland. J Infect Dis. 2015;211:1550–1559. doi: 10.1093/infdis/jiu658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Souza AP, Leitao LA, Luisi F, Souza RG, Coutinho SE, Silva JR, et al. Lack of association between viral load and severity of acute bronchiolitis in infants. J Bras Pneumol. 2016;42:261–265. doi: 10.1590/S1806-37562015000000241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wright PF, Gruber WC, Peters M, Reed G, Zhu Y, Robinson F, et al. Illness severity, viral shedding, and antibody responses in infants hospitalized with bronchiolitis caused by respiratory syncytial virus. J Infect Dis. 2002;185:1011–1018. doi: 10.1086/339822. [DOI] [PubMed] [Google Scholar]
- 45.Franz A, Adams O, Willems R, Bonzel L, Neuhausen N, Schweizer-Krantz S, et al. Correlation of viral load of respiratory pathogens and co-infections with disease severity in children hospitalized for lower respiratory tract infection. J Clin Virol. 2010;48:239–245. doi: 10.1016/j.jcv.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Espinosa Y, San Martín C, Torres AA, Farfán MJ, Torres JP, Avadhanula V, et al. Genomic loads and genotypes of respiratory syncytial virus: viral factors during lower respiratory tract infection in chilean hospitalized infants. Int J Mol Sci. 2017;18:654. doi: 10.3390/ijms18030654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feikin DR, Fu W, Park DE, Shi Q, Higdon MM, Baggett HC, et al. PERCH Study Group. Is higher viral load in the upper respiratory tract associated with severe pneumonia? Findings from the PERCH study. Clin Infect Dis. 2017;64:S337–S346. doi: 10.1093/cid/cix148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Houben ML, Coenjaerts FE, Rossen JW, Belderbos ME, Hofland RW, Kimpen JL, et al. Disease severity and viral load are correlated in infants with primary respiratory syncytial virus infection in the community. J Med Virol. 2010;82:1266–1271. doi: 10.1002/jmv.21771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cohen ES, Scott IC, Majithiya JB, Rapley L, Kemp BP, England E, et al. Oxidation of the alarmin IL-33 regulates ST2-dependent inflammation. Nat Commun. 2015;6:8327. doi: 10.1038/ncomms9327. [DOI] [PMC free article] [PubMed] [Google Scholar]