Abstract
Objectives
To identify predictors of overall (OS) and liver progression-free survival (LPFS) following 90Y radioembolization (RAE) of heavily pretreated patients with colorectal cancer liver metastases (CLM). Create and validate a predictive nomogram for OS.
Materials and Methods
Metabolic, anatomic, laboratory, pathologic, genetic, primary-disease and procedure-related factors, as well as pre- and post-RAE therapies in 103 CLM patients treated with RAE from 9/15/2009 to 3/21/2017 were analyzed. LPFS was defined by RECIST 1.1 and EORTC criteria. Prognosticators of OS and LPFS were selected using univariate Cox regression, adjusted for clustering and competing risk analysis (for LPFS) and subsequently tested in multivariate analysis (MVA). The nomogram was built using R statistical software and internally validated using bootstrap resampling.
Results
Patients received RAE at a median of 30.9 months (range: 3.4–161.7 months) after detection of CLM. Median OS and LPFS were 11.3 (95%CI, 7.9–15.1) and 4 months (95%CI, 3.3–4.8), respectively. Of the 40 parameters tested, six were independently associated with OS in MVA. These baseline parameters included: number of extrahepatic disease sites (p<0.001), carcinoembryonic antigen (CEA) (p<0.001), albumin (p=0.005), alanine aminotransferase (ALT) level (p<0.001), tumor differentiation level (p<0.001) and sum of the two largest tumor diameters (p<0.001). One-year OS of patients with total points of <25 vs. >80 was 90% and 10%, respectively. Bootstrap resampling showed good discrimination (optimism corrected c-index=0.745) and calibration (mean absolute prediction error=0.299) of the nomogram. Only baseline standard uptake value (SUVmax) was significant in MVA for LPFS prediction (p<0.001, SHR=1.06).
Conclusion
The developed nomogram included six pre-RAE parameters and provided good prediction of survival post-RAE in heavily pretreated patients. Baseline SUVmax was the single significant predictor of LPFS.
Keywords: Radioembolization; Nomogram; Yttrium-90; Hepatic Malignancy; Colorectal Cancer Liver Metastases; Arterially directed therapies; Selective Internal Radiation Therapy; SIRT, Liver Tumors
MICROABSTRACT
One-year overall survival prediction nomogram included six easy-to-obtain pre-RAE parameters and provided good prediction of overall survival post-RAE. This can be useful for pre-treatment patient stratification and counseling of heavily pretreated patients with CLM. Baseline SUVmax predicted liver progression-free survival.
INTRODUCTION
90Y radioembolization (RAE) is an FDA approved liver brachytherapy, recommended through the National Comprehensive Cancer Network (NCCN) and the European Society for Medical Oncology (ESMO) guidelines for the treatment of colorectal cancer liver metastases (CLM) in the salvage setting with liver disease progression while on or after second line chemotherapy with encouraging oncologic outcomes (1–18).
RAE performed at this advanced stage of a terminal disease has the goal of prolonging patient survival with limited if any impact on quality of life. Currently a decision for RAE is recommended through a multidisciplinary discussion (7). In clinical practice, recommendations for refractory patients are challenging, because this population often presents with advanced tumor load, unfavorable biologic tumor characteristics, comorbidities or poor performance status. It is thus not surprising, that the range of reported outcomes post-RAE is highly variable. Objective response rates varied between 10%−48% when RAE was applied in the third and subsequent chemotherapy regiment setting (10, 17, 19, 20). Thus, careful patient selection is extremely important to minimize the risk of treatment-related complications and unnecessary hospitalization. Current exclusion criteria do not always provide adequate risk stratification or an optimal estimation of patient survival. Poor outcome may be a consequence of more extensive disease, but may also be associated with RAE-induced adverse events. A predictive scoring system can aid a rational decision with estimation of each patient’s risk/benefit ratio prior to undergoing RAE.
The aim of this study was to analyze metabolic, anatomic, laboratory, pathologic, primary disease-related and genetic biomarkers, procedure-related factors as wells as pre- and post-RAE therapies, that can be associated with liver progression-free and overall survival. In addition, our goal was to create and internally validate a predictive survival nomogram using pre-RAE patient characteristics.
MATERIALS AND METHODS
1. Ethical considerations and patient selection
IRB waiver of approval was obtained for this retrospective review of our prospectively created and maintained HIPAA registered and compliant colorectal cancer liver metastases (CLM) RAE database. All patients with CLM treated with 90Y RAE from 9/15/2009 to 3/21/2017 were included.
2. Inclusion and exclusion criteria
All patients treated with RAE were eligible for inclusion in the study.
Eligibility criteria for RAE in our practice included: age ≥18 years; Eastern Cooperative Oncology Group (ECOG) performance status 0–2; histologically confirmed primary adenocarcinoma of the colon or rectum; CLM considered unresectable or not amenable to percutaneous ablation; adequate blood cell counts (WBC > 1.5 × 109/L, platelet count > 50 × 109/L); adequate renal function (creatinine < 1.5 mg/dL) and total bilirubin level ≤ 1.5 mg/dL.
Exclusion criteria were: prior hepatic radiotherapy; severe cirrhosis; severe portal hypertension; uncorrectable flow to the gastrointestinal tract and/or >20% shunting to the lungs, as determined by technetium-99m labeled macroaggregated albumin (99mTc-MAA) hepatic arterial perfusion scintigram (mapping). All patients at the time of RAE had liver-dominant disease and were considered candidates for RAE even in the face of oligometastatic (up to 5 sites) extrahepatic disease that was stable or controlled by chemotherapy.
3. Pre-procedural work – up and angiographic mapping
All patients were evaluated at a clinic visit within 30 days before RAE. Past medical history was reviewed, physical examination was performed and relevant baseline laboratory values were evaluated. Pre-procedural baseline imaging with liver dynamic (ideally triphasic) CT and 18F-FDG PET/CT was available within 30 days from RAE for accurate re-staging of disease and for calculation of liver and CLM volumes for 90Y dosimetry.
All patients underwent angiographic evaluation and 99mTc-MAA mapping prior to RAE. During arteriography, hepatic arterial anatomy and tumor vascular supply were assessed. Extrahepatic vessels with hepatofugal flow within 3 cm from the desired point of 90Y administration were prophylactically coil-embolized to prevent inadvertent delivery of 90Y in extrahepatic sites. Once the desired location(s) of RAE administration(s) was determined, a total of 4–5 mCi of 99mTc-MAA were injected at desired sites of 90Y arterial infusion and subsequent planar scintigraphy and SPECT/CT imaging were performed to calculate the lung shunt fraction and to detect extrahepatic activity (17).
4. Radioembolization procedure
Approximately two weeks after mapping angiography, patients underwent RAE with SIR-Spheres (Sirtex Medical, Sydney, Australia) or glass microspheres (Therasphere; MDS Nordion, Ottawa, Ontario, Canada). Glass-based microspheres were introduced in March 2015 and were specifically used instead of SIR-spheres in patients with higher risk of developing stasis: in the presence of constricted or small caliber arteries as well as in patients with history of prior non-anatomic liver resections or intra-arterial hepatic infusion pump chemotherapy, who presented with tortuous or constricted vessels at mapping.
As of April 2013 90Y resin microspheres were administered using undiluted contrast medium, which allowed real-time infusion monitoring and resulted in shorter infusion time and reduced fluoroscopy radiation dose as previously described (18).
The total activity of 90Y resin microsphere (in GBq) for each patient was calculated using body surface area (BSA) method with the following formula (21):
SIR-spheres Activity = (treatment liver volume / total liver volume) * [(BSA-0.2) + (fraction of liver with tumor)]
For 90Y glass microspheres, total activity of 90Y was calculated using the following formula (7, 22):
Therasphere activity (GBq)= Desired dose (Gy)×Mass of selected live target lobe/50
In cases where lung shunting was between 10–15%, patients received a 20% reduction of the calculated radiation dose. Adjustments were not made based on prior treatment history.
CLMs confined to one lobe were treated in one session whereas bilobar disease was treated in two sessions separated by 4–8 weeks. If the limited extent of disease permitted sublobar microspheres infusions, the patients with bilobar disease were also treated within one session (as long as not more than 50% of liver parenchyma was exposed to the radiation at each session). Patients with a single hepatic lobe post-resection were treated in lobar or sublobar fashion depending on the extent and location of disease. In general, we aimed to treat as selectively as possible to treat all tumors while sparing uninvolved liver parenchyma.
5. Study objectives
Primary objectives included:
-
1)
Analysis of liver progression-free survival (LPFS) and factors associated with LPFS;
LPFS was defined as the time between the treatment date until disease progression or death/last follow- up. It was calculated using competing risk analysis. If there was no liver disease progression and no imaging available within 6 months before death, then the date of last imaging was included in competing risk analysis. If there was no liver disease progression and there was imaging within 6-months before death available, death date was included in the competing risk analysis. LPFS was evaluated using EORTC and RECIST 1.1 criteria, depending on imaging availability. In cases of differing responses between the criteria, the concordance between both modalities imaging findings was used to determine LPFS;
-
2)
Overall survival (OS) and factors associated with it;
OS was defined as the time from initial RAE to patient death or last follow-up.
Secondary objectives included:
-
1)
Evaluation of local tumor response and objective response rate (ORR);
-
2)
Assessment of radiologic response within the treated hepatic territory.
It was performed using contrast-enhanced CT and 18F-FDG PET/CT at 4–8 and 12–16 weeks post-RAE and compared to the pre-RAE scans. Scans at 4–8 weeks were used as the new baseline for subsequent imaging obtained every 2–4 months thereafter. Radiologic response in the liver was assessed by changes in size and metabolic activity using RECIST 1.1 and European Organization for Research and Treatment of Cancer criteria (EORTC). Using EORTC criteria, 25% threshold was chosen to define response or disease progression, using the sum of up to 5 liver lesions maximum standard uptake values (SUVmax). In patients with two RAE sessions, response was evaluated for each treated region. ORR was defined as the percentage of patients with partial or complete response within 12–16 weeks post-RAE, based on RECIST 1.1 and EORTC criteria.
6. Prognosticators of LPFS and OS
Total of 24 factors were analyzed as potential prognosticators of LPFS. They included primary disease-related variables, liver tumor burden, CEA level, genetic mutations (KRAS, BRAF, PI3KCA), prior and post-RAE systemic regimens for metastatic CRC, procedure-related parameters (sphere type, delivered radiation dose and occurrence of stasis), pre-RAE laboratory parameters {(neutrophil/lymphocyte ratio (NLR) and platelet/lymphocyte ratio (PLR)} and metabolic tumor uptake parameters {(SUVmax, SUVpeak, SUVmean, metabolic tumor volume (MTV) and total lesion glycolysis (TLG)} of the most metabolically active tumor.
Forty factors were analyzed as potential prognosticators of OS. In addition to the factors described above, we included the following factors to overall survival analysis: patients’ age, pre-RAE liver function laboratory parameters, primary disease-related variables, CEA level, pre-and post-RAE therapies, post-RAE NLR and PLR, disease-free survival, time from liver metastases diagnosis to RAE, LPFS and number of extrahepatic disease sites.
7. Statistical analysis
The median follow-up period was calculated for the patients who were alive on last follow-up. Prognosticators of OS and LPFS were selected using univariate Cox regression, adjusted for clustering and competing risk analysis (for LPFS) and subsequently tested in multivariate analysis (MVA). Only pre-treatment easy-to-obtain and well-established parameters were included into MVA as potential predictors of overall survival. All the nomogram factors were included as continuous variables (except for tumor differentiation level) for more accurate estimation of each factor’s predictive value. Liver PFS association with OS was assessed using Cox regression analysis, including only the patients, who had liver disease progression.
The nomogram was built using R statistical software and internally validated using bootstrap resampling. Statistical analysis was performed using STATA 12.1 software. A p-value <0.05 was considered significant.
RESULTS
1. Study population
One hundred and three patients treated with 136 RAE sessions were included in the study.
Median follow-up time was 9.02 months (range, 0.7–42.7 months). Sixty-three/103 (61%) patients were men, 40 (39%) – women, with a median age of 60 years (range, 24–87 years). Seventy-six/103 (74%) patients had synchronous liver metastases. The median time from diagnosis of CLM to RAE was 30.9 months (range: 3.4–161.7 months).
Eighty-one/103 (79%) of patients were treated with resin microspheres, 21/103 (20%) - with glass microspheres, 1/103 (1%) with both (initially with resin and LTP retreatment with glass microspheres almost 3-years later). Seventy-three/103 (71%) of patients were treated in one RAE session, 26/103 (25%)– in two sessions. Seven/103 (7%) of patients underwent repeat RAE for: (1) LTP in previously treated region (in 3 patients); (2) progression of disease in untreated liver lobe (in 3 patients); (3) additional RAE in the same region due to poor tumor coverage during initial RAE (in 1 patient; partial recanalization of embolized vessel precluded flow redirection to a tumor region during initial RAE). Patient characteristics are described in Table 1.
Table 1.
Patient demographics and disease characteristics.
| Patient demographics and disease characteristics | Number of patients |
|---|---|
| Age (median, range) | 60 (range. 24–87 years) |
| Gender, M: F | 63: 40 |
| Synchronous liver disease | 81: 22 |
| Pathologic evidence of vascular invasion from primary tumor (yes/no/unknown) | 44: 33: 26 |
| KRAS mutation (yes/no/unknown) | 29: 33: 16 |
| PIK3CA mutation (yes/no/unknown) | 10: 41: 52 |
| Surgical resection of primary disease (yes/no/unknown) | 82: 20: 1 |
| CEA level before RAE (median, range ng/ml) | 55.3 (1.1–23937.6) |
| Time from diagnosis of CRC to diagnosis of CLM (mean, median, range, in months) | 6.3; 0 (range 0–64.2) |
| Time from diagnosis of CLM to RAE (median, range) | 30.9 (range. 3.4–161.7) |
| Type of microspheres (resin/glass/both) | 81: 21: 1 |
| Prior liver surgery (yes/no) | 49: 54 |
| Prior HAIP chemotherapy (yes/no) | 53: 50 |
| Prior systematic chemotherapy (≥3 lines vs. <3 lines) | 30: 73 |
| Prior bevacizumab (yes/no) | 63: 40 |
| Extent of hepatic replacement by tumor at time of RAE (<25%; ≥25%) | 89: 14 |
| Presence of extrahepatic disease at time of RAE (yes/no) | 74: 29 |
| Number of confirmed metastatic extrahepatic organ systems at time of RAE (0: 1: 2: 3: 4) | 29: 38: 24: 9: 3 |
| ECOG status at time of RE (Grade 0/1/2/unknown) | 33: 26: 2: 42 |
| Distribution of hepatic disease at time of RAE (unilobar/unilobar with one hepatic lobe center/bilobar) | 17: 11: 75 |
| Incidence of stasis in resin microspheres infusions | 39/146 (27%) |
| % of prescribed radiation dose delivered to tumor per procedure | 96% (range. 19–106%) |
| Number of RAE sessions per patients (one/two) | 73: 30 |
| Post-RAE HAIP* therapy (yes/no) | 18: 85 |
| Number of post-RAE systemic chemotherapy lines (0: 1: 2: 3: 4: unknown) | 25: 39: 22: 15: 1 |
| Post-RAE ablation (yes/no) | 5: 98 |
HAIP-hepatic arterial infusion pump therapy; CEA-carcinoembryonic antigen level; CRC-colorectal cancer; CLM-colorectal liver metastases; RAE-radioembolization.
2. Local tumor response, objective response rate and liver progression-free survival
On the first imaging post-RAE at 4–8 weeks, 39% of patients had partial response according to EORTC criteria, whereas 59% had stable disease according to RECIST 1.1. Objective response rate (complete or partial response) at 12–16 weeks post-RAE was 44/84 (52%) by EORTC and 6/94 (6%) by RECIST 1.1 criteria. Local tumor response at initial 4–8 weeks post-RAE imaging is described in Table 2.
Table 2.
Local tumor response on first imaging after RAE (at 4–8 weeks) and at second follow-up (at 12–16 weeks).
| Response | At 4–8weeks | At 12–16 weeks | ||
|---|---|---|---|---|
| EORTC | RECIST 1.1 | EORTC | RECIST 1.1 | |
| Complete response (CR) | 5/84 (6%) | 1/94 (1%) | 3/55 (5%) | 0 |
| Partial response (PR) | 33/84 (39%) | 0 | 9/55 (16%) | 3/76 (4%) |
| Stable disease (SD) | 17/84 (20%) | 55/94 (59%) | 7/55 (13%) | 39/76 (51%) |
| Progression of disease (POD) | 20/84 (24%) | 25/94 (27%) | 35/55 (64%) | 31/76 (41%) |
| Different response in treatment regios: | ||||
| - SD+PR | 4/84 (5%) | 3/94 (3%) | - | - |
| - SD+POD | 2/84 (2%) | 9/94 (9%) | - | 3/76 (4%) |
| - PR+POD | 2 /84(2%) | 1/94 (%) | - | - |
| - SD+CR | 1/84 (1%) | 0 | - | - |
| - CR+POD | - | - | 1/55 (2%) | - |
| Not applicable (absent follow-up or baseline imaging, measurements could not be obtained due technical problems) | 19/103 (18%) | 9/103 (9%) | 48/103 (47%) | 27/103(26%) |
LTP-local tumor progression, CR-complete response, PR-partial response, SD-stable disease, POD-progression of disease.
Despite the lack of statistical significance, the patients who developed early/late stasis in at least one infusion during 90Y RAE, had roughly double the risk for local tumor progression on initial scan, defined by RECIST 1.1 (p= 0.19, OR=1.81) or EORTC criteria (p=0.14, OR=2.2).
Despite the lack of statistical significance, the patients who had ≥ 96% of prescribed radiation dose delivered, had 44% lower risk of local tumor progression at first imaging, compared to patients who received <96% of the dose, when LTP was defined by EORTC criteria (p=0.32, OR=0.56).
Sphere type was neither associated with local tumor progression on first follow-up, evaluated by RECIST1.1 or EORTC criteria (p=0.99 for both), nor with liver progression-free survival (p=0.77, SHR=1.08).
3. Liver progression-free survival (LPFS) and its prognosticators
Median LPFS was 4 months (95%CI, 3.4–4.9 months). Six-months and 1- year LPFS were 27% (95%CI,18–36%) and 9% (95%CI, 3–16%).
Only increased metabolic tumor parameters (SUVmax, SUVpeak, MTV and TLG) and decreased time from CLM diagnosis to RAE were significantly associated with decreased liver progression-free survival on univariate analysis (Table 3). Due to correlation between metabolic parameters, only two variables (SUVmax and time from CLM diagnosis to RAE) were included in the multivariate analysis (MV). Only SUVmax was significant predictor of LPFS on MV analysis (p= 0.003, HR=1.06, 95%CI, 1.02–1.1).
Table 3.
Univariate analysis for prognosticators of overall survival (OS) and liver progression-free survival (LPFS) post-RAE.
| Nr | Variable group | Variable | OS, p-value |
HR | LPFS, p-value |
SHR | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Patient variable | Age | 0.23 | 0.76 | - | - | ||||||
| 2 | Time variable | Time from diagnosis of CLM to RAE | 0.06 | 0.99 | 0.034* | 0.99 | ||||||
| 3 | Disease-free interval (from primary Rx to CLM) | 0.07 | 0.99 | - | - | |||||||
| 4 | Primary disease-related variables | Primary tumor resection (no/yes) | 0.52 | 0.82 | - | - | ||||||
| 5 | Nodal status of the primary (positive/negative) | 0.77 | 0.33 | - | - | |||||||
| 6 | Lymphovascular invasion of primary (yes/no) | 0.03* | 1.74 | 0.35 | 0.79 | |||||||
| 7 | Tumor differentiation level (good/moderate vs. poor) | 0.009* | 2.05 | 0.55 | 0.81 | |||||||
| 8 | Synchronous vs. metachronous liver metastases | 0.24 | 1.31 | 0.31 | 1.27 | |||||||
| 9 | Side of primary (left vs. right; transverse excluded) | 0.33 | 0.77 | 0.45 | 1.25 | |||||||
| 10 | Disease-related variables at time of RAE | Liver tumor burden (≥25% vs. <25%) | 0.056 | 1.77 | 0.74 | 1.12 | ||||||
| 11 | Number of EHD sites (range, 0–4) | 0.03 * | 1.26 | - | - | |||||||
| 12 | Sum of largest diameters of two liver lesions (cm)* | 0.001* | 1.08 | 0.95 | 1 | |||||||
| 13 | CEA level at time of RAE (ng/ml) | <0.001* | 1.0001 | 0.07 | 1.0005 | |||||||
| 14 | Genetic mutations | KRAS (positive/negative) | 0.16 | 1.45 | 0.15 | 1.48 | ||||||
| 15 | PI3KCA (positive/negative) | 0.78 | 1.13 | 0.69 | 0.79 | |||||||
| 16 | BRAF (positive/negative) | 0.88 | 0.89 | 0.2 | 1.38 | |||||||
| 17 | Prior to RAE variables | Prior HAIP (no/yes) | 0.76 | 0.88 | 0.71 | 0.92 | ||||||
| 18 | Prior systemic therapy (≥3 lines vs. < 3 lines) | 0.84 | 1.05 | 0.55 | 0.87 | |||||||
| 19 | Prior liver surgery | 0.36 | 0.82 | 0.75 | 0.93 | |||||||
| 20 | RAE-related variables | % of prescribed radiation dose delivered | 0.43 | 1.004 | 0.6 | 1.003 | ||||||
| 21 | Sphere type (resin vs. glass microspheres) | 0.77 | 0.92 | 0.87 | 0.95 | |||||||
| 22 | Occurrence of stasis (yes/no) | - | - | 0.73 | 0.93 | |||||||
| 23 | Metabolic tumor uptake parameters (of 1– 5 most FDG-avid lesions) | SUV max (continuous) | 0.028* | 1.05 | <0.001* | 1.06 | ||||||
| 24 | SUV peak (continuous) | 0.025* | 1.06 | 0.001* | 1.08 | |||||||
| 25 | SUV mean (continuous) | 0.004* | 1.13 | 0.41 | 1.03 | |||||||
| 26 | MTV (continuous) | <0.001* | 1.001 | <0.001* | 1.0007 | |||||||
| 27 | TLG (continuous) | <0.001* | 1.0002 | 0.046* | 1.0001 | |||||||
| 28 | Pre-RAE laboratory parameters | Pre-RAE NLR | 0.02* | 1.05 | 0.4 | 1.02 | ||||||
| 29 | Pre-RAE PLR | 0.001* | 1.002 | 0.12 | 1.001 | |||||||
| 30 | Albumin | <0.001 * | 0.36 | - | - | |||||||
| 31 | Total bilirubin | 0.4 | 1.26 | - | - | |||||||
| 32 | AST (aspartate aminotransferase) | <0.001 * | 1.02 | - | - | |||||||
| 33 | ALT (alanine aminotransferase) | 0.003* | 1.01 | - | - | |||||||
| 34 | ALP (alkaline phosphatase) | 0.001* | 1.002 | - | - | |||||||
| 35 | Post-RAE laboratory parameters/ therapies | Post-RAE NLR (neutrophil/lymphocyte ratio) | 0.077 | 1.04 | - | - | ||||||
| 36 | Post-RAE PLR (platelet/lymphocyte ratio) | 0.6 | 1.0003 | - | - | |||||||
| 37 | Post-RAE HAIP (hepatic artery infusion pump) | 0.001 * | 0.42 | - | - | |||||||
| 38 | Post-RAE ablation | 0.029* | 0.35 | - | - | |||||||
| 39 | Post-RAE chemotherapy lines (≤1 vs 2–4) | <0.001* | 0.48 | - | - | |||||||
| 40 | Post-RAE bevacizumab | 0.001* | 0.48 | - | - | |||||||
| 41 | Liver progression-free survival (in months) | 0.002* | 0.9 | - | - | |||||||
Statistically significant factors. Analyzed using Cox regression adjusted for clustering: 3 patients had 2 initial radioembolization treatments, thus were clustered. CLM-colorectal liver metastases, RAE-radioembolization, Rx-resection, EHD-extrahepatic disease, CEA-carcinoembryonic antigen level; SUV-standard uptake value, MTV-metabolic tumor volume, TLG-total lesion glycolysis; SHR-sub-hazard ratio
4. Overall survival
Median OS was 11.3 months (95% CI 7.9 – 15.1 months). Six months, 1-, 2- and 3 - year OS was 75% (95%CI, 64–81%), 46% (95%CI, 35–55%), 18% (95%CI, 11–27%) and 15% (95%CI, 8–24%), respectively. There were no deaths within 30 days post-RAE.
Total of 11/103 (11%) patients died within 3 months post- RAE. None of these deaths were considered directly or solely related to the RAE: 7/11 (64%) of the patients had evidence of liver disease progression; 8/11 (73%) patients also developed conditions, that resulted in significant morbidity and were at least partially related to RAE, but did not lead to subsequent death. Analysis of deaths within 3-months post-RAE is presented in Table 4.
Table 4.
Overview of post-procedure course of patients who expired within 3 months post-radioembolization.
| Nr | Baseline patient characteristics |
Post-RAE course/potential reason of death | Death time |
|---|---|---|---|
| 1. | Hx of HAIP; >25%TB, bilobar liver disease; CEA= 766 ng/ml; EHD sites=2 | RAE-related major toxicity (grade 3 −4 chest pain, SOB, pleural effusion in face of stable lung metastases one-month post-RAE); managed conservatively. Death cancer-related, likely due liver failure with evidence of liver POD (one-month post-RAE) and EHD POD (lungs, lymph nodes). | 1.93 months |
| 2. | Hx of HAIP; bilobar liver disease, CEA=1357 ng/ml EHD sites=4 | RAE-related major toxicity: grade 4 SOB (acute hypersensitivity to steroids post-procedure), resolved. Cancer-related death in face of EHD POD one-month post-RAE and liver POD on first follow-up; Hx of hemochromatosis. | 1.53 months |
| 3. | Hx of liver Rx and HAIP; >25% TB, bilobar liver disease, CEA=228 ng/ml EHD sites=2 | Cancer-related death, likely due liver failure (ascites/grade 3 hyperbilirubinemia/anasarca) in face of liver POD (which precluded 2nd RAE session), lung POD and bilateral pulmonary embolism/pleural effusion/IVC and RHV thrombi 1-month post-RAE (treated with anticoagulation). | 2.93 months |
| 4. | Hx of HAIP; bilobar liver disease, CEA=218ng/ml | Unknown death reason; likely liver failure in face of RFA 1-month post-RAE with grade 4 hyperbilirubinemia/fever) | 2.27 months |
| 5. | Hx of liver Rx and HAIP; EHD sites=1; ECOG=1 | RAE contributed to fatal outcome (dramatic increased of LFTs right post-RAE); also, liver POD, secondary biliary obstruction, coagulopathy, colitis, duodenal stenosis, required stenting, HBV. | 1.27 months |
| 6. | Hx of HAIP, mitomycin, ≥3 chemotherapy lines, bilobar disease, CEA=4713ng/ml | Death reason unknown, likely liver failure in patient with history of hemochromatosis (2-months post-RAE admitted for abdominal pain in outside hospital with biliary stricture, cirrhosis, ascites). | 2.9 months |
| 7. | Hx of liver Rx, HAIP, ≥3 chemotherapy lines, bilobar disease, EHD sites=2 | Post-RAE: ECOG decline (from 0 to 3, 5-days post-RAE). Could not proceed with planned 2nd RAE session due grade 3 hyperbilirubinemia in face of liver POD on first follow-up. | 2.47 months |
| 8. | Hx of HAIP, ≥3 chemotherapy lines, bilobar disease, EHD sites=2 | Post-RAE: severe abdominal, back pain/anorexia/ SOB/chest pain/ phlegm 1- week post-RAE; 11 days post-RAE admitted for pneumonia, recovered. Death cancer-related (although no records within 3 weeks before death). | 1.83 months |
| 9. | Bilobar disease, CEA=130 ng/ml, EHD sites=2, ECOG=1 | Death cancer-related in face of untreated liver POD, peritoneal carcinomatosis and not-RAE related adverse event (6 weeks post-RAE obstruction due stomach bleed (patient was on Xarelto) requiring transfusion; significant ECOG decline. | 1.77 months |
| 10. | Bilobar disease, EHD sites=2 ECOG=1 | Grade 3–4 toxicity: Post-embolization syndrome. Six weeks post-RAE urosepsis, 2 months post-RAE: brain radiotherapy for brain metastases, then altered mental status/ hypoxia due fluid overload, leptomeningeal metastases. | 2.13 months |
| 11. | Hx of HAIP; bilobar disease, CEA=404 ng/ml; EHD sites=1 | No report of RAE-related toxicity and other adverse events after RAE. | 2.3 months |
Hx-history, Rx-resection, RAE-radioembolization, TB-tumor burden, CEA-carcinoembryonic antigen level, EHD-extrahepatic disease, POD-progression of disease, RT-radiotherapy, ECOG-performance status, IVC-inferior vena cava, RHV-right hepatic vein, RFA-radiofrequency ablation, LFTs- liver function tests
5. Factors associated with overall survival: creation of predictive nomogram
Twenty-one/40 factors were associated with overall survival post-RAE in univariate analysis (Table 3). Considering the number of deaths during the study period {87/103 (84%)}, only 8 factors with low correlation between each other could be included in MV analysis.
Six/8 pre-RAE parameters included into MV analysis were significantly associated with OS and were subsequently used to build an overall survival prediction nomogram (Table 5). These factors included: (1) baseline CEA level (2) baseline ALT level (3) albumin level (4) sum of sizes of two largest CLM diameters in the treatment region (5) number of extrahepatic disease sites and (6) tumor differentiation level (Figure 1).
Table 5.
Multivariate analysis result of factors associated with overall survival post-RAE.
| Variable included to MVA | p-value | HR | 95%CI | |
|---|---|---|---|---|
| 1 | Tumor differentiation level (good/moderate vs. poor) | <0.0001 | 5.3 | 2.6–10.7 |
| 2 | Sum of largest diameters of two liver target lesions | <0.0001 | 1.15 | 1.09–1.21 |
| 3 | Number of EHD sites (range, 0–4) | 0.016 | 1.41 | 1.07–1.87 |
| 4 | CEA level at time of RAE (ng/ml) | <0.001 | 1.00009 | 1.00006–1.0001 |
| 5 | Albumin | 0.005 | 0.46 | 0.27–0.79 |
| 6 | ALT (alanine aminotransferase) | <0.0001 | 1.02 | 1.01–1.03 |
| 7 | Lymphovascular invasion on pathology of primary (yes/no) | >0.05 | Excluded by stepwise forward selection | |
| 8 | Pre-RAE NLR (neutrophil/lymphocyte ratio) | >0.05 | Excluded by stepwise forward selection | |
EHD-extrahepatic disease; CEA-carcinoembryonic antigen level, RAE-radioembolization.
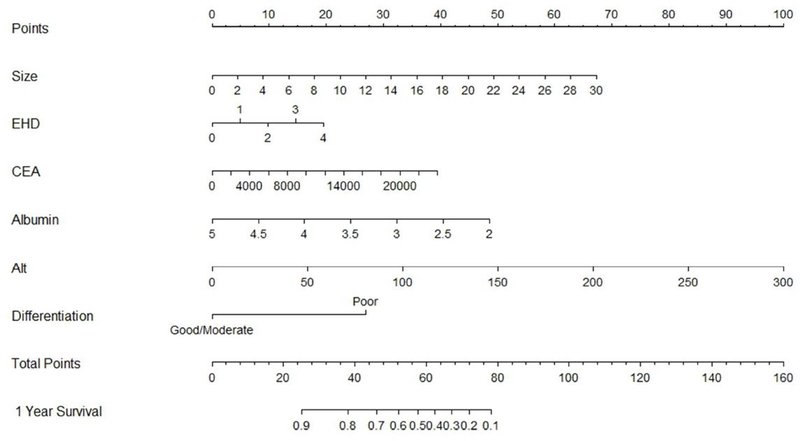
Figure 1.
Nomogram for overall survival prediction after radioembolization of colorectal cancer liver metastases. Size - sum of largest diameters of two largest liver lesions in intended to-treat-regio; EHD-number of extrahepatic disease sites; CEA-carcinoembryonic antigen level (ng/ml); Alt - alanine aminotransferase (U/L); differentiation- tumor differentiation level.
Example 1 of nomogram points calculation. The patient presented for radioembolization with following parameters: sum of two largest liver lesions diameters of 11.1 cm, two sites of extrahepatic disease, CEA 765.6 ng/ml, albumin of 2.3 g/dl, ALT of 27 U/L and poor tumor differentiation level. By drawing a straight line from the 11.1 cm point on the Size axis of the nomogram to the Points axis we find that a lesion of 11.1 cm corresponded to 25 points. Repeating the same for the other variables, we got 10 points for two sites of extrahepatic disease, 1 point for CEA of 765.6 ng/ml, 45 points for albumin of 2.3 g/dl points, 9 points for ALT of 27 U/L and 27 points for poor tumor differentiation. Sum of these points was 117 and again drawing a straight line from 117 on the Total Points axis to the 1 Year Survival prediction axis we obtained <10 % as the predicted probability of 1-year survival for this patient. The patient had actual overall survival of 1.9 months.
Example 2 of nomogram points calculation. The patient had following baseline characteristics: sum of two largest liver lesions diameters of 1.8 cm, no extrahepatic disease, CEA 2.9 ng/ml, albumin of 4.5 g/dl, ALT of 34 U/L and moderate tumor differentiation level. By drawing a straight line from the 1. 8 cm point on the Size axis of the nomogram to the Points axis we find that a sum of lesions diameters of 1.8 cm corresponded to 4 points. Repeating the same for the other variables, we got 0 points for no extrahepatic disease, 0 points for CEA of 2.9 ng/ml, 9 points for albumin of 4.5 g/dl points, 12 points for ALT of 34 U/L and 0 points for moderate tumor differentiation. Sum of these points was 25 and again drawing a straight line from 25 on the Total Points axis to the 1 Year Survival prediction axis we obtained 90% as the predicted probability of 1-year survival for this patient. The patient had actual overall survival of 60.3 months.
One patient had CEA level >20000 ng/ml and was considered as an outlier in our study, that contributed to very high CEA values in the nomogram. It was excluded from internal validation as it was adversely affecting model fit and evaluation.
Each MV analysis factor was assigned points relative to each hazard ratio. The nomogram assigned the probability of 1-year survival by summing the point-scale scores for each variable. The total score projected on the bottom scale indicated the probability of 1-year survival. One-year OS of patients with total points <25 indicated 90% chance of 1-year survival, whereas total sum of points of >80 estimated 10% chance of 1-year survival. Bootstrap resampling showed good discrimination (optimism corrected c- index=0.745) and calibration (mean absolute prediction error=0.299) of the nomogram.
6. Complications
The incidence of Grade 1–2 toxicities was 44/102 (43%) (Table 6). The incidence of Grade 3–4 toxicities was 14/102 (14%) (Table 6). Two patients in our study developed hepatic venous occlusion post-RAE: Budd-Chiari syndrome in one patient and right hepatic vein, inferior vena cava thrombus and bilateral pulmonary embolism in second patient with lung disease progression.
Table 6.
Post-RAE toxicities.
| Grade 1–2 toxicities | ||
| 1. | Fatigue/weakness | 43/102 (42%) |
| 2. | Abdominal pain/distension | 23/102 (23%) |
| 3. | Constipation/ diarrhea | 14/102 (14%) |
| 4. | Fever | 11/102 (11%) |
| 5. | Change in appetite/weight changes | 11/102 (11%) |
| 6. | Nausea | 10/102 (10%) |
| 7. | Light colored stool/dark urine | 10/102 (10%) |
| 8. | Shortness of breath | 10/102 (10%) |
| 9. | Ascites | 5/102 (5%) |
| 10. | Vomiting | 4/102 (4%) |
| 11. | Shoulder/back pain | 4/102 (4%) |
| 12. | Hiccups | 1/102 (1%) |
| 13. | Portal hypertension | 1/102(1%) |
| 14. | Portal hypertension/ascites in patient with prior oxaliplatin administration | 1/102 (1%) |
| TOTAL | 44/102(43%) | |
| Grade 3–4 toxicities | ||
| 1. | Post-radioembolization syndrome (at 1–90 days) | 5/102 (5%) |
| 2. | Grade 3 abdominal pain/distension (at 10–12 days) | 3/102(3%) |
| 3. | Grade 3 fevers (at 3 days) | 1/102 (1%) |
| 4. | Acute liver failure/ failure to thrive/ grade 3 hyperbilirubinemia (at 14 days) | 1/102 (1%) |
| 5. | Grade 3 dyspnea (at 30 days) | 1/102 (1%) |
| 6. | Biliary obstruction + Budd-Chiari syndrome+ stop chemotherapy for 6 weeks (at 1-day/60-days) | 1/102 (1%) |
| 7. | Biloma requiring multiple drainages, liver abscess, E. Faecium sepsis; patient with liver POD (at 5 days) | 1/102 (1%) |
| 8. | Post-mapping brain infarction due anticoagulation break in patient with history of atrial fibrillation. Post-procedure hypertension, recurrent atrial fibrillation, abdominal pain, hypotension, fever, required overnight admission (at 1 day) | 1/102 (1%) |
| TOTAL | 14/102(14%) | |
patient was lost to follow-up post procedure; Multiple complications possible per patient; POD-progression of disease
DISCUSSION
This work provided an extensive, comprehensive analysis of imaging, laboratory, pathologic, genetic, primary-disease-related biomarkers, procedure-related factors as well as prior and post-RAE therapies, associated with overall and liver progression-free survival following RAE of CLMs.
1. Overall survival prediction nomogram
The developed nomogram included six easy-to-obtain pre-RAE parameters and provided good prediction of patient survival post-RAE, based on internal bootstrap validation result: nomogram showed good discrimination (c-index=0.745) and calibration. One-year overall survival of patients with total nomogram points of <25 vs. >80 was 90% and 10%, respectively.
Existing overall survival prediction nomogram, created by Fendler et al (23) potentially suffered from selection bias due to the inclusion of patients with very limited extrahepatic metastases and did not demonstrate any association of extrahepatic metastases with survival, contradictory to the reported negative impact of extrahepatic disease on patient survival post-RAE (24–26). Overall 73% of this study patients had extrahepatic metastases at the time of RAE. Recent multicenter randomized phase 3 trials (FOXFIRE, SIRFLOX, and FOXFIRE-Global) showed no survival benefit when combining 90Y radioembolization with first-line chemotherapy (13). According to existing evidence, patients will continue to present for RAE at latest stages of disease, especially when they progress in the liver while receiving second, third or subsequent chemotherapy regimens. RAE is recommended in the chemo-refractory or salvage setting (1). It is therefore important to identify and describe predictive factors in these settings. An overall survival prognostic score, developed by Damm et al, included heavily pre-treated patients, with a median baseline Karnofsky index of 80% and liver tumor load of 20%. The overall survival post-RAE was 6.7 months. This suggests that radioembolization was a relatively aggressive therapy for this specific compromised population (27). In our study, only 3% of patients had ECOG grade 2 performance status and only 14% had ≥25% baseline liver involvement by tumor.
All increased metabolic tumor uptake parameters of most metabolically active tumor (SUVmax, SUVpeak, SUVmean, MTV, TLG) within the intended-to-treat region were significantly associated with decreased overall survival. Recent studies confirmed that 18F-FDG PET/CT is useful to evaluate treatment response and it is an emerging prognostic tool in patients with CLM undergoing RAE, with semi-quantitative factors (as MTV and TLG) correlating with survival better than RECIST criteria (28, 29). We therefore advocate metabolic imaging to be always performed before RAE. Prior investigations described anatomic biomarkers, such as CLM size, as predictor of decreased survival post-RAE (23, 30–32). Measuring the sum of two largest lesion diameters is also important for RECIST 1.1 evaluations and were part of our nomogram. Both anatomic (lesion size) or metabolic tumor parameters were statistically significant predictors of overall survival in this study. However, both factors could not be included into the nomogram, because lesion size generally correlates bolic uptake. Thus, due to the fact, that 18F-FDG PET/CT is not always available in other institutions for treatment planning, we chose to include anatomic parameters into the nomogram.
ALT (SGPT, alanine aminotransferase), unlike other routine liver panel enzymes, is predominantly found in the liver and released into the bloodstream as the result of liver injury, thus serves as a fairly specific indicator of liver damage. ALT was the most predictive nomogram factor of decreased survival: ALT level >250 U/L alone corresponded to >80 nomogram points, distinguishing patients with >90% risk for death within 1-year after RAE. Other liver enzymes – aspartate aminotransferase and alkaline phosphatase - were also found to be associated with post-RAE survival. Since we could only include a limited number of factors in the MV analysis and the latter factors are less hepatic function-specific biomarkers compared to ALT, they were not included. Transaminase toxicity was predictive of survival post-RAE in prior studies (23, 33), thus it should be emphasized as an important patient selection criterion in patients with CLM. Total bilirubin level was not predictive of survival in our study, most likely due to the strict inclusion criteria of RAE in our center. Only patients with total bilirubin ≤ 1.5 mg/dl were eligible for RAE. Commonly, patients with bilirubin <2 mg/dl are considered eligible for RAE (34).
CEA level differed dramatically in this cohort, with a median of 52 ng/ml, ranging from normal level to >20000 ng/ml, considered as an outlier in our study, that contributed to very high CEA values in the nomogram. Increased CEA level has been associated with poorer survival post-RAE of CLM in multiple studies (4, 28, 33, 35), however, this is influenced by multiple factors, including tumor differentiation level, liver function and the side of primary tumor (35).
The overall survival predictors in this study are similar to a recent study, where higher CEA and AST level, NLR >5, extrahepatic disease, and larger volume of liver metastases were independently associated with increased risk of death on multivariate analysis (36).
Median overall survival in this cohort was 11.3 months post-RAE. This compares favorably to large retrospective studies with reported median survival of 7.2–10.6 months after radioembolization of CLM, mostly in the salvage setting (37–39). It should be noted, that RAE in this cohort was administered at a median of 30.9 months from the initial diagnosis of CLM. This reflects, that most patients were heavily pre-treated and received multiple prior liver-directed therapies, including resection, HAIP and ablation. Pre-RAE therapies were not associated with overall survival, although, the fact, that most patients received loco-regional therapies pre-RAE might have influenced that. The actual liver function and parameters indicating underlying hepatic toxicity (albumin and ALT level), the metastatic disease volume (liver tumor(s) size, extrahepatic disease extent and CEA level) as well as tumor differentiation were significant predictors of post-RAE overall survival.
All analyzed post- RAE therapies (increased number of systemic chemotherapy lines, HAIP, ablation and bevacizumab) were associated with increased overall survival post-RAE. This result is concordant to prior studies, where RAE did not prohibit re-initiation of systemic chemotherapy, hepatic arterial infusion pump therapy or ablation for CLM, that persisted or progressed after initial response to RAE (4, 9, 12, 40–42). This also demonstrated that overall survival post-RAE cannot be attributed solely to RAE, but rather to the overall management of the disease in the salvage setting. Post-RAE therapies were not included in the 1-year overall survival prediction nomogram. This was necessary to allow us to reach our goal of patient stratification before RAE in this setting.
Patients with KRAS or PI3KCA mutations and left-sided primary tumors showed decreased overall survival following RAE in our study, however, the difference was not statistically significant. Patients with BRAF mutation and right-sided primary tumor showed increased post-RAE survival, but again-not statistically significantly.
2. Predictors of local tumor progression and liver progression-free survival
Objective response rate of 52% in this study compares favorably with published data of 10–48% response rates, depending on whether the patients received RAE with second line chemotherapy or at the salvage setting (10, 17, 19, 20, 43). Baseline SUVmax was the only significant predictor of liver progression-free survival on MV analysis. There was no significant difference in liver progression-free survival for those, who received resin or glass microspheres. Although the incidence of stasis, resulting in suboptimal radiation dose delivery to tumor increased the risk of local tumor progression, it did not reach statistical significance. PI3KCA mutation was not a significant predictor of LPFS in this cohort, unlike in prior study (44). This could be related to difference in methodology between studies (response evaluation criteria, evaluation of local tumor progression, rather than LPFS and analysis of different PI3K mutations, associated with gain of function).
3. Safety of 90Y radioembolization
RAE had acceptable safety profile in this series with incidence of grade 3–4 toxicities of 14%, median overall survival of 11.3 months, no death within 30 days post-procedure and no RAE-related death within 3-months post-treatment.
Two patients in this study developed liver venous occlusion post-RAE: Budd-Chiari syndrome in one patient and right hepatic vein, inferior vena cava thrombus and bilateral pulmonary embolism in second patient. Radiation therapy was shown to cause endothelial activation with subsequent pro-thrombotic response and tissue inflammation with subsequent vein external compression, stasis and thrombus formation (45). However, the number of patients with these complications was too small to draw meaningful conclusions regarding liver venous occlusion association with RAE.
4. Study limitations
Study limitations included the single center retrospective study design with its inherited limitations and a relatively short follow-up time. Also, there was a lack of external nomogram validation, that preferably would include prospectively recruited patients at multiple centers. Finally, actual radiation dose delivered to the tumor could not be measured in this study due to technical limitations, thus it could not be analyzed as a prognosticator of RAE.
CONCLUSION
The developed nomogram included six pre-RAE parameters and provided good prediction of patient survival post-RAE. Baseline SUVmax was the single significant predictor of liver progression-free survival on multivariate analysis.
Funding acknowledgements
The research funded by the Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (P30 CA008748).
C.T. Sofocleous: has previously received research support and served as a consultant for SIRTEX. He currently receives research support from BTG.
Footnotes
Disclosure
Ethical Approval and Informed Consent Statements
IRB waiver of approval was obtained for this retrospective cohort study. The database was HIPAA registered and compliant. The patients signed informed consent for the procedure.
Other authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Benson AB 3rd, Venook AP, Cederquist L, Chan E, Chen YJ, Cooper HS, et al. Colon Cancer, Version 1.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15(3):370–98. [DOI] [PubMed] [Google Scholar]
- 2.Khajornjiraphan N, Thu NA, Chow PK. Yttrium-90 microspheres: a review of its emerging clinical indications. Liver Cancer. 2015;4(1):6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray D, McEwan AJ. Radiobiology of systemic radiation therapy. Cancer Biother Radiopharm. 2007;22(1):1–23. [DOI] [PubMed] [Google Scholar]
- 4.Sofocleous CT, Violari EG, Sotirchos VS, Shady W, Gonen M, Pandit-Taskar N, et al. Radioembolization as a Salvage Therapy for Heavily Pretreated Patients With Colorectal Cancer Liver Metastases: Factors That Affect Outcomes. Clin Colorectal Cancer. 2015;14(4):296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell AM, Bailey IH, Burton MA. Analysis of the distribution of intra-arterial microspheres in human liver following hepatic yttrium-90 microsphere therapy. Phys Med Biol. 2000;45(4):1023–33. [DOI] [PubMed] [Google Scholar]
- 6.Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27(8):1386–422. [DOI] [PubMed] [Google Scholar]
- 7.Kennedy A, Nag S, Salem R, Murthy R, McEwan AJ, Nutting C, et al. Recommendations for radioembolization of hepatic malignancies using yttrium-90 microsphere brachytherapy: a consensus panel report from the radioembolization brachytherapy oncology consortium. Int J Radiat Oncol Biol Phys. 2007;68(1):13–23. [DOI] [PubMed] [Google Scholar]
- 8.Sotirchos VS, Petre EN, Brown KT, Brody LA, D’Angelica MI, DeMatteo RP, et al. Safe and Successful Yttrium-90 Resin Microsphere Radioembolization in a Heavily Pretreated Patient with Chemorefractory Colorectal Liver Metastases after Biliary Stent Placement above the Papilla. Case Reports Hepatol. 2014;2014:921406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sofocleous CT, Garcia AR, Pandit-Taskar N, Do KG, Brody LA, Petre EN, et al. Phase I trial of selective internal radiation therapy for chemorefractory colorectal cancer liver metastases progressing after hepatic arterial pump and systemic chemotherapy. Clin Colorectal Cancer. 2014;13(1):27–36. [DOI] [PubMed] [Google Scholar]
- 10.Hendlisz A, Van den Eynde M, Peeters M, Maleux G, Lambert B, Vannoote J, et al. Phase III trial comparing protracted intravenous fluorouracil infusion alone or with yttrium-90 resin microspheres radioembolization for liver-limited metastatic colorectal cancer refractory to standard chemotherapy. J Clin Oncol. 2010;28(23):3687–94. [DOI] [PubMed] [Google Scholar]
- 11.Van Hazel G, Blackwell A, Anderson J, Price D, Moroz P, Bower G, et al. Randomised phase 2 trial of SIR-Spheres plus fluorouracil/leucovorin chemotherapy versus fluorouracil/leucovorin chemotherapy alone in advanced colorectal cancer. J Surg Oncol. 2004;88(2):78–85. [DOI] [PubMed] [Google Scholar]
- 12.Gray B, Van Hazel G, Hope M, Burton M, Moroz P, Anderson J, et al. Randomised trial of SIR-Spheres plus chemotherapy vs. chemotherapy alone for treating patients with liver metastases from primary large bowel cancer. Ann Oncol. 2001;12(12):1711–20. [DOI] [PubMed] [Google Scholar]
- 13.Wasan HS, Gibbs P, Sharma NK, Taieb J, Heinemann V, Ricke J, et al. First-line selective internal radiotherapy plus chemotherapy versus chemotherapy alone in patients with liver metastases from colorectal cancer (FOXFIRE, SIRFLOX, and FOXFIRE-Global): a combined analysis of three multicentre, randomised, phase 3 trials. Lancet Oncol. 2017; 18(9): 1159–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kennedy AS, Nutting C, Coldwell D, Gaiser J, Drachenberg C. Pathologic response and microdosimetry of (90)Y microspheres in man: review of four explanted whole livers. Int J Radiat Oncol Biol Phys. 2004;60(5):1552–63. [DOI] [PubMed] [Google Scholar]
- 15.Sharma RA, Van Hazel GA, Morgan B, Berry DP, Blanshard K, Price D, et al. Radioembolization of liver metastases from colorectal cancer using yttrium-90 microspheres with concomitant systemic oxaliplatin, fluorouracil, and leucovorin chemotherapy. J Clin Oncol. 2007;25(9):1099–106. [DOI] [PubMed] [Google Scholar]
- 16.Vente MA, Wondergem M, van der Tweel I, van den Bosch MA, Zonnenberg BA, Lam MG, et al. Yttrium-90 microsphere radioembolization for the treatment of liver malignancies: a structured meta-analysis. Eur Radiol. 2009;19(4):951–9. [DOI] [PubMed] [Google Scholar]
- 17.Cosimelli M, Golfieri R, Cagol PP, Carpanese L, Sciuto R, Maini CL, et al. Multi-centre phase II clinical trial of yttrium-90 resin microspheres alone in unresectable, chemotherapy refractory colorectal liver metastases. Br J Cancer. 2010;103(3):324–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurilova I, Beets-Tan RGH, Ulaner GA, Boas FE, Petre EN, Yarmohammadi H, et al. (90)Y Resin Microspheres Radioembolization for Colon Cancer Liver Metastases Using Full-Strength Contrast Material. Cardiovasc Intervent Radiol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bester L, Meteling B, Pocock N, Saxena A, Chua TC, Morris DL. Radioembolisation with Yttrium-90 microspheres: an effective treatment modality for unresectable liver metastases. J Med Imaging Radiat Oncol. 2013;57(1):72–80. [DOI] [PubMed] [Google Scholar]
- 20.Seidensticker R, Denecke T, Kraus P, Seidensticker M, Mohnike K, Fahlke J, et al. Matched-pair comparison of radioembolization plus best supportive care versus best supportive care alone for chemotherapy refractory liver-dominant colorectal metastases. Cardiovasc Intervent Radiol. 2012;35(5):1066–73. [DOI] [PubMed] [Google Scholar]
- 21.SIR-Spheres® Y-90 resin microspheres Package Insert. [Google Scholar]
- 22.TheraSphere Yttrium-90 Microspheres Instructions for Use.
- 23.Fendler WP, Ilhan H, Paprottka PM, Jakobs TF, Heinemann V, Bartenstein P, et al. Nomogram including pretherapeutic parameters for prediction of survival after SIRT of hepatic metastases from colorectal cancer. Eur Radiol. 2015;25(9):2693–700. [DOI] [PubMed] [Google Scholar]
- 24.Tohme S, Sukato D, Nace GW, Zajko A, Amesur N, Orons P, et al. Survival and tolerability of liver radioembolization: a comparison of elderly and younger patients with metastatic colorectal cancer. HPB (Oxford). 2014;16(12):1110–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rougier P, Milan C, Lazorthes F, Fourtanier G, Partensky C, Baumel H, et al. Prospective study of prognostic factors in patients with unresected hepatic metastases from colorectal cancer. Fondation Francaise de Cancerologie Digestive. Br J Surg. 1995;82(10):1397–400. [DOI] [PubMed] [Google Scholar]
- 26.Stangl R, Altendorf-Hofmann A, Charnley RM, Scheele J. Factors influencing the natural history of colorectal liver metastases. Lancet. 1994;343(8910):1405–10. [DOI] [PubMed] [Google Scholar]
- 27.Damm R, Seidensticker R, Ulrich G, Breier L, Steffen IG, Seidensticker M, et al. Y90 Radioembolization in chemo-refractory metastastic, liver dominant colorectal cancer patients: outcome assessment applying a predictive scoring system. BMC Cancer. 2016;16:509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fendler WP, Philippe Tiega DB, Ilhan H, Paprottka PM, Heinemann V, Jakobs TF, et al. Validation of several SUV-based parameters derived from 18F-FDG PET for prediction of survival after SIRT of hepatic metastases from colorectal cancer. J Nucl Med. 2013;54(8):1202–8. [DOI] [PubMed] [Google Scholar]
- 29.Shady W, Kishore S, Gavane S, Do RK, Osborne JR, Ulaner GA, et al. Metabolic tumor volume and total lesion glycolysis on FDG-PET/CT can predict overall survival after (90)Y radioembolization of colorectal liver metastases: A comparison with SUVmax, SUVpeak, and RECIST 1.0. Eur J Radiol. 2016;85(6):1224–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dunfee BL, Riaz A, Lewandowski RJ, Ibrahim S, Mulcahy MF, Ryu RK, et al. Yttrium-90 radioembolization for liver malignancies: prognostic factors associated with survival. Journal of vascular and interventional radiology : JVIR. 2010;21(1):90–5. [DOI] [PubMed] [Google Scholar]
- 31.Schonewolf CA, Patel B, Gensure RH, Narra V, Haffty BG, Nosher JL, et al. Patterns of failure in colorectal patients with liver metastases after yttrium-90 radioembolization. Am J Clin Oncol. 2014;37(3):234–40. [DOI] [PubMed] [Google Scholar]
- 32.Abbott AM, Kim R, Hoffe SE, Arslan B, Biebel B, Choi J, et al. Outcomes of Therasphere Radioembolization for Colorectal Metastases. Clin Colorectal Cancer. 2015;14(3):146–53. [DOI] [PubMed] [Google Scholar]
- 33.Fahmueller YN, Nagel D, Hoffmann RT, Tatsch K, Jakobs T, Stieber P, et al. Predictive and prognostic value of circulating nucleosomes and serum biomarkers in patients with metastasized colorectal cancer undergoing Selective Internal Radiation Therapy. BMC Cancer. 2012;12:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boas FE, Bodei L, Sofocleous CT. Radioembolization of Colorectal Liver Metastases: Indications, Technique, and Outcomes. J Nucl Med. 2017;58(Suppl 2):104s–11s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duffy MJ. Carcinoembryonic antigen as a marker for colorectal cancer: is it clinically useful? Clin Chem. 2001;47(4):624–30. [PubMed] [Google Scholar]
- 36.Weiner AA, Gui B, Newman NB, Nosher JL, Yousseff F, Lu SE, et al. Predictors of Survival after Yttrium-90 Radioembolization for Colorectal Cancer Liver Metastases. Journal of vascular and interventional radiology : JVIR. 2018;29(8):1094–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lewandowski RJ, Memon K, Mulcahy MF, Hickey R, Marshall K, Williams M, et al. Twelve-year experience of radioembolization for colorectal hepatic metastases in 214 patients: survival by era and chemotherapy. European journal of nuclear medicine and molecular imaging. 2014;41(10):1861–9. [DOI] [PubMed] [Google Scholar]
- 38.Kennedy AS, Ball D, Cohen SJ, Cohn M, Coldwell DM, Drooz A, et al. Multicenter evaluation of the safety and efficacy of radioembolization in patients with unresectable colorectal liver metastases selected as candidates for (90)Y resin microspheres. Journal of gastrointestinal oncology. 2015;6(2):134–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saxena A, Meteling B, Kapoor J, Golani S, Morris DL, Bester L. Is yttrium-90 radioembolization a viable treatment option for unresectable, chemorefractory colorectal cancer liver metastases? A large single-center experience of 302 patients. Annals of surgical oncology. 2015;22(3):794–802. [DOI] [PubMed] [Google Scholar]
- 40.Sofocleous CT, Petre EN, Gonen M, Brown KT, Solomon SB, Covey AM, et al. CT-guided radiofrequency ablation as a salvage treatment of colorectal cancer hepatic metastases developing after hepatectomy. Journal of vascular and interventional radiology : JVIR. 2011;22(6):755–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang X, Sofocleous CT, Erinjeri JP, Petre EN, Gonen M, Do KG, et al. Margin size is an independent predictor of local tumor progression after ablation of colon cancer liver metastases. Cardiovasc Intervent Radiol. 2013;36(1):166–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoffmann RT, Jakobs TF, Kubisch CH, Stemmler HJ, Trumm C, Tatsch K, et al. Radiofrequency ablation after selective internal radiation therapy with Yttrium90 microspheres in metastatic liver disease-Is it feasible? Eur J Radiol. 2010;74(1):199–205. [DOI] [PubMed] [Google Scholar]
- 43.Lim L, Gibbs P, Yip D, Shapiro JD, Dowling R, Smith D, et al. A prospective evaluation of treatment with Selective Internal Radiation Therapy (SIR-spheres) in patients with unresectable liver metastases from colorectal cancer previously treated with 5-FU based chemotherapy. BMC Cancer. 2005;5:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ziv E, Bergen M, Yarmohammadi H, Boas FE, Petre EN, Sofocleous CT, et al. PI3K pathway mutations are associated with longer time to local progression after radioembolization of colorectal liver metastases. Oncotarget. 2017;8(14):23529–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dorr W. Radiobiology of tissue reactions. Ann ICRP. 2015;44(1 Suppl):58–68. [DOI] [PubMed] [Google Scholar]