Abstract
In recent years, the RNA modification N6-methyladenosine (m6A) has been found to play a role in the life cycles of numerous viruses and also in the cellular response to viral infection. m6A has emerged as a regulator of many fundamental aspects of RNA biology. Here, we highlight recent advances in techniques for the study of m6A, as well as advances in our understanding of the cellular machinery that controls the addition, removal, recognition, and functions of m6A. We then summarize the many newly discovered roles of m6A during viral infection, including how it regulates innate and adaptive immune responses to infection. Overall, the goals of this review are to summarize these roles of m6A on both cellular and viral RNAs and to describe future directions for uncovering new functions of m6A during infection.
Keywords: RNA modifications, N6-methyladenosine, m6A, RNA viruses, DNA viruses, post-transcriptional regulation, innate immunity
1. INTRODUCTION
Post-transcriptional regulation heavily influences RNA fate and function (1, 2). Similarly, viral RNAs are regulated post-transcriptionally to control their function (2, 3). Cellular RNAs are also post-transcriptionally regulated during viral infection to generate either proviral or antiviral states (4, 5). A new post-transcriptional control of viral infection has now emerged: internal N6-methyladenosine (m6A) RNA modification of both viral and cellular RNAs (6–10). In this review, we discuss how m6A modification of both viral and cellular RNAs regulates their function to control infection and immunity.
m6A is one of over 60 known covalent modifications in eukaryotic RNA (11). It is well known that both transfer RNAs and ribosomal RNAs contain many modifications that contribute to their function (12–14). Messenger RNAs (mRNAs) contain terminal modifications, such as the 5′ 7-methylguanosine cap and 2′O methylation of the first and second transcribed bases; this cap structure is critical for mRNA stability and translation (15, 16). mRNA also contains internal modifications, such as pseudouridine, 5-methylcytosine, N4-acetylcytidine, N1-methyladenosine, and m6A, the focus of this review (11, 17–20). Viral RNAs were first described to have specific RNA modifications more than four decades ago (reviewed in 9). These studies identified m6A in host and viral RNA from cells infected with viruses, including influenza A virus (IAV), Rous sarcoma virus, herpes simplex virus 1 (HSV-1), adenovirus, and simian virus 40 (SV40) (21–29). In fact, studies on Rous sarcoma virus RNA even suggested that m6A was added to a consensus motif, [G/A]A*C (A* = m6A) (24–26). However, the functional consequences of m6A on cellular and viral RNA remained unknown for many years. Recently, the discovery of cellular proteins that add, remove, and recognize the modification, as well as the development of sequencing-based methods for transcriptome-wide m6A mapping, has reignited m6A research and advanced our interest in studying m6A during viral infection. While this review focuses on the role of m6A in viral infection, other RNA modifications such as 2′O methylation, terminal uridilyation, and deamination observed during adenosine-to-inosine editing have also been found in viral RNAs, revealing that many RNA modifications could play roles in viral infection (30–35).
2. METHODS FOR THE DETECTION OF m6A-MODIFIED RNA
Determining the cellular and viral RNAs that contain m6A has become feasible due to new techniques to map the modification (reviewed in 36). Prior to the development of these new techniques, modified nucleotides were detected by hydrolysis or nuclease digestion of radiolabeled RNA followed by chromatographic analysis. Now, sequencing-based methods can map m6A across the cellular transcriptome (37–39). These methods can identify specific RNAs that contain m6A and define the approximate position of m6A within those RNAs. The most commonly used technique for transcriptome-wide m6A mapping is MeRIP-seq (methylated RNA immunoprecipitation and sequencing, also known as m6A-seq) (37, 38). In this method, total RNA or mRNA is fragmented (100–200 nucleotides), immunoprecipitated with an m6A-specific antibody, and subjected to next-generation sequencing. m6A-containing fragments are then identified by calculating the enrichment of sequencing reads in the immunoprecipitated sample relative to the input using specific peak calling algorithms such as MACS2 (40). The initial m6A mapping studies identified an m6A sequence motif, DRA*CH (where D = G/A/U, R = G > A, and H = U/C/A, and A* = m6A), with GGACU being the most common motif (37, 38). This DRACH motif agrees with the originally proposed m6A motif ([G/A]A*C) identified in Rous sarcoma virus RNA (24–26). While MeRIP-seq identifies m6A-enriched fragments, it does not define which specific DRACH motifs in these fragments contain m6A. A related technique, PA-m6A-seq (photo-cross-linking-assisted m6A sequencing), defines m6A sites more precisely (41). In this method, RNA labeled with 4-thiouridine is immunoprecipitated with an anti-m6A antibody. This immunoprecipitated, 4-thiouridine-containing RNA is treated with ultraviolet light to cross-link the antibody, and the RNA is then digested to 30-nucleotide fragments. When these fragments are reverse transcribed, the cross-linked antibody adducts introduce mutations into the complementary DNA (cDNA) that ultimately reveal proximal m6A sites. While this technique provides a more accurate m6A map than MeRIP-seq, it does not always identify m6A at single-nucleotide resolution.
Two recently developed methods for single-nucleotide resolution mapping of m6A are miCLIP (m6A individual-nucleotide-resolution-cross-linking and immunoprecipitation) and m6A-CLIP (42–45). In these CLIP-based methods, fragmented RNA is cross-linked to anti-m6A antibodies using ultraviolet light. If m6A is present in the RNA, upon reverse transcription the cross-linked antibody adduct results in characteristic truncations or mutations directly adjacent to the exact m6A site. These methods to detect m6A do have some limitations: They are more labor intensive than MeRIP-seq and require a higher number of unique sequencing reads than traditional RNA-seq due to their reliance on detection of mutations or truncations within the cDNA pool (43).
All current m6A mapping techniques have several shared limitations. They all rely on an antibody, which can lead to detection biases. Indeed, anti-m6A antibodies from different suppliers have different specificities that can be altered by RNA structure (46, 47). Because anti-m6A antibodies can also immunoprecipitate RNA with the similar modification 2′O-dimethyladenosine (m6Am), differentiating between these modifications can be difficult (43, 48–50). Also, while these methods can detect m6A in mRNA, they have difficulty distinguishing condition-induced m6A changes in a given RNA. This is because the current computational methods to call m6A often do not adequately consider changes in transcript abundance or exon usage when mapping m6A, and therefore they can provide divergent results regarding m6A occupancy. In fact, most m6A mapping studies have only been performed with one or two replicates, limiting the robustness of many genome-wide m6A mapping studies. Therefore, more work is needed to develop new reagents, robust computational methods, and rigorous statistical analyses for accurate detection of both static and dynamic m6A sites in the transcriptome. Despite these limitations, current transcriptome-wide m6A mapping methods have provided remarkable information regarding the positioning of m6A in viral and cellular RNA.
3. THE CELLULAR m6A MACHINERY REGULATES RNA FUNCTION
Another major breakthrough that allowed for the study of m6A in viral infection was the discovery of the m6A machinery, which includes the cellular proteins that add, remove, or read m6A (51–63) (Figure 1). Studying the m6A machinery has revealed that m6A regulates many aspects of RNA biology, including structure, splicing, alternative polyadenylation, localization, stability, and translation (64–66). Biologically, this translates into m6A influencing physiological processes including organismal development, stem cell differentiation, hematopoiesis, immune cell function, oncogenesis, circadian rhythms, and neural function (64–66). In this section, we introduce the m6A machinery and how it regulates RNA function.
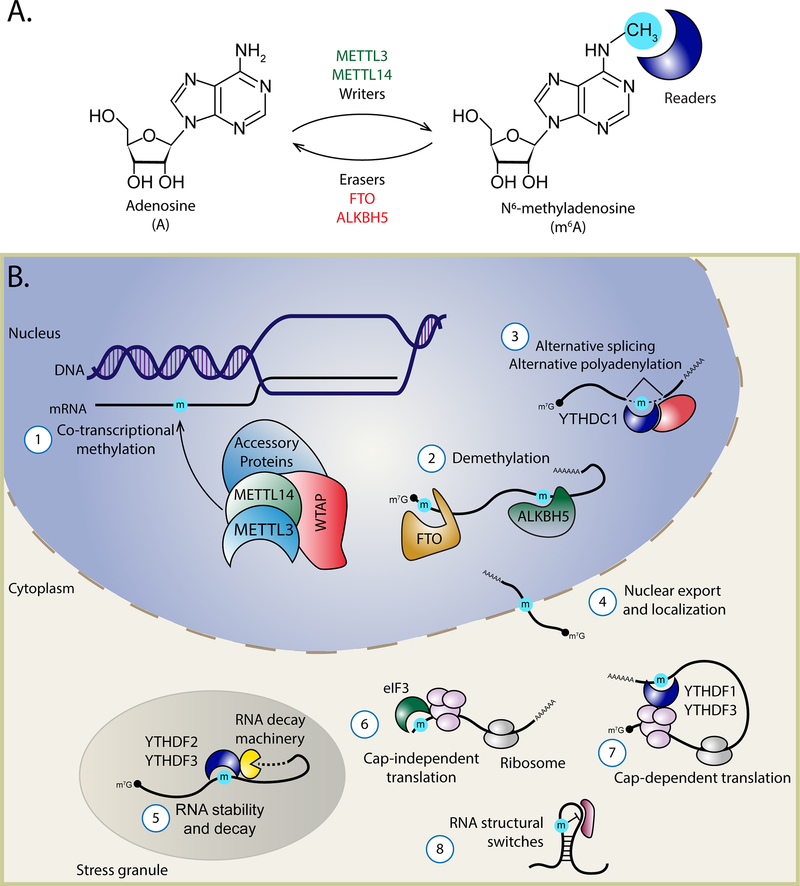
Figure 1.
The cellular m6A machinery and functions of writers, erasers, and readers. (a) Structures of A and m6A. The methyl group is colored blue. METTL3 and METTL14 are the writer proteins that catalyze the covalent conversion of A to m6A on target RNAs. FTO and ALKBH5 are demethylases capable of removing the methylation. The function of m6A bearing RNAs is influenced by interaction with reader proteins. (b) ① m6A is co-transcriptionally added to RNA by a writer complex of proteins, which consists of METTL3, METTL14, and WTAP, as well as accessory factors that can determine RNA targeting. ② m6A can be removed from RNA by the demethylases FTO and ALKBH5. m6A reader proteins, such as the YTHDF proteins, mediate diverse post-transcriptional processes on m6A containing RNA including ③ alternative splicing and polyadenylation, ④ nuclear export and RNA localization, ⑤ alteration of RNA stability, ⑥ cap-independent translation, ⑦ cap-dependent translation, and ⑧ modulation of protein-RNA interactions via structural switches. Abbreviations: A, adenosine; mRNA, messenger RNA; m6A, N6-methyladenosine, m7G, 7-Methylguanosine.
3.1. Cellular Writers of m6A
The primary enzyme that adds, or writes, m6A to mRNA is the methyltransferase METTL3 (Figure 1). METTL3 is predominantly localized in the nucleus and co-transcriptionally methylates adenosine residues within specific DRACH motifs in nascent RNA (45, 52, 67). It can also directly promote of translation (68, 69). METTL3 stability, catalytic efficiency, localization, and RNA targeting are all regulated by numerous interacting RNA-binding proteins. These proteins include METTL14, WTAP, ZC3H13, VIRMA (KIAA1429), and RBM15/15B. METTL14 complexes with METTL3 to stabilize its expression and enhance its methyltransferase activity (52, 58, 70, 71). WTAP localizes METTL3-METTL14 to transcription sites where it promotes RNA binding, while ZC3H13 maintains the nuclear localization of this complex (54, 55, 72). Targeting of METTL3-METTL14 to mRNA occurs through accessory factors such as VIRMA and RBM15/15B (56, 72, 73). The complete set of features in RNA that lead to methylation of specific DRACH motifs, including the RNA structures or secondary m6A recognition motifs that influence this selectivity, are unknown. Elucidating how this selectivity occurs and identifying additional proteins within the methyltransferase complex will be essential for understanding how both cellular and viral RNAs are selected for m6A modification.
In addition to METTL3, three other enzymes have been shown to act as m6A methyltransferases in eukaryotes: METTL16 adds m6A to U6 small nuclear RNAs as well as some mRNAs; ZCCHC4 adds m6A to 28S ribosomal RNA; and PCIF1, a cap-specific m6A methyltransferase, catalyzes the formation of m6Am (48, 49, 74–76). These enzymes have only recently been characterized and have not at present been studied during viral infection; however, it is possible that they may also deposit m6A on viral RNAs.
3.2. Cellular Erasers of m6A
The enzymes that remove, or erase, m6A from mRNA are FTO and ALKBH5 (50, 53, 57, 77, 78) (Figure 1). FTO demethylates both m6A and terminal m6Am, while ALKBH5 specifically demethylates m6A (50, 57, 78). The discovery of these m6A demethylases suggested for the first time that m6A could be added or removed from mRNAs under specific conditions, setting the stage for the study of RNA epigenetics or the epitranscriptome (79, 80).
3.3. Cellular Readers of m6A
The RNA-binding proteins that bind to m6A are referred to as m6A readers. These m6A readers, whose RNA-binding activity can be modulated by the presence of m6A and/or RNA structure, elicit the regulatory functions of m6A on modified RNAs (62, 63, 81, 82; reviewed in 83). These readers can regulate the stability, splicing, polyadenylation, nuclear export, and translation efficiency of their target RNAs (59–61, 84–89) (Figure 1). The most well-described m6A readers—including YTHDF1, YTHDF2, YTHDF3, YTHDC1, and YTHDC2—all possess a YTH domain that contains an m6A-binding pocket (90–93). The YTHDF proteins and YTHDC2 all act as readers of m6A-containing mRNAs in the cytoplasm. In m6A-containing mRNAs, YTHDF1 promotes translation, YTHDF2 increases RNA decay, and YTHDF3 and YTHDC2 can regulate both of these processes (59–61, 85, 94–102). The nuclear reader YTHDC1 regulates the splicing and alternative polyadenylation of specific transcripts (86, 88).
Besides the YTH domain-containing m6A readers, we now know of dozens of additional proteins that preferentially bind to or are repelled by m6A-containing RNA (62, 63, 82, 103). Proteins with enhanced specificity for m6A-modified RNA include eIF3D, FMR1, IGF2BP1, IGF2BP2, IGF2BP3, HNRNPC, HNRNPG, and HNRNPA2B1. Proteins repelled by m6A include G3BP1, G3BP2, and CAPRIN1 (62, 63, 81, 82, 84, 89, 103). The RNA regulatory functions of many of these newly identified m6A readers remain incompletely defined, and an understanding of which m6A-containing RNAs they regulate will undoubtedly reveal new functions for m6A in RNA biology.
4. m6A AND m6A-REGULATORY PROTEINS REGULATE VIRUS INFECTION
While m6A was first identified in viral RNA in the 1970s, the specific roles of m6A in virus replication remained unclear. These roles for m6A in viral infection are now beginning to be uncovered, and the studies that define these roles reveal that m6A is a new regulatory control of viral infection (6–10). In this section we describe the recent work that has defined these regulatory roles for m6A during virus infection, including positive-sense RNA viruses, negative-sense RNA viruses, retroviruses, and DNA viruses (Figure 2).
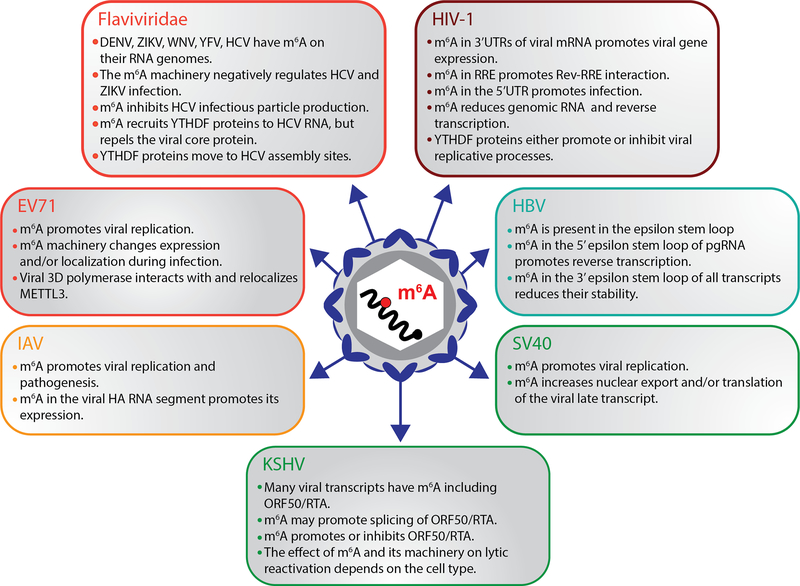
Figure 2.
The cellular m6A machinery impacts the replication of viruses from diverse families. Here we describe the main findings of how m6A on viral RNA regulates infection. Manipulation of cellular writers, erasers, and readers of m6A reshapes virus infection with differential outcomes depending on virus studied and cell type used for experiments. Viruses in the Flaviviridae family (DENV, ZIKV, WNV, YFV, and HCV) are negatively regulated by m6A writers while replication of enterovirus 71 and influenza A virus is promoted by m6A. m6A modification of viral transcripts derived from retroviruses and DNA viruses also bear m6A. The impact of m6A on these viruses is dependent on the stage of the viral replication cycle examined, host tissue, and viral strain studied. Color legend: Orange, positive-sense RNA viruses; Yellow, negative-sense RNA virus; Red, retrovirus; Blue, partially double-stranded DNA virus; Green, double-stranded DNA viruses. Abbreviations: DENV, dengue virus; EV71, enterovirus 71; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV-1, human immunodeficiency virus-1; IAV, influenza A virus; KSHV, Kaposi’s sarcoma-associated herpesvirus; m6A, N6-methyladenosine; pgRNA, pregenomic RNA; RRE, Rev response element; SV40, simian virus 40; WNV, West Nile virus; YFV, yellow fever virus; ZIKV, Zika virus.
4.1. Flaviviridae
The Flaviviridae family of viruses is composed of positive-sense, single-stranded RNA viruses that replicate in the cytoplasm. We and others mapped m6A on the viral RNA genomes of Flaviviridae members, including hepatitis C virus (HCV), Zika virus (ZIKV), dengue virus, yellow fever virus, and West Nile virus (WNV) (104, 105). These viral genomes contain multiple m6A sites along their genomes, as determined by MeRIP-seq, and their presence was validated by mass spectrometry (34, 104, 105). Interestingly, each virus had a high concentration of sites present in the last viral gene (NS5B for HCV or NS5 for the other viruses) (104, 105).
As the canonical m6A methyltransferase complex is predominantly nuclear, it is unclear how these cytoplasmic RNA genomes gain m6A (52). At least some portion of METTL3-METTL14 is in the cytoplasm, where it could interact with and methylate viral RNA (104, 105). In the case of HCV, nuclear pore complex proteins are recruited to the membranous sites of replication, and it is possible that METTL3, which contains a nuclear localization signal, is also recruited to HCV replication sites by these nuclear pore complex proteins (106).
During HCV infection, m6A negatively regulates the production of infectious particles (104). Specifically, m6A in the E1 gene of HCV RNA inhibits the packaging of viral RNA into infectious particles, likely by facilitating competition between YTHDF proteins, which bind to m6A, and the HCV core protein, which is repelled by m6A. Interestingly, during HCV infection these YTHDF proteins relocalize to virion assembly sites at lipid droplets. Together, these findings support the idea that m6A recognition by the YTHDF proteins has a negative effect on viral RNA packaging. Indeed, intracellular HCV RNA contains quantitatively more m6A than extracellular, virion-associated HCV RNA (34, 104). This suggests that the m6A RNA profile on HCV, and likely the other Flaviviridae members, may be different at varying life cycle stages. However, the mechanisms that would underlie this differential modification at different life cycle stages are still unknown. Importantly, while altering expression of the m6A machinery changed the levels of HCV, it did not affect the expression of several known interferon- (IFN) stimulated genes (ISGs), suggesting that manipulation of m6A levels does not alter the overall antiviral response in HCV infection.
Similar to HCV, ZIKV infection is also inhibited by m6A, implying that features of m6A regulation may be shared among Flaviviridae members (105). Some Flaviviridae members are transmitted by mosquitoes, so it is possible that m6A regulates vector-borne transmission. Going forward, more work is required to uncover the role of m6A in other Flaviviridae and establish the function of each m6A site in mammalian and vector models of infection. Further, a kinetic analysis of m6A on viral RNA may give important clues to how m6A regulates Flaviviridae RNA at different stages of the viral life cycle.
4.2. Picornaviridae
Enterovirus 71 (EV71) and poliovirus, both in the family Picornaviridae, are positive-sense, single-stranded RNA viruses that replicate in the cytoplasm and contain m6A (34, 107). While neither the specific location of the m6A sites in the poliovirus RNA nor their importance in replication have been assessed, this has been done for EV71. The m6A sites in the EV71 genome are in genes encoding the VP capsid proteins, the 3D RNA-dependent RNA polymerase, and the nonstructural 2C protein (107). Two m6A-abrogating mutations in VP1 and 2C reduced EV71 replication, suggesting that m6A positively regulates EV71 infection. In support of this, METTL3 depletion reduced viral replication, while depletion of FTO, all three of the YTHDF proteins, or YTHDC1 had the opposite effect (107). Interestingly, in EV71-infected cells, several m6A machinery proteins changed localization: METTL3 and METTL14 were upregulated and moved to the cytoplasm, the cytoplasmic readers YTHDF1 and YTHDF2 were partly relocalized to the nucleus, and the nuclear reader YTHDC1 was also moved to the cytoplasm. Consistent with this change in localization of the m6A writers, METTL3 directly interacted with 3D, indicating that 3D could recruit METTL3 to sites of viral RNA replication.
4.3. Orthomyxoviridae
IAV, in the Orthomyxoviridae family, is a negative-sense, segmented, single-stranded RNA virus that replicates in the nucleus. m6A was first discovered on IAV RNA in the 1970s, but now its role in regulating IAV infection has been more clearly defined (21, 108, 109). Mapping m6A and YTHDF protein binding sites in IAV-infected A549 lung epithelial cells revealed a high density of m6A sites in negative- and positive-sense IAV RNA. The number of m6A sites found in each RNA segment, including HA, is similar to the number of m6A residues per IAV mRNA predicted 30 years ago (108, 109). Unlike in the Flaviviridae viruses already discussed, m6A promotes IAV infection. Overexpression of METTL3 and YTHDF2 during IAV infection increased viral protein expression and viral titer, while mutational inactivation of these m6A sites in either strand of IAV lowered HA mRNA and protein levels (109). These m6A mutant viruses were also attenuated in mouse models of infection. Together, this suggests that m6A enhances HA expression during infection, ultimately promoting viral replication and pathogenesis. Future studies are needed to reveal the molecular mechanisms underlying m6A enhancement of IAV gene expression, such as RNA stability, nuclear export, translation, and how it may regulate the assembly and packaging of the negative-sense genomic RNA segments.
4.4. Retroviridae
m6A has also been identified in retroviruses, including Rous sarcoma virus, feline leukemia virus, and more recently human immunodeficiency virus-1 (HIV-1) (reviewed in 9, 110). Early studies found that m6A sites on RSV RNA were within [G/A]A*C (A* = m6A) motifs within distinct regions of the viral RNA, indicating that there was a mechanism by which m6A could be specifically added to these sites (24–26, 111). These early studies also suggested that m6A may alter the splicing of the RSV Env transcript (112).
Three independent groups have now reported multiple roles for m6A during HIV-1 infection. While they all found that m6A is present in both HIV-1 genomic RNA and mRNA, they uncovered somewhat different mechanisms by which the m6A machinery regulates HIV-1 infection (113–116; reviewed in 110). The first study to examine m6A in HIV-1 found that the m6A machinery regulates infection by controlling the methylation of the Rev response element (RRE). m6A in the RRE increased Rev protein binding to this RNA structure, which is present on and critical for nuclear export of some HIV-1 mRNAs (113). HIV-1 gene expression is also regulated by the YTHDF proteins, which bind to m6A-containing 3′UTRs of viral mRNAs to increase their mRNA levels and protein expression. Consistent with this, YTHDF proteins also positively regulated HIV-1 replication (114). Two more studies found both proviral and antiviral roles for m6A in HIV-1 infection (115, 116). Specifically, YTHDF proteins bound to incoming viral genomic and also reduced HIV-1 reverse transcription products, implying a negative role for YTHDF proteins in these stages of the viral life cycle. Despite this, m6A-abrogating mutations in the 5′UTR of HIV-1 genomic RNA, which prevent binding by YTHDF proteins, reduce infectivity. Additionally, YTHDF proteins promoted Gag protein expression in infected cells. Taken together, these data indicate that m6A and its machinery can have many roles in regulating HIV-1 infection depending on the stage of the viral life cycle and on the position of m6A in both HIV-1 genomic RNA and mRNA. Interestingly, HIV-1 increases m6A in cellular RNA through gp120 protein interacting with the HIV-1 receptor CD4, although its functional consequence for HIV-1 infection remains unclear (113, 117). Further work is needed to fully define the molecular mechanisms that drive m6A regulation of host and viral RNA during HIV-1 infection and define how m6A regulates various aspects of HIV-1 infection.
4.5. Hepadnaviridae
The replication of hepatitis B virus (HBV), a nuclear-replicating, double-stranded DNA virus in the Hepadnaviridae family, is also regulated by m6A. HBV copies its viral DNA using a replication intermediate RNA called the pregenomic RNA (pgRNA), which is reverse transcribed to make viral DNA. Both METTL3-METTL14 and YTHDF2 knockdown increased the stability of viral transcripts, resulting in greater expression of viral proteins (118). All viral transcripts had a single m6A peak within the epsilon stem loop. This stem loop is present at the 3′ end of all viral transcripts, including pgRNA, and also in the 5′ end of the pgRNA. Interestingly, an m6A-abrogating mutation in the 3′ epsilon stem loop increased the stability of viral transcripts, but abrogating the m6A site in the 5′ epsilon stem loop in pgRNA decreased reverse transcription. Taken together, this suggests that m6A can have different effects on the HBV life cycle; therefore, it would be interesting to decipher how the balance of m6A between 3′ and 5′ epsilon stem loop sites facilitates the HBV life cycle.
4.6. Polyomaviridae
The polyomavirus SV40, a small, double-stranded DNA virus that replicates in the nucleus, also contains m6A in its RNA. While the presence of m6A in SV40 mRNA has been known for decades (29, 119), the precise location of m6A on the viral mRNAs and how it may regulate viral replication remained incompletely understood until recently. We now know that m6A is located in both SV40 transcripts; there are 2 m6A sites in the early transcript, while there are 11 m6A sites in the late transcript (120). Loss of m6A from the SV40 early transcript had no effect on infection. Mutation of m6A sites on the late transcript reduced infection. This loss of m6A in the late transcript prevents its nuclear export, resulting in reduced expression of the encoded structural protein VP1 (120). This is in concordance with earlier studies that showed that the SAM inhibitor cycloleucine blocked nuclear export of SV40 late RNAs (119). In support of these results, depletion of METTL3 reduced SV40 replication, while YTHDF2 overexpression increased viral replication, further suggesting that m6A promotes SV40 infection. Future studies are needed to understand the molecular mechanisms by which m6A affects nuclear export of the late transcripts and whether m6A functions similarly in human polyomaviruses.
4.7. Herpesviridae
Herpesviruses have large, double-stranded DNA genomes and replicate in the nucleus. m6A has been studied in HSV-1, human cytomegalovirus (HCMV), and Kaposi’s sarcoma-associated herpesvirus (KSHV) (28, 121–125). While decades ago, HSV-1 was found to contain m6A (28), m6A has now been mapped and studied during HCMV and KSHV infection (121–125). While the studies on HCMV found m6A on multiple viral transcripts, they focused on how m6A impacts antiviral innate immunity through regulating IFN production. Therefore, we discuss these HCMV studies later in Section 5.2, and here we focus on the studies that define how m6A regulates KSHV infection.
Three studies (121–123) found that m6A was present on many KSHV transcripts during lytic reactivation, including the KSHV ORF50/RTA mRNA, which encodes a major transactivator required for the reactivation of latent KSHV. While all three studies showed that m6A regulated this transactivator, they present conflicting roles for how m6A and its machinery control ORF50/RTA RNA levels and/or expression, often depending on the cell type used. In epithelial cells, Hesser et al. (123) found that METTL3 and YTHDF2 depletion suppressed KSHV ORF50/RTA expression, lytic reactivation, and virion release, suggesting that m6A promotes KSHV lytic reactivation. Conversely, Tan et al. (122) found that in similar epithelial cells, YTHDF2 depletion increased the half-life of many viral mRNAs including ORF50/RTA during reactivation, which may or may not change its expression, pointing to a potential inhibitory role for m6A in KSHV lytic reactivation. However, in B cells, Hesser et al. (123) found that m6A negatively regulated ORF50/RTA and KSHV lytic reactivation. On the other hand, in the same cell type, Ye et al. (121) showed that m6A on ORF50/RTA is read by YTHDC1, which acts with the splicing factors SRSF3 and SRSF10 to promote its splicing and expression. Together, these results demonstrate that while m6A clearly plays a role in KSHV lytic reactivation, the whole picture is not yet clear. Further, these seemingly opposing results in different cell lines underscore the importance of studying the effects of m6A in diverse cell lines and limiting conclusions only to the cell line tested.
5. m6A IN INNATE AND ADAPTIVE IMMUNITY
In addition to acting directly on viral RNA to regulate viral replication, m6A can also regulate the immune response to infection. It has been shown to do this by regulating sensing of foreign RNAs, by altering the expression and stability of the transcripts of innate immune signaling molecules, and by affecting adaptive immune responses.
5.1. m6A Regulates Sensing of Foreign Nucleic Acids
Viral RNA present in the cytoplasm can be sensed as foreign or nonself by pattern recognition receptors (PRRs), including Toll-like receptors (TLRs) and RIG-I-like receptors (reviewed in 126). These receptors bind to viral RNAs, often containing specific features, such as a double-stranded region or a 5′ triphosphate, to drive signaling programs that ultimately induce cytokines such as IFNs, leading to an antiviral response. When added to in vitro transcribed RNAs, m6A and other modified nucleotides suppress the activation of these PRRs (127, 128). Indeed, the potential of modified nucleotides for suppressing antiviral innate immune sensing pathways has been harnessed for generation of mRNA vaccines to viruses, including IAV, ZIKV, and HIV-1 (129–132). Therefore, this suggests that m6A addition to viral RNAs could be a strategy that viruses use for innate immune evasion. While this has not been demonstrated yet for m6A in viral infection, it has proven to be true for another RNA modification, 2′O methylation, which normally occurs in mRNA cap (133). Specific viruses (e.g., WNV and vesicular stomatitis virus) use virally encoded methyltransferases to 2′O methylate their RNA caps to prevent sensing by IFIT1, which inhibits the translation of viral RNAs lacking this modification (31, 134). So far, no virus is known to encode an m6A methyltransferase, and at least for flaviviruses, the viral 2′O methyltransferase domain cannot add m6A (135). Interestingly, the cellular methyltransferase FTSJ3 catalyzes internal 2′O methylation in HIV-1 RNA, which prevents IFN activation (35). Therefore, it is also possible that m6A deposited on viral RNA by host methyltransferases could present a pattern that either evades detection by host PRRs or is bound by proviral cellular RNA-binding proteins.
5.2. m6A Regulates the Abundance and Induction of Antiviral Signaling and Effector Molecules
Antiviral gene expression must be tightly controlled to promote effective immunity while preventing cellular toxicity. Indeed, m6A and its machinery contribute to this control by post-transcriptionally regulating the mRNAs of several key molecules that determine the innate immune response to viral infection. Specifically, m6A has been identified in the 3′UTRs of TRAF3, TRAF6, MAVS, and IFNB1 mRNA (124, 125, 136). During innate immune stimulation TRAF3, TRAF6, and MAVS transcripts all lose m6A. This loss of m6A reduces their nuclear export and translation and is catalyzed by the eraser ALKBH5, which is recruited to these mRNAs by DDX46 (136). Interestingly, other DDX family members have been found to interact with ALKBH5, suggesting that this family of proteins may regulate virus infection by altering the abundance and stability of antiviral signaling molecules (137, 138). The IFNB1 transcript is also negatively regulated by m6A in its 3′UTR (124, 125). Loss of m6A in this region due to METTL3/METTL14 depletion increases expression of IFN-β protein during HCMV infection, suggesting again that m6A may be used by the cell to turn off antiviral responses (124, 125). On the other hand, METTL3 promotes the splicing of the TLR signaling adaptor MYD88 and the induction of several cytokines in response to lipopolysaccharide stimulation, which reveals that m6A can also positively regulate innate immunity (139).
The IFN response leads to the transcriptional induction of hundreds of ISGs and is likely regulated by m6A and its machinery. The expression of ISGs is negatively regulated by YTHDF3, which promotes the translation of a transcriptional repressor of ISGs called FOXO3 (140). On the other hand, the expression of some ISGs can be post-transcriptionally regulated by RNA-binding proteins such as G3BP1, G3BP2, and CAPRIN1, which are known to be repelled by m6A-containing RNAs and therefore could regulate ISGs in an m6A-dependent manner (62, 63, 141). Also, some ISG-encoded RNA-binding proteins, including IFIT1, ZAP, and SLFN11, can be directly antiviral (3, 31); others, such as FMR1 and IGF2BP3, selectively recognize m6A (62, 63, 142). Therefore, it is conceivable that uncharacterized IFN-induced RNA-binding proteins could selectively recognize the presence of m6A in viral or cellular transcripts and modulate their function during an antiviral response. An increased understanding of how m6A regulates antiviral innate immunity will have implications for host-directed therapeutics to limit virus infection.
5.3. m6A Regulates Adaptive Immunity
In addition to having several roles in antiviral innate immunity, m6A also regulates adaptive immunity (143). In particular, T cell differentiation and proliferation in mice are regulated by m6A modification of Socs1 and Socs3, which positively regulates their abundance (143). This leads to increased expression of the encoded Socs proteins, thereby reducing T cell differentiation and proliferation (143). Mettl3 also influences T regulatory cell generation and suppressive function (144). Because many diseases are caused by defects in T cell processes, some of which we now know are regulated by m6A-containing mRNAs, m6A in these specific mRNAs may be a therapeutic target.
6. FUTURE FOCUSES IN m6A BIOLOGY DURING VIRAL INFECTION
We anticipate that future work in this rapidly advancing field will paint a more complete picture of the mechanisms by which m6A influences viral infection. Here, we discuss future directions for studying m6A during viral infection, including the impact of m6A on RNA structure, methods for improving m6A mapping techniques, and how the field will move toward understanding the ways m6A and its associated machinery mechanistically impact cellular processes and immune responses during infection. Beyond recognition and regulation of m6A-containing viral RNA by RNA-binding proteins, it is likely that we will discover additional means by which m6A affects viral RNA. m6A has the potential to impact RNA structure, and it is becoming clear that viral RNA structures have a profound effect on viral replication (46, 81, 82, 145–147). Therefore, m6A could affect viral infection by altering viral RNA structures. Indeed, m6A sites that have been shown to regulate HIV-1 and HBV are found within viral RNA structures (113, 118). m6A could also contribute to viral-induced changes to structures in host mRNAs to affect gene expression (148). Therefore, future studies should be aimed at interrogating the role of m6A in altering secondary and tertiary structures in viral and host RNA and defining how these structural changes regulate infection.
Currently, m6A-containing regions on RNA from several families of viruses have been mapped; however, we lack information regarding m6A site occupancy at single-nucleotide resolution in these RNAs. The next steps in determining the m6A landscape during infection will be assessing the phasing and stoichiometry of m6A in viral RNAs. Phasing will reveal if m6A modifications at distinct sites of a particular mRNA species reside in the same or different RNA molecules, while m6A stoichiometry could be used to define both the fraction of each site and RNA species modified by m6A. Determining the phasing and stoichiometry of m6A in viral RNA will be of particular interest, as pools of viral RNAs operate in different stages of viral life cycles, and m6A might allow for the temporal discrimination of such species. Importantly, advances in long-read direct RNA sequencing via nanopores capable of accurately discriminating between unmodified and modified bases will greatly enhance our understanding of both phasing and stoichiometry of m6A in RNA. In the meantime, existing biochemical techniques that measure m6A occupancy and stoichiometry at single sites will be valuable tools for validating m6A sites in viral RNA (149–151).
These evolving methods for identifying m6A sites in different RNA species will also be invaluable for studying the effect of m6A in post-transcriptional regulation of the host response following infection. It is likely that the role of m6A in the host response to infection extends beyond its recently described functions in the antiviral innate immune response. During infection, the host transcriptome is broadly remodeled, and m6A could shape the output of this process (1). One example of m6A regulation during a host transcriptional response is seen during heat shock. Altered m6A patterns on stress-associated cellular mRNAs promote the heat shock response by affecting the localization and translation of these RNAs (95, 98). Similarly, changes in the m6A epitranscriptome could exert a regulatory function on individual transcripts to determine the outcome of infection. It is unknown how these changes in the host epitranscriptome would occur. However, there is evidence that viruses such as EV71 and HCMV increase the expression of METTL3 and METTL14, suggesting that the cellular m6A machinery might be altered during viral infection to catalyze these changes (107, 124, 125). Alternatively, during viral infection the m6A machinery could interact with a new complement of RNA-binding proteins that could target them to specific RNAs to modulate their m6A status. Recently, the m6A methyltransferase complex has been shown to be recruited to the histone modification H3 trimethylation at lysine 36 during active transcription to increase m6A in specific transcripts, which suggests that viral alteration of this epigenetic mark may result in differential m6A modification of host transcripts during infection. Ultimately, it will be important to understand how and which specific transcripts gain or lose m6A in response to infection, what effects m6A has on these RNAs, and ultimately how these changes affect viral infection. However, existing sequencing-based methods for m6A detection are limited in their ability to computationally distinguish between differences in m6A abundance, as opposed to transcript abundance or isoform usage. Better bioinformatic tools, including those that account for variability between samples, to accurately identify differential m6A modification of RNA under diverse conditions will therefore be critical for discovering how m6A regulates gene expression during infection. Indeed, using viral infection as a tool to perturb m6A modification of host transcripts might also allow us to uncover new molecular mechanisms that control the specificity of the m6A methyltransferase complex.
7. CONCLUDING REMARKS
Recent developments in mapping m6A on RNA, coupled with the ability to manipulate the enzymes involved in metabolism of m6A, have shed light on how this modification regulates RNA function to influence biological processes, including viral infection. Here, we have summarized advances in understanding the many roles of m6A in viral infection and immunity. For many viruses that contain m6A in their RNA, depletion of the m6A writer, eraser, or reader proteins affects diverse facets of viral replication that are often mediated by RNA-protein interactions. We note that such phenotypes might derive from the direct function of m6A on viral RNA or from the function of m6A on host transcripts. Overall, it is clear that m6A is neither uniformly proviral nor antiviral but instead regulates many aspects of viral replication by modulating specific RNAs sometimes dependent on tissue or cell type (113–117, 122, 123).
Virus families that employ intrinsically different life cycle strategies can be regulated by m6A and its machinery in diverse ways. Future experiments studying m6A during viral infection should focus on the role of specific modification sites in individual transcripts, temporal dynamics of modification, and the functions of newly identified m6A writers and readers; they should also continue to push these experiments beyond cell culture–based experimental approaches and into animal models. So far, we have only scratched the surface regarding understanding the role of m6A in viral infection. We expect that future work in this field will enable us to learn more about viral replication as well as fundamental functions of m6A in biology.
ACKNOWLEDGMENTS
We would like to thank members of the Horner lab for discussion and review of this manuscript. Research on m6A and viruses in the Horner lab is supported by the US National Institutes of Health (R01 AI125416 and R21 AI129851 to S.M.H.; F32 AI145180 to G.D.W.), the Burroughs Wellcome Fund (S.M.H.), and the American Heart Association Predoctoral Award (17PRE33670017 to N.S.G.).
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Carpenter S, Ricci EP, Mercier BC, Moore MJ, Fitzgerald KA. 2014. Post-transcriptional regulation of gene expression in innate immunity. Nat. Rev. Immunol 14:361–76 [DOI] [PubMed] [Google Scholar]
- 2.Schwerk J, Jarret AP, Joslyn RC, Savan R. 2015. Landscape of post-transcriptional gene regulation during hepatitis C virus infection. Curr. Opin. Virol 12:75–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li MM, MacDonald MR, Rice CM. 2015. To translate, or not to translate: viral and host mRNA regulation by interferon-stimulated genes. Trends Cell Biol. 25:320–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Batra R, Stark TJ, Clark E, Belzile JP, Wheeler EC, et al. 2016. RNA-binding protein CPEB1 remodels host and viral RNA landscapes. Nat. Struct. Mol. Biol 23:1101–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mino T, Takeuchi O. 2018. Post-transcriptional regulation of immune responses by RNA binding proteins. Proc. Jpn. Acad. Ser. B 94:248–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan B, Gao SJ. 2018. RNA epitranscriptomics: regulation of infection of RNA and DNA viruses by N6-methyladenosine (m6A). Rev. Med. Virol 28:e1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brocard M, Ruggieri A, Locker N. 2017. m6A RNA methylation, a new hallmark in virus-host interactions. J. Gen. Virol 98:2207–14 [DOI] [PubMed] [Google Scholar]
- 8.Gonzales-van Horn SR, Sarnow P. 2017. Making the mark: the role of adenosine modifications in the life cycle of RNA viruses. Cell Host Microbe 21:661–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gokhale NS, Horner SM. 2017. RNA modifications go viral. PLOS Pathog. 13:e1006188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kennedy EM, Courtney DG, Tsai K, Cullen BR. 2017. Viral epitranscriptomics. J. Virol 91:e02263–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xuan JJ, Sun WJ, Lin PH, Zhou KR, Liu S, et al. 2018. RMBase v2.0: deciphering the map of RNA modifications from epitranscriptome sequencing data. Nucleic Acids Res. 46:D327–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agris PF, Vendeix FA, Graham WD. 2007. tRNA’s wobble decoding of the genome: 40 years of modification. J. Mol. Biol 366:1–13 [DOI] [PubMed] [Google Scholar]
- 13.Agris PF. 2008. Bringing order to translation: the contributions of transfer RNA anticodon-domain modifications. EMBO Rep. 9:629–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma S, Lafontaine DLJ. 2015. ‘View from a bridge’: a new perspective on eukaryotic rRNA base modification. Trends Biochem. Sci 40:560–75 [DOI] [PubMed] [Google Scholar]
- 15.Shatkin AJ. 1976. Capping of eucaryotic mRNAs. Cell 9:645–53 [DOI] [PubMed] [Google Scholar]
- 16.Ramanathan A, Robb GB, Chan SH. 2016. mRNA capping: biological functions and applications. Nucleic Acids Res. 44:7511–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carlile TM, Rojas-Duran MF, Zinshteyn B, Shin H, Bartoli KM, Gilbert WV. 2014. Pseudouridine profiling reveals regulated mRNA pseudouridylation in yeast and human cells. Nature 515:143–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arango D, Sturgill D, Alhusaini N, Dillman AA, Sweet TJ, et al. 2018. Acetylation of cytidine in mRNA promotes translation efficiency. Cell 175:1872–86.e24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Squires JE, Patel HR, Nousch M, Sibbritt T, Humphreys DT, et al. 2012. Widespread occurrence of 5-methylcytosine in human coding and non-coding RNA. Nucleic Acids Res. 40:5023–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X, Xiong X, Zhang M, Wang K, Chen Y, et al. 2017. Base-resolution mapping reveals distinct m1A methylome in nuclear- and mitochondrial-encoded transcripts. Mol. Cell 68:993–1005.e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krug RM, Morgan MA, Shatkin AJ. 1976. Influenza viral mRNA contains internal N6-methyladenosine and 5′-terminal 7-methylguanosine in cap structures. J. Virol 20:45–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lavi S, Shatkin AJ. 1975. Methylated simian virus 40-specific RNA from nuclei and cytoplasm of infected BSC-1 cells. PNAS 72:2012–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sommer S, Salditt-Georgieff M, Bachenheimer S, Darnell JE, Furuichi Y, et al. 1976. The methylation of adenovirus-specific nuclear and cytoplasmic RNA. Nucleic Acids Res. 3:749–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kane SE, Beemon K. 1987. Inhibition of methylation at two internal N6-methyladenosine sites caused by GAC to GAU mutations. J. Biol. Chem 262:3422–27 [PubMed] [Google Scholar]
- 25.Kane SE, Beemon K. 1985. Precise localization of m6A in Rous sarcoma virus RNA reveals clustering of methylation sites: implications for RNA processing. Mol. Cell. Biol 5:2298–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dimock K, Stoltzfus CM. 1977. Sequence specificity of internal methylation in B77 avian sarcoma virus RNA subunits. Biochemistry 16:471–78 [DOI] [PubMed] [Google Scholar]
- 27.Furuichi Y, Shatkin AJ, Stavnezer E, Bishop JM. 1975. Blocked, methylated 5′-terminal sequence in avian sarcoma virus RNA. Nature 257:618–20 [DOI] [PubMed] [Google Scholar]
- 28.Moss B, Gershowitz A, Stringer JR, Holland LE, Wagner EK. 1977. 5′-Terminal and internal methylated nucleosides in herpes simplex virus type 1 mRNA. J. Virol 23:234–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Canaani D, Kahana C, Lavi S, Groner Y. 1979. Identification and mapping of N6-methyladenosine containing sequences in simian virus 40 RNA. Nucleic Acids Res. 6:2879–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfaller CK, Donohue RC, Nersisyan S, Brodsky L, Cattaneo R. 2018. Extensive editing of cellular and viral double-stranded RNA structures accounts for innate immunity suppression and the proviral activity of ADAR1p150. PLOS Biol. 16:e2006577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hyde JL, Diamond MS. 2015. Innate immune restriction and antagonism of viral RNA lacking 2′-O methylation. Virology 479–480:66–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le Pen J, Jiang H, Di Domenico T, Kneuss E, Kosalka J, et al. 2018. Terminal uridylyltransferases target RNA viruses as part of the innate immune system. Nat. Struct. Mol. Biol 25:778–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dev RR, Ganji R, Singh SP, Mahalingam S, Banerjee S, Khosla S. 2017. Cytosine methylation by DNMT2 facilitates stability and survival of HIV-1 RNA in the host cell during infection. Biochem. J 474:2009–26 [DOI] [PubMed] [Google Scholar]
- 34.McIntyre W, Netzband R, Bonenfant G, Biegel JM, Miller C, et al. 2018. Positive-sense RNA viruses reveal the complexity and dynamics of the cellular and viral epitranscriptomes during infection. Nucleic Acids Res. 46:5776–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ringeard M, Marchand V, Decroly E, Motorin Y, Bennasser Y. 2019. FTSJ3 is an RNA 2′-O-methyltransferase recruited by HIV to avoid innate immune sensing. Nature 565:500–4 [DOI] [PubMed] [Google Scholar]
- 36.Li X, Xiong X, Yi C. 2016. Epitranscriptome sequencing technologies: decoding RNA modifications. Nat. Methods 14:23–31 [DOI] [PubMed] [Google Scholar]
- 37.Dominissini D, Moshitch-Moshkovitz S, Schwartz S, Salmon-Divon M, Ungar L, et al. 2012. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 485:201–6 [DOI] [PubMed] [Google Scholar]
- 38.Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, Jaffrey SR. 2012. Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons. Cell 149:1635–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vandivier LE, Gregory BD. 2017. Reading the epitranscriptome: new techniques and perspectives. Enzymes 41:269–98 [DOI] [PubMed] [Google Scholar]
- 40.Meng J, Lu Z, Liu H, Zhang L, Zhang S, et al. 2014. A protocol for RNA methylation differential analysis with MeRIP-Seq data and exomePeak R/Bioconductor package. Methods 69:274–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen K, Lu Z, Wang X, Fu Y, Luo GZ, et al. 2015. High-resolution N6-methyladenosine (m6A) map using photo-crosslinking-assisted m6A sequencing. Angew. Chem. Int. Ed. Engl 54:1587–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grozhik AV, Linder B, Olarerin-George AO, Jaffrey SR. 2017. Mapping m6A at individual-nucleotide resolution using crosslinking and immunoprecipitation (miCLIP). Methods Mol. Biol 1562:55–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Linder B, Grozhik AV, Olarerin-George AO, Meydan C, Mason CE, Jaffrey SR. 2015. Single-nucleotide-resolution mapping of m6A and m6Am throughout the transcriptome. Nat. Methods 12:767–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ke S, Alemu EA, Mertens C, Gantman EC, Fak JJ, et al. 2015. A majority of m6A residues are in the last exons, allowing the potential for 3′ UTR regulation. Genes Dev. 29:2037–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ke S, Pandya-Jones A, Saito Y, Fak JJ, Vagbo CB, et al. 2017. m6A mRNA modifications are deposited in nascent pre-mRNA and are not required for splicing but do specify cytoplasmic turnover. Genes Dev. 31:990–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu B, Merriman DK, Choi SH, Schumacher MA, Plangger R, et al. 2018. A potentially abundant junctional RNA motif stabilized by m6A and Mg2+. Nat. Commun 9:2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zeng Y, Wang S, Gao S, Soares F, Ahmed M, et al. 2018. Refined RIP-seq protocol for epitranscriptome analysis with low input materials. PLOS Biol. 16:e2006092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Akichika S, Hirano S, Shichino Y, Suzuki T, Nishimasu H, et al. 2019. Cap-specific terminal N6-methylation of RNA by an RNA polymerase II-associated methyltransferase. Science 363:eaav0080. [DOI] [PubMed] [Google Scholar]
- 49.Sun H, Zhang M, Li K, Bai D, Yi C. 2019. Cap-specific, terminal N6-methylation by a mammalian m6Am methyltransferase. Cell Res. 29:80–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mauer J, Luo X, Blanjoie A, Jiao X, Grozhik AV, et al. 2017. Reversible methylation of m6Am in the 5′ cap controls mRNA stability. Nature 541:371–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bokar JA, Shambaugh ME, Polayes D, Matera AG, Rottman FM. 1997. Purification and cDNA cloning of the AdoMet-binding subunit of the human mRNA (N6-adenosine)-methyltransferase. RNA 3:1233–47 [PMC free article] [PubMed] [Google Scholar]
- 52.Liu J, Yue Y, Han D, Wang X, Fu Y, et al. 2014. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat. Chem. Biol 10:93–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jia G, Fu Y, Zhao X, Dai Q, Zheng G, et al. 2011. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat. Chem. Biol 7:885–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ping XL, Sun BF, Wang L, Xiao W, Yang X, et al. 2014. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. 24:177–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wen J, Lv R, Ma H, Shen H, He C, et al. 2018. Zc3h13 regulates nuclear RNA m6A methylation and mouse embryonic stem cell self-renewal. Mol. Cell 69:1028–38.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yue Y, Liu J, Cui X, Cao J, Luo G, et al. 2018. VIRMA mediates preferential m6A mRNA methylation in 3′UTR and near stop codon and associates with alternative polyadenylation. Cell Discov. 4:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zheng G, Dahl JA, Niu Y, Fedorcsak P, Huang CM, et al. 2013. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol. Cell 49:18–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Y, Li Y, Toth JI, Petroski MD, Zhang Z, Zhao JC. 2014. N6-methyladenosine modification destabilizes developmental regulators in embryonic stem cells. Nat. Cell Biol 16:191–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shi H, Wang X, Lu Z, Zhao BS, Ma H, et al. 2017. YTHDF3 facilitates translation and decay of N6-methyladenosine-modified RNA. Cell Res. 27:315–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang X, Zhao BS, Roundtree IA, Lu Z, Han D, et al. 2015. N6-methyladenosine modulates messenger RNA translation efficiency. Cell 161:1388–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang X, Lu Z, Gomez A, Hon GC, Yue Y, et al. 2014. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature 505:117–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arguello AE, DeLiberto AN, Kleiner RE. 2017. RNA chemical proteomics reveals the N6-methyladenosine (m6A)-regulated protein–RNA interactome. J. Am. Chem. Soc 139:17249–52 [DOI] [PubMed] [Google Scholar]
- 63.Edupuganti RR, Geiger S, Lindeboom RGH, Shi H, Hsu PJ, et al. 2017. N6-methyladenosine (m6A) recruits and repels proteins to regulate mRNA homeostasis. Nat. Struct. Mol. Biol 24:870–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Frye M, Harada BT, Behm M, He C. 2018. RNA modifications modulate gene expression during development. Science 361:1346–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang Y, Hsu PJ, Chen YS, Yang YG. 2018. Dynamic transcriptomic m6A decoration: writers, erasers, readers and functions in RNA metabolism. Cell Res. 28:616–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meyer KD, Jaffrey SR. 2014. The dynamic epitranscriptome: N6-methyladenosine and gene expression control. Nat. Rev. Mol. Cell Biol. 15:313–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Slobodin B, Han R, Calderone V, Vrielink J, Loayza-Puch F, et al. 2017. Transcription impacts the efficiency of mRNA translation via co-transcriptional N6-adenosine methylation. Cell 169:326–37.e12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Choe J, Lin S, Zhang W, Liu Q, Wang L, et al. 2018. mRNA circularization by METTL3-eIF3h enhances translation and promotes oncogenesis. Nature 561:556–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lin S, Choe J, Du P, Triboulet R, Gregory RI. 2016. The m6A methyltransferase METTL3 promotes translation in human cancer cells. Mol. Cell 62:335–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang P, Doxtader KA, Nam Y. 2016. Structural basis for cooperative function of Mettl3 and Mettl14 methyltransferases. Mol. Cell 63:306–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sledz P, Jinek M. 2016. Structural insights into the molecular mechanism of the m6A writer complex. eLife 5:e18434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Knuckles P, Lence T, Haussmann IU, Jacob D, Kreim N, et al. 2018. Zc3h13/Flacc is required for adenosine methylation by bridging the mRNA-binding factor Rbm15/Spenito to the m6A machinery component Wtap/Fl(2)d. Genes Dev. 32:415–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Patil DP, Chen CK, Pickering BF, Chow A, Jackson C, et al. 2016. m6A RNA methylation promotes XIST-mediated transcriptional repression. Nature 537:369–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pendleton KE, Chen B, Liu K, Hunter OV, Xie Y, et al. 2017. The U6 snRNA m6A methyltransferase METTL16 regulates SAM synthetase intron retention. Cell 169:824–35.e14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Doxtader KA, Wang P, Scarborough AM, Seo D, Conrad NK, Nam Y. 2018. Structural basis for regulation of METTL16, an S-adenosylmethionine homeostasis factor. Mol. Cell 71:1001–11.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ma H, Wang X, Cai J, Dai Q, Natchiar SK, et al. 2019. N6-Methyladenosine methyltransferase ZCCHC4 mediates ribosomal RNA methylation. Nat. Chem. Biol 15:88–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shen F, Huang W, Huang JT, Xiong J, Yang Y, et al. 2015. Decreased N6-methyladenosine in peripheral blood RNA from diabetic patients is associated with FTO expression rather than ALKBH5. J. Clin. Endocrinol. Metab 100:E148–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wei J, Liu F, Lu Z, Fei Q, Ai Y, et al. 2018. Differential m6A, m6Am, and m1A demethylation mediated by FTO in the cell nucleus and cytoplasm. Mol. Cell 71:973–85.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li S, Mason CE. 2014. The pivotal regulatory landscape of RNA modifications. Annu. Rev. Genom. Hum. Genet 15:127–50 [DOI] [PubMed] [Google Scholar]
- 80.He C 2010. Grand challenge commentary: RNA epigenetics? Nat. Chem. Biol 6:863–65 [DOI] [PubMed] [Google Scholar]
- 81.Liu N, Dai Q, Zheng G, He C, Parisien M, Pan T. 2015. N6-methyladenosine-dependent RNA structural switches regulate RNA-protein interactions. Nature 518:560–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu N, Zhou KI, Parisien M, Dai Q, Diatchenko L, Pan T. 2017. N6-methyladenosine alters RNA structure to regulate binding of a low-complexity protein. Nucleic Acids Res. 45:6051–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Patil DP, Pickering BF, Jaffrey SR. 2018. Reading m6A in the transcriptome: m6A-binding proteins. Trends Cell Biol. 28:113–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Huang H, Weng H, Sun W, Qin X, Shi H, et al. 2018. Recognition of RNA N6-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat. Cell Biol 20:285–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Du H, Zhao Y, He J, Zhang Y, Xi H, et al. 2016. YTHDF2 destabilizes m6A-containing RNA through direct recruitment of the CCR4-NOT deadenylase complex. Nat. Commun 7:12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xiao W, Adhikari S, Dahal U, Chen YS, Hao YJ, et al. 2016. Nuclear m6A reader YTHDC1 regulates mRNA splicing. Mol. Cell 61:507–19 [DOI] [PubMed] [Google Scholar]
- 87.Lesbirel S, Viphakone N, Parker M, Parker J, Heath C, et al. 2018. The m6A-methylase complex recruits TREX and regulates mRNA export. Sci. Rep 8:13827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kasowitz SD, Ma J, Anderson SJ, Leu NA, Xu Y, et al. 2018. Nuclear m6A reader YTHDC1 regulates alternative polyadenylation and splicing during mouse oocyte development. PLOS Genet. 14:e1007412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Meyer KD, Patil DP, Zhou J, Zinoviev A, Skabkin MA, et al. 2015. 5′ UTR m6A promotes cap-independent translation. Cell 163:999–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xu C, Liu K, Ahmed H, Loppnau P, Schapira M, Min J. 2015. Structural basis for the discriminative recognition of N6-methyladenosine RNA by the human YT521-B homology domain family of proteins. J. Biol. Chem 290:24902–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xu C, Wang X, Liu K, Roundtree IA, Tempel W, et al. 2014. Structural basis for selective binding of m6A RNA by the YTHDC1 YTH domain. Nat. Chem. Biol 10:927–29 [DOI] [PubMed] [Google Scholar]
- 92.Zhu T, Roundtree IA, Wang P, Wang X, Wang L, et al. 2014. Crystal structure of the YTH domain of YTHDF2 reveals mechanism for recognition of N6-methyladenosine. Cell Res. 24:1493–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li F, Zhao D, Wu J, Shi Y. 2014. Structure of the YTH domain of human YTHDF2 in complex with an m6A mononucleotide reveals an aromatic cage for m6A recognition. Cell Res. 24:1490–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shi H, Zhang X, Weng YL, Lu Z, Liu Y, et al. 2018. m6A facilitates hippocampus-dependent learning and memory through YTHDF1. Nature 563:249–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Anders M, Chelysheva I, Goebel I, Trenkner T, Zhou J, et al. 2018. Dynamic m6A methylation facilitates mRNA triaging to stress granules. Life Sci. Alliance 1:e201800113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li M, Zhao X, Wang W, Shi H, Pan Q, et al. 2018. Ythdf2-mediated m6A mRNA clearance modulates neural development in mice. Genome Biol. 19:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang C, Chen Y, Sun B, Wang L, Yang Y, et al. 2017. m6A modulates haematopoietic stem and progenitor cell specification. Nature 549:273–76 [DOI] [PubMed] [Google Scholar]
- 98.Zhou J, Wan J, Gao X, Zhang X, Jaffrey SR, Qian SB. 2015. Dynamic m6A mRNA methylation directs translational control of heat shock response. Nature 526:591–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wojtas MN, Pandey RR, Mendel M, Homolka D, Sachidanandam R, Pillai RS. 2017. Regulation of m6A transcripts by the 3′→5′ RNA helicase YTHDC2 is essential for a successful meiotic program in the mammalian germline. Mol. Cell 68:374–87.e12 [DOI] [PubMed] [Google Scholar]
- 100.Hsu PJ, Zhu Y, Ma H, Guo Y, Shi X, et al. 2017. Ythdc2 is an N6-methyladenosine binding protein that regulates mammalian spermatogenesis. Cell Res. 27:1115–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bailey AS, Batista PJ, Gold RS, Chen YG, de Rooij DG, et al. 2017. The conserved RNA helicase YTHDC2 regulates the transition from proliferation to differentiation in the germline. eLife 6:e2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kretschmer J, Rao H, Hackert P, Sloan KE, Hobartner C, Bohnsack MT. 2018. The m6A reader protein YTHDC2 interacts with the small ribosomal subunit and the 5′−3′ exoribonuclease XRN1. RNA 24:1339–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Alarcon CR, Goodarzi H, Lee H, Liu X, Tavazoie S, Tavazoie SF. 2015. HNRNPA2B1 is a mediator of m6A-dependent nuclear RNA processing events. Cell 162:1299–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gokhale NS, McIntyre ABR, McFadden MJ, Roder AE, Kennedy EM, et al. 2016. N6-methyladenosine in Flaviviridae viral RNA genomes regulates infection. Cell Host Microbe 20:654–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lichinchi G, Zhao BS, Wu Y, Lu Z, Qin Y, et al. 2016. Dynamics of human and viral RNA methylation during Zika virus infection. Cell Host Microbe 20:666–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Neufeldt CJ, Joyce MA, Van Buuren N, Levin A, Kirkegaard K, et al. 2016. The hepatitis C virus-induced membranous web and associated nuclear transport machinery limit access of pattern recognition receptors to viral replication sites. PLOS Pathog. 12:e1005428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hao H, Hao S, Chen H, Chen Z, Zhang Y, et al. 2019. N6-methyladenosine modification and METTL3 modulate enterovirus 71 replication. Nucleic Acids Res. 47:362–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Narayan P, Ayers DF, Rottman FM, Maroney PA, Nilsen TW. 1987. Unequal distribution of N6-methyladenosine in influenza virus mRNAs. Mol. Cell. Biol 7:1572–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Courtney DG, Kennedy EM, Dumm RE, Bogerd HP, Tsai K, et al. 2017. Epitranscriptomic enhancement of influenza A virus gene expression and replication. Cell Host Microbe 22:377–86.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Riquelme-Barrios S, Pereira-Montecinos C, Valiente-Echeverria F, Soto-Rifo R. 2018. Emerging roles of N6-methyladenosine on HIV-1 RNA metabolism and viral replication. Front. Microbiol 9:576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Beemon K, Keith J. 1977. Localization of N6-methyladenosine in the Rous sarcoma virus genome. J. Mol. Biol 113:165–79 [DOI] [PubMed] [Google Scholar]
- 112.Stoltzfus CM, Dane RW. 1982. Accumulation of spliced avian retrovirus mRNA is inhibited in S-adenosylmethionine-depleted chicken embryo fibroblasts. J. Virol 42:918–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lichinchi G, Gao S, Saletore Y, Gonzalez GM, Bansal V, et al. 2016. Dynamics of the human and viral m6A RNA methylomes during HIV-1 infection of T cells. Nat. Microbiol 1:16011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kennedy EM, Bogerd HP, Kornepati AV, Kang D, Ghoshal D, et al. 2016. Posttranscriptional m6A editing of HIV-1 mRNAs enhances viral gene expression. Cell Host Microbe 19:675–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lu W, Tirumuru N, St Gelais C, Koneru PC, Liu C, et al. 2018. N6-methyladenosine–binding proteins suppress HIV-1 infectivity and viral production. J. Biol. Chem 293:12992–3005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tirumuru N, Zhao BS, Lu W, Lu Z, He C, Wu L. 2016. N6-methyladenosine of HIV-1 RNA regulates viral infection and HIV-1 Gag protein expression. eLife 5:e15528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tirumuru N, Wu L. 2019. HIV-1 envelope proteins up-regulate N6-methyladenosine levels of cellular RNA independently of viral replication. J. Biol. Chem 294:3249–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Imam H, Khan M, Gokhale NS, McIntyre ABR, Kim GW, et al. 2018. N6-methyladenosine modification of hepatitis B virus RNA differentially regulates the viral life cycle. PNAS 115:8829–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Finkel D, Groner Y. 1983. Methylations of adenosine residues (m6A) in pre-mRNA are important for formation of late simian virus 40 mRNAs. Virology 131:409–25 [DOI] [PubMed] [Google Scholar]
- 120.Tsai K, Courtney DG, Cullen BR. 2018. Addition of m6A to SV40 late mRNAs enhances viral structural gene expression and replication. PLOS Pathog. 14:e1006919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ye F, Chen ER, Nilsen TW. 2017. Kaposi’s sarcoma-associated herpesvirus utilizes and manipulates RNA N6-adenosine methylation to promote lytic replication. J. Virol 91:e00466–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tan B, Liu H, Zhang S, da Silva SR, Zhang L, et al. 2018. Viral and cellular N6-methyladenosine and N6,2′-O-dimethyladenosine epitranscriptomes in the KSHV life cycle. Nat. Microbiol 3:108–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hesser CR, Karijolich J, Dominissini D, He C, Glaunsinger BA. 2018. N6-methyladenosine modification and the YTHDF2 reader protein play cell type specific roles in lytic viral gene expression during Kaposi’s sarcoma-associated herpesvirus infection. PLOS Pathog. 14:e1006995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rubio RM, Depledge DP, Bianco C, Thompson L, Mohr I. 2018. RNA m6A modification enzymes shape innate responses to DNA by regulating interferon β. Genes Dev. 32:1472–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Winkler R, Gillis E, Lasman L, Safra M, Geula S, et al. 2019. m6A modification controls the innate immune response to infection by targeting type I interferons. Nat. Immunol 20:173–82 [DOI] [PubMed] [Google Scholar]
- 126.McFadden MJ, Gokhale NS, Horner SM. 2017. Protect this house: cytosolic sensing of viruses. Curr. Opin. Virol 22:36–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kariko K, Buckstein M, Ni H, Weissman D. 2005. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity 23:165–75 [DOI] [PubMed] [Google Scholar]
- 128.Durbin AF, Wang C, Marcotrigiano J, Gehrke L. 2016. RNAs containing modified nucleotides fail to trigger RIG-I conformational changes for innate immune signaling. mBio 7:e00833–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pardi N, Parkhouse K, Kirkpatrick E, McMahon M, Zost SJ, et al. 2018. Nucleoside-modified mRNA immunization elicits influenza virus hemagglutinin stalk-specific antibodies. Nat. Commun 9:3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pardi N, Hogan MJ, Pelc RS, Muramatsu H, Andersen H, et al. 2017. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature 543:248–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pardi N, Secreto AJ, Shan X, Debonera F, Glover J, et al. 2017. Administration of nucleoside-modified mRNA encoding broadly neutralizing antibody protects humanized mice from HIV-1 challenge. Nat. Commun 8:14630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Richner JM, Himansu S, Dowd KA, Butler SL, Salazar V, et al. 2017. Modified mRNA vaccines protect against Zika virus infection. Cell 168:1114–25.e10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Daffis S, Szretter KJ, Schriewer J, Li J, Youn S, et al. 2010. 2′-O methylation of the viral mRNA cap evades host restriction by IFIT family members. Nature 468:452–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Johnson B, VanBlargan LA, Xu W, White JP, Shan C, et al. 2018. Human IFIT3 modulates IFIT1 RNA binding specificity and protein stability. Immunity 48:487–99.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dong H, Chang DC, Hua MH, Lim SP, Chionh YH, et al. 2012. 2′-O methylation of internal adenosine by flavivirus NS5 methyltransferase. PLOS Pathog. 8:e1002642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zheng Q, Hou J, Zhou Y, Li Z, Cao X. 2017. The RNA helicase DDX46 inhibits innate immunity by entrapping m6A-demethylated antiviral transcripts in the nucleus. Nat. Immunol 18:1094–103 [DOI] [PubMed] [Google Scholar]
- 137.Shah A, Rashid F, Awan HM, Hu S, Wang X, et al. 2017. The DEAD-box RNA helicase DDX3 interacts with m6A RNA demethylase ALKBH5. Stem Cells Int. 2017:8596135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Valiente-Echeverria F, Hermoso MA, Soto-Rifo R. 2015. RNA helicase DDX3: at the crossroad of viral replication and antiviral immunity. Rev. Med. Virol 25:286–99 [DOI] [PubMed] [Google Scholar]
- 139.Feng Z, Li Q, Meng R, Yi B, Xu Q. 2018. METTL3 regulates alternative splicing of MyD88 upon the lipopolysaccharide-induced inflammatory response in human dental pulp cells. J. Cell. Mol. Med 22:2558–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang Y, Wang X, Zhang X, Wang J, Ma Y, et al. 2018. RNA-binding protein YTHDF3 suppresses interferon-dependent antiviral responses by promoting FOXO3 translation. PNAS 116:976–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bidet K, Dadlani D, Garcia-Blanco MA. 2014. G3BP1, G3BP2 and CAPRIN1 are required for translation of interferon stimulated mRNAs and are targeted by a dengue virus non-coding RNA. PLOS Pathog. 10:e1004242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shaw AE, Hughes J, Gu Q, Behdenna A, Singer JB, et al. 2017. Fundamental properties of the mammalian innate immune system revealed by multispecies comparison of type I interferon responses. PLOS Biol. 15:e2004086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Li HB, Tong J, Zhu S, Batista PJ, Duffy EE, et al. 2017. m6A mRNA methylation controls T cell homeostasis by targeting the IL-7/STAT5/SOCS pathways. Nature 548:338–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tong J, Cao G, Zhang T, Sefik E, Vesely MCA, et al. 2018. m6A mRNA methylation sustains Treg suppressive functions. Cell Res. 28:253–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Boerneke ME, JE; Weeks KM. 2019. Physical and functional mapping of viral RNA genomes by SHAPE. Annu. Rev. Virol In press. 10.1146/annurev-virology-092917-043315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Roost C, Lynch SR, Batista PJ, Qu K, Chang HY, Kool ET. 2015. Structure and thermodynamics of N6-methyladenosine in RNA: a spring-loaded base modification. J. Am. Chem. Soc 137:2107–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Spitale RC, Flynn RA, Zhang QC, Crisalli P, Lee B, et al. 2015. Structural imprints in vivo decode RNA regulatory mechanisms. Nature 519:486–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mizrahi O, Nachshon A, Shitrit A, Gelbart IA, Dobesova M, et al. 2018. Virus-induced changes in mRNA secondary structure uncover cis-regulatory elements that directly control gene expression. Mol. Cell 72:862–74.e5 [DOI] [PubMed] [Google Scholar]
- 149.Golovina AY, Dzama MM, Petriukov KS, Zatsepin TS, Sergiev PV, et al. 2014. Method for site-specific detection of m6A nucleoside presence in RNA based on high-resolution melting (HRM) analysis. Nucleic Acids Res. 42:e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Xiao Y, Wang Y, Tang Q, Wei L, Zhang X, Jia G. 2018. An elongation- and ligation-based qPCR amplification method for the radiolabeling-free detection of locus-specific N6-methyladenosine modification. Angew. Chem. Int. Ed. Engl 57:15995–6000 [DOI] [PubMed] [Google Scholar]
- 151.Hengesbach M, Meusburger M, Lyko F, Helm M. 2008. Use of DNAzymes for site-specific analysis of ribonucleotide modifications. RNA 14:180–87 [DOI] [PMC free article] [PubMed] [Google Scholar]