Abstract
Background
Sub-Saharan African adolescents living with HIV face challenges to antiretroviral therapy (ART) adherence. Poor mental health drives non-adherence, but can be improved with cognitive behavioral therapy (CBT). CBT delivered by peers may strengthen effects while building capacity for sustainment in low-income countries. This case study retrospectively applied the Exploration Preparation Implementation Sustainment (EPIS) framework to characterize the execution of the Kigali Imbereheza Project (KIP), a 2-arm individually randomized group controlled trial of Trauma-Informed Adherence-Enhanced CBT (TI-CBTe) delivered by Rwandan youth leaders (YL) to adolescents living with HIV.
Methods
YL (n=14, 43% female, M=22.71 years-old) had confirmed HIV and self-reported ART adherence > 95%. Participants (n=356, 51% female, M=16.78 years-old) living with HIV were randomized to TI-CBTe or usual care. Two YL co-led TI-CBTe sessions over two months for a total of 12 hours, while other YL observed and rated fidelity. Participants reported on YL competence. Additional data evaluated feasibility, acceptability, uptake and fidelity.
Results
In the Exploration phase, focus groups, stakeholder meetings, and individual interviews revealed strong consensus for delivering TI-CBT to reduce adolescent depression and trauma and improve ART adherence. In the Preparation phase, curriculum revisions were made, YL were successfully trained, and a cascading supervision model was established. In the Implementation phase, YL delivered TI-CBTe with close monitoring and supervision. Findings revealed strong feasibility, acceptability, uptake and fidelity, increasing the likelihood of Sustainment.
Conclusions
EPIS can guide implementation planning and delivery and evaluate implementation outcomes.
Keywords: Implementation, adolescents, HIV, peer leaders, mental health
Introduction
Sub-Saharan Africa comprises the largest number of people worldwide living with HIV.1 Despite decreased HIV incidence globally and increased access to antiretroviral therapy (ART), youth ages 15 – 24 are experiencing rising infection rates.2 AIDS-related deaths among youth have tripled since 2000, and AIDS is the leading cause of death among adolescents in Africa.3 With the impending adolescent bulge worldwide, viral suppression in this age group is essential to avoid HIV resistant mutations, virologic failure, and onward HIV transmission.4
ART adherence is essential to achieve viral suppression, but many sub-Saharan African adolescents face adherence challenges.5,6 Depression, anxiety, and trauma are common among youth living with HIV,7–11 and mental health concerns are linked to low ART adherence, poor virologic outcomes,7 and mortality.5,6 Gender-based violence is also associated with low ART adherence12 and increased mental health problems.13 At least two barriers have impeded intervention efforts: a dearth of trained mental health practitioners in Africa and Rwanda specifically14–16 and minimal guidance on how best to deliver evidence-based interventions within the African context. Building in-country capacity by training non-mental health professionals to deliver effective interventions may improve and sustain positive mental health and HIV outcomes.17 Likewise, understanding and documenting factors that impact program uptake and delivery can inform dedicated efforts to address HIV and other health concerns in low-resource settings.
Rwanda is especially well-positioned to lead innovative adolescent-focused HIV-related programs. The Rwandan government and Ministry of Health’s early recognition of the HIV crisis produced numerous successes,18 including reducing incident infections, improving HIV testing uptake and linkage to care, and achieving viral suppression.19 Rwandan national surveys show significant progress toward reaching the 90–90-90 goals.20 Out of the 220,000 individuals estimated to be HIV positive, 88% know their status, 83% are on ART, and 86% are virally suppressed.21 As in other countries, however, these advances have not extended evenly to adolescents.22 The legacy of Rwanda’s genocide has had lasting consequences for nationwide mental health, and many Rwandan youth living with HIV suffer high rates of mental health symptoms, especially depression and trauma.23 To achieve optimal adherence, Rwandan programs must address adolescents’ underlying depression and trauma.23
Few mental health interventions have been evaluated in Africa,24,25 and none have carefully attended to the implementation process. By contrast, Trauma-Informed Cognitive Behavioral Therapy (TI-CBT) is highly promising; it has treated traumatic stress and depression in adolescent survivors of war in Uganda,26 symptoms of grief and post-traumatic stress among orphans in Zambia and Tanzania, and youth victims of intimate partner violence in the US.27,28 Locally adapted cognitive behavioral therapy (CBT) is feasible, acceptable, and effective in low-resource settings.26,29,30
Still, a lack of personnel and other resources are barriers to intervention delivery in low-income countries.31 The Indigenous Leader Outreach Model (ILOM) is an evidence-based approach grounded in social learning principles and social influence theories32–34 with strong potential for sustainability and effectiveness. It has been used extensively in adolescent health promotion,35 including school-based sexual health.36,37 Individuals with similar characteristics to the target population (e.g., age, experience, culture) are trained to deliver interventions.38–40 Peer leaders may increase intervention effectiveness because they are viewed as more legitimate, credible, and empathic to participants’ life circumstances,41–43 and engender trust and comfort about sensitive topics.43–45 Peer leaders can effectively deliver health-related interventions with fidelity,29,30,46,47 as long as there is careful attention to high-quality preparation, ongoing coaching, technical assistance,33,48–51 timely feedback, and continuous monitoring.50 Likewise, CBT can be delivered by lay professionals with adequate supervision and fidelity monitoring.52 Importantly, the ILOM addresses the need to build in-country capacity by training lay individuals to deliver programs.
Implementation science offers a promising opportunity to re-conceptualize how to deliver evidence-based interventions to address adolescent mental health and ART adherence and improve treatment uptake, adoption, fidelity, and acceptability in low-resource settings. Attention to implementation strategies that improve health outcomes while simultaneously building local capacity will lead to more sustainable interventions.53 To address these gaps, we describe a promising case study of the Exploration Preparation Implementation Sustainment (EPIS) framework54 retrospectively applied to the Kigali Imbereheza Project (KIP) conducted in Kigali, Rwanda, and delivered by Rwandan young adults living with HIV. This case study may inform future rigorous applications of implementation science to HIV research in sub-Saharan Africa, as case studies are a useful method to elucidate the complexity of implementation research in global health.55
EPIS comprises four phases,56 and each phase interacts dynamically with various influences (e.g., outer and inner setting contexts, bridging factors) to drive the success and/or failure of the implementation process. In the Exploration phase, key stakeholders and service systems identify existing health needs of the target population and seek out best evidence-based programs (EBPs). Once the most relevant EBP is selected, the team moves to the Preparation phase where potential implementation barriers and facilitators are identified. Stakeholders consider whether and how to adapt the EBP to accommodate the unique individual, cultural, social, and structural factors relevant for the population. An implementation plan is created to address barriers, leverage strengths, and codify important supportive processes, such as training, supervision, and monitoring, that will facilitate success in the final phases. A goal is to establish a positive implementation climate in the setting where the EBP will be delivered so it is supported.
The Implementation phase enacts the supportive processes developed in the Preparation phase and initiates EBP delivery. There is ongoing monitoring of both the implementation process and treatment fidelity, providing opportunities to enhance supports and/or amend strategies as needed. Finally, in the Sustainment phase, the emphasis shifts to maintaining the EBP, using continuous feedback and adjusting as needed to address emergent barriers. Ideally, sustainment has been considered from the beginning (i.e., Exploration), but the phase allows for formal evaluation of planned activities for sustainment.
In addition to the four phases, EPIS describes contextual levels (inner and outer) related to the organization that are instrumental to implementation. The inner context is comprised of internal organization characteristics that drive implementation, such as the institution’s leadership, structures, resources, internal policies, staffing, practices, and provider factors (e.g., attitudes, beliefs). The outer context refers to the external environment that influences organizational implementation. These include policies outside the organization, consumers, relationships across organizations such as funding agencies, health departments, and advocacy groups. These inner and outer setting factors interact with one another and the EBP itself (i.e., its fit in the organization and culture) to influence implementation. Finally, a more recent component of EPIS is bridging factors, representing relationships between the inner and outer contexts to guide implementation (e.g., academic-community partnerships, purveyors).
This paper describes a case study of the retrospective application of the four EPIS phases to the adaptation, delivery, and sustainment of TI-CBT enhanced to support adolescent ART adherence (i.e., TI-CBTe) for Rwandan youth living with HIV. Study activities paid careful attention to feasibility, acceptability, and fidelity and engaged partners from the Rwandan Biomedical Center representing the Ministry of Health; Women’s Equity in Access to Care and Treatment (WE-ACTx), an international non-governmental organization; WE-ACTx for Hope Clinic (WFH) serving people with HIV in Kigali; and the University Teaching Hospital of Kigali (CHUK), housing the public hospital in Rwanda treating children with HIV.
Methods
Overview of Procedures
Study procedures were approved by the Rwandan Biomedical Council, Cook County Health and Hospital System, and the University of Illinois at Chicago ethics committees. KIP is a 2-arm individually randomized controlled trial for youth living with HIV and receiving care at two urban clinics in Kigali, Rwanda. KIP project staff who were also employed at the health clinics invited youth to participate. Consistent with local ethics requirements, staff obtained youth assent and guardian consent for youth participants < 21 years-old and consent for youth > 20 years of age. Youth completed a 2-hour audio-computer-assisted self-interview within one month before the first intervention session. Youth were randomized to TI-CBTe or usual care immediately before the first session to avoid dropout due to group assignment. Clinic staff nominated 18 Rwandan male and female, 19–24 year-olds living with HIV to serve as youth leaders (YL) to be trained to deliver the intervention. YL were interviewed by the second (MC) and fourth (MF) authors and selected based on inclusion criteria (see below).
At both clinics, the intervention and usual care conditions were delivered at the same time over two months depending on clinic space and schedule. TI-CBTe consisted of six 2-hour sessions and usual care consisted of six 2-hour unstructured discussion groups. The discussion groups were led by non-TI-CBTe trained peer educators who met monthly or as needed with the clinic’s psychologist for supervision. Youth completed follow-up assessments at 6-, 12-, and 18-months post-baseline. Youth randomized to TI-CBTe received a booster session immediately following the 12-month assessment. Consistent with the EPIS framework, we report on the phases of implementation and data pertaining to feasibility, uptake, acceptability, and fidelity.
Participants
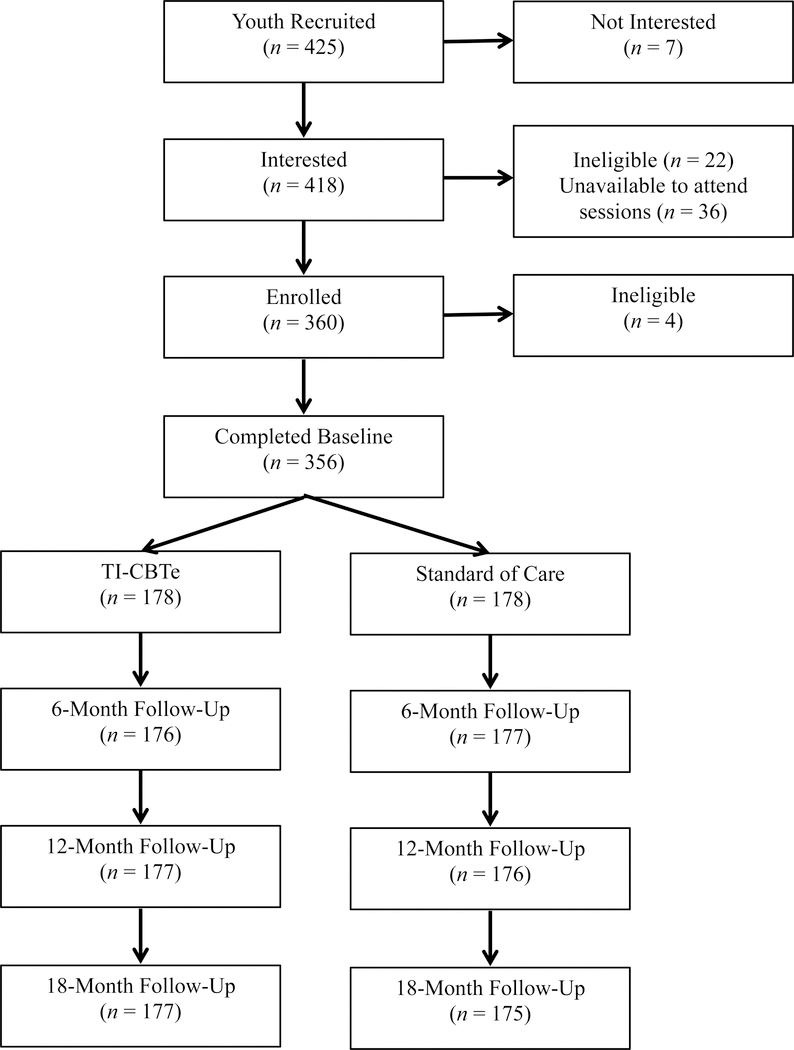
Figure 1 presents a CONSORT map of participant recruitment, randomization, and retention. Inclusion criteria for youth were: biologically confirmed HIV infection, knowledge of HIV status, and prescribed ART. For YL, inclusion criteria were: biologically confirmed HIV infection, ≥ 80% self-reported ART adherence (due to lack of availability of viral load data), able to commit to the study timeline, including training, and completion of ≥ 2 years of secondary school or equivalent skills. Youth (N=356) were 14–21 years old (M=16.78; SD=2.16) and 51% female. YL were 21–24 years-old (M=22.71; SD=1.14) and 43% female.
Figure 1.
Consolidated standards of reporting trials (CONSORT) summary of participant enrollment retention at 6-, 12-, and 18-month follow-up.
Measures
Feasibility
Youth feasibility was evaluated as follows: (1) recruitment, (2) enrollment, (3) session attendance, (4) retention, and (5) booster session attendance. YL feasibility was evaluated according to (1) recruitment, (2) attendance at sessions and supervision, (3) retention, (4) ability to achieve competence in TI-CBTe delivery, (5) reliability (arrived on time, prepared for sessions), and (6) engagement in supervision.
Uptake by Clinics
Uptake was evaluated according to: (1) non-KIP clinic staff support and interest in the intervention; (2) groups conducted as planned; (3) space provided by clinics to deliver the program; (4) close working relationships among clinic and study staff; (5) integration of the study into the clinic programming/schedule; and (6) smooth referral process for study youth needing additional mental health services.
Acceptability
Youth acceptability was evaluated based on three sources: (1) attendance across group sessions; (2) retention; and (3) participant ratings. At the end of each session, participants rated on a scale from 1=Not at all to 5=Extremely: (a) how satisfied they were, (b) how comfortable they felt, (c) how knowledgeable YL group leaders were, and (d) how much YL group leaders valued what they had to say. YL acceptability was evaluated according to four sources: (1) YL turnover; (2) self-reported relationships and support among YL; (3) YL feedback during supervision; and (4) YL reports during a final informal focus group with the study team to identify preliminary themes that will guide future focus groups with formal qualitative analysis.
TI-CBTe Fidelity
Consistent with recommendations by the NIH Behavior Change Consortium,57 the study psychologist, expert trainer (MF) and investigators (first and second authors) monitored treatment fidelity throughout the project. Because coaching and feedback improves post-training efficiency,58 in this case study YL participated in 2-hour twice weekly supervision sessions with the study psychologist. Treatment adherence ratings were completed on 100% of the sessions during direct observations by trained YL, although only 20% is deemed sufficient to ensure fidelity.57 YL observers reported on facilitator adherence and competence for each session activity on a scale from 0=not very well to 4=very well, and provided comments to explain deviations from the curriculum. Mean adherence and competence scores were calculated at each session across activities. In addition to YL observations, during three in-person visits to Rwanda each year, MF and the investigators each observed 3 – 4 group sessions. During these visits, MF provided direct supervision to YL, and conducted two refresher trainings each year (n=9) over the course of the study.
Results
Exploration Phase
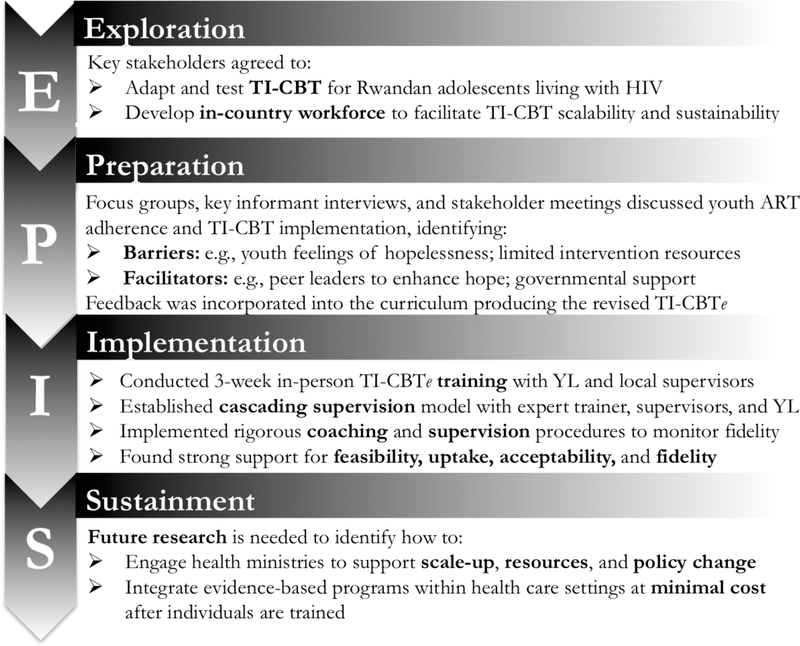
Figure 2 illustrates the EPIS phases and activities. In 2004, We-ACTx For Hope (WFH), a non-governmental organization in Kigali (sister organization of WE-ACTx directed by the second author, MC) was founded to provide care for women and children living with HIV. The organization was a response to calls for help accessing ART following the Rwandan genocide. Since then, MC has worked closely with colleagues at the Rwandan Ministry of Health (RMH) to navigate challenges providing ART. In 2007, WFH identified the need for youth friendly services to address the high numbers of teens on second- and third-line treatment due to adherence barriers and viral resistance to first-line therapy. WFH staff recognized that youth needed opportunities to express themselves, including their concerns about being HIV infected, disclosing their status, feeling angry at their parents, difficulty taking ART, having sex and being safe, and most importantly, about their future. To address these needs, WFH launched weekly support groups delivered on Sunday morning.
Figure 2.
Applying the Exploration, Preparation, Implementation, Sustainment Framework to the Kigali Imbereheza Project.
At the same time, there were ongoing country-wide discussions among HIV providers and the RMH focused on strategies to better meet the needs of adolescents living with HIV. In 2009, MC initiated discussions with the head of the Rwandan Biomedical Center of the RMH (SN, last author) to develop new adolescent services. The RMH ran the Therapy, Research and AIDS Care clinic at the Central Hospital of Kigali (CHUK), and staff at CHUK were also concerned about the high number of youth with poor viral suppression and mental health.
In 2011, MC invited SN to collaborate on a proposal to the Ronald McDonald House Charities to develop a multidisciplinary approach to adolescent care at both clinics. Physicians, psychologists, staff, and leaders of both clinics met several times to discuss the most appropriate intervention, and upon deciding on CBT, to design the intervention. Although the proposal was not funded, the undertaking launched a process of collaboration between WFH and the RMH that paved the way for the current project. In December 2011, MC reached out again to SN to explore a joint application in response to a request for proposals to improve adolescent adherence to antiretroviral therapy. MC invited the first author (GD) to collaborate based on her research experience conducting rigorous randomized clinical trials in adolescent HIV. MC and GD met in person in January 2012 at the University of Illinois at Chicago to discuss the application. Shortly thereafter, the investigative team was established (MC, SN, GD) and other key stakeholders from WFH and RMH were engaged to assist in the planning (e.g., nurses, psychologists, infectious disease physicians).
On January 23, 2012 MC, SN, and WFH staff met in Kigali to discuss the mechanisms responsible for poor adolescent ART adherence. Participants agreed that ART adherence efforts must address untreated trauma and depression and gender-based violence and re-confirmed CBT as the best approach. A second component of the conversation considered how to deliver CBT to increase sustainability and scalability if effective. These concerns were forefront during the Exploration phase and led to strong consensus to build local capacity and create an experienced workforce. Key stakeholders agreed to adapt and test the effectiveness of a trauma-informed CBT approach for Rwandan adolescents living with HIV, and develop an in-country workforce to facilitate intervention scalability and sustainability if effective. Over the following month (January – February 2012), the team met multiple times in Kigali and exchanged email to discuss and flesh out the grant proposal submitted in May 2012.
Preparation Phase
In December 2012, after learning that the grant would be funded, MC and MF conducted approximately five key informant interviews with WFH and CHUK staff and two informal focus groups, one with adolescents and one with young adults to query barriers and facilitators of adolescent ART adherence and intervention implementation. The informal focus groups with adolescents (N=8; 50% male) and young adults (N=10; 50% female) noted similar reasons for non-adherence, including fear of disclosure and accompanying stigma, logistical barriers (too many pills, forgot), hopelessness about the future, and lack of caregiver support. Both groups were uniformly positive about a group-based intervention for adolescents living with HIV to help improve mental health and medication adherence noting a desire to learn more effective ways to cope with stress, and young adults were especially eager to learn how to deliver the intervention.
In March 2013, MC and GD led an in-country 3-day retreat in Prefecture de Kigali attended by 14 stakeholders representing WFH, RMH, and CHUK to discuss the proposed CBT intervention and general project plans. Participants provided feedback about potential barriers and facilitators to program delivery and expressed strong support for a trauma-informed intervention that also addressed depression and medication adherence. They carefully reviewed the materials (curriculum, survey instruments), amended the curriculum to improve cultural relevance, added content related to gender-based violence and ART adherence, and finalized the training manual. Adaptations led to the revised TI-CBTe curriculum used in the study. Participants also offered specific recommendations for implementation, namely involving both CHUK and WFH to achieve the full sample size, training young adults living with HIV to deliver the intervention, and employing clinic psychologists as YL supervisors.
From April to June 2013, MC and MF met with youth and WFH and CHUK staff in Kigali to discuss feasibility, timing, training, space, and clinic resources. They identified potential barriers (few personnel, limited resources, small spaces) and strengths (systematic outreach to retain youth in care, wrap around services, dedicated staff, governmental support). From these inputs, the team developed an implementation plan that detailed rigorous methods to hire and train YL and ensure supervision and fidelity monitoring. The project director (CI, third author) was hired and began preparing for implementation.
Implementation Phase
Table 1 provides descriptive statistics for youth participants and YL at baseline. Across the sample, 82% reported at least one traumatic event in their lifetime and 60% reported two or more events.
Table 1.
Descriptive statistics at baseline among participants (N=356) and youth leaders (N=14)
| Participants |
Youth Leaders |
|
|---|---|---|
| Age, mean (SD) | 16.78 (2.16) | 22.71 (1.14) |
| Female, % (n) | 51% (183) | 43% (6) |
| Abject poverty or very poor, % (n) | 51% (177) | 36% (5) |
| Currently in school, % (n) | 85% (301) | 36% (5) |
| Completed at least grade S1 in school, % (n) | 51% (183) | 100% (14) |
| Orphan, % (n) | 23% (83) | 54% (7) |
| Adherence Wilson Average Score, mean (SD) | 75.01 (14.52) | 84.09 (8.61) |
| Anxiety/depression symptoms (past 6 months), mean (SD) | 0.49 (0.39) | 0.35 (0.27) |
| Trauma symptoms (past 30 days), mean (SD) | 6.41 (5.18) | 3.07 (2.09) |
Note. Adherence was measured with the Adherence Wilson Average Scale Score (range=0–100); higher scores indicated higher adherence (Wilson et al., 2016). Anxiety/depression symptoms were measured with the Youth Self-Report (range = 0–2); higher scores indicated greater anxiety/depression (Achenbach & Rescorla, 2001). Trauma symptoms were measured with an adapted version of the UCLA-PTSD RI (range=0–24); higher scores indicated higher trauma symptoms (Betancourt et al., 2012). All statistics were calculated based on available data.
TI-CBTe training
Shortly after selection and hiring, CI began YL training in research ethics and basic job skills. In September 2013, MF led an in-country TI-CBTe training including group facilitation skills. YL and both study psychologists (one from each clinic) attended training 4-hours per day, five days per week for three weeks at WFH. Of the 18 YL who met inclusion criteria, 14 completed the TI-CBTe training. Week 1 training emphasized basic concepts related to CBT, the importance of ART adherence for viral suppression, knowledge about traumatic stress and depression, and coping strategies. In week 2, MF reviewed the TI-CBTe manual, and YL learned the material and practiced delivering activities in pairs as MF observed. At the end of week 2, MF and the psychologists identified four YL to be the first to deliver TI-CBTe, two at each clinic. An additional four YL were selected to observe the sessions, two at each clinic. During week 3, the four YL chosen to deliver the program practiced the sessions while MF, the psychologists, and remaining YL observed and provided feedback. Final competence was determined by MF who observed each YL directly and confirmed that s/he was: (1) comfortable with the material, (2) followed the intervention manual, (3) demonstrated knowledge and ease delivering the session, (4) worked collaboratively with his/her co-facilitator, and (5) was able to field questions from mock participants during practice. Over the 4-year study, 10 YL facilitated and/or observed the groups, five assisted with assessments and material preparations, and all 14 participated in two refresher trainings each year designed to prevent facilitator drift.
Cascading supervision model
MF led a cascading supervision model, a rigorous approach to monitor YL fidelity. YL facilitators and observers engaged in twice weekly supervision with the WFH and CHUK psychologists. In the first meeting, YL discussed the previous session activities, reviewed feedback from fidelity ratings, and YL shared their observations. In the second meeting, YL practiced delivering the upcoming session and received feedback from the supervisor or additional training as needed. Following weekly supervision sessions with YL, the psychologists participated in weekly 2-hour Skype meetings with MF to discuss YL supervision.
Feasibility
Participant recruitment, enrollment, and attendance were strong (see Figure 1); 86% (n=360) of interested adolescents enrolled, 93% (n=166) attended at least five TI-CBTe sessions, and 76% (n=135) attended all six TI-CBTe sessions. Equal numbers of youth enrolled at each clinic (n=180) and only four participants were dropped due to ineligibility. Retention was 99% at each follow-up assessment (6-, 12-, and 18-months) and 83% at the booster session.
All 18 nominated YL completed the pre-training requirements, and 13 who participated in the TI-CBTe training achieved competence. One YL with difficulty reading was unable to fully deliver the intervention, but he was able to observe sessions. YL attendance at supervision and intervention sessions was > 85%. Where a YL was unavailable to lead a session, another YL served as backup. For > 92% of sessions, there were four YL present - two facilitators and two observers. YL were consistently prepared to lead the sessions, engaged actively in supervision, and were open to feedback. Only two YL missed < 3 supervision sessions due to school internships. In a final informal focus group, YL (n=14) reported increased self-confidence, greater optimism, improved quality of life, better job prospects, and tolerance for diversity as benefits of being part of the study. YL also indicated increased understanding of the connections between thoughts, feelings and behavior, more awareness of gender expectations, and improved condom use skills.
Uptake by Clinics
Non-study clinic staff supported intervention activities across all project years. They assisted with the logistics of implementation, and readily adjusted clinic programming to accommodate study activities. For example, at CHUK, staff increased the frequency of discussion groups to match the TI-CBTe meetings. Clinic staff also triaged referrals for youth in need of mental health services, arranged for ample space to simultaneously deliver the intervention and discussion group sessions, and facilitated medical records abstraction of clinical data, namely CD4 and viral load counts. The usual care group at one clinic was cancelled for two months due to lost funding but resumed once funding was restored in order to meet study requirements.
Acceptability
High youth enrollment (86%) and retention (98%) indicated acceptability, and high attendance across sessions (76% – 93%) suggests the group format was acceptable to youth and caregivers. Additionally, average participant ratings across all sessions of how knowledgeable, comfortable, and much YL valued what participants said ranged from 4.41 to 4.63, corresponding with “very favorable” ratings.
Evidence of acceptability among YL was also strong. Over the 4-year study, there was no YL turnover, and YL reported strong interpersonal connections with one another. Two years after the final group session (February, 2019) all but one YL (who was in school abroad and joined by Skype) attended a luncheon in Kigali with MF, MC, and GD. At the luncheon, YL reported regular get-togethers, a private Facebook page, and a WhatsApp group for ongoing contact among themselves.
Fidelity
Regular supervision and direct observations of multiple sessions by MF and MC twice annually and GD once per year confirmed treatment fidelity. In addition, formal ratings of adherence and competence by YL observers corresponded to an average of “very well” during the intervention sessions (sessions 1– 6 adherence range: M= 3.61–3.68, SD=0.38–0.46; sessions 1– 6 competence range: M=3.57–3.69, SD=0.33–0.40) and booster session (adherence M=3.57, SD=0.42; competence M=3.59, SD=0.36).
Discussion
This paper presents a case study of the Exploration Preparation Implementation and Sustainment framework applied retrospectively to the Kigali Imbereheza Project, a 2-arm randomized controlled trial testing a trauma informed cognitive behavioral intervention enhanced to address ART adherence for Rwandan youth living with HIV. EPIS proved useful to document the implementation process through four phases and identify barriers and facilitators to key implementation outcomes,59 namely feasibility, acceptability, uptake, and fidelity. This study compares favorably to prior applications of EPIS by utilizing multiple phases, planning early for sustainment, operationalizing a novel supervision/fidelity management approach, and targeting a new context with a new population.56
This case study extends previous implementation research on EPIS. A systematic review of peer-reviewed publications from 2011 to 2017 evaluated the use of EPIS components and identified 49 unique projects.56 Only one was implemented in Africa (South Africa),60 and it used EPIS mainly to frame the study. Likewise, most applications were implemented in high to middle-income countries and spanned public sectors but not HIV clinics. No project focused on adolescents living with HIV, the Rwandan context, or a youth-delivered mental health intervention to improve ART adherence. Thus, the current case study offers new insight into the application of EPIS with a unique population, in a novel limited resource context, and focused on an important yet understudied public health problem.
EPIS-related research to date has primarily emphasized the Implementation phase,61 and only 31% of projects reviewed by Moullin et al. (2019)56 evaluated bridging factors. By contrast, this case study describes activity across all four phases with special attention to the Exploration and Preparation phases, and we highlight the role of the research team as “purveyors or intermediaries” in ongoing implementation via training, supervision, support, and consultation. We also describe a relatively innovative approach to quality assurance54 that simultaneously promotes local capacity to deliver an evidence-based mental health intervention. The cascading supervision model outlines a concrete strategy to monitor treatment fidelity while building local expertise to strengthen sustainability.56 Sustainability planning is often ignored in implementation studies62 but important to ensure that effective programs continue after the research ends.63
A key goal of EPIS research is to diffuse the framework across different sectors, new evidence-based programs, and unique users.56 Many of the published reports to date involve one of the developers or their direct mentees.56 This case study diffuses EPIS in three ways; it was applied to Rwandan HIV public health clinics (new sectors), examined for a trauma informed cognitive behavioral intervention to improve ART adherence (new EBP and health concern), and implemented by authors independent of the developers.
In addition to these scientific advances, this case study revealed important lessons that may inform future implementation research. Perhaps most importantly, the Exploration phase was vital to develop a genuine community-engaged approach that involved shared power and decision making, the gold standard of research with marginalized communities (Hermes, 1999). The researchers leveraged the long-term relationship between MC, the founder of WFH, and SN from the RMH to form a cohesive team that helped guide project activities in each EPIS phase. Importantly, we engaged individuals from different stakeholder perspectives, namely HIV health care providers (nurses, psychologists, physicians), youth living with HIV, and staff working at public health clinics to inform implementation. This broad systematic approach during the Exploration phase laid the foundation for widescale buy-in and acceptability of the program by the HIV clinics and RMH. Lastly, engaging the RMH from the start planted the seeds of sustainability and improved the potential for policy change.
Two lessons emerged from the Preparation phase. First, Preparation appeared to directly promote uptake. Researchers and clinic staff worked together to identify a feasible and suitable approach to project implementation, underscoring the role of bridging factors to implementation success.54 During this phase, we clarified study components that could and could not be modified without compromising research integrity.64 For example, learned that changing the day of the intervention, incorporating traditional movement exercises at the start and end of each session, and combining sessions (without losing content) would improve uptake without hampering fidelity. The two clinics had differing schedules for their discussion control groups, so we matched the TI-CBTe groups with the discussion groups at each clinic to align with clinic logistics. In contrast, we ensured that certain activities (e.g., core components, theory-driven activities) were not altered.65 By working together to develop the implementation plan, we were able to facilitate clinic uptake and acceptability while maintaining fidelity.
The Preparation phase was also central to ensure scientific rigor. The implementation plan codified how to carefully evaluate fidelity to study procedures and the intervention, anticipating and adjusting to operational barriers. As an example, we employed a twice weekly supervision schedule to review observer feedback, re-train where needed, and practice upcoming sessions. We avoided delivering intervention groups on the third Saturday of each month to accommodate Rwanda’s “National Cleaning Day”, and we did not run any activities in April when the country formally recognizes the 1994 genocide. During the Preparation phase, we established detailed training and facilitator manuals for participant recruitment, assessment, and retention, intervention delivery, and data transfer to ensure meticulous methods.
Finally, this case study elucidated the importance of attending to broad multilevel factors (inner context, external setting)54 that interact to inform implementation outcomes. On the positive side, HIV clinic support and positive staff attitudes (inner context) toward TI-CBTe contributed to study successes in the ease of uptake and participation among eligible youth, feasibility and acceptability (high session attendance, excellent participant and youth leader retention), and strong intervention fidelity (adherence to the curriculum and participant perceptions of youth leaders as knowledgeable, comfortable and respectful). On the negative side, unanticipated external setting factors impeded study outcome measurement. Changes in ART initiation requirements from 2013 to 2017, shifts in therapeutic regimens from multiple pills to one pill, and decreases in PEPFAR funding to support HIV care and treatment reduced opportunities to evaluate the effects on ART adherence and sustain the intervention.
These lessons underscore future research directions for implementation science in HIV. This case study supports previous research that non-professionals without prior experience can be trained to deliver health interventions with fidelity and careful monitoring,66 and extends these data to young adults living with HIV and TI-CBTe. Reliance on community health workers to deliver mental health and HIV-related services continues to increase,67–69 and evidence for its advantages grow; it can overcome barriers to sustainable ART and scale up in high prevalence, low resource environments,70 address the shortage of health workers, provide employment opportunities, and lower human resource costs.71–73 Employing lay workers to deliver mental health and HIV-related programs may also result in improved patient retention and survival,74,70,75 greater access to HIV care, ART coverage, ART adherence, and virological and immunological outcomes.70 Yet, the impact of task shifting on this unique workforce is not well understood. The positive reports by youth leaders in this case study suggests potentially far reaching benefits, but future formal qualitative research is needed to inform this area.
Despite early preparation, we were unable to fully execute a plan for sustainability. Changes in external factors impeded our best laid plans. Sustainment of effective programs remains challenging in low-income countries,76 particularly as funding for HIV-related services remains flat.77 Training community health workers (see above) may prove cost-effective, but future research should plan explicitly for how to adapt to changes in external and internal factors during implementation. One option is to create a framework with strategies for sustainability that can be regularly evaluated and updated, such as how to engage health ministries at the start to support scale-up with policies and resources. The recently funded PATC3H consortium by the National Institutes of Health is well-poised to inform efforts to engage countrywide partners in program sustainability.
This case study reports on implementation outcomes (feasibility, acceptability, uptake, fidelity) rather than implementation processes. Future research that focuses on processes will build the science of implementation to drive much-needed rigor in methodology that can be generalized broadly. As an example, the Stages of Implementation Change (SIC) aligns with the EPIS framework78,79 and may inform important insights into processes that predict success vs. failure in implementation.79
Minimal attention to implementation factors is implicated in the 17-year gap80 between a scientific innovation and its application in real-life settings. The growing emphasis on implementation science, particularly in low resource settings,55,81 is designed to reduce the gap. This case study applied the EPIS framework to document the implementation process related to TI-CBTe for Rwandan youth living with HIV and demonstrated feasibility, acceptability, uptake, and fidelity, proving useful in key areas and underscoring the need for additional investigation.
Acknowledgements
We thank the Kigali Imbereheza Project staff who carried out the project, our collaborators and clinics, the youth leaders who learned to deliver the intervention with great skill, and the caregivers and young people living with HIV who graciously shared their stories with us and trusted us with their time.
Source of Funding: This study was funded by the National Institute of Child Health and Human Development (R01HD074977).
Footnotes
Conflicts of Interest: None of the authors have a conflict of interest.
ClinicalTrials.gov registration #:
References
- 1.AVERT. Global HIV and AIDS Statistics. 2018; https://www.avert.org/global-hiv-and-aids-statistics. Accessed April 15, 2019.
- 2.UNICEF. Adolescent HIV Prevention. 2018; https://data.unicef.org/topic/hivaids/adolescents-young-people/. Accessed April 15, 2019.
- 3.UNICEF. Children and AIDS: 2015 Statistical Update. https://data.unicef.org/resources/children-aids-2015-statistical-update-2/ Accessed April 15, 2019.
- 4.Cohen M, Chen Y, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. NEJM. 2011;365(6):493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dow D, Shayo A, Cunningham C, Reddy E. Durability of antiretroviral therapy and predictors of virologic failure among perinatally HIV-infected children in Tanzania: a four-year follow-up. BMC Infect Dis. 2014;14:567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nachega J, Hislop M, Nguyen H, et al. Antiretroviral therapy adherence, virologic and immunologic outcomes in adolescents compared with adults in southern Africa. J Acq Immune Def Syn. 2009;51(1):65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dow D, Turner E, Shayo A, Mmbaga B, Cunningham C, O’Donnell K. Evaluating mental health difficulties and associated outcomes among HIV-positive adolescents in Tanzania. AIDS Care. 2016;28(7):825–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Memiah P, Shumba C, Etienne-Mesubi M, et al. The effect of depressive symptoms and CD4 count on adherence to highly active antiretroviral therapy in sub-Saharan Africa. JIAPAC. 2014;13(4):346–352. [DOI] [PubMed] [Google Scholar]
- 9.Kamau J, Kuria W, Mathai M, Atwoli L, Kangethe R. Psychiatric morbidity among HIVinfected children and adolescents in a resource-poor Kenyan urban community. AIDS Care. 2012;24(7):836–842. [DOI] [PubMed] [Google Scholar]
- 10.Lowenthal E, Marukutira T, Chapman J, Mokete K, Riva K, Tshume O. Psychosocial assessments for HIV+ African adolescents: establishing construct validity and exploring underappreciated correlates of adherence. PLoS One. 2014;9(10):e109302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whetten K, Shirey K, Pence B, et al. Trauma history and depression predict incomplete adherence to antiretroviral therapies in a low income country. PLoS One. 2013;8(10):e74771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopez EJ, Jones DL, Villar-Loubet OM, Arheart KL, Weiss SM. Violence, coping, and consistent medication adherence in HIV-positive couples. AIDS Educ Prev. 2010;22(1):61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zunner B, Dworkin SL, Neylan TC, et al. HIV, violence and women: unmet mental health care needs. J Affect Dis. 2015;174:619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Umubyeyi A, Mogren I, Ntaganira J, G K. Help-seeking behaviours, barriers to care and self-efficacy for seeking mental health care: a population-based study in Rwanda. Soc Psych Psych Epid. 2016;51:81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization. Rwanda. 2011. https://www.who.int/countries/rwa/en/. Accessed August 12, 2019.
- 16.World Health Organization. Mental Health Atlas. 2005. https://www.who.int/mental_health/evidence/atlas/global_results.pdf. Accessed August 10, 2019.
- 17.Munetsi E, Simms V, Dzapasi L, et al. Trained lay health workers reduce common mental disorder symptoms of adults with suicidal ideation in Zimbabwe: a cohort study. BMC Public Health. 2018;18(1):227–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nsanzimana S, Prabhu K, McDermott H, et al. Improving health outcomes through concurrent HIV program scale-up and health system development in Rwanda: 20 years of experience. BMC Med. 2015;13:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.UNAIDS. Country factsheets: Rwanda. 2017; http://www.unaids.org/en/regionscountries/countries/rwanda. Accessed April 16, 2019.
- 20.PEPFAR. Rwanda. 2018. https://www.pepfar.gov/documents/organization/199598.pdf. Accessed August 11, 2019.
- 21.Nsanzimana S, Kanters S, Remera E, al. e. HIV care continuum in Rwanda: a cross-sectional analysis of the national programme. Lancet. 2015;2(5):PE208–E215. [DOI] [PubMed] [Google Scholar]
- 22.Lamb MR, Fayorsey R, Nuwagaba-Biribonwoha H, et al. High attrition before and after ART initiation among youth (15–24 years of age) enrolled in HIV care. AIDS. 2014;28(4):559–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith Fawzi MC, Ng L, Kanyanganzi F, et al. Mental Health and Antiretroviral Adherence Among Youth Living With HIV in Rwanda. Pediatrics. 2016;138(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mellins CA, Malee KM. Understanding the mental health of youth living with perinatal HIV infection: lessons learned and current challenges. J Int AIDS Soc. 2013;16:18593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parcesepe AM, Bernard C, Agler R, et al. Mental health and HIV: research priorities related to the implementation and scale up of ‘treat all’ in sub-Saharan Africa. J Virus Erad. 2018;4(Suppl 2):16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bolton P, Bass J, Neugebauer R, et al. Group interpersonal psychotherapy for depression in rural Uganda: a randomized controlled trial. JAMA. 2003;289(23):3117–3124. [DOI] [PubMed] [Google Scholar]
- 27.Cohen J, Mannarino A, Iyengar S. Community treatment of posttraumatic stress disorder for children exposed to intimate partner violence: a randomized controlled trial. Arch Pediatr Adolesc Med. 2011;165(1):16–21. [DOI] [PubMed] [Google Scholar]
- 28.Samuelson K Trauma-focused CBT reduces anxiety and post-traumatic stress disorder in children exposed to intimate partner violence. Evid-Based Men Health. 2011;14(2):56. [DOI] [PubMed] [Google Scholar]
- 29.Bolton P, Bass J, Betancourt T, et al. Interventions for depression symptoms among adolescent survivors of war and displacement in northern Uganda: a randomized controlled trial. JAMA. 2007;298(5):519–527. [DOI] [PubMed] [Google Scholar]
- 30.Rahman A, Malik A, Sikander S, Roberts C, Creed F. Cognitive behaviour therapy-based intervention by community health workers for mothers with depression and their infants in rural Pakistan: a cluster-randomised controlled trial. Lancet. 2008;372(9642):902–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sankoh O, Sevalie S, Weston M. Mental health in Africa. Lancet Glob Health. 2018;6(9):e954–e955. [DOI] [PubMed] [Google Scholar]
- 32.Bandura A Social Foundations of Thought and Action. Upper Saddle River, NJ: Prentice Hall; 1986. [Google Scholar]
- 33.Maticka-Tyndale E, Barnett JP. Peer-led interventions to reduce HIV risk of youth: A review. Eval Program Plann. 2010;33(2):98–112. [DOI] [PubMed] [Google Scholar]
- 34.Wiebel W The Indigenous Leader Outreach Model: Intervention Manual. 1993. Washington, D.C.: U.S. Department of Health and Human Services. [Google Scholar]
- 35.Bernert DJ, Mouzon LD. Peer Education in the’90’s: A Literature Review of Utility and Effectiveness. Health Educator. 2001;33(1):31–37. [Google Scholar]
- 36.Mahat G, Scoloveno MA, De Leon T, Frenkel J. Preliminary Evidence of an Adolescent HIV/AIDS Peer Education Program. J Pediatr Nurs. 2008;23(5):358–363. [DOI] [PubMed] [Google Scholar]
- 37.Kirby D, Korpi M, Adivi C, al e. An impact evaluation of project SNAPP: An AIDS and pregnancy prevention middle school program. AIDS Educ Prev. 1997;9:44–61. [PubMed] [Google Scholar]
- 38.Harden A, Oakley A, Oliver S. Peer-delivered health promotion for young people: A systematic review of different study designs. Health Educ J. 2001;60(4):339–353. [Google Scholar]
- 39.Sciacca J Student peer health education: a powerful yet inexpensive helping strategy. Peer Facilitator Quarterly. 1987;5(2):4–6. [Google Scholar]
- 40.Shiner M Defining peer education. J Adolesc. 1999;22(4):555–566. [DOI] [PubMed] [Google Scholar]
- 41.UNAIDS. Peer Education and HIV/AIDS: Concept, Uses and Challenges. 1999. Joint United Nations Programme on HIV/AIDS. [Google Scholar]
- 42.Milburn K A critical review of peer education with young people with special reference to sexual health. Health Educ Res. 1995;10(4):407–420. [DOI] [PubMed] [Google Scholar]
- 43.Stephenson JM, Strange V, Forrest S, et al. Pupil-led sex education in England (RIPPLE study): cluster-randomised intervention trial. Lancet. 2004;364(9431):338–346. [DOI] [PubMed] [Google Scholar]
- 44.Campbell C, MacPhail C. Peer education, gender and the development of critical consciousness: participatory HIV prevention by South African youth. Soc Sci Med. 2002;55(2):331–345. [DOI] [PubMed] [Google Scholar]
- 45.Helgerson SD, Petersen LR, Group TAES. Acquired Immunodeficiency Syndrome and Secondary School Students: Their Knowledge Is Limited and They Want to Learn More. Pediatrics. 1988;81(3):350–355. [PubMed] [Google Scholar]
- 46.Murray L, Dorsey S, Bolton P, et al. Building Capacity in Mental Health Interventions in Low Resource Countries: An Apprenticeship Model for Training Local Providers. Int J Ment Health Syst. 2011;5(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patel V, Weiss H, Chowdhary N, et al. Effectiveness of an intervention led by lay health counsellors for depressive and anxiety disorders in primary care in Goa, India (MANAS): a cluster randomised controlled trial. Lancet. 2010;376(9758):2086–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Durlak J, DuPre E. Implementation Matters: A Review of Research on the Influence of Implementation on Program Outcomes and the Factors Affecting Implementation. Am J Commun Psychol. 2008;41(3–4):327–350. [DOI] [PubMed] [Google Scholar]
- 49.Fixsen DL, Naoom SF, Blase KA, Friedman RM. Implementation research: a synthesis of the literature. 2005. Tampa, Florida. [Google Scholar]
- 50.Kershner S, Flynn S, Prince M, Potter SC, Craft L, Alton F. Using data to improve fidelity when implementing evidence-based programs. J Adolescent Health. 2014;54(3):S29–S36. [DOI] [PubMed] [Google Scholar]
- 51.Weiner B, Belden C, Bergmire D, Johnston M. The meaning and measurement of implementation climate. Implement Sci. 2011;6(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Donenberg GR, Fabri M, Ingabire C, Nsanzimana S, Emerson E, Cohen M. Empowering Rwandan Young Adults: A Case Example Using Youth Leaders. International Society of Traumatic Stress Studies; November 2015. New Orleans, LA. [Google Scholar]
- 53.Jerene D, Biru M, Teklu A, Rehman T, Ruff A, Wissow L. Factors promoting and inhibiting sustained impact of a mental health task-shifting program for HIV providers in Ethiopia. Glob Ment Health. 2017;4:e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aarons G, Hurlburt M, Horwitz S. Advancing a conceptual model of evidence-based practice implementation in public service sectors. Adm Policy Ment Health. 2011;38(1):4–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Theobald S, Brandes N, Gyapong M, et al. Implementation research: new imperatives and opportunities in global health. Lancet. 2018;392(10160):2214–2228. [DOI] [PubMed] [Google Scholar]
- 56.Moullin JC, Dickson KS, Stadnick NA, Rabin B, Aarons GA. Systematic review of the Exploration, Preparation, Implementation, Sustainment (EPIS) framework. Implement Sci. 2019;14(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Borrelli B The Assessment, Monitoring, and Enhancement of Treatment Fidelity In Public Health Clinical Trials. J Public Health Dent. 2011;71(s1):S52–s63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miller WR, Yahne CE, Moyers TB, Martinez J, Pirritano M. A randomized trial of methods to help clinicians learn motivational interviewing. J Consult Clin Psych. 2004;72(6):1050–1062. [DOI] [PubMed] [Google Scholar]
- 59.Proctor E, Silmere H, Raghavan R, Hovmand P, Aarons G, Bunger A. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Admin Pol Ment Health. 2011;38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peltzer K, Prado G, Horigian V, et al. Prevention of mother-to-child transmission (PMTCT) implementation in rural community health centres in Mpumalanga province, South Africa. J Psych Africa. 2016;26(5):415–418. [PMC free article] [PubMed] [Google Scholar]
- 61.Moullin JC, Sabater-Hernandez D, Fernandez-Llimos F, Benrimoj SI. A systematic review of implementation frameworks of innovations in healthcare and resulting generic implementation framework. Health Res Policy Syst. 2015;13:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shelton RC, Cooper BR, Stirman SW. The Sustainability of Evidence-Based Interventions and Practices in Public Health and Health Care. Annu Rev Public Health. 2018;39:55–76. [DOI] [PubMed] [Google Scholar]
- 63.Wiltsey Stirman S, Kimberly J, Cook N, Calloway A, Castro F, Charns M. The sustainability of new programs and innovations: a review of the empirical literature and recommendations for future research. Implement Sci. 2012;7:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blase K, Fixsen D. Core Intervention Components: Identifying and Operationalizing What Makes Programs Work. 2013. U.S. Department of Health and Human Services. [Google Scholar]
- 65.Castro FG, Barrera M Jr., Martinez CR Jr. The cultural adaptation of prevention interventions: resolving tensions between fidelity and fit. Prev Sci. 2004;5(1):41–45. [DOI] [PubMed] [Google Scholar]
- 66.Naidoo N, Railton JP, Khosa SN, et al. Fidelity of HIV programme implementation by community health workers in rural Mopani district, South Africa: a community survey. BMC Public Health. 2018;18(1):1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Celletti F, Wright A, Palen J, et al. Can the deployment of community health workers for the delivery of HIV services represent an effective and sustainable response to health workforce shortages? Results of a multicountry study. AIDS. 2010;24 Suppl 1:S45–57. [DOI] [PubMed] [Google Scholar]
- 68.Masquillier C, Wouters E, Mortelmans D, van Wyk B, Hausler H, Van Damme W. HIV/AIDS Competent Households: Interaction between a Health-Enabling Environment and Community-Based Treatment Adherence Support for People Living with HIV/AIDS in South Africa. PLoS One. 2016;11(3):e0151379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rasschaert F, Philips M, Van Leemput L, Assefa Y, Schouten E, Van Damme W. Tackling Health Workforce Shortages During Antiretroviral Treatment Scale-up-Experiences From Ethiopia and Malawi. JAIDS. 2011;57:S109–S112. [DOI] [PubMed] [Google Scholar]
- 70.Wouters E, Van Damme W, van Rensburg D, Masquillier C, Meulemans H. Impact of community-based support services on antiretroviral treatment programme delivery and outcomes in resource-limited countries: a synthetic review. BMC Health Serv Res. 2012;12:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Busza J, Dauya E, Bandason T, et al. The role of community health workers in improving HIV treatment outcomes in children: lessons learned from the ZENITH trial in Zimbabwe. Health Policy Plan. 2018;33(3):328–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Torpey K, Kabaso M, LN M, al e. Adherence support workers: a way to address human resource constraints in antiretroviral treatment programs in the public health setting in Zambia. PLoS One. 2008;3:e2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zachariah R, Ford N, M P, al. e. Task shifting in HIV/AIDS: opportunities, challenges and proposed actions for sub-Saharan Africa. Trans R Soc Trop Med Hyg. 2009;103:549–558. [DOI] [PubMed] [Google Scholar]
- 74.Javadi D, Feldhaus I, Mancuso A, Ghaffar A. Applying systems thinking to task shifting for mental health using lay providers: a review of the evidence. Glob Ment Health. 2017;4:e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Franke MF, Kaigamba F, Socci AR, et al. Improved retention associated with community-based accompaniment for antiretroviral therapy delivery in rural Rwanda. Clin Infect Dis. 2013;56(9):1319–1326. [DOI] [PubMed] [Google Scholar]
- 76.Montague BT, Vuylsteke B, Buvé A. Sustainability of programs to reach high risk and marginalized populations living with HIV in resource limited settings: implications for HIV treatment and prevention. BMC Public Health. 2011;11(1):701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kaiser Family Foundation. The U.S. President’s Emergency Plan for AIDS Relief (PEPFAR). 2019. https://www.kff.org/global-health-policy/fact-sheet/the-u-s-presidents-emergency-plan-for/. Accessed August 12, 2019.
- 78.Palinkas LA, Campbell M, Saldana L. Agency Leaders’ Assessments of Feasibility and Desirability of Implementation of Evidence-Based Practices in Youth-Serving Organizations Using the Stages of Implementation Completion. Front Public Health. 2018;6:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Saldana L The stages of implementation completion for evidence-based practice: protocol for a mixed methods study. Implement Sci. 2014;9(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Green LW, Ottoson JM, Garcia C, Hiatt RA. Diffusion theory and knowledge dissemination, utilization, and integration in public health. Annu Rev Public Health. 2009;30:151–17480. [DOI] [PubMed] [Google Scholar]
- 81.Yamey G What are the barriers to scaling up health interventions in low and middle income countries? A qualitative study of academic leaders in implementation science. Globalization Health. 2012;8(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]