Abstract
Purpose:
To propose a new methodology for classifying patient-reported outcomes in glaucoma and for quantifying the amount of visual field damage associated with disability in the disease.
Design:
Cross-sectional study.
Methods:
263 patients with glaucoma were included. Vision-related disability was assessed by the National Eye Institute Visual Function Questionnaire (NEI VFQ-25). A latent class analysis (LCA) model was applied to analyze NEI VFQ-25 data and patients were divided into mutually exclusive classes according to their responses to the questionnaires. Differences in standard automated perimetry (SAP) mean deviation (MD) and integrated binocular mean sensitivity (MS) values between classes were investigated. The optimal number of classes was defined based on goodness-of-fit criteria, interpretability and clinical utility.
Results:
The model with two classes, disabled and non-disabled, had the best fit with an entropy of 0.965, indicating excellent separation of classes. The disabled group had 48 (18%) patients, whereas 215 (82%) patients were classified as non-disabled. The average MD of the better eye in the disabled group was −5.98 dB versus −2.51 dB in the non-disabled group (P < 0.001). For the worse eye, corresponding values were −13.36 dB and −6.05 dB, respectively (P < 0.001).
Conclusion:
Application of a LCA model allowed categorization of patient-reported outcomes and quantification of visual field levels associated with disability in glaucoma. A damage of approximately −6 dB for SAP MD, indicating relatively early visual field loss, may already be associated with significant disability if occurring in the better eye.
INTRODUCTION
Glaucoma is a progressive optic neuropathy that can result in irreversible loss of vision.1,2 As a result of vision loss, individuals with glaucoma may report difficulty with a variety of activities of daily living, such as reading, walking, and driving, with significant impact on quality of life (QoL).3
Staging the severity of glaucoma is important in order to guide management decisions and also to inform prognosis. For that to happen, staging systems should have a meaningful correspondence to clinical outcomes that are directly relevant to patients, such as how the disease affects QoL or the ability to perform daily activities. Glaucoma staging systems have traditionally been based on the severity of visual field loss detected by standard automated perimetry (SAP).4–7 The widely used Hodapp-Parrish-Anderson classification of severity of visual field loss,4 for example, uses cut-offs for mild, moderate and severe glaucoma based on SAP mean deviation (MD) and number of abnormal points in the pattern deviation plot. However, it is not clear whether such classifications are associated with different levels of QoL or disability from the disease.
The impact of glaucoma on QoL has usually been measured using patient-reported outcomes, such as the National Eye Institute Visual Function Questionnaire (NEI VFQ-25).8 The NEI VFQ-25 contains several questions asking patients to rate their ability to perform a variety of tasks. Previous studies have shown significant relationships between visual field loss measured by SAP and summary scores of QoL obtained from the NEI VFQ-25 on a continuous scale. However, although these studies have been important in validating visual field metrics obtained from SAP, it is still not clear how much damage on SAP needs to be present for patients to exhibit a significant decline in vision-related QoL. This can have obvious implications in establishing the prognostic significance of staging systems based on SAP results and how they can guide management decisions.
Latent class analysis (LCA) is a statistical model useful to identify subgroups of individuals who share similar underlying characteristics or hidden patterns. These subgroups can then be used to stage the disease, explain symptoms or levels of disability, or to study differences in prognosis or treatment response.9 For example, using lCa, one could categorize continuous data from a medical test into more easily interpretable categories of diseased versus non-diseased. Although long neglected in Ophthalmology, this modeling approach has been proven useful to identify subgroups (i.e., latent classes) within heterogeneous populations in different fields of medicine, such as psychology, oncology, neurology and others. For example, LCA has been used successfully to identify subgroups of patients with cognitive impairment based on batteries of neuropsychological tests. The subgroups can then be further studied on their relationship to other clinical tests and biomarkers.10–14
In the current study, we propose to use LCA to identify similar patterns of responses to NEI VFQ-25 questionnaire items and thereby identify subgroups of glaucoma patients who are similar in how the disease affects their QoL. We then studied the relationship between these subgroups and levels of visual field damage as measured by SAP. This approach can help address the important question of what amount of visual field loss is associated with disability in glaucoma.
METHODS
This is a cross-sectional study with participants enrolled in a prospective, longitudinal study designed to evaluate functional impairment in glaucoma. The institutional review boards from Duke University and University of California San Diego approved the methods and written informed consent was obtained from all participants. The study adhered to the Health Insurance Portability and Accountability Act, and all study methods complied with the Declaration of Helsinki guidelines for human subject research.
During follow-up, patients underwent comprehensive ophthalmologic examinations, including review of medical history, visual acuity, slit-lamp biomicroscopy, intraocular pressure measurement, gonioscopy, dilated funduscopic examination, stereoscopic optic disc photography, and SAP using 24–2 Swedish interactive threshold algorithm standard (Carl Zeiss Meditec, Inc., Dublin, CA). Only patients with open angles on gonioscopy were included. Patients were excluded if they demonstrated any other ocular or systemic disease that could affect the optic nerve or the visual field in at least one of the eyes. Visual fields were excluded if they had more than 33% fixation losses or more than 15% false-positive errors. Visual fields were also excluded in the presence of eyelid or rim artifacts, fatigue effects, or evidence that the visual field results were caused by a disease other than glaucoma.15
Diagnosis of glaucoma was defined based on the presence of repeatable (at least two consecutive) abnormal SAP results with corresponding optic nerve damage in at least one eye. Abnormal SAP results were defined as a pattern standard deviation with P < 0.05, glaucoma hemifield test results outside normal limits, or both.
To evaluate binocular visual field loss, sensitivities of the monocular SAP threshold sensitivities of the right and left eyes were used to calculate an integrated binocular visual field, according to the binocular summation model described by Nelson-Quigg et al.16
Demographic, Clinical, and Socioeconomic Variables
Socioeconomic questionnaires were also administered to patients. These questionnaires contained a survey about demographics, history of ocular and medical conditions, marital status, health insurance coverage, degree of education, and income. Visual acuity (VA) was measured using an Early Treatment Diabetic Retinopathy chart, and the logarithm of the minimum angle of resolution (logMAR) was used for the analyses.
25-Item National Eye Institute Visual Function Questionnaire
Vision-related QoL was assessed using the NEI VFQ-25.8 The NEI VFQ-25 includes a set of 25 questions representing 11 subscales plus an additional single-item general health rating question. The subscales are: general vision, near and distance vision activities, ocular pain, vision-related social function, vision-related role function, vision-related mental health, vision-related dependency, driving difficulties, color vision and peripheral vision. For the present study, we excluded the items related to dependency, mental health, and role limitations, as they have been previously shown to belong to a separate socioemotional dimension not directly related to visual functioning.17–20 Also, the items of the ocular pain subscale were also excluded because ocular pain would likely induce changes in QoL not directly related to vision loss from glaucoma. The remaining 14 questions were used to assess vision-related disability status. This approach has been used in several previous publications investigating the relationship between SAP and vision-related QoL measured using the NEI VFQ-25.17,20–24
Latent Class Analysis
A latent class analysis (LCA) model was used to characterize vision-related disability from NEI VFQ-25 results. Latent class theory assumes the existence of underlying latent grouping variables that divide the population into two or more mutually exclusive and exhaustive latent classes.25 All individuals in a group are expected to have the same probability of responding to questionnaire items in a particular way.26 The term latent means that an error-free latent variable is postulated: it cannot be directly measured and instead is measured using several fallible indicators, in this case, the NEI VFQ-25 questionnaire items.27 As opposed to the latent variable, the observed variables are subject to errors. The model computes the joint probabilities of observed response patterns and class membership and uses several procedures for maximum likelihood estimation of the parameters. Model constraints were used to indicate the ordered nature of the categorical variables corresponding to the questionnaire answers. A detailed description of LCA has been previously published.28
After the estimation of these parameters for a specified model, we then determined the plausibility of the hypothesized model by computing expected frequencies of each pattern of response on the indicators and comparing these with the observed frequencies. If the expected and observed frequencies are close, then the data are consistent with the model. Multiple indices of model fit have been proposed for comparing models with different numbers of classes, including the Akaike Information Criterion (AIC),29 the Bayesian Information Criterion (BIC),30 the sample-size adjusted BIC (aBIC),31 the bootstrapped likelihood ratio test (BLRT),32 and the Lo-Mendell-Rubin test (LMRT).33 The AIC and BIC are penalized log-likelihood test statistics, where the penalty is two times the number of parameters estimated for the AIC and the log of n times the number of parameters estimated for the BIC. These criteria compare the relative fit of several models under consideration but do not help in determining whether a particular model has sufficiently good fit.26 The BLRT and LMRT test the improvement in fit for each additional estimated class. More recently, entropy calculations have been introduced by Ramaswamy et al34 to measure the uncertainty in classification of individuals to the latent classes based on their pattern of responses. As a summary measure of the estimated posterior class probabilities, entropy evaluates the extent to which the groups identified in the latent class analysis are different from one another. Entropy values close to 1.0 indicate clear delineation of classes, with values >0.8 generally indicating good classification.35 When more than one compared model fits well, the best and simplest model is retained, regarding its parsimony and interpretability.
Statistical analyses were performed with Mplus software version 8 (Muthén & Muthén, Los Angeles, CA) and Stata software version 15 (StataCorp, College Station, TX). The α level (type I error) was set at 0.05.
RESULTS
The study included 263 glaucoma patients with mean age 71.14 ± 11.13 years. 135 (51.3%) of subjects were female. 182 (69.2%) individuals were Caucasian and 81 (30.8%) individuals reported to be African Americans. Average MD in the better eye of all included participants was −3.14 dB (median −2.00 dB, IQR: −4.26 dB to −0.24 dB) and average MD in the worse eye was −7.38 dB (median −4.65 dB, IQR −10.53 to −2.21).
LCA was applied to analyze NEI VFQ-25 data from the 263 glaucoma patients. LCA models from 1 to 5 classes were estimated. The indices measuring goodness-of-fit for these models are shown in Table 1. Although models with larger numbers of latent classes could be estimated, the model with two classes, disabled and non-disabled, had the best fit, with LMRT values showing superiority to the model with one less class and no statistically significant difference to support models with 3, 4 or 5 classes. The entropy of the 2-class model was 0.965.
TABLE 1.
Results of model fit indices for the latent class models investigating National Eye Institute Visual Function (NEI VFQ-25) questionnaire data from 263 glaucoma patients. Results are given for models ranging from 1 to 5 classes.
| 1 class | 2 classes | 3 classes | 4 classes | 5 classes | |
|---|---|---|---|---|---|
| n, by group | 263 | 215; 48 | 35; 72; 155 | 19; 21; 78; 145 | 14; 16; 18; 67; 148 |
| Entropy | - | 0.965 | 0.881 | 0.896 | 0.910 |
| AIC | 5382.889 | 4564.693 | 4409.870 | 4389.380 | 4395.768 |
| BIC | 5525.775 | 4854.037 | 4845.672 | 4971.641 | 5124.487 |
| aBIC | 5398.956 | 4597.229 | 4458.874 | 4454.853 | 4477.710 |
| LMRT | - | 896.273 | 235.791 | 101.944 | 71.220 |
| LMRT, P value | - | <0.001 | 0.001 | 0.195 | 0.539 |
| BLRT, P value | - | <0.001 | <0.001 | <0.001 | 0.333 |
Entropy measures accuracy of classification of participants in latent classes, values closer to 1.0 indicate better classification; AIC: Akaike information criterion, smaller values indicate better fit; BIC: Bayesian information criterion, smaller values indicate better fit; aBIC: BIC adjusted for sample size, smaller values indicate better fit; LMRT: Lo-Mendell-Rubin test and BLRT: Bootstrap likelihood ratio test, P < 0.05 indicates a model superiority to a model with one less latent class. Boldface indicates the retained class solution.
Individuals were then assigned to one of the two latent classes by their highest estimated probability of membership to each class, based on the NEI VFQ-25 responses (Table 2). The disabled group was represented by 48 (18%) patients, whereas 215 (82%) patients were classified as non-disabled. Groups did not show statistically significant differences in gender, race or most of the inquired socioeconomic variables, except marital status (P = 0.042). Average VA in the better eye was +0.05 ± 0.12 logMAR dB in the disabled group and −0.02 ± 0.12 logMAR in the non-disabled group (P < 0.001). Patients in the disabled group were on average significantly older than those in the non-disabled group (74.9 ± 9.8 vs. 70.3 ± 11.3 years, respectively; P=0.015).
TABLE 2.
Demographic and clinical characteristics of disabled and non-disabled subjects as classified according to the latent class model applied to National Eye Institute Visual Function (NEI VFQ-25) questionnaire data from glaucoma patients.
| Characteristic | Non-disabled (n=215) | Disabled (n=48) | P value |
|---|---|---|---|
| Age, years | 70.3 ± 11.3 | 74.9 ± 9.8 | 0.015a |
| Gender, female (%) | 105 (48.8) | 30 (62.5) | 0.087b |
| Race, AA (%) | 68 (31.6) | 13 (27.1) | 0.537b |
| VA of better eye, logMAR | −0.02 ± 0.12 | +0.05 ± 0.12 | <0.001a |
| SAP 24–2 integrated binocular MS, dB* | 28.27 [29.08 (26.88 – 30.60] | 23.61 [26.43 (20.45 – 28.14)] | <0.001a |
| SAP 24–2 MD of better eye, dB* | −2.51 [−1.59 (−3.48 – −0.05)] | −5.97 [−3.89 (−8.31 – −1.99)] | <0.001a |
| SAP 24–2 MD of worse eye, dB* | −6.05 [−4.16 (−8.45 – −1.82)] | −13.36 [−11.52 (−21.44 – −5.04)] | <0.001a |
| Health insurance, % yes | 94.3 | 93.5 | 0.827b |
| Marital status, % married | 56.7 | 40.0 | 0.042b |
| Education, % with at least high school degree | 97.7 | 97.8 | 0.950b |
| Income, % lower than $25,000 | 12.8 | 10.8 | 0.733b |
Values are presented as mean ± standard deviation, unless otherwise noted. AA: African American Descend; VA: Visual acuity; SAP: Standard Automated Perimetry; MS: mean sensitivity; dB: decibel; MD: mean deviation; NEI VFQ-25: 25-item National Eye Institute Visual Function Questionnaire.
Values given as mean [median (interquartile range)]
Wilcoxon rank-sum test;
Fisher’s exact test.
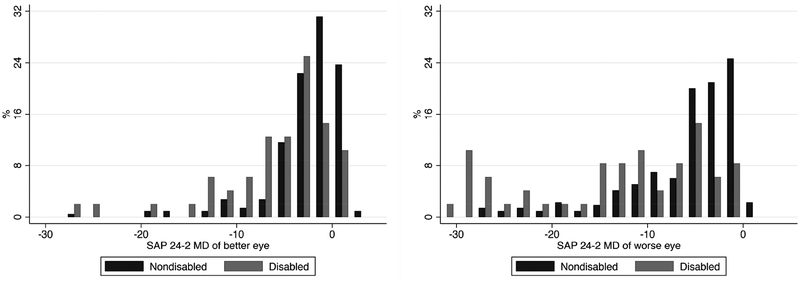
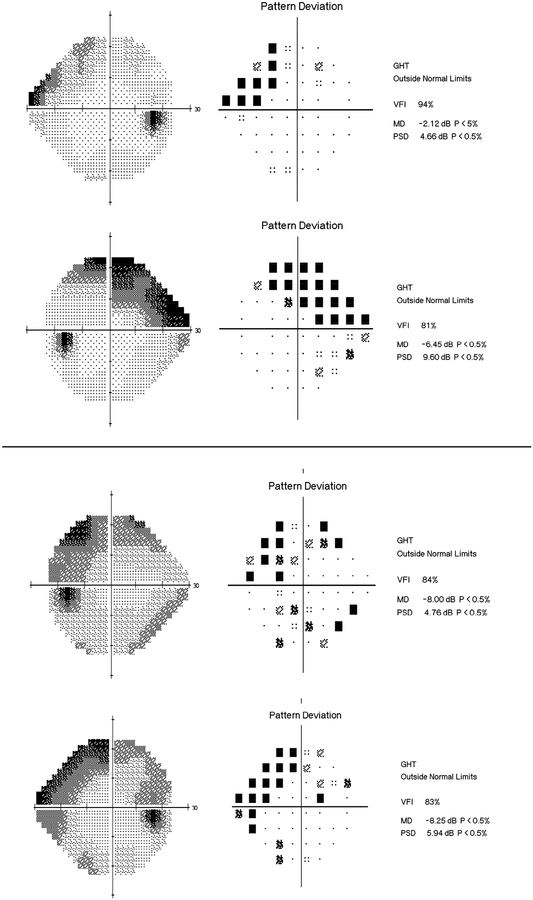
The average MD of the better eye in the disabled group was −5.98 dB (median: −3.89 dB; IQR −8.31 dB to −1.99 dB) versus −2.51 dB (median −1.59 dB; IQR −3.48 dB to −0.05 dB) in the non-disabled group (P < 0.001). For the worse eye, corresponding numbers were −13.36 dB (median: −11.52 dB; IQR −21.44dB to −5.04 dB) and −6.05 dB (median −4.16 dB; IQR −8.45 dB to −1.82 dB), respectively (P < 0.001). The distribution of MD values for the better and worse eye in the two groups are shown in Figure 1. For integrated binocular MS, the disabled group had average value of 23.61 dB (median 26.43 dB, IQR: 20.45 dB to 28.14 dB), whereas the non-disabled group had average of 28.27 dB (median: 29.08 dB, IQR 26.88 dB to 30.60 dB), respectively (P < 0.001). Figure 2 illustrates typical visual fields for subjects assigned to each latent class.
FIGURE 1.
Bar graphs showing the distribution of standard automated perimetry (SAP) mean deviation (MD) values of the better eye (Left) and the worse eye (Right) in glaucoma participants classified as disabled versus non-disabled.
FIGURE 2.
Examples of 24–2 standard automated perimetry results of glaucoma patients assigned to each latent class: (Top) non-disabled; (Bottom) disabled.
We also investigated whether differences in visual field parameters were significant between disabled and non-disabled groups after adjusting for age, gender and visual acuity in an analysis of covariance model. Significant differences between groups were still seen in the adjusted models for the better eye MD, worse eye MD as well as integrated binocular MS (Table 3).
TABLE 3.
Analysis of covariance (ANCOVA) adjusted for age, gender and visual acuity.
| Mean adjusted difference (disabled minus non-disabled) | 95% CI | P value | |
|---|---|---|---|
| Better eye MD | −2.88 | −4.30 to −1.46 | <0.001 |
| Worse eye MD | −6.37 | −8.43 to −4.30 | <0.001 |
| Integrated binocular MS | −3.88 | −5.25 to −2.51 | <0.001 |
MD: mean deviation; MS: mean sensitivity; CI: confidence interval.
DISCUSSION
In the present study, we proposed and described a new methodology, based on latent class models, to classify patient-reported outcomes in glaucoma. We were able to categorize glaucoma patients into groups of disabled and non-disabled based on their responses to the NEI VFQ-25 questionnaire and determine the level of visual field loss associated with disability. We showed that relatively early visual field losses in the better eye may already be associated with disability and provided cutoff levels that may be used as guidelines for assessing the presence or risk of disability.
Finding subjects with underlying similarities within a heterogeneous population, as the one affected by glaucoma, can help to create more practical models to understand true levels of impairment caused by the disease. LCA categorizes patients into phenotypes by taking patterns of questionnaire responses into account rather than just aggregating scores across individuals.27 We identified that, on average, a loss of approximately 6dB in the better eye was associated with disability in glaucoma. For the worse eye, the average loss in the disabled group was approximately 13dB. This is an important finding, as it shows that even relatively early defects can be associated with disability if they occur in the better eye. Curiously, −6dB is also the cut-off value traditionally used to separate mild from moderate cases of glaucoma based on MD according to the HPA classification.4 Although the HPA cutoffs have been arbitrarily set, our findings give some support to such classification by showing they have clinical relevance. Further analysis of the relationship between the HPA classification and the odds of disability from our data shows that patients with severe glaucoma in the better eye had five times higher odds of being classified as disabled (OR = 5.18; 95% CI: 2.50 – 10.73) compared to a patient with mild glaucoma in the better eye. It is also important to emphasize that classifying the severity of disease based on levels of visual field loss should take into account whether one is referring to the better or worse eye. In fact, the non-disabled group had an average loss of 6dB in the worse eye and such loss was not associated with significant decline in patient-reported QoL.
Several previous studies have shown significant relationships between NEI VFQ-25 results and visual field loss in glaucoma. Jampel et al36 showed significant correlations between several visual field metrics and summary scores obtained from the NEI VFQ-25. In a more recent study, McKean-Cowdin and colleagues37 fitted a linear model to assess the relationship between NEI VFQ-25 scores and SAP MD in the better eye in a large population-based study. Of note, due to the nature of the analyses conducted in those studies (i.e., correlational analyses or fit of linear regression models), it is not possible to clearly determine cut-off levels of visual field loss associated with disability. Using LCA, we were able to categorize patients into meaningful clinically relevant categories of disability, which allowed us to determine at what level of visual field loss patients with glaucoma report consistent disability.
Obviously, damage in glaucoma is a continuum, and determining specific cutoff values might seem artificial. However, previous studies have argued that using LCA methodology leads to the most natural and useful group classification.27 In this aspect, LCA classification may be seen as having a role similar to widely used and clinically-relevant categorical classifications of medical results into normal/abnormal. Sorting patients into latent classes helps to understand which characteristics are most associated with poor QoL, assisting in the interpretation of clinical findings and their prognostic significance.
We also found a statistically significant difference of VA between disabled and non-disabled groups as determined by LCA (P < 0.001). One could argue that lower VA was actually the main cause of disability rather than differences in visual field loss and it is possible that some patients with mild degree of cataract may have been included in our study. However, although statistically significant, differences in VA between the groups were of relatively small magnitude, with a mean difference of 0.07 logMAR, which corresponds to only three letters in the same line of the chart. Importantly, differences in visual field MD remained statistically significant after adjustment for differences in visual acuity, age and gender between the two groups.
Correctly identifying the optimal number of latent classes has a pivotal role in LCA, as the number of classes has a strong impact on interpretations of model results. In this study, the model with two classes proved to have superior fit in all model selection methods, except for AIC. AIC, however, has been found to poorly select the correct number of classes, regardless of degree of separation, number of indicators, or sample size.38 Importantly, the 2-class final LCA model had a high entropy value of 0.965, indicating a high degree of certainty in classifying participants. The high entropy shows that individuals belonging to one class are clearly separable from those belonging to the other class based on their responses to the NEI VFQ-25 questionnaires.
One might speculate how different levels of damage in the worse eye would affect QoL in individuals exhibiting similar amounts of damage in the better eye. We therefore investigated whether adding a measure of asymmetry in the amount of visual field damage between both eyes would improve models explaining QoL. In a multivariable model including MD of the better eye, adding asymmetry (MD difference between the two eyes) resulted in some improvement in predicting class membership (i.e. disabled vs. non-disabled), compared to the model including only better eye MD. The areas under the receiver operating characteristic (ROC) curve of these two models were 0.757 vs 0.708, respectively, but the difference did not reach statistical significance (P = 0.204). For comparison, the area under the ROC curve for the model including integrated binocular sensitivity was 0.761. Therefore, it seems that some improvement may be gained by considering the relationship between both eyes in explaining patient-reported QoL measures in glaucoma. It should be noted, however, that as the location of a visual field defect may affect quality of life differently,23 a global metric evaluating binocular integrated visual field sensitivity would not account for differences in the location of field defects between the two eyes. Further studies with larger samples should investigate such relationships considering the presence of different patterns of visual field loss in each eye, their symmetry or asymmetry, and their relationship with classes defined by LCA.
Our study has limitations. Patients in our study were generally aware of their glaucoma diagnoses at the time they responded the NEI VFQ-25 questionnaires. If they were also aware of the severity of their field loss, this may have influenced how they responded to the questions, which could influence the relationship between SAP metrics and disability as determined from the questionnaire results. Such limitation is difficult to avoid unless questionnaires are given to newly diagnosed patients who are still unaware of their disease. It should be noted that although patients may have been aware of their condition, it is unlikely that that they would know precisely the degree of their visual field loss in the better and worse eye to spuriously generate the clear relationships observed in our study. However, further studies should attempt to replicate our findings in newly diagnosed samples of patients. In addition, it will also be important to investigate the relationship between visual field loss and categories of disability as determined from objective tests of patient performance, or from a combination of subjective patient-reported QoL and objective performance.
In conclusion, the current study showed the potential use of a latent class model to analyze patient-reported QoL outcomes in glaucoma. The methodology allowed us to investigate the amount of visual field loss associated with disability, improving the understating of how to use visual field data to infer the impact of the disease on QoL.
Supplementary Material
ACKNOWLEDGMENTS/DISCLOSURE
a. Funding/Support: This work was supported in part by the National Institutes of Health/National Eye Institute [grant numbers EY025056 (FAM) and EY021818 (FAM)].
b. Financial Disclosures: Alessandro A. Jammal, none; Nara G. Ogata, none; Fábio B. Daga, none; Ricardo Y. Abe, none; Vital P. Costa, none; Felipe A. Medeiros, Alcon Laboratories Inc (R), Alcon Laboratories Inc (F), Allergan Inc (F), Allergan Inc (R), Allergan, Inc (c), Bausch & Lomb (F), Carl Zeiss Meditec Inc (F), Carl Zeiss Meditec Inc (R), Carl-Zeiss Meditec, Inc (C), Heidelberg Engineering Inc (F), Merck Inc. (F), National Eye Institute (F), Novartis (C), Reichert Inc (R), Reichert, Inc (F), Topcon Inc (F).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081–2090. [DOI] [PubMed] [Google Scholar]
- 3.Glaucoma Ramulu P. and disability: which tasks are affected, and at what stage of disease? Curr Opin Ophthalmol. 2009;20(2):92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hodapp E, Parrish RK, Anderson DR. Clinical decisions in glaucoma. Mosby; 1993. [Google Scholar]
- 5.Advanced Glaucoma Intervention Study. 2. Visual field test scoring and reliability. Ophthalmology. 1994;101(8):1445–1455. [PubMed] [Google Scholar]
- 6.Mills RP, Budenz DL, Lee PP, et al. Categorizing the stage of glaucoma from pre-diagnosis to end-stage disease. Am J Ophthalmol. 2006;141(1):24–30. [DOI] [PubMed] [Google Scholar]
- 7.Brusini P, Filacorda S. Enhanced Glaucoma Staging System (GSS 2) for classifying functional damage in glaucoma. J Glaucoma. 2006;15(1):40–46. [DOI] [PubMed] [Google Scholar]
- 8.Mangione CM, Lee PP, Gutierrez PR, Spritzer K, Berry S, Hays RD. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol. 2001;119(7):1050–1058. [DOI] [PubMed] [Google Scholar]
- 9.Rindskopf D, Rindskopf W. The value of latent class analysis in medical diagnosis. Stat Med. 1986;5(1):21–27. [DOI] [PubMed] [Google Scholar]
- 10.McCarthy DE, Ebssa L, Witkiewitz K, Shiffman S. Paths to tobacco abstinence: A repeated-measures latent class analysis. J Consult Clin Psychol. 2015;83(4):696–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tucker JS, Orlando M, Ellickson PL. Patterns and correlates of binge drinking trajectories from early adolescence to young adulthood. Health Psychol. 2003;22(1):79–87. [DOI] [PubMed] [Google Scholar]
- 12.Buckner TW, Wang J, DeWalt DA, Jacobs S, Reeve BB, Hinds PS. Patterns of symptoms and functional impairments in children with cancer. Pediatr Blood Cancer. 2014;61(7):1282–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinheiro LC, Tan X, Olshan AF, et al. Examining health-related quality of life patterns in women with breast cancer. Qual Life Res. 2017;26(7):1733–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eppig JS, Edmonds EC, Campbell L, Sanderson-Cimino M, Delano-Wood L, Bondi MW. Statistically Derived Subtypes and Associations with Cerebrospinal Fluid and Genetic Biomarkers in Mild Cognitive Impairment: A Latent Profile Analysis. J Int Neuropsychol Soc. 2017;23(7):564–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sample PA, Girkin CA, Zangwill LM, et al. The African Descent and Glaucoma Evaluation Study (ADAGES): design and baseline data. Arch Ophthalmol. 2009;127(9):1136–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nelson-Quigg JM, Cello K, Johnson CA. Predicting binocular visual field sensitivity from monocular visual field results. Invest Ophthalmol Vis Sci. 2000;41(8):2212–2221. [PubMed] [Google Scholar]
- 17.Fa Medeiros, Gracitelli CP Boer ER, Weinreb RN Zangwill LM, Rosen PN. Longitudinal changes in quality of life and rates of progressive visual field loss in glaucoma patients. Ophthalmology. 2015;122(2):293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmadian L, Massof R. Impact of general health status on validity of visual impairment measurement. Ophthalmic Epidemiol. 2008;15(5):345–355. [DOI] [PubMed] [Google Scholar]
- 19.Massof RW, Fletcher DC. Evaluation of the NEI visual functioning questionnaire as an interval measure of visual ability in low vision. Vision Res. 2001;41 (3):397–413. [DOI] [PubMed] [Google Scholar]
- 20.Marella M, Pesudovs K, Keeffe JE, O’Connor PM, Rees G, Lamoureux EL. The psychometric validity of the NEI VFQ-25 for use in a low-vision population. Invest Ophthalmol Vis Sci. 2010;51(6):2878–2884. [DOI] [PubMed] [Google Scholar]
- 21.Abe RY, Gracitelli CPB, Diniz-Filho A, Zangwill lM, Weinreb RN, Medeiros FA. Frequency Doubling Technology Perimetry and Changes in Quality of Life of Glaucoma Patients: A Longitudinal Study. Am J Ophthalmol. 2015;160(1):114–122.e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gracitelli CP, Abe RY, Tatham AJ, et al. Association between progressive retinal nerve fiber layer loss and longitudinal change in quality of life in glaucoma. JAMA Ophthalmol. 2015;133(4):384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abe RY, Diniz-Filho A, Costa VP, Gracitelli CP, Baig S, Medeiros FA. The Impact of Location of Progressive Visual Field Loss on Longitudinal Changes in Quality of Life of Patients with Glaucoma. Ophthalmology. 2016;123(3):552–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abe RY, Diniz-Filho A, Costa VP, Wu Z, Medeiros FA. Predicting Vision-Related Disability in Glaucoma. Ophthalmology. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clogg CC, Goodman LA. Latent Structure Analysis of a Set of Multidimensional Contingency Tables. J Am Stat Assoc. 1984;79(388):762–771. [Google Scholar]
- 26.Lanza ST, Collins LM. A mixture model of discontinuous development in heavy drinking from ages 18 to 30: the role of college enrollment. J Stud Alcohol. 2006;67(4):552–561. [DOI] [PubMed] [Google Scholar]
- 27.Collins LM, Lanza ST. Latent Class and Latent Transition Analysis: With Applications in the Social, Behavioral, and Health Sciences. Wiley; 2010. [Google Scholar]
- 28.Lanza ST, Flaherty BP, Collins LM. Latent Class and Latent Transition Analysis Handbook of Psychology : John Wiley & Sons, Inc.; 2003. [Google Scholar]
- 29.Akaike H Factor analysis and AIC. Psychometrika. 1987;52(3):317–332. [Google Scholar]
- 30.Schwarz G Estimating the Dimension of a Model. Ann Statist. 1978;6(2):461–464. [Google Scholar]
- 31.Sclove SL. Application of model-selection criteria to some problems in multivariate analysis. Psychometrika. 1987;52(3):333–343. [Google Scholar]
- 32.McLachlan G, Peel D. Finite Mixture Models. John Wiley & Sons, Inc.; 2000. [Google Scholar]
- 33.Lo Y, Mendell NR, Rubin DB. Testing the number of components in a normal mixture. Biometrika. 2001;88(3):767–778. [Google Scholar]
- 34.Ramaswamy V, Desarbo WS, Reibstein DJ, Robinson WT. An Empirical Pooling Approach for Estimating Marketing Mix Elasticities with PIMS Data. Market Sci. 1993;12(1):103–124. [Google Scholar]
- 35.Celeux G, Soromenho G. An entropy criterion for assessing the number of clusters in a mixture model. Journal of Classification. 1996;13(2):195–212. [Google Scholar]
- 36.Jampel HD, Schwartz A, Pollack I, Abrams D, Weiss H, Miller R. Glaucoma patients’ assessment of their visual function and quality of life. J Glaucoma. 2002;11(2):154–163. [DOI] [PubMed] [Google Scholar]
- 37.McKean-Cowdin R, Wang Y, Wu J, Azen SP, Varma R. Impact of Visual Field Loss on Health-Related Quality of Life in Glaucoma: The Los Angeles Latino Eye Study. Ophthalmology. 2008;115(6):941–948.e941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tein J-Y, Coxe S, Cham H. Statistical Power to Detect the Correct Number of Classes in Latent Profile Analysis. Struct Equ Modeling. 2013;20(4):640–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.