Abstract
Purpose
To investigate the role of corneal hysteresis (CH) as a risk factor for development of glaucoma.
Design
Prospective observational cohort study.
Methods
Two-hundred and eighty-seven eyes of 199 patients suspected of having glaucoma followed for an average of 3.9 ± 1.8 years. All eyes had normal visual fields at baseline. Development of glaucoma was defined as occurrence of 3 consecutive abnormal standard automated perimetry tests during follow-up, defined as pattern standard deviation (PSD) <5%, and/or Glaucoma Hemifield Test outside normal limits. Measurements of CH were acquired at baseline using the Ocular Response Analyzer® (ORA). Univariable and multivariable Cox regression models were used to investigate baseline factors associated with development of visual field loss over time.
Results
Fifty-four (19%) eyes developed repeatable visual field defects during follow-up. Measurements of CH at baseline were significant lower in patients who developed glaucoma versus those who did not (9.5 ± 1.5 mmHg vs. 10.2 ± 2.0 mmHg; P=0.012). Each 1 mmHg lower CH was associated with an increase of 21% in the risk of developing glaucoma during follow-up [95% confidence interval (CI): 1.04–1.41; P=0.013]. In a multivariable model adjusting for age, intraocular pressure, central corneal thickness, PSD, and treatment, CH was still predictive of development of glaucoma (hazard ratio=1.20; 95% CI: 1.01–1.42; P=0.040).
Conclusion
Baseline lower CH measurements were significantly associated with increased risk of developing glaucomatous visual field defects over time. The prospective longitudinal design of this study supports a role of CH as a risk factor for developing glaucoma.
INTRODUCTION
Glaucoma is an optic neuropathy characterized by progressive loss of retinal ganglion cells and their axons, potentially leading to irreversible loss of visual function.1 Lowering of the intraocular pressure has been shown to delay or prevent development of glaucomatous damage and its progression. However, as the disease frequently remains asymptomatic until relatively late stages, identification of subjects at high risk for developing glaucoma is important in order to allow early treatment and prevention of irreversible visual loss.
Besides IOP, other factors such as age, central corneal thickness (CCT), disc hemorrhages, and structural and functional measures of the status of the optic nerve have been identified as associated with risk of glaucoma development. More recent investigations have also shown that corneal hysteresis (CH), a measure of the viscoelastic property of the cornea, is associated with glaucoma progression.2–4 In a retrospective study, De Moraes and colleagues3 showed that lower CH measurements were predictive of faster velocity of glaucoma progression. In a prospective investigation, Medeiros and colleagues4 collected baseline CH measurements in patients with glaucoma and showed that those with lower CH measurements showed faster progression of visual field loss during subsequent follow-up. Although those studies have provided compelling evidence for the role of CH in predicting progression,2–4 they focused on patients who had already been diagnosed with glaucoma. It is possible that the factors predicting disease development may be different or carry a different weight than those predicting further progression in subjects who have already been diagnosed with the disease.
The purpose of the present study was to investigate the role of CH in predicting development of glaucoma in a cohort of patients suspected of having the disease followed over time.
METHODS
This was an observational cohort study of participants from a prospective longitudinal study designed to evaluate visual function in glaucoma. This study was registered at http://cilincaltrials.gov (). Approval from the Institutional review board at the University of California San Diego was obtained for this study, and it was conducted in adherence with the Declaration of Helsinki and Health Insurance Portability and Accountability Act. Written informed consent was obtained from all participants after the test procedures were explained.
At baseline and at each visit during follow-up all participants underwent a comprehensive ophthalmologic examination including IOP measured using Goldmann applanation tonometry (GAT; Haag-Streit, Konig, Switzerland), gonioscopy, stereoscopic optic disc examination and visual field testing. Patients also had CCT measurements obtained with ultrasound pachymetry (Pachette GDH 500; DGH Technology, Inc., Philadelphia, PA, USA).
The study included glaucoma suspects (history of IOP greater than 21 mmHg and/or suspicious appearance of the optic nerve, but normal and reliable visual field results at baseline) with open angles on gonioscopy. Subjects were followed every 6 months. A minimum follow-up period of 18 months and a minimum of 4 separate visits were required for inclusion in this study. Subjects were excluded if they presented any other ocular or systemic disease that could affect the optic nerve or the visual field. Standard automated perimetry (SAP) tests were performed using the Swedish Interactive Thresholding Algorithm (SITA) Standard 24–2 strategy on the Humphrey Field Analyzer II-i (Carl Zeiss Meditec, Inc., Dublin, CA, USA). Visual fields with more than 33% fixation losses, or more than 15% false-positive errors, were excluded.
During follow-up, eyes were classified as developing glaucoma if they had repeatable (at least 3 consecutive) abnormal visual field test results. An abnormal visual field was defined as a pattern standard deviation (PSD) with P<0.05 or a Glaucoma Hemifield Test result outside normal limits. Each participant was required to have a minimum of 5 SAP examinations during a minimum 2 years follow-up. Each patient was treated at the discretion of the attending ophthalmologist.
Corneal Hysteresis Measurements
Measurements of CH were acquired at the baseline visit using the Ocular Response Analyzer® (ORA; Reichert Technologies, Inc., Depew, NY, USA). A trained technician obtained three measurements from each eye and the average of three measurements was calculated for analysis. The ORA determines corneal biomechanical properties using an applied force-displacement relationship. Details of its operation have been previously described.5 In summary, this device uses an air puff to deform the cornea into slight concavity and an optical sensor to measure the deflection of the cornea, which is timed to the pressure applied by the air puff. From these data, the pressures at which the cornea flattens inward and outward as the pressure rises and falls are derived. The difference between the two applanation pressures, measured in mmHg, is coined CH, and is related to the viscoelastic property of the cornea. The device provides a waveform score to reflect the quality of measurements. Only measurements associated with a waveform score greater than 4 were considered for inclusion in the study.
Statistical Analysis
Cox proportional hazards models were used to obtain hazard ratios (HRs) and identify baseline factors that predicted which eyes developed glaucomatous visual field loss during the follow-up period.6–10 A frailty model was used to account for potential correlation between two eyes of the same individual. We reported HRs from univariable models, as well as adjusted HRs from the multivariable Cox proportional hazards models. Kaplan-Meier curves showing the cumulative probabilities of developing glaucoma over time were also provided.
All statistical analyses were performed using commercially available software Stata, version 14 (StataCorp LP, College Station, TX). The alpha level (type I error) was set at 0.05.
RESULTS
The study included 287 eyes from 199 patients suspected of having glaucoma, as determined on the baseline visit, and subsequent followed for an average of 3.9 ± 1.8 years. Fifty-four (19%) of the 287 eyes developed repeatable visual field defects during follow-up. Table 1 presents baseline demographic and clinical factors of the study sample. Mean age was 67.6 ± 13.1 years at baseline in patients who developed glaucoma and 63.4 ± 11.6 years at baseline in those who did not (P=0.043). There was no statistically significant difference in gender between the two groups. There was a greater percentage of African Americans in the group that developed glaucoma compared with the one that did not (34.2% vs. 19.0%, respectively; P=0.035). Patients who developed glaucoma had lower SAP mean deviation (MD) values compared with those who did not (−0.6 ± 1.3 dB vs. 0.1 ± 1.3 dB; P<0.001). PSD at baseline was significant higher in patients who developed glaucoma versus those who did not (1.7 ± 0.2 dB vs. 1.5 ± 0.2 dB; P<0.001). CH measurements at baseline were significant lower in patients who developed glaucoma versus those who did not (9.5 ± 1.5 mmHg vs. 10.2 ± 2.0 mmHg; P=0.012). There were no statistically significant differences in mean IOP and mean CCT at baseline between the two groups. Also there was no statistically significant difference in treatment (yes/no) between the two groups.
Table 1.
Baseline characteristics of patients who developed glaucoma and those who did not.
| Developed Glaucoma (n = 54 Eyes, 46 Patients) | Did Not Develop Glaucoma (n = 233 Eyes, 153 Patients) | P Value | |
|---|---|---|---|
| Age (years) | 67.6 ± 13.1 | 63.4 ± 11.6 | 0.043 |
| Gender (% female) | 58.5 | 58.9 | 0.553 |
| Race (%) | |||
| White | 56.1 | 71.5 | |
| African American | 34.2 | 19.0 | 0.035 |
| Other | 9.7 | 9.5 | |
| MD (dB) | −0.6 ± 1.3 | 0.1 ± 1.3 | <0.001 |
| PSD (dB) | 1.7 ± 0.2 | 1.5 ± 0.2 | <0.001 |
| IOP (mmHg) | 17.0 ± 4.1 | 17.6 ± 4.1 | 0.356 |
| CH (mmHg) | 9.5 ± 1.5 | 10.2 ± 2.0 | 0.012 |
| CCT (μm) | 550.6 ± 32.3 | 556.6 ± 40.7 | 0.312 |
| Treatment (% yes) | 63.5 | 66.7 | 0.754 |
MD = mean deviation; dB = decibels; PSD = pattern standard deviation; IOP = intraocular pressure; CH = corneal hysteresis; CCT = central corneal thickness; μm = micrometers.
Values are presented as mean ± standard deviation, unless otherwise noted.
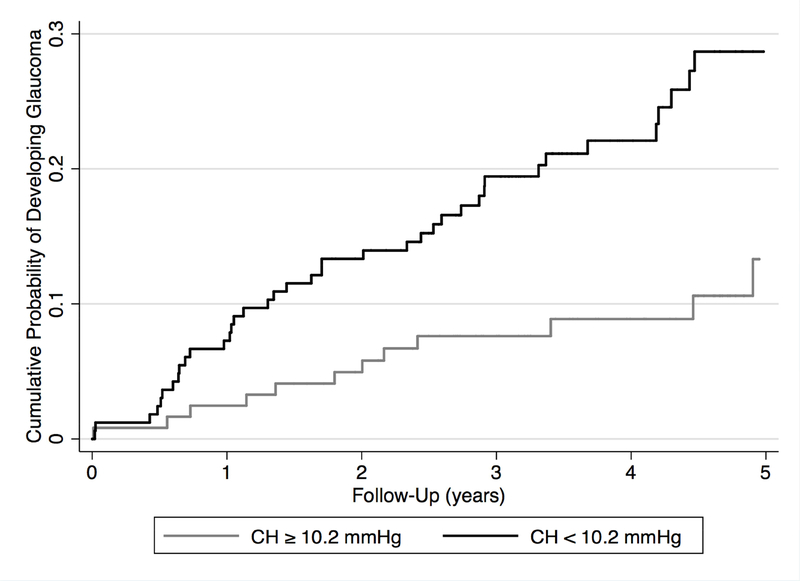
Table 2 presents HRs with 95% confidence interval (CI) for risk factors associated with development of glaucoma. Each 1 mmHg lower CH was associated with an increase of 21% in the risk of developing glaucoma during follow-up [HR=1.21; 95% CI: 1.04–1.41; P=0.013]. In a multivariable model adjusting for age, IOP, CCT, PSD, and treatment, CH was still predictive of development of glaucoma (HR=1.20; 95% CI: 1.01–1.42; P=0.040). Figure 1 shows cumulative probabilities of developing glaucoma in eyes with CH equal or greater than average (10.2 mmHg) and in eyes with CH less than average (P=0.001).
Table 2.
Hazard ratios (HRs) with 95% confidence intervals (CI) for risk factors associated with development of glaucoma.
| Characteristic | Univariable Model |
Multivariable Model |
||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| CH (per 1 mmHg lower) | 1.21 (1.04 – 1.41) | 0.013 | 1.20 (1.01 – 1.42) | 0.040 |
| Age (per 1 decade older) | 1.50 (1.15 – 1.95) | 0.003 | 1.32 (0.99 – 1.76) | 0.053 |
| Gender (male) | 1.21 (0.70 – 2.07) | 0.497 | – | – |
| Race (African American) | 1.59 (0.88 – 2.84) | 0.121 | – | – |
| IOP (per 1 mmHg higher) | 0.96 (0.90 – 1.03) | 0.311 | 0.99 (0.91 – 1.07) | 0.805 |
| CCT (per 40 μm thinner) | 1.15 (0.87 – 1.51) | 0.323 | 0.83 (0.60 – 1.17) | 0.290 |
| MD (per 1 dB lower) | 1.35 (1.12 – 1.65) | 0.002 | – | – |
| PSD (per 0.1 dB higher) | 1.38 (1.21 – 1.56) | <0.001 | 1.36 (1.19 – 1.54) | <0.001 |
| Treatment (yes) | 0.93 (0.40 – 2.14) | 0.863 | 0.90 (0.50 – 1.64) | 0.734 |
CH = corneal hysteresis; IOP = intraocular pressure; CCT = central corneal thickness; μm = micrometers; MD = mean deviation; dB = decibels; PSD = pattern standard deviation.
Figure 1.
Cumulative probability of glaucoma development in suspect eyes with corneal hysteresis (CH) equal or greater than 10.2 mmHg, and in those with CH lesser than 10.2 mmHg.
DISCUSSION
In the current study, eyes with lower baseline CH had a higher probability to develop glaucomatous visual field defects in a cohort of glaucoma suspects followed over time. Such a relationship was present even in the multivariable model adjusting for other factors known to potentially affect risk of glaucoma development. Other studies have already demonstrated low CH values to be associated with glaucoma progression in patients with established disease.2–4 However, to the best of our knowledge, this is the first prospective longitudinal study to evaluate the role of CH as a risk factor for the development of visual field loss in glaucoma suspects. Our findings suggest that evaluation of CH may add significant value to the assessment of risk of disease development in glaucoma suspects followed over time.
In the univariable model, each 1 mmHg lower CH was associated with a 21% increase in the risk of developing glaucoma (HR=1.21; 95% CI: 1.04–1.41; P=0.013). This relationship was still present in the multivariable model adjusting for other factors known to affect glaucoma development, such as age, IOP, CCT, and PSD.11 Each 1 mmHg lower CH was associated with a 20% higher risk to develop visual field defects (HR=1.20; 95% CI: 1.01–1.42; P=0.040). Previous cross-sectional studies have suggested the potential role of CH in glaucoma by showing that glaucomatous eyes tend to have lower CH values than healthy control eyes.2, 12, 13 Others have demonstrated that eyes with more severe damage tend to have lower CH values compared to the fellow eye in asymmetric disease.14 De Moraes and colleagues3 found a correlation between lower CH values and faster glaucomatous progression. Congdon and colleagues2 investigated the relationship between CH levels and visual field progression and found a 20% risk of further functional loss for each 1 mmHg lower CH, a percentage that is similar to the one found in our study. A prospective study by Medeiros and colleagues4 was able to demonstrate that CH was a statistically independent risk factor for glaucoma progression. Eyes with lower hysteresis had faster rates of visual field loss than those with higher hysteresis. However, these studies investigated the association between CH and visual field loss mostly in patients with already established glaucoma, whereas our results demonstrated CH as a baseline risk factor for disease development also in glaucoma suspects. Such assessment is important for development of predictive models assessing risk of glaucoma conversion.
It is still unclear why CH might be related to risk of glaucoma development and progression. In contrast to CCT, CH does not seem to exert a major effect on IOP estimation by Goldmann tonometry.5, 15 Therefore, it seems unlikely that the predictive effect of CH would be related to artifacts associated with IOP measurement. It has been hypothesized that CH might be a surrogate biomarker to the biomechanical properties of tissues located posteriorly in the eye, such as lamina cribrosa and peripapillary sclera. According to this hypothesis, a low CH would increase the risk for glaucomatous damage possibly by being associated with a reduced capacity of relevant posterior ocular structures in dampening IOP peaks or fluctuations.16–20 Supporting this hypothesis, a previous study has shown a significant correlation between CH and anterior lamina cribrosa displacement after reduction of IOP.21
The present study has limitations. There could be uncontrolled confounding by unmeasured factors, such as family history of glaucoma. In addition, we included only baseline values for the covariates included in the predictive models. It is possible that that longitudinal information from these variables would show different predictive effect. However, our main goal was to assess the value of CH as a predictive factor during the initial evaluation of subjects suspected of glaucoma. Future studies should investigate the value of longitudinal measurements obtained from these variables. It should also be noted that high IOP has been clearly shown to be an important risk factor for glaucoma development and progression.11,22 In addition, IOP-lowering therapy has been shown to prevent or delay development of damage.22,23 However, in our multivariable analysis, we were not able to find IOP as a statistically significant risk factor for glaucoma development. This is most likely explained by the fact that our longitudinal study was observational, rather than interventional, and during follow-up subjects were treated at the discretion of the attending ophthalmologist. It is likely that subjects with higher IOP at baseline received more treatment, which decreased their chance of developing damage, artificially weakening the predictive value of baseline IOP measurements. Similarly, since the impact of CCT on risk of glaucoma development is now widely known,11 it is likely that physicians may have treated more aggressively eyes of glaucoma suspects who had thin corneas, also artificially reducing the impact of CCT as a predictive factor in our study. As CH was only obtained as part of our research protocol, it is less likely that information about CH would have been used to guide treatment decisions. Therefore, the higher predictive value of CH compared to CCT in our study should be seen with caution. Future studies including randomization protocols controlling for treatment strategy should be performed to clarify the relative importance of these predictive factors.
In conclusion, lower CH measurements were significantly associated with increased risk of developing glaucoma. Future studies should attempt to incorporate CH measurements along with other known risk factors into models designed to improve risk assessment in glaucoma.
Supplementary Material
ACKNOWLEDGMENTS
A. Funding/Support: Supported in part by National Institutes of Health/National Eye Institute grant EY021818 (F.A.M.).
Footnotes
DISCLOSURE
B. Financial Disclosures: B.N.S. – No financial disclosures; A.D.-F. – No financial disclosures; F.B.D. – No financial disclosures; C.N.S. – No financial disclosures; F.Z. – No financial disclosures; N.G.O. – No financial disclosures; F.A.M. – F: Alcon Laboratories, Bausch & Lomb, Carl Zeiss Meditec, Heidelberg Engineering, Merck, Allergan, Sensimed, Topcon, Reichert, National Eye Institute, R: Alcon Laboratories, Allergan, Carl Zeiss Meditec, Reichert, C: Allergan, Carl Zeiss Meditec, Novartis.
REFERENCES
- 1.Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA 2014;311(18):1901–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Congdon NG, Broman AT, Bandeen-Roche K, Grover D, Quigley HA. Central corneal thickness and corneal hysteresis associated with glaucoma damage. Am J Ophthalmol 2006;141(5):868–875. [DOI] [PubMed] [Google Scholar]
- 3.De Moraes CV, Hill V, Tello C, Liebmann JM, Ritch R. Lower corneal hysteresis is associated with more rapid glaucomatous visual field progression. J Glaucoma 2012;21(4):209–213. [DOI] [PubMed] [Google Scholar]
- 4.Medeiros FA, Meira-Freitas D, Lisboa R, Kuang TM, Zangwill LM, Weinreb RN. Corneal hysteresis as a risk factor for glaucoma progression: A prospective longitudinal study. Ophthalmology 2013;120(8):1533–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luce DA. Determining in vivo biomechanical properties of the cornea with an ocular response analyzer. J Cataract Refract Surg 2005;31(1):156–162. [DOI] [PubMed] [Google Scholar]
- 6.Medeiros FA, Sample PA, Zangwill LM, Bowd C, Aihara M, Weinreb RN. Corneal thickness as a risk factor for visual field loss in patients with preperimetric glaucomatous optic neuropathy. Am J Ophthalmol 2003;136(5):805–813. [DOI] [PubMed] [Google Scholar]
- 7.Mohammadi K, Bowd C, Weinreb RN, Medeiros FA, Sample PA, Zangwill LM. Retinal nerve fiber layer thickness measurements with scanning laser polarimetry predict glaucomatous visual field loss. Am J Ophthalmol 2004;138(4):592–601. [DOI] [PubMed] [Google Scholar]
- 8.Medeiros FA, Sample PA, Weinreb RN. Frequency doubling technology perimetry abnormalities as predictors of glaucomatous visual field loss. Am J Ophthalmol 2004;137(5):863–871. [DOI] [PubMed] [Google Scholar]
- 9.Lalezary M, Medeiros FA, Weinreb RN, et al. Baseline optical coherence tomography predicts the development of glaucomatous change in glaucoma suspects. Am J Ophthalmol 2006;142(4):576–582. [DOI] [PubMed] [Google Scholar]
- 10.Medeiros FA, Weinreb RN, Zangwill LM, et al. Long-term intraocular pressure fluctuations and risk of conversion from ocular hypertension to glaucoma. Ophthalmology 2008;115(6):934–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol 2002;120(6):714–720; discussion 829–30. [DOI] [PubMed] [Google Scholar]
- 12.Sullivan-Mee M, Billingsley SC, Patel AD, Halverson KD, Alldredge BR, Qualls C. Ocular Response Analyzer in subjects with and without glaucoma. Optom Vis Sci 2008;85(6):463–470. [DOI] [PubMed] [Google Scholar]
- 13.Abitbol O, Bouden J, Doan S, Hoang-Xuan T, Gatinel D. Corneal hysteresis measured with the Ocular Response Analyzer in normal and glaucomatous eyes. Acta Ophthalmol 2010;88(1):116–119. [DOI] [PubMed] [Google Scholar]
- 14.Anand A, De Moraes CG, Teng CC, Tello C, Liebmann JM, Ritch R. Corneal hysteresis and visual field asymmetry in open angle glaucoma. Invest Ophthalmol Vis Sci 2010;51(12):6514–6518. [DOI] [PubMed] [Google Scholar]
- 15.Kaushik S, Pandav SS, Banger A, Aggarwal K, Gupta A. Relationship between corneal biomechanical properties, central corneal thickness, and intraocular pressure across the spectrum of glaucoma. Am J Ophthalmol 2012;153(5):840–849.e2. [DOI] [PubMed] [Google Scholar]
- 16.Burgoyne CF, Downs JC, Bellezza AJ, Suh JK, Hart RT. The optic nerve head as a biomechanical structure: a new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog Retin Eye Res 2005;24(1):39–73. [DOI] [PubMed] [Google Scholar]
- 17.Sigal IA, Flanagan JG, Ethier CR. Factors influencing optic nerve head biomechanics. Invest Ophthalmol Vis Sci 2005;46(11):4189–4199. [DOI] [PubMed] [Google Scholar]
- 18.Johnson CS, Mian SI, Moroi S, Epstein D, Izatt J, Afshari NA. Role of corneal elasticity in damping of intraocular pressure. Invest Ophthalmol Vis Sci 2007;48(6):2540–2544. [DOI] [PubMed] [Google Scholar]
- 19.Liu J, He X. Corneal stiffness affects IOP elevation during rapid volume change in the eye. Invest Ophthalmol Vis Sci 2009;50(5):2224–2229. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, Albon J, Jones H, et al. Collagen microstructural factors influencing optic nerve head biomechanics. Invest Ophthalmol Vis Sci 2015;56(3):2031–2042. [DOI] [PubMed] [Google Scholar]
- 21.Lanzagorta-Aresti A, Perez-Lopez M, Palacios-Pozo E, Davo-Cabrera J. Relationship between corneal hysteresis and lamina cribrosa displacement after medical reduction of intraocular pressure. Br J Ophthalmol 2017;101(3):290–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leske MC, Heijl A, Hussein M, Bengtsson B, Hyman L, Komaroff E; Early Manifest Glaucoma Trial Group. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol 2003;121(1):48–56. [DOI] [PubMed] [Google Scholar]
- 23.Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol 2002;120(6):701–713; discussion 829–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.