Abstract
Purpose:
To investigate the incidence and risk factors for glaucomatous visual field progression in eyes with well-controlled intraocular pressure (IOP).
Design:
Prospective cohort.
Participants:
460 eyes of 334 glaucoma patients under treatment.
Methods:
Study subjects had mean follow-up of 4.3 ± 0.8 years. Patients were classified as well-controlled if all IOP measurements were under 18 mmHg. Rates of visual field progression were calculated using ordinary least squares linear regression of standard automated perimetry (SAP) mean deviation (MD) values over time. Progression was defined as a significantly negative MD slope (alpha = 0.05).
Main outcome measures:
Rates of SAP MD change; mean and peak IOP, and IOP fluctuation; corneal biomechanics: corneal hysteresis (CH), central corneal thickness (CCT), and corneal index.
Results:
Of the 179 eyes with well-controlled IOP, 42 (23.5%) demonstrated visual field progression. There was no significant difference between progressing and stable patients in baseline MD (−6.4 ± 7.1 vs. −6.0 ± 6.2 dB; P = 0.346), mean IOP (11.7 ± 2.0 vs. 12.1 ± 2.3 mmHg; P=0.405), IOP fluctuation (1.6 ± 0.6 vs. 1.6 ± 0.5 mmHg; P = 0.402) or peak IOP (14.3 ± 1.9 vs. 14.6 ± 2.1 mmHg; P = 0.926). Progressing eyes had significantly lower CH (8.6 ± 1.3 vs. 9.4 ± 1.6 mmHg; P = 0.014) and thinner CCT (515.1 ± 33.1 vs. 531.1 ± 42.4 μm; P = 0.018, respectively) compared to stable eyes. In the multivariate analysis, a 1 standard deviation lower corneal index, a summation of normalized versions of CH and CCT, resulted in a 68% higher risk of progression (OR: 1.68; 95% CI:1.08 to 2.62; P = 0.021).
Conclusions:
Approximately one-quarter of eyes with well-controlled IOP may show visual field progression over time. Thin cornea and low CH are main risk factors.
Keywords: glaucoma, visual field progression, corneal biomechanics, corneal hysteresis, intraocular pressure
INTRODUCTION
Elevated intraocular pressure (IOP) is the main risk factor for glaucoma progression and currently the primary focus of therapeutic intervention.1 Although IOP lowering may significantly decrease the rate of disease progression, many patients still experience deterioration despite treatment. In the Early Manifest Glaucoma Trial (EMGT), up to 45% of patients had progressive visual field loss despite a 25% reduction of IOP.2 The Normal Tension Glaucoma Study Group (CNTG)3 showed that an IOP reduction by 30% could reduce the number of patients showing progression from 35% to 12% in five years of follow-up, but could not stave off progression in all patients. These findings suggest there may be additional risk factors explaining susceptibility to glaucomatous damage and progression.
Some studies have attempted to identify risk factors for progression in patients with apparently well-controlled IOP. In a retrospective chart review study, De Moraes et al.4 showed that peak IOP, disc hemorrhage, presence of beta-zone parapapillary atrophy and central corneal thickness (CCT) were the main factors related to visual field loss. In their study, a 40 μm thinner cornea was associated with increased odds of 45% of progression during follow-up. Although the association between thin corneas and progression has been suggested by numerous other studies,5, 6 the mechanisms explaining such relationship have not been clarified. It is possible that underestimation of IOP in eyes with thin corneas may lead to a false impression that the IOP is under control, resulting in insufficient treatment. It has also been speculated that CCT may be a true independent risk factor for progression, due to the relationship of scleral thickness and structural properties of the posterior globe.7
Corneal hysteresis (CH), a biomechanical property of the cornea related to its viscous damping, has been indicated as a stronger predictive factor of progression than CCT.8 CH can be estimated by analyzing corneal responses to deformation induced by an air pulse.9 The ability of the cornea to resist deformation might reflect the constitution of its extracellular matrix (ECM). This could be related to the ECM composition of posterior ocular tissues related to glaucomatous damage, such as the lamina cribrosa and peripapillary sclera. An eye with a more deformable cornea could potentially have an optic disc that is more susceptible to IOP damage. Although previous studies have shown that lower CH is significantly associated with faster glaucoma progression,8, 10 no study has yet investigated whether CH is associated with progression in eyes with apparently well-controlled IOP.
The evaluation of predictive factors for progression in eyes with seemingly well-controlled IOP is important in order to identify those eyes in which more aggressive interventions, aimed at even lower IOP levels, may be desired. Therefore, the purpose of the current study was to investigate such predictive factors, including CH, in a prospective cohort of glaucoma subjects followed over time
METHODS
This study included participants from a prospective longitudinal study designed to evaluate optic nerve structure and visual function in glaucoma. Written informed consent was obtained from all participants and the institutional review board and human subjects committee approved all methods. All methods adhered to the tenets of the Declaration of Helsinki for research involving human subjects and the study was conducted in accordance with the regulations of the Health Insurance Portability and Accountability Act.
All participants underwent comprehensive ophthalmologic examination every 6 months, including review of medical history, best-corrected visual acuity, slit-lamp biomicroscopy, IOP measurement using the Goldmann applanation tonometry (GAT; Haag-Streit, Konig, Switzerland) calibrated once a month, gonioscopy, ophthalmoscopy examination, stereoscopic optic disc photography, and standard automated perimetry (SAP) using the Swedish Interactive Threshold Algorithm (SITA) Standard with 24–2 strategy of the Humphrey Field Analyzer II-i, model 750 (Carl Zeiss Meditec, Inc., Dublin, CA, USA). Optic nerve damage was assessed by masked grading of stereophotographs. Central corneal thickness (CCT) measurements were obtained at the baseline visit by a trained technician using the Pachette DGH 500 ultrasound pachymeter (DGH Technology, Inc., Philadelphia, PA).
Participant selection and study outcomes
Patients were included in the study if they had glaucoma and at least one year of follow up with a minimum of 3 reliable visual field tests (≤15% false-positive errors). Visual fields were reviewed for artifacts, fatigue or learning effects, inappropriate fixation, evidence that the visual field results were caused by a disease other than glaucoma (e.g. homonymous hemianopia) and inattention. Tests were excluded if such artifacts were present. Glaucoma was defined by the presence of two or more repeatable glaucomatous visual field defects at baseline, defined as a pattern standard deviation with P < 0.05, or a Glaucoma Hemifield Test result outside normal limits, and corresponding optic nerve damage. Patients were excluded if they presented any other ocular or systemic disease that could affect the optic nerve or visual field, if best-corrected visual acuity was less than 20/40, spherical refraction outside 5.00 diopters, or cylinder correction outside 3.00 diopters. Participants who underwent glaucoma surgeries (i.e. trabeculectomy, tube shunt procedures) after the beginning of the study were also excluded from the analysis.
Patients were classified into groups according to IOP control (well-controlled vs. uncontrolled IOP). Eyes were considered to have well-controlled IOP if all measurements recorded during follow-up were no higher than 18 mmHg. The cutoff of 18 mmHg to determine patients with “well-controlled IOP” was motivated by the Advanced Glaucoma Intervention Study (AGIS),11 which found that eyes with IOP consistently less than 18 mmHg in all visits did not show apparent progression as measured by the AGIS score. Qualified trained personnel obtained IOP measurements at regular clinic hours during follow-up. Baseline IOP was the first measured IOP after enrollment in the study. Mean IOP was calculated as the average of all IOP measurements obtained during follow-up, while peak IOP was the highest value. IOP fluctuation was defined as the standard deviation (SD) of inter-visit IOP measurements.
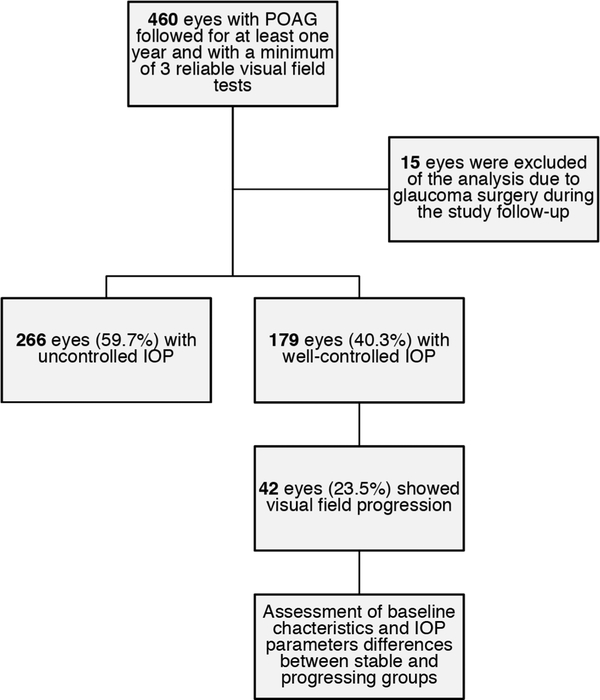
We then compared the baseline characteristics of both IOP groups, examining rates of visual field change by IOP status. We further investigated the group with well-controlled IOP, by separating them in two subgroups based on rates of visual field progression (stable patients versus progressing patients). Finally, we compared baseline characteristics and IOP parameters between groups and investigated which factors were related to visual field progression. (Figure 1)
Figure 1.
The study design flowchart. POAG = primary open angle glaucoma; IOP = intraocular pressure.
Rates of visual field progression were calculated using ordinary least squares linear regression of SAP mean deviation (MD) values over time. Progression was defined as a significantly negative rate of MD change over time (alpha = 0.05). The baseline visual field was defined as the first reliable visual field exam performed after inclusion in the study.
Corneal Hysteresis Measurements
Corneal hysteresis (CH) measurements were acquired at baseline using the Ocular Response Analyzer® (ORA; Reichert Technologies, Inc., Depew, NY, USA). A trained technician obtained three measurements from each eye and the average of these measurements was calculated for analysis. The ORA is a noncontact tonometer that measures IOP by applanation of the cornea with a pulse of air and can also estimate corneal biomechanical properties based on the pressure of applanation on inward corneal motion and on outward motion of the cornea. Details of these calculations have been previously described.9 In summary, at the moment the air reaches the cornea, it exerts an inward pressure that deforms the cornea into slight concavity. Milliseconds later, the airflow ceases, and the outward rebound of the cornea leads to a second corneal applanation. The difference between both applanation pressures is the CH parameter.9
Statistical Analyses
For descriptive analyses, categorical variables were presented as absolute and relative frequencies and continuous variables were summarized using mean and SD. The association between visual field progression and IOP control (well-controlled IOP vs. uncontrolled IOP groups) was verified using Pearson’s chi-squared test.
The effects of potential risk factors to visual field progression, such as age, sex, race, peak IOP, mean IOP, IOP fluctuation, CH, CCT, previous cataract surgery, and baseline SAP 24–2 MD, were evaluated via logistic regression in univariable models. Subsequently, separate multivariable models were used to study corneal properties (i.e., CH, CCT, and “corneal index”) as predictive factors for progression, adjusting for potential confounding factors such as age, race, IOP parameters, previous cataract surgery, and baseline MD. The “corneal index” variable was created by the summation of normalized versions of CH and CCT to address the collinearity issue of those two variables in the same model. Generalized estimating equations (GEE)12 with robust sandwich variance estimator was used to adjust for potential correlations between measurements obtained in the same individual. To evaluate the fit of the three multivariable models proposed above, the quasi-likelihood under the independence model (QIC) was used. QIC is a modification of the Akaike’s information criterion (AIC), and it is used to select the best model for GEE analyses. A smaller value of QIC is preferred.13
All statistical analyses were performed using the commercially available software Stata, version 15 (StataCorp LP, College Station, TX). The alpha level (type I error) was set at 0.05.
RESULTS
This study enrolled 460 eyes of 334 patients with open-angle glaucoma under treatment. Of those, 15 eyes were excluded from the analysis due to glaucoma surgeries during follow-up. The remaining 445 eyes (327 patients) had an average follow-up of 4.3 ± 0.8 years (range, 1.1 to 5.0 years), with a mean of 10 visits (range, 3 to 24). Most patients were women (54.7%) and non-African American (61.5%). Mean age at baseline was 66.2 ± 12.2 years, mean baseline MD was −5.5 ± 6.1 dB, and mean IOP during follow-up was 14.5 ± 3.2 mmHg (Table 1).
Table 1.
Demographic and clinical characteristics of subjects included in the study.
| Characteristic | Overall | Uncontrolled IOP | Well-controlled IOP |
|---|---|---|---|
| (445 eyes of 327 patients) | (266 eyes of 193 patients) | (179 eyes of 134 patients) | |
| Age (years) | 66.2 ± 12.2 | 65.7 ± 12.6 | 66.9 ±11.5 |
| Sex, female (%) | 179 (54.7) | 103 (53.4) | 76 (56.7) |
| Race, African American (%) | 126 (38.5) | 77 (39.9) | 49 (36.6) |
| Baseline SAP 24–2 MD (dB) | −5.5 ± 6.1 | −5.2 ± 5.21 | −6.0 ± 6.4 |
| Peak IOP (mmHg) | 18.7 ± 4.9 | 21.6± 4.0 | 14.5 ± 2.1 |
| Mean IOP (mmHg) | 14.5 ± 3.2 | 16.2 ± 2.7 | 12.0 ± 2.2 |
| IOP fluctuation (mmHg) | 2.4 ± 1.3 | 3.0 ± 1.4 | 1.6 ± 0.5 |
| Corneal hysteresis (mmHg) | 9.1 ± 1.6 | 9.0 ± 1.6 | 9.2 ± 1.5 |
| Central corneal thickness (μn) | 535.1 ± 42.4 | 540.4 ± 42.5 | 527.3 ± 40.9 |
| Rate of MD change (dB/Year) | −0.3 ± 0.8 | −0.4 ± 0.8 | −0.2 ± 0.7 |
| Follow-up period (years) | 4.3 ± 0.8 | 4.4 ± 0.8 | 4.1 ± 0.9 |
SAP = standard automated perimetry; IOP = intraocular pressure; MD = mean deviation. Values are presented as mean ± standard deviation, unless otherwise noted.
From the 445 eyes, 179 had all IOP measurements under 18 mmHg during office visits and were considered to have well-controlled IOP (Figure 1). This group was further evaluated according to progression based on rates of SAP MD change. (Table 2). Forty-two (23.5%) eyes had progressive visual field loss, while 137 (76.5%) remained stable over time (mean rates of MD change of −0.9 ± 0.7 dB/y vs. 0.1 ± 0.5 dB/year, respectively; P = 0.001). When comparing progressive vs. stable eyes in this subgroup, there was no significant difference in age, sex, race, baseline MD, peak IOP, mean IOP, and IOP fluctuation. However, eyes that progressed had lower CH (8.6 ± 1.3 mmHg vs. 9.4 ± 1.6 mmHg; P = 0.014) and thinner CCT (515.1 ± 33.1 μm vs. 531.1 ± 42.4 μm; P = 0.018) than stable eyes. Of note, progressing and stable eyes had a similar number of visits, number of IOP-lowering medications in use, and were submitted to a similar number of glaucoma surgeries and laser procedures before the beginning of the study. Progressing patients had more cataract surgeries before entering the study then stable patients (19 [45.2%] and 27 [19.7%], respectively; P = 0.002). Both groups also presented a similar number of cataract surgeries and laser procedures during follow-up.
Table 2.
Baseline characteristics of subjects who presented well controlled intraocular pressure (IOP; less than 18mmHg) with and without glaucomatous visual field progression.
| Progressive Visual Field Loss | |||
|---|---|---|---|
| No | Yes | P Value | |
| (n=137 eyes, 106 subjects) | (n=42 eyes, 28 subjects) | ||
| Age (years) | 66.1 ± 11.7 | 69.9 ± 10.2 | 0.181a |
| Sex, female (%) | 58 (54.7) | 18 (64.3) | 0.399b |
| Race, African American (%) | 42 (39.6) | 7 (25.0) | 0.189b |
| Baseline MD (dB) | −6.0 ± 6.2 | −6.4 ± 7.1 | 0.346c |
| Peak I0P (mmHg) | 14.6± 2.1 | 14.3 ± 1.9 | 0.926c |
| Mean IOP (mmHg) | 12.1 ± 2.3 | 11.7 ± 2.0 | 0.405c |
| IOP fluctuation (mmHg) | 1.6 ± 0.5 | 1.6 ± 0.6 | 0.402c |
| Corneal hysteresis (mmHg) | 9.4 ± 1.6 | 8.6 ± 1.3 | 0.014c |
| Central corneal thickness (μm) | 531.1 ± 42.4 | 515.1 ± 33.1 | 0.018c |
| Rate of MD change (dB/year) | 0.1 ± 0.5 | −0.9 ± 0.7 | 0.001c |
| Follow-up period, years | 4.0 ± 0.9 | 4.4 ± 0.7 | 0.185a |
| Procedures performed before the beginning of the study, n (%) | |||
| Glaucoma surgery | 19 (13.9) | 8 (19.1) | 0.461b |
| Laser | 30 (21.9) | 11 (26.2) | 0.538b |
| Cataract surgery | 27 (19.7) | 19 (45.2) | 0.002b |
| Procedures performed during follow-up, n (%) | |||
| Laser | 10 (7.3) | 2 (4.8) | 0.735b |
| Cataract surgery | 21 (15.3) | 2 (4.8) | 0.111b |
Values are presented as mean ± standard deviation, unless otherwise noted.
MD = mean deviation; IOP = intraocular pressure.
Wilcoxon rank-sum test
Fisher’s exact test
Generalized Estimating Equation.
Table 3 shows the results from the univariable models investigating putative factors for visual field progression. Only CH (P = 0.014), CCT (P = 0.018) and age (P = 0.017) were significantly associated with progression in the univariable models. Neither baseline MD (P = 0.346) nor any of the IOP parameters were related to visual field loss in our study. We then built separate multivariable models investigating CH and CCT as predictive factors for progression, with adjustment for age, baseline MD previous cataract surgery, and mean IOP. The reason for the separation of CH and CCT in two different multivariable models was due to a statistically large correlation between both parameters (r = 0.447, P < 0.001), which resulted in multicollinearity. Of note, the decrease of 1 SD (1.5 mmHg) in CH was associated with an increase of 65% on the risk of developing visual field loss during follow-up (OR: 1.65; 95% CI: 1.06 to 2.57; P = 0.027). Furthermore, for each 1 SD (41 μm) lower CCT the risk of visual field progression increased by 56%, adjusting for potential confounding factors (OR: 1.56; 95% CI:1.03 to 2.36; P = 0.037). Due to the collinearity, a “corneal index” variable was created that is a summation of normalized versions of CH and CCT. In the multivariable analysis after adjustment for the same potential confounders, a 1 SD decrease in the “corneal index” resulted in an increase in progression by 68% (OR: 1.68; 95% CI:1.08 to 2.62; P = 0.021) (Table 4). This model, which accounts for both corneal measurements, presented a better fit based on the QIC – 188.260 – compared with the multivariable models with either CH (188.726) or CCT (191.422). Additional multivariable models were also built but adjusting for peak IOP instead of mean IOP with very similar results for predictive variables (data not shown).
Table 3.
Estimates of the odds ratio and standard error (SE) for progressive visual field loss in patients with well-controlled intraocular pressure by univariable and multivariable models.
| Variable | Univariable Models | Multivariable Model 1 | Multivariable Model 2 | ||||
|---|---|---|---|---|---|---|---|
| Odds Ratio (SE) |
P Value* | Odds Ratio (SE) | P Value* | Odds Ratio (SE) | P Value* | ||
| Corneal hysteresis (per SD lower) | 1.63 (0.33) | 0.014 | 1.65 (0.37) | 0.027 | -------- | -------- | |
| Central corneal thickness (per SD lower) | 1.57 (0.30) | 0.018 | -------- | -------- | 1.56 (0.33) | 0.037 | |
| Baseline MD (dB) | 0.98 (0.02) | 0.346 | 0.99 (0.03) | 0.788 | 0.99 (0.03) | 0.744 | |
| Age (years) | 1.04 (0.02) | 0.017 | 1.01 (0.02) | 0.630 | 1.02 (0.02) | 0.140 | |
| Sex (female) | 1.10 (0.43) | 0.807 | -------- | -------- | -------- | -------- | |
| Race (African American) | 0.73 (0.30) | 0.443 | 0.72 (0.32) | 0.452 | 0.72 (0.32) | 0.459 | |
| Mean IOP (mmHg) | 0.94 (0.07) | 0.445 | 0.95 (0.09) | 0.632 | 1.00 (0.09) | 0.972 | |
| Peak IOP (mmHg) | 1.01 (0.12) | 0.926 | -------- | -------- | -------- | -------- | |
| IOP fluctuation (mmHg) | 1.42 (0.60) | 0.402 | -------- | -------- | -------- | -------- | |
| Previous cataract surgery | 2.97 (1.15) | 0.005 | 2.45 (1.15) | 0.058 | 1.93 (0.83) | 0.127 | |
| QIC | 188.726 | 191.422 | |||||
Generalized estimating equations
SD = standard deviation; SE = standard error; MD = mean deviation; IOP = intraocular pressure; QIC = quasi-likelihood under the independence model criterion.
Table 4.
Estimates of the corneal index odds ratio and standard error (SE) for progressive visual field loss in patients with well-controlled intraocular pressure after adjustment for potential confounder factors.
| Variable | Multivariable Model 3 | |
|---|---|---|
| Odds Ratio (SE) |
P Value* | |
| Corneal index (per SD lower) | 1.68 (0.38) | 0.021 |
| Baseline MD (dB) | 0.99 (0.03) | 0.789 |
| Age (years) | 1.01 (0.02) | 0.614 |
| Race (African American) | 0.70 (0.31) | 0.430 |
| Mean IOP (mmHg) | 0.96 (0.09) | 0.642 |
| Previous cataract surgery | 2.42 (1.14) | 0.061 |
| QIC | 188.260 | |
Generalized estimating equations
SD = standard deviation; SE = standard error; MD = mean deviation; IOP = intraocular pressure; QIC = quasi-likelihood under the independence model criterion.
DISCUSSION
The current study demonstrated that approximately one-quarter of glaucoma eyes that had all IOP measurements below 18mmHg during follow-up showed visual field progression over time. Thinner CCT and lower CH were risk factors for progression in these patients. Our findings support investigating these characteristics in treated and otherwise progressing patients.
Although high IOP is related to glaucoma damage, reducing IOP may not completely prevent disease progression. This is not new; several previous longitudinal studies in glaucoma acknowledge that a significant percentage of treated eyes still progress, despite a clear effect of IOP reduction with treatment. The EMGT reported that 45% of patients in the treatment group progressed on visual fields over a 6-year period despite an average decrease of 25% in IOP;14 while the CNTG15 has shown that a 30% decrease in IOP was not enough to completely halt the progression rate in individuals with normal tension glaucoma. However, it is still debatable which factors contribute to further visual field loss despite intensive IOP lowering. Due to the multifactorial etiology of glaucomatous damage, it is possible that other characteristics, such as corneal biomechanics, could explain why some patients progress faster than others while maintaining a relatively normal range of IOP.
In our study, we found both lower CH and thinner CCT to be risk factors for progression despite apparently normal IOP measurements. Our findings are in accordance with previous studies, showing faster progression and greater damage in patients with low CH or thin cornea.2, 8, 16–18 Using separate multivariable models, we observed that each 1 SD (1.5 mmHg) decrease in CH was associated with an increase of 65% in risk of visual field progression (OR: 1.65; 95% CI: 1.06 to 2.57; P = 0.027). For each 41 μm thinner CCT, the risk increased by 56% (OR: 1.56; 95% CI:1.03 to 2.36; P = 0.037). The urge to use different multivariable models in this analysis arose from a strong positive correlation between CH and CCT values (r = 0.447, P < 0.001) in this sample, as also found by other studies.19–21 CCT is a geometric property that may confer more stiffness to the cornea and corneoscleral shell,7 so it is expected that higher values of CH would be more common in eyes with thicker central corneas.21 In an attempt to reduce the effect of collinearity between these variables, we created a “corneal index”, which is a summation of normalized values for CH and CCT to represent the effect of the variables ensemble. The model that included the “corneal index” had the lowest QIC, when compared with the multivariable models with CH or CCT alone, meaning it best fitted the data. This indicates that, although CH and CCT may be significantly correlated, there is information from each that is useful for determining progression in patients with well-controlled IOP. In our study, a decrease of 1 SD from the mean of corneal index led to a 68% higher risk of progression (OR: 1.68; 95% CI:1.08 to 2.62; P = 0.021). Hence, although presenting low IOP measurements during follow-up, patients may be at a higher risk of progression if they show low values of CH and CCT.
The mechanisms of how corneal properties are related to susceptibility to glaucomatous damage are still under debate. It has been proposed that they may act as surrogate biomarkers to the biomechanical properties of the lamina cribrosa and peripapillary sclera. Supporting this hypothesis, a recent study demonstrated a significant correlation between CH and anterior lamina cribrosa displacement after reduction of IOP.22 A low CH would be associated with a reduced capacity of relevant posterior ocular structures in dampening IOP peaks and fluctuations,22–26 while a thin CCT would reflect a thin lamina cribrosa7 with greater laminar displacement, leading to increased damage to adjacent axons by different mechanisms.27 A significant interaction has been previously described between IOP and CH,8 with the impact of IOP on progression significantly greater in eyes with low CH levels. In our study we demonstrated that patients with low IOP may be at higher risk for progression if they present low values of CH and CCT, supporting the hypothesis that the effect of IOP on the eye may not be completely independent.
Although both mean and peak IOP have been previously shown to be associated with glaucoma progression,4, 11, 28 neither one of these parameters had a significant effect on visual field progression in the group of eyes with all IOPs during follow-up under 18 mmHg. Average mean IOP in this sample was only 12.0 ± 2.0 mmHg, while average peak IOP was 14.5 ± 2.1 mmHg. However, this should not necessarily be taken as evidence that these parameters would not be important in this group. As IOP measurements were obtained from a limited number of office visits, this may have prevented us from detecting higher IOP levels outside office hours.29, 30 Most importantly, previous studies have suggested that low CCT and CH may lead to underestimation of the transcorneal pressure gradient measured by GAT. Therefore, in eyes with low levels of CH and CCT, the optic nerve would be exposed to higher IOP levels than clinicians were aware of, resulting in progression despite low measured IOPs.31, 32
It has been suggested that target IOP levels in the treatment of patients with glaucoma should be set based on percent reductions relative to untreated IOP levels, taking into account factors such as disease severity, risk factors for progression, life expectancy and potential side effects of treatment.1, 33 However, it is still very common in clinical practice for physicians to adopt absolute target IOP levels, such as 18mmHg or 12mmHg, for eyes with mild or severe disease, respectively. This derives from the fact that no clear algorithm for setting relative target IOP levels has been fully validated in practice. In addition, untreated IOP levels are frequently unavailable for patients who carry a longstanding history of treatment from multiple providers. The results of our study suggest that an absolute target of 18mmHg may still be associated with progression in a significant number of patients, notably those with thin CCT and low CH. It remains to be seen whether the use of relative target IOP taking into consideration CH and CCT as risk factors would completely halt progression in glaucoma.
An interesting observation from our study was that age was a risk factor for visual field progression in the univariable analysis, but not in the multivariable models that also included corneal measures. Previous studies have shown that older patients present lower values of CH and CCT.34, 35 While children present a mean CH of 12.5 mmHg,36 normal adult populations show a mean CH lower than 11 mmHg in all studies.9, 21, 31 Therefore, it is possible that the effect of aging on glaucoma damage may be partially related to its effect on ocular biomechanical characteristics. Further studies are needed to investigate this hypothesis.
Our study had limitations. Clinicians were free to choose treatment options during the study, as no fixed treatment protocol was used. It is likely that patients with more severe stages of the disease underwent more aggressive treatment, which led to lower IOP values, whereas mild cases were allowed higher IOP values. However, groups presented a similar number of procedures and medications at baseline and during follow-up. Also, we limited our definition of progression to a trend-based analysis of MD rates of change. It is possible that patients with a very depressed visual field who reached a “floor” could have been falsely marked as stable, even though progression may continue beyond that. Finally, as mentioned above, IOP measurements were obtained from a limited number of office visits, which may have prevented us from fully characterizing mean and peak IOP values during follow-up and their relationship with progression. Yet, this is commonly the case in clinical practice, and our findings support that other variables should be considered when assessing a patient’s risk of progression in a clinical setting. Notwithstanding, our results should be interpreted with caution in populations with other IOP characteristics and methods of assessment.
In summary, our study demonstrates that corneal properties can help characterize which patients are at greater risk of progression despite apparently well-controlled IOP levels. Hence, it may be reasonable to take into consideration CCT and CH when establishing a therapeutic strategy in patients with glaucoma.
Financial Support:
Supported in part by National Institutes of Health/National Eye Institute grants EY029885 (FAM), EY027651 (FAM), EY021818 (FAM). The sponsor or funding organization had no role in the design or conduct of this research.
Abbreviations:
- AIC
Akaike’s information criterion
- CCT
central corneal thickness
- CH
corneal hysteresis
- ECM
extracellular matrix
- EMGT
Early Manifest Glaucoma Trial
- CNTG
The Normal Tension Glaucoma Study Group
- HFA
Humphrey Field Analyzer
- IOP
intraocular pressure
- GAT
Goldmann applanation tonometry
- GEE
Generalized estimating equations
- MD
mean deviation
- ORA
Ocular Response Analyzer
- QIC
quasi-likelihood under the independence model
- SAP
Standard Automated Perimetry
- SD
standard deviation
- SITA
Swedish Interactive Threshold Algorithm
Footnotes
Conflict of Interest:
Bianca N. Susanna: none; Carolina N. Susanna: none; Nara G. Ogata: none; Alessandro A. Jammal: none; Samuel I. Berchuck: none; Felipe A. Medeiros: Alcon Laboratories (C, L, S), Allergan (C, L), Bausch&Lomb (F), Carl Zeiss Meditec (C, L, S), Heidelberg Engineering (L), Merck (L), nGoggle Inc. (P), Sensimed (C), Topcon (C), Reichert (C, S), National Institutes of Health/National Eye Institute (S).
REFERENCES
- 1.Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA 2014;311(18):1901–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leske MC, Heijl A, Hyman L, et al. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology 2007;114(11):1965–72. [DOI] [PubMed] [Google Scholar]
- 3.Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Collaborative Normal-Tension Glaucoma Study Group. Am J Ophthalmol 1998;126(4):487–97. [DOI] [PubMed] [Google Scholar]
- 4.De Moraes CG, Juthani VJ, Liebmann JM, et al. Risk factors for visual field progression in treated glaucoma. Arch Ophthalmol 2011;129(5):562–8. [DOI] [PubMed] [Google Scholar]
- 5.Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol 2002;120(6):714–20; discussion 829–30. [DOI] [PubMed] [Google Scholar]
- 6.Congdon NG, Broman AT, Bandeen-Roche K, et al. Central corneal thickness and corneal hysteresis associated with glaucoma damage. Am J Ophthalmol 2006;141(5):868–75. [DOI] [PubMed] [Google Scholar]
- 7.Albekioni Z, Joson P, Tello C, et al. Correlation between central corneal thickness and scleral thickness. Investigative Ophthalmology & Visual Science 2003;44(13):U17–U. [Google Scholar]
- 8.Medeiros FA, Meira-Freitas D, Lisboa R, et al. Corneal hysteresis as a risk factor for glaucoma progression: a prospective longitudinal study. Ophthalmology 2013;120(8):1533–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luce DA. Determining in vivo biomechanical properties of the cornea with an ocular response analyzer. J Cataract Refract Surg 2005;31(1):156–62. [DOI] [PubMed] [Google Scholar]
- 10.Zhang C, Tatham AJ, Abe RY, et al. Corneal Hysteresis and Progressive Retinal Nerve Fiber Layer Loss in Glaucoma. Am J Ophthalmol 2016;166:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. The AGIS Investigators. Am J Ophthalmol 2000;130(4):429–40. [DOI] [PubMed] [Google Scholar]
- 12.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 1986;42(1):121–30. [PubMed] [Google Scholar]
- 13.Pan W. Akaike’s information criterion in generalized estimating equations. Biometrics 2001;57(1):120–5. [DOI] [PubMed] [Google Scholar]
- 14.Leske MC, Heijl A, Hussein M, et al. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol 2003;121(1):48–56. [DOI] [PubMed] [Google Scholar]
- 15.The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Collaborative Normal-Tension Glaucoma Study Group. Am J Ophthalmol 1998;126(4):498–505. [DOI] [PubMed] [Google Scholar]
- 16.De Moraes CV, Hill V, Tello C, et al. Lower corneal hysteresis is associated with more rapid glaucomatous visual field progression. J Glaucoma 2012;21(4):209–13. [DOI] [PubMed] [Google Scholar]
- 17.Zhang C, Tatham AJ, Abe RY, et al. Corneal Hysteresis and Progressive Retinal Nerve Fiber Layer Loss in Glaucoma. Am J Ophthalmol 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park JH, Jun RM, Choi KR. Significance of corneal biomechanical properties in patients with progressive normal-tension glaucoma. Br J Ophthalmol 2015;99(6):746–51. [DOI] [PubMed] [Google Scholar]
- 19.Cevik SG, Kivanc SA, Akova-Budak B, Tok-Cevik M. Relationship among Corneal Biomechanics, Anterior Segment Parameters, and Geometric Corneal Parameters. J Ophthalmol 2016;2016:8418613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mangouritsas G, Morphis G, Mourtzoukos S, Feretis E. Association between corneal hysteresis and central corneal thickness in glaucomatous and non-glaucomatous eyes. Acta Ophthalmol 2009;87(8):901–5. [DOI] [PubMed] [Google Scholar]
- 21.Shah S, Laiquzzaman M, Cunliffe I, Mantry S. The use of the Reichert ocular response analyser to establish the relationship between ocular hysteresis, corneal resistance factor and central corneal thickness in normal eyes. Cont Lens Anterior Eye 2006;29(5):257–62. [DOI] [PubMed] [Google Scholar]
- 22.Lanzagorta-Aresti A, Perez-Lopez M, Palacios-Pozo E, Davo-Cabrera J. Relationship between corneal hysteresis and lamina cribrosa displacement after medical reduction of intraocular pressure. Br J Ophthalmol 2017;101(3):290–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang L, Albon J, Jones H, et al. Collagen microstructural factors influencing optic nerve head biomechanics. Invest Ophthalmol Vis Sci 2015;56(3):2031–42. [DOI] [PubMed] [Google Scholar]
- 24.Sigal IA, Flanagan JG, Ethier CR. Factors influencing optic nerve head biomechanics. Invest Ophthalmol Vis Sci 2005;46(11):4189–99. [DOI] [PubMed] [Google Scholar]
- 25.Johnson CS, Mian SI, Moroi S, et al. Role of corneal elasticity in damping of intraocular pressure. Invest Ophthalmol Vis Sci 2007;48(6):2540–4. [DOI] [PubMed] [Google Scholar]
- 26.Burgoyne CF, Downs JC, Bellezza AJ, et al. The optic nerve head as a biomechanical structure: a new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog Retin Eye Res 2005;24(1):39–73. [DOI] [PubMed] [Google Scholar]
- 27.Lesk MR, Hafez AS, Descovich D. Relationship between central corneal thickness and changes of optic nerve head topography and blood flow after intraocular pressure reduction in open-angle glaucoma and ocular hypertension. Arch Ophthalmol 2006;124(11):1568–72. [DOI] [PubMed] [Google Scholar]
- 28.Heijl A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol 2002;120(10):1268–79. [DOI] [PubMed] [Google Scholar]
- 29.Barkana Y, Anis S, Liebmann J, et al. Clinical utility of intraocular pressure monitoring outside of normal office hours in patients with glaucoma. Arch Ophthalmol 2006;124(6):793–7. [DOI] [PubMed] [Google Scholar]
- 30.Drance SM. Diurnal Variation of Intraocular Pressure in Treated Glaucoma. Significance in Patients with Chronic Simple Glaucoma. Arch Ophthalmol 1963;70:302–11. [DOI] [PubMed] [Google Scholar]
- 31.Medeiros FA, Weinreb RN. Evaluation of the influence of corneal biomechanical properties on intraocular pressure measurements using the ocular response analyzer. J Glaucoma 2006;15(5):364–70. [DOI] [PubMed] [Google Scholar]
- 32.Liu J, Roberts CJ. Influence of corneal biomechanical properties on intraocular pressure measurement: quantitative analysis. J Cataract Refract Surg 2005;31(1):146–55. [DOI] [PubMed] [Google Scholar]
- 33.European Glaucoma Society Terminology and Guidelines for Glaucoma, 4th Edition - Chapter 3: Treatment principles and options. Br J Ophthalmol 2017;101(6):130–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hussnain SA, Alsberge JB, Ehrlich JR, et al. Change in corneal hysteresis over time in normal, glaucomatous and diabetic eyes. Acta Ophthalmol 2015;93(8):e627–30. [DOI] [PubMed] [Google Scholar]
- 35.Johannesson G, Hallberg P, Ambarki K, et al. Age-dependency of ocular parameters: a cross sectional study of young and elderly healthy subjects. Graefes Arch Clin Exp Ophthalmol 2015;253(11):1979–83. [DOI] [PubMed] [Google Scholar]
- 36.Kirwan C, O’Keefe M, Lanigan B. Corneal hysteresis and intraocular pressure measurement in children using the reichert ocular response analyzer. Am J Ophthalmol 2006;142(6):990–2. [DOI] [PubMed] [Google Scholar]