Abstract
Introduction
Hyperbaric oxygen therapy (HBOT) involves the risk of central nervous system oxygen toxicity (CNS-OT), including seizures in patients breathing oxygen at pressures ≥ 2 atmospheres absolute. This study aimed to determine the seizure frequency and assess the clinical benefit of a 5-minute air-break (5´-AIRBK).
Methods
Twenty-year (1999–2018) retrospective analysis of all consecutive treatments with HBOT. Medical records were reviewed to determine patient demographics, comorbidities, HBOT indications, and seizure characteristics and timing. Seizure frequency was compared before and after incorporating a 5´-AIRBK in the treatment protocol. Chi-square testing was performed using SPSS (version 24.0); P < 0.05 was accepted as statistically significant.
Results
We evaluated 188,335 HBOT sessions (74,255 before versus 114,080 after introducing a 5´-AIRBK). A total of 43 seizures were observed: 29 before and 14 after the 5´-AIRBK introduction (3.9 versus 1.2 per 10,000 treatments; P < 0.0001). Seizures occurred after a median of 57 (range 15–85) minutes following compression and after a median of 21 HBOT sessions (1–126). Patients experiencing seizures were undergoing treatment for: diabetic ulcer (n = 11); acute traumatic peripheral ischaemia (ATPI) (n = 6); non-diabetic ulcer (n = 5); sudden sensorineural hearing loss (n = 5); chronic refractory osteomyelitis (n = 5); radionecrosis (n = 3); necrotising fasciitis (NF) (n = 2); and haemorrhagic cystitis after allogeneic bone marrow transplantation (n = 1). ATPI and NF had a considerably higher relative frequency of seizures compared to other indications.
Conclusion
A statistically significant lower seizure frequency was achieved with a 5´-AIRBK. Assessing and defining the appropriate patient/treatment profile can be useful to minimise the risk of CNS-OT.
Keywords: Hyperbaric oxygen therapy, Hyperbaric oxygen, Toxicity, Side effects, Hyperoxia, Central nervous system, Seizure
Introduction
Hyperbaric oxygen therapy (HBOT) consists of breathing 100% oxygen while inside a chamber that is pressurised to greater than sea level pressure (1 atmosphere absolute – atm abs, 101.3 kPa). It is used in a number of clinical conditions as well as in professional and military training. Therapeutic HBOT usually involves pressures higher than 1.4 atm abs (141.8 kPa), frequently ranging between 2.0 (202.6 kPa) and 2.5 atm abs (253.3 kPa) for 90 to 120 minutes (min).[ 1 - 4]
Although significant adverse reactions associated with HBOT are unusual, they may influence treatment decisions for eligible patients. Higher tissue availability of oxygen promotes selective, non-hypoxic hyperoxic vasoconstriction, and redistributes peripheral blood volume in favour of hypoxic tissues (Robin-Hood effect).[ 4] This initial process has a protective function in that it avoids abrupt elevation of the partial pressure of oxygen (PO2) at the cerebral level. Subsequently, in a sustained hyperbaric hyperoxic environment, there is a secondary increase in regional cerebral blood flow (rCBF). However, this biphasic response (transient vasoconstriction followed by vasodilation) is less pronounced for total pressures between 2–3 atm abs (303.9 kPa).[ 5 , 6] In “La pression Barométrique, Recherches de Physiologie Expérimentale” Paul Bert, and subsequently others, described the clinical manifestations of central nervous system oxygen toxicity (CNS-OT) ranging from milder and constitutional symptoms such as anxiety, nausea, vomiting, transient vision and hearing disorders to the more severe, as obnubilation or even generalised tonic-clonic seizures.[ 7 - 10] Despite the exact aetiology of these neurological alterations being not yet fully understood, CNS-OT seems to be related to several mechanisms, namely: generation of reactive oxygen species (ROS); central nitric oxide (NO) action; and eventually glutamic acid decarboxylase modulation in the excitatory-inhibitory process.[ 10]
Several studies have shown that intermittent air breaks (AIRBK) can increase tolerance of exposure to hyperoxia, especially if the interruptions were greater than 5 min.[ 11 - 13] However, it is noteworthy that after more than 50 years of research, there are no guidelines that determine the optimal relationship between 100% oxygen and AIRBK periods during therapeutic HBOT.
This study aimed to determine the the seizure frequency during HBOT, their association with baseline patient characteristics, and to assess the clinical impact of the incorporation of a 5-min AIRBK (5´-AIRBK - defined as a 5 min period in which patient was switched from breathing 100% oxygen to chamber air) in the treatment protocol.
Methods
The study was conducted in accordance with Ethics Committee regulations, and after Institutional Review Board approval.
STUDY POPULATION AND INTERVENTION
Patients were referred for treatment at the Centro de Medicina Subaquática e Hiperbárica (CMSH), Lisbon (Portugal) from departments of several hospitals nationwide, and were grouped into categories according to the indication for HBOT. Prior to HBOT, every patient underwent a tympanogram, electrocardiogram, and chest radiograph, followed by a global medical assessment to exclude conditions contraindicating HBOT.
Within each group, patients were assigned to either the routine or the emergency treatment protocol. Both protocols are described in Table 1. The main differences between routine and emergency protocols was use of several initial treatments in a shorter space of time, and occasional use of compressions to higher pressures (for certain diagnoses) in emergencies (Table 1). Indications for the emergency protocol included acute carbon monoxide (CO) poisoning, acute traumatic peripheral ischaemia, life-threatening soft-tissue infections, and central retinal artery occlusion. We excluded paediatric patients (< 6 years), gas embolism and decompression illness because of the differences in treatment tables used for those conditions. Patients with a prior history of seizures or undergoing HBOT sessions with oxygen administered by a hood (which may increase the risk of seizures due to the accumulation of carbon dioxide[ 14]) were also excluded. After July 2008 a single 5´-AIRBK was included into the routine protocol at the 45 min mark.
Table 1. Hyperbaric oxygen therapy protocols. atm abs = atmospheres absolute pressure; O2 = oxygen; 5´-AIRBK = 5-minute air break .
| Routine protocol | January 1999 – July 2008 | O2 100% 2.5 atm abs for 75 min |
| August 2008 – December 2018 | O2 100% 2.5 atm abs for 75 min + 5´-AIRBK | |
| Emergency protocol | Acute carbon monoxide poisoning | O2 100% 2.5 atm abs for 75 min (1 session) |
| Acute traumatic peripheral ischaemia | O2 00% 2.5 atm abs for 75 min (3 sessions) then routine protocol | |
| Life-threatening soft-tissue infection | O2 100% 2.8 atm abs for 110 min (3 sessions) then routine protocol | |
| Central retinal artery occlusion | O2 100% 2.8 atm abs for 110 min (1 session) then routine protocol (2 sessions) |
Patients were administered HBOT in a multiplace hyperbaric chamber Haux-Starmed 2200 (Haux Life Support, Karlsbad-Ittersbach, Germany), annually certified by the European Regulation for Medical Product Manufacturers.
Data from consecutive patients treated at the CMSH, from January 1999 to December 2018 were obtained from clinical records and from the medical and diving supervisor registries that included information on the HBOT protocol administered and clinical data regarding the patients’ characteristics.
During the treatment CNS-OT observed by the clinical staff and HBOT technician was followed by a policy-driven response involving the supervising physician. The staff were trained to identify the signs and symptoms of CNS-OT including nausea, facial twitching, transient vision impairment, tinnitus, dizziness, temporary loss of consciousness and seizures.
COVARIATES, ENDPOINTS, AND STATISTICAL ANALYSIS
Analysed variables included patient gender, age (years), comorbidities, the primary indication for HBOT and type and timing of the seizures within an HBOT session and during the treatment course.
The primary clinical outcome was to determine the seizure frequency during HBOT in the total cohort and assess the impact of a 5´-AIRBK on the rate of seizures.
Data were analysed using Statistical Package for Social Sciences, version 24.0 (SPSS, Chicago, IL, USA). Descriptive statistics were applied (frequencies and proportions for categorical variables and mean, median and percentages for continuous variables) and the Chi-square test was performed to compare the proportion of treatments resulting in a seizure before and after the incorporation of a 5´-AIRBK. A P-value < 0.05 was considered statistically significant.
Results
All HBOT sessions administered from beginning January 1999 to the end of December 2018 were eligible to be included in the analysis. Treatments with missing or data entry errors on key study elements (657 sessions) were excluded from the report, resulting in a final sample size of 188,335 sessions (184,811 routine; 3,524 emergency) and 8,537 patients (Table 2).
Table 2. Clinical indications for hyperbaric oxygen therapy, with number of related sessions, patients, and mean sessions per patient .
| Diagnosis | Sessions n | Patients n | Sessions per patient mean |
| Radionecrosis | 77,055 | 1,521 | 51 |
| Sudden sensorineural hearing loss | 40,155 | 2,673 | 15 |
| Diabetic foot ulcer | 30,398 | 741 | 41 |
| Non-diabetic ulcer | 22,992 | 862 | 27 |
| Chronic refractory osteomyelitis | 11,745 | 295 | 40 |
| Acute carbon monoxide poisoning | 1,988 | 1,982 | 1 |
| Acute traumatic peripheral ischaemia | 1,842 | 102 | 18 |
| Necrotising fasciitis | 852 | 63 | 14 |
| Central retinal artery occlusion | 567 | 174 | 3 |
| Compromised graft/flap | 420 | 21 | 20 |
| Others | 321 | 103 | – |
| Total | 188,335 | 8,537 | 22 |
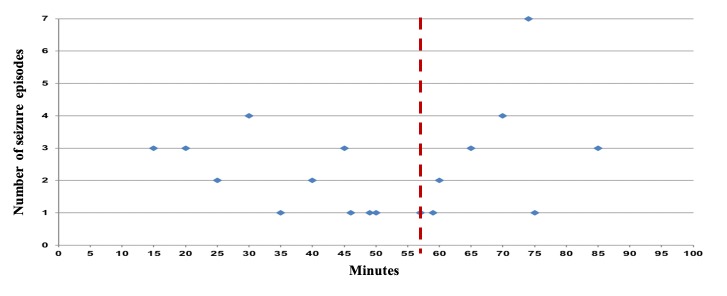
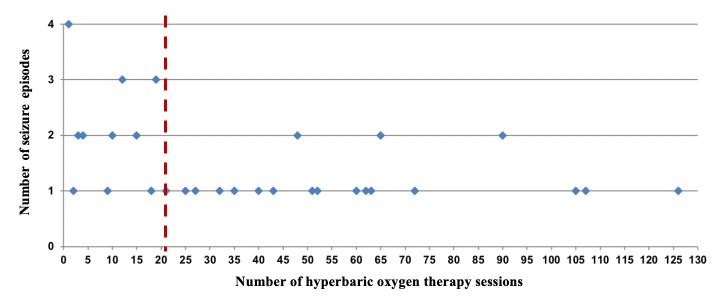
During the 20-year period the overall seizure rate, irrespective of the 5´-AIRBK, was 0.023% (1 in 4,379 or 2.3 per 10,000 treatment sessions) and the risk per patient was 0.45% (4.5 in 1000 patients). We documented 43 cases of seizures occurring in 38 patients (25 males; 65.8%) with a median age of 55 years (range 17–84). All seizures, except in patients treated for necrotising fasciitis and acute traumatic peripheral ischaemia occurred during routine HBOT sessions. Seizures were classified as tonic-clonic generalised (n = 41) and partial (n = 2). Seizure onset occurred after a median of 57 (15–85) min after compression (Figure 1) and after a median of 21 sessions into the treatment course (1–126) (Figure 2). There was a total of 14 episodes in 114,080 sessions (1.2 per 10,000) in patients treated with HBOT with a 5´-AIRBK, compared with 29 episodes in 74,255 sessions (3.9 per 10,000) for patients treated without a 5´-AIRBK (P < 0.0001).
Figure 1.
Timing of the seizures (minutes – X axis) within a hyperbaric oxygen therapy session. Dashed line = median
Figure 2.
Timing of the seizures (session – X axis) during the hyperbaric oxygen treatment course. Dashed line = median
The primary indications for HBOT in patients having a seizure were: diabetic foot ulcer (n = 11), acute traumatic peripheral ischaemia (n = 6), non-diabetic ulcer (n = 5), sudden sensorineural hearing loss (n = 5), chronic refractory osteomyelitis (n = 5), radionecrosis (n = 3), necrotising fasciitis (n = 2) and late-onset haemorrhagic cystitis after allogeneic haematopoietic stem cell transplantation (n = 1). Treatments for acute traumatic peripheral ischaemia (43.4 per 10,000 treatments) and necrotising fasciitis (23.5 per 10,000 treatments) resulted in a considerably higher frequency of seizures compared to other treatment indications (Table 3). Eight patients (2 chronic refractory osteomyelitis, 2 necrotising fasciitis, 1 diabetic foot ulcer, 1 sudden sensorineural hearing loss, 1 radionecrosis, and 1 with late-onset haemorrhagic cystitis after allogeneic haematopoietic stem cell transplantation) developed the convulsive episode in the first five sessions of HBOT.
Table 3. Frequency of oxygen toxicity seizure according to the primary indication for hyperbaric oxygen therapy. *Late-onset haemorrhagic cystitis after allogeneic haematopoietic stem cell transplantation (frequency not determined by being included in a very heterogenous diagnostic subgroup) .
| Diagnosis (number of patients) | Frequency | Rate per 10,000 | Seizure rate (%) |
| Radionecrosis (n = 3) | 3 in 77,055 | 0.4 | 0.004 |
| Sudden sensorineural hearing loss (n = 5) | 5 in 40,155 | 1.2 | 0.012 |
| Diabetic foot ulcer (n = 11) | 12 in 30,398 | 3.9 | 0.039 |
| Non-diabetic ulcer (n = 5) | 7 in 22,992 | 3 | 0.03 |
| Chronic refractory osteomyelitis (n = 5) | 5 in 11,745 | 4.3 | 0.043 |
| Acute carbon monoxide poisoning (n = 0) | – | – | – |
| Acute traumatic peripheral ischaemia (n = 6) | 8 in 1,842 | 43.4 | 0.43 |
| Necrotising fasciitis (n = 2) | 2 in 852 | 23.5 | 0.23 |
| Central retinal artery occlusion (n = 0) | – | – | – |
| Compromised graft/flap (n = 0) | – | – | – |
| Others* (n = 1) | – | – | – |
| Total | 43 in 188,335 | 2.3 | 0.023 |
Another four patients (2 non-diabetic ulcer, 1 diabetic foot ulcer, and 1 with acute traumatic peripheral ischaemia) had a recurrence of seizures. More than half of the patients experiencing seizures (n = 21; 55%) did not complete the treatment course as a result. Among patients suffering a seizure recorded comorbidities were: diabetes mellitus (n = 15), arterial hypertension (n = 15), high intensity trauma (n = 8, 1 head trauma), heavy smoker (n = 6), dyslipidaemia (n = 6), chronic renal disease (n = 5), chronic heart failure (n = 3), chronic obstructive pulmonary disease (n = 3), cerebrovascular disease (n = 2), autoimmune disease (n = 2), active metastatic disease (n = 2), drepanocytosis (n = 1), deep venous thrombosis (n = 1); medicated regularly with analgesics (n = 10, 1 including opioids), antidepressants (n = 4) and recent high-dose intensity chemotherapy (n = 1).
Discussion
In this 20-year analysis of more than 180,000 HBOT sessions at CMSH, the overall seizure rate was less than 0.03% and introduction of the 5´-AIRBK was associated with a significant decrease in seizure frequency.
The results of this study are consistent with prior studies confirming the rarity of this event. However, in the past 15–20 years, there seems to have an increase in frequency to approximately one case in 2,000–5,000 treatment sessions. This may be related to patient selection (with more comorbidities) and modifications in HBOT protocols. Overall, there are considerable variations between the different studies reporting the seizure frequency (Table 4).[ 14 - 24] It is difficult to compare the different series that have used various hyperbaric protocols (PO2 pressures, AIRBK, duration of exposures and number of HBOT sessions), varied techniques for oxygen delivery (hood, mask, monoplace), changeable HBOT chamber settings (CO2 removal, equipment resistance), and variable indications and patient status (in respect of comorbidities, medications, etc.). Every one of these factors could contribute to the considerable variation in reported seizure rates.
Table 4. Frequency of oxygen toxicity seizures reported by previous studies .
| Study | Frequency | Rate per 10,000 | Seizure rate (%) | Tx pressure atm abs |
| Hart 1987[15] | 1 in 12,253 | 0.8 | 0.008 | 2–3 atm abs |
| Davis 1989[16] | 1 in 10,552 | 0.95 | 0.009 | 2.4 atm abs |
| Welslau 1996[17] | 1 in 6,704 | 1.5 | 0.015 | 2.4–2.8 atm abs |
| Plafki 2000[18] | 1 in 2,844 | 3.5 | 0.035 | 2.4–2.5 atm abs |
| Hampson 2003[14] | 1 in 3,388 | 3 | 0.029 | 2–2.8 atm abs |
| Yildiz 2004[19] | 1 in 40,339 | 0.25 | 0.002 | 2–2.8 atm abs |
| Banham 2011[20] | 1 in 1,651 | 6 | 0.061 | 1.9–4 atm abs |
| Heyboer 2014[21] | 1 in 2,121 | 5 | 0.047 | 2–2.8 atm abs |
| Hadanny 2016[22] | 1 in 8,945 | 1.1 | 0.011 | 1.5–2.8 atm abs |
| Jokinen-Gordon 2017[23] | 1 in 5,730 | 1.7 | 0.017 | 2–2.5 atm abs |
| Sherlock 2018[24] | 1 in 3,718 | 2.7 | 0.027 | 2.4 atm abs |
| Alpuim Costa 2019 | 1 in 4,379 | 2.3 | 0.023 | 2.5–2.8 atm abs |
Because of the differences in treatment tables among some group of patients, we excluded paediatric age (< 6 years), gas embolism and decompression illness to obtain reasonable treatment protocol homogeneity in our sample. A lower seizure frequency was associated with the incorporation of a 5´-AIRBK in the treatment protocol, validating previous findings that the risk of CNS-OT occurrence is often mitigated by interspersing short periods of air-breathing between 100% oxygen periods at increased pressure. The major problem is to define the limit of duration of exposure to HBOT, as there are no identifiable biomarkers that indicate the transition from the beneficial effect to the toxicity.
In this study, patients who had seizures (43 seizures in 38 patients), the majority had their convulsive episode after the first half hour of treatment (Figure 1). This fact may reflect the continued action of several factors, including an imbalance of the vaso-modulation and antioxidant defence processes.
In prolonged HBOT protocols for chronic disorders, the repeated exposure to oxygen could increase susceptibility to hyperoxic seizure. In experimental studies with mice exposed to 4 atm abs, latency to seizure shortened and severity increased after prolonged intermittent therapy. It was found that for exposures at sub-convulsive minor pressures (2 atm abs) for 2 hours, the sensitivity for subsequent exposures to higher pressures (4 atm abs–405.2 kPa) increased significantly. This was present even after 5 sessions and persisted for at least 10 days (possibly related to NO and increased NO synthetase function).[ 25 , 26]
In our series, seizure frequency increased longitudinally throughout the treatment course. However, eight patients developed the convulsive episode in the first five sessions of HBOT (four in the first treatment session). Another four patients experienced more than one seizure episode (all before the 5´-AIRBK was introduced and even after interruption of treatment for more than one month), including one patient with three non-consecutive episodes (middle-aged, a heavy smoker, non-diabetic ulcer with arterial hypertension, dyslipidaemia, hyperuricaemia, and chronic pancreatitis). This may indicate greater individual susceptibility and or even modifications acquired in the previously mentioned mechanisms. In our centre, we generally recommend interruptions of one month after 40 or more consecutive sessions to diminish the pulmonary and CNS-OT risk. In general, the number of treatment-sessions administered to patients with seizure episodes was shorter than those without (comparing with our historical controls and protocols), indicating that when a seizure occurs, a treatment course is more likely to be ended early.
HBOT-induced seizures are accepted to be generalised, although several studies suggested specific susceptible foci for the initiation of the epileptic activity. The local sensitivity during HBOT may be related to regional variations between different brain areas with respect to rCBF, amino acids and ammonia levels, lipid peroxidation, and antioxidant enzymes distribution[ 6 , 27 - 32] We documented two episodes of partial seizures, possibly reflecting early abnormal changes in cortical electrical activity, that completely resolved with reduction of the inspired PO2. Notably, none of the patients with seizures had a history of epilepsy or another neurological disease.
Environmental and personal factors may modify the sensitivity to CNS-OT, thus shortening the duration of the latent period, and lowering the threshold pressure for the development of seizures. It has been observed that age could increase susceptibility to CNS-OT (higher sensitivity to ROS and lower level of the neurotransmitter GABA) and that gender might be relevant.[ 33 - 35] In our series of seizing patients, the median age was 55 years, and the majority were male. Prior research demonstrates gender differences in healthcare utilisation behaviours; women are more likely to use preventive services and to seek care early in the disease process.[ 36 , 37] Thus, female patients may experience fewer severe comorbid conditions and may be more likely to adhere to other prescribed treatments, reducing the likelihood of treatment complications.[ 23]
In addition to gender and age, individual day-to-day variation, circadian rhythm, physical activity, diet, alcohol dependence, narcotic withdrawal, various drugs (opioids, analgesics, antidepressants, antibiotics, etc.), fever, chronic obstructive pulmonary disease, heart failure, acute ischaemic events and trauma may contribute to wide-ranging physiological variability in the sensitivity to CNS-OT.[ 9 , 19 , 23 , 24 , 38 , 39] The patients reported in this series suffered from a diversity of comorbidities, some of which have previously been associated with seizure risk, and some which have a plausible basis for being considered risk factors. However, our data do not allow any conclusions to be drawn on this issue.
It is noteworthy that the seizure frequency was significantly higher in patients with acute traumatic peripheral ischaemia (43.4 per 10,000) and necrotising fasciitis (23.5 per 10,000) (Table 3) even though treatment of these diagnoses took place over fewer treatment sessions per patient (Table 2). The reasons for this increased risk, despite the shorter duration of treatment are uncertain and may be related to several factors, namely: higher pressure and duration of oxygen exposure in the first three treatment sessions for necrotising fasciitis (the episodes occurred in the inaugural session of HBOT), less time for patient education concerning the potential risk factors, acute oxidative stress, CO2 retention, fever, high intensity trauma and some drugs (opioids and antibiotics).
The current analysis is limited by several factors, including its retrospective nature, the single institution source of data, the exclusion of patients < 6 years age and with gas embolism or decompression illness, and the diversity of the population included in the 20-year period of the study. Even though the authors believe that the current findings represent an accurate depiction of seizure occurrence associated with HBOT, including critical care patients underrepresented in other studies, our medical and supervisor records must be improved.
Conclusions
In this cohort of patient-treatments (the largest reported to date), the overall seizure rate was similar to previous studies confirming the rarity of this event. Acute traumatic peripheral ischaemia and necrotising fasciitis patients exhibited a higher rate of events compared to other indications treated. The incorporation of a 5´-AIRBK was associated with a significantly lower seizure frequency. Assessing and defining the appropriate patient/treatment profile can be useful to minimise the risk of CNS-OT. We believe that a meta-analysis may provide helpful further information.
Footnotes
Acknowledgements
The authors are very grateful to the secretariat and clinical staff of the Centro de Medicina Subaquática e Hiperbárica (CMSH) for the efficiency of their work in identifying and collecting the data necessary to perform this retrospective analysis.
Conflicts of interest and funding: nil
Contributor Information
Diogo A Costa, Centro de Medicina Subaquática e Hiperbárica (CMSH), Portuguese Navy, Portugal; Centro de Investigação Naval (CINAV), Portuguese Navy, Portugal; Department of Haematology and Oncology, CUF Instituto de Oncologia, Lisbon, Portugal.
José S Ganilha, Centro de Medicina Subaquática e Hiperbárica (CMSH), Portuguese Navy, Portugal; Centro de Investigação Naval (CINAV), Portuguese Navy, Portugal.
Pedro C Barata, Department of Internal Medicine, Section of Hematology and Medical Oncology, Tulane Medical School, New Orleans, United States.
Francisco G Guerreiro, Centro de Medicina Subaquática e Hiperbárica (CMSH), Portuguese Navy, Portugal; Centro de Investigação Naval (CINAV), Portuguese Navy, Portugal.
References
- Undersea and Hyperbaric Medical Society. Available from: https://www.uhms.org/resources/hbo-indications.html [January 31 2019]
- Tibbles PM, Edelsberg JS. Hyperbaric-oxygen therapy . N Engl J Med. 1996;334:1642–8. doi: 10.1056/NEJM199606203342506. [DOI] [PubMed] [Google Scholar]
- Fernandes TD. Hyperbaric medicine . Acta Med Port. 2009;22:323–34. [PubMed] [Google Scholar]
- Albuquerque e Sousa JG. Oxigenoterapia hiperbárica (OTHB). Perspectiva histórica, efeitos fisiológicos e aplicações clínicas. Revista da Sociedade Portuguesa de Medicina Interna. 2007;14:219–27. [Google Scholar]
- Lambertsen CJ, Dough RH, Cooper DY, Emmel GL, Loeschcke HH, Schmidt CF. Oxygen toxicity. Effects in man of oxygen inhalation at 1 and 3.5 atmospheres upon blood gas transport, cerebral circulation and cerebral metabolism . J Appl Physiol. 1953;5:471–86. doi: 10.1152/jappl.1953.5.9.471. [DOI] [PubMed] [Google Scholar]
- Demchenko IT, Boso AE, O’Neill TJ, Bennett PB, Piantodosi CA. Nitric oxide and cerebral blood flow responses to hyperbaric oxygen. J Appl Physiol (1985). 2000;88:1381–9. doi: 10.1152/jappl.2000.88.4.1381. [DOI] [PubMed] [Google Scholar]
- Bert P. La pression barométrique. Recherches de physiologie expérimentale . Paris: Masson; 1878. (Fre). [Google Scholar]
- Donald KW. Oxygen poisoning in man. Br Med J. 1947;1(4506):667. [PMC free article] [PubMed] [Google Scholar]
- Lambertsen CJ. Effects of oxygen at high partial pressure. In: Fenn WO, editor. Handbook of physiology: Respiration. Bethesda (MD): American Physiological Society; 1965. [Google Scholar]
- Bitterman N. CNS oxygen toxicity. Undersea Hyperb Med. 2004;31:63–72. [PubMed] [Google Scholar]
- Harabin AL, Survanshi SS, Weathersby PK, Hays JR, Homer LD. The modulation of oxygen toxicity by intermittent exposure. Toxicol Appl Pharmacol. 1988;93:298–311. doi: 10.1016/0041-008x(88)90130-5. [DOI] [PubMed] [Google Scholar]
- Lambertsen CJ. Extension of oxygen tolerance in man: philosophy and significance. Exp Lung Res. 1988;14(Suppl):1035–58. doi: 10.3109/01902148809064191. [DOI] [PubMed] [Google Scholar]
- Chavko M, McCarron RM. Extension of brain tolerance to hyperbaric O2 by intermittent air breaks is related to the time of CBF increase. Brain Res. 2006;1084:196–201. doi: 10.1016/j.brainres.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Hampson N, Atik D. Central nervous system oxygen toxicity during routine hyperbaric oxygen therapy. Undersea Hyperb Med. 2003;30:147–53. [PubMed] [Google Scholar]
- Hart GB, Strauss MB. Central nervous system oxygen toxicity in a clinical setting. In: Bove AA, Bachrach AJ, Greenbaum LJ, editors. Undersea and hyperbaric physiology IX. Proceedings of the ninth international symposium on underwater and hyperbaric physiology Bethesda (MD): Undersea Hyperbaric Medical Society; 1987. p. 695- 9. [Google Scholar]
- Davis JC. Hyperbaric oxygen therapy. J Intensive Care Med. 1989;4:55–7. doi: 10.1177/088506668900400203. [DOI] [Google Scholar]
- Welslau W, Almeling M. Toxicity of hyperbaric oxygen (HBO)–incidence of major CNS intoxications. Strahlenther Onkol. 1996;172(Suppl2):10–2. [PubMed] [Google Scholar]
- Plafki C, Peters P, Almeling M, Welslau W, Busch R. Complications and side effects of hyperbaric oxygen therapy. Aviat Space Environ Med. 2000;71:119–24. [PubMed] [Google Scholar]
- Yildiz S, Aktas S, Cimsit M, Ay H, Toğrol E. Seizure incidence in 80,000 patient treatments with hyperbaric oxygen. Aviat Space Environ Med. 2004;75:992–4. [PubMed] [Google Scholar]
- Banham ND. Oxygen toxicity seizures: 20 years’ experience from a single hyperbaric unit. Diving Hyperb Med. 2011;41:202–10. [PubMed] [Google Scholar]
- Heyboer M 3rd, Jennings S, Grant WD, Ojevwe C, Byrne J, Wojcik SM. Seizure incidence by treatment pressure in patients undergoing hyperbaric oxygen therapy. Undersea Hyperb Med. 2014;41:379–85. [PubMed] [Google Scholar]
- Hadanny A, Meir O, Bechor Y, Fishlev G, Bergan J, Efrati S. Seizures during hyperbaric oxygen therapy: retrospective analysis of 62,614 treatment sessions. Undersea Hyperb Med. 2016;43:21–8. [PubMed] [Google Scholar]
- Jokinen-Gordon H, Barry RC, Watson B, Covington DS. A retrospective analysis of adverse events in hyperbaric oxygen therapy (2012-2015): Lessons learned from 1.5 million treatments. Adv Skin Wound Care. 2017;30:125–9. doi: 10.1097/01.ASW.0000508712.86959.c9. [DOI] [PubMed] [Google Scholar]
- Sherlock S, Way M, Tabah A. Audit of practice in Australasian hyperbaric units on the incidence of central nervous system oxygen toxicity. Diving Hyperb Med. 2018;48:73–8. doi: 10.28920/dhm48.2.73-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton LH, Robinson MB. Repeated exposure to hyperbaric oxygen sensitizes rats to oxygen-induced seizures. Brain Res. 1993;632:143–9. doi: 10.1016/0006-8993(93)91149-M. [DOI] [PubMed] [Google Scholar]
- Chavko M, Xing G, Keyser DO. Increased sensitivity to seizures in repeated exposures to hyperbaric oxygen: role of NOS activation. Brain Res. 2001;900:227–33. doi: 10.1016/s0006-8993(01)02301-0. [DOI] [PubMed] [Google Scholar]
- Torbati D, Parolla D, Lavy S. Blood flow in rat brain during exposure to high oxygen pressure. Aviat Space Environ Med. 1978;49:963–7. [PubMed] [Google Scholar]
- Bergö GW, Tyssebotn I. Cerebral blood flow distribution during exposure to 5 bar oxygen in awake rats. Undersea Biomed Res. 1992;19:339–54. [PubMed] [Google Scholar]
- Mialon P, Gibey R, Bigot JC, Barthelemy L. Changes in striatal and cortical amino acid and ammonia levels of rat brain after one hyperbaric oxygen-induced seizure. Aviat Space Environ Med. 1992;63:287–91. [PubMed] [Google Scholar]
- Noda Y, McGeer PL, McGeer EG. Lipid peroxide distribution in brain and the effect of hyperbaric oxygen. J Neurochem. 1983;40:1329–32. doi: 10.1152/jappl.1990.69.5.1761. [DOI] [PubMed] [Google Scholar]
- Piantadosi CA, Tatro LG. Regional H2O2 concentration in rat brain after hyperoxic convulsions. J Appl Physiol. 1990;69:1761–6. doi: 10.1152/jappl.1990.69.5.1761. [DOI] [PubMed] [Google Scholar]
- Torley LW, Weiss HS. Effect of age and magnesium ions on oxygen toxicity in the neonate chicken. Undersea Biomed Res. 1975;2:223–7. [PubMed] [Google Scholar]
- Wood JD. Seizures induced by hyperbaric oxygen and cerebral gamma-aminobutyric acid in chicks during development. J Neurochem. 1970;17:573–9. doi: 10.1111/j.1471-4159.1970.tb00536.x. [DOI] [PubMed] [Google Scholar]
- Wood JD, Radomski MW, Watson WJ. A study of possible biochemical mechanisms involved in hyperbaric oxygen-induced changes in cerebral gamma-aminobutyric acid levels and accompanying seizures. Can J Biochem. 1971;49:543–7. doi: 10.1139/o71-081. [DOI] [PubMed] [Google Scholar]
- Troy SS, Ford DH. Hormonal protection of rats breathing oxygen at high pressure. Acta Neurol Scand. 1972;48:231–42. doi: 10.1111/j.1600-0404.1972.tb07544.x. [DOI] [PubMed] [Google Scholar]
- Pinkhasov RM, Wong J, Kashanian J, Lee M, Samadi DB, Pinkhasov MM, et al. Are men shortchanged on health? Perspective on health care utilization and health risk behaviour in men and women in the United States. Int J Clin Pract. 2010;64:475–87. doi: 10.1111/j.1742-1241.2009.02290.x. [DOI] [PubMed] [Google Scholar]
- Vaidya V, Partha G, Karmarkar M. Gender differences in utilization of preventive care services in the United States. J Womens Health (Larchmt). 2012;21:140–5. doi: 10.1089/jwh.2011.2876. [DOI] [PubMed] [Google Scholar]
- Seidel R, Carroll C, Diem RG, Yeboah K, Hayes AJ, et al. Risk factors for oxygen toxicity seizures in hyperbaric oxygen therapy: case reports from multiple institutions. Undersea Hyperb Med. 2013;40:515–9. [PubMed] [Google Scholar]
- Heyboer M, Sharma D, Santiago W, McCulloch N. Hyperbaric oxygen therapy: Side effects defined and quantified. Adv Wound Care (New Rochelle) 2017;6:210–24. doi: 10.1089/wound.2016.0718. [DOI] [PMC free article] [PubMed] [Google Scholar]