Abstract
Introduction
Delayed wound healing indicates wounds that have failed to respond to more than 4–6 weeks of comprehensive wound care. Wounds with delayed healing are a major source of morbidity and a major cost to hospital and community healthcare providers. Hyperbaric oxygen therapy (HBOT) is a treatment designed to increase the supply of oxygen to wounds and has been applied to a variety of wound types. This article reviews the place of HBOT in the treatment of non-healing vasculitic, calcific uremic arteriolopathy (CUA), livedoid vasculopathy (LV), pyoderma gangrenosum (PG) ulcers.
Methods
We searched electronic databases for research and review studies focused on HBOT for the treatment of delayed healing ulcers with rare etiologies. We excluded HBOT for ulcers reviewed elsewhere.
Results
We included a total of three case series and four case reports including 63 participants. Most were related to severe, non-healing ulcers in patients with vasculitis, CUA, LV, and PG. There was some evidence that HBOT may improve the healing rate of wounds by increasing nitric oxide (NO) levels and the number of endothelial progenitor cells in the wounds. HBOT may also improve pain in these ulcers.
Conclusion
We recommend the establishment of comprehensive and detailed wound care registries to rapidly collect prospective data on the use of HBOT for these problem wounds. There is a strong case for appropriately powered, multi-centre randomized trials to establish the true efficacy and cost-effectiveness of HBOT especially for vasculitis ulcers that have not improved following immunosuppressive therapy.
Keywords: Calciphylaxis, Economics, Epidemiology, Hyperbaric medicine, Review article, Skin, Nitric oxide
Introduction
The definition of a ‘problem’ or ‘non-healing’ ulcer or wound varies considerably depending on the context and geography. For the purposes of the current review, we have used the following definitions.
Problem wound: any wound where there are one or more local complicating factors, such as exudate, infection and/or systemic comorbidities, such as diabetes or polypharmacy.
Delayed wound healing: applies to any wound that fails to respond to 4–6 weeks of comprehensive wound care, does not heal or recurs.[ 1] This is often a consequence of being a problem wound where the complicating factors have been poorly appreciated. While wound healing does not need to be complete within 4–6 weeks, a wound healing trajectory should be established within that time frame.
Wounds are a major source of morbidity and a major cost to hospital and community healthcare providers. There appears to be a knowledge deficit on how to adequately manage problem wounds, given the low healing rates reported; for example, 50% of venous leg ulcers remaining unhealed after one year of treatment.[ 2]
Many problem wound types have been reviewed in the context of HBOT over the past ten years. The tenth European Consensus Conference on Hyperbaric Medicine in 2016 reviewed burns, compromised skin grafts and flaps, and diabetic foot ulcers (DFU).[ 3] The Cochrane Collaboration® have published reviews of acute surgical and traumatic wounds (2013) and chronic wounds due to diabetes, venous ulcers and arterial ulcers (2015).[ 4 , 5] These types of wounds have been excluded in the current review.
Review
EPIDEMIOLOGY
Chronic wounds constitute a significant health problem. They are common and reduce the quality of life of those affected. The true incidence, cost and health impact are difficult to assess accurately given the wide range of disease, the fact that much care is delivered at home and that many wound care products are purchased directly in some countries. Evidence on the total number of patients receiving wound treatment in a local population is limited. The prevalence of open wounds could be estimated as being from 120–320 per 100,000 (0.12–0.32%) in the western population.[ 6] Applying these rates to the population of Europe (in 2018)[ 7] suggests that between 615,000 and 1.64 million individuals have an open wound at any one time.
Epidemiologic studies show that up to 80% of leg ulcers have a vascular etiology (venous, peripheral arterial disease, or mixed).[ 8] Epidemiologic studies conducted in dedicated wound healing clinics have found that 6.6–23.0% (average 14.8) of ulcers are associated with autoimmune disease including vasculitis (Table 1), rheumatoid arthritis, scleroderma, systemic lupus erythematous, psoriasis, and pyoderma gangrenosum.[ 9 - 11] A study from the Mayo Clinic found that ulcers occurred at a rate of 1.8 leg ulcers per 100 person-years in a population of 813 rheumatoid arthritis patients followed for 9,771 person-years.[ 12] In that study, 6% of ulceration episodes ultimately required amputation and in the rheumatoid arthritis population, leg ulcers were associated with increased mortality (Hazard Ratio (HR) 2.42; 95% CI 1.71 to 3.42). Leg ulcers in this population were associated with age (HR 1.73 per 10-year increase; 95 % CI 1.47 to 2.04), rheumatoid factor positivity (HR 1.63; 95% CI 1.05, 2.53), presence of rheumatoid nodules (HR 2.14; 95% CI 1.39 to 3.31) and venous thromboembolism (HR 2.16; 95% CI 1.07 to 4.36).
Table 1. Prevalence, annual incidence and cost of wounds related to systemic connective tissue disorders (vasculitis) in Europe (28 member countries) .
| Adult population (2018) = 510,381,379[7] | ||
| Vasculitic wounds | ||
| Prevalence* (adult population) | 11.5 per 100,000 | 59,006 patients |
| Cost per wound** (mean) | €8,8505[52] | |
| Indicative annual cost (mean) | €522 million per year | |
| *The prevalence increases significantly with age (up to 60 per 100,000 women aged 75–79) | ||
| ** up to €13,800 per wound with a persistence time longer than two years | ||
Most wound care costs arise in the hospital sector: 27–30% of acute hospital beds are likely to be occupied on any day by patients with a wound.[ 6] With regard to surgical wounds with surgical site infections (SSIs), the standardized infection ratio is influenced by many factors including the type of operation and age. In Europe, in 2013–2016, the overall cumulative incidence of SSI was highest in open colonic surgery (10.4%, range 5.8–18.4) and lowest in knee prosthesis (0.5%, range 0.1–1.4). The incidence density was also highest in open colonic surgery (6 SSI per 1,000 post-operative patient days) and lowest in knee prosthesis (0.1 in-hospital SSI per 1,000 post-operative patient-days). It is important to note an increase of the percentage of SSIs in laminectomy operations in 2012–2015 (0.9%, range 0.2–2.4).[ 13] SSIs are twice as common in patients over 64 (RR 1.6; 95% CI 1.2 to 2.3).[ 14] An acute hospital performing 10,000 surgical procedures annually may have 300–400 surgical wounds with SSI at a cost of 3,300–4,400 excess bed-days or 1.74–2.32 million Euros.[ 15 , 16] Surveillance of surgical wounds with SSI and prevention are considered very important. Burns exert a catastrophic influence in terms of human life, suffering, disability, and financial loss. Burns are estimated to cause approximately 180,000 deaths annually worldwide, mostly in low- to middle-income countries. Burns accounted for the primary diagnosis in 424,000 visits to emergency departments in the United States in 2014, while in 2016 there were approximately 40,000 burn-related hospitalizations in the United States, 30,000 of which were at specialized burn centers. Work-related burns account for 20–25% of all serious burns.[ 17]
This review describes the delayed healing ulcers associated with rare etiologies such as autoimmune disease including vasculitis, calcific uremic arteriolopathy (CUA), livedoid vasculopathy (LV) and pyoderma gangrenosum (PG).
WOUND PATHOPHYSIOLOGY
Chronic wounds are often associated with poor perfusion and one near-universal characteristic of chronic wounds is that the wound tissues are pathologically hypoxic.[ 1 , 18 , 19] Normal wound healing proceeds through an orderly sequence of steps involving control of contamination and infection, resolution of inflammation, regeneration of the connective tissue matrix, angiogenesis and epithelialization. Several of these steps are critically dependent upon adequate perfusion and oxygen availability.
The result of this process is a sustained restoration of anatomical continuity and functional integrity. Problem wounds are those that have failed to proceed through this orderly sequence of events and have therefore failed to establish an anatomic and functional result. This failure of wound healing is usually the result of one or more local wound or systemic host factors inhibiting the normal tissue response to injury, including persistent infection, poor perfusion and hypoxia, cellular failure and unrelieved pressure or recurrent trauma.[ 18]
Not all non-healing wounds are hypoxic but pathological hypoxia is correlated with impaired wound healing and increased rates of wound infection.[ 19] Fibroblast replication, collagen deposition, angiogenesis, resistance to infection and intracellular leukocyte bacterial killing are oxygen sensitive responses essential to normal wound healing.[ 4] Steep oxygen gradients from the normally perfused wound edge to the hypoxic wound center may also play a role in stimulating normal wound healing.[ 20]
Pressure ulcers are localized injuries to the skin and/or underlying tissue, usually over a bony prominence, due to pressure or pressure in combination with shear forces. Common areas involved are over the sacrum, calcaneus and ischium. The superficial skin is less susceptible to pressure-induced damage than deeper tissues, and the external appearance may underestimate the extent of injury. Pressure ulcers are typically related to immobility but can also result from poorly fitting shoes, casts or other medical equipment.[ 21]
STANDARD MANAGEMENT OPTIONS
Many factors are associated with chronic ulceration and a multidisciplinary approach is required in the assessment of these patients. The goals are to ascertain the pathogenesis, make a definitive diagnosis and choose the optimal treatments to achieve healing within a given time. A correct diagnosis is essential to avoid inappropriate treatment that may cause deterioration of the wound, delay in wound healing, or harm to the patient.[ 1] The primary determinant of specific management strategies is the basic etiology of the wound in question.
The general principle in treating problem wounds is to simultaneously address the underlying pathology and institute both systemic and local treatments designed to improve the local wound environment. A wide range of therapies are available, including pressure-relieving mattresses, negative pressure wound therapy, growth factor therapies and tissue-engineered dermal substitutes.[ 22] In practice, wound management is often a sequential search for a successful combined approach. HBOT should be one element of such a combined approach.
RATIONALE FOR HBOT USE
HBOT involves breathing 100% oxygen in a compression chamber and is designed to increase the serum partial pressure of oxygen and oxygen diffusion into the target tissue. HBOT is increasingly used as an adjunctive treatment in many problem wounds. Regardless of the primary etiology, a common contributor to delayed healing is hypoperfusion. Evidence exists to demonstrate that intermittent oxygenation of these wounds, achievable only during HBOT exposure, can encourage normalization of wound healing processes and hasten the healing trajectory.[ 23 , 24]
HBOT produces an increase in plasma oxygen content directly proportional to the increase in alveolar oxygen tension in accordance with Henry’s Law. This greatly increases the effective diffusion distance for oxygen down a steep pressure gradient, making more oxygen available for cellular metabolism. The availability of oxygen is an important rate-limiting factor for several aspects of wound healing.
Local hypoperfusion and hypoxia may also be the result of an interaction between vascular endothelium damaged by trauma, infection or hypoxia and circulating leucocytes. Neutrophils are activated by damaged endothelium and chemo-attraction results in microvascular plugging and hypoperfusion. In a series of elegant experiments, Thom has clearly demonstrated that HBOT (and not normobaric oxygen) inhibits the adherence and sequestration of neutrophils by inhibiting β2 integrin function while also inducing antioxidant enzymes and anti-inflammatory proteins.[ 25 , 26]
Neutrophils, fibroblasts and macrophages are all dependent upon oxygen to achieve specific inflammatory or repair functions. Both improved leukocyte-mediated bacterial killing and antibiotic potentiation have been demonstrated.[ 23 , 24] In addition, high oxygen tensions inhibit the production of a range of bacterial toxins, allowing increased host resistance to infection.[ 23] Hyperoxia increases synthesis of reactive species derived from inducible Nitric Oxide Synthase (iNOS) and myeloperoxidase, leading to excessive S-nitrosylation of cytoskeletal β actin.[ 25]
Importantly, HBOT does not reduce neutrophil viability and functions such as degranulation and phagocytosis remain intact.[ 26] A separate anti-inflammatory pathway for HBOT involves impaired pro-inflammatory cytokine production by monocyte-macrophages and has been shown in animal models and humans.[ 27] This may be the basis for reduced levels of circulating pro-inflammatory cytokines under stress conditions.[ 28] The molecular mechanism is unknown, but could be related to HBOT-mediated enhancement of haemeoxygenase-1 and heat shock proteins (e.g. HSP 70).[ 29]
Control of healing has been described as taking place via 'waves' of reactive oxygen species (ROS), lactate and nitric oxide (NO) production. In chronic, non-healing wounds HBOT has been shown to produce a persistent increase in NO in wound fluid associated with increased granulation tissue formation and wound closure.[ 30] ROS appear to be among the most important signals that control the healing process. Oxidative stress plays a positive role during angiogenesis and involves hypoxia-inducible factor (HIF) and vascular endothelial growth factor (VEGF) signaling. The full picture is emerging, but recent studies have identified several pathways that are VEGF-independent.[ 31]
As well as the above, HBOT has been associated with a suite of other effects that may play an important role in stimulating wound healing. HBOT and lipoic acid supplementation can downregulate an existing chronic inflammatory state, changing the protease/anti-protease levels within the wound microenvironment. A concomitant decrease in matrix metalloproteinase-9 expression, together with increased levels of platelet derived growth factor contribute significantly to acceleration of the dermal wound repair process.[ 32]
HBOT also produces a transient increase in basic fibroblast growth factor production by fibroblasts and may inhibits transforming growth factor beta-1 production. Daily HBOT (202.6 kPa, 2 atmospheres absolute pressure (atm abs)) selectively stimulates fibroblast proliferation after seven days. Lower or higher levels of HBOT do not appear to have this effect.[ 33]
Finally, HBOT augments the release of stem/progenitor Cell (SPCs) from bone marrow through a NO dependent mechanism associated with the wound repair process.[ 34] The population of CD34 haematopoietic progenitor cells in peripheral circulation doubled in response to a single HBOT exposure at 202.6 kPa and increased eight-fold over the course of 20 treatments.[ 35] The net result of these mechanisms is improved local host immune response, clearance of infection, enhanced tissue growth, angiogenesis and a positive healing trajectory.
EVIDENCE REVIEW OF HBOT USE
Our review of HBOT in ulcers of rare etiology assessed the evidence from January 2002 to March 2016 and was originally undertaken for the tenth ECHM European Consensus Conference on Hyperbaric Medicine in Lille (France).[ 3] We undertook a Medline search using appropriate MeSH terms finding a total of 284, but after elimination of those investigating wound etiologies reviewed elsewhere, studies not relevant to HBOT, reviews or guidelines and duplicates, we were left with four case series and three case reports.[ 36 - 43] These include differing treatment of 63 individuals with non-healing vasculitic ulcers that had not improved following immunosuppressive therapy, in patients with CUA, LV, and PG[ 36 - 43] (see Figure 1).
Figure 1.
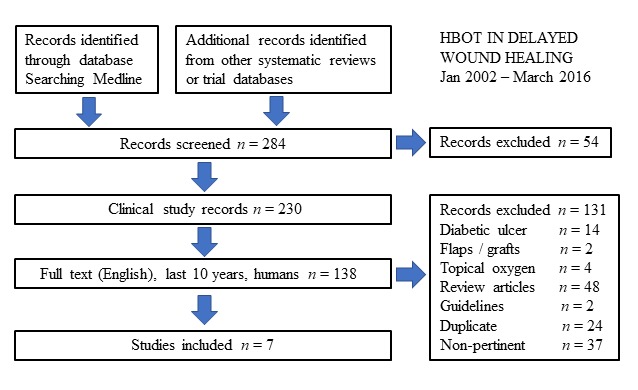
Flow diagram. Literature analysis, between January 2002 and March 2016, for Hyperbaric Oxygen Therapy in the treatment of refractory wounds of rare etiology. Seven publications have been included with different treatment of 63 individuals from three rare etiologies for chronic wounds: non-healing vasculitic ulcers that had not improved following immunosuppressive therapy, patients with calcific uremic arteriolopathy (CUA), livedoid vasculopathy (LV) and pyoderma gangrenosum (PG)
In general, positive responses were reported with the use of HBOT. In the first case series 11 patients with end-stage renal disease and dialyzed for an average of 163 (SD 84) months had treatment for distal ulcers with an average of 40 sessions at 2.5 atm abs (for details see Table 2). Two patients could not be evaluated (one patient interrupted HBOT after 10 sessions, one patient died due to ventricular arrhythmia after eight sessions). Eight completely healed and survived to 1-year follow up, with no recurrence of skin lesions. One deteriorated leading to foot amputation.[ 36] In the second case series reporting 35 patients with severe, non-healing, vasculitic wounds not improved following immunosuppressive therapy, 20 sessions of HBOT at 2 atm abs were associated with complete healing in 28 (78%), partial healing in four (11.4%), no improvement in 3 (8.6%).[ 37] In the third case series, an average of 28 sessions of HBOT at 2 atm abs was associated with healing in 11 of 12 patients with wounds due to CUA in end-stage renal failure.[ 38 , 39]
Table 2. Literature published January 2002 to March 2016, for HBOT in refractory wounds of rare etiology. This review does not include the wounds already considered in the tenth European Consensus Conference on Hyperbaric Medicine in 2016 (burns, compromised skin grafts and flaps, diabetic foot ulcers),[ 3] or analyzed by The Cochrane Collaboration® reviews on acute surgical and traumatic wounds (2013) and chronic wounds due to diabetes, venous ulcers and arterial ulcers (2015).[ 4 , 5]) .
| Non-healing vasculitic ulcers that had not improved following immunosuppressive therapy | ||||||
| Ref number | n | Outcome measure | Patients | HBOT protocol | Results | Comment |
| 37 | 35 | Healing | Aged ≥ 18 years with severe, vasculitis-induced ulcers | 202.6 kPa (2 atm abs), 90 min, 20 sessions, 5 times per week | Complete healing 28 (80%), partial healing 4 (11.4%), no improvement 3 (8.6%) | Favours HBOT Moderate |
| 42 | 1 | Healing | 14-year-old girl with refractory vasculitic ulcer (systemic lupus) of the toe over three months | 263.4 kPa (2.6 atm abs), 90 min, 16 sessions | Ulcer healed after 16 sessions | Favours HBOT |
| Patients with calcific uremic arteriolopathy (CUA), livedoid vasculopathy (LV) and pyoderma gangrenosum (PG) | ||||||
| 40 | 1 | Healing | 43-year-old male with CUA 2 years after kidney transplant. Despite intensive standard treatment his wounds progressed. Treated with iloprost + HBOT+ skin substitute | 253.3 kPa (2.5 atm abs), 90 min, 19 sessions | Complete healing 7 months after finishing treatment. Remained healed during the 4-year follow-up | Favours HBOT combined with iloprost and autologous cultured skin substitutes |
| 36 | 11 | Healing | Patients with end-stage renal disease (dialysis for mean 163 months) and distal ulcers | 253.3 kPa (2.5 atm abs), 90 min, average 40 sessions (range: 20–108). | Two did not finish treatment. Eight completely healed with no recurrence at 1 year. One deteriorated (foot amputation) | Favours HBOT Moderate |
| 41 | 2 | Healing, Pain, QoL | LV with recurrent multiple non-healing ulcers involving feet and ankles and severe pain | 253.3 kPa (2.5 atm abs), 60 minutes, 10 and 17 daily sessions for the 2 patients, 6 days a week. | Healing of the ulcers, pain relief | Favours HBOT |
| 38, 39 | 12 | Healing | Patients with CUA and skin ulcers, in end-stage renal disease | 202.6 kPa (2 atm abs), 130 minutes. Average 28 sessions (range: 7–41) | 11/12 demonstrated healing of wounds over an average of 6 weeks. The average duration of survival following successful treatment was 25.5 months (range 1.5–82) | Favours HBOT |
| 43 | 1 | Healing, pain, QoL | 15-year-old female with PG; multiple ulcers in inguinal and suprapubic region, and right upper limb | 253.3 kPa (2.5 atm abs), 90 minutes, 10 daily sessions | Complete healing | Favours HBOT |
In the first case report, in a 43-year-old patient presented with deteriorating CUA two years after kidney transplantation. The authors started iloprost (a synthetic analogue of prostacyclin PGI2 used as a vasodilator) and HBOT at 2.5 atm abs for a total of 19 sessions. The wounds became clean and there was no further necrosis. Transcutaneous oxygen pressure (PtcO2) around the wounds improved from 18 to 25 mmHg. Two large wounds were then covered with cultivated autologous skin cells (keratinocytes and fibroblasts) to further enhance epithelialization. Seven months later the wounds were healed and remained so the four-year follow-up period. The authors recommended robust trials in a larger sample of patients to verify these findings.[ 40]
In the second case report, two patients with LV were treated with HBOT at 2.5 atm abs for 10 and 17 daily sessions respectively with wound healing and pain relief for recurrent multiple non-healing ulcers involving the feet and ankles.[ 41]
In the third case report a refractory vasculitic toe wound, in a 14-year-old girl suffering from systemic lupus erythematosus (SLE), was treated with HBOT at 2.6 atm abs for 16 sessions with subsequent healing of that wound.[ 42]
Finally, in the fourth case report a 15 year-old girl with PG affecting the inguinal and suprapubic region and the right upper limb was treated with HBOT at 2.5 atm abs for 10 sessions with complete healing and relief of pain.[ 43]
While we have made every effort to locate further unpublished data, it is very likely this review is subject to a positive publication bias, with generally favorable cases more likely to be reported.
PATIENT SELECTION AND TREATMENT PROTOCOLS FOR HBOT
Assessment
HBOT is unlikely to accelerate tissue repair in wounds with normal oxygen tensions and it is essential to demonstrate reversible tissue hypoxia in the absence of surgically improvable arterial or venous disease before considering the use of HBOT.[ 44 , 45]
Transcutaneous oximetry (TCOM) is a simple non-invasive diagnostic technique that provides an objective assessment of local tissue perfusion and oxygenation. It can be used for serial assessment of the soft tissue envelope surrounding the problem wound. The role of TCOM in predicting wound healing remains a work in progress and the poor quality of the available clinical studies limits the interpretations of the available evidence.[ 46 , 47] Almost all data is obtained from patients with DFUs and the applicability to other wounds has not yet been systematically assessed. Currently, TCOM is a tool that will help predict if hypoxia is a factor contributing to poor wound healing in diabetic ulcers. In principle, a low transcutaneous oxygen (PtcO2 < 40 mmHg) while breathing air must show an adequate response to either or both of normobaric oxygen and HBOT administration.[ 48] The thresholds for what constitutes an adequate response is a matter of some controversy. While low PtcO2 values breathing air confirm wound hypoxia, they do not predict outcome with HBOT. A PtcO2 > 200 mmHg breathing HBO (253.3 kPa or 2.5 atm abs) is the best single discriminator between success and failure of HBOT (74% reliable).[ 48] Clinical practice guidelines are provided to use the available data to assist in identifying which patients will not heal spontaneously.[ 46]
CURRENT PROTOCOLS
The evidence to support HBOT is clearly very sparse for the target wounds in this review. No robust dose-finding trials exist for HBOT, therefore many different protocols are used for delivery of oxygen in clinical practice. Hammarlund and Sundberg used a treatment session of 243.1 kPa (or 2.4 atm abs) for 90 minutes, five days a week to a total of 30 sessions over six weeks.[ 49] With some minor variations, this is a common schedule for chronic wound management, although in some countries a schedule employing 202.6 kPa (or 2 atm abs) pressure is more common.
HBOT is associated with some risk of adverse effects including barotrauma to ears, sinuses and lungs, temporary worsening of short-sightedness, claustrophobia and oxygen toxicity.[ 5] Although serious adverse events are rare, HBOT cannot be regarded as an entirely benign intervention.
COST IMPACT OF HBOT
The cost of wound care has been estimated at 2.5–3.9 million € per 100,000 individuals.6 A typical course of treatment with standard wound care for six to eight weeks may cost €1,744 (USD 2,000) per week, with an estimated €10,463 to €13,951 (USD 12,000–16,000) for dressings alone; €1,308 (USD 1,500) in materials and supplies, €436 (USD 500) in fees to the hospital or wound care center. Among patients with partial foot amputation wounds up to the trans-metatarsal level with evidence of adequate perfusion, fulfilling 8 weeks of treatment, the average weekly total cost for patient treated by negative pressure wound therapy is €2,994 (USD 3,338) compared to €4,353 (USD 4,853) for standard moist wound therapy.[ 50]
Our best estimate is that approximately 11.5 wounds per 100 thousand inhabitants are related to rare etiology such as systemic connective tissue disorders (vasculitis) and CUA.[ 9 - 11] This suggests that in Europe’s 28 member countries (2018), there is an average of 59,000 patients at any one time suffering from a vasculitis-related wound and the corresponding costs for these wounds are likely to be €522 million per year.[ 9 , 12 , 51] Of these wounds, 31.3% have a healing time of 12 months or longer, and over 24 months for 16.4%.[ 52] The average cost per wound (healing time average cost) for a patient with 2 or more comorbidities is €3,900. The longer the wound healing time, the higher the treatment cost, that is up to the average cost of €13,800 per wound with a healing time of 24 months or longer.[ 52] Assuming that HBOT maintains the wound healing trajectory within average times (15 weeks, 107 days; SD 150), based on the Fife 2012 data, we can claim that HBOT could lead to an average healing cost saving of €9,900 (€13,800–€3,900) per each refractory wound of rare etiology treated by HBOT as part of a multidisciplinary treatment plan.
The high cost of wound care has stimulated the development of combined care models designed to diagnose and treat such wounds efficiently in the primary care and outpatient setting, with care escalating to inpatient when required. HBOT administered in an outpatient hyperbaric and wound care center may significantly reduce the number and cost of inappropriate admissions.[ 51]
Caring for a patient with chronic ulceration is complex and necessitates multidisciplinary collaboration to achieve the goal of providing comprehensive wound care. The combined use of HBOT with other advanced wound healing modalities may be a useful synergy in the armamentarium of wound healing. Niezgoda reported improvements in treatment of compromised post-surgical and arterial wounds. The combination of negative pressure wound therapy and HBOT produced results better than when either was used alone.[ 53]
Unfortunately, there is little direct evidence on the cost-effectiveness of HBOT in the treatment of acute and chronic wounds.[ 54] Although there is some evidence suggesting effectiveness, none of the studies in this review measured utilities or expressed their health outcomes as QALYs.
The lack of available evidence on economic endpoints is striking, given the fact that HBOT is widely applied in the problem wounds settings and is reimbursed by insurance companies in Europe and the USA for the treatment of chronic wounds.[ 55] Further studies should include economic outcomes in large clinical studies of strong methodological quality to make recommendations on the cost-effectiveness of applying HBOT in wound care.
While the gold standard for evidence will remain large, well-constructed RCTs, there is an important supportive role for epidemiological studies and clinical registries to confirm and quantify benefit.[ 52] To this end, we believe the initiation of a European register of wounds would be a valuable advance in improving our knowledge about in general wound management and the use of HBOT in particular.
Conclusions
Based on the case series and case reports available, from January 2002 to March 2016, HBOT may be effective in ulcers exhibiting delayed healing and associated with rare etiologies such as vasculitis, CUA, LV and PG. Assessment for HBOT should be recommended for problem wounds, especially those that have failed to respond to more than four weeks of comprehensive wound care or when healing is not predicted within four to six weeks of standard of care.
In clinical practice, physicians should set clear clinical targets and rigorously measure patient-based outcomes to better understand the impact of adding HBOT to any established wound care regimen. In assessing patients for treatment, physicians should provide documentation of vascular screening and evidence that appropriate action has been taken to optimize vascular flow prior to the institution of HBOT. TCOM is a useful tool to predict whether hypoxia is contributory to delayed wound healing.
Trials of high methodological rigor are required to properly establish the place of HBOT in the therapeutic pathways of these wounds. These trials should include common agreed HBOT protocols and a robust cost-utility analysis. While waiting for the completion of appropriate RCTs, we recommend the establishment of comprehensive wound registries to improve clinical practice.
Footnotes
Acknowledgements
The authors wish to thank participating investigators: Elisa Casadei and Ilenia De Cesero (Centro iperbarico – Hyperbaric and Wound Care Centre, Ravenna, Italy) who participated in the writing of the manuscript; Marta Milandri (Unit of Organizational Development, Training and Evaluation of AUSL Romagna; clinician librarian of the hospitals of Cesena, Forlì and Rimini, Italy) who collected data; Gladiol Zenunaj MD (consultant in vascular surgery of the Unit of Vascular and Endovascular Surgery, University Hospital of Ferrara, Italy, and Vice Director, Professor Vincenzo Gasbarro) for the collected data.
Conflicts of interest and funding: nil
Contributor Information
Pasquale Longobardi, Centro Iperbarico (Hyperbaric Medicine and Wound Care Centre), Ravenna, Italy.
Klarida Hoxha, Centro Iperbarico (Hyperbaric Medicine and Wound Care Centre), Ravenna, Italy.
Michael H Bennett, Department of Anaesthesia, University of New South Wales, Sydney, Australia.
References
- Agale SV. Chronic leg ulcers: epidemiology, aetiopathogenesis, and management . Ulcers. 2013:1–9. doi: 10.1155/2013/413604. [DOI] [Google Scholar]
- Scottish Intercollegiate Guidelines Network (SIGN) . SIGN Guideline 120: Management of chronic venous leg ulcers [Internet]. Available from: https://www.sign.ac.uk/assets/sign120.pdf [cited 2019 March 10].
- Mathieu D, Marroni A, Kot J. Tenth European consensus conference on hyperbaric medicine: recommendations for accepted and non-accepted clinical indications and practice of hyperbaric oxygen treatment . Diving Hyperb Med. 2017;47:131–32. doi: 10.28920/dhm47.2.131-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskes A, Vermeulen H, Lucas C, Ubbink DT. Hyperbaric oxygen therapy for treating acute surgical and traumatic wounds . Cochrane Database Syst Rev. 2013;(12):CD008059. doi: 10.1002/14651858.CD008059.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranke P, Bennett MH, Martyn-St James M, Schnabel A, Debus SE, Weibel S. Hyperbaric oxygen therapy for chronic wounds . Cochrane Database Syst Rev. 2015;(6):CD004123. doi: 10.1002/14651858.CD004123.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posnett J, Gottrup F, Lundgren H, Saal G. The resource impact of wounds on health-care providers in Europe . J Wound Care. 2009;18:154–61. doi: 10.12968/jowc.2009.18.4.41607. [DOI] [PubMed] [Google Scholar]
- Eurostat . Avaiable from: http://appsso.eurostat.ec.europa.eu/nui/show.do?dataset=demo_gind&lang=en. [cited 2019 March 06].
- Körber A, Klode J, Al-Benna S, Wax C, Schadendorf D, Steinstraesser L, et al. Etiology of chronic leg ulcers in 31,619 patients in Germany analyzed by an expert survey . Dtsch Dermatol Ges. 2011;9:116–21. doi: 10.1111/j.1610-0387.2010.07535.x. [DOI] [PubMed] [Google Scholar]
- Shanmugam VK, Schilling A, Germinario A, Mete M, Kim P, Steinberg J, et al. Prevalence of immune disease in patients with wounds presenting to a tertiary wound healing centre . Int Wound J. 2012;9:403–11. doi: 10.1111/j.1742-481X.2011.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bruin A, Voorrips L, Ploemacher J, Van Gool C Poos R, Gommer M. Netherlands pilot project on morbidity statistics. (Eurostat Grant #:10501.2009.004-2009.513). The Hague/Bilthoven: Statistics Netherlands and National Institute for Public Health and the Environment; 2011. Available from: https://www.cbs.nl/en-gb/background/2012/11/netherlands-pilot-project-on-morbidity-statistics. [cited 2019 March 10]. [Google Scholar]
- Italian Association of Skin Ulcers (AIUC) . I.S.U.S. Project. Italian Skin Ulcer Study (1 January 2015–31 December 2016). Final data. Italian Journal of Wound Care. 2017. Available from: http://woundcarejournal.it/index.php/ijwc/article/view/1/1. [cited 2019 March 10]. [Google Scholar]
- Jebakumar AJ, Udayakumar PD, Crowson CS, Gabriel SE, Matteson EL. Occurrence and effect of lower extremity ulcer in rheumatoid arthritis – a population-based study. J Rheumatol. 2014;41:437–43. doi: 10.3899/jrheum.130392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Centre for Disease Prevention and Control (ECDC) . Surgical site infections. In: ECDC . Annual epidemiological report for 2016. Stockholm: ECDC; 2018. Available from: https://ecdc.europa.eu/en/publications-data/healthcare-associated-infections-surgical-site-infections-annual-0. [cited 2019 March 10]. [Google Scholar]
- Moro ML, Morsillo F, Tangenti M, Mongardi MC, Ragni P, Pirazzini MC, et al. Rates of surgical-site infection: an international comparison. ICN Regional Group. Infect Control Hosp Epidemiol. 2005;26:442–8. doi: 10.1086/502565. [DOI] [PubMed] [Google Scholar]
- Shepard J, Ward W, Milstone A, Carlson T, Frederick J, Hadhazy E, et al. Financial impact of surgical site infections on hospitals: the hospital management perspective. JAMA Surg. 2013;148:907–14. doi: 10.1001/jamasurg.2013.2246. [DOI] [PubMed] [Google Scholar]
- Defez C, Fabbro-Peray P, Cazaban M, Boudemaghe T, Sotto A, Daurès JP. Additional direct medical costs of nosocomial infections: an estimation from a cohort of patients in a French university hospital. J Hosp Infect. 2008;68:130–6. doi: 10.1016/j.jhin.2007.11.005. [DOI] [PubMed] [Google Scholar]
- American Burn Association . Burn incidence fact sheet. Available from: http://ameriburn.org/who-we-are/media/burn-incidence-fact-sheet/. [cited 2019 March 10].
- Mustoe T. Understanding chronic wounds: a unifying hypothesis on their pathogenesis and implications for therapy. Am J Surg. 2004;187(5A):65S–70S. doi: 10.1016/S0002-9610(03)00306-4. [DOI] [PubMed] [Google Scholar]
- Sen CK. Wound healing essentials: let there be oxygen . Wound Repair Regen. 2009;17:1–18. doi: 10.1111/j.1524-475X.2008.00436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf HW, Gibson JJ, Angeles AP, Constant JS, Feng JJ, Rollins MD, et al. Hyperoxia and angiogenesis. Wound Repair Regen. 2005;13:558–64. doi: 10.1111/j.1524-475X.2005.00078.x. [DOI] [PubMed] [Google Scholar]
- Cushing CA, Phillips LG. Evidence-based medicine: pressure sores . Plast Reconstr Surg. 2013;132:1720–32. doi: 10.1097/PRS.0b013e3182a808ba. [DOI] [PubMed] [Google Scholar]
- Zielins ER, Brett EA, Luan A, Hu MS, Walmsley GG, Paik K, et al. Emerging drugs for the treatment of wound healing. Expert Opin Emerg Drugs. 2015;20:235–46. doi: 10.1517/14728214.2015.1018176. [DOI] [PubMed] [Google Scholar]
- Hopf HW, Rollins MD. Wounds: an overview of the role of oxygen. Antioxid Redox Signal. 2007;9:1183–92. doi: 10.1089/ars.2007.1641. [DOI] [PubMed] [Google Scholar]
- Camporesi EM, Bosco G. Mechanisms of action of hyperbaric oxygen therapy. Undersea Hyperb Med. 2014;41:247–52. [PubMed] [Google Scholar]
- Thom SR, Bhopale VM, Mancini JD, Milovanova TN. Actin S-nitrosylation inhibits neutrophil beta-2 integrin function. J Biol Chem. 2008;283:10822–34. doi: 10.1074/jbc.M709200200. [DOI] [PubMed] [Google Scholar]
- Thom SR. Oxidative stress is fundamental to hyperbaric oxygen therapy. J Appl Physiol (1985). 2009;106:988–95. doi: 10.1152/japplphysiol.91004.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihara K, Ueno S, Sakoda M, Aikou T. Effects of hyperbaric oxygen exposure on experimental hepatic ischemia reperfusion injury: relationship between its timing and neutrophil sequestration. Liver Transpl. 2005;11:1574–80. doi: 10.1002/lt.20533. [DOI] [PubMed] [Google Scholar]
- Fildissis G, Venetsanou K, Myrianthefs P, Karatzas S, Zidianakis V, Baltopoulos G. Whole blood pro-inflammatory cytokines and adhesion molecules post-lipopolysaccharides exposure in hyperbaric conditions . Eur Cytokine Netw . 2004;15:217–21. [PubMed] [Google Scholar]
- Thom SR. Hyperbaric oxygen: its mechanisms and efficacy . Plast Reconstr Surg . 2011;127(Suppl 1):131S–141S. doi: 10.1097/PRS.0b013e3181fbe2bf. (Ger). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boykin JV, Baylis C. Hyperbaric oxygen therapy mediates increased nitric oxide production associated with wound healing: a preliminary study . Adv Skin Wound Care . 2007;20:382–8. doi: 10.1097/01.ASW.0000280198.81130d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YW, Byzova TV. Oxidative stress in angiogenesis and vascular disease . Blood . 2014;123:625–31. doi: 10.1182/blood-2013-09-512749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alleva R, Tomasetti M, Sartini D, Emanuelli M, Nasole E, Di Donato F, et al. α-Lipoic acid modulates extracellular matrix and angiogenesis gene expression in non-healing wounds treated with hyperbaric oxygen therapy . Mol Med. 2008;14:175–83. doi: 10.2119/2007-00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang TS, Gorti GK, Quan SY, Ho M, Koch RJ. Effect of hyperbaric oxygen on the growth factor profile of fibroblasts . Arch Facial Plast Surg. 2004;6:31–5. doi: 10.1001/archfaci.6.1.31. [DOI] [PubMed] [Google Scholar]
- Fosen KM, Thom SR. Hyperbaric oxygen, vasculogenic stem cells, and wound healing . Antioxid Redox Signal . 2014;21:1634–47. doi: 10.1089/ars.2014.5940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom SR, Bhopale VM, Velazquez OC, Goldstein LJ, Thom LH, Buerk DG. Stem cell mobilization by hyperbaric oxygen . Am J Physiol Heart Circ Physiol . 2006;290:H1378–86. doi: 10.1152/ajpheart.00888.2005. [DOI] [PubMed] [Google Scholar]
- Basile C, Montanaro A, Masi M, Pati G, De Maio P, Gismondi A. Hyperbaric oxygen therapy for calcific uremic arteriolopathy: a case series . J Nephrol . 2002;15:676–80. [PubMed] [Google Scholar]
- Efrati S, Bergan J, Fishlev G, Tishler M, Golik A, Gall N. Hyperbaric oxygen therapy for nonhealing vasculitic ulcers . Clin Exp Dermatol. 2007;32:12–7. doi: 10.1111/j.1365-2230.2006.02240.x. [DOI] [PubMed] [Google Scholar]
- Rogers NM, Chang SH, Teubner DJ, Coates PT. Hyperbaric oxygen as effective adjuvant therapy in the treatment of distal calcific uraemic arteriolopathy . NDT Plus. 2008;1:244–9. doi: 10.1093/ndtplus/sfn036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers NM, Coates PT. Calcific uremic arteriolopathy - the argument for hyperbaric oxygen and sodium thiosulfate . Semin Dial. 2010;23:38–42. doi: 10.1111/j.1525-139X.2009.00656.x. [DOI] [PubMed] [Google Scholar]
- Alikadic N, Kovac D, Krasna M, Lindic J, Sabovic M, Tomazic J, et al. Review of calciphylaxis and treatment of a severe case after kidney transplantation with iloprost in combination with hyperbaric oxygen and cultured autologous fibrin-based skin substitutes . Clin Transplant. 2009;23:968–74. doi: 10.1111/j.1399-0012.2009.01062.x. [DOI] [PubMed] [Google Scholar]
- Bhutani S, Verma R, Verghese G. Livedoid vasculopathy managed with hyperbaric oxygen therapy . Med J Armed Forces India. 2012;68:389–91. doi: 10.1016/j.mjafi.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivieri AN, Mellos A, Duilio C, Meglio M, Mauro A, Perrone L. Refractory vasculitic ulcer of the toe in an adolescent suffering from systemic lupus erythematosus treated successfully with hyperbaric oxygen therapy . Ital J Pediatr. 2010;36:72. doi: 10.1186/1824-7288-36-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira WA, Barbosa LR, Martin LM. Hyperbaric oxygen therapy as an adjuvant treatment for pyoderma gangrenosum . An Bras Dermatol. 2011;86:1193–6. doi: 10.1590/s0365-05962011000600022. [DOI] [PubMed] [Google Scholar]
- Undersea and Hyperbaric Medical Society . Arterial inefficiencies: enhancement of healing in selected problem wounds. In: Weaver L, editor. . Hyperbaric oxygen therapy indications. 13th ed. North Palm Beach (FL): Best Publishing; 2014. [Google Scholar]
- Goldman RJ. Hyperbaric oxygen therapy for wound healing and limb salvage: a systematic review . PMR. 2009;1:471–89. doi: 10.1016/j.pmrj.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Fife CE, Smart DR, Sheffield PJ, Hopf HW, Hawkins G, Clarke D. Transcutaneous oximetry in clinical practice: consensus statements from an expert panel based on evidence . Undersea Hyperb Med. 2009;36:43–53. [PubMed] [Google Scholar]
- Blake DF, Young DA, Brown LH. Retraction of three papers investigating transcutaneous oxygen tensions in healthy volunteers . Diving Hyperb Med. 2016;46:57. [PubMed] [Google Scholar]
- Smart DR, Bennett MH, Mitchell SJ. Transcutaneous oximetry, problem wounds and hyperbaric oxygen therapy . Diving Hyperb Med. 2006; 36: 72- 86. Available from: https://www.researchgate.net/profile/Michael_Bennett6/publication/223131847_Transcutaneous_oximetry_problem_wounds_and_hyperbaric_oxygen_therapy/links/0fcfd5101c3f188cd2000000/Transcutaneous-oximetry-problem-wounds-and-hyperbaric-oxygen-therapy.pdf. [cited 2019 March 10]. [Google Scholar]
- Hammarlund C, Sundberg T. Hyperbaric oxygen reduced size of chronic leg ulcers: a randomized double-blind study . Plast Reconstr Surg. 1994;93:829–33. [PubMed] [Google Scholar]
- Apelqvist J, Armstrong DG, Lavery LA, Boulton AJ. Resource utilization and economic costs of care based on a randomized trial of vacuum-assisted closure therapy in the treatment of diabetic foot wounds . Am J Surg. 2008;195:782–8. doi: 10.1016/j.amjsurg.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Longobardi P, Piccinini E, Poddie D, Caruso B, Laghi F, Aimola A, et al. Clinical and economic impact of an outpatient problem wound care centre . Acta Vulnologica. 2007; 5: 89- 103. Available from: https://www.minervamedica.it/en/journals/acta-vulnologica/article.php?cod=R45Y2007N03A0089. [cited 2019 March 10]. [Google Scholar]
- Fife CE, Carter MJ. Wound care outcomes and associated cost among patients treated in US outpatient wound centers: data from the US wound registry . Wounds. 2012;24:10–7. [PubMed] [Google Scholar]
- Niezgoda JA. Combining negative pressure wound therapy with other wound management modalities . Ostomy Wound Manage. 2005;51:36S–38S. [PubMed] [Google Scholar]
- Henry AJ, Hevelone ND, Belkin M, Nguyen LL. Socioeconomic and hospital-related predictors of amputation for critical limb ischemia . J Vasc Surg. 2011;53:330–9.e1. doi: 10.1016/j.jvs.2010.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkin L, Bućko Z, Conde Montero E, Cutting K, Moffatt C, Probst A, et al. Implementing TIMERS: the race against hard-to-heal wounds . J Wound Care. 2019;23(Sup3a):S1–S50. doi: 10.12968/jowc.2019.28.Sup3a.S1. [DOI] [PubMed] [Google Scholar]


