Abstract
Microbes often augment their metabolism by conditionally constructing proteinaceous organelles, known as bacterial microcompartments (BMCs), that encapsulate enzymes to degrade organic compounds or assimilate CO2. BMCs self-assemble and are spatially delimited by a semi-permeable shell made up of hexameric, trimeric, and pentameric shell proteins. Bioengineers aim to recapitulate the organization and efficiency of these complex biological architectures by redesigning the shell to incorporate non-native enzymes from biotechnologically relevant pathways. To meet this challenge, a diverse set of synthetic biology tools are required, including methods to manipulate the properties of the shell as well as target and organize cargo encapsulation. We designed and determined the crystal structure of a synthetic shell protein building block with an inverted sidedness of its N- and C-terminal residues relative to its natural counterpart; the inversion targets genetically fused protein cargo to the lumen of the shell. Moreover, the titer of fluorescent protein cargo encapsulated using this strategy is controllable using an inducible tetracycline promoter. These results expand the available set of building blocks for precision engineering of BMC-based nanoreactors and are compatible with orthogonal methods which will facilitate the installation and organization of multi-enzyme pathways.
Keywords: bacterial microcompartments, protein design, metabolic engineering, synthetic biology
1. Introduction
Many bacteria compartmentalize segments of their metabolism within self-assembling proteinaceous nanoscale structures known as bacterial microcompartments (BMCs).[1,2] A semi-permeable polyhedral shell composed of cyclic hexamers (BMC-H), pseudohexameric trimers (BMC-T), and pentamers (BMC-P), serves as both a scaffold to organize encapsulated enzymes as well as a physical boundary to reduce diffusive loss of volatile or toxic intermediates and restrict crosstalk with cytosolic pathways.[3–5] BMCs carry out a range of different metabolic reactions, including carbon fixation and the degradation of small carbon compounds (e.g. choline, 1,2-propanediol, ethanolamine). Many of the enzymes central to these anabolic and catabolic pathways are targeted to the BMC lumen via encapsulation peptides (EPs), typically located at their N- or C- termini, that interact non-covalently with the luminal surface of the shell.[6–9] However, formation of BMC shells is not dependent on the presence of EP-possessing enzymes; empty intact shells can assemble in the absence of native cargo when BMC-H, BMC-T, and BMC-P proteins are either expressed heterologously,[10–12] or combined in vitro.[13] The ability to construct empty shells provides the possibility to target non-native enzymes and ultimately construct new subcellular nanoreactors to augment the metabolism of a bacterial host.
Unfortunately, repurposing EPs from BMC-associated enzymes has been only modestly successful in directing non-native cargo to the lumen of heterologous shells, in part due to the propensity for EPs to promote aggregation.[14,15] An alternative strategy is the translational fusion of cargo directly to individual shell proteins. However, this approach requires both knowledge and control of the sidedness and composition of the BMC shell in order to facilitate cargo placement and loading titers. For example, in the recent atomic-level crystal structure of the empty 6.5 MDa BMC shell from Haliangium ochraceum (HO shell), the N-and C-termini of most shell proteins are located on the outside of the shell;[16] translational fusion of protein cargo to the termini of any of these building blocks would lead to display on the outer surface rather than encapsulation within the BMC lumen. Furthermore, as in other BMC shells,[17] BMC-H are the numerically dominant component of the HO shell and provide an attractive target for maximizing the number and/or distribution of encapsulated proteins if fusions with the individual BMC-H protomers could be directed toward the lumen.[16] Here we designed and report the structure of a circularly permuted version of the BMC-H protein (CPH) from the HO shell that oligomerizes into hexamers and has new N- and C-termini, relocated to its luminal face. CPH, along with BMC-T and BMC-P, assemble into shells similar in size and morphology to the unmodified HO shell. GFP fused to the C-terminus of CPH incorporates into the shell, is localized to the lumen, and its titer can be controlled using an inducible tetracycline promoter. Our rationally designed circularly permuted building block provides precise control over sidedness and quantitative cargo incorporation for the construction of new BMC nanoreactors.
2. Results and Discussion
2.1. Design and structure of a circularly permuted shell protein that assembles into shells
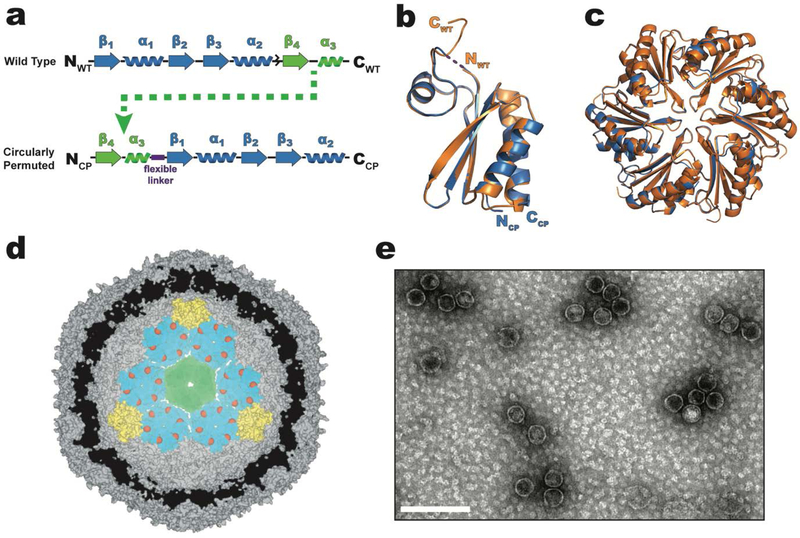
All BMC-H proteins contain a single BMC domain (pfam 00936) that consists of three alpha-helices, four beta-strands, and is ~100 amino acids in length.[18] Cyclic hexamers of BMC-H proteins form discoids; hexagons approximately 7 nm across and 2 nm thick with a distinct convex and concave face. The N- and C-termini of the wild type BMC-H (WTH) from the HO shell are located on the concave side of the hexamer and therefore on the outer surface of the shell.[16] The ~10 C-terminal residues of WTH appear to lack any secondary structure and, in the crystal structure (pdb 5djb), the distance between the N- and C-termini is only ~13 Å.[19] The potential flexibility of the C-terminal residues and the spatial proximity of the N- and C-termini suggest WTH is amenable to circular permutation, a rearrangement of secondary structure elements that, in this case, serves the purpose of creating new N- and C-termini on the luminal face of the hexamer. To circularly permute the WTH protein, its primary structure is first separated into two parts: residues Ala2-Gly68 (beta-strand 1 to alpha-helix 2) (Figure 1a, blue) and residues Glu69-Ala99 (beta-strand 4 to alpha-helix 3) (Figure 1a, green). Moving the latter segment to the start of the polypeptide and joining the original N- and C-termini (between alpha-helix 3 and beta-strand 1) with a flexible (Gly-Ser)3 linker introduces new N- and C-termini in a short loop region located on the convex side which faces the inside when incorporated in a shell (Figure 1a). The 1.6 Å x-ray crystal structure of the individual CPH protomer (pdb 6nlu, Table S1) has a rmsd of 0.2 Å when superimposed onto the alpha carbons of the unpermuted WTH structure (pdb 5djb); no electron density was observed for the majority of the (Gly-Ser)3 linker, indicating that it is flexible (Figure 1b). Moreover, CPH forms a cyclic hexamer like WTH (Figure 1c), suggesting it could assemble into shells with luminal-facing N- and C-termini if expressed in the presence of BMC-T and BMC-P proteins (Figure 1d). To test if CPH can substitute for WTH in the context of the HO shell, CPH shells were isolated using the rapid complementary affinity-based purification (CAP) technique recently developed for HO shells consisting of BMC-H (WTH), BMC-T1(T1) and strep-II-tagged BMC-P (PSII).[20] When CPH, T1, and PSII are co-expressed in E. coli, negative-stained transmission electron microscopy (TEM) shows intact shells with similar morphology and size (d = 37 ± 3 nm, n = 245) to the shell with the unpermuted BMC-H can be purified from the cell lysate using CAP (Figure 1e), indicating that CPH can structurally substitute for WTH.
Figure 1.
Design and structure of a circularly permuted CPH protein that substitutes for the WTH in intact HO shells. a) primary structure of WTH (top) and circularly permuted CPH (bottom). The flexible (Gly-Ser)3 linker used to join the original NFWT- and CWT- termini of WTH is represented as a purple box. b) Structural alignment of WTH (orange, pdb 5djb) and CPH (blue, pdb 6nlu) protomers. No electron density was observed for the Gly-Ser linker and is therefore drawn as a dashed line. c) Alignment of the hexameric assemblies of WTH (orange) and CPH (blue). View is top-down looking at the convex side of the hexamers. d) Cutaway model (derived from pdb 5v74) of a minimal shell made of CPH (teal), T1 (green), and PSII (yellow) proteins showing the predicted luminal orientation of the CPH C-termini (red spheres). e) TEM micrograph of purified CPH-T1-PSII shells. Scale bar is 100 nm.
In contrast, two reports of the circular permutation of PduA (CP-PduA), one of the predominant BMC-H proteins in the propanediol utilization (PDU) BMC,[17] had different structural and morphological outcomes despite using similar designs. In one report, the crystal structure of CP-PduA revealed a pentameric rather than hexameric oligomeric state that assembles into small (d ~ 13 nm) nanocages in the absence of other shell proteins.[21] In the second study, although no direct measurement of the oligomeric state was reported, CP-PduA was able to incorporate into PDU shells in E. coli, and when expressed alone, formed extended structures consistent with a hexameric state. However, because CP-PduA (or PduA) is not required for the formation of heterologous PDU shells due the presence of additional BMC-H homologs (i.e. PduJ, PduK, PduU), the final number of incorporated CP-PduA proteins remain unclear. Moreover, the incorporation of CP-PduA into PDU shells prevented their purification using standard protocols used for isolation of PDU shells.[22]
2.2. Incorporation of genetically fused cargo into CPH shells
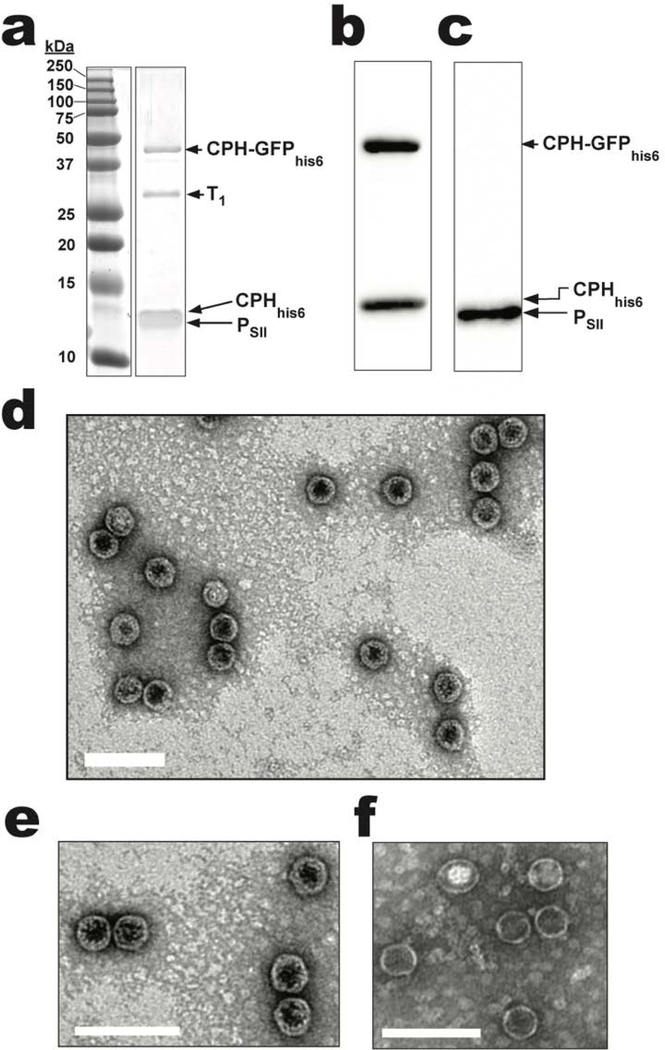
The ability to assemble and purify CPH-T1-PSII shells from E. coli enables screening for successful incorporation of genetically fused cargo by SDS-PAGE, western blot, and TEM analyses. As a proof-of-principle, a C-terminal hexahistidine (his6) tagged superfolder GFP[23] (GFP) fused to the C-terminus of CPH (CPH-GFPhis6) was co-expressed with a CPHhis6-T1- PSII shell (a hexahistidine tag was appended to the C-terminus of CPH (CPHhis6) to enable western blot detection). Retaining a copy of CPHhis6 and regulating expression of CPH-GFPhis6 from a separate plasmid prevents steric clashes that would arise from too many GFP molecules on the shell lumen; indeed, when only CPH-GFPhis6 is co-expressed with T1 and PSII, no shells are formed (data not shown). The presence of a ~40 kDa band on SDS-PAGE of purified CPHhis6-T1-PSII /CPH-GFPhis6 shells provides support for the incorporation of CPH-GFPhis6 (~38.4 kDa) (Figure 2a) and is further substantiated by the visibly green color of the sample. The presence of CPH-GFPhis6 as well as CPHhis6 and PSII are confirmed by western blotting using anti-his (Figure 2b) and anti-Strep (Figure 2c) antibodies. TEM analysis of this sample confirms the presence of shells with a diameter of 40 ± 3 nm (n = 130) (Figure 2d). In addition, a higher magnification view of the TEM micrograph of shells shows a thicker shell wall (Figure 2e) when compared to a similarly magnified view of the CPH-T1- PSII-shells shown in Figure 1e (Figure 2f). A thicker shell is consistent with the presence of GFPhis6 fused to some of the CPH. While it is possible that staining differences during TEM sample preparation could account for this increased shell thickness, similar morphological differences have previously been observed when fluorescent proteins are targeted to the lumen of the HO shell by covalent attachment to T1,[20] or non-covalent attachment to WTH.[13]
Figure 2.
CPH-GFPhis6 incorporation into CPHhis6-T1-PSII shells. a) SDS-PAGE analysis of purified CPHhis6-T1-PSII/CPH-GFPhis6 shells (see Figure S1 for the uncropped SDS-PAGE image). b) Anti-his or c) anti-strep western blots of purified shells from (a). d) TEM micrograph of purified CPHhis6-T1-PsII/CPH-GFPhis6 shells. e) Further magnified TEM micrograph of CPHhis6-T1-PSII/CPH-GFPhis6 shells. f) For comparison, magnified TEM micrograph of CPH-T1-PsII shells without GFP cargo from Figure 1e. Scale bars are 100 nm.
2.3. GFP cargo is targeted to the luminal face of intact CPH shells
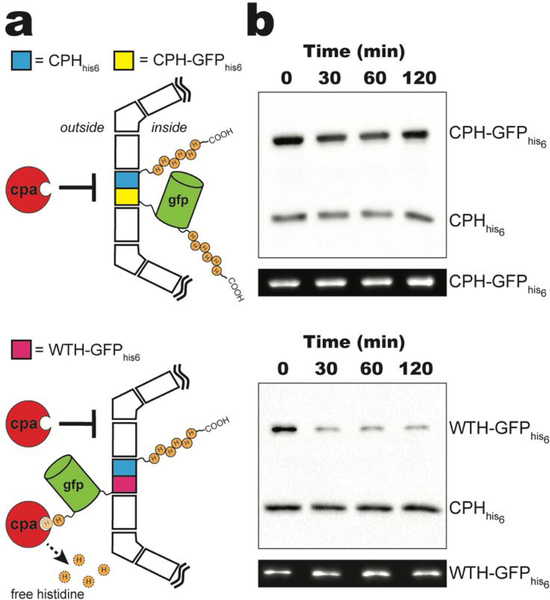
To confirm the orientation of the C-terminus of CPH, we probed the susceptibility of the C-terminal his-tags of CPH-GFPhis6 as well as CPHhis6 to proteolysis by bovine carboxypeptidase A (CPA). CPA catalyzes the removal of a broad range of C-terminal aromatic and aliphatic residues, including histidine.[24,25] The sidedness of the C-terminal his-tags can therefore be tracked by monitoring the anti-his detected western blot signals from samples following exposure to CPA. C-terminal his-tags located within the shell’s lumen are protected from proteolysis because the ~35 kDa CPA enzyme is too large to pass through the central pores of hexamer, trimer, or pentamer shell subunits; while a surface exposed his-tag is susceptible to proteolysis (Figure 3a). Throughout 120 minutes of CPA exposure, the majority of the anti-his western blot signals from both CPHhis6 and CPH-GFPhis6 appear to be retained, suggesting protection from proteolysis (Figure 3b, top). CPHhis6-T1-PSII shells incorporating WTH-GFPhis6 (Figure S2), which has an outward facing C-terminal his-tag, were used as a control. The western blot signal for WTH-GFPhis6 incorporated into CPHhis6-T1-PsII shells was almost completely lost after 30 minutes of exposure to CPA while the CPHhis6 signal remained relatively unchanged throughout the time course (Figure 3b, bottom). Interestingly, the WTH-GFPhis6 anti-his western blot signal is not completely lost even after 120 minutes of exposure to CPA. This fraction of WTH-GFPhis6 may represent a small population either stochastically encapsulated within the lumen or with sterically occluded C-termini due to their position on the surface of the shell. These results confirm that the we successfully generated a BMC-H that localizes translationally fused cargo on the luminal side of intact BMC shells.
Figure 3.
In vitro protease protection assay. a) Schematic of carboxypeptidase A (CPA) protection assay for purified CPHhis6-T1-PSII shells incorporating CPH-GFPhis6 (top) or WTH-GFPhis6 (bottom). For clarity, only one CPHhis6 (cyan) and CPH-GFPhis6 (yellow) or WTH-GFPhis6 (pink) is shown per shell. b) Western blot of a CPA time course of purified CPHhis6-T1-PSII shells incorporating CPH-GFPhis6 (top) or WTH-GFPhis6 (bottom) using anti-his antibodies. Representative in-blot intrinsic GFP fluorescence is shown below each western blot. See Figure S3 for uncropped fluorescence images.
2.4. Control and quantification of the number of encapsulated GFP molecules
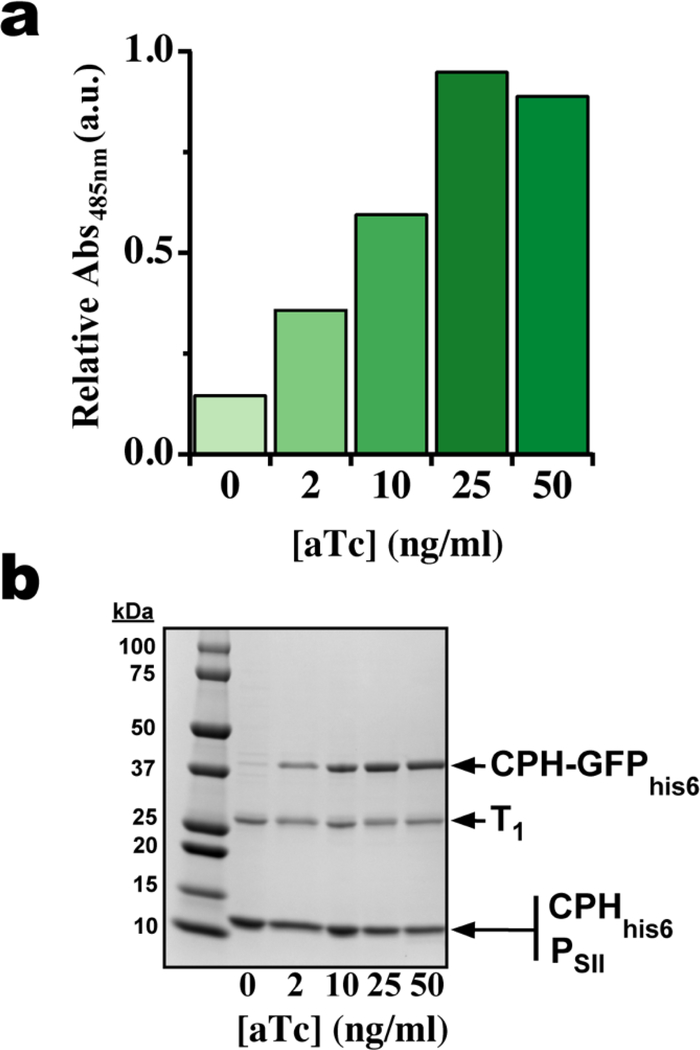
An advantage of engineering BMC-H proteins is that they constitute the majority of the surface, or potential scaffolding, of the shell; HO shells consist of 60 hexamers, 20 trimers and 12 pentamers.[16] Using our two-plasmid expression system we determined the maximum loading capacity of GFP molecules per hexamer and shell by titrating the induction level of CPH-GFPhis6 with anhydrotetracycline (aTc) while supplying a constant amount of IPTG to induce the CPHhis6-T1-PSII shell components. Absorption spectra of shells purified from E. coli cells induced with different amounts of aTc were normalized to total protein content (Figure S4) and the absorbance at 485 nm used to track relative GFP content. As the aTc concentration is raised from 0 to 25 ng Ml−1, a linear ~7-fold increase in absorbance is observed indicative of increasing GFP encapsulation. When the aTc concentration is raised further to 50 ng mL−1, a slight decrease in absorbance is observed suggesting that encapsulation is saturated between 25 and 50 ng mL−1 aTc (Figure 4a). A similar trend is also observed when the same purified shell samples are analyzed by SDS-PAGE (Figure 4b). Densitometry can be used to quantify the amount CPH-GFPhis6 incorporated in shells by measuring the ratio of intensity between CPH-GFPhis6 and T1 bands (assuming 20 trimers or 60 copies of T1 protein per shell). At the maximum GFP absorbance, the average ratio of CPH-GFPhis6 to T1 is ~1.3, or ~1.3 GFP molecules per CPH hexamer and ~80 CPH-GFPhis6 per shell (out of a total of 360 CPH chains). The length and flexibility of the linker between CPH and GFP may play an important role in determining the final titer of encapsulated cargo. In this case, the (Gly-Ser)3 linker connecting CPH and GFP may be long and flexible enough to sterically preclude the incorporation of more than two fusion proteins into an individual hexamer, or, alternatively, the integration of a hexamer with more than two CPH-GFP fusions into the shell; adapting this strategy for the encapsulation of cargo larger than GFP may therefore require a smaller and/or more rigid intervening linker. Targeting cargo to the BMC lumen using CPH-fusions also has the potential to be applied in conjunction with orthogonal encapsulation methods that use T1 as a scaffold. For example, a recently developed strategy for inserting a split adhesion domain, SpyTag or SpyCatcher,[26] within a luminal-facing loop of T1[20] could be implemented in a CPH shell. This would allow covalent attachment of one type of cargo to T1 using SpyTag/SpyCatcher as well as the incorporation and control over the relative titer of a second cargo directly fused to CPH. In addition, T1 and CPH are located right next to each other, making an ideal configuration for two enzymes that catalyze sequential reactions. The combination of these strategies would extend our ability to engineer more complex BMCs by offering control not only over localization and abundance but also the relative stoichiometry of encapsulated enzymes.
Figure 4.
CPH-GFPhis6 content of purified CPHhis6-T1-PSII shells isolated from cells induced with different concentrations of aTc. a) GFP absorption at 485 nm from shell samples normalized by total protein content at 280 nm, a darker color indicates greater relative GFP absorbance (See Figure S4 for full spectra). b) Coomassie-stained SDS-PAGE of the same samples in (a). 2.5 μg protein loaded in each lane.
2.5. Conclusions
Diversity of synthetic building blocks for construction and functionalization of BMCs is essential for realizing their metabolic potential as modular intracellular nanoreactors. Toward this goal, we designed and structurally characterized a circularly permuted BMC-H protein. CPH forms hexamers equivalent to its unpermuted form with the exception that the N- and C-termini of the individual protomers are reoriented toward the luminal face. Shells are efficiently formed in vivo by co-expressing CPH, T1, and PSII and purified similar to HO shells. In vitro protease protection assays confirm the luminal orientation of C-terminally fused GFP cargo, and by varying its induction level, the amount of CPH-GFPhis6 protein incorporated into purified CPHhis6-T1-PSII shells can be titrated over a range of about 10 to 80 cargo molecules per shell. The structurally defined assembly of BMC shells with an inverted orientation of hexamer terminal residues and titratable composition. The synthetic HO shells containinshells are selectively permeable, composed of trimers and Ps too, expands the tractable parameter space available to metabolic engineers seeking to design new subcellular compartmentalized metabolic pathways with a level of precision previously limited to containers based on a single protein such as virus-like particles[27] and lumazine synthase.[28]
3. Materials and Methods
3.1. Cloning and purification of CPH
The E. coli codon optimized CPH gene was synthesized (Integrated DNA technologies) and cloned into pET11b using Ndel and BamHI restriction sites (see Table S2 and S3 for a list of plasmids and amino acid sequences respectively). The resulting plasmid (pBF27) was transformed into E. coli BL21 (DE3) cells and induced with 0.4 mM IPTG at mid-exponential phase (OD600 nm = 0.6–0.8) and grown for 4 h at 37 °C. Cells were harvested by centrifugation and resuspended in buffer containing 0.05 M Tris, 0.1 M NaCl, and 0.01 M MgCl2 at a density of 0.5 g Ml−1 and stored at −20 C until lysis. CPH was purified following the protocol for WTH from H. ochraceum (locus tag HO_5815) using iterative centrifugation steps and triton washes as described in the literature.[19]
3.2. X-ray Crystallography
CPH at 10.6 mg mL−1 (as measured by BCA) in buffer containing 0.01 M Tris pH 7.8 and 0.05 M NaCl was crystallized by vapor diffusion against a solution of 1.0 M ammonium tartrate and 0.1 M sodium acetate pH 5.1 at a protein to precipitant ratio of 2 μL:2 μL. Hexagonal crystals were looped in drop solution containing 12.5% PEG400 as a cryoprotectant after four months and frozen in liquid nitrogen. Diffraction data were collected at ALS beam line 5.0.2, integrated with XDS[29] and scaled with SCALA (CCP4).[30] Molecular replacement with phenix.phaser[31] was used with a search model of the HO-BMC- H (HO_5815, pdb 5djb) to obtain phase information. The resulting model was refined and rebuilt using phenix.refine[31] and COOT.[32] Statistics for diffraction data collection, structure determination and refinement are summarized in Table S1.
3.3. Shell expression and purification
CPH-T1-PSII (pBF36) and CPHhis6-T1-PSII (pBF53) shells were expressed from IPTG-inducible pETDuet-based plasmids, and when indicated, coexpressed with CPH-GFPhis6 (pBF64) or WTH-GFPhis6 (pBF71) from a tetracycline-inducible Bglbrick pBbA2k vector.[33] 2 L of cells were grown to mid-exponential phase (OD600 nm = 0.6–0.8) at 37 C in lysogeny broth supplemented with 10 μg Ml−1 ampicillin (for pBF53) and 50 μg mL−1 kanamycin (when co-transformed with pBF64 or pBF71) and induced with 0.25 mM IPTG and 0, 2, 10, 25, or 50 ng mL−1 aTc, briefly cold-shocked on ice for 15 min, and then grown for 20–22 h at 18 C. Cells were harvested by centrifugation and resuspended in 0.05 M Tris pH 8.0, 0.2 M NaCl to a density of 0.5 g L−1 and stored at −20 C until lysis. Cells were lysed by French Press at 2×104 psi in the presence of SigmaFast protease inhibitor (Sigma Aldrich, St. Louis USA), 0.1 mg mL−1 lysozyme (Sigma Aldrich, St. Louis USA), and 1 mg mL−1 DNase (Sigma Aldrich, St. Louis USA). Lysates were clarified by centrifugation at 27,000 × g for 30 min at 4 C. Shells were isolated from clarified lysates using CAP[20] with the addition of an initial 30% sucrose cushion (as described in Sutter et al.[16]) to remove unincorporated PSII subunits prior to applying cell lysates onto the strep column. Eluates from the strep column were further polished with a strong anion exchange column using the method described in Sutter et al.[16] Anti-his and anti-strep western blots were carried out as described for the CPA assays with the exception that the anti-strep antibody (Millipore, Burlington USA) was applied at a titer of 1:4000.
3.4. Transmission Electron Microscopy
Purified shells were imaged by negative stained TEM on a JEOL 100CXII microscope operated at an accelerating voltage of 100 kV using a Gatan Orius SC200 CCD camera. Purified shells were diluted 10-fold in HPLC-grade water and 5 μL of each sample was applied to 150 mesh carbon-coated copper grids (Electron Microscopy Sciences, Hatfield USA) for 30 s, wicked dry, stained for 15 s with 1% uranyl acetate, and again wicked dry before imaging.
3.5. Carboxypeptidase A assay
0.09 U of bovine carboxypeptidase A (Sigma Aldrich, St. Louis USA) was added per μg of total protein in a master mix of purified shells in buffer containing 0.05 M Tris pH 8.0, 0.2 M NaCl and incubated at room temperature for 120 minutes. At each time point, an equivalent volume was removed from the master mix to which SDS-PAGE sample buffer was added. The sample was immediately boiled in water for 10 min, briefly centrifuged, and placed at −20 C until samples from all time points were collected. Gels were run with equivalent volumes loaded per lane, including a T = 0 control that was prepared before the addition of CPA to the master mix; buffer (0.05 M Tris pH 8.0, 0.2 M NaCl) was added to this sample to maintain the same final concentration of protein in the SDS-PAGE sample. Following SDS-PAGE, proteins were transferred onto a nitrocellulose membrane and blocked for 4 h at room temperature with a 5% (w/v) solution of dry milk in 0.02 M Tris pH 7.8, 0.15 M NaCl, 0.3% (v/v) TWEEN 20. Membranes were incubated with HRP-conjugated anti-his antibodies at a titer of 1:3333 (Invitrogen, Carlsbad USA) for 1 h and imaged using ECL-detection reagents (General Electric, Boston USA) and a Bio-rad Chemidoc-MP imager (Bio-rad, Hercules USA). intrinsic fluorescence from GFP was recorded using the same Bio-rad Chemidoc-MP imager using the built-in GFP excitation/emission filter set to confirm equal loading between wells.
Supplementary Material
Acknowledgements
We would like to thank Andrew Hagen for helpful discussions and creating and sharing WT HO shell constructs that were used to generate the constructs in this paper. We also thank Aiko Turmo for help collecting preliminary TEM data and the entire Kerfeld lab for insightful discussions.
Funding
This work was supported by the Office of Science of the US Department of Energy DE-FG02–91ER20021. CAK and MS also acknowledge support from the National Institutes of Health, National Institute of Allergy and Infectious Diseases (NIAID) grant 5 R01 AI114975–05. This work used resources of the Advanced Light Source which is supported by the Office of Science, Office of Basic Energy Sciences, of the U.S. DOE under Contract No. DE-AC02–05CH11231. The x-ray crystallographic coordinates and structure-factor files have been deposited in the Protein Data Bank (PDB) with the accession number: 6nlu.
Abbreviations
- BMC
bacterial microcompartment
- EP
encapsulation peptide
- CP
circularly permuted
- CPA
bovine carboxypeptidase-A
- HO-shell
Haliangium ochraceum BMC shell
4. References
- [1].Axen SD, Erbilgin O, Kerfeld CA, PLoS Comput Biol 2014, 10, e1003898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kerfeld CA, Aussignargues C, Zarzycki J, Cai F, Sutter M, Nat Rev Microbiol 2018, 16, 277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Brinsmade SR, Paldon T, Escalante-Semerena JC, J Bacteriol 2005, 187, 8039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sampson EM, Bobik TA, J. Bacteriol 2008, 190, 2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jakobson CM, Tullman-Ercek D, Slininger MF, Mangan NM, PLoS Comput. Biol 2017, 13, e1005525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fan C, Cheng S, Liu Y, Escobar CM, Crowley CS, Jefferson RE, Yeates TO, Bobik TA, Proc Natl Acad Sci USA 2010, 107, 7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Fan C, Cheng S, Sinha S, Bobik TA, Proc. Natl. Acad. Sci. U.S.A 2012, 109, 14995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Jakobson CM, Kim EY, Slininger MF, Chien A, Tullman-Ercek D, J. Biol. Chem 2015, 290, 24519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Aussignargues C, Paasch BC, Gonzalez-Esquer R, Erbilgin O, Kerfeld CA, Commun Integr Biol 2015, 8, e1039755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Parsons JB, Frank S, Bhella D, Liang M, Prentice MB, Mulvihill DP, Warren MJ, Molecular Cell 2010, 38, 305. [DOI] [PubMed] [Google Scholar]
- [11].Lassila JK, Bernstein SL, Kinney JN, Axen SD, Kerfeld CA, J. Mol. Biol 2014, 426, 2217. [DOI] [PubMed] [Google Scholar]
- [12].Choudhary S, Quin MB, Sanders MA, Johnson ET, Schmidt-Dannert C, PLoS ONE 2012, 7, e33342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hagen AR, Plegaria JS, Sloan N, Ferlez B, Aussignargues C, Burton R, Kerfeld CA, Nano Letters 2018, DOI 10.1021/acs.nanolett.8b02991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lee MJ, Brown IR, Juodeikis R, Frank S, Warren MJ, Metab. Eng 2016, 36, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tobimatsu T, Kawata M, Toraya T, Biosci. Biotechnol. Biochem 2005, 69, 455. [DOI] [PubMed] [Google Scholar]
- [16].Sutter M, Greber B, Aussignargues C, Kerfeld CA, Science 2017, 356, 1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Havemann GD, Bobik TA, J. Bacteriol 2003, 185, 5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kerfeld CA, Sawaya MR, Tanaka S, Nguyen CV, Phillips M, Beeby M, Yeates TO, Science 2005, 309, 936. [DOI] [PubMed] [Google Scholar]
- [19].Sutter M, Faulkner M, Aussignargues C, Paasch BC, Barrett S, Kerfeld CA, Liu L-N, Nano Lett. 2016, 16, 1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hagen A, Sutter M, Sloan N, Kerfeld CA, Nature Communications 2018, 9, 2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jorda J, Leibly DJ, Thompson MC, Yeates TO, Chem. Commun. (Camb.) 2016, 52, 5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lee MJ, Mantell J, Brown IR, Fletcher JM, Verkade P, Pickersgill RW, Woolfson DN, Frank S, Warren MJ, Nat Commun 2018, 9, 3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Pedelacq J-D, Cabantous S, Tran T, Terwilliger TC, Waldo GS, Nat. Biotechnol 2006, 24, 79. [DOI] [PubMed] [Google Scholar]
- [24].Gardell SJ, Craik CS, Clauser E, Goldsmith EJ, Stewart CB, Graf M, Rutter WJ, J. Biol. Chem 1988, 263, 17828. [PubMed] [Google Scholar]
- [25].Lilius G, Persson M, Bülow L, Mosbach K, Eur. J. Biochem 1991, 198, 499. [DOI] [PubMed] [Google Scholar]
- [26].Zakeri B, Fierer JO, Celik E, Chittock EC, Schwarz-Linek U, Moy VT, Howarth M, Proc. Natl. Acad. Sci. U.S.A 2012, 109, E690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sharma J, Uchida M, Miettinen HM, Douglas T, Nanoscale 2017, 9, 10420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Seebeck FP, Woycechowsky KJ, Zhuang W, Rabe JP, Hilvert D, J. Am. Chem. Soc 2006, 128, 4516. [DOI] [PubMed] [Google Scholar]
- [29].Kabsch W, Acta Crystallogr D Biol Crystallogr 2010, 66, 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Winn MD, Ballard CC, Cowtan KD, Dodson EJ, Emsley P, Evans PR, Keegan RM, Krissinel EB, Leslie AGW, McCoy A, McNicholas SJ, Murshudov GN, Pannu NS, Potterton EA, Powell HR, Read RJ, Vagin A, Wilson KS, Acta Crystallogr. D Biol. Crystallogr 2011, 67, 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Afonine PV, Grosse-Kunstleve RW, Echols N, Headd JJ, Moriarty NW, Mustyakimov M, Terwilliger TC, Urzhumtsev A, Zwart PH, Adams PD, Acta Crystallogr. D Biol. Crystallogr 2012, 68, 352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Emsley P, Cowtan K, Acta Crystallogr. D Biol. Crystallogr 2004, 60, 2126. [DOI] [PubMed] [Google Scholar]
- [33].Lee TS, Krupa RA, Zhang F, Hajimorad M, Holtz WJ, Prasad N, Lee SK, Keasling JD, J Biol Eng 2011, 5, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.