Abstract
Background:
Recent data suggest increases in methamphetamine potency, affordability, and availability in the US. Other data indicate rising rates of methamphetamine use among patients seeking treatment for opioid use disorder. The extent to which similar increases in methamphetamine use have occurred for populations outside of a treatment context and for those reporting other substance use is unknown.
Purpose:
The current analysis used a nationally representative data source to evaluate recent trends in past month methamphetamine use based on opioid use history.
Methods:
Data from the 2015-2017 National Survey on Drug Use and Health (NSDUH) were analyzed for yearly variations in past month methamphetamine use by opioid use history. Sensitivity analyses assessed if these trends were specific to methamphetamine use and to persons reporting opioid use.
Results:
Significant increases in past month methamphetamine use were observed for persons reporting past month heroin use, past year heroin use disorder, and past year prescription opioid use disorder. Among individuals reporting past month heroin use, for example, methamphetamine use tripled from 9.0% in 2015 to 30.2% in 2017. These associations were specific to methamphetamine with little change in other substance use. Similar increases in methamphetamine use were not observed for populations reporting other illicit substance use with the exception of prescription tranquilizers.
Conclusions:
These results provide data corroborating evidence of emergent concerns related to methamphetamine use in the US. Such findings highlight the importance of considering global drivers of substance use to avoid cyclic waves of new and emerging substance use crises.
Keywords: Heroin, Methamphetamine, NSDUH, Opioid, Polysubstance, Stimulant
1. Introduction
Rising rates of overdose deaths associated with opioid use have highlighted public health concerns posed by prescription opioid use disorder (POUD) and heroin use disorder (HUD) (CDC, 2018; Manchikanti et al., 2012). However, closely following these increases is a corresponding growth in overdose fatalities related to psychomotor stimulants. Methamphetamine has seen the largest proportional increase in fatal overdose over the past decade behind synthetic opioids (like fentanyl) with a 7.5-fold increase from 2007 to 2017 (CDC, 2018). Concerns relevant to methamphetamine are particularly striking when placed in the context of comorbid methamphetamine-opioid use for which overdose rates have risen 11-fold with any opioid and 73-fold when combined with synthetic opioids (CDC, 2018).
In this timeframe, increases in methamphetamine potency, affordability, and availability have been documented by several US federal agencies (e.g., ~300% increase in methamphetamine drug seizures from 2014-2018) (DEA, 2018; U.S. Customs and Border Protection, 2019). Increased methamphetamine accessibility combined with the widespread use of supply-side prevention efforts (i.e., policies designed to reduce drug supply) may have created ideal conditions for the proliferation of methamphetamine use and an emergent methamphetamine crisis.
Ellis and colleagues (2018) recently summarized this potential for “twin epidemics” by describing specific increases in methamphetamine use among persons seeking treatment for OUD in a national treatment dataset. That analysis found that prevalence of past-month methamphetamine use among patients with OUD nearly doubled from 19% in 2011 to 34% in 2017. These changes were pharmacologically specific given that increases for other substance use were substantively smaller over the same time period. The extent to which similar changes in methamphetamine use have occurred in populations outside of treatment contexts and reporting other illicit substance use is unknown.
The current analysis was designed to address these gaps by evaluating temporal trends in recent methamphetamine and opioid use using a nationally representative data source. Data from the National Survey on Drug Use and Health (NSDUH) were analyzed for yearly variations in past month methamphetamine use based on NMPO and heroin use. Sensitivity analyses assessed if national trends were 1) specific to methamphetamine and 2) specific to persons reporting opioid use.
2. Materials and Methods
2.1. Data Source
Data from the 2015, 2016, and 2017 NSDUH were used (Center for Behavioral Health Statistics, 2018). Years prior to 2015 were not included due to changes for methamphetamine and prescription drug variables in 2015 that made comparisons to earlier years non-analogous (SAMSHA, 2015).1 The NSDUH population consists of non-institutionalized US residents, aged 12 years and older, with dwelling units including households, homeless shelters, and other non-institutional group quarters. The NSDUH conducts large independent multi-stage area probability samples of this population annually. Additional information is described previously (Parker and Anthony, 2014; Seedall and Anthony, 2013). All NSDUH protocols are approved by Institutional Review Boards. The University of Kentucky Medical Institutional Review Board considered this analysis exempt.
2.2. Measures
The primary dependent measure was past month methamphetamine use. The primary independent measures were past month NMPO use, past month heroin use, past year POUD, and past year HUD. Variables in sensitivity analyses were past month cannabis, cocaine, prescription stimulant2, and prescription tranquilizer3 use and past year substance use disorder for these substances. Substance use disorder was evaluated via questions indexing criteria for DSM-IV substance abuse or dependence specified by substance. Demographic covariate models used age, sex, race, education, employment, and income variables selected a priori as common sociodemographic factors associated with opioid use (Becker et al., 2008).
2.3. Data Analysis
Logistic regression models evaluated temporal trends in past month methamphetamine use. These models included the interaction of opioid use (e.g., past month heroin use) with year as a categorical variable (results with year as continuous were qualitatively identical). Significant interactions between opioid use and year indicated different temporal trends among individuals endorsing the opioid use behavior compared to those observed in the population without endorsement. Sensitivity analyses were conducted using a similar modeling approach to evaluate 1) changes in other illicit substance use based on opioid use and 2) changes in methamphetamine use based on endorsement of other substance use. Significant effects were also analyzed using demographic-adjusted models. Data analysis was conducted in R using the survey package (Lumley, 2010).4 Estimates incorporated sampling weights as well as the complex survey design for design-based variance estimation and used a type I error rate of .05.
3. Results
3.1. Changes in Methamphetamine Use by Opioid History
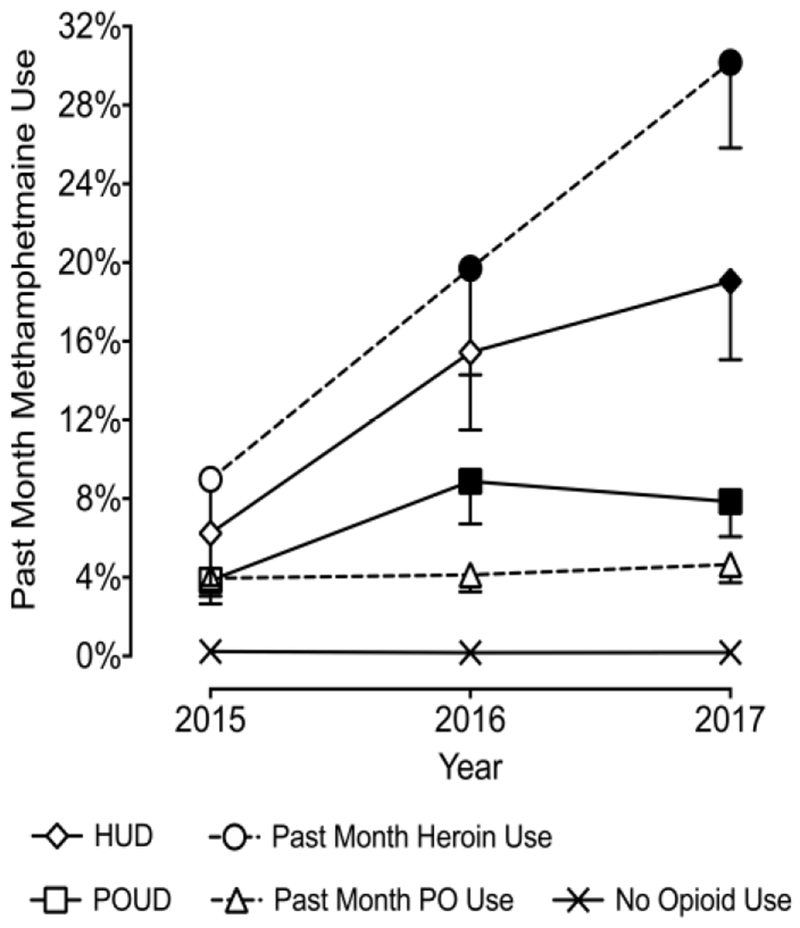
Figure 1 presents past month methamphetamine use by opioid use variables (also see Table 1). Logistic regression models indicated significant interactions involving past month heroin use, HUD, and OUD, p values < .05. These effects each indicated increases in past month methamphetamine use from 2015 to 2017 for these populations. For example, among individuals reporting past month heroin use, past month methamphetamine use tripled from 9.0% in 2015 to 19.7% in 2016 to 30.2% in 2017.
Figure 1.
Past Month Methamphetamine Use by Opioid Use History. Plotted are weighted prevalence estimates for past month methamphetamine use from the 2015-2017 NSDUH. Values are presented for past month heroin use (circles), past year heroin use disorder (diamonds), past month non-medical prescription opioid use (triangles), and past year non-medical prescription opioid use disorder (squares). Filled symbols are significantly different from 2015. Dotted lines represent substance use disorder variables and solid lines represent past month variables. Also plotted are estimates for individuals with no past month heroin or non-medical prescription opioid use (crosses). Error bars represent standard error of the mean. HUD = heroin use disorder; POUD = prescription opioid use disorder.
Table 1.
Prevalence of recent substance use by substance use variables 2015-2017.
| Predictors | 2015 | 2016 | 2017 |
|---|---|---|---|
| Past Month Methamphetamine Use | |||
| Heroin Month Use | 9.0% (0.1, 17.9) | 19.7% (9.1, 30.3) | 30.2% (17.5, 42.9)* |
| HUD | 6.2% (1.0, 11.5) | 15.4% (7.7, 23.2)* | 19.1% (11.2, 26.9)** |
| PO Month Use | 4.0% (2.2, 5.8) | 4.1% (2.4, 5.8) | 4.7% (2.9, 6.5) |
| POUD | 3.8% (1.5, 6.2) | 8.9% (4.6, 13.1)* | 7.9% (4.4, 11.4)* |
| Use Disorder | |||
| Cannabis | 3.4% (2.2, 4.6) | 2.7% (1.0, 4.4) | 3.5% (2.0, 4.9) |
| Cocaine | 7.6% (2.8, 12.4) | 6.7% (1.5, 11.9) | 8.9% (5.1, 12.7) |
| Rx Tranquilizer | 2.0% (0.3, 3.7) | 6.6% (1.9, 11.2)* | 13.4% (6.3, 20.5)*** |
| Rx Stimulant | 13.9% (3.7, 24.1) | 9.9% (2.7, 17.2) | 11.5% (5.4, 17.5) |
| Past Month Use | |||
| Cannabis | 2.2% (1.7, 2.8) | 1.5% (1.0, 2.0) | 2.0% (1.5, 2.6) |
| Cocaine | 7.0% (4.1, 9.8) | 4.9% (1.1, 8.7) | 5.7% (2.7, 8.8) |
| Rx Tranquilizer | 5.3% (2.9, 7.9) | 4.8% (2.5, 7.1) | 10.6% (6.7, 14.5)* |
| Rx Stimulant | 8.7% (4.7, 12.6) | 4.1% (1.5, 6.7) | 4.2% (2.0, 6.4) |
| Past Month Cannabis Use | |||
| Heroin Month Use | 56.9% (43.2, 70.7) | 67.8% (56.2, 79.3) | 55.0% (44.2, 65.8) |
| HUD | 48.5% (38.5, 58.5) | 61.1% (52.2, 70.0) | 42.6% (34.4, 50.8) |
| PO Month Use | 39.7% (34.6, 44.8) | 42.1% (36.2, 47.9) | 44.7% (39.8, 49.7) |
| POUD | 35.0% (28.5, 41.5) | 37.7% (32.1, 43.3) | 42.7% (36.6, 48.8) |
| Past Month Cocaine Use | |||
| Heroin Month Use | 29.8% (17.9, 41.6) | 39.2% (26.4, 52.1) | 34.9% (22.2, 47.6) |
| HUD | 16.9% (9.4, 24.4) | 27.6% (18.1, 37.1) | 16.7% (9.9, 23.4) |
| PO Month Use | 9.4% (6.6, 12.3) | 8.3% (5.7, 11.0) | 7.6% (5.3, 9.9) |
| POUD | 10.0% (5.5, 14.5) | 12.1% (8.1, 16.0) | 8.6% (5.5, 11.7) |
| Past Month Prescription Stimulant Use | |||
| Heroin Month Use | 16.7% (7.9, 25.5) | 10.1% (2.4, 17.8) | 9.6% (3.5, 15.7) |
| HUD | 9.4% (4.0, 14.8) | 10.8% (4.9, 16.8) | 6.6% (2.9, 10.4) |
| PO Month Use | 10.6% (7.9, 13.2) | 9.0% (6.4, 11.6) | 10.6% (8.0, 13.2) |
| POUD | 10.1% (6.4, 13.8) | 9.9% (6.2, 13.5) | 11.6% (8.2, 15.0) |
| Past Month Prescription Tranquilizer Use | |||
| Heroin Month Use | 28.0% (16.2, 39.9) | 27.7% (17.1, 38.4) | 24.6% (13.6, 35.6) |
| HUD | 19.7% (12.1, 27.4) | 25.0% (16.6, 33.4) | 21.0% (12.3, 29.6) |
| PO Month Use | 18.8% (15.0, 22.6) | 20.2% (16.1, 24.4) | 16.3% (12.6, 20.1) |
| POUD | 18.6% (14.0, 23.2) | 18.1% (12.7, 23.4) | 21.8% (15.7, 27.9) |
Note. Bold values showed statistically significant use history by year interactions. Asterisked values indicate significant differences from the year 2015
p < .05;
p < .01.
3.2. Sensitivity Analyses
No significant changes in other illicit substance use were observed based on opioid use (Table 1). The only other variable with temporal changes in past month methamphetamine use was prescription tranquilizer use (5.3% to 10.6%) and prescription tranquilizer use disorder (2.0% to 13.4%). Inclusion of demographic covariates did not change the results of primary or sensitivity analyses.
4. Discussion
The current analysis was designed to evaluate national trends in methamphetamine use based on opioid use history and to determine the pharmacological specificity of this relationship. Nationally representative data demonstrated increases in methamphetamine use among persons reporting opioid use, broadly, and heroin use, specifically, with rates that far exceeded national averages during this period (0.29%). These associations were specific to methamphetamine use with little change in consumption of other illicit compounds based on opioid use history. These relationships were also largely isolated to persons reporting opioid use because little change in methamphetamine use was observed among persons reporting other illicit substance use with the exception of prescription tranquilizers. Collectively, these findings provide clear and convincing corroborating evidence for emergent concerns related to methamphetamine use in the US.
This analysis was motivated by independent, but converging, information implicating methamphetamine use as a growing public health concern, including anecdotal media reports, overdose records and treatment admissions, and drug seizures and purity data. Particularly germane are recent data describing steady increases in methamphetamine use among patients with OUD who entered treatment programs in the US from 2011 to 2017 (Ellis et al., 2018). The results of the current analysis are remarkably consistent with this report and suggest that increases in methamphetamine use are not isolated to treatment-seeking populations.
Supply-side efforts have remained a major public policy response to reduce opioid-related harms (e.g., prescription drug monitoring programs; Clark and Schumacher, 2017; Fink et al., 2018). Tighter regulation of prescription opioid availability, however, may have had an unintended consequence of pushing individuals into illicit markets that were recently dominated by the more affordable and directly substitutable heroin (Cicero et al., 2014; Compton et al., 2016; Jones, 2013; Mars et al., 2014). Increases in potency, affordability, and availability of methamphetamine, however, might have provided an alternative inexpensive replacement. In this way, transitions to methamphetamine may be driven less by a specific pharmacological mechanism and more by a behavioral economic mechanism by which the reduced costs of methamphetamine make for an attractive alternative commodity. Regulation of prescription opioids that increased cost and accessibility could have in this way first lead to transitions to heroin and, more recently, to methamphetamine following increasing scrutiny of heroin markets.
This substitution hypothesis is in part supported by qualitative evidence in a sub-sample of individuals in the aforementioned treatment dataset (Ellis et al., 2018). These patients indicated that major motivations for using opioids and methamphetamine included to balance effects (38.6%) and as an opioid substitute (15.2%). Similar anecdotal evidence is evident in Internet forums dedicated to recreational substance use (e.g., bluelight.org), which contain discussions describing methamphetamine use to curb opioid withdrawal when other opioids are unavailable or undesirable. Consistent data is found in case reports (Rosen et al. 1992) and human laboratory studies (Kosten, 1990) showing that stimulants can reduce or postpone opioid withdrawal severity. On the other hand, preclinical work also indicates that opioid exposure can enhance the behavioral and reinforcing effects of psychomotor stimulants (Lacy et al., 2019; Leri et al., 2003; Wade-Galuska et al., 2011). It is possible that such reinforcement-enhancement mechanisms may explain the 51% of patients in the study by Ellis and colleagues (2018) who also endorsed seeking methamphetamine for “high-seeking” reasons such as synergistic effects or a “fabulous high”. Controlled laboratory work linking opioid exposure specifically to methamphetamine-relevant behaviors are nevertheless lacking, and future work is needed to clarify these potential mechanisms.
Increases in recent methamphetamine use among persons reporting opioid use were specific to methamphetamine and not observed for other recent illicit substance use consistent with the prior treatment population (Ellis et al., 2018). We extended these findings by documenting that increases in methamphetamine use were also largely isolated to persons reporting opioid use. The only other population to show similar increases in methamphetamine use were persons reporting prescription tranquilizer use (i.e., benzodiazepines). Temporal trends by prescription tranquilizer history were similarly alarming given an approximate 2- and 6- fold increase in methamphetamine use within recent use and use disorder groups, respectively. It is possible that this increase is due to similar supply-side efforts at reducing prescription drug use resulting in analogous seeking of alternatives, although this explanation fails to explain why increases were not observed in prescription stimulant populations. It is also possible that increases related to prescription tranquilizers are related to the co-prescription and comorbidity between individuals reporting opioid and benzodiazepine use (e.g., Han et al., 2017; Jones et al., 2012; Stein et al., 2016; Votaw et al., 2019). Monitoring of these trends will be important given similar overdose risks relevant to benzodiazepines.
Limitations of this analysis should be noted. Key populations reporting illicit substance use are not included in the NSDUH (e.g., institutionalized individuals). However, this limitation provides greater weight to a likely growth of methamphetamine use given that the current estimates are a probable underestimate. These data are also cross-sectional so cannot provide specific information about the directionality of opioid-to-methamphetamine associations. There is also likely meaningful overlap between persons reporting heroin and NMPO use. The current data suggest that increases in methamphetamine prevalence are likely driven by persons reporting heroin use. However, without time-series data it is difficult to determine the contributing role NMPO use may have had along a NMPO-to-heroin-to-methamphetamine pathway. Although the temporal trends reported in this analysis seem consistent and stable, we also had to rely on three years of data due to changes implemented in the 2015 NSDUH. This limited sample size reduced power and precision in some cases.
5. Conclusion
This report independently replicates and emphasizes recent reports of methamphetamine use among persons reporting opioid use. Multiple sources have now provided converging evidence of an unsettling conclusion that problems relevant to opioid use may soon be compounded by resurgent problems relevant to methamphetamine use. These findings highlight the importance of considering global drivers of drug-taking behavior even in the context of increases in a specific substance’s prevalence-related harms. Although the development of substance-specific interventions is relevant and important, there remains a paramount concern of developing and disseminating prevention and treatment efforts that address broader, systemic contributors to substance use disorder to avoid cyclic waves of new and emerging substance use crises.
Highlights.
We evaluated temporal trends in methamphetamine use based on opioid use history.
Methamphetamine use has increased among persons reporting opioid use 2015 to 2017.
Increases were 3-fold among persons reporting heroin use (9% to 30% 2015 to 2017).
Increases were specific to methamphetamine with little change in other substance use.
These data corroborate evidence of emergent concerns related to methamphetamine.
Acknowledgments
Role of Funding Source
This research was supported by the National Institute on Drug Abuse (NIDA) of the National Institutes of Health (T32 DA07209). This funding source had no role in analysis concept, data analysis, or preparation and submission of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Briefly, methamphetamine was previously considered a prescription stimulant in the NSDUH and revisions created an independent module from prescription stimulant use. Similarly, a variety of changes were made to NMPO use questions such as redefinition of misuse to “in any way a doctor did not direct you to use it/them”. For specific details see SAMSHA, 2015.
Defined in the NSDUH as amphetamine products, methylphenidate products, anorectic stimulants (e.g., phentermine), and modafinil.
Defined in the NSDUH as alprazolam products, lorazepam products, clonazepam products, diazepam products, and muscle relaxants (cyclobenzaprine (Flexeril®) and Soma®).
All code and documentation needed to reproduce this analysis is available at https://osf.io/dbc9m/?view_only=d8da68e9ef9d4428a41d4c9d6a599909
Conflict of Interest
The authors have no financial conflicts of interest in regard to this research.
References
- Becker WC, Sullivan LE, Tetrault JM, Desai RA, Fiellin DA, 2008. Non-medical use, abuse, and dependence on prescription opioids among U.S. adults: Psychiatric, medical and substance use correlates. Drug Alcohol Depend. 94, 38–47. [DOI] [PubMed] [Google Scholar]
- Center for Behavioral Health Statistics, 2018. 2017 National Survey on Drug Use and Health: Detailed tables. Substance Abuse and Mental Health Services Administration, Rockville, MD. [Google Scholar]
- Center for Disease Control and Prevention, 2018. Multiple Cause of Death 1999-2017 on CDC WONDER Online Database. Center for Disease Control and Prevention, Atlanta. [Google Scholar]
- Cicero TJ, Ellis MS, Surratt HL, Kurtz SP, 2014. The changing face of heroin use in the United States: A retrospective analysis of the past 50 years. JAMA Psychiatry. 71, 821–826. [DOI] [PubMed] [Google Scholar]
- Clark DJ, Schumacher MA, 2017. America’s opioid epidemic: Supply and demand considerations. Anesth. Analg 125, 1667–1674. [DOI] [PubMed] [Google Scholar]
- Compton WM, Jones CM, Baldwin GT, 2016. Relationship between nonmedical prescription-opioid use and heroin use. N. Engl. J. Med 374, 154–163. [DOI] [PubMed] [Google Scholar]
- Drug Enforcement Administration, 2018. National Drug Threat Assessment. U.S. Department of Justice, Falls Church, VA. [Google Scholar]
- Ellis MS, Kasper ZA, Cicero TJ, 2018. Twin epidemics: The surging rise of methamphetamine use in chronic opioid users. Drug Alcohol Depend. 193, 14–20. [DOI] [PubMed] [Google Scholar]
- Fink DS, Schleimer JP, Sarvet A, Grover KK, Delcher C, Castillo-Carniglia A, Kim JH, Rivera-Aguirre AE, Henry SG, Martins SS, 2018. Association between prescription drug monitoring programs and nonfatal and fatal drug overdoses: A systematic review. Ann. Intern. Med 168, 783–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B, Compton WM, Blanco C, Crane E, Lee J, Jones CM, 2017. Prescription opioid use, misuse, and use disorders in US adults: 2015 National Survey on Drug Use and Health. Ann. Intern. Med 167, 293–301. [DOI] [PubMed] [Google Scholar]
- Jones CM, 2013. Heroin use and heroin use risk behaviors among nonmedical users of prescription opioid pain relievers— United States, 2002-2004 and 2008-2010. Drug Alcohol Depend. 132, 95–100. [DOI] [PubMed] [Google Scholar]
- Jones JD, Mogali S, Comer SD, 2012. Polydrug abuse: A review of opioid and benzodiazepine combination use. Drug Alcohol Depend. 125, 8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TA, 1990. Cocaine attenuates the severity of naloxone-precipitated opioid withdrawal. Life Sci. 47, 1617–1623. [DOI] [PubMed] [Google Scholar]
- Lacy RT, Austin BP, Strickland JC, 2019. The influence of sex and estrous cyclicity on cocaine and remifentanil demand in rats. Addict. Biol Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leri F, Flores J, Rajabi H, Stewart J, 2003. Effects of cocaine in rats exposed to heroin. Neuropsychopharmacol. 28, 2102–2116. [DOI] [PubMed] [Google Scholar]
- Manchikanti L, Helm S 2nd, Fellows B, Janata JW, Pampati V, Grider JS, Boswell MV, 2012. Opioid epidemic in the United States. Pain Physician. 15, ES9–38. [PubMed] [Google Scholar]
- Mars SG, Bourgois P, Karandinos G, Montero F, Ciccarone D, 2014. “Every ‘never’ I ever said came true”: Transitions from opioid pills to heroin injecting. Int. J. Drug Policy 25, 257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker MA, Anthony JC, 2014. Should anyone be riding to glory on the now-descending limb of the crack-cocaine epidemic curve in the United States? Drug Alcohol Depend. 138, 225–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen MI, Wallace EA, Sullivan MC, Stine S, Kosten TR, 1992. Use of cocaine to prevent opiate withdrawal. Am. J. Psychiatry 149, 1609. [DOI] [PubMed] [Google Scholar]
- Seedall RB, Anthony JC, 2013. Risk estimates for starting tobacco, alcohol, and other drug use in the United States: Male-female differences and the possibility that ‘limiting time with friends’ is protective. Drug Alcohol Depend. 133, 751–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MD, Kanabar M, Anderson BJ, Lembke A, Bailey GL, 2016. Reasons for benzodiazepine use among persons seeking opioid detoxification. J. Subst. Abuse Treat 68, 57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration, 2015. National Survey on Drug Use and Health: 2014 and 2015 Redesign Changes. Substance Abuse and Mental Health Services Administration, Rockville, MD. [PubMed] [Google Scholar]
- U.S. Customs and Border Protection, 2019. CBP Enforcement Statistics FY2018. U.S. Department of Homeland Security, Washington, D.C. [Google Scholar]
- Votaw VR, Geyer R, Rieselbach MM, McHugh RK, 2019. The epidemiology of benzodiazepine misuse: A systematic review. Drug Alcohol Depend. 200, 95–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade-Galuska T, Galuska CM, Winger G, 2011. Effects of daily morphine administration and deprivation on choice and demand for remifentanil and cocaine in rhesus monkeys. J. Exp. Anal. Behav 95, 75–89. [DOI] [PMC free article] [PubMed] [Google Scholar]