Abstract
Objective
Growing evidence indicates exposure to air pollution contributes to obesity and cardiometabolic disease risk in children and adults, however studies are lacking in young adulthood, an important transitional period in the life course. The aim of this study was to examine the associations of short- and long-term regional ambient and near-roadway air pollution (NRAP) exposures on adiposity and cardiometabolic health in young adults aged 17–22 years.
Methods
From 2014–2018, a subset of participants (n=158) were recruited from the Children’s Health Study to participate in the Meta-AIR (Metabolic and Asthma Incidence Research) study to assess obesity (body composition and abdominal adiposity) and cardiometabolic health (fasting glucose, fasting insulin and lipid profiles) measures. Prior 1-month and 1-year average air pollution exposures were calculated from residential addresses. This included nitrogen dioxide (NO2), ozone (O3), particulate matter with aerodynamic diameter <10 μm (PM10), particulate matter with aerodynamic diameter <2.5 μm (PM2.5)) and NRAP (freeway, non-freeway, and total nitrogen oxides (NOx)) exposures. Linear regression models examined associations of prior 1-month (short-term) and 1-year (long-term) air pollution exposures on obesity and cardiometabolic factors adjusting for covariates and past childhood air pollution exposures.
Results
In the Meta-AIR study, we conducted a comprehensive analysis with short- and long-term regional ambient and NRAP exposures (in both single- and multi-pollutant models) and obesity- and cardiometabolic-related outcomes and found associations with a few outcomes. A 1 standard deviation (SD) change in long-term NO2 exposure was associated with a 11.3 mg/dL higher level of total cholesterol (p=0.04) and 9.4 mg/dL higher level of low-density lipoproteins (LDL)-cholesterol (p=0.04). Among obese participants, associations between long-term NO2 and total cholesterol and LDL-cholesterol were 4.5 and 9 times larger than the associations in non-obese participants (pinteraction=0.008 and 0.03, respectively). Additionally, we observed a statistically significant association with increased short-term O3 exposure and higher triglyceride and very-low-density lipoprotein (VLDL) cholesterol levels (p=0.04), lower high-density lipoprotein (HDL) cholesterol levels (p=0.03), and higher hepatic fat levels (p=0.02). Amongst glucose-related factors, long-term PM2.5 exposure was associated with higher levels of insulin area under the curve (p=0.03). There were no other statistically significant associations with short- or long-term air pollutants and BMI, other measures of adiposity, and cardiometabolic outcomes.
Conclusion
Higher exposure to regional air pollutants, namely prior 1-year average NO2, was associated with higher fasting serum lipid measures. These associations were more pronounced in obese participants, suggesting obesity may exacerbate the effects of air pollution exposure on lipid levels in young adults. This study did not find any other associations between short- and long-term ambient and NRAP exposures across a range of other obesity and cardiometabolic indicators. Further studies in young adults are warranted as our study suggests potential deleterious associations of both short- and long-term air pollution exposures and lipid metabolism.
Keywords: ambient air pollution, near-roadway air pollution, obesity, cardiometabolic health, young adult
1. Introduction
New data shows significant increasing obesity trends in both youth and adults from 1999–2016 (1). Early onset of detrimental health effects is a concern as obesity can influence the development of type 2 diabetes (T2DM) (2–5) and cardiovascular disease (CVD) (6, 7) later in life. As obese children are likely to become obese adults (8), it is important to characterize obesity and cardiometabolic profiles in young adults as they are at the forefront of the obesity epidemic and have greater risk to obesity-related health consequences. Apart from traditional obesity risk factors of poor diet, low physical activity, and low socioeconomic status (SES), epidemiological evidence has shown that air pollution may contribute to increased risk for obesity (9–12) and cardiometabolic disease (13–17). Furthermore, several studies have shown stronger associations with air pollution and adverse cardiovascular health in obese subjects compared to normal weigh subjects suggesting that obesity status may exacerbate the effects of air pollution (15, 18). Research on the effects of air pollution on obesity and cardiometabolic outcomes focused on the young adulthood period, however, is lacking in the literature.
Our current study, the Meta-AIR (Metabolic and Asthma Incidence Research) study, is a subset of young adults aged 17–22 years from the larger Southern California Children’s Health Study (CHS). We examined associations of prior 1-month (short-term) and prior 1-year (long-term) air pollution exposures on various indicators of obesity and cardiometabolic health. Regional ambient pollutants explored include nitrogen dioxide (NO2), ozone (O3), particulate matter with aerodynamic diameter <10 μm (PM10), and particulate matter with aerodynamic diameter <2.5 μm (PM2.5), and near-roadway air pollution (NRAP) include freeway, non-freeway, and total nitrogen oxides (NOx). The aim of this study was to determine if prior 1-month or 1- year ambient and NRAP exposures are associated with obesity measures and cardiometabolic outcomes in young adults. We hypothesized that increased exposure to prior 1-month and 1-year ambient and NRAP will be associated with higher levels of adiposity measures and adverse levels of cardiometabolic outcomes.
2. Material and Methods
2.1. Study Recruitment
The Meta-AIR study is a subset of young adults aged 17–22 years who were originally part of the larger CHS. Details of the CHS have been described previously (19). Briefly, in 2002 a cohort of kindergarten and first grade children were recruited from public schools across Southern California communities and followed through their high school years. Meta-AIR subjects were selected based on their high school overweight or obese status in 2011–2012 of CHS as well as predicted NOx exposures from their respective residential addresses in CHS towns. Potential participants were oversampled from “low” and “high” predicted NOx exposures to ensure maximum exposure contrast amongst study subjects within each CHS Southern California community. This recruitment strategy allowed for a wide range of air pollution exposures amongst potentially overweight and obese CHS young adults. Inclusion criteria included age- and sex-specific BMI percentiles ≥ 85th percentile measured by CHS staff in school year 2011–2012. Exclusion criteria were as follows: ineligible if using any medications known to influence body composition and insulin action/secretion, any diagnosis of diseases that may influence insulin or body composition including Type 1 and Type 2 diabetes, and any major illness since birth. Eligible participants, who are now young adults, were contacted and invited to enroll in the Meta-AIR study between 2014–2018. Written informed assents and consents were obtained from study participants. The Institutional Review Board at the University of Southern California approved this study.
2.2. Study Design
The Meta-AIR study visit included several questionnaires as well as extensive phenotyping of obesity and cardiometabolic outcomes conducted at the University of Southern California Diabetes and Obesity Research Institute and the Clinical Trials Unit from 2014–2018. The study visit flow is shown in Figure 1. In short, we administered questionnaires detailing sociodemographic characteristics, parental health and education, smoking history including e-cigarette use, self-reported physical activity, residential history, and 24-hour diet recalls (20). The first dietary recall was completed in person at the study visit, and the second was conducted by phone. A third phone recall was conducted if one of the first two recalls was either “more than usual” or “less than usual” from what the participant usually consumes day to day. These diet data were processed using the Nutrition Data System for Research (version 2014, University of Minnesota). Details of adiposity and cardiometabolic measures obtained are below.
Figure 1. Meta-AIR Studya Flow.
aMeta-AIR subjects were recruited between 2014–2018 from Children’s Health Study to examine the effects of short- and long-term ambient and near-roadway air pollution exposures on obesity and cardiometabolic health in young adults.
2.3. Adiposity and Cardiometabolic Outcomes
2.3.1. Adiposity
Several anthropometric and body composition measures were taken to estimate adiposity: 1) height and weight to determine body mass index (BMI) where BMI= weight/height2 (kg/m2), 2) dual-energy X-ray absorptiometry (DEXA) scan to determine total body fat percent, and 3) 3T magnetic resonance imaging (MRI) abdominal scan to determine subcutaneous abdominal adipose tissue (SAAT), visceral adipose tissue (VAT), and hepatic fat fraction (HFF). Obesity was defined as BMI≥ 30 kg/m2 and nonobesity defined as BMI <30.0 kg/m2.
2.3.2. Glucose and lipid metabolism
Following a minimum 10-hour fast, a 2-hour oral glucose tolerance test (OGTT) was administered using a load of anhydrous glucose dissolved in water for 1.75 grams per kilogram of body weight with a max dose of 75 grams. All participants received the maximum glucose load. Blood glucose and insulin samples were collected at fasting (pre-glucose load) and then post glucose challenge at 30-, 60-, 90-, and 120-minutes. Glucose-related outcomes included fasting glucose, fasting insulin, insulin area under the curve (AUC), homeostatic model assessment for insulin resistance (HOMA-IR), and the Matsuda Index. Insulin AUC was calculated using the trapezoidal method using all time points from the OGTT. HOMA-IR gives estimates of insulin resistance (IR) from fasting insulin and glucose concentrations where (21). The Matsuda Index gives an approximation of whole-body insulin sensitivity using all times points from the OGTT were the ratio of plasma glucose to insulin concentrations are calculated.
Matsuda index is defined as such that and (22). Fasting lipid-related outcomes included triglycerides, total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), and very-low-density lipoprotein (VLDL) cholesterols.
2.4. Assays
Blood samples from the OGTT were collected in potassium oxalate, sodium fluoride 2mL tubes and centrifuged for 15 minutes at 1500 RCF. These plasma samples were then assayed for glucose concentration by hexokinase-mediated reaction assay run on Roche Covas C501. Additional OGTT samples were collected in sodium heparin 2mL tubes for insulin and centrifuged at 2500 RPM for 10 minutes. Plasma samples were stored at −80°C and later assayed for insulin in duplicate by Human Insulin ELISA Kit (EZHI-14BK). Fasting blood for lipids was collected in serum separator 4mL tube, inverted several times, placed in room temperature for 60 minutes for clotting and centrifuged at 2000 RPM for 10 minutes. Serum lipid samples were stored at −80°C and later assayed in duplicate by Fujifilm Wako Diagnostics enzymatic assay.
2.5. Air Pollution Exposures
Residential history was collected from all Meta-AIR participants at their study visit including move in and move out dates for each respective residence. Residential addresses were geocoded using the Texas A&M geocoder (23) and assigned latitude and longitude coordinates that reflect each subject’s housing unit or building. Monthly air pollution data was averaged for prior 1-month and 1-year regional ambient and near-roadway air pollution (NRAP) exposures to reflect short-term and long-term exposures prior to each participant’s study visit. Exposures were weighted by time spent at each different residential address by month since some of our Meta-AIR participants were college students who lived between two residences during the year. In these instances, short-term and long-term exposures prior to the study visit accounted for both college and parental home residences using move in and move out months to appropriately weigh time spent at each respective residence. Additionally, our analysis included historic air pollution exposures or cumulative childhood exposures that were obtained from the parent study CHS, which account for past exposures beyond our periods of interests: prior 1-month and 1-year. For regional ambient pollutants, historic air pollution was defined as average childhood exposures for each participant from birth through year 2011. For NRAP, historic air pollution exposure was defined as average childhood exposures from CHS study entry (May 2003) through year 2011 where all our study participants had NRAP data available.
2.5.1. Ambient Air Pollution Exposures
Regional exposures were obtained from ambient monitoring stations by downloading hourly air quality data from the U.S. Environmental Protection Agency’s Air Quality System (http://www.epa.gov/ttn/airs/airsaqs). Daily averages for four regional ambient air pollutants, NO2, O3, PM10, and PM2.5, were calculated. For O3 only, levels were characterized as the eight-hour average daily maximum concentrations. Air monitoring stations in California are spaced 20–30 kilometers (km) apart, which provides good characterization of air pollution gradients across the region. Gaseous pollutants like NO2 and O3 are measured by the Federal Reference Method (FRM) monitors while particulates like PM10 and PM2.5 are measured through FRM and Federal Equivalent Method (FEM) monitors. Monthly averages were calculated from daily data using 75% completeness criteria. To calculate monthly ambient exposures, parcel level data was used in the inverse distance-squared weighting algorithm which spatially interpolated air quality data from up to four monitoring stations within a 50 km radius of the participant’s residence (24).
2.5.2. Near-Roadway Air Pollution Exposures
NRAP exposures were estimated by the California Line Source Dispersion Model (CALINE4) through detailed residential history where each participant’s residential addresses were geocoded. CALINE4 line-source dispersion model then estimated concentrations of near-road NOx at each latitude and longitude for freeway and non-freeway roads using traffic emissions (calculated within 5-km buffer of the residence), traffic volume, roadway geometry and meteorological conditions including wind speed and direction, pollution mixing heights, and atmospheric stability (25). Traffic counts and road geometry were obtained from Caltrans and TeleAtlas/GDT, and average daily traffic volumes were assigned based on year. Monthly near-road freeway, non-freeway and total NOx (sum of freeway and non-freeway) were then calculated for 1-month (short-term) and 1-year (long-term) average NRAP exposures prior to the study visit
2.6. Statistical Methods
Physical and cardiometabolic characteristics of the cohort were compared by obesity status (non-obese vs obese) using chi-square or t-tests. Non-obesity was defined as BMI <30.0 kg/m2 and obesity as BMI≥ 30.0 kg/m2. All outcomes were assessed for normality and skewed measures were log transformed to fit a normal distribution. Triglycerides, VLDL-cholesterol, fasting insulin, HOMA-IR, Matsuda Index were log transformed to meet assumptions of the linear regression. One subject was removed from this analysis due to undiagnosed diabetes; another subject was removed from the glucose-related cardiometabolic analysis due to a high fasting insulin that was greater than 4 standard deviations (SDs) above the mean.
Linear regression models were used to estimate effects of short-term (1-month) and long-term (1-year) air pollution exposures prior to study visit on obesity and cardiometabolic measures. Models were adjusted for age, sex, race/ethnicity, occupational status of participant (SES surrogate), parental education (SES surrogate), self-reported exercise, current cigarette smoking, e-cigarette use, body fat percent, diet (average total calories per day), season of study visit (warm or cool), historic air pollution exposure, and baseline CHS town as a random effect. Historic air pollution data, or cumulative childhood exposures, allows us to evaluate the effects of short- and long-term exposures on obesity- and cardiometabolic-related outcomes independent of past childhood air pollution exposures. Given we had this data available from the parent CHS study, we included these historic exposures to be able to account for the more recent short-term or long-term exposures of interest. Besides near-road non-freeway and total NOx, historic air pollution exposures have low to median correlation with prior 1-month or 1-year average air pollution exposures (all spearman correlation coefficient ≤0.7, Supplement Table 2). Association estimates of air pollution exposure and obesity- and cardiometabolic-related outcomes are reported for a 1 SD in air pollution exposure for prior 1-month and 1-year average regional ambient and NRAP exposures. We also investigated whether the associations between air pollution exposure and metabolic outcomes differed by sex, race/ethnicity and obesity status by testing the interaction terms in the full model. Additionally, we further explored associations with multipollutant models with additional short-term pollutants in short-term associations as well as additional long-term pollutants in long-term air pollution exposure associations. A two-sided p-value < 0.05 was considered statistically significant for all models. All analyses were performed in SAS, version 9.4 (SAS, Institute, Cary, NC).
3. Results
From 2014–2018, the Meta-AIR study enrolled 158 young adults who underwent extensive obesity and cardiometabolic phenotyping. General study characteristics are presented in Table 1. Briefly, mean age of participants was 19.7 years (SD=1.2, range=17.6–22.9). There were slightly more males than females (52.5% vs 47.5%), and 60% of participants were Hispanic, 28% were Non-Hispanic White and remaining 13% of participants were Asian, African American or other/mixed races. Generally, participants were full-time college students, students with part time/full time jobs, or working full time. Approximately 80% of participant’s parents had education levels of high school graduation and beyond. About 6% of participants were current smokers who have smoked 20 cigarettes or more in the past month, and e-cigarette ever use was about 15% amongst study participants. Our participants consumed an average of 2050 kcal (SD=632) per day obtained from the dietary recalls. Sociodemographic characteristics did not differ by obesity status (non-obese vs obese) across all variables (all p>0.1, Table 1).
Table 1.
Sociodemographic Characteristics by Obesity Status of 158 Participants Enrolled in the Meta-AIR Study from 2014–2018.
| Totala | Non-Obeseb | Obesec | p-valued | |
|---|---|---|---|---|
| Sex | n (%) | n (%) | n (%) | 0.24 |
| Male | 83 (52.5) | 56 (56.6) | 27 (45.8) | |
| Female | 75 (47.5) | 43 (43.4) | 32 (54.2) | |
| Race/Ethnicity | 0.11 | |||
| White | 44 (27.9) | 33 (33.3) | 11 (18.6) | |
| Hispanic | 94 (59.5) | 53 (53.5) | 41 (69.5) | |
| Othere | 20 (12.7) | 13 (13.1) | 7 (11.9) | |
| Occupational Status | 0.44 | |||
| Student only | 53 (33.5) | 33 (33.3) | 20 (33.9) | |
| Full or part time work only | 32 (20.3) | 22 (22.2) | 10 (17.0) | |
| Student + full/part time | 65 (41.2) | 41 (41.4) | 24 (40.7) | |
| Unemployed + other | 8 (5.1) | 3 (3.0) | 5 (8.5) | |
| Parental Education | ||||
| Less than high school | 31 (20.3) | 20 (20.4) | 11 (20.0) | 0.28 |
| High school graduate | 24 (15.7) | 12 (12.2) | 12 (21.8) | |
| Some college and beyond | 98 (64.1) | 66 (67.3) | 32 (58.2) | |
| Self-Reported Exercise | 0.24 | |||
| Yes | 121 (76.5) | 79 (79.8) | 42 (71.2) | |
| No | 37 (23.4) | 20 (20.2) | 17 (28.9) | |
| Current Smokerf | 0.49 | |||
| Yes | 9 (5.7) | 7 (7.1) | 2 (3.4) | |
| No | 149 (94.3) | 92 (92.9) | 57 (96.6) | |
| E-cigarette Use | 0.31 | |||
| Ever | 20 (15.4) | 11 (12.8) | 9 (20.5) | |
| Never | 110 (84.6) | 75 (87.2) | 35 (79.5) |
Variable denominators may differ due to missing values.
Non-Obese= BMI<30.
Obese= BMI ≥ 30.
Chi-square (non-obese vs obese) p-value.
Other races= Asian (n=10), African-American (n=6), Other/Mixed Races=(n=4).
Current smoker= smoked more than 20 cigarettes (1 pack) in the past month.
Mean adiposity- and cardiometabolic-related outcomes amongst all Meta-AIR participants as well as by obesity status are shown in Table 2. Of the 158 participants, 37% were obese (n=59) with BMI ≥ 30 kg/m2, 47% were overweight (n=75) with 25 kg/m2 ≤ BMI< 30 kg/m2, and 15% had normal BMI (n=24) with BMI<25 kg/m2. Amongst all study participants, mean BMI was 29.9 kg/m2 (SD=5.1) and mean body fat percent was 34.9% (SD=8.5). As expected, obesity-related measures (BMI, total body fat percent, SAAT, VAT, and HFF) were higher in obese compared to non-obese participants, all p<0.0001 (Table 2). Cardiometabolic measures were classified into two groups: lipid and glucose metabolism. For lipid metabolism, means for fasting lipid measures are presented in Table 2. Higher levels of triglycerides and VLDL-cholesterol are seen in obese versus non-obese subjects (p=0.0004); furthermore, lower HDL-cholesterol levels were seen in obese compared to non-obese subjects (p=0.006). Total cholesterol and LDL-cholesterol levels were similar across non-obese and obese subjects (p=0.6 and 0.7, respectively). Details of glucose metabolism measures are found in Table 2. Higher levels of glucose-related metabolic measures, like fasting glucose and fasting insulin, are seen in obese compared to non-obese participants, all p<0.01. Compared to the non-obese participants, obese participants show early signs of insulin resistance with higher HOMA-IR (3.2 in obese vs 1.5 in non-obese) and lower Matsuda index levels (3.9 in obese vs 7.3 in non-obese) (Table 2). In adults, the HOMA cutoff point for IR is >2.5 (21); however studies in children and adolescents have proposed higher cut off points >3.16 (26) and >4.0 (27). The Matsuda Index ≤2.5 has been proposed as the cut off for IR (28).
Table 2.
Obesity and Cardiometabolic Measures by Obesity Status in 158 Participants Enrolled in the Meta-AIR Study from 2014–2018.
| Total | Non-Obesea | Obeseb | p-valuec | |||||||
| Obesity Measures: | n | Mean | SD | n | Mean | SD | n | Mean | SD | |
| BMI (kg/m2) | 158 | 29.9 | 5.1 | 99 | 26.7 | 2.1 | 59 | 35.1 | 4.2 | <0.0001 |
| Total Body fat (%) | 158 | 34.9 | 8.5 | 99 | 31.4 | 7.8 | 59 | 40.9 | 6.0 | <0.0001 |
| Abdominal MRI Measures | ||||||||||
| SAAT (L) | 152 | 5.2 | 2.8 | 95 | 3.7 | 1.6 | 57 | 7.7 | 2.5 | <0.0001 |
| VAT (L) | 152 | 1.4 | 1 | 95 | 0.9 | 0.6 | 57 | 2.1 | 1.2 | <0.0001 |
| HFF (%) | 152 | 4.5 | 3.8 | 95 | 3.4 | 2.7 | 57 | 6.6 | 4.5 | <0.0001 |
| Cardiometabolic Measures: Lipid Metabolism | n | Mean | SD | n | Mean | SD | n | Mean | SD | |
| Triglycerides (mg/dL) | 145 | 85.8 | 47.7 | 91 | 75.2 | 43.0 | 54 | 103.5 | 50.1 | 0.0004 |
| Total Cholesterol (mg/dL) | 145 | 158.9 | 37.6 | 91 | 157.8 | 38.1 | 54 | 160.8 | 37.1 | 0.63 |
| HDL-Cholesterol (mg/dL) | 145 | 39.6 | 9.7 | 91 | 41.3 | 9.8 | 54 | 36.7 | 9.1 | 0.006 |
| LDL-Cholesterol (mg/dL) | 145 | 102.2 | 32.2 | 91 | 101.4 | 32.6 | 54 | 103.5 | 32.0 | 0.71 |
| VLDL-Cholesterol (mg/dL) | 145 | 17.2 | 9.5 | 91 | 15.0 | 8.6 | 54 | 20.7 | 10.0 | 0.0004 |
| Cardiometabolic Measures: Glucose Metabolism | n | Mean | SD | n | Mean | SD | n | Mean | SD | |
| Clinical fasting glucose (mg/dL) | 154* | 90.1 | 7.6 | 98 | 88.8 | 6.8 | 56 | 92.3 | 8.4 | 0.006 |
| Fasting insulin (μU/mL) | 141*† | 9.4 | 9.2 | 89 | 6.8 | 6.6 | 52 | 13.8 | 11.2 | 0.0001 |
| Insulin AUC | 141*† | 212.2 | 151.0 | 89 | 183.5 | 140.8 | 52 | 261.5 | 156.4 | 0.0003 |
| HOMA-IR | 141*† | 2.1 | 2.2 | 89 | 1.5 | 1.4 | 52 | 3.2 | 2.8 | 0.0001 |
| Matsuda Index | 141*† | 6.1 | 5.5 | 89 | 7.3 | 5.8 | 52 | 3.9 | 4.1 | 0.0003 |
SD= standard deviation; BMI=body mass index; MRI=magnetic resonance imaging; SAAT= subcutaneous abdominal adipose tissue; VAT=visceral adipose tissue; HFF= Hepatic (Liver) Fat Fraction; AUC= area under the curve; HOMA-IR= homeostatic model assessment-insulin resistance; HDL= high-density lipoprotein; LDL= low-density lipoprotein; VLDL= very low-density lipoprotein.
Non-Obese= BMI<30 where 24 participants have normal BMI (BMI<25) and 75 participants were overweight (25≤BMI<30).
Obese= BMI ≥ 30.
T-test (non-obese vs obese) p-value.
one diabetic subject was removed
one subject with high fasting insulin removed.
Prior to study visit, 1-month and 1-year average regional ambient and NRAP exposures are shown in Table 3, and historic exposures are show in Supplemental Table 1. To avoid potential collinearity of 1-month vs historic or 1-year vs historic air pollution exposures in the same model, Spearman correlations between 1-month vs historic exposures and 1-year vs historic exposures are shown in Supplemental Table 2. All regional ambient models (NO2, O3, PM10, and PM2.5) for 1-month and 1-year average air pollution exposures included the historic exposures (all correlations<0.7). For NRAP models (freeway, non-freeway, total NOx), freeway NOx was the only NRAP exposure that included the historic exposures in the 1-month and 1-year NRAP models (correlation<0.7).
Table 3.
Short- and Long-Term Regional Ambient and Near-Roadway Air Pollution Exposures Among 158 Participants Enrolled in the Meta-AIR Study from 2014–2018.
| 1-Month (Short-term) Exposurea | Mean | SD | IQR |
| Regional Ambient Air Pollutants | |||
| NO2 (ppb) | 16.1 | 5.7 | 12.6–20.0 |
| O3 (ppb) | 48.8 | 14.1 | 38.5–58.1 |
| PM10 (μg/m3) | 30.3 | 9.7 | 22.8–36.7 |
| PM2.5 (μg/m3) | 12.4 | 4.3 | 9.2–15.2 |
| Near-Roadway Air Pollutants | |||
| Freeway NOx (ppb) | 5.6 | 6.4 | 2.1–6.3 |
| Non-freeway NOx (ppb) | 1.7 | 1.3 | 0.8–2.2 |
| Total NOx (ppb) | 6.9 | 6.9 | 3.0–8.0 |
| 1-Year (Long-term) Exposureb | Mean | SD | IQR |
| Regional Ambient Air Pollutants | |||
| NO2 (ppb) | 16.0 | 3.9 | 14.3–18.7 |
| O3 (ppb) | 48.7 | 6.5 | 42.2–53.3 |
| PM10 (μg/m3) | 30.9 | 7.9 | 26.1–35.0 |
| PM2.5 (μg/m3) | 12.4 | 2.5 | 10.3–14.5 |
| Near-Roadway Air Pollutants | |||
| Freeway NOx (ppb) | 6.0 | 6.3 | 2.0–7.9 |
| Non-freeway NOx (ppb) | 1.8 | 1.4 | 0.8–2.3 |
| Total NOx (ppb) | 7.3 | 6.9 | 3.3–9.3 |
SD=standard deviation; IQR=interquartile range; NO2= nitrogen dioxide; O3=ozone 8-hour maximum daily; PM10= particulate matter with aerodynamic diameter <10 μm; PM2.5=particulate matter with aerodynamic diameter <2.5 μm; ppb=parts per billion; NOx=nitrogen oxides.
1-month average air pollution exposure prior to the Meta-AIR visit date.
1-year average air pollution exposure prior to the Meta-AIR visit date.
3.1. Associations of Short- and Long-Term Ambient Air Pollution and Obesity-Related Outcomes
Associations of short-term (1-month) and long-term (1-year) average ambient air pollution exposures and obesity-related outcomes are shown in Table 4. All models reflect association estimates for one obesity outcome and one short-term or long-term ambient air pollutant adjusting for age, sex, race/ethnicity, occupational status of participant, parental education, self-reported exercise, current cigarette smoking, e-cigarette use, total body fat % (not included in SAAT, VAT, HFF models), diet, season of visit, and respective historic air pollution exposures. In models pertaining to adiposity measures of total body fat percent and abdominal adiposity (SAAT, VAT, and HFF), we found prior 1-month O3 exposure was statistically, significantly associated with HFF, liver fat. A 1 SD (14.1 ppb) increase in prior 1-month O3 exposure was associated with a 20% higher liver fat levels after adjusting for covariates (p=0.02) (Table 4). We further explored the association between liver fat and short-term O3 exposure in multipollutant models where short-term NO2 or short-term PM2.5 were added separately to the model as these pollutants were not highly correlated with short-term O3 (Supplemental Table 3). Association estimates were slightly attenuated by adding short-term NO2 or PM2.5, however associations remained statistically significant (Supplemental Table 5). The association between HFF and short-term O3 was not modified by sex (male vs female), Hispanicity (non-hispanic white vs hispanic), or obesity status (obese vs non-obese) (all pinteraction>0.1). We did not find any other statistically significant associations of BMI, total body fat percent, SAAT, or VAT and short- or long-term ambient measures of NO2, O3, PM10, and PM2.5.
Table 4.
Associationsa of Short- and Long-Term Ambient Air Pollution Exposures with Obesity Measures in 158 Participants Enrolled in the Meta-AIR Study from 2014–2018.
| Short-Term Exposures | ||||||||
| Obesity Measures | 1-Month NO2 | 1-Month O3 | 1-Month PM10 | 1-Month PM2.5 | ||||
| βb | p-value | βb | p-value | βb | p-value | βb | p-value | |
| BMI | 0.64 | 0.16 | 0.93 | 0.07 | 0.30 | 0.42 | −0.02 | 0.96 |
| Total Body Fat Percent | −0.66 | 0.43 | −0.02 | 0.98 | −0.024 | 0.97 | 0.41 | 0.57 |
| SAAT | −0.005 | 0.63 | 0.008 | 0.52 | 0.004 | 0.70 | 0.007 | 0.47 |
| VAT | 0.002 | 0.64 | 0.007 | 0.19 | 0.003 | 0.50 | 0.003 | 0.51 |
| HFF† | 0.11 | 0.14 | 0.18 | 0.02* | 0.098 | 0.12 | 0.06 | 0.37 |
| Long Term Exposures | ||||||||
| Obesity Measures | 1-Year NO2 | 1-Year O3 | 1-Year PM10 | 1-Year PM2.5 | ||||
| βb | p-value | βb | p-value | βb | p-value | βb | p-value | |
| BMI | 0.34 | 0.44 | −0.062 | 0.90 | −0.52 | 0.22 | −0.34 | 0.38 |
| Total Body Fat Percent | −1.49 | 0.06 | −0.91 | 0.31 | 0.039 | 0.96 | −0.24 | 0.74 |
| SAAT | −0.013 | 0.21 | −0.004 | 0.73 | 0.006 | 0.58 | 0.001 | 0.94 |
| VAT | −0.007 | 0.10 | −0.003 | 0.61 | −0.003 | 0.43 | −0.002 | 0.57 |
| HFF† | 0.044 | 0.53 | 0.024 | 0.76 | 0.063 | 0.36 | 0.068 | 0.29 |
NO2= nitrogen dioxide; O3=ozone 8-hour maximum daily; PM10= particulate matter with aerodynamic diameter <10 μm; PM2.5=particulate matter with aerodynamic diameter <2.5 μm; BMI=body mass index; SAAT= subcutaneous abdominal adipose tissue volume/ total abdominal volume; VAT=visceral adipose tissue volume/ total abdominal volume; HFF= hepatic fat fraction.
Associations reflect change in outcome measure (association estimate (β)) scaled to 1 standard deviation of prior 1-month average ambient NO2 with 5.7 ppb, O3 with 14.1 ppb, PM10 with 9.7 μg/m3, and PM2.5 with 4.3 μg/m3 OR prior 1-year average ambient NO2 with 3.9 ppb, O3 with 6.5 ppb, PM10 with 7.9 μg/m3, and PM2.5 with 2.5 μg/m3.
Linear regression model was used to estimate the associations of prior 1-month or 1-year NO2, O3, PM10, and PM2.5 average exposures with obesity related outcomes after adjusting for age, sex, race/ethnicity, occupational status of subject, parental education, self-reported exercise, current cigarette smoking, e-cigarette use (ever/never), total body fat % (not included in SAAT, VAT, HFF models), diet (total calories), season (warm/cool), and historic air pollution exposure.
log-transformed variable
p<0.05.
3.2. Associations of Short-Term Ambient Air Pollution and Cardiometabolic-Related Outcomes
Associations with short-term, prior 1-month, ambient pollutant exposures and cardiometabolic measures are shown in Table 5. Amongst lipid metabolism measures, statistically significant associations with higher short-term O3 exposures and higher triglycerides, higher VLDL-cholesterol, and lower HDL-cholesterol levels were found after adjusting for covariates (all p<0.05, Table 5). For example, a 1 SD (14.1 ppb) increase in prior 1-month O3 exposure was associated with a 18% higher triglyceride levels (p=0.04) and a 18% higher VLDL-cholesterol levels (p=0.04). Additionally, a 1 SD increase in prior 1-month O3 exposure was associated with a 3.02 mg/dL lower HDL levels after adjusting for covariates (p=0.03).
Table 5.
Associationsa of Short-Term Ambient Air Pollution Exposures with Cardiometabolic Measures in 158 Participants Enrolled in the Meta-AIR Study from 2014–2018.
| Cardiometabolic Measures | 1-Month NO2 | 1-Month O3 | 1-Month PM10 | 1-Month PM2.5 | ||||
| Lipid Metabolism | Estimateb | p-value | Estimateb | p-value | Estimateb | p-value | Estimateb | p-value |
| Triglycerides† | 0.059 | 0.40 | 0.17 | 0.04* | 0.058 | 0.33 | 0.083 | 0.19 |
| Total Cholesterol | 5.98 | 0.27 | −3.067 | 0.61 | 3.049 | 0.48 | 5.92 | 0.21 |
| HDL-Cholesterol | 0.29 | 0.82 | −3.017 | 0.03* | −0.41 | 0.69 | 0.43 | 0.69 |
| LDL-Cholesterol | 5.22 | 0.25 | −3.36 | 0.51 | 2.43 | 0.51 | 3.63 | 0.36 |
| VLDL-Cholesterol† | 0.059 | 0.40 | 0.17 | 0.04* | 0.058 | 0.33 | 0.083 | 0.19 |
| Glucose Metabolism | Estimateb | p-value | Estimateb | p-value | Estimateb | p-value | Estimateb | p-value |
| Fasting Glucose | 0.19 | 0.85 | −0.45 | 0.69 | −0.51 | 0.52 | −0.85 | 0.32 |
| Fasting Insulin† | 0.18 | 0.22 | 0.11 | 0.52 | 0.023 | 0.85 | −0.19 | 0.14 |
| Insulin AUC | 30.1 | 0.08 | 6.02 | 0.76 | 12.99 | 0.35 | 22.21 | 0.14 |
| HOMA-IR† | 0.18 | 0.23 | 0.01 | 0.56 | 0.017 | 0.89 | −0.2 | 0.12 |
| Matsuda Index† | −0.15 | 0.13 | −0.037 | 0.74 | −0.037 | 0.65 | 0.059 | 0.51 |
NO2= nitrogen dioxide; O3=ozone 8-hour maximum daily; PM10= particulate matter with aerodynamic diameter <10 μm; PM2.5=particulate matter with aerodynamic diameter <2.5 μm; HDL= high-density lipoprotein; LDL= low-density lipoprotein; VLDL= very low-density lipoprotein; AUC= area under the curve; HOMA-IR= Homeostatic model assessment-insulin resistance.
Associations reflect change in outcome measure (effect estimate (β)) scaled to 1 standard deviation of prior 1-month average ambient NO2 with 5.7 ppb, O3 with 14.1 ppb, PM10 with 9.7 μg/m3, and PM2.5 with 4.3 μg/m3.
Linear regression model used to estimate the associations of prior 1-month NO2, O3, PM10, and PM2.5 exposures with glucose- and lipid-related measures after adjusting for age, sex, race/ethnicity, occupational status of participant, parental education, self-reported exercise, current cigarette use, e-cigarette use (ever/never), total body fat %, diet (total calories), season (warm/cool), and childhood air pollution exposure.
log-transformed variable
p<0.05.
We further explored short-term O3 and lipid associations in multipollutant models by adding short-term NO2 or short-term PM2.5 (Supplemental Table 5). In multipollutant models with short-term O3 and NO2, there was slight attenuation in association estimates across triglycerides, HDL-cholesterol and VLDL-cholesterol but all associations remained statistically significant (all p<0.05). With short-term O3 and PM2.5, association estimates were also attenuated, however triglyceride and VLDL-cholesterol models were no longer statistically significant (p=0.09 and p=0.09, respectively). Effect modification by sex, Hispanicity, and obesity status for triglycerides, HDL-cholesterol, and VLDL-cholesterol models were further explored however there were no statistically significant interactions were found (all pinteraction>0.1). No other statistically significant associations were found with short-term ambient pollution exposures, NO2, PM10, and PM2.5, and lipid metabolism measures. Glucose metabolism measures of fasting glucose, fasting insulin, insulin AUC, HOMA-IR and Matsuda Index were not associated with short-term ambient pollutants.
3.3. Associations of Long-Term Ambient Air Pollution and Cardiometabolic-Related Outcomes
Associations of long-term, prior 1-year, ambient pollution exposures and cardiometabolic measures are shown in Table 6. Amongst lipid metabolism measures, higher long-term ambient NO2 exposure was associated with higher fasting total cholesterol and LDL-cholesterol levels in this cohort of young adults (Table 6). For example, a 1 SD (3.9 ppb) increase in 1-year average NO2 exposure was associated with 11.3 mg/dL higher total cholesterol levels after adjusting for covariates (p=0.04). Similarly, a 1 SD increase in 1-year NO2 exposure was associated with a 9.4 mg/dL higher LDL-cholesterol levels (p=0.04). Additional exploration with multipollutant models was completed by adding long-term O3 or long-term PM2.5. (Supplemental Table 4). In multipollutant models with long-term NO2 and long-term O3, associations were slightly attenuated in total cholesterol and LDL-cholesterol models with marginal significance (p=0.05 and p=0.06, respectively) (Supplemental Table 6). In multipollutant models with long-term NO2 and long-term PM2.5, associations strengthened when adding long-term PM2.5 in total cholesterol and LDL-cholesterol models maintaining statistical significance p<0.05 (Supplemental Table 6).
Table 6.
Associationsa of Long-Term Regional Ambient Air Pollution Exposures with Cardiometabolic Measures in 158 Participants Enrolled in the Meta-AIR Study from 2014–2018.
| Cardiometabolic Measures | 1-Year NO2 | 1-Year O3 | 1-Year PM10 | 1-Year PM2.5 | ||||
| Lipid Metabolism | Estimateb | p-value | Estimateb | p-value | Estimateb | p-value | Estimateb | p-value |
| Triglycerides† | 0.11 | 0.11 | 0.085 | 0.35 | 0.022 | 0.78 | 0.043 | 0.51 |
| Total Cholesterol | 11.25 | 0.04* | 4.23 | 0.54 | 8.89 | 0.13 | 1.39 | 0.78 |
| HDL-Cholesterol | 0.12 | 0.93 | −1.27 | 0.43 | 0.63 | 0.65 | −0.72 | 0.53 |
| LDL-Cholesterol | 9.37 | 0.04* | 3.98 | 0.49 | 7.48 | 0.14 | 0.82 | 0.84 |
| VLDL-Cholesterol† | 0.11 | 0.11 | 0.085 | 0.35 | 0.022 | 0.78 | 0.043 | 0.51 |
| Glucose Metabolism | βb | p-value | βb | p-value | βb | p-value | βb | p-value |
| Fasting Glucose | −0.10 | 0.92 | −0.81 | 0.51 | −1.49 | 0.12 | −0.031 | 0.97 |
| Fasting Insulin† | 0.17 | 0.26 | −0.049 | 0.79 | −0.21 | 0.18 | −0.13 | 0.34 |
| Insulin AUC | 29.93 | 0.09 | 37.50 | 0.08 | 20.51 | 0.27 | 33.57 | 0.03* |
| HOMA-IR† | 0.17 | 0.26 | −0.054 | 0.78 | −0.23 | 0.16 | −0.12 | 0.36 |
| Matsuda Index† | −0.16 | 0.14 | −0.064 | 0.63 | 0.064 | 0.57 | −0.018 | 0.85 |
NO2= nitrogen dioxide; O3=ozone 8-hour maximum daily; PM10= particulate matter with aerodynamic diameter <10 μm; PM2.5=particulate matter with aerodynamic diameter <2.5 μm; HDL= high-density lipoprotein; LDL= low-density lipoprotein; VLDL= very low-density lipoprotein; AUC= area under the curve; HOMA-IR= homeostatic model assessment-insulin resistance.
Associations reflect change in outcome measure (effect estimate (β)) scaled to 1 standard deviation of prior 1-year average ambient NO2 with 3.9 ppb, O3 with 6.5 ppb, PM10 with 7.9 μg/m3, and PM2.5 with 2.5 μg/m3.
Linear regression model was used to estimate the associations of prior 1-year NO2, O3, PM10, and PM2.5 exposures with glucose- and lipid-related measures after adjusting for age, sex, race/ethnicity, occupational status of participant, parental education, self-reported exercise, current cigarette use, e-cigarette use (ever/never), total body fat %, diet (total calories), season (warm/cool), and historic air pollution exposure.
log-transformed variable
p<0.05.
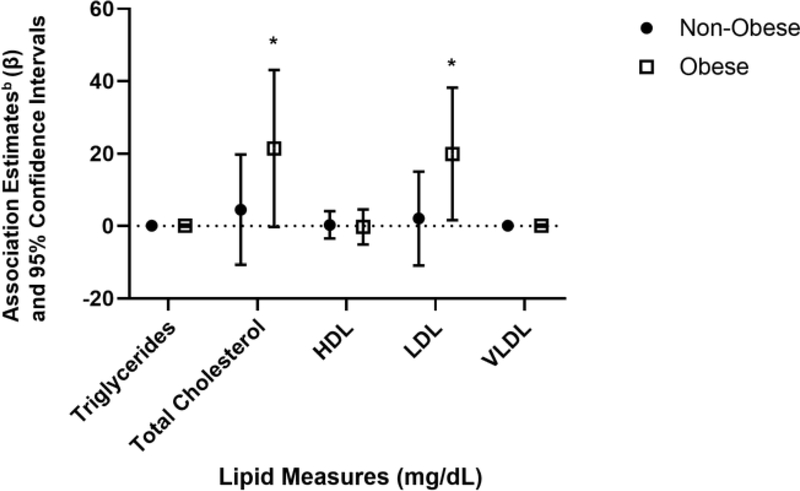
Associations of long-term NO2 exposure and total cholesterol and LDL-cholesterol were further assessed for effect modification by sex, Hispanicity, and obesity status. There were no statistically significant interactions of sex and hispancity (all p>0.1); however, the interaction for obesity status (non-obese vs obese) and NO2 were statistically significant for total cholesterol (p=0.008) and LDL-cholesterol (p=0.03). These results suggest differences in the effect of long-term NO2 exposure on lipid levels by obesity status, so associations were stratified by obesity status. In obese subjects, the association estimate of prior 1-year NO2 exposure on total cholesterol and LDL-cholesterol were substantially higher compared to non-obese subjects (Figure 2). The association estimates between prior 1-year NO2 exposure and total cholesterol among obese participants were nearly 5-fold larger (21.4 mg/dL vs 4.7 mg/dL) than non-obese participants. Likewise, the association estimates between prior 1-year NO2 exposure and LDL-cholesterol among obese participants were 9-fold larger (19.9 mg/dL vs 2.2 mg/dL) than non-obese participants.
Figure 2. Associationsa of Prior 1-Year NO2 Exposures and Lipid Metabolism Measures by Obesity Status in 158 Participants Enrolled in the Meta-AIR Study from 2014–2018.
NO2= nitrogen dioxide; HDL= high-density lipoprotein; LDL= low-density lipoprotein; VLDL= very low-density lipoprotein; obese= BMI ≥ 30.0 kg/m2, non-obese= BMI< 30.0 kg/m2.
aAssociations reflect change in outcome measure (association estimate (β)) scaled to 1 standard deviation of prior 1-year average ambient NO2 with 3.9 ppb stratified by obesity status (non-obese vs obese).
bLinear regression model was used to estimate the associations of 1-year NO2 and lipid metabolism outcomes after adjusting for age, sex, race/ethnicity, occupational status of subject, parental education, self-reported exercise, current cigarette smoking, e-cigarette use, total body fat percent, diet, season, and historic air pollution exposure.
*p<0.05
Amongst glucose metabolism measures, higher long-term PM2.5 exposure was associated with higher insulin AUC such that a 1 SD (2.5 μg/m3) increase in prior 1-year PM2.5 exposure was associated with 33.6 higher units of insulin AUC (p=0.03). Further exploration was conducted with multipollutant models adding long-term NO2 or long-term O3 (Supplemental Table 4). In the multipollutant model with long-term PM2.5 and NO2, the association with insulin AUC was attenuated and was no longer statistically significant (p=0.09, Supplemental Table 7). Similarly, the multipollutant model with long-term PM2.5 and O3, further attenuation was found with loss in significance (p=0.17, Supplemental Table 7). There was no effect modification with insulin AUC and long-term PM2.5 exposure by sex, Hispanicity, and obesity status (pinteraction>0.1). There were no other statistically significant associations between long-term ambient exposures, NO2, O3, PM10, and PM2.5, and glucose metabolism measures of fasting glucose, fasting insulin, insulin AUC, HOMA-IR, and Matsuda Index.
3.4. Associations of Long-Term and Short-Term NRAP and Obesity- and Cardiometabolic-Related Outcomes
Like regional ambient air pollution exposures, associations of short-term and long-term NRAP exposures with obesity- and cardiometabolic-related outcomes were explored (Supplemental Table 8–9). There were no statistically significant associations with prior 1-month and 1-year average NRAP exposures of non-freeway, freeway and total NOx with obesity- or cardiometabolic-related outcomes.
4. Discussion
In the Meta-AIR study, we conducted a comprehensive analysis with short- and long-term regional ambient and NRAP exposures (in both single- and multi-pollutant models) and obesity- and cardiometabolic-related outcomes and found associations with only a few outcomes. These associations include liver fat (with short-term O3), lipid profiles (with short-term O3 and long-term NO2) and insulin-related phenotype (with long-term PM2.5). Though we did not find statistically significant associations with short-term or long-term ambient and NRAP exposures and BMI and other adiposity measures, we showed a positive association where higher short-term (prior 1-month) O3 exposure was associated with higher liver fat in young adults. Increasing incidence of non-alcoholic fatty liver disease (NAFLD), an accumulation of liver fat, has been strongly liked to obesity, and many with NAFLD are obese and insulin resistant which draws concerns (29). Additionally, there is some evidence of the role of air pollution on NAFLD (30).
Our findings suggest that higher short-term O3 and higher long-term NO2 exposures may increase risk of dyslipidemia in young adults. Though it is not clear why higher short-term O3 and higher long-term NO2 exposures affects different lipid types, perhaps some ambient pollutants like O3 elicit acute or short-term effects whereas NO2 exposures may have more chronic or long-term effects on lipid profiles. Several reported associations support our study findings, however most studies have focused effects of air pollution and lipid abnormalities in adult and elderly populations (31–34). Similar to our findings, a recent study of 15,000 Chinese adults (aged 18–74 years) detected statistically significant associations with increased long-term ambient air pollution exposures and altered lipid measures with stronger associations in obese participants (34). Studies in young adults are lacking, though one study in youth has shown deleterious effects of poor air quality and elevated levels of total cholesterol and triglycerides in Irani adolescents (35). Additionally, an experimental model has shown that increased air pollution exposure may perturb lipid levels (36), though exact biological mechanism remains uncertain. One proposed mechanism is inflammatory responses from air pollution exposure which induces macrophage infiltration in adipose tissue (37). Macrophage infiltration then cues expression of proinflammatory cytokines inducing uncontrolled lipolysis which may lead to elevated levels of circulating nonesterified fatty acids (38). These fatty acids are transported to the liver for upregulation of triglycerides synthesis, VLDL production and ultimately dyslipidemia.
Lastly, associations of air pollution and glucose metabolism in children and adolescents have been shown previously (13, 39, 40); yet again, studies are limited in young adults. One study from Southern California showed adverse effects of higher NO2 and PM2.5 exposures on insulin sensitivity and beta-cell function in overweight and obese children (aged 8–15 years) after an average 3 year follow up period (13). Though we did not find statistically significant associations with short-term ambient and NRAP exposures and glucose metabolism measures, our study suggests higher long-term (prior 1-year) PM2.5 exposure may be associated with higher insulin AUC levels. Several animal models have proposed mechanisms by which air pollution may affect glucose metabolism (37, 41).
The Meta-AIR study has notable strengths. Unlike most air pollution studies, our study had life-time residential history on our participants by which we were able to incorporate past, childhood air pollution exposures. We used the well-established CHS to recruit our Meta-AIR participants where we sampled across high and low air pollution exposures across CHS communities to ensure a wide range of air pollution exposures in both regional ambient and NRAP exposures. An extensive number of exposure metrics (both short- and long-term regional and local traffic pollutants) alongside various measures of adiposity and cardiometabolic health were carried out in this study. A rigorous collection of adiposity measures using gold-standards such as DEXA and MRI and cardiometabolic-related outcomes were performed prospectively. We collected many different measures of modifiable risk factors such as diet, physical activity, current smoking and e-cigarette use, and non-modifiable risk factors age, race/ethnicity, sex, occupational status of participant and parental education via questionnaires. Our air pollution exposure estimates incorporated multiple residences as our study population included college-aged subjects who resided at their parent’s residence as well as their school residence giving appropriate weights to each respective residence.
Despite the strengths, there were limitations to this current study. First, we cannot draw causal relationships between air pollution exposures and obesity- and cardiometabolic-related outcomes as our study outcomes were only collected at one time point. We were limited in our sample size as we only had 158 young adults in this study; therefore, various interactions tested should be interpreted with caution. Given our study design, our participants were primed for potentially adverse levels of obesity and cardiometabolic outcomes, however given the standard deviations of our clinical measures, there was an adequate range of outcomes. There were no contextual variables in our analysis given that ~70% of our participants were students (full time or part time), about half lived in multiple residences due to school addresses and parental addresses (time spent in summer and winter break months). Contextual covariates would need to incorporate several different addresses, weighting time spent at in each neighborhood as most student who lived in college dorms or apartment were not in their hometowns. Though this may be feasible, we collected a rather robust list of individual-level covariates that may capture this information. Despite incorporating multiple residences, we were unable to incorporate other locations such as work locations that our subjects may have frequently visited or indoor exposures. We also acknowledge our variables of occupational status of the participant and parental education may not capture SES fully. Our study findings may be only generalizable to young adults with similar demographic data (primarily Hispanic or White), with similar range of air pollution exposures, and those with a history of overweight/obesity during mid-teenage years. Additionally, markers of oxidation were not available in this current study. Finally, our findings cannot determine the precise mechanism behind the association of air pollution and obesity and cardiometabolic health, however our results indicate potential pathways that may involve disrupted lipid metabolism, increased liver fat and increased insulin production.
5. Conclusion
Findings from the Meta-AIR study suggests that differential ambient regional air pollution exposures, NO2, O3 and PM2.5, may contribute to poor cardiometabolic health in young adults aged 17–22 years. Notably, the association between long-term NO2 and fasting lipid measures may adversely affect obese young adults compared to non-obese young adults. Differences in association by obesity status suggest that obese young adults may be more susceptible to adverse effects of long-term air pollution exposure, and this may exacerbate indicators of cardiometabolic health. Additional longitudinal studies in young adults are warranted as to verify associations of air pollution and adverse obesity and cardiometabolic outcomes.
Supplementary Material
Highlights.
We assessed measures of adiposity, cardiometabolic health in 158 young adults.
Ambient air pollution was associated with some cardiometabolic risk factors.
Long-term NO2 was associated higher total cholesterol and LDL-C levels.
Associations between NO2 and lipids were more pronounced in obese participants.
Short-term O3 was associated with higher triglycerides and VLDL-C and lower HDL-C.
Acknowledgments
Funding: This work was funded by the following agencies: Southern California Environmental Health Sciences Center from NIH NIEHS (grants 5P30ES07048 and P30ES007048), T32 Environmental Genomics Training grant from NIH NIEHS (grant T32ES013678), Southern California Children’s Environmental Health Center from NIH NIEHS and EPA (grant P01ES022845 and RD-83544101-0), NIH NIEHS (grants R00ES027853 and R00ES027870), and the Hastings Foundation.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hales CM, Carroll MD, Fryar CD, et al. Prevalence of Obesity Among Adults and Youth: United States, 2015–2016. NCHS Data Brief 2017(288):1–8. [PubMed] [Google Scholar]
- 2.Hannon TS, Rao G, Arslanian SA. Childhood obesity and type 2 diabetes mellitus. Pediatrics 2005;116(2):473–80. [DOI] [PubMed] [Google Scholar]
- 3.Buchanan TA. Pancreatic beta-cell loss and preservation in type 2 diabetes. Clin Ther 2003;25 Suppl B:B32–46. [DOI] [PubMed] [Google Scholar]
- 4.Weiss R, Kaufman FR. Metabolic complications of childhood obesity: identifying and mitigating the risk. Diabetes Care 2008;31 Suppl 2:S310–6. [DOI] [PubMed] [Google Scholar]
- 5.Sinha R, Fisch G, Teague B, et al. Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. N Engl J Med 2002;346(11):802–10. [DOI] [PubMed] [Google Scholar]
- 6.Juonala M, Magnussen CG, Berenson GS, et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med 2011;365(20):1876–85. [DOI] [PubMed] [Google Scholar]
- 7.Freedman DS, Mei Z, Srinivasan SR, et al. Cardiovascular risk factors and excess adiposity among overweight children and adolescents: the Bogalusa Heart Study. J Pediatr 2007;150(1):12–7.e2. [DOI] [PubMed] [Google Scholar]
- 8.Ward ZJ, Long MW, Resch SC, et al. Simulation of Growth Trajectories of Childhood Obesity into Adulthood. N Engl J Med 2017;377(22):2145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jerrett M, McConnell R, Wolch J, et al. Traffic-related air pollution and obesity formation in children: a longitudinal, multilevel analysis. Environ Health 2014;13:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McConnell R, Shen E, Gilliland FD, et al. A longitudinal cohort study of body mass index and childhood exposure to secondhand tobacco smoke and air pollution: the Southern California Children’s Health Study. Environ Health Perspect 2015;123(4):360–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim JS, Alderete TL, Chen Z, et al. Longitudinal associations of in utero and early life near-roadway air pollution with trajectories of childhood body mass index. Environ Health 2018;17(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rundle A, Hoepner L, Hassoun A, et al. Association of childhood obesity with maternal exposure to ambient air polycyclic aromatic hydrocarbons during pregnancy. Am J Epidemiol 2012;175(11):1163–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alderete TL, Habre R, Toledo-Corral CM, et al. Longitudinal Associations Between Ambient Air Pollution With Insulin Sensitivity, beta-Cell Function, and Adiposity in Los Angeles Latino Children. Diabetes 2017;66(7):1789–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brook RD, Rajagopalan S, Pope CA 3rd, et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 2010;121(21):2331–78. [DOI] [PubMed] [Google Scholar]
- 15.Qin XD, Qian Z, Vaughn MG, et al. Gender-specific differences of interaction between obesity and air pollution on stroke and cardiovascular diseases in Chinese adults from a high pollution range area: A large population based cross sectional study. Sci Total Environ 2015;529:243–8. [DOI] [PubMed] [Google Scholar]
- 16.Brook RD, Sun Z, Brook JR, et al. Extreme Air Pollution Conditions Adversely Affect Blood Pressure and Insulin Resistance: The Air Pollution and Cardiometabolic Disease Study. Hypertension 2016;67(1):77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J, Zhou C, Xu H, et al. Ambient Air Pollution Is Associated With HDL (High-Density Lipoprotein) Dysfunction in Healthy Adults. Arterioscler Thromb Vasc Biol 2019;39(3):513–22. [DOI] [PubMed] [Google Scholar]
- 18.Weichenthal S, Hoppin JA, Reeves F. Obesity and the cardiovascular health effects of fine particulate air pollution. Obesity (Silver Spring) 2014;22(7):1580–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Z, Salam MT, Eckel SP, et al. Chronic effects of air pollution on respiratory health in Southern California children: findings from the Southern California Children’s Health Study. J Thorac Dis 2015;7(1):46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffmann K, Boeing H, Dufour A, et al. Estimating the distribution of usual dietary intake by short-term measurements. Eur J Clin Nutr 2002;56 Suppl 2:S53–62. [DOI] [PubMed] [Google Scholar]
- 21.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28(7):412–9. [DOI] [PubMed] [Google Scholar]
- 22.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999;22(9):1462–70. [DOI] [PubMed] [Google Scholar]
- 23.Goldberg D. Texas A&M University Geoservices; Available online at http://geoservices.tamu.edu. 2016. (Accessed June 1 2016). [Google Scholar]
- 24.Wong DW, Yuan L, Perlin SA. Comparison of spatial interpolation methods for the estimation of air quality data. J Expo Anal Environ Epidemiol 2004;14(5):404–15. [DOI] [PubMed] [Google Scholar]
- 25.Benson P. CALINE4 – A Dispersion Model for Predicting Air Pollutant Concentrations Near Roadways. 1989;Report No. FHWA/CA/TL-84/15.(Prepared by the California Department of Transportation, Sacramento, CA.). [Google Scholar]
- 26.Keskin M, Kurtoglu S, Kendirci M, et al. Homeostasis model assessment is more reliable than the fasting glucose/insulin ratio and quantitative insulin sensitivity check index for assessing insulin resistance among obese children and adolescents. Pediatrics 2005;115(4):e500–3. [DOI] [PubMed] [Google Scholar]
- 27.Reinehr T, Andler W. Changes in the atherogenic risk factor profile according to degree of weight loss. Arch Dis Child 2004;89(5):419–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kernan WN, Inzucchi SE, Viscoli CM, et al. Pioglitazone improves insulin sensitivity among nondiabetic patients with a recent transient ischemic attack or ischemic stroke. Stroke 2003;34(6):1431–6. [DOI] [PubMed] [Google Scholar]
- 29.Moore JB. Non-alcoholic fatty liver disease: the hepatic consequence of obesity and the metabolic syndrome. Proc Nutr Soc 2010;69(2):211–20. [DOI] [PubMed] [Google Scholar]
- 30.Tomaru M, Takano H, Inoue K, et al. Pulmonary exposure to diesel exhaust particles enhances fatty change of the liver in obese diabetic mice. Int J Mol Med 2007;19(1):17–22. [PubMed] [Google Scholar]
- 31.Wu XM, Broadwin R, Basu R, et al. Associations between fine particulate matter and changes in lipids/lipoproteins among midlife women. Sci Total Environ 2019;654:1179–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bind MA, Peters A, Koutrakis P, et al. Quantile Regression Analysis of the Distributional Effects of Air Pollution on Blood Pressure, Heart Rate Variability, Blood Lipids, and Biomarkers of Inflammation in Elderly American Men: The Normative Aging Study. Environ Health Perspect 2016;124(8):1189–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chuang KJ, Yan YH, Cheng TJ. Effect of air pollution on blood pressure, blood lipids, and blood sugar: a population-based approach. J Occup Environ Med 2010;52(3):258–62. [DOI] [PubMed] [Google Scholar]
- 34.Yang BY, Bloom MS, Markevych I, et al. Exposure to ambient air pollution and blood lipids in adults: The 33 Communities Chinese Health Study. Environ Int 2018;119:485–92. [DOI] [PubMed] [Google Scholar]
- 35.Poursafa P, Mansourian M, Motlagh ME, et al. Is air quality index associated with cardiometabolic risk factors in adolescents? The CASPIAN-III Study. Environ Res 2014;134:105–9. [DOI] [PubMed] [Google Scholar]
- 36.Takano H, Yanagisawa R, Inoue K, et al. Nitrogen dioxide air pollution near ambient levels is an atherogenic risk primarily in obese subjects: a brief communication. Exp Biol Med (Maywood) 2004;229(4):361–4. [DOI] [PubMed] [Google Scholar]
- 37.Sun Q, Yue P, Deiuliis JA, et al. Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation 2009;119(4):538–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gutierrez DA, Puglisi MJ, Hasty AH. Impact of increased adipose tissue mass on inflammation, insulin resistance, and dyslipidemia. Curr Diab Rep 2009;9(1):26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toledo-Corral CM, Alderete TL, Habre R, et al. Effects of air pollution exposure on glucose metabolism in Los Angeles minority children. Pediatr Obes 2018;13(1):54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thiering E, Markevych I, Bruske I, et al. Associations of Residential Long-Term Air Pollution Exposures and Satellite-Derived Greenness with Insulin Resistance in German Adolescents. Environ Health Perspect 2016;124(8):1291–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu Z, Xu X, Zhong M, et al. Ambient particulate air pollution induces oxidative stress and alterations of mitochondria and gene expression in brown and white adipose tissues. Part Fibre Toxicol 2011;8:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.