Abstract
Growth plate cartilage resides near the ends of long bones and is the primary driver of skeletal growth. During growth, both intrinsically and extrinsically generated mechanical stresses act on chondrocytes in the growth plate. Although the role of mechanical stresses in promoting tissue growth and homeostasis has been strongly demonstrated in articular cartilage of the major skeletal joints, effects of stresses on growth plate cartilage and bone growth are not as well established. Here, we review the literature on mechanobiology in growth plate cartilage at macroscopic and microscopic scales, with particular emphasis on comparison of results obtained using different methodological approaches, as well as from whole animal and in vitro experiments. To answer these questions, macroscopic mechanical stimulators have been developed and applied to study mechanobiology of growth plate cartilage and chondrocytes. However, the previous approaches have tested a limited number of stress conditions, and the mechanobiology of a single chondrocyte has not been well studied due to limitations of the macroscopic mechanical stimulators. We explore how microfluidics devices can overcome these limitations and improve current understanding of growth plate chondrocyte mechanobiology. In particular, microfluidic devices can generate multiple stress conditions in a single platform and enable real-time monitoring of metabolism and cellular behavior using optical microscopy. Systematic characterization of the chondrocytes using microfluidics will enhance our understanding of how to use mechanical stresses to control the bone growth and the properties of tissue-engineered growth plate cartilage.
Keywords: Growth plate chondrocyte, bone growth, mechanobiology, microfluidics
I. Bone growth and growth plate chondrocytes
Bones are an essential support in vertebrates that primarily allow movement and protect internal organs. These functions require growth of hundreds of individual bones to achieve ideal size and shape, which is accomplished through the process of endochondral ossification of the growth plate cartilage (Fig. 1) [1–3].
Fig. 1. Endochondral bone generation (reproduced from [1] with permission).
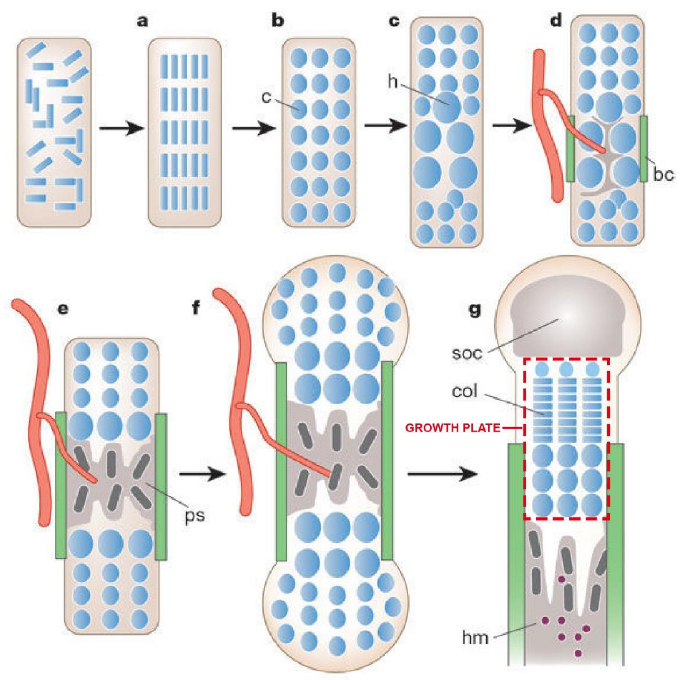
(a) Condensation of mesenchymal cells (blue). (b) Condensed cells developed into chondrocytes. (c) Hypertrophic chondrocytes are generated at the center of condensation. (d) Bone collar is formed, and hypertrophic chondrocytes induce mineralized extracellular matrix (ECM) and invasion of blood vessels. (e) Primary spongiosa is generated by osteoblasts. (f) Osteoblasts of bone collar and primary spongiosa become cortical bone and trabecular bone, respectively. (g) Secondary ossification center is formed at the end of bone and columns of chondrocytes are generated in the proliferation zone of growth plate. Haematopoietic marrow is generated in bone marrow space. c: chondrocytes, h: hypertrophic chondrocytes, bc: bone collar, ps: primary spongiosa, soc: secondary ossification center, col: columns of proliferating chondrocytes, hm: Haematopoietic marrow.
Endochondral bone generation starts with the condensation of loosely associated, fibroblast-like mesenchymal stem cells (MSCs) (Fig. 1 (a)) [1,4]. This condensation process involves cell-cell contact that produces regions of high cell density, prior to differentiating into chondrocytes. Chondrocytes secrete and assemble a complex extracellular matrix (ECM) containing type II collagen and proteoglycans such as aggrecan (Fig. 1 (b)) to form cartilage. This cartilage anlage provides an initial template for growth and development of bones of the appendicular skeleton and much of the axial skeleton, except for the head. Endochondral ossification converts these cartilage templates into bone. During endochondral ossification, chondrocytes progressively undergo hypertrophy [1–3,5], a state marked by volumetric swelling and production of type X collagen (Fig. 1 (c)). These hypertrophic chondrocytes induce mineralization of the surrounding ECM, release vascular endothelial growth factor to guide blood vessels into an adjacent ECM, and promote formation of the bone collar (Fig. 1 (d)). This mineralized cartilage is replaced by bone through coordinated removal of hypertrophic chondrocytes by cell death and by recruitment of osteoblasts and osteoclasts (Fig. 1 (e)). As the bone grows, osteoblasts of the bone collar and primary spongiosa (initially formed bone) become cortical bone and trabecular bone, respectively (Fig. 1 (f)). A secondary ossification center is formed at the end of the bone, and the region between the secondary ossification and the primary spongiosa is called the growth plate (Fig. 1 (g)).
The rate of bone growth is controlled by the growth plate cartilage, and elongation of bones ceases when the remaining cartilage is removed at the end of adolescence. A microscopic image of the rabbit tibial growth plate shown in Fig. 2 (a) illustrates key features of the growth plate that are expanded on in the accompanying schematic (Fig. 2 (b)). The growth plate consists of four different zones [6,7,3,8,5]: resting zone (= reserve zone), proliferative zone, prehypertrophic zone, and hypertrophic zone.
Fig. 2. Growth plate structure.
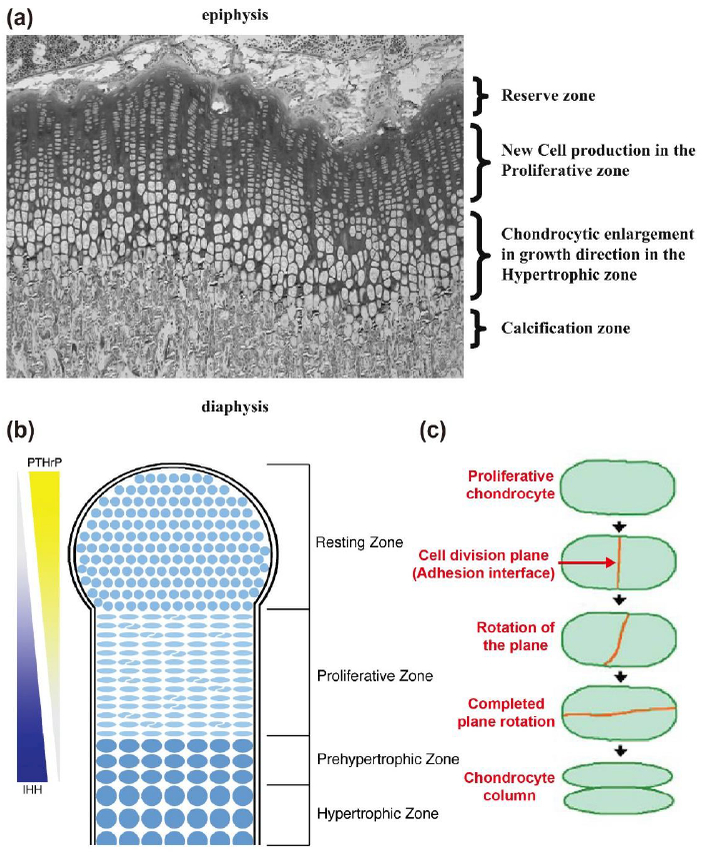
(a) An image of the rat tibial growth plate shows reserve zone (or resting zone), proliferative zone, and hypertrophic zone (reproduced from [8] with permission). (b) A schematic of growth plate structure (reproduced from [7] with permission). The function of growth plate is regulated by molecular signals of parathyroid hormone-related protein (PTHrP) and Indian Hedgehog (IHH). (c) Process of chondrocyte column formation (reproduced from [6] with permission).
Chondrocytes in the resting zone provide a continuous supply of cells to feed the growth process. Resting chondrocytes are progressively recruited into the proliferative zone. In this zone, the cell division rate increases, and the formerly unorganized ellipsoidal cells generate tight columns of discoidal chondrocytes, which involves rotation of the daughter cell pair (Fig. 2 (b) and (c)) [6,7]. Orientation of the chondrocyte columns determines the direction of bone growth. Following the proliferative phase, chondrocytes withdraw from the cell cycle and begin to enlarge in the prehypertrophic zone. Prehypertrophic chondrocytes form hypertrophic chondrocytes that greatly enlarge and secrete collagen X. Thus, the direction and extent of bone growth are determined by controlled proliferation, enlargement, and ECM secretion of chondrocytes in the growth plate.
Preservation of growth potential until the end of adolescence requires tight control over the rates of cell proliferation and chondrocyte hypertrophy, as well as the rate at which cells are recruited to subsequent zones. This chondrocyte development process is known as chondrocyte maturation. A complex network of interrelated signaling pathways regulates chondrocyte maturation [1,3,9]. At the core of this network lies a feedback interaction between the parathyroid hormone-related peptide (PTHrP) and the Indian hedgehog (IHH) secreted factor signaling pathways (Fig. 2 (b)).
PTHrP is generated by the chondrocytes in the superficial resting zone near the joint surface. PTHrP signaling preserves the resting population and promotes chondrocyte proliferation. In addition, PTHrP inhibits formation of hypertrophic chondrocytes and directly antagonizes IHH signaling. IHH is expressed by prehypertrophic chondrocytes on the other side of the growth plate. IHH signaling promotes hypertrophy of chondrocytes in the absence of PTHrP and cell proliferation in the presence of low levels of PTHrP. In addition, IHH maintains PTHrP production in resting chondrocytes. Thus, bone growth results from position-dependent signaling interactions that are generated by gradients of PTHrP and IHH. The signaling interactions establish zones of chondrocyte maturation and regulate the rate at which cells transit through each phase of maturation (Fig. 2 (b)).
This spatiotemporal growth factor control model of bone elongation has been the paradigm that has guided research in growth plate cartilage for the past two decades [1,3,10,11,9]. However, advances in articular cartilage biology have turned the attention of many to the potential regulation of growth by mechanical forces. Indeed, several recent papers have presented evidence supporting a primary role of mechanical forces in promoting normal growth plate cartilage function [12–14]. However, a strong counter-argument is provided by clinical observations of fetuses with paralyzing neuromuscular disease, in which muscular forces do not transfer to bones, show near normal bone growth but low bone density [15]. These clinical observations are consistent with mechanical forces being more crucial in process the of bone formation than in growth plate cartilage function.
To identify the missing link between mechanical forces and growth plate chondrocyte development, it is crucial to understand the current experimental approaches and results. In the next two sections, we first address various experimental tools and methods that have been employed in growth plate chondrocyte mechanobiology and then discuss what these methods revealed about how mechanical stress affects the structure and function of growth plate chondrocytes.
II. Experimental tools for mechanical stimulation of chondrocytes
In this section, the experimental tools for mechanically stimulating chondrocytes will be introduced. Because many tools have also been used on articular chondrocytes, we will include the mechanical stress generating systems used in mechanobiology studies for both growth plate chondrocytes and articular chondrocytes.
1. External fixator
Originally a surgical tool immobilizing the damaged bones, the external fixator has been used to exert static compression and static tension on growth plate cartilage in-vivo (Fig. 3). To accomplish this, two rings of the external fixator are fixed perpendicular to the axial direction of the growing bone with transfixing pins. The amount of static compression or tension is adjusted by controlling the length of the calibrated spring between the two fixed rings of the external fixator (Fig. 3 (a)) [13,16].
Fig. 3. External fixators.
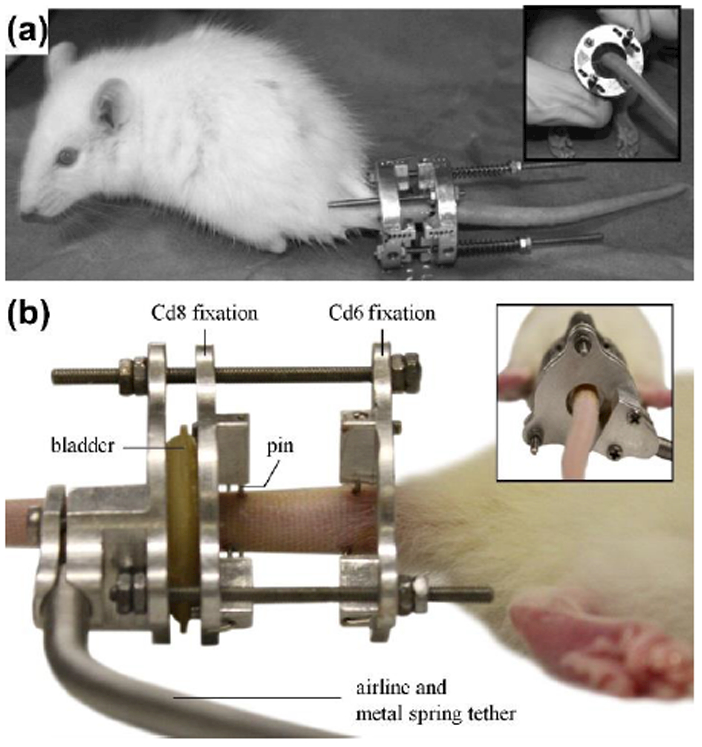
(a) An external fixator actuated by a calibrated spring. The magnitude of the applied force is proportional to the change in the spring length (reproduced from [16] with permission). (b-c) An external fixator actuated by pneumatic pressure (reproduced from [17] from permission). (b) The bladder operated by pressurized air pushes the one of the fixation to generate compression on the sample.
One caveat of using surgical fixators is the inability of the device to generate dynamic stress conditions. To address this issue, external fixators operated by pneumatic pressure were developed and applied (Fig. 3 (b)) [17,18]. These devices use pressurized air to inflate the latex bladder that pushes one of the fixation rings attached to the bone sample. The magnitude and frequency of stresses are manipulated by pressure changes in the latex bladder.
Although external fixators are useful tools for examining the effect of mechanical stress on cartilage, it is difficult to dissect the molecular mechanisms of mechanotransduction in chondrocytes due to the complex mechanical and chemical stimuli present in vivo. Thus, other tools have been developed for in-vitro and ex-vivo experiments.
2. Compression device
Various instruments have been developed to stimulate chondrocytes with compressive stress because compressive stress is one of the major forces acting on the chondrocytes in the growth plate and articular cartilage. Examples of compression devices are shown in Fig. 4.
Fig. 4. Macroscopic compression device to compress hydrogel-chondrocyte constructs or cartilage samples.
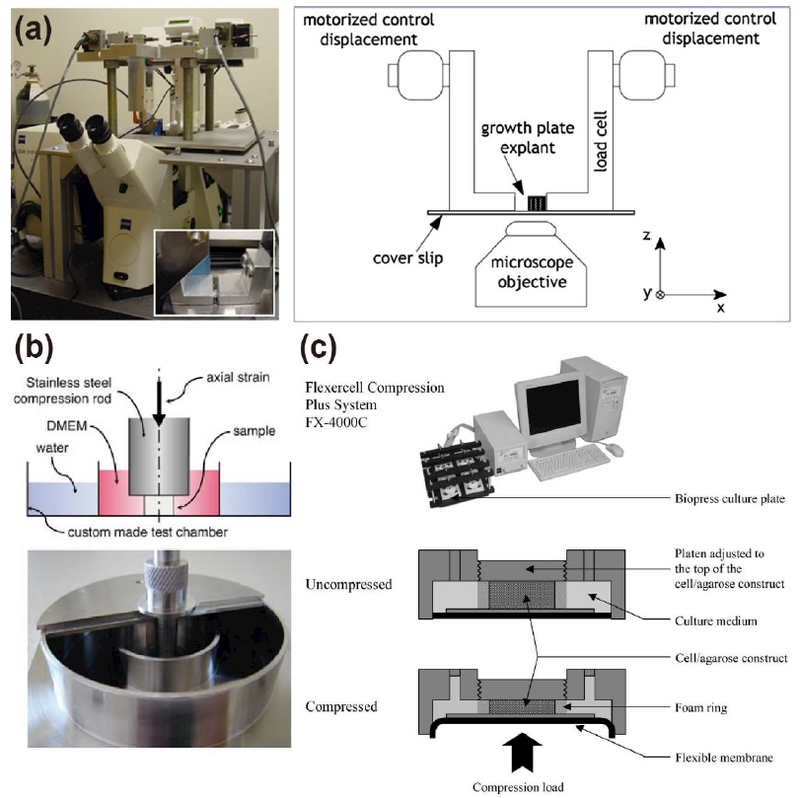
(a) A sample is compressed by two plungers actuated by a motorized actuator (reproduced from [19] with permission). (b) Mechanical testing equipment for unconfined compression test of a sample (reproduced from [23] with permission). (c) Bioreactor (FX-4000C™ Flexcell® Compression Plus™ System) actuated by air pressure to compress multiple samples (reproduced from [26]).
To visualize deformation of chondrocytes due to compression (Fig. 4 (a)), hydrogels with chondrocytes or whole cartilage explants are prepared in a hemi-cylindrical shape. The processed specimen is placed between two loading plates (Fig. 4 (a), inset), and the semi-confined compression is applied to the specimen with known displacement or stress [19–22]. Although this device simplifies the process for visualizing chondrocyte morphology, it may be inefficient for stimulating multiple samples for a long period of time due to equipment configuration.
The second type of compression device, which applies unconfined compression to the specimen, is shown in Fig. 4 (b). A cylindrical cartilage explant or hydrogel-chondrocyte construct in a culture medium is placed between the parallel plates of mechanical testing equipment, and the specimen is compressed with known magnitude of stress (or displacement) and frequency [23,24].
The above types of compression device cannot test multiple specimens at the same time. To test various samples simultaneously, the commercialized cell compression system (FX-4000C, Flexcell® International Corporation, NC) can be used instead (Fig. 4 (c)) [25,26]. The specimen is placed between top and bottom plates, and then the specimen is confined in lateral direction by foam ring. By inflating the flexible membrane underneath the bottom plate with pressurized air, the sample is compressed with specific magnitude and frequency. Although the device can accommodate multiple samples, a large amount of specimen, culture media and petri dishes is needed for the compression experiment.
3. Two-dimensional (2D) cell stretching device
Tensile stress is known to regulate the chondrocytes’ behaviors such as ECM production, proliferation, and their mechanical properties [25,27,28]. The effects of tensile stress on growth plate or articular chondrocytes were tested with 2D cell stretching devices. Figure 5 (a) shows 2D cell stretcher (Flexcell® Tension Systems, Flexcell® International Corporation, NC) [28]. The cells are cultured on thin elastic membrane, and the tension is applied to the cells by stretching the membrane. The magnitude and frequency of tension can be controlled by modulating vacuum pressure.
Fig. 5. Two-dimensional (2D) cell stretching device.
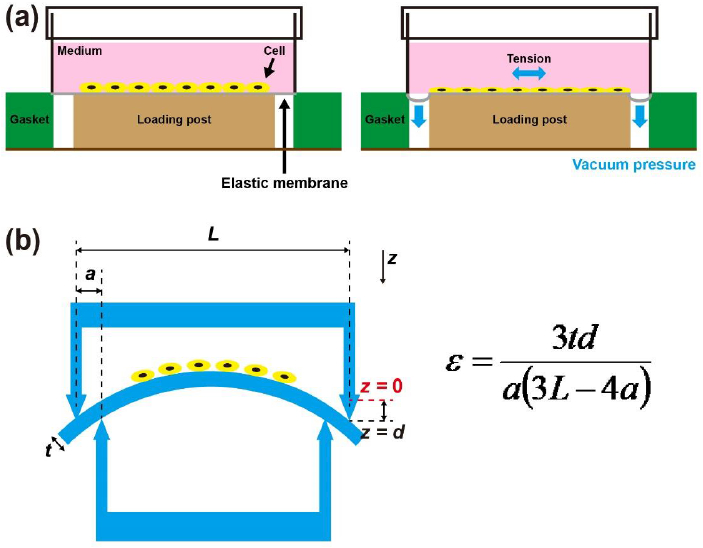
(a) 2D cell stretcher actuated by vacuum pressure. The cells are cultured on thin elastic membrane, and the tension is applied to the cells by pulling the thin elastic membrane with vacuum pressure (modified from Liu et al. [28]). (b) The elastic membrane bending system with four points. Depending on the relative height between inner and outer supporting points, the strain on the cell seeded elastic membrane can be manipulated. The strain (ε) can be calculated with the equation shown above. t is the thickness of elastic membrane, d is the displacement of loading part of the device, a is the distance between the inner and outer contact points, and L is the distance between outer contact points (modified from Sun et al. [27]).
Alternatively, tension can be applied to cells on an elastic membrane with a 4-points bending system (Fig. 5 (b)) [27,29]. Depending on the relative height difference between inner and outer supporting points, the strain on the cell seeded elastic membrane can be manipulated.
III. Mechanical stimuli and growth plate chondrocyte biology
Mechanical stresses have long been considered to be a major factor regulating bone growth. According to the Hueter-Volkmann law, additional compressive stress prohibits bone growth, whereas reducing compressive stress enhances bone growth [13,8]. There is growing evidence that mechanical stimulation affects bone growth by manipulating the growth plate function (Table 1). These experiments yielded varying results as expected because they were conducted under different in vivo, ex vivo, and in vitro conditions. Additionally, the experiments differed in type of stresses, frequency and magnitude of stresses, species, and bone of origin. However, several important trends were observed despite the many differences in experimental approach.
Table 1. Mechanical stimulations on growth plate chondrocytes.
↑: increase relative to control (or sham) group. ↓: decrease relative to control (or sham) group. —: negligible changes (or same) relative to control (or sham) group.
| Year | Reference | Subject | Samples | Experiment type | Tools | Type of stress | Parameters | Brief results [relative to control (or sham) group] | Findings |
|---|---|---|---|---|---|---|---|---|---|
| 1993 | Alberty et al. [30] | New Zealand white rabbits 5–6 weeks | Left distal femoral physis | in-vivo | External fixator | Static compression |
d = 0.5 mm/day (day ≤ 7 days) d = 3.5 mm (day ≥ 8 days) t = 3–21 days |
The height of proliferative and hypertrophic zones: ↓ The height of resting zone: - The orientation of chondrocyte columns: distorted The number of proliferating chondrocytes:↓ |
1. Static compression suppresses growth plate proliferative activity and change growth plate morphology. 2. The role of static tension on bone growth is not clear. |
| Static tension |
d = 0.7 mm/day, t = 3–21 days |
The Hight of proliferative and hypertrophic zones: ↑ The number of proliferating chondrocytes: - |
|||||||
| 2001 | Robling et al. [34] | Male Sprague Dawley rat | Ulna | in-vivo | Compression device | Static compression |
F = 17 N t = 10 min/day (days 1–5, 8–12) |
Length of ulna: ↓ 4% | 1. Peak load magnitude affects the growth suppression regardless of the type of compression and average load magnitude. 2. Thicker distal growth plate and accumulation of hypertrophic cell lacunae are related with growth suppression accumulation of hypertrophic cell lacunae are related with growth suppression. |
|
F = 8.5 N t = 10 min/day (days 1–5, 8–12) |
Length of ulna: ↓ 2% | ||||||||
| Dynamic compression |
F = 17 N f = 2 Hz t = 10 min/day (days 1–5, 8–12) |
Length of ulna: ↓ 4% | |||||||
| 2002 | Wang and Mao [36] | New Zealand white rabbits 6 weeks | Cranial base growth plate | in-vivo | Mechanical testing equipment connected to maxillary central incisors | Static tension |
F = 2 N t = 20 minutes/day (12 days) |
Height of growth plate: ↑ 16% Height of proliferation zone: ↑ 117% The number of proliferative chondrocytes: - |
1. Dynamic tension promotes chondral growth. |
| Dynamic tension |
F = 2 N f = 1 Hz t = 20 minutes/day (12 days) |
Height of growth plate: ↑ 54% Height of proliferation zone: ↑ 193% The number of proliferative chondrocytes: ↑ 65% |
|||||||
| 2002 | Stokes et al.[13] | Sprague Dawley rat | Caudal vertebra | in-vivo | External fixator | Static compression |
F = 60% of body weight (~ 0.736 N) t = 4 weeks |
Growth rate: ↓ 48% Height of hypertrophic zone: ↓ 13% Mean chondrocyte height: ↓ 15% |
1. The effect of static compression is greater than static tension in bone growth. 2. The change in height of hypertrophic chondrocytes is related to growth rate. |
| Static tension |
F = 60% of body weight (~ 0.736 N) t = 4 weeks |
Growth rate: ↑ 13% Height of hypertrophic zone:- Mean chondrocyte height: - |
|||||||
| 2006 | Akyuz et al. [32] | Sprague Dawley rat | Caudal vertebra | in-vivo | External fixator | Static compression |
F = 55% of body weight t = 3 weeks Asymmetric load |
Average wedge deformity: 10.3 ± 3.7° (sham / control group: 1.1 ± 20°/ 0.0 ± 1.0°) Longitudinal growth: 0.46 ± 0.19 mm (concavity) / 0.83 ± 0.32 mm (convexity) |
1. Dynamic compression has greater effects on the growth regulation. |
| Dynamic compression |
F = 55% of body weight f = 1.0 Hz t = 3 weeks Asymmetric load |
Average wedge deformity: 15.2 ± 6.4° Longitudinal growth: 0.34 ± 0.23 mm (concavity) / 0.86 ± 0.23 mm (convexity) |
|||||||
| 2007 | Stokes et al. [31] | Rat, 38 days Rabbit, 41 days Cattle, 48 days | Proximal tibia (Rat, cattle and rabbit) Caudal vertebra (Rat and cattle) | in-vivo | External fixator | Static compression |
P = 0.1 MPa/0.2 MPa t = 7 days |
Growth rate: ↓ | 1. The height of hypertrophic chondrocytes have a greater effect on growth rate than the number of proliferative chondrocytes. |
| Static tension |
P = 0.1 MPa t = 7 days |
Growth rate: ↑ | |||||||
| 2008 | Ueki et al. [25] | Male Wistar strain rat 4 weeks | Chondrocytes from rib growth plate | in-vitro | 2D cell stretcher | Dynamic tension (2D) |
P = 2 kPa f = 0.03 Hz t = 12 or 24 hours |
Chondrocyte proliferation: - Collagen and proteoglycan syntheses: - |
1. Chondrocyte metabolism improved due to increased frequency of tension. |
|
P = 2 kPa f = 0.5 Hz t = 12 or 24 hours |
Chondrocyte proliferation: ↑ Collagen and proteoglycan syntheses: ↑ |
||||||||
|
P = 2 kPa f = 2.5 Hz t = 12 or 24 hours |
Chondrocyte proliferation: ↑ Collagen and proteoglycan syntheses:↑ |
||||||||
| 2009 | Cancel et al. [16] | Male Sprague Dawley rat | 7th Caudal vertebra (Cd7) | in-vivo | External fixator | Static compression |
P = 0.2 MPa t = 2 weeks |
Longitudinal growth rate: ↓ 28% Growth plate thickness: ↓ 25% Amount of MMP-3 expression: ↑ Type II and X collagen: ↓ |
1. The growth of caudal vertebrae was suppressed by 2-week static compression. |
| 2011 | Valteau et al. [17] | Male Sprague Dawley rat | 7th Caudal vertebra (Cd7) | in-vivo | External fixator with pneumatic loading system | Static compression |
P = 0.2 MPa t = 2 weeks |
Growth rate: ↓ 21% Growth plate height: ↓ 20% Hypertrophic chondrocyte height: ↓ 25% The number of proliferative chondrocytes per column: ↓ 32% | 1. Both static and dynamic compression modulate bone growth effectively. 2. Dynamic compression is less harmful on growth plate morphology than static compression. |
| Dynamic compression |
P = 0.2 ± 0.06 MPa f = 0.1 Hz t = 2 weeks |
Growth rate: ↓ 21% Growth plate height: ↓ 11% Hypertrophic chondrocyte height: ↓ 15% The number of proliferative chondrocytes per column: ↓ 19% |
|||||||
| 2011 | Sergerie et al. [23] | Swine 4 weeks | Distal ulnae (cylindrical explant, D = 6 mm, h = 3.04 ± 0.54 mm) | ex-vivo | Compression device with parallel plate | Static compression |
ε = 10% t = 2 days |
Chondrocyte column: structure is reserved Aggrecan, type II collagen, type X collagen, and MMP13:↓ |
1. Growth plate behaves differently depending on the type of compressive stress. 2. Dynamic compression increases the expression of extracellular matrix components. |
| Dynamic compression |
ε = 7 and 13% f = 0.1 Hz t = 2 days |
Chondrocyte column: structure is distorted Aggrecan and type II collagen:↑ |
|||||||
| 2014 | Menard et al. [35] | Male Sprague Dawley rat 4 weeks | 7th Caudal vertebra (Cd7) | in-vivo | External fixator with pneumatic loading system | Dynamic compression |
P = 0.2 ± 0.06 MPa f = 1.0 Hz t = 15 days |
Growth rate: ↓ 16% Growth plate height: ↓ 17% Hypertrophic chondrocyte height: ↓ 14% The number of proliferative chondrocytes per column: ↓15% |
1. Increase in both magnitude and frequency of the stress destroys the integrity of growth plate. |
|
P = 0.2 ± 0.2 MPa f = 0.1 Hz t = 15 days |
Growth rate: ↓ 17% Growth plate height: ↓ 8% Hypertrophic chondrocyte height: ↓ 11% The number of proliferative chondrocytes per column: ↓ 13% |
||||||||
|
P = 0.2 ± 0.14 MPa f = 1.0 Hz t = 15 days |
Tissues were damaged. | ||||||||
| 2015 | Kaviani et al. [24] | Swine 4 weeks | Distal ulnae (cylindrical explant) D = 6 mm h = 9 mm | ex-vivo | Compression device with parallel plates | Static compression | P = 0.1 MPa / t = 12 hours | High stress:↓ viability of chondrocytes High frequency:↓ viability of chondrocytes Long loading time: ↓ viability of chondrocytes |
1. Chondrocyte viability significantly decreases under the static compression with long loading time. 2. Proliferative and hypertrophic chondrocytes are susceptible to the stress. |
| P = 0.2 MPa / t = 12 hours | |||||||||
| P = 0.1 MPa / t = 24 hours | |||||||||
| Dynamic compression | P = 0.1 ± 0.03 MPa /f = 0.1 Hz t = 12 hour | ||||||||
| P = 0.2 ± 0.06 MPa /f = 0.1 Hz t = 12 hour | |||||||||
| P = 1.0 ± 0.03 MPa /f = 0.1 Hz t = 24 hour | |||||||||
| P = 0.1 ± 0.03 MPa /f = 0.1 Hz t = 12 hour | |||||||||
| P = 0.1 ± 0.1 MPa /f = 0.1 Hz t = 12 hour | |||||||||
| 2017 | Zimmermann et al. [20] | Male Sprague Dawley rat 27–30 days | Growth plate explants from tibia | ex-vivo | Compression device with parallel plate | Static compression |
P = 0.2 MPa t = 2 hours |
Hypertrophic chondrocytes compressed more in lateral direction than axial direction | 1. Axial strains of hypertrophic chondrocytes are similar in all stress conditions. 2. Volumetric strains of cells decrease when the frequency becomes higher. |
| Dynamic compression |
P = 0.2 ± 0.06 MPa f = 0.1 Hz t = 2 hours |
Hypertrophic chondrocytes maintained their shape under dynamic compression | |||||||
|
P = 0.2 ± 0.06 MPa f = 1.0 Hz t = 2 hours |
Hypertrophic chondrocytes maintained their shape under dynamic compression | ||||||||
| 2017 | Sun et al. [27] | Human (children with thumb duplication) | Chondrocytes from the growth plate of multi-fingered phalange | in-vitro | 2D cell stretcher | Dynamic 2D stretching |
ε= 0.2% f = 0.5 Hz t = 6 hours |
Type 2 collagen, Type 10 collagen, and PTHrP: ↑ | 1. Proper amount of stretch promotes proliferation and differentiation of chondrocytes. 2. Excessive amount of stretch inhibits proliferation and differentiation of chondrocytes. 3. Excessive amount of stretch may induce cell death. |
|
ε= 0.4% f = 0.5 Hz t = 6 hours |
Type 2 collagen, Type 10 collagen, and PTHrP: ↓ |
1. Static stimulation
Alberty et al. examined the effect of static compression and tension on the structural changes in the growth plates of distal femur of rabbits [30]. Static compression suppressed proliferation of chondrocytes, shortened the proliferative and hypertrophic zones, and disrupted tissue architecture; all three of which could reduce longitudinal growth. In contrast, tension increased the height of the proliferative and hypertrophic zones initially. However, the effect of tension progressively decreased with time, and ultimately, the height of both proliferative and hypertrophic zones did not show significant differences from controls.
Stokes et al. tested the effects of static compression and tension on the growth plate of rats’ vertebrae [13]. The growth rate of the vertebrae decreased by 48% with static compression and increased by 13% with static tension as compared to the growth of untreated vertebrae. Static compression also decreased the height of the hypertrophic zone by decreasing the size of individual hypertrophic chondrocytes. Neither hypertrophic zone height nor hypertrophic cell size changed significantly under the static tension. The fact that height of hypertrophic chondrocytes had greater impact on bone growth than the number of proliferative chondrocytes is in strong agreement with earlier studies demonstrating that chondrocyte hypertrophy is the primary driver of growth [13,31].
Together, these studies (Table 1) reveal two interesting findings. First, reduction in height of the growth plate [13,16,14], height of hypertrophic chondrocytes [19,31], number of chondrocytes in proliferative columns [31], and misalignment of chondrocytes columns [30,16] parallels changes in bone growth rate [32,33,16,31], supporting the idea that the growth plate cartilage is the primary mediator of mechanical effects on growth. Second, because growth plate cartilage is affected by additional compression but is not significantly altered by tension, it is thought that compression from gravitational force is below the effective threshold for negatively affecting growth plate cartilage. It remains to be determined whether normal gravitational force is a growth stimulator; but if this were the case, it might be expected that tension would decrease growth.
2. Static stimulation vs. dynamic stimulation
However, one has to be careful not to over-interpret these data sets because static compression and static tension do not model normal activity of the growth plate or articular cartilages. During normal activity, growth plate cartilage and articular cartilage undergo alternating periods of mechanical stress and relaxation (dynamic compression).
Valteau et al. compared effects of two week-long static (0.2 MPa) and dynamic compression (0.2 ± 0.06 MPa, 0.1 Hz) on the caudal vertebra of the rat [17]. The height of the growth plate and hypertrophic chondrocytes decreased to a greater extent in conditions of static compression than in dynamic compression. Moreover, the number of proliferative chondrocytes per column also decreased more under static compression than dynamic compression. Furthermore, Sergerie et al. showed that dynamic compression distorted alignment of chondrocyte columns [23]. Together, these data suggest that compressive stress is detrimental to growth plate cartilage whether applied statically or dynamically in vivo. It remains to be determined whether dynamic compression is less damaging because it stimulates anabolic production of cartilage ECM proteins [23].
The parametric studies of static and dynamic compressions have revealed several other interesting results. First, the peak magnitude of compression results in the growth suppression regardless of type of compression and average magnitude of compression [34]. Second, increase in magnitude, frequency, and loading duration of compression destroys the integrity of the growth plate and lowers the viability of the growth plate chondrocytes [35,24]. Last, the volumetric strains of growth plate chondrocytes decrease more under higher frequency compression [20].
Regarding the effects of dynamic tension, Wang et al. revealed that the dynamic tension promoted chondral growth because the height of growth plate and the number of proliferative chondrocytes increased more under dynamic tension than static tension [36]. The frequency of dynamic tension is an also important factor in bone growth modulation. The chondrocyte synthesized more collagen and proteoglycan under the dynamic tension of higher frequency [25]. However, extreme magnitude of dynamic tension inhibited proliferation and differentiation of growth plate chondrocytes and induced cell death [27].
Collectively, data obtained from different in vivo, ex vivo, and in vitro systems using growth plate cartilage from different species and bones strongly argue that compressive forces negatively affect growth plate cartilage function. Paradoxically, mechanical loading appears important in the postnatal mouse as paralysis (e.g., without mechanical loading on limbs) leads to small but significant differences in limb growth. It remains to be determined whether the differences between these mouse studies and the large number of studies described in this review result from peculiarities of the mouse or to the various methods for loading/unloading limbs.
By contrast, review of the literature clearly demonstrates that tension, dynamic tension in particular, stimulates anabolic metabolism and promotes growth in growth plate chondrocytes. While interesting and possibly applicable to tissue engineering, it is not obvious that tensile stresses are relevant in the context of the growth plate cartilage. Although the cited studies reveal important general principles of mechanobiology, little is known about the molecular pathways of mechanotransduction in growth plate cartilage.
3. Mechanotransduction pathways
Some classical molecular pathways have been tested to understand growth plate biology. For example, Rho GTPases modify the actin cytoskeleton network by controlling the activity of actin polymerizing (e.g., ARP2/3) and depolymerizing (e.g., Cofilin) proteins via various kinases (e.g., ROCK and LIM kinase). RhoA is one of the Rho GTPases family members, and RhoA/ROCK signaling promotes chondrocyte proliferation and decreases chondrocyte hypertrophy [37]. In addition, cell adhesion surface plays an important role in growth plate cartilage formation because cell-cell adhesion through N-cadherin guides chondrogenesis which converts MSCs into chondrocytes [38], and cell adhesion surfaces with cadherins and β-catenin are essential for chondrocyte column formation in the proliferative zone [6]. Although the parts of classical molecular pathways were identified, there are still significant gaps in knowledge on how mechanical signals are converted into biochemical signals for maintaining growth plate function and architecture. Thus, it is critical to examine the chondrocyte behaviors quantitatively in the controlled mechanical environment.
IV. Potential approaches for studying chondrocytes’ mechanobiology
Although previous studies have revealed valuable information regarding the mechanobiology of the growth plate and the growth plate chondrocytes as summarized in Section III, the tested stress conditions are not enough to fully understand mechanobiological responses of chondrocytes as few detailed studies have been performed on single chondrocyte behaviors under mechanical stimulation. Moreover, composition of the cellular environment might alter chondrocyte response to mechanical stimuli. The following questions cannot be definitively determined using the experimental methodology of previous studies: how cells sense mechanical stresses, what signaling pathways transduce the signals, and how the collective information is integrated and interpreted to determine cell response.
Macroscopic mechanical stimulation devices have been widely used to understand the growth plate chondrocyte mechanobiology as summarized in Section II. However, they are not suitable for rapid tests of various stress conditions on multiple samples because the conventional mechanical testing equipment can examine limited numbers of specimen and stress conditions in a single test. Also, many of the previous mechanical stimulation methods do not provide a way to examine the behavior of living growth plate chondrocytes in real time. Those limitations in conventional approaches can be improved by using micro-engineered devices such as microfluidic devices.
A microfluidic device, which manipulates small volumes of fluid to achieve a desired function, has been used in many research areas including mechanobiology studies, disease detection, particle separations, and material synthesis because of its unique advantages over the conventional experimental tools. First, the microfluidic device is more cost-effective than the conventional macroscale equipment because its micron-size channel dimension requires a small amount of a sample to achieve its function. Second, various magnitudes of mechanical stresses can be generated on a single device by changing the channel dimensions and fluid motions. Third, regulating the concentration gradient of chemicals in the microfluidic device is feasible because the mixing in the microfluidic device is limited. Last, various biological materials including cells and artificial ECM can be integrated into the microfluidic device. Above characteristics of the microfluidic device are useful in generating various mechanical and chemical stimulations on cells or tissues in physiological environment for cell biology studies.
Microfluidic devices can generate different types of mechanical stresses as shown in Fig. 6. Different magnitudes of tensile stresses were generated in a single microfluidic platform simultaneously for the high throughput test of cellular behavior under tension (Fig. 6 (a)) [39]. The amounts of tensile stresses were adjusted by injecting pressurized air into actuation cavities of various sizes. This approach is significantly more efficient than the conventional 2D cell stretching devices shown in Fig. 5. Moreover, the microfluidic device can generate controlled magnitudes of compression on a fine structure of the cell. As an example, the axonal injury model was generated by compressing axons with microfluidic valve systems (Fig. 6 (b)) [40]. This cell compression device is a valuable tool for examining the detailed mechanism of axon damage. Modulation of the intensity of shear stress is easily performed with microfluidic devices by changing the dimensions of channels and volume flow rate of working fluid. With the microfluidic shear stress generator, adhesion strength of endothelial cells on different ECM molecules was tested in parallel (Fig. 6 (c) [41]. With this microfluidic device, both mechanical stresses and the mechanical properties of an artificial ECM can be controlled independently. For example, a precise gradient of ECM stiffness was generated on a polyacrylamide hydrogel by controlled mixing monomers and cross-linkers in the microfluidic platform (Fig. 6 (d) [42].
Fig. 6. Examples of microfluidic devices used in cell biology studies.

(a) Microfluidic device generating multiple tensions in a single platform. (reproduced from [39] with permission). (b) Singe axon injury model generated in a microfluidic device. (reproduced from [40] with permission). (c) Multi channel shear stress generator. (reproduced from [41] with permission). (d) Stiffness gradient generator on a hydrogel (reproduced from [42] with permission). (e) Lung-on-a-chip device which mimics the physiology of alveolar-capillary interface of lung (reproduced from [44] with permission).
One of the emerging technologies achieved with the microfluidic device is mimicking the organ-level structures and physiological stresses on a chip (organ-on-a-chip) [43]. The basic structure of human lung (alveolar-capillary interface) was mimicked in the lung-on-a-chip device (Fig. 6 (e)) [44]. The multiple mechanical (e.g., shear stress and tensile stress), chemical (e.g., inflammatory cytokines), biological (e.g., living Escherichia coli) and nanotoxicological (e.g., nanoparticles) stimuli were generated in the lung-on-a-chip device, and the artificial lung in the microfluidic device was proven to respond as a human lung.
The aforementioned versatility of the microfluidic devices makes them become important tools in mechanobiology studies of cells and tissues. It is possible to create an in-vivo like environment [43–48] and to scale down mechanical testing to look at single cell mechanics with the microfluidic devices. Also, various levels of mechanical stimuli can be generated on multiple samples simultaneously in a single microfluidic platform [39,49–53]. Moreover, various mechanical cellular environments (e.g., substrate stiffness and geometric confinement) can be incorporated into the microfluidics. Those capabilities are not usually accessible with conventional macroscopic experimental tools used in mechanical stimulations on growth plate chondrocytes. Therefore, the details of growth plate chondrocytes behavior under the mechanical stimuli can be studied better by applying the microfluidic platform.
ACKNOWLEDGMENT
This study was supported by Bioengineering for Human Health grant from the University of Nebraska-Lincoln (UNL) and the University of Nebraska Medical Centre (UNMC), and grant AR070242 from the NIH/NIAMS.
Footnotes
The authors declare no competing financial interest.
REFERENCES
- 1.Kronenberg HM (2003) Developmental regulation of the growth plate. Nature 423 (6937):332. [DOI] [PubMed] [Google Scholar]
- 2.DeLise AM, Fischer L, Tuan RS (2000) Cellular interactions and signaling in cartilage development. Osteoarthr Cartil 8 (5):309–334 [DOI] [PubMed] [Google Scholar]
- 3.Roselló-Díez A, Joyner AL (2015) Regulation of long bone growth in vertebrates; it is time to catch up. Endocr Rev 36 (6):646–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall BK, Miyake T (2000) All for one and one for all: condensations and the initiation of skeletal development. Bioessays 22 (2):138–147 [DOI] [PubMed] [Google Scholar]
- 5.Yılmaz G (2016) Growth Plate In: Musculoskeletal Research and Basic Science. Springer, pp 357–366 [Google Scholar]
- 6.Romereim SM, Conoan NH, Chen B, Dudley AT (2014) A dynamic cell adhesion surface regulates tissue architecture in growth plate cartilage. Development 141 (10):2085–2095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Romereim SM, Dudley AT (2011) Cell polarity. Organogenesis 7 (3):217–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villemure I, Stokes IAF (2009) Growth plate mechanics and mechanobiology. A survey of present understanding. J Biomech 42 (12):1793–1803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kozhemyakina E, Lassar AB, Zelzer E (2015) A pathway to bone: signaling molecules and transcription factors involved in chondrocyte development and maturation. Development 142 (5):817–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mak KK, Kronenberg HM, Chuang P-T, Mackem S, Yang Y (2008) Indian hedgehog signals independently of PTHrP to promote chondrocyte hypertrophy. Development 135 (11):1947–1956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao B, Song H, Bishop K, Elliot G, Garrett L, English MA, Andre P, Robinson J, Sood R, Minami Y, Economides AN, Yang Y (2011) Wnt signaling gradients establish planar cell polarity by inducing Vangl2 phosphorylation through Ror2. Dev Cell 20 (2):163–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Killion CH, Mitchell EH, Duke CG, Serra R (2017) Mechanical loading regulates organization of the actin cytoskeleton and column formation in postnatal growth plate. Mol Biol Cell 28 (14):1862–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stokes IA, Mente PL, Iatridis JC, Farnum CE, Aronsson DD (2002) Enlargement of growth plate chondrocytes modulated by sustained mechanical loading. J Bone Joint Surg Am 84 (10):1842–1848 [DOI] [PubMed] [Google Scholar]
- 14.Reich A, Jaffe N, Tong A, Lavelin I, Genina O, Pines M, Sklan D, Nussinovitch A, Monsonego-Ornan E (2005) Weight loading young chicks inhibits bone elongation and promotes growth plate ossification and vascularization. J Appl Physiol 98 (6):2381–2389 [DOI] [PubMed] [Google Scholar]
- 15.Rodríguez JI, Palacios J, García-Alix A, Pastor I, Paniagua R (1988) Effects of immobilization on fetal bone development. A morphometric study in newborns with congenital neuromuscular diseases with intrauterine onset. Calcif Tissue Int 43 (6):335–339 [DOI] [PubMed] [Google Scholar]
- 16.Cancel M, Grimard G, Thuillard-Crisinel D, Moldovan F, Villemure I (2009) Effects of in vivo static compressive loading on aggrecan and type II and X collagens in the rat growth plate extracellular matrix. Bone 44 (2):306–315 [DOI] [PubMed] [Google Scholar]
- 17.Valteau B, Grimard G, Londono I, Moldovan F, Villemure I (2011) In vivo dynamic bone growth modulation is less detrimental but as effective as static growth modulation. Bone 49 (5):996–1004 [DOI] [PubMed] [Google Scholar]
- 18.Walsh AJL, Lotz JC (2004) Biological response of the intervertebral disc to dynamic loading. J Biomech 37 (3):329–337 [DOI] [PubMed] [Google Scholar]
- 19.Amini S, Veilleux D, Villemure I (2010) Tissue and cellular morphological changes in growth plate explants under compression. J Biomech 43 (13):2582–2588 [DOI] [PubMed] [Google Scholar]
- 20.Zimmermann EA, Bouguerra S, Londoño I, Moldovan F, Aubin C-É, Villemure I (2017) In situ deformation of growth plate chondrocytes in stress-controlled static vs dynamic compression. J Biomech 56:76–82 [DOI] [PubMed] [Google Scholar]
- 21.Guilak F (1995) Compression-induced changes in the shape and volume of the chondrocyte nucleus. J Biomech 28 (12):1529–1541 [DOI] [PubMed] [Google Scholar]
- 22.Knight MM, Ghori SA, Lee DA, Bader DL (1998) Measurement of the deformation of isolated chondrocytes in agarose subjected to cyclic compression. Med Eng Phys 20 (9):684–688 [DOI] [PubMed] [Google Scholar]
- 23.Sergerie K, Parent S, Beauchemin P-F, Londoño I, Moldovan F, Villemure I (2011) Growth plate explants respond differently to in vitro static and dynamic loadings. J Orthop Res 29 (4):473–480 [DOI] [PubMed] [Google Scholar]
- 24.Kaviani R, Londono I, Parent S, Moldovan F, Villemure I (2015) Compressive mechanical modulation alters the viability of growth plate chondrocytes in vitro. J Orthop Res 33 (11):1587–1593 [DOI] [PubMed] [Google Scholar]
- 25.Ueki M, Tanaka N, Tanimoto K, Nishio C, Honda K, Lin Y-Y, Tanne Y, Ohkuma S, Kamiya T, Tanaka E, Tanne K (2008) The effect of mechanical loading on the metabolism of growth plate chondrocytes. Ann Biomed Eng 36 (5):793–800 [DOI] [PubMed] [Google Scholar]
- 26.Bougault C, Paumier A, Aubert-Foucher E, Mallein-Gerin F (2008) Molecular analysis of chondrocytes cultured in agarose in response to dynamic compression. BMC Biotechnol 8 (1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun K, Liu F, Wang J, Guo Z, Ji Z, Yao M (2017) The effect of mechanical stretch stress on the differentiation and apoptosis of human growth plate chondrocytes. In Vitro Cell Dev Biol-Animal 53 (2):141–148 [DOI] [PubMed] [Google Scholar]
- 28.Liu Q, Hu X, Zhang X, Duan X, Yang P, Zhao F, Ao Y (2016) Effects of mechanical stress on chondrocyte phenotype and chondrocyte extracellular matrix expression. Sci Rep 6:37268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Draper ERC, Goodship AE (2003) A novel technique for four-point bending of small bone samples with semi-automatic analysis. J Biomech 36 (10):1497–1502 [DOI] [PubMed] [Google Scholar]
- 30.Alberty A, Peltonen J, Ritsilä V (1993) Effects of distraction and compression on proliferation of growth plate chondrocytes: A study in rabbits. Acta Orthop Scand 64 (4):449–455 [DOI] [PubMed] [Google Scholar]
- 31.Stokes IAF, Clark KC, Farnum CE, Aronsson DD (2007) Alterations in the growth plate associated with growth modulation by sustained compression or distraction. Bone 41 (2):197–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akyuz E, Braun JT, Brown NAT, Bachus KN (2006) Static versus dynamic loading in the mechanical modulation of vertebral growth. Spine 31 (25):E952–E958 [DOI] [PubMed] [Google Scholar]
- 33.Aronsson DD, Stokes IAF, Rosovsky J, Spence H (1999) Mechanical modulation of calf tail vertebral growth: implications for scoliosis progression. Clin Spine Surg 12 (2):141–146 [PubMed] [Google Scholar]
- 34.Robling AG, Duijvelaar KM, Geevers JV, Ohashi N, Turner CH (2001) Modulation of appositional and longitudinal bone growth in the rat ulna by applied static and dynamic force. Bone 29 (2):105–113 [DOI] [PubMed] [Google Scholar]
- 35.Ménard A-L, Grimard G, Valteau B, Londono I, Moldovan F, Villemure I (2014) In vivo dynamic loading reduces bone growth without histomorphometric changes of the growth plate. J Orthop Res 32 (9):1129–1136 [DOI] [PubMed] [Google Scholar]
- 36.Wang X, Mao JJ (2002) Accelerated chondrogenesis of the rabbit cranial base growth plate by oscillatory mechanical stimuli. J Bone Miner Res 17 (10):1843–1850 [DOI] [PubMed] [Google Scholar]
- 37.Wang G, Woods A, Sabari S, Pagnotta L, Stanton L-A, Beier F (2004) RhoA/ROCK signaling suppresses hypertrophic chondrocyte differentiation. J Biol Chem 279 (13):13205–13214 [DOI] [PubMed] [Google Scholar]
- 38.Delise AM, Tuan RS (2002) Analysis of N-cadherin function in limb mesenchymal chondrogenesis in vitro. Dev Dyn 225 (2):195–204 [DOI] [PubMed] [Google Scholar]
- 39.Moraes C, Chen J-H, Sun Y, Simmons CA (2010) Microfabricated arrays for high-throughput screening of cellular response to cyclic substrate deformation. Lab Chip 10 (2):227–234 [DOI] [PubMed] [Google Scholar]
- 40.Hosmane S, Fournier A, Wright R, Rajbhandari L, Siddique R, Yang IH, Ramesh KT, Venkatesan A, Thakor N (2011) Valve-based microfluidic compression platform: single axon injury and regrowth. Lab Chip 11 (22):3888–3895 [DOI] [PubMed] [Google Scholar]
- 41.Young EWK, Wheeler AR, Simmons CA (2007) Matrix-dependent adhesion of vascular and valvular endothelial cells in microfluidic channels. Lab Chip 7 (12):1759–1766 [DOI] [PubMed] [Google Scholar]
- 42.Byfield FJ, Wen Q, Levental I, Nordstrom K, Arratia PE, Miller RT, Janmey PA (2009) Absence of filamin a prevents cells from responding to stiffness gradients on gels coated with collagen but not fibronectin. Biophys J 96 (12):5095–5102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huh D, Hamilton GA, Ingber DE (2011) From 3D cell culture to organs-on-chips. Trends Cell Biol 21 (12):745–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE (2010) Reconstituting organ-level lung functions on a chip. Science 328 (5986):1662–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moraes C, Mehta G, Lesher-Perez SC, Takayama S (2012) Organs-on-a-chip: a focus on compartmentalized microdevices. Ann Biomed Eng 40 (6):1211–1227 [DOI] [PubMed] [Google Scholar]
- 46.Huh D, Kim HJ, Fraser JP, Shea DE, Khan M, Bahinski A, Hamilton GA, Ingber DE (2013) Microfabrication of human organs-on-chips. Nat Protocols 8 (11):2135–2157 [DOI] [PubMed] [Google Scholar]
- 47.Bhatia SN, Ingber DE (2014) Microfluidic organs-on-chips. Nat Biotech 32 (8):760–772 [DOI] [PubMed] [Google Scholar]
- 48.Lee JS, Romero R, Han YM, Kim HC, Kim CJ, Hong J-S, Huh D (2016) Placenta-on-a-chip: a novel platform to study the biology of the human placenta. J Matern Fetal Neonatal Med 29 (7):1046–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moraes C, Wang G, Sun Y, Simmons CA (2010) A microfabricated platform for high-throughput unconfined compression of micropatterned biomaterial arrays. Biomaterials 31 (3):577–584 [DOI] [PubMed] [Google Scholar]
- 50.Moraes C, Zhao R, Likhitpanichkul M, Simmons C,A Sun Y (2011) Semi-confined compression of microfabricated polymerized biomaterial constructs. J Micromech Microeng 21 (5):054014 [Google Scholar]
- 51.Birukov KG, Birukova AA, Dudek SM, Verin AD, Crow MT, Zhan X, DePaola N, Garcia JGN (2002) Shear stress-mediated cytoskeletal remodeling and cortactin translocation in pulmonary endothelial cells. Am J Respir Cell Mol Biol 26 (4):453–464 [DOI] [PubMed] [Google Scholar]
- 52.Wojciak-Stothard B, Ridley AJ (2003) Shear stress–induced endothelial cell polarization is mediated by Rho and Rac but not Cdc42 or PI 3-kinases. J Cell Biol 161 (2):429–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lu H, Koo LY, Wang WM, Lauffenburger DA, Griffith LG, Jensen KF (2004) Microfluidic shear devices for quantitative analysis of cell adhesion. Anal Chem 76 (18):5257–5264 [DOI] [PubMed] [Google Scholar]


