Abstract
Indoleamine 2,3-dioxygenase (IDO), an intracellular enzyme responsible for catalyzing the rate limiting step of tryptophan catabolism, plays a critical role in immune cell suppression and tolerance. Indoleamine 2,3-dioxygenase-mediated depletion of the essential amino acid tryptophan increases susceptibility of T cells to apoptosis, while kynurenine and its downstream metabolites, such as 3-hydroxyanthranilic acid and quinolinic acid, have a direct cytotoxic effect on conventional effector T cells. Additionally, IDO-expressing antigen presenting cells (APCs) induce proliferation of regulatory T cells. When expressed by an APC, the immunosuppressive effects of IDO may act directly on the APC as well as indirectly upon local T cells. One approach to elicit immune tolerance or reduce inflammation therefore is to promote expression of IDO. However, this approach is constrained by several factors including the potential for deleterious biologic effects of conventional IDO-inducing agents such as interferon gamma (IFNγ), and the potential limitations of constitutive gene transfection. Alternatively, direct action of recombinant IDO enzyme supplied exogenously as a potential therapeutic in the extracellular space has not been investigated previously, and is the focus of this work. Results indicate exogenous recombinant human IDO supplementation influences murine dendritic cell (DC) maturation and ability to suppress antigen specific T cell proliferation. Following treatment, DCs were refractory to maturation by LPS as defined by co-stimulatory molecule expression (CD80 and CD86) and major histocompatibility complex II (MHC-II) expression. Dendritic cells exhibited skewing toward an anti-inflammatory cytokine release profile, with reduced secretion of IL-12p70 and maintained basal level of secreted IL-10. Notably, IDO-treated DCs suppressed proliferation of ovalbumin (OVA) antigen-specific CD4+ and CD8+ T cells in the presence of cognate antigen presentation in a manner dependent on active enzyme, as introduction of IDO inhibitor 1-methyl-tryptophan, restored T cell proliferation. Defined media experiments indicate a cumulative role for both tryptophan depletion and kynurenine presence, in the suppressive programming of DCs. In sum, we report that exogenously supplied IDO maintains immunoregulatory function on DCs, suggesting that IDO may have potential as a therapeutic protein for suppressive programming with application toward inflammation and tolerance.
Keywords: Immunometabolism; Tryptophan; Kynurenine; Suppression; Indoleamine 2,3-Dioxygenase; Dendritic cells
Summary sentence:
Indoleamine 2,3-dioxygenase functions as an extracellular immunomodulator
INTRODUCTION
Dendritic cells (DCs) are professional antigen presenting cells (APCs) and key regulators of the immune system. They continually encounter, process, and present antigen to naïve T cells in a variety of contexts, shaping T and B cell responses through surface bound and soluble factors [1]. While DCs play a crucial role in the initiation of inflammatory responses, they are also able to induce tolerogenic or anti-inflammatory outcomes. DCs induce tolerance through various mechanisms, including the increased secretion of anti-inflammatory cytokines, decreased secretion of pro-inflammatory cytokines, downregulation of stimulatory and co-stimulatory molecules and the upregulation of the intracellular enzyme indoleamine 2,3-dioxygenase (IDO) [2]. Therefore, agents which modulate DC phenotype may redirect the immune system toward suppressive response, an approach particularly advantageous for inflammation and autoimmune diseases. A key feature of one type of tolerogenic DC is increased expression of IDO [3, 4], the enzyme catalyzing the rate limiting step of catabolic tryptophan conversion to kynurenines.
Indoleamine 2,3-dioxygenase was first discovered, and is expressed in the placenta, where it contributes to tolerance of the fetus by the maternal immune system [3]. In vivo inhibition of IDO with 1-methyl-tryptophan (MT), a competitive inhibitor for catabolism of L-tryptophan, has been shown to induce fetal rejection in a murine model [5]. IDO is found at low levels, particularly in lymphoid organs, the spleen, thymus, lungs and digestive tract in healthy individuals, and increases during resolution of infection, and inflammation [6]. Expression of IDO can be induced by lipopolysaccharides (LPS), interferon-γ (IFN-γ) and other agents [3, 7] as well as through gene transfection [8]. Indoleamine 2,3-dioxygenase participates in modulation of T cell responses toward a suppressive lineage [9–14] by initiation of apoptosis, induction of anergy and limitations on the activity of effector T cells, and by the induction of regulatory T cells (Tregs) [12–17]. Two proposed mechanisms for IDO-mediated suppressive effects have emerged: (i) depletion of tryptophan suppresses T cell proliferation by activating the general control nonderepressible 2 (GCN2) stress-response kinase which controls transcriptional and translational processes coupling cell growth to amino acid availability, known as the integrated stress response [18–20]; and (ii) downstream metabolites (collectively referred to as kynurenines) directly interact with immune cells through the aryl hydrocarbon receptor (AhR) [21, 22] [14] and/or the inhibition of IL-2 signaling, crucial to T cell survival [23].
The effects of IDO-expressing cells have been well characterized and documented [5, 9, 13, 24, 25], however, exogenously supplied IDO in the extracellular space has not been explored. In this study, we demonstrate that murine DCs treated with exogenous human recombinant IDO maintain an immature phenotype and provide robust suppression of antigen-specific T cell proliferation in vitro. Results are consistent with a mechanism of suppression involving both the aspects of tryptophan depletion as well as kynurenine accumulation. This work establishes that IDO maintains immunomodulatory capacity in the extracellular environment and that such exogenous supply of IDO programs DCs toward a suppressive phenotype.
MATERIALS AND METHODS
IDO characteristics and activity assay.
Recombinant human IDO expressed in Escherichia coli was purchased from R&D Systems (Minneapolis, MN) with a predicted molecular mass of 46 kDa, purity >95% by SDS-PAGE. Endotoxin levels were determined using the ChromoLAL method according to manufacturer’s instructions (Associates of Cape Cod) at < 0.1 EU/μg of protein. Briefly, samples were incubated with Limulus Amebocyte Lysate (LAL) at 37°C and absorbance measurements collected over 100 minutes using a Synergy HT plate reader (BioTek) in kinetic acquisition mode. The time taken for a sample to reach a specified absorbance is calculated and compared against a standard curve. The specific activity of IDO was established at >500 pmoles/min/μg as measured by its ability to oxidize L-tryptophan to N-formyl-kynurenine. The specific activity was measured before experiments to ensure maximal effect at the beginning of the assay following an adapted procedure from Valladares et al. [26]. The reaction substrate contained 200 μM tryptophan, 20 mM ascorbic acid, 10 μM methylene blue, 225 U catalase and 50 mM MES buffer (pH 6.5). Recombinant human IDO at 16 ng/mL was loaded onto a flat bottom 96-well plate and the reaction started by mixture in 1:1 ratio with reaction substrate. Absorbance was measured in kinetic mode for 5 minutes at 321 nm.
Dendritic cell culture and extracellular enzyme treatment.
Dendritic cells were generated by isolating the bone marrow from femurs and tibias of 8-12-week-old C57BL6/j female mice euthanized by CO2 asphyxiation followed by cervical dislocation in accordance with approved protocols by the University of Florida Institutional Animal Care and Use Committee. The marrow cells were obtained by flushing the shaft of the bones with a 25G5/8 needle using RPMI 1640 (Corning, Corning, NY) containing 10% fetal bovine serum (Lonza, Walkersville, MD) and 1% penicillin-streptomycin (Hyclone, Logan, UT) and mixed homogenously. The cell suspension was strained using a 70 μm cell strainer (Becton, Dickinson, NJ, USA) and collected after centrifugation at 1600 rpm for 5 minutes at 4°C. The red blood cells were then removed by lysing with ACK Lysing buffer (Lonza, Walkersville, MD) followed by centrifugation at 1600 rpm for 5 minutes at 4°C to recover leukocyte progenitors. Remaining cells were re-suspended in DC media: DMEM/F-12 with L-glutamine (Cellgro, Herndon, VA) containing 10% fetal bovine serum, 1% sodium pyruvate (Lonza, Walkersville, MD), 1% non-essential amino acids (Lonza, Walkersville, MD), 1% penicillin-streptomycin, 20 ng/ml of GM-CSF (R&D Systems, MN, USA) and were plated on tissue culture flasks for 2 d in order to remove adherent cells. After 2 d, floating cells were transferred to low attachment tissue culture plates with fresh DC media for the expansion of DC precursors. Half media change was performed on day 4 of isolation. On day 6 floating cells were carefully removed and plated on tissue culture plates at an appropriate cell density for the adhesion and proliferation of DCs. On day 8 media was removed, cells washed with PBS and fresh media provided. On day 10 dendritic cells were either treated with 15 μg/mL of recombinant human IDO (rhIDO - R&D Systems, Minneapolis, MN) cultured in DC media containing tryptophan, or cultured in tryptophan free media supplemented with 500 μM L-kynurenine without rhIDO (Sigma-Aldrich, St. Louis, MO) for 24 h at 37°C to mimic conditions induced by the enzyme.
Dendritic cell viability and maturation resistance.
DC viability was inspected through fluorescence microscopy imaging via Zeiss AxioVision 200M using LIVE/DEAD imaging stain kit (ThermoFisher Scientific, Waltham, VA), and quantified via flow cytometry using LIVE/DEAD Fixable Near-IR Dead Cell Stain Kit (ThermoFisher Scientific, Waltham, VA), according to manufacturer’s instructions. DC maturation was evaluated by the surface expression of stimulatory and co-stimulatory markers (MHC II, CD80 and CD86) as well as cytokine release (IL-10 and IL-12p70) by flow cytometry and enzyme-linked immunosorbent assay (ELISA), respectively. Following IDO incubation, DCs were challenged with 1 μg/mL of lipopolysaccharide (LPS) (Sigma-Aldrich, St. Louis, MO) and supernatant was collected and stored at −20°C for subsequent analysis using BD OptEIA ELISA kits (BD Biosciences, Franklin Lakes, NJ) following manufacturer’s instructions. Adherent cells were incubated with 5 mM Na2EDTA in PBS solution at 37°C for 30 minutes and lifted using a cell scraper. Cells were then washed with 1% fetal bovine serum in PBS and incubated with viability dyes mentioned above, followed by washing and incubation with antibodies (BD Bioscience, San Jose, CA) against CD16/32 (Fcγ III/II receptor) (clone 2.4G2) for 30 mins on ice to block Fcγ receptors on DCs. Cells were washed and stained with antibodies against CD11c (clone HL3) CD80 (clone 16-10A1), CD86 (clone GL1) and MHC II (clone M5/114.15.2).
T cell isolation and proliferation assay.
T cells were isolated from splenocytes of OT-I and OT-II female mice (Jackson Laboratories, Bar Harbor, ME) 8-12 weeks of age. The OT-I mouse model carries a transgene insert for rearranged TCR α and β genes on CD8+ T cells, that assemble to allow specific recognition of ovalbumin peptide residue 257-264 in the context of H2kb. The OT-II mouse model carries a transgene insert for rearranged TCR α and β genes on CD4+ T cells that is specific for ovalbumin 323-339 in the context of I-Ab. Animals were euthanized by CO2 asphyxiation followed by cervical dislocation as a secondary method in accordance with guidelines approved by the University of Florida. Spleens were excised and homogenized with RPMI 1640 medium (MP Biomedicals, OH, USA) containing 10% fetal bovine serum (Lonza, Walkersville, MD) and 1% penicillin-streptomycin (Hyclone, Logan, UT). The cell suspension was then filtered through a 70 μm cell strainer and centrifuged at 1600 rpm for 5 minutes at 4°C. Red blood cells were lysed with ACK Lysing buffer (Lonza, Walkersville, MD) followed by centrifugation to recover lymphocytes. CD4+ and CD8+ T cells were purified using a negative selection isolation kit (MACS Miltenyi Biotec, Bergisch Gladbach, Germany) following the manufacturer’s instructions. Resulting T cells were then labeled with 10 μM carboxyfluorescein succinimidyl ester (CFSE) to track proliferation. For antigen specific proliferation, DCs were treated with extracellular IDO either in the presence or absence of 1 mM 1-methyl-tryptophan (MT) for 24 h, washed to remove IDO in the media, then pulsed with 1 μg/mL ovalbumin peptide, 323-339 for CD4+ T cell assays and 257-264 for CD8+ assays, for 3 h or cultured in tryptophan free media supplemented with kynurenine. DCs and T cells were co-cultured for 4 d. After day 4, cells were centrifuged and washed with 1% fetal bovine serum in PBS and incubated with LIVE/DEAD Fixable Near-IR Dead Cell Stain kit for 10 mins at room temperature. Dye was then removed and cells incubated with antibodies against CD16/32 (Fcγ III/II receptor) (clone 2.4G2) for 30 mins on ice. Cells were washed and stained with antibodies against either CD4 (clone RM4-5) or CD8 (clone 53-6.7) for 30 mins on ice.
Statistical Analysis
Statistical analyses were performed using ANOVA followed by Tukey’s significance test to make pair-wise comparisons. Differences were considered significant when p≤0.05 using GraphPad Prism.
RESULTS
Extracellularly supplied recombinant human IDO does not induce murine bone marrow-derived dendritic cell death.
The specific activity of IDO and endotoxin levels were quantified and determined to be >500 pmoles/min/μg and <0.1 EU/μg protein, respectively. Effects of IDO supplementation on DCs were investigated by supplementing the culture media with an IDO amount sufficient to deplete the volume of tryptophan over the 24 h culture time period. Viability of DCs was then inspected by live/dead staining through microscopy and quantified via flow cytometry (Figure 1). Dendritic cells were cultured in DC media (untreated) or with Triton X-100 (dead cell), to serve as controls. Additionally, DCs were either cultured 24 h with IDO in DC media, in tryptophan free media, in DC media supplemented with kynurenine, or in tryptophan free media supplemented with kynurenine (Fig. 1A). Live cells are indicated by membrane-permeable calcein AM dye (green) staining in Fig. 1A, whereas staining by the membrane-impermeable dye (red) indicates the availability of dye to bind DNA via disrupted cell membrane, signifying dead and dying cells. Fluorescence microscopy imaging indicates that in contrast to the dead cell control, high viability was maintained for all treatments at a similar level to the untreated control. Flow cytometry, in conjunction with a fixable near-IR dead cell stain, was used for quantification of viability (Fig. 1B and Fig. 1C). All groups, apart from Triton X-100 (dead cell control), maintained 60% viability after 24 h, equivalent to the untreated control, and consistent with microscopic imaging observations. This indicates that the conditions of IDO treatment, tryptophan depletion, and kynurenine presence are all compatible with the maintenance of DC viability.
Figure 1. Treatment with exogenous IDO maintains dendritic cell viability.
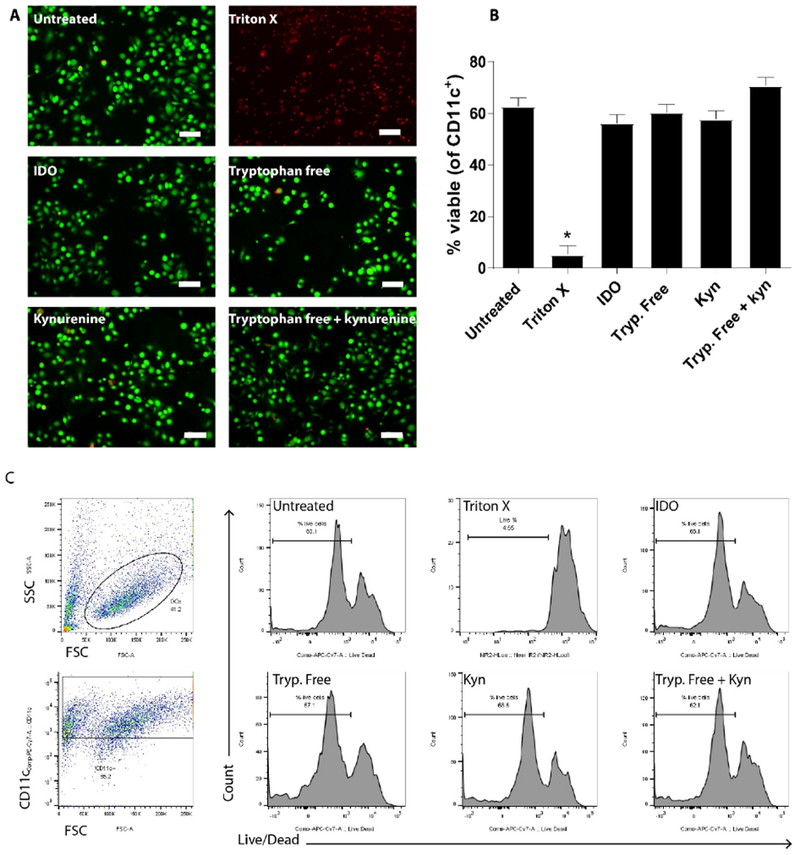
Dendritic cells were cultured 24 h in DC media or Triton X to serve as untreated and dead cell controls, respectively, as well as in the presence of IDO, in tryptophan free media, in tryptophan-containing media supplemented with kynurenine or in tryptophan free media supplemented with kynurenine. A. Following treatment, cells were stained with membrane permeable dye (green) and membrane-impermeant dye (red) to denote live and dead cells, respectively, and imaged via fluorescence microscopy. Scale bars = 50 μm. B. Following treatment, cells were stained with live/dead fixable NIR dye and viability assessed through flow cytometry. Shown is the mean ± SEM of three separate experiments, each conducted in triplicate. * denotes pair-wise significant differences (p ≤ 0.05) from all other groups by ANOVA, with Tukey’s post-hoc test. C. Representative plots of gating strategy used for flow cytometry analysis.
IDO-treated DCs resist LPS-induced maturation.
To quantitate the capacity of IDO to suppress DC activation in response to LPS, the expression levels of stimulatory (MHC II) and co-stimulatory (CD80, CD86) molecules were analyzed by flow cytometry (Figure 2). A low frequency of untreated DCs (negative control) expressed the maturation markers CD80 (Fig. 2A, 9% ± 0.6), CD86 (Fig. 2B, 3% ± 0.2) and MHC II (Fig. 2C, 7% ± 0.8). In contrast, upon LPS treatment (positive control) expression of all three activation markers significantly increased to 47% ± 0.5 (CD80), 18% ± 0.2 (CD86) and 33% ± 0.8 (MHC II) of cells. When DCs were treated with IDO for 24 h, maturation markers remained comparable to immature untreated cells with 10% ± 0.5 (CD80), 3% ± 0.2 (CD86) and 7% ± 0.7 (MHC II). When DCs were cultured with IDO followed by LPS challenge, maturation markers were lower than LPS alone, with 24% ± 0.5 (CD80), 5% ± 0.2 (CD86) and 13% ± 0.7 (MHC II), confirming cells treated with IDO are significantly resistant to LPS-induced maturation. Similarly, comparison of mean fluorescence intensity (MFI) values indicates IDO treatment as well as IDO followed by LPS challenge also resulted in significantly reduced levels of CD80, CD86 and MHC II (Fig. 2D,E,F). In contrast to the cell frequency analysis, when comparisons were made based on MFI, all marker expression levels were equivalent to untreated immature DC controls. Together, phenotypic marker data indicates IDO treatment directs DCs to maintain a relatively immature state. Additionally, data suggests the presence of exogenous IDO did not appear to act as a damage associated molecular pattern, which can be a concern for DCs extracellularly exposed to a normally cytosolic protein.
Figure 2. Dendritic cells treated with soluble IDO resist LPS maturation.
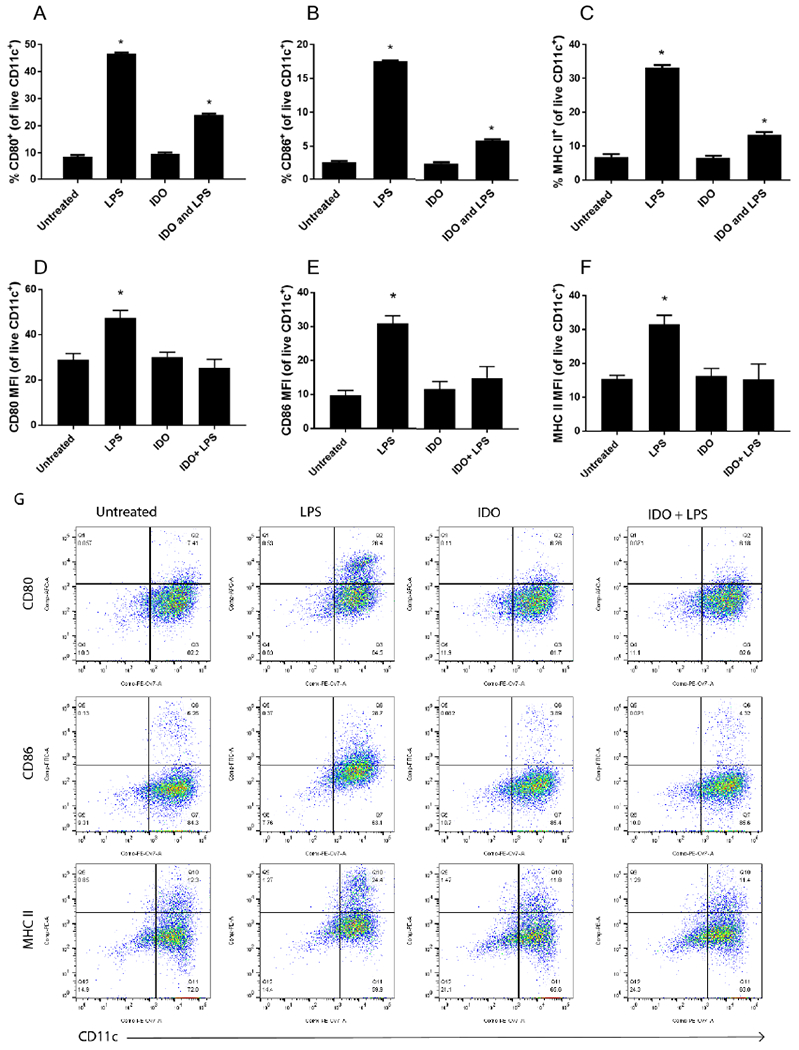
Dendritic cells were incubated with soluble IDO for 24 h and challenged with LPS overnight. Untreated and LPS groups were included for comparison. Cells were immunostained for maturation marker CD80, CD86 and MHC II. Viable cells expressing CD11c and the marker of interest were assed via flow cytometry and shown as percent positive (A-C) or mean fluorescence intensity (MFI – D-F). Shown is the mean ± SEM of three separate experiments, each conducted in triplicate. * denotes pair-wise significant differences (p ≤ 0.05) from all other groups by ANOVA, with Tukey’s post-hoc test. Representative flow cytometry plots shown in G.
Next, release of inflammatory and anti-inflammatory cytokines, IL-12p70 and IL-10, was evaluated after 24 h through ELISA (Figure 3). IL-12p70 secretion levels for untreated cells were determined to be 38 ± 8 pg/mL and 1920 ± 7 pg/mL for LPS treated cells. When IDO was introduced to the culture, IL-12p70 secretion remained low (61 ± 8 pg/mL), equivalent to untreated control. Furthermore, when cells were treated with IDO and then challenged with LPS, IL-12p70 secretion significantly diminished (65 pg/mL ± 8 pg/mL) compared to the LPS group (Fig. 3A) and was also equivalent to the untreated control. Conversely, at 24 h the basal IL-10 secretion level for the untreated cells (134 ± 13 pg/mL) was higher than the LPS treated control (52 ± 13 pg/mL) (Fig. 3B). The value for the IDO treatment (181 ± 13 pg/mL) remained equivalent to the untreated group, and notably, the IDO and LPS treatment group (157 ± 11 pg/mL) also remained equivalent to the untreated group. Together, cytokine data indicates IDO treatment blocks LPS-induced secretion of inflammatory IL-12 while maintaining basal levels of anti-inflammatory IL-10.
Figure 3. Treatment of dendritic cells with exogenous IDO inhibits IL-12 secretion and maintains IL-10 production.
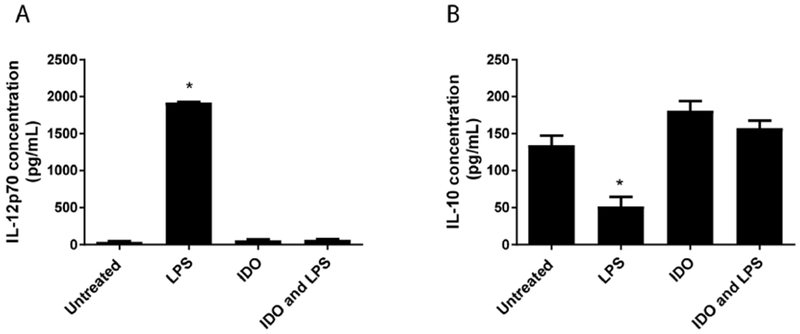
Dendritic cells were incubated with IDO for 24 h and challenged with LPS overnight. Untreated and LPS groups were included for comparison. A. IL-12p70 secretion and B. IL-10 secretion were evaluated via ELISA of the supernatant for each condition. Shown is the mean ± SEM of three separate experiments, each conducted in triplicate. * denotes pair-wise significant differences (p ≤ 0.05) from all other groups by ANOVA, with Tukey’s post-hoc test.
IDO-treated DCs suppress antigen-specific proliferation, and suppressive programming is active enzyme dependent.
To evaluate whether IDO-treated DCs can attenuate antigen specific T cell proliferation, DCs were co-cultured 4 d with CFSE labeled T cells isolated from splenocytes of either the T cell receptor (TCR) transgenic OT-II or OT-I mice. The CD4+ and CD8+ T cells of these mice proliferate in response to OVA peptide 323-339 and 257-264, respectively, when presented by MHC molecules (MHC II or MHC I, respectively) on antigen presenting cells. Data (Figure 4) represent the percent proliferation of viable CD4+ or CD8+ T cells, with the gating scheme used to quantify proliferation for each treatment (Fig. 4A-D). T cells only were cultured and included as a control with proliferation at 8% ± 1 for CD4+ T cells and 11% ± 2 for CD8+ T cells (Fig. 4E,F). In cultures where no antigen or treatment were present, and when IDO alone was introduced, minimal proliferation was observed: 11% ± 1 and 4% ± 1, respectively, for CD4+ T cells (Fig. 4E) and 17% ± 1 and 35% ± 3, respectively, for CD8+ T cells (Fig. 4F). When DCs were pulsed with the corresponding TCR specific peptide and co-cultured, T cells were activated, and proliferation was 95% ± 1 for CD4+ T cells (Fig. 4E) and 83% ± 0.8 for CD8+ T cells (Fig. 4F) after 4 d. However, when DCs were cultured with IDO for 24 h, washed (all groups were treated with identical wash steps), pulsed with OVA peptide and then co-cultured, DC capacity to stimulate T cell proliferation was reduced to basal or greatly reduced levels: 16% ± 1 for CD4+ T cells (Fig. 4E) and 36% ± 2 for CD8+ T cells (Fig. 4F). These data indicate a strongly suppressive programming of DCs by IDO treatment.
Figure 4. IDO treated DCs suppress antigen specific T cell proliferation, and suppression is active enzyme dependent.
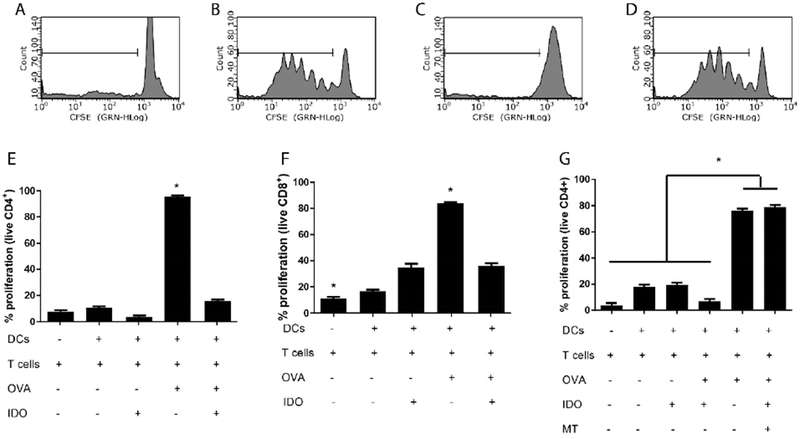
Dendritic cells were incubated with IDO in the presence or absence of 1-methyl tryptophan (MT) for 24 h, then washed and pulsed with the corresponding ovalbumin peptide (OVA) for 3 h. Dendritic cells were then washed and co-cultured with CD4+ or CD8+ CFSE labeled T cells isolated from OT-II and OT-I mice respectively, for 4 d. T cell proliferation was quantified through CFSE dilution via flow cytometry. Representative histograms for live CD4+ T cells, stimulated with: A. Untreated DCs, B. DCs pulsed with OVA, C. IDO-treated DCs pulsed with OVA, D. IDO-MT-treated DCs pulsed with OVA. T cell proliferation was quantified for E. CD4+ and F. CD8+ populations where “+” and “−” represent presence or absence of a specific component. G. CD4+ T cell proliferation assay with the introduction of MT during IDO treatment of DCs. Shown is the mean ± SEM of three separate experiments, each conducted in triplicate. * denotes pair-wise significant differences (p ≤ 0.05) from all other groups by ANOVA, with Tukey’s post-hoc test.
This experiment was next repeated with the introduction of 1-methyl tryptophan (MT), an IDO inhibitor [27], as a treatment to determine the necessity of IDO enzymatic activity on suppressive conditioning. In the presence of IDO plus MT-treated DCs, both CD4+ (Fig. 4G) and CD8+ (data not shown) T cell proliferation was restored to over 70%, equivalent to positive controls, demonstrating that active enzyme is required for suppressive conditioning of DCs.
Lastly, mechanisms of action for exogenous IDO-induced suppression were evaluated (Figure 5), comparing tryptophan starvation, kynurenine accumulation, and the combination by altering the relevant components in defined culture media. T cells alone were cultured in all media conditions (data not shown) and did not differ significantly from control T cells in tryptophan containing media (5% ± 2 proliferation). Dendritic cells and T cells were co-cultured without OVA peptide antigen and percent proliferating cells assessed in tryptophan containing media (31% ± 0.5), in tryptophan depleted media (21% ± 2.3), or in tryptophan depleted media supplemented with kynurenine (30% ± 2.3) respectively. T cells cultured with OVA-pulsed DCs exhibited proliferating cell frequencies of 81% ± 2 in tryptophan containing media (positive control), 31% ± 2.3 in tryptophan free media, and 39% ± 3 in tryptophan containing media supplemented with kynurenine, which was equivalent to the antigen-free negative controls. Notably, the largest suppression of T cell proliferation, at 15% ± 3, was observed when DCs were pulsed in OVA and cultured in tryptophan free media supplemented with kynurenine. This level of proliferation was equivalent to the negative control of T cells alone, and indicates effective suppressive DC programming through the combination of both tryptophan depletion and kynurenine supplementation.
Figure 5. Tryptophan depletion and kynurenine accumulation combine to maximally suppress antigen specific proliferation.
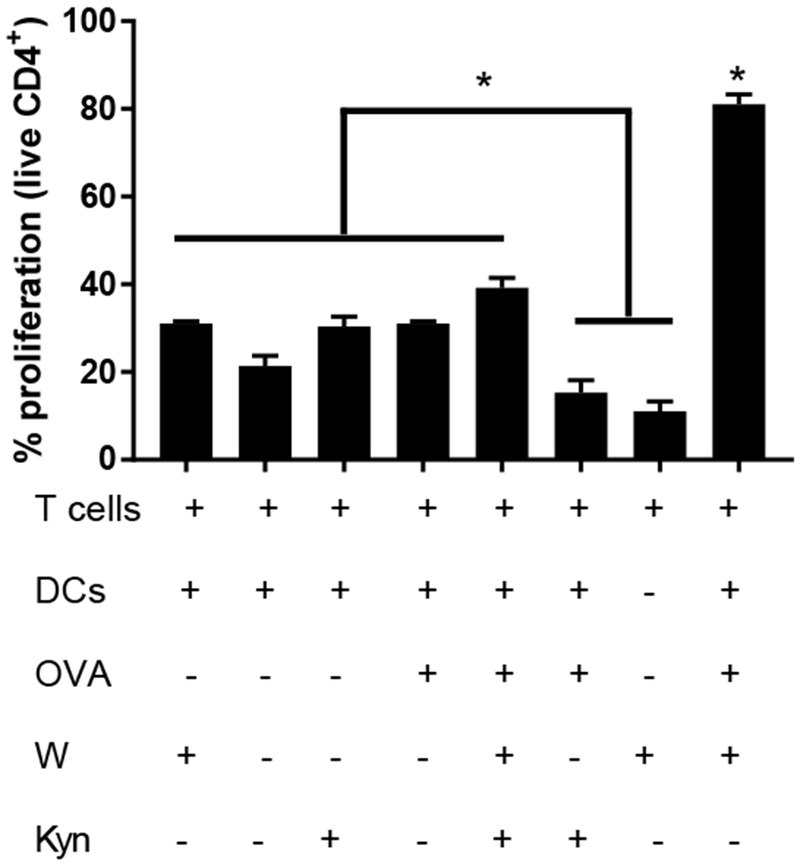
Dendritic cells were cultured in tryptophan (W) -free media supplemented with kynurenine (Kyn) or relevant controls for 24 h then pulsed with ovalbumin peptide (OVA) for an additional 3 h. Cells were washed and co-cultured with CD4+ CFSE labeled T cells isolated from OT-II mice for 4 d. “+” and “−” denote the presence or absence of a particular component during assay. Proliferation of live T cells was quantified through CFSE dilution via flow cytometry. Shown is the mean ± SEM of three separate experiments, each conducted in triplicate. * denotes pair-wise significant differences (p ≤ 0.05) from all other groups, while * above brackets indicates pair-wise significant differences (p ≤ 0.05) from indicated groups by ANOVA, with Tukey’s post-hoc test.
DISCUSSION
Suppression of inflammatory processes would be beneficial for the treatment of inflammatory and autoimmune diseases. Current treatments are limited to non-specific immunosuppressive drugs which carry harmful off-target effects [28]. To address these limitations, scientists have focused on reprogramming patients own immune systems to take advantage of naturally occurring suppressive mechanisms such as the upregulation of IDO. Since Munn and Mellor first demonstrated that IDO at the maternal-fetal interface played a pivotal role in maternal T cell tolerance to fetal tissue in mammals [5]. IDO has become established as a potent immunomodulator and promoter of tolerance.
In recent years, therapeutic application of IDO has primarily focused on overexpression of the gene in transplant cells/tissues, shown to prolong graft survival in a number of models [15, 29–32], or inhibition of its enzymatic activity for the treatment of cancer [33–36]. However, genetic overexpression to promote IDO levels through the use of viral and non-viral vectors faces several obstacles, such as viral vector-induced inflammation and malignancies [37, 38], which may hinder its clinical application [39]. For example, Tan et al. demonstrated DCs transduced with commonly used high efficiency viral-vectors upregulate expression of stimulatory co-stimulatory molecules and in the case of adenovirus, induced the production of Th1 and pro-inflammatory cytokines [40]. Additionally, infected cells demonstrated altered function and inability to properly stimulate allogeneic lymphocytes.
Follow up studies by Munn and Mellor indicated that suppression of T cell proliferation could be controlled by depletion of the essential amino acid tryptophan. T cells stimulated in the presence of tryptophan activated normally, whereas T cells stimulated without tryptophan experienced growth arrest at the G1 phase [11]. The tryptophan starvation theory was later challenged by Terness et al. by investigating human DCs transduced to express IDO, co-cultured with allogeneic T cells [14]. The authors concluded suppression of T cell proliferation was driven by the accumulation of tryptophan metabolites, particularly kynurenine, 3-hydroxykunureine, 3-hydroxyanthranilic and quinolinic acid which largely induced activated T cell death. More recently, Pallotta et al. demonstrated IDO catalytic activity is not required for certain IDO-mediated immunoregulatory effects of plasmacytoid DCs conditioned with TGF-β. Instead, TGF-β activates tyrosine phosphatases, SHP-1 and SHP-2, which lead to initiation of the non-canonical NF-κB pathway [12]. However, no studies to date have investigated the immunomodulatory properties of exogenously supplied IDO in the extracellular space, which is expected to replicate the native aspects of tryptophan depletion and kynurenine production, while lacking the intracellular functions of SHP-1/2 signaling and the inhibitory pathway induced by IL-6 and CD28, in which SOCS3 binding targets IDO for proteasomal degradation [41].
Dendritic cells are key regulators of the immune system and play a crucial role in the initiation of inflammatory responses as well as anti-inflammatory outcomes. The potential for DCs to activate tolerance-inducing mechanisms has been shown previously to be closely related to their maturation state [42]. T cell tolerance can be induced by immature DCs, which can be characterized by low expression of stimulatory and co-stimulatory molecules, and secretion of suppressive cytokines [2, 43, 44]. Multiple approaches have been used to generate suppressive DCs in vitro and in vivo, primarily by pharmacological agents [45–49] as well as gene silencing of pro-inflammatory molecules and cytokines [50]. Our investigation is the first to deliver exogenous IDO to DCs and demonstrate induction of a potent suppressive phenotype (summary schematic, Figure 6). Dendritic cells treated with IDO, without affecting viability, showed significant resistance to LPS-induced upregulation of MHC II and CD80/86 molecules. Consistent with our findings, reports have shown that while IDO-mediated T cell arrest can induce apoptosis of T cells, DC viability was not affected [13, 51, 52]. Additionally, IL-12p70 production was greatly diminished while IL-10 was maintained in IDO plus LPS conditions. Furthermore, treatment with exogenous IDO administered in the extracellular milieu effectively suppressed DC ability to stimulate both CD4 and CD8 T cells in vitro even when DCs were challenged by inflammatory stimulus. Finally, we found DC conditioning was mediated by the enzymatic action of IDO, and that DC-mediated suppression of T cells was directed both by a tryptophan starvation and presence of kynurenines, which cumulatively combined to more effectively to abrogate T cell stimulation.
Figure 6. Indoleamine 2,3-dioxygenase (IDO) functions as an extracellular immunomodulator.
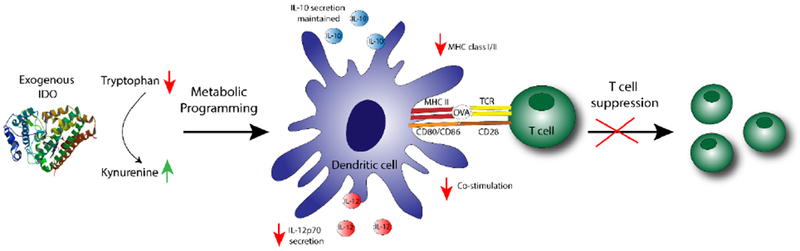
Dendritic cells treated with exogenously supplied IDO enzyme resist LPS activation as quantified by the reduction in IL-12p70 secretion, a maintained level of IL-10 secretion, and a downregulation in surface expression of stimulatory and co-stimulatory markers, generating a phenotype suppressive to T cells.
While the intracellular signaling pathways by which extracellular IDO acts upon DCs were not defined, it is expected that depletion of tryptophan in the extracellular space starves local availability of tryptophan for transport through the large-neutral amino acid transporter 1 (LAT-1). Such depletion has been shown to lead to intracellular accumulation of uncharged transfer RNA, which binds to general control nonderepressible 2 kinase (GCN2) causing its autophosphorylation and subsequent phosphorylation of the alpha subunit of the eukaryotic translation initiation factor 2 (eIF2a), resulting in the activation of the integrated stress response downregulating global protein synthesis [18, 53]. Additionally, the product of tryptophan catabolism, kynurenine, is also capable of transport via LAT1 [54], and can engage the DC aryl hydrocarbon receptor (AhR) in the cytosol [55]. It has been reported that endogenous AhR ligands can inhibit expression of stimulatory and co-stimulatory molecules in DCs as well as promote production of anti-inflammatory cytokines [56]. Finally, AhR-kynurenine interaction can induce the production of intracellular IDO through a positive feedback loop [57], and previous reports indicate AhR signaling is required for expression of IDO in DCs [21, 22].
In conclusion, we provide evidence to establish exogenous IDO as a potent immunomodulator in the extracellular space, as the enzyme potently programs suppressive DCs which inhibit T cell stimulation. This serves as a foundation for the use of exogenously supplied IDO as a biologically active therapeutic protein, insensitive to the intracellular signaling pathways which normally regulate its function. This demonstration therefore supports the future development of new IDO delivery strategies as an approach to suppress pathologic inflammation and autoimmunity.
Dendritic cells treated with extracellularly supplied IDO maintain viability
IDO treated dendritic cells resist LPS activation
IDO treated dendritic cells suppress antigen-specific T cell proliferation
Suppressive programming is dependent on enzyme activity
ACKNOWLEDGMENT
Support is acknowledged by grants from the National Institute of Diabetes and Digestive and Kidney Diseases, R01 DK091658, R01 DK098589, National Institute of Dental and Craniofacial Research R01 DE027301 and National Institute of Allergy and Infection Diseases R01 AI133623 (to BGK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ABBREVIATIONS
- APCs
antigen presenting cells
- CFSE
carboxyfluorescein succinimidyl ester
- DC
dendritic cells
- ELISA
enzyme-linked immunosorbent assay
- IDO
indoleamine 2,3-dioxygenase
- IFN-γ
Interferon γ
- LPS
lipopolysaccharides
- MHC I and II
major histocompatibility complex class I and II
- MT
1-methyl tryptophan
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST DISCLOSURE
The authors declare no conflict of interest.
REFERENCES
- [1].Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K, Immunobiology of dendritic cells, Annual review of immunology 18 (2000) 767–811. [DOI] [PubMed] [Google Scholar]
- [2].Steinman RM, Hawiger D, Nussenzweig MC, Tolerogenic dendritic cells, Annual review of immunology 21 (2003) 685–711. [DOI] [PubMed] [Google Scholar]
- [3].Harden JL, Egilmez NK, Indoleamine 2,3-dioxygenase and dendritic cell tolerogenicity, Immunological investigations 41(6-7) (2012) 738–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mellor AL, Munn DH, IDO expression by dendritic cells: tolerance and tryptophan catabolism, Nature reviews. Immunology 4(10) (2004) 762–74. [DOI] [PubMed] [Google Scholar]
- [5].Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL, Prevention of allogeneic fetal rejection by tryptophan catabolism, Science (New York, N.Y.) 281(5380) (1998) 1191–3. [DOI] [PubMed] [Google Scholar]
- [6].Dai X, Zhu BT, Indoleamine 2,3-Dioxygenase Tissue Distribution and Cellular Localization in Mice: Implications for Its Biological Functions, Journal of Histochemistry and Cytochemistry 58(1) (2010) 17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Watcharanurak K, Zang L, Nishikawa M, Yoshinaga K, Yamamoto Y, Takahashi Y, Ando M, Saito K, Watanabe Y, Takakura Y, Effects of upregulated indoleamine 2, 3-dioxygenase 1 by interferon gamma gene transfer on interferon gamma-mediated antitumor activity, Gene therapy (2014). [DOI] [PubMed] [Google Scholar]
- [8].Löb S, Königsrainer A, Role of IDO in Organ Transplantation: Promises and Difficulties, International Reviews of Immunology 28(3-4) (2009) 185–206. [DOI] [PubMed] [Google Scholar]
- [9].Fallarino F, Grohmann U, Puccetti P, Indoleamine 2,3-dioxygenase: from catalyst to signaling function, European journal of immunology 42(8) (2012) 1932–7. [DOI] [PubMed] [Google Scholar]
- [10].Lim JY, Lee SE, Park G, Choi EY, Min CK, Inhibition of indoleamine 2,3-dioxygenase (IDO) by stereoisomers of 1-methyl tryptophan in an experimental graft-versus-tumor model, Experimental hematology (2014). [DOI] [PubMed] [Google Scholar]
- [11].Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL, Inhibition of T cell proliferation by macrophage tryptophan catabolism, The Journal of experimental medicine 189(9) (1999) 1363–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Pallotta MT, Orabona C, Volpi C, Vacca C, Belladonna ML, Bianchi R, Servillo G, Brunacci C, Calvitti M, Bicciato S, Mazza EM, Boon L, Grassi F, Fioretti MC, Fallarino F, Puccetti P, Grohmann U, Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells, Nature immunology 12(9) (2011) 870–8. [DOI] [PubMed] [Google Scholar]
- [13].Sun J, Yu J, Li H, Yang L, Wei F, Yu W, Liu J, Ren X, Upregulated expression of indoleamine 2, 3-dioxygenase in CHO cells induces apoptosis of competent T cells and increases proportion of Treg cells, Journal of experimental & clinical cancer research : CR 30 (2011) 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Terness P, Bauer TM, Röse L, Dufter C, Watzlik A, Simon H, Opelz G, Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites, The Journal of experimental medicine 196(4) (2002) 447–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].do Prado KM, Correa-Silva S, Oliveira LG, Camara NO, Ono E, Sandri S, Tourino MC, Campa A, de Sa Lima L, Scavone C, Bevilacqua E, Indoleamine 2,3-dioxygenase (IDO) activity in placental compartments of renal-transplanted pregnant women, American journal of reproductive immunology (New York, N.Y. : 1989) 72(1) (2014) 45–56. [DOI] [PubMed] [Google Scholar]
- [16].Johnson TS, Munn DH, Host indoleamine 2,3-dioxygenase: contribution to systemic acquired tumor tolerance, Immunological investigations 41(6-7) (2012) 765–97. [DOI] [PubMed] [Google Scholar]
- [17].Stubbendorff M, Deuse T, Hua X, Phan TT, Bieback K, Atkinson K, Eiermann TH, Velden J, Schroder C, Reichenspurner H, Robbins RC, Volk HD, Schrepfer S, Immunological properties of extraembryonic human mesenchymal stromal cells derived from gestational tissue, Stem cells and development 22(19) (2013) 2619–29. [DOI] [PubMed] [Google Scholar]
- [18].Zhang P, McGrath BC, Reinert J, Olsen DS, Lei L, Gill S, Wek SA, Vattem KM, Wek RC, Kimball SR, Jefferson LS, Cavener DR, The GCN2 eIF2α Kinase Is Required for Adaptation to Amino Acid Deprivation in Mice, Molecular and Cellular Biology 22(19) (2002) 6681–6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, Orabona C, Bianchi R, Belladonna ML, Volpi C, Santamaria P, Fioretti MC, Puccetti P, The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells, Journal of immunology (Baltimore, Md. : 1950) 176(11) (2006) 6752–61. [DOI] [PubMed] [Google Scholar]
- [20].Mellor AL, Munn DH, Tryptophan catabolism and T-cell tolerance: immunosuppression by starvation?, Immunology today 20(10) (1999) 469–73. [DOI] [PubMed] [Google Scholar]
- [21].Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA, An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells, Journal of immunology (Baltimore, Md. : 1950) 185(6) (2010) 3190–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Nguyen NT, Kimura A, Nakahama T, Chinen I, Masuda K, Nohara K, Fujii-Kuriyama Y, Kishimoto T, Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism, Proceedings of the National Academy of Sciences of the United States of America 107(46) (2010) 19961–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Dagenais-Lussier X, Aounallah M, Mehraj V, El-Far M, Tremblay C, Sekaly RP, Routy JP, van Grevenynghe J, Kynurenine Reduces Memory CD4 T-Cell Survival by Interfering with lnterleukin-2 Signaling Early during HIV-1 Infection, Journal of virology 90(17) (2016) 7967–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Funeshima N, Fujino M, Kitazawa Y, Hara Y, Hayakawa K, Okuyama T, Kimura H, Li XK, Inhibition of allogeneic T-cell responses by dendritic cells expressing transduced indoleamine 2,3-dioxygenase, The journal of gene medicine 7(5) (2005) 565–75. [DOI] [PubMed] [Google Scholar]
- [25].Huang L, Baban B, Johnson BA 3rd, Mellor AL, Dendritic cells, indoleamine 2,3 dioxygenase and acquired immune privilege, Int Rev Immunol 29(2) (2010) 133–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Valladares R, Bojilova L, Potts AH, Cameron E, Gardner C, Lorca G, Gonzalez CF, Lactobacillus johnsonii inhibits indoleamine 2,3-dioxygenase and alters tryptophan metabolite levels in BioBreeding rats, FASEB journal : official publication of the Federation of American Societies for Experimental Biology 27(4) (2013) 1711–20. [DOI] [PubMed] [Google Scholar]
- [27].Cady SG, Sono M, 1-Methyl-DL-tryptophan, beta-(3-benzofuranyl)-DL-alanine (the oxygen analog of tryptophan), and beta-[3-benzo(b)thienyl]-DL-alanine (the sulfur analog of tryptophan) are competitive inhibitors for indoleamine 2,3-dioxygenase, Archives of biochemistry and biophysics 291(2) (1991) 326–33. [DOI] [PubMed] [Google Scholar]
- [28].Wiseman AC, Immunosuppressive Medications, Clinical Journal of the American Society of Nephrology : CJASN 11(2) (2016) 332–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Brandacher G, Cakar F, Winkler C, Schneeberger S, Obrist P, Bosmuller C, Werner-Felmayer G, Werner ER, Bonatti H, Margreiter R, Fuchs D, Non-invasive monitoring of kidney allograft rejection through IDO metabolism evaluation, Kidney international 71(1) (2007) 60–7. [DOI] [PubMed] [Google Scholar]
- [30].Cook CH, Bickerstaff AA, Wang JJ, Nadasdy T, Della Pelle P, Colvin RB, Orosz CG, Spontaneous renal allograft acceptance associated with “regulatory” dendritic cells and IDO, Journal of immunology (Baltimore, Md. : 1950) 180(5) (2008) 3103–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Miki T, Sun H, Lee Y, Tandin A, Kovscek AM, Subbotin V, Fung JJ, Valdivia LA, Blockade of tryptophan catabolism prevents spontaneous tolerogenicity of liver allografts, Transplantation proceedings 33(1-2) (2001) 129–30. [DOI] [PubMed] [Google Scholar]
- [32].Guillonneau C, Hill M, Hubert FX, Chiffoleau E, Herve C, Li XL, Heslan M, Usal C, Tesson L, Menoret S, Saoudi A, Le Mauff B, Josien R, Cuturi MC, Anegon I, CD40lg treatment results in allograft acceptance mediated by CD8CD45RC T cells, IFN-gamma, and indoleamine 2,3-dioxygenase, The Journal of clinical investigation 117(4) (2007) 1096–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Friberg M, Jennings R, Alsarraj M, Dessureault S, Cantor A, Extermann M, Mellor AL, Munn DH, Antonia SJ, Indoleamine 2,3-dioxygenase contributes to tumor cell evasion of T cell-mediated rejection, International journal of cancer. Journal international du cancer 101(2) (2002) 151–5. [DOI] [PubMed] [Google Scholar]
- [34].Johnson BA 3rd, Baban B, Mellor AL, Targeting the immunoregulatory indoleamine 2,3 dioxygenase pathway in immunotherapy, Immunotherapy 1(4) (2009) 645–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Muller AJ, Prendergast GC, Marrying immunotherapy with chemotherapy: why say IDO?, Cancer research 65(18) (2005) 8065–8. [DOI] [PubMed] [Google Scholar]
- [36].Munn DH, Mellor AL, Indoleamine 2,3-dioxygenase and tumor-induced tolerance, Journal of Clinical Investigation 117(5) (2007) 1147–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hacein-Bey-Abina S, von Kalle C, Schmidt M, Le Deist F, Wulffraat N, McIntyre E, Radford I, Villeval JL, Fraser CC, Cavazzana-Calvo M, Fischer A, A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency, N Engl J Med, United States, 2003, pp. 255–6. [DOI] [PubMed] [Google Scholar]
- [38].Couzin J, Kaiser J, Gene therapy. As Gelsinger case ends, gene therapy suffers another blow, Science (New York, N.Y.), United States, 2005, p. 1028. [DOI] [PubMed] [Google Scholar]
- [39].Rezakhanlou AM, Habibi D, Lai A, Jalili RB, Ong CJ, Ghahary A, Highly Efficient Stable Expression of Indoleamine 2,3 Dioxygenase Gene in Primary Fibroblasts, Biological Procedures Online 12 (2010) 107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Tan PH, Beutelspacher SC, Xue SA, Wang YH, Mitchell P, McAlister JC, Larkin DF, McClure MO, Stauss HJ, Ritter MA, Lombardi G, George AJ, Modulation of human dendritic-cell function following transduction with viral vectors: implications for gene therapy, Blood 105(10) (2005) 3824–32. [DOI] [PubMed] [Google Scholar]
- [41].Orabona C, Pallotta MT, Volpi C, Fallarino F, Vacca C, Bianchi R, Belladonna ML, Fioretti MC, Grohmann U, Puccetti P, SOCS3 drives proteasomal degradation of indoleamine 2,3-dioxygenase (IDO) and antagonizes IDO-dependent tolerogenesis, Proceedings of the National Academy of Sciences of the United States of America 105(52) (2008) 20828–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Schmidt SV, Nino-Castro AC, Schultze JL, Regulatory dendritic cells: there is more than just immune activation, Frontiers in Immunology 3 (2012) 274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Barratt-Boyes SM, Thomson AW, Dendritic cells: tools and targets for transplant tolerance, American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 5(12) (2005) 2807–13. [DOI] [PubMed] [Google Scholar]
- [44].Li H, Shi B, Tolerogenic dendritic cells and their applications in transplantation, Cellular & molecular immunology 12(1) (2015) 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Azzi J, Tang L, Moore R, Tong R, El Haddad N, Akiyoshi T, Mfarrej B, Yang S, Jurewicz M, Ichimura T, Lindeman N, Cheng J, Abdi R, Polylactide-cyclosporin A nanoparticles for targeted immunosuppression, FASEB journal : official publication of the Federation of American Societies for Experimental Biology 24(10) (2010) 3927–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Sauma D, Fierro A, Mora JR, Lennon-Dumenil AM, Bono MR, Rosemblatt M, Morales J, Cyclosporine preconditions dendritic cells during differentiation and reduces IL-2 and IL-12 production following activation: a potential tolerogenic effect, Transplantation proceedings 35(7) (2003) 2515–7. [DOI] [PubMed] [Google Scholar]
- [47].Hackstein H, Taner T, Zahorchak AF, Morelli AE, Logar AJ, Gessner A, Thomson AW, Rapamycin inhibits IL-4--induced dendritic cell maturation in vitro and dendritic cell mobilization and function in vivo, Blood 101(11) (2003) 4457–63. [DOI] [PubMed] [Google Scholar]
- [48].Fischer R, Turnquist HR, Taner T, Thomson AW, Use of rapamycin in the induction of tolerogenic dendritic cells, Handbook of experimental pharmacology (188) (2009) 215–32. [DOI] [PubMed] [Google Scholar]
- [49].Barragan M, Good M, Kolls JK, Regulation of Dendritic Cell Function by Vitamin D, Nutrients 7(9) (2015) 8127–8151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Li M, Zhang X, Zheng X, Lian D, Zhang ZX, Ge W, Yang J, Vladau C, Suzuki M, Chen D, Zhong R, Garcia B, Jevnikar AM, Min WP, Immune modulation and tolerance induction by RelB-silenced dendritic cells through RNA interference, Journal of immunology (Baltimore, Md. : 1950) 178(9) (2007) 5480–7. [DOI] [PubMed] [Google Scholar]
- [51].Fallarino F, Grohmann U, Vacca C, Bianchi R, Orabona C, Spreca A, Fioretti MC, Puccetti P, T cell apoptosis by tryptophan catabolism, Cell death and differentiation 9(10) (2002) 1069–77. [DOI] [PubMed] [Google Scholar]
- [52].Hayashi T, Beck L, Rossetto C, Gong X, Takikawa O, Takabayashi K, Broide DH, Carson DA, Raz E, Inhibition of experimental asthma by indoleamine 2,3-dioxygenase, The Journal of clinical investigation 114(2) (2004) 270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Pakos-Zebrucka K, Koryga I, Mnich K, Ljujic M, Samali A, Gorman AM, The integrated stress response, EMBO reports 17(10) (2016) 1374–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Sekine A, Kuroki Y, Urata T, Mori N, Fukuwatari T, Inhibition of Large Neutral Amino Acid Transporters Suppresses Kynurenic Acid Production Via Inhibition of Kynurenine Uptake in Rodent Brain, Neurochemical research 41(9) (2016) 2256–66. [DOI] [PubMed] [Google Scholar]
- [55].Nguyen NT, Nakahama T, Le DH, Van Son L, Chu HH, Kishimoto T, Aryl Hydrocarbon Receptor and Kynurenine: Recent Advances in Autoimmune Disease Research, Frontiers in Immunology 5 (2014) 551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Wang C, Ye Z, Kijlstra A, Zhou Y, Yang P, Activation of the aryl hydrocarbon receptor affects activation and function of human monocyte-derived dendritic cells, Clinical and experimental immunology 177(2) (2014) 521–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Li Q, Harden JL, Anderson CD, Egilmez NK, Tolerogenic Phenotype of IFN-gamma-Induced IDO+ Dendritic Cells Is Maintained via an Autocrine IDO-Kynurenine/AhR-IDO Loop, Journal of immunology (Baltimore, Md. : 1950) 197(3) (2016) 962–70. [DOI] [PubMed] [Google Scholar]


