Abstract
It is widely accepted that an effective HIV-1 preventative vaccine must elicit antibodies that can block virus acquisition. Although, anti-HIV-1 broadly neutralizing antibodies (bnAbs) have been isolated, unfortunately, no vaccine immunogens have been designed that can elicit these bnAbs in uninfected at-risk individuals. Some studies have suggested that other antibody functionalities, besides neutralization, such as antibody-dependent cellular cytotoxicity (ADCC), may prevent HIV-1 acquisition. In contrast to bnAbs, ADCC-inducing antibodies may be more amenable to elicitation by current vaccine technologies. This review will provide clarity about the role of nAbs and ADCC-inducing antibodies in preventing transmission, highlight mechanisms that potentially explain how ADCC-mediating antibodies may work, and speculate about the generation of these novel protective antibodies. Anti-HIV-1 ADCC-inducing antibodies may provide a new avenue for developing an effective HIV-1 vaccine.
Keywords: HIV-1, antibodies, transmission, antibody dependent cellular cytotoxicity
INTRODUCTION
Developing an HIV-1 vaccine remains a major public health priority despite the availability of highly effective ways to prevent acquisition. For instance, antiretrovirals for either an infectious individual or a person at high risk due to repeated exposure, circumcision, barrier protection, and needle exchange programs reduce HIV-1 transmission [1, 2]. These protective modalities, however, may be cost prohibitive, require stable medical infrastructure, and can fail in the absence of adherence. Thus, developing an HIV-1 vaccine is likely going to be the most effective way to halt the ongoing epidemic. HIV-1 vaccine development will require the identification of immune factors that protect against HIV-1 acquisition. In general, all effective vaccines against infectious diseases elicit antibodies [3, 4]. Antibodies against infectious organisms are either of a neutralizing or of a non-neutralizing variety. Neutralizing antibodies (nAbs) bind to the invading organism, presumably blocking the early steps required to establish infection [5, 6]. Non-neutralizing antibodies likely do not block events such as receptor attachment and fusion necessary for cell-host entry. Thus, it is generally believed that nAbs are the humoral component responsible for a vaccine’s ability to provide sterilizing protection [7, 8]. As a result, extensive effort has been devoted to elicit nAbs against HIV-1 with limited success.
Broadly neutralizing antibodies (bnAbs) have provided a tangible goal for vaccinology
All HIV-1-infected individuals develop antibodies early after virus acquisition, but it is generally believed that the earliest antibodies cannot inhibit virus replication [9]. Inhibitory nAbs generally develop more than six weeks after acquisition, and because HIV-1 evolves at a rapid rate, nAbs primarily block cell entry of previously circulating viruses but not contemporaneous strains [10–12]. Furthermore, most HIV-1-infected individuals develop nAbs that are able to block the virus circulating in their own body (termed autologous variants), but in general, these strain-specific nAbs cannot block viruses isolated from other individuals (classified as heterologous variants). While the strain-specific antibodies conclusively demonstrate that HIV-1 can be neutralized, they are of limited value for a vaccine. Ultimately, vaccine-elicited antibodies will need to block a wide variety of viruses, not just a small number of specific strains, in the hope of providing sterilizing protection. In this respect, HIV-1 extensive genetic diversity presents a formidable challenge for any vaccine that aims to elicit nAbs.
Over the past 5–10 years, numerous bnAbs have been identified which can potently block a large number of diverse HIV-1 variants [13–19]. BnAbs attach to various HIV-1 envelope (Env) domains, such as the apex, high mannose patch, CD4 binding site (bs), surface unit (gp120) – transmembrane (gp41) interface, and gp41 membrane proximal external region (MPER). Importantly, these Env structures are highly conserved, and thus antibody binding to these domains confers neutralization breadth against a diverse range of HIV-1 strains. Unfortunately, to date, no designed immunogens can elicit these bnAbs, and thus innovative technologies, such as antibody producing virus vectors, are being designed and imagined to generate these antibodies prior to HIV-1 exposure [20–22].
It has been difficult to elicit bnAbs using standard vaccine approaches. Natural history studies and investigations that have tracked the generation of nAbs and virus evolution suggest that bnAbs develop after repeated exposure to a continuously evolving antigen [23–29]. Indeed, it is generally believed that bnAbs only develop among a small number of individuals that have high plasma virus level and prolonged duration of infection. A few infants, however, have developed HIV-1 bnAbs relatively early after virus acquisition, suggesting that these type of antibodies can develop in some special cases without prolonged HIV-1 antigen exposure [30]. Repeated exposure to continuously changing strains within an infected host forces antibody evolution [31, 32]. Some of the highly evolved antibodies eventually display neutralization breath and potency and are deemed as bnAbs. With this prolonged evolution, bnAbs have extensive sequence changes compared to the unmutated ancestor germline sequence [33–35]. This somatic hypermutation is one of the greatest barriers to eliciting bnAbs through standard vaccine approaches. Current vaccine efforts are contemplating using multiple sequential exposures to different HIV envelope antigens over time to elicit antibodies that harbor extensive somatic hypermutation [36, 37]. It remains uncertain if this vaccine strategy or the use of highly specific envelope antigens will yield broadly potent antibodies.
Neutralizing antibodies may not provide protection against HIV-1 acquisition
Animal models have clearly demonstrated that passive infusion of nAbs, especially bnAbs, prior to virus exposure prevent infection against cell-free virus challenge [38–53]. These highly conclusive results from various different animal models using high and low dose parenteral, vaginal, rectal and/or oral challenge, however, do not automatically imply that even if bnAbs could be successfully elicited in individuals at risk for acquiring HIV-1, the nAbs would impart sterilizing protection. There are a number of differences among animal models and human HIV-1 acquisition that, at a minimum, suggest animal model results may not be perfectly predictive for human modes of HIV-1 acquisition.
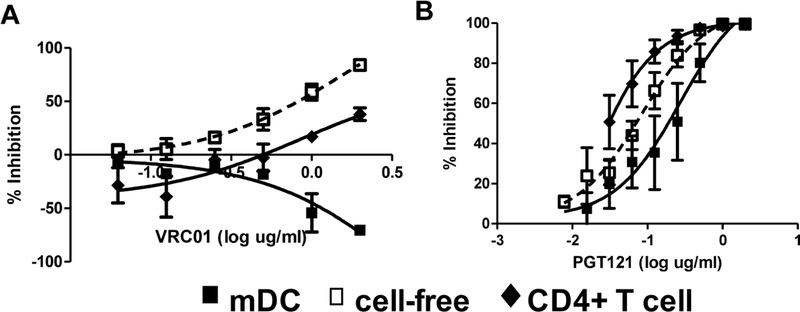
Laboratory-based challenge models mostly utilize cell-free virus but human infectious material, such as genital secretions, breast milk, and blood, contain infected cells with infectious virus [54–58]. Studies from our group and others suggest that nAbs, even bnAbs, are relatively inefficient in preventing virus transfer from infected cells to naïve targets [59–62]. For instance, the CD4bs bnAb, VRC01 demonstrates excellent efficacy in preventing virus acquisition in animal models, but it is relatively poor at preventing virus transfer from a virus-bearing cell to a naïve target (Figure 1). On the other hand, PGT121 demonstrates equivalent inhibition efficacy against all modes of virus transfer (Figure 1). Interestingly, passive infusion of a potent bnAb (PGT121) only showed partial efficacy in protecting animals from challenge with cell-associated infectious virus even though it is highly efficacious against cell-free virus [58]. Animal-based transmission studies also, invariably, use challenge strains that are sensitive to the passively infused antibody under investigation. In humans, however, it is quite possible that individuals may be exposed to viruses that are resistant to the antibodies present during exposure. Furthermore, antibodies are often present at supraphysiologic levels, especially at the time of virus challenge, in pre-exposure passive infusion animal models. Among at risk individuals, nAbs titers may be inadequate to neutralize incoming virus inoculum. These important caveats raise the possibility that HIV-1 transmission, as opposed to virus acquisition in animal challenge models, may occur even in the presence of bnAbs.
Figure 1. Some broadly neutralizing antibodies (bnAbs) are less efficient in blocking cell to cell virus transfer.
Representative examples of neutralization against cell-free (hollow squares), mature dendritic cell (DC) associated (filled squares) and infected CD4+ T cells (diamonds). Neutralization is against primary HIV-1 variant, Q23. The x-axis shows the amount of input VRC01 (A) and PGT121 (B) in log µg/ml, and the y-axis shows the percent inhibition relative to infection without any antibody. Each point represents an average of at least three independent experiments performed in triplicate and is the mean percent inhibition with standard errors of mean.
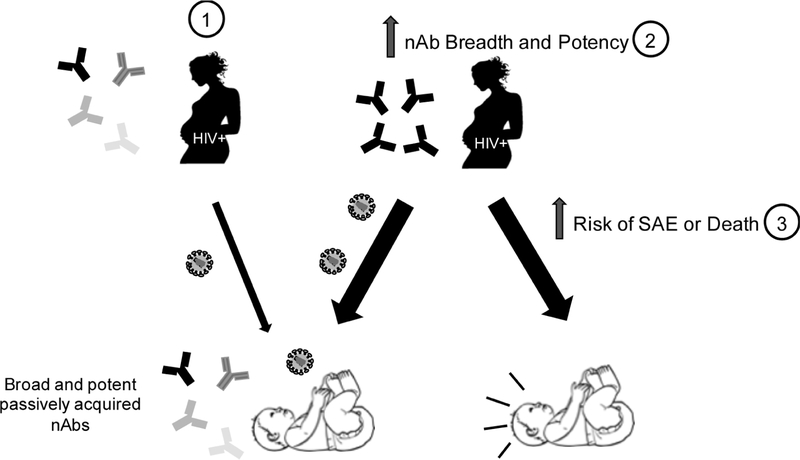
As stated, to date no vaccine immunogen has elicited bnAbs, and thus, it has not been possible to test the ability of bnAbs to prevent human HIV-1 acquisition. Clinical trials are underway examining the safety and efficacy of a passively infused bnAb (VRC01) in reducing HIV-1 acquisition among at-risk individuals (National Clinical Trial (NCT) 02568215 and NCT02716675). In the absence of these results, mother to child transmission (MTCT) cohorts present an ideal way to examine the role of nAbs in preventing HIV-1 transmission (Figure 2). Infected mothers harbor antibodies against their autologous virus, and they can pass these antibodies to their infant during both gestation and breast feeding. Even though infants are consistently exposed to their mother’s virus during gestation and breast feeding, in the absence of antiretroviral therapy, only around 30% of babies eventually become infected [63–67]. Although, diverse factors, such as maternal virus levels, human leukocyte antigen concordance, and localized mammary gland inflammation, influence the likelihood of virus transmission, maternal antibodies circulating in the mother and infant may also protect against HIV-1 transmission [55, 67–76]. Thus, MTCT cohorts may be ideal to examine the role of antibodies during HIV-1 acquisition because the exposed infants harbor maternal antibodies prior to infection, infants are definitively exposed to their mother’s virus, infant samples are available around the time of transmission, and there are relatively high transmission rates in the absence of antiretroviral therapy [77].
Figure 2. Mother to child transmission (MTCT) can be used to examine the impact of pre-infection antibodies in preventing HIV-1 acquisition.
(1) Infants are exposed to HIV-1-infected mother’s virus and acquire maternal antibodies. (2 and 3) In our studies, we showed that infants born to mothers with broad and potent neutralizing antibodies (nAbs) were more likely to acquire HIV-1 through breast feeding and more likely to have a severe adverse outcome (SAE) or death.
Interestingly, examinations of MTCT cohorts have often failed to confirm the animal challenge model findings. We and others have shown that exposed infants harbor HIV-1-directed nAbs prior to infection and during exposure [78, 79]. In some instances, they often have broad and potent humoral responses similar to those observed with passive bnAb infusions in animal challenge models. Multiple studies, including ours, have clearly shown, however, that this nAb breadth and potency does not correlate with protection against HIV-1 MTCT [78–84]. Indeed, we have shown that infected mothers with broad and potent nAbs are more likely to transmit the virus to their infants (Figure 2). Surprisingly, infants born to mothers with relatively broad and potent neutralization responses were more likely to have serious adverse outcomes, such as death, meningitis, or pneumonia. In aggregate, human transmission studies, using perhaps the best available methodology at this time, have failed to conclusively confirm findings from animal or in-vitro models that pre-existing broad and potent neutralization response will prevent virus acquisition. The reasons for this discrepancy between animal models and natural history human cohorts remain uncertain; the presence of infected cells, neutralization-resistant virus, and inadequate antibody levels during the entire exposure period are some potential explanations.
Other antibody functionalities may prevent transmission
In addition to MTCT cohorts, the one and only HIV-1 vaccine trial that demonstrated modest efficacy also showed that protection was not correlated with the nAb titers or cytotoxic CD8+ T cell responses [85, 86]. Surprisingly, protection was directly correlated with envelope variable loop 1 and 2 (V1-V2)-directed binding antibodies. In addition, vaccinees that resisted infection had lower IgA antibody levels compared to vaccinated individuals that were eventually infected. These results led to the hypothesis that antibodies with the ability to elicit effector functions, such as antibody-dependent cellular cytotoxicity (ADCC), antibody-dependent cellular viral inhibition (ADCVI), or complement deposition protected against HIV-1 acquisition. Furthermore, it was hypothesized that increased IgA antibody levels interfered with these effector functions, which accounted for the observed inverse correlation. Further comparisons have been done among vaccine recipients in this trial (RV144) compared to enrollees in a vaccine study that failed to show any protection (VAX003) [87, 88]. These comparisons were done to examine differences in antibody functionality that potentially correlated with protection. Importantly, these 2 clinical trials (RV144 and VAX003) used the same envelope immunogen boost although there were differences in the inoculation schedule and vector used to initially prime the immune response [86, 89]. Comparison of these studies showed that the RV144 as opposed to the VAX003 vaccine regimen elicited higher IgG3 antibody responses; IgG3 as opposed to other IgG antibody subclasses have been associated with an enhanced ability to induce effector cell function [90, 91]. Indeed, the subsequent comparisons showed that these IgG3 differences correlated with ADCC differences, which potentially accounts for the difference in vaccine efficacy among the 2 clinical trials. MTCT studies have also shown that infected mothers with high ADCC or specific polymorphisms in the receptors that bind to the antibody Fc segment and subsequently induce effector cell function, such as ADCC, are less likely to transmit HIV-1 to their infants [92–95]. Surprisingly, in direct contrast to nAbs, higher levels of ADCC responses have been associated with improved survival among infants born to HIV-1-infected mothers [93]. Greater ADCC has also been linked to protection from heterosexual transmission among discordant couples [96]. In aggregate, these studies imply that antibody functionalities other than neutralization, such as IgG3-mediated ADCC, potentially provide protection against HIV-1 acquisition.
The role of ADCC responses remains controversial primarily because lab-based models have failed to validate that passive immunization with non-neutralizing monoclonal or polyclonal antibodies that medicate effector functions like ADCC provide protection [97–102]. However, the data from lab-based challenge models do not imply that ADCC is not important, as both neutralization and effector cell functions are needed for sterilizing protection and virus clearance [38, 39, 43, 50, 103, 104]. Nabs capable of engaging specific FcRs are significantly better at protecting against virus challenge and preventing the establishment of a systemic infection compared to nAbs that lack this function. Interestingly, it has been argued that the most broad and potent bnAbs may not require Fc functionality to prevent an infection from being established in animal challenge models [105]. Collectively, human and animal studies suggest that other antibody functions beyond neutralization, although likely in conjunction with neutralization, are important correlates of protection. Indeed, the most successful vaccine strategy to date, RV144, elicited antibodies that mediated effector cell function, but not necessarily broad and potent neutralization. Importantly, this suggests that protective antibodies may be relatively easier to elicit compared to generating bnAbs.
ADCC-inducing antibodies may work by eliminating cells with infectious virus
Antibodies with effector cell function may prevent transmission by eliminating cells with infectious virus from both the transmission source and the exposed individual [104, 106, 107]. Indeed, non-human primate models have suggested that some bnAbs prevent systemic infection in challenged animals by clearing infected cells present far from the site of inoculation [43]. Furthermore, frequency of MTCT has been positively correlated with quantity of HIV-1 DNA in breast milk cells [55, 57], which implies that transmission potentially occurs from infected cells. ADCC responses can recognize non-self cells implying that pre-existing antibodies could help eliminate incoming cells with infectious virus [108]. It should be noted, however, that the majority of infected cells in viremic individuals contain defective genomes, and these cells are unable to generate replication-competent transmissible virus [109, 110]. The number of cells with infectious virus cannot be estimated merely by quantifying total HIV-1 DNA and the association between the number of cells with infectious virus and transmission frequency has not been studied. An observed association may provide a mechanistic understanding for the association between ADCC and protection.
Novel methods are needed to adequately assess the role of ADCC during human HIV-1 transmission
ADCC as opposed to nAbs have not been adequately examined as a correlate of protection in human natural history cohorts primarily due to assay limitations. For instance, studies have not examined ADCC responses against variants present in the infected mother (the exposure strain) among transmitting and non-transmitting HIV-1-infected mothers or their HIV-1 exposed uninfected (HEU) or eventually infected infants. This investigation would highlight if ADCC responses against autologous strains influences transmission. Furthermore, studies have not adequately examined ADCC capacity against a diverse range of heterologous HIV-1 variants. Documenting ADCC breadth and potency differences among transmitting versus non-transmitting mothers and among HEU versus infected infants would suggest that heterologous ADCC responses impact subsequent transmission. The groups highlighted above have been compared for neutralization responses against HIV-1 primarily because of the availability of a reliable high throughput neutralization assay, namely the TZM-bl cell method.
ADCC investigations have primarily examined responses against a single or small number of heterologous unrelated Envs due to assay limitations [92, 93, 95, 111–115]. Studies have often used gp120 monomers to coat cells and then subsequently measure ADCC [92, 93, 95, 116, 117]. This method can only be conducted with a restricted number of Envs because gp120 monomers cannot be generated in a high throughput manner for diverse strains especially those present in infected mothers. Newer ELISA-based assays can measure ADCC against diverse viral variants, but the ADCC estimates from these methods are not based on actual cell killing [118, 119]. Methods that estimate ADCC by either using Env protein coating a target cell, a non-physiologic condition, or by not directly measuring the killing of infected cells potentially generate skewed results because of bystander cell effects [107, 118–121]. Indeed, assays that measure lysis of infected cells have demonstrated that a non-neutralizing antibody (A32) has no significant ADCC activity, even though other assays that measure surrogates of NK activity using gp120 pulsed target cells implies that A32 has broad ADCC function [107, 120, 121]. This may be another potential reason why antibodies that mediated ADCC but with minimal neutralization capacity were ineffective in preventing infection in animal challenge models [97, 98]. Investigations that have examined the relationship between ADCC and transmission have primarily used the CEM natural killer (NK)-resistant (NKR) cell line in their assays [85, 92, 93]. Unfortunately, these cells do not support replication of a large variety of primary CCR5-using variants rendering them unusable for infection-based ADCC assays [115, 117, 122]. Assays that use primary cells cannot be conducted in a high throughput manner for the required large scale investigations of diverse HIV-1 variants and multiple plasma samples [120]. A new method is needed, which should be somewhat analogous to the TZM-bl neutralization assay because the TZM-bl technique was critically important for identifying neutralization breadth and potency and the subsequent bnAb isolations [24, 25, 123–125].
CONCLUSION
In summary, ADCC activity in conjunction with nAb responses, may be an immune correlate of protection against HIV-1 acquisition. Animal and in-vitro challenge studies potentially do not support this scientific premise because of inherent problems with these models, such as the challenge stock lacking infected cells, the consistent use of neutralization susceptible virus, and the presence of non-physiologic high antibody levels especially around the time of virus exposure. ADCC investigations among human cohorts, however, suggest that this antibody functionality is important for sterilizing protection. Further studies examining antibody-mediated cytotoxicity are needed to provide greater clarity into this issue. The role of nAbs has been examined among exposed individuals against potential exposure strains and by assessing pre-infection anti-HIV-1 neutralization breadth and potency. These investigations have been made possible by the high throughput neutralization assays, such as those that employ the TZM-bl cells. Unfortunately, similar high throughput reliable assays are not available to examine pre-existing antibody ADCC responses against exposure strains or the extent of ADCC breadth and potency. Development of assays and examination of ADCC responses in samples from pre-infection but exposed individuals, such as infants in MTCT cohorts, will provide important information about the role of ADCC in providing protection. Furthermore, these studies will potentially highlight the mechanisms of protection. Importantly, potentially novel antibodies can be isolated from samples that harbor broad and potent ADCC activity. Characterization of these broadly potent ADCC-mediating antibodies may reveal that they have unique characteristics compared to bnAbs. Current vaccine strategies aim to elicit bnAbs, but most bnAbs contain a high level of somatic hypermutation, which is difficult to engineer with traditional immunogen strategies. Characterization of the broadly potent ADCC-mediating antibodies may suggest that they are more amenable for elicitation using traditional immunogens. While efforts to elicit bnAbs remain highly useful, future isolation of antibodies that have relatively limited neutralization potential but extensive ability to mediate cellular cytotoxicity may yield new more attainable goals for vaccinology to prevent HIV-1 transmission.
Footnotes
CONFLICT OF INTEREST STATEMENT
No author has a commercial or other association that might pose a conflict of interest.
REFERENCES
- 1.Eakle R, Venter F and Rees H 2018, Retrovirology, 15, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stover J, Bollinger L, Izazola JA, Loures L, DeLay P and Ghys PD 2016, PLoS One, 11, e0154893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Plotkin SA 2001, Pediatr. Infect. Dis. J, 20, 63. [DOI] [PubMed] [Google Scholar]
- 4.Plotkin SA 2008, Clinical Infectious Diseases, 47, 401. [DOI] [PubMed] [Google Scholar]
- 5.Mascola JR 2003, Curr. Mol. Med, 3, 209. [DOI] [PubMed] [Google Scholar]
- 6.Zwick MB and Burton DR 2007, Current HIV Research, 5, 608. [DOI] [PubMed] [Google Scholar]
- 7.Walker BD and Burton DR 2008, Science, 320, 760. [DOI] [PubMed] [Google Scholar]
- 8.Barouch DH 2008, Nature, 455, 613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomaras GD, Yates NL, Liu P, Qin L, Fouda GG, Chavez LL, Decamp AC, Parks RJ, Ashley VC, Lucas JT, Cohen M, Eron J, Hicks CB, Liao HX, Self SG, Landucci G, Forthal DN, Weinhold KJ, Keele BF, Hahn BH, Greenberg ML, Morris L, Karim SS, Blattner WA, Montefiori DC, Shaw GM, Perelson AS and Haynes BF 2008, Journal of Virology, 82, 12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richman DD, Wrin T, Little SJ and Petropoulos CJ 2003, Proceedings of the National Academy of Sciences of the United States of America, 100, 4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei X, Decker JM, Wang S, Hui H, Kappes JC, Wu X, Salazar-Gonzalez JF, Salazar MG, Kilby JM, Saag MS, Komarova NL, Nowak MA, Hahn BH, Kwong PD and Shaw GM 2003, Nature, 422, 307. [DOI] [PubMed] [Google Scholar]
- 12.Sagar M, Wu X, Lee S and Overbaugh J 2006, Journal of Virology, 80, 9586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walker LM, Phogat SK, Chan-Hui PY, Wagner D, Phung P, Goss JL, Wrin T, Simek MD, Fling S, Mitcham JL, Lehrman JK, Priddy FH, Olsen OA, Frey SM, Hammond PW, Kaminsky S, Zamb T, Moyle M, Koff WC, Poignard P and Burton DR 2009, Science, 326, 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scheid JF, Mouquet H, Ueberheide B, Diskin R, Klein F, Oliveira TY, Pietzsch J, Fenyo D, Abadir A, Velinzon K, Hurley A, Myung S, Boulad F, Poignard P, Burton DR, Pereyra F, Ho DD, Walker BD, Seaman MS, Bjorkman PJ, Chait BT and Nussenzweig MC 2011, Science, 333, 1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, Julien JP, Wang SK, Ramos A, Chan-Hui PY, Moyle M, Mitcham JL, Hammond PW, Olsen OA, Phung P, Fling S, Wong CH, Phogat S, Wrin T, Simek MD, Koff WC, Wilson IA, Burton DR and Poignard P 2011, Nature, 477, 466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu X, Yang ZY, Li Y, Hogerkorp CM, Schief WR, Seaman MS, Zhou T, Schmidt SD, Wu L, Xu L, Longo NS, McKee K, O’Dell S, Louder MK, Wycuff DL, Feng Y, Nason M, Doria-Rose N, Connors M, Kwong PD, Roederer M, Wyatt RT, Nabel GJ and Mascola JR 2010, Science, 329, 856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mouquet H, Scharf L, Euler Z, Liu Y, Eden C, Scheid JF, Halper-Stromberg A, Gnanapragasam PN, Spencer DI, Seaman MS, Schuitemaker H, Feizi T, Nussenzweig MC and Bjorkman PJ 2012, Proceedings of the National Academy of Sciences of the United States of America, 109, E3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang J, Ofek G, Laub L, Louder MK, Doria-Rose NA, Longo NS, Imamichi H, Bailer RT, Chakrabarti B, Sharma SK, Alam SM, Wang T, Yang Y, Zhang B, Migueles SA, Wyatt R, Haynes BF, Kwong PD, Mascola JR and Connors M 2012, Nature, 491, 406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang J, Kang BH, Pancera M, Lee JH, Tong T, Feng Y, Imamichi H, Georgiev IS, Chuang GY, Druz A, Doria-Rose NA, Laub L, Sliepen K, van Gils MJ, de la Pena AT, Derking R, Klasse PJ, Migueles SA, Bailer RT, Alam M, Pugach P, Haynes BF, Wyatt RT, Sanders RW, Binley JM, Ward AB, Mascola JR, Kwong PD and Connors M 2014, Nature, 515, 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson PR, Schnepp BC, Zhang J, Connell MJ, Greene SM, Yuste E, Desrosiers RC and Clark KR 2009, Nature Medicine, 15, 901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balazs AB, Chen J, Hong CM, Rao DS, Yang L and Baltimore D 2011, Nature, 481, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balazs AB, Ouyang Y, Hong CM, Chen J, Nguyen SM, Rao DS, An DS and Baltimore D 2014, Nature Medicine, 20, 296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doria-Rose NA, Klein RM, Manion MM, O’Dell S, Phogat A, Chakrabarti B, Hallahan CW, Migueles SA, Wrammert J, Ahmed R, Nason M, Wyatt RT, Mascola JR and Connors M 2009, Journal of Virology, 83, 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sather DN, Armann J, Ching LK, Mavrantoni A, Sellhorn G, Caldwell Z, Yu X, Wood B, Self S, Kalams S and Stamatatos L 2009, Journal of Virology, 83, 757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simek MD, Rida W, Priddy FH, Pung P, Carrow E, Laufer DS, Lehrman JK, Boaz M, Tarragona-Fiol T, Miiro G, Birungi J, Pozniak A, McPhee DA, Manigart O, Karita E, Inwoley A, Jaoko W, Dehovitz J, Bekker LG, Pitisuttithum P, Paris R, Walker LM, Poignard P, Wrin T, Fast PE, Burton DR and Koff WC 2009, Journal of Virology, 83, 7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piantadosi A, Panteleeff D, Blish CA, Baeten JM, Jaoko W, McClelland RS and Overbaugh J 2009, Journal of Virology, 83, 10269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Gils MJ, Euler Z, Schweighardt B, Wrin T and Schuitemaker H 2009, AIDS, 23, 2405. [DOI] [PubMed] [Google Scholar]
- 28.Deeks SG, Schweighardt B, Wrin T, Galovich J, Hoh R, Sinclair E, Hunt P, McCune JM, Martin JN, Petropoulos CJ and Hecht FM 2006, Journal of Virology, 80, 6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mikell I, Sather DN, Kalams SA, Altfeld M, Alter G and Stamatatos L 2011, PLoS Pathogens, 7, e1001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goo L, Chohan V, Nduati R and Overbaugh J 2014, Nature Medicine, 20, 655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonsignori M, Zhou T, Sheng Z, Chen L, Gao F, Joyce MG, Ozorowski G, Chuang GY, Schramm CA, Wiehe K, Alam SM, Bradley T, Gladden MA, Hwang KK, Iyengar S, Kumar A, Lu X, Luo K, Mangiapani MC, Parks RJ, Song H, Acharya P, Bailer RT, Cao A, Druz A, Georgiev IS, Kwon YD, Louder MK, Zhang B, Zheng A, Hill BJ, Kong R, Soto C, Mullikin JC, Douek DC, Montefiori DC, Moody MA, Shaw GM, Hahn BH, Kelsoe G, Hraber PT, Korber BT, Boyd SD, Fire AZ, Kepler TB, Shapiro L, Ward AB, Mascola JR, Liao HX, Kwong PD and Haynes BF 2016, Cell, 165, 449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doria-Rose NA, Schramm CA, Gorman J, Moore PL, Bhiman JN, DeKosky BJ, Ernandes MJ, Georgiev IS, Kim HJ, Pancera M, Staupe RP, Altae-Tran HR, Bailer RT, Crooks ET, Cupo A, Druz A, Garrett NJ, Hoi KH, Kong R, Louder MK, Longo NS, McKee K, Nonyane M, O’Dell S, Roark RS, Rudicell RS, Schmidt SD, Sheward DJ, Soto C, Wibmer CK, Yang Y, Zhang Z, Mullikin JC, Binley JM, Sanders RW, Wilson IA, Moore JP, Ward AB, Georgiou G, Williamson C, Abdool Karim SS, Morris L, Kwong PD, Shapiro L and Mascola JR 2014, Nature, 509, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sok D, Laserson U, Laserson J, Liu Y, Vigneault F, Julien JP, Briney B, Ramos A, Saye KF, Le K, Mahan A, Wang S, Kardar M, Yaari G, Walker LM, Simen BB, St John EP, Chan-Hui PY, Swiderek K, Kleinstein SH, Alter G, Seaman MS, Chakraborty AK, Koller D, Wilson IA, Church GM, Burton DR and Poignard P 2013, PLoS Pathogens, 9, e1003754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haynes BF, Kelsoe G, Harrison SC and Kepler TB 2012, Nat. Biotechnol, 30, 423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonsignori M, Kreider EF, Fera D, Meyerhoff RR, Bradley T, Wiehe K, Alam SM, Aussedat B, Walkowicz WE, Hwang KK, Saunders KO, Zhang R, Gladden MA, Monroe A, Kumar A, Xia SM, Cooper M, Louder MK, McKee K, Bailer RT, Pier BW, Jette CA, Kelsoe G, Williams WB, Morris L, Kappes J, Wagh K, Kamanga G, Cohen MS, Hraber PT, Montefiori DC, Trama A, Liao HX, Kepler TB, Moody MA, Gao F, Danishefsky SJ, Mascola JR, Shaw GM, Hahn BH, Harrison SC, Korber BT and Haynes BF 2017, Science Translational Medicine, 9, pii: eaai7514.28298420 [Google Scholar]
- 36.Doria-Rose NA and Joyce MG 2015, Current Opinion in Virology, 11, 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sheward DJ, Marais J, Bekker V, Murrell B, Eren K, Bhiman JN, Nonyane M, Garrett N, Woodman ZL, Abdool Karim Q, Abdool Karim SS, Morris L, Moore PL and Williamson C 2018, Cell Host & Microbe, 24, 593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bournazos S, Klein F, Pietzsch J, Seaman MS, Nussenzweig MC and Ravetch JV 2014, Cell, 158, 1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Halper-Stromberg A, Lu CL, Klein F, Horwitz JA, Bournazos S, Nogueira L, Eisenreich TR, Liu C, Gazumyan A, Schaefer U, Furze RC, Seaman MS, Prinjha R, Tarakhovsky A, Ravetch JV and Nussenzweig MC 2014, Cell, 158, 989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang Y, Yu J, Lanzi A, Yao X, Andrews CD, Tsai L, Gajjar MR, Sun M, Seaman MS, Padte NN and Ho DD 2016, Cell, 165, 1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Julg B, Sok D, Schmidt SD, Abbink P, Newman RM, Broge T, Linde C, Nkolola J, Le K, Su D, Torabi J, Pack M, Pegu A, Allen TM, Mascola JR, Burton DR and Barouch DH 2017, Journal of Virology, 91, pii: e01187–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Julg B, Liu PT, Wagh K, Fischer WM, Abbink P, Mercado NB, Whitney JB, Nkolola JP, McMahan K, Tartaglia LJ, Borducchi EN, Khatiwada S,, Kamath M, LeSuer JA, Seaman MS, Schmidt SD, Mascola JR, Burton DR, Korber BT and Barouch DH 2017, Science Translational Medicine, 9, pii: eaao4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu J, Ghneim K, Sok D, Bosche WJ, Li Y, Chipriano E, Berkemeier B, Oswald K, Borducchi E, Cabral C, Peter L, Brinkman A, Shetty M, Jimenez J, Mondesir J, Lee B, Giglio P, Chandrashekar A, Abbink P, Colantonio A, Gittens C, Baker C, Wagner W, Lewis MG, Li W, Sekaly RP, Lifson JD, Burton DR and Barouch DH 2016, Science, 353, 1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moldt B, Rakasz EG, Schultz N, Chan-Hui PY, Swiderek K, Weisgrau KL, Piaskowski SM, Bergman Z, Watkins DI, Poignard P and Burton DR 2012, Proceedings of the National Academy of Sciences of the United States of America, 109, 18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pegu A, Yang Z-Y, Boyington JC, Wu L, Ko S-Y, Schmidt SD, McKee K, Kong W-P, Shi W, Chen X, Todd J-P, Letvin NL, Huang J, Nason MC, Hoxie JA, Kwong PD, Connors M, Rao SS, Mascola JR and Nabel GJ 2014, Science Translational Medicine, 6, 243ra88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shingai M, Donau OK, Plishka RJ, Buckler-White A, Mascola JR, Nabel GJ, Nason MC, Montefiori D, Moldt B, Poignard P, Diskin R, Bjorkman PJ, Eckhaus MA, Klein F, Mouquet H, Cetrulo Lorenzi JC, Gazumyan A, Burton DR, Nussenzweig MC, Martin MA and Nishimura Y 2014, The Journal of Experimental Medicine, 211, 2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu L, Pegu A, Rao E, Doria-Rose N, Beninga J, McKee K, Lord DM, Wei RR, Deng G, Louder M, Schmidt SD, Mankoff Z, Wu L, Asokan M, Beil C, Lange C, Leuschner WD, Kruip J, Sendak R, Kwon YD, Zhou T, Chen X, Bailer RT, Wang K, Choe M, Tartaglia LJ, Barouch DH, O’Dell S, Todd JP, Burton DR, Roederer M, Connors M, Koup RA, Kwong PD, Yang ZY,, Mascola JR and Nabel GJ 2017, Science, 358, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hessell AJ, Jaworski JP, Epson E, Matsuda K, Pandey S, Kahl C, Reed J, Sutton WF, Hammond KB, Cheever TA, Barnette PT, Legasse AW, Planer S, Stanton JJ, Pegu A, Chen X, Wang K, Siess D, Burke D, Park BS, Axthelm MK, Lewis A, Hirsch VM, Graham BS, Mascola JR, Sacha JB and Haigwood NL 2016, Nature Medicine, 22, 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shingai M, Nishimura Y, Klein F, Mouquet H, Donau OK, Plishka R, Buckler-White A, Seaman M, Piatak M Jr., Lifson JD, Dimitrov DS, Nussenzweig MC and Martin MA 2013, Nature, 503, 277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hessell AJ, Poignard P, Hunter M, Hangartner L, Tehrani DM,, Bleeker WK, Parren PW, Marx PA and Burton DR 2009, Nature Medicine, 15, 951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gautam R, Nishimura Y, Pegu A, Nason MC, Klein F, Gazumyan A, Golijanin J, Buckler-White A, Sadjadpour R, Wang K, Mankoff Z, Schmidt SD, Lifson JD, Mascola JR, Nussenzweig MC and Martin MA 2016, Nature, 533, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gautam R, Nishimura Y, Gaughan N, Gazumyan A, Schoofs T, Buckler-White A, Seaman MS, Swihart BJ, Follmann DA, Nussenzweig MC and Martin MA 2018, Nature Medicine, 24, 610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moldt B, Shibata-Koyama M, Rakasz EG, Schultz N, Kanda Y, Dunlop DC, Finstad SL, Jin C, Landucci G, Alpert MD, Dugast AS, Parren PW, Nimmerjahn F, Evans DT, Alter G, Forthal DN, Schmitz JE, Iida S, Poignard P, Watkins DI, Hessell AJ and Burton DR 2012, Journal of Virology, 86, 6189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anderson DJ, Politch JA, Nadolski AM, Blaskewicz CD, Pudney J and Mayer KH 2010, AIDS, 24, 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rousseau CM, Nduati RW, Richardson BA, John-Stewart GC, Mbori-Ngacha DA, Kreiss JK and Overbaugh J 2004, The Journal of Infectious Diseases, 190, 1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sagar M 2014, The Journal of Infectious Diseases, 210(Suppl. 3), S667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ndirangu J, Viljoen J, Bland RM, Danaviah S, Thorne C, van de Perre P and Newell ML 2012, PLoS One, 7, e51493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parsons MS, Lloyd SB, Lee WS, Kristensen AB, Amarasena T, Center RJ, Keele BF, Lifson JD, LaBranche CC, Montefiori D, Wines BD, Hogarth PM, Swiderek KM, Venturi V, Davenport MP and Kent SJ 2017, Science Translational Medicine, 9, pii: eaaf1483. [DOI] [PubMed] [Google Scholar]
- 59.Sagar M, Akiyama H, Etemad B, Ramirez N, Freitas I and Gummuluru S 2012, The Journal of Infectious Diseases, 205, 1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reh L, Magnus C, Schanz M, Weber J, Uhr T, Rusert P and Trkola A 2015, PLoS Pathogens, 11, e1004966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Malbec M, Porrot F, Rua R, Horwitz J, Klein F, Halper-Stromberg A, Scheid JF, Eden C, Mouquet H, Nussenzweig MC and Schwartz O 2013, The Journal of Experimental Medicine, 210, 2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Duncan CJ, Williams JP, Schiffner T, Gartner K, Ochsenbauer C, Kappes J, Russell RA, Frater J and Sattentau QJ 2014, Journal of Virology, 88, 2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Coovadia H 2004, The New England Journal of Medicine, 351, 289. [DOI] [PubMed] [Google Scholar]
- 64.Coutsoudis A, Dabis F, Fawzi W, Gaillard P, Haverkamp G, Harris DR, Jackson JB, Leroy V, Meda N, Read JS and Wiktor S 2004, The Journal of Infectious Diseases, 189, 2154. [DOI] [PubMed] [Google Scholar]
- 65.Miotti PG, Taha TE, Kumwenda NI, Broadhead R, Mtimavalye LA, van der Hoeven L, Chiphangwi JD, Liomba G and Biggar RJ 1999, JAMA, 282, 744. [DOI] [PubMed] [Google Scholar]
- 66.Nduati R, John G, Mbori-Ngacha D, Richardson B, Overbaugh J, Mwatha A, Ndinya-Achola J, Bwayo J, Onyango FE, Hughes J and Kreiss J 2000, JAMA, 283, 1167. [DOI] [PubMed] [Google Scholar]
- 67.Taha TE, Hoover DR, Kumwenda NI, Fiscus SA, Kafulafula G, Nkhoma C, Chen S, Piwowar E, Broadhead RL, Jackson JB and Miotti PG 2007, The Journal of Infectious Diseases, 196, 10. [DOI] [PubMed] [Google Scholar]
- 68.Embree JE, Njenga S, Datta P, Nagelkerke NJ, Ndinya-Achola JO, Mohammed Z, Ramdahin S, Bwayo JJ and Plummer FA 2000, AIDS, 14, 2535. [DOI] [PubMed] [Google Scholar]
- 69.Eshleman SH, Lie Y, Hoover DR, Chen S, Hudelson SE, Fiscus SA, Petropoulos CJ, Kumwenda N, Parkin N and Taha TE 2006, The Journal of Infectious Diseases, 193, 1512. [DOI] [PubMed] [Google Scholar]
- 70.Fawzi W, Msamanga G, Spiegelman D, Renjifo B, Bang H, Kapiga S, Coley J, Hertzmark E, Essex M and Hunter D 2002, Journal of Acquired Immune Deficiency Syndromes, 31, 331. [DOI] [PubMed] [Google Scholar]
- 71.John GC, Nduati RW, Mbori-Ngacha DA, Richardson BA, Panteleeff D, Mwatha A, Overbaugh J, Bwayo J, Ndinya-Achola JO and Kreiss JK 2001, The Journal of Infectious Diseases, 183, 206. [DOI] [PubMed] [Google Scholar]
- 72.Mwapasa V, Rogerson SJ, Kwiek JJ, Wilson PE, Milner D, Molyneux ME, Kamwendo DD, Tadesse E, Chaluluka E and Meshnick SR 2006, AIDS, 20, 1869. [DOI] [PubMed] [Google Scholar]
- 73.Pillay K, Coutsoudis A, York D, Kuhn L and Coovadia HM 2000, Journal of Acquired Immune Deficiency Syndromes, 24, 330. [DOI] [PubMed] [Google Scholar]
- 74.Rousseau CM, Nduati RW, Richardson BA, Steele MS, John-Stewart GC, Mbori-Ngacha DA, Kreiss JK and Overbaugh J 2003, The Journal of Infectious Diseases, 187, 741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Semba RD, Kumwenda N, Hoover DR, Taha TE, Quinn TC, Mtimavalye L, Biggar RJ, Broadhead R, Miotti PG, Sokoll LJ, van der Hoeven L and Chiphangwi JD 1999, The Journal of Infectious Diseases, 180, 93. [DOI] [PubMed] [Google Scholar]
- 76.Coovadia HM, Rollins NC, Bland RM, Little K, Coutsoudis A, Bennish ML and Newell ML 2007, Lancet, 369, 1107. [DOI] [PubMed] [Google Scholar]
- 77.Aldrovandi GM and Kuhn L 2010, The Journal of Infectious Diseases, 202(Suppl. 3), S366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ghulam-Smith M, Olson A, White LF, Chasela CS, Ellington SR, Kourtis AP, Jamieson DJ, Tegha G, van der Horst CM and Sagar M 2017, mBio, 8, pii: e01373–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lynch JB, Nduati R, Blish CA, Richardson BA, Mabuka JM, Jalalian-Lechak Z, John-Stewart G and Overbaugh J 2011, Journal of Virology, 85, 5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guevara H, Casseb J, Zijenah LS, Mbizvo M, Oceguera LF 3rd., Hanson CV, Katzenstein DA and Hendry RM 2002, Journal of Acquired Immune Deficiency Syndromes, 29, 435. [DOI] [PubMed] [Google Scholar]
- 81.Pancino G, Leste-Lasserre T, Burgard M, Costagliola D, Ivanoff S, Blanche S, Rouzioux C and Sonigo P 1998, The Journal of Infectious Diseases, 177, 1737. [DOI] [PubMed] [Google Scholar]
- 82.Lallemant M, Baillou A, Lallemant-Le Coeur S, Nzingoula S, Mampaka M, M’Pele P, Barin F and Essex M 1994, Lancet, 343, 1001. [DOI] [PubMed] [Google Scholar]
- 83.Mann DL, Hamlin-Green G, Willoughby A, Landesman SH and Goedert JJ 1994, Journal of Acquired Immune Deficiency Syndromes, 7, 617. [PubMed] [Google Scholar]
- 84.Baan E, de Ronde A, Stax M, Sanders RW, Luchters S, Vyankandondera J, Lange JM, Pollakis G and Paxton WA 2013, PLoS One, 8, e69274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao HX, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL and Kim JH 2012, The New England Journal of Medicine, 366, 1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P and Kim JH 2009, The New England Journal of Medicine, 361, 2209. [DOI] [PubMed] [Google Scholar]
- 87.Chung AW, Ghebremichael M, Robinson H, Brown E, Choi I, Lane S, Dugast AS, Schoen MK, Rolland M, Suscovich TJ, Mahan AE, Liao L, Streeck H, Andrews C, Rerks-Ngarm S, Nitayaphan S, de Souza MS, Kaewkungwal J, Pitisuttithum P, Francis D, Michael NL, Kim JH, Bailey-Kellogg C, Ackerman ME and Alter G 2014, Science Translational Medicine, 6, 228ra38. [DOI] [PubMed] [Google Scholar]
- 88.Yates NL, Liao HX, Fong Y, deCamp A, Vandergrift NA, Williams WT, Alam SM, Ferrari G, Yang ZY, Seaton KE, Berman PW, Alpert MD, Evans DT, O’Connell RJ, Francis D, Sinangil F, Lee C, Nitayaphan S, Rerks-Ngarm S, Kaewkungwal J, Pitisuttithum P, Tartaglia J, Pinter A, Zolla-Pazner S, Gilbert PB, Nabel GJ, Michael NL, Kim JH, Montefiori DC, Haynes BF and Tomaras GD 2014, Science Translational Medicine, 6, 228ra39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pitisuttithum P, Gilbert P, Gurwith M, Heyward W, Martin M, van Griensven F, Hu D, Tappero JW and Choopanya K 2006, The Journal of Infectious Diseases, 194, 1661. [DOI] [PubMed] [Google Scholar]
- 90.Jefferis R 2012, Arch. Biochem. Biophys, 526, 159. [DOI] [PubMed] [Google Scholar]
- 91.Ackerman ME, Dugast AS and Alter G 2012, Annu. Rev. Med, 63, 113. [DOI] [PubMed] [Google Scholar]
- 92.Mabuka J, Nduati R, Odem-Davis K, Peterson D and Overbaugh J, 2012, PLoS Pathogens, 8, e1002739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Milligan C, Richardson BA, John-Stewart G, Nduati R and Overbaugh J 2015, Cell Host & Microbe, 17, 500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lassauniere R, Musekiwa A, Gray GE, Kuhn L and Tiemessen CT 2016, Retrovirology, 13, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tiemessen CT, Shalekoff S, Meddows-Taylor S, Schramm DB, Papathanasopoulos MA, Gray GE, Sherman GG, Coovadia AH and Kuhn L 2009, Journal of Immunology (Baltimore, Md : 1950), 182, 5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ruiz MJ, Salido J, Abusamra L, Ghiglione Y, Cevallos C, Damilano G, Rodriguez AM, Trifone C, Laufer N, Giavedoni LD, Sued O, Salomon H, Gherardi MM and Turk G 2017, EBioMedicine, 26, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dugast AS, Chan Y, Hoffner M, Licht A, Nkolola J, Li H, Streeck H, Suscovich TJ, Ghebremichael M, Ackerman ME, Barouch DH and Alter G 2014, PLoS One, 9, e97229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Burton DR, Hessell AJ, Keele BF, Klasse PJ, Ketas TA, Moldt B, Dunlop DC, Poignard P, Doyle LA, Cavacini L, Veazey RS and Moore JP 2011, Proceedings of the National Academy of Sciences of the United States of America, 108, 11181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Santra S, Tomaras GD, Warrier R, Nicely NI, Liao HX, Pollara J, Liu P, Alam SM, Zhang R, Cocklin SL, Shen X, Duffy R, Xia SM, Schutte RJ, Pemble CW Iv., Dennison SM, Li H, Chao A, Vidnovi K, Evans A, Klein K, Kumar A, Robinson J, Landucci G, Forthal DN, Montefiori DC, Kaewkungwal J, Nitayaphan S, Pitisuttithum P, Rerks-Ngarm S, Robb ML, Michael NL, Kim JH, Soderberg KA, Giorgi EE, Blair L, Korber BT, Moog C, Shattock RJ, Letvin NL, Schmitz JE, Moody MA, Gao F, Ferrari G, Shaw GM and Haynes BF 2015, PLoS Pathogens, 11, e1005042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Astronomo RD, Santra S, Ballweber-Fleming L, Westerberg KG, Mach L, Hensley-McBain T, Sutherland L, Mildenberg B, Morton G, Yates NL, Mize GJ, Pollara J, Hladik F, Ochsenbauer C, Denny TN, Warrier R, Rerks-Ngarm S, Pitisuttithum P, Nitayapan S, Kaewkungwal J, Ferrari G, Shaw GM, Xia SM, Liao HX, Montefiori DC, Tomaras GD, Haynes BF and McElrath JM 2016, EBioMedicine, 14, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gomez-Roman VR, Patterson LJ, Venzon D, Liewehr D, Aldrich K, Florese R and Robert-Guroff M 2005, Journal of Immunology (Baltimore, Md : 1950), 174, 2185. [DOI] [PubMed] [Google Scholar]
- 102.Moog C, Dereuddre-Bosquet N, Teillaud JL, Biedma ME, Holl V, Van Ham G, Heyndrickx L, van Dorsselaer A, Katinger D, Vcelar B, Zolla-Pazner S, Mangeot I, Kelly C Shattock RJ and Le Grand R 2014, Mucosal Immunology, 7, 46. [DOI] [PubMed] [Google Scholar]
- 103.Hessell AJ, Hangartner L, Hunter M, Havenith CE, Beurskens FJ, Bakker JM, Lanigan CM, Landucci G, Forthal DN, Parren PW, Marx PA and Burton DR 2007, Nature, 449, 101. [DOI] [PubMed] [Google Scholar]
- 104.Lu CL, Murakowski DK, Bournazos S, Schoofs T, Sarkar D, Halper-Stromberg A, Horwitz JA, Nogueira L, Golijanin J, Gazumyan A, Ravetch JV, Caskey M, Chakraborty AK and Nussenzweig MC 2016, Science, 352, 1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Parsons MS, Lee WS, Kristensen AB, Amarasena T, Khoury G, Wheatley AK, Reynaldi A, Wines BD, Hogarth PM, Davenport MP and Kent SJ 2019, The Journal of Clinical Investigation, 129, 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Overbaugh J and Morris L 2012, Cold Spring Harb Perspect Med, 2, a007039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Richard J, Prevost J, Baxter AE, von Bredow B, Ding S, Medjahed H, Delgado GG, Brassard N, Sturzel CM, Kirchhoff F, Hahn BH, Parsons MS, Kaufmann DE, Evans DT and Finzi A 2018, mBio, 9, e00358–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gooneratne SL, Richard J, Lee WS, Finzi A, Kent SJ and Parsons MS 2015, Journal of Virology, 89, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cohn LB, Silva IT, Oliveira TY, Rosales RA, Parrish EH, Learn GH, Hahn BH, Czartoski JL, McElrath MJ, Lehmann C, Klein F, Caskey M, Walker BD, Siliciano JD, Siliciano RF, Jankovic M and Nussenzweig MC 2015, Cell, 160, 420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bruner KM, Murray AJ, Pollack RA, Soliman MG, Laskey SB, Capoferri AA, Lai J, Strain MC, Lada SM, Hoh R, Ho YC, Richman DD, Deeks SG, Siliciano JD and Siliciano RF 2016, Nature Medicine, 22, 1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Forthal DN, Landucci G and Daar ES 2001, Journal of Virology, 75, 6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Forthal DN, Gilbert PB, Landucci G and Phan T 2007, Journal of Immunology (Baltimore, Md : 1950), 178, 6596. [DOI] [PubMed] [Google Scholar]
- 113.Forthal DN, Landucci G, Haubrich R, Keenan B, Kuppermann BD, Tilles JG and Kaplan J 1999, The Journal of Infectious Diseases, 180, 1338. [DOI] [PubMed] [Google Scholar]
- 114.Ruppach H, Nara P, Raudonat I, Elanjikal Z, Rubsamen-Waigmann H and Dietrich U 2000, Journal of Virology, 74, 5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pollara J, McGuire E, Fouda GG, Rountree W, Eudailey J, Overman RG, Seaton KE, Deal A, Edwards RW, Tegha G, Kamwendo D, Kumwenda J, Nelson JA, Liao HX, Brinkley C, Denny TN, Ochsenbauer C, Ellington S, King CC, Jamieson DJ, van der Horst C, Kourtis AP, Tomaras GD, Ferrari G and Permar SR 2015, Journal of Virology, 89, 9952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gomez-Roman VR, Florese RH, Patterson LJ, Peng B, Venzon D, Aldrich K and Robert-Guroff M 2006, Journal of Immunological Methods, 308, 53. [DOI] [PubMed] [Google Scholar]
- 117.Orlandi C, Flinko R and Lewis GK 2016, Journal of Immunological Methods, 433, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wines BD, Billings H, McLean MR, Kent SJ and Hogarth PM 2017, Current HIV Research, 15, 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Parekh BS, Berger E, Sibley S, Cahya S, Xiao L, LaCerte MA, Vaillancourt P, Wooden S and Gately D 2012, mAbs, 4, 310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Smalls-Mantey A, Doria-Rose N, Klein R, Patamawenu A, Migueles SA, Ko SY, Hallahan CW, Wong H, Liu B, You L, Scheid J, Kappes JC, Ochsenbauer C, Nabel GJ, Mascola JR and Connors M 2012, Journal of Virology, 86, 8672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.von Bredow B, Arias JF, Heyer LN, Moldt B, Le K, Robinson JE, Zolla-Pazner S, Burton DR and Evans DT 2016, Journal of Virology, 90, 6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Trkola A, Matthews J, Gordon C, Ketas T and Moore JP 1999, Journal of Virology, 73, 8966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.McCoy LE and Burton DR 2017, Immunological Reviews, 275, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li Y, Svehla K, Louder MK, Wycuff D, Phogat S, Tang M, Migueles SA, Wu X, Phogat A, Shaw GM, Connors M, Hoxie J, Mascola JR and Wyatt R 2009, Journal of Virology, 83, 1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Montefiori DC 2009, Methods Mol. Biol, 485, 395. [DOI] [PubMed] [Google Scholar]