Abstract
OBJECTIVES:
Preoperative decision-making for differentiating malignant from benign lesions in the gallbladder remains challenging. We aimed to create a diagnostic nomogram to identify gallbladder cancer (GBC), especially for incidental GBC (IGBC), before surgical resection.
METHODS:
A total of 587 consecutive patients with pathologically confirmed gallbladder lesions from a hospital were randomly assigned to a training cohort (70%) and an internal validation cohort (30%), with 287 patients from other centers as an external validation cohort. Radiological features were developed by the least absolute shrinkage and selection operator logistic regression model. Significant radiological features and independent clinical factors, identified by multivariate analyses, were used to construct a nomogram.
RESULTS:
A diagnostic nomogram was established by age, CA19.9, and 6 radiological features. The values of area under the curve in the internal and external validation cohorts were up to 0.91 and 0.89, respectively. The calibration curves for probability of GBC showed optimal agreement between nomogram prediction and actual observation. Compared with previous methods, it demonstrated superior sensitivity (91.5%) and accuracy (85.1%) in the diagnosis of GBC. The accuracy using the nomogram was significantly higher in GBC groups compared with that by radiologists in the training cohort (P < 0.001) and similarly in each cohort. Notably, most of the IGBC, which were misdiagnosed as benign lesions, were successfully identified using this nomogram.
DISCUSSION:
A novel nomogram provides a powerful tool for detecting the presence of cancer in gallbladder masses, with an increase in accuracy and sensitivity. It demonstrates an unprecedented potential for IGBC identification.
INTRODUCTION
Gallbladder mass lesions are detected with an approximate incidence rate of 5% and 9.9% in Western and eastern countries (1–3), respectively. They consist of 2 categories, benign and malignant lesions. The benign gallbladder mass lesions (BGMLs) include cholesterol or inflammatory, adenomyosis, and adenomatous polyps, and the most common malignant gallbladder mass is gallbladder cancer (GBC). Although the incidence of GBC is low, it is one of the most aggressive biliary tract diseases with poor prognosis and high mortality (4–6). The 5-year survival rate of advanced GBC is less than 5% (7).
At present, resection by surgery is the most effective and only curative treatment for GBC if diagnosed early. The majority of the incidental GBC (IGBC) cases are discovered accidentally during a pathological examination after a simple laparoscopic cholecystectomy (8–10). Although warning signs, such as the size of the mass and irregularity of the gallbladder wall, may help diagnose, none of them has been widely accepted for the diagnosis of IGBC (11). Patients who are older than 50 years and whose masses are greater than 10 mm in size are recommended for surgical management (12). A minimally invasive and simple laparoscopic cholecystectomy is recommended as a first choice for benign lesions and tumors limited to the lamina propria (T1a), and open extended cholecystectomy (cholecystectomy including a rim of liver tissue) should be performed for T1b diseases (tumors that invade the muscular layer) and T2 tumors that invade the perimuscular connective tissue without involvement of the serosa (13–15). As for early GBC, many patients present the early lymphatic metastases involvement. Although extensive lymphadenectomy for early GBC remains major controversy, local lymph dissection and biopsy are recommended for early GBC, especially for T2 GBC (16,17). Therefore, it is particularly important and critical for the differential diagnosis of mass lesions (benign or malignant) preoperatively to avoid an unnecessary second surgery for IGBC and obtain better outcomes (18,19).
Various methods are used to differentiate GBC from BGML before surgery. Ultrasonography is a common tool used for the discovery of gallbladder lesions with low accuracy, which depends largely on the operator's skills (20,21). For example, among polypoid lesions, the gallbladder sludge ball may mimic true gallbladder polyps, appearing as immobile mass lesion (22). More accurate and objective features can be achieved in a computed tomography (CT) scan. The imaging procedure helps distinguish the benign from malignant, despite with some limitations in identifying the cause of gallbladder wall thickening (23). The imaging features by contrast-enhanced CT demonstrate the possibility of distinguishing adenomyomatosis from the malignant tumors in the gallbladder wall thickening (24). MRI and endoscopic ultrasonography are other methods used in practice (25,26). Compared with CT, MRI is more expensive and has no advantages in the sensitivity and specificity of detection (25). In addition, endoscopic ultrasonography combined with fine-needle aspiration is an invasive procedure, with the theoretic risk of tumor seeding (23). In other words, CT is superior in terms of detection accuracy and has advantages in the differential diagnosis of neoplastic from nonneoplastic small lesions in the gallbladder.
In this study, we aimed to build a diagnostic tool that incorporated the clinical factors and image features of CT for early identification of BGMLs and GBC (mainly T1-2). This could provide useful information to clinicians for presurgery and next treatment decision-making.
METHODS
Study population
A total of 587 patients pathologically diagnosed with gallbladder mass lesions between January 2008 and April 2018 were identified and selected from the Sir Run-Run Shaw Hospital as a primary cohort. Next, based on a random split-sample approach, patients were randomly assigned to a training cohort (n = 411) or an internal validation cohort (n = 176). In addition, 287 patients coming from 3 other centers (Jinhua Municipal Central Hospital, Shaoxing People's Hospital, and Longyou People's Hospital) were regarded as an external cohort. The inclusion criteria for the patients with gallbladder mass lesions were as follows: (i) patients underwent surgical treatment and had pathologically confirmed diagnosis and (ii) examination with ultrasonography and upper abdominal enhanced CT scan was performed within 1 month before surgery. The exclusion criteria were: (i) patients who were younger than 18 years, (ii) those only diagnosed with gallbladder stones, (iii) with liver metastasis and/or adjacent organ involvement or distant metastasis, (iv) comorbid with other cancers, (v) comorbid with bile duct abnormalities such as choledochal cyst and gallbladder abnormalities such as porcelain gallbladder, and (vi) patients who had abdominal surgery history. A standardized data form was created to collect all relevant information on the patients' demographic characteristics such as age, sex, body mass index, smoking, years of gallbladder stone, and tumor markers (CA19.9, CA125, CA15.3, and carcinoembryonic antigen [CEA]).
Radiological features
All patients received an upper abdominal enhanced CT scan preoperatively. This included unenhanced, arterial, portal, and delayed phases, and the images were recorded on picture archiving and communication system work stations. Radiological features were evaluated independently by 2 senior radiologists in abdominal CT-scanning techniques, and they were blinded to clinical and pathological outcomes. The radiological features consisted of: (i) mass characteristics: size, location, number, CT value, and ΔCT; (ii) gallbladder wall characteristics: mucosal smoothness, thickness and enhancement of the GB wall, and layered patterns; and (iii) other characteristics: regional lymph nodes and gallbladder stone. However, whether it was associated with gallbladder stones would be confirmed by an ultrasound to avoid invisible stones. In addition, some radiological features of the mass, including CT values, could be automatically extracted using MaZda software (http://eletel.eu/mazda). MaZda is a 2- or 3-dimensional image texture analysis software program that is widely used for image analysis tasks (27).
Statistical analysis
A pathological diagnosis (benign or malignant) was the primary prerequisite in this study. To avoid human error, the interoperator agreement and intraoperator agreement in each radiological feature were estimated by Cohen's kappa. The most significant radiological features were identified by the least absolute shrinkage and selection operator (LASSO) with 10-fold cross-validation methods. Clinical continuous variables (age and all tumor markers) were transformed into categorical variables based on recognized cutoff values. Those variables between benign and malignant gallbladder mass lesions were estimated and compared using the χ2 test or Fisher exact test as appropriate. If those clinical variables showed statistical significance in the univariate analysis (P < 0.05), they were taken into multivariate logistic regression analysis with significant radiological features together. A P value of less than 0.05 was considered as statistical significance. According to the above statistical analyses, a nomogram was performed using R software packages (http://www.r-project.org). A final model was selected using a backward step-down process, which used the Akaike information criterion as a stopping rule. The performance of the nomogram was evaluated with the area under the receiver operating characteristic curve (AUC) and calibration curve. We compared this novel model with previous diagnostic methods (12,23,24,28–30) using the receiver operating characteristic.
RESULTS
Demographic and clinical characteristics of patients
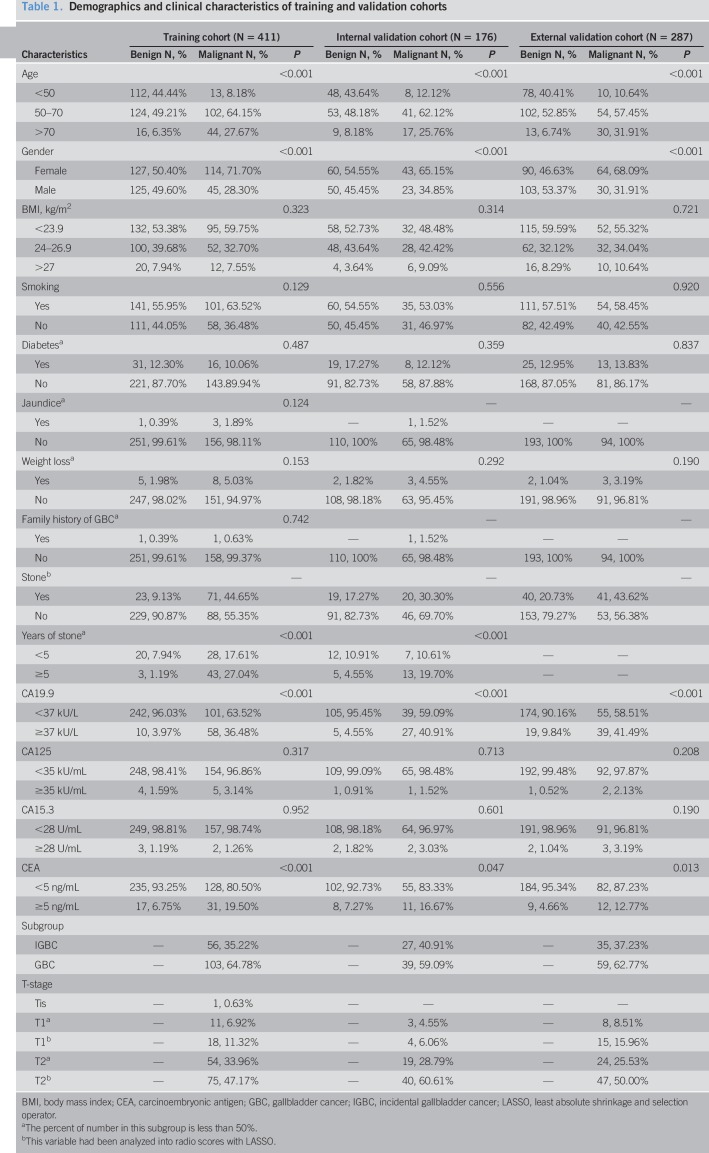
Demographic and clinical characteristics of the training cohort and validation cohort are shown in Table 1. Among 411 patients in the training cohort, the median age was 56 years, ranging from 22 to 88 years; and 241 patients (58.6%) were women. There were 252 benign gallbladder lesions and 159 cases of GBC by pathological examination. The gallbladder stones were present in 9.1% of benign and 44.7% of malignant lesions, respectively. Moreover, duration (years) of gallbladder stones in GBC was longer than that in BGML. An elevation of CA19.9 was found in 58/159 (36.5%) of patients with GBC compared with 10/252 (4.0%) of benign lesions (P < 0.001) and abnormal CEA in 19.5% of patients with GBC relative to 6.7% in those with benign lesions. However, the normal level of CA125 and CA15.3 was found in most of the patients with GBC and benign lesion, respectively. Overall, similar results were observed in the internal and external validation cohorts.
Table 1.
Demographics and clinical characteristics of training and validation cohorts
Radiological features selection
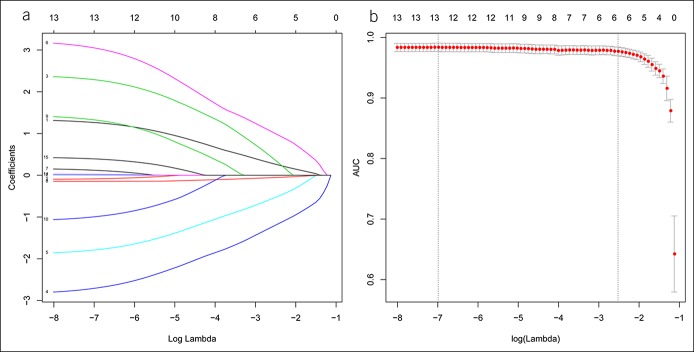
Radiological features were identified by 2 radiologists with more than 10 years' experience in abdominal CT on reviewing the enhanced CT. The results of the interoperator and intraoperator agreement in each of the radiological features were listed in Table 2. Kappa coefficients, which were between 0.76 and 0.97, were all greater than 0.75. Therefore, these demonstrated good interoperator and intraoperator agreements. Next, 6 significant radiological features were identified and selected, according to the results of the LASSO logistic regression (Figure 1). The 6 most significant radiological features of training cohorts (mass size, gallbladder stone, mucosal smoothness, enhanced gallbladder wall, layered patterns of gallbladder wall on the portal vein phase [PVP], and ΔCT value) were summarized in Table S1 (Supplementary Digital Content 1, http://links.lww.com/CTG/A111).
Table 2.
Interoperator and intraoperator agreements in each radiological feature
Figure 1.
Radiological features of computed tomography scan selection using the least absolute shrinkage and selection operator (LASSO) logistic regression model. (a) Identification of the optimal penalization coefficient lambda in the LASSO model with 10-fold cross-validation. (b) Optimal lambda resulted in 6 nonzero coefficients. AUC, area under the receiver operating characteristic curve.
Independent clinical and radiological diagnostic factors
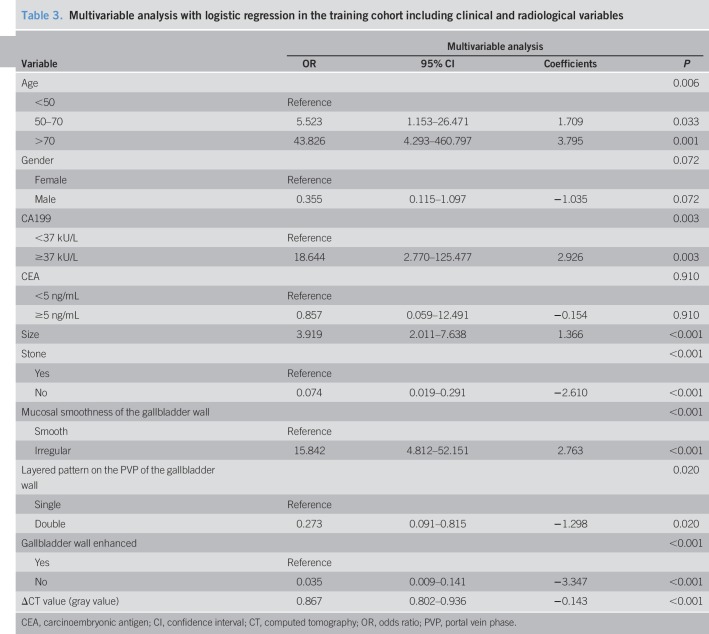
As shown in Table 1, no significant differences were detected between benign and malignant cases in body mass index, CA125, and CA15.3 by the univariate analysis. Despite the significance of univariate analysis, the duration of the presence of gallbladder stones was not taken to multivariate analysis owing that there were less than 50% of patients with gallstones in both training and validation cohorts. The results of multivariable analyses with regression models in the training cohort were summarized in Table 3. Eight factors, including age, CA19.9, size, gallbladder stone, mucosal smoothness, enhanced gallbladder wall, layered patterns of gallbladder wall on the PVP, and ΔCT value, were identified as the independent diagnostic factors by the multivariable analysis.
Table 3.
Multivariable analysis with logistic regression in the training cohort including clinical and radiological variables
Establishment and validation of the diagnostic nomogram
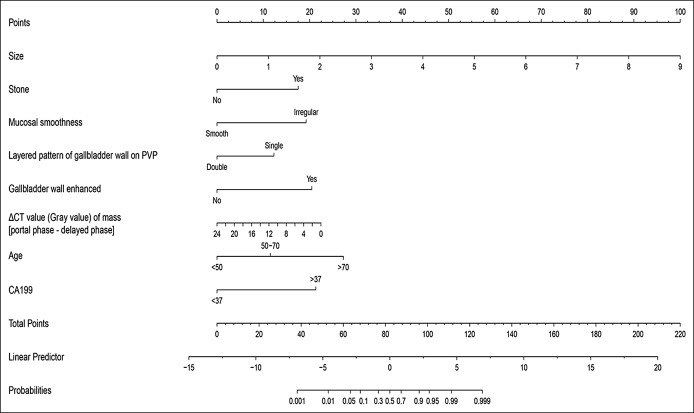
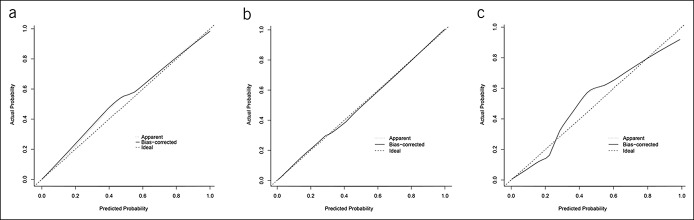
A novel model to estimate the possibility of malignancy was established with the 8 factors (Figure 2). To use this nomogram, one needs to draw a vertical line over the graph from the top point row to the bottom of the probabilities row to obtain points of each variable. Then, it was added up to the total points from each intersecting point of the vertical line for each of the 8 factors. It was the total points with the cutoff of 82 that determine the possibility of benign lesion or malignancy status. The software on how to use the nomogram was provided in Figure S1 (Supplementary Digital Content 1, http://links.lww.com/CTG/A110). The nomogram was validated by the AUC and calibration curve. Discrimination, as measured by the bootstrap-corrected AUC, was 0.95 in the training cohort, 0.91 in the internal validation cohort, and 0.89 in the external validation cohort. The calibration plots for the probability of the GBC had a good agreement between the nomogram predicted and actual probability among cohorts (Figure 3).
Figure 2.
Nomogram for estimating the probabilities of gallbladder cancer. PVP, portal vein phase.
Figure 3.
Calibration curve demonstrating how predictions from the model to the actual observed probability: (a) training cohort; (b) internal validation cohort; and (c) external validation cohort.
Performance of the diagnostic nomogram
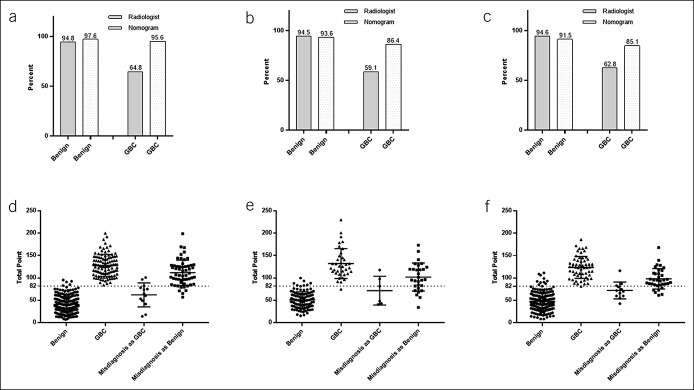
Compared to previous methods (see Table S2, Supplementary Digital Content 1, http://links.lww.com/CTG/A112), our nomogram demonstrated better sensitivity (95.6%) and accuracy (95.2%) in the diagnosis of GBC (probability of malignancy, 50%) in the training cohort. The similar result was obtained in the validation cohort with an AUC of 0.89. The AUC for radiological features alone was 0.87, and that for previous methods was 0.76 (clinical factors), 0.65 (ΔCT value), or 0.82 (combination of clinical factors and ΔCT value) (Figure 4). Notably, the accuracy using the nomogram was significantly higher in GBC groups compared with that by radiologists in the training cohort (P < 0.001) and similarly in the internal and external validation cohorts (Figure 5a–c). Interestingly, most of the IGBC, which were misdiagnosed as benign lesion by radiologists, were identified using this nomogram (Figure 5d–f).
Figure 4.
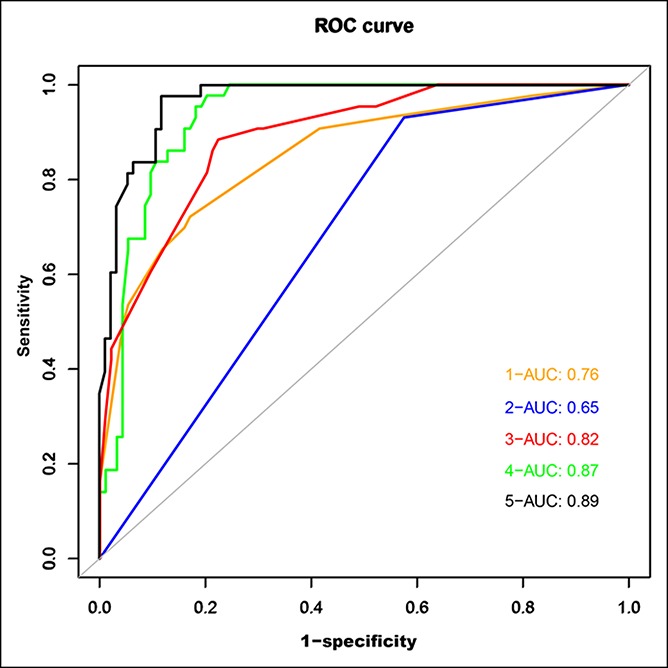
Receiver operating characteristic curve for malignancy detection sensitivity and specificity in the external validation cohort: (1) ROC curve of clinical factors alone; (2) ROC curve of the ΔCT value (Zhou et al. (23)); (3) ROC curve of the ΔCT value combined with clinical factors; (4) ROC curve of radiological features; and (5) ROC curve of radiological features combined with clinical factors (our novel diagnostic model). AUC, area under the receiver operating characteristic curve; CT, computed tomography; ROC, receiver operating characteristic.
Figure 5.
Radiologist and nomogram accuracy in the benign, GBC groups of the training, internal, and external cohorts: (a) in training cohort; (b) in internal validation cohort; and (c) in external validation cohort; total scores of the diagnostic nomogram with the cutoff value (82) in training and internal and external validation cohorts: (d) in training cohort; (e) in internal validation cohort; and (f) in external validation cohort (All P < 0.001). GBC, gallbladder cancer.
DISCUSSION
The presence of a mass in the gallbladder is one of the most challenging and common findings in the field of hepatobiliary surgery. A simple cholecystectomy is routinely performed with the assumption that most are BGMLs. However, a majority of the localized GBC are diagnosed incidentally during a pathological examination after surgery. Unfortunately, this situation renders patients to undergo completion resection depending on the cancer stage, except those with T1a, which may be treated by simple cholecystectomy without surgical reintervention (19). Thus, preoperative differentiation of patients who are classified as high-risk group for GBC is critical. This allows the general surgeons to consider referral to a team of more specialized hepatobiliary surgeons upfront. In addition, this knowledge helps the surgeons to further define T-stage and status of the cystic duct margin as well as their decision to perform a partial hepatectomy, lymphadenectomy, and/or excision of the extrahepatic biliary tree. Finally, early proper preoperative planning may allow to cut down the risk of bile spillage, a life-threatening event in the case of GBC.
There has been an increasing use of nomograms as diagnostic and prognostic tools to help guide clinical decision-making in oncology (31–33). They incorporated multiple factors with more accuracy given the complex nature and biological processes of malignancy (34). Recently, nomograms by CT have been shown to be superior to the traditional methods (34,35). Unlike a biopsy, a CT scan is noninvasive. However, its clinical significance was limited because of the subjective interpretations and qualitative in nature. Leijenaar et al. (35) developed a signature feature to predict human papillomavirus status from standard CT imaging. Larue et al. (36) used pretreatment CT image to predict 3-year overall survival after chemoradiotherapy of esophageal cancer. In the present study, we have developed a diagnostic nomogram with a combination of the multicomponent radiological features and independent clinical factors. We demonstrated that the AUC (0.89) was higher than that of the previous method (23). It helped to rule out some benign diseases such as chronic granulomatous cholecystitis, adenomyomatosis, and acute and chronic cholecystitis, which can also cause similar single- or double-layer patterns on an enhanced CT (37).
In this study, the nomogram was established by 6 radiological features selected by LASSO statistics and 2 clinical factors. The radiological features such as the size of the mass, with or without gallbladder stones, mucosal smoothness, and enhanced gallbladder wall were widely recognized as the diagnostic characteristics of GBC. The anatomic layers of the gallbladder wall are similar to those of the gastrointestinal wall and, so, are the progression of GBC and that of gastrointestinal tract adenocarcinoma. The enhancement patterns of diffusing gastric cancers may be caused mainly by fibrous stroma in tumors (38). Layered patterns of the gallbladder wall on the PVP may also be attributed to intratumoral fibrous stroma (39). It is worthy to mention that most gallbladder carcinomas are associated with heterogeneous or indistinguishable signal intensity without layering (single-layer pattern) (31,40). We speculated that widespread infiltrative tumor cells with the interstitial fiber components could have contributed to the feature. Therefore, when the contrast agent passes the interface, it diffuses slower from vessels to the fibrous stroma than normal (41), showing a single-layer and enhanced gallbladder wall. In addition, acute cholecystitis usually has a mixture of mature fibrosis (abundant collagen fibers combined with scattered vessels and cells) and immature fibrosis (plenteous neovascularization and fibroblasts) in the gallbladder wall so that they are with both weak enhancement and good enhancement, respectively, on the PVP. Also, the value of ΔCT (portal phase − delayed phase) is critical to identify a malignancy. It can reflect the biological processes of tumor including its formation and accumulation of fibrosis more intuitively. However, molecular changes that arose from the progression and intratumoral events are less well detected and characterized than those in cancer (42). In this study, we identified 2 significant clinical factors—age and CA19.9 levels in addition to image factors. The possibility of malignancy increased with age (high, >70; medium, 50–70; and low, <50) and higher CA19.9 levels, a widely accepted tumor marker for GBC. The establishment of nomogram with both clinical and radiological factors added significant strength for early detection of malignancy in the gallbladder, especially for T1-2 tumors.
In fact, most previous studies (12,23,24,28–30) presented some main factors, such as “cotton ball sign” and wall thickness and enhancement. Compared with them, Zhou's model, based on the ΔCT value for GBC, showed a higher sensitivity and specificity. Therefore, we compared our model with Zhou's model, even combined with clinical factors. In addition, to know whether our model (clinical factors combined with radiological features) was superior to either of them alone, we compared our model with each one. Notably, the external validation cohort was used to compare each model/method, and our model got superior sensitivity and accuracy.
Patients with T4 disease and/or with liver metastasis can be staged adequately with routine scanning. However, it is extremely challenging to diagnose early-stage GBC, usually with T1/T2 stages, that accounts for more than 40% of GBC misdiagnosis (43). Fortunately, our novel nomogram can assist in distinguishing nearly 60% of misdiagnosed IGBC. According to the cutoff value of 82 (probability of malignancy, 50%), we classified the patients into low- and high-risk subgroups. Using this nomogram, most benign lesions were classified as low-risk with diagnostic accuracy not inferior to that of radiologists, and more than 85% of GBC as high risk as a whole. This indicates that the diagnostic accuracy of GBC had been improved greatly with our model. Interestingly, using the nomogram, we enabled to detect malignant gallbladder mass lesions preoperatively; in other words, we could conduct preoperative qualitative analysis of mass lesions (benign or malignant).
The strengths of this study are as follows. First, this is the first multicenter study performed to distinguish BGMLs and GBC. We evaluated and analyzed the layered patterns of the gallbladder wall using contrast-enhanced CT and assessed its significance in the differentiation of benign and malignant diseases. Besides, our radiological features were extracted from 3 parts, which consisted of the mass, gallbladder wall characteristics, and outside of the gallbladder. By contrast, only the single parts had been used in previous studies. In addition, we used the ΔCT value to evaluate the enhancement of the gallbladder mass more intuitively.
There are some limitations in the present study. First, it is a retrospective study, which consisted of a small number of patient samples, and we have not used liver invasion, bile duct invasion, and hepatic artery invasion in the model. Therefore, the model needs to be validated by larger studies with adjacent organ invasion to some extent. Second, patients with rare diseases such as bile duct abnormalities and gallbladder abnormalities were excluded from the study due to the fact that most patients with few basic diseases diagnosed as gallbladder mass lesions were referred to ambulatory surgery in our center. With more patients comorbid with rare diseases in our center, we would include more patients to improve the diagnostic accuracy of the model. Besides, although the value of the AUC reached to 0.89 and all kappa coefficients were greater than 0.76, some features were not recommended to extracted by CT scan. To avoid some errors from CT scan, Doppler ultrasound was used to double check the suspected gallbladder characteristics, such as the absence of gallbladder stones. Finally, the detective model constructed by retrospective data should be validated by prospective randomized clinical trials. Nonetheless, it is a useful tool for clinical decision-making given a lack of randomized controlled trial data.
In conclusion, our study demonstrates a novel diagnostic nomogram using specific radiological features and clinical factors. It enables clinicians to obtain an individual probability of GBC and may assist clinicians in preoperative decision-making.
CONFLICTS OF INTEREST
Guarantor of the article: Xiujun Cai, MD, PhD, FACS, FRCS.
Specific author contributions: Mingyu Chen, MD, PhD, and Jiasheng Cao, MD, contributed equally to this work. M.Y.C., J.S.C., and X.J.C. in planning and conducting the study; J.S.C., Y.B., C.H.T., and J.L. in collecting and interpreting data; V.J., L.C.B., S.N., S.X.Y, A.P., F.P., A.A., K.K., R.M., and T.B.S. in helping analyze data and all authors in drafting the manuscript, and all authors have approved the final draft submitted.
Financial support: This research was funded by the Opening Fund of Engineering Research Center of Cognitive Healthcare of Zhejiang Province (grant number 2018KFJJ09), a project supported by Scientific Research Fund of Zhejiang Provincial Education Department (Y201941406), and a project supported by Scientific Research Fund of Zhejiang University, which sponsors in the study of collection, analysis, and interpretation of the data and in the writing of the report.
Potential competing interests: None to report.
Study Highlights.
WHAT IS KNOWN
✓ Preoperative decision-making for differentiating gallbladder lesions remains challenging.
WHAT IS NEW HERE
✓ This study developed and validated a diagnostic nomogram to identify GBC.
✓ Compared with previous methods, this nomogram demonstrated superior sensitivity and accuracy.
✓ This nomogram provides useful information to clinicians for presurgery decision-making.
TRANSLATIONAL IMPACT
✓ The novel diagnostic nomogram enables clinicians to obtain an individual probability of GBC preoperatively and treat them timely to improve patient outcomes.
Supplementary Material
ACKNOWLEDGMENTS
We thank Tunan Yu, Xu Feng, Yifan Tong, Lian Duan, and Jiliang Shen for efforts spent in checking all image features for the study. We are grateful to Yun Cai, Qihong Lai, and all our colleagues for assistance in this study.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A110, http://links.lww.com/CTG/A111, http://links.lww.com/CTG/A112
REFERENCES
- 1.Myers RP, Shaffer EA, Beck PL. Gallbladder polyps: Epidemiology, natural history and management. Can J Gastroenterol 2002;16:187–94. [DOI] [PubMed] [Google Scholar]
- 2.Stinton LM, Shaffer EA. Epidemiology of gallbladder disease: Cholelithiasis and cancer. Gut Liver 2012;6:172–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mao YS, Mai YF, Li FJ, et al. Prevalence and risk factors of gallbladder polypoid lesions in Chinese petrochemical employees. World J Gastroenterol 2013;19:4393–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hardy MA, Volk H. Primary carcinoma of the gallbladder. A ten year review. Am J Surg 1970;120:800–3. [DOI] [PubMed] [Google Scholar]
- 5.Ferretti S, Gafa L. Upper gastrointestinal tract cancers: Oesophagus, stomach, liver, gallbladder and biliary ducts, pancreas. Epidemiol Prev 2004;28:34–42. [PubMed] [Google Scholar]
- 6.Takebe N, Yang SX. Sonic hedgehog signaling pathway and gallbladder cancer: Targeting with precision medicine approach. Chin Clin Oncol 2016;5:1. [DOI] [PubMed] [Google Scholar]
- 7.Kondo S, Takada T, Miyazaki M, et al. Guidelines for the management of biliary tract and ampullary carcinomas: Surgical treatment. J Hepatobiliary Pancreat Surg 2008;15:41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Varshney S, Butturini G, Gupta R. Incidental carcinoma of the gallbladder. Eur J Surg Oncol 2002;28:4–10. [DOI] [PubMed] [Google Scholar]
- 9.Shih SP, Schulick RD, Cameron JL, et al. Gallbladder cancer: The role of laparoscopy and radical resection. Ann Surg 2007;245:893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Konstantinidis IT, Deshpande V, Genevay M, et al. Trends in presentation and survival for gallbladder cancer during a period of more than 4 decades: A single-institution experience. Arch Surg 2009;144:441–7. [DOI] [PubMed] [Google Scholar]
- 11.Isambert M, Leux C, Metairie S, et al. Incidentally-discovered gallbladder cancer: When, why and which reoperation? J Visc Surg 2011;148:e77–84. [DOI] [PubMed] [Google Scholar]
- 12.Guo J, Wu G, Zhou Z. Polypoid lesions of the gallbladder: Report of 160 cases with special reference to diagnosis and treatment in China. Int J Clin Exp Pathol 2015;8:11569–78. [PMC free article] [PubMed] [Google Scholar]
- 13.Coburn NG, Cleary SP, Tan JC, et al. Surgery for gallbladder cancer: A population-based analysis. J Am Coll Surg 2008;207:371–82. [DOI] [PubMed] [Google Scholar]
- 14.Wakai T, Shirai Y, Yokoyama N, et al. Early gallbladder carcinoma does not warrant radical resection. Br J Surg 2001;88:675–8. [DOI] [PubMed] [Google Scholar]
- 15.Wright BE, Lee CC, Iddings DM, et al. Management of T2 gallbladder cancer: Are practice patterns consistent with national recommendations? Am J Surg 2007;194:820–5. [DOI] [PubMed] [Google Scholar]
- 16.Chen M, Cao J, Zhang B, et al. A nomogram for prediction of overall survival in patients with node-negative gallbladder cancer. J Cancer 2019;10:3246–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen M, Lin J, Cao J, et al. Development and validation of a nomogram for survival benefit of lymphadenectomy in resected gallbladder cancer. Hepatobiliary Surg Nutr 2019;8:480–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beal EW, Lyon E, Kearney J, et al. Evaluating the American College of Surgeons National Surgical Quality Improvement project risk calculator: Results from the U.S. Extrahepatic biliary malignancy consortium. HPB (Oxford) 2017;19:1104–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zaidi MY, Maithel SK. Updates on gallbladder cancer management. Curr Oncol Rep 2018;20:21. [DOI] [PubMed] [Google Scholar]
- 20.Mariani PJ, Hsue A. Adenomyomatosis of the gallbladder: The “good omen” comet. J Emerg Med 2011;40:415–8. [DOI] [PubMed] [Google Scholar]
- 21.Hammad AY, Miura JT, Turaga KK, et al. A literature review of radiological findings to guide the diagosis of gallbladder adenomyomatosis. HPB (Oxford) 2016;18:129–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim SW, Kim HC, Yang DM, et al. Gallbladder carcinoma: Causes of misdiagnosis at CT. Clin Radiol 2016;71:e96–109. [DOI] [PubMed] [Google Scholar]
- 23.Zhou W, Li G, Ren L. Triphasic dynamic contrast-enhanced computed tomography in the differentiation of benign and malignant gallbladder polypoid lesions. J Am Coll Surg 2017;225:243–8. [DOI] [PubMed] [Google Scholar]
- 24.Yang HK, Lee JM, Yu MH, et al. CT diagnosis of gallbladder adenomyomatosis: Importance of enhancing mucosal epithelium, the “cotton ball sign”. Eur Radiol 2018;28:3573–82. [DOI] [PubMed] [Google Scholar]
- 25.Hu Y, Jia D, Xu Z, et al. [Value of diffusion-weighted MRI in differentiation between benign and malignant polypoid gallbladder lesions]. Zhonghua Yi Xue Za Zhi 2015;95:3201–4. Chinese. [PubMed] [Google Scholar]
- 26.Chantarojanasiri T, Hirooka Y, Kawashima H, et al. The role of endoscopic ultrasound in the diagnosis of gallbladder diseases. J Med Ultrason (2001) 2017;44:63–70. [DOI] [PubMed] [Google Scholar]
- 27.Szczypinski PM, Strzelecki M, Materka A, et al. MaZda—A software package for image texture analysis. Comput Methods Programs Biomed 2009;94:66–76. [DOI] [PubMed] [Google Scholar]
- 28.Corwin MT, Khera SS, Loehfelm TW, et al. Incidentally detected focal fundal gallbladder wall thickening at contrast-enhanced computed tomography: Prevalence and computed tomography features of malignancy. J Comput Assist Tomogr 2019;43:149–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kong WT, Shen HY, Qiu YD, et al. Application of contrast enhanced ultrasound in gallbladder lesion: Is it helpful to improve the diagnostic capabilities? Med Ultrason 2018;20:420–6. [DOI] [PubMed] [Google Scholar]
- 30.Wang YF, Feng FL, Zhao XH, et al. Combined detection tumor markers for diagnosis and prognosis of gallbladder cancer. World J Gastroenterol 2014;20:4085–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jung SE, Lee JM, Lee K, et al. Gallbladder wall thickening: MR imaging and pathologic correlation with emphasis on layered pattern. Eur Radiol 2005;15:694–701. [DOI] [PubMed] [Google Scholar]
- 32.Jang JY, Park T, Lee S, et al. Proposed nomogram predicting the individual risk of malignancy in the patients with branch duct type intraductal papillary mucinous neoplasms of the pancreas. Ann Surg 2017;266:1062–8. [DOI] [PubMed] [Google Scholar]
- 33.Freedman AN, Seminara D, Gail MH, et al. Cancer risk prediction models: A workshop on development, evaluation, and application. J Natl Cancer Inst 2005;97:715–23. [DOI] [PubMed] [Google Scholar]
- 34.Jiang Y, Chen C, Xie J, et al. Radiomics signature of computed tomography imaging for prediction of survival and chemotherapeutic benefits in gastric cancer. EBioMedicine 2018;36:171–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leijenaar RT, Bogowicz M, Jochems A, et al. Development and validation of a radiomic signature to predict HPV (p16) status from standard CT imaging: A multicenter study. Br J Radiol 2018;91:20170498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Larue RTHM, Klaassen R, Jochems A, et al. Pre-treatment CT radiomics to predict 3-year overall survival following chemoradiotherapy of esophageal cancer. Acta Oncol 2018;57:1475–481. [DOI] [PubMed] [Google Scholar]
- 37.Kim SJ, Lee JM, Lee JY, et al. Analysis of enhancement pattern of flat gallbladder wall thickening on MDCT to differentiate gallbladder cancer from cholecystitis. AJR Am J Roentgenol 2008;191:765–71.. [DOI] [PubMed] [Google Scholar]
- 38.Tsurumaru D, Miyasaka M, Muraki T, et al. Diffuse-type gastric cancer: Specific enhancement pattern on multiphasic contrast-enhanced computed tomography. Jpn J Radiol 2017;35:289–95. [DOI] [PubMed] [Google Scholar]
- 39.Tongdee R, Maroongroge P, Suthikeree W. The value of MDCT scans in differentiation between benign and malignant gallbladder wall thickening. J Med Assoc Thai 2011;94:592–600. [PubMed] [Google Scholar]
- 40.Zissin R, Osadchy A, Shapiro-Feinberg M, et al. CT of a thickened-wall gall bladder. Br J Radiol 2003;76:137–43. [DOI] [PubMed] [Google Scholar]
- 41.Misra S, Chaturvedi A, Misra NC, et al. Carcinoma of the gallbladder. Lancet Oncol 2003;4:167–76. [DOI] [PubMed] [Google Scholar]
- 42.Lazcano-Ponce EC, Miquel JF, Muñoz N, et al. Epidemiology and molecular pathology of gallbladder cancer. CA Cancer J Clin 2001;51:349–64. [DOI] [PubMed] [Google Scholar]
- 43.Shindoh J, de Aretxabala X, Aloia TA, et al. Tumor location is a strong predictor of tumor progression and survival in T2 gallbladder cancer: An international multicenter study. Ann Surg 2015;261:733–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.