Abstract
OBJECTIVES:
There is a significant unmet need for a blood test with adequate sensitivity to detect colorectal cancer (CRC) and adenomas. We describe a novel circulating tumor cell (CTC) platform to capture colorectal epithelial cells associated with CRC and adenomas.
METHODS:
Blood was collected from 667 Taiwanese adults from 2012 to 2018 before a colonoscopy. The study population included healthy control subjects, patients with adenomas, and those with stage I–IV CRC. CTCs were isolated from the blood using the CellMax platform. The isolated cells were enumerated, and an algorithm was used to determine the likelihood of detecting adenoma or CRC. Nominal and ordinal logistic regression demonstrated that CTC counts could identify adenomas and CRC, including CRC stage.
RESULTS:
The CellMax test demonstrated a significant association between CTC counts and worsening disease status (Cuzick's P value < 0.0001) with respect to the adenoma-carcinoma sequence. The test showed high specificity (86%) and sensitivity across all CRC stages (95%) and adenomatous lesions (79%). The area under the curve was 0.940 and 0.868 for the detection of CRC and adenomas, respectively.
DISCUSSION:
The blood-based CTC platform demonstrated high sensitivity in detecting adenomas and CRC, as well as reasonable specificity in an enriched symptomatic patient population.
TRANSLATIONAL IMPACT:
If these results are reproduced in an average risk population, this test has the potential to prevent CRC by improving patient compliance and detecting precancerous adenomas, eventually reducing CRC mortality.
INTRODUCTION
Colorectal cancer (CRC) remains a common lethal disease, ranking third for incidence and second for cancer-related mortality in the United States (1). High incidence and mortality rates can be reduced significantly by the early detection of precursor adenomatous colorectal lesions or CRC (2).
Observations from the National Polyp Study in 2,602 individuals with advanced and nonadvanced colorectal adenomas over a median of 15.8 years showed that polyp removal resulted in a 53% reduction in CRC-related mortality (2). Other studies have found that for a 1% increase in the adenoma detection rate, there is an associated 3% decrease in the risk of cancer (3). Hence, the screening of an average risk population for colorectal adenomas and CRC is the principal path for CRC prevention. US medical guidelines, including the United States Preventive Services Task Force, National Comprehensive Cancer Network, and American College of Gastroenterology, recommend routine screening for individuals aged above 50 years. The goals of CRC screening in an average risk population are (i) detection and removal of precancerous polyps to reduce incidence rates and (ii) detection of CRC at an early stage when the disease is still curable, to decrease CRC-associated mortality.
Unfortunately, 60% of CRC is detected only after the cancer has spread beyond the colon and rectum, when survival rates have decreased (4). This could be attributed partly to low compliance with current CRC screening modalities. Colonoscopy is the gold standard screening method for the detection of colorectal polyps and CRC, but adherence rates are estimated to be as low as 38% (5). In addition to colonoscopy, US guidelines also recommend using noninvasive, stool-based tests such as the fecal immunochemical test (FIT) for occult blood and the multitarget DNA-FIT test (6). However, these tests have low sensitivity for adenomatous lesions (FIT: 7.6%–40% and DNA-FIT: 17%–42%) (6–8), possibly due to the absence of the targeted biomarkers in colorectal polyps (6,9,10). Due to easy accessibility and patient preference, the peripheral blood is an ideal analyte for cancer biomarkers. Methylated septin 9 is currently the only blood test for CRC screening approved by the Food and Drug Administration, although multiple studies of circulating biomarkers for the detection of colorectal neoplasms are underway. Although methylated septin 9 achieves an acceptable sensitivity and specificity for CRC at 69% and 86% respectively, it lacks the ability to detect colorectal adenomas due to its lower sensitivity for adenomatous lesions (18%–24%) (11).
The serial enumeration of circulating tumor cells (CTCs) has enabled the monitoring of cancer progression in real time, via minimally invasive blood collection across several solid tumor types including CRC (12). Tracking CTCs in the peripheral blood may have the potential to radically alter cancer detection, treatment, and disease outcome (13–15). CTCs are shed into the bloodstream by primary and metastatic lesions and are thought to be one of the mechanisms by which cancer metastasizes. There is emerging scientific evidence to suggest that tumor cells can intravasate into the peripheral circulation not only at the invasive stage of cancer but also from preinvasive or in situ lesions. It has been shown that the epithelial cells derived from ductal carcinoma in situ of breast can pass the basement membrane and reach the bone marrow via hematogenous spread (16). Hardingham et al. (17) reported detection of epithelial cells in 3 patients with colorectal adenomas almost 2 decades ago. However, given that CTC detection is a rare event even at the metastatic stage, it is significantly more challenging to detect cells released by in situ colorectal carcinomas or adenomas (18–20). Addressing these challenges requires the development of newer, more sensitive technologies and the establishment of models to study rare cell populations in the context of CRC precursor lesions.
The goal of the proof-of-concept study described herein was to explore CTC isolation and enumeration in patients with CRC and colorectal adenomas using the ultrasensitive CellMax (CMx) CTC microfluidic technology platform, which was optimized to capture and characterize rare circulating colorectal epithelial cells from the peripheral blood. We also developed a statistical model to evaluate the performance of this CTC detection platform in predicting the likelihood of adenomas and CRC.
METHODS
Ethics
The study protocol was reviewed and approved by the Chang Gung Memorial Hospital's Institutional Review Board (No. 100–4274B, 103–3787C) for clinical utility, statistical methodology, and ethical considerations in compliance with the Declaration of Helsinki. Written informed consent was obtained from all study participants.
Study design
Patients were prospectively enrolled into a single-center, double-blind exploratory clinical study that was conducted from 2012 to 2018 at the Chang Gung Memorial Hospital, Taiwan. The study was exploratory given that neither the predictive algorithm nor the clinical thresholds were predefined. To increase statistical power, the study was enriched for patients with CRC stages I–IV and advanced adenomatous (AA) lesions. During a 6-year period, the study enrolled subjects scheduled for routine screening colonoscopy as well as symptomatic patients scheduled for diagnostic colonoscopy and referral patients scheduled for endoscopic mucosal resection.
Statistical analyses involving logistic regression and weighted maximum likelihood estimation methods were used to account for enrichment of the study with patients having CRC and adenoma and to enable approximations to prevalence rates expected in US and Taiwanese populations.
Briefly, approximately 8 mL of peripheral blood was collected on the day of colonoscopy from every subject undergoing the procedure. De-identified blood samples without clinical and demographic information were submitted for testing on the CMx Platform for CTC enumeration. The CMx CTC detection assay had been validated analytically before subject enrollment, and the corresponding standard operating procedure was followed.
Enrolling physicians conducting colonoscopy procedures were blinded to the CTC test results, and laboratory personnel running the CTC enumeration assay were blinded to the clinical status of study subjects. CMx test results as well as clinical diagnoses and other relevant information were disclosed to an independent group of researchers for statistical analyses after database review and lock.
Study participants
This study enrolled a total of 667 Taiwanese subjects. The control group contained 235 healthy subjects and the group with CRC or adenomas included 432 subjects (Table 1, see Figure S1, Supplementary Digital Content 1, http://links.lww.com/CTG/A108). Healthy subjects (aged 50–87 years) underwent a screening or diagnostic colonoscopy. The screening colonoscopy indication was routine CRC screening for average-risk individuals. Subjects with no findings on colonoscopy or with distal hyperplastic polyps less than 1 cm in size were considered to have a normal colonoscopy result. Within the disease group, there were 312 patients with untreated CRC diagnoses distributed across cancer stage as follows: stage I = 65 patients, stage II = 93 patients, stage III = 115 patients, and stage IV = 39 patients (Table 1). Staging information was not available for an additional 13 patients.
Table 1.
Distribution of age and CTC counts by disease stage
Among 107 subjects with adenomatous colorectal polyps, information on adenoma subtypes was available for 67 subjects, including 32 with small adenomas (≤5 mm adenomas without dysplasia), 13 with intermediate adenomas (6–9 mm adenomas without dysplasia), 14 with AA (≥1 cm in size, with or without high-grade dysplasia, and villous histology), 6 with large adenomas (>2 cm, high-grade dysplasia), and 2 with carcinomas in situ.
Study subjects who had undergone surgery in the preceding month or who had a history of any type of cancer, autoimmune disease, chronic inflammatory bowel disease, or acute inflammatory/infectious disease in the prior 3 months were excluded.
Study intervention
Colonoscopy and diagnosis.
Colonoscopy is the gold standard structural examination procedure for the detection of CRC and colorectal adenomas and was therefore chosen as the reference standard for this study. All subjects undergoing colonoscopy had bowel cleansing performed based on the standard clinical practice in Taiwan. Briefly, subjects started bowel preparation 3 days before the procedure, taking a low residual diet for 2 days and then a clear liquid diet for 1 day before colonoscopy, followed by the administration of a single dose of polyethylene glycol or split dose of Phospho-Soda before examination. The colonoscopy procedure was considered complete if all standard quality indicators were met. The quality measures of colonoscopy included adequate bowel preparation, optimal cecal intubation with visualization of the cecum and ileocecal valve, and withdrawal time of >6 minutes. Patients with incomplete colonoscopies caused by various factors were excluded from this study. The location, size, and gross description of colonic lesions were recorded in detail during the procedure. All endoscopists were skilled specialists with more than 10 years of experience, who perform at least 200 colonoscopies annually.
Biopsy specimens were submitted for standard pathology diagnosis. CRC staging was performed according to the American Joint Committee on Cancer guidelines.
Blood sample collection and preanalytics.
Peripheral blood was drawn from the median decubitus vein and collected in 10 mL EDTA vacutainer tubes (BD Biosciences) by a licensed nurse or a phlebotomist. A proprietary cell preservative (0.5 mL) was added per 2 mL aliquot of blood within 2 hours from the blood collection and mixed by inverting the tube gently approximately 10 times. Blood samples were de-identified, and tubes were assigned unique artificial barcodes and then securely placed in a previously validated vibration-resistant transportation box and shipped at room temperature. On arrival in the laboratory, the blood samples were evaluated for gross hemolysis and clotting and rejected if severely hemolyzed or clotted. In total, rejected samples comprised less than 5% of all samples/tests. Single blood aliquots (2.5 mL) were processed within 48 hours from the blood drawn for CTC enumeration. The maximum capacity for the laboratory during the study was 20 samples when 2 pumps were operated.
CTC detection platform
Analytic validation.
The CMx CTC detection platform was validated per standard requirements for a laboratory developed test before the initiation of the clinical study, and the corresponding standard operating procedure was implemented. Currently, the test is listed as a laboratory developed test by the College of American Pathologists-accredited clinical laboratory in Taiwan (CAP ID: 9258554).
Blood processing and CTC enumeration procedure.
The CMx CTC detection platform is a microfluidic chip-based assay, which uses a proprietary antibody to enrich rare circulating epithelial cells (referred to as CTCs) from the peripheral blood and uses a tissue- and cell-specific antibody cocktail to identify captured cells. The CMx proprietary capture antibody EpCAM (epithelial cell adhesion molecule) clones (OC98-1 and EpAb4-1) have demonstrated 2–5 times higher binding affinity than commercial clones by flow cytometry and Western blot (21), resulting in a 6-fold higher recovery rate for low EpCAM expressing cell lines (22,23). CMx microfluidic chip design and cell capture is shown in the Appendix (Supplementary Digital Content 2, http://links.lww.com/CTG/A109).
The CMx microfluidic chip captures CTCs in 2.5 mL of peripheral blood with preservative. Cytokeratin 20 (CK20) (EPR1622Y, AbCAM) and CD45 (F10-89-4, AbCAM) antibodies are used to stain epithelial cells and white blood cells (WBCs), respectively. These antibodies are then fluorescently tagged with different secondary antibodies (Alexa Fluor 568-A-11011, Alexa Fluor 488-A-21131; Thermo Fisher). Excitation and emission of the fluorescent tags at different wavelengths provide a mechanism for visual identification as well as computer intensity quantification on acquired images. Finally, stained cells are mounted with an antifade solution containing DAPI (4′, 6-diamidino-2-phenylindole; Thermo Fisher, P36931) for nuclear staining, sealed and preserved in the dark before image capture.
One hundred fluorescent images per channel (300 images in total) are taken to cover the entire membrane area (10 × 10 mm) and then stitched together for 3 separate channels (DAPI-nucleus, Alexa Fluor 488-CD45, and Alexa Fluor 568-CK20). Cell Finder software is used to automatically analyze the 3-channel images and save the identified regions of interest (ROIs). Subsequently, a trained operator loads the images and identified ROIs into Cell Reviewer software and applies intensity cutoffs to generate a list of cell candidates for additional morphologic evaluation. The ROIs are then subjected to additional criteria to exclude cells with WBC characteristics. A cell confirmed as a CTC must pass both quantitative exclusion criteria and qualitative testing of the staining pattern. WBC exclusions and CTC calls are confirmed by 2 independent operators. The details of the assay workflow are depicted in Figure 1.
Figure 1.
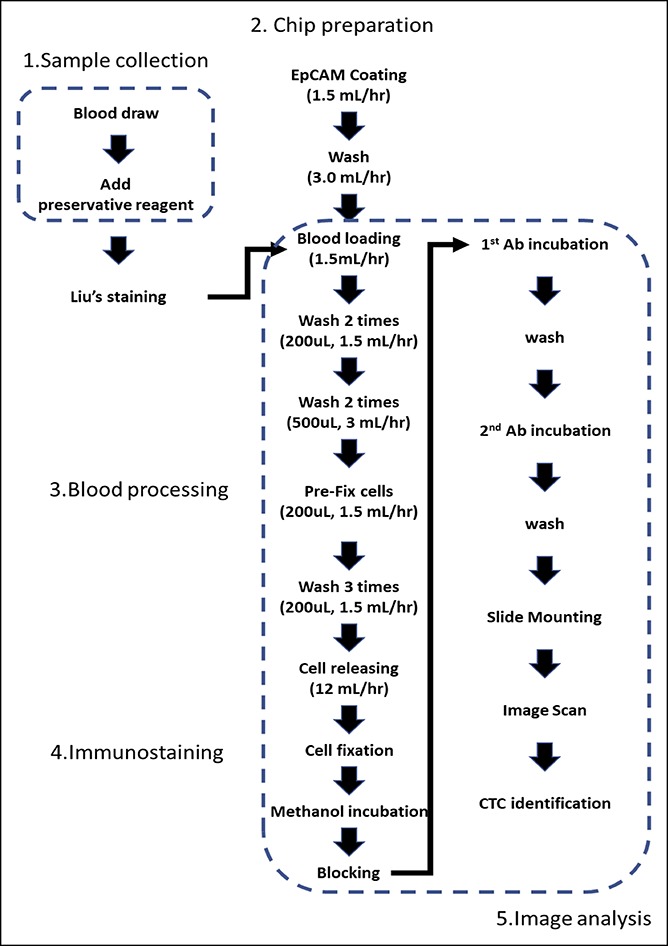
CMx CTC platform assay workflow including sample collection, cell enrichment, staining, imaging, and analysis. CMx, CellMax; CTC, circulating tumor cell.
Statistical methods
Logistic regression analyses.
To assess the contribution of CTC counts in the prediction of adenomas or CRC, nominal logistic regression was used to model cancer state (either cancer/adenomas vs healthy subjects) as a function of CTC counts and ordinal regression was used to model cancer stage as a function of CTC counts. Given that the study was enriched with patients having CRC and some patients having adenoma, statistical adjustments were necessary to account for differences in prevalence rates affecting Taiwanese and US average-risk populations. Consequently, statistical analyses involving weighted maximum likelihood estimation methods were applied to account for variations in CRC and adenoma prevalence rates (24). Subsequent to each analysis, sensitivity analyses were performed to ensure that study results were insensitive to modest changes in these sample weights. Thirteen patients with CRC without staging information were included in the analyses comparing cancer/adenomas vs healthy subjects but excluded in the analyses of disease stage.
Cuzick's trend test.
Statistical analyses were performed to test for a trend in the (log10) CTC counts as a function of CRC stage. P value < 0.01 for Cuzick's trend test was considered significant.
Likelihood ratio analyses.
Likelihood ratio (LR) analyses were performed to test the hypothesis that (log10) CTC counts provide additional predictive information above and beyond patient age and other clinical factors alone. For this purpose, the presence or absence of adenomas/cancers was modeled using logistic regression as a function of the full model of (log10) patient age and (log10) CTC count vs the reduced model of (log10) patient age alone. Quadratic terms for (log10) CTC count and sample source were included in these models to account for nonlinearity and potential sample bias. Logarithmic transformations for patient age were used for the development of age-adjusted CTC measurements and consistency with subsequent regression analyses. P value < 0.05 for the LR test for CTC counts was considered significant.
Similarly, ordinal logistic regression was used to model increasing severity of the disease (namely, healthy vs adenoma vs CRC stage I vs CRC stage II vs CRC stage III vs CRC stage IV) as a function of the full model of (log10) patient age and (log10) CTC count. The full model was compared with the reduced model of patient age alone. P value < 0.01 for the corresponding LR test for (log10) CTC count was considered significant.
Sensitivity and specificity analyses.
Overall estimates of sensitivity and specificity, associated confidence intervals, mean CTC counts, and standard errors were obtained along with estimates for each cancer stage. Given that the algorithms and clinical thresholds were not prespecified, the test was rigorously verified using statistical internal validation methods, including 10-fold cross-validation and bootstrapping methods.
RESULTS
Observed CTC characteristics
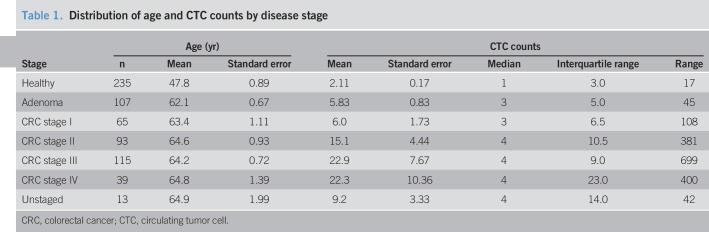
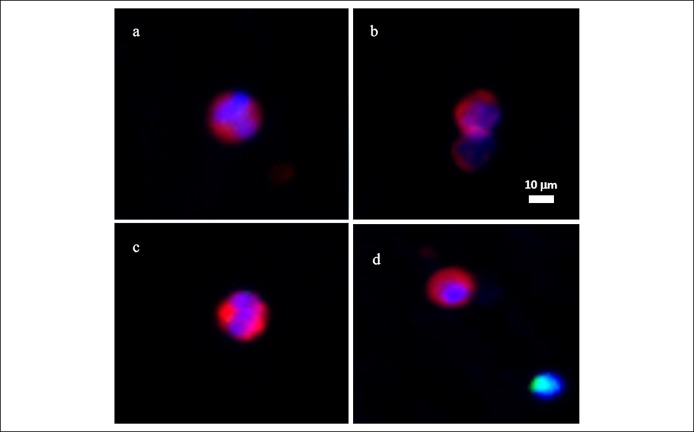
CTCs were observed in the peripheral blood from the subjects affected with CRC and colorectal adenomas. Observed CTCs ranged from 8 to 25 μm in diameter, with a nuclear-cytoplasmic ratio of ≥50%. The nuclear shape of the observed CTCs varied across the spectrum of round oval to multilobate, with differing shape patterns. CTCs observed in our affected subjects always display cytoplasmic staining of CK20 intercalating with nuclear DAPI staining, with the fluorescent intensity higher than the cutoff. Observed CTCs displayed no CD45 staining. There were no unique features displayed by the CTCs observed in patients with CRC vs those with adenoma (Figures 2 and 3).
Figure 2.
CTCs (a–d) isolated from patients with CRC. Images taken with Leica DM6B microscope with 10× objective (red, CK20; green, CD45; blue, DAPI). CRC, colorectal cancer; CTC, circulating tumor cell; DAPI, 4', 6-diamidino-2-phenylindole.
Figure 3.
CTCs (a–d) isolated from patients with colorectal adenomas. Images taken with Leica DM6B microscope with 10× objective (red, CK20; green, CD45; blue, DAPI). CTC, circulating tumor cell; DAPI, 4', 6-diamidino-2-phenylindole.
Sensitivity and specificity of the CTC assay
Table 2 and Figure 5 present the overall sensitivity and specificity of the CTC detection assay. The CTC test identified 307 of 325 patients with CRC at an optimal decision cutoff of 0.53 from the logistic model for a sensitivity of 95.2% (95% confidence interval [CI]: 92.2%–97.3%). For internal validation purposes, the corresponding bootstrap estimate of sensitivity for stages I–IV based on 2,000 replicate samples was 94.2% (95% CI: 90.4%–96.8%). A sensitivity of >89% was maintained across all stages of cancer. Among the 107 subjects with adenomas, the test detected 87 for a sensitivity of 79.2% (95% CI: 70.8%–86.0%). The area under the receiver operating characteristic (ROC) curve for the detection of CRC was 0.940, whereas the area under the ROC curve for adenomas was 0.868 (Figure 5). For 22 patients with AA, large/high-grade lesions, or other high-risk lesions, the CMx test had 71.4% sensitivity (95% CI: 47.8%, 88.7%) and 84.7% specificity (95% CI: 79.4%, 89.0%), using a cutoff of 0.09. By contrast, for 46 subjects with small and intermediate adenomas, the CMx test had a sensitivity of 60.9% (95% CI: 45.4%, 74.9%) and a specificity of 86.8% (95% CI: 81.8%, 90.9%),at a cutoff of 0.23. Further adenoma subgroup analysis was not performed due to inadequate sample sizes.
Table 2.
Sensitivity of CMx test and CTCa counts grouped by disease stage
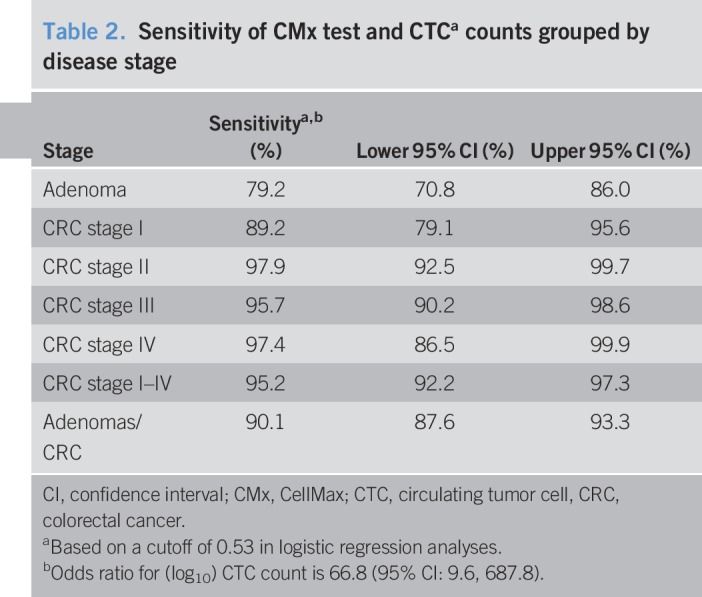
Figure 5.
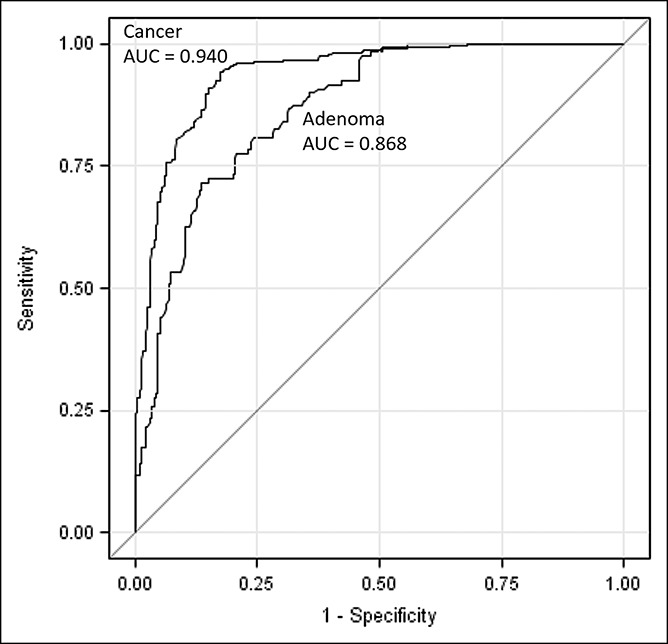
ROC curve comparison for CRC and adenomas. CRC, colorectal cancer; ROC, receiver operating characteristic.
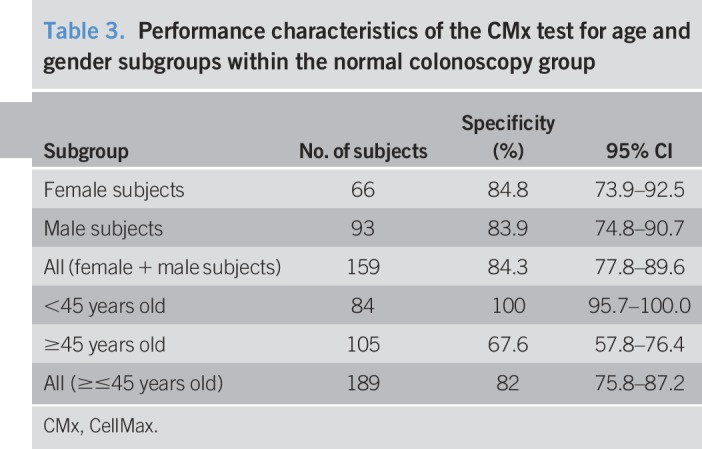
Among 235 subjects with normal colonoscopy, the specificity of the test was 82.1% (95% CI: 76.6%–86.8%). Within the normal colonoscopy group, there was no difference in specificity by gender, male (83.9%) vs female (84.8%) for the 159 subjects analyzed. Specificity was 100% for the subgroup aged <45 years, whereas it was 67.6% for the subgroup aged >45 years. The latter could be explained by the strong association of CRC with older age (Table 3). Consistent with the estimate of specificity from the overall data, the corresponding bootstrap estimate of specificity based on 2,000 replicate samples was 83.8% (95% CI: 78.7%, 89.0%).
Table 3.
Performance characteristics of the CMx test for age and gender subgroups within the normal colonoscopy group
The prevalence rate of adenomas in Taiwanese population aged 50 years and older is approximately 16%, leading to an estimated positive predictive value and negative predictive value (NPV) of 51.4% (95% CI: 43.9%–58.7%) and 97.8% (95% CI: 96.2%–99.0%), respectively (25). By comparison, the prevalence rate of adenomas in US men and women aged 50 years and older is approximately 39% (24), leading to an estimated positive predictive value and NPV of 78.1% (95% CI: 72.9%–82.6%) and 93.0% (95% CI, 89.8%–95.3%), respectively. Based on a prevalence rate of 4.23 CRC cases per 1,000 subjects (26), the NPV for CRC was calculated to be 99.9% (95% CI 99.0%–100.0%).
Relationship of age and CTC count with CRC stage
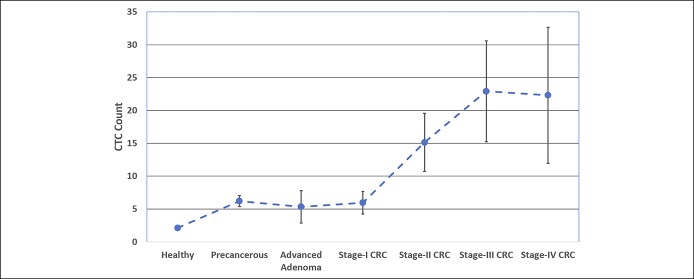
Table 1 shows the distribution of age and CTC counts as the functions of disease status. There was no significant difference in the age distribution of the subjects with adenomas vs those with CRC (P > 0.05). However, a significant association was found between CTC counts and worsening disease stage (Cuzick's P value < 0.0001). Figure 4 depicts mean CTC count as a function of cancer stage.
Figure 4.
Plot of mean CTC count ± standard error of the mean vs CRC (CTC) stage. Based on mean CTC counts, healthy subjects (2.11 CTCs) could be distinguished from patients with adenomas (5.83 CTCs; P < 0.0001) or CRC (16.99 CTCs; P < 0.0001). Mean CTC counts for subjects with late-stage CRC (III–IV; >22 CTCs) were also significantly different from mean CTC counts for subjects with early stage-I CRC (6.0 CTCs; P = 0.015) as well as adenomas (5.8 CTCs; P = 0.016). CRC, colorectal cancer; CTC, circulating tumor cell.
Based on mean CTC counts, healthy subjects (2.11 CTCs) could be distinguished from patients with adenomas (5.83 CTCs; P < 0.0001) or CRC (16.99 CTCs; P < 0.0001). The mean (log10) CTC counts were significantly different between subjects with adenomas and early stage I CRC compared with healthy subjects (P < 0.001). The mean CTC counts for patients with late-stage CRC (III–IV; >22 CTCs) were also significantly different from mean CTC counts for subjects with early stage-I CRC (6.0 CTCs; P = 0.015) as well as adenomas (5.8 CTCs; P = 0.016), which may be of prognostic significance. These results were further supported by proportional odds logistic regression results, which revealed a significant proportional relationship between age-adjusted (log10) CTC counts and cancer stage defined as healthy, adenoma, or stage I–IV CRC (LR P value < 0.0001).
Predictive value of age and CTC count
Patient age alone is a significant predictor of CRC (LR P value <0.0001), with younger patients tending to have a lower probability of CRC compared with older patients. The mean age of the healthy control subjects was 47.8 years compared with patients with CRC or adenomas, who were aged 63.7 years on average. However, nominal logistic regression showed a significant relationship between cancer status (defined as healthy subjects vs those with adenomas or cancer) and (log10) CTC count after adjusting for patient age (LR P value < 0.0001). For all 667 patient analyses, the resulting odds ratio for (log10) CTC counts for a 50-year-old individual was 66.8 (95% CI: 9.6, 687.8). Consequently, the observed CTC count provides significant additional predictive value for CRC above and beyond patient age alone (LR P value < 0.0001).
DISCUSSION
In this proof-of-concept study, we have evaluated CTCs as an alternative blood-based analyte for the early detection of CRC and colorectal adenomas, using a novel ultrasensitive CTC detection technology (CMx platform). The CMx platform is capable of detecting EpCAM(+), CK20(+), CD45(−) epithelial cells, simply referred to as CTCs, in the peripheral blood of patients with CRC or colorectal adenomas. For the latter group of patients, the CMx platform demonstrated 78% sensitivity. Furthermore, we found that CTC counts are associated with CRC disease stage (Figure 4), with sensitivity ranging from 89% to 97% across the spectrum of disease severity.
In 1869, Ashworth was the first to suggest that the cells from solid tumors can intravasate into the blood stream (27). Almost a century later, CTCs were detected in the peripheral blood of patients with liver cancer (28). More recently, Sastre et al. (29) reported quantitative CTC correlation with CRC stage using CellSearch technology.
Although the presence of CTCs in metastatic CRC is widely accepted, studies reporting tumor cell dissemination in the peripheral circulation at the in situ (17,30,31) or precancerous stage (17) have not gained much attention. “Bloodborne” epithelial cells from patients with colorectal adenomas have been reported (17), and the authors hypothesized that such adenomas may harbor foci with a malignant phenotype that shed epithelial cells into the peripheral blood. Certain studies describe disseminating tumor cells that are shed by early in situ lesions and reach bone marrow; these cells seem to differ genetically from their potentially malignant CTC counterparts (31). Isolating rare and fragile CTCs in the early stages of cancer has been a particularly difficult challenge. On the CellSearch Food and Drug Administration-cleared CTC platform, inter-rater agreement of a cell being not a CTC was shown to be higher than the agreement on being a CTC (32).
In studies using the CellSearch EpCAM cell enrichment strategy, the authors concluded that the many processing steps of the blood resulted in the loss of CTCs (19) and suggested that gentler methods could reduce this loss and enable the detection of CTCs at earlier stages of disease (13). In the current study, we used a novel ultrasensitive CTC detection CMx platform for this purpose. The cells referred to as CTCs in our study are nucleated, DAPI positive, CD45 negative epithelial cells with EpCAM and CK20 expression. The latter allows discrimination of epithelial cells originating from the gastrointestinal tract vs other organ systems. The CMx test demonstrated higher sensitivity for adenoma detection than the values reported for noninvasive stool and blood tests currently used in clinical practice, although this trial did not include a direct comparison (6,7,9,12). The CMx platform has unique technical attributes that provide an advantage over other CTC capture technologies. These include a biomimetic surface coating on the microfluidic chip that enables capture of CTCs with 3-fold greater sensitivity and 6-fold greater purity, proprietary antibodies with a 6-fold greater affinity for cancer cells, and a gentle air-foam release mechanism, capturing low EpCAM expressing cells with 97% efficiency (22,23,33). This allows for the isolation of rare EpCAM(+), CK20(+), CD45(−) epithelial cells in 2 mL of blood from patients with CRC or individuals with adenomatous polyps.
The CMx platform for CTC detection could help meet a significant need for a noninvasive test that is sensitive enough to identify patients with adenomas that can be safely removed via a diagnostic colonoscopy, thereby helping to prevent CRC. In a published survey of individuals who opted out of colonoscopy, 97% would have accepted a noninvasive screening test, and 83% of this group preferred a blood test over a stool test (34). However, the study described here was exploratory and designed to estimate the performance characteristics of the new CTC platform in a population enriched with cohorts of patients with adenoma and CRC. As such, it has several limitations, and generalization to an average-risk Taiwanese or US-based CRC screening population may result in systematic study bias. The model coefficients and clinical thresholds were not prespecified before data analyses. Furthermore, small sample size for the adenoma group and lack of information on colorectal adenoma subtypes for all 107 subjects precluded powered analyses. Due to these study limitations, large-scale, adequately powered, multicenter clinical validation studies that use prespecified algorithms, clinical thresholds, and statistical methods are being planned to target average-risk patient populations.
In conclusion, this exploratory proof-of-concept study performed on a Taiwanese population, although limited in scope, demonstrated that the CMx blood-based CTC test is capable of detecting precancerous adenomas (advanced and nonadvanced) and CRC (stage I–IV) with high sensitivity and adequate specificity.
CONFLICTS OF INTEREST
Guarantors of the article: The first author, W.-S.T., assumes full responsibility about the study, including patient enrollment, eligibility, and clinical information accuracy. The corresponding author, A.N., assumes full responsibility for the study, has had access to the data, has control of the decision to publish, and guarantees that all authors have reviewed the final manuscript.
Specific author contributions: W.-S.T., J.-F.Y., H.-Y.H. and P.-S.H. were involved in study conceptualization, supervision, acquisition of data, and review of the manuscript. B.H., R.M., M.J., H.-J.S., J.-W., J.-M.L., and S.-E.C. were involved in technology development, material support, and manuscript review. G.I., S.F., H.-J.L., and J.Y.-J.P. reviewed the manuscript. D.W. was involved in providing statistical analysis, interpretation of data, and manuscript review. W.-S.T., M.J. and A.N. were involved in the interpretation of data, material support, and drafting and critical revision of the manuscript for important intellectual content. All authors have read and approved this manuscript.
Financial support: This work was supported by the Taiwan Ministry of Health and CellMax Life Inc. (No. CMRPG3C1141, MOHW103-TD-B-111-09) and the CellMax Life Clinical Research Program (CMx-CTC-CRC-001).
Potential competing interests: B.H., D.W., H.-J.S., J.-W., J.-M.L., M.J., R.M. and S.-E.C. are employed by CellMax Life Inc. on a part-time or full-time basis. A.N. and H.-J.L. are advisors to CellMax Life. All other authors have no conflicts of interest to declare.
Study Highlights.
WHAT IS KNOWN
✓ CRC-related mortality could be reduced if precursor lesions such as colorectal adenomas are detected and removed early.
✓ Colonoscopy is the gold standard for CRC and colorectal polyp detection, but the reported compliance rate is low.
✓ Current noninvasive stool and blood tests have low sensitivity for precancerous colorectal adenomas.
✓ Individuals opting out of colonoscopy would prefer a blood test to a stool test.
WHAT IS NEW HERE
✓ Circulating epithelial cells, commonly referred to as CTCs, can be detected in the blood of patients with colorectal adenomas by an ultrasensitive CTC platform.
✓ An ultrasensitive CTC detection platform can distinguish patients with colorectal adenomas and cancer from healthy individuals.
TRANSLATIONAL IMPACT
✓ If validated in an adequately powered study in an average-risk patient population, this test has the potential to prevent CRC by facilitating improved patient compliance and detecting precancerous adenomas, thus ultimately reducing CRC-related mortality.
✓ In a clinical setting, this test can serve as a stratification tool for patients needing a colonoscopy.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Sumedha Sinha for assistance in the preparation of the manuscript.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A108, http://links.lww.com/CTG/A109
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin 2015;65(1):5–29. [DOI] [PubMed] [Google Scholar]
- 2.Zauber AG, Winawer SJ, O'Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med 2012;366(8):687–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corley DA, Jensen CD, Marks AR, et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med 2014;370(14):1298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Cancer Society. Colorectal Cancer Facts & Figures 2017-2019. American Cancer Society: Atlanta, GA: 2019. [Google Scholar]
- 5.Inadomi JM, Vijan S, Janz NK, et al. Adherence to colorectal cancer screening: A randomized clinical trial of competing strategies. Arch Intern Med 2012;172:575–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med 2014;370(14):1287–97. [DOI] [PubMed] [Google Scholar]
- 7.Imperiale TF, Gruber RN, Stump TE, et al. Performance characteristics of fecal immunochemical tests for colorectal cancer and advanced adenomatous polyps: A systematic review and meta-analysis. Ann Intern Med 2019;170(5):319–29. [DOI] [PubMed] [Google Scholar]
- 8.Berger BM, Levin B, Hilsden RJ. Multitarget stool DNA for colorectal cancer screening: A review and commentary on the United States preventive services draft guidelines. World J Gastrointest Oncol 2016;8(5):450–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okugawa Y, Grady WM, Goel A. Epigenetic alterations in colorectal cancer: Emerging biomarkers. Gastroenterology 2015;149(5):1204–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osborn NK, Ahlquist DA. Stool screening for colorectal cancer: Molecular approaches. Gastroenterology 2005;128(1):192–206. [DOI] [PubMed] [Google Scholar]
- 11.Potter NT, Hurban P, White MN, et al. Validation of a real-time PCR-based qualitative assay for the detection of methylated SEPT9 DNA in human plasma. Clin Chem 2014;60(9):1183–91. [DOI] [PubMed] [Google Scholar]
- 12.Cohen SJ, Punt CJ, Iannotti N, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol 2008;26:3213–21. [DOI] [PubMed] [Google Scholar]
- 13.Parkinson DR, Dracopoli N, Petty BG, et al. Considerations in the development of circulating tumor cell technology for clinical use. J Transl Med 2012;10:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burz C, Pop VV, Buiga R, et al. Circulating tumor cells in clinical research and monitoring patients with colorectal cancer. Oncotarget 2018;9(36):24561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ren C, Han C, Wang D, et al. Detection of circulating tumor cells: Clinical relevance of a novel metastatic tumor marker. Exp Ther Med 2011;2(3):385–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gruber IV, Hartkopf AD, Hahn M, et al. Relationship between hematogenous tumor cell dissemination and cellular immunity in DCIS patients. Anticancer Res 2016;36:2345–51. [PubMed] [Google Scholar]
- 17.Hardingham JE, Hewett PJ, Sage RE, et al. Molecular detection of blood-borne epithelial cells in colorectal cancer patients and in patients with benign bowel disease. Int J Cancer 2000;89(1):8–13. [PubMed] [Google Scholar]
- 18.Li P, Stratton ZS, Dao M, et al. Probing circulating tumor cells in microfluidics. Lab Chip 2013;13(4):602–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flores LM, Kindelberger DW, Ligon AH, et al. Improving the yield of circulating tumour cells facilitates molecular characterisation and recognition of discordant HER2 amplification in breast cancer. Br J Cancer 2010;102(10):1495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gertler R, Rosenberg R, Fuehrer K, et al. Detection of circulating tumor cells in blood using an optimized density gradient centrifugation. Recent Results Cancer Res 2003;162:149–55. [DOI] [PubMed] [Google Scholar]
- 21.Wu JC, Tseng PY, Tsai WS, et al. Antibody conjugated supported lipid bilayer for capturing and purification of viable tumor cells in blood for subsequent cell culture. Biomaterials 2013;34(21):5191–9. [DOI] [PubMed] [Google Scholar]
- 22.Tsai WS, Chen JS, Shao HJ, et al. Circulating tumor cell count correlates with colorectal neoplasm progression and is a prognostic marker for distant metastasis in non-metastatic patients. Sci Rep 2016;6:24517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen JY, Tsai WS, Shao HJ, et al. Sensitive and specific biomimetic lipid coated microfluidics to isolate viable circulating tumor cells and microemboli for cancer detection. PLoS One 2016;11(3):e0149633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corley DA, Jensen CD, Marks AR, et al. Variation of adenoma prevalence by age, sex, race, and colon location in a large population: Implications for screening and quality programs. Clin Gastroenterol Hepatol 2013;11(2):172–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang FW, Hsu PI, Chuang HY, et al. Prevalence and risk factors of asymptomatic colorectal polyps in Taiwan. Gastroenterol Res Pract 2014;2014:98520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Noone AM, Howlader N, Krapcho M, et al. SEER Cancer Statistics Review: 1975-2015. National Cancer Institute: Bethesda, MD, 2017. [Google Scholar]
- 27.Ashworth T. A case of cancer in which cells similar to those in the tumours were seen in the blood after death. Med J Aust 1869;14:146–147. [Google Scholar]
- 28.Engell HC. Cancer cells in the circulating blood: A clinical study on the occurrence of cancer cells in the peripheral blood and in venous blood draining the tumour area at operation. Ugeskr Laeger 1955;117:822–3. [PubMed] [Google Scholar]
- 29.Sastre J, Maestro ML, Puente J, et al. Circulating tumor cells in colorectal cancer: Correlation with clinical and pathological variables. Ann Oncol 2008;19:935–8. [DOI] [PubMed] [Google Scholar]
- 30.Braun S, Vogl FD, Naume B, et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med 2005;353:793–802. [DOI] [PubMed] [Google Scholar]
- 31.Sänger N, Effenberger KE, Riethdorf S, et al. Disseminated tumor cells in the bone marrow of patients with ductal carcinoma in situ. Int J Cancer 2011;129:2522–6. [DOI] [PubMed] [Google Scholar]
- 32.Zeune LL, de Wit S, Berghuis AMS, et al. How to agree on a CTC: Evaluating the consensus in circulating tumor cell scoring. Cytometry A 2018;93(12):1202–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lai JM, Shao HJ, Wu JC, et al. Efficient elusion of viable adhesive cells from a microfluidic system by air foam. Biomicrofluidics 2014;8(5):052001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adler A, Geiger S, Keil A, et al. Improving compliance to colorectal cancer screening using blood and stool based tests in patients refusing screening colonoscopy in Germany. BMC Gastroenterol 2014;14:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.