Abstract
The effect of repetitive sub-concussive head impact exposure in contact sports like American football on brain health is poorly understood, especially in the understudied populations of youth and high school players. These players, aged 9–18 years old may be particularly susceptible to impact exposure as their brains are undergoing rapid maturation. This study helps fill the void by quantifying the association between head impact exposure and functional connectivity, an important aspect of brain health measurable via resting-state fMRI (rs-fMRI). The contributions of this paper are three fold. First, the data from two separate studies (youth and high school) are combined to form a high-powered analysis with 60 players. These players experience head acceleration within overlapping impact exposure making their combination particularly appropriate. Second, multiple features are extracted from rs-fMRI and tested for their association with impact exposure. One type of feature is the power spectral density decomposition of intrinsic, spatially distributed networks extracted via independent components analysis (ICA). Another feature type is the functional connectivity between brain regions known often associated with mild traumatic brain injury (mTBI). Third, multiple supervised machine learning algorithms are evaluated for their stability and predictive accuracy in a low bias, nested cross-validation modeling framework. Each classifier predicts whether a player sustained low or high levels of head impact exposure. The nested cross validation reveals similarly high classification performance across the feature types, and the Support Vector, Extremely randomized trees, and Gradboost classifiers achieve F1-score up to 75%.
1. INTRODUCTION
Understanding the effects of repetitive sub-concussive head impacts in youth (ages 9–13), high school (ages 14–19) football players on brain development is of growing concern, and yet the association is challenging to understand. Players sustaining a concussion frequently complain of sensitivity to visual stimuli. Therefore, we hypothesized that the visual networks would contain discriminatory information. Recently, Zhu et al [1] demonstrated the ability of functional connectivity of the default mode network (DMN) to serve as a potential biomarker to monitor dynamic changes in brain function after sports related concussion [2]. There is increasing evidence that the hippocampus, a core region for human memory, should be included in the DMN. Since traumatic brain injury (TBI) often compromises memory we also hypothesized that the DMN would have telltale features that characterize injury level [3].
In this study we included subjects from both high school and youth and studied the changes in the power spectrum of the resting state networks and the functional network connectivity between AAL regions of DMN, Hippocampal and Visual regions to differentiate high impact exposed players form those who are expose to light impact. The accuracy of our classification is an indicator of the level of association and the features used by the classifier reveal the aspects of functional brain connectivity most effected from the exposure.
2. MATERIALS AND METHODS
2.1. Combining youth and high school datasets and player selection
The data used in this research is part of IRB-approved iTAKL [4] studies on the effect of repetitive sub-concussive head impacts in youth and high school football players. In each study, players were instrumented with Head Impact Telemetry System (HITS) [5] during all practices and games. In this system, accelerometers are mounted inside the helmet to measure skull acceleration. The risk of concussion from each impact, was computed from the combined probability of concussion from measured linear and rotation accelerations [6]. The risks from each impact are summed to compute each football player’s Risk of concussion-Weighted cumulative Exposure (RWECP) for the season. The 36 players with lowest impact exposure (mean RWE=0.65 ± 0.03) and the 24 subjects with highest impact exposure (mean RWE=1.91 ± 0.67) out of total 138 subjects were selected to study the effect of subconcussive head impact exposure on brain connectivity (Fig.2). Resting state fMRI (rs-fMRI) was acquired on a Siemens 3T scanner. The rs-fMRI scans were acquired with an echo planar sequence covering the entire brain (FOV = 224 × 224 mm, flip angle = 90 deg, TR = 2 sec, TE = 25 msec) over a 6-minute period before and after the season for each player. The participants were instructed to keep their eyes open and cross hair fixated. The fMRI data was preprocessed using an in house developed processing pipeline that includes steps for motion correction, spatially smoothing and spatial normalization to a common atlas space (MNI) in order to facilitate group ICA.
Figure 2:
Distribution of Risk Weighted cumulative Exposure from combined probability (RWECP)
2.2. Construction of resting state network features
Two types of features were constructed. For the first type, thirty independent components were extracted from the pre- and post-season rs-fMRI data using temporal concatenation group ICA using the InfoMax algorithm (GIFT toolbox [7]). The subject specific time course of three components of DMN including the posterior and frontal DMN, and posterior cingulate cortex (PCC) and Visual Medial were converted to their power spectrum through power spectral density decomposition. This formed four network specific features: Posterior DMNPSD, Frontal DMNPSD, PCCPSD and VMPSD. This frequency-based representation is invariant to phase and facilitates intersubject comparison of non phase locked, time based activity, which characterizes resting state experiments. The difference of the discrete (binned) PSD vectors, post-season minus pre-season, was calculated for baseline correction, Fig 3(left). This allowed us to focus on functional changes in a single season of football.
Figure 3:
Representative examples of the two types features used as input to the machine learning algorithms. (left) ΔPSD, the changes in power/frequency and (right) Seasonal ΔFNC matrix (upper triangular portion).
For the second feature type, a mean fMRI activation time series was extracted from gray matter voxels that comprise the DMN, Visual Network and the hippocampus. The DMN regions included L&R inferior parietal lobule, L&R medial orbitofrontal cortex, L&R posterior cingulate cortex, L&R superior frontal gyrus. The visual medial regions included L&R superior occipital gyrus, L&R middle occipital gyrus, L&R inferior occipital, and L&R fusiform gyrus. The hippocampal regions included L&R hippocampus, L&R para hippocampus gyrus, L&R amygdala. Inter-regional connectivity was measured using Pearson’s correlation coefficient of the mean time series between pairs of regions. These pairwise connectivity values were used to form the entries in a symmetric matrix, called the functional network connectivity matrix. The difference of two FNC matrices, post-season minus pre-season, was computed. The upper triangular portion, Fig 3(right) is vectorized and used as the feature vector. In all three feature vectors were formed and were denoted ΔDMNFNC for the DMN regions, ΔDHFNC for the combined DMN and hippocampus regions, and, ΔVisualFNC for the visual medial regions.
2.3. Classifier training, evaluation and model selection methodology
Next, multiple classifiers were systematically trained to test their ability to distinguish between the high and light impact exposure groups using each feature type. The classifiers included Adaboost, Gradient boost, Support Vector, Random Forest and ERT(Extremely Randomized Trees) classifiers [8, 9]. Nested cross-validation was performed with stratified 3-fold cross-validation in the outer layer and 3-fold cross-validated grid search in the inner layer. Nested cross-validation holds out test data from training data and allows to compute an unbiased estimate of real world performance [10]. The mean of the means of training and cross validation (CV) score across the nested folds are shown in Table 1. The inner layer performed grid search for model (hyper parameter) selection within each classifier category (e.g. Adaboost, Gradboost, Support vector). The F1 score was chosen as the performance metric because it provides an unbiased measure of performance when classes are unbalanced.
Table 1:
Nested Cross validation mean F1-scores (percentages) for Training, Cross Validation and Test data
| Classifiers | Train | CV | Test | |
|---|---|---|---|---|
| Δ VMPSD | Adaboost | 88.1 | 53.1± 16.8 | 64.6 ± 23.2 |
| Gradboost | 81.9 | 73.6 ± 2.9 | 75.0 ± 0.0 | |
| Support Vector | 82.2 | 70.9 ± 7.2 | 72.2 ± 4.8 | |
| Random Forest | 90.4 | 70.7 ± 5.5 | 68.3 ± 5.1 | |
| ERT | 89.3 | 73.9 ± 3.6 | 70.6 ± 11.1 | |
| Δ Posterior DMN PSD | Adaboost | 83 | 54.2 ± 18.1 | 70.0 ± 5.3 |
| Gradboost | 81.6 | 71.2 ± 7.0 | 70.5 ± 7.8 | |
| Support Vector | 81.1 | 75.0 ± 1.6 | 75.0 ± 0.0 | |
| Random Forest | 88.8 | 69.4 ± 6.9 | 59.9 ± 19.4 | |
| ERT | 88.7 | 71.6 ± 3.8 | 62.6 ± 9.1 | |
| Δ PCC PSD | Adaboost | 86.6 | 52.5 ±13.5 | 58.8±16.9 |
| Gradboost | 82 | 72.0 ± 4.5 | 75.0 ± 0.0 | |
| Support Vector | 83.2 | 63.2 ± 17.1 | 73.8 ± 2.1 | |
| Random Forest | 90.2 | 62.8 ± 8.9 | 62.7 ± 9.2 | |
| ERT | 90.1 | 67.5 ± 6.4 | 73.3 ± 3.8 | |
| Δ Frontal DMN PSD | Adaboost | 83.2 | 58.1 ± 10.8 | 65.5 ± 10.3 |
| Gradboost | 81.7 | 74.4 ± 1.9 | 75.0 ± 0.0 | |
| Support Vector | 81.4 | 68.3 ± 6.8 | 73.0 ± 5.6 | |
| Random Forest | 89.5 | 64.1 ± 9.8 | 58.1 ± 24.3 | |
| ERT | 89 | 69.4 ± 6.6 | 53.7 ± 36.9 | |
| Δ DMN FNC | Adaboost | 88.5 | 66.6±11.0 | 72.0±9.0 |
| Gradboost | 81.8 | 75.0 ± 1.6 | 70.5±7.8 | |
| Support Vector | 92.4 | 73.7 ±4.0 | 73.2±4.2 | |
| Random Forest | 90.3 | 64.7±6.5 | 77.5±2.5 | |
| ERT | 89.2 | 75.3±3.0 | 72.7±3.2 | |
| Δ DH FNC | Adaboost | 88.6 | 62.8±9.1 | 72.0±9.0 |
| Gradboost | 82.2 | 75.3±1.3 | 72.2±4.8 | |
| Support Vector | 93.5 | 72.0±6.6 | 70.2±4.3 | |
| Random Forest | 90.5 | 63.7±5.4 | 72.1±13.2 | |
| ERT | 89.3 | 72.9±7.2 | 72.4±13.5 | |
| Δ Visual FNC | Adaboost | 87.1 | 62.8±11.9 | 67.2±11.0 |
| Gradboost | 82.1 | 73.1±4.0 | 70.3±8.2 | |
| Support Vector | 92.1 | 73.7±3.0 | 73.7±2.3 | |
| Random Forest | 90.2 | 69.5±6.6 | 71.0±4.2 | |
| ERT | 89.1 | 70.8±2.6 | 75.8±1.4 | |
3. RESULTS
For each feature, several models performed much better than chance (F1-score=50%) as shown in Table 1. For example both the Gradboost (F1-score=75.0%) and the Support Vector (F1-score=72.2%) classifiers performed well for the feature ΔVMPSD. Similarly, performing classifiers were found for each feature. To assess the reliability of the learning models accuracy via the notion of statistical significance, the approach of [11] was used from which the top performing classifiers have p-values≈ 0.0001.
3.1. Classification based on RSN power spectral density features
For the features ΔVMPSD, ΔPCCPSD and ΔFrontal DMNPSD the Gradboost classifier was stable across the nested cross-validation and generalized well to the held out test data, as shown in Table 1(left). For the feature, ΔPosterior DMNPSD, the support vector classifier was stable and generalized well to the test data with F1-score=75.0%. Further, changes in power from pre to post changes in high impact players showed significant increase in posterior DMN, frontal DMN and significant decrease in PCC compared to light impact players as shown in Table 2. A decrease in Visual medial network power is also observed which trend towards significant with p-value 0.1
Table 2:
Comparison of PSD in High Impact compared to Light Impact players
| ΔPSD | Change | P-Value |
|---|---|---|
| Visual Medial | Decrease | 0.1 |
| Posterior DMN | Increase | 0.0201 |
| PCC | Decrease | 0.0263 |
| Frontal DMN | Increase | 0.0002 |
3.2. Classification based on regional connectivity features
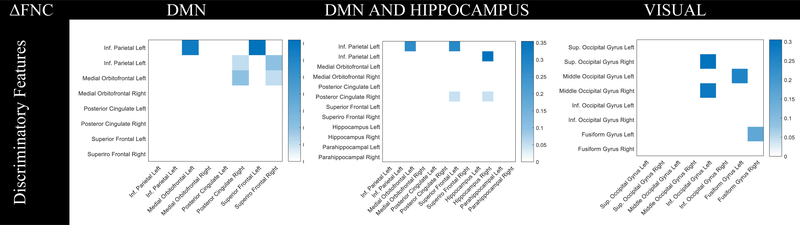
For functional network connectivity features: ΔDMNFNC, ΔDHFNC and, ΔVisualFNC the support vector, ERT, and Gradboost classifiers performed well in the nested cross validation, as shown in Table 1(right). These classifiers have similar F1-scores between 70.2 and 75.3% and overlapping distributions with generally small standard deviations. Since these classifiers have similar discriminatory power when hyperparameters are properly tuned, their similar performance make sense. Discriminatory features to classify the head impact exposure of the players is identified for Gradboost classifier as it was stable across the nested cross-validation iterations. Connectivity changes of inferior parietal with superior frontal left and medial orbitofrontal left is identified as top two discriminatory features for ΔDMNFNC. Changes of connectivity between hippocampus right and inferior parietal right is identified as top discriminatory feature after adding hippocampus regions to DMN regions in ΔDHFNC followed by connectivity changes of inferior parietal with superior frontal left and medial orbitofrontal left.
4. DISCUSSION
There are several important meta results from the current study. First, is that more than one machine learning algorithm performed well for each feature type. This bolsters our belief in the existence of a true association between functional connectivity and head impact exposure during a single season of play. If only one classifier performed well, it might be attributed to chance or bias in that model; however, when several perform well this is unlikely to be due to chance. Second, the use of nested cross validation computes an unbiased estimate of real world performance and the confidence in that estimate which facilitates model comparison. As expected, we observed the cross-validation F1-score is slightly inflated for nearly every model relative to the model’s test F1-score [10]. Thus, the additional outer layer in the nested cross validation appropriately corrected the inherent inflation of model selection in the inner layer. Lastly, we note that adding the hippocampal regions to DMN regions did not improve these results, suggesting that the hippocampus regions are correlated to the existing features.
4.1. Classification based on RSN power spectral density features
An increase in activity of frontal and posterior DMN subcomponents, and a decrease in PCC is observed in this study, which has also been reported in several other diseases such as in epilepsy, Alzheimer’s and mTBI [12]. Suggesting that frontoposterior DMN components are not only intrinsically independent but are also highly complementary in its function [3, 12, 13]. Increase in the DMN activity in players subjected to high head impact exposure, may attribute to compensatory mechanism of neuroplasticity in response to neuronal repair [12]. A decreased connectivity in the PCC is observed in this study and was also shown by Abbas et al [2] in high school football players, exposed to subconcussive head imapcts. The observed decrease in PCC activity may have subsequent effects in functional brain health as PCC serves to play a role in processing external/internal stimuli, emotional processing related to episodic memory and frontal regions that has been associated with social cognition [14].
4.2. Classification based on regional connectivity features
Our results supports our hypothesis that head impact exposure effects functional connectivity that is manifest in important resting state fMRI networks and the hippocampal regions. Discriminatory features derived from ΔFNC analysis of DMN and hippocampus regions show connectivity changes from the inferior parietal region to the superior frontal,medial orbital frontal left and the connectivity with the hippocampus left was the top discriminatory feature for identifying the head impact exposure (Fig.4). Our results corroborates previous findings that head injury often affects memory with changes in hippocampal and frontal regions [14]. For the ΔVM FNC feature, the inferior occipital gyrus left’s connectivity between medial and superior occipital gyrus right was identified as a discriminatory feature. In the future, we aim to further study the interactions between the regions, and add subjects and features to enrich the analyses.
Figure 4:
Discriminatory features derived from Gradient Boosting algorithm
5. CONCLUSION
In this study, we examined whether single season changes in resting state fMRI features, including the power spectral density of resting state networks and the functional connectivity between gray matter regions, can discriminate the football players with different levels of sub concussive head impact exposure. We employed a robust model evaluation methodology to compare suitable classifiers, which gives unbiased estimates of real world performance. Our results supports the notion of compensatory role of DMN in response to injury. The consistent results that were found demonstrate the utility of using such a machine learning approach to study connectivity changes in youth and high school football players and provides strong support to the growing body of evidence that there are detectable changes in brain health from playing a single season of football.
Figure 1:
A) Processing steps including preprocessing, feature construction and classifier training. B) Intrinsic resting state networks
Acknowledgement
Support for this research was provided by NIH R01NS082453 (JAM, JS), and R01NS091602 provided support for this research (JAM, CW, JS). This material is also based upon work supported by the NSF Graduate Research Fellowship under Grant #DGE-0907738. Any opinion, findings, and conclusions or recommendations expressed in this material are those of the authors(s) and do not necessarily reflect the views of the NSF.
Note: This work has not been submitted for publication or presentation elsewhere.
6. REFERENCE
- 1.Zhu DC, Covassin T, Nogle S et al. (2015) A potential biomarker in sports-related concussion: Brain functional connectivity alteration of the default-mode network measured with longitudinal resting-state fMRI over thirty days. J Neurotrauma 32(5): 327–341. doi: 10.1089/neu.2014.3413 [DOI] [PubMed] [Google Scholar]
- 2.Abbas K, Shenk TE, Poole VN et al. (2015) Effects of repetitive sub-concussive brain injury on the functional connectivity of Default Mode Network in high school football athletes. Dev Neuropsychol 40(1): 51–56. doi: 10.1080/87565641.2014.990455 [DOI] [PubMed] [Google Scholar]
- 3.Johnson B, Neuberger T, Gay M et al. (2014) Effects of subconcussive head trauma on the default mode network of the brain. J Neurotrauma 31(23): 1907–1913. doi: 10.1089/neu.2014.3415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davenport EM, Whitlow CT, Urban JE et al. (2014) Abnormal white matter integrity related to head impact exposure in a season of high school varsity football. J Neurotrauma 31(19): 1617–1624. doi: 10.1089/neu.2013.3233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crisco JJ, Fiore R, Beckwith JG et al. (2010) Frequency and location of head impact exposures in individual collegiate football players. J Athl Train 45(6): 549–559. doi: 10.4085/1062-6050-45.6.549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Urban JE, Davenport EM, Golman AJ et al. (2013) Head impact exposure in youth football: high school ages 14 to 18 years and cumulative impact analysis. Ann Biomed Eng 41(12): 2474–2487. doi: 10.1007/s10439013-0861-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calhoun VD, Adali T, Pearlson GD et al. (2001) A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp 14(3): 140–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geurts P, Ernst D, Wehenkel L (2006) Extremely randomized trees. Mach Learn 63(1): 3–42. doi: 10.1007/s10994-006-6226-1 [DOI] [Google Scholar]
- 9.Abraham A, Pedregosa F, Eickenberg M et al. Machine Learning for Neuroimaging with Scikit-Learn [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cawley Gavin C., Talbot Nicola L.C. (2010) On Over-fitting in Model Selection and Subsequent Selection Bias in Performance Evaluation. Journal of Machine Learning Research 11: 2079–2107 [Google Scholar]
- 11.Combrisson E, Jerbi K (2015) Exceeding chance level by chance: The caveat of theoretical chance levels in brain signal classification and statistical assessment of decoding accuracy. J Neurosci Methods 250: 126–136.doi: 10.1016/j.jneumeth.2015.01.010 [DOI] [PubMed] [Google Scholar]
- 12.Zhou Y, Milham MP, Lui YW et al. (2012) Default-mode network disruption in mild traumatic brain injury. Radiology 265(3): 882–892. doi: 10.1148/radiol.12120748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson B, Zhang K, Gay M, Horovitz S, Hallett M, Sebastianelli W, Slobounov S. Alteration of brain default network in subacute phase of injury in concussed individuals: resting-state fMRI study. Neuroimage [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castellanos FX, Margulies DS, Kelly C et al. (2008) Cingulate-precuneus interactions: A new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol Psychiatry 63(3): 332–337. doi: 10.1016/j.biopsych.2007.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]