SUMMARY
To review the outcome of vagus nerve stimulation (VNS) therapy in all implanted Slovenian patients with drug-resistant epilepsy, data on 48 patients implanted between 2001 and 2015 were obtained retrospectively from medical records. The outcome was assessed in 2016. Out of 48 patients, 39 responded at follow up. The seizure frequency was reduced in 18 (46.2%) patients; 13 (33.3%) of them reported ≥50% reduction after 12 months of therapy. The responder rate was higher among patients implanted before the age of six years. Ictal severity decreased in 22 (56.4%), seizure duration in 19 (48.7%) and post-ictal recovery time in 22 (56.4%) patients. Favorable effects on the quality of life (QOL) were improved alertness in 33.3%, concentration in 41.0%, energy and mood in 38.5%, and memory in 17.9% of patients. Reduced seizure burden and improved QOL were more often observed in patients implanted at a younger age. Shorter duration of epilepsy was significantly associated with QOL improvement. Adverse effects were transient. Overall positive effects showed VNS to be a safe, well-tolerated and effective adjunctive treatment in most severe drug-resistant epilepsy patients. Implantation at a younger age and shorter duration of epilepsy before implantation could be important predictors of better outcome.
Key words: Vagus nerve stimulation, Drug resistant epilepsy, Quality of life, Follow-up studies, Seizures, Slovenia
Introduction
Epilepsy is one of the most prevalent neurological conditions and a significant cause of disability and mortality. It is estimated to affect 50-70 million people worldwide (1). Despite many antiepileptic drugs (AEDs), 20%-30% of patients have drug-resistant epilepsy (2, 3).
Vagus nerve stimulation (VNS) therapy was approved in 1997 as an adjunctive treatment for patients with drug-resistant epilepsy, who are not suitable candidates for epilepsy surgery or had insufficient benefit from it (4, 5). Compared to the new AEDs, VNS therapy has similar or even better long-term efficacy, with continued improvement in seizure reduction for up to two years after implantation (6). VNS therapy is associated with significantly greater improvement in health-related quality of life (QOL) over best medical practice alone in patients with drug-resistant focal epilepsy (7). Jennum et al. in a case-control study of 101 patients showed that implantation of VNS was associated with reduction of hospital services and epilepsy-related prescription medications (8). Not only may VNS reduce the seizure frequency without negatively affecting behavior or cognitive functions, it also reduces seizure severity, duration and post-ictal recovery time (9, 10). Some studies suggest that the effectiveness of VNS increases with treatment duration (9-13). However, VNS treatment is also known to cause some adverse effects (9-17).
The aim of our study was to determine the outcome of VNS therapy with emphasis on seizure burden, QOL and adverse effects, focusing in particular on the impact of age at VNS implantation and duration of epilepsy before the implantation as possible predictors of treatment efficacy.
Patients and Methods
We reviewed the files of all 48 Slovenian patients who had a VNS device implanted until 2015. Since VNS implantation in Slovenia started in January 2005, three of our patients were implanted abroad before 2005, while 45 were implanted in Slovenia from January 2005 to December 2015. All patients were implanted and regularly followed up at the University Medical Centre Ljubljana. All implantations were done by two neurosurgeons, trained by official product manager (Cyberonics) supervisior, so that correct surgical technique was achieved. The VNS-related data, possible adverse effects and AEDs were routinely documented at regular outpatient visits.
We collected the following data from medical records: gender, date of birth, age at seizure onset, epilepsy etiology, dominant seizure type, age at VNS implantation, duration of VNS treatment, time elapsed from the first seizure to VNS implantation, epilepsy-related surgery, diagnosis of behavioral disorders, and verbal communication status. The etiology of epilepsy was reclassified according to the ILAE latest guidelines as genetic, structural, metabolic, immune, infectious or unknown (18). Regarding the dominant or most disabling seizure type, patients were divided into two groups: focal and generalized.
During the follow up in 2016, the questionnaire was sent to all patients, their parents or guardians. Eight of the 48 patients or their parents declined to participate, one patient died from a cause unrelated to epilepsy. The questionnaire included questions on the following characteristics: most disabling/dominant seizure type frequency, seizure severity and duration, post-ictal recovery time after VNS treatment – whether the characteristics improved, worsened, or did not change. The monthly seizure frequency after VNS implantation was calculated from the patient seizure diaries before and at three, six and twelve months after the onset of stimulation, if available. With the purpose of comparing our results to other studies, we assessed the rate of overall seizure reduction after 12 months of VNS implantation. The information was collected from medical records. Patients were considered responders if they had ≥50% reduction in seizure frequency.
The QOL was assessed by the following parameters: alertness, concentration, energy, memory, mood and progress in schoolwork. Parents or guardians assessed QOL changes as better, unchanged or worse than before VNS.
To study the impact of age at implantation and duration of epilepsy before implantation on seizure-related parameters and QOL, we divided patients into different age groups to check where significant differences appeared. We reported results only if the observation was positive.
Data on adverse effects immediately after VNS implantation were gathered from the questionnaire. Information on their presence in the following months was obtained from medical records.
The Slovenian National Medical Ethics Committee approved the study and a written informed consent was obtained from all participants and their parents or legal guardians before inclusion in the study.
Data were presented as a mean, standard deviation and range for continuous variables, and as frequencies for categorical variables. For graphical demonstration of the possible association between treatment duration and efficacy, the change in seizure frequency over time was calculated with generalized linear modeling using a bootstrap method. When determining statistical significance of proportions, we used the χ2 proportions test. Values of p<0.050 were considered statistically significant. All statistical analyses were carried out by free accessible statistical program R+ (version 3.3.2).
Results
In total, 45 patients with drug-resistant epilepsy were implanted VNS in Slovenia from January 2005 to December 2015, while three our patients had been implanted before 2005 abroad. Epilepsy etiologies in all 48 patients are enlisted in Table 1.
Table 1. Epilepsy etiologies in all VNS implanted Slovenian patients (N=48).
| Etiology of epilepsy | n |
|---|---|
| Perinatal hypoxic ischemic encephalopathy | 7 |
| Congenital cerebrovascular insult | 2 |
| Meningoencephalitis | 5 |
| Focal cortical dysplasia | 4 |
| Dravet syndrome | 2 |
| CDKL5 mutation | 1 |
| Unspecified genetic mutation | 1 |
| Post-traumatic | 4 |
| Tuberous sclerosis | 1 |
| Bilateral pachygyria | 1 |
| Hamartoma | 1 |
| Hippocampal sclerosis | 1 |
| Cortical atrophy | 3 |
| Mitochondrial disorder | 2 |
| Unverricht-Lundborg syndrome | 2 |
| Brain tumor | 2 |
| Unknown | 9 |
VNS = vagus nerve stimulation
In the follow up study in 2016, 39 (81.2%) patients agreed to participate. Their clinical features are shown in Table 2.
Table 2. Demographic and clinical features of VNS patients (N=39).
| Clinical feature | n (%) | Mean ± SD | Range | |
|---|---|---|---|---|
| Gender | Male | 21 (53.8%) | ||
| Female | 18 (46.2%) | |||
| Age at seizure onset (years) | 4.8±6.3 | 0-24 | ||
| Age at VNS implantation (years) | 18.1±14.2 | 3-56 | ||
| Time elapsed from first seizure to VNS implantation (years) | 13.4±11.3 | 2.5-53.5 | ||
| Time elapsed from VNS implantation to follow up (years) | 7.2±3.6 | 1.6-15 | ||
| Etiology | Genetic | 11 (28.2%) | ||
| Structural | 19 (48.7%) | |||
| Infectious | 6 (15.4%) | |||
| Unknown | 3 (7.7%) | |||
| Dominant seizure type | Focal | 27 (69.2%) | ||
| Generalized | 12 (30.8%) | |||
| History of epilepsy-related surgical procedure | Yes | 6 (15.4%) | ||
| No | 33 (84.6%) | |||
| Number of different AEDs prior to VNS implantation | ≤5 | 10 (25.6%) | ||
| 6-9 | 20 (51.3%) | |||
| ≥10 | 9 (23.1%) | |||
| Behavioral disorders prior to VNS | Yes | 17 (43.6%) | ||
| No | 21 (53.8%) | |||
| Unknown | 1 (2.6%) | |||
| Verbal communication status prior to VNS | Appropriately developed for chronologic age | 12 (30.8%) | ||
| Not appropriately developed for chronologic age | 13 (33.3%) | |||
| Not capable of verbal communication | 12 (30.8%) | |||
| Unknown | 2 (5.1%) |
VNS = vagus nerve stimulation; AED = antiepileptic drug
The effect of VNS on seizure burden
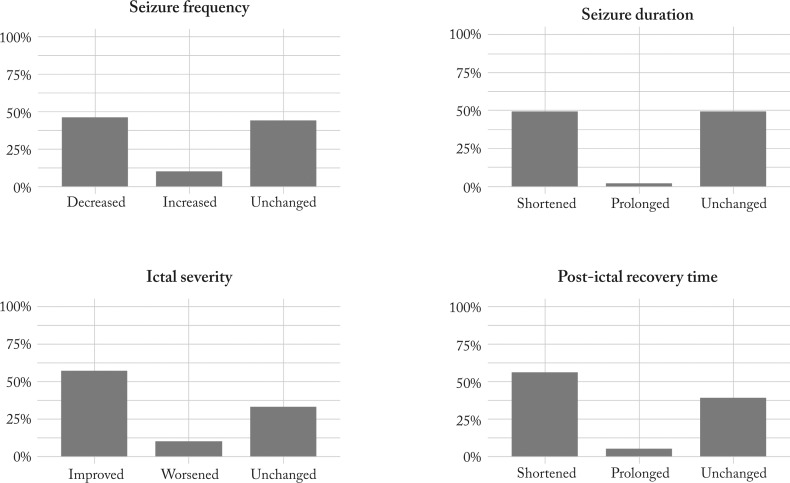
At follow up in 2016, an overall decrease in seizure frequency was seen in 18 (46.2%), ictal severity improved in 22 (56.4%), seizure duration in 19 (48.7%) and post-ictal recovery time decreased in 22 (56.4%) patients (Fig. 1).
Fig. 1.
Percentages of patients with different seizure-related parameter outcomes after VNS implantation as reported at follow up in 2016. VNS = vagus nerve stimulation
Out of 39 patients, 13 (33.3%) had ≥50% reduction in seizure frequency 12 months after VNS implantation. The responder rate was higher in those implanted before the age of six years (71.4%) compared to patients implanted later (25.0% of responders).
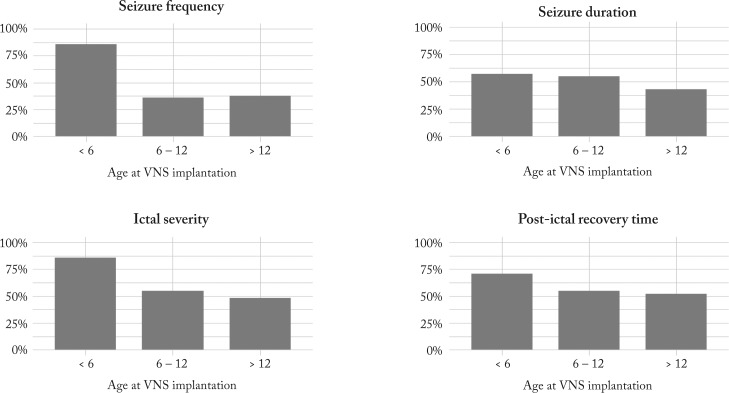
The impact of age at VNS implantation on different seizure-related parameter outcomes among the three age groups is illustrated in Figure 2. Reduction in all four parameters was more frequently reported in the youngest group.
Fig. 2.
Percentages of patients with improvement of seizure-related parameters in three different age groups at VNS implantation. VNS = vagus nerve stimulation
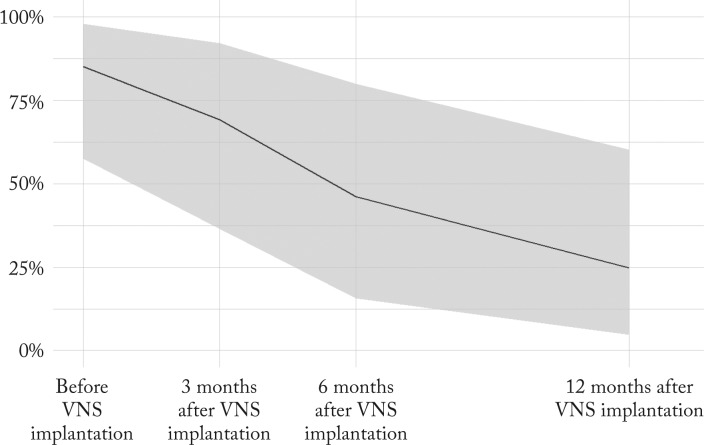
Positive correlation was found between longer VNS treatment and its efficacy. In 16 patients having kept precise seizure diaries after VNS therapy onset, seizure frequencies were analyzed as a proportion of their maximum reported seizure frequency. The reported seizure frequencies before VNS and 3, 6 and 12 months after the onset of stimulation were compared (Fig. 3). The overall mean seizure frequency 3 months after implantation fell to 69.3% (95% CI, 34.3%-92.3%), after 6 months to 46.3% (95% CI, 14.6%-79.5%) and after 12 months to 24.8% (95% CI, 4.5%-59.0%) of the baseline value measured before VNS. The decline in seizure frequency after VNS was statistically significant (χ2=21.3, df=3, p<0.001).
Fig. 3.
Seizure frequencies in 16 patients before and 3, 6 and 12 months after the onset of VNS (line represents the mean seizure frequency; shaded area denotes 95% confidence interval; the change in seizure frequency over time was calculated with generalized linear modeling using a bootstrap method). VNS = vagus nerve stimulation
The effect of VNS on QOL
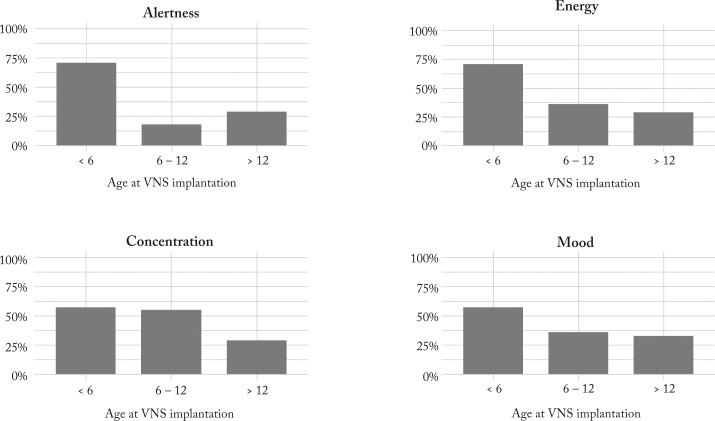
According to the questionnaires, the following changes were reported by parents: increased alertness in 13 (33.3%), better concentration in 16 (41.0%), improved energy and mood in 15 (38.5%), progress in schoolwork in nine (23.1%) and memory in seven (17.9%) of 39 patients. Four QOL parameters (alertness, concentration, energy and mood) were more frequently improved in patients implanted before the age of six years (Fig. 4).
Fig. 4.
Percentages of patients having showed improvement in QOL parameters in different age groups at VNS implantation. VNS = vagus nerve stimulation; QOL = quality of life
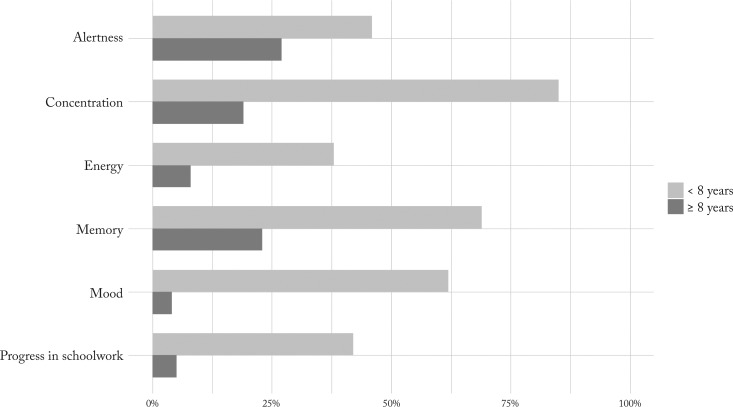
Epilepsy duration before VNS therapy also seemed to affect QOL parameters in favor of those with earlier implantation (Fig. 5). A statistically significant difference between the two groups was observed in concentration (χ2=12.7, df=1, p<0.001), energy (χ2=14.7, df=1, p<0.001), mood (χ2=6.0, df=1, p<0.01) and progress in schoolwork (χ2=13.2, df=1, p<0.001).
Fig. 5.
Percentages of patients having showed improvement in QOL parameters. Comparison of two groups according to duration of epilepsy before VNS implantation (<8 years and ≥8 years) revealed notable differences. VNS = vagus nerve stimulation; QOL = quality of life
Adverse effects
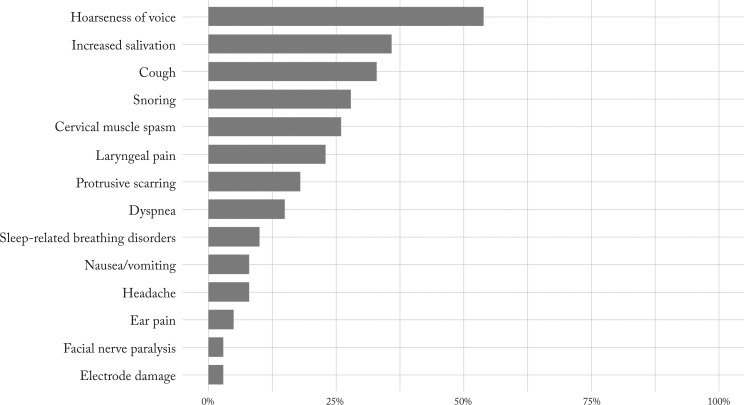
The following adverse effects were reported in medical records a few months after VNS implantation: hoarseness in 21 (53.8%), increased salivation in 14 (35.9%), cough in 13 (33.3%), snoring in 11 (28.2%), cervical muscle spasm in ten (25.6%) and laryngeal pain in nine (23.1%) patients (Fig. 6). At follow up in 2016, all effects except for hoarseness during stimulation were much milder or gone.
Fig. 6.
Percentages of all adverse effects reported.
Discussion
We found that VNS therapy contributed to notable reduction of seizure burden in nearly half of patients (Fig. 1), and 33% of our patients responded with ≥50% reduction of seizure frequency after 12 months of therapy, which is similar to many other studies (9, 10, 12, 17, 19), while some authors have reported better efficacy (16, 20-23). The response rate in our patients should be considered from two aspects: firstly, the majority of our patients had severe drug-resistant epilepsy for a very long time before VNS, and some studies indicated that longer duration of epilepsy could be negatively correlated with the efficacy of VNS (24). Secondly, intellectual disability prior to VNS therapy was present in 64% of our patients, and it has been published recently that VNS is less effective in children with intellectual disability (25).
At follow up, the mean duration of VNS therapy in our group was 7.2 (SD 3.6, range 1.6-15) years, long enough to cover most responders, as patients may have seizure reduction 3 to 5 years after VNS implantation (26).
In addition to seizure frequency, we found improvements in other seizure-related parameters in 46% to 56% of our patients (Fig. 1), which is comparable to other studies (9, 27).
Correlation between the age at VNS implantation and the outcome in our study showed that younger patients had better outcomes in all seizure parameters. There are some contradictory data published regarding this association. Elliott et al. (22) and Murphy et al. (28) did not find any significant correlation, whereas Ghaemi et al. (29), Lagae et al. (24) and Yu et al. (23) report that a younger age at VNS implantation might be a positive predictive factor for VNS efficacy. In our study, the percentage of patients with reduction in seizure frequency in the youngest age group was 71.4%, similar to the youngest group in a Belgian study with 77% responder rate (24). However, the number of cases in each age group in our study was rather small and with different etiology, therefore, statistical analysis was not feasible. Additional studies should elucidate the impact of age at VNS implantation and/or etiology itself on efficacy.
Lagae et al. found that shorter duration of epilepsy before VNS might be an important predictor of efficacy (24). Better results with earlier implantation during the first decade might also reflect the possible role of younger age with ongoing brain maturation interacting with the epileptogenic processes, while VNS mechanisms, although not well understood yet, might raise the level of functional inhibitory networks (30). In our study, shorter duration of epilepsy seemed to affect most QOL parameters with significant difference between the two groups observed in concentration, mood and schoolwork (Fig. 4).
We found a positive correlation between longer VNS treatment and efficacy with reference to reduction in seizure frequency (Fig. 3). Similar results have been reported by several authors (9, 10, 12, 17, 20, 31-33), but not by some others (21). Yu et al. (23) showed this correlation to depend on the baseline median seizure frequency; those patients with high baseline seizure frequency achieved significant reduction at 12-month follow up, whereas in the low baseline seizure frequency group, the seizure frequency reduction was not significant. VNS has repeatedly been claimed to exert neuro-modulatory effects with prolonged treatment rather than as an immediate consequence of stimulation. Long-term effectiveness suggests that VNS may work through re-modulation of networks towards a less epilepsy-prone state (34).
At the beginning of VNS therapy era, the seizure frequency reduction was the only measure of its efficacy. Later, focus on QOL parameters has become an additional important issue in the management of drug-resistant epilepsy patients. We found positive effects of VNS therapy on most QOL parameters in one-third of patients, which is consistent with some other studies (9-11, 14, 21, 27, 35). Longer duration of poorly managed drug resistant epilepsy is a major factor in cognitive decline (36). We found better results in the youngest age group with shorter duration of epilepsy, as positive changes in most parameters were noticed in patients implanted less than eight years after the onset of epilepsy. It remains unclear whether improvement in QOL is a result of stimulation itself or a beneficial effect of seizure alleviation. With better control of seizures with VNS therapy in school and other public places, social acceptance of people with epilepsy might improve and reduce the level of stigma in our societies (37).
Among VNS adverse effects (Fig. 6), voice change was most frequently reported, and it serves as a marker of VNS function. The proportion of other adverse effects found in our group was quite similar to the figures published elsewhere (9-12, 14-16, 23, 28, 33, 35). Most adverse effects in our patients were transitory and present mainly during the first three months after the onset of VNS stimulation, which is comparable to other studies (11, 12, 14, 21). None of our patients had VNS device removed due to adverse effects, but it was removed in five patients due to its ineffectiveness, which is the most common reason for VNS device removal (28, 38).
Limitations
Our study had several limitations, the most important being the small and very heterogeneous sample size, as well as retrospective and observational nature of the study. The medical records did not always provide detailed information on the frequency of several seizure types, severity and post-ictal recovery time, and this part of information was covered by questionnaires at follow up study.
Improvements in QOL parameters in VNS treated patients could also be a consequence of natural evolution of epilepsy, and not exclusively a positive effect of VNS. However, it is not common that patients with long lasting severe drug-resistant epilepsy would achieve remission or important seizure frequency reduction by another AED.
Conclusions
VNS therapy is a well-tolerated treatment option for drug-resistant epilepsy, which may reduce the burden of seizures and improve the overall QOL. The adverse effects are mild and mostly transitory. It seems that the seizures and QOL might improve more among patients with VNS implanted at a younger age and earlier after the onset of epilepsy. VNS therapy should therefore be considered early in the course of disease, including patients with intellectual disabilities, who are too often left with no further treatment option.
Acknowledgments
The authors acknowledge other colleagues of VNS team for their contribution in clinical follow up of VNS patients: Mirjana Perković Benedik, MD, PhD, pediatric neurologist; Matevž J. Kržan, MSc and Marko Korošec, MD, PhD, neurophysiologists; and Assist. Prof. Marjan Koršič, MD, PhD, neurosurgeon. We thank Jure Demšar and David Gosar for valuable feedback and advisory assistance regarding statistical analysis.
References
- 1.Abramovici S, Bagić A. Epidemiology of epilepsy. Handb Clin Neurol. 2016;138:159–71. 10.1016/B978-0-12-802973-2.00010-0 [DOI] [PubMed] [Google Scholar]
- 2.Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen Hauser W, Mathern G, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51(6):1069–77. 10.1111/j.1528-1167.2009.02397.x [DOI] [PubMed] [Google Scholar]
- 3.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342(5):314–9. 10.1056/NEJM200002033420503 [DOI] [PubMed] [Google Scholar]
- 4.Schachter SC, Saper CB. Vagus nerve stimulation. Epilepsia. 1998;39(7):677–86. 10.1111/j.1528-1157.1998.tb01151.x [DOI] [PubMed] [Google Scholar]
- 5.Penry JK, Dean JC. Prevention of intractable partial seizures by intermittent vagal stimulation in humans: preliminary results. Epilepsia. 1990;31 Suppl 2:S40–3. 10.1111/j.1528-1157.1990.tb05848.x [DOI] [PubMed] [Google Scholar]
- 6.Ben-Menachem E, French JA. VNS therapy versus the latest antiepileptic drug. Epileptic Disord. 2005;7 Suppl 1:S22–6. [PubMed] [Google Scholar]
- 7.Ryvlin P, Gilliam FG, Nguyen DK, Colicchio G, Iudice A, Tinuper P, et al. The long-term effect of vagus nerve stimulation on quality of life in patients with pharmacoresistant focal epilepsy: the PuLsE (Open Prospective Randomized Long-term Effectiveness) trial. Epilepsia. 2014;55(6):893–900. 10.1111/epi.12611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jennum P, Sabers A, Christensen J, Ibsen R, Kjellberg J. Socioeconomic evaluation of vagus stimulation: a controlled national study. Seizure. 2016;42:15–9. 10.1016/j.seizure.2016.08.011 [DOI] [PubMed] [Google Scholar]
- 9.Orosz I, McCormick D, Zamponi N, Varadkar S, Feucht M, Parain D, et al. Vagus nerve stimulation for drug-resistant epilepsy: a European long-term study up to 24 months in 347 children. Epilepsia. 2014;55(10):1576–84. 10.1111/epi.12762 [DOI] [PubMed] [Google Scholar]
- 10.Ulate-Campos A, Cean-Cabrera L, Petanas-Argemi J, Garcia-Fructuoso G, Aparicio J, Lopez-Sala A, et al. Vagus nerve stimulator implantation for epilepsy in a paediatric hospital: outcomes and effect on quality of life. Neurologia. 2015;30(8):465–71. 10.1016/j.nrl.2014.04.014 [DOI] [PubMed] [Google Scholar]
- 11.Helmers SL, Wheless JW, Frost M, Gates J, Levisohn P, Tardo C, et al. Vagus nerve stimulation therapy in pediatric patients with refractory epilepsy: retrospective study. J Child Neurol. 2001;16(11):843–8. 10.1177/08830738010160111101 [DOI] [PubMed] [Google Scholar]
- 12.Ryzí M, Brazdil M, Novak Z, Chrastina J, Oslejskova H, Rektor I, et al. Long-term vagus nerve stimulation in children with focal epilepsy. Acta Neurol Scand. 2013;127(5):316–22. 10.1111/ane.12009 [DOI] [PubMed] [Google Scholar]
- 13.Englot DJ, Chang EF, Auguste KI. Efficacy of vagus nerve stimulation for epilepsy by patient age, epilepsy duration, and seizure type. Neurosurg Clin N Am. 2011;22(4):443–8. 10.1016/j.nec.2011.07.002 [DOI] [PubMed] [Google Scholar]
- 14.Chen CY, Lee HT, Chen CC, Kwan SY, Chen SJ, Hsieh LP, et al. Short-term results of vagus nerve stimulation in pediatric patients with refractory epilepsy. Pediatr Neonatol. 2012;53(3):184–7. 10.1016/j.pedneo.2012.04.005 [DOI] [PubMed] [Google Scholar]
- 15.Kersing W, Dejonckere PH, van der Aa HE, Buschman HP. Laryngeal and vocal changes during vagus nerve stimulation in epileptic patients. J Voice. 2002;16(2):251–7. 10.1016/S0892-1997(02)00094-2 [DOI] [PubMed] [Google Scholar]
- 16.Khurana DS, Reumann M, Hobdell EF, Neff S, Valencia I, Legido A, et al. Vagus nerve stimulation in children with refractory epilepsy: unusual complications and relationship to sleep-disordered breathing. Childs Nerv Syst. 2007;23(11):1309–12. 10.1007/s00381-007-0404-8 [DOI] [PubMed] [Google Scholar]
- 17.Morris GL, 3rd, Mueller WM. Long-term treatment with vagus nerve stimulation in patients with refractory epilepsy. The Vagus Nerve Stimulation Study Group E01-E05. Neurology. 1999;53(8):1731–5. 10.1212/WNL.53.8.1731 [DOI] [PubMed] [Google Scholar]
- 18.Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L, et al. ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):512–21. 10.1111/epi.13709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hilderink J, Tjepkema-Cloostermans MC, Geertsema A, Glastra-Zwiers J, de Vos CC. Predicting success of vagus nerve stimulation (VNS) from EEG symmetry. Seizure. 2017;48:69–73. 10.1016/j.seizure.2017.03.020 [DOI] [PubMed] [Google Scholar]
- 20.Gurbani S, Chayasirisobhon S, Cahan L, Choi S, Enos B, Hwang J, et al. Neuromodulation therapy with vagus nerve stimulation for intractable epilepsy: a 2-year efficacy analysis study in patients under 12 years of age. Epilepsy Res Treat. 2016;2016:9709056. 10.1155/2016/9709056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.You SJ, Kang HC, Kim HD, Ko TS, Kim DS, Hwang YS, et al. Vagus nerve stimulation in intractable childhood epilepsy: a Korean multicenter experience. J Korean Med Sci. 2007;22(3):442–5. 10.3346/jkms.2007.22.3.442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elliott RE, Rodgers SD, Bassani L, Morsi A, Geller EB, Carlson C, et al. Vagus nerve stimulation for children with treatment-resistant epilepsy: a consecutive series of 141 cases. J Neurosurg Pediatr. 2011;7(5):491–500. 10.3171/2011.2.PEDS10505 [DOI] [PubMed] [Google Scholar]
- 23.Yu C, Ramgopal S, Libenson M, Abdelmoumen I, Powell C, Remy K, et al. Outcomes of vagal nerve stimulation in a pediatric population: a single center experience. Seizure. 2014;23(2):105–11. 10.1016/j.seizure.2013.10.002 [DOI] [PubMed] [Google Scholar]
- 24.Lagae L, Verstrepen A, Nada A, Van Loon J, Theys T, Ceulemans B, et al. Vagus nerve stimulation in children with drug-resistant epilepsy: age at implantation and shorter duration of epilepsy as predictors of better efficacy? Epileptic Disord. 2015;17(3):308–14. 10.1684/epd.2015.0768 [DOI] [PubMed] [Google Scholar]
- 25.Sourbron J, Klinkenberg S, Kessels A, Schelhaas HJ, Lagae L, Majoie M. Vagus nerve stimulation in children: a focus on intellectual disability. Eur J Paediatr Neurol. 2017;21(3):427–40. 10.1016/j.ejpn.2017.01.011 [DOI] [PubMed] [Google Scholar]
- 26.Pakdaman H, Amini Harandi A, Abbasi M, Karimi M, Arami MA, Mosavi SA, et al. Vagus nerve stimulation in drug-resistant epilepsy: the efficacy and adverse effects in a 5-year follow-up study in Iran. Neurol Sci. 2016;37(11):1773–8. 10.1007/s10072-016-2661-3 [DOI] [PubMed] [Google Scholar]
- 27.Shahwan A, Bailey C, Maxiner W, Harvey AS. Vagus nerve stimulation for refractory epilepsy in children: more to VNS than seizure frequency reduction. Epilepsia. 2009;50(5):1220–8. 10.1111/j.1528-1167.2008.01940.x [DOI] [PubMed] [Google Scholar]
- 28.Murphy JV, Torkelson R, Dowler I, Simon S, Hudson S. Vagal nerve stimulation in refractory epilepsy: the first 100 patients receiving vagal nerve stimulation at a pediatric epilepsy center. Arch Pediatr Adolesc Med. 2003;157(6):560–4. 10.1001/archpedi.157.6.560 [DOI] [PubMed] [Google Scholar]
- 29.Ghaemi K, Elsharkawy AE, Schulz R, Hoppe M, Polster T, Pannek H, et al. Vagus nerve stimulation: outcome and predictors of seizure freedom in long-term follow-up. Seizure. 2010;19(5):264–8. 10.1016/j.seizure.2010.03.002 [DOI] [PubMed] [Google Scholar]
- 30.Matsuo T, Kawai K, Kunii N, Ibayashi K, Saito N. Vagus nerve stimulation activates inhibitory neuronal network in human cerebral cortex. Epilepsia. 2015;56:161–2. [Google Scholar]
- 31.Morris GL, 3rd, Gloss D, Buchhalter J, Mack KJ, Nickels K, Harden C. Evidence-based guideline update: vagus nerve stimulation for the treatment of epilepsy: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;81(16):1453–9. 10.1212/WNL.0b013e3182a393d1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeGiorgio CM, Schachter SC, Handforth A, Salinsky M, Thompson J, Uthman B, et al. Prospective long-term study of vagus nerve stimulation for the treatment of refractory seizures. Epilepsia. 2000;41(9):1195–200. 10.1111/j.1528-1157.2000.tb00325.x [DOI] [PubMed] [Google Scholar]
- 33.Panebianco M, Zavanone C, Dupont S, Restivo DA, Pavone A. Vagus nerve stimulation therapy in partial epilepsy: a review. Acta Neurol Belg. 2016;116(3):241–8. 10.1007/s13760-016-0616-3 [DOI] [PubMed] [Google Scholar]
- 34.Schulze-Bonhage A. Brain stimulation as a neuromodulatory epilepsy therapy. Seizure. 2017;44:169–75. 10.1016/j.seizure.2016.10.026 [DOI] [PubMed] [Google Scholar]
- 35.Terra VC, Furlanetti LL, Nunes AA, Thome U, Nisyiama MA, Sakamoto AC, et al. Vagus nerve stimulation in pediatric patients: is it really worthwhile? Epilepsy Behav. 2014;31:329–33. 10.1016/j.yebeh.2013.10.011 [DOI] [PubMed] [Google Scholar]
- 36.Rampp S, Pauli E, Stefan H. Cognition and epilepsy: neuroimaging findings. Acta Clin Croat. 2011;50 Suppl 2:75–7. https://hrcak.srce.hr/106119 [Google Scholar]
- 37.Vodopić S, Vujisić S. Public awareness, understanding and attitudes towards epilepsy in Montenegro. Acta Clin Croat. 2017;56:399–405. 10.20471/acc.2017.56.03.06 [DOI] [PubMed] [Google Scholar]
- 38.Aalbers MW, Rijkers K, Klinkenberg S, Majoie M, Cornips EMJ. Vagus nerve stimulation lead removal or replacement: surgical technique, institutional experience, and literature overview. Acta Neurochir (Wien). 2015;157(11):1917–24. 10.1007/s00701-015-2547-9 [DOI] [PMC free article] [PubMed] [Google Scholar]