Abstract
Observational human data and several lines of animal experimental data indicate that maternal obesity impairs offspring health. Here, we comprehensively tested the model that maternal obesity causes defects in the next three generations of oocytes and embryos. We exposed female F0 mice to a high-fat/high-sugar (HF/HS) diet for 6 weeks before conception until weaning. Sires, F1 offspring and all subsequent generations were fed control chow diet. Oocytes from F1, F2 and F3 offspring of obese mothers had lower mitochondrial mass and less ATP and citrate than oocytes from offspring of control mothers. F0 blastocysts from HF/HS-exposed mice, but not F1 and F2 blastocysts, had lower mitochondrial mass and membrane potential, less citrate and ATP and smaller total cell number than F0 blastocysts from control mothers. Finally, supplementation of IVF media with the anti-oxidant mito-esculetin partially prevented the oocyte mitochondrial effects caused by maternal HF/HS diet. Our results support the idea that maternal obesity impairs offspring oocyte quality and suggest that antioxidant supplementation should be tested as a means to improve IVF outcomes for obese women.
Keywords: HF/HS diet, oocytes, blastocyst, mitochondria, mito-esculetin
Introduction
According to the World Health Organization, in 2016, more than 1.3 billion adults were overweight and 650 million were obese. The prevalence of obesity is especially alarming given that, in addition to harming the health of the individual, obesity appears to impair health in the next generation. For example, maternal obesity is associated with increased risk of various neurodevelopmental and psychiatric morbidities in offspring, including intellectual disability, autism spectrum disorders, attention deficit hyperactivity disorder, cerebral palsy, anxiety, depression, schizophrenia and eating disorders (Edlow, 2017).
Determining the effects of parental obesity in humans is challenging because offspring are often exposed to the same obesogenic diet and lifestyle as their parents. To model the effects of parental obesity on offspring in the absence of obesogenic environment, many researchers have used models in which female or male rodents are fed an obesogenic (e.g. high fat or high fat/high sugar [HF/HS]) diet for 4–10 weeks. After mating and weaning, the offspring are then fed a control diet. In such a model, Luzzo et al. (2012) found that F1 offspring of obese female mice had higher rates of growth retardation and brain deformation than offspring of mice fed a control diet. Similarly, Keleher et al. (2018) reported that the F1 offspring of obese female mice were at increased risk of obesity, and Masuyama et al. (2016) reported that paternal HF diet exposure altered metabolism and body weight of offspring.
Some data suggest that the effects of maternal obesity are passed to offspring via effects on oocytes. For example, in the Luzzo et al. (2012) study, effects were observed even when the embryos were transferred to the uterus of control-fed mice. Additionally, several animal studies have suggested that maternal obesity impairs oocyte function and leads to inheritance of defective mitochondria. For example, both genetic- and diet-induced maternal obesity impaired oocyte quantity and quality, as indicated by disrupted meiotic spindle morphology, increased oxidative stress, increased mitochondrial malformation and dysfunction and poor oocyte fertilization (Wu et al., 2015; Hou et al., 2016). Additionally, the oocytes and embryos derived from females exposed to an HF/HS diet had decreased mitochondrial membrane potential, decreased ATP and increased expression of mitochondrial dynamics proteins (Boudoures et al., 2017). Moreover, Saben et al. (2016) found that F1, F2 and F3 offspring born to HF/HS-fed F0 females had deformed mitochondria and altered expression of mitochondria function-related genes in their skeletal muscles and oocytes.
Despite this evidence, no single study has comprehensively tested the model that maternal obesity causes defects in F1, F2 and F3 oocytes and embryos. Here, we sought to do so by exposing F0 mice to HF/HS diet and then performing a battery of analyses on the oocytes of F1, F2 and F3 mice. Additionally, we performed IVF of oocytes from F0, F1 and F2 mice and performed the same battery of tests on the resulting blastocysts. Finally, we asked whether addition of the antioxidant mito-esculetin during IVF could rescue the effects of HF/HS diet exposure on blastocysts.
Materials and Methods
Animals and diet
All procedures involving the use of animals were approved by the Institutional Animal Care and Use Committee at Washington University School of Medicine (protocol number 20140088 and 20170072) and conformed to NIH guidelines. Four-week-old female C57Bl/6J mice (The Jackson Laboratory, Bar Harbor, USA) were fed either standard chow diet (PicoLab Rodent diet 20; 13% fat, 62% carbohydrates [3.2% sucrose] and 25% protein) or HF/HS (TestDiet® Formula 58R3: 59% fat, 26% carbohydrates [17% sucrose] and 15% protein) for 6 weeks. To produce F1 generation mice, estrus or pro-estrus chow- and HF/HS-fed females were mated to chow-fed males. F1 mice were fed standard chow diet after weaning. Eight-week-old F1 or F2 females were housed with chow-fed males to produce F2 or F3 generation mice, respectively. F2 and F3 mice were fed standard chow diet.
Meiosis II oocyte collection
Ten-week-old F1, F2 and F3 females were injected intraperitoneally with 7.5 IU pregnant mare serum gonadotropin (provided by Dr A. F. Parlow, NHPP, Torrance, CA) diluted in rabbit anti-inhibin serum. Forty-eight hours later, the mice were administered 7.5 IU hCG (Sigma, St. Louis, MO). Mice were sacrificed by cervical dislocation 15–17 h after hCG injection. For meiosis II oocyte analysis, cumulus–oocyte complexes (COCs) from ampulla were collected into M2 media (Sigma, St. Louis, MO) supplemented with 300 μg/ml hyaluronidase (Sigma, St. Louis, MO) for 5 min. Denuded oocytes were then washed three times in M2 media, and 30 oocytes from four to six mice per diet from each generation were used for further analysis.
IVF and embryo culture
IVF was performed as previously described (Takeo and Nakagata, 2015) with slight modifications. In brief, proven fertile male mice (2 to 6 months old) were sacrificed by cervical dislocation, and the cauda epididymides were collected. Clots of sperm were collected and transferred to a 150 μl drop of sperm preincubation medium modified from Krebs-Ringer solution (Alfa Aesar, Tewksbury, MA) supplemented with 1.19 mM KH2PO4, 0.75 mM methyl-b-cyclodextrin, 1 mg/ml polyvinyl alcohol and 4 mg/ml bovine serum albumin (BSA). The sperm were allowed to capacitate in a 37°C incubator with 5% (v/v) CO2 in humidified air for 1 h. COCs were collected as above into IVF media (research vitro fert [RVF] media from Cook Medical LLC, Bloomington, IN, supplemented with 0.25 mM reduced glutathione) and incubated at 37°C under 5% (v/v) CO2 in humidified air for 30–60 min.
At the time of fertilization, 10 μL of preincubated spermatozoa were transferred into IVF media containing COCs. Five to six hours later, oocytes were washed with and then incubated in RVF media supplemented with 0.1 mM EDTA at 37°C under 5% (v/v) CO2 in humidified air. At 24 h after fertilization, two-cell embryos were transferred into M16 media (Sigma, St. Louis, MO) and further incubated until 96 h after fertilization. At this point, blastocysts were counted, and 30 blastocysts per diet from each generation, collected from four to six mice, were used for further analysis. Fertilization rates were calculated as (total number of two-cell embryos/total number of inseminated oocytes) ×100. Blastocyst rates were calculated as (total number of expanded blastocysts at 96 h/total number of two-cell embryos) ×100. In experiments shown in Fig. 8, before COC collection, IVF media were supplemented with the indicated concentrations of mito-esculetin (provided by Dr S. Kotamraju, Centre for Chemical Biology, Hyderabad, India).
Figure 8.
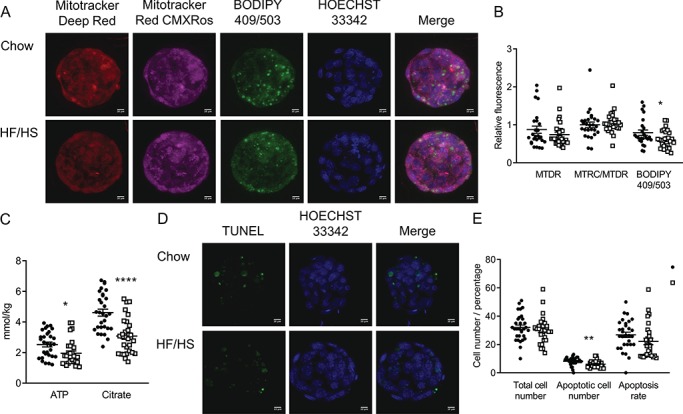
Effect of F0 diet (chow versus HF/HS) on blastocyst mitochondrial parameters and fat content with 1 μM mito-esculetin supplementation during IVF. (A and D) Representative images of blastocysts derived from chow and HF/HS groups. (B) Quantification of mitochondrial mass (MitoTracker Deep Red), mitochondrial membrane potential (MitoTracker CMXRos) and lipid droplets (BODIPY 409/503) in blastocysts derived from chow (n = 27 blastocysts from five mice) and HF/HS (n = 30 blastocysts from five mice) groups. (C) Quantification of ATP and citrate in blastocysts derived from F0 oocytes (n = 30 blastocysts derived from five mice/treatment). (E) Total cell number, apoptotic cell number and apoptosis rate in blastocysts derived F0 oocytes (n = 28–31 blastocysts derived from five mice/treatment). Scale bar = 10 μm. Data are represented as mean ± SEM. *P < 0.05, **P < 0.01, ****P < 0.0001.
Total mitochondrial mass, membrane potential and lipid droplet quantification
Denuded oocytes or blastocysts were incubated at 37°C for 30 min in a mixture of 200 nM MitoTracker™ deep red (MTDR; Life Technologies Corporation, Grand Island, NY) and 200 nM MitoTracker™ red CMXRos (MTRC; Life Technologies Corporation, Grand Island, NY). After three washes in 1% (w/v) BSA-supplemented phosphate buffered saline (PBS), the samples were fixed in 4% (v/v) paraformaldehyde for 10 min and permeabilized with 0.25% (v/v) triton X-100 for 20 min. The samples were then incubated with 5 μM BODIPY™ 493/503 (Life Technologies Corporation, Grand Island, NY) for 1 h. In the last 15 min, Hoechst 33342 (Sigma, St. Louis, MO) was added at a final concentration of 10 μg/ml. The samples were then washed three times with 1% (w/v) BSA-supplemented PBS and mounted in VectaShield (Vector Labs, Burlingame, CA) on 12-well, 5 mm diameter teflon-printed slides (Electron Microscope Sciences, Hatfield, PA). Mitochondrial mass was indicated by MTDR staining intensity, and mitochondrial membrane potential was indicated by the ratio between MTDR and MTRC staining intensities (Pendergrass et al., 2004). Lipid droplets were quantified by measuring the intensity of BODIPY™ 493/503 signal.
Immunofluorescence
Denuded oocytes or blastocysts were fixed with 4% (v/v) paraformaldehyde for 10 min and permeabilized in 0.25% (v/v) triton X-100 for 20 min. The samples were then blocked with 3% (w/v) BSA-supplemented PBS for 1 h, then incubated overnight at 4°C in 3% (w/v) BSA-supplemented PBS containing one of the following primary antibodies: anti-Oma1 (1100, Biorbyt LLC, San Francisco, CA), anti-H3K9me2/3 (1:100, Cell Signaling Technology, Danvers, MA) or anti-5mC (1100, Cell Signaling Technology, Danvers, MA). After three washes in 1% (w/v) BSA-supplemented PBS, the samples were incubated with appropriate fluorescent dye-conjugated secondary antibody for 1 h. In the last 15 min, Hoechst 33342 was added at a final concentration of 10 μg/ml. The samples were then washed three times with 1% (w/v) BSA-supplemented PBS and mounted as described above.
Apoptosis and total cell count
The terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay was performed by using the in situ cell death detection kit, fluorescein (Sigma, St. Louis, MO). Briefly, blastocysts were fixed in 4% (v/v) paraformaldehyde for 10 min, permeabilized with 0.25% (v/v) triton X-100 for 20 min and then incubated in TUNEL reaction mixture at 37°C for 1 h. After three washes in 1% (w/v) BSA-supplemented PBS, the samples were incubated in 1 μg/ml Hoechst 33342 for 15 min, then washed and mounted as described above. The numbers of total and apoptotic cells were counted in 13–15 z-series images with 5 μm spacing between z-sections. Apoptosis rate was calculated as (total number of apoptotic cells/total cell number) ×100.
Confocal microscopy and image quantification
Slides were imaged with a Leica DMI 4000B microscope (Leica Microsystems, Buffalo Grove, IL) under 63× oil immersion objective with 1.5× zoom at 512 × 512 pixels resolution. Typically, oocyte images were of a single section, and blastocyst images were collected as a complete z-stack of 13–15 images at 5 μm spacing. Except for apoptosis analysis, images were split into single channels before setting a threshold as described (Otsu, 1979). In addition, z-series images from one blastocyst were converted into single images by using sum slice projection before threshold processing (Saben et al., 2016). Total mean gray value for each channel was normalized to total area of measurement (Parry and Hemstreet, 1988). Relative fluorescence intensities from individual samples from the HF/HS-fed group were normalized to the average of mean gray area from samples from the control-fed group.
ATP and citrate analysis
Individual denuded oocytes or blastocysts were frozen on a glass slide by dipping in isopentane equilibrated with liquid nitrogen. After freeze-drying overnight under vacuum at −35°C, the oocytes or blastocysts were extracted in microdrops overlaid with mineral oil. The ATP and citrate content in alkaline-extracted oocytes or embryos were measured by performing an enzyme-linked assay as previously described (Chi et al., 2002).
Statistical analysis
Unless otherwise stated, all quantitative data are presented as mean ± standard error of the mean. Statistical significance was analyzed by either t-test or one-way ANOVA (GraphPad Prism version 7.00 for Windows, GraphPad Software, La Jolla, CA).
Results
Effects of F0 HF/HS diet exposure on F1, F2 and F3 oocyte mitochondria and metabolism
As previously reported (Saben et al., 2016), the F0 HF/HS-fed mice were 15.6–19.5% heavier than the chow-fed mice at the time of mating, whereas there were no differences in offspring (F1–F3) body weights between the chow and HF/HS groups at 8 weeks of age (Supplementary Table SI). In addition, F0 diet did not affect litter sizes in F0, F1 or F2. However, in the third parity, F1 and F2 progeny of HF/HS-fed F0s delivered litters that contained significantly more female than male pups (Supplementary Table SI).
To determine the effect of HF/HS exposure on oocyte mitochondrial mass, we stained meiosis II stage oocytes from F1, F2 and F3 mice with Mitotracker Deep Red, which stains all mitochondria. Additionally, to assess membrane potential, we stained the oocytes with Mitotracker Red CMXRos, a derivate of CMX rosamine that is sensitive to mitochondrial membrane potential changes and oxidative stress in live cells (Pendergrass et al., 2004). By combining these two dyes, we could assess mitochondrial morphology, mass and number in one assay. Representative images of F1 oocytes are shown in Fig. 1A. Similar to previous studies of F0 germinal vesicle stage oocytes (Boots et al., 2016), we noted that mitochondria appeared to be aggregated instead of perinuclear in the F1 meiosis II stage oocytes.
Figure 1.
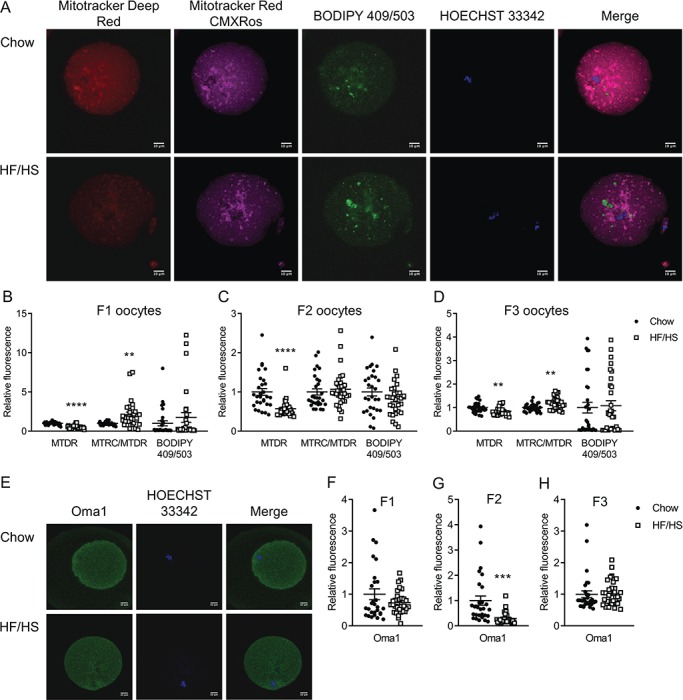
Effect of F0 diet on mitochondrial mass and membrane potential and Oma1 protein expression in offspring oocytes. (A and E) Representative images of oocytes derived from chow and HF/HS groups. Quantification of mitochondrial mass (MTDR), mitochondrial membrane potential (MTRC/MTDR) and lipid droplets (BODIPY 409/503) in oocytes derived from F1 (B), F2 (C) and F3 (D) generations (n = 29–30 oocytes derived from five mice/diet/generation). Quantification of Oma1 protein expression in F1 (F), F2 (G) and F3 (H) oocytes (n = 28–30 oocytes derived from five mice/diet/generation). Scale bar = 10 μm. Data are represented as mean ± SEM. **P < 0.01, ***P < 0.001, ****P < 0.0001.
Meiosis II stage oocytes from all three generations of offspring of HF/HS-fed mice (F1-HF/HS, F2-HF/HS and F3-HF/HS) had less Mitotracker Deep Red staining than did oocytes from offspring of chow-fed mice (F1-chow, F2-chow and F3-chow; Fig. 1B–D). Oocytes from F1-HF/HS and F3-HF/HS mice had higher mitochondrial membrane potential than did oocytes from F1-chow and F3-chow mice, respectively. However, this difference was not observed in oocytes from F2 offspring. The oocytes from F2-HF/HS mice had less of the mitochondrial protein Oma1 than did oocytes from F2-chow mice (Fig. 1G), but we saw no differences in Oma1 expression between oocytes from the chow and HF/HS groups in the F1 and F3 generations (Fig. 1F and H). Consistent with the effects on total mitochondria, the oocytes from F1-HF/HS and F2-HF/HS offspring had lower ATP and citrate concentrations than the oocytes of F1-chow and F2-chow offspring (Fig. 2A and B). In F3, the concentration of citrate but not of ATP was affected by F0 diet exposure (Fig. 2C). We also examined lipid content by staining the oocytes with Bodipy and found no differences between HF/HS- and chow-fed groups in F1, F2 or F3 (Fig. 1B–D). Together, these findings indicate that HF/HS diet exposure in the F0 generation leads to generally, but not completely, consistent impairments of mitochondrial mass and metabolism in oocytes of the next three generations.
Figure 2.
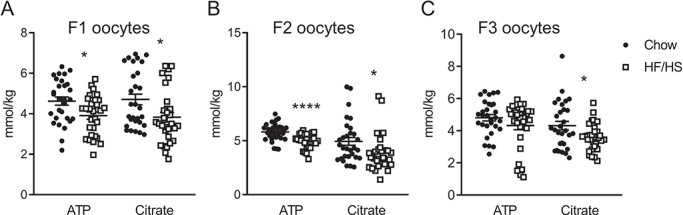
Effect of F0 diet (chow versus HF/HS) on ATP and citrate in offspring oocytes. Quantification of ATP and citrate in oocytes derived from F1 (A), F2 (B) and F3 (C) mice (n = 29–30 oocytes derived from five mice/diet/generation). Data are represented as mean ± SEM. *P < 0.05, ****P < 0.0001.
Effects of F0 HF/HS diet exposure on chromatin marks in F1, F2 and F3 oocytes
Two groups reported that exposure to HF diet altered gene expression in offspring by altering regulation of chromatin and DNA methylation (Masuyama et al., 2016;Keleher et al., 2018). In addition, oocytes from obese mice had less 5-methyl-cytosine (5mC) and more lysine-9-methylated histone H3 (H3K9me2) than oocytes from lean mice (Hou et al., 2016). Thus, we wondered whether 5mC and H3K9me2 in oocytes were affected by F0 diet exposure. We found that F1-HF/HS oocytes had more 5mC than F1-chow oocytes, F2-HF/HS oocytes had more H3K9me2/3 and less 5mC than F2-chow oocytes and F3-HF/HS oocytes had more H3K9me2/3 than F3-chow oocytes (Fig. 3). These results suggest that maternal HF/HS diet exposure alters epigenetic regulation in the oocytes of offspring.
Figure 3.
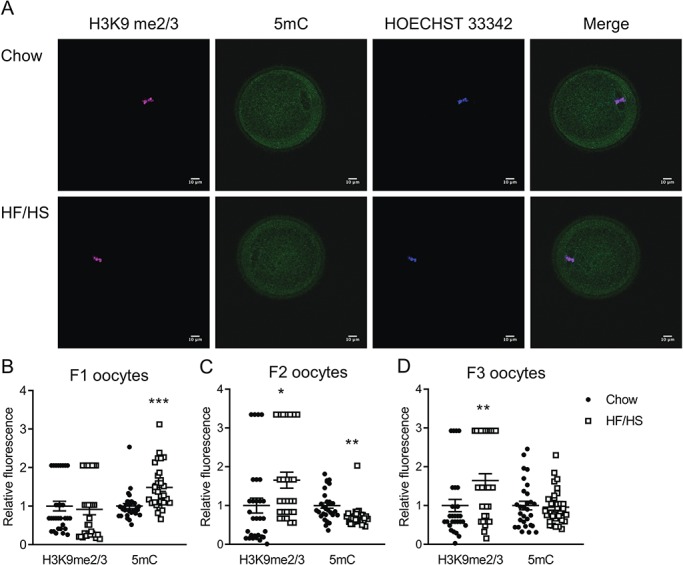
Effect of F0 diet (chow versus HF/HS) on H3K9me2/3 and 5mC in offspring oocytes. (A) Representative images of oocytes derived from chow and HF/HS groups. Quantification of H3K9me2/3 and 5mC in oocytes derived from F1 (B), F2 (C) and F3 (D) generations (n = 29–32 oocytes derived from five mice/diet/generation). Scale bar = 10 μm. Data are represented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001.
Effects of F0 HF/HS diet exposure on F0, F1 and F2 blastocysts
Given the effects we observed in oocytes, we wondered whether early embryos were also affected by F0 diet exposure. To answer this question, we collected meiosis II-stage oocytes from F0, F1 and F2 mice and performed IVF. F0 HF/HS exposure had no effect on the number of ovulated oocytes, fertilization rate or blastocyst rate (Supplementary Table SII). Blastocysts derived from F0-HF/HS oocytes had lower mitochondrial mass but higher membrane potential (Fig. 4B), more Oma1 (Fig. 4F), less ATP and citrate (Fig. 5A) and more H3K9me2/3 (Fig. 6B) than blastocysts derived from F0-chow oocytes. Blastocysts from F1-HF/HS oocytes had more lipid (Fig. 4C) and Oma-1 (Fig. 4G) and less citrate (Fig. 5B) than blastocysts from F1-chow oocytes. Blastocysts from F2-HF/HS oocytes had more mitochondria, less lipid (Fig. 4D) and less citrate (Fig. 5C) than blastocysts from F2-chow oocytes.
Figure 4.
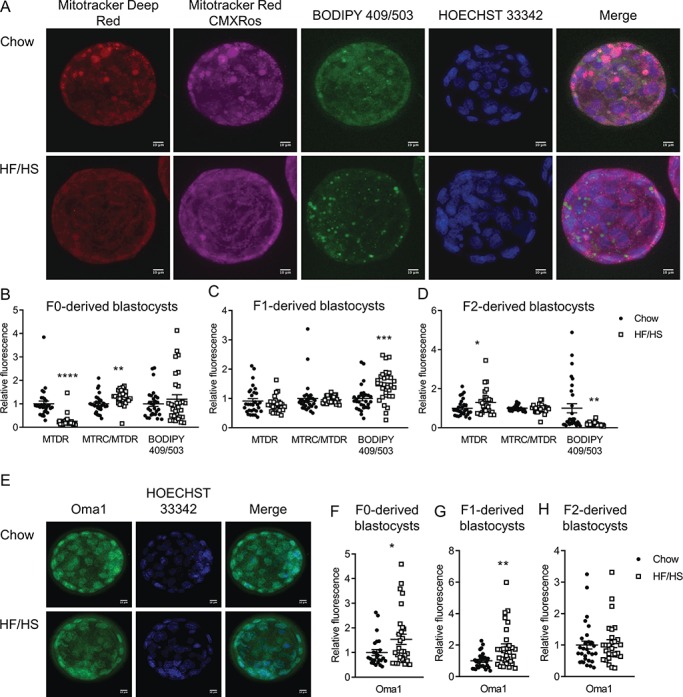
Effect of F0 diet (chow versus HF/HS) on mitochondria and lipid in offspring blastocysts. (A and E) Representative images of blastocysts derived from chow and HF/HS groups. Quantification of mitochondrial mass (MTDR), mitochondrial membrane potential (MTRC/MTDR) and lipid droplets (BODIPY 409/503) in blastocysts derived from F0 (B), F1 (C) and F2 (D) oocytes (n = 29–30 blastocysts derived from five mice/diet/generation). Quantification of Oma1 protein expression in blastocysts derived from F0 (F), F1 (G) and F2 (H) oocytes (n = 25–30 blastocysts derived from five mice/diet/generation). Scale bar = 10 μm. Data are represented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Figure 5.
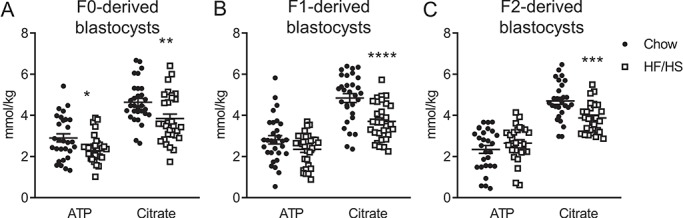
Effect of F0 (chow versus HF/HS) diet on ATP and citrate in offspring blastocysts. Quantification of ATP and citrate in blastocysts derived from F0 (A), F1 (B) and F2 (C) oocytes (n = 27–30 blastocysts derived from five mice/diet/generation). Data are represented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Figure 6.
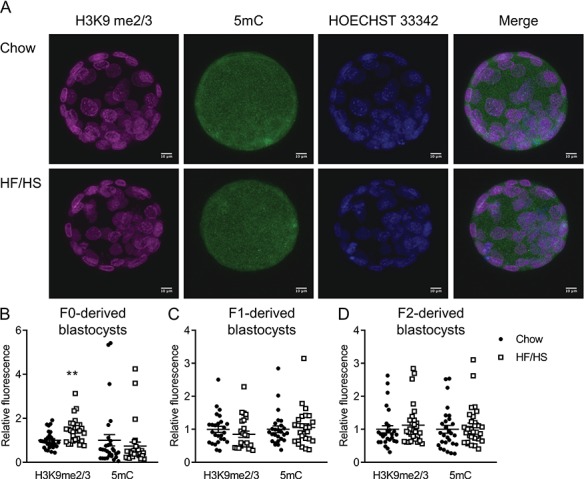
Effect of F0 diet (chow versus HF/HS) on H3K9 me2/3 and 5mC in offspring blastocysts. (A) Representative images of blastocysts derived from chow and HF/HS groups. Quantification of H3K9me2/3 and 5mC in blastocysts derived from F0 (B), F1 (C) and F2 (D) oocytes (n = 28–30 blastocysts derived from five mice/diet/generation). Scale bar = 10 μm. Data are represented as mean ± SEM. **P < 0.01.
We next examined total cell number and the number of apoptotic cells in blastocysts, as these indicators are often used to determine embryo quality (Van Soom et al., 2003; Irani et al., 2017). Moreover, mitochondria play an important role in regulating cell proliferation, cell cycle arrest and cell death (Rustin, 2002; Mason and Rathmell, 2011). Thus, we counted cell number and performed terminal deoxynucleotidyl transferase dUTP nick end labeling at 96 h post-fertilization. F0-HF/HS blastocysts had fewer cells and fewer apoptotic cells than F0-chow blastocysts (Fig. 7B). No differences were seen between F1-HF/HS and F1-chow blastocysts (Fig. 7C), whereas F2-HF/HS blastocysts had more cells and more apoptotic cells than F2-chow blastocysts (Fig. 7D). Together, these data indicate that F0, and to a lesser extent, F1 and F2 blastocysts, are impaired by F0 HF/HS diet exposure
Figure 7.
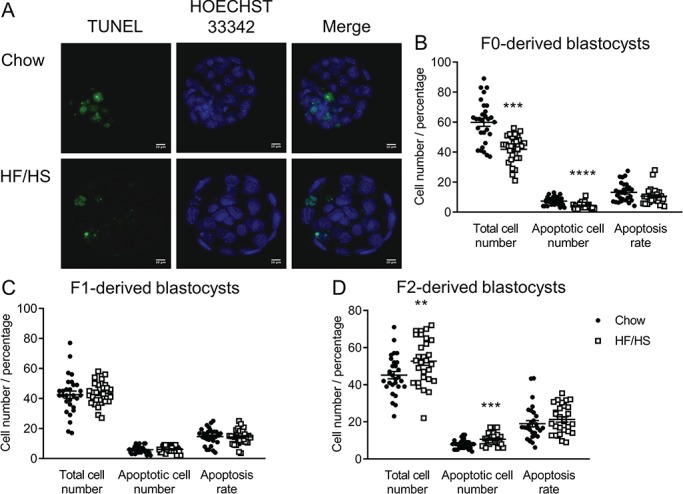
Effect of F0 diet (chow versus HF/HS) on cell number and apoptosis in IVF-derived offspring blastocysts. (A) Representative images of blastocysts derived from chow and HF/HS groups. Total cell number, apoptotic cell number and apoptosis rate in blastocysts derived from F0 (B), F1 (C) and F2 (D) oocytes (n = 29–30 blastocysts derived from five mice/diet/generation). Scale bar = 10 μm. Data are represented as mean ± SEM. **P < 0.01, ***P < 0.001, ****P < 0.0001.
Effects of the antioxidant mito-esculetin on F0-HF/HS embryo development
In previous work (Reynolds et al., 2015), we tried preventing oocyte mitochondrial defects by feeding mice a control diet for 8 weeks after consuming an HF diet for 6 weeks. Although the mice had similar body weight, cholesterol and glucose as mice fed a control diet for 14 weeks, their oocytes still had spindle defects, abnormal lipid droplet distribution, disrupted mitochondrial membrane potential and reduced metabolites. Thus, here, we instead asked whether we could improve F0-HF/HS blastocyst quality by adding an antioxidant during IVF. Antioxidants appear to be important for sperm, oocyte and embryo development, and the antioxidant l-carnitine has been used to improve human sperm function and mouse IVF success (Lenzi et al., 2004; Truong and Gardner, 2017). We chose to use mito-esculetin, a form of the anti-oxidant esculetin that is modified by coupling to the lipophilic triphenylphosphonium cation tag to promote mitochondrial uptake (Murphy and Smith, 2000). In vitro treatment of human aortic endothelial cells with mito-esculetin inhibited hydrogen peroxide- and angiotensin II-induced cell death, promoted mitochondrial biogenesis and reversed hydrogen peroxide-induced inhibition of mitochondrial respiration (Karnewar et al., 2016).
Initially, we supplemented IVF media with 0, 0.5, 1, 2.5 or 5 μM mito-esculetin and found that it had no effect on fertilization and blastocyst rates in F0-chow or F0-HF/HS (Supplementary Tables SIII and SIV). We next compared F0-chow and F0-HF/HS blastocysts derived from IVF in the presence of 1 μM mito-esculetin. Mito-esculetin normalized the difference in mitochondrial number and membrane potential but caused F0-HF/HS blastocysts to have less lipid than F0-chow blastocysts (compare Fig. 8B to Fig. 4B). Mito-esculetin did not normalize the differences in ATP or citrate (compare Fig. 8C to Fig. 5A) or number of apoptotic cells, but did minimize the difference in total cell number (compare Fig. 8E to Fig. 7B). These findings suggest that mito-esculetin supplementation during IVF somewhat improved development of F0-HF/HS embryos.
Discussion
Together, the data presented here indicate that maternal consumption of HF/HS diet contributes to mitochondrial and metabolic dysfunction in oocytes and embryos of the next three generations. Furthermore, we report that the antioxidant mito-esculetin can partially reverse these effects when provided during IVF. Our finding that at least some effects of maternal obesity were evident in the F3 generation is important, as it indicates that the effects were transgenerational. Whereas the F1 fetuses and the oocytes that gave rise to the F2 generation were exposed to the HF/HS diet in utero, the F3 generation was not (Skinner et al., 2008; Dias and Ressler, 2014; Klengel et al., 2015). Thus, the mitochondrial and metabolic phenotypes were likely caused by epigenetic changes. This idea is consistent with a previous finding that both paternal and maternal exposure to HF diet resulted in offspring with increased insulin resistance and decreased expression of adiponectin and leptin genes and that this was caused by epigenetic regulation of these genes in offspring adipose tissue (Masuyama et al., 2016).
Our data here are consistent with our lab’s previous demonstration that maternal consumption of HF/HS diet led to abnormally shaped mitochondria and altered concentrations of the mitochondria-related proteins Opa1, Drp1 and OxPhos in the skeletal muscle of F1, F2 and F3 offspring and oocytes of F1 and F2 offspring (Saben et al., 2016). Additionally, in models of genetic- and diet-induced obesity, offspring had low mitochondrial number, decreased membrane potential, lower viability oocytes and impaired embryo development (Luzzo et al., 2012; Wu et al., 2015; Boudoures et al., 2017). We report that oocytes from F1, F2 and F3 offspring of HF/HS-fed F0 mice had low mitochondrial number and altered mitochondrial membrane potential.
Our findings that maternal HF/HS exposure led to variable alterations in H3K9me2/3 and 5mC in F1, F2 and F3 oocytes and F0, F1 and F2 blastocysts are somewhat consistent with a previous study in which genetic- and diet-induced obesity led to decreased 5mC and H3K27me2 and increased H3K9me2 in oocytes (Hou et al., 2016). Additionally, Keleher et al. (2018) found that maternal exposure to HF diet led to differential methylation and altered expression of almost 30% of genes in the livers of F1 offspring. Likewise, Masuyama et al. (2016) found that HF diet exposure led to decreased H3K9ac1 and increased H4K20me1 marks on the adiponectin and leptin, respectively, promoters in offspring adipose tissue. Why the epigenetic marks varied across generations is currently unclear. However, one possibility is that the F0, F1 and F2 were all directly exposed to the diet but in different ways: the F0 consumed the diet, the F1 animals were exposed in utero and the germ cells that would give rise to the F2 were exposed during F1 fetal development. In contrast, the F3 was not directly exposed. Similarly, dynamic changes in epigenetic traits across generations have been noted in harlequin flies, mice and rats (Li et al., 2012; Lilley et al., 2012; Manikkam et al., 2012; Burggren, 2015). Future work should seek to fully define the maternal obesity-induced epigenetic and gene expression changes in offspring oocytes and embryos.
Previous papers have reported that oocytes derived from mice exposed to HF diet had low mitochondrial DNA content and membrane potential, poor quality and low fertilization rate (Reynier et al., 2001). Additionally, authors have noted that maternal obesity impaired pre- and post-implantation embryo development (Wai et al., 2010; Finger et al., 2015; Boudoures et al., 2017). Similarly, blastocysts from a genetic model of obesity (bbb/bbb) had fewer cells than their wild-type counterparts (Wu et al., 2015). Such findings make sense, as adequate mitochondrial number and function is essential for fertilization and embryo development. For example, Thouas et al. used photosensitization to cause oocyte mitochondrial dysfunction, characterized by altered mitochondrial morphology and metabolic function before fertilization. The resulting blastocysts had low ATP and NADH–NADPH content, increased apoptosis rate and reduced blastocyst formation rate (Thouas et al., 2004). However, in our experiments here, we saw no effect on fertilization or blastocyst development in offspring of HF/HS-fed mice. This could mean that our diet exposure model did not result in mitochondria with function below a critical threshold (Wai et al., 2010). Future experiments could assess whether a longer HF/HS diet exposure would lead to defects in fertilization or blastocyst development. Nonetheless, blastocysts from HF/HS-exposed F0 mice had decreased mitochondrial mass and membrane potential; increased expression of Oma1, which negatively regulates mitochondrial health and fusion (Head et al., 2009); decreased ATP and citrate concentrations; and decreased total cell number in blastocysts. It is important to note that in this study, the blastocysts were generated by IVF and thus were not exposed to maternal obesity in utero. Additionally, some, but not all, of these phenotypes are also evident in later generations, providing evidence for compensatory mechanisms.
Others have associated poor oocyte quality and embryo development in obese mice with elevated production of reactive oxygen species (Boots et al., 2016; Hou et al., 2016). Supplementing culture media with antioxidants reduced reactive oxygen species production and improved oocyte maturation, fertilization and embryo development (Truong et al., 2016; Liang et al., 2017; Sovernigo et al., 2017). Furthermore, supplementing fertilization media with the combination of antioxidants Acetyl-L-Carnitine, N-Acetyl-L-Cysteine and α-Lipoic Acid increased total cell number in blastocysts and kinetics of embryo development (Truong and Gardner, 2017), two key markers in choosing embryos for transfer (Prados et al., 2012; Aparicio-Ruiz et al., 2018). We tested mitochondria-targeted esculetin (mito-esculetin) because it was shown to counteract oxidant-induced deregulation of mitochondrial function and biogenesis in human endothelial cells (Karnewar et al., 2016). We supplemented IVF media with mito-esculetin and found that it had no effect on fertilization and embryo development. Although mito-esculetin had no effect on citrate and ATP concentration in blastocysts, it partially prevented the HF/HS diet-induced mitochondrial dysfunction and lower total cell number in F0-derived blastocysts. Whether the next generations (F1 and F2) would also benefit from mito-esculetin treatment of F0 oocytes is an important question for future work. Nevertheless, our data support the idea that supplementing fertilization media, and possibly embryo culture media (Bellver et al., 2015), with antioxidants could improve IVF success rates for obese women.
Supplementary Material
Acknowledgements
The authors thank Deborah J. Frank (Obstetrics and Gynecology, Washington University in St. Louis) for critical reading and editing of this manuscript.
Authors’ roles
E.A. conceived the study, performed the experiments and wrote and edited the manuscript. K.H.M. helped design the study and interpret the findings, reviewed the manuscript and acquired the funding. M. R and W.Z. provided considerable technical assistance.
Funding
National Institutes of Health (RO1HD065435 and RO1HD083896 to K.H.M.).
Conflict of interest
The authors declare no conflict of interests.
References
- Aparicio-Ruiz B, Romany L, Meseguer M. Selection of preimplantation embryos using time-lapse microscopy in in vitro fertilization: state of the technology and future directions. Birth Defects Res 2018;110:648–653. [DOI] [PubMed] [Google Scholar]
- Bellver J, De los Santos MJ, Alamá P, Castelló D, Privitera L, Galliano D, Labarta E, Vidal C, Pellicer A, Domínguez F. Day-3 embryo metabolomics in the spent culture media is altered in obese women undergoing in vitro fertilization. Fertil Steril 2015;103:1407, e1401–1415. [DOI] [PubMed] [Google Scholar]
- Boots C, Boudoures A, Zhang W, Drury A, Moley K. Obesity-induced oocyte mitochondrial defects are partially prevented and rescued by supplementation with co-enzyme Q10 in a mouse model. Hum Reprod 2016;31:2090–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudoures AL, Saben J, Drury A, Scheaffer S, Modi Z, Zhang W, Moley KH. Obesity-exposed oocytes accumulate and transmit damaged mitochondria due to an inability to activate mitophagy. Dev Biol 2017;426:126–138. [DOI] [PubMed] [Google Scholar]
- Burggren WW. Dynamics of epigenetic phenomena: intergenerational and intragenerational phenotype ‘washout’. J Exp Biol 2015;218:80–87. [DOI] [PubMed] [Google Scholar]
- Chi MM-Y, Hoehn A, Moley KH. Metabolic changes in the glucose-induced apoptotic blastocyst suggest alterations in mitochondrial physiology. Am J Physiol Endocrinol Metab 2002;283:E226–E232. [DOI] [PubMed] [Google Scholar]
- Dias BG, Ressler KJ. Experimental evidence needed to demonstrate inter- and trans-generational effects of ancestral experiences in mammals. Bioessays 2014;36:919–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlow AG. Maternal obesity and neurodevelopmental and psychiatric disorders in offspring. Prenat Diagn 2017;37:95–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger BJ, Harvey AJ, Green MP, Gardner DK. Combined parental obesity negatively impacts preimplantation mouse embryo development, kinetics. morphology and metabolism. Hum Reprod 2015;30:2084–2096. [DOI] [PubMed] [Google Scholar]
- Head B, Griparic L, Amiri M, Gandre-Babbe S, van der Bliek AM. Inducible proteolytic inactivation of OPA1 mediated by the OMA1 protease in mammalian cells. J Cell Biol 2009;187:959–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y-J, Zhu C-C, Duan X, Liu H-L, Wang Q, Sun S-C. Both diet and gene mutation induced obesity affect oocyte quality in mice. Sci Rep 2016;6:18858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irani M, Reichman D, Robles A, Melnick A, Davis O, Zaninovic N, Xu K, Rosenwaks Z. Morphologic grading of euploid blastocysts influences implantation and ongoing pregnancy rates. Fertil Steril 2017;107:664–670. [DOI] [PubMed] [Google Scholar]
- Karnewar S, Vasamsetti SB, Gopoju R, Kanugula AK, Ganji SK, Prabhakar S, Rangaraj N, Tupperwar N, Kumar JM, Kotamraju S. Mitochondria-targeted esculetin alleviates mitochondrial dysfunction by AMPK-mediated nitric oxide and SIRT3 regulation in endothelial cells: potential implications in atherosclerosis. Sci Rep 2016;6:24108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keleher MR, Zaidi R, Shah S, Oakley ME, Pavlatos C, El Idrissi S, Xing X, Li D, Wang T, Cheverud JM. Maternal high-fat diet associated with altered gene expression, DNA methylation, and obesity risk in mouse offspring. PLoS One 2018;13:e0192606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klengel T, Dias BG, Ressler KJ. Models of intergenerational and transgenerational transmission of risk for psychopathology in mice. Neuropsychopharmacology 2015;41:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzi A, Sgro P, Salacone P, Paoli D, Gilio B, Lombardo F, Santulli M, Agarwal A, Gandini L. A placebo-controlled double-blind randomized trial of the use of combined l-carnitine and l-acetyl-carnitine treatment in men with asthenozoospermia. Fertil Steril 2004;81:1578–1584. [DOI] [PubMed] [Google Scholar]
- Li J, Huang J, Li JS, Chen H, Huang K, Zheng L. Accumulation of endoplasmic reticulum stress and lipogenesis in the liver through generational effects of high fat diets. J Hepatol 2012;56:900–907. [DOI] [PubMed] [Google Scholar]
- Liang L-F, Qi S-T, Xian Y-X, Huang L, Sun X-F, Wang W-H. Protective effect of antioxidants on the pre-maturation aging of mouse oocytes. Sci Rep 2017;7:1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley TM, Ruokolainen L, Pikkarainen A, Laine VN, Kilpimaa J, Rantala MJ, Nikinmaa M. Impact of tributyltin on immune response and life history traits of Chironomus riparius: single and multigeneration effects and recovery from pollution. Environ Sci Technol 2012;46:7382–7389. [DOI] [PubMed] [Google Scholar]
- Luzzo KM, Wang Q, Purcell SH, Chi M, Jimenez PT, Grindler N, Schedl T, Moley KH. High fat diet induced developmental defects in the mouse: oocyte meiotic aneuploidy and fetal growth retardation/brain defects. PLoS One 2012;7: e49217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner MK. Dioxin (TCDD) induces epigenetic transgenerational inheritance of adult onset disease and sperm epimutations. PLoS One 2012;7: e46249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason EF, Rathmell JC. Cell metabolism: an essential link between cell growth and apoptosis. Biochim Biophys Acta 2011;1813:645–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuyama H, Mitsui T, Eguchi T, Tamada S, Hiramatsu Y. The effects of paternal high-fat diet exposure on offspring metabolism with epigenetic changes in the mouse adiponectin and leptin gene promoters. Am J Physiol Endocrinol Metab 2016;311:E236–E245. [DOI] [PubMed] [Google Scholar]
- Murphy MP, Smith RA. Drug delivery to mitochondria: the key to mitochondrial medicine. Adv Drug Deliv Rev 2000;41:235–250. [DOI] [PubMed] [Google Scholar]
- Otsu NA. Threshold selection method from gray-level histograms. IEEE Trans Syst Man Cybern 1979;9:62–66. [Google Scholar]
- Parry WL, Hemstreet GP. Cancer detection by quantitative fluorescence image analysis. J Urol 1988;139:270–274. [DOI] [PubMed] [Google Scholar]
- Pendergrass W, Wolf N, Poot M. Efficacy of MitoTracker Green and CMXrosamine to measure changes in mitochondrial membrane potentials in living cells and tissues. Cytometry A 2004;61:162–169. [DOI] [PubMed] [Google Scholar]
- Prados FJ, Debrock S, Lemmen JG, Agerholm I. The cleavage stage embryo. Hum Reprod 2012;27:i50–i71. [DOI] [PubMed] [Google Scholar]
- Reynier P, May-Panloup P, Chrétien MF, Morgan CJ, Jean M, Savagner F, Barrière P, Malthièry Y. Mitochondrial DNA content affects the fertilizability of human oocytes. Mol Hum Reprod 2001;7:425–429. [DOI] [PubMed] [Google Scholar]
- Reynolds KA, Boudoures AL, Chi MM, Wang Q, Moley KH. Adverse effects of obesity and/or high-fat diet on oocyte quality and metabolism are not reversible with resumption of regular diet in mice. Reprod Fertil Dev 2015;27:716–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustin P. Mitochondria, from cell death to proliferation. Nat Genet 2002;30:352. [DOI] [PubMed] [Google Scholar]
- Saben JL, Boudoures AL, Asghar Z, Thompson A, Drury A, Zhang W, Chi M, Cusumano A, Scheaffer S, Moley KH. Maternal metabolic syndrome programs mitochondrial dysfunction via germline changes across three generations. Cell Rep 2016;16:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner MK, Anway MD, Savenkova MI, Gore AC, Crews D. Transgenerational epigenetic programming of the brain transcriptome and anxiety behavior. PLoS One 2008;3: e3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sovernigo T, Adona P, Monzani P, Guemra S, Barros F, Lopes F, Leal C. Effects of supplementation of medium with different antioxidants during in vitro maturation of bovine oocytes on subsequent embryo production. Reprod Domest Anim 2017;52:561–569. [DOI] [PubMed] [Google Scholar]
- Takeo T, Nakagata N. Superovulation using the combined administration of inhibin antiserum and equine chorionic gonadotropin increases the number of ovulated oocytes in C57BL/6 female mice. PLoS One 2015;10: e0128330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thouas GA, Trounson AO, Wolvetang EJ, Jones GM. Mitochondrial dysfunction in mouse oocytes results in preimplantation embryo arrest in vitro. Biol Reprod 2004;71:1936–1942. [DOI] [PubMed] [Google Scholar]
- Truong T, Gardner DK. Antioxidants improve IVF outcome and subsequent embryo development in the mouse. Hum Reprod 2017;32:2404–2413. [DOI] [PubMed] [Google Scholar]
- Truong TT, Soh YM, Gardner DK. Antioxidants improve mouse preimplantation embryo development and viability. Hum Reprod 2016;31:1445–1454. [DOI] [PubMed] [Google Scholar]
- Van Soom A, Mateusen B, Leroy J, de Kruif A. Assessment of mammalian embryo quality: what can we learn from embryo morphology? Reprod Biomed Online 2003;7:664–670. [DOI] [PubMed] [Google Scholar]
- Wai T, Ao A, Zhang X, Cyr D, Dufort D, Shoubridge EA. The role of mitochondrial DNA copy number in mammalian fertility. Biol Reprod 2010;83:52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LL, Russell DL, Wong SL, Chen M, Tsai TS, St John JC, Norman RJ, Febbraio MA, Carroll J, Robker RL. Mitochondrial dysfunction in oocytes of obese mothers: transmission to offspring and reversal by pharmacological endoplasmic reticulum stress inhibitors. Development 2015;142:681–691. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


