Abstract
Nitrite-oxidizing bacteria (NOB) catalyze the second step of nitrification, nitrite oxidation to nitrate, which is an important process of the biogeochemical nitrogen cycle. NOB were traditionally perceived as physiologically restricted organisms and were less intensively studied than other nitrogen-cycling microorganisms. This picture is contrasted by new discoveries of an unexpected high diversity of mostly uncultured NOB and a great physiological versatility, which includes complex microbe-microbe interactions and lifestyles outside the nitrogen cycle. Most surprisingly, close relatives to NOB perform complete nitrification (ammonia oxidation to nitrate) and this finding will have far-reaching implications for nitrification research. We review recent work that has changed our perspective on NOB and provides a new basis for future studies on these enigmatic organisms.
NOB: the ‘Big Unknown’ of the Nitrogen Cycle
Nitrogen is essential for all living organisms on Earth. In the biogeochemical nitrogen cycle, microorganisms mediate essential conversions of nitrogen compounds, such as N2 fixation into organic molecules and the recycling of nitrogen from decaying biomass into the atmosphere. A key step of the nitrogen cycle is the sequential oxidation of ammonia via nitrite to nitrate, a process termed ‘nitrification’ (Figure 1). More than a century ago Sergei Winogradsky isolated the first chemolithoautotrophic bacteria that grew by nitrification, using ammonia or nitrite as their energy source and electron donor [1, 2]. In the course of his monumental work, Winogradsky found the two nitrification steps to be catalyzed by distinct bacteria, ammonia-oxidizing bacteria (AOB; see Glossary) and nitrite-oxidizing bacteria (NOB) whose cooperation was needed to achieve complete nitrification. He also noticed that both groups were slow-growing organisms, whose cultivation in the lab required patience [2]. In the following century, nitrification research made great progress by illuminating the biochemistry of nitrifiers from the genera Nitrosomonas (AOB) and Nitrobacter (NOB) (reviewed in [3]). This research was driven by scientific curiosity and the practical importance of nitrification. In agriculture, the conversion of fertilizer ammonium to nitrate leads to substantial nitrogen loss from soils. In contrast, nitrification is beneficial as a key step of biological wastewater treatment for eliminating excess nitrogen from sewage.
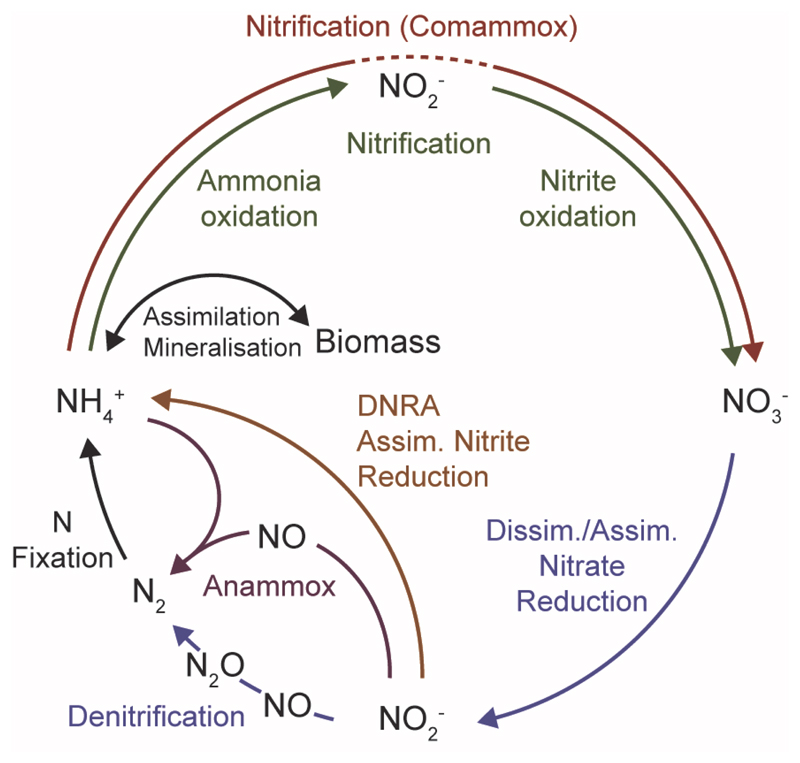
Figure 1. Nitrite, a Key Intermediate of the Biogeochemical Nitrogen Cycle.
Schematic illustration of the key processes of the nitrogen cycle. Note that the fate of nitrite determines whether nitrogen remains fixed (as nitrite, nitrate or ammonium) or is lost to the atmosphere (as NO, N2O or N2). The stippled line for comammox indicates that nitrite is an intermediate but oxidized to nitrate by the same organism. Abbreviations: Anammox, anaerobic ammonium oxidation; DNRA, dissimilatory nitrite reduction to ammonia; assim., assimilatory; dissim., dissimilatory.
Glossary.
Ammonia monooxygenase (AMO): the key enzyme of all known bacterial and archaeal ammonia oxidizers (including comammox), which oxidizes ammonia to hydroxylamine.
Ammonia-oxidizing archaea (AOA) and bacteria (AOB): mediate the first step of nitrification by oxidizing ammonia to nitrite.
Complete ammonia oxidizer (Comammox): an organism that is capable of performing complete nitrification on its own. Comammox was a hypothetical microbe until the recent discovery of completely nitrifying bacteria in the genus Nitrospira.
Extracellular polymeric substances (EPS): are secreted by microorganisms and are considered to be of key importance for the integrity and function of microbial biofilms and flocs.
Fluorescence in situ hybridization (FISH): FISH with rRNA-targeted probes is a widely used cultivation-independent method to detect and identify microorganisms in environmental samples.
Hydroxylamine dehydrogenase (HAO): a key enzyme of bacterial ammonia oxidizers (including comammox), which oxidizers hydroxylamine to nitrite. Formerly known as hydroxylamine oxidoreductase.
Nitrite oxidoreductase (NXR): the key enzyme of nitrite oxidizers (including comammox) that catalyzes nitrite oxidation to nitrate, but can also reduce nitrate to nitrite.
Nitrite-oxidizing bacteria (NOB): catalyze the second step of nitrification by oxidizing nitrite to nitrate.
Oxygen minimum zone (OMZ): oceanic regions where intense respiration of organic matter (exported from overlying productive waters) and a poor ventilation of water masses cause a depletion of dissolved oxygen.
Wastewater treatment plant (WWTP): modern WWTPs include a combination of the nitrification and denitrification processes to eliminate excess nitrogen from sewage.
For decades, nitrification research has focused mainly on ammonia oxidizers. The reasons for this include that ammonia oxidation has been considered the rate-limiting nitrification step [4], research on ammonia oxidizers has received an impetus from the discovery of ammonia-oxidizing archaea (AOA) [5], and NOB have a reputation for being even more difficult to grow in the lab than AOA or AOB. Since nitrite does not accumulate in most ecosystems, the nitrite oxidation process receives less attention although low standing pools of a substrate do not allow conclusions on the importance of the consuming reactions. In addition, NOB were perceived as obligate chemolithoautotrophs with a very limited physiological repertoire and thus little potential for the discovery of new physiologies. In consequence, progress in NOB-related research lagged behind the knowledge increase on other nitrogen-cycling microbes. However, the fate of nitrite determines whether fixed nitrogen remains in an ecosystem or is lost to the atmosphere (Figure 1). NOB counteract nitrogen loss by converting nitrite to nitrate, which is utilized as a nitrogen source by many microbes and plants and represents an impressive 88% of the fixed nitrogen in the oceans [6]. Hence, NOB have an important regulatory function in the nitrogen cycle. Furthermore, nitrite is toxic to eukaryotes [7] and inhibits bacterial growth [8]. NOB activity in wastewater treatment plants (WWTPs) tends to be unstable (in particular in industrial systems), and breakdowns of nitrite oxidation can cause tremendous ecological damage if nitrite from WWTPs leaks into natural waters. Considering the ecological importance of NOB and our limited knowledge on their biology, NOB are a ‘big unknown’ of the nitrogen cycle. Fortunately, this situation is now improving as metagenomics and other ‘meta-omics’ approaches [9, 10], single-cell isotope labeling and genomic techniques [11, 12], and refined cultivation methods [13, 14] offer new opportunities to study NOB. This review provides an overview of recent insights and discoveries that have changed our perspective on these fascinating organisms. We focus on the chemolithoautotrophic NOB and skip the phototrophic NOB [15, 16], which use light as energy source and utilize nitrite only as electron donor for biosynthesis.
A Highly Diverse Functional Group
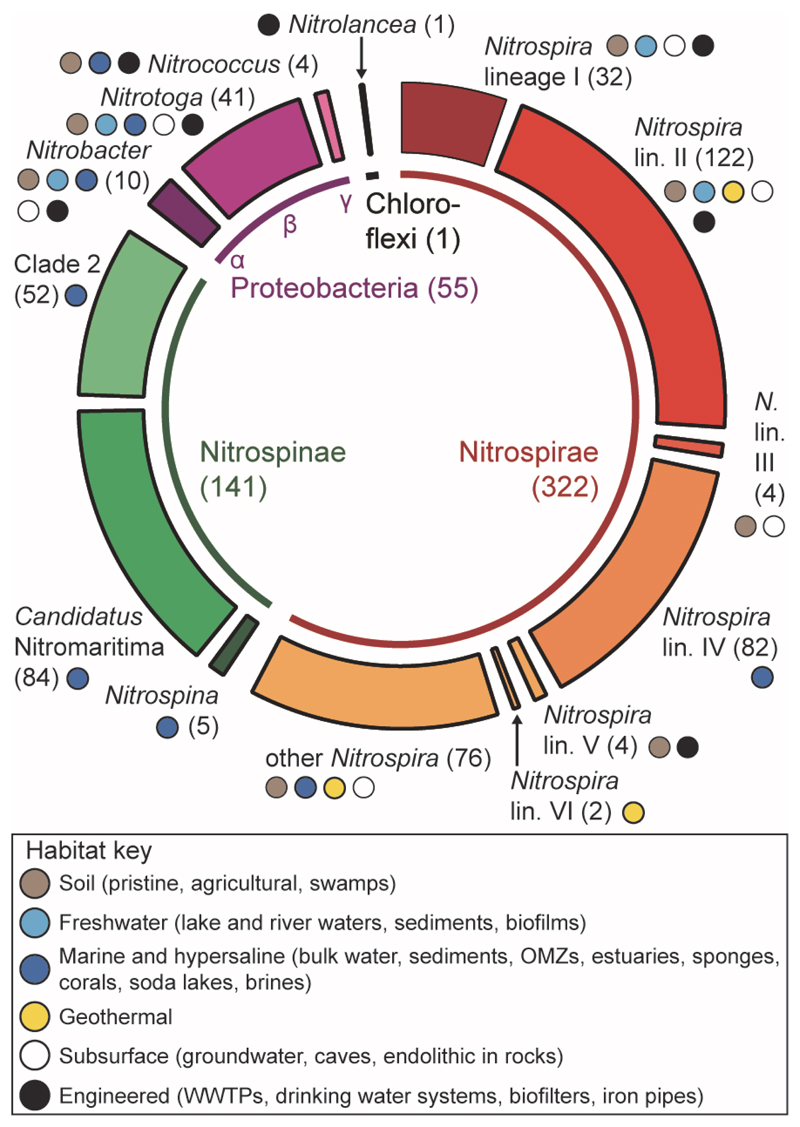
The known NOB belong to seven genera in four bacterial phyla (Figure 2) including a new candidate genus of uncultured marine NOB, ‘Candidatus Nitromaritima’, which is related to Nitrospina and was proposed based on comparative analyses of single-cell amplified genomes [17]. All NOB have Gram-negative cell envelopes except Nitrolancea hollandica which stains Gram-positive and forms thick cell wall layers [18]. The NOB lineages are unequally distributed in the environment (Figure 2). In particular, the Nitrospinae are the predominant marine NOB and can reach high abundances (up to ~10% of the microbial community) in the mesopelagic zone, oxygen minimum zones (OMZs), deep-sea waters, and sediments [19–22]. Nitrospira is the most diverse NOB genus and consists of at least six phylogenetic sublineages [23–25], which together are ubiquitous in nature (Figure 2). Nitrospira in geothermal springs are the only known thermophilic NOB and have a temperature limit of 60-65 °C [25–27].
Figure 2. Phylogenetic Affiliation, Species-Level Diversity, and Habitats of NOB.
The known nitrite oxidizers belong to the genera Nitrobacter [1, 103], Nitrotoga [40], Nitrococcus [104, 105], Nitrospira [68, 72], Nitrospina [58, 104, 106], Nitrolancea [18], and ‘Candidatus Nitromaritima’ [17]. Nitrospinae ‘Clade 2’ is a monophyletic group without a defined taxonomic status [17]. The inner ring indicates which bacterial phyla contain these genera. To assess the species-level diversity within each genus, the 16S rRNA sequences of known NOB and closely related bacteria were retrieved from the SILVA Ref NR 99 database (release 123, July 2015) [107] and clustered in operational taxonomic units (OTUs) using a sequence identity threshold of 98.7% [108]. The obtained OTU numbers are indicated in parentheses for each NOB group or bacterial phylum as an estimate of the NOB species numbers represented in the sequence database. Wedges in the outer ring are drawn proportionally to the OTU numbers. The six Nitrospira lineages are shown separately, whereas ‘other Nitrospira’ does not refer to one coherent lineage but instead comprises all Nitrospira sequences outside the already established lineages of this genus. Colored small circles indicate the known major habitats of each NOB group. Most of the collected 16S rRNA sequences stem from uncultured organisms.
NOB are difficult to cultivate and the purification of isolates took up to twelve years [24]. Sorting single cells with optical tweezers [28] or separating cell aggregates by flow cytometry [13] facilitate the isolation of NOB, but growing enough biomass for follow-up studies often remains a challenge. Thus, molecular methods are essential for studying the majority of the uncultured or merely enriched NOB and have revealed an unexpected diversity of these organisms. For example, 16S rRNA gene surveys discovered hundreds of distinct marine Nitrospina populations with seasonal and depth-related distribution patterns in the upper water column and OMZ waters [21, 29].
The key enzyme of NOB is nitrite oxidoreductase (NXR; Box 1). The nxrA and especially the nxrB gene are powerful functional and phylogenetic markers to detect and identify uncultured NOB [30–32]. An overwhelming diversity (richness) of terrestrial Nitrospira was discovered by nxrB amplicon high-throughput sequencing and phylogenetic analyses. Based on 95% nxrB nucleotide sequence identity as species-level cut-off, 109 to 764 Nitrospira species were found in soils from Namibia [32]. Moreover, the nxrB amplicon sequencing approach detected at least 120 co-occurring Nitrospira species in activated sludge from a full-scale WWTP [33]. Some of these populations were detected and distinguished by fluorescence in situ hybridization (FISH) with highly specific oligonucleotide probes targeting single nucleotide polymorphisms in the 16S rRNA of these closely related organisms. FISH revealed that the complex Nitrospira community had persisted for at least four years in the WWTP, suggesting a stable coexistence of many Nitrospira strains [33].
Box 1. Nitrite Oxidoreductase (NXR).
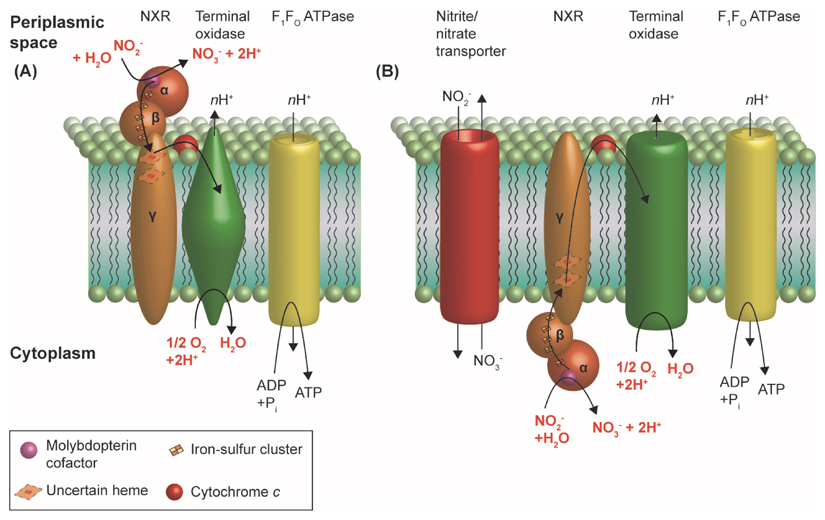
NXR, the key enzyme of NOB, oxidizes nitrite to nitrate and shuttles two electrons per reaction into the respiratory chain (Figure I). It belongs to the type II DMSO reductase-like family of molybdopterin-binding enzymes [55, 58]. NXR is associated with the cytoplasmic membrane and probably consists of three subunits NxrA (α), NxrB (β), and NxrC (γ) [55, 86]. The substrate-binding subunit NxrA [55, 86] is located in the periplasmic space in Nitrospira [54, 55, 87], Nitrospina [58] and ‘Candidatus Nitromaritima’ [17], but in the cytoplasm in Nitrobacter, Nitrococcus and Nitrolancea [18, 56, 88] (Figure I). With a periplasmic NXR, protons are derived from water during nitrite oxidation in the periplasmic space, where they contribute to proton motive force (PMF) and thus to the cell’s energy budget [55] (Figure IA). However, with a cytoplasmic NXR the protons do not contribute to PMF (Figure IB). Due to the low energy yield of nitrite oxidation (ΔG°′ = -74 kJ mol-1 NO2-), this subtle difference may distinguish highly economical from less optimized nitrite oxidation pathways. Furthermore, NOB with a cytoplasmic NXR must transport nitrite and nitrate over the cytoplasmic membrane (Figure IB). This could become another physiological bottleneck depending on the substrate affinity and turnover rate of the transporter. The cytoplasmic NXRs are phylogenetically affiliated with membrane-bound cytoplasmic nitrate reductases (NARs), whereas the periplasmic NXRs belong to a distinct phylogenetic lineage within the type II enzymes of the DMSO reductase family [55, 58]. The two NXR types evolved independently and likely spread by lateral gene transfer into different organisms, giving rise to the large phylogenetic diversity of NOB (Figure 2) [18, 55, 58].
Figure I. Schematic Illustration of the NXR Types and the Assumed Electron Flow During Nitrite Oxidation.
The critical reactions, which release or consume protons on either side of the cytoplasmic membrane, are highlighted in red. Arrows indicate the electron flow through the NXR subunits via cytochrome c and towards the terminal oxidase. nH+ indicates that the numbers of protons translocated per reaction have not been determined yet in NOB. The numbers and types of heme cofactors in the NxrC subunits are unknown. Candidates are one or two b- or c-type cytochromes in Nitrospira [55], and cytochrome a1 and/or cytochrome c1 in Nitrobacter [3]. (A) Periplasmic NXR, (B) cytoplasmic NXR.
It is unlikely that such complex NOB communities would develop if all these organisms were physiologically restricted to nitrite oxidation and shared highly similar ecological niches. Indeed, recent studies have revealed a surprising ecophysiological versatility of NOB that could at least partly explain the observed complexity by niche specialization and niche partitioning. It comprises different adaptations to environmental parameters and ecological strategies, microbe-microbe interactions, metabolisms other than nitrite oxidation, and even complete nitrification.
Environmental Adaptations and Ecological Strategies
Owing to the biotechnological importance of nitrification and the ease of sampling activated sludge, WWTPs are often used as model systems to study NOB biology. The key NOB in WWTPs are members of Nitrospira lineages I and II (e.g., [23, 34, 35]). Only recently, Nitrotoga has been recognized as another important NOB group in engineered systems [36–39]. Interestingly, Nitrospira and Nitrotoga populations coexist in some WWTPs [36, 37]. Similar to Nitrotoga arctica from permafrost-affected soil [40], Nitrotoga strains enriched from a WWTP preferred low temperatures between 10 and 17 °C [36]. Uncultured Nitrotoga were mostly abundant in WWTPs operated at low temperatures, although CO2 fixation by these organisms was still detected at 27 °C [37]. Nitrospira strains grew in a broad temperature range (10 to 28 °C), but outcompeted Nitrotoga in nitrite-oxidizing enrichment cultures only at the higher temperatures [36]. A recently enriched Nitrotoga strain had temperature and pH optima of 22 °C and 6.8, whereas Nitrospira defluvii (a member of Nitrospira lineage I from a WWTP [41]) grew best at 32 °C and pH 7.3 [39]. The coexistence of mesophilic Nitrospira and cold-adapted Nitrotoga in WWTPs may be supported by seasonal temperature shifts, which provide temporally optimal conditions for either group, and by other niche-defining properties like microbe-microbe interactions or alternative metabolisms (see below). The dissolved oxygen concentration might be another important environmental factor for NOB niche partitioning. Varying aeration intensities affected the relative abundances of lineage I and II Nitrospira in bioreactors [42] and it appears likely that oxygen gradients in biofilms, sediments and soils provide niches for different coexisting NOB. In this context it is interesting to note that compared to the nitrite oxidation activities in OMZ waters at >10 µM O2, as much as 36-59% activity was still measured at <1 µM O2, suggesting that NOB in this habitat have an exceptionally high affinity for oxygen [19, 43]. It has also been hypothesized that NOB in OMZ might utilize alternative terminal electron acceptors for nitrite oxidation such as iodate, Mn(IV) or Fe(III) [44, 45].
Fundamental ecophysiological differences between major NOB groups result from the kinetics and biochemistry of nitrite oxidation. First, NOB differ in their affinity for nitrite (Box 2). Second, the subcellular localization of NXR affects the energetic efficiency of nitrite oxidation (Box 1). NOB with a high nitrite affinity and a periplasmic NXR should be optimally adapted to low nitrite concentrations. This molecular adaptation likely contributes to the wide distribution of Nitrospira in natural ecosystems, where nitrite rarely accumulates, and in continuously operated WWTPs, where the nitrite levels in the activated sludge mixed liquor are low and equal the low nitrite concentrations in the effluent (like in a chemostat). It may also facilitate the growth of Nitrospina in nitrite-depleted marine waters. We assume that NOB with a cytoplasmic NXR, such as Nitrobacter, benefit from locally or seasonally occurring nitrogen-rich conditions, for example close to decaying cadavers, after leaf fall, in fertilized soils, in organic aggregates like marine snow, and in sequencing batch reactors [23, 46, 47]. This high degree of niche specialization may cause complex spatial distribution patterns and temporal population dynamics of coexisting NOB, of which we have only gained snapshot-like insights [21, 29, 32, 33, 48].
Box 2. The ‘K/r Hypothesis’ for Nitrite Oxidizers.
Measured Km(NO2-) values of Nitrospira strains from WWTPs and freshwater ranged from 9 to 27 µM [89, 90] and were below the Km(NO2-) values of Nitrobacter strains from sewage, soils, and other habitats (49 to 544 µM) [90]. Based on their high affinity for nitrite, it was hypothesized that Nitrospira may be K-strategists, which can reach high population densities despite nitrite limitation. In contrast, Nitrobacter could be r-strategists that prefer higher nitrite levels and grow faster than Nitrospira under such conditions [89]. Consistent with this hypothesis, the relative abundance of Nitrobacter compared to Nitrospira increased in bioreactors only under nitrite-rich conditions [46, 91] and after nitrogen addition to soil mesocosms [47]. At low nitrite concentrations, Nitrospira were the dominant NOB populations. However, the very broad range of Km(NO2-) values of Nitrobacter strains [90] shows that these NOB differ in their adaptation to lower or higher nitrite levels. The nitrite optima of Nitrospira are generally lower but also differ within this genus [33, 48, 90], and coexisting Nitrospira inhabit different zones along microscale nitrite concentration gradients in biofilms [48]. In summary, experimental results indicate that the ‘K/r hypothesis’ is true for Nitrospira and Nitrobacter, although adaptations to nitrite levels are strain dependent. In this framework ‘Candidatus Nitrotoga arctica’ (from soil) with a Km(NO2-) of 58 µM is placed between Nitrospira and Nitrobacter [90], and Nitrolancea hollandica (from sewage) with a Km(NO2-) of 1 mM is an r-strategist [18]. Kinetic data for other NOB are missing, and the roles of the other key substrates O2 and CO2 in the ecological strategies of NOB remain to be studied in detail.
Microbe-Microbe Interactions of NOB
The tight interactions of ammonia oxidizers with NOB (e.g., [49]) are reflected by a close spatial co-aggregation of these nitrifiers, which is often observed in biofilms and activated sludge flocs and known as the ‘nitrification aggregate’ [50] (Box 3). While this symbiosis might be surprisingly more complex (Box 3), the canonical model dictates that nitrification itself must be initiated by the ammonia oxidizers (Figure 3, Key Figure, panel A). However, recent studies showed that this is not always true. Many, but not all, ammonia oxidizers can utilize urea for nitrification because they possess the enzyme urease that hydrolyses urea to NH3 and CO2 [51]. This feature is important for example in acidic soils, where free ammonia is scarce and urea is a key substrate for nitrification [52], and possibly in oceanic habitats where indications exist that urea may be an important substrate [53]. A ureolytic activity of NOB was unknown and not anticipated as relevant, because NOB would not utilize ammonia from urea as energy source. Surprisingly, genes encoding urea transporters and cytoplasmic ureases were recently found in several Nitrospira genomes, and the capability of Nitrospira moscoviensis to cleave urea was experimentally confirmed [54]. This led to the discovery of a new interaction between nitrifiers called ‘reciprocal feeding’: urease-positive NOB can provide urease-negative AOB such as Nitrosomonas europaea with ammonia from urea degradation. Subsequently, the NOB obtain nitrite from the AOB (Figure 3B). Reciprocal feeding was demonstrated when mixed cultures of N. moscoviensis and N. europaea formed nitrate from urea [54]. Here nitrification was initiated by Nitrospira, which indirectly used urea as an energy source by recruiting Nitrosomonas. Analyses of metagenomes and single-cell genomes revealed that uncultured Nitrospira in soil and freshwater habitats and Nitrospina-like marine NOB also encode ureases, indicating that urease-positive NOB occur widespread and reciprocal feeding could be a common lifestyle of nitrifiers [54].
Box 3. The Nitrification Aggregate.
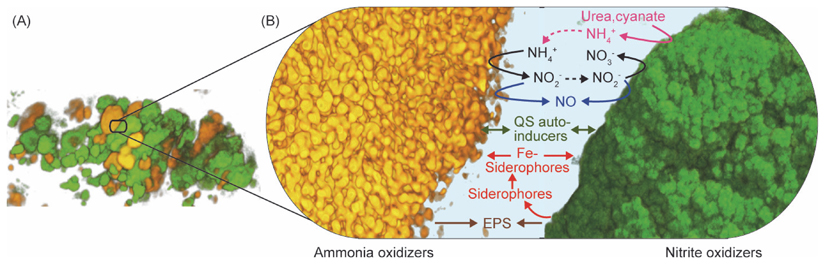
The classical concept of nitrification describes a mutualistic symbiosis where NOB depend on nitrite produced by ammonia oxidizers, which benefit from nitrite detoxification by NOB [92]. This results in a close juxtaposition of nitrifiers in biofilms (Figure I). However, the interactions in these aggregates may be more complex. Nitrifying bacteria have a high demand for iron as cofactor of their heme- and [FeS] cluster-containing enzymes, which is satisfied by iron uptake with siderophores. Surprisingly, the genome of the AOB Nitrosomonas europaea contains no siderophore biosynthesis pathways but encodes siderophore receptors [93]. In contrast, the NOB Nitrobacter and Nitrospira can produce siderophores [55, 56, 94]. Hence, for iron acquisition N. europaea likely utilizes siderophores secreted by NOB or other organisms [50, 93] (Figure I). However, smaller genetic inventories for iron transport and the presence of putative siderophore biosynthesis genes in other AOB [95, 96] indicate that they possess different iron acquisition strategies. Other interactions might result from nitrite reduction to nitric oxide (NO). As NO triggers biofilm formation in N. europaea [97], NOB releasing NO might recruit AOB cells to form nitrification aggregates [50]. Genes of copper-dependent dissimilatory nitrite reductase (nirK) occur in all sequenced Nitrobacter, Nitrococcus, Nitrospira and Nitrospina genomes, suggesting that these NOB can produce NO. Interestingly, NO might act as electron flux regulator in Nitrobacter [98]. In the metabolism of ammonia oxidizers, NO is formed during nitrifier denitrification [99] and NO is an essential intermediate of ammonia oxidation in AOA [100]. Thus, NO released in nitrification aggregates might modulate the mode of growth and the metabolism of both symbionts in favor of the mutualistic interaction (Figure I). Recently, Nitrobacter winogradskyi was found to produce quorum sensing autoinducers (N-acyl homoserine lactones) depending on the cell density and growth phase [101]. Quorum sensing could trigger physiological changes in Nitrobacter at the high cell densities in nitrification aggregates. Diffusible autoinducers might even enable interspecies communication between nitrifiers [101] (Figure I). The EPS matrix around a nitrification aggregate likely is produced by both partners [50] (Figure I). Transcriptomics revealed differential gene expression in co-cultured N. europaea and N. winogradskyi compared to single cultures and suggested multiple physiological effects in the presence of both partners, such as reduced NO2- stress in N. europaea and less NH4+ stress in N. winogradskyi [102]. Interactions between nitrifiers have been studied for a few AOB and NOB strains. Future research should include different nitrifiers (especially AOA) to determine whether the known interactions are generally representative and to possibly reveal novel aspects of this symbiosis.
Figure I.
Visualization of a nitrification aggregate formed by AOB and NOB cell clusters in biofilm (A) and a close-up with interactions as described in [50] and reciprocal feeding with urea or cyanate [54, 57] (B). QS, quorum sensing.
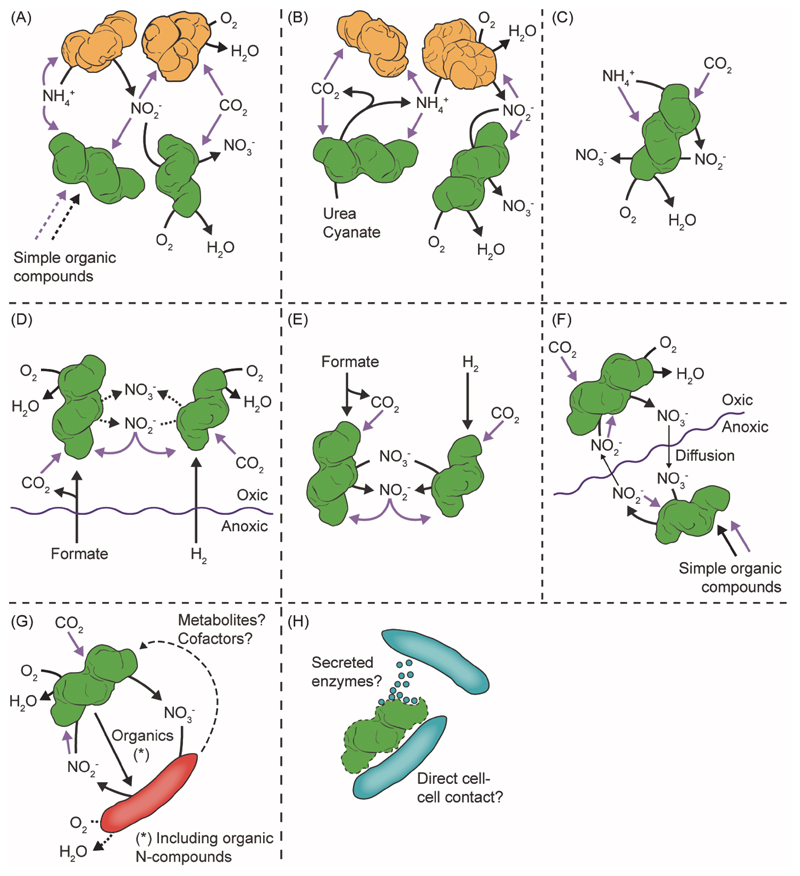
Figure 3. Potential Lifestyles and Microbe-Microbe Interactions of NOB.
Depicted are the known physiologies and interactions of Nitrospira (green). Black arrows indicate catabolic reactions or the exchange of compounds, purple arrows indicate assimilation. Dotted arrows indicate facultative reactions. Curled lines depict the oxycline. In all panels except (F), multiple nitrifier cells are only shown to facilitate illustration and the respective reactions may occur in every AOB or NOB cell. AOB are drawn in orange. (A) Canonical nitrification with possible mixotrophy. (B) Nitrification by reciprocal feeding. (C) Complete nitrification by comammox Nitrospira. Cells probably do not release nitrite at low ammonia concentrations [79]. (D) Utilization of formate or H2 (produced by fermenting anaerobes) at oxic-anoxic interfaces. With formate, nitrate can be used as electron acceptor in addition to O2. Nitrite can be oxidized in parallel to formate or H2 oxidation. Formate degradation yields CO2 as carbon source (chemoorganoautotrophy). (E) Utilization of formate or H2 in anoxia with nitrate as electron acceptor. (F) Aerobic nitrite oxidation supported by anaerobic nitrate reduction (and vice versa) by NOB sub-populations living on either side of the oxycline (‘ping-pong mechanism’). Nitrate reduction is fueled by organic substrates. (G) Interaction of NOB with nitrate-reducing heterotrophs (red). NOB may utilize metabolites from heterotrophs to compensate for lacking biosynthetic pathways and to reduce costs for biosynthesis. Heterotrophs may respire nitrate and O2 under microoxic conditions. (H) Nitrospira attacked by predatory Micavibrio-like bacteria (blue). It is unknown whether the predators destroy Nitrospira cells with exoenzymes or feed on Nitrospira through direct cell-cell contact.
Intriguingly, this phenomenon is not restricted to urea as substrate. The known NOB possess the enzyme cyanase that converts cyanate ([OCN]-) to NH3 and CO2 [55–58]. Within cells cyanate is formed mainly from spontaneous degradation of carbamoylphosphate [59]. Environmental cyanate pools result from the physicochemical decomposition of urea or cyanide [60]. In addition, cyanate formation during enzymatic degradation of thiocyanate [61] and possibly cyanide has been reported. As revealed by a new method to quantify cyanate, its concentrations in oligotrophic seawater can reach the same nanomolar range as the ammonium concentrations [60]. Since all known aerobic ammonia oxidizers for which genome sequences have been published except one AOA strain [57] lack cyanase, reciprocal feeding with cyanate may occur frequently (Figure 3B). Indeed, a co-culture of the AOB Nitrosomonas nitrosa with cyanase-positive N. moscoviensis nitrified cyanate to nitrate by reciprocal feeding and formed nitrification aggregates [57]. Future research should address the ecological significance of reciprocal feeding and of cyanate as a substrate for nitrification.
Organic compounds produced by the chemolithoautotrophic NOB are substrates for heterotrophic microorganisms [62, 63] (Figure 3G). For example, a Nitrospira population fed a multispecies heterotrophic community during years of cultivation in mineral nitrite medium [64]. NOB may also benefit from the presence of heterotrophs, for example via heterotrophic nitrate reduction (Figure 3G). In granular sludge, nitrate produced by NOB may be reduced back to nitrite by heterotrophs in anoxic zones of the granules. After diffusion into the oxic zone, this nitrite could be used by NOB in addition to nitrite from ammonia oxidation [65]. In marine OMZ the in situ nitrite oxidation rates exceeded the ammonia oxidation rates at some depths, suggesting that nitrite oxidation was coupled to nitrate reduction [19, 21]. The reoxidation of nitrite back to nitrate by NOB may reduce the amount of nitrogen loss through the anammox and denitrification pathways in OMZ [19]. If the NOB themselves are facultative nitrate reducers, sub-populations living on either side of the oxycline in narrow oxic-anoxic transition zones (e.g., in biofilms, particles and sediments) can even provide each other with nitrite or nitrate in a ‘ping-pong’ interaction [65, 66] (Figure 3F). The supply with additional nitrite from nitrate reduction may sometimes explain higher in situ abundances of NOB than of ammonia oxidizers (e.g., [65]). Difficulties to purify NOB from enrichments containing also heterotrophs in mineral nitrite media [28] suggest additional but unknown interactions. For example, we hypothesize that NOB utilize cofactors released by heterotrophs (Figure 3G). However, NOB-heterotroph symbioses are not always peaceful: a highly specific antagonistic interaction was described for Nitrospira and an alphaproteobacterial predator related to the genus Micavibrio. In activated sludge, the putative predator attacked and destroyed cell aggregates of lineage I Nitrospira (Figure 3H). Lineage II Nitrospira and other nitrifiers were not affected [67].
Alternative Metabolisms of NOB
Until recently, only few metabolic activities of NOB were known aside from chemolithoautotrophic nitrite oxidation. Some Nitrobacter and Nitrospira members benefit from the presence of simple organic compounds in addition to nitrite and CO2 [23, 68, 69] (Figure 3A), and some Nitrobacter strains can grow completely heterotrophically [70]. Still, the presence of NOB in the environment has generally been linked to nitrification.
This picture changed when new genome analyses revealed the true metabolic potential of environmentally widespread NOB. The genome of N. moscoviensis encodes a group 2a [NiFe] hydrogenase and accessory proteins involved in the maturation and transcriptional regulation of hydrogenases [71]. Based on this finding, N. moscoviensis was incubated with H2 as sole energy source and electron donor, O2 as electron acceptor, and CO2 as carbon source. The organism grew by aerobic respiration of H2, and the first chemolithoautotrophic lifestyle of a nitrifier outside the nitrogen cycle was discovered [71] (Figure 3D). Hydrogen was consumed at environmentally relevant nanomolar concentrations [71], but the lowest H2 level utilized by N. moscoviensis remains to be determined. N. moscoviensis consumed H2 also anaerobically with nitrate as electron acceptor, but growth was not detected [72] (Figure 3E).
Consistent with its genetic inventory for the uptake and oxidation of formate, N. moscoviensis grew aerobically on formate [54] (Figure 3D). Under anoxic conditions, nitrate was reduced to nitrite with formate as electron donor [54] (Figure 3E). Since N. moscoviensis lacks any canonical nitrate reductase, NXR must operate in the reverse direction to reduce nitrate [54]. However, nitrate reduction by the periplasmic NXR of Nitrospira (Box 1) consumes protons in the periplasmic space and does not contribute to energy conservation, unless NXR would contain a protonmotive quinone-cycle mechanism as predicted for archaeal periplasmic nitrate reductases [73] or would receive electrons not from quinol but from cytochrome c, so that respiratory complex III could contribute to energy conservation. More insight into the composition of the NXR protein complex is needed to assess its bioenergetic properties. N. moscoviensis should gain energy from anaerobic formate oxidation at least by the proton-pumping activity of respiratory complex I, because its soluble formate dehydrogenase likely uses NAD+ as electron acceptor [54]. Formate-driven nitrate reduction occurred even in oxic incubations, and the produced nitrite was re-oxidized to nitrate with O2 as the electron acceptor [54] (Figure 3D). Thus, N. moscoviensis simultaneously performed three metabolic reactions: formate oxidation with O2 and nitrate as electron acceptors, and aerobic nitrite oxidation. Such versatility should be advantageous for organisms that thrive in oxic-anoxic transition zones as found in biofilms, flocs, sediments, and soils. Close to the oxycline, aerobic and anaerobic processes occur next to each other and multiple substrates are available (Figure 3D). Oxygen-sensitive key pathways, such as the reverse tricarboxylic acid cycle for CO2 fixation [55], indicate a microaerophilic lifestyle of Nitrospira. This could explain the tendency of Nitrospira to form flocs and biofilms, which may offer protection against oxygen in aerated laboratory cultures and WWTPs [41, 74, 75].
N. moscoviensis belongs to the Nitrospira lineage II, a diverse and globally widespread NOB group [23] (Figure 2). Another lineage II member, Nitrospira japonica, also grew on formate [13] and uncultured Nitrospira in activated sludge utilized formate in the presence or absence of nitrite [33]. Growth on formate was also observed for Nitrobacter and Nitrolancea hollandica [18, 76]. Moreover, loci encoding group 3b or group 1h/5 [NiFe] hydrogenases [77] occur in the genomes of Nitrospina gracilis and Nitrolancea hollandica, respectively [18, 54, 58]. We hypothesize that metabolic versatility, such as growth on H2 or formate, is a common feature of NOB. Keeping this in mind, it is tempting to speculate that nitrite oxidation is not the primary lifestyle of all NOB and that at least some of these organisms have other major activities, also depending on the current environmental conditions in their habitat. For example, high cell densities of Nitrospina and Nitrococcus were found in the Namibian OMZ at sites with very low in situ nitrite oxidation activities, indicating that these organisms may not be obligate aerobic NOB [19] although their energy source, electron donor, and electron acceptor remain to be identified.
Discovery of Comammox: a Paradigm Shift
The catalysis of the two nitrification steps by two separate groups of organisms - the ammonia oxidizers and the NOB - has been a central hypothesis of nitrification research and textbook knowledge in microbiology. However, it is a puzzling phenomenon. The higher energy yield of complete nitrification (ΔG°′=-349 kJ mol−1 NH3) compared to either single step (ΔG°′=-275 kJ mol−1 NH3 and ΔG°′=-74 kJ mol−1 NO2-) should enable a complete nitrifier, which converts ammonia to nitrate, to outcompete the canonical ‘incomplete’ nitrifiers. A theoretical analysis [78] predicted hypothetical complete ammonia oxidizers tentatively called ‘comammox’ to be especially competitive in biofilms with limited substrate influx. However, no such organism was found since Winogradsky’s discovery of the first nitrifying bacteria in the late 19th century.
This situation changed with the recent, surprising discovery of members of the genus Nitrospira that catalyze complete nitrification [79, 80] (Figure 3C). In contrast to other Nitrospira and all other known organisms, these comammox bacteria possess the full genetic complement for both ammonia and nitrite oxidation. Like the canonical AOB, they utilize the enzymes ammonia monooxygenase (AMO) and hydroxylamine dehydrogenase (HAO) for ammonia oxidation although their forms are distinct from the homologs in AOB [79, 80]. The NXR of comammox is highly similar to the NXRs of strictly nitrite-oxidizing Nitrospira. The first identified comammox Nitrospira were enriched from a trickling filter connected to an aquaculture system [80] and from a moderately thermophilic biofilm submerged in hot groundwater from a 1,200 m deep abandoned oil borehole [79]. The presence of comammox-like amoA, amoB, amoC and hao genes in many published metagenomic datasets, and analyses of new metagenomes, suggested that comammox Nitrospira are widespread in soil and freshwater ecosystems, WWTPs, and drinking water treatment systems [79–82]. Intriguingly, comammox turned out to be the dominant ammonia oxidizer in a full-scale WWTP [79]. These results, and the phylogenetic placement of the known comammox in the widespread Nitrospira lineage II [79–81], suggest that comammox Nitrospira are common nitrifiers in natural and engineered microbial communities. The presence of comammox might explain unexpected observations, for example that DNA from Nitrospira was much stronger 13C-labeled than DNA from AOB and AOA after incubations of paddy soils under nitrifying conditions with 13CO2 [83]. Surprisingly high abundances of Nitrospira, which exceed the amounts of AOB and AOA in the same habitat, can be an indication of comammox [79–82] or might result from growth of Nitrospira by alternative metabolisms (see above). Comammox activity might be one reason for low environmental nitrite concentrations if comammox Nitrospira do not release nitrite during complete nitrification. However, low nitrite levels could also result from a tight coupling of canonical ammonia and nitrite oxidation (like in the nitrification aggregate, Box 3) and from nitrite consumption by denitrification and anaerobic ammonium oxidation (Figure 1).
The discovery of comammox raised many fundamental questions. For example, why does complete nitrification occur in these organisms but not in the other known nitrifiers? Was the activity of canonical nitrifiers in various environments overestimated over decades because comammox had been overlooked? Does comammox contribute to N2O formation in natural and engineered systems? Under which conditions does comammox outcompete other nitrifiers because of the higher energy yield per ammonia oxidized completely to nitrate? Physiological data, including the kinetics and the growth rate and yield of comammox, are urgently needed for understanding the ecological niche of comammox and for modeling nitrification in microbial communities with different relative proportions of comammox and incomplete nitrifiers. In parallel, the abundance of comammox and its contribution to nitrification in various ecosystems must be assessed. Insights into the biochemistry and regulation of complete nitrification may reveal molecular adaptations needed to enable the full pathway in one organism. Earlier studies on nitrification, which could not take comammox into account, might need reevaluation in the light of the new results. From a practical perspective, the effects of nitrification-inhibiting or toxic compounds on comammox should be studied to optimize nitrification management in agriculture and WWTPs. So far, comammox has been identified in environmental samples by metagenomics. To facilitate further research, methods are needed to more efficiently detect and quantify comammox Nitrospira, which cannot be distinguished from the strictly nitrite-oxidizing Nitrospira in 16S rRNA-, nxrA- or nxrB-based phylogenies [79–81]. We also need tools to specifically measure the nitrification activity of comammox in environmental samples.
Concluding Remarks
Recent advances in nitrification research have dramatically changed our perspective on NOB by revealing new and unexpected biological features. These findings suggest that the presence of NOB in environmental samples cannot simply be associated with nitrite oxidation. One of the core questions for future studies is whether nitrite oxidation really is the primary lifestyle of these organisms (see Outstanding Questions). The discovery of comammox further complicates the picture, and collectively the new results call for a reevaluation of the ecological roles played by NOB. Human activities severely affect the nitrogen cycle, which has already been altered more than any other basic element cycle on Earth [84]. The nitrogen cycle is recognized as one of the critical Earth system processes, for which a high-risk level of anthropogenic perturbation has been reached [85]. However, we still face an increasing use of nitrogen fertilizers to feed the growing human population and increasing loads of nitrogen-polluted sewage in urban areas. It will be essential to understand how the core microbial drivers and regulators of the nitrogen cycle, such as NOB, respond to these alterations and possible countermeasures. Therefore, we urgently need much more insight into the niche specialization, population dynamics, physiological limits, and interactions of NOB. The recent progress outlined in this review, and the modern molecular tools of microbial ecology, are a perfect starting point to bridge our knowledge gaps and eventually make NOB a main focus of nitrification research.
Trends Box.
Nitrite-oxidizing bacteria (NOB) are key players in the biogeochemical nitrogen cycle. They are a phylogenetically diverse guild with pronounced ecological niche specialization and differ from each other in fundamental physiological and molecular traits.
NOB are involved in complex symbioses with ammonia-oxidizing and heterotrophic microorganisms. In a new type of interaction called reciprocal feeding, NOB recruit ammonia oxidizers to use urea or cyanate as energy source.
NOB are surprisingly versatile and can switch between nitrite oxidation and alternate metabolisms such as H2 or formate oxidation. Thus, NOB may have diverse ecological functions within and beyond the nitrogen cycle.
The unexpected discovery of complete ammonia oxidizers (‘comammox’) in the NOB genus Nitrospira will have broad implications for future research on nitrification and the nitrogen cycle.
Acknowledgements
We thank Elena Lebedeva, Eberhard Bock, Andreas Schramm, Per Nielsen, Eva Spieck, Marcel Kuypers, Hanna Koch, Christiane Gruber-Dorninger, and Maartje van Kessel for long-term collaboration. The authors’ research described in this review was supported by the Austrian Science Fund, FWF (grants no. P25231-B21, P27319-B21, and P24101-B22), the Vienna Science and Technology Fund (WWTF, grants no. LS09-40 and LS216), the European Research Council Advanced Grant project NITRICARE (no. 294343), and the Netherlands Organization for Scientific Research (NWO, grant no. 863.14.019).
References
- 1.Winogradsky S. Arch Sci Biol. Vol. 1. St. Petersb: 1892. Contributions a la morphologie des organismes de la nitrification; pp. 88–137. [Google Scholar]
- 2.Dworkin M. Sergei Winogradsky: a founder of modern microbiology and the first microbial ecologist. FEMS Microbiol Rev. 2012;36:364–379. doi: 10.1111/j.1574-6976.2011.00299.x. [DOI] [PubMed] [Google Scholar]
- 3.Bock E, Wagner M. Oxidation of inorganic nitrogen compounds as an energy source. In: Dworkin M, et al., editors. The Prokaryotes: A Handbook on the Biology of Bacteria. 3. edn. Springer Science+Business Media; 2001. pp. 457–495. [Google Scholar]
- 4.Kowalchuk GA, Stephen JR. Ammonia-oxidizing bacteria: a model for molecular microbial ecology. Annu Rev Microbiol. 2001;55:485–529. doi: 10.1146/annurev.micro.55.1.485. [DOI] [PubMed] [Google Scholar]
- 5.Könneke M, et al. Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature. 2005;437:543–546. doi: 10.1038/nature03911. [DOI] [PubMed] [Google Scholar]
- 6.Gruber N. The marine nitrogen cycle: Overview and challenges. In: Capone DG, et al., editors. Nitrogen in the marine environment. 2 edn. Academic Press; 2008. pp. 1–50. [Google Scholar]
- 7.Lewis WM, Morris DP. Toxicity of nitrite to fish: A review. Trans Am Fish Soc. 1986;115:183–195. [Google Scholar]
- 8.Castellani AG, Niven CF. Factors affecting the bacteriostatic action of sodium nitrite. Appl Environ Microbiol. 1955;3:154–159. doi: 10.1128/am.3.3.154-159.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albertsen M, et al. Genome sequences of rare, uncultured bacteria obtained by differential coverage binning of multiple metagenomes. Nat Biotechnol. 2013;31:533–538. doi: 10.1038/nbt.2579. [DOI] [PubMed] [Google Scholar]
- 10.Wilmes P, et al. A decade of metaproteomics: Where we stand and what the future holds. Proteomics. 2015;15:3409–3417. doi: 10.1002/pmic.201500183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wagner M. Single-cell ecophysiology of microbes as revealed by Raman microspectroscopy or secondary ion mass spectrometry imaging. Annu Rev Microbiol. 2009;63:411–429. doi: 10.1146/annurev.micro.091208.073233. [DOI] [PubMed] [Google Scholar]
- 12.Stepanauskas R. Single cell genomics: an individual look at microbes. Curr Opin Microbiol. 2012;15:613–620. doi: 10.1016/j.mib.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Ushiki N, et al. Isolation of Nitrospira belonging to sublineage II from a wastewater treatment plant. Microbes Environ. 2013;28:346–353. doi: 10.1264/jsme2.ME13042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vekeman B, et al. A generally applicable cryopreservation method for nitrite-oxidizing bacteria. Syst Appl Microbiol. 2013;36:579–584. doi: 10.1016/j.syapm.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Griffin BM, et al. Nitrite, an electron donor for anoxygenic photosynthesis. Science. 2007;316:1870. doi: 10.1126/science.1139478. [DOI] [PubMed] [Google Scholar]
- 16.Hemp J, et al. Genomics of a phototrophic nitrite oxidizer: insights into the evolution of photosynthesis and nitrification. ISME J. 2016 doi: 10.1038/ismej.2016.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ngugi DK, et al. Diversification and niche adaptations of Nitrospina-like bacteria in the polyextreme interfaces of Red Sea brines. ISME J. 2015 doi: 10.1038/ismej.2015.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sorokin D, et al. Nitrification expanded: Discovery, physiology, and genomics of a nitrite-oxidizing bacterium from the phylum Chloroflexi. ISME J. 2012;6:2245–2256. doi: 10.1038/ismej.2012.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Füssel J, et al. Nitrite oxidation in the Namibian oxygen minimum zone. ISME J. 2012;6:1200–1209. doi: 10.1038/ismej.2011.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jorgensen SL, et al. Correlating microbial community profiles with geochemical data in highly stratified sediments from the Arctic Mid-Ocean Ridge. Proc Natl Acad Sci U S A. 2012;109:E2846–E2855. doi: 10.1073/pnas.1207574109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beman JM, et al. Nitrite oxidation in the upper water column and oxygen minimum zone of the eastern tropical North Pacific Ocean. ISME J. 2013;7:2192–2205. doi: 10.1038/ismej.2013.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nunoura T, et al. Hadal biosphere: Insight into the microbial ecosystem in the deepest ocean on Earth. Proc Natl Acad Sci U S A. 2015;112:E1230–E1236. doi: 10.1073/pnas.1421816112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daims H, et al. In situ characterization of Nitrospira-like nitrite-oxidizing bacteria active in wastewater treatment plants. Appl Environ Microbiol. 2001;67:5273–5284. doi: 10.1128/AEM.67.11.5273-5284.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lebedeva EV, et al. Physiological and phylogenetical characterization of a new lithoautotrophic nitrite-oxidizing bacterium 'Candidatus Nitrospira bockiana'. Int J Syst Evol Microbiol. 2008;58:242–250. doi: 10.1099/ijs.0.65379-0. sp. nov. [DOI] [PubMed] [Google Scholar]
- 25.Lebedeva EV, et al. Isolation and characterization of a moderately thermophilic nitrite-oxidizing bacterium from a geothermal spring. FEMS Microbiol Ecol. 2011;75:195–204. doi: 10.1111/j.1574-6941.2010.01006.x. [DOI] [PubMed] [Google Scholar]
- 26.Edwards TA, et al. Cultivation and characterization of thermophilic Nitrospira species from geothermal springs in the US Great Basin, China, and Armenia. FEMS Microbiol Ecol. 2013;85:283–292. doi: 10.1111/1574-6941.12117. [DOI] [PubMed] [Google Scholar]
- 27.Marks CR, et al. Nitrospira-dominated biofilm within a thermal artesian spring: a case for nitrification-driven primary production in a geothermal setting. Geobiology. 2012;10:457–466. doi: 10.1111/j.1472-4669.2012.00335.x. [DOI] [PubMed] [Google Scholar]
- 28.Nowka B, et al. Improved isolation strategies allowed the phenotypic differentiation of two Nitrospira strains from widespread phylogenetic lineages. FEMS Microbiol Ecol. 2015;91 doi: 10.1093/femsec/fiu031. fiu031. [DOI] [PubMed] [Google Scholar]
- 29.Levipan HA, et al. Nitrospina-like bacteria are the main drivers of nitrite oxidation in the seasonal upwelling area of the Eastern South Pacific (Central Chile ~ 36°S) Environ Microbiol Rep. 2014;6:565–573. doi: 10.1111/1758-2229.12158. [DOI] [PubMed] [Google Scholar]
- 30.Vanparys B, et al. The phylogeny of the genus Nitrobacter based on comparative rep-PCR, 16S rRNA and nitrite oxidoreductase gene sequence analysis. Syst Appl Microbiol. 2007;30:297–308. doi: 10.1016/j.syapm.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 31.Poly F, et al. First exploration of Nitrobacter diversity in soils by a PCR cloning-sequencing approach targeting functional gene nxrA. FEMS Microbiol Ecol. 2008;63:132–140. doi: 10.1111/j.1574-6941.2007.00404.x. [DOI] [PubMed] [Google Scholar]
- 32.Pester M, et al. NxrB encoding the beta subunit of nitrite oxidoreductase as functional and phylogenetic marker for nitrite-oxidizing Nitrospira. Environ Microbiol. 2014;16:3055–3071. doi: 10.1111/1462-2920.12300. [DOI] [PubMed] [Google Scholar]
- 33.Gruber-Dorninger C, et al. Functionally relevant diversity of closely related Nitrospira in activated sludge. ISME J. 2015;9:643–655. doi: 10.1038/ismej.2014.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Juretschko S, et al. Combined molecular and conventional analyses of nitrifying bacterium diversity in activated sludge: Nitrosococcus mobilis and Nitrospira-like bacteria as dominant populations. Appl Environ Microbiol. 1998;64:3042–3051. doi: 10.1128/aem.64.8.3042-3051.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kruse M, et al. The nitrite-oxidizing community in activated sludge from a municipal wastewater treatment plant determined by fatty acid methyl ester-stable isotope probing. Syst Appl Microbiol. 2013;36:517–524. doi: 10.1016/j.syapm.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 36.Alawi M, et al. Temperature influences the population structure of nitrite-oxidizing bacteria in activated sludge. Environ Microbiol Reports. 2009;1:184–190. doi: 10.1111/j.1758-2229.2009.00029.x. [DOI] [PubMed] [Google Scholar]
- 37.Lücker S, et al. Nitrotoga-like bacteria are previously unrecognized key nitrite oxidizers in full-scale wastewater treatment plants. ISME J. 2015;9:708–720. doi: 10.1038/ismej.2014.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saunders AM, et al. The activated sludge ecosystem contains a core community of abundant organisms. ISME J. 2016;10:11–20. doi: 10.1038/ismej.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hüpeden J, et al. Relative abundance of Nitrotoga in a biofilter of a cold freshwater aquaculture plant appears to be stimulated by slightly acidic pH. Appl Environ Microbiol. 2016;82:1838–1845. doi: 10.1128/AEM.03163-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alawi M, et al. Cultivation of a novel cold-adapted nitrite oxidizing betaproteobacterium from the Siberian Arctic. ISME J. 2007;1:256–264. doi: 10.1038/ismej.2007.34. [DOI] [PubMed] [Google Scholar]
- 41.Spieck E, et al. Selective enrichment and molecular characterization of a previously uncultured Nitrospira-like bacterium from activated sludge. Environ Microbiol. 2006;8:405–415. doi: 10.1111/j.1462-2920.2005.00905.x. [DOI] [PubMed] [Google Scholar]
- 42.Park HD, Noguera DR. Nitrospira community composition in nitrifying reactors operated with two different dissolved oxygen levels. J Microbiol Biotechnol. 2008;18:1470–1474. [PubMed] [Google Scholar]
- 43.Kalvelage T, et al. Nitrogen cycling driven by organic matter export in the South Pacific oxygen minimum zone. Nat Geosci. 2013;6:228–234. [Google Scholar]
- 44.Casciotti KL, Buchwald C. Insights on the marine microbial nitrogen cycle from isotopic approaches to nitrification. Front Microbiol. 2012;3:356. doi: 10.3389/fmicb.2012.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lam P, Kuypers MMM. Microbial nitrogen cycling processes in oxygen minimum zones. In: Carlson CA, Giovannoni SJ, editors. Annu Rev Mar Sci. Annual Reviews; 2011. pp. 317–345. [DOI] [PubMed] [Google Scholar]
- 46.Kim DJ, Kim SH. Effect of nitrite concentration on the distribution and competition of nitrite-oxidizing bacteria in nitratation reactor systems and their kinetic characteristics. Water Res. 2006;40:887–894. doi: 10.1016/j.watres.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 47.Simonin M, et al. Coupling between and among ammonia oxidizers and nitrite oxidizers in grassland mesocosms submitted to elevated CO2 and nitrogen supply. Microb Ecol. 2015;70:809–818. doi: 10.1007/s00248-015-0604-9. [DOI] [PubMed] [Google Scholar]
- 48.Maixner F, et al. Nitrite concentration influences the population structure of Nitrospira-like bacteria. Environ Microbiol. 2006;8:1487–1495. doi: 10.1111/j.1462-2920.2006.01033.x. [DOI] [PubMed] [Google Scholar]
- 49.Daebeler A, et al. Interactions between Thaumarchaea, Nitrospira and methanotrophs modulate autotrophic nitrification in volcanic grassland soil. ISME J. 2014;8:2397–2410. doi: 10.1038/ismej.2014.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arp D, Bottomley PJ. Nitrifiers: More than 100 years from isolation to genome sequences. Microbe. 2006;1:229–234. [Google Scholar]
- 51.Pommerening-Röser A, Koops HP. Environmental pH as an important factor for the distribution of urease positive ammonia-oxidizing bacteria. Microbiol Res. 2005;160:27–35. doi: 10.1016/j.micres.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 52.Burton SAQ, Prosser JI. Autotrophic ammonia oxidation at low pH through urea hydrolysis. Appl Environ Microbiol. 2001;67:2952–2957. doi: 10.1128/AEM.67.7.2952-2957.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alonso-Saez L, et al. Role for urea in nitrification by polar marine Archaea. Proc Natl Acad Sci U S A. 2012;109:17989–17994. doi: 10.1073/pnas.1201914109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koch H, et al. Expanded metabolic versatility of ubiquitous nitrite-oxidizing bacteria from the genus Nitrospira. Proc Natl Acad Sci U S A. 2015;112:11371–11376. doi: 10.1073/pnas.1506533112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lücker S, et al. A Nitrospira metagenome illuminates the physiology and evolution of globally important nitrite-oxidizing bacteria. Proc Natl Acad Sci U S A. 2010;107:13479–13484. doi: 10.1073/pnas.1003860107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Starkenburg SR, et al. Genome sequence of the chemolithoautotrophic nitrite-oxidizing bacterium Nitrobacter winogradskyi Nb-255. Appl Environ Microbiol. 2006;72:2050–2063. doi: 10.1128/AEM.72.3.2050-2063.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Palatinszky M, et al. Cyanate as an energy source for nitrifiers. Nature. 2015;524:105–108. doi: 10.1038/nature14856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lücker S, et al. The genome of Nitrospina gracilis illuminates the metabolism and evolution of the major marine nitrite oxidizer. Frontiers Microbiol. 2013;4:27. doi: 10.3389/fmicb.2013.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Suzuki I, et al. Regulation by cyanate of the genes involved in carbon and nitrogen assimilation in the cyanobacterium Synechococcus sp strain PCC 7942. J Bacteriol. 1996;178:2688–2694. doi: 10.1128/jb.178.9.2688-2694.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Widner B, et al. Chromatographic determination of nanomolar cyanate concentrations in estuarine and sea waters by precolumn fluorescence derivatization. Anal Chem. 2013;85:6661–6666. doi: 10.1021/ac400351c. [DOI] [PubMed] [Google Scholar]
- 61.Watts MP, Moreau JW. New insights into the genetic and metabolic diversity of thiocyanate-degrading microbial consortia. Appl Microbiol Biotechnol. 2016;100:1101–1108. doi: 10.1007/s00253-015-7161-5. [DOI] [PubMed] [Google Scholar]
- 62.Rittmann BE, et al. Nitrification as a source of soluble organic substrate in biological treatment. Water Sci Technol. 1994;30:1–8. [Google Scholar]
- 63.Okabe S, et al. Fate of 14C-labeled microbial products derived from nitrifying bacteria in autotrophic nitrifying biofilms. Appl Environ Microbiol. 2005;71:3987–3994. doi: 10.1128/AEM.71.7.3987-3994.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dolinšek J, et al. Depletion of unwanted nucleic acid templates by selective cleavage: LNAzymes open a new window for detecting rare microbial community members. Appl Environ Microbiol. 2013;79:1534–1544. doi: 10.1128/AEM.03392-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Winkler MKH, et al. Unravelling the reasons for disproportion in the ratio of AOB and NOB in aerobic granular sludge. Appl Microbiol Biotechnol. 2012;94:1657–1666. doi: 10.1007/s00253-012-4126-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Freitag A, et al. Growth of Nitrobacter by dissimilatoric nitrate reduction. FEMS Microbiol Lett. 1987;48:105–109. [Google Scholar]
- 67.Dolinšek J, et al. Interactions of nitrifying bacteria and heterotrophs: Identification of a Micavibrio-like, putative predator of Nitrospira. Appl Environ Microbiol. 2013;79:2027–2037. doi: 10.1128/AEM.03408-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Watson SW, et al. Nitrospira marina gen. nov. sp. nov.: a chemolithotrophic nitrite-oxidizing bacterium. Arch Microbiol. 1986;144:1–7. [Google Scholar]
- 69.Bock E, et al. A new facultatively nitrite oxidizing bacterium, Nitrobacter vulgaris sp. nov. Arch Microbiol. 1990;153:105–110. [Google Scholar]
- 70.Bock E. Growth of Nitrobacter in the presence of organic matter. II. Chemoorganotrophic growth of Nitrobacter agilis. Arch Microbiol. 1976;108:305–312. doi: 10.1007/BF00454857. [DOI] [PubMed] [Google Scholar]
- 71.Koch H, et al. Growth of nitrite-oxidizing bacteria by aerobic hydrogen oxidation. Science. 2014;345:1052–1054. doi: 10.1126/science.1256985. [DOI] [PubMed] [Google Scholar]
- 72.Ehrich S, et al. A new obligately chemolithoautotrophic, nitrite-oxidizing bacterium, Nitrospira moscoviensis sp. nov. and its phylogenetic relationship. Arch Microbiol. 1995;164:16–23. doi: 10.1007/BF02568729. [DOI] [PubMed] [Google Scholar]
- 73.Martinez-Espinosa RM, et al. Look on the positive side! The orientation, identification and bioenergetics of 'Archaeal' membrane-bound nitrate reductases. FEMS Microbiol Lett. 2007;276:129–139. doi: 10.1111/j.1574-6968.2007.00887.x. [DOI] [PubMed] [Google Scholar]
- 74.Okabe S, et al. In situ analysis of nitrifying biofilms as determined by in situ hybridization and the use of microelectrodes. Appl Environ Microbiol. 1999;65:3182–3191. doi: 10.1128/aem.65.7.3182-3191.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Almstrand R, et al. New methods for analysis of spatial distribution and coaggregation of microbial populations in complex biofilms. Appl Environ Microbiol. 2013;79:5978–5987. doi: 10.1128/AEM.01727-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Van Gool A, Laudelout H. Formate utilization by Nitrobacter winogradskyi. Biochim Biophys Acta. 1966;127:295–301. doi: 10.1016/0304-4165(66)90384-9. [DOI] [PubMed] [Google Scholar]
- 77.Greening C, et al. Genomic and metagenomic surveys of hydrogenase distribution indicate H2 is a widely utilised energy source for microbial growth and survival. ISME J. 2016;10:761–777. doi: 10.1038/ismej.2015.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Costa E, et al. Why is metabolic labour divided in nitrification? Trends Microbiol. 2006;14:213–219. doi: 10.1016/j.tim.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 79.Daims H, et al. Complete nitrification by Nitrospira bacteria. Nature. 2015;528:504–509. doi: 10.1038/nature16461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.van Kessel MAHJ. Complete nitrification by a single microorganism. Nature. 2015;528:555–559. doi: 10.1038/nature16459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pinto AJ, et al. Metagenomic evidence for the presence of comammox Nitrospira-like bacteria in a drinking water system. mSphere. 2015;1:e00054–00015. doi: 10.1128/mSphere.00054-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Palomo A, et al. Metagenomic analysis of rapid gravity sand filter microbial communities suggests novel physiology of Nitrospira spp. ISME J. 2016 doi: 10.1038/ismej.2016.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang BZ, et al. Differential contributions of ammonia oxidizers and nitrite oxidizers to nitrification in four paddy soils. ISME J. 2015;9:1062–1075. doi: 10.1038/ismej.2014.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fields S. Global nitrogen - Cycling out of control. Environ Health Perspect. 2004;112:A556–A563. doi: 10.1289/ehp.112-a556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Steffen W, et al. Planetary boundaries: Guiding human development on a changing planet. Science. 2015 doi: 10.1126/science.1259855. [DOI] [PubMed] [Google Scholar]
- 86.Sundermeyer-Klinger H. Membrane-bound nitrite oxidoreductase of Nitrobacter: evidence for a nitrate reductase system. Arch Microbiol. 1984;140:153–158. [Google Scholar]
- 87.Spieck E, et al. Isolation and immunocytochemical location of the nitrite-oxidizing system in Nitrospira moscoviensis. Arch Microbiol. 1998;169:225–230. doi: 10.1007/s002030050565. [DOI] [PubMed] [Google Scholar]
- 88.Spieck E, et al. Immunocytochemical detection and location of the membrane-bound nitrite oxidoreductase in cells of Nitrobacter and Nitrospira. FEMS Microbiol Lett. 1996;139:71–76. [Google Scholar]
- 89.Schramm A, et al. Microscale distribution of populations and activities of Nitrosospira and Nitrospira spp. along a macroscale gradient in a nitrifying bioreactor: quantification by in situ hybridization and the use of microsensors. Appl Environ Microbiol. 1999;65:3690–3696. doi: 10.1128/aem.65.8.3690-3696.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nowka B, et al. Comparison of oxidation kinetics of nitrite-oxidizing bacteria: nitrite availability as a key factor in niche differentiation. Appl Environ Microbiol. 2015;81:745–753. doi: 10.1128/AEM.02734-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nogueira R, Melo LF. Competition between Nitrospira spp. and Nitrobacter spp. in nitrite-oxidizing bioreactors. Biotechnol Bioeng. 2006;95:169–175. doi: 10.1002/bit.21004. [DOI] [PubMed] [Google Scholar]
- 92.Stein LY, Arp DJ. Loss of ammonia monooxygenase activity in Nitrosomonas europaea upon exposure to nitrite. Appl Environ Microbiol. 1998;64:4098–4102. doi: 10.1128/aem.64.10.4098-4102.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chain P, et al. Complete genome sequence of the ammonia-oxidizing bacterium and obligate chemolithoautotroph Nitrosomonas europaea. J Bacteriol. 2003;185:2759–2773. doi: 10.1128/JB.185.9.2759-2773.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Starkenburg SR, et al. Complete genome sequence of Nitrobacter hamburgensis X14 and comparative genomic analysis of species within the genus Nitrobacter. Appl Environ Microbiol. 2008;74:2852–2863. doi: 10.1128/AEM.02311-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stein LY, et al. Whole-genome analysis of the ammonia-oxidizing bacterium, Nitrosomonas eutropha C91: implications for niche adaptation. Environ Microbiol. 2007;9:2993–3007. doi: 10.1111/j.1462-2920.2007.01409.x. [DOI] [PubMed] [Google Scholar]
- 96.Norton JM, et al. Complete genome sequence of Nitrosospira multiformis, an ammonia-oxidizing bacterium from the soil environment. Appl Environ Microbiol. 2008;74:3559–3572. doi: 10.1128/AEM.02722-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schmidt I, et al. Physiologic and proteomic evidence for a role of nitric oxide in biofilm formation by Nitrosomonas europaea and other ammonia oxidizers. J Bacteriol. 2004;186:2781–2788. doi: 10.1128/JB.186.9.2781-2788.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Starkenburg SR, et al. Expression of a putative nitrite reductase and the reversible inhibition of nitrite-dependent respiration by nitric oxide in Nitrobacter winogradskyi Nb-255. Environ Microbiol. 2008;10:3036–3042. doi: 10.1111/j.1462-2920.2008.01763.x. [DOI] [PubMed] [Google Scholar]
- 99.Stein LY. Heterotrophic nitrification and nitrifier denitrification. In: Ward BB, et al., editors. Nitrification. ASM Press; 2011. pp. 95–114. [Google Scholar]
- 100.Kozlowski JA, et al. Pathways and key intermediates required for obligate aerobic ammonia-dependent chemolithotrophy in bacteria and Thaumarchaeota. ISME J. 2016 doi: 10.1038/ismej.2016.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mellbye BL, et al. Nitrite-oxidizing bacterium Nitrobacter winogradskyi produces N-acyl-homoserine lactone autoinducers. Appl Environ Microbiol. 2015;81:5917–5926. doi: 10.1128/AEM.01103-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Perez J, et al. Interactions of Nitrosomonas europaea and Nitrobacter winogradskyi grown in co-culture. Arch Microbiol. 2015;197:79–89. doi: 10.1007/s00203-014-1056-1. [DOI] [PubMed] [Google Scholar]
- 103.Woese CR, et al. The phylogeny of the purple bacteria: the alpha subdivision. System Appl Microbiol. 1984;5:315–326. doi: 10.1016/s0723-2020(84)80034-x. [DOI] [PubMed] [Google Scholar]
- 104.Watson SW, Waterbury JB. Characteristics of two marine nitrite oxidizing bacteria, Nitrospina gracilis nov. gen. nov. sp. and Nitrococcus mobilis nov. gen. nov. sp. Arch Mikrobiol. 1971;77:203–230. [Google Scholar]
- 105.Woese CR, et al. The phylogeny of the purple bacteria: the gamma subdivision. System Appl Microbiol. 1985;6:25–33. doi: 10.1016/s0723-2020(84)80034-x. [DOI] [PubMed] [Google Scholar]
- 106.Spieck E, et al. Characterization of a new marine nitrite oxidizing bacterium, Nitrospina watsonii sp nov., a member of the newly proposed phylum "Nitrospinae". Syst Appl Microbiol. 2014;37:170–176. doi: 10.1016/j.syapm.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 107.Quast C, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41:D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stackebrandt E, Ebers J. Taxonomic parameters revisited: tarnished gold standards. Microbiology Today. 2006;33:152–155. [Google Scholar]