Natural killer (NK) cell-mediated antibody-dependent cellular cytotoxicity (ADCC) through CD16 plays a critical role in anti-human immunodeficiency virus (HIV) responses.1–3 CD300a is a surface receptor highly expressed on NK cells that has the capacity to inhibit NK cell-mediated cytotoxicity in healthy donors.4 The CD300a molecule has been related to several viral infections and is able to diminish the NK cell killing of pseudorabies-infected cells through interactions with its ligands phosphatidylserine and phosphatidylethanolamine.5 In addition, CD300a expression on B and CD4+ T lymphocytes is altered during HIV-1 infection, and combined antiretroviral therapy (cART) does not restore nonpathological expression levels.5,6 However, the expression and function of CD300a on NK cells during HIV-1 infection is still unknown. We have determined the surface expression of CD300a on different NK cell subsets and the capacity of this receptor to inhibit CD16-induced NK cell effector functions in healthy and HIV-1 infected individuals.
As HIV-1 infection modulates CD300a expression on some immune cells,5,6 we first analyzed the expression of the CD300a receptor on different NK cell subpopulations from healthy donors, untreated HIV-1-infected subjects, and patients on cART by flow cytometry. The samples were provided by the HIV BioBank integrated in the Spanish AIDS Research Network (RIS) (see Supplementary Material). Clinical data from HIV-1-infected patients are shown in Table S1. Three different NK cell subsets were studied: CD56bright (CD56++NKp80+), CD56dim (CD56+NKp80+), and CD56neg (CD56−NKp80+) (Fig. S1). When we examined the CD300a expression, no significant differences were observed between the groups, with the exception of CD56neg NK cells, which displayed a higher frequency of CD300a+ cells in untreated HIV-1 infected patients (Fig. S2). Ongoing HIV replication induces the expansion of a dysfunctional CD56neg NK cell subset1,7,8 (Fig. S1). Thus, we suggest that the overexpression of the CD300a inhibitory receptor on the CD56neg NK cell subset may contribute to the dysfunctionality observed in this expanded population in HIV-1 infected patients, which is partially restored with cART.
We also examined CD300a expression on different NK cell subsets selected according to the expression of NKG2A, NKG2C, CD57, and NKp46 (Fig. S3). These receptors are altered during HIV-1 infection,7,8 and some of them are commonly used to distinguish NK cell maturation stages.9 In general, we observed that different CD300a expression levels were associated with the expression of these markers in all subjects (Fig. S4). Specifically, higher CD300a expression was found on NKG2A+ CD56dim NK cells, while CD57+ cells displayed lower CD300a expression levels (Fig. S4), indicating that CD300a is more expressed on immature CD56dim NK cells, a cell subset that is significantly decreased in HIV-1 infected patients.3,7
NK cells express the FcγRIIIA (CD16) surface receptor, which is responsible for ADCC.1,2 To investigate the capacity of CD300a to inhibit CD16-mediated NK cell activation in HIV-1-infected patients, we performed a redirected lysis assay (Fig. S5). We cocultured NK cells with the Fc receptor-bearing cell line P815. CD16 and CD300a from NK cells were triggered with specific mAbs, and the MOPC-21 isotype control was utilized as a negative control (Fig. S5). To study NK cell effector functions, we determined the percentage of NK cells positive for the degranulation marker CD107a, the cytokines interferon (IFN)γ and tumor necrosis factor (TNF), and the chemokine macrophage inflammatory protein (MIP)-1β utilizing flow cytometry-based procedures (see Supplementary Material).
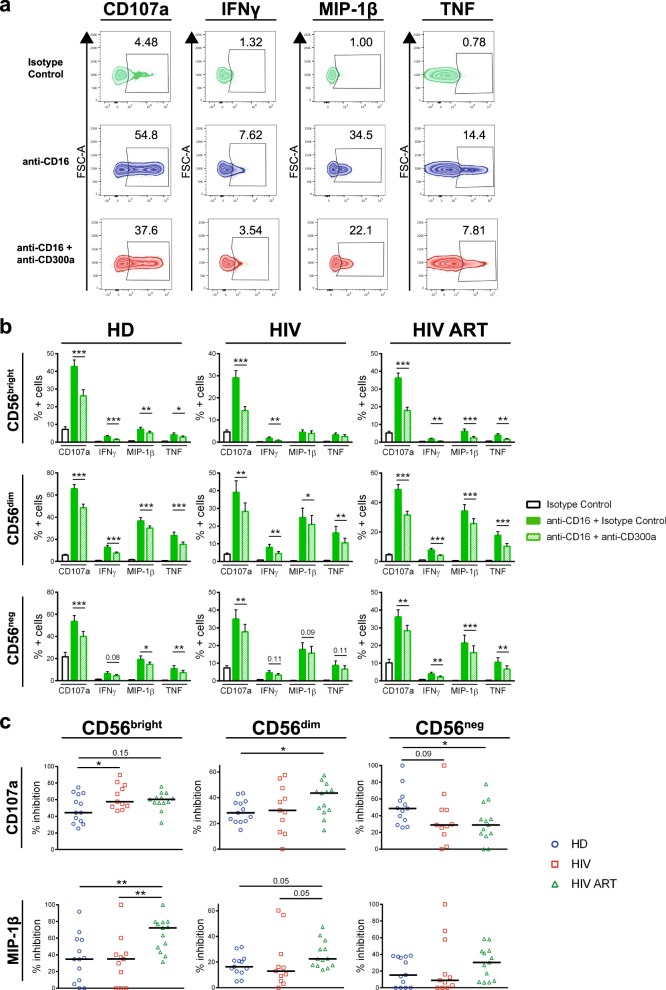
In agreement with the literature,1,3,7 the CD56dim NK cell subset displayed the highest response to CD16-mediated stimulation, and NK cell effector functions were significantly diminished in HIV-1 infected patients (Fig. S6). Very importantly, we observed that all NK cell subsets from the three groups exhibited lower effector functions after the CD16-mediated stimulation and cross-linking of CD300a with specific mAbs (Fig. 1a, b). Moreover, when we compared the degranulation and MIP-1β production by different NK cell subsets, we observed that CD56bright cells were the most inhibited subset after CD300a cross-linking in all donors (Fig. S7), consistent with the higher CD300a expression in this NK cell subpopulation (Fig. S2). Finally, we observed a higher CD300a-mediated inhibition of degranulation and MIP-1β production by CD56bright and CD56dim NK cells from HIV-1 infected patients, particularly from those who were under cART (Fig. 1c).
Fig. 1.
Degranulation and cytokine production by NK cells in response to the cocrosslinking of CD16 and CD300a. a Zebra plots showing the percentage of CD56dim NK cells positive for CD107a, IFNγ, MIP-1β, and TNF from a representative untreated HIV-1 infected patient after stimulation with isotype control, anti-CD16 plus isotype control, or anti-CD16 plus anti-CD300a mAbs. b Bar graphs showing the percentage of CD56bright, CD56dim, and CD56neg NK cells positive for CD107a, IFNγ, MIP-1β, and TNF from healthy donors (HD), untreated HIV-1 infected subjects (HIV), and subjects under cART (HIV ART) after stimulation with isotype control, anti-CD16 plus isotype control, or anti-CD16 plus anti-CD300a mAbs. The mean with the SEM is represented. c Dot plots showing the percentage of CD300a-mediated inhibition of degranulation (CD107a) and MIP-1β production by CD56bright, CD56dim, and CD56neg NK cells, comparing HD, HIV, and HIV ART patients. Each dot represents a subject, and the median is shown. *p < 0.05, **p < 0.01, ***p < 0.001
ADCC has been demonstrated as an important factor for the long-term control of HIV-1 infection that subsequently results in better disease prognosis.1–3,7 Furthermore, the relevance of ADCC in new anti-HIV therapies has been emphasized with the introduction of broadly neutralizing antibodies.2,3 Nevertheless, decreased HIV-specific effector antibody responses have been found in HIV-1-infected individuals, including those receiving cART.7,10 Our results suggest that CD300a might decrease ADCC-mediated NK cell killing of HIV-infected cells by inhibiting degranulation and cytokine and chemokine production by NK cells. Previous findings have indicated that successful cART is not enough to achieve effective antibody-mediated HIV-1 control,7,10 highlighting the importance of our study in the search for new strategies to achieve more effective ADCC in HIV-1-infected patients. Therefore, similar to other therapeutic strategies in which NKG2A and KIR inhibitory receptors are targeted with monalizumab and lirilumab, respectively, targeting CD300a could represent a new strategy to improve NK cell functions. Nonetheless, further experiments are required to confirm our hypothesis.
Supplementary information
Acknowledgements
This study was supported by grants to F.B. from Instituto de Salud Carlos III (ISCIII)-Subdirección de Evaluación y Fondo Europeo de Desarrollo Regional (FEDER) (Grant PI13/00889) and Marie-Curie Actions, Career Integration Grant, European Commission (Grant CIG 631674). J.V. and I.T. are recipients of a predoctoral contract funded by the Department of Education, Basque Government (PRE_2018_2_0211 and PRE_2018_1_0032). I.T. is the recipient of a fellowship from the Jesús de Gangoiti Barrera Foundation (FJGB17/003). O.Z. is the recipient of a postdoctoral contract funded by ISCIII-Contratos Sara Borrell (CD17/0128) and the European Social Fund. F.B. is an Ikerbasque Research Professor, Ikerbasque, Basque Foundation for Science. We would like to thank the patients in this study for their participation and the HIV BioBank integrated in the Spanish AIDS Research Network (RIS) and collaborating centers for the generous gifts of clinical samples. The HIV BioBank is supported by ISCIII, Spanish Health Ministry (Grant no. RD06/0006/0035, RD12/0017/0037, and RD16/0025/0019) as part of the Plan Nacional R+D+I and cofinanced by ISCIII-Subdirección General de Evaluación y FEDER. This study would not have been possible without the collaboration of all the patients, medical and nursery staff, and data managers who have taken part in the project. The RIS Cohort (CoRIS) is funded by the ISCIII through the RIS (RIS C03/173, RD12/0017/0018, and RD16/0002/0006) as part of the Plan Nacional R+D+I and cofinanced by ISCIII-Subdirección General de Evaluación y FEDER.
Author contributions
J.V. designed and performed the experiments, analyzed and interpreted the data, designed the figures, and wrote the manuscript. I.T. analyzed the results and made the figures. A.O. participated in the interpretation of the data. R.P.-G. determined the CMV serology and the levels of CRP and B2M. F.V., J.A.I., C.R., A.M.L.L., and E.B. clinically characterized the patients and participated in the interpretation of the data. O.Z. participated in the design of the study and interpreted the data. F.B. conceived and designed the study, interpreted the data, and wrote the manuscript. All the authors critically reviewed, edited, and approved the final manuscript.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary information
The online version of this article (10.1038/s41423-019-0275-4) contains supplementary material.
References
- 1.Scully E, Alter G. NK cells in HIV disease. Curr. HIV/AIDS Rep. 2016;13:85–94. doi: 10.1007/s11904-016-0310-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee WS, Kent SJ. Anti-HIV-1 antibody-dependent cellular cytotoxicity: is there more to antibodies than neutralization? Curr. Opin. HIV AIDS. 2018;13:160–166. doi: 10.1097/COH.0000000000000439. [DOI] [PubMed] [Google Scholar]
- 3.Flórez-Álvarez L, Hernandez JC, Zapata W. NK cells in HIV-1 infection: from basic science to vaccine strategies. Front. Immunol. 2018;9:1–13. doi: 10.3389/fimmu.2018.02290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zenarruzabeitia O, Vitallé J, Eguizabal C, Simhadri VR, Borrego F. The biology and disease relevance of CD300a, an inhibitory receptor for phosphatidylserine and phosphatidylethanolamine. J. Immunol. 2015;194:5053–5060. doi: 10.4049/jimmunol.1500304. [DOI] [PubMed] [Google Scholar]
- 5.Vitallé J, Terrén I, Orrantia A, Zenarruzabeitia O, Borrego F. CD300 receptor family in viral infections. Eur. J. Immunol. 2019;49:364–374. doi: 10.1002/eji.201847951. [DOI] [PubMed] [Google Scholar]
- 6.Vitallé J, et al. Altered expression of CD300a inhibitory receptor on CD4+ T cells from human immunodeficiency virus-1-infected patients: association with disease progression markers. Front. Immunol. 2018;9:1709. doi: 10.3389/fimmu.2018.01709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ansari AW, et al. Natural killer cell heterogeneity: cellular dysfunction and significance in HIV-1 immuno-pathogenesis. Cell Mol. Life Sci. 2015;72:3037–3049. doi: 10.1007/s00018-015-1911-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mikulak J, Oriolo F, Zaghi E, Di Vito C, Mavilio D. Natural killer cells in HIV-1 infection and therapy. AIDS. 2017;31:2317–2330. doi: 10.1097/QAD.0000000000001645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freud AG, Mundy-Bosse BL, Yu J, Caligiuri MA. The broad spectrum of human natural killer cell diversity. Immunity. 2017;47:820–833. doi: 10.1016/j.immuni.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nabatanzi R, Cose S, Joloba M, Jones SR, Nakanjako D. Effects of HIV infection and ART on phenotype and function of circulating monocytes, natural killer, and innate lymphoid cells. AIDS Res. Ther. 2018;15:1–8. doi: 10.1186/s12981-018-0194-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.