Abstract
The dehydrogenative aryl C-H/N-H cross-coupling is a powerful synthetic methodology to install nitrogen functionalities into aromatic compounds. Herein, we report an electrochemical oxidation induced intermolecular cross-coupling between aromatics and sulfonimides with high regioselectivity through N-radical addition pathway under external-oxidant-free and catalyst-free conditions. A wide variety of arenes, heteroarenes, alkenes and sulfonimides are applicable scaffolds in this transformation. In addition, aryl sulfonamides or amines (aniline derivatives) can be obtained through different deprotection process. The cyclic voltammetry mechanistic study indicates that the N-centered imidyl radicals are generated via proton-coupled electron transfer event jointly mediated by tetrabutylammonium acetate and anode oxidation process.
Subject terms: Synthetic chemistry methodology, Electrocatalysis
The dehydrogenative C-H/N-H cross-coupling serves to install nitrogen functionalities into arenes with the highest atom economy. Here, the authors report an electrochemical cross-coupling between aromatics and sulfonimides through an N-radical addition pathway under oxidant- and catalyst-free conditions.
Introduction
The ubiquity of nitrogen-containing aromatics in natural products, pharmaceuticals, agrochemicals, and functional materials makes the exploration of efficient methodologies to construct aryl C–N bonds of great importance1. Compared with the well-established Buchwald−Hartwig and Ullmann amination2–8, the direct amination of aryl C–H bonds, especially the dehydrogenative aryl C–H/N–H cross-coupling, represents a more straightforward and atom-economical strategy to access these essential molecules, which can circumvent the pre-functionalization of the aromatic substrates9–24.
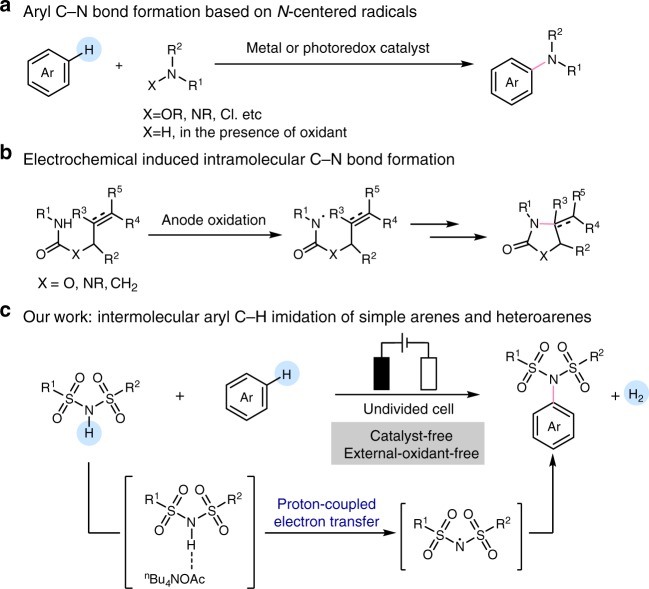
Although significant progress has been made in nucleophilic or electrophilic amination reactions of aromatic compounds, the formation of aryl C–N bonds based on N-centered radicals remains more challenging25–29. The lack of convenient methods to produce N-centered radical species is one of the major challenges in this field. Mostly, the N-centered radical intermediates were generated via the cleavage of reactive N–X (X = oxygen, nitrogen or halogen atom) bond30–35. Recent advances in this class have been propelled by the direct oxidative cleavage of the N–H bond (Fig. 1a)36–45. Itami46, Yu47, and Lee48 reported the dehydrogenative aromatic C–H imidation under visible-light irradiation conditions, respectively. More recently, Leonori’s group disclosed a regioselective amination of arenes using alkyl amino radicals generated by photo-redox catalysis49. However, the stoichiometric oxidants were required in these transformations. In this context, developing an efficient method to generate N-centered radicals from N–H reagent under external oxidant-free and catalyst-free conditions to realize dehydrogenative aryl C–H/N–H cross-coupling would be significantly appealing.
Fig. 1.
C–N bond formation via radical addition of N-centered radical to unsaturated bonds. a Aryl C–N bond formation based on N-centered radicals. b Electrochemical-induced intramolecular C–N bond formation. c Our work: intermolecular aryl C–H imidation of simple arenes and heteroarenes.
Electrochemical synthesis offers a powerful alternative to conventional chemical approaches in oxidative C–H functionalization reactions50–65. Particularly, anode oxidation has been proved as a convenient approach to generate N-centered radicals by cleavage of the strong N–H bonds from N–H precursors66. Although the electrochemical-oxidation-induced amidyl and sulfamidyl radicals have been successfully applied in the intramolecular C–N bond formation (Fig. 1b)42–45, the N-centered radical-mediated intermolecular aminations of simple arenes and heteroarenes are still limited. Based on our previous research, we found that the intermolecular H-bonding between sulfonamide and acetate would assist the single electron oxidation and deprotonation of the sulfonamide to generate the sulfamidyl radicals67. Furthermore, the sulfonimidyl radicals possess higher electrophilicity than sulfamidyl radicals. We hypothesized that the electrochemical oxidation concert deprotonation would be an efficient method to generate an electrophilic N-centered radical from sulfonimides, which tend to react with electron-rich aromatics through radical addition pathway. Followed by the sequent electrochemically oxidative aromatization process, the direct electrochemical intermolecular oxidative aryl C(sp2)-H amination can be achieved, accompanying with the cathodic hydrogen evolution process (Fig. 1c). Here, we report an electrochemically oxidative intermolecular cross-coupling of simple arenes and heteroarenes with sulfonimides under external-oxidant-free and catalyst-free conditions.
Results
Investigation of reaction conditions
Initially, naphthalene (1aa) was chosen as a model substrate for the electro-oxidative C–H imidation with dibenzenesulfonimide (2aa) as the nitrogen source. To our delight, the cross-coupling product 3aa can be afforded in acetonitrile with a 43% isolated yield by using a carbon rod anode and a platinum plate cathode in an undivided cell under 10 mA constant current for 4 h at ambient temperature (Table 1, Entry 2). Tetrabutylammonium acetate was used as the electrolyte, simultaneously as a base. An obviously increasing yield can be obtained when fluorine-containing hexafluoroisopropanol (HFIP) was added as co-solvent (Table 1, Entry 3). Further investigation of the solvent revealed that a mix-solvent system, DCM/MeCN/HFIP = 20:4:1, gave the best result (76% isolated yield, Table 1, Entry 1). A significant decline of yield was observed when a platinum plate anode and carbon rod cathode were used, or a nickel plate cathode was used instead of the platinum plate cathode (Table 1, Entries 4–5). However, when the electrolyte was changed to nBu4NBF4, nBu4NPF6, or nBu4NClO4, no reaction can be observed (Table 1, Entry 6). The imidation product 3aa can be formed with a 46% yield by using NaOAc instead of nBu4NOAc (Table 1, Entry 7). These results demonstrated that acetate plays an important role in this transformation. Decreasing the loading of nBu4NOAc to 0.5 equivalent or 2aa to 1.2 equivalent provided reduced yields (Table 1, Entries 8–9). The reaction occured smoothly when decreasing the operating current to 5 mA, while increasing the current to 15 mA led to a slightly lower yield (Table 1, Entries 10–11). It should be noted that the reaction proceeded even when it was exposed to the air (Table 1, Entry 12). As expected, no product 3aa can be observed without electric current (Table 1, Entry 13).
Table 1.
Investigation of the reaction conditions.
| Entry | Variation from standard condition | Yield (%)b |
|---|---|---|
| 1 | None | 79(76)c |
| 2 | CH3CN as solvent | 45(43)c |
| 3 | CH3CN/HFIP = 12 mL/0.5 mL as solvent | 68(65)c |
| 4 | Pt(+) | C(−) instead of C(+) | Pt(−) | 59 |
| 5 | C(+) | Ni(−) instead of C(+) | Pt(−) | 58 |
| 6 | nBu4NBF4 or nBu4NPF6 or nBu4NClO4 instead of nBu4NOAc | N.D. |
| 7a | NaOAc instead of nBu4NOAc | 46 |
| 8 | 0.5 equiv. nBu4NOAc | 67 |
| 9 | 1.2 equiv. 2aa instead of 1.5 equiv. 2aa | 63 |
| 10 | 5 mA, 8 h instead of 10 mA, 4h | 76 |
| 11 | 15 mA, 2.67h instead of 10 mA, 4h | 59 |
| 12 | Reaction in the air | 70 |
| 13 | Without current | N.D. |
Reaction conditions: carbon rod anode(Φ 6 mm), platinum plate cathode (15 mm × 15 mm × 0.3 mm), constant current = 10 mA, 1aa (0.40 mmol), 2aa (0.60 mmol), nBu4NOAc (0.40 mmol), solvent (DCM/CH3CN/HFIP = 10 mL/2 mL/0.5 mL), undivided cell, N2, 4 h.
HFIP hexafluoroisopropanol, N. D. not determined.
a1.0 equiv. nBu4NBF4 was used as an electrolyte.
bYield of 3aa was determined by HPLC analysis with 1,3,5-trimethoxybenzene as the internal standard.
cIsolated yields are shown in parentheses.
Substrate scope
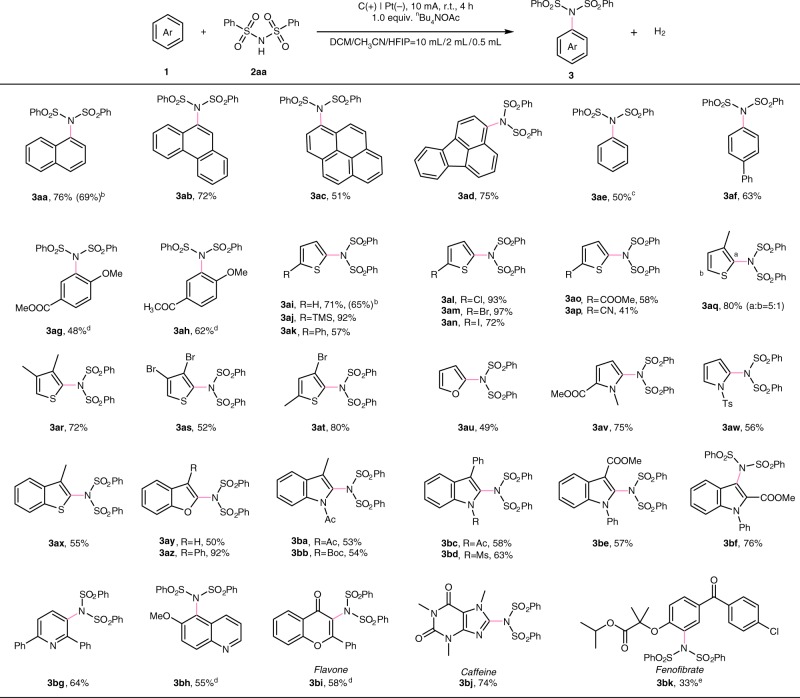
With the optimized reaction conditions in hand, the substrate scope of this electro-oxidative aryl C(sp2)-H imidation was explored (Fig. 2). A range of polycyclic aromatic hydrocarbons, such as naphthalene (3aa, 76%), phenanthrene (3ab, 72%), pyrene (3ac, 51%), and fluoranthene (3ad, 75%), could couple with dibenzenesulfonimide (2aa), giving the corresponding imidation product in moderate to good yields with excellent regioselectivity by constant current electrolysis. To our delight, benzene, a simple electron-neutral aromatic compound, can also be adapted to this electro-oxidative system albeit an excess amount of benzene was used (3ae). The imidation of biphenyl proceeded at the para-position to give 3af in 63% yield. When ester or carbonyl functional group was introduced at the para-position of anisole, the imidation would occur at the ortho-position to the methoxy substituent (3ag-3ah). This strategy was not only applicable to aromatic hydrocarbons but also heteroaromatics. Thiophene (3ai), furan (3au), N-methyl pyrrole derivatives (3av), as well as tosyl-protected pyrrole (3aw) underwent regioselective C–H imidation at the α-position. The thiophene deriveatives bearing various functional groups such as silyl, phenyl, halide, ester, cyano groups in the C2 position were also well tolerated in moderate to good yields (3aj-3ap). Notably, 3al, 3am, and 3an could be obtained without decomposition of the halide group, which can be applied in the subsequent transformation. 3-Methylthiophene gave the corresponding product as a mixture of regioisomers with ratio of 5:1 (3aq, 80%). Besides mono-substituted thiophenes, di-substituted thiophenes also provided the desired products with good yields (3ar-3at). Other 5-membered heteroaromatics, including 3-methylbenzothiophene, benzofuran, N-acetyl indole, N-methanesulfonyl indole, and N-butoxycarbonyl indole derivatives readily underwent C(sp2)-H imidation with 2aa, thus giving the corresponding adducts (3ax-3az, 3ba-3bd). Methyl 1-phenyl-1H-indole-3-carboxylate and methyl 1-phenyl-1H-indole-2-carboxylate would be selectively imidated at the C2 or C3 position (3be-3bf). Six-membered heterocycles bearing electron-donating substitution are competent substrates. 2,6-Diphenylpyridine and 6-methoxyquinoline gave the imidation products 3bg and 3bh as the single regioisomer, respectively. Importantly, the modification of natural products flavone and caffeine could be conducted smoothly with this strategy, yielding the imidated products 3bi and 3bj, respectively. Small-molecule drug such as fenofibrate could also give the corresponding imidation product 3bk. Using the above conditions, this electrochemical oxidation-induced C(sp2)-H imidation can be performed in gram-scale. The constant current electrolysis of 5 mmol of 1aa and 1ai can be conducted to produce the desired imidation product (3aa, 3ai) in 69% and 65% isolated yield, respectively.
Fig. 2.
Substrate scope of aromatic compounds. Reaction conditions: carbon rod anode, platinum plate cathode, constant current = 10 mA, 1 (0.40 mmol), 2aa (0.60 mmol), nBu4NOAc (0.40 mmol), solvent (DCM/ CH3CN/ HFIP = 10 mL/2 mL/0.5 mL), undivided cell, N2, 4 h. Isolated yields. b5 mmol scale. c1 mL benzene and 0.40 mmol 2aa was used. d5 mA, 8 h. eSolvent (DCE/TFE = 8 mL/4 mL).
Next, the scope of the reaction with respect to sulfonimides was also investigated by using naphthalene as the coupling-partner (Fig. 3). Various functional groups on the phenyl rings of symmetrical diaryl sulfonimides were tolerated well (3bl-3bm). The sulfonimide with two thienyl substituents could be used as a viable imination reagent to generate the desired product 3bn in an acceptable yield (43%). Furthermore, N-(methylsulfonyl)benzenesulfonamide derivatives could react with naphthalene in good yields, and several substituents in the para-position of the phenyl rings were compatible in the reaction system (3bo-3bq). Besides, N-(methylsulfonyl)thiophene-2-sulfonamide and N-(methylsulfonyl)naphthalene-1-sulfonamide produced the aryimines 3br in 74% yield and 3bs in 66% yield, respectively. It is worth noting that the coupling of saccharin and naphthalene can be achieved successfully by using this method (3bt). To our delight, When 3-phenylbenzofuran was used as a coupling-partner, N-((4-methoxyphenyl)sulfonyl)acetamide and N-(thiophen-2-ylsulfonyl)acetamide were found to be available imidation reagents through the optimization of the reaction conditions (3bu-3bv). In addition, we have tried to expand the types of amine source. We found that N-(4-methoxyphenyl)sulfonamide derivatives could couple with N-acetyl indoles (Fig. 4), thus giving the corresponding amination products in moderate to good yields (Fig. 4, 5aa-5ah).
Fig. 3.
Substrate scope of sulfonimides. Reaction conditions: carbon rod anode, platinum plate cathode, constant current = 10 mA, 1aa (0.40 mmol), 2 (0.60 mmol), nBu4NOAc (0.40 mmol), solvent ((DCM/CH3CN/HFIP = 10 mL/2 mL/0.5 mL), undivided cell, N2, 4 h. Isolated yields. b Reaction conditions: carbon cloth anode, platinum plate cathode, constant current = 3 mA, 1az (0.40 mmol), 2 (0.60 mmol), nBu4NBF4 (0.40 mmol), NaOAc (0.8 mmol), solvent ((DCM/CH3CN/HFIP = 10 mL/2 mL/1 mL), undivided cell, N2, 8h.
Fig. 4.
Substrate scope of sulfonamides. Reaction conditions: carbon rod anode, platinum plate cathode, constant current = 10 mA, 1aa (0.40 mmol), 4 (0.60 mmol), nBu4NBF4 (0.40 mmol), HOAc (0.4 mmol), solvent (DCM/ CH3CN/ HFIP = 10 mL/2 mL/2 mL), undivided cell, N2, 4 h. Isolated yields.
The dehydrogenative C–H/N–H cross-coupling between alkenes with amines to construct enamines, especially under the external oxidant-free conditions, is of great significance. And the further studies revealed that this electrochemical oxidation-induced system is also applicable for the imidation of alkenes (Fig. 5). And the desired product 7aa was produced in 70% yield under 5 mA constant current for 6 h at ambient temperature when 1,1-diphenylethylene was employed as the radical acceptor. The 1,1-diphenylethylene derivatives bearing electron-donating and electron-withdrawing substituents both underwent the reaction smoothly with dibenzenesulfonimide (7ab-7af). In addition, the procedure was also compatible with trisubstituted aryl alkenes to form corresponding imidation product (7ag-7ah). The allylimine could be obtained as the only isomer when methylenecyclohexane was used as the coupling-partner (7ai).
Fig. 5.
Electrochemical oxidation-induced dehydrogenative imidation of alkenes. Reaction conditions: carbon rod anode, platinum plate cathode, constant current = 5 mA, 6 (0.40 mmol), 2aa (0.60 mmol), nBu4NOAc (0.40 mmol), solvent (DCM/CH3CN/HFIP = 10 mL/2 mL/0.5 mL), undivided cell, N2, 6 h. Isolated yields.
Deprotection of arylimines
Since sulfonyl groups are useful and removable protecting groups for amino groups, above C(sp2)-H imidation reaction can be further utilized in the synthesis of substituted sulfonamide and aryl amines (Fig. 6). Treating 3aa with magnesium in methanol at refluxed temperature, N-(naphthalen-1-yl)benzenesulfonamide (8aa) can be prepared in quantitative yield with high selectivity through the mono-desulfonation process (Fig. 6a). In addition, the double deprotection of sulonimides 3 can be achieved by adding 10 equivalent of magnesium, 2 equivalent of titanium isopropoxide, and 3 equivalent of trimethylsilyl chloride (Fig. 6b). Several aromatic primary amines (9aa, 9ab, 9ad, 9af) can be obtained.
Fig. 6.
Deprotection of arylimines. a Mono-desulfonation of the product. b Double-desulfonation of the product.
Discussion
To gain a deeper insight into the mechanism of this transformation, some mechanistic experiments were performed. By adding 2,6-di-tert-butyl-4-methylphenol (BHT, 2.0 equivalent) as the radical scavenger, the reaction was completely suppressed, and the benzylic imidation product 10aa was obtained in 35% isolated yield (Fig. 7a). Furthermore, in the presence of stoichiometric amount of 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO), the reaction with radical clock substrate 11aa gave rise to ring-opening and radical trapping product 13aa in 13% yield (Fig. 7b), which should be resulted from a radical cross-coupling of intermediate 12aa-B with TEMPO. These results revealed that radical process was involved in this transformation. Furthermore, parallel reaction and intermolecular competitive reaction kinetic isotope effect (KIE) experiments were also performed (Figs. 7c and d), thus giving KIE values of 1.06 and 0.82, respectively. This interesting secondary inverse KIE provided important information for the understanding of the mechanism. Although the inverse secondary KIE is rarely reported, it can be observed in electrophilic nitration of benzene and carbonyl addition reactions, in which sp2 → sp3 rehybridization of C–H (C–D) bond is considered to be the rate-determining step68. Moreover, the Ritter group69 and Itami group70 have reported quite distinct KIE values in the aromatic C–H imidation. These results implicated that the addition of the imidyl radical to the arenes might be involved in the rate-determining step of the transformation.
Fig. 7.
Mechanistic experiments. a Radical trapping experiment. b Radical clock experiment. c Parallel kinetic isotopic effect experiment. d Competition kinetic isotopic effect experiment.
To investigate the interaction between acetate anion and sulfonimide, thus understanding the elementary steps for the generation of N-centered imidyl radical, the cyclic voltammetry studies were performed (Fig. 8). We found that the oxidation potential of dibenzenesulfonimide alone is relatively high (E1/2 = +3.28 V vs Ag/AgCl in acetonitrile). The oxidation potential of nBu4NOAc (tetrabutylammonium acetate) could be observed at 1.78 V (vs Ag/AgCl in acetonitrile). Specifically, we also carried out cyclic voltammetry studies on dibenzenesulfonimide in acetonitrile containing 0.1 M nBu4NBF4 in the presence of 1 equivalent of nBu4NOAc. The oxidation peaks of the dibenzenesulfonimide and nBu4NOAc disappeared with the generation of a new oxidation peak. Compared with dibenzenesulfonimide, the oxidation potential and onset potential of the new peak were shifted to less positive potentials. Importantly, we found that the current response increased with increasing concentrations of nBu4NOAc. These outcomes were consistent with a proton-coupled electron transfer process in this transformation.
Fig. 8.
Cyclic voltammograms studies. a Cyclic voltammograms of dibenzenesulfonimide (2 mM), nBu4NOAc (2 mM), and equivalent mixture of the dibenzenesulfonimide with nBu4NOAc in acetonitrile containing 0.1 M nBu4NBF4. b Cyclic voltammograms of dibenzenesulfonimide (2 mM) in acetonitrile containing 0.1 M nBu4NBF4 and varying concentrations of nBu4NOAc.
A plausible mechanism for the electrochemical oxidation-induced dehydrogenative imidation of arenes is outlined in Fig. 9. The tetrabutylammonium acetate would first form a hydrogen bonded complex I with the dibenzenesulfonimide N–H bond. The resulting adduct I would undergo a concerted proton-coupled electron transfer event with the single electron oxidation on the anode, leading the homolysis of the N–H bond and generation of the key sulfonimidyl radical intermediate II. The subsequent radical addition to naphthalene furnished a new C–N bond and a vicinal carbon-centered radical III. Then the radical species underwent further oxidation to furnish a carbon cation intermediate IV, which finally was aromatized to provide the aryl C(sp2)–H imidation product through proton elimination. Concomitant cathodic reduction of generated protons would generate H2 during the reaction process, which can avoid the requirement of external oxidant in the transformation.
Fig. 9.
Proposed mechanism of electrochemical oxidation-induced intermolecular aromatic C–H imidation. A plausible mechanism involves the homolysis of the N–H bond to afford intermediate II, subsequent radical addition, single electron oxidation, and deprotonation to form the desired product 3aa. Protons undergo cathodic reduction to generate hydrogen.
In summary, we have developed a method for the electrochemical oxidation-induced dehydrogenative C(sp2)-H/N-H cross-coupling between arenes/alkenes and sulfonimides. Neither transition metal catalyst nor stoichiometric additional oxidant was required in this transformation. The electro-synthetic method exhibited a broad substrate scope and provided access to various aryl sulfonimides and alkenyl sulfonimides. The reaction can be easily scaled up, demonstrating the synthetic potential of this method. The cyclic voltammetry confirmed that the N-centered imidyl radical intermediate was generated through a concerted proton-coupled electron transfer process, demonstrating the potential benefits of concerted proton-coupled electron transfer activation in electrochemical synthesis.
Methods
General procedure
The synthesis of 3aa is representative: naphthalene (0.4 mmol, 1 equiv.), diphenylsulfonimide (0.6 mmol, 1.5 equiv.), nBu4NOAc (0.40 mmol, 1 equiv.) were placed in an oven-dried undivided three-necked bottle (25 mL). The bottle was equipped with a stir bar, a carbon rod (Φ 6 mm) anode and a platinum plate (15 mm × 15 mm × 0.3 mm) cathode. The bottle was flushed with nitrogen. Degased dry dichloromethane (DCM, 10 mL), degased dry acetonitrile (MeCN, 2 mL) and commercially available hexafluoroisopropanol (HFIP, 0.5 mL) were added. The reaction mixture was stirred and electrolyzed at a constant current of 10 mA at room temperature for 4 h. After completion of the reaction, the product was identified by TLC. The solvent was removed under reduced pressure by an aspirator, then the pure product was obtained by flash column chromatography on silica gel (eluent: petroleum ether/ethyl acetate = 10:1). Full experimental details can be found in the Supplementary Methods.
Supplementary information
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21520102003) and the Hubei Province Natural Science Foundation of China (2017CFA010). The Program of Introducing Talents of Discipline to Universities of China (111 Program) is also appreciated. We are grateful to the guidance and advice from Dr. Yi-Hung Chen in this work.
Author contributions
A.L. and X.H. conceived the project and designed the experiments. X.H., G.Z., L.N., and T.K. performed the experiments, analyzed the data, and discussed the results. X.H., G.Z., and A.L. wrote the paper, Supplementary Methods, and related materials.
Data availability
The authors declare that the data supporting the findings of this study are available within the article and its supplementary information files and from the authors upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks Hai-Chao Xu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Xia Hu, Guoting Zhang.
Supplementary information
Supplementary information is available for this paper at 10.1038/s41467-019-13524-4.
References
- 1.Ricci, A. Amino Group Chemistry: From Synthesis to the Life Sciences (Wiley, Hoboken, 2008).
- 2.Bariwal J, Van der Eycken E. C–N bond forming cross-coupling reactions: an overview. Chem. Soc. Rev. 2013;42:9283–9303. doi: 10.1039/c3cs60228a. [DOI] [PubMed] [Google Scholar]
- 3.Ruiz-Castillo P, Buchwald SL. Applications of palladium-catalyzed C–N cross-coupling reactions. Chem. Rev. 2016;116:12564–12649. doi: 10.1021/acs.chemrev.6b00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hartwig JF. Evolution of a fourth generation catalyst for the amination and thioetherification of aryl halides. Acc. Chem. Res. 2008;41:1534–1544. doi: 10.1021/ar800098p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beletskaya IP, Cheprakov AV. The complementary competitors: palladium and copper in C–N cross-coupling reactions. Organometallics. 2012;31:7753–7808. doi: 10.1021/om300683c. [DOI] [Google Scholar]
- 6.Fischer C, Koenig B. Palladium- and copper-mediated N-aryl bond formation reactions for the synthesis of biological active compounds. Beilstein J. Org. Chem. 2011;7:59–74. doi: 10.3762/bjoc.7.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sambiagio C, Marsden SP, Blacker AJ, McGowan PC. Copper catalysed Ullmann type chemistry: from mechanistic aspects to modern development. Chem. Soc. Rev. 2014;43:3525–3550. doi: 10.1039/C3CS60289C. [DOI] [PubMed] [Google Scholar]
- 8.Creutz SE, Lotito KJ, Fu GC, Peters JC. Photoinduced Ullmann C-N coupling: demonstrating the viability of a radical pathway. Science. 2012;338:647–651. doi: 10.1126/science.1226458. [DOI] [PubMed] [Google Scholar]
- 9.Roane J, Daugulis O. A general method for aminoquinoline-directed, copper-catalyzed sp(2) C-H bond amination. J. Am. Chem. Soc. 2016;138:4601–4607. doi: 10.1021/jacs.6b01117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang HW, et al. Ligand-promoted rhodium(III)-catalyzed ortho-C-H amination with free amines. Angew. Chem. Int. Ed. 2017;56:7449–7453. doi: 10.1002/anie.201703300. [DOI] [PubMed] [Google Scholar]
- 11.Tsang WC, Munday RH, Brasche G, Zheng N, Buchwald SL. Palladium-catalyzed method for the synthesis of carbazoles via tandem C-H functionalization and C-N bond formation. J. Org. Chem. 2008;73:7603–7610. doi: 10.1021/jo801273q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shrestha R, Mukherjee P, Tan Y, Litman ZC, Hartwig JF. Sterically controlled, palladium-catalyzed intermolecular amination of arenes. J. Am. Chem. Soc. 2013;135:8480–8483. doi: 10.1021/ja4032677. [DOI] [PubMed] [Google Scholar]
- 13.Matsubara T, Asako S, Ilies L, Nakamura E. Synthesis of anthranilic acid derivatives through iron-catalyzed ortho amination of aromatic carboxamides with N-chloroamines. J. Am. Chem. Soc. 2014;136:646–649. doi: 10.1021/ja412521k. [DOI] [PubMed] [Google Scholar]
- 14.Monguchi D, Fujiwara T, Furukawa H, Mori A. Direct amination of azoles via catalytic C-H, N-H coupling. Org. Lett. 2009;11:1607–1610. doi: 10.1021/ol900298e. [DOI] [PubMed] [Google Scholar]
- 15.Wang Q, Schreiber SL. Copper-mediated amidation of heterocyclic and aromatic C-H bonds. Org. Lett. 2009;11:5178–5180. doi: 10.1021/ol902079g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kantak AA, Potavathri S, Barham RA, Romano KM, DeBoef B. Metal-free intermolecular oxidative C–N bond formation via tandem C–H and N–H bond functionalization. J. Am. Chem. Soc. 2011;133:19960–19965. doi: 10.1021/ja2087085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim HJ, Kim J, Cho SH, Chang S. Intermolecular oxidative C–N bond formation under metal-free conditions: control of chemoselectivity between aryl sp2 and benzylic sp3 C–H bond imidation. J. Am. Chem. Soc. 2011;133:16382–16385. doi: 10.1021/ja207296y. [DOI] [PubMed] [Google Scholar]
- 18.Antonchick AP, Samanta R, Kulikov K, Lategahn J. Organocatalytic, oxidative, intramolecular C-H bond amination and metal-free cross-amination of unactivated arenes at ambient temperature. Angew. Chem. Int. Ed. 2011;50:8605–8608. doi: 10.1002/anie.201102984. [DOI] [PubMed] [Google Scholar]
- 19.Romero NA, Margrey KA, Tay NE, Nicewicz DA. Site-selective arene C-H amination via photoredox catalysis. Science. 2015;349:1326–1330. doi: 10.1126/science.aac9895. [DOI] [PubMed] [Google Scholar]
- 20.Niu L, et al. Photo-induced oxidant-free oxidative C–H/N–H cross-coupling between arenes and azoles. Nat. Commun. 2017;8:14226–14232. doi: 10.1038/ncomms14226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyer AU, Berger AL, König B. Metal-free C–H sulfonamidation of pyrroles by visible light photoredox catalysis. Chem. Commun. 2016;52:10918–10921. doi: 10.1039/C6CC06111G. [DOI] [PubMed] [Google Scholar]
- 22.Jiao J, Murakami K, Itami K. Catalytic methods for aromatic C–H amination: an ideal strategy for nitrogen-based functional molecules. ACS Catal. 2016;6:610–633. doi: 10.1021/acscatal.5b02417. [DOI] [Google Scholar]
- 23.Louillat ML, Patureau FW. Oxidative C–H amination reactions. Chem. Soc. Rev. 2014;43:901–910. doi: 10.1039/C3CS60318K. [DOI] [PubMed] [Google Scholar]
- 24.Park Y, Kim Y, Chang S. Transition metal-catalyzed C–H amination: scope, mechanism, and applications. Chem. Rev. 2017;117:9247–9301. doi: 10.1021/acs.chemrev.6b00644. [DOI] [PubMed] [Google Scholar]
- 25.Stella, L. Nitrogen-centered radicals. in Radicals in Organic Synthesis, Vol. 2 (eds Renaud, P. & Sibi, M. P.) 407–426 (Wiley-VCH, Weinheim, 2001).
- 26.Jiang, H. & Studer, A. Chemistry with N-centered radicals generated by single-electron transfer-oxidation using photoredox catalysis. CCS Chem.1,38–49 (2019).
- 27.Chen JR, Hu XQ, Lu LQ, Xiao WJ. Visible light photoredox-controlled reactions of N-radicals and radical ions. Chem. Soc. Rev. 2016;45:2044–2056. doi: 10.1039/C5CS00655D. [DOI] [PubMed] [Google Scholar]
- 28.Xiong T, Zhang Q. New amination strategies based on nitrogen-centered radical chemistry. Chem. Soc. Rev. 2016;45:3069–3087. doi: 10.1039/C5CS00852B. [DOI] [PubMed] [Google Scholar]
- 29.Kärkäs MD. Photochemical generation of nitrogen-centered amidyl, hydrazonyl, and imidyl radicals: methodology developments and catalytic applications. ACS Catal. 2017;7:4999–5022. doi: 10.1021/acscatal.7b01385. [DOI] [Google Scholar]
- 30.Allen LJ, Cabrera PJ, Lee M, Sanford MS. N-acyloxyphthalimides as nitrogen radical precursors in the visible light photocatalyzed room temperature C–H amination of arenes and heteroarenes. J. Am. Chem. Soc. 2014;136:5607–5610. doi: 10.1021/ja501906x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davies J, Svejstrup TD, Fernandez RD, Sheikh NS, Leonori D. Visible-light-mediated synthesis of amidyl radicals: transition-metal-free hydroamination and N-arylation reactions. J. Am. Chem. Soc. 2016;138:8092–8095. doi: 10.1021/jacs.6b04920. [DOI] [PubMed] [Google Scholar]
- 32.Greulich TW, Daniliuc CG, Studer A. N-aminopyridinium salts as precursors for N-centered radicals—direct amidation of arenes and heteroarenes. Org. Lett. 2015;17:254–257. doi: 10.1021/ol503338b. [DOI] [PubMed] [Google Scholar]
- 33.Qin Q, Yu S. Visible-light-promoted redox neutral C–H amidation of heteroarenes with hydroxylamine derivatives. Org. Lett. 2014;16:3504–3507. doi: 10.1021/ol501457s. [DOI] [PubMed] [Google Scholar]
- 34.Kim H, Kim T, Lee DG, Roh SW, Lee C. Nitrogen-centered radical-mediated C-H imidation of arenes and heteroarenes via visible light induced photocatalysis. Chem. Commun. 2014;50:9273–9276. doi: 10.1039/C4CC03905J. [DOI] [PubMed] [Google Scholar]
- 35.Foo K, Sella E, Thome I, Eastgate MD, Baran PS. A mild, ferrocene-catalyzed C-H imidation of (hetero)arenes. J. Am. Chem. Soc. 2014;136:5279–5282. doi: 10.1021/ja501879c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gentry EC, Knowles RR. Synthetic applications of proton-coupled electron transfer. Acc. Chem. Res. 2016;49:1546–1556. doi: 10.1021/acs.accounts.6b00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi GJ, Zhu Q, Miller DC, Gu CJ, Knowles RR. Catalytic alkylation of remote C-H bonds enabled by proton-coupled electron transfer. Nature. 2016;539:268–271. doi: 10.1038/nature19811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chu JC, Rovis T. Amide-directed photoredox-catalysed C-C bond formation at unactivated sp(3) C-H bonds. Nature. 2016;539:272–275. doi: 10.1038/nature19810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu XQ, et al. Photocatalytic generation of N-centered hydrazonyl radicals: a strategy for hydroamination of β,γ-unsaturated hydrazones. Angew. Chem. Int. Ed. 2014;53:12163–12167. doi: 10.1002/anie.201406491. [DOI] [PubMed] [Google Scholar]
- 40.Shu W, Genoux A, Li Z, Nevado C. gamma-Functionalizations of amines through visible-light-mediated, redox-neutral C-C bond cleavage. Angew. Chem. Int. Ed. 2017;56:10521–10524. doi: 10.1002/anie.201704068. [DOI] [PubMed] [Google Scholar]
- 41.Zhou Z, Li Y, Han B, Gong L, Meggers E. Enantioselective catalytic beta-amination through proton-coupled electron transfer followed by stereocontrolled radical-radical coupling. Chem. Sci. 2017;8:5757–5763. doi: 10.1039/C7SC02031G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hou ZW, et al. Electrochemical C-H/N-H functionalization for the synthesis of highly functionalized (aza)indoles. Angew. Chem. Int. Ed. 2016;55:9168–9172. doi: 10.1002/anie.201602616. [DOI] [PubMed] [Google Scholar]
- 43.Zhu L, et al. Electrocatalytic generation of amidyl radicals for olefin hydroamidation: use of solvent effects to enable anilide oxidation. Angew. Chem. Int. Ed. 2016;55:2226–2229. doi: 10.1002/anie.201510418. [DOI] [PubMed] [Google Scholar]
- 44.Xiong P, Xu HH, Xu HC. Metal- and reagent-free intramolecular oxidative amination of tri- and tetrasubstituted alkenes. J. Am. Chem. Soc. 2017;139:2956–2959. doi: 10.1021/jacs.7b01016. [DOI] [PubMed] [Google Scholar]
- 45.Hou ZW, Mao ZY, Melcamu YY, Lu X, Xu HC. Electrochemical synthesis of imidazo-fused N-heteroaromatic compounds through a C-N bond-forming radical cascade. Angew. Chem. Int. Ed. 2018;57:1636–1639. doi: 10.1002/anie.201711876. [DOI] [PubMed] [Google Scholar]
- 46.Ito E, Fukushima T, Kawakami T, Murakami K, Itami K. Catalytic dehydrogenative C–H imidation of arenes enabled by photo-generated hole donation to sulfonimide. Chem. 2017;2:383–392. doi: 10.1016/j.chempr.2017.02.006. [DOI] [Google Scholar]
- 47.Tong K, Liu X, Zhang Y, Yu S. Visible-light-induced direct oxidative C−H amidation of heteroarenes with sulfonamides. Chem. Eur. J. 2016;22:15669–15673. doi: 10.1002/chem.201604014. [DOI] [PubMed] [Google Scholar]
- 48.Kim H, Kim T, Lee DG, Roh SW, Lee C. Nitrogen-centered radical-mediated C–H imidation of arenes and heteroarenes via visible light induced photocatalysis. Chem. Commun. 2014;50:9273–9276. doi: 10.1039/C4CC03905J. [DOI] [PubMed] [Google Scholar]
- 49.Ruffoni A, et al. Practical and regioselective amination of arenes using alkyl amines. Nat. Chem. 2019;11:426–433. doi: 10.1038/s41557-019-0254-5. [DOI] [PubMed] [Google Scholar]
- 50.Amatore C, Cammoun C, Jutand A. Electrochemical recycling of benzoquinone in the Pd/benzoquinone-catalyzed heck-type reactions from arenes. Adv. Synth. Catal. 2007;349:292–296. doi: 10.1002/adsc.200600389. [DOI] [Google Scholar]
- 51.Yoshida J, Kataoka K, Horcajada R, Nagaki A. Modern strategies in electroorganic synthesis. Chem. Rev. 2008;108:2265–2299. doi: 10.1021/cr0680843. [DOI] [PubMed] [Google Scholar]
- 52.Feng R, Smith JA, Moeller KD. Anodic cyclization reactions and the mechanistic strategies that enable optimization. Acc. Chem. Res. 2017;50:2346–2352. doi: 10.1021/acs.accounts.7b00287. [DOI] [PubMed] [Google Scholar]
- 53.Yan M, Kawamata Y, Baran PS. Synthetic organic electrochemical methods since 2000: on the verge of a renaissance. Chem. Rev. 2017;117:13230–13319. doi: 10.1021/acs.chemrev.7b00397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jiang Y, Xu K, Zeng C. Use of electrochemistry in the synthesis of heterocyclic structures. Chem. Rev. 2018;118:4485–4540. doi: 10.1021/acs.chemrev.7b00271. [DOI] [PubMed] [Google Scholar]
- 55.Wiebe A, et al. Electrifying organic synthesis. Angew. Chem. Int. Ed. 2018;57:5594–5619. doi: 10.1002/anie.201711060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morofuji T, Shimizu A, Yoshida J. Electrochemical C–H amination: synthesis of aromatic primary amines via N-arylpyridinium ions. J. Am. Chem. Soc. 2013;135:5000–5003. doi: 10.1021/ja402083e. [DOI] [PubMed] [Google Scholar]
- 57.Fu N, Sauer GS, Saha A, Loo A, Lin S. Metal-catalyzed electrochemical diazidation of alkenes. Science. 2017;357:575–579. doi: 10.1126/science.aan6206. [DOI] [PubMed] [Google Scholar]
- 58.Gao WJ, et al. Electrochemically initiated oxidative amination of benzoxazoles using tetraalkylammonium halides as redox catalysts. J. Org. Chem. 2014;79:9613–9618. doi: 10.1021/jo501736w. [DOI] [PubMed] [Google Scholar]
- 59.Broese T, Francke R. Electrosynthesis using a recyclable mediator–electrolyte system based on ionically tagged phenyl iodide and 1,1,1,3,3,3-hexafluoroisopropanol. Org. Lett. 2016;18:5896–5899. doi: 10.1021/acs.orglett.6b02979. [DOI] [PubMed] [Google Scholar]
- 60.Zhang L, et al. Photoelectrocatalytic arene C-H amination. Nat. Catal. 2019;2:266–373. doi: 10.1038/s41929-019-0344-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Siu T, Yudin AK. Practical olefin aziridination with a broad substrate scope. J. Am. Chem. Soc. 2002;124:530–531. doi: 10.1021/ja0172215. [DOI] [PubMed] [Google Scholar]
- 62.Li J, et al. Electrochemical aziridination by alkene activation using a sulfamate as the nitrogen source. Angew. Chem. Int. Ed. 2018;57:5695–5698. doi: 10.1002/anie.201801106. [DOI] [PubMed] [Google Scholar]
- 63.Sauermann N, Mei R, Ackermann L. Electrochemical C−H amination by cobalt catalysis in a renewable solvent. Angew. Chem. Int. Ed. 2018;57:5090–5094. doi: 10.1002/anie.201802206. [DOI] [PubMed] [Google Scholar]
- 64.Yang Q-L, et al. Copper-catalyzed electrochemical C–H amination of arenes with secondary amines. J. Am. Chem. Soc. 2018;140:11487–11494. doi: 10.1021/jacs.8b07380. [DOI] [PubMed] [Google Scholar]
- 65.Feng P, et al. Azolation of phenol and aniline derivatives. Angew. Chem. Int. Ed. 2019;58:8400–8404. doi: 10.1002/anie.201901762. [DOI] [PubMed] [Google Scholar]
- 66.Zhao Y, Xia W. Recent advances in radical-based C–N bond formation via photo-/electrochemistry. Chem. Soc. Rev. 2018;47:2591–2608. doi: 10.1039/C7CS00572E. [DOI] [PubMed] [Google Scholar]
- 67.Hu X, Zhang G, Bu F, Nie L, Lei A. Electrochemical-oxidation-induced site-selective intramolecular C(sp3)–H amination. ACS Catal. 2018;8:9370–9375. doi: 10.1021/acscatal.8b02847. [DOI] [Google Scholar]
- 68.Do AL, Bull HG, Cordes EH. Secondary deuterium isotope effects for carbonyl addition reactions. J. Am. Chem. Soc. 1972;94:7579–7580. doi: 10.1021/ja00776a051. [DOI] [Google Scholar]
- 69.Boursalian GB, Ngai M-Y, Hojczyk KN, Ritter T. Pd-catalyzed aryl C–H imidation with arene as the limiting reagent. J. Am. Chem. Soc. 2013;135:13278–13281. doi: 10.1021/ja4064926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kawakami T, Murakami K, Itami K. Catalytic C–H imidation of aromatic cores of functional molecules: ligand-accelerated Cu catalysis and application to materials- and biology-oriented aromatics. J. Am. Chem. Soc. 2015;137:2460–2463. doi: 10.1021/ja5130012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the article and its supplementary information files and from the authors upon reasonable request.