Abstract
Post-translational modifications, like phosphorylation, ubiquitylation, and sumoylation, have been shown to impact on synaptic neurotransmission by modifying pre- and postsynaptic proteins and therefore alter protein stability, localization, or protein-protein interactions. Previous studies showed that post-translational modifications are essential during the induction of synaptic plasticity, defined by a major reorganization of synaptic proteins. We demonstrated before that neddylation, a post-translational modification that covalently binds Nedd8 to lysine-residues, strongly affects neuronal maturation and spine stability. We now analysed the consequences of inhibiting neddylation on excitatory synaptic transmission and plasticity, which will help to narrow down possible targets, to make educated guesses, and test specific candidates. Here, we show that acute inhibition of neddylation impacts on synaptic neurotransmission before morphological changes occur. Our data indicate that pre- and postsynaptic proteins are neddylated since the inhibition of neddylation impacts on presynaptic release probability and postsynaptic receptor stabilization. In addition, blocking neddylation during the induction of long-term potentiation and long-term inhibition abolished both forms of synaptic plasticity. Therefore, this study shows the importance of identifying synaptic targets of the neddylation pathway to understand the regulation of synaptic transmission and plasticity.
Subject terms: Molecular neuroscience, Synaptic transmission
Introduction
Neuronal communication requires presynaptic neurotransmitter release and subsequent postsynaptic receptor activation. To modify synaptic strength, pre- and postsynaptic proteins regulating synaptic transmission are fine-tuned by numerous post-translational modifications1,2. Besides the commonly known modifications such as phosphorylation, glycosylation, ubiquitylation, and sumoylation, the conjugation of Nedd8 has been described. We showed earlier that neddylation of non-cullin proteins in neurons is essential for synaptic function3. Neddylation describes the process of attaching the ubiquitin-like protein Nedd8 covalently to a specific substrate4. Similar to other ubiquitin-like proteins, Nedd8 is covalently bound to lysine residues by an enzymatic cascade consisting of the heterodimeric E1-activating enzyme NAE1 (Nedd8 activating enzyme), the conjugating enzyme Ubc12 and yet to be identified E3-ligases3–6. The best-documented function of Nedd8 is to target cullin scaffold proteins, thereby increasing the activity of cullin-RING E3 ubiquitin-ligase complexes (CRLs), which are mainly involved in the control of cell cycle and cellular proliferation7,8. Recent reports indicate that neddylation also influences the enzymatic activity, transcriptional function, protein stability, and partner interaction of several non-cullin substrates, suggesting additional functions of Nedd8 conjugation beyond CRLs3,4,9,10.
Although Nedd8 was discovered in neurons, only very few neddylated neuronal proteins have been described11. Besides Parkin12 we discovered that the synaptic protein PSD-95 is neddylated3. The discovery of additional targets has been difficult. Several aspects complicate the identification of neddylated proteins, mostly the relative abundance of a neddylated protein is fairly low as target proteins are constantly neddylated and de-neddylated4. Unfortunately, a de-neddylase inhibitor has not been discovered. Therefore, analysing the consequences of inhibiting neddylation helps to narrow down possible targets, to make educated guesses, and test specific candidates.
We reported earlier that long-lasting neddylation inhibition in neuronal cultures and genetic mouse models strongly impairs spine development and morphology3. We now used acute hippocampal slices and acutely inhibited neddylation for up to 120 min with the specific NAE1 inhibitor MLN-492410. This allowed us to studying functional changes independent of morphological changes, as our initial data indicated that several pre- and postsynaptic proteins are neddylated3.
We could show that blocking neddylation in acute brain slices for 120 min does not impact on neuronal excitability or spine morphology. Thus, acute brain slices are ideally suited to investigating the effects of inhibiting neddylation on neurotransmitter release, postsynaptic function, and neuronal plasticity. We show that neddylation is involved in the localization of AMPA and NMDA receptors at the postsynapse. In addition, neddylation regulates presynaptic neurotransmitter release by changing vesicular release probability. Interestingly, blocking de-novo neddylation just during the induction time of LTP and LTD blocks both paradigms for synaptic plasticity. Thus, we could show that protein neddylation is required for synaptic integrity and de-novo neddylation is necessary to induce synaptic plasticity.
Results
It was previously reported that blocking neddylation of proteins by the NAE1-specific inhibitor MLN-492410 or by genetic ablation of NAE1 specifically in forebrain excitatory neurons decreases spine size and density thus reducing synaptic transmission. We now wanted to study, whether neddylation inhibition impacts on synaptic transmission before morphological changes occur. In addition, we wanted to test whether neddylation of synaptic proteins is involved in the induction of synaptic plasticity by blocking de-novo neddylation during induction time; addressing the question whether neddylation is not only important for neuronal and spine morphology but also for the regulation of synaptic strength.
To address these questions, we used MLN-4924 to inhibit neddylation. The original study describing MLN-492410 shows an IC50 for blocking NAE of about 5 nM in a purified enzyme assay. To inhibit neddylation effectively in acute brain slice, we increased the concentration to 1 µM. In our hands, 1 µM MLN-4924 had no side-effect on Ubiquitin-activating enzymes (Supplemental Fig. S1).
Acute neddylation inhibition does not alter neuronal morphology or excitability in hippocampal brain slices
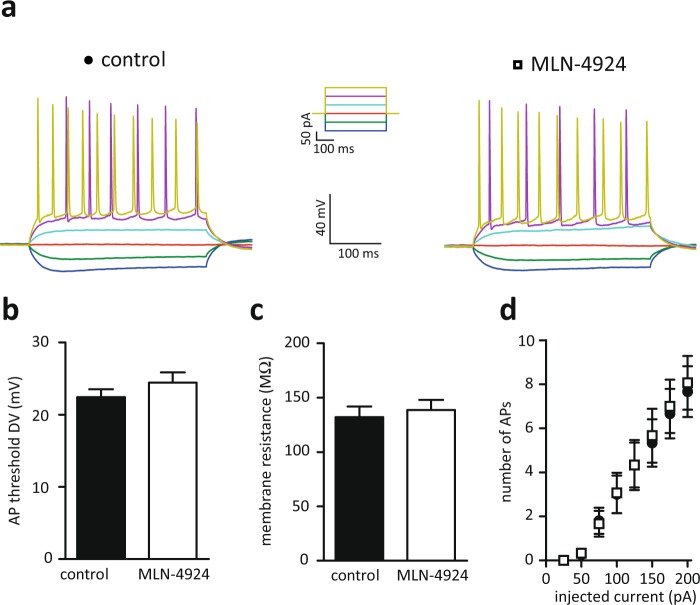
In this study, we used acute hippocampal brain slices to investigate the effect of neddylation inhibition on neuronal function. It was essential to exclude changes in spine morphology and neuronal excitability that could influence synaptic transmission. Previous experiments showed that neddylation inhibition in neuronal cultures strongly impacts on spine morphology after 120 min3. Therefore, we assessed spine morphology in acute brain slices incubated for 120 min in 1 μM MLN-4924 using two-photon imaging. Acute brain slices were obtained from Thy-1-GFP mice that sparsely express green fluorescent protein (GFP) in hippocampal pyramidal neurons13. GFP intensity of spine heads, as a measure for spine size, was monitored over a time course of 120 min after adding MLN-4924. Over the course of 120 minutes, we did not detect any changes in spine size neither in control nor MLN-4924 treated hippocampal slices (Fig. 1a,b, application (Ctrl. Spines n = 115, from 8 slices from 3 animals; MLN Spines n = 135, from 9 slices from 3 animals, values 5 min: Ctrl. 102 ± 4%, MLN 104 ± 5%, 10 min: Ctrl. 104 ± 3%, MLN 103 ± 6%, 30 min: Ctrl. 103 ± 3%, MLN 99 ± 4%, 60 min: Ctrl. 108 ± 2%, MLN 102 ± 2, 120 min: Ctrl. 104 ± 2%, MLN 101 ± 1%, p > 0.05, ANOVA). This finding clearly showed, that spine morphology in acute brain slices is less vulnerable to neddylation inhibition than in neuronal cultures. To avoid morphological effects, we performed all following experiments investigating neuronal transmission within 120 min of MLN-4924 treatment.
Figure 1.
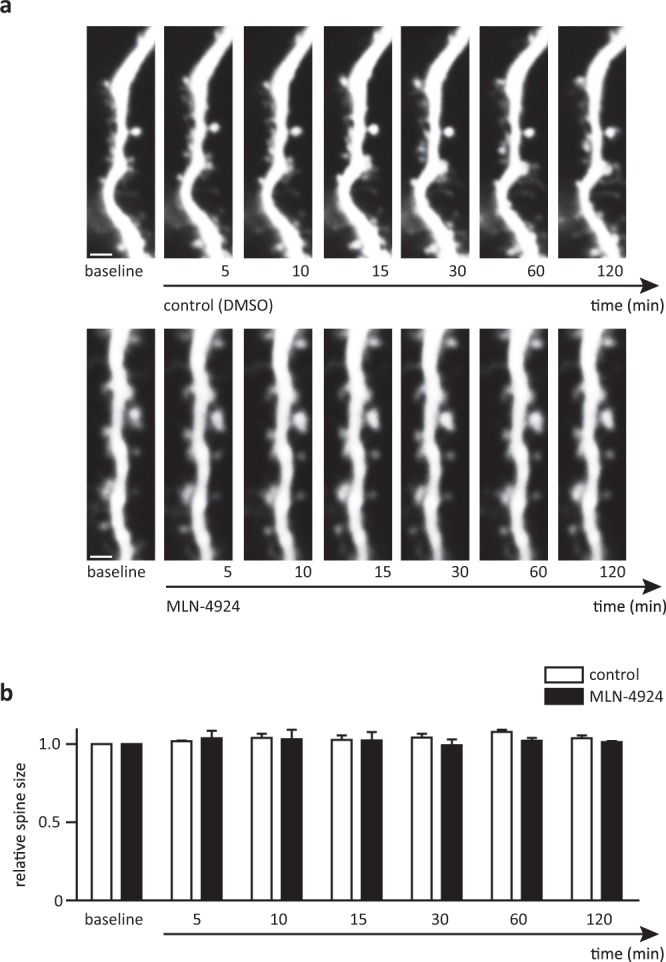
Spine size does not change within 120 min of MLN-4924 treatment. (a) Maximum projections of a stretch of dendrite in acute hippocampal slices from Thy-1 GFP mice under control conditions (top) and treated with MLN-4924 (bottom), scale bar 5 µm. (b) Relative spine size normalized to the individual spine size before and during MLN-4924 treatment.
Next, we tested the resting membrane potential and neuronal excitability, parameters that would affect synaptic transmission, of pyramidal hippocampal neurons after 60 min of MLN-4924 treatment. Neddylation inhibition neither affected input resistance nor the action potential threshold. Consistently, the number of action potentials elicited by a super threshold current injection was not changed (Fig. 2b–d, Ctrl. n = 17 cells, MLN n = 15 cells, membrane resistance Ctrl. 139 ± 10 MΩ, MLN 144 ± 14 MΩ, p = 0.754, t-test, AP-threshold Ctrl. 22.5 ± 1 ΔmV, MLN 24.4 ± 2 ΔmV, p = 0.429, t-test). These data indicate that the general health and intrinsic electrophysiological properties of pyramidal hippocampal neurons were not affected by blocking neddylation for 60 minutes.
Figure 2.
Intrinsic membrane properties are unaltered after MLN-4924 treatment. (a) Sample traces of current-clamp recordings; left control (DMSO treated), right after 60 min MLN-4924 treatment. (b) the AP threshold is not affected by MLN-4924. (c) membrane resistance is not altered by MLN-4924. (d) 1 μM MLN-4924 treatment for 60 min does not affect the number of action potentials generated by increasing current injections.
Neddylation is required for AMPA and NMDA mediated neurotransmission
To test whether blocking neddylation affects synaptic transmission at CA3-CA1 synapses, we recorded input-output curves in acute hippocampal brain slices treated with DMSO or 1 μM MLN-4924 for at least 30 minutes. We compared the size of the presynaptic fiber volley (input) with the slope of the field EPSP (output) in stratum radiatum. Neddylation inhibition reduced synaptic transmission by ~30% at fiber volleys greater than 0.15 mV (Fig. 3a,b, Ctrl. 0.033 ± 0.006 mV/s, MLN 0.033 ± 0.004 mV/s, p > 0.05, Ctrl. 0.107 ± 0.013 mV/s, MLN 0.087 ± 0.010 mV/s, p > 0.05, Ctrl. 0.186 ± 0.021 mV/s, MLN 0.140 ± 0.017 mV/s, p > 0.05, Ctrl. 0.251 ± 0.026 mV/s, MLN 0.181 ± 0.020 mV/s, p < 0.05, Ctrl. 0.306 ± 0.028 mV/s, MLN 0.193 ± 0.017 mV/s, p < 0.01, 2way ANOVA, Ctrl. n = 9 from 3 animals. MLN n = 10 from 4 animals), indicating that the synaptic transmission is acutely regulated by protein neddylation.
Figure 3.
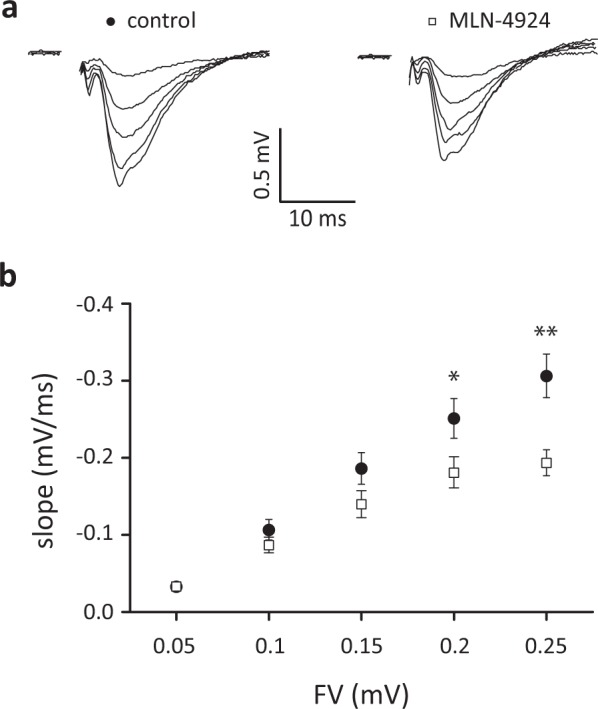
Basal synaptic transmission is reduced in MLN-4924 treated hippocampal brain slices. Input-output curves recorded at the CA3-CA1 synapse show reduced fEPSP responses after 60 min of 1 μM MLN-4924 treatment. (a) Representative traces of fEPSCs from control (left) or MLN-4924 (right) treated hippocampal slices using different stimulus intensities. Please note, stimulus artefacts have been omitted for clarity. (b) Input-output curve shows fEPSP-slope as a function of stimulus intensity in CA1. fEPSP slopes are significantly lower in MLN-4924 treated slices (Control closed circles, MLN-4924 treated open squares).
Two major effects could cause the reduction in neurotransmission: 1. a decrease in presynaptic vesicle fusion and consequently decreased neurotransmitter release or 2. a decrease in post-synaptic receptor density. To get a more detailed image of the changes in synaptic transmission upon neddylation inhibition, we recorded evoked EPSCs (eEPSCs) from hippocampal CA1 pyramidal cells, which allowed us to analysing the effect of neddylation inhibition on AMPA or NMDA receptor mediated currents separately. After obtaining a stable baseline of AMPA or NMDA currents, 1 µM MLN-4924 was added to the bath solution. Inhibiting neddylation for 60 min decreased both, AMPA and NMDA receptors currents to the same extend (Fig. 4c,d, relative AMPA current 47 ± 5%, p = 0.0002, paired t-test, n = 9; relative NMDA currents 45 ± 5%, p = 0.0031, paired t-test, n = 10). We exclude a direct inhibitory effect of MLN-4924 on the receptors, as changes of the evoked AMPA or NMDA currents did only occur after a longer application of MLN-4924; if MLN-4924 would directly block the receptors, the evoked currents should be immediately reduced. To further corroborate changes in synaptic transmission, we recorded miniature EPSCs (mEPSCs). These recordings showed that, mEPSC amplitude and frequency were significantly reduced after incubation in MLN-4924 (Fig. 4e,f, mean mEPSC amplitude: Ctrl. 15.1 ± 0.6 pA, n = 11; MLN, 10.9 ± 0.4 pA, n ± 10, p < 0.001; mean mEPSC frequency: Ctrl. 2.9 ± 0.5 Hz, n = 11; MLN, 1.6 ± 0.3 Hz, n = 10, p < 0.001, Kolmogorov-Smirnov test). A change in the size of these events is thought to reflect a postsynaptic change in the response to the neurotransmitter. In contrast, a change in frequency is thought to represent a change in neurotransmitter release14.
Figure 4.
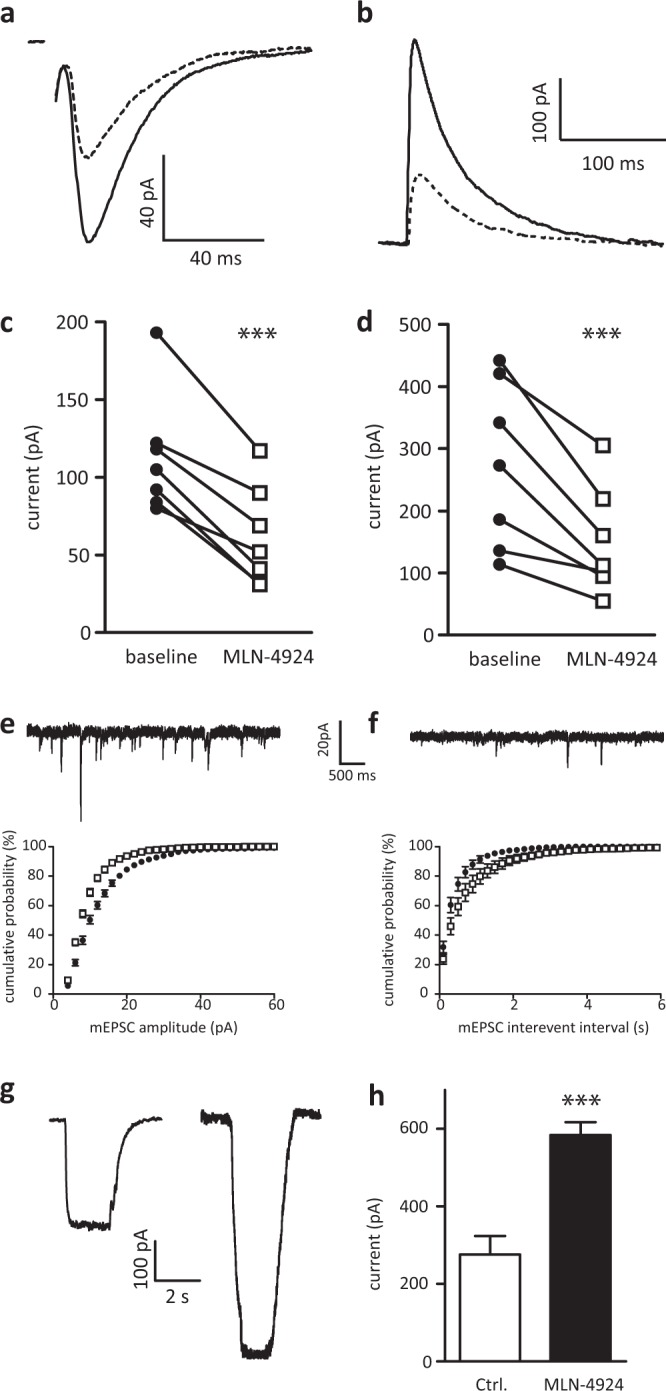
MLN-4924 treatment reduces post-synaptic AMPA and NMDA currents. (a) Sample traces of evoked AMPA currents of the same cell without (solid line) and with MLN-4924 (dashed line). (b) Sample traces of evoked NMDA currents of the same cell without (solid line) and with MLN-4924 (dashed line). Please note, stimulus artefacts have been omitted for clarity. (c) Recordings from several cells show that AMPA currents decrease after >30 min wash in of MLN-4924 in all cells recorded. (d) Recordings from several cells show that NMDA currents decreased after >30 min of MLN-4924 treatment in all cells recorded. (e) sample trace of mEPSC recorded from control cells. (f) sample trace of mEPSC recorded from cells incubated in MLN-4924 for 60 min. bottom panels show cumulative distributions of mEPSC amplitude (left) and mEPSC inter-event interval (right); ctrl; closed circles, MLN-4924 open squares. (g) Traces of AMPA-evoked currents from outside-out somatic patches in control and MLN-4924 treated cells. MLN-4924 treatment significantly increases extrasynaptic AMPA-R responses.
Previously, we showed that the synaptic scaffolding protein PSD-95 is neddylated. Further, we demonstrated that a non-neddylated variant of PSD-95 does not anchor AMPA receptors at the synapse3. As knock-down of PSD-95 by shRNA redistributed AMPA receptors from synapses to extrasynaptic membranes15, it is tempting to speculate that blocking neddylation of PSD-95 has a similar effect. To measure AMPA receptor density at extrasynaptic membranes, we performed somatic (non-synaptic) outside-out patches from cells treated with MLN-4924 and compared them to control conditions. MLN-4924 treatment resulted in increased AMPA receptor mediated currents (Fig. 4g,h, Ctrl. 276 ± 48 pA, n = 6; MLN 584 ± 33 pA, n = 5, t-test, p = 0.0007). Together, these data suggest that blocking neddylation by MLN-4924 decreases the number of AMPA and NMDA receptors at postsynaptic sites, which could be explained via the interaction of both receptors with PSD-95.
Neddylation controls presynaptic vesicular release probability
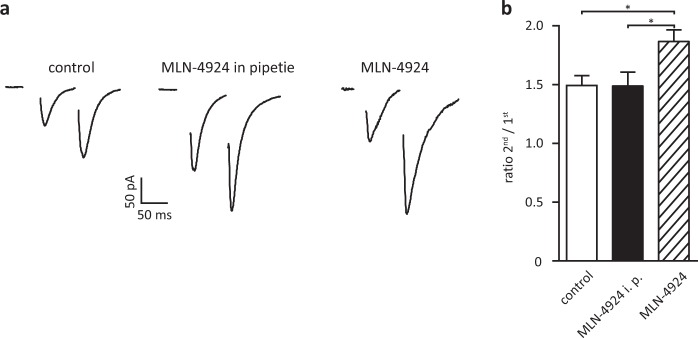
Our previous biochemical data indicated that also presynaptic proteins are neddylated3. To test whether blocking neddylation affects presynaptic vesicular release, we recorded paired-pulse facilitation (PPF), a sensitive measure of changes in the probability of transmitter release, from CA1 neurons in acute hippocampal brain slices. PPF was increased in slices that were incubated in 1 µM MLN-4924 for 60 minutes. An increase in PPF correlates with a decrease in the probability of vesicular release from the presynapse. To limit the action of MLN-4924 only to the postsynapse, we added MLN-4924 to the pipette solution. This configuration did not affect PPF and proves that changes in PPF are specific for MLN-4924 action at the presynaptic side (Fig. 5a,b, PPF Ratio Ctrl. 1.52 ± 0.08, n = 10, MLN in pipette 1.49 ± 0.12, n = 9, MLN 1.87 ± 0.09, n = 9, Ctrl. vs. MLN p = 0.012, Ctrl. vs. MLN in pipette p = 0.83, t-test for each pair). These data demonstrate that blocking neddylation affects transmitter release.
Figure 5.
Release probability of presynaptic vesicles is decreased when neddylation is blocked. Release probability of excitatory synapses is decreased after MLN-4924 treatment. (a) Representative traces of typical EPSCs for control conditions (left), no effect when MLN-4924 is added only to the pipette solution (middle), increase in PPF when MLN-4924 is added to the bath (right). (b) Mean values of PPF in the different conditions. Please note, stimulus artefacts have been omitted for clarity.
Neddylation is required to induce synaptic plasticity
So far, we demonstrated that basal synaptic transmission depends on neddylation. However, if neddylation of synaptic proteins is an important regulatory mechanism, activity dependent changes, e.g. synaptic plasticity, could be affected by neddylation. To test whether de-novo neddylation of synaptic proteins is required for synaptic plasticity, we applied MLN-4924 during the induction of LTP or LTD.
We recorded extracellular field potentials (fEPSPs) from stratum radiatum of the CA1 area and induced LTP by applying a brief tetanic stimulation of 100 Hz for 1 sec or induced LTD by stimulating with 1 Hz for 15 min. To block de-novo neddylation during the induction of synaptic plasticity, we added 1 µM MLN-4924 to the bath 5 min before the induction of plasticity until the end of induction. To monitor effects of MLN-4924 on synaptic transmission, we recorded from slices without inducing synaptic plasticity; here we never observed any changes in the slope of extracellular field potentials after these short times of neddylation inhibition.
When neddylation was inhibited 5 min before the induction of plasticity until the end of induction by 1 µM MLN-4924, the expression of LTP was significantly reduced. In contrast, blocking neddylation right after LTP induction for 5 min by 1 µM MLN-4924 did not affect LTP (Fig. 6a, Ctrl. LTP 1.46 ± 0.01, n = 10; MLN-4924 after induction 1.41 ± 0.01, n = 8; MLN-4924 during induction 1.06 ± 0.01, n = 10; no LTP induction 0.99 ± 0.01, n = 7; Ctrl. vs MLN-4924 after p = 0.21, Ctrl. vs MLN-4924 during p < 0.0001, Ctrl. vs no LTP p < 0.0001, ANOVA). The magnitude of LTD was strongly reduced when induced in the presence of MLN-4924 (Fig. 6b, Ctrl. 0.78 ± 0.02, n = 10; MLN during induction 0.89 ± 0.03 n = 10; no LTD induction 0.98 ± 0.03, n = 6; Ctrl. vs. MLN-4924 p < 0.0001, Ctrl. vs. no LTD p < 0.0001, ANOVA). To exclude that MLN-4924 directly blocks NMDA receptors within the time applied, which would prevent the induction of synaptic plasticity, we recorded evoked NMDA currents by whole cell patch recordings for CA1 pyramidal cells. NMDA currents were not inhibited by washing in 1 µM MLN-4924 (Fig. 6c normalized NMDA currents, 5 min MLN 111 ± 14%, p = 0.74, n = 7; 20 min MLN 91 ± 7%, p = 0.93, n = 5; ANOVA). Please note, the effects of MLN-4924 described in Fig. 4b were obtained after 60 min MLN-4924 application. These recordings clearly demonstrate that de-novo neddylation of target proteins during the induction phase of synaptic plasticity is required for the expression of hippocampal LTP and LTD.
Figure 6.
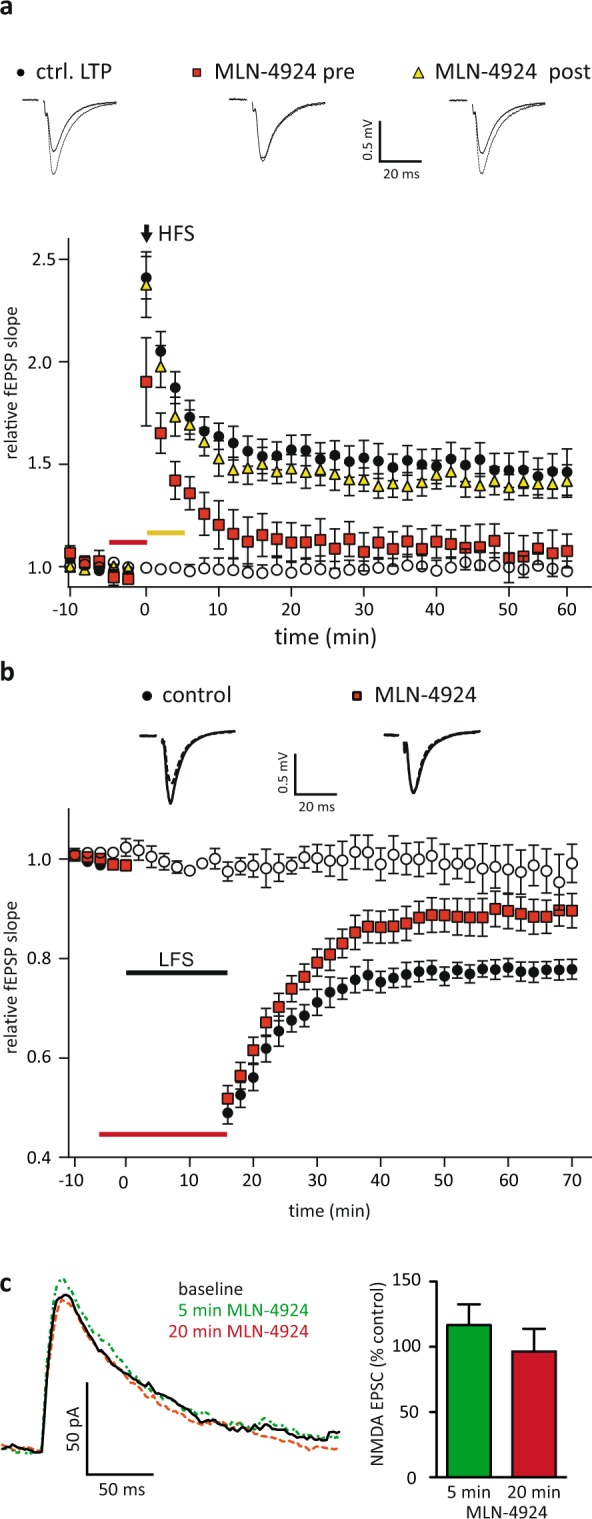
Neddylation is required for synaptic plasticity induction. (a) LTP was induced in acute hippocampal brain slices by high frequency stimulation (HFS). Application of 1 µM MLN-4924 5 min before starting induction (red bar) significantly decreased LTP compared to controls, while MLN-4924 added after the induction (yellow bar) did not affect LTP magnitude. (closed circles: control, red squares: MLN-4924 added before induction, yellow triangles: MLN-4924 after induction, open circles: MLN-4924 no LTP induction). (b) LTD could be induced under control conditions by low frequency stimulation (closed circles) and was strongly reduced when induced in the presence of MLN-4924 (red squares). Open circles show control fEPSP recordings from slices treated with MLN-4924 without inducing plasticity demonstrating that a short application of MLN-4924 does not directly affect synaptic transmission. Red bar indicates 1 µM MLN-4924 application. Insets on top show sample traces; solid line before induction, dashed line after induction. (closed circles: control, open squares: MLN-4924 before induction, open circles: MLN-4942 no LTD induction). (c) MLN-4924 does not directly inhibit NMDA receptor mediated currents. Please note, stimulus artefacts have been omitted for clarity.
Discussion
Besides PSD-95, parkin, and PINK1 no other neddylated neuron specific proteins have been identified so far3,12. To get a better picture of neuronal functions affected by neddylation and thereby guiding the search for neddylated neuronal proteins, we characterized the effect of neddylation on neurotransmission. In contrast to dissociated neuronal cultures, spine size did not shrink within 120 min of MLN-4924 treatment in acute hippocampal slices.
Post-translational modifications of synaptic proteins are described to tightly regulate synaptic strength by modifying protein stability and function16. In a previous study, we reported that beyond classical post-translational modifications like phosphorylation or ubiquitylation, pre- and postsynaptic proteins are neddylated3. In that study, we found that neddylation of synaptic proteins is essential for spine development and spine stability in mature neurons. Similar results were obtained by the Patrick lab, showing that neddylation inhibition by pharmacological blocking of the activating enzyme NAE1 of the neddylation pathway by MLN-4924 affected spine morphology and synaptic strength17. Both studies used neuronal cultures to examine basal synaptic transmission and analysed neuronal transmission at time points when neddylation inhibition already affected spine morphology.
We here demonstrate that neddylation of synaptic proteins is essential for basal synaptic neurotransmission and synaptic plasticity. We have functional evidence that proteins on either side of the synapse are neddylated since blocking neddylation affects pre-synaptic release probability and post-synaptic receptor density. Our results indicate that neddylation of presynaptic proteins impacts on vesicular release probability. Therefore, proteins involved in vesicle fusion are good candidates18, which can be individually tested for neddylation by co-purification experiments. The situation at the postsynaptic side is slightly different, as PSD-95 was identified as the first neddylated synaptic protein in a previous study3. PSD-95 binds directly NMDA receptors and interacts via Stargazin with AMPA receptors. Changes in PSD-95 expression strongly regulate synaptic transmission, and overexpression of PSD-95 blocks LTP and increases LTD19. This central function of PSD-95 in postsynaptic function could directly explain the reduction of evoked AMPA EPSCs and the impaired LTP. Further, our results show that LTP and LTD were blocked by the application of MLN-4924 during the induction of synaptic plasticity. Importantly, applying MLN-4924 after the induction of LTP did not affect LTP magnitude, indicating that acute neddylation of synaptic proteins is required for the expression of activity dependent changes of synaptic transmission. Interestingly, blocking neddylation for up to 60 min did not affect neuronal excitability, which mainly depends on the proper function of voltage gated K+ and Na+ channels20. These data suggest that an effect of neddylation on voltage-gated ion channels is unlikely.
The reasons why no other neuronal proteins besides PSD-95, parkin and PINK1 have been identified as neddylated are manifold. The identification of neddylated proteins is challenging. Mainly, neddylation and de-neddylation are a rapid process, leading to a small amount of the neddylated form of a protein, which makes detection difficult. Unfortunately, a de-neddylase inhibitor has not been found so far; it would facilitate the search for neddylated proteins as substances like MG132 helped to identify ubiquitylated proteins. Next, the identification of neddylated lysines can be identified by mass spectrometry by detecting the mass shift of modified peptides caused by di-Gly overhangs after trypsin digestion; however, ubiquitin and Nedd8 generate the same di-Gly remnant, precluding discrimination between ubiquitin- and Nedd8-modified peptides3,21,22.
We sought here to get a more detailed understanding of what is functionally regulated by neddylation before spine morphology deteriorates. We showed evidence for pre- and postsynaptic regulation by neddylation and can exclude voltage gated ion channels that regulate membrane potential and excitability. The sensitivity of synaptic plasticity suggests that neddylation of synaptic proteins plays an important role in the activity dependent regulation of synaptic transmission.
Based on the findings presented here and based on the biochemical analysis of pre- and postsynaptic proteins presented before3, we conclude that several synaptic proteins are neddylated. Further, we show that de-novo neddylation is required for synaptic plasticity, as inhibiting neddylation before the induction of synaptic plasticity blocks the expression of synaptic plasticity while adding MLN-4924 after the induction of LTP has no effect on the magnitude of LTP.
So far, only little is known about neddylation beyond cullin-RING ligases. Leading to one obvious question: Could an indirect effect of reduced ubiquitylation caused by reduced neddylation of E3-ligases explain our observations? Cullin-RING ligases, the largest class of RING ubiquitin E3 ligases, are the best-characterized and fully validated neddylation substrates23. Hence, blocking neddylation could decrease the activity of these E3 ligases and in turn reduce ubiquitylation. If proteasomal degradation is the main regulator of synaptic function, blocking the ubiquitin-proteasome system (UPS) should have similar effects as blocking neddylation. We observed a decrease in both frequency and amplitude of mEPSC; however, it was shown that blocking the UPS by MG132 or inhibiting the ubiquitin E1 by ziram24 lead to an increase in mEPSC frequency and had no effect on mEPSC amplitude25. It was also discussed hat ubiquitylation has additional functions to protein degradation via the UPS and could regulate protein function or protein-protein interaction25, similar to the proposed function of neddylation4. In summary, our knowledge about the function of neddylation and ubiquitylation is still sparse. Therefore, identifying additional substrates, analysing changes in post-translational modifications, and their consequences on protein function will be necessary to further understand the complex regulation not only of synaptic function.
Experimental Procedures
Methods
Ethical statement
All animal experiments were performed in accordance with the relevant guidelines and regulations. Protocols were approved by the Landesamt für Natur, Umwelt und Verbraucherschutz Nordrhein-Westfalen.
Animals
C57BL/6 mice were group-housed under standard laboratory conditions with 12 hours light-dark cycle with food and water ad libitum. For tissue preparation, animals were handled in agreement with the European Union and local guidelines.
Hippocampal slice preparation for electrophysiological recordings and imaging
Acute hippocampal brain slices were prepared from P14-P19 C57BL/6 wildtype mice or expressing eGFP (GFP-M line13) for imaging. Animals were anesthetized with Isoflurane (Baxter), decapitated, brains were removed and placed into chilled and carbogen (95% O2, 5% CO2) gassed artificial cerebrospinal fluid (ACSF) containing in mM: 125 NaCl; 2.6 KCl; 1.4 MgSO4; 2.5 CaCl2; 1.1 NaH2PO4; 27.5 NaHCO3 and 11.1 D-glucose; pH 7.2, 310 mosm/kg. The hippocampus was transversally cut into 400 µm slices (VT1200S, Leica). Afterwards slices were equilibrated in ACSF for 30 min at 32 °C and thereafter kept at room temperature until recording or imaging.
Imaging
Imaging was performed using a custom built two-photon-microscope, operated with ScanImage26 and a Ti:sapphire Laser (Chameleon Vision-S, Coherent) tuned to 910 nm for GFP excitation. Slices were perfused with ACSF and image stacks of 2nd or 3rd order dendrites from pyramidal neurons were taken every 5 min with a 40x objective (LUMPlanFl, Olympus). After baseline imaging MLN-4924 treatment was started and images were obtained for 120 min. Spine sizes were analysed using custom-written software (MATLAB, Mathworks) and imageJ (NIH). Briefly, image stacks were maximum projected and for each spine the brightest pixels were averaged, divided by the average pixel values of their parent dendrite and normalized to the baseline images.
Electrophysiological recordings
Recordings were performed as described earlier27. Field excitatory postsynaptic potentials (fEPSP) were recorded from acute hippocampal brain slices. To avoid recurrent excitation, Schaffer collaterals were severed between CA3 and CA1. Synaptic responses were evoked by stimulating Schaffer collaterals at 0.33 Hz with 0.2 ms pulses and recorded in the stratum radiatum of CA1. fEPSP data were acquired using a Multiclamp 700B amplifier (Axon Instruments) and digitized with a Digidata 1440 A (Axon Instruments). All experiments were conducted at room temperature. The recording chamber was continuously perfused with carbogenated ACSF. Whole-cell patch recordings were obtained in ACSF supplemented with picrotoxin (100 µM) to block GABAA receptors. Glass electrodes were filled with an internal solution containing (in mM): 150 Cs-gluconate, 8 NaCl, 2 MgATP, 10 HEPES, 0.2 EGTA, 0.1 spermine, and 5 QX-314, pH 7.2. NMDA currents in CA1 pyramidal cells were obtained by evoking eEPSC at + 40 mV, the current being taken 70 ms after stimulus. Series resistances were monitored during the experiment and typically ranged from 8 to 12 MΩ. Leak current was typically below 50 pA. Recordings were terminated if the series resistance exceeded 14 MΩ or the leak current exceeded 75 pA. Miniature EPSCs (mEPSCs) were recorded at −70 mV in ACSF supplemented with TTX (0.2 µM), PTX (100 µM), and trichlormethiazide (250 µM) to increase mEPSC frequency. Somatic outside-out patches were clamped at −70 mV. AMPA-R currents were evoked by local application of 500 mM S-AMPA for 2 sec in the presence of 100 mM trichlormethiazid. For current-clamp recordings pipette solutions contained the following (in mM): 150 K-methylsulphonate, 4 KCl, 4 NaCl, 4 MgATP, 0.4 MgGTP, and 10 HEPES. Current injections ranged from −100 to + 250 pA, increased in 25 pA steps. AP threshold in ΔmV was calculated as the difference between the resting membrane potential and the potential at which the first derivative of single APs became maximal.
Induction protocols for synaptic plasticity
After 10 min of stable baseline LTP was induced by tetanic stimulation (100 Hz for 1 s), LTD was induced by low frequency stimulation (1 Hz for 15 min). MLN-4924 treatment was started 5 min before synaptic plasticity induction and was stopped at the end of the induction protocol.
Western-Blot analysis
HEK cells treated for 60 minutes with either DMSO or MLN were briefly washed with PBS, harvested, lysed with hot non-reducing sample buffer and homogenized using microtip-aided sonication. SDS-polyacrylamide gel electrophoresis and immunoblotting were performed using standard procedures. The following antibodies were used: Rabbit anti-UBE2M 1:1,000 (clone EPR5333, abcam), mouse anti-UBE2C 1:1,000 (clone 1F5D3, abcam) and fluorescence-linked secondary antibodies 1:2,000 (Li-cor). Fluorescence signals were detected using an Odissey Fc System (Li-cor, Lincoln, Nebraska USA).
Statistical analysis
For each experiment, including recordings and imaging experiments, data were collected from at least three independent animal preparations. Data were tested for normal distribution and statistical significance using Prism 5 (GraphPad, San Diego, CA). Error bars represent SEM. We used paired student’s t-tests to compare paired data and unpaired t-tests for comparison the means of different groups. We used ANOVA to compare multiple groups. Significance levels are denoted as follows: *p < 0.05; **p < 0.01; ***p < 0.001.
Supplementary information
Acknowledgements
We thank Dorit Glass for technical support. This work was supported by the DFG SPP1365 grant (D.R.), the Max Planck Society (D.R.), the Volkswagen Stiftung (D.R.), the Agencia Nacional de Promoción Científica y Tecnológica, Argentina (D.R.), and the Fondo para la Convergencia Estructural del Mercosur-FOCEM (D.R.).
Author contributions
M.M.B. conducted voltage clamp recordings and field recordings. M.D. conducted field recordings. U.E. conducted current clamp recordings and performed Western Blots. N.K. conducted two photon imaging experiment. V.S. and M.M.B. wrote the manuscript and prepared Figures. V.S. and D.R. directed the study. All authors reviewed the manuscript.
Data availability
Data generated in this study are available from the corresponding author upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-019-54182-2.
References
- 1.Kawabe H, Brose N. The role of ubiquitylation in nerve cell development. Nat Rev Neurosci. 2011;12:251–268. doi: 10.1038/nrn3009. [DOI] [PubMed] [Google Scholar]
- 2.Yi JJ, Ehlers MD. Ubiquitin and protein turnover in synapse function. Neuron. 2005;47:629–632. doi: 10.1016/j.neuron.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 3.Vogl AM, et al. Neddylation inhibition impairs spine development, destabilizes synapses and deteriorates cognition. Nat Neurosci. 2015;18:239–251. doi: 10.1038/nn.3912. [DOI] [PubMed] [Google Scholar]
- 4.Rabut G, Peter M. Function and regulation of protein neddylation. ‘Protein modifications: beyond the usual suspects’ review series. EMBO Rep. 2008;9:969–976. doi: 10.1038/embor.2008.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gong L, Yeh ET. Identification of the activating and conjugating enzymes of the NEDD8 conjugation pathway. J Biol Chem. 1999;274:12036–12042. doi: 10.1074/jbc.274.17.12036. [DOI] [PubMed] [Google Scholar]
- 6.Liakopoulos D, Doenges G, Matuschewski K, Jentsch S. A novel protein modification pathway related to the ubiquitin system. EMBO J. 1998;17:2208–2214. doi: 10.1093/emboj/17.8.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petroski MD, Deshaies RJ. Function and regulation of cullin-RING ubiquitin ligases. Nat Rev Mol Cell Biol. 2005;6:9–20. doi: 10.1038/nrm1547. [DOI] [PubMed] [Google Scholar]
- 8.Hori T, et al. Covalent modification of all members of human cullin family proteins by NEDD8. Oncogene. 1999;18:6829–6834. doi: 10.1038/sj.onc.1203093. [DOI] [PubMed] [Google Scholar]
- 9.Xirodimas DP. Novel substrates and functions for the ubiquitin-like molecule NEDD8. Biochemical Society transactions. 2008;36:802–806. doi: 10.1042/bst0360802. [DOI] [PubMed] [Google Scholar]
- 10.Soucy TA, et al. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature. 2009;458:732–736. doi: 10.1038/nature07884. [DOI] [PubMed] [Google Scholar]
- 11.Kumar S, Tomooka Y, Noda M. Identification of a set of genes with developmentally down-regulated expression in the mouse brain. Biochem Biophys Res Commun. 1992;185:1155–1161. doi: 10.1016/0006-291X(92)91747-E. [DOI] [PubMed] [Google Scholar]
- 12.Choo Y. S., Vogler G., Wang D., Kalvakuri S., Iliuk A., Tao W. A., Bodmer R., Zhang Z. Regulation of parkin and PINK1 by neddylation. Human Molecular Genetics. 2012;21(11):2514–2523. doi: 10.1093/hmg/dds070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng G, et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/S0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- 14.Oliet SH, Malenka RC, Nicoll RA. Bidirectional control of quantal size by synaptic activity in the hippocampus. Science. 1996;271:1294–1297. doi: 10.1126/science.271.5253.1294. [DOI] [PubMed] [Google Scholar]
- 15.Elias GM, et al. Synapse-specific and developmentally regulated targeting of AMPA receptors by a family of MAGUK scaffolding proteins. Neuron. 2006;52:307–320. doi: 10.1016/j.neuron.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 16.Yokoi N, Fukata M, Fukata Y. Synaptic plasticity regulated by protein-protein interactions and posttranslational modifications. Int Rev Cell Mol Biol. 2012;297:1–43. doi: 10.1016/b978-0-12-394308-8.00001-7. [DOI] [PubMed] [Google Scholar]
- 17.Scudder Samantha L., Patrick Gentry N. Synaptic structure and function are altered by the neddylation inhibitor MLN4924. Molecular and Cellular Neuroscience. 2015;65:52–57. doi: 10.1016/j.mcn.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sudhof TC. Neurotransmitter release: the last millisecond in the life of a synaptic vesicle. Neuron. 2013;80:675–690. doi: 10.1016/j.neuron.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stein V, House DR, Bredt DS, Nicoll RA. Postsynaptic density-95 mimics and occludes hippocampal long-term potentiation and enhances long-term depression. J Neurosci. 2003;23:5503–5506. doi: 10.1523/JNEUROSCI.23-13-05503.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Armstrong CM, Hille B. Voltage-gated ion channels and electrical excitability. Neuron. 1998;20:371–380. doi: 10.1016/S0896-6273(00)80981-2. [DOI] [PubMed] [Google Scholar]
- 21.Kirkpatrick DS, Denison C, Gygi SP. Weighing in on ubiquitin: the expanding role of mass-spectrometry-based proteomics. Nat Cell Biol. 2005;7:750–757. doi: 10.1038/ncb0805-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Witze ES, Old WM, Resing KA, Ahn NG. Mapping protein post-translational modifications with mass spectrometry. Nat Methods. 2007;4:798–806. doi: 10.1038/nmeth1100. [DOI] [PubMed] [Google Scholar]
- 23.Enchev Radoslav I., Schulman Brenda A., Peter Matthias. Protein neddylation: beyond cullin–RING ligases. Nature Reviews Molecular Cell Biology. 2014;16(1):30–44. doi: 10.1038/nrm3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chou AP, et al. Ziram causes dopaminergic cell damage by inhibiting E1 ligase of the proteasome. J Biol Chem. 2008;283:34696–34703. doi: 10.1074/jbc.M802210200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rinetti GV, Schweizer FE. Ubiquitination acutely regulates presynaptic neurotransmitter release in mammalian neurons. J Neurosci. 2010;30:3157–3166. doi: 10.1523/jneurosci.3712-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pologruto TA, Sabatini BL, Svoboda K. ScanImage: flexible software for operating laser scanning microscopes. Biomed Eng Online. 2003;2:13. doi: 10.1186/1475-925X-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rinke I, Artmann J, Stein V. ClC-2 Voltage-Gated Channels Constitute Part of the Background Conductance and Assist Chloride Extrusion. Journal of Neuroscience. 2010;30:4776–4786. doi: 10.1523/jneurosci.6299-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data generated in this study are available from the corresponding author upon request.