Abstract
The present study was undertaken to assess the antimicrobial activity of Lactobacillus spp. (L. salivarius, L. johnsonii, L. reuteri, L. crispatus, and L. gasseri) against Campylobacter jejuni as well as their immunomodulatory capabilities. The results demonstrated that lactobacilli exhibit differential antagonistic effects against C. jejuni and vary in their ability to elicit innate responses in chicken macrophages. All lactobacilli exerted inhibitory effects on C. jejuni growth, abrogated the production of the quorum sensing molecule autoinducer-2 (AI-2) by C. jejuni and inhibited the invasion of C. jejuni in human intestinal epithelial cells. Additionally, all lactobacilli, except L. reuteri, significantly reduced the expression of virulence-related genes in C. jejuni, including genes responsible for motility (flaA, flaB, and flhA), invasion (ciaB), and AI-2 production (luxS). All lactobacilli enhanced C. jejuni phagocytosis by macrophages and increased the expression of interferon (IFN)-γ, interleukin (IL)-1β, IL-12p40, IL-10, and chemokine (CXCLi2) in macrophages. Furthermore, L. salivarius, L. reuteri, L. crispatus, and a mixture of all lactobacilli significantly increased expression of the co-stimulatory molecules CD40, CD80, and CD86 in macrophages. In conclusion, these findings demonstrate that lactobacilli possess anti-Campylobacter and immunomodulatory activities. Further studies are needed to assess their protective efficacy against intestinal colonization by C. jejuni in broiler chickens.
Subject terms: Bacterial infection, Antimicrobials
Introduction
In recent years, there has been an increasing interest in the use of antimicrobial alternatives to reduce the burden of foodborne pathogens in poultry. To date, several antimicrobial alternatives have been identified, including prebiotics, probiotics and their by-products, herbs and their extract, enzymes, organic acids, essential oils, bacteriophages, antimicrobial peptides, nanoparticles and other immune-stimulants such as Toll-like receptor (TLR) ligands1–4. Among these, probiotics have gained increasing attention due to their actions not only in reducing enteric pathogen burden but also their ability to modulate immune responses and thereby, enhancing gut health in poultry.
Probiotics are a group of microorganisms, often referred to as beneficial bacteria that when added to poultry feed or water confer various health benefits4,5. In addition to their role in improving poultry growth performance6 and gut health7, numerous studies have demonstrated that probiotic supplementation, with either single or multiple species, can prevent or reduce intestinal colonization by foodborne pathogens in chickens, such as Salmonella8,9 and Campylobacter10. Growing evidence indicates that probiotic bacteria exert their antimicrobial effects through various mechanisms, including competitive exclusion of potentially pathogenic microorganisms by competing for nutrients and mucosal adhesion sites, production of antimicrobial metabolites such as volatile fatty acids and bacteriocins11,12, and modulation of the immune system13–17 and microbiota composition18,19. In the context of bacterial infections, previous studies have shown that the addition of Lactobacillus salivarius to chicken feed or drinking water can prevent Salmonella enterica serovar Enteritidis colonization8,9. In another study, oral administration of a mixture of probiotics (Lactobacillus acidophilus, Bifidobacterium bifidum, and Streptococcus faecalis) reduced cecal colonization by Salmonella enterica serovar Typhimurium in chickens15.
Despite their ability to reduce Salmonella colonization, the use of probiotics to control enteric C. jejuni colonization has shown variable success. For example, a 6 log10 reduction in C. jejuni colonization was observed in broiler chickens that received multispecies probiotics, consisting of Enterococcus, Pediococcus, Lactobacillus, and Bifidobacterium10. In contrast, another study reported that a probiotic mixture of L. acidophilus, Bacillus subtilis, and Enterococcus faecium could not significantly reduce cecal colonization of C. jejuni in broiler chickens20. The variability observed in these studies may be due to large variations in the antimicrobial and/or immune-stimulatory activities of the different probiotics. For instance, a recent study demonstrated that of 117 isolates of Bacillus and Lactobacillus spp., only 26 exhibited inhibitory activity against C. jejuni in vitro21. Furthermore, a number of studies have demonstrated differential abilities of Lactobacillus spp. (L. acidophilus, L. reuteri and L. salivarius) to enhance phagocytosis and modulate immune responses of chicken macrophage16,17,22.
Thus, prior to evaluating the protective efficacy of probiotics against C. jejuni colonization in chickens, a rigorous investigation of the anti-C. jejuni potentials of probiotic candidates, as well as characterization of their immunomodulatory properties are warranted. Therefore, the present study was undertaken to assess the anti-C. jejuni activities of five Lactobacillus spp. (L. salivarius, L. reuteri, L. crispatus, L. johnsonii, L. gasseri) of chicken origin and their immunomodulatory capabilities.
Results
Lactobacilli inhibited the growth of C. jejuni
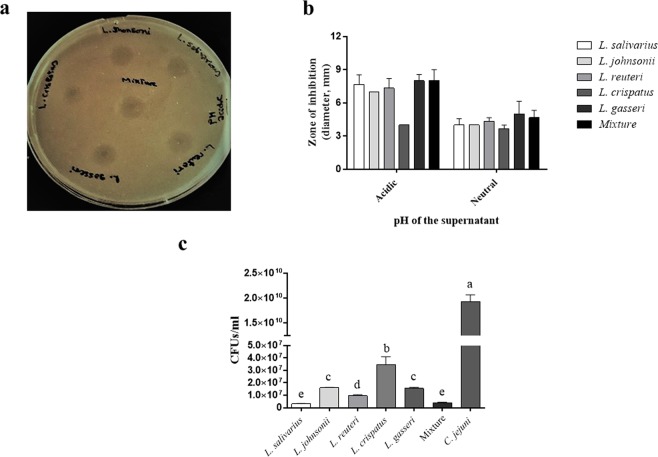
An agar radial diffusion assay was used to evaluate the inhibitory activity of the cell-free supernatants of the five Lactobacillus spp., either as a single species or in combination, against C. jejuni. The results revealed that both the naturally-acidic and neutralized cell-free supernatants of all Lactobacillus spp. were capable of inhibiting C. jejuni growth; however, varying levels of C. jejuni inhibition were observed. The inhibition zones induced by the naturally-acidic supernatants of all Lactobacillus spp. were not significantly different from their respective neutralized supernatants. There was no significant increase in the size of the inhibition zone when the supernatants were combined (Fig. 1a,b).
Figure 1.
Inhibitory and bactericidal activity of both the live culture and cell-free supernatants of lactobacilli against C. jejuni. (a) Agar gel diffusion assay: 108 CFUs C. jejuni were mixed with 30 mL of MH agar and poured into a round Petri dish. Approximately 3 mm diameter holes were punched in the agar and 20 μL of the cell-free supernatant of each Lactobacillus spp., or a mixture of all lactobacilli was added to the holes. Plates were overlaid with 10 mL of MH agar and incubated at 41 °C under microaerophilic conditions. After an incubation period of 40–48 h, the dimeters of the zones of inhibition (a clear ring around the well) were measured. (b) Comparison between the diameter of the inhibition zones induced by natural-acidic and neutralized lactobacilli supernatants. This assay was conducted in triplicate with similar results. (c) Killing assay: equal volumes of 107 CFUs of each Lactobacillus spp. or the mixture of all species in MRS and 107 CFUs of C. jejuni in MH broth were co-incubated overnight under microaerophilic conditions. Untreated C. jejuni culture was used as a positive control. Subsequently, 100 µL of each culture was serially diluted and streaked onto MH agar. Plates were incubated at 41 °C under microaerophilic conditions and CFUs of C. jejuni were enumerated after 40–48 h of incubation. Graphical data are presented as mean ± standard error of the mean (SEM). Bars (within a time point) which are marked by the same letter did not differ significantly (Duncan’s multiple range tests, P > 0.05). This assay was conducted in triplicate and repeated twice.
The killing assay was used to quantitatively measure the bactericidal activity of both the live culture and cell-free supernatants of lactobacilli against C. jejuni. The results showed that both the naturally-acidic and the neutralized supernatants of each Lactobacillus sp. or their combination completely inhibited the growth of C. jejuni (data not shown). With respect to the live cultures, each Lactobacillus sp. or their combination significantly reduced the number of C. jejuni compared to the untreated culture of C. jejuni. Treatment effects with either L. salivarius or the lactobacilli mixture was found to be superior as determined by a significantly higher reduction in C. jejuni numbers compared to the other treatment groups (Fig. 1c).
Lactobacilli down-regulated the expression of virulence-related genes in C. jejuni
To identify the suitable internal reference genes, the expression patterns and PCR amplification efficiencies of 6 housekeeping genes of C. jejuni were screened using LightCycler® 480 Software. The results revealed constant expression levels of ilvC, rpoA, thuC, and rrs following exposure to a single or a mixture of lactobacilli, compared to the other housekeeping genes. In regard to gyrA and sylD, there were low to undetectable levels of expression and, therefore, these two genes were excluded from subsequent analysis. The efficiency of ilvC, rpoA, thiC and rrs was 0.85, 0.89, 0.92, and 0.92, respectively. Further analysis was conducted using SASqPCR to assess and rank the expression stability of these genes revealing that thiC and rrs were the most stable genes, whereas ilvC and rpoA were the least stable genes (Table 1). Based on these findings, rrs was selected as a housekeeping gene to normalize the expression of target genes.
Table 1.
PCR amplification efficiency and stability of C. jejuni reference genes.
| Gene | Slope | Intercept | RSQ | E | Rank | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Value | StdErr | P value | Value | StdErr | P value | |||||
| ilvc | −3.7365 | 0.0755 | 3.08E-11 | 37.64 | 0.1849 | 3.80E-16 | 0.996 | 0.851 | 1 |  |
| rpoA | −3.6185 | 0.0499 | 1.47E-12 | 37.641 | 0.1224 | 1.40E-17 | 0.998 | 0.889 | 2 | |
| rrs | −3.521 | 0.0115 | 1.46E-17 | 30.066 | 0.0282 | 6.72E-22 | 0.999 | 0.923 | 3 | |
| thic | −3.5155 | 0.0862 | 1.45E-10 | 35.182 | 0.2113 | 1.89E-15 | 0.995 | 0.925 | 4 | |
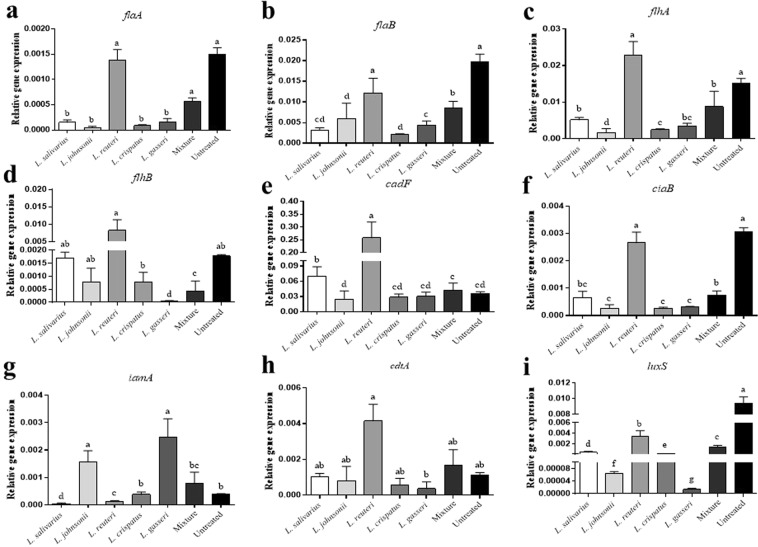
To gain insight into the role of the lactobacilli in the attenuation of C. jejuni virulence, the expression of several virulence genes of C. jejuni (flaA, flaB, flhA, flhB, cadF, ciaB, iamA, cdtA, and luxS) were measured following exposure to either a single or a mixture of Lactobacillus spp. In general, lactobacilli varied in their ability to attenuate the expression of these genes.
Expression of flaA and flaB was downregulated at 24 h following exposure to all Lactobacillus spp., except L. reuteri, as compared to the unexposed C. jejuni group. No significant changes were observed in flaA and flaB expression between L. reuteri-exposed C. jejuni and unexposed C. jejuni group (Fig. 2a,b). Treatment with the mixture of lactobacilli significantly reduced the expression of flaB but not flaA. Expression of levels of flhA were significantly decreased following exposure to the lactobacilli mixture and all single Lactobacillus spp. except for L. reuteri (Fig. 2c). For flhB, expression levels were significantly reduced following exposure to L. gasseri and the lactobacilli mixture, whereas no significant differences were observed between the rest of Lactobacillus spp.-exposed groups and the unexposed C. jejuni group (Fig. 2d).
Figure 2.
Relative gene expression of C. jejuni virulence-related genes. Equal volumes of 107 CFUs of C. jejuni in MH broth and 108 CFUs of each Lactobacillus sp., or the mixture of all species, or MRS medium were incubated at 41 °C for 24 h under microaerophilic conditions. Quantitative real time-PCR was used to measure the relative expression of genes responsible for motility such as flaA (a), flab (b), flhA (c), and flhB (d), adhesion such as cadF (e), invasion such as ciaB (f) and iamA (g), cytotoxin production such as cdtA (h) and autoinducer production such as luxS (i). Expression levels of all genes were calculated relative to the reference gene rrs (16S RNA ribosomal subunit) using 2−ΔΔCT method. Graphical data are presented as mean ± standard error of the mean (SEM). Bars (within a time point) which are marked by the same letter did not differ significantly (Duncan’s multiple range tests, P > 0.05). This assay was conducted in triplicate.
Expression of cadF was significantly enhanced following exposure to L. salivarius and L. reuteri, whereas no significant changes were observed after exposure to L. johnsonii, L. gasseri, L. crispatus, and the mixture of lactobacilli (Fig. 2e).
Expression of ciaB was significantly decreased following exposure to the lactobacilli mixture and all single Lactobacillus spp., except L. reuteri, compared to the unexposed C. jejuni group (Fig. 2f). iamA expression levels were significantly elevated following exposure to L. johnsonii and L. gasseri, whereas exposure to L. salivarius and L. reuteri significantly reduced the expression of iamA as compared to the unexposed C. jejuni group. Exposure to L. crispatus and the lactobacilli mixture, however, did not alter cadf expression compared to the unexposed C. jejuni group (Fig. 2g).
Expression of cdtA was significantly elevated following exposure to L. reuteri, whereas no significant changes were observed following exposure to the rest of Lactobacillus spp. and the lactobacilli mixture compared to the unexposed C. jejuni group (Fig. 2h).
Expression of luxS was significantly reduced following exposure to all Lactobacillus spp., either singly or as a mixture, compared to the unexposed C. jejuni group. Additionally, L. gasseri resulted in the highest reduction of luxS expression of all treatment groups (Fig. 2i).
Lactobacilli inhibited the quorum sensing signals of C. jejuni by suppressing the production of AI-2
Quantitative measurement of the quorum sensing molecule AI-2 was performed by monitoring V. harveyi luminescence following treatment with the culture supernatants of C. jejuni, done in the presence or absence of lactobacilli for various time points. The results showed a consistent increase in AI-2 production across the time course in the untreated C. jejuni group, whereas treatment with the supernatants of Lactobacillus spp., either singly or as a mixture, resulted in abrogation of AI-2 production to levels comparable to those of the negative control group. At 8 h post-incubation, there were no significant differences in AI-2 production between treatment groups, whereas different effects between the treatment supernatants were observed at 24 and 48 h post-incubation (Fig. 3).
Figure 3.
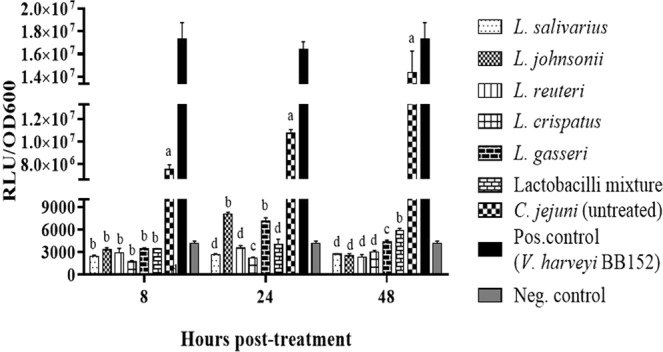
Levels of extracellular AI-2 produced by C. jejuni following co-incubation with lactobacilli at 41 °C for 24 h under microaerophilic conditions. V. harveyi bioluminescence assay was used to measure luminescence production. In a 96-well black clear bottom plate, 90 µL of the diluted V. harveyi BB170 and 10 µL of the filtered cell-free culture supernatant of both the treated and non-treated culture of C. jejuni were added to the wells. The supernatant from V. harveyi BB152 was used as a positive control and AB medium was used as a negative control. Maximal bioluminescence was observed at 13 hours after incubation with the lactobacilli supernatants. Data are presented as mean ± standard error of the mean (SEM) of the relative light unit (RLU) per unit of absorbance (cell density; OD 600 nm). Bars (within a time point) which are marked by the same letter did not differ significantly (Wilcoxon signed-rank test, P > 0.05). This assay was conducted in triplicate and repeated twice with similar results.
Lactobacilli reduced C. jejuni adherence to and invasion of human intestinal epithelial cells
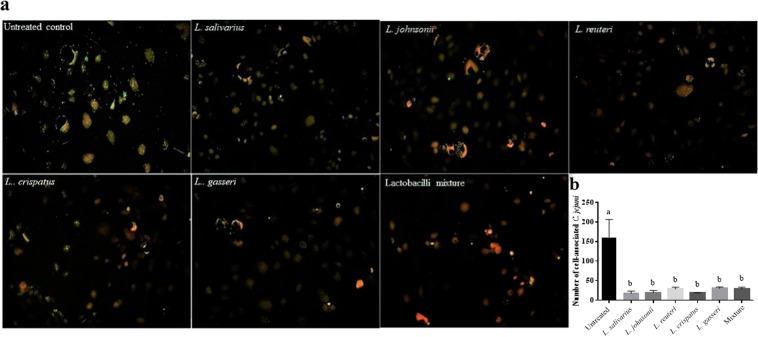
The capacity of Lactobacillus spp. to inhibit the invasion of C. jejuni in Caco-2 cells was investigated by incubating the cells with fluorescein isothiocyanate (FITC)-labelled C. jejuni alone or in combination with lactobacilli and visualizing the invaded fluorescent C. jejuni. The results indicated that co-incubation of each Lactobacillus sp. or their combination with C. jejuni resulted in a significant reduction of C. jejuni adherence to and invasion of Caco-2 cells as demonstrated by fewer number of C. jejuni associated with cells co-cultured with C. jejuni and lactobacilli, compared to cells co-cultured with C. jejuni alone (Fig. 4a,b).
Figure 4.
Lactobacilli reduced C. jejuni adherence to or invasion of human intestinal epithelial cells (Caco-2 cells). Caco-2 cells were seeded in 6-well plates at density 4 × 105 cells/well in EMEM and incubated at 37 °C in a humidified 5% CO2 environment until reaching 90% confluency followed by 107 CFUs/mL of FITC-labelled C. jejuni were added alone or simultaneously with 107 CFUs/mL of each Lactobacillus sp. or a mixture of all species to cultured cells and incubated for 2, 5 and 8 h. ImageJ software was used to count the number of C. jejuni associated with Caco-2 cells. The green color represents the C. jejuni, while the red color represents the nucleus. This assay was conducted in triplicate and repeated twice with similar results.
Lactobacilli enhanced nitric oxide (NO) production in chicken macrophages
The immunomodulatory activity of lactobacilli on chicken macrophages was evaluated by measuring NO production, which is an indicator of macrophage activation, following exposure to either a single or a mixture of heat-killed lactobacilli. Treatment of macrophages with a single heat-killed Lactobacillus sp., at a dose of 10 MOI, did not significantly increase levels of the NO relative to the untreated cells. The combination of Lactobacillus spp., however, synergistically enhanced the production of NO, as compared to the untreated cells. Treatment of macrophages with 100 MOI of a single species of all heat-killed lactobacilli, except L. reuteri, significantly enhanced NO production by macrophages compared to the untreated cells. The treatment of macrophages with L. salivarius induced a significantly higher NO production as compared to the other single species of Lactobacillus and their combination, whereas treatment with L. reuteri significantly reduced NO production compared to the untreated control. There were no statistically significant differences in the NO levels between the lactobacilli mixture and L. johnsonii or L. crispatus alone (Fig. 5).
Figure 5.
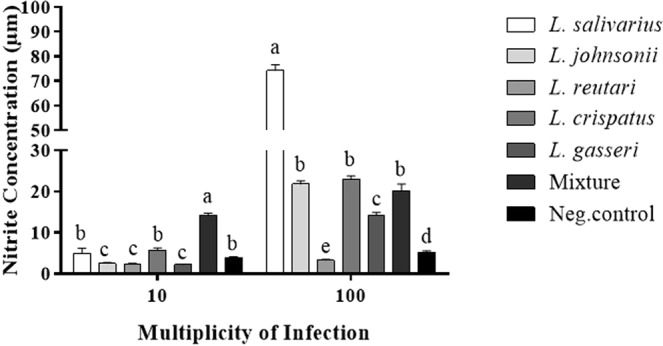
Lactobacilli enhanced the production of nitric oxide by macrophages. MQ-NCSU cells were seeded in 24-well plates at density 4 × 105 cells/well in LM-HAHN medium and incubated at 40 °C in a humidified 5% CO2 environment. Following incubation for 3 h, cells were stimulated with 10 or 100 MOI of a single or a mixture of heat-killed Lactobacillus spp. in DMEM and incubated at 41 °C in a humidified 5% CO2 environment for 24 h. Supernatants were collected, and NO production was measured by Griess assay. Graphical data are presented as mean ± standard error of the mean (SEM). Bars (within a time point) which are marked by the same letter did not differ significantly (Wilcoxon signed-rank test, P > 0.05). This assay was carried out using five replicates.
Lactobacilli enhanced the phagocytic activity of chicken macrophage-like cells
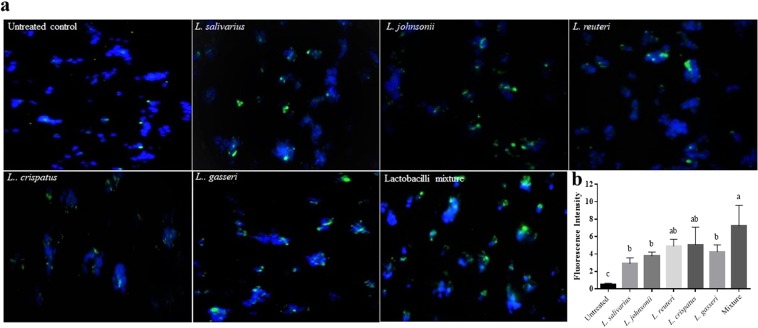
The effects of lactobacilli on the phagocytic activity of macrophages were evaluated using latex beads and FITC-labelled C. jejuni. Fluorescent latex beads were used to determine the optimal MOI of lactobacilli required for the enhancement of macrophage phagocytosis. The results indicated that 100 MOI of each Lactobacillus sp. or their mixture, significantly enhanced the phagocytic capacity of macrophages compared to 10 MOI (Fig. 6). Therefore, MOI of 100 was chosen as an optimal dose to enhance C. jejuni uptake by macrophages. Pre-treatment of macrophages with 100 MOI heat-killed Lactobacillus, either singly or as a mixture, significantly enhanced phagocytosis of FITC-labelled C. jejuni by macrophages as demonstrated by the high number of C. jejuni associated with cells that were pre-treated with lactobacilli compared to the untreated cells (Fig. 7a,b). Macrophages treated with the mixture of lactobacilli exhibited a higher phagocytic activity than those treated with L. salivarius and L. johnsonii. and L. gasseri, whereas no significant differences were observed between the other treatments and the mixture of lactobacilli.
Figure 6.
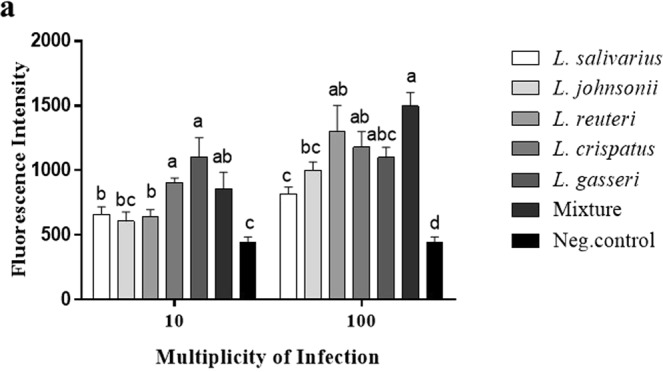
Lactobacilli enhanced macrophage phagocytosis of IgG-FITC coated latex beads. MQ-NCSU cells were seeded, in 6 replicates, into 96-well black clear bottom polystyrene plates at density 1 × 105 cells/well in LM-HAHN medium and incubated for 3 h. Cells were then stimulated with 10 or 100 MOI of a single or a mixture of heat-killed Lactobacillus spp. in DMEM along with the Latex BeadsRabbit IgG-FITC complex and incubated at 41 °C in a humidified 5% CO2 environment. The fluorescence intensity was read in a fluorescence plate reader at an excitation of 485 nm and an emission of 535 nm. Graphical data are presented as mean ± standard error of the mean (SEM). Bars (within a time point) which are marked by the same letter did not differ significantly (Duncan’s multiple range tests, P > 0.05). This assay was carried out using six replicates.
Figure 7.
Lactobacilli enhanced macrophage phagocytosis of FITC-labelled C. jejuni. MQ-NCSU cells were seeded into 6-well plates containing glass coverslips at density 8 × 105 cells/well in LM-HAHN medium as described above. After treatment with 100 MOI of heat-killed lactobacilli, cells were incubated at 41 °C in a humidified 5% CO2 environment for 2 h. Subsequently, cells were infected with 8 × 107 CFUs of C. jejuni/well in DMEM medium. The invasion of fluorescent C. jejuni was visualized by fluorescence microscopy (b). The fluorescence intensity of the phagocytosed C. jejuni was measured by imageJ (c). This assay was conducted in triplicate and repeated twice with similar results. Graphical data are presented as mean ± standard error of the mean (SEM). Bars (within a time point) which are marked by the same letter did not differ significantly (Duncan’s multiple range tests, P > 0.05).
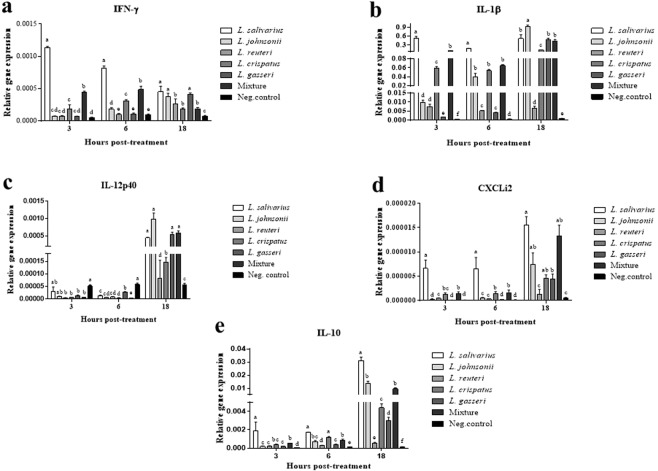
Lactobacilli modulated cytokine gene expression in chicken macrophages
We explored the kinetics of cytokine and chemokine gene expression in chicken macrophages following exposure to lactobacilli. Irrespective of the time point, treatment with either a single or a mixture of lactobacilli significantly enhanced the expression of IFN-γ (Fig. 8a), pro-inflammatory cytokines (IL-1β, IL-12p40, Fig. 8b,c), a pro-inflammatory chemokine (CXCLi2, Fig. 8d), and a regulatory cytokine (IL-10, Fig. 8e). Importantly, treatment with L. salivarius, L. johnsonii, and the mixture of lactobacilli consistently induced substantially higher levels of expression of these cytokines and chemokine followed by L. gasseri and L. crispatus, compared to the untreated control group. The expression of all cytokines and chemokines in the group treated with L. reuteri were statistically higher than the untreated control group, but significantly lower than the other treatment groups.
Figure 8.
Relative gene expression of IFN-γ (a), IL-1β (b), IL-12p40 (c), CXCLi2 (d), and IL-10, (e) in macrophages at 3, 6, and 18 h following exposure to heat-killed lactobacilli. Expression levels of all target genes were calculated relative to the housekeeping gene β-actin using 2−ΔΔCT method. Graphical data are presented as mean ± standard error of the mean (SEM). Bars (within a time point) which are marked by the same letter did not differ significantly (Duncan’s multiple range tests, P > 0.05).
Lactobacilli enhanced the expression of costimulatory and antigen presentation molecules of macrophages
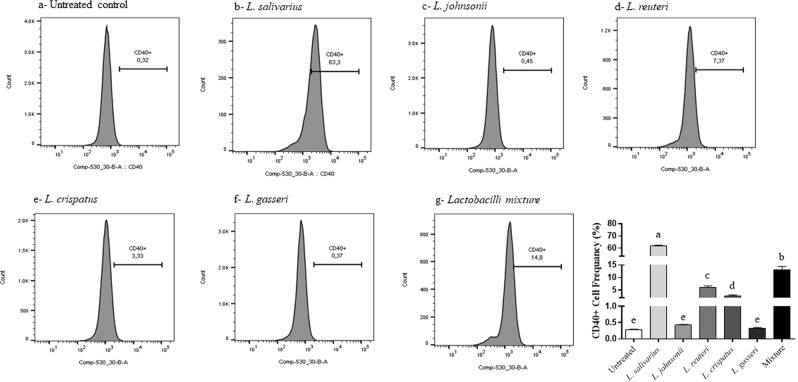
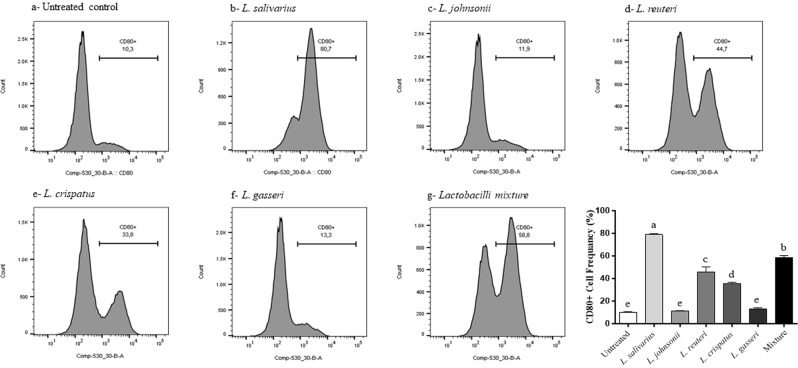
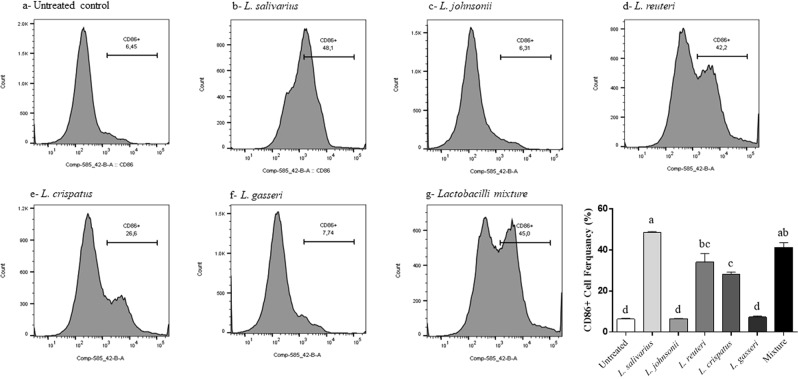
In this study, we explored the role of lactobacilli in enhancing the expression of costimulatory molecules that play a critical role in T cell activation by measuring the expression of CD40 CD80, CD86, and MHC-II following treatment with heat-killed lactobacilli. The results showed that treatment of macrophages with L. salivarius, L. reuteri, L. crispatus, and the mixture of the five species significantly increased the expression of CD40 (Fig. 9), CD80 (Fig. 10), and CD86 (Fig. 11), whereas no significant changes were observed following treatment with L. johnsonii or L. gasseri, compared to the untreated controls. None of the treatments induced significant alterations in the surface expression of MHC-II molecules (data not shown).
Figure 9.
Expression of macrophage costimulatory molecule CD40 following treatment with heat-killed lactobacilli. MQ-NCSU cells were seeded, in triplicates, into 24-well plates and then treated with 100 MOI of heat-killed lactobacilli. After 24 hours of incubation, cells were harvested and stained with unlabelled anti-CD40 followed by labelled anti-mouse IgG1 secondary antibodies. Data analysis was performed using FlowJo software. Graphical data are presented as mean ± standard error of the mean (SEM). Bars (within a time point) which are marked by the same letter did not differ significantly (Duncan’s multiple range tests, P > 0.05).
Figure 10.
Expression of macrophage costimulatory molecule CD80 following treatment with heat-killed lactobacilli. MQ-NCSU cells were seeded, in triplicates, into 24-well plates and then treated with 100 MOI of heat-killed lactobacilli. After 24 hours of incubation, cells were harvested and stained with unlabelled anti-CD80 followed by labelled anti-mouse IgG1 secondary antibodies. Data analysis was performed using FlowJo software. Graphical data are presented as mean ± standard error of the mean (SEM). Bars (within a time point) which are marked by the same letter did not differ significantly (Duncan’s multiple range tests, P > 0.05).
Figure 11.
Expression of macrophage costimulatory molecule CD86 following treatment with heat-killed lactobacilli. MQ-NCSU cells were seeded, in triplicates, into 24-well plates and then treated with 100 MOI of heat-killed lactobacilli. After 24 hours of incubation, cells were harvested and stained with anti-CD86 followed by anti-mouse IgG2a secondary antibodies. Data analysis was performed using FlowJo software. Graphical data are presented as mean ± standard error of the mean (SEM). Bars (within a time point) which are marked by the same letter did not differ significantly (Duncan’s multiple range tests, P > 0.05).
Discussion
Over the last few decades, probiotics have emerged as a promising alternative to antibiotics. In poultry, lactic acid bacteria (Lactobacillus and Bifidobacterium species) are the most commonly used probiotic organisms for prevention or control of many enteric diseases4,5. In addition to their roles in modulation of growth performance, probiotics have been shown to possess antimicrobial activity against various foodborne bacterial pathogens, such as E. coli, Salmonella spp., and C. jejuni10,23,24. Probiotics exhibit these activities either directly, through the production of bactericidal molecules, including organic acids, hydrogen peroxide, and bacteriocins, or indirectly, through competitive exclusion and/or the induction of innate mucosal immunity25–27.
Although there have been many studies examining the protective effects of lactobacilli against C. jejuni, the mechanisms underlying their protective activity and their interactions with the chicken immune system are not yet completely understood. A better understanding of these mechanisms may help reveal potential probiotic properties of lactobacilli and could also help identify the most effective probiotic strain(s), potentially leading to their use as feed additives to prevent Campylobacter colonization in broiler chickens. Therefore, the present study was undertaken to examine the probiotic potential of five Lactobacillus spp. for growth inhibition and virulence attenuation of C. jejuni and to evaluate their immunomodulatory properties.
The ability of lactobacilli to inhibit the growth of C. jejuni is thought to occur through the secretion of organic acids, such as lactic and acetic acids, which, in turn, alter the local pH to a level that makes the environment unsuitable for C. jejuni growth and/or causes disruption of the integrity of the C. jejuni membrane28,29. It has been shown previously that the cell-free supernatant of L. fermentum can inhibit the growth of C. jejuni in vitro; however, no such effect was found when the pH was increased to 6.330. Strikingly, in the present study, both acidic and neutralized lactobacilli supernatants showed comparable levels of inhibition, indicating that the inhibitory capability of lactobacilli is not strongly pH-dependent, rather other antimicrobial substances may contribute to growth inhibition. Live lactobacilli, however, varied in their antagonistic activities against C. jejuni growth. It is noteworthy that L. salivarius displayed significant levels of bactericidal activity against C. jejuni, comparable to that of the mixture of the five Lactobacillus spp., indicating the differential abilities of Lactobacillus spp. to antagonize C. jejuni.
In addition to their bactericidal activity, lactobacilli may be able to attenuate C. jejuni virulence. Indeed, C. jejuni expresses several virulence factors, including those related to motility such as flaA, flaB31,32, flhA, and flhB33, adhesion such as cadF34,35, invasion such as ciaB36 and iamA37 cytotoxin such as cdtA38 and autoinducer production luxS39. Down-regulation of expression of virulence-associated genes in C. jejuni has been shown to attenuate its survival, colonization and invasion ability into chicken and human intestinal epithelial cells40,41. In the present study, all Lactobacillus spp., except L. reuteri, downregulated motility-associated genes. Since motility is required for C. jejuni to escape from stressful changes in the environment and to colonize the mucus layer lining the intestinal epithelium35,42, the observed reduction in the expression of motility-related genes and subsequent impairment of flagellar motility apparatus may consequently result in longer exposure of C. jejuni to antimicrobial substances produced by lactobacilli, thus suppressing its growth.
Invasion of human enterocytes is regarded as an important virulence factor of C. jejuni. Activation of invasion-related genes is required for translocation of C. jejuni across human enterocytes43. In view of this, the observed reduction in the expression of invasion-related genes following exposure to lactobacilli may subsequently reduce C. jejuni invasion of human enterocytes. This observation was further confirmed by examining the ability of C. jejuni to invade human intestinal epithelial cells in the presence or absence of lactobacilli. Our findings are consistent with those reported by Wine and colleagues in that lactobacilli have the potential to attenuate the invasive ability of C. jejuni against human intestinal epithelial cells44.
The expression of virulence genes that are involved in the pathogenesis of C. jejuni is coordinated by a quorum sensing regulatory system45,46. Quorum sensing is the process of cellular communication that allows bacteria to regulate their gene expression in accordance with cell density45,47. This regulation is mediated by diffusible signaling molecules, known as autoinducers45,47. AI-2 is an extracellular signaling molecule produced by many bacteria, including C. jejuni. In C. jejuni, the biosynthesis of AI-2 is catalyzed by the luxS gene product (S-ribosylhomocysteinase) and reaches a threshold level during the mid-exponential growth phase48. Alterations in luxS gene expression can, therefore, alter the production of AI-2. In the present study, we observed a concomitant reduction in the expression of luxS gene and AI-2 levels following exposure to lactobacilli, which is indicative of a disruption of bacterial quorum sensing signals. In addition to these effects, previous studies have also shown that the luxS gene is involved in the regulation of other virulence factors, including the transcription of the flagellin and cytolethal-distending toxin genes, biofilm formation and adherence to and invasion of human intestinal epithelial cells46,49. Therefore, it is conceivable to speculate that the administration of probiotic lactobacilli to chickens may not only reduce C. jejuni colonization, but could also attenuate the ability of C. jejuni to survive, produce cytotoxins, and invade human intestinal epithelial cells, thereby reducing the incidence of human illness. To evaluate this hypothesis, further studies are needed to investigate the ability of C. jejuni, harvested from lactobacilli-treated chickens, to invade human intestinal epithelial cells in vitro or the intestine of a suitable animal model in-vivo.
The second aim of this study was to evaluate the immune-stimulatory effects of lactobacilli on chicken macrophages. Macrophages are known as the main effector cells of the innate immune system and represent the first cellular line of defense against invading pathogens50. Upon activation, macrophages can eliminate pathogens directly through phagocytosis and production of nitric oxide, or indirectly through antigen presentation and secretion of cytokines and other mediators51 which, in turn, initiate a cascade of events leading to activation of other cells of the immune system. In this context, mounting evidence indicates that intestinal macrophages can be activated by the commensal microbes and their metabolites52.
Due to the difficulty of obtaining sufficient numbers of macrophages from the chicken intestine, macrophage-like cells (MQ-NCSU) were used as an in vitro model to study the interplay between lactobacilli and the host immune system. Even though these cells may not be a true representation of chicken macrophages, several research groups, including our group, have shown that these cells possess similar features of the mononuclear phagocyte lineage and exhibit chicken macrophage biology and function17,22,53–55.
Given the prominent role of NO in the disruption of bacterial membrane integrity and eventual killing of bacteria, this study sought to determine the capacity of different Lactobacillus spp. to induce NO production in chicken macrophages. Consistent with a previous study showing that L. acidophilus and L. salivarius, but not L. reuteri, enhance production of NO by chicken macrophages16, we demonstrated that all Lactobacillus spp., except L. reuteri, enhanced the production of NO by macrophages. It should be noted that a higher concentration of L. reuteri significantly inhibited NO production by macrophages, which may explain the absence of synergy of the lactobacilli mixture, as compared with the untreated group.
A series of studies have indicated that probiotics can enhance macrophage phagocytosis in vitro and in vivo. Here, we extend previous findings demonstrating that in vitro treatment of macrophages with lactobacilli enhances the uptake of latex beads into macrophages16,17,22. Further, our results show that pre-treatment of chicken macrophages with lactobacilli enhances their phagocytic activity against C. jejuni, as demonstrated by the high number of internalized C. jejuni as compared to the untreated cells. In the context of enteric infection, a previous study in mice reported that oral administration of probiotic lactobacilli mediates Candida albicans phagocytosis and clearance by intestinal macrophages56. Another study in chickens postulated that intestinal macrophages activated by probiotics may contribute to protection against colonization by Salmonella enterica serovar Enteritidis57.
Collectively, the notable increase in NO levels coupled with the enhancement of macrophage phagocytosis suggests that oral administration of these lactobacilli spp. to chickens can potentially reduce intestinal colonization by C. jejuni, at least in part, through activation of tissue-resident gut macrophages.
The findings of the present study demonstrated that lactobacilli differentially altered cytokine expression profiles in macrophages. The simultaneous induction of both pro-and anti-inflammatory cytokines by lactobacilli is indicative of the unique immunomodulatory functions of these probiotic bacteria, as well as their potential to maintain immune system homeostasis in the host. We have previously shown that the induction of pro-inflammatory cytokines and chemokines in chicken ileum and cecal tonsil contributes to protection against colonization by C. jejuni58,59. Previous studies have also shown that protection against Salmonella enterica serovar Typhimurium correlates with probiotic-induced cytokine and antimicrobial peptide gene expression in cecal tonsils15,25,60. In view of these facts, it is tempting to speculate that the administration of probiotics to chickens can provide protection against colonization of C. jejuni through the induction of cytokine expression in intestinal innate immune system cells.
Recognition and elimination of pathogens requires an effective communication between the innate and adaptive immune systems61. For example, T cell activation is orchestrated by the cellular components of antigen presenting cells such as costimulatory molecules62. Thus, the enhanced cell-surface expression of CD40, CD80, and CD86 molecules in response to lactobacilli will, in turn, lead to a series of interactions between macrophages and T-cell surface molecules that ultimately culminate in adaptive immunity against invading pathogens.
Inconclusion, these findings demonstrated that lactobacilli exhibit differential antagonistic effects against C. jejuni and vary in their ability to stimulate innate responses in chicken macrophages. The failure of L. reuteri to attenuate C. jejuni virulence and to induce NO production in macrophages, indicates that not all lactobacilli possess desirable probiotic properties and may also explain the lack of synergistic effects of the lactobacilli mixture. Nevertheless, L. reuteri has demonstrated the ability to inhibit the growth of C. jejuni and to promote various sets of effector molecules present during innate responses. Despite these promising results, the in vitro models, used in this study, may not accurately mimic the intestinal environment. Thus, further research is warranted to ascertain the effects of these lactobacilli in a complex cellular interaction in an in vivo setting. Future studies will aim to assess the immune responses in gut lymphoid and epithelial cells following administration of probiotic lactobacilli and the potential use of these bacteria, either as single or multiple species, as feed additives to prevent Campylobacter colonization in broiler chickens.
Materials and Methods
Evaluation of antagonistic activity of lactobacilli against C. jejuni
The inhibitory activity of the cell-free supernatant of lactobacilli against C. jejuni was performed using radial diffusion assay as described previously by Arsi et al.21, with minor modifications. Briefly, 108 CFUs of C. jejuni was mixed with 30 mL of MH agar and poured into a 100 mm round Petri dish. Approximately 3 mm diameter holes were punched in the agar and 20 μL of the naturally-acidic or neutralized cell-free supernatant of each Lactobacillus sp., or a mixture of all lactobacilli was added to the holes. After the supernatants were fully absorbed, plates were overlaid with 10 mL of MH agar and incubated at 41 °C under microaerophilic conditions. After an incubation period of 40–48 h, the diameters of the zones of inhibition were measured.
The killing assay was used to determine the bactericidal activity of both the live culture and cell-free supernatants of lactobacilli against C. jejuni. Briefly, equal volumes of the naturally-acidic or neutralized cell-free culture supernatant of each Lactobacillus sp., or the mixture of the supernatants and 107 CFUs of C. jejuni in MH broth were co-incubated at 41 °C overnight under microaerophilic conditions of 10% CO2, 5% O2, and 85% N2. Similarly, equal volumes of 107 CFUs of each Lactobacillus sp. or the mixture of all species in MRS and 107 CFUs of C. jejuni in MH broth were co-incubated overnight under microaerophilic conditions. The C. jejuni culture alone was used as a positive control. Subsequently, 100 µL of each culture was serially-diluted and streaked onto MH agar. Plates were incubated at 41 °C under microaerophilic conditions and the CFUs of C. jejuni enumerated after 40–48 h of incubation.
Effects of lactobacilli on the expression of virulence-related genes in C. jejuni
Equal volumes (500 µL) of 107 CFUs of C. jejuni in MH broth and 108 CFUs of each Lactobacillus sp., or 108 CFUs of the mixture of all species, or MRS medium were incubated at 41 °C for 24 h under microaerophilic conditions.
Prior to RNA extraction, RNAprotect Bacteria Reagent (Qiagen 76506) was added to each culture (2:1) for stabilization of RNA in bacterial cultures and total RNA was subsequently extracted using TRIzol reagent (Invitrogen) according to the manufacturer’s protocol. cDNA synthesis was performed as previously described by Koolman et al.35.
Quantitative real time-PCR (qPCR) was used to measure the transcripts levels of six different housekeeping genes of C. jejuni (gyrA, ilvC, rpoA, slyD, thiC and rrs {16 s RNA}) as previously described by Ritz et al.63. Sequences of all primers are outlined in Table 2. A standard curve was created for each gene and the efficiencies of the primers were calculated using LightCycler® 480 Software (Roche Diagnostics GmbH, Mannheim, DE). SASqPCR was used to assess the PCR amplification efficiency and rank the stability of these genes as previously described64.
Table 2.
Genes and primer sequences used for C. jejuni reference genes and virulence-related genes.
| Target gene | Primer sequence | Reference |
|---|---|---|
| gyrA |
F: GTTATTATAGGTCGTGCTTT R: CTATGAGGTGGGATGTTTGT |
63 |
| ilvC |
F: GCATGCAGAACGCAAAAATA R: TGATCCAAGGCATCATAGCA |
63 |
| rpoA |
F: CGAGCTTGCTTTGATGAGTG R: AGTTCCCACAGGAAAACCTA |
63 |
| slyD |
F: TACGATGAAAATGCCGTTCA R: TTCGCCAAAAAGCTCCATAC |
63 |
| rrs |
F: AAGGGCCATGATGACTTGAC R: AGCGCAACCCACGTATTTAG |
63 |
| thiC |
F: TTATCTTTGGGCGATGCTTT R: CATCCCAAGCCCTTTGAGTA |
63 |
| flaA |
F: GGATGGCGATAGCAGATAGTTT R: CTCATCCATAGCCTTATCAGCA |
67 |
| flaB |
F: ACACCAACATCGGTGCATTA R: CATCCCTGAAGCATCATCTG |
67 |
| flhA |
F: GGAGCGATTAAAGGCCCCAA R: AGTGGTGGCACTTGTCCAAA |
35 |
| flhB |
F: CAGGTGCGGATGTGGTGATC R: CACTCCTTTGGCAACAACCCT |
35 |
| cadF |
F: TTCTATGGTTTAGCAGGTGGAG R: TTACACCCGCGCCATAAT |
67 |
| iamA |
F: GAAGATGCACTTGCTTTGCG R: ATACCGCCACTAAGTTCGCT |
35 |
| ciaB |
F: AAAAGCTTGGCAAGAAGCTG R: ATGCCACCGCATGAGTATAA |
67 |
| cdtA |
F: GGATTTGGCGATGCTAGAGTT R: CATTTGTGCGTGATTGCTTG |
67 |
| luxS |
F: AAAATGCCAGCTCCTGCTGT R: GTGCGACAACCCATAGGTGA |
35 |
Quantitative real time-PCR was used to measure the relative expression of genes responsible for motility such as flaA, flaB, flhA, and flhB, adhesion such as cadF, invasion such as ciaB and iamA, cytotoxin production such as cdtA and autoinducer production luxS. The PCR reactions and cycling conditions were previously described35 and sequences for all primers are outlined in Table 2. A standard curve was created for each gene and the efficiencies of the primers were calculated using LightCycler® 480 Software (Roche Diagnostics GmbH, Mannheim, DE). Expression levels of all genes were calculated relative to the selected reference gene, rrs (16S RNA ribosomal subunit), using the 2−ΔΔCT method (LightCycler® 480 Software, Roche Diagnostics GmbH, Mannheim, DE).
Effect of probiotics on C. jejuni autoinducer-2 production (quorum sensing)
The levels of extracellular AI-2 produced by C. jejuni were measured using the Vibrio harveyi bioluminescence assay as described by Carter et al.65. Briefly, the reporter strain V. harveyi BB170 and the positive control V. harveyi BB152 were grown overnight at 30 °C and diluted 1: 5,000 into autoinducer bioassay (AB) medium consisting of a mixture of two buffers (Buffer1: 0.3 M NaCl, 0.05 M MgSO4, 2% vitamin free casamino acids, pH 7.5; Buffer 2: 1 M KPH4, 0.1M L-arginine hydrochloride, 50% glycerol, pH 7.0). Equal volumes (500 µL) of 107 CFUs of the mid-log culture of C. jejuni in MH broth and the mid-log culture of 107 Lactobacillus sp., or the mixture of lactobacilli in MRS broth were incubated at 41 °C for 8, 24 and 48 h under microaerophilic conditions. In a 96-well black clear bottom plate, 90 µL of the diluted V. harveyi BB170 and 10 µL of the filtered cell-free culture supernatant of both the treated and untreated culture of C. jejuni were added to the wells. The supernatant from V. harveyi BB152 was used as a positive control and AB medium was used as a negative control. Subsequently, the plate was incubated at 30 °C for 24 h with continuous shaking using VictorTM Multilabel plate counter (Wallac, PerkinElmer Life Sciences Canada, Woodbridge, Ontario, Canada). Luminescence production was measured every hour and the maximal bioluminescence was observed at 13 h after incubation with the lactobacilli. Data were presented as the relative light unit (RLU) per unit of absorbance (cell density; OD 600 nm).
Effects of lactobacilli on C. jejuni associated with Caco-2 cells
Caco-2 cells were seeded in 6-well plates at a density of 4 × 105 cells/well in EMEM and incubated at 37 °C in a humidified 5% CO2 environment until 90% confluency was reached. Afterward, 107 CFUs of FITC-labelled C. jejuni in EMEM were added alone or simultaneously with 107 CFUs of each Lactobacillus sp. or a mixture of all species to the Caco-2 cells and incubated for 2, 5 and 8 h. Adherent cells were washed with DPBS (pH 7.4) and fixed with 4% paraformaldehyde. Cells were permeabilized with 0.1% NP40 in PBS and the nucleus was stained with 7-AAD dye. The cell-associated fluorescent C. jejuni was visualized by fluorescence microscopy.
Effects of lactobacilli on nitric oxide (NO) production
MQ-NCSU cells were seeded in a 24-well plate at density 4 × 105 cells/well in LM-HAHN medium and incubated for 3 h. Afterward, cells were stimulated with either a single or a mixture of heat-killed Lactobacillus spp. at a multiplicity of infection (MOI) of 10 or 100 in DMEM and incubated at 41 °C in a humidified 5% CO2 environment for 24 h. Supernatants were collected and NO production was measured by Griess assay (Promega, USA), according to the manufacturer’s protocol.
Effects of lactobacilli on phagocytic activity of chicken macrophage-like cells
Using fluorescent latex beads
A phagocytosis assay kit (IgG FITC; Cayman Chemical Michigan, USA) was used to quantitatively assess the phagocytic activity of macrophages. Briefly, MQ-NCSU cells were seeded, in 6 replicates, into 96-well black clear bottom polystyrene plates at a density 1 × 105 cells/well in LM-HAHN medium and incubated for 3 h at 41 °C in a humidified 5% CO2 environment. Cells were then stimulated with either a single or a mixture of heat-killed Lactobacillus spp. at a MOI of 10 of 100 in DMEM along with the Latex BeadsRabbit IgG-FITC complex (according to manufacturer’s instruction) and incubated at 41 °C in a humidified 5% CO2 environment. After a 2 h incubation, cells were centrifuged at 400 × g for 10 min and the supernatant was discarded. Afterward, 50 μL of trypan blue solution was added to the cells, followed by incubation for 1–2 min at room temperature. Cells were then centrifuged at 400 × g for 10 min and excess trypan blue was removed. Fluorescence intensity was read using a fluorescence plate reader (PerkinElmer multimode plate reader, USA) at an excitation of 485 nm and an emission of 535 nm.
Using FITC-labelled C. jejuni
MQ-NCSU cells were seeded into 6-well plates containing glass coverslips at density 8 × 105 cells/well in LM-HAHN medium as described above. After treatment with 100 MOI of either a single or a mixture of heat-killed lactobacilli, cells were incubated at 41 °C in a humidified 5% CO2 environment for 2 h. Subsequently, cells were washed three times with DPBS prior to infection with 8 × 107 CFUs of FITC-labeled C. jejuni/well in DMEM medium. Cells were incubated for an additional 2 h and then washed three times with PBS. Adherent cells were washed with DPBS (pH 7.4) and fixed with 4% paraformaldehyde. Cells were permeabilized with 0.1% NP40 in PBS and the nucleus was stained with 4′,6-diamidino-2-phenylindole (DAPI). The internalization of fluorescent C. jejuni was visualized by fluorescence microscopy.
Effects of lactobacilli on cytokine gene expression
MQ-NCSU cells were seeded in 24-well plates as described above. Cells were harvested at 3, 6 and 18 h post-treatment and RNA was extracted, and reverse transcribed to cDNA as previously described59. Expression levels of all target genes were calculated relative to the housekeeping gene β-actin using the 2−ΔΔCT method (LightCycler® 480 Software, Roche Diagnostics GmbH, Mannheim, DE). The primers used in this study are outlined in Table 3. The PCR reactions and cycling conditions have been previously described66.
Table 3.
Genes and primer sequences used for cytokines gene expression.
| Target gene | Primer sequence (5′-3′) | Annealing Temp (°C) | Reference |
|---|---|---|---|
| IFN-γ |
F:ACACTGACAAGTCAAAGCCGCACA R:AGTCGTTCATCGGGAGCTTGGC |
60 | 26 |
| IL-1β |
F:GTGAGGCTCAACATTGCGCTGTA R:TGTCCAGGCGGTAGAAGATGAAG |
64 | 68 |
| IL-12p40 |
F: CCAAGACCTGGAGCACACCGAAG R: CGATCCCTGGCCTGCACAGAGA |
60 | 68 |
| CXCLi2 |
F:CCAAGCACACCTCTCTTCCA R:GCAAGGTAGGACGCTGGTAA |
64 | 68 |
| IL-10 |
F:TTTGGCTGCCAGTCTGTGTC R:CTCATCCATCTTCTCGAACGTC |
64 | 58 |
| β-actin |
F:CAACACAGTGCTGTCTGGTGGTA R:ATCGTACTCCTGCTTGCTGATCC |
60 | 68 |
Effects of lactobacilli on the expression of macrophage cell surface proteins
Flow cytometry was used to determine the expression of CD40, CD80, CD86, and major histocompatibility complex (MHC)-II molecules following treatment with heat-killed lactobacilli as previously described22. Briefly, MQ-NCSU cells were seeded, in triplicates, into 24-well plates followed by being treated with 100 MOI of either a single or a mixture of heat-killed lactobacilli. After a 24 h incubation, the cells were stained in two different panels due to the paucity of having multi-colors in our staining panel. First panel used anti-MHC-II antibodies (directly labelled with Fluorescein isothiocyanate, FITC) and anti-CD40 (indirectly stained with anti-mouse IgG1 labelled with phycoerythrin, PE secondary antibodies). The second panel used anti-CD80 and anti-CD86 that were indirectly stained with anti-mouse IgG1- PE and anti-mouse IgG2a- FITC secondary antibodies, respectively. All the antibodies were purchased from AbD Serotec, NC. Dead cells were stained with the viable dye, Live/Dead stain near IR (infrared), purchased from Invitrogen, CA. Briefly, cells were re-suspended in staining buffer (PBS with 1% BSA) with the antibodies added and cells were stained for 30 min on ice and then washed and fixed with 2% paraformaldehyde before data acquisition on BD Canto-II flow cytometer. The gating strategy included excluding doublet cells through forward and side scatters height and width followed by gating on live cells excluding dead cells. Data analysis was carried out using the FlowJo software (Tree Star, Ashland, OR).
Statistical analysis
All analyses were carried out using SAS version 9.3 (SAS, Cary, NC). The effects of lactobacilli on the growth of C. jejuni, virulence-related gene expression, C. jejuni associated with Caco-2 cells, phagocytic activity of macrophages, cytokine gene expression, and the expression of macrophage surface proteins were analyzed using SAS Proc GLM (General Linear Model), followed by Duncan’s multiple range test. The effects of lactobacilli on AI-2 and NO production were analyzed using Kruskal-Wallis followed by Wilcoxon. ImageJ software (https://imagej.net/Downloads) was used to measure the fluorescence intensity of the phagocytosed C. jejuni and to count the number of C. jejuni associated with Caco-2 cells. Data are presented as mean ± standard error of the mean (SEM) using GraphPad Prism V5.0 (GraphPad software, San Diego, CA, USA). P < 0.05 was considered significant for all statistical tests.
Acknowledgements
This research was supported by the Ontario Ministry of Agriculture, Food and Rural Affairs, the Natural Sciences and Engineering Research Council of Canada (NSERC), the Poultry Industry Council and the Canadian Poultry Research Council.
Author contributions
K.T.A. conceived of the study, carried out the experimental work, and drafted the manuscript. K.T.A. and J.A. performed the gene expression experiments. K.T.A., R.K. and L.R. performed the flow cytometry work and data analysis. K.T.A., A.N. and J.F. performed the quorum sensing experiments. S.S. participated in study design and coordination, and helped draft the manuscript. All authors critically reviewed and approved the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Veldhuizen, E. J. A., Brouwer, E. C., Schneider, V. A. F. & Fluit, A. C. Chicken Cathelicidins Display Antimicrobial Activity against Multiresistant Bacteria without Inducing Strong Resistance. PLoS One8 (2013). [DOI] [PMC free article] [PubMed]
- 2.Cheng G, et al. Antibiotic alternatives: The substitution of antibiotics in animal husbandry? Front. Microbiol. 2014;5:1–15. doi: 10.3389/fmicb.2014.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehdi Y, et al. Use of antibiotics in broiler production: Global impacts and alternatives. Anim. Nutr. 2018;4:170–178. doi: 10.1016/j.aninu.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taha-Abdelaziz K, Hodgins DC, Lammers A, Alkie TN, Sharif S. Effects of early feeding and dietary interventions on development of lymphoid organs and immune competence in neonatal chickens: A review. Vet. Immunol. Immunopathol. 2018;201:1–11. doi: 10.1016/j.vetimm.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Patterson JA, Burkholder KM. Application of prebiotics and probiotics in poultry production. Poult. Sci. 2008;4:627–631. doi: 10.1093/ps/82.4.627. [DOI] [PubMed] [Google Scholar]
- 6.Bai SP, et al. Effects of probiotic-supplemented diets on growth performance and intestinal immune characteristics of broiler chickens. Poult. Sci. 2013;92:663–670. doi: 10.3382/ps.2012-02813. [DOI] [PubMed] [Google Scholar]
- 7.Aliakbarpour HR, Chamani M, Rahimi G, Sadeghi AA, Qujeq D. The Bacillus subtilis and lactic acid bacteria probiotics influences intestinal mucin gene expression, histomorphology and growth performance in broilers. Asian-Australasian J. Anim. Sci. 2012;25:1285–1293. doi: 10.5713/ajas.2012.12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pascual, N., Hugas, M., Badiola, J. I., Monfort, J. M. & Garriga, M. Colonization in Chickens. 65, 4981–4986 (1999). [DOI] [PMC free article] [PubMed]
- 9.Zhang G, Ma L, Doyle MP. Salmonellae reduction in poultry by competitive exclusion bacteria Lactobacillus salivarius and Streptococcus cristatus. J. Food Prot. 2007;70:874–878. doi: 10.4315/0362-028X-70.4.874. [DOI] [PubMed] [Google Scholar]
- 10.Ghareeb K, et al. Evaluating the efficacy of an avian-specific probiotic to reduce the colonization of Campylobacter jejuni in broiler chickens. Poult. Sci. 2012;91:1825–1832. doi: 10.3382/ps.2012-02168. [DOI] [PubMed] [Google Scholar]
- 11.Gagnon, M., Zihler A., Chassard, C. & Lacroix, C. Probiotic Bacteria and Enteric Infections-Cytoprotection by probiotic bacteria (ed. Malago, J. J., Koninkx, J. F. J. G. & Marinsek-Logar, R) 65–85 (Springer, 2011).
- 12.Papadimitriou K, et al. Discovering probiotic microorganisms: In vitro, in vivo, genetic and omics approaches. Front. Microbiol. 2015;6:1–28. doi: 10.3389/fmicb.2015.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haghighi, H. R. et al. Modulation of Antibody-Mediated Immune Response by Probiotics in Chickens. 12, 1387–1392 (2005). [DOI] [PMC free article] [PubMed]
- 14.Haghighi HR, et al. Probiotics stimulate production of natural antibodies in chickens. Clin. Vaccine Immunol. 2006;13:975–980. doi: 10.1128/CVI.00161-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haghighi HR, Abdul-Careem MF, Dara RA, Chambers JR, Sharif S. Cytokine gene expression in chicken cecal tonsils following treatment with probiotics and Salmonella infection. Vet. Microbiol. 2008;126:225–233. doi: 10.1016/j.vetmic.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 16.Brisbin JT, Davidge L, Roshdieh A, Sharif S. Characterization of the effects of three Lactobacillus species on the function of chicken macrophages. Res. Vet. Sci. 2015;100:39–44. doi: 10.1016/j.rvsc.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Quinteiro-Filho WM, Brisbin JT, Hodgins DC, Sharif S. Lactobacillus and Lactobacillus cell-free culture supernatants modulate chicken macrophage activities. Res. Vet. Sci. 2015;103:170–175. doi: 10.1016/j.rvsc.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Brisbin JT, Gong J, Sharif S. Interactions between commensal bacteria and the gut-associated immune system of the chicken. Anim. Health Res. Rev. 2008;9:101–110. doi: 10.1017/S146625230800145X. [DOI] [PubMed] [Google Scholar]
- 19.Hemarajata P, Versalovic J. Effects of probiotics on gut microbiota: mechanisms of intestinal immunomodulation and neuromodulation. Therap. Adv. Gastroenterol. 2012;1:39–51. doi: 10.1177/1756283X12459294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomrongsuwannakij T, Chuanchuen R, Chansiripornchai N. Identification of competitive exclusion and its ability to protect against Campylobacter jejuni in broilers. Thai J. Vet. Med. 2016;46:279–286. [Google Scholar]
- 21.Arsi K, Donoghue AM, Woo-Ming A, Blore PJ, Donoghue DJ. The efficacy of selected probiotic and prebiotic combinations in reducing Campylobacter colonization in broiler chickens. J. Appl. Poult. Res. 2015;24:327–334. doi: 10.3382/japr/pfv032. [DOI] [Google Scholar]
- 22.Shojadoost B, et al. Interactions between lactobacilli and chicken macrophages induce antiviral responses against avian influenza virus. Res. Vet. Sci. 2017;17:30043–7. doi: 10.1016/j.rvsc.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 23.Tellez G, Pixley C, Wolfenden RE, Layton SL, Hargis BM. Probiotics/direct fed microbials for Salmonella control in poultry. Food Res. Int. 2012;45:628–633. doi: 10.1016/j.foodres.2011.03.047. [DOI] [Google Scholar]
- 24.Wang S, et al. Prevention of Escherichia coli infection in broiler chickens with Lactobacillus plantarum B1. Poult. Sci. 2017;96:2576–2586. doi: 10.3382/ps/pex061. [DOI] [PubMed] [Google Scholar]
- 25.Akbari MR, et al. Expression of antimicrobial peptides in cecal tonsils of chickens treated with probiotics and infected with Salmonella enterica serovar typhimurium. Clin. Vaccine Immunol. 2008;15:1689–1693. doi: 10.1128/CVI.00242-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brisbin JT, Gong J, Parvizi P, Sharif S. Effects of lactobacilli on cytokine expression by chicken spleen and cecal tonsil cells. Clin. Vaccine Immunol. 2010;17:1337–1343. doi: 10.1128/CVI.00143-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bermudez-Brito M, Plaza-Díaz J, Muñoz-Quezada S, Gómez-Llorente C, Gil A. Probiotic mechanisms of action. Ann. Nutr. Metab. 2012;61:160–174. doi: 10.1159/000342079. [DOI] [PubMed] [Google Scholar]
- 28.Chang MH, Chen TC. Reduction of Campylobacter jejuni in a Simulated Chicken Digestive Tract by Lactobacilli Cultures. J. Food Prot. 2000;63:1594–1597. doi: 10.4315/0362-028X-63.11.1594. [DOI] [PubMed] [Google Scholar]
- 29.Neal-McKinney Jason M., Lu Xiaonan, Duong Tri, Larson Charles L., Call Douglas R., Shah Devendra H., Konkel Michael E. Production of Organic Acids by Probiotic Lactobacilli Can Be Used to Reduce Pathogen Load in Poultry. PLoS ONE. 2012;7(9):e43928. doi: 10.1371/journal.pone.0043928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lehri B, Seddon AM, Karlyshev AV. Lactobacillus fermentum 3872 as a potential tool for combatting Campylobacter jejuni infections. Virulence. 2017;8:1753–1760. doi: 10.1080/21505594.2017.1362533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wassenaar TM, Bleumink-Pluym NM, van der Zeijst BA. Inactivation of Campylobacter jejuni flagellin genes by homologous recombination demonstrates that flaA but not flaB is required for invasion. EMBO J. 1991;10:2055–2061. doi: 10.1002/j.1460-2075.1991.tb07736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grant CC, Konkel ME, Cieplak W, Jr., Tompkins LS. Role of flagella in adherence, internalization, and translocation of Campylobacter jejuni in nonpolarized and polarized epithelial cell cultures. Infect Immun. 1993;61:1764–1771. doi: 10.1128/iai.61.5.1764-1771.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carrillo CD, et al. Genome-wide expression analyses of Campylobacter jejuni NCTC11168 reveals coordinate regulation of motility and virulence by flhA. J. Biol. Chem. 2004;279:20327–20338. doi: 10.1074/jbc.M401134200. [DOI] [PubMed] [Google Scholar]
- 34.Konkel ME, Garvis SG, Tipton SL, Anderson DE, Jr., Cieplak W., Jr. Identification and molecular cloning of a gene encoding a fibronectin-binding protein (CadF) from Campylobacter jejuni. Mol. Microbiol. 1997;24:953–963. doi: 10.1046/j.1365-2958.1997.4031771.x. [DOI] [PubMed] [Google Scholar]
- 35.Koolman L, Whyte P, Burgess C, Bolton D. Virulence gene expression, adhesion and invasion of Campylobacter jejuni exposed to oxidative stress (H2O2) Int. J. Food Microbiol. 2016;220:33–38. doi: 10.1016/j.ijfoodmicro.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Konkel ME, Kim BJ, Rivera-Amill V, Garvis SG. Bacterial secreted proteins are required for the internalization of Campylobacter jejuni into cultured mammalian cells. Mol. Microbiol. 1999;32:691–702. doi: 10.1046/j.1365-2958.1999.01376.x. [DOI] [PubMed] [Google Scholar]
- 37.Carvalho ACT, et al. Molecular characterization of invasive and non-invasive Campylobacter jejuni and Campylobacter coli isolates. J. Clin. Microbiol. 2001;39:1353–1359. doi: 10.1128/JCM.39.4.1353-1359.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pickett CL, et al. Prevalence of cytolethal distending toxin production in Campylobacter jejuni and relatedness of Campylobacter sp. cdtB genes. Infect. Immun. 1996;64:2070–2078. doi: 10.1128/iai.64.6.2070-2078.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elvers KT, Park SF. Quorum sensing in Campylobacter jejuni: detection of a luxS encoded signalling molecule. Microbiology. 2002;148:1475–1481. doi: 10.1099/00221287-148-5-1475. [DOI] [PubMed] [Google Scholar]
- 40.Hermans D, et al. Colonization factors of Campylobacter jejuni in the chicken gut. Vet. Res. 2011;42:82. doi: 10.1186/1297-9716-42-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mohan V. The role of probiotics in the inhibition of Campylobacter jejuni colonization and virulence attenuation. Eur. J. Clin. Microbiol. Infect. Dis. 2015;34:1503–1513. doi: 10.1007/s10096-015-2392-z. [DOI] [PubMed] [Google Scholar]
- 42.Guerry P. Campylobacter flagella: not just for motility. Trends Microbiol. 2007;15:456–461. doi: 10.1016/j.tim.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 43.Backert S, Boehm M, Wessler S, Tegtmeyer N. Transmigration route of Campylobacter jejuni across polarized intestinal epithelial cells: Paracellular, transcellular or both? Cell Commun. Signal. 2013;11:72. doi: 10.1186/1478-811X-11-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wine E, Gareau MG, Johnson-Henry K, Sherman PM. Strain-specific probiotic (Lactobacillus helveticus) inhibition of Campylobacter jejuni invasion of human intestinal epithelial cells. FEMS Microbiol. Lett. 2009;300:146–152. doi: 10.1111/j.1574-6968.2009.01781.x. [DOI] [PubMed] [Google Scholar]
- 45.Zhu J, et al. Quorum-sensing regulators control virulence gene expression in Vibrio cholerae. Proc. Natl. Acad. Sci. 2002;99:3129–3134. doi: 10.1073/pnas.052694299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Plummer PJ. LuxS and quorum-sensing in Campylobacter. Front. Cell Infect. Microbiol. 2012;2:1–9. doi: 10.3389/fcimb.2012.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khmel IA. Quorum-sensing regulation of gene expression: Fundamental and applied aspects and the role in bacterial communication. Microbiology. 2006;75:390–397. doi: 10.1134/S0026261706040047. [DOI] [PubMed] [Google Scholar]
- 48.Cloak OM, Solow BT, Briggs CE, Chen CY, Fratamico PM. Quorum sensing and production of autoinducer-2 in Campylobacter spp., Escherichia coli O157:H7, and Salmonella enterica serovar Typhimurium in foods. Appl. Environ. Microbiol. 2002;68:4666–4671. doi: 10.1128/AEM.68.9.4666-4671.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ligowska M, Cohn MT, Stabler RA, Wren BW, Brøndsted L. Effect of chicken meat environment on gene expression of Campylobacter jejuni and its relevance to survival in food. Int. J. Food Microbiol. 2011;145:111–15. doi: 10.1016/j.ijfoodmicro.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 50.Smith PD, Ochsenbauer-Jambor C, Smythies LE. Intestinal macrophages: Unique effector cells of the innate immune system. Immunol. Rev. 2005;206:149–159. doi: 10.1111/j.0105-2896.2005.00288.x. [DOI] [PubMed] [Google Scholar]
- 51.Kaufmann SHE, Dorhoi A. Molecular determinants in phagocyte-bacteria interactions. Immunity. 2016;44:476–491. doi: 10.1016/j.immuni.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 52.Bain CC, Mowat AM. Macrophages in intestinal homeostasis and inflammation. Immunol. Rev. 2014;260:102–117. doi: 10.1111/imr.12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qureshi MA. Avian macrophage and immune response: an overview. Poult. Sci. 2003;82:691–8. doi: 10.1093/ps/82.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qureshi MA, Miller L, Lillehoj HS, Ficken MD. Establishment and characterization of a chicken mononuclear cell line. Vet. Immunol. Immunopathol. 1990;26:237–250. doi: 10.1016/0165-2427(90)90094-9. [DOI] [PubMed] [Google Scholar]
- 55.Barjesteh N, Taha-Abdelaziz K, Kulkarni RR, Sharif S. Innate antiviral responses are induced by TLR3 and TLR4 ligands in chicken tracheal epithelial cells: Communication between epithelial cells and macrophages. Virology. 2019;534:132–142. doi: 10.1016/j.virol.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 56.Marranzino G, Villena J, Salva S, Alvarez S. Stimulation of macrophages by immunobiotic Lactobacillus strains: Influence beyond the intestinal tract. Microbiol. Immunol. 2012;56:771–781. doi: 10.1111/j.1348-0421.2012.00495.x. [DOI] [PubMed] [Google Scholar]
- 57.Higgins SE, et al. Effect of probiotic treatment in broiler chicks on intestinal macrophage numbers and phagocytosis of Salmonella enteritidis by abdominal exudate cells. Poult. Sci. 2007;86:2315–2321. doi: 10.3382/ps.2007-00123. [DOI] [PubMed] [Google Scholar]
- 58.Taha-Abdelaziz K, et al. Gene expression profiling of chicken cecal tonsils and ileum following oral exposure to soluble and PLGA-encapsulated CpG ODN, and lysate of Campylobacter jejuni. Vet. Microbiol. 2017;212:67–74. doi: 10.1016/j.vetmic.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 59.Taha-Abdelaziz K, et al. Oral administration of PLGA-encapsulated CpG ODN and Campylobacter jejuni lysate reduces cecal colonization by Campylobacter jejuni in chickens. Vaccine. 2018;36:388–394. doi: 10.1016/j.vaccine.2017.11.073. [DOI] [PubMed] [Google Scholar]
- 60.Hu JL, et al. Modulation of cytokine gene expression by selected Lactobacillus isolates in the ileum, caecal tonsils and spleen of Salmonella-challenged broilers. Avian Pathol. 2015;44:463–469. doi: 10.1080/03079457.2015.1086725. [DOI] [PubMed] [Google Scholar]
- 61.Clark R, Kupper T. Old meets new: The interaction between innate and adaptive immunity. J. Invest. Dermatol. 2005;125:629–637. doi: 10.1111/j.0022-202X.2005.23856.x. [DOI] [PubMed] [Google Scholar]
- 62.Lim TS, et al. CD80 and CD86 differentially regulate mechanical interactions of T-cells with antigen-presenting dendritic cells and B-cells. PLoS One. 2012;7:1–8. doi: 10.1371/annotation/f0a21e28-7f3c-4b76-870e-128dd89d0e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ritz M, Garenaux A, Berge M, Federighi M. Determination of rpoA as the most suitable internal control to study stress response in C. jejuni by RT-qPCR and application to oxidative stress. J. Microbiol. Methods. 2009;76:196–200. doi: 10.1016/j.mimet.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 64.Ling Daijun. SASqPCR: Robust and Rapid Analysis of RT-qPCR Data in SAS. PLoS ONE. 2012;7(1):e29788. doi: 10.1371/journal.pone.0029788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carter GP, Purdy D, Williams P, Minton NP. Quorum sensing in Clostridium difficile: Analysis of a luxS-type signalling system. J. Med. Microbiol. 2005;54:119–127. doi: 10.1099/jmm.0.45817-0. [DOI] [PubMed] [Google Scholar]
- 66.Taha-abdelaziz K, Alkie TN, Hodgins DC, Shojadoost B, Sharif S. Characterization of host responses induced by Toll-like receptor ligands in chicken cecal tonsil cells. Vet. Immunol. Immunopathol. 2016;174:19–25. doi: 10.1016/j.vetimm.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 67.Chaisowwong W, et al. Physiological Characterization of Campylobacter jejuni under cold stresses conditions: Its Potential for Public Threat. J. Vet. Med. Sci. 2012;74:43–50. doi: 10.1292/jvms.11-0305. [DOI] [PubMed] [Google Scholar]
- 68.St. Paul M, et al. Characterization of chicken thrombocyte responses to toll-like receptor ligands. PLoS One. 2012;7:2–10. doi: 10.1371/journal.pone.0043381. [DOI] [PMC free article] [PubMed] [Google Scholar]