Abstract
Exposure to early psychosocial deprivation as a result of institutional care disrupts typical brain development. The Bucharest Early Intervention Project (BEIP) is the first longitudinal study to investigate the neurodevelopment of institutionalized infants randomized to a foster care (FCG) intervention versus care as usual (CAUG). Here, we present findings from a follow-up assessment of brain electrical activity as indexed by resting EEG at age 16 years. In addition, we examined the effects of disruption of foster care placement, (e.g. the number of moves among foster care placements), on brain electrical activity. Resting-state EEG was collected from 48 CAUG, 46 FCG and 48 never institutionalized (NIG) control participants. Absolute (µV2) and relative (proportion) power were computed from eyes closed, resting EEG data for theta, alpha, and beta frequency bands. The CAUG displayed higher relative theta and lower relative alpha power compared to the FCG at age 16 years. The FCG showed brain activity comparable to the NIG. The results further showed that disruptions following the original foster care placement had an adverse effect on brain electrical activity. Within the foster care group, there were no effects of age of placement on EEG power. Placement of children who have experienced early institutional rearing into stable foster care settings ensures long-term improvement in brain functioning.
Keywords: institutional care, foster care intervention, BEIP, EEG, alpha power, theta power
Introduction
Throughout the world, a large number of abandoned and orphaned children live in institutions. Although the quality of care varies among and within these settings, many institutions do not provide typical environments necessary for healthy human development (Mason & Narad, 2005; Nelson et al., 2007). Given the challenge of meeting the needs of young children at many levels, institutions, even in the best circumstances, are characterized by suboptimal rearing environments and psychosocial deprivation (Gunnar, Bruce, & Grotevant, 2000; Nelson, 2007). Institutions often have a high child-to-caregiver ratio, strict regimented schedules, a lack of sensitivity to children’s need, and inadequate linguistic, cognitive, and sensory stimulation (Nelson, 2007). Studies suggest that exposure to severe early psychosocial deprivation results in long-term detrimental effects across various developmental domains, including reduced physical growth, lower IQ, poor executive skills, emotion dysregulation, elevated symptoms of attention-related disorders, and altered brain structure and function (Beckett et al., 2006; McLaughlin et al., 2010; Smyke et al., 2007).
The deleterious developmental effects across multiple domains in institutionalized children might be shaped by disrupted or perturbed brain development. Some neuroimaging studies have shown that early institutional deprivation alters the typical development of brain structure and function (Behen et al., 2009; Chugani et al., 2001; Eluvathingal et al., 2006; Mehta et al., 2009). For example, studies using diffusion tensor imaging (DTI) found decreased white matter brain connectivity of the uncinate fasciculus (Eluvathingal et al., 2006) in children exposed to early institutional deprivation compared to children reared in families. Also, structural MRI studies have shown a significant reduction of grey matter volume in the prefrontal cortex, hippocampus (Hodel et al., 2015) and cerebellum (Bauer, Hanson, Pierson, Davidson, & Pollak, 2009) in children with a history of institutional rearing compared to children raised with their biological families. However, none of the neuroimaging studies other than the Bucharest Early Intervention Project (BEIP) have examined the long-term effects on brain development as a result of prolong institutional care and early foster care intervention among previously institutionalized children. The unique aspect of the BEIP study is that it is a randomized control trial and thereby has the ability to experimentally examine the impact and placement timing of a foster care intervention on brain development among previously institutionalized children as well as the impact of an extended institutional care.
The BEIP study is the only randomized control trial of foster care as an alternative to institutional care for previously institutionalized children (Zeanah et al., 2003). The BEIP follows a group of institutionalized infants who either received care as usual (CAUG) or were randomized to high quality foster care (FCG), and a group of demographically matched never institutionalized group (NIG) of comparison children. This study aimed to investigate the physical, psychological and neural development related to institutional care, as well as the effects of foster care intervention beginning early in life (Zeanah et al., 2003).
Previous findings from the BEIP have shown profound detrimental effects of institutional care and improvement because of foster care intervention on brain development. At the baseline assessment of resting EEG power at age 22 months, institutionalized children showed a higher proportion of theta power and a lower proportion of alpha and beta power compared to a never institutionalized control group (Marshall, Fox, & BEIP Core Group, 2004). This pattern of reduced higher frequency power (typically in the alpha range) combined with elevated lower frequency power (e.g., in the delta and theta ranges) has been associated with ADHD, learning disorders, disruptive behavior disorders, and psychosocial risk factors, which are prevalent among children exposed to early institutional rearing (Barry, Clarke, & Johnstone, 2003; Chabot, di Michele, & Prichep, 2005; McLaughlin et al., 2010; Otero, Pliego-Rivero, Fernández, & Ricardo, 2003). After foster care placement, when children were 54 months old, there was a trend for a positive effect of the foster care placement intervention, with earlier age of placement associated with increased alpha power (Marshall, Reeb, Fox, Nelson, & Zeanah, 2008). The impact of foster care and the timing of intervention were clearly evident when the participants were examined at age 8 years, as earlier foster care placement was associated with greater alpha power. The 8-year assessment further showed that children randomized to foster care before 24 months of age displayed identical alpha activity to the NIG, whereas children placed into foster care after 24 months were indistinguishable from the CAUG (Vanderwert, Marshall, Nelson, Zeanah, & Fox, 2010). The positive effects of the foster care intervention remained at age 12 years, as the FCG continued to show decreased theta power and increased alpha power compared to CAUG; alpha power of the FCG and NIG were comparable, whereas the CAUG continued to display a higher proportion of theta power compared to the other groups (Vanderwert, Zeanah, Fox, & Nelson, 2016).
In the current study, we examine data from follow-up measures of resting-state EEG in the BEIP at 16 years of age. The main goal of this study was to examine the continuous effect of foster care intervention on brain activity in children removed from institutions and placed into foster care in infancy. Our primary hypothesis was that the intervention effect, as evidenced in lower theta and higher alpha power at age 12 in the FCG compared the CAUG, would persist through age 16, the current assessment. Our previous report at age 12 showed that the EEG power of the FCG and NIG were comparable. As such, we expected no statistically significant differences of EEG power between the FCG and the NIG in the current assessment. At 8 years (Vanderwert et al., 2010), we showed earlier placement into foster care resulted in greater alpha power. However, in the 12 years assessment (Vanderwert et al., 2016), the timing effect in the foster care had waned (i.e., no longer statistically significant). Nevertheless, given the possibility of “sleeper effects” (Mauer, Mondloch, & Lewis, 2007) we examined whether placement timing (i.e., age at original placement into foster care) played a role in recovery.
We also wanted to assess the effects of disruptions in foster care placement on brain activity. Across several recent papers we have observed that children who experienced multiple placement disruptions showed less recovery than those experiencing fewer placement disruptions (Almas et al., 2018; Fox, Almas, Degnan, Nelson, & Zeanah, 2011; Humphreys et al., 2015). Children living in foster care often experience disruption or instability in the caregiving environment. The disruption can include moves among foster care placements or reunification with biological family (Almas et al., 2018). Caregiver disruptions have been associated with decreases in IQ and higher rates of psychopathology including increased internalizing disorders, externalizing disorders and attention-deficit hyperactivity disorder (ADHD) in the BEIP cohort (Almas et al., 2018; Fox, Almas, Degnan, Nelson, & Zeanah, 2011; Humphreys et al., 2015), as well as adverse behavioral and academic outcomes in other groups of children in foster care (Newton, Litrownik, & Landsverk, 2000; Rubin, O’Reilly, Luan, & Localio, 2007). However, no studies to date have examined whether disruptions in the caregiving environment influences brain function among post-institutionalized children. Hence, the effect of placement disruptions on the neuronal outcome is not known. We hypothesized that more caregiver disruptions would result in reduced alpha and increased theta power.
Method
Participants
At the inception of the BEIP study, 187 infants and young children were initially assessed from six institutions in Bucharest, Romania. 51 infants were excluded for serious medical and genetic disorders, resulting in 136 institutionalized infants selected for the study (for details about the trial design and participant selection see Zeanah et al., 2003). At baseline, the age of these institutionalized infants ranged from 6 to 31 months and had spent more than half of their life in institutions care at the time of recruitment for the study. After baseline assessment, 68 infants remained in institutions receiving care as usual (CAUG) and 68 infants were randomized to foster care (FCG). The mean age of placement into foster care was 22.63 months (range = 6.81 to 33.01 months). An age- and gender-matched group of 72 typically developing children who never spent time in institutions (NIG) were also recruited at baseline assessment.
At the 16-year assessment, resting-state EEG was collected from 48 CAUG (22 female; M age= 16.68, SD= .54), 46 FCG (23 female; M age= 16.75, SD=.62) and 48 NIG (30 female; M age= 17.03, SD= .55) participants. After preprocessing of EEG data, 4 participants were excluded from the analysis because they had too few (< 60) artifact free epochs (CAUG: n= 1, FCG: n = 1, NIG: n = 2). Of the original sample, 20 CAUG, 22 FCG and 24 NIG participants were not available for the 16-year assessment.
Over the years since of the inception of the BEIP study, Romania has undergone a number of cultural, political and policy changes, which led to the formation of a public foster care program to reduce the number of children living in institutions. As a result of these policies, many of the CAUG and FCG children from BEIP study were removed from institutions or the BEIP project’s foster care and placed into government foster care or reunited with their biological families. Although many of the CAUG and FCG children were no longer in their randomized placements at age 16, we adopted an intent-to-treat approach for the data presented in this paper. The intent-to-treat approach considers each participant as an affiliate to the initial randomly assigned group and allows direct assessment of foster care intervention by comparing the intervention (FCG) and institutionalized (CAUG) groups.
The University Institutional Review Boards of the principal investigators (Fox, Nelson & Zeanah) and of the University of Bucharest, Romania approved the 16-year study protocol. Informed consent was obtained from each subject for participation in the study.
EEG recording and analysis
EEG was recorded while the participants sat quietly in a chair using a 64-channel HydroCel Geodesic Sensor Net and a NetAmps 300 amplifier (Electrical Geodesics, Inc., Eugene, OR). The vertex (Cz) electrode was used as online reference. EEG data were sampled at 500Hz and impedances were kept below 50 kΩ. The resting EEG was recorded for 6 minutes, alternating 1 minute of eyes open and eyes closed. During the eyes open condition, the subjects were instructed to fixate on a small white cross on the center of a computer screen.
EEG data were exported to a Matlab (Mathworks, Natick, MA) compatible format using Net Station software for offline processing with EEGLAB toolbox (Delorme and Makeig, 2004) and Matlab scripts. EEG data were down sampled to 250Hz and bandpass filtered (0.3 to 40Hz). Artifact-laden channels were identified and removed using the EEGLAB plug-in FASTER (Nolan et al., 2010). To further remove ocular artifacts and generic noise, independent component analysis (ICA) was performed on continuous EEG data. Artifactual independent components (ICs) were identified and removed from the data using the ADJUST plugin (Mognon et al., 2011) of EEGLAB and by visual inspection of individual ICs. EEG data were then segmented into 2 sec epochs with 1 sec (50%) overlap for both eyes open and eyes closed conditions. Epochs containing artifact were removed from the analysis using a voltage threshold (±150µV) rejection. After artifact rejection, missing channels were interpolated using spherical interpolation and epoched data were re-referenced to an average of all channels. Across all participants, on average 2.39 channels were interpolated (M. 2.39, STD. 1.01, range 1–5). After preprocessing, participants with less than 60 artifact-free epochs were excluded from further spectral power analysis.
Spectral power computation
The artifact-free epoched data were spectrally analyzed using a Fast Fourier transform (FFT) with a 2 sec Hanning window. Spectral power (µV2) was computed in the eyes closed condition for theta (4–7 Hz), alpha (8–13 Hz), and beta (14–25 Hz) frequency band. The eyes closed condition was analyzed as EEG power is stronger when eyes are closed in a resting condition (Barry, Clarke, Johnstone, & Brown, 2009; Barry, Clarke, Johnstone, Magee, & Rushby, 2007). For each frequency band, we computed both absolute and relative power. Absolute power was computed by taking the natural logarithm of spectral power in each frequency band. Absolute power reflects the total energy intensity of a certain brain region as measured by scalp electrodes at different frequencies (Machado et al., 2007). Relative power is a measure of how much a particular frequency band contributes to the total power at a given scalp site and is computed as the proportion of power in a specific frequency band relative to the total power in the EEG power spectrum (Marshall, Fox, & Group, 2004). For a given scalp electrode, EEG power can be measured as absolute or relative power and their relative merit as a representation of EEG band power has been debated in the context of brain maturation (Somsen, van’t Klooster, van der Molen, van Leeuwen, & Licht, 1997). Since relative power values are proportion scores, an increase or decrease in absolute power in one frequency band affects the relative power in other bands. Thus, relative power reduces the individual differences in the magnitude of absolute power, which results largely from interindividual variation in anatomical factors such as skull thickness (Marshall et al., 2004; Marshall, Reeb, Fox, Nelson, & Zeanah, 2008). Moreover, because of higher test-retest reliability, relative power is considered as a more reliable measure of EEG power than absolute power (Fernández et al., 1993). However, because of the relative merit of both metrics, the previous assessment from BEIP study reported both absolute as well as relative power and current study adopted this approach. Relative power was computed as the proportion of power in each frequency band at a given electrode site relative to the total power (4–25 Hz) at that electrode site. The absolute and relative power in each frequency band was averaged over clusters of electrodes overlying approximately the left and right frontal (F3, F4), central (C3, C4), parietal (P3, P4) and occipital (O1, O2) scalp sites according to the international 10/20 system (Figure. 1).
Figure. 1.
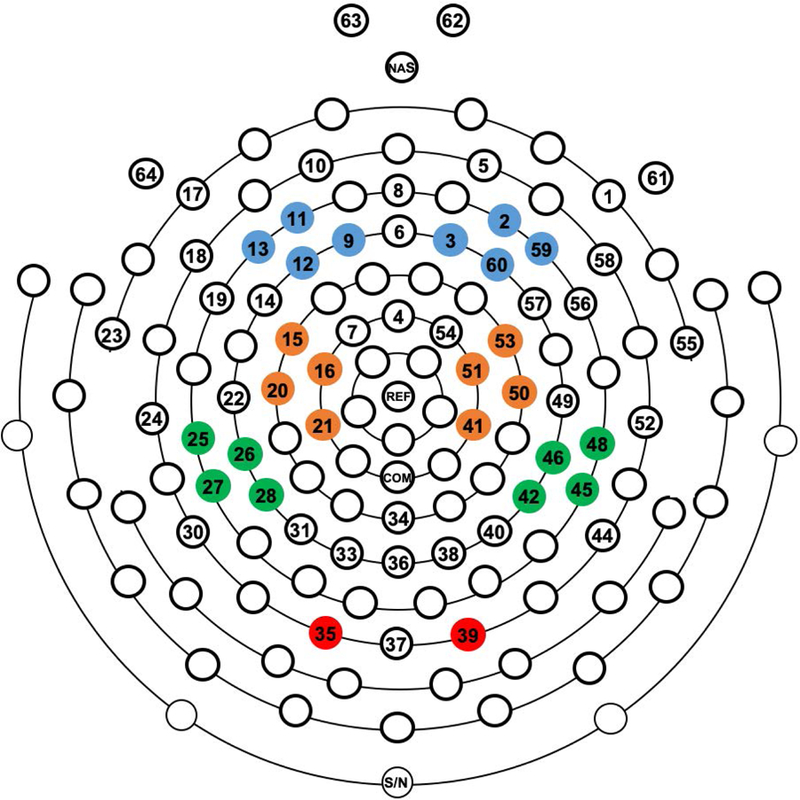
Example of the 64-channel EEG net with electrode clusters highlighted in color. Electrode clusters, from top to bottom, for Frontal, Central, Parietal, and Occipital scalp locations.
Statistical analysis approach
In order to test our primary hypothesis that there was an intervention effect, we adopted the intent-to-treat analysis approach and first directly compared the spectral power between FCG and CAUG separately for each frequency band. We performed separate mixed-design analysis of variance (mixed-ANOVA) for each frequency band of absolute and relative power with Region (frontal, central, parietal, occipital) and Hemisphere (left, right) as within-subject factors, and Group (CAUG, FCG) as the between-subjects factor. Greenhouse–Geisser correction was applied for violations of sphericity. Post-hoc comparisons were performed for a significant main effect of Group. Independent samples t-test was used for decomposing interactions involving Group. Any main and interaction effects not involving Group were not followed-up. Bonferroni correction was applied for all post-hoc comparisons.
We then examined whether the spectral power of the two intervention groups (FCG, CAUG) was comparable with the never institutionalized control (NIG) participants. As the intent-to-treat analysis did not show any interaction involving Group, we computed average power of all electrode clusters across scalp for comparison with the NIG. We performed separate one-way ANOVA for absolute and relative power in each frequency band with averaged spectral power as the dependent variable and three groups (CAUG, FCG, NIG) as the between-subjects variable.
We next explored the effect of timing of foster care placement on brain electrical activity within the FCG. We performed a series of Pearson correlations between averaged spectral power of each frequency band with the age at foster care placement. To further probe whether there is a timing threshold linked to foster care placement, we adopted an approach described in previous BEIP studies (Nelson et al., 2007; Vanderwert, Marshall, Iii, Zeanah, & Fox, 2010). We split the FCG group into those placed into foster care before 24 months (N=20) and those after 24 months (N=22) of age and performed independent samples t-test to assess the group differences.
Finally, we examined the effect of placement disruption on EEG power in each frequency band for both absolute and relative power. We performed a series of Pearson correlations between spectral power averaged across all electrode clusters in each frequency band with the number of disruptions in foster care placement.
Results
Intent-to-treat analysis
Following the intent-to-treat analysis approach, first we directly compared the spectral power between FCG and CAUG separately for each frequency band.
Absolute power
The intent-to-treat analysis did not reveal significant main effects or interactions involving group (FCG and CAUG) for absolute power in theta (4–7 Hz), alpha (8–13 Hz) or beta (14–25 Hz) frequency bands (Figure. 2).
Figure. 2.
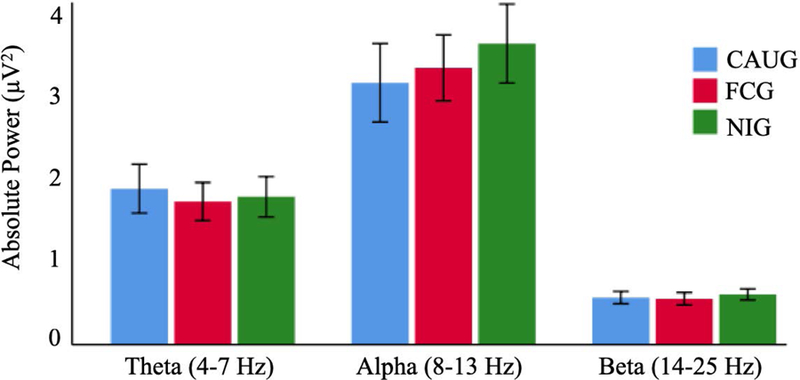
Mean absolute power (μV2) in theta, alpha and beta frequency band for the care-as-usual group (CAUG), foster care group (FCG) and never-institutionalized group (NIG). Error bars indicate +/− 2 standard error.
Relative power
Examination of relative alpha (8–13 Hz) power showed a significant main effect of Group (F(1,90) = 6.972, p = .010). Follow-up comparisons revealed that the FCG (M = .588, SE = .016) had greater relative alpha power than the CAUG (M = .530, SE = .015) (Figure. 3). There were also main effects of Region (F(2.274, 204.697) = 32.070, p < .001) and Hemisphere (F(1,90) = 4.633, p = .034). There were no interaction effects involving Group. Similar analysis for relative beta (14–25 Hz) power did not show a significant main effect of Group (F(1,90) = .644, p = .424) (Figure. 3) or interactions involving Group. The examination of intervention effects in the relative theta (4–7 Hz) power revealed a significant main effect of Group (F(1,90) = 7.385, p = .008). Follow-up comparisons showed that the CAUG (M = .369, SE = .013) had greater relative theta power compared to the FCG (M = .318, SE = .013) (Figure. 3). The ANOVA revealed a main effect of Region (F(2.144, 192.964) = 80.232, p < .001) and Hemisphere (F(1,90) = 4.495, p = .037). There was no interaction involving Group.
Figure. 3.
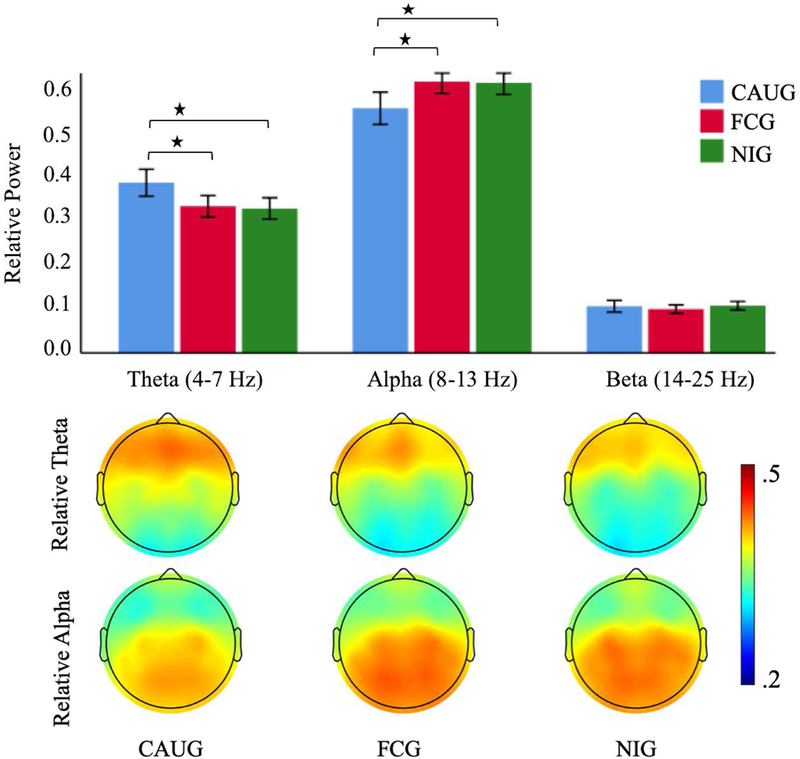
Mean relative power (top) in theta, alpha and beta frequency band for the care-as-usual group (CAUG), foster care group (FCG) and never-institutionalized group (NIG). Error bars indicate +/− 2 standard error. * p < .05. Topographic maps (bottom) display distribution of relative theta and alpha power across the scalp for the CAUG, FCG and NIG.
Institutional care and foster care compared to typically developing children
We examined whether the spectral power of the two intervention groups (FCG, CAUG) was comparable with the never institutionalized control (NIG) participants.
Absolute power
The comparison of three groups did not show a main effect of Group for absolute power in any frequency band (Figure. 2).
Relative power
The comparison of relative alpha power (Figure. 3) revealed a significant main effect of Group (F(2,135) = 5.051, p = .008). Post hoc comparison showed that compared to CAUG (M =.530, SE = .018), the NIG (M =.585, SE = .013, p = .024) had significantly greater relative alpha power. There was no difference between NIG and FCG (M = .588, SE = .013, p = 1.000). The three group analysis of relative beta power (Figure. 3) did not reveal a main effect of Group (F(2,135) = .558, p = .574). The three group analysis for relative theta power (Figure. 3) revealed a main effect of Group (F(2,135) = 5.950, p = .003). Post hoc analysis showed that CAUG (M = .369, SE = .016, p = .007) had significantly greater relative theta power compared to the NIG (M = .312, SE = .012). There was no difference between NIG and FCG (M = .318, SE = .012, p=1.000).
Timing effects
As we observed at age 12, no significant correlations were found between age at foster care placement and spectral power in any frequency band for either absolute or relative power (all p > .05). Likewise, there was no significant difference between the the FCG children placed into foster care before and after 24 months of age (FCG<24M, FCG>24M) for either absolute or relative power in any frequency band.
Effect of disruption of placement
There were no significant correlations between the disruption of foster care placement and absolute power in any frequency band. However, examination of relative power revealed significant relations with disruptions of foster care placement. The disruption of foster care placement was significantly related to relative alpha power (r(45) = −.329, p =.028), such that more disruptions were associated with lower alpha power (Figure. 4). However, there was no significant correlation between relative beta power and the placement disruption (r(45) = .127, p =.404). There also was a significant correlation between the number of placement disruptions and relative theta power (r(45) = .303, p =.043), such that more disruptions were associated with greater theta power (Figure. 4).
Figure. 4.
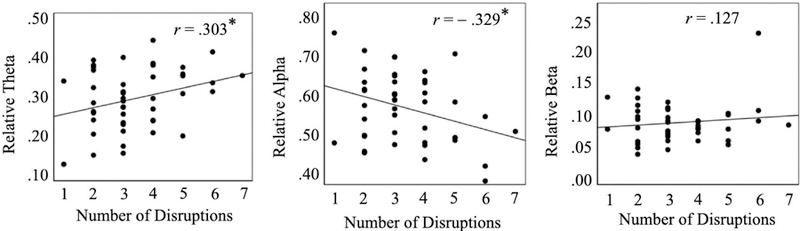
Scatterplots showing 16-year relative power in theta, alpha and beta band against number of disruptions of placement from birth to age 16 years for foster care group. The Pearson correlation coefficients for the relation between placement disruption and EEG power are also shown. * p < .05.
Discussion:
Previous findings from BEIP have shown that early placement of young institutionalized children into high quality foster care normalized the pattern of EEG power to be comparable to typically developing never institutionalized children. In this 16-year follow-up study, we examined the continuing effect of foster care placement on brain activity in institutionalized children placed into foster care. The results demonstrated the continuous positive effects of the foster care intervention. The foster care group (FCG) showed greater alpha and lower theta power than those who received care as usual (CAUG) as was found at 12-year assessment. The FCG showed comparable power with the NIG whereas the CAUG showed an immature pattern of brain activity with lower power in alpha and higher power in theta frequency band compared to both the FCG and NIG. Similar to age 12, we found no effect of timing of foster placement in early childhood on brain activity at age 16. While the FCG as a group was not different from NIG, those who experienced more caregiving disruptions had less alpha and higher theta power a pattern of brain activity pattern similar to that observed in the CAUG. Altogether the present findings suggest that high-quality foster care and a stable caregiving environment are essential for long-term optimal brain functioning of previously institutionalized children.
Reduced alpha power and elevated theta power suggest a deficit in cortical maturation in CAUG group. This pattern of immature neuronal activity, characterized by greater power in the slower frequency band combined with reduced power in a faster frequency, has been observed in children reared in social isolation and deprivation (Gendreau, Freedman, Wilde, & Scott, 1972; Zubek, Welch, & Saunders, 1963), extreme stress (Montes, Alcántara, Cedeño, García, & Rojas, 2015), poor environmental stimulation (Otero, 1994, 1997) and adverse family environment (Bick, Palmwood, Zajac, Simons, & Dozier, 2018). Bick et al. (2018) examined the relations between early family adversity and brain electrical activity and found that early family adversity was associated with greater power in theta frequency band and lower power in alpha frequency band in middle childhood. The immature cortical activity may be the result of deficits in the structural and functional development of the brain. Previous studies have reported that institutional rearing and adversity in early life lead to a reduction in cortical thickness (Kelly et al., 2013; McLaughlin et al., 2014), decreased white matter volume (Sheridan, Fox, Zeanah, McLaughlin, & Nelson, 2012) and white matter connectivity (Tendolkar, Mårtensson, Kühn, Klumpers, & Fernández, 2018). Sheridan et al. (2012) used structural MRI to examine brain structure and function in the CAUG, FCG and NIG from the BEIP cohort between the ages of 8 and 11 years. They found that the cortical white matter was no different for the FCG than the NIG but was significantly smaller for the CAUG children. In the present study, the atypical brain activity of the CAUG might reflect continued deficits in the structural and functional development of the brain of this group.
The findings of the current 16-year follow-up study converge with the effect of the foster care intervention that was reported at the 12-year assessment (Vanderwert et al., 2016). At age 12, the FCG showed comparable brain activity with the NIG in all frequency bands in both absolute and relative power (Vanderwert et al., 2016). The FCG and NIG displayed comparable brain activity in the current 16-year assessment in all frequency bands of absolute and relative power. Also, at both age 12 and now 16, in the more fine grained analysis of differences based on timing of placement into foster care, there were no associations between the age at placement and EEG power. But since the oldest children in the study were randomized at 31 months of age, the differences in EEG power between the FCG and CAUG are strong evidence for the importance of altering early trajectories. Together these findings suggest that early intervention and continued high quality care can improve and normalize the cortical activity of individuals who have experienced severe early psychosocial deprivation. Our findings are consistent with a number of studies that have shown that early intervention altered and normalized neural activity of deprived children (Bick et al., 2018; Raine et al., 2001).
Of note, researchers have consistently reported the adverse effects of placement disruptions on behavioral (Rubin et al., 2007), socio-emotional (Lewis, Dozier, Ackerman, & Sepulveda-Kozakowski, 2007), physical (Johnson et al., 2018), and cognitive development (Almas et al., 2018) for children living in foster care. Almas et al. (2018) examined the impact of disruptions in caregiving on cognitive, behavioral, and social outcomes at age 12 in the BEIP cohort and found that caregiving disruptions predicted increases in externalizing and internalizing behavior problems, even after controlling for internalizing and externalizing problems in early childhood. In this study, we examined the effect of disruption of foster care placement on neural activity of the brain. Our result showed a clear association between placement disruptions and brain activity in children who received foster care intervention. A higher number of disruptions was associated with increased relative theta power and decreased relative alpha power, a pattern of brain activation often observed in children living in impoverished environments (Bick et al., 2018) and in the CAUG. On the other hand, fewer disruptions were associated with decreased relative theta power and increased relative alpha power, a pattern typically observed in never institutionalized children (Marshall et al., 2004; Vanderwert et al., 2016). While our results do not indicate a causal connection between caregiver disruptions and brain development, they do illustrate the benefits of a stable high-quality foster care intervention for children who have experienced early institutional rearing. Given that caregiver disruptions are common, these findings along with those reported by others have significant implications on social policy to ensure stable foster care placements and healthy development of previously institutionalized children.
In summary, the current study demonstrates the long-term impacts of institutionalization and early foster care intervention on brain function. At 16 years, the children placed into foster care showed the pattern of brain activation, which is comparable to never institutionalized children whereas the children who remained in the institution showed immature brain activation. Our findings demonstrate the long-term positive effects of early foster care intervention as an alternative to institution rearing. In addition, this study reveals new information about the effect of disruption of foster care placement on brain activity. Our results suggest that disruption of placement is detrimental for children who are living in foster care. Together the present findings suggest that early foster care intervention and stability of placement can alter the brain function and yield long-term improvements in neural activity for children living in institutions.
Highlights.
Children with a history of institutionalization, placed into foster care, showed normative pattern of brain activation at age 16 years.
The children who remained in institutions showed immature brain activation.
Disruption of placement is detrimental for children who are living in foster care.
Early placement in foster care and stability of placement can improve brain function and yield long term improvements in brain activity for children living in institutions.
Acknowledgement
We thank all the families and children who participated in this study. We also acknowledge the invaluable contributions of our research staff in Bucharest for their contributions to this work. This work was supported by the Binder Family Foundation, the Jacobs Foundation, and the National Institute of Mental Health of the National Institutes of Health (R01MH091363 to CAN, CHZ & NAF).
References
- Almas AN, Papp LJ, Woodbury MR, Nelson CA, Zeanah CH, & Fox NA (2018). The Impact of Caregiving Disruptions of Previously Institutionalized Children on Multiple Outcomes in Late Childhood. Child Development, 00(0), 1–14. 10.1111/cdev.13169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry RJ, Clarke AR, Johnstone SJ, & Brown CR (2009). EEG differences in children between eyes-closed and eyes-open resting conditions. Clinical Neurophysiology, 120(10), 1806–1811. 10.1016/j.clinph.2009.08.006 [DOI] [PubMed] [Google Scholar]
- Barry RJ, Clarke AR, Johnstone SJ, Magee CA, & Rushby JA (2007). EEG differences between eyes-closed and eyes-open resting conditions. Clinical Neurophysiology, 118(12), 2765–2773. 10.1016/j.clinph.2007.07.028 [DOI] [PubMed] [Google Scholar]
- Barry RJ, Clarke AR, & Johnstone SJ (2003). A review of electrophysiology in attention-deficit/hyperactivity disorder: I. Qualitative and quantitative electroencephalography. Clinical Neurophysiology, 114(2), 171–183. 10.1016/S1388-2457(02)00362-0 [DOI] [PubMed] [Google Scholar]
- Bauer PM, Hanson JL, Pierson RK, Davidson RJ, & Pollak SD (2009). Cerebellar Volume and Cognitive Functioning in Children Who Experienced Early Deprivation. Biological Psychiatry, 66(12), 1100–1106. 10.1016/j.biopsych.2009.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckett C, Maughan B, Rutter M, Castle J, Colvert E, Groothues C, … Sonuga-Barke EJS (2006). Do the Effects of Early Severe Deprivation on Cognition Persist Into Early Adolescence? Findings From the English and Romanian Adoptees Study. Child Development, 77(3), 696–711. 10.1111/j.1467-8624.2006.00898.x [DOI] [PubMed] [Google Scholar]
- Behen ME, Muzik O, Saporta ASD, Wilson BJ, Pai D, Hua J, & Chugani HT (2009). Abnormal Fronto-striatal Connectivity in Children with Histories of Early Deprivation: A Diffusion Tensor Imaging Study. Brain Imaging and Behavior, 3(3), 292–297. 10.1007/s11682-009-9071-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bick J, Palmwood EN, Zajac L, Simons R, & Dozier M (2018). Early Parenting Intervention and Adverse Family Environments Affect Neural Function in Middle Childhood. Biological Psychiatry 10.1016/j.biopsych.2018.09.020 [DOI] [PMC free article] [PubMed]
- Chugani HT, Behen ME, Muzik O, Juhász C, Nagy F, & Chugani DC (2001). Local Brain Functional Activity Following Early Deprivation: A Study of Postinstitutionalized Romanian Orphans. NeuroImage, 14(6), 1290–1301. 10.1006/nimg.2001.0917 [DOI] [PubMed] [Google Scholar]
- Delorme A, & Makeig S (2004). EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134(1), 9–21. 10.1016/j.jneumeth.2003.10.009 [DOI] [PubMed] [Google Scholar]
- Eluvathingal TJ, Chugani HT, Behen ME, Juhász C, Muzik O, Maqbool M, … Makki M (2006). Abnormal Brain Connectivity in Children After Early Severe Socioemotional Deprivation: A Diffusion Tensor Imaging Study. Pediatrics, 117(6), 2093–2100. 10.1542/peds.2005-1727 [DOI] [PubMed] [Google Scholar]
- Fernández T, Harmony T, Rodríguez M, Reyes A, Marosi E, & Bernal J (1993). Test-Retest Reliability of EEG Spectral Parameters During Cognitive Tasks: I Absolute and Relative Power. International Journal of Neuroscience, 68(3–4), 255–261. 10.3109/00207459308994280 [DOI] [PubMed] [Google Scholar]
- Fox NA, Almas AN, Degnan KA, Nelson CA, & Zeanah CH (2011). The effects of severe psychosocial deprivation and foster care intervention on cognitive development at 8 years of age: findings from the Bucharest Early Intervention Project. Journal of Child Psychology and Psychiatry, 52(9), 919–928. 10.1111/j.1469-7610.2010.02355.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendreau P, Freedman NL, Wilde GJ, & Scott GD (1972). Changes in EEG alpha frequency and evoked response latency during solitary confinement. Journal of Abnormal Psychology, 79(1), 54–59. 10.1037/h0032339 [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Bruce J, & Grotevant HD (2000). International adoption of institutionally reared children: Research and policy. Development and Psychopathology, 12(4), 677–693. [DOI] [PubMed] [Google Scholar]
- Hodel AS, Hunt RH, Cowell RA, Van Den Heuvel SE, Gunnar MR, & Thomas KM (2015). Duration of early adversity and structural brain development in post-institutionalized adolescents. NeuroImage, 105, 112–119. 10.1016/j.neuroimage.2014.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys KL, Gleason MM, Drury SS, Miron D, Nelson CA, Fox NA, & Zeanah CH (2015). Effects of institutional rearing and foster care on psychopathology at age 12 years in Romania: follow-up of an open, randomised controlled trial. The Lancet Psychiatry, 2(7), 625–634. 10.1016/S2215-0366(15)00095-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DE, Tang A, Almas AN, Degnan KA, McLaughlin KA, Nelson CA, … Drury SS (2018). Caregiving Disruptions Affect Growth and Pubertal Development in Early Adolescence in Institutionalized and Fostered Romanian Children: A Randomized Clinical Trial. The Journal of Pediatrics 10.1016/j.jpeds.2018.07.027 [DOI] [PMC free article] [PubMed]
- Kelly PA, Viding E, Wallace GL, Schaer M, De Brito SA, Robustelli B, & McCrory EJ (2013). Cortical Thickness, Surface Area, and Gyrification Abnormalities in Children Exposed to Maltreatment: Neural Markers of Vulnerability? Biological Psychiatry, 74(11), 845–852. 10.1016/j.biopsych.2013.06.020 [DOI] [PubMed] [Google Scholar]
- Lewis EE, Dozier M, Ackerman J, & Sepulveda-Kozakowski S (2007). The effect of placement instability on adopted children’s inhibitory control abilities and oppositional behavior. Developmental Psychology, 43(6), 1415–1427. 10.1037/0012-1649.43.6.1415 [DOI] [PubMed] [Google Scholar]
- Machado S, Portella CE, Silva JG, Velasques B, Terra P, Vorkapic CF, … Ribeiro P (2007). Changes in quantitative EEG absolute power during the task of catching an object in free fall. Arquivos de Neuro-Psiquiatria, 65(3A), 633–636. 10.1590/S0004-282X2007000400017 [DOI] [PubMed] [Google Scholar]
- Marshall PJ, Fox NA, & Group BC (2004). A Comparison of the Electroencephalogram between Institutionalized and Community Children in Romania. Journal of Cognitive Neuroscience, 16(8), 1327–1338. 10.1162/0898929042304723 [DOI] [PubMed] [Google Scholar]
- Marshall PJ, Reeb BC, Fox NA, Nelson CA, & Zeanah CH (2008). Effects of early intervention on EEG power and coherence in previously institutionalized children in Romania. Development and Psychopathology, 20(3), 861–880. 10.1017/S0954579408000412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason P, & Narad C (2005). International Adoption: A Health and Developmental Prospective. Seminars in Speech and Language, 26(1), 1–9. 10.1055/s-2005-864211 [DOI] [PubMed] [Google Scholar]
- Maurer D, Mondloch CJ, & Lewis TL (2007). Sleeper effects. Developmental Science, 10(1), 40–47. 10.1111/j.1467-7687.2007.00562.x [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Fox NA, Zeanah CH, Sheridan MA, Marshall P, & Nelson CA (2010). Delayed Maturation in Brain Electrical Activity Partially Explains the Association Between Early Environmental Deprivation and Symptoms of Attention-Deficit/Hyperactivity Disorder. Biological Psychiatry, 68(4), 329–336. 10.1016/j.biopsych.2010.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Winter W, Fox NA, Zeanah CH, & Nelson CA (2014). Widespread Reductions in Cortical Thickness Following Severe Early-Life Deprivation: A Neurodevelopmental Pathway to Attention-Deficit/Hyperactivity Disorder. Biological Psychiatry, 76(8), 629–638. 10.1016/j.biopsych.2013.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta MA, Golembo NI, Nosarti C, Colvert E, Mota A, Williams SCR, … Sonuga-Barke EJS (2009). Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: The English and Romanian Adoptees Study Pilot. Journal of Child Psychology and Psychiatry, 50(8), 943–951. 10.1111/j.1469-7610.2009.02084.x [DOI] [PubMed] [Google Scholar]
- Mognon A, Jovicich J, Bruzzone L, & Buiatti M (2011). ADJUST: An automatic EEG artifact detector based on the joint use of spatial and temporal features. Psychophysiology, 48(2), 229–240. 10.1111/j.1469-8986.2010.01061.x [DOI] [PubMed] [Google Scholar]
- Montes LG, Alcántara H, Cedeño BA, García AO, & Rojas PE (2015). Persistent decrease in alpha current density in fully remitted subjects with major depressive disorder treated with fluoxetine: A prospective electric tomography study. International Journal of Psychophysiology, 96(3), 191–200. 10.1016/j.ijpsycho.2015.03.010 [DOI] [PubMed] [Google Scholar]
- Nelson CA (2007). A Neurobiological Perspective on Early Human Deprivation. Child Development Perspectives, 1(1), 13–18. 10.1111/j.1750-8606.2007.00004.x [DOI] [Google Scholar]
- Nelson CA, Zeanah CH, Fox NA, Marshall PJ, Smyke AT, & Guthrie D (2007). Cognitive Recovery in Socially Deprived Young Children: The Bucharest Early Intervention Project. Science, 318(5858), 1937–1940. 10.1126/science.1143921 [DOI] [PubMed] [Google Scholar]
- Newton RR, Litrownik AJ, & Landsverk JA (2000). Children and youth in foster care: disentangling the relationship between problem behaviors and number of placements. Child Abuse & Neglect, 24(10), 1363–1374. 10.1016/S0145-2134(00)00189-7 [DOI] [PubMed] [Google Scholar]
- Nolan H, Whelan R, & Reilly RB (2010). FASTER: fully automated statistical thresholding for eeg artifact rejection. Journal of Neuroscience Methods, 192(1), 152–162. 10.1016/j.jneumeth.2010.07.015 [DOI] [PubMed] [Google Scholar]
- Otero GA (1994). Eeg spectral analysis in children with sociocultural handicaps. International Journal of Neuroscience, 79(3–4), 213–220. 10.3109/00207459408986082 [DOI] [PubMed] [Google Scholar]
- Otero GA (1997). Poverty, cultural disadvantage and brain development: a study of pre-school children in Mexico. Electroencephalography and Clinical Neurophysiology, 102(6), 512–516. 10.1016/S0013-4694(97)95213-9 [DOI] [PubMed] [Google Scholar]
- Otero GA, Pliego-Rivero FB, Fernández T, & Ricardo J (2003). EEG development in children with sociocultural disadvantages: a follow-up study. Clinical Neurophysiology, 114(10), 1918–1925. 10.1016/S1388-2457(03)00173-1 [DOI] [PubMed] [Google Scholar]
- Raine A, Venables PH, Dalais C, Mellingen K, Reynolds C, & Mednick SA (2001). Early educational and health enrichment at age 3–5 years is associated with increased autonomic and central nervous system arousal and orienting at age 11 years: Evidence from the Mauritius Child Health Project. Psychophysiology, 38(2), 254–266. [PubMed] [Google Scholar]
- Rubin DM, O’Reilly ALR, Luan X, & Localio AR (2007). The Impact of Placement Stability on Behavioral Well-being for Children in Foster Care. Pediatrics, 119(2), 336–344. 10.1542/peds.2006-1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan MA, Fox NA, Zeanah CH, McLaughlin KA, & Nelson CA (2012). Variation in neural development as a result of exposure to institutionalization early in childhood. Proceedings of the National Academy of Sciences, 109(32), 12927–12932. 10.1073/pnas.1200041109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyke AT, Koga SF, Johnson DE, Fox NA, Marshall PJ, Nelson CA, … the BEIP Core Group. (2007). The caregiving context in institution-reared and family-reared infants and toddlers in Romania. Journal of Child Psychology and Psychiatry, 48(2), 210–218. 10.1111/j.1469-7610.2006.01694.x [DOI] [PubMed] [Google Scholar]
- Somsen RJM, van’t Klooster BJ, van der Molen MW, van Leeuwen HMP, & Licht R (1997). Growth spurts in brain maturation during middle childhood as indexed by EEG power spectra. Biological Psychology, 44(3), 187–209. 10.1016/S0301-0511(96)05218-0 [DOI] [PubMed] [Google Scholar]
- Tendolkar I, Mårtensson J, Kühn S, Klumpers F, & Fernández G (2018). Physical neglect during childhood alters white matter connectivity in healthy young males. Human Brain Mapping, 39(3), 1283–1290. 10.1002/hbm.23916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwert RE, Marshall PJ, Iii CAN, Zeanah CH, & Fox NA (2010). Timing of Intervention Affects Brain Electrical Activity in Children Exposed to Severe Psychosocial Neglect. PLOS ONE, 5(7), e11415 10.1371/journal.pone.0011415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwert RE, Zeanah CH, Fox NA, & Nelson CA (2016). Normalization of EEG activity among previously institutionalized children placed into foster care: A 12-year follow-up of the Bucharest Early Intervention Project. Developmental Cognitive Neuroscience, 17, 68–75. 10.1016/j.dcn.2015.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeanah CH, Nelson CA, Fox NA, Smyke AT, Marshall P, Parker SW, & Koga S (2003). Designing research to study the effects of institutionalization on brain and behavioral development: The Bucharest Early Intervention Project. Development and Psychopathology, 15(4), 885–907. 10.1017/S0954579403000452 [DOI] [PubMed] [Google Scholar]
- Zubek JP, Welch G, & Saunders MG (1963). Electroencephalographic Changes during and after 14 Days of Perceptual Deprivation. Science, 139(3554), 490–492. 10.1126/science.139.3554.490 [DOI] [PubMed] [Google Scholar]


