Advances in therapy for childhood cancer and resulting improved survival over time have led to a greater focus on the long-term health of childhood cancer survivors and risks for therapy-related adverse effects.1 Therapy-related myelodysplastic syndrome/acute myeloid leukemia (tMDS/AML) are rare yet serious complications of cytotoxic cancer therapies.2 Studies primarily conducted in adult cancer patients have demonstrated that tMDS/AML risk varies by type of chemotherapy, with the highest risks for certain epipodophyllotoxins, alkylating agents, and platinum compounds.2
The limited data available on tMDS/AML risk after childhood cancer derive primarily from case-control and cohort studies, conducted predominantly in patients treated in the 1980s3–6 and 1990s.7 However, chemotherapy regimens for many first primary cancers have evolved since the 1990s.8,9 Clinical trials also have reported tMDS/AML after childhood cancer,10–13 but most lack sufficient sample size to quantify rare adverse effects such as tMDS/AML and cannot compare risks by childhood cancer type. Two population-based studies reported increasing tAML risk after childhood cancer.14,15 However, those studies included a small number of cases (N=36 and 54 cases), only included follow-up through the early 2000s, and did not evaluate risks separately for those treated with chemotherapy.
We utilized data from the US population-based cancer registries of the Surveillance, Epidemiology and End Results (SEER) program to evaluate calendar year trends in tAML risk after chemotherapy for childhood cancer during 1975-2015 in nine registries and then to broadly investigate the current landscape of tMDS/AML risk among childhood cancer survivors during 2000-2015 within the expanded set of 17 SEER registries. The outcome for the SEER 9 analyses was restricted to tAML because MDS was not systematically reported to SEER prior to 2001. For all analyses, we included individuals who were diagnosed with a non-myeloid first primary cancer at age <20 years (Supplementary Table-S1), received initial chemotherapy, and survived ≥6 months after diagnosis without developing a second cancer. tMDS/AML cases were identified using International Classification of Diseases for Oncology, Third Edition (ICD-O-3) morphology codes and the WHO classification of tumors of haematopoietic and lymphoid tissues.
Patients treated with initial chemotherapy were followed beginning 6-months after first primary cancer diagnosis until the first of: second cancer diagnosis, death, loss to follow-up, or end of the study (December 31, 2015). Standardized incidence ratios (SIRs) and accompanying exact 95% confidence intervals (CIs) quantified the relative risk of tAML or tMDS/AML compared with the general population. We also estimated excess absolute risk [EAR=(observed-expected)×10,000/person-years]. Multivariable Poisson regression models were constructed to test for heterogeneity in the SIRs by patient characteristics. Lastly, we calculated cumulative incidence of tMDS/AML with death and diagnosis of other second primary malignancies considered as competing risks, and we calculated median overall survival following tMDS/AML diagnosis. More details on methods can be found in supplementary materials.
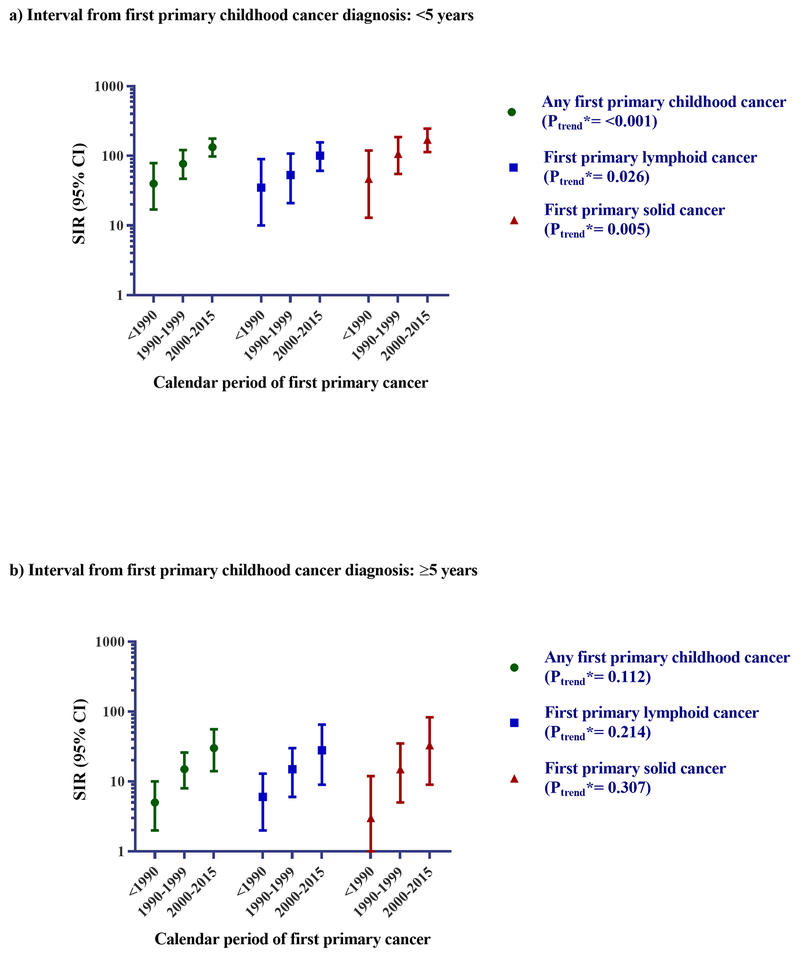
Initial analyses focused on 25,933 patients who received initial chemotherapy for first primary childhood cancers diagnosed during 1975-2015 from 9 SEER registries (Supplementary Table-S2, Figure-1). Analyses by calendar year revealed significantly higher tAML risks within <5 years from initial diagnosis in more recent calendar years for lymphoid (SIR<1990=35; 95%CI=10-90; SIR1990-1999=53; 95%CI=21-108; SIR2000-2015=101; 95%CI=61-157; Ptrend = 0.026) or solid cancers (SIR<1990=47; 95%CI=13-120; SIR1990-1999=107; 95%CI=55-187; SIR2000-2015=171; 95%CI=114-247; Ptrend= 0.005). Similar but nonsignificant temporal patterns were observed for tAML risk ≥5 years after diagnosis.
Figure 1: Risk for tAML by calendar period <5 years (A) and ≥5 years (B) following diagnosis of first primary childhood cancer among ≥6 month survivors who received initial chemotherapy, 1975-2015.
Abbreviations: confidence interval (CI); standardized incidence ratio (SIR); therapy-related acute myeloid leukemia (tAML)
* P-trend: Multivariable Poisson regression models adjusted through stratification for age at first primary cancer diagnosis were used to conduct a two-sided test for trend in SIRs by calendar period of first primary cancer diagnosis (modeled as an ordinal variable) using a likelihood ratio statistic, where inclusion of the log of the expected number of cases as an offset indirectly adjusts for attained age and calendar year. The model for tAML ≥5 years following diagnosis was additionally adjusted for time since first primary cancer (5-9 versus ≥10 years).
Population characteristics and SIRs are provided in Supplementary Table S2.
Subsequent analyses focused on 36,975 childhood cancer survivors diagnosed during 2000-2015 and treated with initial chemotherapy from 17 SEER registries (Table-1). We observed 186 tMDS/AML cases (18 tMDS, 168 tAML) compared with 2.3 MDS/AML cases expected, representing an approximately 80-fold increased risk (95%CI=69-92) and an excess of 8.3 cases/10,000 person-years. Over half (N=106) were diagnosed after initial chemotherapy for a solid tumor, most commonly sarcoma (N=62, SIR=204; 95%CI=156-261; EAR=22.3). Despite the absence of data on specific chemotherapy drugs in SEER, these risks are consistent with the use of cisplatin/doxorubicin-(osteosarcoma), cyclophosphamide/ifosfamide/doxorubicin/etoposide-(Ewing sarcoma), and cyclophosphamide/doxorubicin-based (rhabdomyosarcoma) regimens as a mainstay of treatment for sarcomas.8 Significantly elevated tMDS/AML risks also were observed after initial chemotherapy for neuroblastoma and hepatic, CNS, and germ-cell tumors, with SIRs ranging from 46 to >100. These results are also consistent with the use of leukemogenic agents, including cyclophosphamide/doxorubicin/etoposide/cisplatin-(neuroblastoma), cisplatin/doxorubicin-(hepatic), cyclophosphamide/cisplatin/temozolomide-(CNS), and etoposide/cisplatin-based-(germ cell) regimens.8
Table 1.
Selected patient characteristics and the risk for tMDS/AML, overall and by interval from first primary childhood cancer diagnosis, among 36,975 ≥6 month survivors who received initial chemotherapy, 17 SEER registries,* 2000-2015
| First primary childhood cancer | Patient characteristics and
overall risk |
Interval from first primary
childhood cancer |
P‖ | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Na | Mean age at diagnosis years | Mean person-years at
risk† |
Female % | Also received radiation % | Nb | SIR‡ | 95% CI | EAR§ | <5 years |
≥5 years |
||||||||
| Nb | SIR‡ | 95% CI | EAR§ | Nb | SIR‡ | 95% CI | EAR§ | |||||||||||
| Total¶ | 36,975 | 9.3 | 6.0 | 43 | 30 | 186** | 80 | (69, 92) | 8.3 | 147 | 119 | (100, 140) | 12.0 | 39 | 36 | (26, 49) | 3.8 | <0.001 |
| Lymphoid cancer | 19,371 | 9.8 | 6.5 | 42 | 20 | 80 | 60 | (48, 75) | 6.2 | 61 | 90 | (69, 116) | 8.9 | 19 | 29 | (17, 45) | 3.1 | <0.001 |
| ALL | 12,622 | 7.5 | 6.4 | 43 | 12 | 58 | 74 | (56, 95) | 7.1 | 43 | 106 | (77, 142) | 9.7 | 15 | 39 | (22, 65) | 4.0 | <0.001 |
| B-cell ALL | 9,565 | 7.1 | 6.1 | 45 | 7 | 38 | 69 | (49, 94) | 6.5 | 26 | 86 | (56, 126) | 7.8 | 12 | 48 | (25, 83) | 4.7 | 0.076 |
| T-cell ALL | 1,760 | 10.5 | 5.9 | 28 | 38 | 14 | 126 | (69, 212) | 13.3 | 13 | 231 | (123, 394) | 22.6 | <3 | ||||
| Unknown lineage ALL | 1,297 | 6.9 | 9.7 | 43 | 11 | 6 | 49 | (18, 106) | 4.7 | 4 | 82 | (22, 209) | 7.4 | <3 | ||||
| Hodgkin lymphoma | 3,974 | 15.4 | 7.0 | 47 | 52 | 15 | 43 | (24, 71) | 5.3 | 11 | 65 | (32, 116) | 7.3 | 4 | 23 | (6, 58) | 2.9 | 0.050 |
| Other lymphoid neoplasms | 2,775 | 12.0 | 6.5 | 32 | 10 | 7 | 35 | (14, 71) | 3.8 | 7 | 70 | (28, 144) | 7.3 | 0 | ||||
| Solid cancer | 17,604 | 8.9 | 5.3 | 44 | 42 | 106 | 107 | (88, 130) | 11.2 | 86 | 153 | (123, 189) | 16.0 | 20 | 47 | (29, 72) | 4.8 | <0.001 |
| Neuroblastoma†† | 1,834 | 3.0 | 5.2 | 47 | 36 | 11 | 119 | (59, 213) | 11.4 | 10 | 164 | (79, 302) | 17.9 | <3 | ||||
| Renal tumors | 1,915 | 4.0 | 6.4 | 52 | 50 | <3 | <3 | 0 | ||||||||||
| Hepatic tumors | 663 | 4.1 | 5.3 | 37 | 2 | 3 | 85 | (17, 248) | 8.5 | <3 | <3 | |||||||
| CNS tumors | 4,173 | 7.6 | 4.7 | 45 | 65 | 20 | 102 | (63, 158) | 10.2 | 14 | 126 | (69, 211) | 12.3 | 6 | 71 | (26, 155) | 7.2 | 0.238 |
| Medulloblastoma | 1,173 | 7.7 | 5.6 | 37 | 82 | 6 | 92 | (34, 200) | 9.1 | 4 | 115 | (31, 293) | 10.7 | <3 | ||||
| Glioma | 1,528 | 9.0 | 3.3 | 48 | 69 | 4 | 79 | (22, 203) | 7.8 | <3 | <3 | |||||||
| PNET | 533 | 8.3 | 5.5 | 47 | 67 | 4 | 131 | (36, 336) | 13.5 | 3 | 196 | (40, 573) | 19.1 | <3 | ||||
| Other CNS tumors | 939 | 4.8 | 5.3 | 48 | 34 | 6 | 122 | (45, 266) | 12.0 | 5 | 168 | (54, 391) | 17.3 | <3 | ||||
| Sarcoma (bone and soft-tissue) | 5,416 | 11.6 | 5.1 | 43 | 42 | 62 | 204 | (156, 261) | 22.3 | 52 | 310 | (232, 407) | 32.1 | 10 | 73 | (35, 135) | 8.5 | <0.001 |
| Osteosarcoma | 1,663 | 13.6 | 5.3 | 45 | 4 | 21 | 210 | (130, 322) | 24.0 | 16 | 302 | (173, 491) | 31.8 | 5 | 107 | (35, 249) | 13.4 | 0.026 |
| Ewing’s tumor‡‡ | 969 | 12.7 | 5.2 | 39 | 50 | 12 | 210 | (109, 367) | 23.7 | 11 | 352 | (175, 629) | 36.9 | <3 | ||||
| Rhabdomyosarcoma | 1,565 | 8.3 | 5.2 | 42 | 68 | 11 | 135 | (67, 241) | 13.4 | 8 | 176 | (76, 348) | 17.0 | 3 | 83 | (17, 242) | 8.5 | 0.241 |
| Other | 1,219 | 12.3 | 4.8 | 45 | 53 | 18 | 275 | (163, 434) | 31.0 | 17 | 446 | (260, 715) | 49.0 | <3 | ||||
| GCT | 2,254 | 14.9 | 6.3 | 38 | 21 | 8 | 46 | (20, 90) | 5.5 | 7 | 78 | (31, 161) | 9.0 | <3 | ||||
Abbreviations: acute lymphoblastic leukemia/lymphoma (ALL); confidence interval (CI); central nervous system (CNS); excess absolute risk (EAR); germ cell and trophoblastic tumors and neoplasm of gonads (GCT); total number of patients (Na); observed number of tMDS/AML cases (Nb); primitive neuroectodermal tumor (PNET); standardized incidence ratio (SIR); Surveillance, Epidemiology, and End Results (SEER); therapy-related myelodysplastic syndrome/acute myeloid leukemia (tMDS/AML).
17 SEER registries included metropolitan areas of Atlanta, Detroit, Seattle-Puget Sound; states of Connecticut, Hawaii, Iowa, New Mexico, California (San Francisco-Oakland, Los Angeles, San Jose-Monterey, Greater California), Georgia (Rural Georgia, Greater Georgia), Utah, Kentucky, Louisiana, and New Jersey.
Patients were followed from 6 months after first primary cancer diagnosis until the first of: second cancer diagnosis, death, age 85 years, loss to follow-up or end of the study period (December 31, 2015).
SIR=observed/expected. Expected numbers of cases were derived from incidence rates for MDS/AML in the total population of the same 17 SEER registries, stratified by age (5-year groups), race (white/unknown, black, other), sex, and calendar year (5-year groups), multiplied by the appropriate person-years at risk.
EAR=(observed-expected)×10,000/person-years.
P-heterogeneity: Multivariable Poisson regression models adjusted for age at first primary cancer diagnosis were used to conduct a two-sided test for heterogeneity in SIRs by time since first primary childhood cancer using a likelihood ratio statistic, where inclusion of the log of the expected number of cases as an offset indirectly adjusts for attained age and calendar year. Models were not constructed when one group had <3 cases due to insufficient sample size.
Total includes all entities in the table and International Classification of Childhood Cancer (ICCC) categories V (Retinoblastoma), XI (Other malignant epithelial neoplasms and malignant melanomas) and XII (Other and unspecified malignant neoplasms).
Of the 186 tMDS/AML cases observed after all childhood cancers, 24.7% were MDS/AML not otherwise specified (NOS) (ICD-O-3 morphology code: 9861: Acute myeloid leukemia, NOS = 38 cases; 9989: Myelodysplastic syndrome, unclassifiable or NOS = 8 cases), 47.9% were therapy-related MDS/AML (ICD-O-3 morphology code: 9920: Therapy-related (acute) myeloid neoplasm = 79 cases; 9987: Therapy related myelodysplastic syndrome, NOS = 10 cases), while the remaining 27.4% belonged to various other MDS/AML subtypes.
Includes neuroblastoma and other peripheral nervous cell tumors
Includes Ewing’s tumor and related sarcomas of bone
Note: Exact cell counts with <3 observed cases are suppressed to protect patient confidentiality.
The remaining tMDS/AML cases (N=80) occurred among 19,371 patients treated with initial chemotherapy for lymphoid malignancies. Elevated risks were observed after ALL (N=58, SIR=74; 95%CI=56-95), particularly T-ALL (N=14, SIR=126; 95%CI=69-212) versus B-ALL (N=38, SIR=69; 95%CI=49-94) (Pheterogeneity=0.047). For several decades, patients treated for childhood ALL have received combination chemotherapy including cyclophosphamide and anthracyclines.9 Increased risks in more recent years could reflect improved ascertainment of tAML after lymphoid malignancies since the 1980s or a true increase in risk, possibly due to dose intensification for high-risk patients, which could explain the higher risks we observed for T-cell than B-cell ALL.12 Significantly elevated risks also were observed after initial chemotherapy for Hodgkin lymphoma (N=15, SIR=43; 95%CI=24-71) and other lymphoid malignancies (N=7, SIR=35; 95%CI=14-71). Chemotherapy for Hodgkin lymphoma evolved in the 1980s to reduce the use of highly-leukemogenic regimens (e.g., MOPP: mechlorethamine, vincristine, procarbazine, and prednisone) in favor of less toxic ones (e.g., ABVD: doxorubicin, bleomycin, vinblastine, and dacarbazine).9 However, our data suggest that the elevated tMDS/AML risks after Hodgkin lymphoma persist, possibly due to aggressive treatment for relapse/progressive disease.
Nearly 80% of tMDS/AML cases (N=147) occurred within the first 5 years following childhood cancer diagnosis. Of these, 100 cases occurred within the first 3 years after diagnosis (Supplementary Table-S3). These results are consistent with the use of topoisomerase-II inhibitors for pediatric cancers,8,9 which has been associated with earlier onset leukemias compared with alkylating agents.2 For all first primary childhood cancers combined, tMDS/AML risks were significantly higher at <5 (SIR=119; 95%CI=100-140) versus ≥5 (SIR=36; 95%CI=26-49) years after diagnosis (Pheterogeneity<0.001). SIRs continued to decrease in the period of ≥5 years after diagnosis, but the overall SIR remained significantly elevated ≥7 years after diagnosis (N=11, SIR=15; 95%CI=7-27), suggesting the effect of subsequent chemotherapy for relapsed/progressive disease.
In exploratory analyses by patient subgroup, tMDS/AML risk did not statistically significantly vary by age at diagnosis of childhood cancer (Supplementary Table-S4), in contrast with adult studies that report higher SIRs for individuals diagnosed at younger ages. Additional studies with detailed treatment data are needed to clarify whether risks do, indeed, vary by age at exposure. Alternatively, observed risk differences by age in adult studies may be attributed to the low baseline MDS/AML risk at younger ages in the general population or differences in treatment approaches for patients of different ages with varying comorbidities. Finally, SIRs for tMDS/AML were similar among patients who received initial chemotherapy and radiotherapy versus initial chemotherapy without known radiotherapy (Supplementary Table-S5). These findings support previous literature that has suggested a weak effect, if any, of radiotherapy, beyond that from chemotherapy, on leukemia risk.7
Despite the strikingly elevated SIRs for tMDS/AML, the 10-year cumulative incidence of tMDS/AML following chemotherapy was generally <1% for all first primary childhood cancers, with the exception of sarcoma (10-year cumulative incidence=1.44%; 95%CI=1.11-1.84%) (Supplementary Figure-1). Survival following tMDS/AML was poor (median overall survival=16 months). High prevalence of adverse cytogenetics and suboptimal response to standard treatment approaches may adversely affect prognosis for tMDS/AML compared with de novo MDS/AML.2 Further studies, are needed to characterize the cytogenetic profiles of tMDS/AML occurring after chemotherapy for childhood cancer and to identify optimal treatment strategies.
The major limitation of our study was the lack of information in SEER on specific chemotherapeutic agents, doses and duration of initial treatment, and subsequent therapy. Receipt of initial chemotherapy is also under-ascertained in SEER, therefore we could not directly compare tMDS/AML risks among childhood cancer survivors treated with and without initial chemotherapy. Future studies with detailed data on initial and subsequent courses of therapy including agents, doses, and duration are critical to identify patients at highest risk of developing tMDS/AML based on their first cancer treatment exposures and perhaps genetic susceptibility. Additionally, we had insufficient sample size for our analyses of patient subgroups, which should be interpreted cautiously due to limited statistical power.
In summary, our data enabled us to examine patterns in tMDS/AML risks in a large, population-based cohort across a broad range of childhood cancer survivors with systematic long-term follow-up. We demonstrate that tAML risk after initial chemotherapy for childhood cancer increased significantly during 1975-2015 and was strikingly elevated after most common childhood cancer types during 2000-2015. These results represent the first comprehensive assessment of the current landscape of tMDS/AML risk after chemotherapy for childhood cancer, following substantial evolution in treatment approaches for many types of childhood cancer over the last several decades.8,9 Due to the expansion of SEER in 2000 (~28% of the US population versus 9-13% during 1975-1999), the increasing use of initial chemotherapy to treat certain childhood cancers more recently, and the higher tMDS/AML risks associated with current treatment approaches, our study includes a larger number of cases than previous studies of tMDS/AML after childhood cancers. Although tMDS/AML is rare, the poor prognosis underscores the importance of counseling patients and their families regarding the risks and benefits of specific treatments. Future research should be directed toward more precisely identifying susceptible individuals.
Supplementary Material
Acknowledgements:
This research was supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health, Department of Health and Human Services. The authors acknowledge the efforts of the National Cancer Institute and SEER Program tumor registries in the creation of the SEER database. Please note that the opinions and information in this article are those of the authors, and do not represent the views and/or policies of the U.S. Food and Drug Administration.
Footnotes
Conflict-of-interest disclosure: The authors declare no conflict of interest.
Presentation: These data were presented in part at the 2018 Conference on Radiation and Health, in Chicago, Illinois, September 23-25, 2018.
REFERENCES
- 1.Robison LL, Hudson MM. Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nat Rev Cancer. 2014;14(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McNerney ME, Godley LA, Le Beau MM. Therapy-related myeloid neoplasms: when genetics and environment collide. Nat Rev Cancer. 2017;17(9):513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haddy N, Le Deley MC, Samand A, Diallo I, Guérin S, Guibout C, et al. Role of radiotherapy and chemotherapy in the risk of secondary leukaemia after a solid tumour in childhood. Eur J Cancer 2006;42(16):2757–64. [DOI] [PubMed] [Google Scholar]
- 4.Hawkins M, Wilson L, Stovall M, Marsden H, Potok M, Kingston J, et al. Epipodophyllotoxins, alkylating agents, and radiation and risk of secondary leukaemia after childhood cancer. BMJ. 1992;304(6832):951–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nottage K, Lanctot J, Li Z, Neglia JP, Bhatia S, Hammond S, et al. Long-term risk for subsequent leukemia after treatment for childhood cancer: a report from the Childhood Cancer Survivor Study. Blood. 2011;117:6315–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tucker MA, Meadows AT, Boice JD Jr, Stovall M, Oberlin O, Stone BJ, et al. Leukemia after therapy with alkylating agents for childhood cancer. J Natl Cancer Inst. 1987;78(3):459–64. [PubMed] [Google Scholar]
- 7.Allodji RS, Schwartz B, Veres C, Haddy N, Rubino C, Le Deley M-C, et al. Risk of subsequent leukemia after a solid tumor in childhood: impact of bone marrow radiation therapy and chemotherapy. Int J Radiat Oncol Biol Phys. 2015;93(3):658–67. [DOI] [PubMed] [Google Scholar]
- 8.Green DM, Kun LE, Matthay KK, Meadows AT, Meyer WH, Meyers PA, et al. Relevance of historical therapeutic approaches to the contemporary treatment of pediatric solid tumors. Pediatr Blood Cancer. 2013;60(7):1083–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hudson MM, Neglia JP, Woods WG, Sandlund JT, Pui CH, Kun LE, et al. Lessons from the past: opportunities to improve childhood cancer survivor care through outcomes investigations of historical therapeutic approaches for pediatric hematological malignancies. Pediatr Blood Cancer. 2012;58(3):334–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhatia S, Krailo MD, Chen Z, Burden L, Askin FB, Dickman PS, et al. Therapy-related myelodysplasia and acute myeloid leukemia after Ewing sarcoma and primitive neuroectodermal tumor of bone: A report from the Children’s Oncology Group. Blood. 2007;109(1):46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ingram L, Mott M, Mann J, Raafat F, Darbyshire P, Jones PM. Second malignancies in children treated for non-Hodgkin’s lymphoma and T-cell leukaemia with the UKCCSG regimens. Br J Cancer. 1987;55(4):463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pui C-H, Behm FG, Raimondi SC, Dodge RK, George SL, Rivera GK, et al. Secondary acute myeloid leukemia in children treated for acute lymphoid leukemia. N Engl J Med. 1989;321(3):136–42. [DOI] [PubMed] [Google Scholar]
- 13.Winick NJ, McKenna RW, Shuster JJ, Schneider NR, Borowitz MJ, Bowman WP, et al. Secondary acute myeloid leukemia in children with acute lymphoblastic leukemia treated with etoposide. J Clin Oncol. 1993;11(2):209–17. [DOI] [PubMed] [Google Scholar]
- 14.Inskip PD, Curtis RE. New malignancies following childhood cancer in the United States, 1973–2002. Int J Oncol. 2007;121(10):2233–40. [DOI] [PubMed] [Google Scholar]
- 15.Rihani R, Bazzeh F, Faqih N, Sultan I. Secondary hematopoietic malignancies in survivors of childhood cancer: An analysis of 111 cases from the Surveillance, Epidemiology, and End Result‐9 registry. Cancer. 2010;116(18):4385–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.