Abstract
We recently reported that diabetes negates the cerebrovascular protection typically seen in adult female rats resulting in cognitive impairment, which is worsened by increased parenchymal bleeding and edema after ischemic stroke. Although women experience more severe diabetes and suffer from a higher rate of diabetic complications, including stroke and cognitive impairment, underlying mechanisms contributing to sex differences are limited. Emerging evidence suggests interleukin (IL)-17 contributes to cerebrovascular pathologies: 1) high salt diet-mediated expansion of IL-17-producing T cells (Th17) in the gut microbiome promotes cerebrovascular dysfunction and cognitive impairment in male mice, 2) increased IL-17-producing γδTCR cells exacerbates stroke injury in male mice, and 3) IL-17 promotes rupture of cerebral aneurysms in female mice. Based on these premises, we investigated the potential involvement of IL-17-producing inflammatory cells in cerebrovascular dysfunction and post-stroke vascular injury in diabetes by measuring intestinal, circulating, or cerebral T-cell profiles as well as in plasma IL-17 in both sexes. Cell suspensions prepared from naive or stroked (3 days after stroke) diabetic and control rats were analyzed by flow cytometry, and IL-17 levels were measured in plasma using ELISA. Diabetes deferentially promoted the expansion of cerebral Th17 cells in females. In response to stroke, diabetes had a sexually dimorphic effect on the expansion of numerous T cell profiles. These results suggest that a better understanding of the role of IL-17-producing cells in diabetes may identify potential avenues in which the molecular mechanisms contributing to these sex differences can be further elucidated.
Keywords: Diabetes, Stroke, IL-17, Female, Brain, Blood
Introduction
Over 422 million adults globally suffer from diabetes and associated complications including cognitive impairment/dementia and acute ischemic stroke (Flores-Gomez et al. 2019). Diabetes not only increases the risk of these diseases, but patients also experience higher morbidity and mortality (Bertoni et al. 2002). Interestingly, both type 2 diabetes (T2D) and ischemic stroke are sexually dimorphic diseases that affect women disproportionately (Arnetz et al. 2014). Women experience more severe diabetes and higher allcause mortality (Arnetz et al. 2014). While young women are protected from the cerebrovascular consequences of ischemic stroke before menopause (Arnetz et al. 2014; Janghorbani et al. 1993; Carr 2003; Barrett-Connor 1997), after menopause the risk increases to equal to that in men, resulting in more severe strokes, higher short- term mortality, and a worse quality of life than men (Bushnell et al. 2014; Gall et al. 2012; Ward et al. 2018). After a stroke, women are also more likely to enter a nursing home due to worsened cognitive impairment (Bushnell et al. 2014; Gall et al. 2012; Ward et al. 2018). The fact that women are more likely to have a comorbid condition such as diabetes additionally contributes to the increased prevalence of stroke in women compared to men (Gall et al. 2010). Diabetes, as the fastest growing risk factor for stroke, has been shown to negate the protective effect of estrogen in young women and severely increase risk, morbidity and mortality from stroke (Arnetz et al. 2014; Li et al. 2017). Lack of understanding of the mechanisms contributing to diabetes-mediates cerebrovascular complications hamper the development of targeted therapeutic strategies for women.
Animal models offer an opportunity to investigate the underlying mechanisms and exploit potential new targets for the development of treatment. Unfortunately, preclinical studies in cognitive impairment and ischemic stroke in diabetic female animals are also limited. A recent study reported that similar to that observed in women patients, diabetes negates the neuroprotection typically seen in adult female rats and increases the infarct size (Li et al. 2017). Diabetes also increases the risk and severity of vascular injury including increased parenchymal bleeding, also referred to as hemorrhagic transformation (HT), and edema after ischemic stroke, worsening sensorimotor and cognitive neurological deficits as well as recovery (Li et al. 2018; Abdul et al. 2018; Li et al. 2017; Hafez et al. 2016; Zhang et al. 2018). Studies showed that diabetes magnifies HT in female rats depending on the duration of ischemia and exacerbates outcomes (Li et al. 2017; Li et al. 2018). A recent study also showed that hippocampal neurodegeneration after stroke is greater in diabetic female rats and associated with amplified inflammation (Ward et al. 2018). Emerging evidence suggests that interleukin 17 (IL-17) is a critical player in chronic inflammatory conditions and cerebrovascular pathologies. First, it has been reported that a high salt diet-induced expansion of IL-17-producing T cells (Th17) in the gut microbiome leads to cerebrovascular dysfunction and cognitive impairment in male mice (Faraco et al. 2018). Second, an elegant study showed that migration of IL-17-producing γδTCR cells from the gut to the meninges exacerbates ischemic injury in male mice (Benakis et al. 2016). Third, IL-17 was shown to play a key role in the rupture of cerebrovascular aneurysms in estrogen-deficient female mice by altering endothelial cell tight junction proteins providing a mechanistic explanation of increased cerebral aneurysm rupture in women. Cells that secrete IL-17 such as Th17 and γδTCR cells span both innate and adaptive immunity (Holley and Kielian 2012; Khader et al. 2009). Both Th17 and γδTCR cells can positively contribute to innate immunity through bacterial clearance and impact neutrophil and macrophage recruitment and activation status (Holley and Kielian 2012). Leukocytes, epithelial cells, mesothelial cells, vascular endothelial cells, keratinocytes and fibroblasts express the receptor for IL-17 and respond to IL-17R-mediated signaling by production of granulocyte colony stimulating factor (G-CSF), IL-6, IL-8. Through these pathways they mediate neutrophil recruitment and inflammatory responses within the innate immunity and span into their infamous pro-inflammatory role in the adaptive immunity (Khader et al. 2009). Based on these grounds, we postulated that 1) circulating and/or cerebrovascular IL-17 producing cells are elevated in high fat diet (HFD)-induced diabetic rats, 2) diabetes promotes Th17 expansion in the gut, and 3) ischemic stroke further promotes the expansion of Th17 cells.
Methods
Rats were housed in the animal care facility at Augusta University, which is approved by the American Association for Accreditation of Laboratory Animal Care. All experiments were conducted in accordance with the National Institute of Health (NIH) guidelines for the care and use of animals in research. Furthermore, all protocols were approved by the institutional animal care and use committee.
Animal model
In order to evaluate the impact of diabetes on the T cell profiles diabetes was induced in male and female Wistar rats (Envigo RMS, Inc., Indianapolis, IN) using a high-fat diet/low-dose streptozotocin (HFD/STZ) combination. Diabetic rats were received at 4 weeks of age and immediately started and maintained on a 45% kcal fat diet for the remainder of the study (Research Diets Inc., New Brunswick, NJ). A single dose STZ injection (35 mg/kg; Cayman Chemical, Ann Arbor, MI) was administered intraperitoneally (ip) at 6 weeks of age. If blood glucose was not above 150 mg/dL 5 days post-injection, a second small dose (20 mg/kg) was administered. Control rats were received at 10-11 weeks of age and maintained on regular chow with 4% kcal fat. Body weight and blood glucose were measured twice a week until euthanasia. All female rats underwent surgery during the diestrus phase after careful monitoring of the estrus cycle by vaginal swab.
Experiment 1 included the following groups: control naive (no intervention) females (N=6), control naive males (N= 6), diabetic naive females (N=6), and diabetic naive males (N=6). Experiment 2 included control stroked females (N=5), control stroked males (N=6), diabetic stroked females (N=4), and diabetic stroked males (N=3). While this experiment also started with N=6 in each group, 4 diabetic animals (2 male and 2 female) died prior to surgery resulting in lower animal numbers in the diabetic stroke groups. 1 control female and 1 diabetic male died right after surgery.
Middle Cerebral Artery Occlusion (MCAO) surgery
Stroke was induced by transient (60 min) focal cerebral ischemia (MCAO) at 12-15 weeks of age as previously described (Li et al. 2017). In the postoperative period blood glucose was monitored daily. Rats were then sacrificed at day 3.
Euthanasia and specimen collection
Rats were euthanized 3 days post-MCAO using isoflurane overdose and cardiac puncture. Blood (100 μL) was collected via cardiac puncture. Brain homogenates as well as the small intestines were additionally collected. Brain homogenates consisted of the prefrontal cortex through the hippocampus sampled from the ipsilateral side of the injury. Small intestine sections were sampled from the ileum.
Flow cytometry analysis
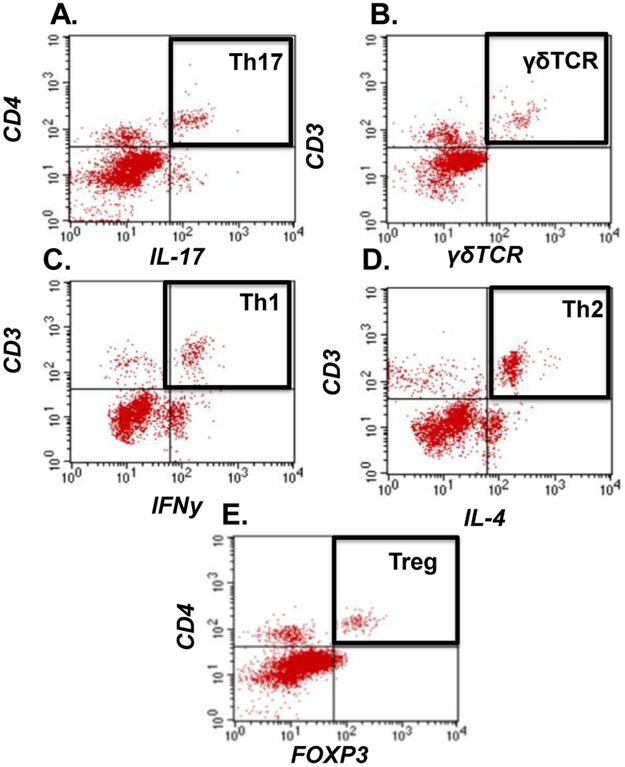
Freshly harvested brains and small intestines were sieved through a 100 μM cell strainer (BD Bio- sciences, San Diego, CA), followed by centrifugation (1400 rpm, 5 min). The pelleted cells were then incubated with Ack lysing buffer (Quality Biological, Gaithersburg, MD), washed and centrifugation was repeated to prepare single-cell suspensions. Blood was collected via cardiac puncture, as above. Cells were incubated with antibodies against cell surface markers CD3, CD4, γδTCR (eBioSciences, San Diego, CA). Following a PBS wash, cells were fixed and permeabilized using a fixation/permeabilization concentrate (Affymatrix eBioscience), and then incubated with antibodies for intracellular labeling of IFNy, IL4, IL-17A and FOXP3. After a final wash, cells were analyzed using a 4-color flow cytometer (FACSCalibur, BD Biosciences, San Diego, CA), and CellQuest software (BD Biosciences, San Jose, CA), as previously described (Braun et al. 2017). Isotype-matched controls were analyzed to set the appropriate gates for each sample. For each marker, samples were analyzed in duplicate. To minimize false-positive events, the number of double-positive events detected with the isotype controls was subtracted from the number of double-positive cells stained with corresponding antibodies (not isotype control), respectively. Cells expressing a specific marker were reported as a percentage of the number of gated events. The gating strategy is shown in Fig. 1. Inflammatory cells were identified as Th17 (CD4+, IL-17A+), γδTCR (CD3+, γδTCR+), and Th1 (CD3+, IFNy+). Anti-inflammatory cells were identified by Th2 (CD3+, IL-4+), and regulatory cells were identified by Treg (CD4+, FOXP3+).
Fig. 1: Flow cytometric gating.
Representative images from brain tissue to depict the gating strategy used to identify A) Th17 (CD4+, IL-17A+), B) γδTCR (CD3+, γδTCR+), C) Th1 (CD3+, IFNy+), D) Th2 (CD3+, IL-4+), and E) Treg (CD4+, FOXP3+).
Plasma IL-17A ELISA
The concentration of IL-17A in the rat plasma was measured by enzyme-linked immunosorbent assay (ELISA) using rat IL-17A Platinum ELISA kit from Affymetrix (E Bioscience, La Jolla, CA, catalog #BMS635). According the manufacture protocol, 100 μl of each plasma sample was measured in the 96-well plate. The level of IL-17A was calculated on triplicate wells for each sample.
Statistical Analyses
Prism7 was used for all analyses. The main effect of sex or diabetes as well as the interaction between diabetes and sex on the T-cell response to stroke was determined by two-way ANOVA (Figs 2 and 3). Sidak’s multiple comparisons post-hoc test was used to compare means within a sex from significant ANOVAs. Only main effects were given if results were significant. Statistical significance was determined at alpha<0.05.
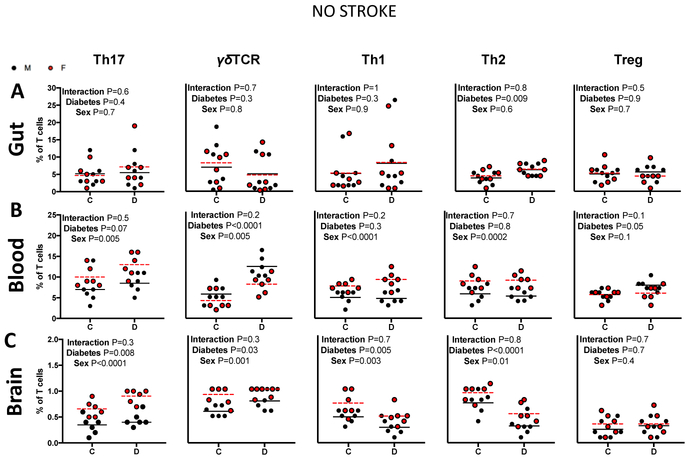
Fig. 2: The impact of sex and diabetes on T-cell profiles in naive rats.
A) Intestinal T-cell profiles were similar in all four groups. B) There was a sex effect in all circulating T-cells with the exception of Tregs and diabetes effect was seen only in the γδTCR cells. C) Diabetes promoted the expansion of cerebral Th17 cells only in females. N=6, two-way ANOVA (control × diabetes) × (male × female) followed by Sidak’s multiple comparisons post-hoc test.
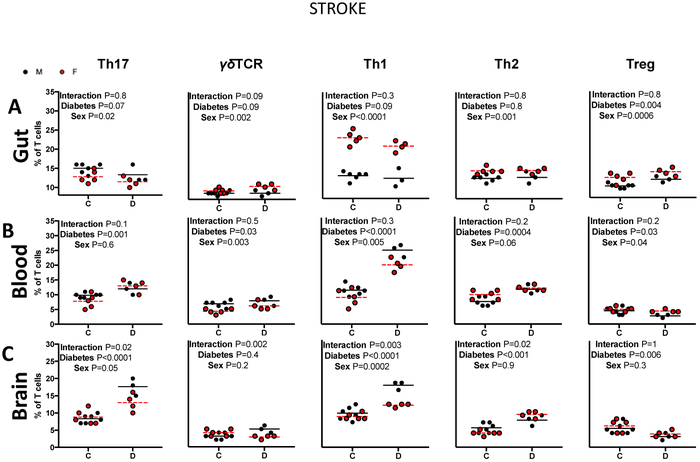
Fig. 3: The impact of sex and diabetes on T-cell profiles in stroked rats.
A) Diabetes upregulated intestinal Treg cells in response to stroke. B) Diabetes altered levels of all T-cells. Post-hoc analyses indicated that diabetes promoted the expansion of circulating Th17 cells in females and Th2 cells in males in response to stroke. C) Diabetes promoted a sexually dimorphic response of cerebral Th17, γδTCR, Th1 and Th2 cells to stroke. Diabetes led to a larger increase of cerebral Th17, γδTCR and Th1 in males, and a larger increase in Th2 in females. N=3-6, two-way ANOVA (control × diabetes) × (male × female) followed by Sidak’s multiple comparisons post-hoc test.
Results
Diabetes promoted the expansion of cerebral Th17 cells in female rats
The impact of diabetes on gut, blood and brain T cell profiles in the absence of a stroke was evaluated by comparing control and diabetic male and female naive rats (Fig. 2). There were no differences within any of the intestinal T cell subtypes between males and females (Fig. 2A). However, there were several differences within the circulating T cell subtypes (Fig. 2B). Regardless of disease (control vs diabetes), females exhibited a higher percentage of both circulating inflammatory and anti-inflammatory cells: Th17, Th1 and Th2 cells (P=0.005, P<0.0001, P=0.002), while male animals exhibited higher circulating γδTCR cells (P=0.005). Regardless of sex, diabetes promoted the expansion of circulating γδTCR cells (P<0.0001) in naive animals.
Within the brain a similar pattern was observed, where female rats exhibited a higher percentage of both inflammatory and anti-inflammatory cells, Th17, Th1, and Th2 cells (P<0.0001, P=0.003, P=0.01) (Fig. 2C). Female animals also exhibited higher percentages of cerebral γδTCR cells (P=0.001). Regardless of sex, diabetes promoted the expansion of Th17 and γδTCR while lowering Th1 and Th2 cells (P<0.0001, P= 0.001, P=0.003, P=0.01). Interestingly, post-hoc analysis indicated that diabetes promoted the expansion of cerebral Th17 and Th1 cells only in female rats (P=0.02, P=0.04).
Diabetes promoted a sexually dimorphic T cell response to ischemic stroke
Next, T cell profiles were assessed in response to an ischemic stroke (Fig. 3). Within the gut, stroke females exhibited a higher percentage of intestinal γδTCR, Th1, Th2 and Treg cells (P=0.002, P<0.0001, P=0.001, P=0.0006), but lower intestinal Th17 cells (P=0.02) compared to stroke males (Fig. 3A). Regardless of sex, diabetes increased Treg cells in response to stroke (P=0.004).
In the blood, stroke males exhibited a higher percentage of circulating γδTCR and Th1 cells (P=0.003, P=0.005) compared to stroke females, while females exhibited a higher percentage of Treg cells (P=0.04) in response to a stroke (Fig. 3B). Regardless of sex, diabetes enhanced the expansion of circulating Th17, γδTCR, Th1 and Th2, but downregulated Treg cells (P=0.001, P=0.03, P<0.0001, P=0.0004, P=0.03). Post-hoc analysis indicated that diabetes enhanced the expansion of Th17 cells in stroke females, but not males (P=0.002), while changing Th2 and Treg cell profiles in stroke males, but not females (P=0.002, 0.04).
Within the brain, stroke males exhibited a higher percentage of Th1 cells (Fig. 3C). Regardless of sex, diabetes promoted an upregulation of Th17, Th1 and Th2, and downregulation of Treg cells in response to stroke (P<0.0001, P=0.0001, P=0.0006). Most notably, an interaction of sex and diabetes only occurred within the brain. Diabetes had a sexually dimorphic effect on the upregulation of cerebral Th17, γδTCR, Th1 and Th2 cells in response to stroke (Interaction P=0.02, P=0.0002, P=0.003, P=0.02). Diabetes exerted a larger effect on the expansion of cerebral Th17 and Th1 cells in stroke males, while exerting a larger effect on the expansion of cerebral Th2 cells in females. Interestingly, while diabetes promoted the expansion of γδTCR cells in stroke males, it downregulated γδTCR cells in females.
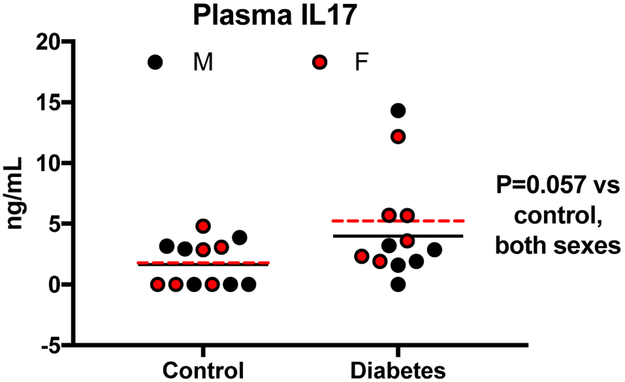
Plasma IL-17 was again evaluated to elucidate its role in the sexually dimorphic cerebrovascular complications of diabetes (Fig. 4). Despite the expansion of Th17 and γδTCR cells under diabetic conditions, plasma IL-17 levels remained undetectable. IL-17 was detectable after stroke and there was a trend for increased IL-17 with diabetes (P = 0.06). However, it did not significantly differ with sex nor diabetes.
Fig. 4: Stroke upregulated plasma IL-17A.
Plasma IL-17A, which was very low and even undetectable in all naive rats, was elevated in response to stroke. There was a trend for increased plasma IL-17A in both sexes with diabetes (p=0.057).
Discussion
The objectives of this study were to investigate whether and the extent to which diabetes promotes expansion of IL-17 producing inflammatory cells, especially after a stroke event, and whether there are sex differences in this response. The study was developed as a result of our interest in exploring emerging concepts that may explain our previous findings that diabetic animals develop cognitive deficits within 6-8 weeks of diabetes onset and exhibit greater vascular injury after ischemic stroke, especially in female animals, leading to poor outcomes. Th17 cells secrete IL-17, a cytokine that has been shown to promote cognitive impairment and worsen stroke injury in male mice and cause cerebrovascular aneurysm rupture in female animals. IL-17-producing cells have been shown to exacerbate ischemic injury by increasing the infarct size (Benakis et al. 2016). The fact that IL-17 has also been shown to specifically induce neuronal apoptosis through its increased expression in activated microglia in the acute period after ischemic injury is likely to underlie its role in increasing infarct size (Lv et al. 2016). This additionally leads to increased BBB breakdown and immune cell infiltration (Wojkowska et al. 2017). Additionally, IL-17 was shown to exhibit an increased expression in samples derived from cerebral aneurysms (Chalouhi et al. 2013). It was specifically implicated in the alteration of endothelial cell tight junction proteins leading to the rupture of cerebrovascular aneurysms in estrogen-deficient female mice, providing a mechanistic explanation of increased cerebral aneurysm rupture in women (Hoh et al. 2018; Wojkowska et al. 2017). In this study, we provide evidence that before stroke, female rats have a higher percentage of circulating and cerebral Th17 cells than males, of which diabetes promotes a dimorphic expansion. On the other hand, after stroke the expansion of IL-17-producing Th17 and γδTCR cells is greater in male diabetic rats. The interaction of diabetes, stroke and sex in the control of these inflammatory cells may identify sex and/or disease-specific targets for intervention.
We have previously shown that multiple models of diabetes develop early cerebrovascular dysfunction and neurovascular uncoupling with associated cognitive deficits in male rats (Li et al. 2017; Ward et al. 2018; Li et al. 2018; Kelly-Cobbs et al. 2012). It was also reported by us and others that HFD/STZ-induced diabetes causes cognitive impairment in both male and female rats (Pedicino et al. 2013; Ward et al. 2018). An interesting study showed that high salt diet (HSD) causes cognitive impairment in male mice via cerebrovascular dysfunction mediated by the expansion of intestinal Th17 cells (Faraco et al. 2018). We hypothesized that HFD may have a similar effect. However, in this current study, we did not observe differences in intestinal Th17 cells. HFD/STZ may differ from HSD by exerting the bulk of its effects on the expansion of circulating and cerebral Th17 cells, highlighting potentially different mechanisms of HSD and HFD induced cerebrovascular and cognitive deficits. It may also be possible that the 8-week duration of HFD/STZ was not long enough to observe intestinal Th17 differences.
Similar to Th17 cells, γδTCR cells have also been associated with inflammation (Mehta et al. 2015). In this study we demonstrate that while naive males have higher circulating γδTCR cells, females have higher cerebral γδTCR cells. Diabetes further promotes the expansion of circulating and cerebral γδTCR cells in both sexes, without altering the intestinal percentages of either. A subpopulation of γδTCR cells that secrete IL-17 were shown to migrate from the intestines to the meninges after stroke and exacerbate ischemic injury (Benakis et al. 2016). Interestingly we have also demonstrated here that females have higher intestinal γδTCR cells than males after stroke, and that diabetes actually further promotes the expansion of cerebral γδTCR cells in males but downregulates it in females. The fact that female rats have more intestinal γδTCR cells than males, which are further expanded with diabetes, yet they have a downregulation of γδTCR cells in response to stroke when compounded with diabetes brings into question whether diabetic female rats display a more gradual and chronic trafficking of γδTCR to the meninges after stroke.
Treg cells are regulatory T cells which suppress inflammation and the expansion of immune cells such as Th17 and γδTCR to promote self-tolerance. After stroke, we demonstrate that diabetes decreased the percentages of circulating and cerebral Treg cells. Interestingly, diabetes actually increased the percentage of intestinal Treg cells, especially in females. This is accompanied by a decrease in intestinal Th17 cells in response to stroke when compounded with diabetes. To this extent, one would expect diabetic rats to be afforded neuroprotection from the gut-brain axis, but this was not the case. Despite the observation that in response to stroke diabetes tilts the gut T cell profile toward a more immune tolerable state, the neuroprotection is still lost, and diabetic females still experience worsened stroke outcomes. This suggests that perhaps diabetes-induced cerebrovascular and cognitive deficits are independent of intestinal changes in our model.
Although diabetes increased the percentage of all circulating T-cells in response to stroke, multiple comparisons within these results indicated that it only significantly increased Th1 cells in naive females, without the paired upregulation of Th2 or Treg cells as seen in males. In compilation with this, diabetes only upregulated circulating Th17 cells in naive females. This may point to a mechanism by which neuroprotection is lost in diabetic females: diabetes may result in a loss of neuroprotection by heightening the circulating inflammatory response and simultaneously dampening the anti-inflammatory and regulatory responses leading to enhanced IL-17 production. Although plasma IL-17 levels in naive animals were undetectable, stroke profoundly upregulated its expression and diabetes trended to upregulate the expression even further. The inability to detect differences within IL-17 may be due to the sensitivity of the ELISA. Seeing as diabetes had a larger impact on the cerebral Th17 cells, investigation of cerebral IL-17 versus plasma IL-17 may manifest larger and more easily detectable differences.
There are limitations of the study that need to be recognized. First, this was an initial study to establish whether there is dysregulation of IL-17 producing inflammatory cells in diabetes and diabetic stroke, which may contribute to compromised cerebrovascular function and integrity, especially in females. As such, this study did not include any interventions to tease out molecular mechanisms. However, it is important to note that the upregulation of IL-17 has been identified as a mechanism that compromises vascular structural integrity promoting aneurysm formation and rupture in females via the activation of matrix metallopreinases (MMPs) (Hoh et al. 2018). MMP-3 is upregulated by hyperglycemia and has also been shown to be activated by IL-17 (Hafez et al. 2016; Gelderblom et al. 2012). We have preliminary evidence that diabetes activates MMP-3 to a greater extent in the cerebral vessels of female rats than in male diabetic rats. Regulation of MMP-3 activity by IL17-producing inflammatory cells at the neurovascular interface in both sexes warrants further investigation. Second, the study was completed in two phases. In phase one, T cell profiles were assessed in naive male and female control and diabetic animals. In phase two, T cell profiles were assessed in male and female control and diabetic animals after stroke. Since flow cytometry measurements were conducted separately in naive vs stroked rats, we did not analyze how stroke affected T cell profiles as compared to naive rats in male or female groups but rather contrasted sex and disease differences in naive (Fig. 2) or stroked rats (Fig. 3) separately. Third, due to greater mortality in diabetic stroke, the animal numbers were lower in this cohort. While this study is limited in the fact that it is primarily explorative and descriptive in nature, studies of this kind guide and inform translational research. T cells are well known to play a critical role in diabetes and in stroke, yet this study is the first to explore the sex differences within their response to diabetic stroke.
Acknowledgments
Funding This study was supported by Veterans Affairs (VA) Merit Review (BX000347), VA Senior Research Career Scientist Award, National Institute of Health (NIH) R01NS083559 and R01 NS104573 (multi-PI, Susan C. Fagan as co-PI) to Adviye Ergul; a TL1 award TL1 TR002382 and UL1TR002378 to Ladonya Jackson; and Diabetic Complications Research Consortium DiaComp awards 17AU3831/18AU3903 (DK076169/115255) to Dr. Weiguo Li.
Footnotes
Conflict of interest. On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical Approval and Compliance All rats were housed in the animal care facility at Augusta University, which is approved by the American Association for Accreditation of Laboratory Animal Care. All experiments were conducted in accordance with the National Institute of Health (NIH) guidelines for the care and use of animals in research. Furthermore, all protocols were approved by the institutional animal care and use committee.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- Abdul Y, Abdelsaid M, Li W, Webb RC, Sullivan JC, Dong G, et al. (2018). Inhibition of Toll-Like Receptor-4 (TLR-4) Improves Neurobehavioral Outcomes After Acute Ischemic Stroke in Diabetic Rats: Possible Role of Vascular Endothelial TLR-4. Mol Neurobiol, doi: 10.1007/s12035-018-1184-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnetz L, Ekberg NR, & Alvarsson M (2014). Sex differences in type 2 diabetes: focus on disease course and outcomes. Diabetes Metab Syndr Obes, 7, 409–420, doi: 10.2147/DMSO.S51301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett-Connor E (1997). Sex differences in coronary heart disease. Why are women so superior? The 1995 Ancel Keys Lecture. Circulation, 95(1), 252–264. [DOI] [PubMed] [Google Scholar]
- Benakis C, Brea D, Caballero S, Faraco G, Moore J, Murphy M, et al. (2016). Commensal microbiota affects ischemic stroke outcome by regulating intestinal gammadelta T cells. Nat Med, 22(5), 516–523, doi: 10.1038/nm.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoni AG, Krop JS, Anderson GF, & Brancati FL (2002). Diabetes-related morbidity and mortality in a national sample of U.S. elders. Diabetes Care, 25(3), 471–475. [DOI] [PubMed] [Google Scholar]
- Braun M, Vaibhav K, Saad N, Fatima S, Brann DW, Vender JR, et al. (2017). Activation of Myeloid TLR4 Mediates T Lymphocyte Polarization after Traumatic Brain Injury. J Immunol, 198(9), 3615–3626, doi: 10.4049/jimmunol.1601948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell CD, Reeves MJ, Zhao X, Pan W, Prvu-Bettger J, Zimmer L, et al. (2014). Sex differences in quality of life after ischemic stroke. Neurology, 82(11), 922–931, doi: 10.1212/WNL.0000000000000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr MC (2003). The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab, 88(6), 2404–2411, doi: 10.1210/jc.2003-030242. [DOI] [PubMed] [Google Scholar]
- Chalouhi N, Points L, Pierce GL, Ballas Z, Jabbour P, & Hasan D (2013). Localized increase of chemokines in the lumen of human cerebral aneurysms. Stroke, 44(9), 2594–2597, doi: 10.1161/STROKEAHA.113.002361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraco G, Brea D, Garcia-Bonilla L, Wang G, Racchumi G, Chang H, et al. (2018). Dietary salt promotes neurovascular and cognitive dysfunction through a gut-initiated TH17 response. Nat Neurosci, 21(2), 240–249, doi: 10.1038/s41593-017-0059-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Gomez AA, de Jesus Gomez-Villalobos M, & Flores G (2019). Consequences of diabetes mellitus on neuronal connectivity in limbic regions. Synapse, 73(3), e22082, doi: 10.1002/syn.22082. [DOI] [PubMed] [Google Scholar]
- Gall SL, Donnan G, Dewey HM, Macdonell R, Sturm J, Gilligan A, et al. (2010). Sex differences in presentation, severity, and management of stroke in a population-based study. Neurology, 74(12), 975–981, doi: 10.1212/WNL.0b013e3181d5a48f. [DOI] [PubMed] [Google Scholar]
- Gall SL, Tran PL, Martin K, Blizzard L, & Srikanth V (2012). Sex differences in long-term outcomes after stroke: functional outcomes, handicap, and quality of life. Stroke, 43(7), 1982–1987, doi: 10.1161/STROKEAHA.111.632547. [DOI] [PubMed] [Google Scholar]
- Gelderblom M, Weymar A, Bernreuther C, Velden J, Arunachalam P, Steinbach K, et al. (2012). Neutralization of the IL-17 axis diminishes neutrophil invasion and protects from ischemic stroke. Blood, 120(18), 3793–3802, doi: 10.1182/blood-2012-02-412726. [DOI] [PubMed] [Google Scholar]
- Hafez S, Abdelsaid M, El-Shafey S, Johnson MH, Fagan SC, & Ergul A (2016). Matrix Metalloprotease 3 Exacerbates Hemorrhagic Transformation and Worsens Functional Outcomes in Hyperglycemic Stroke. Stroke, 47(3), 843–851, doi: 10.1161/STROKEAHA.115.011258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoh BL, Rojas K, Lin L, Fazal HZ, Hourani S, Nowicki KW, et al. (2018). Estrogen Deficiency Promotes Cerebral Aneurysm Rupture by Upregulation of Th17 Cells and Interleukin-17A Which Downregulates E-Cadherin. J Am Heart Assoc, 7(8), doi: 10.1161/JAHA.118.008863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley MM, & Kielian T (2012). Th1 and Th17 cells regulate innate immune responses and bacterial clearance during central nervous system infection. J Immunol, 188(3), 1360–1370, doi: 10.4049/jimmunol.1101660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janghorbani M, Hedley AJ, Jones RB, Zhianpour M, & Gilmour WH (1993). Gender differential in all-cause and cardiovascular disease mortality. Int J Epidemiol, 22(6), 1056–1063. [DOI] [PubMed] [Google Scholar]
- Kelly-Cobbs AI, Prakash R, Coucha M, Knight RA, Li W, Ogbi SN, et al. (2012). Cerebral myogenic reactivity and blood flow in type 2 diabetic rats: role of peroxynitrite in hypoxia-mediated loss of myogenic tone. J Pharmacol Exp Ther, 342(2), 407–415, doi: 10.1124/jpet.111.191296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khader SA, Gaffen SL, & Kolls JK (2009). Th17 cells at the crossroads of innate and adaptive immunity against infectious diseases at the mucosa. Mucosal Immunol, 2(5), 403–411, doi: 10.1038/mi.2009.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Valenzuela JP, Ward R, Abdelbary M, Dong G, Fagan SC, et al. (2018). Post-stroke neovascularization and functional outcomes differ in diabetes depending on severity of injury and sex: Potential link to hemorrhagic transformation. Exp Neurol, 311, 106–114, doi: 10.1016/j.expneurol.2018.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Ward R, Valenzuela JP, Dong G, Fagan SC, & Ergul A (2017). Diabetes Worsens Functional Outcomes in Young Female Rats: Comparison of Stroke Models, Tissue Plasminogen Activator Effects, and Sexes. Transl Stroke Res, doi: 10.1007/s12975-017-0525-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv M, Zhang D, Dai D, Zhang W, & Zhang L (2016). Sphingosine kinase 1/sphingosine-1-phosphate regulates the expression of interleukin-17A in activated microglia in cerebral ischemia/reperfusion. Inflamm Res, 65(7), 551–562, doi: 10.1007/s00011-016-0939-9. [DOI] [PubMed] [Google Scholar]
- Mehta P, Nuotio-Antar AM, & Smith CW (2015). gammadelta T cells promote inflammation and insulin resistance during high fat diet-induced obesity in mice. J Leukoc Biol, 97(1), 121–134, doi: 10.1189/jlb.3A0414-211RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedicino D, Liuzzo G, Trotta F, Giglio AF, Giubilato S, Martini F, et al. (2013). Adaptive immunity, inflammation, and cardiovascular complications in type 1 and type 2 diabetes mellitus. J Diabetes Res, 2013, 184258, doi: 10.1155/2013/184258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward R, Valenzuela JP, Li W, Dong G, Fagan SC, & Ergul A (2018). Post Stroke Cognitive Impairment and Hippocampal Neurovascular Remodeling: The Impact of Diabetes and Sex. Am J Physiol Heart Circ Physiol, doi: 10.1152/ajpheart.00390.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojkowska DW, Szpakowski P, & Glabinski A (2017). Interleukin 17A Promotes Lymphocytes Adhesion and Induces CCL2 and CXCL1 Release from Brain Endothelial Cells. Int J Mol Sci, 18(5), doi: 10.3390/ijms18051000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, He M, Xu Y, Li X, Cai Z, Guo Z, et al. (2018). Hemoglobin A1c predicts hemorrhagic transformation and poor outcomes after acute anterior stroke. Eur J Neurol, 25(12), 1432–e1122, doi: 10.1111/ene.13747. [DOI] [PubMed] [Google Scholar]