Abstract
Objective
We evaluated the 6-month postpartum risk of metabolic syndrome (MetS), a marker of future cardiovascular disease (CVD) risk, comparing women whose most recent pregnancies were complicated with gestational hypertension (GH) or preeclampsia (PE) versus those who had normotensive pregnancies.
Study design
This was a prospective cohort study in which women with GH or PE and normotensive women were actively enrolled during the first 12 weeks after delivery in Nairobi, Kenya. Participants were interviewed, blood pressures and anthropometric measurements including waist circumference obtained at enrollment and 6 months postpartum. Fasting lipid profile and plasma glucose were measured at 6 months postpartum. A generalized linear regression model with Poisson distribution was used to estimate crude relative risk (RR) of 6-month postpartum MetS and adjusted RR (ARR) after adjusting for apriori potential confounders.
Results
Among 194 postpartum, 63 (32%) had experienced GH or PE. Prevalence of MetS at 6 months postpartum was higher among women whose pregnancies were complicated with GH or PE (34.9%) compared to those who were normotensive (11.5%). GH and PE was associated with a 3-fold or greater risk of MetS (ARR) 3.01; 95% Confidence interval [CI] 1.58, 5.71; p<0.001) overall and three of the five components, namely hypertension (ARR 3.35 [2.04, 5.51], p<0.001), hypertriglyceridemia (ARR 3.25 [1.16–9.10], p=0.01), and fasting hyperglycemia (ARR 6.20 [1.07–35.76], p=0.03), compared to having normal blood pressures during pregnancy.
Conclusion
At 6 months postpartum, GH and PE were associated with three-fold or higher risk of MetS and especially hypertension, fasting hypertriglyceridemia, and fasting hyperglycemia.
Introduction
Metabolic syndrome (MetS) is defined by the presence of 5 cardiovascular disease (CVD) risk factors: atherogenic dyslipidemia (elevated triglycerides [TG] and reduced high-density lipoprotein cholesterol [HDL-C] levels), elevated blood pressure (BP), central obesity and elevated fasting plasma glucose (FPG)1. MetS is associated with an estimated 2-fold increase in risk of developing ischemic heart disease and cerebrovascular disease, and has been associated with increased CVD-related mortality, as well as all-cause mortality2. Whereas each of the five components of MetS independently increases CVD risk, when present together the risk is multiplied. Although the underlying mechanisms linking the development of MetS and CVD have not been conclusively described, MetS and CVD have genetic and environmental origins3,4 but progression of MetS to CVD may result from insulin resistance, neurohormonal activation and chronic inflammation, processes that can be delayed by lifestyle changes, risk factor modification and pharmaceutical therapy5. Therefore, individuals with MetS and at high risk for future CVD should be identified for targeted prevention and therapy.
Pregnancies complicated by gestational hypertension (GH) or preeclampsia (PE) are associated with increased risk of MetS postpartum6–11 in high income countries. GH is new onset hypertension (after twenty weeks of pregnancy or within 12 weeks postpartum) without evidence of end organ damage such as proteinuria, renal, liver, cardiac or neurological complications while PE is new onset hypertension with end organ damage12. GH and PE have both been associated with a 2-fold or greater risk of future CVD, premature CVD related deaths and type 2 diabetes compared with normotensive pregnancies13–16. The increased risk for MetS and CVD after GH or PE suggests a shared pathway9,17,18. Thus, GH and PE have been recognized as cardiac risk factors19 for closer postpartum follow-up in high-income countries10,20,21. While CVD-mortality has declined in both men and women in the United States, stagnation in younger adults, especially women under the age of 55 years22, partly as a result of female-specific CVD risk factors such as GH and PE has been observed23. The association between GH and PE and CVD has not been well documented in low and middle-income countries (LMIC) especially in sub-Saharan Africa (SSA), a region heavily burden by CVD and CVD-related deaths24. In SSA this association may be stronger due to the high burden of PE and GH. Understanding the risk of postpartum MetS and risk of CVD can inform care after GH and PE in LMIC and globally.
In this prospective cohort study in Nairobi, Kenya, we compared the prevalence of MetS and that of its components between women whose most recent pregnancies were complicated with GH or PE versus those who had normotensive pregnancies. We hypothesized that women with recent GH or PE would have a higher risk of MetS at six months postpartum when compared to women without these pregnancy complications.
Methods
Study Design and Setting
This was a prospective cohort study to compare the risk of MetS among women with versus those without GH or PE at 6 months postpartum at Kenyatta National Hospital (KNH), the largest national teaching and referral hospital in Nairobi, Kenya. KNH provides low and high-risk obstetric care and conducts more than 10,000 deliveries annually, 5% of which are complicated by GH and PE. The study was approved by the University of Nairobi/KNH Ethical Review Committee and University of Washington’s Institutional Review Board. All participants signed written informed consent.
Study population
Postpartum women with or without GH or PE, who were stable and ready for discharge were screened for eligibility. Women were eligible if they were: HIV uninfected, not intending to become pregnant for at least 3 years, aged 28 years or older and did not have conditions suggestive of prepregnancy MetS such as pregestational diabetes, chronic hypertension, malignancy, renal, hepatic or biliary disease. We excluded women who used statins, insulin, oral hypoglycemic agents, or antihypertensives before onset of pregnancy. Only women who had delivered at 28 weeks or greater, had medical records, were planning to live within 50 km from the hospital so as to be followed for at least 6 months and to undergo physical examination and blood sample collection were recruited.
Definition of exposure
Potential participants were categorized as exposed if they had GH or PE and unexposed if they were normotensive using in-patient records including BP measurements at screening prior to discharge. We used the International Society for the Study of Hypertension in Pregnancy (ISSHP) 2018 classification guidelines to define PE as new onset elevation of blood pressure ([BP] ≥ 140/90 mmHg at least 2 hours apart at rest, after 20 weeks of pregnancy and before 12 weeks postpartum) with evidence of end organ damage such as proteinuria, renal, liver, cardiac or neurological complications and GH as new onset elevation of BP without evidence of end organ damage12. We excluded women who were known to have gestational diabetes mellitus.
Enrollment procedures
We enrolled women who returned to the study clinic within 12 weeks postpartum. Each participant underwent a structured interview to collect sociodemographic, family and past medical history followed by measurements of BP (mmHg), weight (kilograms), height (centimeters), hip circumference (centimeters), and waist circumference ([WC]) centimeters). The BP was measured using a calibrated automatic BP recording device, OMRON®, at least 10 minutes after the participant arrived at the clinic. As per the recommendations of the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High BP, three readings were taken at least 10 minutes apart using a cuff that encircles at least 80% of the arm while seated at rest and then averaged25. We measured weight using a calibrated electronic scale with subjects in light clothing and without shoes, height using a dropdown ruler, WC using a non-stretchable tape at the approximate midpoint between the lower margin of the last palpable rib and the top of the iliac crest and widest hip circumference. Measurements were repeated twice and averaged if within 1 cm but repeated if exceeded 1 cm (discrepant). We computed the waist-hip-ratio and the body mass index (BMI). All study procedures were conducted by trained research study nurses. A trained community health worker and research study nurse called enrolled participants monthly to update contacts and addresses and remind them of the appointment at 6 months postpartum.
Postpartum follow-up and study procedures
Study subjects underwent structured interviews, measurements of BP, weight, height and WC and blood draws. Blood samples were collected from the antecubital vein 9–12 hours after fasting into evacuated blood collection tubes with ethylenediaminetetraacetic acid anticoagulant (BD Vacutainer®) for lipids or a serum separator gel for glucose. Samples were inverted gently 5–10 times, stored on ice, centrifuged in a refrigerated centrifuge at 1,500 rpm for 30 minutes to isolate the plasma fraction at 4°C and then placed into an ice bath at 2–4°C within 2 hours of collection. Plasma and serum aliquots were kept frozen at −80°C at the University of Nairobi laboratory, then batched and shipped to Seattle, USA for testing by the University of Washington Department of Laboratory Medicine’s Research Testing Service. On the day of analysis, the specimens were thawed and mixed thoroughly. Serum lipids and glucose were quantified using the Beckman Coulter AU5812 automated chemistry and immunochemistry analyzer using enzymatic assays.
Outcome
The primary outcome, postpartum MetS was diagnosed if a participant had 3 or more of the 5 components of the 2009 MetS consensus criteria: 1) abdominal obesity (WC≥ 88 cm), 2) elevated fasting TG (≥150 mg/dL) or its treatment, 3) low HDL-cholesterol (<50 mg/dL) or its treatment, 4) elevated BP (systolic BP ≥130 or diastolic BP ≥85 mm Hg or treatment for hypertension), and 5) elevated FPG ≥100 mg/dL or its treatment26.
Sample size and statistical analysis
We assumed the risk of MetS to be 20% in hypertensive versus 5% in normotensive women and estimated that we would require 180 women (60 exposed versus 120 unexposed) to detect the 15% difference in risk of MetS with 80% power and alpha level of 0.05. To compare the baseline sociodemographic, physical and reproductive characteristics and the 6-month postpartum risk of MetS between women with and without GH or PE, we used Student t-tests and nonparametric Wilcoxon rank sum tests for continuous variables and Pearson’s chi-square tests or Fisher’s exact tests for categorical variables. We obtained crude and adjusted relative risk estimates using a generalized linear model with a Poisson distribution link and adjusted for potential confounding variables that were determined a priori to be related to both the exposure and the outcome. These potential confounding variables included maternal age, level of education, body mass index, hormonal contraception, breastfeeding, and marital and employment status. P-values <0.05 were considered statistically significant. All analyses were conducted using STATA® version 13. College Station, TX: StataCorp LP.
Results
Baseline characteristics of study participants
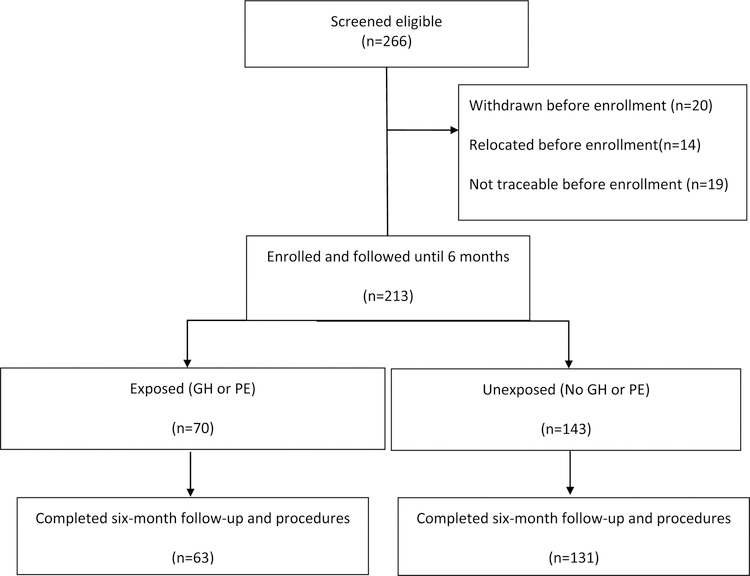
From November 2016 to July 2018, we followed 213 women from enrollment until 6 months postpartum. Overall, 194 (91%) completed all the study procedures (Figure 1). Of these, 63 (32%) had either GH or PE while 131 (68%) had normal BP during their most recent pregnancies. Most baseline sociodemographic, reproductive characteristics and physical measurements at enrollment were similar between the two groups (Table 1). However, we observed some differences; specifically, women with prior GH or PE were more likely to have delivered an infant with a lower mean birthweight (2516.1g versus 3323.6 g, p<0.001) and at a lower mean gestational age (36.1 versus 38.8 weeks, p<0.001), and were more likely to have a stillbirth or neonatal death (28.6% versus 6.1%, p<0.001) higher parity (mean 4 versus 3, p=0.049), higher body mass index (BMI) ≥ 30 kg/m2 (60.7% versus 43.8%, p=0.03) and to have exclusively breastfed their infant (75.6 % versus 54.5%, p=0.01). The mean systolic blood pressure (BP) and diastolic BP were also significantly higher among the exposed compared to the unexposed women (134.0 versus 116.8 and 90.1 versus 78.7 mmHg respectively, p<0.001) (Table 1).
Figure 1: Study participants.
GH, Gestational hypertension, new onset hypertension after twenty weeks of pregnancy or within 12 weeks postpartum without symptoms or signs of end organ damage such as proteinuria, renal, liver, cardiac or neurological complications; PE, Preeclampsia, new onset hypertension after twenty weeks of pregnancy or within 12 weeks postpartum with symptoms or signs of end organ damage such as proteinuria, renal, liver, cardiac or neurological complications
Table 1:
Baseline characteristics of study participants
| GH¶ or PE§ N=63 | Normotensive N=131 | P value | ||
|---|---|---|---|---|
| Characteristic | n (%) or mean ± SD | n (%) or mean ± SD | ||
| Age, years | 33.4 ± 3.6 | 32.8 ± 4.1 | 0.33 | |
| ≥ 35 | 18 (28.6) | 45(34.3) | 0.42 | |
| Married | 61(96.8) | 124(94.7) | 0.50 | |
| Secondary or higher education | 41(65.1) | 95(72.5) | 0.33 | |
| Employed | 45(71.4) | 103(78.6) | 0.27 | |
| Preconception hormonal contraception | 33(52.4) | 7 (58.8) | 0.40 | |
| Parity | 4 ± 2 | 3 ± 1 | 0.049 | |
| Gestation (weeks) | 36.1 ± 3.7 | 38.8 ± 2.0 | <0.001* | |
| Maternal weight, Kg | 74.5 ± 14.1 | 72.2 ± 13.1 | 0.25 | |
| Height (cm) | 141.4 ± 42.1 | 141.8 ± 41.1 | 0.94 | |
| Waist circumference, cm | 97.0 ±17.0 | 94.5 ± 9.4 | 0.29 | |
| Hip circumference (cm) | 109.3 ± 16.2 | 107.2 ± 9.5 | 0.35 | |
| Waist-hip ratio >0.85, cm | 44 (68.6) | 98 (76.2) | 0.41 | |
| Infant birthweight, grams | 2516.1 ± 995.3 | 3323.6 ± 617.7 | <0.001* | |
| Male infant sex | 33 (52.4) | 69 (52.7) | 0.97 | |
| Stillbirths or neonatal deaths | 18 (28.6) | 8 (6.1) | <0.001* | |
| Exclusive breastfeeding | 34 (75.6) | 67 (54.5) | 0.01* | |
| SBP, mm Hg | 134.0 ± 21.7 | 116.8 ± 13.9 | <0.001* | |
| DBP, mm Hg | 90.1 ± 15.8 | 78.7 ± 10.7 | <0.001* | |
| Body mass index (kg/m2) | 31.9 ± 5.7 | 30.7 ± 5.9 | 0.19 | |
| ≥30 (obese) | 37 (60.7) | 56 (43.8) | 0.03* | |
Values are in n (%) or mean ± SD. SD, standard deviation;
GH, Gestational hypertension, hypertension occurring after twenty weeks of pregnancy or within 12 weeks postpartum without symptoms or signs of end organ damage such as proteinuria, renal, liver, cardiac or neurological complications;
PE, Preeclampsia, hypertension and symptoms or signs of end organ damage such as proteinuria, renal, liver, cardiac or neurological complications; SBP, Systolic blood pressure; DBP, Diastolic blood pressure;
p<0.05
Physical and biochemical characteristics of participants at six months postpartum
At the six-month follow-up visit, exposed women had significantly higher mean systolic (132.6 versus 119.1 mmHg) and diastolic (89.2 versus 79.1 mmHg) BP compared to normotensive women (p<0.001) (Table 2). Nearly one quarter (n=15 out of 63[23.8%] of exposed women versus less than one tenth of unexposed women (n=12 out of 131 [9.1%], p=0.006) had elevated BP≥ 140/90 mmHg, p<0.001. Exposure to GH or PE was associated with a trend towards higher mean serum triglycerides (TG) levels (95.7 versus 80.2 mg/dl, p=0.05) and higher mean serum remnant cholesterol levels (19.1 versus 16.1 mg/dl, p=0.05) compared to the unexposed. There was no difference in mean levels of serum total cholesterol, HDL-cholesterol, FPG, weight, WC, BMI and proportion with BMI ≥30 Kg/m2 between the exposed and normotensive women.
Table 2:
Physical and biochemical characteristics at six months postpartum
| Characteristic | GH¶ or PE§ N=63 | Normotensive N=131 | P value |
|---|---|---|---|
| SBP, mmHg | 132.6 ± 18.4 | 119.1 13.7 | <0.001* |
| DBP, mmHg | 89.2 ± 13.1 | 79.1 10.3 | <0.001* |
| BP≥140/90 mmHg | 15 (23.8) | 12 (9.2) | 0.006* |
| Total cholesterol | 177.8 ± 36.6 | 174.6 ± 31.9 | 0.56 |
| Triglycerides | 95.7 ± 50.6 | 80.2 ± 53.5 | 0.053 |
| HDL-cholesterol | 50.6 ± 11.1 | 49.9 ± 10.1 | 0.65 |
| LDL-cholesterol | 108.0 ± 30.4 | 108.7 ± 26.7 | 0.88 |
| Remnant-cholesterol | 19.1 ± 10.1 | 16.1 ± 10.7 | 0.053 |
| Fasting plasma glucose (mg/dl) | 81.2 ± 18.8 | 77.1 ± 8.1 | 0.10 |
| Weight (kg) | 76.8 ± 15.3 | 74.7 ± 13.4 | 0.34 |
| Waist circumference (cm) | 95.6 ± 12.4 | 95.2 ± 11.4 | 0.79 |
| Body mass index (kg/m2) | 32.8 ± 6.2 | 31.9 ± 6.0 | 0.31 |
| ≥30 (obese) | 41 (65.1) | 76 (58.9) | 0.30 |
Values are in n (%) or mean ± SD. SD, standard deviation;
GH, Gestational hypertension, hypertension occurring after twenty weeks of pregnancy or within 12 weeks postpartum without symptoms or signs of end organ damage such as proteinuria, renal, liver, cardiac or neurological complications;
PE, Preeclampsia, hypertension and symptoms or signs of end organ damage such as proteinuria, renal, liver, cardiac or neurological complications; SBP, Systolic blood pressure; DBP, Diastolic blood pressure; HDL, high density lipoprotein, LDL-low density lipoprotein;
p<0.05
Prevalence of postpartum metabolic syndrome and its components at six months postpartum
The prevalence of MetS was significantly higher in women who were exposed to GH or PE (n=22 of 63, 34.9%) compared to those who were not ([n=15 of 131, 11.5%], p<0.001) (Figure 2). Compared to the unexposed, exposed women were significantly more likely to have elevated BP (systolic BP≥ 130 or diastolic BP ≥ 85 mmHg or treatment for hypertension [n=28, 44.4% versus n=20, 15.6%, p<0.001]), TG ≥150 mg/dl (n=10, 15.9% versus n=7, 5.5%, p=0.02) and FPG≥ 100 mg/dl (n=5, 7.9% versus n=2, 1.6%, p=0.03). WC ≥ 88 cm and HDL-cholesterol < 50 mg/dl were most common MetS criteria, however they were not statistically significantly different between women with and without GH or PE.
Figure 2. Prevalence of the metabolic syndrome among women with and without gestational hypertension or preeclampsia at 6 months postpartum.
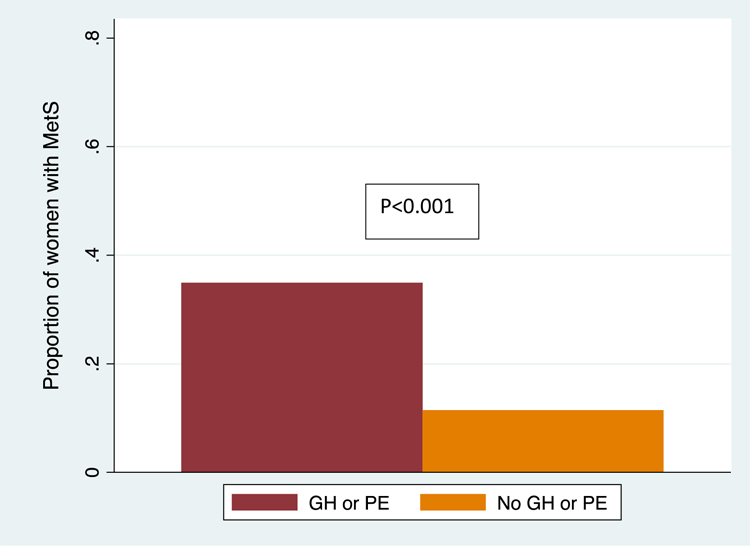
MetS, Metabolic syndrome; GH, Gestational hypertension, new onset hypertension after twenty weeks of pregnancy or within 12 weeks postpartum without evidence of end organ damage such as proteinuria, renal, liver, cardiac or neurological complications; PE, Preeclampsia, new onset hypertension after twenty weeks of pregnancy or within 12 weeks postpartum with evidence of end organ damage.
In unadjusted analysis, the risk of MetS was three times greater in women exposed compared to those who were not exposed to GH or PE (Relative Risk (RR) 3.05, 95% Confidence interval (CI) [1.70,5.47], P<0.001 (Table 3). The associations persisted when adjusted for maternal age, level of education, hormonal contraception, breastfeeding, body mass index, marital and employment status. In adjusted analysis, the risk remained three times or greater for MetS (RR 3.01, 95% CI [1.58,5.71], p=0.001, BP ≥130/85 mmHg or treatment for hypertension (RR 3.35 95% CI [2.04,5.51], p<0.001, hypertriglyceridemia (RR 3.25 95% CI [1.16,9.10] p=0.01, and fasting hyperglycemia (RR 6.20 95% CI [1.07,35.76], p=0.03). There was no statistically significant difference in the prevalence of low HDL-cholesterol and high WC.
Table 3:
Risk of metabolic syndrome and its components at 6 months postpartum
| GH¶ or PE§ N=63 | Normotensive N=131 | P value | RR | 95% CI | aRR† | 95% CI | P value | |
|---|---|---|---|---|---|---|---|---|
| MetS (3 or more components) | 22 (34.9) | 15 (11.5) | <0.001* | 3.05 | 1.70–5.47 | 3.01 | 1.58–5.71 | 0.001* |
| BP ≥130/85 mmHg or treatment | 28 (44.4) | 20 (15.6) | <0.001* | 3.14 | 1.97–5.01 | 3.35 | 2.04–5.51 | <0.001* |
| Triglycerides ≥150 mg/dl | 10 (15.9) | 7 (5.3) | 0.02* | 2.97 | 1.19–7.44 | 3.25 | 1.16–9.10 | 0.03* |
| HDL-cholesterol ≤40 md/dl | 32 (50.8) | 66 (50.4) | 0.96 | 1.01 | 0.75–1.36 | 0.97 | 0.72–1.31 | 0.85 |
| Fasting plasma glucose ≥100 mg/dl | 5 (7.9) | 2 (1.5) | 0.03* | 5.20 | 1.04–26.06 | 6.20 | 1.07–35.76 | 0.04* |
| Waist circumference ≥88 cm | 47 (74.6) | 96 (73.3) | 0.85 | 1.02 | 0.85–1.22 | 0.91 | 0.77–1.06 | 0.22 |
Values are in n (%);
GH, Gestational hypertension, hypertension occurring after twenty weeks of pregnancy or within 12 weeks postpartum without symptoms or signs of end organ damage such as proteinuria, renal, liver, cardiac or neurological complications;
PE, Preeclampsia, hypertension and symptoms or signs of end organ damage such as proteinuria, renal, liver, cardiac or neurological complications; SBP, Systolic blood pressure; DBP, Diastolic blood pressure;
p<0.05 MetS, Metabolic syndrome; BP, Blood pressure; HDL, high density lipoprotein; *p<0.05 RR, relative risk; aRR; adjusted relative risk; MetS, metabolic syndrome; HDL, high density lipoprotein
Adjusted for age, education, body mass index, marital status, employment status, hormonal contraception and breastfeeding, * P<0.05
Discussion
In this prospective study, we found that at six-months postpartum, relatively young women exposed to new onset hypertension after the first twenty weeks of pregnancy (gestational hypertension [GH] or preeclampsia [PE]) had more than three times greater risk of metabolic syndrome (MetS) compared to women who had normal blood pressures during pregnancy in this low- and middle-income countries (LMIC) setting. We observed three-fold or greater risk of 3 of the 5 components of MetS at 6 months postpartum after GH or PE compared with normotensive pregnancies. Women with history of GH or PE were also more than two times as likely to have hypertension at six months postpartum.
The prevalence of MetS of 35% among women exposed to GH or PE in this setting is higher than those reported in previous prospective cohorts in high-income settings that used similar criteria. Van Rijin et al reported prevalence of MetS of 15.2% versus 4.3% after early onset PE versus normotensive pregnancies at six months postpartum6. Although this and our analysis were conducted at six month postpartum, they exluded late onset preeclampsia and gestational hypertension which are also associated with increased future CVD risk. Whereas our prevalence of postaprtum MetS is higher, the relative risk is similar between the two studies. In Canada, Smith et al observed a prevalence of MetS of 18%–18.6% in exposed compared to 5.7–7% in unexposed women20,27. However, their analysis was at one year postpartum and their study population excluded GH. The Utrecht and Hypitat Risk Assessment Study cohorts found a prevalence of 14–16% for MetS among exposed women21,28. There are no published studies on the prevalence of MetS among postpartum women in SSA for comparison. The high prevalence of MetS in our study, is consistent with the 40% or greater prevalence of MetS among non-pregnant adult female population regionally, in Kenya and Ghana29–31. These studies and our findings suggest that insulin resistance, dyslipidemia, and associated inflammation, the risk factors for MetS, are highly prevalent after GH or PE in this setting. As the MetS is a known risk factor for CVD2,32, our study suggests that without intervention women exposed to GH or PE in SSA are at exceedingly high risk of CVD.
The components of MetS that were elevated after GH or PE were hypertriglyceridemia, high BP and high FPG. In a recent metanalysis of 24 case control and 5 cohort studies, hypertriglyceridemia was associated with preeclampsia, suggesting a role of TG in the development of PE, MetS and CVD33–35. Since hypertriglyceridemia may also reflect hyperglycemia, studies using glycated hemoglobin may evaluate this association and identify women with long standing insulin resistance postpartum. Apart from low HDL-cholesterol, elevated serum TG may play a significant role in CVD and a dose dependent increase in risk of CVD and all-cause mortality from hypertriglyceridemia has been described33–35. The dyslipidemia in postpartum MetS in other settings have been from low HDL-cholesterol and high triglycerides in addition to high total cholesterol. We did not observe a similar trend probably due to the high prevalence of hypercholesterolemia in our population. The slight increase in remnant cholesterol an independent risk factor of ischemic heart disease warrants further evaluation36–40. Postpartum hypertension and fasting hyperglycemia have been reported in multiple studies estimating the risk of MetS from 3 months to decades postpartum17,41,42. Our results therefore suggest that hypertriglyceridemia, hypertension and insulin resistance are the most affected components of MetS after GH or PE in this setting and should be considered in monitoring and potential interventions to reduce risk of CVD in these women. We will follow this cohort until 3 years postpartum to for these outcomes.
As the first study of postpartum MetS in SSA setting, our study supports the need for structured care and interventions to reduce CVD risk after GH or PE6–8,11,17,20,23,42,43 in similar settings and globally. Even though the evidence GH and PE increase the risk of CVD continues to grow in high income settings, this burden has not previously been estimated in sub-Saharan Africa (SSA), where three quarters of global annual CVD-related deaths occur24,44. This is especially important because these women are relatively young and younger adults, especially younger women below 55 years, continue to experience high CVD morbidity and mortality rates22 partly due to female-specific CVD risk factors, including GH or PE2,23,45.
Our study had several strengths. First, to the best of our knowledge, this is the first study to assess the risk of MetS among a cohort of postpartum women following exposure to GH or PE in Kenya and the rest of SSA. Secondly, we studied the risk of MetS after GH and PE and not PE alone. Since GH has also been associated with increased CVD risk, our findings are more representative of the risk of MetS after new onset of hypertension in pregnancy.
The major limitation of our study was that by enrolling women postpartum and not before 20 weeks of pregnancy, we may have not completely excluded preexisting MetS or misclassified some cases of preexisting hypertension as GH or PE. Also, due to lack of universal screening for gestational diabetes, we were not able to completely identify women with gestational diabetes among hypertensive and normotensive women and this should be considered in future studies. Due to their rarity in our study population, we did not include other CVD risk behaviors such as smoking, alcohol consumption, physical activity and dietary patterns in our adjusted models.
Conclusion
The MetS and its components, elevated blood pressure, fasting hypertriglyceridemia and fasting hyperglycemia were highly prevalent at six months postpartum among women with GH or PE in this LMIC setting of SSA. Since MetS suggests an increased risk of future CVD, there is an urgent need for additional longitudinal incidence, mechanistic and intervention studies targeting CVD prevention, such as blood pressure and lipid lowering medications for women with GH or PE to lower their risk of CVD.
Highlights.
Gestational hypertension and preeclampsia are associated with three-fold or greater risk of metabolic syndrome, a marker of future cardiovascular disease at six months postpartum
Gestational hypertension and preeclampsia are associated with three-fold or greater risk of 3 of the 5 components of metabolic syndrome, elevated blood pressure and hypertension, fasting hypertriglyceridemia and fasting hyperglycemia at six months postpartum
There is a high risk of metabolic syndrome and therefore future cardiovascular disease risk among women who experience gestational hypertension and preeclampsia in Kenya, Sub-Saharan Africa, a region overburdened by cardiovascular disease burden
This study informs the need for structured postpartum care, as well as longitudinal mechanistic and intervention studies that focus on lowering cardiovascular disease risk after gestational hypertension and preeclampsia.
Acknowledgment(s)
We gratefully acknowledge the contribution of the study staff, study participants and the National Institute of Health through the Emerging Global Leader Award (K43) for making this study possible.
This project was supported by the Fogarty International Center and the Office of Research on Women’s Health (ORWH) of the National Institutes of Health under Award Number K43 TW010363. A. O. was supported by NIH Research Grant D43 TW009580, funded by the Fogarty International Center, National Institute on Drug Abuse, and National Institute of Mental Health. The NIH was not involved in the study design, collection, analysis and interpretation of data, writing of the report or in the decision to submit the article for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- 1.Grundy SM, Brewer HB, Cleeman JI, et al. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 2004;109(3):433–438. [DOI] [PubMed] [Google Scholar]
- 2.Gami AS, Witt BJ, Howard DE, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol 2007;49(4):403–414. [DOI] [PubMed] [Google Scholar]
- 3.Jermendy G, Horváth T, Littvay L, et al. Effect of genetic and environmental influences on cardiometabolic risk factors: a twin study. Cardiovasc Diabetol 2011;10:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elder SJ, Lichtenstein AH, Pittas AG, et al. Genetic and environmental influences on factors associated with cardiovascular disease and the metabolic syndrome. J Lipid Res 2009;50(9):1917–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rochlani Y, Pothineni NV, Kovelamudi S, Mehta JL. Metabolic syndrome: pathophysiology, management, and modulation by natural compounds. Ther Adv Cardiovasc Dis 2017;11(8):215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Rijn BB, Nijdam ME, Bruinse HW, et al. Cardiovascular disease risk factors in women with a history of early-onset preeclampsia. Obstet Gynecol 2013;121(5):1040–1048. [DOI] [PubMed] [Google Scholar]
- 7.Mangos GJ, Spaan JJ, Pirabhahar S, Brown MA. Markers of cardiovascular disease risk after hypertension in pregnancy. J Hypertens 2012;30(2):351–358. [DOI] [PubMed] [Google Scholar]
- 8.Giguère Y, Charland M, Thériault S, et al. Linking preeclampsia and cardiovascular disease later in life. Clin Chem Lab Med 2012;50(6):985–993. [DOI] [PubMed] [Google Scholar]
- 9.Forest JC, Girouard J, Massé J, et al. Early occurrence of metabolic syndrome after hypertension in pregnancy. Obstet Gynecol 2005;105(6):1373–1380. [DOI] [PubMed] [Google Scholar]
- 10.Cusimano MC, Pudwell J, Roddy M, Cho CK, Smith GN. The maternal health clinic: an initiative for cardiovascular risk identification in women with pregnancy-related complications. Am J Obstet Gynecol 2014;210(5):438.e431–439. [DOI] [PubMed] [Google Scholar]
- 11.Carson MP. Society for maternal and fetal medicine workshop on pregnancy as a window to future health: Clinical utility of classifying women with metabolic syndrome. Semin Perinatol 2015;39(4):284–289. [DOI] [PubMed] [Google Scholar]
- 12.Brown MA, Magee LA, Kenny LC, et al. The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens 2018;13:291–310. [DOI] [PubMed] [Google Scholar]
- 13.Lykke JA, Langhoff-Roos J, Sibai BM, Funai EF, Triche EW, Paidas MJ. Hypertensive pregnancy disorders and subsequent cardiovascular morbidity and type 2 diabetes mellitus in the mother. Hypertension 2009;53(6):944–951. [DOI] [PubMed] [Google Scholar]
- 14.Hermes W, Tamsma JT, Grootendorst DC, et al. Cardiovascular risk estimation in women with a history of hypertensive pregnancy disorders at term: a longitudinal follow-up study. BMC Pregnancy Childbirth 2013;13:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Breimer AY, Koster WP, Hermes W, et al. OS023. Postpartum cardiovascular disease risk factors in women with ahistory of early onset preeclampsia, late onset preeclampsia and pregnancy induced hypertension. Pregnancy Hypertens 2012;2(3):188. [DOI] [PubMed] [Google Scholar]
- 16.Mongraw-Chaffin ML, Cirillo PM, Cohn BA. Preeclampsia and cardiovascular disease death: prospective evidence from the child health and development studies cohort. Hypertension 2010;56(1):166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hooijschuur MC, Ghossein-Doha C, Al-Nasiry S, Spaanderman ME. Maternal metabolic syndrome, preeclampsia, and small for gestational age infancy. Am J Obstet Gynecol 2015;213(3):370.e371–377. [DOI] [PubMed] [Google Scholar]
- 18.Al-Nasiry S, Ghossein-Doha C, Polman S, et al. Metabolic syndrome after pregnancies complicated by pre-eclampsia or small-for-gestational-age: a retrospective cohort. BJOG 2015;122(13):1818–1823. [DOI] [PubMed] [Google Scholar]
- 19.Mosca L, Benjamin EJ, Berra K, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women--2011 update: a guideline from the American Heart Association. J Am Coll Cardiol 2011;57(12):1404–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith GN, Pudwell J, Walker M, Wen SW. Risk estimation of metabolic syndrome at one and three years after a pregnancy complicated by preeclampsia. J Obstet Gynaecol Can 2012;34(9):836–841. [DOI] [PubMed] [Google Scholar]
- 21.Verbeek AL, Verbeek AJ. Timely assessment of cardiovascular risk after preeclampsia. Womens Health (Lond Engl) 2014;10(6):557–559. [DOI] [PubMed] [Google Scholar]
- 22.Wilmot KA, O’Flaherty M, Capewell S, Ford ES, Vaccarino V. Coronary Heart Disease Mortality Declines in the United States From 1979 Through 2011: Evidence for Stagnation in Young Adults, Especially Women. Circulation 2015;132(11):997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilkins-Haug L, Celi A, Thomas A, Frolkis J, Seely EW. Recognition by Women’s Health Care Providers of Long-Term Cardiovascular Disease Risk After Preeclampsia. Obstet Gynecol 2015;125(6):1287–1292. [DOI] [PubMed] [Google Scholar]
- 24.Roth GA, Huffman MD, Moran AE, et al. Global and Regional Patterns in Cardiovascular Mortality From 1990 to 2013. Circulation 2015;132(17):1667–1678. [DOI] [PubMed] [Google Scholar]
- 25.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003;289(19):2560–2572. [DOI] [PubMed] [Google Scholar]
- 26.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120(16):1640–1645. [DOI] [PubMed] [Google Scholar]
- 27.Smith GN, Walker MC, Liu A, et al. A history of preeclampsia identifies women who have underlying cardiovascular risk factors. Am J Obstet Gynecol 2009;200(1):58.e51–58. [DOI] [PubMed] [Google Scholar]
- 28.Veerbeek JH, Hermes W, Breimer AY, et al. Cardiovascular disease risk factors after early-onset preeclampsia, late-onset preeclampsia, and pregnancy-induced hypertension. Hypertension 2015;65(3):600–606. [DOI] [PubMed] [Google Scholar]
- 29.Akpalu J, Akpalu A, Ofei F. The metabolic syndrome among patients with cardiovascular disease in Accra, Ghana. Ghana Med J 2011;45(4):161–166. [PMC free article] [PubMed] [Google Scholar]
- 30.Kaduka LU, Kombe Y, Kenya E, et al. Prevalence of metabolic syndrome among an urban population in Kenya. Diabetes Care 2012;35(4):887–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arthur FK, Adu-Frimpong M, Osei-Yeboah J, Mensah FO, Owusu L. The prevalence of metabolic syndrome and its predominant components among pre-and postmenopausal Ghanaian women. BMC Res Notes 2013;6:446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McNeill AM, Rosamond WD, Girman CJ, et al. The metabolic syndrome and 11-year risk of incident cardiovascular disease in the atherosclerosis risk in communities study. Diabetes Care 2005;28(2):385–390. [DOI] [PubMed] [Google Scholar]
- 33.Klempfner R, Erez A, Sagit BZ, et al. Elevated Triglyceride Level Is Independently Associated With Increased All-Cause Mortality in Patients With Established Coronary Heart Disease: Twenty-Two-Year Follow-Up of the Bezafibrate Infarction Prevention Study and Registry. Circ Cardiovasc Qual Outcomes 2016;9(2):100–108. [DOI] [PubMed] [Google Scholar]
- 34.Jiao ZY, Li XT, Li YB, et al. Correlation of triglycerides with myocardial infarction and analysis of risk factors for myocardial infarction in patients with elevated triglyceride. J Thorac Dis 2018;10(5):2551–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bodnar LM, Ness RB, Harger GF, Roberts JM. Inflammation and triglycerides partially mediate the effect of prepregnancy body mass index on the risk of preeclampsia. Am J Epidemiol 2005;162(12):1198–1206. [DOI] [PubMed] [Google Scholar]
- 36.Nakajima K, Nakano T, Tanaka A. The oxidative modification hypothesis of atherosclerosis: the comparison of atherogenic effects on oxidized LDL and remnant lipoproteins in plasma. Clin Chim Acta 2006;367(1–2):36–47. [DOI] [PubMed] [Google Scholar]
- 37.Varbo A, Freiberg JJ, Nordestgaard BG. Remnant Cholesterol and Myocardial Infarction in Normal Weight, Overweight, and Obese Individuals from the Copenhagen General Population Study. Clin Chem 2018;64(1):219–230. [DOI] [PubMed] [Google Scholar]
- 38.Varbo A, Benn M, Nordestgaard BG. Remnant cholesterol as a cause of ischemic heart disease: evidence, definition, measurement, atherogenicity, high risk patients, and present and future treatment. Pharmacol Ther 2014;141(3):358–367. [DOI] [PubMed] [Google Scholar]
- 39.Varbo A, Nordestgaard BG. Remnant cholesterol and ischemic heart disease. Curr Opin Lipidol 2014;25(4):266–273. [DOI] [PubMed] [Google Scholar]
- 40.Varbo A, Benn M, Tybjærg-Hansen A, Jørgensen AB, Frikke-Schmidt R, Nordestgaard BG. Remnant cholesterol as a causal risk factor for ischemic heart disease. J Am Coll Cardiol 2013;61(4):427–436. [DOI] [PubMed] [Google Scholar]
- 41.Stekkinger E, Zandstra M, Peeters LL, Spaanderman ME. Early-onset preeclampsia and the prevalence of postpartum metabolic syndrome. Obstet Gynecol 2009;114(5):1076–1084. [DOI] [PubMed] [Google Scholar]
- 42.Bartha JL, González-Bugatto F, Fernández-Macías R, González-González NL, Comino-Delgado R, Hervías-Vivancos B. Metabolic syndrome in normal and complicated pregnancies. Eur J Obstet Gynecol Reprod Biol 2008;137(2):178–184. [DOI] [PubMed] [Google Scholar]
- 43.Wenger NK. Prevention of cardiovascular disease in women: highlights for the clinician of the 2011 American Heart Association Guidelines. Adv Chronic Kidney Dis 2013;20(5):419–422. [DOI] [PubMed] [Google Scholar]
- 44.Mensah GA, Roth GA, Sampson UK, et al. Mortality from cardiovascular diseases in sub-Saharan Africa, 1990–2013: a systematic analysis of data from the Global Burden of Disease Study 2013. Cardiovasc J Afr 2015;26(2 Suppl 1):S6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stranges S, Guallar E. Cardiovascular disease prevention in women: a rapidly evolving scenario. Nutr Metab Cardiovasc Dis 2012;22(12):1013–1018. [DOI] [PubMed] [Google Scholar]