Abstract
Ion channels are critical to kidney function, and their dysregulation leads to several distinct kidney diseases. Of the diversity of ion channels in kidney cells, the TRP (transient receptor potential) superfamily of proteins plays important and varied roles in both maintaining homeostasis, as well as in causing disease. Recent work showed that TRPC5 blockers could successfully protect critical components of the kidney filter both in vitro and in vivo, thus revealing TRPC5 as a tractable therapeutic target for focal and segmental glomerulosclerosis (FSGS), a common cause of kidney failure. Human genetics point to three additional TRP channels as plausible therapeutic targets: TRPC6 in FSGS, PKD2 in polycystic kidney disease, and TRPM6 in familial hypomagnesemia with secondary hypocalcemia. We conclude that targeting TRP channels could pave the way for much needed therapies for kidney diseases.
Keywords: FSGS, TRPC5, TRPC6, AC1903, Podocyte, ion channels
Ion channels in kidney disease
Ion channels play important roles in maintaining normal kidney function, most prominently in the homeostasis of ionic concentrations in the bloodi. In support of their importance for kidney homeostasis, the disruption of ion channel activity due to genetic mutations or chemically-mediated dysregulation can lead to kidney diseases [1, 2]. The renal outer-medullary K+ channel (ROMK) is the major mediator of potassium secretion in the kidney and is mutated in type 2 Bartter syndrome, a salt-wasting disease [3]. Mutations in the C LC family of chloride channels also lead to Bartter syndrome [4]. Apical epithelial sodium channels (ENaC) are responsible for Na+ reabsorption and are hyperactive in Liddle syndrome, while deficient in autosomal recessive pseudohypoaldosteronism (PHA1) [5]. Dysfunction of the water channel aquaporin-2 (AQP2) causes nephrogenic diabetes insipidus in which the kidney is unable to concentrate urine [6]. While these diseases, among others, illuminated the mechanisms by which the kidney achieves ionic balance between the blood, the urine and the intracellular space, there are channelopathies that result in kidney dysfunction via molecular and cellular mechanisms that we are just beginning to understand.
Transient Receptor Potential channels in the kidney
The transient receptor potential (TRP) superfamily of ion channels are important in renal physiology [7]. First described in Drosophila, the TRP channel family consists of 28 genes in mammals [1, 8, 9]. TRP channels are functional when four gene products (subunits) assemble into a tetramer. Each subunit contains intracellular N- and C-termini, six transmembrane domains (S1–S6), and a pore loop between the S5 and S6 segments. TRP channels are most often non-selective cation channels with high Ca2+ permeability [8]. The TRP superfamily can be further divided into six subfamilies based on sequence homology and function: TRPC (Canonical), TRPV (Vanilloid), TRPA1 (Ankyrin), TRPM (Melastatin), TRPML (Mucolipin), and TRPP (Polycystin). There are several TRP proteins reported to be expressed in various cells of the kidney including TRPC1, TRPC3, TRPC4, TRPC5, TRPC6, TRPV1, TRPV4, TRPV5, TRPV6, TRPM2, TRPM3, TRPM4, TRPM6, and PKD2 [7, 10–13]. Here, we focus on TRP channels that may be targeted for therapeutic benefit. We thus explore the role TRPC5 and TRPC6 channels play at the kidney filter in health and disease, and we end with a brief perspective on PKD2 and TRPM6, two additional, attractive TRP channel targets supported by human genetics.
TRPC5 and TRPC6 channels in glomerular disease
The kidney’s primary function is to filter the blood, removing wastes and regulating homeostasis. The filtration unit of the kidney, the glomerulus, is a highly specialized corpuscle of capillaries capable of modulating hydrostatic ultrafiltration of blood plasma, allowing the passage of solutes, but retaining vital proteins [14]. Each cycle of cardiac output delivers blood that the kidney glomerulus converts to an ultrafiltrate, the precursor to urinei. The glomerulus (also known as the renal corpuscle) consists of a glomerular tuft encompassed by Bowman’s capsule, and comprises four resident cell types: endothelial cells, mesangial cells, parietal epithelial cells of Bowman’s capsule, and podocytes. The primary architectural scaffold of the structure is the glomerular basement membrane (GBM). Fenestrated endothelial cells line the capillaries and smooth muscle-like mesangial cells provide capillary support, while podocytes are attached to the outer aspect of the GBM [14].
Podocytes are post-mitotic, pericyte-like cells with complex morphology that can be divided into a cell body, major processes and foot processes. The foot processes of adjacent podocytes form a unique form of cell junction called the slit diaphragm that covers the outer aspect of the GBM. Podocyte dysfunction and cytoskeletal disorganization leads to a disruption of these foot processes in a phenomenon known as foot process effacement [14]. This disruption of the slit diaphragm causes proteinuria, the spilling of essential proteins into the urine. Thus, damage to the filtration barrier, and specifically to podocytes, is the hallmark of many progressive (chronic) kidney diseases which affect about 750 million people worldwide, and whose prevalence continue to grow [15, 16].
Focal and segmental glomerulosclerosis (FSGS) is the leading histopathology underlying progressive (chronic) kidney diseases and is characterized by proteinuria and podocyte loss [17–19]. It is challenging to determine the precise prevalence of FSGS due to the large variations in indications and diagnosis, but estimates range from 0.2–1.8/100,000 [20]. In its most severe form, FSGS is associated with nephrotic syndrome, a disease whose hallmarks are proteinuria, severe swelling throughout the body and progression to kidney failure, with scarring in large segments of the glomerulus observed by histopathologic analysis [18]. Scarring in FSGS-afflicted kidneys is due to injury and loss of the glomerular podocytes [14]. Beyond severe edema and shortness of breath, FSGS increases the risk of kidney failure, heart failure and premature death [18]. Current therapies for FSGS involve off-label use of non-specific medications, which are significantly toxic and do not alter disease progression [18]. Therefore, specific podocyte-protective therapies are urgently needed in the clinic.
The involvement of TRPC6 in glomerular disease, and specifically in FSGS, is well-established. High-penetrance genetic causes of FSGS have been associated with at least 38 genes, including TRPC6. There are 20 missense/nonsense, 4 small deletion, and 1 small insertion mutations in TRPC6 annotated in the Human Gene Mutation Databaseii as disease causing for glomerulosclerosis and nephrotic syndrome. In a study of 1783 families with steroid-resistant nephrotic syndrome, TRPC6 mutations were identified as causative in 9 families (0.5%) [21]. In all cases for which biopsy data was available, individuals with identified TRPC6 mutations displayed FSGS [21]. Of note, both gain- and loss-of-function mutations in TRPC6 have been identified [14, 22–24].
FSGS-associated TRPC6 mutations have been mostly characterized by overexpressing TRPC6 channels heterologously and observing either calcium influx via calcium imaging, or current amplitude as measured by electrophysiology. Several of these mutations have been designated as gain-of-function by these experiments, leading to the hypothesis that TRPC6 inhibition may be beneficial in glomerular disease. Recently, a specific mechanism by which at least some TRPC6 FSGS mutations lead to gain-of-function was shown to involve disruption of calmodulin-mediated calcium-dependent inactivation [25]. The prevailing model is that increased calcium influx through TRPC6 leads to podocyte damage [26]. However, it has also been proposed that TRPC6 can regulate podocyte homeostasis independent of its channel activity, through binding partners such as calpain [27].
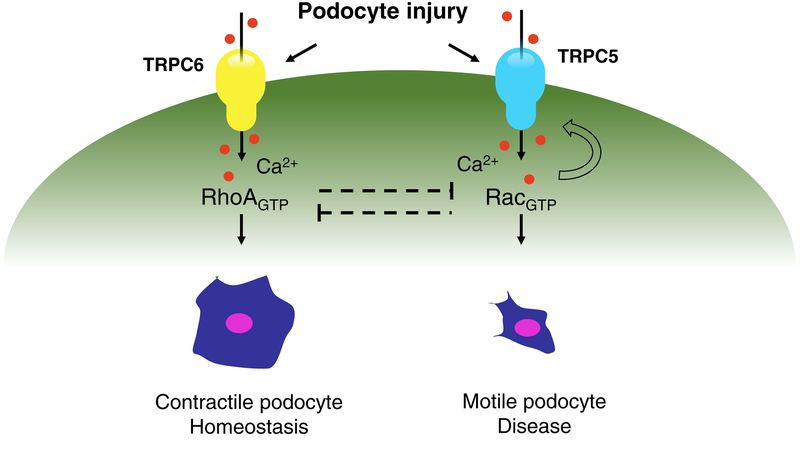
TRPC channels are activated by G-protein coupled receptors (GPCRs) which is particularly relevant given that the GPCR angiotensin type II receptor 1 (AT1R) has been shown to induce podocyte apoptosis [28]. Linking these two notions, in an in vitro podocyte model, AT1R induced calcium influx was shown to be dependent on both TRPC6 as well as TRPC5, a receptor operated ion channel similar to TRPC6 [13]. Further insight into a potentially disease relevant podocyte cellular pathway came from the observation that inherited and sporadic forms of FSGS are caused by mutations in genes involved in actin cytoskeleton regulation. A significant number of known mutations converge on modulators of Rac1, namely ARHGAP24 [29], ARHGDIA [30] and ARHGEF17 [31]. These mutations generally result in excess Rac1 signaling in podocytes [32, 33] leading to vesicular insertion of TRPC5 into the podocyte plasma membrane and more TRPC5 channels available for activation by receptors such as the AT1R [13, 32]. The subsequent increase in transient Ca2+ influx into the podocyte leads to further Rac1 activation, thus completing a runaway feedforward circuit that promotes podocyte cytoskeletal remodeling [13, 34, 35]. Despite this insight into podocyte cytoskeletal dysregulation, it was previously unknown whether TRPC5 activity mediated the onset and progression of FSGS, and whether blocking this activity could provide therapeutic benefit.
To this end, the first promising set of experiments showed that treatment with ML204, a small molecule tool compound that blocks TRPC5 (and also TRPC4) ion channels reversed podocyte loss and proteinuria in a rat model of FSGS which specifically expresses human AT1R in podocytes [36, 37]. ML204 also blocked TRPC5 channels in single channel recordings from podocytes on isolated glomeruli from rats with progressive kidney disease [36]. Thus, increased TRPC5 channel activity was correlated with kidney disease, and blocking this activity emerged as a candidate therapeutic target [36]. Of interest, TRPC6 channel activity could be recorded at all times, and was found to be unchanged in control rats as well as in rats with progressive kidney disease, indicating that TRPC6 channel activity in this animal model was not correlated with kidney dysfunction. This is further supported by human genetics: both gain and loss of function mutations in TRPC6 lead to FSGS, suggesting that the channel is required for podocyte homeostasis [22, 24, 38].
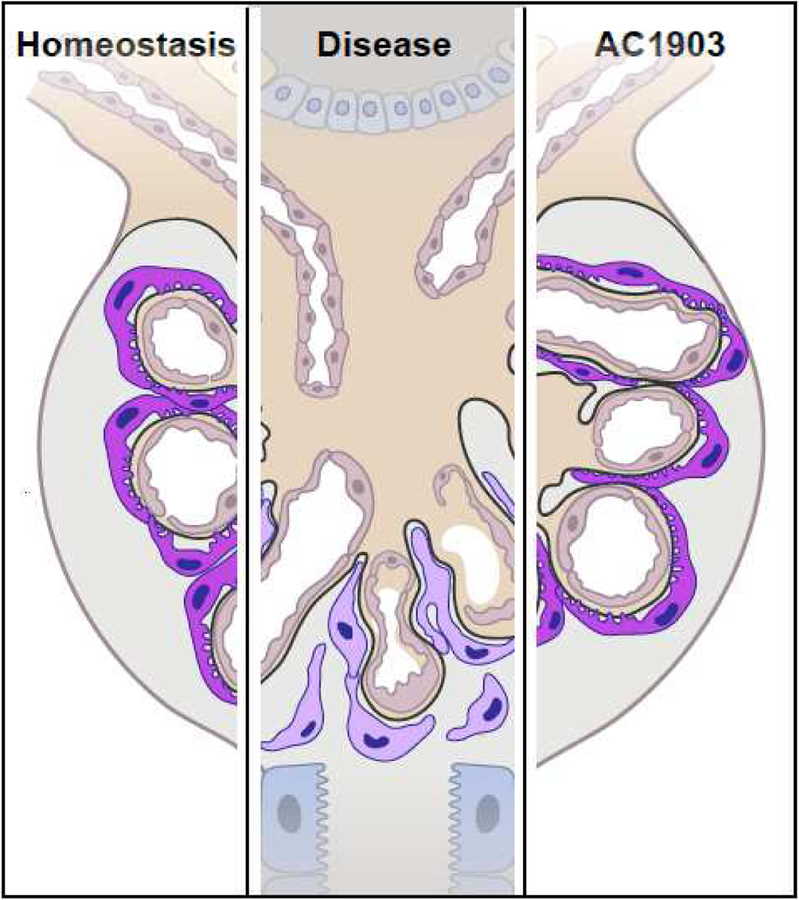
Since ML204 was a tool compound with poor specificity, medicinal chemistry studies were aimed at developing a specific TRPC5 blocker. The result of this work was the discovery of AC1903, a compound that specifically blocks TRPC5 channels, with much reduced potency against TRPC4 and essentially no effect on TRPC6 channels [36, 39]. This is particularly relevant given the high sequence homology between TRPC4 and TRPC5, and previous difficulty in finding compounds that preferentially inhibited TRPC5 [37, 40]. Single channel recordings in acutely isolated rat glomeruli showed that AC1903 was effective in blocking TRPC5 channel activity in the glomeruli of proteinuric rats [36]. Importantly, chronic administration of AC1903 suppressed severe proteinuria and prevented podocyte loss (Figure 1), thus demonstrating TRPC5 inhibition as a podocyte-preserving therapeutic strategy. The efficacy of AC1903 was further demonstrated in another animal model of kidney disease: Dahl salt sensitive (Dahl S) rats. Dahl S rats exhibit progressive kidney injury as they age, with moderate hypertension when raised on a low-salt diet. On a high-salt diet, these rats develop progressive proteinuria and decline in kidney function with significant angiotensin II-mediated hypertension. This situation in Dahl S rats likely mirrors the systemic conditions leading to progressive proteinuric kidney diseases in patients with severe hypertension. Crucially, pharmacological block of TRPC5 with AC1903 at a time of severe, established proteinuria -- but before creatinine is elevated (indicating irreversible kidney damage) -- rescued podocytes and attenuated the progression of morphologic and molecular changes that characterize FSGS [36]. Taken together, data with two distinct chemical compounds (AC1903 and ML204) in two different rat models of disease showed that TRPC5 ion channel activity is induced to drive disease, and TRPC5 inhibitors may thus be a novel mechanism-based strategy for the treatment of progressive kidney diseases.
Figure 1. The TRPC5 inhibitor AC1903 is a podocyte-protective therapeutic strategy for progressive kidney diseases.
Healthy podocytes (purple), the only post-mitotic cells in the kidney, are attached to the glomerular basement membrane (GBM) under homeostatic conditions. In disease, podocytes detach from the GBM. This podocyte loss is the hallmark of many progressive kidney diseases. The TRPC5 blocker AC1903 prevents podocyte loss and preserves kidney filter function.
To place this in the appropriate context, it is important to recognize previous efforts focused on TRPC6 channels. These were based on the discovery of familial FSGS gain-of-function mutations in TRPC6, leading to the logical conclusion that TRPC6 inhibition may be a good therapeutic strategy for FSGS. This picture is complicated by the discovery that TRPC6 loss of function mutations also cause FSGS [24]. Early work indicated that TRPC6 deficient mice have mild reductions in albuminuria in an angiotensin II infused model of renal injury [41]. More recently, investigations of TRPC6 deficiency in the context of kidney disease indicate that much of the protective effect could be due to a role for TRPC6 in cells outside of the glomerulus. Immune cell infiltration, fibrosis and activation of inflammatory markers were less pronounced in Trpc6−/− animals than in wild-type controls after unilateral ureteral obstruction or chronic puromycin aminonucleoside administration in rodent models (mouse and rat respectively) [10, 42]. The effects on glomerular damage were surprisingly variable, particularly given that these models represent whole-body constitutive knockouts of TRPC6 and thus the theoretical ceiling for a maximally effective and available inhibitor. In a streptozotocin-treated Dahl S model of diabetic kidney disease, the effects of TRPC6 deficiency were minimal [43]. Other experiments with this rodent model demonstrate the benefit of knocking out Nox4, an NADPH oxidase that generates reactive oxygen species (ROS), but the subsequent linkage to TRPC6 channels is unclear due to a lack of genetic or pharmacological validation to identify the electrical activity induced by ROS (H2O2) application [44]. Further complicating the picture is a recent study on a mouse model of type 1 diabetes. In these mice, TRPC6 knockout only transiently inhibited albuminuria [45] and instead, TRPC6 loss led to exacerbation of progressive kidney disease by increasing podocyte apoptosis in the setting of insulin resistance [45]. Assessment of TRPC5, if any, in most studies is limited to mRNA expression analysis, and thus does not address whether TRPC5 function is upregulated by patch clamp electrophysiology, the gold standard for the assessment of all ion channels. Moving forward, the availability of new inhibitors with improved specificity should allow for pharmacological dissection of the contributions of different TRPC channels in various models of renal pathology.
Curiously, in a study that overexpressed TRPC5 in a transgenic mouse model, no effect was observed on proteinuria or glomerular damage [46]. As also commented by others [47], the sequestration of TRPC5 in intracellular vesicles could mean that overexpression of TRPC5 would not lead to increased channel abundance at the cell membrane. Here again the situation could be clarified by electrophysiological recording of podocytes, which were absent in this study. Wang and colleagues also administered englerin A (a compound known to be a nanomolar activator of TRPC4 and TRPC5 in vitro [48]) to mice to assess the effect of TRPC5 activation. However, englerin A is extremely unstable in rodent plasma and likely rapidly converted in vivo to englerin B which is not a TRPC5 activator [49]. Therefore, the negative study by Wang et al. may be due to lack of available TRPC5 channels in the plasma membrane and/or the absence of activating compound.
Work in cultured podocytes has shown that TRPC6 drives RhoA activity to secure a homeostatic, contractile cytoskeleton, while TRPC5 drives Rac1 activity (and Rac1 conversely activates TRPC5) to mediate disease-associated podocyte motility [13, 32, 35, 36, 50]. This provides further cell biological evidence that TRPC5 may be an attractive therapeutic target (Figure 2). In support of this notion, Zhou et al. showed that real-time measurements of single channel TRPC6 activity in isolated glomeruli seem relatively unchanged through disease onset and progression, whereas increased TRPC5 activity is associated with proteinuric disease progression. We can also speculate that TRPC6 may have become a less attractive target after preclinical studies conducted over the past 15 years: though there could be several factors involved, we are unaware of a therapeutic kidney disease program targeting TRPC6 inhibition that has reached the clinic despite reports of potent and selective TRPC6 inhibitors [51, 52].
Figure 2.
A model for how different channels in the same disease setting could lead to different outcomes.
The efficacy of AC1903 in hypertensive Dahl S rats supports a broader applicability of TRPC5 inhibition as a therapeutic strategy. Rac1 activation emerges as a nodal event, a convergence point for multiple podocyte injury pathways [53]. We speculate that Rac1-activating mutations may more generally underlie susceptibility to disease in patients presenting with proteinuria and progressive kidney failure. If so, this offers the opportunity to either (1) genetically stratify patients based on Rac1 activating mutations, and/or (2) select patients based on detection of (urinary) biomarkers relevant to Rac1 activation for future clinical trials. Indeed, pairing the patient with the right genetic background or relevant biomarker profile with the appropriate TRPC5-targeted therapy may be the closest this field has come to a precision medicine approach to date.
Several convergent data were reassuring regarding the safety of TRPC5 inhibitors in humans. Rats treated with TRPC5 inhibitor for up to 14 days showed no detectable toxicity [36]. Moreover, TRPC5 knockout mice (where the channel is absent in utero and beyond) have no gross abnormalities, except an attenuated fear response due to a developmental defect in the mouse amygdala [54] that may translate into a possible anxiolytic on-target side-effect of TRPC5 inhibition in the human brain. Pharmacokinetic/pharmacodynamic studies have also demonstrated that the efficacy of AC1903 in the podocyte may be achievable with low circulating concentrations of drug [36], well below what may reach the brain. Thus, the salutary effects of treatment with a TRPC5 inhibitor may form the basis for much needed therapies aimed at treating progressive chronic kidney diseases at their mechanistic source. At the time this review went to press, TRPC5 inhibitors were being tested in a Phase I study in the clinic (Clinical Trial Numberiii: ), so we are likely to soon learn much more about the safety and efficacy of this therapeutic strategy for the treatment of TRPC5/Rac1-mediated proteinuric kidney diseases.
PKD2 and TRPM6 in kidney disease
Efforts at targeting TRPC channels in glomerular disease are embedded in a larger landscape of TRP channels playing crucial and likely complex roles in kidney disease. Autosomal dominant polycystic kidney disease (ADPKD) is one of the most common monogenic disorders with a 1:400–1:1000 prevalence [2]. Mutations in PKD1 (~85%) and PKD2 (~15%) account for ADPKD cases [55]. PKD1 encodes a putative 11 transmembrane domain protein that resembles GPCRs, while PKD2 encodes a TRP channel (PKD2, formerly TRPP2, now TRPP1 based on recently revised nomenclature). The majority of PKD2 is found on the endoplasmic reticulum (ER) although its role there is still incompletely understood largely due to discrepancies in observations from manipulating its function [56]. The general hypothesis is that PKD2 serves to prevent calcium leak across the ER membrane. Importantly, the PKD2 protein is also found in the cilia of kidney tubular epithelial cells. Cilia are cellular organelles with specialized protein composition, likely providing a spatially constrained signal-sensing micro-environment. It has been postulated that cilia act as mechanosensors, and that PKD2 transduces a mechanically activated calcium signal. However other studies do not detect ciliary calcium influx in primary cilia even upon supra-physiological mechanical stimulation [57]. Of interest, PKD2 was shown to mediate an ionic current in the primary cilia of kidney epithelial cell lines, although, to add to the mystery, this current was not specifically carried by calcium ions [58, 59]. Thus the physiological role of PKD2 remains unclear and may involve the unique signaling micro-environment of the cilium [60].
Moving forward, understanding the cell biology of disease-associated kidney ion channels will likely yield important therapeutic insights. For instance, though it was recently established that PKD2 does indeed contribute to an ionic conductance in the renal collecting duct [58, 59], it is still unknown how exactly PKD2 mutations lead to polycystic kidney disease. Nevertheless, it is important to note that progress has been recently made by addressing the sequelae of disrupted PKD1/PKD2 function: the V2-receptor antagonist tolvaptan decreases cyst formation and total kidney volume, and has been approved for the treatment of ADPKD [61]. Fully understanding the precise nature of the signaling pathways triggered by PKD1/PKD2 may allow for PKD2-targeted approaches.
TRPM 6 mutations are associated with familial hypomagnesemia with secondary hypocalcemia (HSH) [62, 63]. Explanations for TRPM 6’s role in HSH have mostly emphasized Mg2+ conductance through the channel which is disrupted by disease-causing mutations. TRPM 6 is expressed in both the intestine and the distal tubule of kidney nephrons, both thought to be important sites in maintaining Mg2+ homeostasis, but the mechanisms contributing to this disease are not fully understood. TRPM 6 is one of a small group of “chanzymes”, channel proteins that also encode enzymes. The carboxy-terminus of TRPM 6 is a serine/threonine kinase that has been shown to be cleaved from the channel in an activity-dependent manner and regulate gene transcription after translocation to the nucleus [64]. The potential breadth of roles of the TRPM 6 chanzyme could mean that there is still much to be discovered about the mechanisms leading to HSH.
Concluding Remarks
Ion channels are intuitively druggable targets due to our clear biophysical understanding of how they function, and their relatively accessible cellular location on the plasma membrane. As with the Rac1 and TRPC5 “disease circuit” in FSGS, the dysfunction caused by human mutations in ion channels will likely extend to as yet unknown signaling events mediated by disrupted ionic fluxes (see Outstanding Questions). Specific pharmacological agents targeting ion channels will therefore not only be important tool compounds for elucidating disease pathology, but may also lead to new, mechanism-based therapies for diseases spanning all tissues, including the kidney, the brain, and beyond.
Outstanding questions.
What are the precise molecular pathways linking TRP channel dysfunction to disease in the human kidney?
Will insights from cellular and animal models translate successfully to the clinic?
Can we target TRP channels and the molecular pathways they mediate in podocytes with other therapeutic modalities, beyond small molecules?
Highlights.
TRP channels may be excellent drug targets for the development of much needed therapies for kidney diseases
TRPC6 has been genetically linked to a rare form of familial glomerular disease
Rac1 activating mutations in patients revealed a Rac1-TRPC5 disease pathway in podocytes
Additional electrophysiology and cell biology studies revealed TRPC5 as a promising target for progressive kidney diseases
Newly developed TRPC5-selective inhibitors have shown significant efficacy in reversing kidney disease progression in animal models
Acknowledgements
We would like to acknowledge all the extensive work in this field that due to space constraints we are unable to cite. We would like to thank Leslie Gaffney, Broad Institute of MIT and Harvard for help with Figure 1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement:
A.G. has a financial interest in Goldfinch Biopharma, which was reviewed and is managed by Brigham and Women’s Hospital and Partners HealthCare and the Broad Institute of MIT and Harvard in accordance with their conflict of interest policies.
Resources:
- i).Taal MW, Brenner BM, and Rector FC, Brenner & Rector’s the kidney. 9th ed 2012, Philadelphia, PA: Elsevier/Saunders. [Google Scholar]
- ii). http://www.hgmd.org.
- iii). https://clinicaltrials.gov/
References
- 1.Zhou Y and Greka A, Calcium-permeable ion channels in the kidney. Am J Physiol Renal Physiol, 2016. 310(11): p F1157–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loudon KW and Fry AC, The renal channelopathies. Ann Clin Biochem, 2014. 51(Pt 4): p 441–58. [DOI] [PubMed] [Google Scholar]
- 3.Welling PA and Ho K, A comprehensive guide to the ROMK potassium channel: form and function in health and disease. Am J Physiol Renal Physiol, 2009. 297(4): p F849–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stolting G, Fischer M, and Fahlke C, CLC channel function and dysfunction in health and disease. Front Physiol, 2014. 5: p 378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanukoglu I and Hanukoglu A, Epithelial sodium channel (ENaC) family: Phylogeny, structure-function, tissue distribution, and associated inherited diseases. Gene, 2016. 579(2): p 95–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deen PM, van Balkom BW, and Kamsteeg EJ, Routing of the aquaporin-2 water channel in health and disease. Eur J Cell Biol, 2000. 79(8): p 523–30. [DOI] [PubMed] [Google Scholar]
- 7.Woudenberg-Vrenken TE, Bindels RJM, and Hoenderop JGJ, The role of transient receptor potential channels in kidney disease. Nature Reviews Nephrology, 2009. 5(8): p 441–449. [DOI] [PubMed] [Google Scholar]
- 8.Ramsey IS, Delling M, and Clapham DE, An introduction to TRP channels. Annu Rev Physiol, 2006. 68: p 619–47. [DOI] [PubMed] [Google Scholar]
- 9.Montell C, Drosophila visual transduction. Trends Neurosci, 2012. 35(6): p 356–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kong W, et al. , Renal Fibrosis, Immune Cell Infiltration and Changes of TRPC Channel Expression after Unilateral Ureteral Obstruction in Trpc6−/− Mice. Cell Physiol Biochem, 2019. 52(6): p 1484–1502. [DOI] [PubMed] [Google Scholar]
- 11.Ambrus L, et al. , Human podocytes express functional thermosensitive TRPV channels. Br J Pharmacol, 2017. 174(23): p 4493–4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marko L, et al. , Renoprotection: focus on TRPV1, TRPV4, TRPC6 and TRPM2. Acta Physiol (Oxf), 2017. 219(3): p 589–612. [DOI] [PubMed] [Google Scholar]
- 13.Tian D, et al. , Antagonistic regulation of actin dynamics and cell motility by TRPC5 and TRPC6 channels. Sci Signal, 2010. 3(145): p ra77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greka A and Mundel P, Cell biology and pathology of podocytes. Annu Rev Physiol, 2012. 74: p 299–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jha V, et al. , Chronic kidney disease: global dimension and perspectives. Lancet, 2013. 382(9888): p 260–72. [DOI] [PubMed] [Google Scholar]
- 16.Coresh J, et al. , Prevalence of chronic kidney disease in the United States. JAMA, 2007. 298(17): p 2038–47. [DOI] [PubMed] [Google Scholar]
- 17.D’Agati VD, Pathobiology of focal segmental glomerulosclerosis: new developments. Curr Opin Nephrol Hypertens, 2012. 21(3): p 243–50. [DOI] [PubMed] [Google Scholar]
- 18.D’Agati VD, Kaskel FJ, and Falk RJ, Focal segmental glomerulosclerosis. N Engl J Med, 2011. 365(25): p 2398–411. [DOI] [PubMed] [Google Scholar]
- 19.Jefferson JA and Shankland SJ, The pathogenesis of focal segmental glomerulosclerosis. Adv Chronic Kidney Dis, 2014. 21(5): p 408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenberg AZ and Kopp JB, Focal Segmental Glomerulosclerosis. Clin J Am Soc Nephrol, 2017. 12(3): p 502–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sadowski CE, et al. , A single-gene cause in 29.5% of cases of steroid-resistant nephrotic syndrome. J Am Soc Nephrol, 2015. 26(6): p 1279–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winn MP, et al. , A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science, 2005. 308(5729): p 1801–4. [DOI] [PubMed] [Google Scholar]
- 23.Reiser J, et al. , TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat Genet, 2005. 37(7): p 739–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riehle M, et al. , TRPC6 G757D Loss-of-Function Mutation Associates with FSGS. J Am Soc Nephrol, 2016. 27(9): p 2771–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polat OK, et al. , Contribution of Coiled-Coil Assembly to Ca(2+)/Calmodulin-Dependent Inactivation of TRPC6 Channel and its Impacts on FSGS-Associated Phenotypes. J Am Soc Nephrol, 2019. 30(9): p 1587–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang L, et al. , Gq signaling causes glomerular injury by activating TRPC6. J Clin Invest, 2015. 125(5): p 1913–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farmer LK, et al. , TRPC6 Binds to and Activates Calpain, Independent of Its Channel Activity, and Regulates Podocyte Cytoskeleton, Cell Adhesion, and Motility. J Am Soc Nephrol, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsu HH, et al. , Downregulation of the antioxidant protein peroxiredoxin 2 contributes to angiotensin II-mediatedpodocyte apoptosis. Kidney Int, 2011. 80(9): p 959–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akilesh S, et al. , Arhgap24 inactivates Rac1 in mouse podocytes, and a mutant form is associated with familial focal segmental glomerulosclerosis. J Clin Invest, 2011. 121(10): p 4127–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gee HY, et al. , ARHGDIA mutations cause nephrotic syndrome via defective RHO GTPase signaling. J Clin Invest, 2013. 123(8): p 3243–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu H, et al. , A role for genetic susceptibility in sporadic focal segmental glomerulosclerosis. J Clin Invest, 2016. 126(3): p 1067–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greka A and Mundel P, Balancing calcium signals through TRPC5 and TRPC6 in podocytes. J Am Soc Nephrol, 2011. 22(11): p 1969–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shibata S, et al. , Modification of mineralocorticoid receptor function by Rac1 GTPase: implication in proteinuric kidney disease. Nat Med, 2008. 14(12): p 1370–6. [DOI] [PubMed] [Google Scholar]
- 34.Yu H, et al. , Rac1 activation in podocytes induces rapid foot process effacement and proteinuria. Mol Cell Biol, 2013. 33(23): p 4755–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schaldecker T, et al. , Inhibition of the TRPC5 ion channel protects the kidney filter. J Clin Invest, 2013. 123(12): p 5298–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou Y, et al. , A small-molecule inhibitor of TRPC5 ion channels suppresses progressive kidney disease in animal models. Science, 2017. 358(6368): p 1332–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller M, et al. , Identification of ML204, a novel potent antagonist that selectively modulates native TRPC4/C5 ion channels. J Biol Chem, 2011. 286(38): p 33436–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dryer SE, Roshanravan H, and Kim EY, TRPC channels: Regulation, dysregulation and contributions to chronic kidney disease. Biochim Biophys Acta Mol Basis Dis, 2019. 1865(6): p 1041–1066. [DOI] [PubMed] [Google Scholar]
- 39.Sharma SH, et al. , Design, synthesis and characterization of novel N-heterocyclic-1-benzyl-1H-benzo[d]imidazole-2-amines as selective TRPC5 inhibitors leading to the identification of the selective compound, AC1903. Bioorg Med Chem Lett, 2019. 29(2): p 155–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richter JM, Schaefer M, and Hill K, Clemizole hydrochloride is a novel and potent inhibitor of transient receptor potential channel TRPC5. Mol Pharmacol, 2014. 86(5): p 514–21. [DOI] [PubMed] [Google Scholar]
- 41.Eckel J, et al. , TRPC6 enhances angiotensin II-induced albuminuria. J Am Soc Nephrol, 2011. 22(3): p 526–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim EY, Yazdizadeh Shotorbani P, and Dryer SE, Trpc6 inactivation confers protection in a model of severe nephrosis in rats. J Mol Med (Berl), 2018. 96(7): p 631–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spires D, et al. , Protective role of Trpc6 knockout in the progression of diabetic kidney disease. Am J Physiol Renal Physiol, 2018. 315(4): p F1091–F1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ilatovskaya DV, et al. , A NOX4/TRPC6 Pathway in Podocyte Calcium Regulation and Renal Damage in Diabetic Kidney Disease. J Am Soc Nephrol, 2018. 29(7): p 1917–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang L, et al. , Knockout of TRPC6 promotes insulin resistance and exacerbates glomerular injury in Akita mice. Kidney Int, 2019. 95(2): p 321–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang X, et al. , TRPC5 Does Not Cause or Aggravate Glomerular Disease. J Am Soc Nephrol, 2018. 29(2): p 409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chung JJ and Shaw AS, TRP’ing up chronic kidney disease. Science, 2017. 358(6368): p 1256–1257. [DOI] [PubMed] [Google Scholar]
- 48.Akbulut Y, et al. , (−)−Englerin A is a potent and selective activator of TRPC4 and TRPC5 calcium channels. Angew Chem Int Ed Engl, 2015. 54(12): p 3787–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carson C, et al. , Englerin A Agonizes the TRPC4/C5 Cation Channels to Inhibit Tumor Cell Line Proliferation. PLoS One, 2015. 10(6): p e0127498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wieder N and Greka A, Calcium, TRPC channels, and regulation of the actin cytoskeleton in podocytes: towards a future of targeted therapies. Pediatr Nephrol, 2016. 31(7): p 1047–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Urban N, et al. , Identification and Validation of Larixyl Acetate as a Potent TRPC6 Inhibitor. Mol Pharmacol, 2016. 89(1): p 197–213. [DOI] [PubMed] [Google Scholar]
- 52.Sharma S and Hopkins CR, Review of Transient Receptor Potential Canonical (TRPC5) Channel Modulators and Diseases. J Med Chem, 2019. 62(17): p 7589–7602. [DOI] [PubMed] [Google Scholar]
- 53.Fujita T, Mechanism of salt-sensitive hypertension: focus on adrenal and sympathetic nervous systems. J Am Soc Nephrol, 2014. 25(6): p 1148–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Riccio A, et al. , Essential role for TRPC5 in amygdala function and fear-related behavior. Cell, 2009. 137(4): p 761–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu G and Somlo S, Molecular genetics and mechanism of autosomal dominant polycystic kidney disease. Mol Genet Metab, 2000. 69(1): p 1–15. [DOI] [PubMed] [Google Scholar]
- 56.Chebib FT, et al. , Vasopressin and disruption of calcium signalling in polycystic kidney disease. Nat Rev Nephrol, 2015. 11(8): p 451–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Delling M, et al. , Primary cilia are not calcium-responsive mechanosensors. Nature, 2016. 531(7596): p 656–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu X, et al. , Polycystin-2 is an essential ion channel subunit in the primary cilium of the renal collecting duct epithelium. Elife, 2018. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kleene SJ and Kleene NK, The native TRPP2-dependent channel of murine renal primary cilia. Am J Physiol Renal Physiol, 2016: p ajprenal 00272 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pablo JL, DeCaen PG, and Clapham DE, Progress in ciliary ion channel physiology. J Gen Physiol, 2017. 149(1): p 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Torres VE, Pro: Tolvaptan delays the progression of autosomal dominant polycystic kidney disease. Nephrol Dial Transplant, 2019. 34(1): p 30–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schlingmann KP, et al. , Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat Genet, 2002. 31(2): p 166–70. [DOI] [PubMed] [Google Scholar]
- 63.Walder RY, et al. , Mutation of TRPM6 causes familial hypomagnesemia with secondary hypocalcemia. Nat Genet, 2002. 31(2): p 171–4. [DOI] [PubMed] [Google Scholar]
- 64.Krapivinsky G, et al. , Histone phosphorylation by TRPM6’s cleaved kinase attenuates adjacent arginine methylation to regulate gene expression. Proc Natl Acad Sci U S A, 2017. 114(34): p E7092–E7100. [DOI] [PMC free article] [PubMed] [Google Scholar]