Abstract
Background:
Schizophrenia and autism share many behavioral and neurological similarities, including altered white matter tract structure. However, because schizophrenia and autism are rarely compared directly, it is difficult to establish whether white matter abnormalities are disorder-specific or are common across these disorders that share some symptomatology.
Methods:
In the current study, we compared white matter water diffusion using tensor imaging in 25 adults with autism, 15 adults with schizophrenia, all with IQ scores above 88, and 19 neurotypical adults.
Results:
Although the three groups evinced no statistically significant differences in measures of fractional anisotropy (FA), the schizophrenia group showed significantly greater mean diffusivity (MD; Cohen’s d>0.77), due to greater radial diffusivity (RD; Cohen’s d>0.92), compared to both the autism and control groups. This effect was evident across the brain rather than specific to a particular tract.
Conclusions:
The greater MD and RD in schizophrenia appears to be diagnosis-specific. The altered diffusion may reflect subtle abnormalities in myelination, which could be a potential mechanism underlying the widespread behavioral deficits associated with schizophrenia.
Keywords: autism, schizophrenia, DTI, white matter, mean diffusivity, TBSS
Introduction
Schizophrenia and autism are both characterized by problems with social and communication abilities and by sensory abnormalities (Eack et al., 2017; Ciaramidaro et al., 2018; Mançe et al., 2018; Noel, Stevenson & Wallace, 2018). Half of the individuals with autism satisfy the criteria for schizophrenia reflecting the similarities across the two profiles (Konstantareas & Hewitt, 2001; Ghaziuddin, Tsai & Ghaziuddin, 1992). The overlap in these disorders extends to the neuropsychological profiles, which are nearly identical when the groups are matched on IQ scores (Eack et al., 2013). Recent research has shown that 3.6% to 12.8% of individuals with autism develop schizophrenia as adults (see review (Chisholm et al., 2015)). Despite these similarities and overlaps, the disorders have a very different age of onset and are further distinguished by the absence of psychosis in autism. As a result, these two conditions have been demarcated as separate conditions since the DSM-II.
In addition to the behavioral similarities, brain imaging studies in schizophrenia and autism highlight several abnormalities that are common across the two conditions (Chisholm et al., 2015). One meta-analysis showed reduced grey matter volume in right limbic-striato-thalamic circuitry in both conditions (Cheung et al., 2010), while a separate review paper reported reduced fractional anisotropy (FA), one possible measure of white matter structure derived from diffusion tensor imaging, indicating impairments in the white matter structure in both conditions (Mueller et al., 2012), although in both studies, there were more dissociations among the conditions than similarities. A third study of functional imaging reported under-activity in emotion-related neural circuits in both schizophrenia and autism (see review by Sugranyes et al., 2011; Abdi & Sharma, 2005).
Most of the studies alluded to above investigated each disorder separately rather than directly comparing the two disorders. A few functional imaging studies have directly compared individuals with schizophrenia and autism and have typically found abnormal functioning, for example, under-activation of fMRI responses in the social cognitive network (Pinkham et al., 2008), but often the direction of the abnormal functioning differs. One such study examining fMRI responses during social situations reported increased connectivity between right posterior superior temporal sulcus and ventral medial prefrontal cortex in schizophrenia but decreased connectivity in autism (Ciaramidaro et al., 2015). Another study found reduced signal to noise ratios in all sensory modalities (visual, auditory, and somatosensory) in the schizophrenia and autism groups compared to age- and gender-matched controls (Haigh et al., 2016). However, the cause of the reduced SNR differed between schizophrenia and autism: adults with schizophrenia tended to under-respond to the sensory stimulation, whereas the adults with autism exhibited more variable responses from one trial to the next (intra-trial inconsistency).
Both schizophrenia and autism have long been associated with abnormalities in white matter tracts (as mentioned above). However, it should be noted that, in autism, the majority of the studies showing abnormal diffusion properties indicating weaker white matter structure in autism were conducted in children (Cheon et al., 2011; Weinstein et al., 2011; Billeci et al., 2012; Ameis et al., 2013; Abdel, Mazroa & Baz, 2014; Cheung et al., 2009; Pryweller et al., 2014; Lazar et al., 2014; Kirkovski et al., 2015; Noriuchi et al., 2010; Shukla et al., 2011a), with a minority focusing on adults (Shukla et al., 2011b; Jou et al., 2015; Gibbard et al., 2013; Roine et al., 2015; Libero et al., 2015). Of those that did focus on adults with autism, there was no clear indication as to the specific mechanism underlying the deficit. Some showed widespread abnormalities (Roine et al., 2015; Libero et al., 2015), or specific deficits in tracts such as in the forceps minor (Gibbard et al., 2013), or in anterior thalamic radiation and cingulum (Haigh et al., in press). Other studies have found no significant differences in diffusion properties between adults with autism and neurotypical individuals (Roine et al., 2015; Libero et al., 2015), highlighting the debate as to where and how severe white matter alterations are in autism.
On the other hand, diffusion studies in schizophrenia have been conducted in adults, as the first episode of psychosis that diagnostically defines the onset of schizophrenia typically occurs in late adolescence and early adulthood. Early-onset schizophrenia that occurs during childhood and adolescence may have a different etiology to schizophrenia that occurs in adulthood (for a review, see Tamnes & Agartz, 2016). Measures of diffusion appear to be impacted even before the first-episode (for a review see Samartzis et al., 2013), suggesting that changes in diffusion could be a risk marker for schizophrenia. However, there is also a disagreement as to whether atypical diffusion in those with chronic schizophrenia is present across the brain (Asami et al., 2014; Fujino et al., 2014; Nakamura et al., 2012; Nakamura et al., 2012; Roalf et al., 2013; Roalf 2015; Sasamoto et al., 2014; Scheel et al., 2013) or is restricted to certain tracts (Kochunov et al., 2014; Levitt et al., 2012; Kelly et al., 2018 (FA but not MD) Nazeri et al., 2013; Ohtani et al., 2014; Prasad et al., 2015; Wagner et al., 2015).
A couple of studies have directly examined the structural correlates of autism compared to schizophrenia. One study showed autism-specific differences in grey matter volume (compared to schizophrenia) in the insula and amygdala that correlated with symptoms (Radeloff et al., 2014). A second study also found differences between autism and schizophrenia in grey matter volume specific to prefrontal cortex and anterior cingulate, and found similar reductions in fractional anisotropy measures of white matter, particularly in the left inferior fasciculus compared to neurotypical controls (Katz et al., 2016). A third study found increased metabolic rate in white matter in schizophrenia but more so in autism in a variety of regions of interest across the brain (Mitelman et al., 2018), emphasizing abnormal white matter structures as potential biomarkers.
One point to consider is that all of the studies of altered diffusion discussed above focused on fractional anisotropy (FA) which has been suggested to reflect a variety of factors that contribute to tract structure (Beaulieu, 2002): the higher the FA, the more intact the tract. However, water diffusion can also be measured by mean diffusivity, which reflects the microstructure of white matter tracts: the lower the MD, the greater hinderance of water diffusion, indicative of more robust microstructure (Winklewski et al., 2018). Several studies report increased MD in chronic schizophrenia compared to neurotypical controls (Narr et al., 2009; Lee et al., 2009; only MD Ardekani et al., 2011; Leroux, Delcroix & Dollfus, 2014; Knöchel et al., 2012; Spalletta et al., 2015; Spoletini et al., 2011), whereas this result is less common in adults with autism (Gibbard et al., 2013; Itahashi et al., 2015). In autism, there is some evidence that MD normalizes by adulthood (for example, Kleinhans et al., 2012), suggesting differences in the developmental trajectory and, thus, MD may be a dissociable marker between adults with autism or schizophrenia.
We focused on differences in measures of diffusion, to indicate differences in white matter tract structure, between individuals with schizophrenia, individuals with autism, and neurotypical controls. Specifically, we used Diffusion Tensor Imaging (DTI) to measure fractional anisotropy (FA) and mean diffusivity (MD) across the brain using Tract-Based Spatial Statistics (TBSS). We focused on adults with schizophrenia or autism with IQ scores in the normal range, as both conditions persist throughout their lifetime, but have different developmental trajectories – the onset of schizophrenia typically occurs in late adolescence to early adulthood, whereas autism is generally diagnosed in early childhood. Therefore, we were able to match all participants on age and ensure that they were stable on their medication. We predict that schizophrenia and autism will exhibit different abnormalities in diffusion that will be specific to their diagnosis. Deficits specific to one condition might highlight structural biomarkers that are unique and diagnostically relevant. However, if white matter tract abnormalities are common across schizophrenia and autism, then this could highlight a possible transdiagnostic mechanism that might be related to their shared behavioral characteristics.
Methods and Materials
Participants
Fifteen individuals with schizophrenia (10 males; mean age 25, range 19-33 years), 25 individuals with Autism Spectrum Disorder (ASD) (21 males; mean age 29, range 19-42 years), and 19 neurotypical controls were compared (14 males; mean age 26, range 21-40 years).
The individuals in the schizophrenia group were either diagnosed with schizophrenia or schizoaffective disorder (diagnosed using the Structured Clinical Interview for DSM-IV (First et al., 2002) and symptoms were measured using the Brief Psychiatric Rating Scale (BPRS; Lukoff, Nuechterlein & Ventura, 1986, by an expert diagnostician). Thirteen of the individuals with schizophrenia were taking antipsychotics (see Table 1 for demographic and diagnostic information), and all had IQ above 88. There is a potential link between larger doses of antipsychotic medication and greater reductions in white matter volume (Emsley et al., 2017) and so, here, we conducted an exploratory correlation on the relationship between medication dosage and diffusion measures.
Table 1.
Demographic and medication information for the individuals with autism.
| Participant | Gender | Age (years) |
ADOS social |
ADOS comm |
ADOS stereotypical |
ADI social |
ADI comm | ADI stereotypical |
Full scale IQ |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 35 | |||||||
| 2 | M | 20 | 4 | 3 | 3 | 11 | 10 | 6 | 129 |
| 3 | M | 21 | 8 | 4 | 2 | 21 | 17 | 6 | 123 |
| 4 | M | 23 | 6 | 4 | 1 | 21 | 18 | 8 | 123 |
| 5 | F | 19 | 7 | 5 | 3 | 27 | 20 | 6 | 107 |
| 6 | M | 41 | 6 | 4 | 2 | 24 | 18 | 10 | 96 |
| 7 | M | 19 | 7 | 3 | 3 | 22 | 15 | 5 | 96 |
| 8 | M | 20 | 8 | 5 | 1 | 27 | 22 | 5 | 124 |
| 9 | M | 33 | 5 | 3 | 3 | 26 | 18 | 12 | 131 |
| 10 | M | 27 | 6 | 2 | 3 | 20 | 16 | 7 | 104 |
| 11 | F | 31 | 10 | 6 | 3 | 15 | 9 | 6 | 121 |
| 12 | F | 31 | 7 | 2 | 4 | 10 | 8 | 6 | 123 |
| 13 | M | 44 | 9 | 4 | 3 | 24 | 19 | 5 | 108 |
| 14 | M | 32 | 9 | 4 | 0 | 18 | 18 | 9 | 92 |
| 15 | M | 36 | 8 | 2 | 1 | 20 | 11 | 3 | 125 |
| 16 | M | 39 | 7 | 4 | 1 | 21 | 16 | 8 | 116 |
| 17 | M | 24 | 13 | 6 | 3 | 10 | 16 | 3 | 118 |
| 18 | M | 22 | 11 | 5 | 3 | 20 | 15 | 3 | 107 |
| 19 | M | 30 | 13 | 5 | 3 | 20 | 13 | 3 | 134 |
| 20 | M | 30 | 19 | 13 | 5 | 121 | |||
| 21 | M | 27 | 9 | 4 | 3 | 20 | 17 | 7 | 100 |
| 22 | M | 29 | 6 | 3 | 1 | 15 | 12 | 2 | 116 |
| 23 | M | 31 | 7 | 4 | 2 | 25 | 9 | 8 | 117 |
| 24 | M | 30 | 10 | 6 | 2 | 23 | 17 | 6 | 128 |
| Mean/Count | F=3; | 28.92 | 8.00 | 4.00 | 2.27 | 19.96 | 15.09 | 6.04 | 115.61 |
| SD | M=21 | 7.03 | 2.35 | 1.23 | 1.03 | 4.97 | 3.81 | 2.44 | 12.07 |
The individuals in the autism group all met DSM-IV criteria for autism and had IQ scores above 88. Clinical diagnosis was confirmed with the Autism Diagnostic Observation Schedule (ADOS) (Lord et al., 1989) and Autism Diagnostic Interview (ADI) (Le Couteur et al., 1989; Lord, Rutter & Le Couteur, 1994) assessments carried out by expert clinicians at the Center For Excellence in Autism Research at the University of Pittsburgh (see Table 2 for demographic and diagnostic information). A study comparing the DSM-IV and the DSM-V criteria showed that the participants who met the criteria for autism under the DSM-IV also met the criteria for autism under the DSM-V (Mazefsky et al., 2013). One individual with autism was taking an antipsychotic medication, and 5 were taking medication for depression.
Table 2.
Demographic and medication information for the individuals with schizophrenia. BPRS=Brief Psychiatric Rating Scale; CPZ=chlorpromazine equivalents.
| Participant | Gender | Age (years) | BPRS Score | Medication CPZ (mg/day) |
Full-Scale IQ |
|---|---|---|---|---|---|
| 1 | F | 24 | 28 | 93.3 | 96 |
| 2 | M | 33 | 47 | 75.0 | 94 |
| 3 | M | 34 | 30 | 200.0 | 95 |
| 4 | F | 31 | 28 | 33.3 | 96 |
| 5 | M | 23 | 32 | 266.7 | 100 |
| 6 | M | 24 | 36 | 0.0 | 102 |
| 7 | M | 19 | 23 | 100.0 | 117 |
| 8 | M | 25 | 29 | 50.0 | 102 |
| 9 | F | 30 | 33 | 507.1 | 112 |
| 10 | M | 25 | 33 | 968.1 | 97 |
| 11 | M | 22 | 28 | 33.3 | 129 |
| 12 | F | 19 | 33 | 783.3 | 101 |
| 13 | M | 28 | 29 | 0.0 | 113 |
| 14 | F | 24 | 44 | 100.0 | 89 |
| 15 | M | 26 | 18 | 100.0 | 109 |
| Mean/Count | F=5; M=10 | 25.80 | 31.40 | 220.67 | 103.47 |
| SD | 4.59 | 7.22 | 297.33 | 10.61 |
Groups did not differ from each other on age or gender. However, the ASD group had higher IQ than the schizophrenia group (t(32.8)=3.26, p=.003). IQ was not collected for the control group, although all of the control participants were students at Carnegie Mellon University. All participants gave informed consent to take part in the 90-minute study and were paid $75 for their time. The Institutional Review Boards at Carnegie Mellon University (CMU) and the University of Pittsburgh approved the experimental procedures, which were in compliance with the safety guidelines for MRI research.
DTI Data Acquisition
A 3T Siemens MRI scanner at the Carnegie Mellon University Scientific Imaging and Brain Research Center was used to acquire diffusion data. A diffusion-weighted, single-shot, spin-echo, echo-planar imaging sequence was used with TR = 5300 ms, TE = 95 ms, bandwidth = 1860 Hz/voxel, FOV = 200 mm, and matrix size = 96 × 96. There were 50 2.4-mm thick slices (no slice gap) with no diffusion-weighting (b = 0 s/mm2, 8 repetitions equally spaced during acquisition), and with diffusion-weighting gradients applied in 128 orthogonal directions (b = 2000 s/mm2). The diffusion data took 24 min to acquire. A gradient echo field map was also collected for correction of distortions in the diffusion-weighted images. The acquisition of this field map used an EPI sequence with TR = 550 ms, TE1 = 5 ms, TE2 = 7.46 ms, bandwidth = 300 Hz/Voxel, FOV = 230 mm, matrix size = 128 × 128, and slices were acquired in the same planes as the diffusion data.
Data Processing and Analysis
Diffusion-weighted data were preprocessed with a scripted pipeline calling tools from the FMRIB Software Library (Jenkinson et al., 2012; http://www.fmrib.ox.ac.uk/fsl). The images with no diffusion weighting (b=0) were motion-corrected and averaged to serve as an initial reference for further processing. The gradient echo field map images were used to correct for geometric distortions in these images and in the diffusion weighted images using the prelude and fugue tools. All images were then corrected for motion and eddy currents using the eddy_correct tool. The vectors specifying the diffusion-weighted gradient directions were then rotated to compensate from head motion prior to fitting the diffusion tensor model with the dtifit tool.
Participant movement in the scanner was calculated as a z-score of Euclidean distance for each individual and then compared across groups. There were no significant overall nor pairwise differences in absolute head motion (F(2,55)=1.33, p=.273), or normalized brain volume (F(2,55)=1.77, p=.181) across the ASD group, the schizophrenia group, or the neurotypical controls. Voxelwise statistical analysis of the fractional anisotropy (FA) and mean diffusivity (MD) data were carried out using TBSS (Tract-Based Spatial Statistics, Smith et al., 2006), part of FSL (Smith et al., 2004), using the following approach.
First, FA images were created by fitting a tensor model to raw diffusion data using FMRIB’s Diffusion Toolbox (FDT), and second, by extracting the brain using the Brain Extracted Toolbox (BET; Smith, 2002). All FA data were then aligned into a common space using the nonlinear registration tool FNIRT (Andersson, Jenkinson & Smith, 2007; Andersson, Jenkinson & Smith, 2007), which uses a b-spline representation of the registration warp field (Rueckert et al., 1999). Next, the mean FA image was created and thinned to create a mean FA skeleton which represents the centers of all tracts common to all participants regardless of group identity (threshold at 0.2). Each aligned FA data was then projected onto this skeleton and the resulting data entered voxelwise cross-subject statistics. Finally, the randomise method was used to compare schizophrenia, ASD, and neurotypical groups (Winkler et al., 2014) using 500 permutations to compute the null distribution. Familywise corrected Threshold-Free Cluster Enhancement p-values (TFCE; corrected by using the null distribution of the max voxelwise test statistic across the image; Smith & Nichols, 2009) were used to identify clusters where there was a significant difference in FA between the experimental groups. MD data were similarly registered into a common nonlinear space and projected onto the mean FA skeleton. A random permutation testing method was used, using the randomise tool included in FSL. The permutation test was used to compare schizophrenia, ASD, and control groups, and the multiple comparison corrected p-values (corrected using Threshold-Free Cluster Enhancement) were used examine the significance of any between-group differences.
The mean FA, MD, axial (L1 direction), and radial (averaged L2 and L3 directions) diffusion were calculated for each participant across the whole white matter skeleton (see Figure 1). Results were the same when median was used as the summary statistic. Group effects were analyzed using one-way ANOVAs and independent-samples t-tests were used for post-hoc comparisons.
Figure 1.
The white matter skeleton common across all individuals with schizophrenia, autism, and neurotypical controls. The skeleton was inflated for illustration.
Results
Comparing Measures of Fractional Anisotropy (FA) and Mean Diffusivity (MD)
We compared the schizophrenia, autism and control groups on measures of diffusion to indicate white matter structure across the brain. First, we focused on FA as a measure of efficient diffusion along tracts and found that there was no statistically significant difference between the three groups (F(2,55)=1.47, p=.074). However, when we focused on MD, which provides a measure of the white matter microstructure, individuals with schizophrenia exhibited greater MD (F(2,55)=3.29, p=.045), compared to ASD individuals (t(26)=3.37, p=.002), and neurotypical controls (t(20)=2.48, p=.022), and there was no difference between ASD and controls (t(39)=0.45, p=.658; Figure 2). The mean diffusivity measures were calculated using the mean of the axial (AD) and radial (RD) diffusion directions. Therefore, to uncover the source of the group differences, we compared groups on AD and RD, and found that whereas individuals with schizophrenia show greater diffusivity in the radial direction (F(2,55)=4.01, p=.024; Figure 3), there was no group difference in the axial direction (F(2,55)=2.73, p=.074). Despite there being no significant difference between groups on age or gender, we included age and gender as covariates to ensure that there were no unexpected effects on MD. There was no significant main effect of age (F(1,52)=0.10, p=.752) or gender (F(2,52)=0.03, p=.971), and the effect of group still held (F(2,52)=3.12, p=.053).
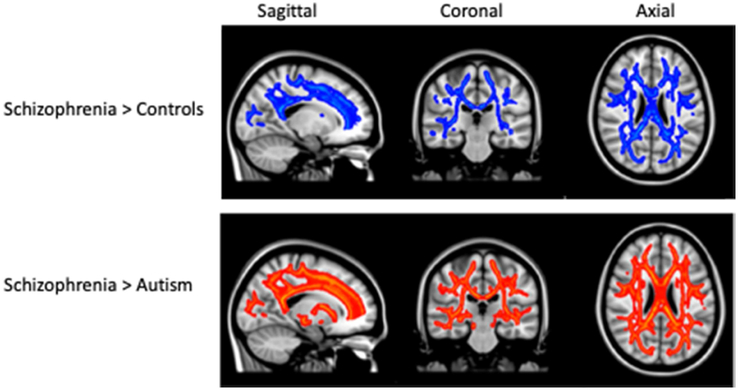
Figure 2.
White matter tracts with greater mean diffusivity in schizophrenia compared to controls (top row in blue) and compared to individuals with autism (bottom row in red), shown in sagittal, coronal, and axial slices across the brain. Differences in TFCE- selected clusters significant at p < .05 by permutation testing and, corrected for multiple comparisons. Significant clusters were inflated for illustration.
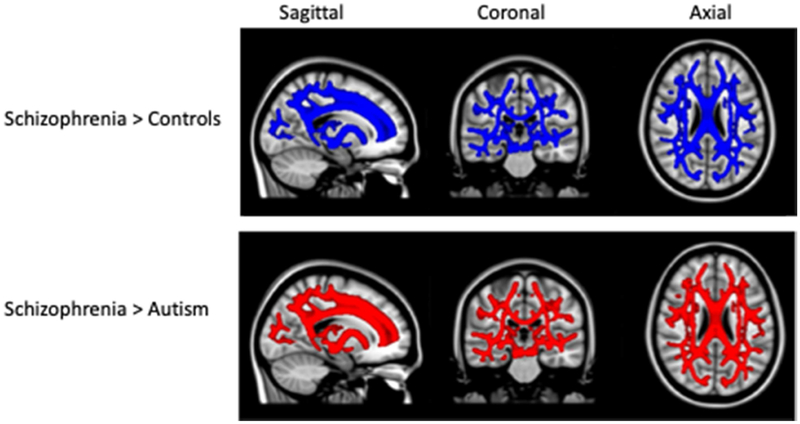
Figure 3.
White matter tracts with greater radial diffusivity in schizophrenia compared to controls (top row in blue) and compared to individuals with autism (bottom row in red), shown in sagittal, coronal, and axial slices across the brain. Differences in TFCE- selected clusters significant at p < .05 by permutation testing and, corrected for multiple comparisons. Significant clusters were inflated for illustation.
Due to the smaller sample size in the schizophrenia group, two additional analyses were conducted. First, Cohen’s d effect sizes were calculated for all group comparisons and for FA, MD, AD, and RD measures of diffusion (Table 3). The effects sizes for the increased MD, AD, and RD in schizophrenia compared to autism and control groups were by far the largest (despite the group comparisons only trending to be statistically significant in the axial). However, the effect sizes comparing autism to schizophrenia and control groups on FA were in the medium range (>0.2), suggesting that there may be decreased FA in autism, but the smaller sample sizes for the schizophrenia and control groups may have prevented this effect from reaching significance.
Table 3.
Effect sizes (Cohen’s d) comparing group differences on fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD). Group comparisons are the effect size of (1) the autism group (ASD) having increased diffusion compared to controls (HC), (2) the schizophrenia group (Sz) having increased diffusion compared to the autism group, and (3) the schizophrenia group having increased diffusion compared to controls. Negative effect sizes denote the effect in the opposite direction. Significant comparisons are in bold.
| Group Comparison | Fractional Anisotropy (FA) |
Mean Diffusivity (MD) |
Axial Diffusivity (AD) |
Radial Diffusivity (RD) |
|---|---|---|---|---|
| ASD > HC | −0.47 | −0.14 | −0.21 | −0.03 |
| Sz > ASD | 0.34 | 0.89 | 0.82 | 0.93 |
| Sz > HC | −0.19 | 0.77 | 0.61 | 0.92 |
Second, a subset of 15 individuals with autism and 15 control participants who still matched the schizophrenia group on age and gender were selected and the analyses recalculated. The results were the same. There was no significant difference between the groups on FA (F(2,42)=1.50, p=.235), but there was for MD (F(2,420=3.43, p=.042), and in the radial direction (F(2,42)=4.02, p=.025), with a trend toward significance in the axial direction (F(2,42)=2.89, p=.067).
Including Measures of Head Motion and Brain Volume
Despite the fact that there were no group differences in measures of head motion and brain volume, it is possible that these physiological measures were indirectly related to the group differences in MD. Therefore, we used an ANCOVA to show that when normalized brain volume was accounted for, there was still a significant group difference in MD (F(2,52)=3.15, p=.051), no significant effect of brain volume (F(1,52)=0.01, p=.916), and no significant interaction between group and brain volume (F(2,52)=0.34, p=.713). However, when head motion was accounted for in an ANCOVA, there was still a significant effect of group (F(2,52)=6.09, p=.004), but there was also a significant effect of absolute head motion on MD (F(1,52)=47.09, p<.001). Despite this, there was no significant interaction between group identity and head motion (F(2,55)=1.36, p=.265). Together, this suggests that head motion does not account for the differences in mean diffusivity between groups (as the interaction is not significant) but does highlight the importance of accounting for head motion in DTI analyses.
For FA, even when accounting for head motion and brain volume, there was no significant difference between groups (head motion: F(2,52)=2.29, p=.112; brain volume: F(2,52)=1.54, p=.225). Therefore, there is no evidence in this sample that there are significant differences in FA measures of white matter structure between schizophrenia, autism and controls.
Correlations with Symptom Measures
Finally, measures of MD were correlated with symptom scores in the schizophrenia and ASD groups separately. In the schizophrenia group, there was no significant correlation between MD and Brief Psychotic Rating Score (BPRS; r(13)=.10, p=.721) but there was a significant correlation with amount of antipsychotic medication (r(13)=−.60, p=.018), suggesting that those on higher dosage had reduced MD. This appears to be primarily driven by two individuals who were on high levels of medication. The higher dosage may have helped improve MD over time, but there is no evidence here that this is the case. In the ASD group, the only correlation that was significant was between MD and ADOS measures of communication (r(22)=−.48, p=.024), suggesting that poorer communicative behaviors were associated with less MD, which is in the opposite direction to our hypothesis. Correlations were not significant for ADOS social or stereotypical behavior scores, or with any ADI scores. There were no significant correlations with IQ in either the ASD or the schizophrenia group.
Discussion
In this study, we investigated measures of diffusion to investigate white matter tract structure in adults with schizophrenia and in adults with autism compared to age- and gender-matched controls. IQ was not measured in the controls. There were no significant group differences in fractional anisotropy (FA) across the brain, but the adults with schizophrenia did show greater mean diffusivity (MD). Specific deficits in MD but not FA (despite normal brain volume) have been suggested to reflect abnormal myelination (Song et al., 2005; Winklewski et al., 2018), which could impact the efficiency of the tracts when transferring information across the brain. The abnormal diffusion was specific to schizophrenia and was not evident in the autism group. One other study is known to have previously focused on diffusion measures in autism and schizophrenia and found FA reductions in autism and schizophrenia groups compared to controls, but this reduction was specific to left fronto-occipital inferior fasciculus (Katz et al., 2016). The current study found reductions in MD that appear to be specific to schizophrenia.
A review of the literature reveals several studies reporting increased MD in schizophrenia compared to controls (Narr et al., 2009; Lee et al., 2009; only MD Ardekani et al., 2011; Leroux et al., 2014; Knöchel et al., 2012; Spalletta et al., 2015; both Spalletta et al., 2015). There were relatively few studies reporting a MD difference in adults with autism versus controls (Gibbard et al., 2013; Itashashi et al., 2015), perhaps consistent with the claim that greater MD may be a specific abnormality in schizophrenia.
Interestingly, the greater MD in schizophrenia was due to significantly greater radial diffusivity, and slightly greater axial diffusivity. However, when these measures were normalized to calculate FA, the overall diffusion from the white matter tracts were unimpaired. Increased radial diffusivity (perpendicular to the length of the tract) has been associated with demyelination (Song et al., 2005; Winklewski et al., 2018), although this conclusion will need to be verified with in vivo studies as measures of radial diffusivity alone can be misleading when inferring myelination (Wheeler-Kingshott & Cercignani, 2009; Jones & Cercignani, 2010; see Jones, Knösche & Turner, 2013, for a review of the difficulties in interpreting structural properties from DTI measures). This finding of greater radial diffusivity in schizophrenia has been reported previously (Scheel et al., 2013), and evidence of demyelination has been found in structural MRI scans focusing on myelin water fractions (Flynn et al., 2003).
Impaired myelination impacts the transfer of information across the brain (Fields, 2008), and reduced processing speed has been shown to be related to impaired diffusion properties in schizophrenia (Wright et al., 2015). However, both schizophrenia and autism are associated with slower processing speeds (Eack et al., 2013), and yet the individuals with autism in this study did not evince with the same white matter abnormalities as schizophrenia. Furthermore, we have shown in another study that FA is not associated with processing speed in adults with autism (Haigh et al., in press). Therefore, the functional impairments in schizophrenia and autism may be differentially impacted by structural abnormalities.
There were no significant correlations between MD and symptom measures in autism or in schizophrenia, except in the autism group where worse MD correlated with better scores on ADOS social communication. However, this correlation is in the opposite direction to what would be predicted and will need to be replicated before any conclusions can be drawn. Future studies should correlate MD with the same measure of social communication across both groups to gain better insight into the functional impact of increased MD.
The lack of significant differences between groups in FA is somewhat surprising, considering the wealth of studies showing reduced FA in both schizophrenia and in autism compared to neurotypical individuals. This is in direct contrast to Katz and colleagues (2016) who reported that both autism and schizophrenia exhibited reduced FA that was specific to the left fronto-occipital inferior fasciculus compared to neurotypical individuals. The current study did not show any significant FA reductions along any part of the white matter skeleton. There is a possibility that this lack of significant effect may have been due to smaller sample sizes in the schizophrenia group, although the additional analyses equating the groups on sample size resulted in the same effects of greater MD in schizophrenia (but of course reduced statistical power). Effect sizes showed that there was a medium effect in the direction of weaker FA in autism compared to the neurotypical control group, but also compared to the adults with schizophrenia. However, the effect sizes comparing MD in schizophrenia to autism and control groups were much larger, suggesting that weaker FA in autism may not be as fundamental to distinguishing between schizophrenia and autism as the MD differences.
The main limitation of this study is the somewhat smaller sample size for the schizophrenia group, relative to the other two groups. Increasing the sample size in the schizophrenia group is, however, unlikely to alter the results: a main result was greater MD in schizophrenia compared to autism and control groups and these comparisons had large effect sizes, demonstrating that even with a small sample, greater MD was a significant result. A larger sample size may have generated a significant effect of weaker FA in autism, as this comparison had a medium effect size although we note that a group of 25 ASD participants is larger than many of the groups in existing studies. Critically, even if this latter result did differ with a larger group, this would not alter the concluding finding that the main effect of MD when comparing the three groups is a defining feature distinguishing schizophrenia from autism. One caveat is that the ASD group had higher IQ than the schizophrenia group. This may have impacted the groups differences in their diffusion measures of white matter tract structure. However, there were no significant correlations between IQ and MD in either group, suggesting that IQ differences would not have had a large impact on the results.
Conclusions
The functional impact of the differences in diffusivity in schizophrenia and autism are unclear but illustrate potential endophenotypic distinctions that may be diagnostically specific. Building a more complete picture of how schizophrenia and autism are related but differ in their neurological manifestations can help to create individualized treatments. What is clear is that the impacted water diffusion in schizophrenia is evident across the brain and is not located in specific areas of the brain. Future studies investigating the behavioral impact will help to ascertain the value of the increase in MD and RD as a biomarker of schizophrenia.
Highlights.
Schizophrenia and autism share many behavioral and neurological similarities
We compared their white matter tract integrity using FSL’s TBSS
Schizophrenia showed greater radial diffusivity compared to autism and controls
Greater radial diffusivity is associated with abnormal myelination
This was evident across the brain and is diagnosis specific
Acknowledgements
This research was supported by a grant from the Simons Foundation Autism Research Initiative (177638) to MB, a National Institutes of Health/National Institute of Child Health and Human Development grant (HD055748) to NJM, a NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation (26282) to SMH, and an NSF EPSCoR grant (1632749) that SMH is a Co-I on.
Footnotes
Disclosures
Declarations of interest: none
References
- Abdel Razek A, Mazroa J, Baz H, 2014. Assessment of white matter integrity of autistic preschool children with diffusion weighted MR imaging. Brain Dev. 36, 28–34. [DOI] [PubMed] [Google Scholar]
- Abdi Z, Sharma T, 2004. Social Cognition and Its Neural Correlates in Schizophrenia and Autism. CNS Spectr. 9, 335–343. [DOI] [PubMed] [Google Scholar]
- Ameis SH, Fan J, Rockel C, Soorya L, Wang AT, Anagnostou E, 2013. Altered cingulum bundle microstructure in autism spectrum disorder. Acta Neuropsychiatr. 25, 275–282. 10.1017/neu.2013.2 [DOI] [PubMed] [Google Scholar]
- Andersson JLR, Jenkinson M, Smith S, 2007a. Non-linear optimisation. FMRIB technical report TR07JA1. [Google Scholar]
- Andersson JLR, Jenkinson M, Smith S, 2007b. Non-linear registration, aka Spatial normalisation FMRIB technical report TR07JA2. [Google Scholar]
- Ardekani BA, Tabesh A, Sevy S, Robinson DG, Bilder RM, Szeszko PR, 2011. Diffusion Tensor Imaging Reliably Differentiates Patients With Schizophrenia from Healthy Volunteers. Hum. Brain Mapp 32, 1–9. 10.1002/hbm.20995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asami T, Lee SH, Bouix S, Rathi Y, Whitford TJ, Niznikiewicz M, Nestor P, McCarley RW, Shenton ME, Kubicki M, 2014. Cerebral White Matter Abnormalities and Their Associations with Negative but not Positive Symptoms of Schizophrenia. Psychiatry Res. 222, 52–59. 10.1016/j.pscychresns.2014.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C, 2002. The basis of anisotropic water diffusion in the nervous system – a technical review. NMR Biomed. 15, 435–455. 10.1002/nbm.782 [DOI] [PubMed] [Google Scholar]
- Billeci L, Calderoni S, Tosetti M, Catani M, Muratori F, 2012. White matter connectivity in children with autism spectrum disorders: a tract-based spatial statistics study. BMC Neurol. 12, 148 10.1186/1471-2377-12-148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheon K-A, Kim Y-S, Oh S-H, Park S-Y, Yoon H-W, Herrington J, Nair A, Koh Y-J, Jang D-P, Kim Y-B, Leventhal BL, Cho Z-H, Castellanos FX, Schultz RT, 2011. Involvement of the anterior thalamic radiation in boys with high functioning autism spectrum disorders: A Diffusion Tensor Imaging study. Brain Res. 1417, 77–86. [DOI] [PubMed] [Google Scholar]
- Cheung C, Chua SE, Cheung V, Khong PL, Tai KS, Wong TKW, Ho TP, McAlonan GM, 2009. White matter fractional anisotrophy differences and correlates of diagnostic symptoms in autism. J. Child Psychol. Psychiatry 50, 1102–1112. 10.1111/j.1469-7610.2009.02086.x [DOI] [PubMed] [Google Scholar]
- Cheung C, Yu K, Fung G, Leung M, Wong C, Li Q, Sham P, Chua S, McAlonan G, 2010. Autistic Disorders and Schizophrenia: Related or Remote? An Anatomical Likelihood Estimation. PLoS One 5, e12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm K, Lin A, Abu-Akel A, Wood SJ, 2015. The association between autism and schizophrenia spectrum disorders: A review of eight alternate models of co-occurrence. Neurosci. Biobehav. Rev. 55, 173–183. [DOI] [PubMed] [Google Scholar]
- Ciaramidaro A, Bölte S, Schlitt S, Hainz D, Poustka F, Weber B, Bara BG, Freitag C, Walter H, 2015. Schizophrenia and Autism as Contrasting Minds: Neural Evidence for the Hypo-Hyper-Intentionality Hypothesis. Schizophr. Bull 41, 171–179. 10.1093/schbul/sbu124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaramidaro A, Bölte S, Schlitt S, Hainz D, Poustka F, Weber B, Freitag C, Walter H, 2018. Transdiagnostic deviant facial recognition for implicit negative emotion in autism and schizophrenia. Eur. Neuropsychopharmacol 28, 264–275. [DOI] [PubMed] [Google Scholar]
- Eack SM, Bahorik AL, McKnight SAF, Hogarty SS, Greenwald DP, Newhill CE, Phillips ML, Keshavan MS, Minshew NJ, 2013. Commonalities in social and non-social cognitive impairments in adults with autism spectrum disorder and schizophrenia. Schizophr. Res 148, 24–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eack SM, Hogarty SS, Greenwald DP, Litschge MY, Shannondora, Porton A, Mazefsky CA, Minshew NJ, 2017. Cognitive enhancement therapy for adult autism spectrum disorder: Results of an 18-month randomized clinical trial. Autism Res. 11, 519–530. 10.1002/aur.1913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley R, Asmal L, du Plessis S, Chiliza B, Phahladira L, Kilian S, 2017. Brain volume changes over the first year of treatment in schizophrenia: relationships to antipsychotic treatment. Psychol. Med 47, 2187–2196. 10.1017/S0033291717000642 [DOI] [PubMed] [Google Scholar]
- Fields RD, 2008. White matter in learning, cognition and psychiatric disorders. Trends Neurosci. 31, 361–370. 10.1016/j.tins.2008.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW, 2002. Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition. SCID-I/P. [Google Scholar]
- Flynn SW, Lang DJ, Mackay AL, Goghari V, Vavasour IM, Whittall KP, Smith GN, Arango V, Mann JJ, Dwork AJ, Falkai P, Honer WG, 2003. Abnormalities of myelination in schizophrenia detected in vivo with MRI, and post-mortem with analysis of oligodendrocyte proteins. Mol. Psychiatry 8, 811. [DOI] [PubMed] [Google Scholar]
- Fujino J, Takahashi H, Miyata J, Sugihara G, Kubota M, Sasamoto A, Fujiwara H, Aso T, Fukuyama H, Murai T, 2014. Impaired empathic abilities and reduced white matter integrity in schizophrenia. Prog. Neuro-Psychopharmacology Biol. Psychiatry 48, 117–123. [DOI] [PubMed] [Google Scholar]
- Ghaziuddin M, Tsai L, Ghaziuddin N, 1992. Comorbidity of autistic disorder in children and adolescents. Eur. Child Adolesc. Psychiatry 1, 209–213. 10.1007/BF02094180 [DOI] [PubMed] [Google Scholar]
- Gibbard CR, Ren J, Seunarine KK, Clayden JD, Skuse DH, Clark CA, 2013. White matter microstructure correlates with autism trait severity in a combined clinical–control sample of high-functioning adults. Neuroimage (Amst). 3, 106–114. 10.1016/j.nicl.2013.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigh SM, Gupta A, Barb SM, Glass SAF, Minshew NJ, Dinstein I, Heeger DJ, Eack SM, Behrmann M, 2016. Differential sensory fMRI signatures in autism and schizophrenia: Analysis of amplitude and trial-to-trial variability. Schizophr. Res 175, 12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigh SM, Keller TA, Minshew NJ, Eack SM, n.d. Reduced White Matter Integrity and Deficits in Neuropsychological Functioning in Adults with Autism Spectrum Disorder. J. Autism Dev. Disord [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itahashi T, Yamada T, Nakamura M, Watanabe H, Yamagata B, Jimbo D, Shioda S, Kuroda M, Toriizuka K, Kato N, Hashimoto R, 2015. Linked alterations in gray and white matter morphology in adults with high-functioning autism spectrum disorder: A multimodal brain imaging study. Neuroimage (Amst). 7, 155–169. 10.1016/j.nid.2014.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM, 2012. FSL. Neuroimage 62, 782–790. [DOI] [PubMed] [Google Scholar]
- Jones DK, Cercignani M, 2010. Twenty-five pitfalls in the analysis of diffusion MRI data. NMR Biomed. 23, 803–820. 10.1002/nbm.1543 [DOI] [PubMed] [Google Scholar]
- Jones DK, Knösche TR, & Turner R (2013). White matter integrity, fiber count, and other fallacies: The do’s and don’ts of diffusion MRI. NeuroImage, 73, 239–254. [DOI] [PubMed] [Google Scholar]
- Jou RJ, Reed HE, Kaiser MD, Voos AC, Volkmar FR, Pelphrey KA, 2015. White Matter Abnormalities in Autism and Unaffected Siblings. J. Neuropsychiatry Clin. Neurosci 28, 49–55. 10.1176/appi.neuropsych.15050109 [DOI] [PubMed] [Google Scholar]
- Katz J, d’Albis M-A, Boisgontier J, Poupon C, Mangin J-F, Guevara P, Duclap D, Hamdani N, Petit J, Monnet D, Le Corvoisier P, Leboyer M, Delorme R, Houenou J, 2016. Similar white matter but opposite grey matter changes in schizophrenia and high-functioning autism. Acta Psychiatr. Scand 134, 31–39. 10.1111/acps.12579 [DOI] [PubMed] [Google Scholar]
- Kelly S, Jahanshad N, Zalesky A, Kochunov P, Agartz I, Alloza C, Andreassen OA, Arango C, Banaj N, Bouix S, Bousman CA, Brouwer RM, Bruggemann J, Bustillo J, Cahn W, Calhoun V, Cannon D, Carr V, Catts S, Chen J, Chen J-X, Chen X, Chiapponi C, Cho KK, Ciullo V, Corvin AS, Crespo-Facorro B, Cropley V, De Rossi P, Diaz-Caneja CM, Dickie EW, Ehrlich S, Fan F-M, Faskowitz J, Fatouros-Bergman H, Flyckt L, Ford JM, Fouche J-P, Fukunaga M, Gill M, Glahn DC, Gollub R, Goudzwaard ED, Guo H, Gur RE, Gur RC, Gurholt TP, Hashimoto R, Hatton SN, Henskens FA, Hibar DP, Hickie IB, Hong LE, Horacek J, Howells FM, Hulshoff Pol HE, Hyde CL, Isaev D, Jablensky A, Jansen PR, Janssen J, Jönsson EG, Jung LA, Kahn RS, Kikinis Z, Liu K, Klauser P, Knöchel C, Kubicki M, Lagopoulos J, Langen C, Lawrie S, Lenroot RK, Lim KO, Lopez-Jaramillo C, Lyall A, Magnotta V, Mandl RCW, Mathalon DH, McCarley RW, McCarthy-Jones S, McDonald C, McEwen S, McIntosh A, Melicher T, Mesholam-Gately RI, Michie PT, Mowry B, Mueller BA, Newell DT, O’Donnell P, Oertel-Knöchel V, Oestreich L, Paciga SA, Pantelis C, Pasternak O, Pearlson G, Pellicano GR, Pereira A, Pineda Zapata J, Piras F, Potkin SG, Preda A, Rasser PE, Roalf DR, Roiz R, Roos A, Rotenberg D, Satterthwaite TD, Savadjiev P, Schall U, Scott RJ, Seal ML, Seidman LJ, Shannon Weickert C, Whelan CD, Shenton ME, Kwon JS, Spalletta G, Spaniel F, Sprooten E, Stäblein M, Stein DJ, Sundram S, Tan Y, Tan S, Tang S, Temmingh HS, Westlye LT, Tønnesen S, Tordesillas-Gutierrez D, Doan NT, Vaidya J, van Haren NEM, Vargas CD, Vecchio D, Velakoulis D, Voineskos A, Voyvodic JQ, Wang Z, Wan P, Wei D, Weickert TW, Whalley H, White T, Whitford TJ, Wojcik JD, Xiang H, Xie Z, Yamamori H, Yang F, Yao N, Zhang G, Zhao J, van Erp TGM, Turner J, Thompson PM, Donohoe G, 2018. Widespread white matter microstructural differences in schizophrenia across 4322 individuals: results from the ENIGMA Schizophrenia DTI Working Group. Mol. Psychiatry 23, 1261–1269. 10.1038/mp.2017.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkovski M, Enticott PG, Maller JJ, Rossell SL, Fitzgerald PB, 2015. Diffusion tensor imaging reveals no white matter impairments among adults with autism spectrum disorder. Psychiatry Res. Neuroimaging 233, 64–72. [DOI] [PubMed] [Google Scholar]
- Kleinhans NM, Pauley G, Richards T, Neuhaus E, Martin N, Corrigan NM, Shaw DW, Estes A, Dager SR, 2012. Age-related abnormalities in white matter microstructure in autism spectrum disorders. Brain Res. 1479, 1–16. 10.1016/j.brainres.2012.07.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knöchel C, Oertel-Knöchel V, Schönmeyer R, Rotarska-Jagiela A, van de Ven V, Prvulovic D, Haenschel C, Uhlhaas P, Pantel J, Hampel H, Linden DEJ, 2012. Interhemispheric hypoconnectivity in schizophrenia: Fiber integrity and volume differences of the corpus callosum in patients and unaffected relatives. Neuroimage 59, 926–934. [DOI] [PubMed] [Google Scholar]
- Kochunov P, Chiappelli J, Wright SN, Rowland LM, Patel B, Wijtenburg SA, Nugent K, McMahon RP, Carpenter WT, Muellerklein F, Sampath H, Hong LE, 2014. Multimodal white matter imaging to investigate reduced fractional anisotropy and its age-related decline in schizophrenia. Psychiatry Res. 223, 148–156. 10.1016/j.pscychresns.2014.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantareas MM, Hewitt T, 2001. Autistic Disorder and Schizophrenia: Diagnostic Overlaps. J. Autism Dev. Disord 31, 19–28. 10.1023/A:1005605528309 [DOI] [PubMed] [Google Scholar]
- Lazar M, Miles LM, Babb JS, Donaldson JB, 2014. Axonal deficits in young adults with High Functioning Autism and their impact on processing speed. Neuroimage (Amst). 4, 417–425. 10.1016/j.nicl.2014.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Couteur A, Rutter M, Lord C, Rios P, Robertson S, Holdgrafer M, McLennan J, 1989. Autism diagnostic interview: a standardized investigator-based instrument. J. Autism Dev. Disord 19, 363–387. [DOI] [PubMed] [Google Scholar]
- Lee K, Yoshida T, Kubicki M, Bouix S, Westin C-F, Kindlmann G, Niznikiewicz M, Cohen A, McCarley RW, Shenton ME, 2009. Increased Diffusivity in Superior Temporal Gyrus in Patients with Schizophrenia: A Diffusion Tensor Imaging Study. Schizophr. Res 108, 33–40. 10.1016/j.schres.2008.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroux E, Delcroix N, Dollfus S, 2014. Left fronto-temporal dysconnectivity within the language network in schizophrenia: An fMRI and DTI study. Psychiatry Res. Neuroimaging 223, 261–267. 10.1016/j.pscychresns.2014.06.002 [DOI] [PubMed] [Google Scholar]
- Levitt JJ, Alvarado JL, Nestor PG, Rosow L, Pelavin PE, McCarley RW, Kubicki M, Shenton ME, 2012. Fractional anisotropy and radial diffusivity: Diffusion measures of white matter abnormalities in the anterior limb of the internal capsule in schizophrenia. Schizophr. Res 136, 55–62. 10.1016/j.schres.2011.09.009 [DOI] [PubMed] [Google Scholar]
- Libero LE, DeRamus TP, Lahti AC, Deshpande G, Kana RK, 2015. Multimodal neuroimaging based classification of autism spectrum disorder using anatomical, neurochemical, and white matter correlates. Cortex. 66, 46–59. 10.1016/j.cortex.2015.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, Goode S, Heemsbergen J, Jordan H, Mawhood L, Schopler E, 1989. Austism diagnostic observation schedule: A standardized observation of communicative and social behavior. J. Autism Dev. Disord 19, 185–212. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A, 1994. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J. Autism Dev. Disord 24, 659–685. [DOI] [PubMed] [Google Scholar]
- Lukoff D, Nuechterlein KH, Ventura J, 1986. Manual for the expanded brief psychiatric rating scale. Schizophr Bull 12, 594–602. [Google Scholar]
- Mançe Çalişir Ö, Atbaşoğlu EC, Devrimci Özgüven H, Ölmez Ş, 2018. Cognitive Features of High-functioning Adults with Autism and Schizophrenia Spectrum Disorders. Turk Psikiyatri Derg. 29, 1–10. [PubMed] [Google Scholar]
- Mazefsky CA, Herrington J, Siegel M, Scarpa A, Maddox BB, Scahill L, White SW, 2013. The Role of Emotion Regulation in Autism Spectrum Disorder RH: Emotion Regulation in ASD. J. Am. Acad. Child Adolesc. Psychiatry 52, 679–688. 10.1016/j.jaac.2013.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitelman SA, Buchsbaum MS, Young DS, Haznedar MM, Hollander E, Shihabuddin L, Hazlett EA, Bralet M-C, 2018. Increased white matter metabolic rates in autism spectrum disorder and schizophrenia. Brain Imaging Behav. 12, 1290–1305. 10.1007/s11682-017-9785-9 [DOI] [PubMed] [Google Scholar]
- Mueller S, Keeser D, Reiser MF, Teipel S, Meindl T, 2012. Functional and Structural MR Imaging in Neuropsychiatric Disorders, Part 2: Application in Schizophrenia and Autism. Am. J. Neuroradiol 33, 2033 LP – 2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Kawasaki Y, Takahashi T, Furuichi A, Noguchi K, Seto H, Suzuki M, 2012. Reduced white matter fractional anisotropy and clinical symptoms in schizophrenia: A voxel-based diffusion tensor imaging study. Psychiatry Res. Neuroimaging 202, 233–238. 10.1016/j.pscychresns.2011.09.006 [DOI] [PubMed] [Google Scholar]
- Narr KL, Hageman N, Woods RP, Hamilton LS, Clark K, Phillips O, Shattuck DW, Asarnow RF, Toga AW, Nuechterlein KH, 2009. Mean diffusivity: A biomarker for CSF-related disease and genetic liability effects in schizophrenia. Psychiatry Res. 171, 20–32. 10.1016/j.pscychresns.2008.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazeri A, Chakravarty MM, Felsky D, Lobaugh NJ, Rajji TK, Mulsant BH, Voineskos AN, 2013. Alterations of Superficial White Matter in Schizophrenia and Relationship to Cognitive Performance. Neuropsychopharmacology 38, 1954–1962. 10.1038/npp.2013.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel J-P, Stevenson RA, Wallace MT, 2018. Atypical audiovisual temporal function in autism and schizophrenia: similar phenotype, different cause. Eur. J. Neurosci 47, 1230–1241. 10.1111/ejn.13911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noriuchi M, Kikuchi Y, Yoshiura T, Kira R, Shigeto H, Hara T, Tobimatsu S, Kamio Y, 2010. Altered white matter fractional anisotropy and social impairment in children with autism spectrum disorder. Brain Res. 1362, 141–149. [DOI] [PubMed] [Google Scholar]
- Ohtani T, Bouix S, Hosokawa T, Saito Y, Eckbo R, Ballinger T, Rausch A, Melonakos E, Kubicki M, 2014. Abnormalities in white matter connections between orbitofrontal cortex and anterior cingulate cortex and their associations with negative symptoms in Schizophrenia: A DTI Study. Schizophr. Res 157, 190–197. https://doi.Org/10.1016/j.schres.2014.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkham AE, Hopfinger JB, Pelphrey KA, Piven J, Penn DL, 2008. Neural bases for impaired social cognition in schizophrenia and autism spectrum disorders. Schizophr. Res [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad KM, Upton CH, Nimgaonkar VL, Keshavan MS, 2015. Differential susceptibility of white matter tracts to inflammatory mediators in schizophrenia: An integrated DTI study. Schizophr. Res. 161, 119–125. 10.1016/j.schres.2014.09.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryweller JR, Schauder KB, Anderson AW, Heacock JL, Foss-Feig JH, Newsom CR, Loring WA, Cascio CJ, 2014. White matter correlates of sensory processing in autism spectrum disorders. Neuroimage (Amst). 6, 379–387. 10.1016/j.nicl.2014.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roalf DR, Gur RE, Verma R, Parker WA, Quarmley M, Ruparel K, Gur RC, 2015. White matter microstructure in schizophrenia: Associations to neurocognition and clinical symptomatology. Schizophr. Res 161, 42–49. 10.1016/j.schres.2014.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roalf DR, Ruparel K, Verma R, Elliott MA, Gur RE, Gur RC, 2013. White matter organization and neurocognitive performance variability in schizophrenia. Schizophr. Res. 143, 172–178. 10.1016/j.schres.2012.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roine U, Salmi J, Roine T, Wendt TN, Leppämäki S, Rintahaka P, Tani P, Leemans A, Sams M, 2015. Constrained spherical deconvolution-based tractography and tract-based spatial statistics show abnormal microstructural organization in Asperger syndrome. Mol. Autism 6, 4 10.1186/2040-2392-6-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueckert D, Sonoda LI, Hayes C, Hill DLG, Leach MO, Hawkes DJ, 1999. Nonrigid registration using free-form deformations: application to breast MR images. Med. Imaging, IEEE Trans. 18, 712–721. 10.1109/42.796284 [DOI] [PubMed] [Google Scholar]
- Samartzis L, Dima D, Fusar-Poli P, Kyriakopoulos M, 2013. White Matter Alterations in Early Stages of Schizophrenia: A Systematic Review of Diffusion Tensor Imaging Studies. J. Neuroimaging 24, 101–110. 10.1111/j.1552-6569.2012.00779.x [DOI] [PubMed] [Google Scholar]
- Sasamoto A, Miyata J, Kubota M, Hirao K, Kawada R, Fujimoto S, Tanaka Y, Hazama M, Sugihara G, Sawamoto N, Fukuyama H, Takahashi H, Murai T, 2014. Global Association Between Cortical Thinning and White Matter Integrity Reduction in Schizophrenia. Schizophr. Bull 40, 420–427. 10.1093/schbul/sbt030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheel M, Prokscha T, Bayerl M, Gallinat J, Montag C, 2013. Myelination deficits in schizophrenia: evidence from diffusion tensor imaging. Brain Struct. Funct 218, 151–156. 10.1007/s00429-012-0389-2 [DOI] [PubMed] [Google Scholar]
- Shukla DK, Keehn B, Müller R-A, 2011a. Tract-Specific Analyses of Diffusion Tensor Imaging Show Widespread White Matter Compromise in Autism Spectrum Disorder. J. Child Psychol. Psychiatry 52, 286–295. 10.1111/j.1469-7610.2010.02342.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla DK, Keehn B, Smylie DM, Müller R-A, 2011b. Microstructural abnormalities of short-distance white matter fiber tracts in autism spectrum disorder. Neuropsychologia 49, 1378–1382. 10.1016/j.neuropsychologia.2011.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, 2002. Fast robust automated brain extraction. Hum. Brain Mapp 17, 143–155. 10.1002/hbm.10062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TEJ, 2006. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage 31, 1487–1505. 10.1016/j.neuroimage.2006.02.024 [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM, 2004. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23, Supple, S208–S219. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE, 2009. Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 44, 83–98. [DOI] [PubMed] [Google Scholar]
- Song S-K, Yoshino J, Le TQ, Lin S-J, Sun S-W, Cross AH, Armstrong RC, 2005. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage 26, 132–140. [DOI] [PubMed] [Google Scholar]
- Spalletta G, De Rossi P, Piras F, lorio M, Dacquino C, Scanu F, Girardi P, Caltagirone C, Kirkpatrick B, Chiapponi C, 2015. Brain white matter microstructure in deficit and nondeficit subtypes of schizophrenia. Psychiatry Res. Neuroimaging 231, 252–261. 10.1016/j.pscychresns.2014.12.006 [DOI] [PubMed] [Google Scholar]
- Spoletini I, Cherubini A, Banfi G, Rubino IA, Peran P, Caltagirone C, Spalletta G, 2011. Hippocampi, Thalami, and Accumbens Microstructural Damage in Schizophrenia: A Volumetry, Diffusivity, and Neuropsychological Study. Schizophr. Bull 37, 118–130. 10.1093/schbul/sbp058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugranyes G, Kyriakopoulos M, Corrigall R, Taylor E, Frangou S, 2011. Autism Spectrum Disorders and Schizophrenia: Meta-Analysis of the Neural Correlates of Social Cognition. PLoS One 6, e25322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamnes CK, Agartz I, 2016. White matter microstructure in early-onset schizophrenia: a systematic review of diffusion tensor imaging studies. J. Am. Acad. Child Adolesc. Psychiatry 55, 269–279. [DOI] [PubMed] [Google Scholar]
- Wagner G, De la Cruz F, Schachtzabel C, Güllmar D, Schultz CC, Schlösser RG, Bär K-J, Koch K, 2015. Structural and functional dysconnectivity of the fronto-thalamic system in schizophrenia: A DCM-DTI study. Cortex 66, 35–45. [DOI] [PubMed] [Google Scholar]
- Weinstein M, Ben-Sira L, Levy Y, Zachor DA, Itzhak E. Ben, Artzi M, Tarrasch R, Eksteine PM, Hendler T, Bashat D. Ben, 2011. Abnormal white matter integrity in young children with autism. Hum. Brain Mapp. 32, 534–543. 10.1002/hbm.21042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler-Kingshott CAM, Cercignani M, 2009. About “axial” and “radial” diffusivities. Magn. Reson. Med 61, 1255–1260. 10.1002/mrm.21965 [DOI] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE, 2014. Permutation inference for the general linear model. Neuroimage 92, 381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winklewski PJ, Sabisz A, Naumczyk P, Jodzio K, Szurowska E, Szarmach A, 2018. Understanding the Physiopathology Behind Axial and Radial Diffusivity Changes—What Do We Know? . Front. Neurol . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright SN, Hong LE, Winkler AM, Chiappelli J, Nugent K, Muellerklein F, Du X, Rowland LM, Wang DJJ, Kochunov P, 2015. Perfusion Shift from White to Gray Matter May Account for Processing Speed Deficits in Schizophrenia. Hum. Brain Mapp 36, 3793–3804. 10.1002/hbm.22878 [DOI] [PMC free article] [PubMed] [Google Scholar]