Abstract
Background
A question of epidemiological relevance in Chagas disease studies is to understand Trypanosoma cruzi transmission cycles and trace the origins of (re)emerging cases in areas under vector or disease surveillance. Conventional parasitological methods lack sensitivity whereas molecular approaches can fill in this gap, provided that an adequate sample can be collected and processed and a nucleic acid amplification method can be developed and standardized. We developed a duplex qPCR assay for accurate detection and quantification of T. cruzi satellite DNA (satDNA) sequence in samples from domestic and sylvatic mammalian reservoirs. The method incorporates amplification of the gene encoding for the interphotoreceptor retinoid-binding protein (IRBP), highly conserved among mammalian species, as endogenous internal amplification control (eIAC), allowing distinction of false negative PCR findings due to inadequate sample conditions, DNA degradation and/or PCR interfering substances.
Results
The novel TaqMan probe and corresponding primers employed in this study improved the analytical sensitivity of the assay to 0.01 par.eq/ml, greater than that attained by previous assays for Tc I and Tc IV strains. The assay was tested in 152 specimens, 35 from 15 different wild reservoir species and 117 from 7 domestic reservoir species, captured in endemic regions of Argentina, Colombia and Mexico and thus potentially infected with different parasite discrete typing units. The eIACs amplified in all samples from domestic reservoirs from Argentina and Mexico, such as Canis familiaris, Felis catus, Sus scrofa, Ovis aries, Equus caballus, Bos taurus and Capra hircus with quantification cycles (Cq’s) between 23 and 25. Additionally, the eIACs amplified from samples obtained from wild mammals, such as small rodents Akodon toba, Galea leucoblephara, Rattus rattus, the opossums Didelphis virginiana, D. marsupialis and Marmosa murina, the bats Tadarida brasiliensis, Promops nasutus and Desmodus rotundus, as well as in Conepatus chinga, Lagostomus maximus, Leopardus geoffroyi, Lepus europaeus, Mazama gouazoubira and Lycalopex gymnocercus, rendering Cq’s between 24 and 33.
Conclusions
This duplex qPCR assay provides an accurate laboratory tool for screening and quantification of T. cruzi infection in a vast repertoire of domestic and wild mammalian reservoir species, contributing to improve molecular epidemiology studies of T. cruzi transmission cycles.
Keywords: Trypanosoma cruzi, Chagas disease, Mammalian reservoirs, Molecular epidemiology, Internal amplification standard, Multiplex qPCR, Parasite load
Background
Chagas disease, a neglected tropical disease caused by the protozoan parasite Trypanosoma cruzi is endemic in Latin America, where it is mainly transmitted by hematophagous insects belonging to the genera Triatoma, Rhodnius, Pastrongylus and Mepraia. Other transmission routes, such as congenital (from mother to child), oral (by consuming contaminated food) and through blood transfusions and organ transplantation, are also important. Approximately seven million people are estimated to suffer from Chagas disease and hundreds of thousands of infected individuals have migrated to non-endemic countries [1].
The natural cycles of transmission involve sylvatic, domestic and peridomestic habitats. Opossums, armadillos and rodents are major sylvatic reservoir hosts, whereas humans, dogs, cats and commensal (synanthropic) rodents are the main hosts in domestic or peridomestic habitats [2–4]. A major question of epidemiological relevance is whether these types of transmission cycles are connected or independent. Characterizing the level of interconnection/ independence of these transmission cycles is paramount to trace the origins of (re)emerging cases in areas under vector or disease surveillance [5, 6].
Assessing the infection status of potential mammalian reservoirs is essential. Molecular techniques, such as qPCR have much greater sensitivity than conventional parasitological methods [7–11]. However, the diverse composition of biological samples collected from different mammalian reservoir species may affect amplification accuracy, making it difficult to compare prevalence of infection among different species within a same area under study and/or between different geographical regions. Blood samples may contain substances acting as qPCR inhibitors, leading to false negative results and sub-estimated prevalence rates. The quality of the sample may be altered during transportation from the site of blood collection to the molecular biology laboratory and/or during DNA purification. Accordingly, an accurate method must include an internal amplification control. In this context, we aimed to develop a duplex qPCR assay which would allow for simultaneous amplification of a T. cruzi DNA specific target and an endogenous internal control (eIAC) as amplification standard. The design of novel TaqMan probe and primers targeting a satellite DNA (satDNA) sequence allowed for improved analytical sensitivity, beyond that of other previously developed assays based on the same target [12, 13], especially for TcI and TcIV strains [14]. The eIAC was based on a gene fragment encoding the interphotoreceptor retinoid-binding protein (IRBP), which is highly conserved among mammalian species and its usefulness as a DNA integrity control was previously reported in a conventional PCR assay [15]. Once standardized, the duplex assay has been evaluated in a panel of biological samples collected from different sylvatic and domestic mammalian species captured in field studies at endemic areas of Argentina, Colombia and Mexico.
Methods
Mammalian reservoir samples
Analysis of a standard panel of samples
A first evaluation of the duplex T. cruzi satDNA/IRBP qPCR assay (index test) was carried out using archival DNA from blood samples of well-characterized domestic and sylvatic mammalian species previously tested using standardized qPCR procedures (comparator test, [13]) in order to estimate their agreement.
Analysis of field samples
The index method was assayed using DNA extracted from peripheral blood samples preserved in guanidine hydrochloride 6M, EDTA 0.2 M (GE), pH 8.00 (blood:GE proportion of 1:3) and collected from domestic and sylvatic mammalian reservoirs captured in endemic regions from Argentina, Colombia and Mexico.
Argentinean wild and domestic samples were provided by Andrea Gomez-Bravo (Fundación Mundo Sano, Buenos Aires, Argentina) from Añatuya, Santiago del Estero, Argentina. Colombian samples were provided by Felipe Guhl (Universidad de los Andes, Bogotá, Colombia). Mexican samples were collected from mammalian reservoirs captured in an endemic region for Chagas disease in Yucatán, Mexico and kindly provided by Janine M. Ramsey (Centro Regional de Investigación en Salud Pública, Chiapas, Mexico).
DNA extraction methods
DNA was extracted from 300 µl of whole blood/GE samples (blood:GE proportion of 1:3) using phenol-chloroform based purification (for Mexican samples) or the High Pure PCR Template Preparation Kit (Roche Diagnostic Corp., Indiana, USA), following manufacturer instructions (for Argentinean and Colombian samples).
Design of an endogenous internal amplification standard for duplex qPCR
A pair of primers and a TaqMan probe complementary to a highly conserved region within the amplified zone of the highly conserved mammalian IRBP gene were designed. Primer IRBP2 Fw was modified with respect to primer IRBP-CF-FWD reported by Ferreira et al. [15] for molecular diagnosis of leishmaniasis. Primer IRBP3Rv and probe IRBPTq were designed from a consensus sequence obtained after the alignment of IRBP sequences, available from 9 domestic and 8 wild mammalian reservoir species on GenBank (Table 1, Fig. 1).
Table 1.
Primer and probe sequences and concentrations used in duplex TaqMan qPCR assay for detection of T. cruzi DNA in mammalian reservoir species
| Gene target | Primer/probe name | Primer/probe sequence (5’-3’) | Reaction concentration (µM) | Amplicon size (bp) | Source |
|---|---|---|---|---|---|
| Satellite repeat unit from T. cruzi genome | Primer Cruzi 1c | TGAATGGYGGGAGTCAGAG | 0.75 | 98 | Ramírez et al. [14] |
| Primer Cruzi 2c | ATTCCTCCAAGMAGCGGAT | 0.75 | |||
| Probe Cruzi 3 | FAM-CACACACTGGACACCAA-NFQ-MGB | 0.05 | |||
| IRBP gene from mammalian genome | Primer IRBP2 Fw | CCAAYACVACCACTGAGATCTG | 0.60 | 140 | Present study |
| Primer IRBP3 Rv | GCGCATCTGYTTGAGGATGTARG | 0.60 | |||
| Probe IRBP Tq | HEX-TGGTGGTCCTCACCAG-NFQ-MGB | 0.05 |
Fig. 1.
IRBP sequence alignment for different wild and domestic reservoir species. Primer and probe annealing sequences are highlighted in bold letters. Nucleotides that differ from the primer or probe sequences are underlined
Duplex TaqMan qPCR assay
The reaction was performed in a final volume of 20 µl with FastStart Universal Probe Master Mix (Roche Diagnostics, Mannheim, Germany) and 5 µl of DNA, in a Rotor-Gene 3000 (Corbett Life Science, Cambridge, UK) or an ABI 7500 (Applied Biosystems, Foster City, CA) device. For T. cruzi DNA amplification, new primers Cruzi1c, Cruzi2c [14] and probe Cruzi3 were used to enhance sensitivity with respect to a previous satDNA qPCR [12], in particular for Tc I and Tc IV strains. An internal amplification standard was amplified using primers IRBP Fw and Rv and IRBP probe. Their sequences and final concentrations in the qPCR reaction are given in Table 1. Cycling conditions were an initial step of 10 min at 95 °C and 45 cycles at 95 °C for 15 s and 56 °C for 1 min.
Analytical parameters of duplex T. cruzi satDNA/IRBP qPCR assay
The satDNA single qPCR reaction was inclusive for strains belonging to discrete typing units DTUs TcI to TcVI, as previously reported [14]. To assess analytical sensitivity of the duplex format, blood from non-infected dogs was spiked with cultured epimastigotes of CL Brener and Silvio X10 T. cruzi strains (TcVI and TcI, respectively) to a final concentration of 107 parasite equivalents/ml (par.eq/ml) and treated with three volumes of guanidine hidrochloride 6 M-EDTA 0.2 M (pH 8.00) (GE). Next, 10-fold serial dilutions were performed to cover a range between 0.001 to 106 par.eq/ml. DNA of each dilution was purified and amplified in duplicate by duplex qPCR. Theoretical versus measured Cq values were converted to log10 par.eq/ml and plotted for linear regression analysis. Analytical sensitivity was estimated using triplicate dilutions of the above-mentioned spiked samples for both parasites and analytical specificity was estimated using DNA from T. rangeli, Leishmania major, L. donovani and L. amazonensis.
Quality controls of the duplex T. cruzi satDNA/IRBP qPCR assay
Each DNA extraction round included one blood sample from a seronegative dog as a negative extraction control. Each amplification round included two positive DNA controls containing 1 par.eq/ml and 100 par.eq/ml of CL Brener spiked dog samples and one non-template control.
The satDNA/IRBP qPCR results were considered as valid when the Cq of IRBP was within the expected range according to the criteria of Tukey: Cq’s.75th percentile + 1.5 × interquartile distance of median Cq, which would indicate inhibition or material loss in samples from the same experiment with n > 10 [16].
Trypanosoma cruzi DNA quantification of satDNA/IRBP qPCR positive samples
A panel of 22 satDNA/IRBP qPCR-positive samples was quantified for estimation of parasite load. For this, a standard quantification curve was constructed. Given that satDNA qPCR positive samples were genotyped as TcI [17, 18], DNA was obtained from non-infected dog blood spiked with 107 par.eq/ml of Silvio X10 clone (TcI) cultured parasites, and serially diluted in DNA obtained from blood collected from non-infected dogs aiming to cover a range of standards containing 10−1 to 105 par.eq/ml.
Data analysis
To compare the agreement between the index duplex qPCR assay with comparator standardized qPCR procedures in a panel of characterized samples, inter-observer kappa coefficients were calculated using GraphPad Software online statistical calculators (http://www.graphpad.com/quickcalcs/kappa1.cfm). Kappa values < 0.01 indicate no agreement, those between 0.1 and 0.4 indicate weak agreement, those between 0.41 and 0.60 indicate clear agreement, those between 0.61 and 0.80 indicate strong agreement, and those between 0.81 and 1.00 indicate nearly perfect agreement.
Results
Design and analytical performance of duplex T.cruzi satDNA/IRBP qPCR assay
IRBP primer and probe sequences were designed from a consensus IRBP sequence obtained after alignment of orthologous sequences from different mammalian species, available in the GenBank database (Table 1, Fig. 1). The reportable range of T. cruzi satDNA/IRBP qPCR assay was assessed in single and duplex formats (Additional file 1: Figure S1). No significant differences between single T. cruzi satDNA qPCR and duplex T. cruzi satDNA /IRBP qPCR were observed when comparing the Cq values obtained for different T. cruzi DNA concentrations ranging between 10 and 105 par.eq/ml (Additional file 1: Figure S1).
The duplex T. cruzi sat DNA/IRBP qPCR analytical sensitivity was evaluated in dog blood samples spiked with cultured parasites from the Silvio X10 (TcI) and CL Brener (TcVI) stocks. The reportable range was from 0.1 to 105 par.eq./ml and from 1 to 104 par.eq./ml for CL Brener and Silvio X10 stocks, respectively. Analytical sensitivities were 0.01 par.eq/ml for both T. cruzi stocks.
The assay amplified exclusively T. cruzi DNA samples; in contrast, it did not amplify DNA from different Leishmania species and T. rangeli (Table 2). Moreover, we compared the agreement between the duplex T. cruzi sat DNA/IRBP qPCR assay with previously reported PCR procedures in a panel of well-characterized blood samples from domestic and sylvatic mammal reservoirs (Table 3). An almost perfect agreement (% of agreement: 97.83%; Cohen’s k: 0.95) was obtained.
Table 2.
Analytical parameters of the duplex T.cruzi satDNA/IRBP qPCR assay
| Analytical parameter | satDNA/IRBP qPCR |
|---|---|
| Analytical sensitivity, par.eq./ml | |
| TcI (Silvio X10) | 0.01 |
| TcVI (CL Brener) | 0.01 |
| Inclusivity (fg/µl) | |
| Tc I (Silvio X10) | 0.25 |
| Tc II (Y) | 0.125 |
| Tc III (M5631 cl5) | 0.0625 |
| Tc IV (4167) | 0.0625 |
| Tc V (MnCl2) | 0.0625 |
| Tc VI (CL Brener) | 0.0625 |
| Exclusivity (non detectable qPCR) pg/µl | |
| T. rangeli | 100 |
| L. major | 1000 |
| L. donovani | 1000 |
| L. amazonensis | 100 |
| Reportable range | |
| Cl Brener |
0.1–105 par.eq/ml; y = − 2.48X + 22.69; R2 = 0.99 |
| Silvio X10 |
1–104 par.eq/ml; y = − 2.64 + 27.28; R2 = 0.98 |
Table 3.
Comparison of T. cruzi DNA detection by means of T. cruzi satDNA/IRBP qPCR assay (index test) and standardized qPCR (comparator test)
| Panel of well-characterized samples | n | Index test | |
|---|---|---|---|
| Positive | Negative | ||
| Comparator qPCR test-positive | |||
| Canis familiaris | 2 | 2 | 0 |
| Felis catus | 5 | 5 | 0 |
| Ovis aries | 3 | 3 | 0 |
| Didelphis sp. | 6 | 6 | 0 |
| Total | 16 | 16 | 0 |
| Comparator qPCR test-negative | |||
| Canis familiaris | 9 | 0 | 9 |
| Felis catus | 1 | 0 | 1 |
| Ovis aries | 3 | 1 | 2 |
| Capra hircus | 9 | 0 | 9 |
| Promops nasutus | 6 | 0 | 6 |
| Conepatus chinga | 1 | 0 | 1 |
| Lagostomus maximus | 1 | 0 | 1 |
| Total | 30 | 1 | 29 |
Note: Comparator qPCR test was reported in Ramirez et al. [13]
Evaluation of blood samples from wild and domestic reservoirs
Blood sample panels from different mammalian species captured in three endemic regions for Chagas disease (Santiago del Estero, Argentina; Maní, Colombia; and Yucatán, México) were tested for simultaneous detection of T. cruzi infection and IRBP amplification (Table 4).
Table 4.
IRBP (eIAC) gene amplification in duplex satDNA/IRBP qPCR assay for T.cruzi DNA detection in samples from reservoir species
| n | satDNA/IRBP qPCR | ||||
|---|---|---|---|---|---|
| IRBP control | T cruzi positive/total | ||||
| Valid samples | Mean Cq | SD | |||
| Wild reservoirs (n = 35) | |||||
| Small rodents | |||||
| Akodon toba, Argentina | 5 | 5 | 26.29 | 2.22 | 0/5 |
| Galea leucoblephara, Argentina | 1 | 1 | 26.88 | nd | 0/1 |
| Rattus rattus, Argentina | 1 | 1 | 24.72 | nd | 0/1 |
| Marsupials | |||||
| Didelphis virginiana, México | 4 | 4 | 24.29 | 1.27 | 0/4 |
| Didelphis marsupialis, Colombia | 3 | 3 | 26.28 | 0.27 | 3/3 |
| Marmosa murina, Colombia | 3 | 3 | 29.73 | 4.91 | 3/3 |
| Bats | |||||
| Tadarida brasiliensis, Argentina | 1 | 1 | 24.48 | nd | 0/1 |
| Promops nasutus, Argentina | 4 | 4 | 33.60 | 1.97 | 0/4 |
| Desmodus rotundus (vampire), Argentina | 2b | 1 | 27.90 | nd | 0/1 |
| Other mammals | |||||
| Conepatus chinga (skunk), Argentina | 3 | 3 | 24.86 | 1.65 | 0/3 |
| Lagostomus maximus (viscacha), Argentina | 3 | 3 | 28.38 | 0.20 | 0/3 |
| Leopardus geoffroyi (wildcat), Argentina | 1 | 1 | 22.71 | nd | 0/1 |
| Lepus europaeus (hare), Argentina | 1 | 1 | 27.88 | nd | 0/1 |
| Mazama gouazoubira (brown brocket deer), Argentina | 1 | 1 | 26.78 | nd | 0/1 |
| Lycalopex gymnocercus (Pampas fox), Argentina | 2 | 2 | 25.08 | 0.60 | 0/2 |
| Domestic reservoirs (n =117) | |||||
| Bos taurus (cow), Argentina | 12 | 12 | 24.84 | 0.31 | 0/12 |
| Canis lupus familiaris (dog), Argentina | 27 | 27 | 24.52 | 1.10 | 0/27 |
| Canis lupus familiaris (dog), Méxicoa | 4 | 4 | 28.85 | 4.50 | 4/4 |
| Capra hircus (goat), Argentina | 24 | 24 | 23.75 | 1.13 | 0/24 |
| Equus caballus (horse), Argentina | 2 | 2 | 23.40 | 0.49 | 0/2 |
| Felis catus (cat), Argentina | 4 | 4 | 26.21 | 0.37 | 0/4 |
| Felis catus (cat), México | 10 | 10 | 26.45 | 0.61 | 10/10 |
| Ovis aries (sheep), Argentina | 29 | 29 | 25.16 | 0.92 | 0/29 |
| Ovis aries (sheep), Méxicoa | 2 | 2 | 32.67 | 1.51 | 2/2 |
| Sus scrofa domesticus (pig), Argentina | 3 | 3 | 22.72 | 0.98 | 0/3 |
a Phenol chloroform DNA extraction
b One sample was IRBP negative
Note: IRBP amplification and detection of T.cruzi positive cases are shown
Abbreviations: SD, standard deviation; Cq, quantification cycle; nd, not determined
The eIAC amplified in all samples from domestic reservoirs from Argentina and Mexico, such as Canis familiaris, Felis catus, Sus scrofa, Ovis aries, Equus caballus, Bos taurus and Capra hircus with Cq’s between 23 and 25. It also amplified samples from wild mammals from Argentina, Colombia and Mexico, such as small rodents Akodon toba, Galea leucoblephara, Rattus rattus, the opossums Didelphis virginiana, D. marsupialis and Marmosa murina, the bats, Tadarida brasiliensis, Promops nasutus and Desmodus rotundus, as well as in Conepatus chinga (skunk), Lagostomus maximus (viscacha), Leopardus geoffroyi (wildcat), Lepus europaeus (hare), Mazama gouazoubira (brown brocket deer) and Lycalopex gymnocercus (Pampas fox) rendering Cq’s between 24 and 33 (Table 4). The Tukey’s criterion [16] was used to detect samples with outlier Cq values for the eIAC, which would indicate PCR inhibition or material loss in samples from the same experiment with n > 10. Only one sample of Desmodus rotundus was considered invalid.
Samples from Colombian Didelphis marsupials, Marmosa murina, Mexican dogs, cats and sheep that showed amplification of T. cruzi satDNA were considered positive (Table 4, [14]).
Quantification of parasite load
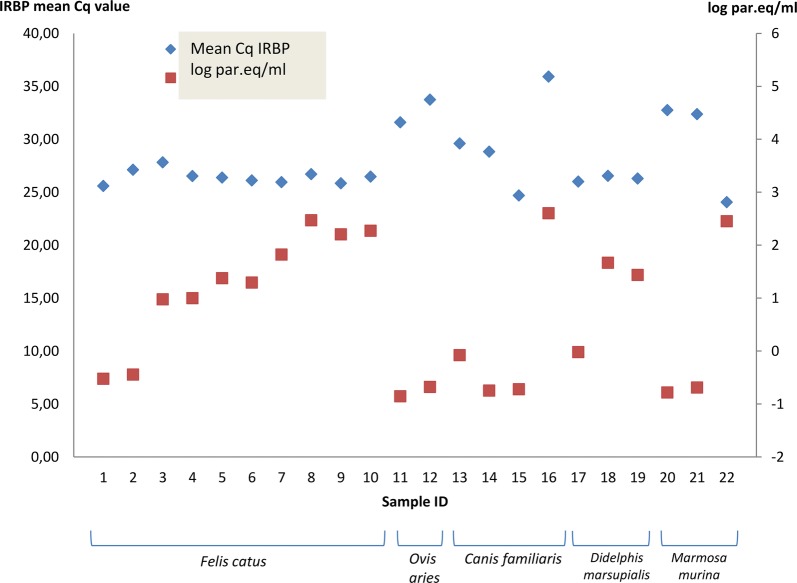
TaqMan qPCR allows for quantification of parasitic burden in infected samples. Parasite loads were quantitated in infected sheep, dog and cat samples, as well as in Didelphis marsupialis and Marmosa murina (n = 22) (Fig. 2, Table 5). Except for both Ovis aries specimens, individuals from the other species presented heterogeneity in their parasite loads, ranging from 0.14 to 4.02 102 par.eq/ml.
Fig. 2.
Quantification of parasite loads by means of duplex T. cruzi satDNA/IRBP qPCR in infected specimens. Quantification is expressed in parasite equivalents/ml of blood
Table 5.
Parasite loads in satDNA/IRBP qPCR-positive samples from mammalian reservoir hosts
| Sample ID | Mammalian species | Duplex SatDNA/IRBP qPCR | DTU | |||
|---|---|---|---|---|---|---|
| Mean Cq IRBP | Mean Cq cruzi | par.eq/ml | log par.eq/ml | |||
| 1 | Felis catus | 25.59 | 28.70 | 0.30 | − 0.522878745 | TcI |
| 2 | Felis catus | 27.13 | 28.52 | 0.36 | − 0.443697499 | TcI |
| 3 | Felis catus | 27.82 | 24.78 | 9.49 | 0.977266212 | TcI |
| 4 | Felis catus | 26.53 | 24.73 | 9.94 | 0.997386384 | TcI |
| 5 | Felis catus | 26.39 | 23.75 | 23.72 | 1.375114685 | TcI |
| 6 | Felis catus | 26.11 | 23.96 | 19.67 | 1.29380436 | TcI |
| 7 | Felis catus | 25.96 | 25.58 | 66.40 | 1.822168079 | TcI |
| 8 | Felis catus | 26.70 | 20.89 | 296.45 | 2.471951455 | TcI |
| 9 | Felis catus | 25.84 | 21.58 | 160.17 | 2.204581176 | TcI |
| 10 | Felis catus | 26.48 | 21.40 | 187.50 | 2.273001272 | TcI |
| 11 | Ovis ariesa | 31.61 | 29.45 | 0.14 | − 0.853871964 | TcI/TcVI |
| 12 | Ovis ariesa | 33.74 | 29.13 | 0.21 | − 0.677780705 | TcI/TcVI |
| 13 | Canis familiarisa | 29.60 | 27.47 | 0.84 | − 0.075720714 | TcVI |
| 14 | Canis familiarisa | 28.83 | 29.31 | 0.18 | − 0.744727495 | TcI/TcVI |
| 15 | Canis familiarisa | 24.69 | 29.27 | 0.19 | − 0.721246399 | TcI/TcVI |
| 16 | Canis familiaris | 35.92 | 18.23 | 402.73 | 2.60500859 | TcI |
| 17 | Didelphis marsupialis | 26.01 | 26.80 | 0.96 | − 0.017728767 | nd |
| 18 | Didelphis marsupialis | 26.54 | 21.29 | 46.41 | 1.666564777 | nd |
| 19 | Didelphis marsupialis | 26.30 | 22.04 | 27.39 | 1.437592032 | nd |
| 20 | Marmosa murina | 32.76 | 29.28 | 0.17 | − 0.782516056 | nd |
| 21 | Marmosa murina | 32.37 | 29.17 | 0.21 | − 0.688246139 | nd |
| 22 | Marmosa murina | 24.07 | 18.72 | 284.38 | 2.453891414 | nd |
a DNA extracted with phenol-chloroform
Abbreviation: nd, not determined
Discussion
Trypanosoma cruzi transmission cycles in sylvatic and domestic mammals have been studied in different eco-epidemiological settings in endemic regions [5]. Initially, microscopic analysis, blood culture or xenodiagnosis were used for detection and isolation of T. cruzi strains from mammalian reservoirs [19, 20]. Later, studies developed in-house conventional amplification procedures for direct detection and genotyping of T. cruzi in domestic and wildlife reservoirs [6–9, 11, 21, 22] whereas in dogs qPCR assays were carried out [23, 24]. However, methods lacking internal amplification controls could not discriminate between absence of infection and inadequate samples. Here, we have developed a TaqMan-based duplex qPCR procedure useful for detection and quantification of parasite loads in biological samples from wild and domestic mammalian reservoirs, coupled with an IRBP-DNA-based internal amplification standard. This enables a distinction between true negative samples and false negative samples due to the presence of PCR interfering substances and/or degradation of DNA.
The assay was evaluated using 35 blood samples from 15 different wild reservoir species and 117 samples from 7 domestic mammalian species. The IRBP-DNA-based integrity control performed adequately in all above mentioned specimens except in one DNA sample from Desmodus rotundus. The IRBP Cq-values were variable in different species, in particular in wild reservoirs, which may arise from different concentrations of nucleated cells in the blood of the different species [25, 26] and/or a different yield in blood-based DNA. In the case of column-based DNA extraction procedures, the pigs and wildcats presented the lowest IRBP Cq-values (22.72 and 22.71, respectively) whereas the bat (Promops nasutus) showed the highest Cq-values (32.86). Nevertheless, in some cases, samples of the same species extracted using different DNA purification methods yielded different Cq-values for IRBP-DNA. In particular, phenol-chloroform-extracted DNA samples from Mexico yielded higher between-sample mean Cq- and SD-values than those obtained using column-based DNA extraction (Table 4). Previous comparison of DNA extraction methods from blood samples showed that those based in organic solvents rendered a higher degree of PCR inhibition [27, 28]. Thus, in comparative studies of parasitic burden among individuals from the same species and/or among other reservoir species, the same DNA extraction procedure should be used and the acceptable range of IRBP Cq-values should be estimated for each round and method of DNA extraction in order to detect outlier Cq-values that allow distinction of false negative samples [16]. We have explored the capacity of the IRBP internal control to detect DNA degradation by performing the following experiments: incubation of DNA samples for 48 hours at room temperature and exposition of DNA samples to UV light. In both cases the Cq-values of IRBP increased compared to outlier values (unpublished results).
On the other hand, high parasite loads may inhibit amplification of IRBP. This will not be a problem for qualitative detection of T. cruzi infection, but if accurate parasite load quantifications are required, it is recommended to dilute the clinical sample and repeat the qPCR assay to achieve IRBP Cq-values within the acceptable range.
The high analytical sensitivity for satDNA amplification for both CL Brener and Silvio X10 stocks obtained with our assay had not been previously achieved, especially for TcI and TcIV strains [12, 13]. Probably, the exhaustive alignment for satDNA sequences performed in this study, including a higher number of strains for each T. cruzi stock, contributed in this regard [14]. Indeed, to our knowledge, this is the first time that this set of satDNA-based PCR primers and probe have been used in biological specimens.
Conclusions
Our results indicate this novel assay is useful for T. cruzi infection screening in samples from different mammalian species, either in prospective studies or employing archival DNA. Sample quality can be inferred by means of eIAC amplification. Moreover, the quantification of parasite load may be indicative of the severity and the stage of infection in these reservoir species and their “transmission potential” in their habitats, thus contributing to epidemiological knowledge of factors involved in T. cruzi transmission cycles.
Supplementary information
Additional file 1: Figure S1. Comparison of single and duplex T. cruzi satDNA qPCR reportable ranges for detection and quantification of T. cruzi DNA.
Acknowledgments
We are grateful to María de los Angeles Curto for culturing parasites to construct the quantification curve and to Paula Beati for her assistance in editing figures.
Abbreviations
- qPCR
real time polymerase chain reaction
- satDNA
satellite DNA
- IRBP
interphotoreceptor retinoid-binding protein
- eIAC
endogenous internal amplification control
- Cq
quantification cycle
- par.eq/ml
parasite equivalents per milliliter
- UV light
ultraviolet light
- SD
standard deviation
- DTU
discrete typing unit
- GE
guanidine hydrochloride/EDTA
Authors’ contributions
DW, JCR and AGS conceived and designed the qPCR assay. DW performed DNA extraction and qPCR experiments. AGB and CC collected biological samples from Argentina, APM and JR collected samples from Mexico and FG from Colombia. CC, APM and FG performed identification of DTUs. DW and AGS wrote the first version of the manuscript and all authors revised it. AGS, MA and FG conceived the study. AGS and MA provided financial support. AGS supervised molecular diagnosis related work. All authors read and approved the final manuscript.
Funding
This study was possible thanks to the financial support from ANPCyT through PICT 2014-0274 to AGS and funds from the Fundación Mundo Sano, Argentina.
Availability of data and materials
Data supporting the conclusions of this article are included within the article and its additional file. All raw data are available upon request to the corresponding authors or at http://ingebi-conicet.gov.ar/biologia-molecular-de-la-enfermedad-de-chagas/.
Ethics approval and consent to participate
All mammalian samples were collected according to the national or provincial guidelines of each State.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Diana P. Wehrendt, Email: dwehrendt@gmail.com
Andrea Gómez-Bravo, Email: agomez@mundosano.org.
Juan C. Ramirez, Email: juankrg@yahoo.es
Carolina Cura, Email: cura.carolina@gmail.com.
Angélica Pech-May, Email: apechmay@gmail.com.
Janine M. Ramsey, Email: janineramseyw@gmail.com
Marcelo Abril, Email: mabril@mundosano.org.
Felipe Guhl, Email: fguhl@uniandes.edu.co.
Alejandro G. Schijman, Email: schijman@dna.uba.ar, Email: aleschijman@gmail.com
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13071-019-3817-9.
References
- 1.WHO Chagas disease in Latin America: an epidemiological update based on 2010 estimates. Wkly Epidemiol Rec. 2010;2015(90):33–44. [PubMed] [Google Scholar]
- 2.Jansen AM, Xavier SC, Roque AL. The multiple and complex and changeable scenarios of the Trypanosoma cruzi transmission cycle in the sylvatic environment. Acta Trop. 2015;151:1–15. doi: 10.1016/j.actatropica.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 3.Minter DM. Epidemiology of Chagasʼ disease. Trans R Soc Trop Med Hyg. 1976;70:124. doi: 10.1016/0035-9203(76)90170-X. [DOI] [PubMed] [Google Scholar]
- 4.Noireau F, Diosque P, Jansen AM. Trypanosoma cruzi: adaptation to its vectors and its hosts. Vet Res. 2009;40:26. doi: 10.1051/vetres/2009009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miles MA, Llewellyn MS, Lewis MD, Yeo M, Baleela R, Fitzpatrick S, et al. The molecular epidemiology and phylogeography of Trypanosoma cruzi and parallel research on Leishmania: looking back and to the future. Parasitology. 2009;136:1509–1528. doi: 10.1017/S0031182009990977. [DOI] [PubMed] [Google Scholar]
- 6.Argibay HD, Orozco MM, Cardinal MV, Rinas MA, Arnaiz M, Mena Segura C, et al. First finding of Trypanosoma cruzi II in vampire bats from a district free of domestic vector-borne transmission in northeastern Argentina. Parasitology. 2016;143:1358–1368. doi: 10.1017/S0031182016000925. [DOI] [PubMed] [Google Scholar]
- 7.Orozco MM, Enriquez GF, Alvarado-Otegui JA, Cardinal MV, Schijman AG, Kitron U, et al. New sylvatic hosts of Trypanosoma cruzi and their reservoir competence in the humid Chaco of Argentina: a longitudinal study. Am J Trop Med Hyg. 2013;88:872–882. doi: 10.4269/ajtmh.12-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Enriquez GF, Cardinal MV, Orozco MM, Schijman AG, Gürtler RE. Detection of Trypanosoma cruzi infection in naturally infected dogs and cats using serological, parasitological and molecular methods. Acta Trop. 2013;126:211–217. doi: 10.1016/j.actatropica.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monje-Rumi MM, Brandán CP, Ragone PG, Tomasini N, Lauthier JJ, Alberti DʼAmato AM, et al. Trypanosoma cruzi diversity in the Gran Chaco: mixed infections and differential host distribution of TcV and TcVI. Infect Genet Evol. 2015;29:53–59. doi: 10.1016/j.meegid.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Falla A, Herrera C, Fajardo A, Montilla M, Vallejo GA, Guhl F. Haplotype identification within Trypanosoma cruzi I in Colombian isolates from several reservoirs, vectors and humans. Acta Trop. 2009;110:15–21. doi: 10.1016/j.actatropica.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Cardinal MV, Lauricella MA, Ceballos LA, Lanati L, Marcet PL, Levin MJ, et al. Molecular epidemiology of domestic and sylvatic Trypanosoma cruzi infection in rural northwestern Argentina. Int J Parasitol. 2008;38:1533–1543. doi: 10.1016/j.ijpara.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duffy T, Cura CI, Ramirez JC, Abate T, Cayo NM, Parrado R, et al. Analytical performance of a multiplex real-time PCR assay using TaqMan probes for quantification of Trypanosoma cruzi satellite DNA in blood samples. PLoS Negl Trop Dis. 2013;7:e2000. doi: 10.1371/journal.pntd.0002000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramírez JC, Cura CI, da Cruz Moreira O, Lages-Silva E, Juiz N, Velázquez E, et al. Analytical validation of quantitative real-time PCR methods for quantification of Trypanosoma cruzi DNA in blood samples from Chagas disease patients. J Mol Diagn. 2015;17:605–615. doi: 10.1016/j.jmoldx.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramírez JC, Torres C, Curto MLA, Schijman AG. New insights into Trypanosoma cruzi evolution, genotyping and molecular diagnostics from satellite DNA sequence analysis. PLoS Negl Trop Dis. 2017;11:e0006139. doi: 10.1371/journal.pntd.0006139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferreira EC, Gontijo CM, Cruz I, Melo MN, Silva AM. Alternative PCR protocol using a single primer set for assessing DNA quality in several tissues from a large variety of mammalian species living in areas endemic for leishmaniasis. Mem Inst Oswaldo Cruz. 2010;105:895–898. doi: 10.1590/S0074-02762010000700009. [DOI] [PubMed] [Google Scholar]
- 16.Burns MJ, Nixon GJ, Foy CA, Harris N. Standardisation of data from real-time quantitative PCR methods - evaluation of outliers and comparison of calibration curves. BMC Biotechnol. 2005;5:31. doi: 10.1186/1472-6750-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cura CI, Duffy T, Lucero RH, Bisio M, Péneau J, Jimenez-Coello M, et al. Multiplex real-time PCR assay using TaqMan probes for the identification of Trypanosoma cruzi DTUs in biological and clinical samples. PLoS Negl Trop Dis. 2015;9:e0003765. doi: 10.1371/journal.pntd.0003765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.López-Cancino SA, Tun-Ku E, De la Cruz-Felix HK, Ibarra-Cerdeña CN, Izeta-Alberdi A, Pech-May A, et al. Landscape ecology of Trypanosoma cruzi in the southern Yucatan Peninsula. Acta Trop. 2015;151:58–72. doi: 10.1016/j.actatropica.2015.07.021. [DOI] [PubMed] [Google Scholar]
- 19.Yaeger RG. The prevalence of Trypanosoma cruzi infection in armadillos collected at a site near New Orleans, Louisiana. Am J Trop Med Hyg. 1988;38:323–326. doi: 10.4269/ajtmh.1988.38.323. [DOI] [PubMed] [Google Scholar]
- 20.Barr SC, Brown CC, Dennis VA, Klei TR. The lesions and prevalence of Trypanosoma cruzi in opossums and armadillos from southern Louisiana. J Parasitol. 1991;77:624–627. doi: 10.2307/3283170. [DOI] [PubMed] [Google Scholar]
- 21.Martínez MF, Kowalewski MM, Salomón OD, Schijman AG. Molecular characterization of trypanosomatid infections in wild howler monkeys (Alouatta caraya) in northeastern Argentina. Int J Parasitol Parasites Wildl. 2016;5:198–206. doi: 10.1016/j.ijppaw.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curtis-Robles R, Lewis BC, Hamer SA. High Trypanosoma cruzi infection prevalence associated with minimal cardiac pathology among wild carnivores in central Texas. Int J Parasitol Parasites Wildl. 2016;5:117–123. doi: 10.1016/j.ijppaw.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaimes-Dueñez J, Triana-Chávez O, Cantillo-Barraza O, Hernández C, Ramírez JD, Góngora-Orjuela A. Molecular and serological detection of Trypanosoma cruzi in dogs (Canis lupus familiaris) suggests potential transmission risk in areas of recent acute Chagas disease outbreaks in Colombia. Prev Vet Med. 2017;141:1–6. doi: 10.1016/j.prevetmed.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 24.Tahir D, Davoust B, Heu K, Lamour T, Demar M, Marié JL, et al. Molecular and serological investigation of Trypanosoma cruzi infection in dogs in French Guiana. Vet Parasitol Reg Stud Reports. 2017;12:106–109. doi: 10.1016/j.vprsr.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Weiss DJ, Wardrop KJ. Schalm’s Veterinary hematology. 6. Hoboken: Wiley-Blackwell; 2010. [Google Scholar]
- 26.Latimer KS. Duncan and Prasse’s veterinary laboratory medicine: clinical pathology. 5. Hoboken: Wiley-Blackwell; 2011. [Google Scholar]
- 27.Marcet PL, Duffy T, Cardinal MV, Burgos JM, Lauricella MA, Levin MJ, et al. PCR-based screening and lineage identification of Trypanosoma cruzi directly from faecal samples of triatomine bugs from northwestern Argentina. Parasitology. 2006;132:57–65. doi: 10.1017/S0031182005008772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duffy T, Bisio M, Altcheh J, Burgos JM, Diez M, Levin MJ, et al. Accurate real-time PCR strategy for monitoring bloodstream parasitic loads in Chagas disease patients. PLoS Negl Trop Dis. 2009;3:e41. doi: 10.1371/journal.pntd.0000419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Comparison of single and duplex T. cruzi satDNA qPCR reportable ranges for detection and quantification of T. cruzi DNA.
Data Availability Statement
Data supporting the conclusions of this article are included within the article and its additional file. All raw data are available upon request to the corresponding authors or at http://ingebi-conicet.gov.ar/biologia-molecular-de-la-enfermedad-de-chagas/.