Abstract
Cancer cells with strong immunogenicity are susceptible for elimination by cancer immunoediting, while the subpopulations with weak immunogenicity survive. As a result, a subset of cancer cells evade the immune attack and evolve into overt clinical lesions. During cancer evolution, it has been well established that multiple alterations such as the dysfunction of antigen presentation machinery and the upregulation of immunosuppressive signals (e.g. PD-L1) play important roles in immune escape. Recently, promoter hypermethylation of neoantigen genes has been proposed to be a vital mechanism of immunoediting. This epigenetically mediated immune evasion enriches the mechanisms of carcinogenesis.
Keywords: Immunoediting, Neoantigens, Immune evasion, Hypermethylation, Epigenetics
Neoantigens arise from somatic mutations and are exclusively expressed in cancer cells. These tumor-associated antigens are the ideal targets for immune recognition and attack. Derived by immune pressures, cancer cells down-regulate the recognizable targets on their surfaces and evolve into weakly immunogenic subclones [1]. It is generally believed that the loss of complex formation between neopeptide and major histocompatibility complex (MHC) in cancer cells is responsible for the acquired dysfunction of antigen processing and presentation [2].
Recently, Rosenthal et al. found that the hypermethylation of the promoter of neoantigen genes participated in the decreased cancer immunogenicity [3]. In this study, Rosenthal et al. analyzed immune infiltration statuses of untreated non-small cell lung cancer (NSCLC) patients by RNA-sequencing and tumor infiltrating lymphocyte (TIL) histopathology estimates [3]. The study showed that just 33% clonal neoantigens were ubiquitously expressed in every region of a given tumor [3]. Further investigation revealed that the proportion of ubiquitously expressed clonal neoantigens was significantly decreased in tumors with abundant TILs compared to tumors with scarce TILs (41% vs. 29%, P = 0.01) [3]. At the transcription level, the researchers observed immune pressure-caused neoantigen depletions [3]. Using the multi-region reduced representation bisulfite sequencing, it was detected that the genes carrying neoantigenic mutations harbored 11.4-fold increase in hypermethylation of promoters when compared to other genes (P = 0.00016) [3]. To verify whether this increased hypermethylation was neoantigen-specific or not, the researchers compare the methylation statuses between neoantigens and corresponding wild type genes. The results indicated that these non-expressed neoantigens were more likely to possess increased promoter methylation (odds ratio = 2.33, P = 0.045) [3].
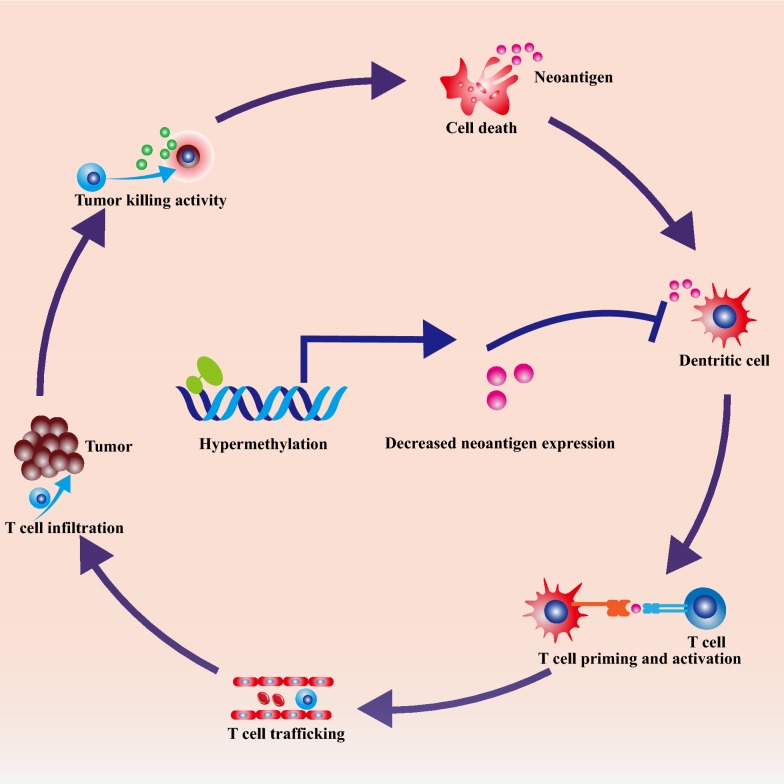
These findings demonstrated that the neoantigen silencing was the result of immune pressures via promoter hypermethylation. The loss of neoantigens is a core event of immunoediting and immune evasion. Abundant neoantigens released from cancer cells initiate robust anti-cancer immune responses [4]. Then, professional antigen presentation cells (APCs) take in and process these neoantigens [4]. Subsequently, in peripheral lymphoid organs, the naïve T lymphocytes are primed and activated by APCs [4]. These activated T cells could migrate and infiltrate into tumors. Eventually, TILs recognize and kill cancer cells [4]. As a result, the release of more neoantigens propagate the anti-cancer immune response [4]. It is well accepted that cancer cells can adopt multiple manners to counteract immune clearance such as secreting anti-inflammation cytokines, upregulating immune checkpoint signals, counter-attacking TILs via increasing Fas ligand (Fas-L) expression, and disabling antigen presentation machinery (Fig. 1) [5, 6]. As the hallmark of cancer cells, neoantigens are generated as the by-products of accumulated somatic mutations [7]. Theoretically, tumor-associated neoantigens are ideal targets for immunotherapies with chimeric antigen receptor T cells (CAR-T) and bi-specific antibodies [8, 9], though in reality, resistance to these cancer neoantigen-targeted immunotherapies still remains a major challenge [10]. The results of Rosenthal et al. provide a novel perspective to the understanding of carcinogenesis and cancer evolution under immune pressure. Moreover, this study suggests that combination of hypomethylating agents with immunotherapy might offer double attack on neoantigen-rich cancers.
Fig. 1.
Promoter hypermethylation-mediated neoantigen downregulation leads to evasion of cancer immune response. Release of abundant neoantigens initiate anti-cancer immune response. Then, professional antigen presentation cells (APCs) take in and process these neoantigens. Subsequently, in peripheral lymphoid organs, the naïve T lymphocytes are primed and activated by APCs. These activated T cells migrate and infiltrate into tumors (TILs). These TILs recognize and destroy cancer cells. As a result, more neoantigens propagate the anti-cancer immune response. Under these immune pressure, cancer cells downregulate neoantigen expression by promoter hypermethylation and evolve into weakly immunogenic subclones
Acknowledgements
We thank Dr. Shuang Qin and Dr. Shengnan Yu of Tongji Hospital for helpful discussion and language editing assistance.
Abbreviations
- MHC
major histocompatibility complex
- NSCLC
non-small cell lung cancer
- TIL
tumor infiltrating lymphocyte
- APC
antigen presentation cell
- Fas-L
Fas ligand
- CAR-T
chimeric antigen receptor T cell
Authors’ contributions
MY, BD, QC, and KW contributed to drafting and revising the article and agree to be accountable for all aspects of the work. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No’s. 81874120, 81572608, 81672984), Wuhan Science and Technology Bureau (No. 2017060201010170), Natural Science Foundation of Henan (No. 162300410266).
Availability of data and materials
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
This article is a comment on 10.1038/s41586-019-1032-7
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ming Yi, Email: 1978135000@qq.com.
Bing Dong, Email: dongbing2015@126.com.
Qian Chu, Email: qianchu@tjh.tjmu.edu.cn.
Kongming Wu, Email: kmwu2005@yahoo.com.
References
- 1.Yamamoto TN, Kishton RJ, Restifo NP. Developing neoantigen-targeted T cell-based treatments for solid tumors. Nat Med. 2019 doi: 10.1038/s41591-019-0596-y. [DOI] [PubMed] [Google Scholar]
- 2.Yi M, Qin S, Zhao W, et al. The role of neoantigen in immune checkpoint blockade therapy. Exp Hematol Oncol. 2018;7:28. doi: 10.1186/s40164-018-0120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenthal R, Cadieux EL, Salgado R, et al. Neoantigen-directed immune escape in lung cancer evolution. Nature. 2019;567:479–485. doi: 10.1038/s41586-019-1032-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 5.Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321–330. doi: 10.1038/nature21349. [DOI] [PubMed] [Google Scholar]
- 6.Yi M, Jiao D, Xu H, et al. Biomarkers for predicting efficacy of PD-1/PD-L1 inhibitors. Mol Cancer. 2018;17:129. doi: 10.1186/s12943-018-0864-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bobisse S, Foukas PG, Coukos G, Harari A. Neoantigen-based cancer immunotherapy. Ann Transl Med. 2016;4:262. doi: 10.21037/atm.2016.06.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu S, Li A, Liu Q, et al. Chimeric antigen receptor T cells: a novel therapy for solid tumors. J Hematol Oncol. 2017;10:78. doi: 10.1186/s13045-017-0444-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu S, Liu Q, Han X, et al. Development and clinical application of anti-HER2 monoclonal and bispecific antibodies for cancer treatment. Exp Hematol Oncol. 2017;6:31. doi: 10.1186/s40164-017-0091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Majzner RG, Mackall CL. Clinical lessons learned from the first leg of the CAR T cell journey. Nat Med. 2019;25:1341–1355. doi: 10.1038/s41591-019-0564-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.