Abstract
Background:
Endoscopic endonasal transsphenoidal surgery (EES) is the gold standard for pituitary adenoma (PA) resection. The sphenoid sinus (SS), a highly variable anatomic structure, is located in the center of the cranial base. It has previously been reported that poor pneumatization of the lateral recess of the SS (LRSS) increases the difficulty level of the surgery and the risk of neural and vascular injury. However, to date no studies have evaluated the association between LRSS volume and PAs removal rate by EES.
Methods:
The present study analyzed 23 consecutive patients with new-onset PAs categorized as Knosp Grades 3 and 4 who underwent EES. A retrospective radiographic analysis was conducted on patients undergoing magnetic resonance imaging and high-resolution computed tomography scans.
Results:
Among PA cases categorized as Knosp 3 and 4, no significant association was found between the whole tumor’s resection rate and LRSS volume (R = 0.08, P = 0.70). However, a significant association was found between cavernous sinus (CS) tumors’ removal rate and LRSS volume (R = 0.52, P = 0.011). The same results were achieved in PAs with a Knosp Grade 4, with a stronger correlation (R = 0.60, P = 0.014).
Conclusion:
The development of LRSS pneumatization affects the removal rate of CS tumors in PAs. Preoperative analysis of LRSS development should be considered when planning EES against PA with CS invasion.
Keywords: Cavernous sinus, Endoscopic endonasal transsphenoidal surgery, Lateral recess of the sphenoid sinus, Pituitary adenoma

INTRODUCTION
Pituitary gland and sellar region tumors account for approximately 15% of all brain tumors, with pituitary adenomas (PAs) being the most common. PAs are benign neuroendocrine neoplasms confined to the sella.[21] However, some may infiltrate adjacent tissues such as the sphenoid sinus (SS), diaphragma sellae, and cavernous sinus (CS), with approximately 10% invading the CS.[6,15] The endoscopic transsphenoidal approach to the CS was first performed in the 1990s,[8,10] and comprises an excellent logical route to remove CS tumors, especially PAs, through the medial CS wall. In the transsphenoidal approach, tumors invading the CS through its medial wall are approached inferomedially following the direction of tumor growth, which spares the cranial nerves. However, this approach is limited by the narrow surgical corridor and insufficient visualization. The development of new endoscopic instruments providing enhanced visualization has expanded the limitations of the traditional microscopic transsphenoidal approach, thereby facilitating a safe resection of CS lesions.[26]
The SS, a highly variable anatomic structure, is located in the center of the cranial base, surrounded by numerous neurovascular structures. Its pneumatization provides a dilating natural cavity through which the wide areas of the cranial base may be accessed. The sinus is bordered anteriorly by the ethmoidal air cells, posteriorly by the clivus, laterally by the CS, superiorly by the pituitary fossa and planum sphenoidale, and inferiorly by the choana.[28] Congdon classified the SS into three types, based on the degree of pneumatization relative to the sella turcica: conchal, presellar, and sellar.[5] Approximately 2% of cases with sellar lesions present no SS pneumatization, which increases the difficulty level of endoscopic endonasal transsphenoidal surgery (EES).[9,17-19,23,24] SS pneumatization, both in the anteroposterior and the lateral (i.e., the lateral recess of the SS [LRSS]) directions, is important for EES. Poor LRSS pneumatization increases the surgery’s difficulty level, as well as the risk of neural and vascular injury. According to our experience with EES to resect tumors invading the CS, less developed LRSS makes CS tumor resection more difficult due to limited manipulation of the surgical instruments.[13] We hypothesized that the removal rate of PAs in the CS was correlated with the volume of LRSS. In the present study, we quantitatively evaluate the relationship between LRSS and the removal rate of PAs with CS invasion. Preoperative evaluation of LRSS helps the neurosurgeon to plan an appropriate surgical strategy for PAs with CS invasion.
MATERIALS AND METHODS
Patients
The Institutional Review Board of the Keio University School of Medicine approved this retrospective study. New-onset PA patients who underwent surgery through EES were retrospectively evaluated. CS invasion was reported according to the Knosp criteria, and only PAs with Knosp Grades 3 and 4 were included in the study.[15] Knosp grading[15] was used to predict invasion into the medial wall of the CS, which was evaluated based on the coronal section of magnetic resonance imaging (MRI): Grade 0, PAs without extending the medial carotid line; Grade 1, PAs crossing the medial carotid line; Grade 2, PAs extending beyond the median line; Grade 3, PAs extending beyond the lateral line of the internal carotid artery; and Grade 4, PAs totally wrapping around the internal carotid artery in the CS.
Surgical procedure
EES was performed with otolaryngologists using a binostril, two-surgeon technique.[14,16,22] A surgical corridor was achieved through an outfracture of the bilateral middle turbinate. Following resection of the nasal septum’s posterior segment, a wide sphenoidotomy was performed. The sellar floor was opened and the bone of the ventral CS was removed as necessary, depending on the tumor location. For lesions occupying both the sella and the CS, a transsellar approach to the CS was used.
Measurements and data analysis
The volumes of the whole tumor, the tumor located in the bilateral CS, and the bilateral LRSS were measured. All pre and postoperative MRI and preoperative computed tomography (CT) images were retrospectively reviewed by three authors (Kenzo Kosugi, Taro Mase, and Haruka Tamura), who calculated the whole tumor and CS tumor removal rate [Figure 1]. Volumetric analysis was performed using the SYNAPSE VINCENT imaging system (Fujifilm Medical Co., Tokyo, Japan), and the segmentation tool function was used on gadolinium-enhanced T1-weighted images obtained close to the surgery date. LRSS was examined using the coronal planes from each patient. On each coronal plane, the Vidian canal and the rotundum foramen were connected, and the SS area in the lateral line side was measured. The volume of both LRSS sides was calculated by integrating each area from end to end of the lateral recess in the coronal plane. The pre- and post-surgery volumes of the whole tumor volume and the bilateral CS tumor were also measured on the coronal plane.
Figure 1:
The volumetric analysis of the lateral recess of the sphenoid sinus (a and b) and representative cases (c-e) using coronal computed tomography. The upper yellow arrow shows the foramen of rotundum, and the lower arrow shows the Vidian canal (a). A line was drawn through the two points and the area in the sphenoid sinus lateral side of the line on each slice was measured (b). Well-pneumatized case (c), moderately pneumatized case (d), and poorly pneumatized case (e).
Statistical analysis
Correlations between two ordinal parameters were investigated using the Pearson correlation test. A linear regression model was fitted for trend analysis. P < 0.05 was considered statistically significant. All statistical analyses were performed with the Statistical Package for the Social Sciences (SPSS) version 20.0 software (SPSS, Chicago, IL).
RESULTS
Patient characteristics
EES was performed for new-onset PA in 31 patients at the Keio University Hospital between July 2012 and March 2018. Among these patients, 23 with Knosp 3 and 4 PA who had available pre and postoperative MRI studies, including gadolinium-enhanced T1-weighted images and high-resolution CT studies, were included in the study. The patients’ average age was 59 (range 38−81) years and the study population had almost equal sex distribution (12 men and 11 women). Among 23 patients, 7 had only right side CS invasion, 6 had only left side CS invasion, and 10 had CS invasion on both sides. Four tumors were endocrinologically active with elevated plasma levels of growth hormone and prolactin [Table 1]. The mean duration of the surgeries was 228 min and no major complications were observed. The endocrinological and radiological outcomes were satisfactory in patients with both functioning and non-functioning PAs. An illustrative case is shown in Figure 2.
Table 1:
Summary of pituitary adenomas removed with endoscopic transsphenoidal surgery.
Figure 2:
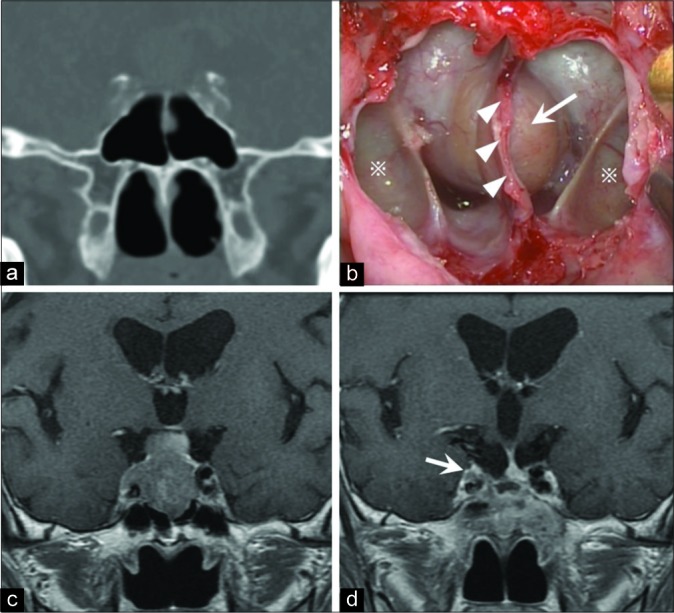
Illustrative case with images and operation view. (a) Preoperative computed tomography in a coronal section showing the developed lateral recess of the sphenoid sinus (LRSS). (b) This intraoperative view shows the posterior wall of the SS from an endoscopic endonasal transsphenoidal approach, including the LRSS (※), septum of the SS (head arrow), and sella turcica (arrow). (c) Preoperative gadolinium-enhanced T1-weighted magnetic resonance imaging (Gd-T1WI MRI) in a coronal section showing a pituitary adenoma with cavernous sinus (CS) invasion. (d) Postoperative Gd-T1WI MRI showing that almost all of the tumor was removed but there was a small residual tumor on the internal carotid artery in the right CS (arrow).
Association between the degree of tumor resection and the volume of the LRSS
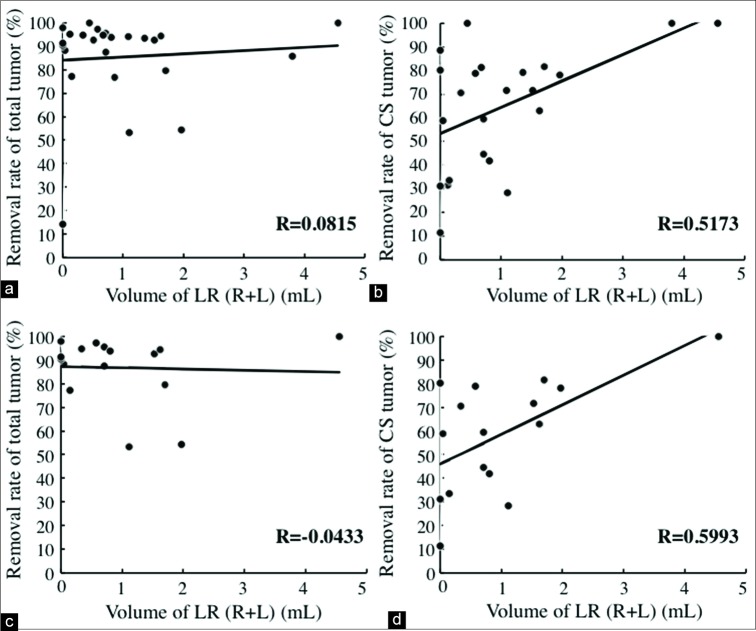
Among cases with PAs categorized as Knosp Grades 3 and 4, no significant association was found between the whole tumor’s resection rate and the bilateral LRSS volume (R = 0.08, P = 0.70) [Figure 3a]. In contrast, a significant correlation was found between the CS tumors’ removal rate and the bilateral LRSS volume (R = 0.52, P = 0.011) [Figure 3b]. The same results were achieved for PA categorized as Knosp Grade 4. No significant association was found between the whole tumor resection rate and the bilateral LRSS volume (R = −0.043, P = 0.87) [Figure 3c]. In contrast, the CS tumor removal rate was significantly correlated with the bilateral LRSS volume (R = 0.60, P = 0.014) [Figure 3d]. A stronger correlation was observed between the removal rate of Knosp Grade 4 tumors and volume of the LRSS than between the removal of Knosp Grades 3 and 4 tumors (R(Knosp4) = 0.60>R(Knosp3-4) = 0.52) and LRSS volume. No significant association was found between surgical time and LRSS volume (Knosp Grades 3–4: P = 0.20 and Knosp Grade 4: P = 0.43).
Figure 3:
Correlation between the removal rates of pituitary adenomas and the volume of the lateral recess of the sphenoid sinus (LRSS). Linear regression models revealed no correlation between the whole tumor removal rate for tumors categorized as both Knosp Grades 3 and 4 (a) and Grade 4 (c), and the volume of LRSS (a: P = 0.70, c: P = 0.87). A significant correlation was found between the removal rate of the cavernous sinus tumor and the volume of the LRSS both in Knosp Grades 3 and 4 (b) and in Knosp Grade 4 (d) (b: R = 0.52, P < 0.05, d: R = 0.60, P < 0.05).
DISCUSSION
Advances in optics, endoscopic cameras and video monitors resolution, and computer-assisted navigation have significantly enhanced the resection potential of various cranial base lesions using minimal access surgery.[1,4,11,25] The use of the endoscope enabled direct visualization of the area under surgery, along with extreme close-ups and with changeable endoscopes with different view angles.[2,3,7,20] However, the difficulty level of EES depends on the patients’ nasal cavity and septum anatomy. SS pneumatization is one of the most important anatomical features to consider during preoperative planning. Determining SS pneumatization, both in the anteroposterior and lateral (i.e., LRSS) directions, is essential for EES.[9,24,29] Vaezi et al. reported a correlation between the extent of LRSS pneumatization and the difficulty level of the surgery, the amount of drilling, and the need to sacrifice the Vidian nerve in poor pneumatization cases when approaching the middle cranial fossa.[27] They categorized SS pneumatization in the coronal plane into three distinct types, arguing that the classification could potentially improve the preoperative planning and allow a better evaluation of the risk for neurovascular EES complications.[27] However, they did not investigate the relationship between LRSS pneumatization and clinical parameters, such as the tumor removal rate. The present study revealed an association between LRSS volume and CS invasion removal rate in PAs.
In the present study, cases with substantial pneumatization tended to have a higher CS tumors’ removal rate, since LRSS pneumatization influences the manipulation of surgical instruments during resection. Furthermore, in cases with a poorly pneumatized sinus, the direct visualization of the internal carotid artery’s location and its relationship to the sella is basically impossible. Our results show that LRSS volumes are not associated with whole tumor removal rate and that LRSS pneumatization may not influence the removal of sellar tumors. In the present study, most cases presented with CS tumors in a small portion of the total tumor. Therefore, the whole tumor resection rate was not associated with LRSS volume. We believe that analyzing LRSS development preoperatively is useful to plan EES for CS tumor resection in PA patients.
Limitations of the present study include the retrospective design and the relatively small sample size. Prospective studies with a larger number of subjects are warranted to confirm the present findings. In the present study, several cases achieved a nearly total removal of the CS tumor despite a narrow LRSS [Table 1]. This suggests that the level of difficulty in CS tumor resection may be influenced by other factors, such as the nature of the pathology (i.e., fibrous consistency and vascularity), nasal structures, the surgeon’s skill, and expertise and institutional resources (i.e., support specialists and equipment).[12] The outcome of endoscopic endonasal skull base surgery has been dramatically improved by the advancements in surgical instruments. Future technological innovations might overcome the disadvantages arising from the poor pneumatization of the LRSS.
CONCLUSION
The present study reveals a correlation between LRSS volume and tumor removal rate. Narrow LRSS is associated with difficulty in performing surgical procedures, resulting in a lower removal rate of the cavernous region of PAs. Preoperative evaluation of LRSS may help the neurosurgeon to plan an appropriate surgical strategy, including the selection of endoscopic instruments for PAs with CS invasion.
Footnotes
How to cite this article: Kosugi K, Tamura R, Mase T, Tamura H, Jinzaki M, Yoshida K, et al. Relationship between pneumatization of lateral recess in the sphenoid sinus and removal of cavernous sinus invasion in pituitary adenomas by endoscopic endonasal surgery. Surg Neurol Int 2019;10:222.
Contributor Information
Kenzo Kosugi, Email: kensan03977@yahoo.co.jp.
Ryota Tamura, Email: moltobello-r-610@hotmail.co.jp.
Taro Mase, Email: mstr.komed05241996@gmail.com.
Haruka Tamura, Email: rovin124@icloud.com.
Masahiro Jinzaki, Email: jinzaki@rad.med.keio.ac.jp.
Kazunari Yoshida, Email: kazrmky@z3.keio.jp.
Masahiro Toda, Email: todam@keio.jp.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Cappabianca P, Alfieri A, Colao A, Ferone D, Lombardi G, de Divitiis E, et al. Endoscopic endonasal transsphenoidal approach: An additional reason in support of surgery in the management of pituitary lesions. Skull Base Surg. 1999;9:109–17. doi: 10.1055/s-2008-1058157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cappabianca P, Cavallo LM, de Divitiis E. Endoscopic endonasal transsphenoidal surgery. Neurosurgery. 2004;55:933–40. doi: 10.1227/01.neu.0000137330.02549.0d. [DOI] [PubMed] [Google Scholar]
- 3.Cappabianca P, de Divitiis E. Endoscopy and transsphenoidal surgery. Neurosurgery. 2004;54:1043–8. doi: 10.1227/01.neu.0000119325.14116.9c. [DOI] [PubMed] [Google Scholar]
- 4.Cappabianca P, Kelly DF, Laws ER Jr. Endoscopic transnasal versus open transcranial cranial base surgery: The need for a serene assessment. Neurosurgery. 2008;63:240–1. doi: 10.1227/01.NEU.0000327038.09638.37. [DOI] [PubMed] [Google Scholar]
- 5.Congdon ED. The distribution and mode of origin of septa and walls of the sphenoid sinus. Anat Rec. 1920;18:97–123. [Google Scholar]
- 6.Cottier JP, Destrieux C, Brunereau L, Bertrand P, Moreau L, Jan M, et al. Cavernous sinus invasion by pituitary adenoma: MR imaging. Radiology. 2000;215:463–9. doi: 10.1148/radiology.215.2.r00ap18463. [DOI] [PubMed] [Google Scholar]
- 7.Elwany S, Elsaeid I, Thabet H. Endoscopic anatomy of the sphenoid sinus. J Laryngol Otol. 1999;113:122–6. doi: 10.1017/s0022215100143361. [DOI] [PubMed] [Google Scholar]
- 8.Fraioli B, Esposito V, Santoro A, Iannetti G, Giuffrè R, Cantore G, et al. Transmaxillosphenoidal approach to tumors invading the medial compartment of the cavernous sinus. J Neurosurg. 1995;82:63–9. doi: 10.3171/jns.1995.82.1.0063. [DOI] [PubMed] [Google Scholar]
- 9.Hamid O, El Fiky L, Hassan O, Kotb A, El Fiky S. Anatomic variations of the sphenoid sinus and their impact on trans-sphenoid pituitary surgery. Skull Base. 2008;18:9–15. doi: 10.1055/s-2007-992764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hashimoto N, Kikuchi H. Transsphenoidal approach to infrasellar tumors involving the cavernous sinus. J Neurosurg. 1990;73:513–7. doi: 10.3171/jns.1990.73.4.0513. [DOI] [PubMed] [Google Scholar]
- 11.Kabil MS, Eby JB, Shahinian HK. Fully endoscopic endonasal vs. Transseptal transsphenoidal pituitary surgery. Minim Invasive Neurosurg. 2005;48:348–54. doi: 10.1055/s-2005-915635. [DOI] [PubMed] [Google Scholar]
- 12.Kasemsiri P, Carrau RL, Ditzel Filho LF, Prevedello DM, Otto BA, Old M, et al. Advantages and limitations of endoscopic endonasal approaches to the skull base. World Neurosurg. 2014;82:S12–21. doi: 10.1016/j.wneu.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 13.Kikuchi R, Toda M, Tomita T, Ogawa K, Yoshida K. Analysis of sphenoid sinus lateral pneumatization for endonasal endoscopic surgery. Surg Neurol Int. 2015;6:166. doi: 10.4103/2152-7806.168313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kikuchi R, Toda M, Tomita T, Ogawa K, Yoshida K. Surgical outcome of endoscopic endonasal surgery for non-functional pituitary adenoma by a team of neurosurgeons and otolaryngologists adenoma by a team of neurosurgeons and otolaryngologists. Turk Neurosurg. 2017;27:1–7. doi: 10.5137/1019-5149.JTN.14354-15.0. [DOI] [PubMed] [Google Scholar]
- 15.Knosp E, Steiner E, Kitz K, Matula C. Pituitary adenomas with invasion of the cavernous sinus space: A magnetic resonance imaging classification compared with surgical findings. Neurosurgery. 1993;33:610–7. doi: 10.1227/00006123-199310000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Kuga D, Toda M, Ozawa H, Ogawa K, Yoshida K. Endoscopic endonasal approach combined with a simultaneous transcranial approach for giant pituitary tumors. World Neurosurg. 2019;121:173–9. doi: 10.1016/j.wneu.2018.10.047. [DOI] [PubMed] [Google Scholar]
- 17.Liu JK, Christiano LD, Patel SK, Eloy JA. Surgical nuances for removal of retrochiasmatic craniopharyngioma via the endoscopic endonasal extended transsphenoidal transplanum transtuberculum approach. Neurosurg Focus. 2011;30:E14. doi: 10.3171/2011.1.FOCUS10297. [DOI] [PubMed] [Google Scholar]
- 18.Liu JK, Christiano LD, Patel SK, Tubbs RS, Eloy JA. Surgical nuances for removal of tuberculum sellae meningiomas with optic canal involvement using the endoscopic endonasal extended transsphenoidal transplanum transtuberculum approach. Neurosurg Focus. 2011;30:E2. doi: 10.3171/2011.3.FOCUS115. [DOI] [PubMed] [Google Scholar]
- 19.Liu JK, Eloy JA. Endoscopic endonasal transplanum transtuberculum approach for resection of retrochiasmatic craniopharyngioma. Neurosurg Focus. 2012;32(Suppl 1):E2. doi: 10.3171/2012.V2.FOCUS11299. [DOI] [PubMed] [Google Scholar]
- 20.Magro F, Solari D, Cavallo LM, Samii A, Cappabianca P, Paternò V, et al. The endoscopic endonasal approach to the lateral recess of the sphenoid sinus via the pterygopalatine fossa: Comparison of endoscopic and radiological landmarks. Neurosurgery. 2006;59:ONS237–42. doi: 10.1227/01.NEU.0000233977.79721.17. [DOI] [PubMed] [Google Scholar]
- 21.Mete O, Lopes MB. Overview of the 2017 WHO classification of pituitary tumors. Endocr Pathol. 2017;28:228–43. doi: 10.1007/s12022-017-9498-z. [DOI] [PubMed] [Google Scholar]
- 22.Saito K, Toda M, Tomita T, Ogawa K, Yoshida K. Surgical results of an endoscopic endonasal approach for clival chordomas. Acta Neurochir (Wien) 2012;154:879–86. doi: 10.1007/s00701-012-1317-1. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt RF, Choudhry OJ, Raviv J, Baredes S, Casiano RR, Eloy JA, et al. Surgical nuances for the endoscopic endonasal transpterygoid approach to lateral sphenoid sinus encephaloceles. Neurosurg Focus. 2012;32:E5. doi: 10.3171/2012.3.FOCUS1267. [DOI] [PubMed] [Google Scholar]
- 24.Song Y, Wang T, Chen J, Tan G. Endoscopic transsphenoidal resection of sellar tumors with conchal sphenoid sinus: A report of two cases. Oncol Lett. 2015;9:713–6. doi: 10.3892/ol.2014.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spencer WR, Das K, Nwagu C, Wenk E, Schaefer SD, Moscatello A, et al. Approaches to the sellar and parasellar region: Anatomic comparison of the microscope versus endoscope. Laryngoscope. 1999;109:791–4. doi: 10.1097/00005537-199905000-00020. [DOI] [PubMed] [Google Scholar]
- 26.Toda M, Kosugi K, Ozawa H, Ogawa K, Yoshida K. Surgical treatment of cavernous sinus lesion in patients with nonfunctioning pituitary adenomas via the endoscopic endonasal approach. J Neurol Surg B Skull Base. 2018;79:S311–5. doi: 10.1055/s-0038-1667123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vaezi A, Cardenas E, Pinheiro-Neto C, Paluzzi A, Branstetter BF, 4th, Gardner PA, et al. Classification of sphenoid sinus pneumatization: Relevance for endoscopic skull base surgery. Laryngoscope. 2015;125:577–81. doi: 10.1002/lary.24989. [DOI] [PubMed] [Google Scholar]
- 28.Wang J, Bidari S, Inoue K, Yang H, Rhoton A Jr. Extensions of the sphenoid sinus: A new classification. Neurosurgery. 2010;66:797–816. doi: 10.1227/01.NEU.0000367619.24800.B1. [DOI] [PubMed] [Google Scholar]
- 29.Zada G, Agarwalla PK, Mukundan S, Jr, Dunn I, Golby AJ, Laws ER, Jr, et al. The neurosurgical anatomy of the sphenoid sinus and sellar floor in endoscopic transsphenoidal surgery. J Neurosurg. 2011;114:1319–30. doi: 10.3171/2010.11.JNS10768. [DOI] [PMC free article] [PubMed] [Google Scholar]