Abstract
Background
The objective of this study was to investigate the plasma pharmacokinetic profiles, intratumoral concentration and tissue distribution of arsenic trioxide (ATO) by drug-eluting beads (DEB)-transcatheter arterial chemoembolization (TACE) compared with conventional TACE (cTACE) in a rabbit liver tumor model.
Methods
Sixty-four rabbits with VX2 liver tumor were established and randomly assigned to four groups equally. The calliSpheres microspheres (CSM)-ATO group received DEB-TACE treatment using ATO-loaded CSM; the cTACE-ATO group received cTACE treatment using ATO mixed with lipiodol; the CSM-normal control (NC) group received DEB-TACE treatment using blank CSM; the TAE-lipiodol group received cTACE treatment using saline mixed with lipiodol. ATO concentration in plasma, tumor and normal tissues, and liver and kidney function indexes were evaluated.
Results
The CSM-ATO group exhibited lower plasma ATO concentrations at 10 minutes and 20 minutes post treatment compared with the cTACE-ATO group. Meanwhile, intratumoral ATO concentrations were higher in the CSM-ATO group compared with the cTACE-ATO group at 3-, 7- and 14-days post treatment. In normal liver tissue, heart and muscle tissues, ATO concentrations between the CSM-ATO and cTACE groups were similar at each time point; in kidney and lung tissues, ATO concentrations were lower in the CSM-ATO group at 1-day post treatment while they were similar at 3, 7 and 14 days post treatment. Also, liver or kidney function indexes were of no difference at each time point between CSM-ATO and cTACE-ATO groups.
Conclusion
Administration of ATO via DEB-TACE decreases systemic concentration while increasing intratumoral concentration of ATO without increasing liver or kidney toxicity compared with cTACE.
Keywords: liver cancer, Arsenic trioxide, Transcatheter arterial chemoembolization, Drug-eluting beads, pharmacokinetics
Introduction
Liver cancer is reported to be the sixth most prevalent cancer and the fourth leading cause of cancer deaths all over the world, with approximately 841,000 new cases and 782,000 deaths every year.1 Despite that there are a considerable number of treatment strategies for liver cancer, the 5-year survival rate is still less than 20%, indicating a great challenge for liver cancer treatment.2,3 Arsenic trioxide (ATO), an inorganic compound which is used as a traditional Chinese medicine, has been permitted for acute promyelocytic leukemia treatment by the US Food and Drug Administration since 2000.4 ATO is also discovered to play anti-tumor effects in various solid tumors including liver cancer.5–8 In a previous in vitro study, the administration of ATO in liver cancer cells (HepG2 cells) resulted in cell apoptosis via activation of caspase-3 and phosphatidylserine externalization.9 In another study, intraperitoneal administration of ATO in HCC rats induced tumor necrosis through decreasing vascular endothelial growth factor (VEGF) expression and microvessel density (MVD) in tumor tissues.10 These studies indicate the clear anti-tumor activity of ATO and its wide clinical application potential in liver cancer treatment. However, systemic administration of ATO might cause intolerant adverse events due to its hypertoxicity (such as bleeding, liver function lesion and cardiovascular diseases), and it could hardly achieve effective drug concentration in tumor tissues owing to the high serum protein binding rate and rapid renal clearance, which impede its clinical utility significantly.11–15
Transcatheter arterial chemoembolization (TACE), a locoregional therapy which is able to pronouncedly increase intratumoral drug concentration while decreasing systemic drug concentration in liver cancer patients, might help to maximize the anti-tumor efficacy of ATO while minimizing its systemic adverse events.16,17 A few animal studies have investigated the efficacy of conventional TACE (cTACE) treatment using ATO iodized oil emulsion in a rabbit VX2 liver tumor model, which reveal that administration of ATO via cTACE greatly suppresses liver tumor growth without causing severe liver or kidney toxicity.18,19 However, these studies do not comprehensively explore the pharmacokinetics or tissue distribution of ATO during cTACE treatment, thus whether administration of ATO via TACE is effective and safe in treating liver cancer patients remains unclear. Drug-eluting beads (DEB)-TACE is a novel TACE approach which utilizes microspheres as both drug carrier and embolic agent.20 Accumulating evidences have revealed that DEB-TACE exhibits more stable and sustained drug delivery compared with cTACE, hence the administration of ATO via DEB-TACE provides better pharmacokinetics of ATO compared with cTACE, which might enhance treatment efficacy of ATO while diminishing its systemic adverse events more obviously.21–23 However, no relevant study is reported until now. Therefore, we conducted the current study to investigate the plasma pharmacokinetic profiles, intratumoral concentration and tissue distribution of ATO by DEB-TACE treatment compared with cTACE in a rabbit model with VX2 liver tumor.
Materials And Methods
Animals And Ethics
Adult Japanese white rabbits (weighting 2.8 kg~3.4 kg without sex preference or pregnancy) used in the current study were provided by Experimental Animal Center of Henan Province. All the rabbits were bred in our Laboratory Animal Center and had free access to standard pellet diets and water during the experimental period. The study was approved by the Animal Care and Use Committee of Henan Province, and all related experiments were conducted in accordance with the “Code for the Care and Use of Animals for Scientific Purposes” statement and under the principles of 3R (replacing, refining and reducing).
Tumor Implantation
To obtain solid liver tumor in experimental rabbits, active VX2 tumors derived from the donor rabbits (donated by Tongji Medical College Animal Experimental Center of Huazhong University of Science and Technology) were inoculated into the experimental rabbits according to previous methods.19,20,24,25 In brief, experimental rabbits were anesthetized with intramuscular injections of sodium pentobarbital (30 mg/kg) and immobilized on a surgical table. Next, the abdomens were shaved, and livers were exposed through a midline subxiphoid incision under aseptic conditions, then the minced active VX2 tumors were implanted directly into the left lobe of the exposed livers. After surgery, the experimental rabbits were monitored and bred for 16 days before TACE treatment to make sure the growth of the VX2 tumors, then contrast-enhanced computed tomography (CT) was utilized to obtain tumor location and size.
Randomization And Grouping
A total of 64 eligible rabbits were randomly assigned into four groups (CSM-ATO group, cTACE-ATO, TAE-CSM group and TAE-lipiodol group) using a simple randomization method (Random Number Remainder Grouping Method) with 16 rabbits in each group. Briefly, randomization was performed as follows: (1) numbering rabbits by body weight from 1 to 64; (2) 64 random numbers were created by SAS 9.0 (SAS Institute, Inc., Cary, NC, USA); (3) dividing the random number by the number of groups (n=4) to get the remainders. If the random number was divided by 4 with no remainder generated, the “4” was used as remainder; (4) grouping by remainder; (5) if a total of N cases need to be adjusted due to unbalanced grouping, N random numbers were generated by SAS again, which then were divided by the “N”, and the obtained remainder was considered as the serial number of the selected animals. If the random number was divided by N with no remainder generated, the “N” was used as “remainder” to select animals for adjustment.
Treatment
All these 64 rabbits in 4 groups received treatment through the TACE procedure, and the detailed treatment procedures were as follows: (1) the CSM-ATO group received hepatic intraarterial administration of ATO-loaded CalliSpheres (CSM) (100–300 μm, Jiangsu Hengrui Medicine Co. Ltd., Jiangsu, China) (0.5 mg/kg of ATO); (2) the cTACE-ATO group received hepatic intraarterial administration of a mixture of ATO and lipiodol (LaboratoireGuerbet, Aulnay-Sous-Bois, France) (0.5 mg/kg of ATO); (3) the TAE-CSM group received hepatic intraarterial administration of blank CSM (100–300 μm, Jiangsu Hengrui Medicine Co. Ltd., Jiangsu, China); (4) the TAE-lipiodol group received hepatic intraarterial administration of 10 mL saline mixed with lipiodol (LaboratoireGuerbet, Aulnay-Sous-Bois, France).
CSM Preparation
CSM preparation was conducted in an aseptic environment. The loading procedures were performed as follows: (1) 15 mg of ATO was firstly diluted with saline; (2) one vial of CSM solution (containing 1 g CSM) was shaken up and the supernatant was extracted then added to the ATO solution; (3) the mixture was shaken up for 30 mins at room temperature and then nonionic isotonic contrast agent was added, the mixture was then vibrated for further used.
TACE Procedure
Hepatic arteries in the CSM-ATO and TAE-CSM groups were embolized with CSM, while the hepatic arteries in the cTACE-ATO and TAE-lipiodol groups were embolized with lipiodol. All rabbits were anesthetized using the aforementioned method in tumor implantation, then the right femoral artery was exposed, and a 4-F sheath (Terumo, Tokyo, Japan) was placed into the artery; after that, a 4-F catheter (Terumo) was inserted into the common hepatic artery to identify the hepatic artery anatomy. A 2.7-F coaxial microcatheter (Terumo) was then catheterized to the left hepatic artery, and the following treatments were conducted respectively according to the method previously described in grouping and treatment. After the treatment, the sheath and the catheter were removed from the artery and the wound was sewn up.
Measurement Of ATO Concentration In Plasma
Blood samples (0.5 mL) from the CSM-ATO and cTACE-ATO groups were collected at 10 minutes, 20 minutes, 40 minutes, 1 hour, 3 hours, 6 hours, 12 hours, 1 day and 3 days post treatment via the ear vein, and then were added to centrifuge tubes (containing 2 μL heparin) and centrifuged for 10 minutes (3000 rpm/minute at 4°C), respectively. After that, the upper layer (plasma) from each tube was obtained and stored at −80°C until further analysis. 0.1 g of upper layer was added to the flask containing 5 mL 67% HNO3, then the mixture was sealed and vibrated overnight. After that, 2 mL 30% H2O2 was added and incubated at 100°C for 3 hours and then cooled to room temperature. The solution was added to the 10 mL volumetric flask and diluted with 2% HNO3 to 10 mL.26 Subsequently, the solution was centrifuged at 3000 g for 5 minutes, then the upper layer (containing arsenic ion) was obtained to determine the ATO concentration using the atomic fluorescence method. In addition, alanine transaminase (ALT), aspartate transaminase (AST), blood urea nitrogen (BUN) and serum creatinine (Scr) levels in plasma were also evaluated before treatment, at 1-day, 3-days and 7-days post treatment.
Measurement Of ATO Concentration In Tissues
Four rabbits in each group were humanely sacrificed at 1-day, 3-days, 7-days and 14-days post treatment, and active VX2 tumor tissue, normal liver tissue (taken from the right lobe of the liver, farthest from the tumor), heart tissue, lung tissue and kidney tissue were harvested and frozen at −80°C until analysis. Then 0.5–1.0 g tissue samples were obtained and the ATO concentration in each tissue sample was determined according to the method described in the plasma ATO concentration measurement section.26
Statistical Analysis
Statistical analysis was performed using SPSS 19.0 software (IBM Corporation, Armonk, NY, USA) and GraphPad Prism 6.01 software (GraphPad Software Inc., San Diego, USA). Data were expressed as mean ± standard deviation. Comparison between two groups at the same time point was determined by t-test. Comparison among 4 groups at same time point and among different time points in the same group was determined by One-way ANOVA, followed by Tukey’s multiple comparisons test. P < 0.05 was considered significant.
Results
Animal Model Construction And Transarterial Embolization
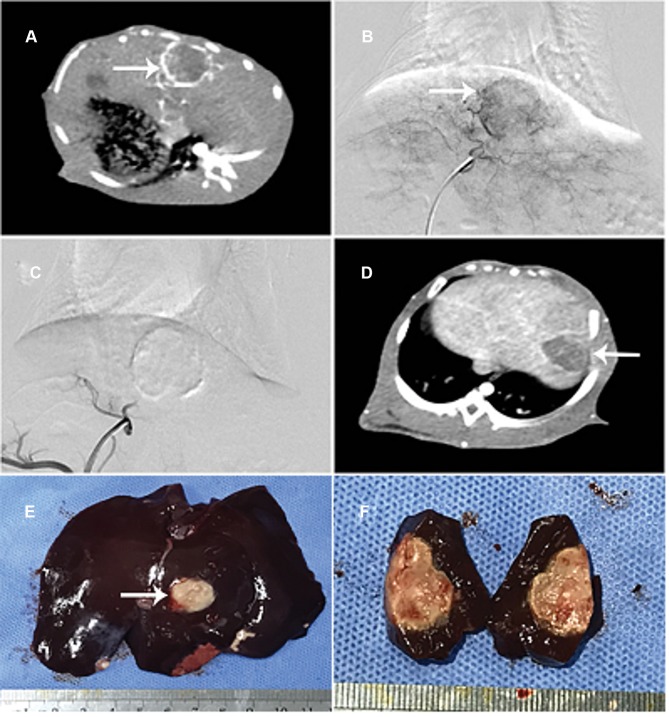
All of the 64 rabbits were incubated with VX2 tumor successfully at 16-days post implantation (Figure 1A and B). Subsequently, the transarterial embolization procedure in each group (the CSM-ATO, cTACE-ATO, TAE-CSM and TAE-lipiodol groups) was performed, and the embolization effect in the CSM-ATO group was utilized as an example, and is displayed in Figure 1C–F as follows. After transarterial embolization, the blood supply was blocked completely without obvious reflux, indicating that transarterial embolization was successful (Figure 1C). At 7-days post treatment, contrast-enhanced CT showed that the tumor volume of rabbits was not increased (Figure 1D), and then a tumor tissue sample was obtained and dissected (Figure 1E), showing that the tumor was completely necrotic (Figure 1F). These analyses indicated the good treatment efficacy of CSM-ATO in VX2 tumor rabbits.
Figure 1.
Construction of animal model and transarterial embolization. All 64 rabbits were incubated with VX2 tumor successfully at 16 days post implantation (A, B). Transarterial embolization in the CSM-ATO group was exemplified as follows: the blood supply was embolized completely after transarterial embolization (C); at 7-days post treatment, tumor volume of rabbits was not increased (D), then the tumor tissue sample was obtained and dissected (E), showing that the tumor was necrotic throughout (F).
ATO Concentration In Plasma
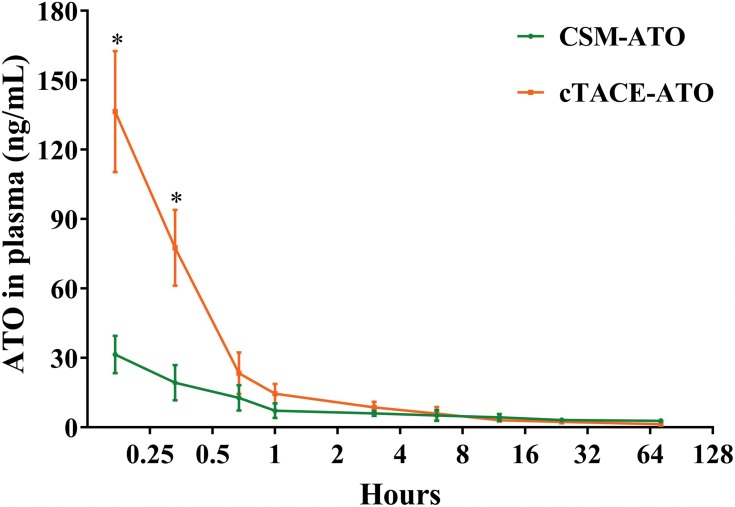
Plasma ATO concentration reached a maximum at 10 minutes post treatment, and then decreased over time both in the CSM-ATO group and the cTACE-ATO group (Figure 2). At 10 minutes (31.46±8.12 ng/mL vs 136.43±26.12 ng/mL, P = 0.002) and 20 minutes (19.34±7.61 ng/mL vs 77.60±16.35 ng/mL, P = 0.006) post treatment, the CSM-ATO group had lower plasma ATO concentration compared with the cTACE-ATO group (Figure 2). The CSM-ATO group also had numerically lower plasma ATO concentration compared to the cTACE-ATO group at 40-minutes (P = 0.566), 1-hour (P = 0.256) and 3-hours (P = 0.604) post treatment although statistically insignificant. At 6-hours-, 12-hours-, 24-hours and 72-hours post treatment, plasma ATO concentration was similar between the two groups (all P > 0.05). These data indicated that administration of ATO via DEB-TACE exhibited lower and more stable plasma ATO concentration compared with cTACE, which might decrease the systemic adverse events of ATO.
Figure 2.
Plasma ATO concentration. Plasma concentration of ATO was highest at 10-minutes post treatment then decreased over time both in the CSM-ATO and cTACE-ATO groups. Meanwhile, plasma concentration of ATO in CSM-ATO group was decreased at 10=minutes and 20-minutes post treatment compared with the cTACE-ATO group. Comparison between two groups at the same time point was determined by t test. P < 0.05 was considered significant. *P < 0.05.
Abbreviations: ATO, arsenic trioxide; CSM, CalliSpheres Microspheres; cTACE, conventional transcatheter arterial chemoembolization.
ATO Concentration In Tumor
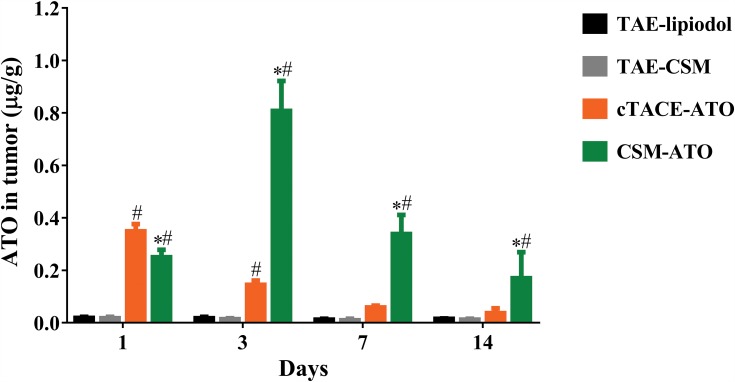
The CSM-ATO group had higher intratumoral ATO concentration compared with the TAE-CSM group at each time point post treatment, and the cTACE-ATO group had higher intratumoral ATO concentration compared with the TAE-lipiodol group at 1-day and 3-days post treatment (all P < 0.05, Figure 3). Meanwhile, intratumoral ATO concentration was lower at 1-day (0.2501±0.0279 μg/g vs 0.3486±0.0278 μg/g, P < 0.001) post treatment but higher at 3-days (0.8075±0.1145 μg/g vs 0.1447±0.0163 μg/g, P < 0.001), 7-days (0.3383±0.0731 μg/g vs 0.0583±0.0072 μg/g, P < 0.001) and 14-days (0.1701±0.0993 μg/g vs 0.0363±0.0186 μg/g, P = 0.010) post treatment in the CSM-ATO group compared with the cTACE-ATO group. Notably, intratumoral ATO concentration in the CSM-ATO group still remained at a high level at 14-days post treatment, whereas in cTACE-ATO group, the concentration was decreased to baseline level at 7-days post treatment. These data suggested that administration of ATO via DEB-TACE achieved higher intratumoral ATO concentration and more sustained ATO release compared to cTACE (Figure 3).
Figure 3.
Comparison of ATO concentration in tumor. At 1-day post treatment, intratumoral ATO concentration in the CSM-ATO group was lower compared with the cTACE-ATO group, whereas at 3-days, 7-days and 14-days post treatment, intratumoral ATO concentration was higher in the CSM-ATO compared with the cTACE-ATO group. Comparison among the four groups at the same time point and among different time points in the same group was determined by One-way ANOVA, followed by Tukey’s multiple comparisons test. P < 0.05 was considered significant. *Comparison between the CSM-ATO and cTACE-ATO groups was significant. #Comparison between the CSM-ATO and TAE-CSM groups was significant, or comparison between the cTACE-ATO and TAE-lipiodol groups was significant.
Abbreviations: ATO, arsenic trioxide; CSM, CalliSpheres Microspheres; cTACE, conventional transcatheter arterial chemoembolization; TAE, transcatheter arterial embolization; NC, negative control.
ATO Concentration In Normal Liver Tissue
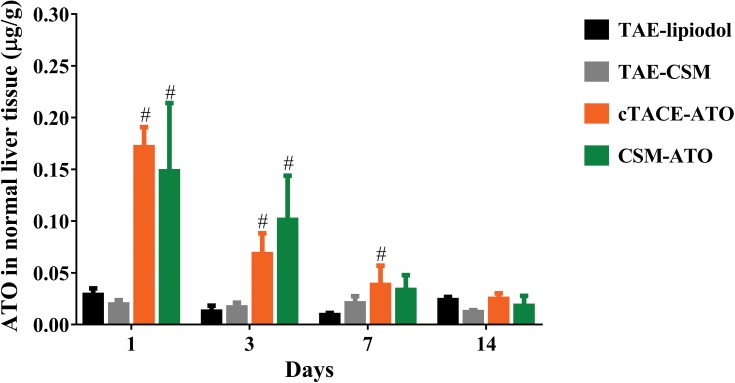
ATO concentration in normal liver tissue was higher at 1-day, 3-days or 7days post treatment (P < 0.05), while it was similar at 14-days post treatment (all P > 0.05) in the CSM-ATO group compared with the TAE-CSM group or in the cTACE-ATO group compared with the TAE-lipiodol group (Figure 4), suggesting that the potential liver injury of ATO would be attenuated at 14-days post-DEB-TACE and post-cTACE treatment. What’s more, there was no difference of ATO concentration in normal liver tissue between the CSM-ATO and cTACE-ATO groups at each time point (all P > 0.05, Figure 4), implying that administration of ATO via DEB-TACE was similar to that of cTACE regarding underlying liver toxicity.
Figure 4.
Comparison of ATO concentration in normal liver tissue. ATO concentration in normal liver tissue between the CSM-ATO and cTACE-ATO groups was of no difference at each time point. Comparison among the four groups at the same time point and among different time points in the same group was determined by One-way ANOVA, followed by Tukey’s multiple comparisons test. P < 0.05 was considered significant. #Comparison between the CSM-ATO and TAE-CSM groups was significant, or comparison between the cTACE-ATO and TAE-lipiodol groups was significant.
Abbreviations: ATO, arsenic trioxide; CSM, CalliSpheres Microspheres; cTACE, conventional transcatheter arterial chemoembolization; TAE, transcatheter arterial embolization; NC, negative control.
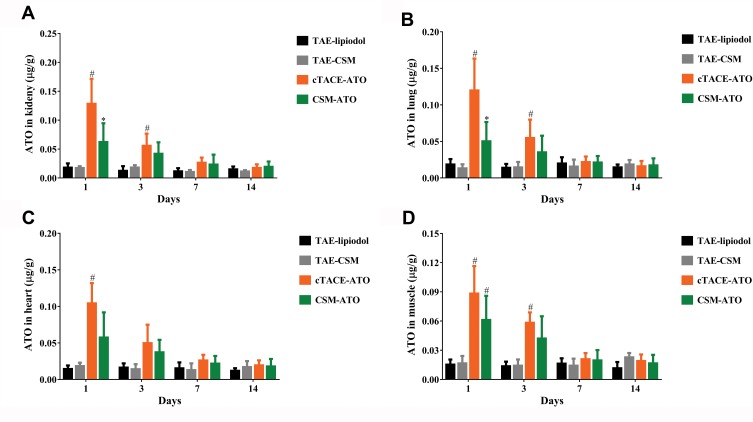
ATO Concentration In Kidney, Lung, Heart And Muscle Tissues
ATO concentration in the kidney (Figure 5A), lung (Figure 5B), heart (Figure 5C) and muscle (Figure 5D) was increased at 1-day or 3-days post treatment in the CSM-ATO group compared with the TAE-CSM group, and increased in the cTACE-ATO group compared to the TAE-lipiodol group (P < 0.05). While at 7-days or 14-days post treatment, no difference was observed between either of the two groups (P > 0.05). More importantly, ATO concentration in kidney (P = 0.020) and lung (P = 0.010) was decreased in the CSM-ATO group compared with the cTACE-ATO group at 1-day post treatment, whereas at 3-days, 7-days or 14-days post treatment, there was no difference in kidney or lung between the CSM-ATO and cTACE-ATO groups (all P > 0.05) (Figure 5A and B). No difference of ATO concentration in heart or muscle was observed between the two groups at each time point, either (all P>0.05) (Figure 5C and D), suggesting that administration of ATO via DEB-TACE was equally safe compared with cTACE in terms of potential kidney, lung, heart and muscle toxicity.
Figure 5.
Comparison of ATO concentrations in kidney, lung, heart and muscle. ATO concentration in kidney (A), lung (B), heart (C) and muscle (D) was at the highest level at 1-day post treatment, then declined to baseline levels within 7-days post treatment both in the CSM-ATO and cTACE-ATO groups. ATO concentration of kidney and lung in the CSM-ATO group was decreased compared with the cTACE-ATO group at 1-day post treatment, while at 3-days, 7-days or 14-days post treatment, no difference was observed between the two groups; no difference of ATO concentration in heart or muscle was discovered between the two groups at the same time point either. Comparison among the four groups at the same time point and among different time points in the same group was determined by One-way ANOVA, followed by Tukey’s multiple comparisons test. P < 0.05 was considered significant. *Comparison between the CSM-ATO and cTACE-ATO groups was significant. #Comparison between the CSM-ATO and TAE-CSM groups was significant, or comparison between the cTACE-ATO and TAE-lipiodol groups was significant.
Abbreviations: ATO, arsenic trioxide; CSM, CalliSpheres Microspheres; cTACE, conventional transcatheter arterial chemoembolization; TAE, transcatheter arterial embolization; NC, negative control.
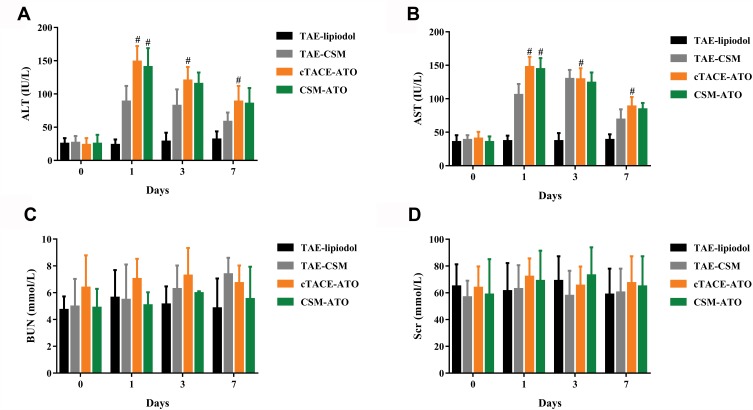
Liver And Kidney Function Indexes
ALT (Figure 6A) and AST (Figure 6B) levels were increased at 1-day, 3-days or 7-days post treatment in the CSM-ATO group compared with the TAE-CSM group and in the cTACE-ATO group compared with the TAE-lipiodol group (P < 0.05), while they were similar between the CSM-ATO and cTACE-ATO groups at each time point post treatment (all P > 0.05), indicating that administration of ATO via DEB-TACE did not deteriorate liver function. Meanwhile, BUN (Figure 6C) and Scr (Figure 6D) levels remained similar among the four groups at each time point (all P > 0.05), implying that administration of ATO via DEB-TACE did not deteriorate kidney function compared with cTACE, either.
Figure 6.
Comparison of liver and kidney function indexes. ALT (A) and AST (B) levels were increased at 1-day post treatment and then decreased over time both in the CSM-ATO and cTACE-CSM groups, and no difference of ALT or AST level between the two groups was observed at each time point. As for BUN (C) and Scr (D), they remained at baseline levels in the CSM-ATO and cTACE-ATO groups post treatment and no difference was observed between the two groups either. Comparison among the four groups at the same time point and among different time points in the same group was determined by One-way ANOVA, followed by Tukey’s multiple comparisons test. P < 0.05 was considered significant. #Comparison between the CSM-ATO and TAE-CSM groups was significant, or comparison between the cTACE-ATO and TAE-lipiodol groups was significant.
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; BUN, blood urea nitrogen; Scr, serum creatinine; ATO, arsenic trioxide; CSM, CalliSpheres Microspheres; cTACE, conventional transcatheter arterial chemoembolization; TAE, transcatheter arterial embolization; NC, negative control.
Discussion
ATO has been used as a palliative treatment for late-stage liver cancer patients in China for its great anti-hepatoma activity. It is reported that ATO is able to induce cell apoptosis in a dose- and time-dependent manner and bring in considerable reactive oxygen species (ROS), while adding the antioxidant N-acetylcysteine could suppress ATO-induced cell apoptosis in HepG2 cells, indicating that ATO stimulating cell apoptosis might be through producing ROS.27 ATO is also discovered to inhibit activity of DNA methyltransferase dose-dependently and then promotes cell apoptosis in HepG2 and Huh-7 cells.28 Also, ATO enhances G2/M arrest in HepG2 and SMMC7721 cells by up regulating the expression of a tumor suppressor (phosphatase and tensin homologue deleted on chromosome 10 (PTEN)) thereby inhibiting cell proliferation.8 Another animal study found that ATO decreases VEGF expression and MVD as well as cancer cell proliferation while increases cancer cell apoptosis and tumor necrosis in experimental liver cancer rats.10 Despite the superb anti-hepatoma activity of ATO, its clinical application is strictly restrained due to the hypertoxicity and poor pharmacokinetic profile after systemic treatment of ATO.29
TACE brings in a high intratumoral drug concentration while a low systemic concentration in liver cancer patients, which enhances treatment efficacy and alleviates adverse events.16,17 Such good properties make it one of the first-line treatments for unresectable liver cancer. Generally, the chemotherapeutic drugs used for cTACE and DEB-TACE include doxorubicin, cisplatin and so on, which have been demonstrated to be successful in liver cancer treatment.30,31 Recently, ATO has also been applied for cTACE treatment and is observed to be effective in a rabbit model with VX2 liver cancer by a few studies, suggesting the feasibility of ATO via TACE in liver cancer treatment.18,19 However, there are two problems remained unsolved: firstly, the pharmacokinetic profile and tissue distribution of ATO after TACE treatment are rarely known; secondly, whether administration of ATO via DEB-TACE is superior to cTACE also remains unclear.
In the current study, we found that administration of ATO via DEB-TACE produced lower and more stable plasma ATO concentration compared with cTACE, indicating that DEB-TACE might alleviate systemic adverse events of ATO compared with cTACE. Our study was consistent with a previous study. In that previous study, administration of doxorubicin via DEB-TACE produces lower plasma drug concentration at 6-hours post treatment compared with cTACE.23 The possible reasons for our result might derive from the better properties of DEB-TACE than that of cTACE: on one hand, cTACE uses lipiodol as drug carrier, which is not stable enough and will release ATO rapidly, leading to a higher plasma concentration post treatment compared with DEB-TACE. On the other hand, the lipiodol might escape via arterial branches whereas CSM blocks the tumor blood supply permanently.
According to a recent study, administration of doxorubicin (4 mg) via DEB-TACE yields an intratumoral doxorubicin concentration of 196.5±312.8 ng/mg at 1-day post treatment, while administering the same dose of doxorubicin via cTACE only yields a concentration of 61±4.9 ng/mg at the same time in a rabbit model with VX2 liver cancer. Besides, intratumoral concentration of doxorubicin remains at a high level (43.88±23.2 ng/mg) at 14-days post DEB-TACE treatment, while it is decreased to the negligible level from 7-days post cTACE treatment.21 Similar results are observed in another animal study that intratumoral concentration of doxorubicin (5 mg) maintains at a high level (23.1 nM/g) at 7-days post DEB-TACE treatment in a rabbit model with VX2 liver cancer.32 In addition, a more recent study shows that doxorubicin (4 mg) concentration remains at 28.09±5.10 μg/mL at 1-month post DEB-TACE treatment in rabbit liver.23 These studies indicate the higher drug intratumoral concentration and more sustained drug release of DEB-TACE compared with cTACE. In line with these previous studies, we discovered that administration of ATO via DEB-TACE exhibited higher intratumoral ATO concentration at 3-, 7- and 14-days post treatment compared with cTACE, and the concentration maintained at the high level at 14-days post DEB-TACE treatment, suggesting that administration of ATO via DEB-TACE produced higher intratumoral ATO concentration and more long-lasting ATO release than that of cTACE, which might bring in better treatment efficacy. The possible reason for our results might be due to that (1) ATO is tightly encapsulated in CSM whereas the lipiodol emulsion is not stable enough, thus CSM releases ATO more stably and sustainably than that of lipiodol; (2) CSM embolizes tumor blood supply permanently, whereas lipiodol would escape from the hepatic artery over time.
Although a lot of studies have investigated the safety of ATO in the animal model, the tissue distribution of ATO post DEB-TACE treatment remains unilluminated. Besides, liver and kidney function indexes of ATO post DEB-TACE treatment also need to be further explored.13,18 To this end, tissue distribution of ATO, liver function indexes and kidney function indexes were evaluated in this study, which revealed that administration of ATO via DEB-TACE exhibited reduced ATO concentration at 1-day post treatment while similar ATO concentration at 3-days, 7-days and 14-days post treatment in kidney and lung tissues compared with administration of ATO via cTACE. In addition, administration of ATO via DEB-TACE also had similar ATO concentration in normal liver, heart and muscle tissues, as well as similar ALT, AST, BUN and Scr levels compared with cTACE. Our studies implied that administration of ATO via DEB-TACE was as safe as cTACE. These data were in consistent with a previous study that ALT and AST levels were elevated at 1 day, but nearly decreased to normal range at 7 days post DEB-TACE or cTACE treatment, and no difference of ALT or AST level was observed between the DEB-TACE and cTACE groups.20 The possible explanation for the results might be that: Since the plasma ATO concentration in the CSM-ATO group is lower at an early stage but is similar at a later stage compared with cTACE-ATO, and ATO in normal tissues is derived from blood circulation, thus ATO concentration in kidney and lung tissues might also be lower at an early stage (1-day post treatment) but similar at a later stage (3 days, 7 days and 14 days) compared to cTACE.
As far as we know, it was the first study to investigate the pharmacokinetic profiles and tissue distribution of ATO post-TACE treatments, which highlighted the advantages of DEB-TACE over cTACE in ATO delivery, and provided novel insights for future liver cancer treatment. However, there were several limitations which should not be ignored. To begin with, the size of CSM in this study was 100–300 μm, while other sized CSM might exhibit different pharmacokinetic profiles, which needed further investigation. Also, an ATO dose of 0.5 mg/kg was applied in the current study in order to minimize the potential sufferings of rabbits, whereas it might not be the optimal dose, thus further studies with dose gradient needed to be conducted. Besides, there could be biological differences between male and non-pregnant female rabbits that might bring in confounding bias to the results. Finally, the experiments lacked spatial analysis of ATO distribution in the tumor, since ATO in tumor tissue might be unevenly distributed.
In conclusion, administration of ATO via DEB-TACE decreases systemic concentration but increases intratumoral concentration of ATO without increasing liver or kidney toxicity compared with cTACE.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 81401494) and the Henan Province Medical Science and Technology Project (No. 201403059).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Ward EM, Johnson CJ, et al. Annual report to the nation on the status of cancer, 1975–2014, featuring survival. J Natl Cancer Inst. 2017;109(9):djx030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]
- 4.Antman KH. Introduction: the history of arsenic trioxide in cancer therapy. Oncologist. 2001;6(suppl 2):1–2. doi: 10.1634/theoncologist.6-suppl_2-1 [DOI] [PubMed] [Google Scholar]
- 5.Leung LL, Lam S-K, Li -Y-Y, Ho JC-M. Tumour growth-suppressive effect of arsenic trioxide in squamous cell lung carcinoma. Oncol Lett. 2017;14(3):3748–3754. doi: 10.3892/ol.2017.6646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stevens JJ, Graham B, Dugo E, et al. Arsenic trioxide induces apoptosis via specific signaling pathways in HT-29 colon cancer cells. J Cancer Sci Ther. 2017;9(1):298–306. doi: 10.4172/1948-5956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi Y, Cao T, Huang H, et al. Arsenic trioxide inhibits cell growth and motility via up-regulation of let-7a in breast cancer cells. Cell Cycle. 2017;16(24):2396–2403. doi: 10.1080/15384101.2017.1387699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang X, Jia S, Yang S, et al. Arsenic trioxide induces G2/M arrest in hepatocellular carcinoma cells by increasing the tumor suppressor PTEN expression. J Cell Biochem. 2012;113(11):3528–3535. doi: 10.1002/jcb.v113.11 [DOI] [PubMed] [Google Scholar]
- 9.Alarifi S, Ali D, Alkahtani S, Siddiqui MA, Ali BA. Arsenic trioxide-mediated oxidative stress and genotoxicity in human hepatocellular carcinoma cells. Onco Targets Ther. 2013;6:75–84. doi: 10.2147/OTT.S38227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan B, Huang J-F, Wei Q, Zhang H, Ni R-Z. Anti-hepatoma effect of arsenic trioxide on experimental liver cancer induced by 2-acetamidofluorene in rats. World J Gastroenterol. 2005;11(38):5938–5943. doi: 10.3748/wjg.v11.i38.5938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin CC, Hsu C, Hsu CH, et al. Arsenic trioxide in patients with hepatocellular carcinoma: a phase II trial. Invest New Drugs. 2007;25(1):77–84. doi: 10.1007/s10637-006-9004-9 [DOI] [PubMed] [Google Scholar]
- 12.Barbey JT. Cardiac toxicity of arsenic trioxide. Blood. 2001;98(5):1632. doi: 10.1182/blood.V98.5.1632 [DOI] [PubMed] [Google Scholar]
- 13.GA R, El-Gareib AW. Toxic effects of gestational arsenic trioxide on the neuroendocrine axis of developing rats. Food Chem Toxicol. 2018. [DOI] [PubMed] [Google Scholar]
- 14.Swindell EP, Hankins PL, Chen H, Miodragovic DU, O’Halloran TV. Anticancer activity of small-molecule and nanoparticulate arsenic(III) complexes. Inorg Chem. 2013;52(21):12292–12304. doi: 10.1021/ic401211u [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song X, You J, Shao H, Yan C. Effects of surface modification of As2O3-loaded PLGA nanoparticles on its anti-liver cancer ability: an in vitro and in vivo study. Colloids Surf B Biointerfaces. 2018;169:289–297. doi: 10.1016/j.colsurfb.2018.05.024 [DOI] [PubMed] [Google Scholar]
- 16.Gallkowski U, Low A, Hirner A. Regional chemotherapy and transcatheter arterial chemoembolization. Digestion. 1998;59(suppl 2):83–85. doi: 10.1159/000051433 [DOI] [PubMed] [Google Scholar]
- 17.Liapi E, Georgiades CC, Hong K, Geschwind J-FH. Transcatheter arterial chemoembolization: current technique and future promise. Tech Vasc Interventional Radiol. 2007;10(1):2–11. doi: 10.1053/j.tvir.2007.08.008 [DOI] [PubMed] [Google Scholar]
- 18.Kim HJ, Shin JH, Kim TH, et al. Efficacy of transarterial embolization with arsenic trioxide oil emulsion in a rabbit VX2 liver tumor model. J Vasc Interv Radiol. 2009;20(10):1365–1370. doi: 10.1016/j.jvir.2009.07.005 [DOI] [PubMed] [Google Scholar]
- 19.Li H, Gong J, Jiang X, Shao H. Arsenic trioxide treatment of rabbit liver VX-2 carcinoma via hepatic arterial cannulation-induced apoptosis and decreased levels of survivin in the tumor tissue. Croat Med J. 2013;54(1):12–16. doi: 10.3325/cmj.2013.54.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi JW, Cho HJ, Park JH, et al. Comparison of drug release and pharmacokinetics after transarterial chemoembolization using diverse lipiodol emulsions and drug-eluting beads. PLoS One. 2014;9(12):e115898. doi: 10.1371/journal.pone.0115898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta S, Wright KC, Ensor J, et al. Hepatic arterial embolization with doxorubicin-loaded superabsorbent polymer microspheres in a rabbit liver tumor model. Cardiovasc Intervent Radiol. 2011;34(5):1021–1030. doi: 10.1007/s00270-011-0154-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong K, Khwaja A, Liapi E, et al. New intra-arterial drug delivery system for the treatment of liver cancer: preclinical assessment in a rabbit model of liver cancer. Clin Cancer Res. 2006;12(8):2563–2567. doi: 10.1158/1078-0432.CCR-05-2225 [DOI] [PubMed] [Google Scholar]
- 23.Zhang S, Huang C, Li Z, et al. Comparison of pharmacokinetics and drug release in tissues after transarterial chemoembolization with doxorubicin using diverse lipiodol emulsions and CalliSpheres beads in rabbit livers. Drug Deliv. 2017;24(1):1011–1017. doi: 10.1080/10717544.2017.1344336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parvinian A, Casadaban LC, Hauck ZZ, van Breemen RB, Gaba RC. Pharmacokinetic study of conventional sorafenib chemoembolization in a rabbit VX2 liver tumor model. Diagn Interv Radiol. 2015;21(3):235–240. doi: 10.5152/dir [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu HT, Yao QJ, Meng YL, et al. Arsenic trioxide intravenous infusion combined with transcatheter arterial chemoembolization for the treatment of hepatocellular carcinoma with pulmonary metastasis: long-term outcome analysis. J Gastroenterol Hepatol. 2017;32(2):295–300. doi: 10.1111/jgh.2017.32.issue-2 [DOI] [PubMed] [Google Scholar]
- 26.Li SW, He Y, Zhao HJ, et al. Assessment of 28 trace elements and 17 amino acid levels in muscular tissues of broiler chicken (Gallus gallus) suffering from arsenic trioxide. Ecotoxicol Environ Saf. 2017;144:430–437. doi: 10.1016/j.ecoenv.2017.06.061 [DOI] [PubMed] [Google Scholar]
- 27.Jiang L, Wang L, Chen L, et al. As(2)O(3) induces apoptosis in human hepatocellular carcinoma HepG2 cells through a ROS-mediated mitochondrial pathway and activation of caspases. Int J Clin Exp Med. 2015;8(2):2190–2196. [PMC free article] [PubMed] [Google Scholar]
- 28.Cui X, Wakai T, Shirai Y, et al. Arsenic trioxide inhibits DNA methyltransferase and restores methylation-silenced genes in human liver cancer cells. Hum Pathol. 2006;37(3):298–311. doi: 10.1016/j.humpath.2005.10.013 [DOI] [PubMed] [Google Scholar]
- 29.Shooshtary S, Behtash S, Nafisi S. Arsenic trioxide binding to serum proteins. J Photochem Photobiol B. 2015;148:31–36. doi: 10.1016/j.jphotobiol.2015.03.001 [DOI] [PubMed] [Google Scholar]
- 30.Song MJ, Park CH, Kim JD, et al. Drug-eluting bead loaded with doxorubicin versus conventional Lipiodol-based transarterial chemoembolization in the treatment of hepatocellular carcinoma: a case-control study of Asian patients. Eur J Gastroenterol Hepatol. 2011;23(6):521–527. doi: 10.1097/MEG.0b013e328346d505 [DOI] [PubMed] [Google Scholar]
- 31.Seki A, Hori S. Switching the loaded agent from epirubicin to cisplatin: salvage transcatheter arterial chemoembolization with drug-eluting microspheres for unresectable hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2012;35(3):555–562. doi: 10.1007/s00270-011-0176-0 [DOI] [PubMed] [Google Scholar]
- 32.Lee KH, Liapi EA, Cornell C, et al. Doxorubicin-loaded QuadraSphere microspheres: plasma pharmacokinetics and intratumoral drug concentration in an animal model of liver cancer. Cardiovasc Intervent Radiol. 2010;33(3):576–582. doi: 10.1007/s00270-010-9794-1 [DOI] [PMC free article] [PubMed] [Google Scholar]