Abstract
Prostate cancer remains one of the top common cancers in terms of incidence and cancer-related deaths. Approximately 1/3rd cases develop biochemical recurrence during surveillance post-definite therapy. Multiple imaging modalities, including computed tomography (CT), magnetic resonance imaging (MRI) (including multiparametric prostate MRI), bone scan, and positron emission tomography (PET) using different tracers are being used for the characterization of the prostate cancer recurrence. CT and MRI do not provide physiological information, thus have lower sensitivity in detecting the metastasis. A bone scan has low sensitivity (depending on the prostate-specific antigen level) with low specificity as well. Among different PET tracers, Axumin PET appears to be the most promising tool. Axumin PET is Food and Drug Administration approved for the evaluation of prostate cancer biochemical recurrence. Several studies have shown that Axumin PET findings played a key role in treatment modification by finding otherwise undetected lesions. We briefly discuss the salient characteristics, imaging protocol and image interpretation criteria for Axumin PET in the workup of prostate cancer biochemical recurrence.
Keywords: Prostate cancer, Biochemical recurrence, Prostate-specific antigen, Axumin (18F-fluciclovine), Positron emission tomography
INTRODUCTION
Prostate cancer is one of the top-ranking malignancies in terms of incidence and cancer-related mortality. After definite treatment, approximately 1/3rd develop recurrence detected by rising prostate-specific antigen (PSA) level. Accurate characterization of the recurrence is pivotal in planning further management. Multiple imaging modalities can be used to characterize the extent of the recurrence with their own advantages and limitations. A novel positron emission tomography (PET) tracer using 18F-fluciclovine (Axumin, Blue Earth Diagnostics, Bracco, Burlington, Massachusetts, USA) is approved by the Food and Drug Administration (FDA) for workup of the prostate cancer biochemical recurrence.
CASE REPORT
Case 1
An 85-year-old male was diagnosed as T2 prostate adenocarcinoma with Gleason score 6 and PSA 4 ng/mL in 2006. The patient underwent radiation therapy with excellent response to therapy and PSA nadir of 0.1 ng/mL. PSA level increased to 4.6 ng/mL in November 2018 and then further to 6.1 ng/mL in April 2019, indicative of biochemical recurrence. Magnetic resonance imaging (MRI) pelvis performed in April 2019 showed an 8 cc prostate gland with brachytherapy seeds and a new 1.7 cm × 1.3 cm enlarged left internal iliac lymph node. The new enlarged lymph node was concerning for local recurrence. Axumin PET scan was ordered to confirm the isolated recurrence at the left internal iliac lymph node so that local radiation therapy can be administered.
Subsequently, Axumin PET showed abnormal radiotracer uptake with a standardized uptake value maximum (SUVmax) of 5.3 only in the enlarged left internal iliac lymph node, consistent with metastatic lymphadenopathy [Figure 1]. However, no other suspicious uptake concerning for local or distant recurrence was noted. Hence, the patient is undergoing local pelvic radiation therapy with further confidence.
Figure 1:
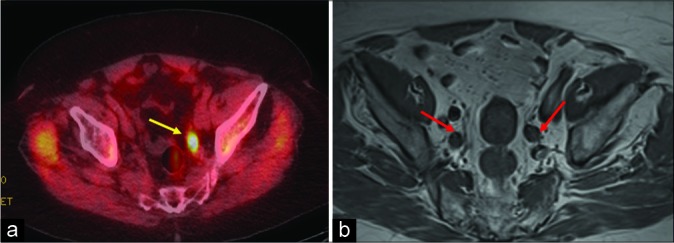
An 85-year-old male with history of prostate cancer status post radiation treatment presented with rising prostate- specific antigen level of 6.1 ng/mL. (a) Axumin positron emission tomography-computed tomography axial image showing increased radiotracer uptake (standardized uptake value maximum of 5.3) in the left internal iliac lymph node (yellow arrow). (b) Magnetic resonance imaging pelvis T1 weighted axial image depicting a few enlarged bilateral internal iliac lymph nodes (red arrow) which were otherwise inconclusive for recurrence.
Case 2
A 76-year male with a history of prostate adenocarcinoma had brachytherapy in the past with non detectable PSA post treatment. On surveillance, the PSA level was found to be elevated to 6.4 ng/mL, suspicious for biochemical recurrence. Thus, the patient was referred to our center for Axumin PET scan.
Axumin PET revealed focal asymmetric tracer activity with SUVmax 2.1 in the central prostate, slightly right to the midline [Figure 2]. The focal asymmetric activity in the prostate was higher than that of marrow activity (SUVmax 1.4). Hence, the prostatic hot focus was suspicious for local recurrence.
Figure 2:
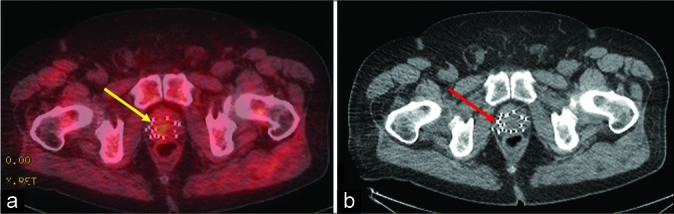
A 76-year-old male with history of prostate cancer status post brachytherapy with rising prostate-specific antigen level of 6.4 ng/mL. (a) Axumin positron emission tomography-computed tomography (CT) axial image showing focal asymmetric tracer activity in the prostate with standardized uptake value maximum (SUVmax) 2.1 (yellow arrow) in contrast to marrow activity (SUVmax 1.4). (b) Non-contrast CT of pelvis for radiation planning axial image showing brachytherapy seeds but no visible mass (red arrow).
Case 3
An 88-year male with a history of prostate adenocarcinoma had prior prostatectomy with non detectable PSA post treatment. On surveillance, computed tomography (CT) abdomen pelvis in February 2019 revealed soft tissue in prostatic bed suspicious for recurrence. Furthermore, the PSA level was found to be 6.9 ng/mL and the patient complained of progressive back pain for a few weeks duration. The bone scan did not reveal any suspicious bony metastasis. Thereafter, the patient was referred to our center for Axumin PET scan.
Axumin PET showed mild activity (SUVmax 1.5) in the prostate bed, but not significantly higher than the blood pool activity (SUVmax 1.4), thus not suspicious for recurrence at the prostatic bed per the guideline. However, a focus of intense tracer uptake (SUVmax 6.4) was noted in a 2.2 cm sclerotic lesion in the posterolateral aspect of the T8 vertebral body, consistent with osteoblastic metastasis [Figure 3].
Figure 3:
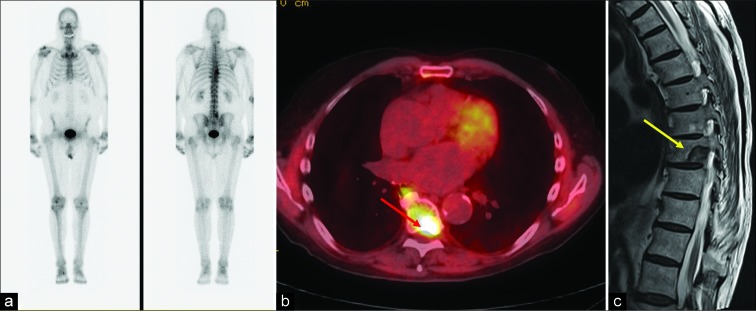
An 88-year-old male with history of prostate cancer status post prostatectomy presented with progressive back pain and prostate- specific antigen level of 6.9 ng/mL concerning for recurrence. (a) Bone scan was negative. (b) Axumin positron emission tomography- computed tomography axial image demonstrating intense tracer uptake (standardized uptake value maximum 6.4) in the left posterolateral aspect of the T8 vertebral body (red arrow). (c) Pre-biopsy magnetic resonance imaging performed showing T2 hypointense lesion (yellow arrow) measuring 2.2 cm which was consistent with osteoblastic metastasis on biopsy.
DISCUSSION
Prostate cancer is the most common site of new cancer cases (20%) and the second most common site of new cancer deaths (10%) in the US.[1] After definitive therapy for localized prostate cancer, patients are followed up with the PSA level. At least one-third of these patients later develop recurrence, detected by rising PSA level.[2] In rising PSA cases, only local recurrence is seen in 25–35%, only distant metastasis is seen in 20–25%, and both local and distant metastasis in 45–55%.[3] Additional information about the location (local versus systemic) and extent of tumor recurrence is required to plan further management.
MRI of the pelvis and CT abdomen pelvis with contrast have limitations in accurately depicting the disease extent. Although multiparametric MRI of the prostate is the diagnostic method of choice for T component of tumor staging, assessment of the extraprostatic spread is difficult with MRI. MRI evaluation of the prostatic bed is limited also due to brachytherapy seeds leading to artifacts. Both MRI and CT scans are not able to identify small but metastatic lymph nodes as abnormal since they do not reflect tumor physiology. A bone scan has low sensitivity and low specificity. Since bone scan shows the osteoblastic activity, bone scan is not sensitive in detecting the tumor proliferation in the soft tissues (prostate bed, extraprostatic extension, lymph nodes, and distant metastasis). Sensitivity is low especially at biochemical relapse with low PSA level as evidenced by <5% of positivity in bone scan when PSA level was <7 ng/mL.[4] The specificity of bone scan is low since it cannot differentiate the bony metastasis versus flare phenomenon or other nonspecific osteoblastic activity.
Given the limitations of conventional anatomical imaging modalities to accurately characterize the cause for biochemical relapse, PET imaging with novel tracers is an emerging and promising solution. The different tracers being used for prostate cancer evaluation are 18F-fluorodeoxyglucose (FDG) (glucose metabolism), 18F-sodium fluoride (osteoblastic activity), 11C-choline/18F-choline (membrane turnover), 68Ga-prostate-specific membrane antigen (PSMA) and 18F-fluciclovine (amino acid).
18F-FDG is the most commonly used PET tracer in the oncology world. However, it is of limited use in prostate cancer biochemical relapse due to low sensitivity. Low sensitivity might be attributed to (a) small size of recurrent lesion, (b) most prostate cancer utilizing non-glucose metabolic pathway instead,[5] and (c) adjacent intense urinary bladder activity. Choline PET appears to be inferior to multiparametric MRI for local recurrence,[6] but lymph nodal and distant metastatic lesions can be reliably assessed.[7] A metaanalysis in 2013 revealed that choline PET detected lymph nodal metastasis with 100% sensitivity and with 81.8% specificity.[8] Detection is highly dependent on PSA value, with a study showing PSA value of 1.4 ng/mL predicting positive scan.[9] NCCN guideline recommends consideration of choline PET in cases of biochemical recurrence.[10] 68Ga-PSMA is another emerging tracer, which has better tumor- to-background ratio and higher detection rate compared to choline PET, especially at relatively lower PSA level.[7] However, 68Ga-PSMA is not FDA approved and not widely available (given that the half-life of 68Ga is short at only 68 min).
18F-fluciclovine (Axumin) is a novel tracer which is leucine amino acid analog. Axumin was approved by the FDA on May 27, 2016, for the imaging of suspected prostate cancer recurrence based on elevated PSA level following treatment.[11] Axumin PET has high diagnostic accuracy in post-treatment cases, particularly compared to 11C-Choline PET.[12] A study showed detection rates of 72.0%, 83.3%, and 100% at PSA levels <1, 1–2, and equal or more than 2 ng/ mL, respectively.[13] A study showed negative predictive value (NPV) of 100% with PSA value of more than 1.05 ng/mL, but 80% with PSA value of equal or <1.05 ng/ mL.[14] Unsurprisingly, the performance of Axumin PET is also dependent on PSA level, but the notable fact of 100% NPV at PSA level more than 1.05 ng/mL is remarkable. Unlike bone scan which only shows osteoblastic process, Axumin PET shows both bony and soft tissue lesions indicative of tumor cellular proliferation. The findings on Axumin PET have been crucial in treatment modification (e.g., widening radiation field because more local lesions are seen or avoiding radiation altogether because distant metastasis is seen, which were not picked up by other modalities or other PET tracers).[13]
The amino acid movement across the tumor cells occur fairly rapidly, with peak time ranging from 4 to 10 min.[2] Thus, the Axumin is injected while the patient is lying on the scanner and the images are obtained approximately 3–5 min post- injection, whereas in FDG PET, FDG is injected in a quiet room and scanned 45–90 min post-injection. The scan is obtained caudocranially to obtain high target to background ratio (target being the pelvis). Fasting is recommended for at least 4 h before the scan, to reduce the free amino acid levels in the blood that might compete with tracer (amino acid analog) uptake by the tumor cells.[15] Contrary to FDG PET (which is performed with empty bladder), Axumin PET is performed with full bladder as full bladder will decrease the urinary excretion of the contrast and dilute any excreted contrast. Thus, it is recommended to avoid emptying the bladder at least 30 min before the scan.[16]
It has remarkably favorable biodistribution for prostate cancer imaging because it has very minimum renal excretion and urinary bladder activity, thus avoiding the high activity in urinary bladder (seen in other PET tracers) interfering with interpretation of prostatic bed/pelvic lymph nodes. The most intense uptake is noted in the pancreas and liver (liver being the critical organ). Variable uptake is noted in salivary glands, bone marrow, and muscles. Unlike FDG, uptake in brain is minimal. The degree of uptake in a lesion is categorized compared to uptake in the blood pool, bone marrow (L3 vertebral body as reference), and liver as (a) mild: Uptake equal or greater than blood pool, but less than marrow; (b) moderate: Uptake equal or greater than marrow, but less than liver; and (c) intense: Uptake equal or greater than liver.
Visual subjective assessment is primarily used rather than SUV measurement to identify the abnormal uptake. The significance of uptake intensity depends on the size of the anatomical correlate. Lesion <1 cm has significant uptake even if it is “mild” uptake (uptake equal or greater than blood pool, but less than marrow). However, lesion 1 cm or larger needs at least “moderate” uptake (uptake equal or greater than marrow, but less than liver) to be considered significant. If SUV measurement is required to make a decision about significant pathologic uptake, SUVmax 1.2 times the SUVmax of the L3 vertebral body can also be considered significant.[2] In non-prostatectomy cases, “moderate” focal asymmetric and “moderate” heterogeneous uptake are concerning for local recurrence; but homogenous uptake usually is not. However, similar to the other tracers, it is non-specific as well showing uptake in infection, inflammation, benign prostatic hyperplasia, and other malignancies.
CONCLUSION
Currently, Axumin is FDA approved only for biochemical recurrence of prostate cancer after definitive therapy. Axumin is not approved for routine initial workup of prostate cancer as yet. Since Axumin is better at characterizing the lymph nodal and distant metastasis of prostate cancer, Axumin is likely to be used for initial workup and restaging of prostate cancer in the future. The information obtained from Axumin PET directly correlates with the target or field for surgical or radiation therapy.[17] Nuclear medicine technologists and reporting radiologists should be aware of the characteristics, imaging protocol, and interpretation criteria for Axumin PET to best utilize this novel exciting diagnostic tool for optimal patient management.
Footnotes
How to cite this article: Songmen S, Nepal P, Olsavsky T, Sapire J. Axumin positron emission tomography: Novel agent for prostate cancer biochemical recurrence. J Clin Imaging Sci 2019;9:49.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.American Cancer Society Cancer Facts and Figures. 2019 Available from: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2019.html [Last accessed on 2019 Jun 25]
- 2.Gusman M, Aminsharifi JA, Peacock JG, Anderson SB, Clemenshaw MN, Banks KP, et al. Review of 18F-fluciclovine PET for detection of recurrent prostate cancer. Radiographics. 2019;39:822–41. doi: 10.1148/rg.2019180139. [DOI] [PubMed] [Google Scholar]
- 3.Carroll P. Rising PSA after a radical treatment. Eur Urol. 2001;40(Suppl 2):9–16. doi: 10.1159/000049879. [DOI] [PubMed] [Google Scholar]
- 4.Gomez P, Manoharan M, Kim SS, Soloway MS. Radionuclide bone scintigraphy in patients with biochemical recurrence after radical prostatectomy: When is it indicated? BJU Int. 2004;94:299–302. doi: 10.1111/j.1464-410X.2004.04927.x. [DOI] [PubMed] [Google Scholar]
- 5.Liu Y, Zuckier LS, Ghesani NV. Dominant uptake of fatty acid over glucose by prostate cells: A potential new diagnostic and therapeutic approach. Anticancer Res. 2010;30:369–74. [PubMed] [Google Scholar]
- 6.Vees H, Buchegger F, Albrecht S, Khan H, Husarik D, Zaidi H, et al. 18F-choline and/or 11C-acetate positron emission tomography: Detection of residual or progressive subclinical disease at very low prostate-specific antigen values (<1 ng/ mL) after radical prostatectomy. BJU Int. 2007;99:1415–20. doi: 10.1111/j.1464-410X.2007.06772.x. [DOI] [PubMed] [Google Scholar]
- 7.Wallitt KL, Khan SR, Dubash S, Tam HH, Khan S, Barwick TD, et al. Clinical PET imaging in prostate cancer. Radiographics. 2017;37:1512–36. doi: 10.1148/rg.2017170035. [DOI] [PubMed] [Google Scholar]
- 8.Evangelista L, Zattoni F, Guttilla A, Saladini G, Zattoni F, Colletti PM, et al. Choline PET or PET/CT and biochemical relapse of prostate cancer: A systematic review and meta-analysis. Clin Nucl Med. 2013;38:305–14. doi: 10.1097/RLU.0b013e3182867f3c. [DOI] [PubMed] [Google Scholar]
- 9.Giovacchini G, Picchio M, Coradeschi E, Bettinardi V, Gianolli L, Scattoni V, et al. Predictive factors of [(11)C]choline PET/CT in patients with biochemical failure after radical prostatectomy. Eur J Nucl Med Mol Imaging. 2010;37:301–9. doi: 10.1007/s00259-009-1253-3. [DOI] [PubMed] [Google Scholar]
- 10.National Comprehensive Cancer Network NCCN Guidelines Version 2.2019 Prostate Cancer. doi: 10.6004/jnccn.2019.0034. Available from: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf [Last accessed on 2019 Jun 25] [DOI] [PMC free article] [PubMed]
- 11.US Food and Drug Administration FDA Approves New Diagnostic Imaging Agent to Detect Recurrent Prostate Cancer. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-new-diagnostic-imaging-agent-detect-recurrent-prostate-cancer [Last accessed on 2019 Jun 25]
- 12.Nanni C, Zanoni L, Pultrone C, Schiavina R, Brunocilla E, Lodi F, et al. (18)F-FACBC (anti1-amino-3-(18)F-fluorocyclobutane-1-carboxylic acid) versus (11)C-choline PET/CT in prostate cancer relapse: Results of a prospective trial. Eur J Nucl Med Mol Imaging. 2016;43:1601–10. doi: 10.1007/s00259-016-3329-1. [DOI] [PubMed] [Google Scholar]
- 13.Akin-Akintayo OO, Jani AB, Odewole O, Tade FI, Nieh PT, Master VA, et al. Change in salvage radiotherapy management based on guidance with FACBC (Fluciclovine) PET/CT in postprostatectomy recurrent prostate cancer. Clin Nucl Med. 2017;42:e22–e28. doi: 10.1097/RLU.0000000000001379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schuster DM, Nieh PT, Jani AB, Amzat R, Bowman FD, Halkar RK, et al. Anti-3-[(18)F]FACBC positron emission tomography-computerized tomography and (111)In-capromab pendetide single photon emission computerized tomography-computerized tomography for recurrent prostate carcinoma: Results of a prospective clinical trial. J Urol. 2014;191:1446–53. doi: 10.1016/j.juro.2013.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oka S, Kanagawa M, Doi Y, Schuster DM, Goodman MM, Yoshimura H, et al. Fasting enhances the contrast of bone metastatic lesions in18 F-fluciclovine-PET: Preclinical study using a rat model of mixed osteolytic/Osteoblastic bone metastases. Int J Mol Sci. 2017;18:E934. doi: 10.3390/ijms18050934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Savir-Baruch B, Zanoni L, Schuster DM. Imaging of prostate cancer using fluciclovine. PET Clin. 2017;12:145–57. doi: 10.1016/j.cpet.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Incerti E, Mapelli P, Gianolli L, Picchio M. PET imaging for lymph node dissection in prostate cancer. World J Urol. 2017;35:507–15. doi: 10.1007/s00345-016-1954-8. [DOI] [PubMed] [Google Scholar]