Abstract
Objective:
The objective of the study was to determine the positive predictive value (PPV) of architectural distortions (AD) observed on digital breast tomosynthesis (DBT) and without an ultrasound (US) correlate.
Materials and Methods:
In this single-institution, retrospective study, patients who underwent DBT-guided biopsies of AD without any associated findings on digital mammography (DM) or DBT, and without a correlate on targeted US exam, over a 14-month period were included in this study. All patients had DM and DBT and targeted US exams. The PPV was computed along with the exact 95% confidence limits (CL) using simple binomial proportions, with histopathology as the reference standard.
Results:
A total of 45 ADs in 45 patients met the inclusion criteria. Histopathology indicated 6/45 (PPV: 13.3%, CL: 5.1–26.8%), ADs were malignant, including one high-risk lesion that was upgraded at surgery. ADs were appreciated only on DBT in 12/45 (26.7%) patients, and on both DBT and DM in 33/45 (73.3%) patients, and the corresponding PPV was 25% (3/12, CL: 5.5–57.2%) and 9.1% (3/33, CL: 1.9–24.3%), respectively. In all analyses, the observed PPV significantly exceeded the 2% probability of malignancy for Breast Imaging Reporting and Data System-3 diagnostic categories (P < 0.004).
Conclusions:
The PPV of malignancy in DBT detected AD without an US correlate in our series of 45 cases was 6/45 (13.3%). In the absence of an US correlate, the PPV of AD is lower than that mentioned in prior literature but exceeds the 2% threshold to justify DBT-guided biopsy.
Keywords: Breast, Cancer, Digital breast tomosynthesis, Architectural distortion, Positive predictive value
INTRODUCTION
Architectural distortion (AD) is defined by the Breast Imaging Reporting and Data System (BI-RADS®)[1] as “distortion of normal parenchyma with no definite mass visible. This includes thin straight lines or spiculations radiating from a point and focal retraction, distortion, or straightening at the edges of the parenchyma.” AD is observed in up to 6% of screening mammograms.[2] AD is often subtle and is the third most common cause of missed non- palpable breast cancer, accounting for 12–45% of missed breast cancers.[2-6] The advent of digital breast tomosynthesis (DBT) has allowed radiologists to identify AD more often and more confidently.[3,5,7-10] DBT continues to gain wider acceptance due to increased cancer detection and lower recall rates.[11-13]
Multiple benign and malignant etiologies can manifest as AD on a mammogram. The common benign causes are radial scar/complex sclerosing lesion (CSL), dense stromal fibrosis, sclerosing adenosis, post-operative scar, and fat necrosis. Malignant causes include invasive ductal carcinoma (including the tubular carcinoma subtype), invasive lobular carcinoma, and ductal carcinoma in situ. It is difficult to distinguish malignancies associated with AD based on imaging morphology.[2-4,7-9,14-16] The workup of AD includes full-field, spot compression or magnification, two-dimensional digital mammography (DM), full-field or spot-compression DBT, targeted ultrasound (US), problem- solving magnetic resonance imaging (MRI), and/or imaging- guided biopsy.[3,7] Although DBT-guided percutaneous biopsies and needle localizations are increasingly utilized, this capability is not yet widely available.[17] Durand et al. highlighted the lack of evidence-based guidelines for management of DBT-detected AD with no correlate with other imaging modalities. They suggest either excisional biopsy or short term follow up (BI-RADS 3) if a DBT-based guidance system was unavailable.[3]
An AD with an US correlate has an increased likelihood of malignancy.[7,8,18] In their large series of 181 ADs, Alshafeiy et al.[18] found only 26 with no US correlate. Only 2 of these 26 cases were malignant (7.7%). Partyka et al.[5] in a smaller study noted no malignancies in ADs without an US correlate. However, Freer et al.[19] noted a 47% malignancy rate (17/36) in their study of 36 ADs that were surgically excised. Thus, there is a wide reported range for the positive predictive value (PPV) of DBT-detected AD without an US correlate. There are also limited data on the outcome of malignancy in ADs without an US correlate.
A DBT-based guidance system for performing percutaneous biopsies became available a few years ago. In this study, we focused on malignant outcomes of a subset of DBT- detected ADs without an US correlate and without associated findings on DM or DBT such as a mass, asymmetry, or microcalcifications. The intent of this study was to determine if short-term follow-up, an option suggested by Durand et al.[3] is still appropriate considering the availability of DBT-guided biopsy systems.
MATERIALS AND METHODS
This single-institution, retrospective study was conducted in accordance with our institutional review board-approved and Health Insurance Portability and Accountability Act – compliant protocol with waiver of informed consent. All imaging and procedures were performed as standard of care.
Inclusion/exclusion criteria
The inclusion criteria were: (1) Women who underwent image-guided biopsies using an upright biopsy guidance system (Affirm®, Hologic Inc., Marlborough, MA) used in conjunction with a DBT system (Selenia® Dimensions®, Hologic Inc., Marlborough, MA) between January 2016 and February 2017; (2) an indication of AD without an US correlate; (3) women who had undergone imaging evaluation with all three modalities – DM, DBT, and targeted US; (4) retrospective imaging evaluation by two fellowship-trained breast imagers to confirm the indication of AD without an US correlate; and (5) availability of biopsy pathology results in electronic records. Women who did not satisfy all of the above inclusion criteria were excluded from this study.
Retrospective data were retrieved from the electronic radiology database. During this period 66 upright DBT-guided biopsies were performed using the standard technique with six samples obtained using a 9G E-Viva needle (Hologic Inc., Marlborough, MA). On review of the electronic medical record, the indication for biopsy in 47/66 cases was AD without an US correlate. One of three breast-certified US technologists (10–20 years experience), who perform only breast sonograms, did all the scans. A fellowship-trained breast radiologist simultaneously evaluates the images and if necessary, personally scans and obtains additional images. The 19/66 cases where the indication for biopsy was not AD were excluded and the remaining 47/66 cases were eligible to be considered for the study. The images of these 47 cases were reviewed by two fellowship- trained breast imagers (study authors 1 and 2) to ensure that all patients had undergone imaging evaluation with all three modalities - DM, DBT, and targeted US. Lack of an obvious mass or suspicious microcalcifications on tomosynthesis images and absence of a correlate on US were confirmed. During this review, two (2/47) cases demonstrated the question of a central asymmetry on DBT images and were, therefore, excluded from the analysis. Biopsy pathology results were available for all 45 patients. Thus, 45 patients satisfied inclusion criteria and none were excluded from this study.
Reference standard
All retrieved biopsy samples and surgically excised tissues underwent histopathological evaluation by one of two pathologists specializing in breast cancer with 10 and 20 years of experience. Since selective surgical excision of high-risk lesions could contribute to bias, two reference standards were used for the presence of malignancy: (1) Histopathology results from core-needle biopsy alone and (2) all available histopathology results that include the results from core-needle biopsy and from subsequent surgical excision, if the patient underwent surgery. A 2-year negative imaging follow-up was considered truth for the absence of malignancy.
Statistical analysis
The primary aim of this study was to determine the PPV of AD with no associated findings on DM or DBT and without a correlate on targeted US. With histopathology results serving as the truth, the PPV was computed and the exact (Clopper–Pearson) 95% confidence limits (CL) were determined by simple binomial proportions. Fisher’s exact test was used to compare the outcomes from independent groups. The effects associated with P < 0.05 were considered significant. All analyses were performed using statistical software (SAS® version 9.4., SAS Institute Inc., Cary, NC).
RESULTS
Demographics
Forty-five patients with 45 ADs met the inclusion criteria. Mean (±standard deviation) age was 58.1 ± 10.7 years (range: 28–78.7 years). Of the 45 ADs in the study, 31 were noted on screening exams and 14 were seen on diagnostic imaging. None of the 14 diagnostic cases were symptomatic. The indications for these diagnostic exams were short interval follow-up of a previously noted AD or routine annual evaluation in a patient with history of treated breast cancer. The incidence of AD did not vary with laterality in our cohort, with 21 and 24 lesions in the right and left breasts, respectively. In 12 of 45 (26.7%) patients, AD was seen only on DBT and not appreciated on DM. ADs were noted in both DM and DBT in the remaining 33 of 45 (73.3%) patients, with the DBT depicting the lesion better than the DM in all 33 patients. ADs were seen in both views in 40/45 (88.9%) patients, on craniocaudal view only in 3/45 (6.7%) patients, and on mediolateral oblique view only in 2/45 (4.4%) patients.
Pathology of core needle biopsy and subsequent follow-up
The pathology outcomes from core-needle biopsy are outlined in Table 1 and subsequent follow-up is outlined in Table 2. Histopathology results from DBT-guided core- needle biopsies were available for all 45 ADs, of which 5 were malignant. Among patients with malignancies, 4/5 were initially recalled from a screening examination and 1/5 was from a diagnostic examination (assigned a BI-RADS 3 on a prior exam). Figure 1 shows an example of a patient recalled from screening. On reviewing subsequent surgery and follow-up information, 13/45 patients underwent surgery at our institution and have been followed for 2 years; 28/45 cases did not undergo surgery post-biopsy and have had imaging follow-up for 2 years; and 4/45 (8.9%) had breast surgery at outside facilities and were lost to follow-up. All radial scars with atypia are recommended surgery at our institution. For radial scars without atypia, patient preference for surgery or imaging follow-up guided management and 6/12 patients declined surgery.
Table 1:
Histopathology of architectural distortions without associated findings such as a mass, focal asymmetry, or microcalcifications on DM and DBT, and without an ultrasound correlate (*One complex sclerosing lesion with atypical ductal hyperplasia was upgraded to ductal carcinoma in situ after surgical excision and is included in the malignant category).
| Histology | Either DM or DBT (n=45) | Both DM and DBT (n=33) | DBT only (n=12) |
|---|---|---|---|
| Malignant | |||
| Invasive lobular carcinoma | 2 | 1 | 1 |
| Invasive ductal carcinoma | 1 | 1 | 0 |
| Invasive ductal-lobular carcinoma | 1 | 1 | 0 |
| Ductal carcinoma in situ* | 2 | 0 | 2 |
| Total (including 1 surgical upgrade) | 6 | 3 | 3 |
| High-risk lesions | |||
| Radial scar/complex sclerosing lesion | 12 | 10 | 2 |
| Atypical ductal hyperplasia | 3 | 2 | 1 |
| Complex sclerosing lesion+atypical lobular hyperplasia | 1 | 1 | 0 |
| Complex sclerosing lesion+atypical ductal hyperplasia* | 2 | 1 | 1 |
| Total | 18 | 14 | 4 |
| Benign | |||
| Dense stromal fibrosis | 18 | 13 | 5 |
| Pseudoangiomatous stromal hyperplasia | 1 | 1 | 0 |
| Focal sclerosing lesion | 1 | 1 | 0 |
| Papillary lesion | 1 | 1 | 0 |
| Total | 21 | 16 | 5 |
DM: Digital mammography, DBT: Digital breast tomosynthesis
Table 2:
Summary of post-biopsy follow-up (n=45). Post-biopsy, 17/45 underwent surgery, of which 13/17 were at our institution and the remainder (4/17) outside our institution and hence were lost to follow-up. The remaining 28/45 cases (22 benign and 6 radial scars/ complex sclerosing lesions without atypia) have had 2-year imaging follow-up to date that was negative.
| Malignant | Radial scar/complex sclerosing lesion with atypia | Radial scar/complex sclerosing lesion without atypia | Benign | Total | |
|---|---|---|---|---|---|
| Biopsy outcomes | 5 | 6 | 12 | 22 | 45 |
| Post-biopsy surgery at our institution | 3 | 4 (1 upgrade) | 6 | 0 | 13 |
| Post-biopsy surgery outside our institution and lost to follow-up | 2 | 2 | 0 | 0 | 4 |
Figure 1:
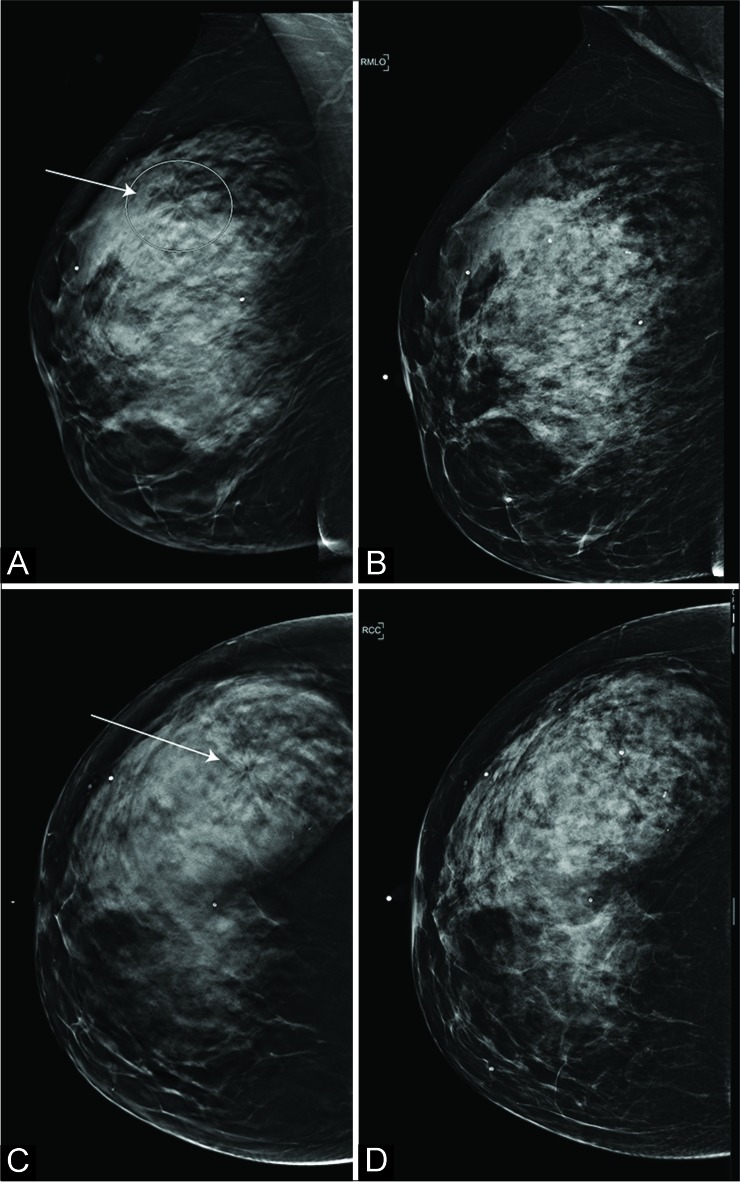
A 59-year-old woman recalled from screening mammography for the right upper outer quadrant architectural distortion (AD). The AD (arrows in A and C; ellipse in A) was well appreciated in digital breast tomosynthesis (DBT) (A: Mediolateral oblique view and C: Craniocaudal view) compared to digital mammography (B: Mediolateral oblique view and D: Craniocaudal view). No correlate was identified on the targeted ultrasound exam. Subsequent DBT-guided biopsy indicated invasive lobular carcinoma.
Surgical outcomes for 3/5 cancers were available and final surgical histopathology was concordant with imaging and with the histopathology from DBT-guided biopsy. The remaining two patients sought treatment elsewhere. Among 18 patients with radial scar/CSL, 6 patients had associated atypia (atypical ductal hyperplasia, n = 5 and atypical lobular hyperplasia, n = 1), of which 4 underwent surgery at our institution. One patient, who had CSL with atypical ductal hyperplasia on DBT-guided biopsy was upgraded to ductal carcinoma in situ following surgical excision. In the 2/6 patients who sought surgical treatment at another institution, surgical concordance with biopsy results could not be verified. Among the remaining 12 patients with CSL/ radial scar without atypia, 6 patients opted for surgery, and histopathology from surgery was concordant with core- needle biopsy in all patients. The remaining 6 (who opted for imaging follow-up) have had a 2-year follow-up with no significant interval change on imaging. Figure 2 shows an example.
Figure 2:
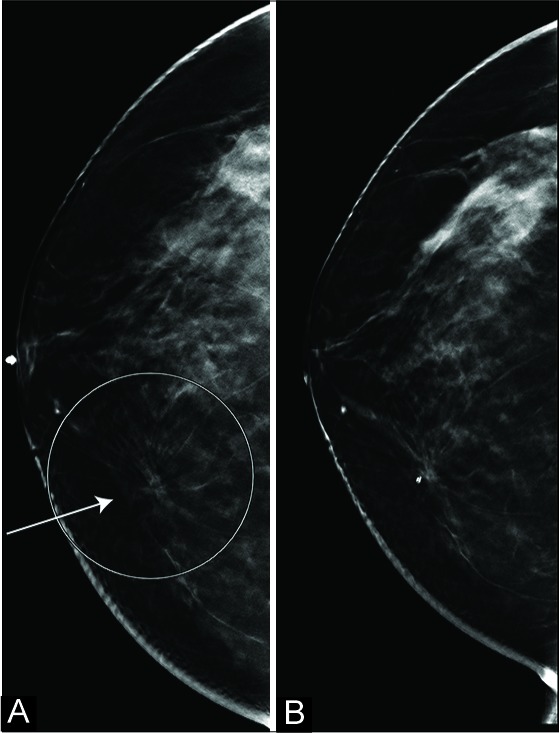
A 50-year-old woman recalled from screening mammography for right lower inner quadrant architectural distortion (AD). The AD (ellipse and arrow in A; craniocaudal view) was well appreciated in digital breast tomosynthesis (DBT) (A). No correlate was identified on targeted ultrasound. Histopathology from biopsy indicated radial scar/complex sclerosing lesion without atypia. 2-year follow-up DBT (B) shows biopsy changes and adjacent biopsy-marker.
PPV
Thus, a total of 6/45 AD without associated findings on DM or DBT and without an US correlate were malignant, resulting in PPV of 13.3% (CL: 5.1–26.8%). When the histopathology results from core-needle biopsy alone are considered as the reference standard, 5/45 were malignant, resulting in PPV of 11.1% (CL: 3.7–24.1%). If surgical pathology is considered the reference standard, i.e., after excluding 4 patients (2 biopsy- proven malignancies and 2 radial scars/CSL with atypia) who were lost to follow-up, 4/41 (9.8%; CL: 2.7–23.1%) were malignant. Irrespective of the reference standard, the cancer rate of DBT-detected AD without an US correlate exceeded the 2% threshold of BI-RADS 3 (P < 0.0002). Among the 33 AD seen on both DM and DBT, 3/33 were malignant, resulting in a PPV of 9.1% (CL: 1.9–24.3%). DM missed 12/45 (26.7%; CL: 14.6–41.9%) ADs and these were detected only on DBT. Three of these 12 ADs were malignant, resulting in PPV of 25% (CL: 5.5–57.2%) for AD seen only on DBT. None of the ADs were seen only on DM, but not on DBT.
DISCUSSION
In our series of 45 DBT-guided biopsy of AD, 5/45 were malignant on biopsy pathology (an additional cancer was noted at a subsequent surgical biopsy of a high-risk lesion), 18/45 documented a high-risk lesion, and 22/45 had benign pathology. The PPV of malignancy in our cohort of 45 cases was 9.8–13.3%, depending on the reference standard. Importantly, irrespective of the reference standard, the PPV exceeded the 2% threshold of BI-RADS 3 (P < 0.0002). Considering the availability of DBT-guided biopsy systems, we do not support a BI-RADS 3 recommendation as mentioned in earlier literature.[3] We recommend biopsy as the next step.
The likelihood of a malignant outcome in biopsies of ADs varies from 25% to 75%.[4,5,8,9,14,15,18,19] Most studies were before the introduction of DBT and were biopsied under DM, US, or MRI guidance.[4,5,8] AD is being diagnosed more often and confidently in DBT.[3,5,7-10] In the initial years after the introduction of DBT, a DBT-based biopsy guidance system was unavailable. Hence, in several studies after the introduction of DBT, most of the ADs had correlates on other modalities, which were used for biopsy guidance.[5,9,19] Our study differs in that every subject had all three imaging exams (acquired DM, DBT, and US) and all ADs were detected by DBT. Synthesized mammography is not used in our institution. There were no associated findings on DM or DBT, there was no US correlate, and biopsies were performed under DBT guidance. Hence, direct comparison with prior studies is challenging, where appropriate, selective cohorts from prior studies were used for comparison.
An early study using pre-operative DBT-guided needle localization followed by surgical excision of DBT-detected lesions that were occult on other modalities (DM, US, and MRI, if performed), reported 17/36 (47%) were malignant.[19] In this study, 12 ADs were detected by DBT alone, of which 3 were malignant, and the cancer rate 25% (3/12) was not significantly different (P = 0.311, Fisher’s exact test). The DBT-guided pre-surgical needle localization required the expertise of radiologists, surgeons, and anesthesiologists and involved operating room time.[15] This makes needle localization less cost-effective.
Bahl et al. reported that AD is seen more often on DBT than on DM and that 31/106 (29.2%) DBT-detected AD without an US correlate were malignant.[8] Our study also observed that more AD was detected on DBT than on DM (n = 45 vs. n = 33), and the PPV in our study was marginally different (P = 0.0404, Fisher’s exact test). However, in Bahl et al.,[8] some of the patients had associated findings on DM, whereas there were none in our study. Subsequent to the availability of DBT-based biopsy guidance system, there have been a few studies.[18,20,21] Schrading et al. reported on the technical comparison between DBT-guided and stereotactic biopsies and noted a reduction in procedure time with DBT-guided biopsies.[21] Waldherr et al. reported on outcomes from DBT-guided biopsies of findings without a US correlate that included 24 patients with ADs, of which 13 were malignant (54%).[20] The PPV significantly differed from our study (P = 0.0005, Fisher’s exact test). It is unclear if the ADs included in their study had associated findings on DM or DBT. None of the ADs in our study had associated findings on DM or DBT.
In a study comparing outcomes for DM-detected and DBT- detected AD, when lesions without a US correlate alone are considered, 5/45 (11.1%) were malignant, of which 2/26 (7.7%) were detected by DBT alone.[18] In our study, 3/33 (9.1%) DM-detected AD and 3/12 (25%) DBT-detected AD were malignant and the PPVs were not significantly different (P > 0.301, Fisher’s exact test). One would expect a higher PPV in DM-detected ADs; however, DM-detected ADs have higher likelihood of an US correlate,[18] and patients with US correlate were excluded in our study. Patel et al. reported that 9/34 DBT-detected ADs without US correlate were malignant on DBT-guided core-needle biopsy and the PPV (26%) reported in that study[22] was not significantly different from our study (P = 0.135, Fisher’s exact test).
To the best of our knowledge, our cohort, though relatively small, of 45 DBT-detected ADs without any associated findings such as a mass, a focal asymmetry, or microcalcifications on DM or DBT and without an US correlate and biopsied under DBT-guidance may be the largest to date. Literature on pathology outcomes of ADs seen on DBT without associated findings on DM or DBT and without an US correlate is relatively sparse. In our cohort of 45 ADs seen on DBT, the cancer rate was 6/45 (13.3%). The 95% CLs (5.1–26.8%) indicate that the likelihood of malignancy may be as high as 27%.
A high-risk lesion was demonstrated in 18/45 (40%) biopsies, as outlined in Tables 1 and 2. Patients with high-risk lesions are selectively recommended for surgery based on imaging and core-needle histopathology at most institutions. The upgrade rates vary from <2% to 18%.[15,16,23,24] In our opinion, excluding patients who did not undergo surgery could contribute to statistical bias by preferentially decreasing the denominator and hence, resulting in higher PPV. Hence, for completeness, the PPVs are reported with histopathology results from core-needle biopsy alone and from all available histopathology that include the results from both core- needle biopsy and from surgery (if performed). We conclude that the probability of cancer would be no <5.1% (lower bound of 95% CL). The management of radial scar is evolving and debatable. Current literature suggests that not all radial scars merit surgical excision. The cancer upgrade rates are low.[15,16] The PPV of DBT guided biopsies of AD at our center exceeds the 2% threshold of BI-RADS 3. Since differentiating benign from malignant causes of AD on imaging is difficult, we recommend DBT-guided biopsy of AD as the next step in the management of AD at our institute.
Our study had limitations. Evaluation of AD is compounded by interobserver variability.[25] We only included patients biopsied with a DBT guidance system with an initial diagnosis of AD. There is a possibility that additional patients with AD may have been excluded if the initial diagnosis was different. The study reflects the initial outcomes at a single institution. Some patients (8/18) with radial scars in our series did not have the benefit of surgical pathology. The patients with benign histology (22/45) have had a 2-year imaging follow-up to date that was negative, but long-term follow-up would be useful.
CONCLUSIONS
The PPV of DBT-detected ADs without a correlate on targeted US was 13% (CL: 5.1–26.8%). Our findings corroborate prior literature with a smaller sample size. There is need for follow- up studies with larger sample size to confirm the PPV of AD detected on DBT and without an US correlate.
Acknowledgments
This work was supported in part by grants R01 CA195512 and R01 CA199044 from the National Cancer Institute (NCI) of the National Institutes of Health (NIH). The contents are solely the responsibility of the authors and do not reflect the official views of the NCI or the NIH.
Footnotes
How to cite this article: Vijayaraghavan GR, Newburg A, Vedantham S. Positive predictive value of tomosynthesis-guided biopsies of architectural distortions seen on digital breast tomosynthesis and without an ultrasound correlate. J Clin Imaging Sci 2019;9:53.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.ACR Breast Imaging Reporting and Data Systems (BI- RADS): Breast Imaging Atlas. 5th ed. Reston, VA: American College of Radiology; 2013. ACR. [Google Scholar]
- 2.Gaur S, Dialani V, Slanetz PJ, Eisenberg RL. Architectural distortion of the breast. AJR Am J Roentgenol. 2013;201:W662–70. doi: 10.2214/AJR.12.10153. [DOI] [PubMed] [Google Scholar]
- 3.Durand MA, Wang S, Hooley RJ, Raghu M, Philpotts LE. Tomosynthesis-detected architectural distortion: Management algorithm with radiologic-pathologic correlation. Radiographics. 2016;36:311–21. doi: 10.1148/rg.2016150093. [DOI] [PubMed] [Google Scholar]
- 4.Venkatesan A, Chu P, Kerlikowske K, Sickles EA, Smith-Bindman R. Positive predictive value of specific mammographic findings according to reader and patient variables. Radiology. 2009;250:648–57. doi: 10.1148/radiol.2503080541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Partyka L, Lourenco AP, Mainiero MB. Detection of mammographically occult architectural distortion on digital breast tomosynthesis screening: Initial clinical experience. AJR Am J Roentgenol. 2014;203:216–22. doi: 10.2214/AJR.13.11047. [DOI] [PubMed] [Google Scholar]
- 6.Suleiman WI, McEntee MF, Lewis SJ, Rawashdeh MA, Georgian-Smith D, Heard R, et al. In the digital era, architectural distortion remains a challenging radiological task. Clin Radiol. 2016;71:e35–40. doi: 10.1016/j.crad.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 7.Bahl M, Baker JA, Kinsey EN, Ghate SV. Architectural distortion on mammography: Correlation with pathologic outcomes and predictors of malignancy. AJR Am J Roentgenol. 2015;205:1339–45. doi: 10.2214/AJR.15.14628. [DOI] [PubMed] [Google Scholar]
- 8.Bahl M, Lamb LR, Lehman CD. Pathologic outcomes of architectural distortion on digital 2D versus tomosynthesis mammography. AJR Am J Roentgenol. 2017;209:1162–7. doi: 10.2214/AJR.17.17979. [DOI] [PubMed] [Google Scholar]
- 9.Mariscotti G, Durando M, Houssami N, Zuiani C, Martincich L, Londero V, et al. Digital breast tomosynthesis as an adjunct to digital mammography for detecting and characterising invasive lobular cancers: A multi-reader study. Clin Radiol. 2016;71:889–95. doi: 10.1016/j.crad.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Ray KM, Turner E, Sickles EA, Joe BN. Suspicious findings at digital breast tomosynthesis occult to conventional digital mammography: Imaging features and pathology findings. Breast J. 2015;21:538–42. doi: 10.1111/tbj.12446. [DOI] [PubMed] [Google Scholar]
- 11.Vedantham S, Karellas A, Vijayaraghavan GR, Kopans DB. Digital breast tomosynthesis: State of the art. Radiology. 2015;277:663–84. doi: 10.1148/radiol.2015141303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedewald SM, Rafferty EA, Rose SL, Durand MA, Plecha DM, Greenberg JS, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA. 2014;311:2499–507. doi: 10.1001/jama.2014.6095. [DOI] [PubMed] [Google Scholar]
- 13.Butler R, Philpotts L, Conant EF. Digital breast tomosynthesis: What have we learned? J Breast Imaging. 2019;1:9–22. doi: 10.1093/jbi/wby008. [DOI] [PubMed] [Google Scholar]
- 14.Young JJ, Joines M. Benign to malignant spectrum of architectural distortions on digital breast tomosynthesis. Contemp Diagn Radiol. 2016;39:1–6. doi: 10.1097/01.CDR.0000504575.58842.27. [DOI] [Google Scholar]
- 15.Cohen MA, Newell MS. Radial scars of the breast encountered at core biopsy: Review of histologic, imaging, and management considerations. AJR Am J Roentgenol. 2017;209:1168–77. doi: 10.2214/AJR.17.18156. [DOI] [PubMed] [Google Scholar]
- 16.Ha SM, Cha JH, Shin HJ, Chae EY, Choi WJ, Kim HH, et al. Radial scars/complex sclerosing lesions of the breast: Radiologic and clinicopathologic correlation. BMC Med Imaging. 2018;18:39. doi: 10.1186/s12880-018-0279-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choudhery S, Simmons C, Harper L, Lee CU. Tomosynthesis-guided needle localization of breast and axillary lesions: Our initial experience. AJR Am J Roentgenol. 2019;212:943–6. doi: 10.2214/AJR.18.20363. [DOI] [PubMed] [Google Scholar]
- 18.Alshafeiy TI, Nguyen JV, Rochman CM, Nicholson BT, Patrie JT, Harvey JA, et al. Outcome of architectural distortion detected only at breast tomosynthesis versus 2D mammography. Radiology. 2018;288:38–46. doi: 10.1148/radiol.2018171159. [DOI] [PubMed] [Google Scholar]
- 19.Freer PE, Niell B, Rafferty EA. Preoperative tomosynthesis-guided needle localization of mammographically and sonographically occult breast lesions. Radiology. 2015;275:377–83. doi: 10.1148/radiol.14140515. [DOI] [PubMed] [Google Scholar]
- 20.Waldherr C, Berclaz G, Altermatt HJ, Cerny P, Keller P, Dietz U, et al. Tomosynthesis-guided vacuum-assisted breast biopsy: A feasibility study. Eur Radiol. 2016;26:1582–9. doi: 10.1007/s00330-015-4009-4. [DOI] [PubMed] [Google Scholar]
- 21.Schrading S, Distelmaier M, Dirrichs T, Detering S, Brolund L, Strobel K, et al. Digital breast tomosynthesis-guided vacuum-assisted breast biopsy: Initial experiences and comparison with prone stereotactic vacuum-assisted biopsy. Radiology. 2015;274:654–62. doi: 10.1148/radiol.14141397. [DOI] [PubMed] [Google Scholar]
- 22.Patel BK, Covington M, Pizzitola VJ, Lorans R, Giurescu M, Eversman W, et al. Initial experience of tomosynthesis-guided vacuum-assisted biopsies of tomosynthesis-detected (2D mammography and ultrasound occult) architectural distortions. AJR Am J Roentgenol. 2018;210:1395–400. doi: 10.2214/AJR.17.18802. [DOI] [PubMed] [Google Scholar]
- 23.Lamb LR, Bahl M, Hughes KS, Lehman CD. Pathologic upgrade rates of high-risk breast lesions on digital two-dimensional vs tomosynthesis mammography. J Am Coll Surg. 2018;226:858–67. doi: 10.1016/j.jamcollsurg.2017.12.049. [DOI] [PubMed] [Google Scholar]
- 24.Menes TS, Rosenberg R, Balch S, Jaffer S, Kerlikowske K, Miglioretti DL, et al. Upgrade of high-risk breast lesions detected on mammography in the breast cancer surveillance consortium. Am J Surg. 2014;207:24–31. doi: 10.1016/j.amjsurg.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee AY, Wisner DJ, Aminololama-Shakeri S, Arasu VA, Feig SA, Hargreaves J, et al. Inter-reader variability in the use of BI-RADS descriptors for suspicious findings on diagnostic mammography: A multi-institution study of 10 academic radiologists. Acad Radiol. 2017;24:60–6. doi: 10.1016/j.acra.2016.09.010. [DOI] [PubMed] [Google Scholar]



