Abstract
Across the animal kingdom, social interactions rely on sound production and perception. From simple cricket chirps to more elaborate bird songs, animals go to great lengths to communicate information critical for reproduction and survival via acoustic signals. Insects produce a wide array of songs to attract a mate, and the intended receivers must differentiate these calls from competing sounds, analyze the quality of the sender from spectrotemporal signal properties, and then determine how to react. Insects use numerically simple nervous systems to analyze and respond to courtship songs, making them ideal model systems for uncovering the neural mechanisms underlying acoustic pattern recognition. We highlight here how the combination of behavioral studies and neural recordings in three groups of insects—crickets, grasshoppers, and fruit flies—reveals common strategies for extracting ethologically relevant information from acoustic patterns and how these findings might translate to other systems.
Keywords: auditory circuits, courtship songs, auditory processing, temporal pattern recognition, animal communication
INTRODUCTION
Communication between individuals is paramount to finding food, avoiding predators, resolving conflicts, and selecting mates. Communication signals consist of chemical, visual, mechanical, electrical, or auditory cues and can often be elaborate (Bradbury & Vehrencamp 2011, Eisenberg & Kleiman 1972, Hill 2001, Hopkins 1988, Osorio & Vorobyev 2008). Here we focus on acoustic courtship cues. The intended receivers of courtship songs must extract information about sender species, sex, and quality and then respond accordingly. However, unintended receivers may also use these signals to drive predation, parasitism, or aggression (Halfwerk et al. 2014, Reichert & Gerhardt 2014, Tuttle & Ryan 1981, Zuk & Kolluru 1998). The mechanisms by which neural circuits extract patterns from sounds and transform the resulting representations into appropriate behavioral outputs remain largely mysterious. However, numerous insights have come from studying courtship song recognition in insects, which have evolved exceptional abilities to extract information from acoustic cues despite having relatively small brains. We focus our review on three groups in which recent and significant advances have been made in revealing song pattern recognition mechanisms: crickets, grasshoppers, and fruit flies. In all three systems, males produce songs with time-varying spectrotemporal features, and females rely on these songs to inform mate choices.
Decades of neuroethological studies of crickets and grasshoppers have provided invaluable insights into how nervous systems detect and analyze songs (Hartbauer & Römer 2014, Hildebrandt et al. 2015, Pollack et al. 2016). However, the absence of genetic tools in these insects hampers neuronal circuit tracing and manipulation. Studies of auditory processing in Drosophila are more recent, but progress has been accelerated due to the extensive toolkit associated with this model system. For instance, neural activation (e.g., via optogenetics) allows for direct tests of a neuron’s role in behavior (Inagaki et al. 2014). Imaging of genetically encoded calcium or voltage indicators permits the recording of responses in identifiable neurons and intact circuits (Chen et al. 2013, Yang & St-Pierre 2016). Combining optogenetics with neural imaging enables the confirmation of functional connectivity within circuits (Franconville et al. 2018). CRISPR (clustered regularly interspaced short palindromic repeats) technology will allow for comparative studies of song recognition by facilitating transgenesis in other Drosophila (Seeholzer et al. 2018, Stern et al. 2017) and insect (Chaverra-Rodriguez et al. 2018, Sun et al. 2017) species. We posit that studies of auditory processing in Drosophila will greatly benefit from interpreting results in light of mechanisms uncovered in larger insect species.
INSECT ACOUSTIC SIGNALS AND THE IMPORTANCE OF TEMPORAL PATTERN RECOGNITION
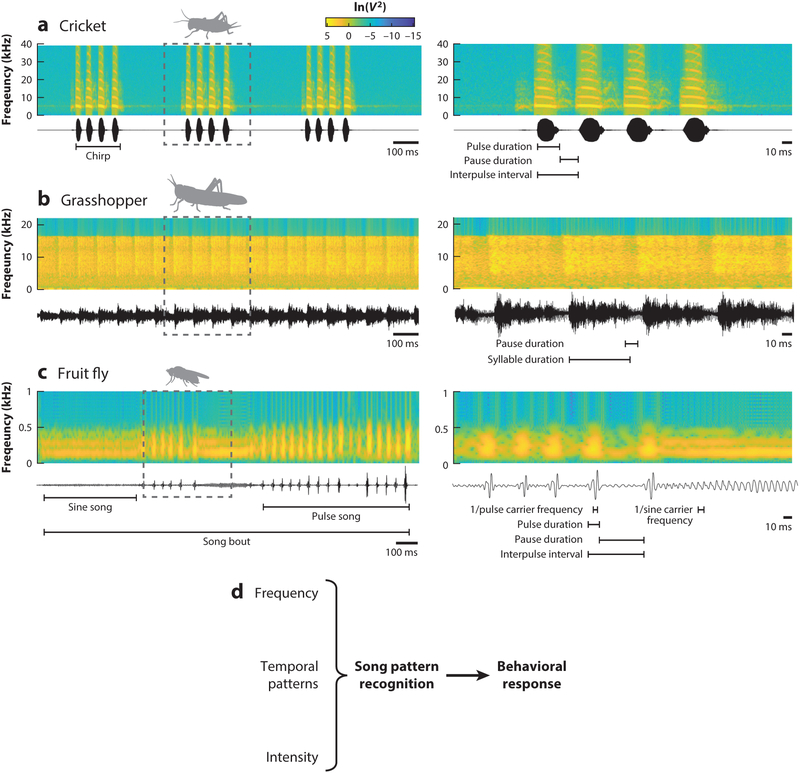
Although insect songs vary widely in complexity, many insects stick to a common bauplan of trains of impulses (Hedwig 2016, Markow & O’Grady 2005, Ronacher 2014) (Figure 1). The sound pulse carrier frequency can be species specific and is often amplitude modulated, with loud pulses or syllables interleaved by soft or silent pauses. Trains of pulses are then sequenced into chirps or bouts, which can also vary across species. Accordingly, recognition of pulsed songs consists of filtering for conspecific carrier frequency and then evaluating the temporal pattern. These commonalities facilitate comparing behavioral algorithms and neural mechanisms of song recognition across species (Clemens & Hennig 2013).
Figure 1.
Examples of insect calling and courtship songs. (a) An example of cricket calling song from Gryllus bimaculatus. The 1.5-s sound recording is shown at the bottom (black), and the constituent sound frequencies over time are shown in the corresponding spectrogram (top). Time bins of 20 ms were used in the spectrogram. Cricket songs often consist of a series of pure-tone sound pulses grouped into chirps, which are then repeated at a regular rate. (b) An example of 1.5 s of grasshopper calling song from Chorthippus biguttulus. Time bins of 22.7 ms were used in the spectrogram. Grasshopper songs often contain broadband pulses grouped into syllables. (c) An example of 1.75 s of fly courtship song from Drosophila melanogaster. Time bins of 40 ms were used in the spectrogram. Fly songs often consist of nearly pure-tone sine song interleaved with trains of brief pulses (pulse song). In panels a–c, the dotted box on the left indicates the region enlarged on the right. (d) The intended receivers of these songs must detect and analyze frequency, timing, and intensity cues to gain information about the sender, such as species, sex, and quality, and then decide whether and how to respond.
Crickets and grasshoppers sing long-range calling songs to attract mates (Hedwig 2016, Ronacher 2014). Cricket calling song pulses have carrier frequencies in the narrow range of 3–6 kHz. Whereas some species produce continuous pulse trains called trills, others group several pulses into chirps and then repeat a few chirps each second (Otte 1992) (Figure 1a). Song structure, including pulse carrier frequency, pulse durations and pauses, and number of pulses per chirp, varies across species. In contrast to cricket songs, grasshopper calling songs often have a broadband carrier frequency (5–30 kHz) (Meyer & Elsner 1996) and are structured into syllables and pauses, with a series of several sound pulses repeated at regular intervals constituting a syllable (Klappert & Reinhold 2003) (Figure 1b). The duration of syllables and intervening pauses is species specific. In grasshopper species in which both sexes sing, temporal song patterns distinguish female from male songs (Ronacher et al. 2015, von Helversen & von Helversen 1997).
Male crickets and grasshoppers also sing a short-range courtship song when close to a female. Cricket courtship songs usually have a higher carrier frequency (11–16 kHz) and/or a different temporal pattern than the calling song (Libersat et al. 1994, Zuk et al. 2008). Courtship songs tend to be more variable than calling songs, leading to the hypothesis that stereotyped calling songs contribute to conspecific recognition, whereas variable courtship songs contribute to mate assessment (Zuk et al. 2008).
Male fruit flies chase females and sing only short-range courtship songs that vary widely across species and are dynamic over time (Coen et al. 2014, Markow & O’Grady 2005). In many species, the courtship song consists of brief pulses repeated at regular intervals (pulse song) interspersed with near-constant-frequency song (sine song) (Figure 1c). The carrier frequencies of both pulse and sine songs are comparatively low, usually between 100 and 800 Hz. In contrast to sine song, pulse song may include more than one distinct pulse type (Clemens et al. 2018a, Riabinina et al. 2011). Features on multiple timescales, including carrier frequencies, pulse durations, and intervals between pulses (Figure 1c), are species specific (Cowling & Burnet 1981). Thus, females likely need to assess multiple temporal patterns to recognize and evaluate individual songs. Drosophila melanogaster males structure their songs into bouts, which usually consist of switches between pulse and sine modes, and they use sensory feedback from the female to rapidly modulate bout structure and overall song intensity (Coen et al. 2014, 2016). During aggressive encounters, males also produce agonistic pulse songs that are distinct from courtship songs (Jonsson et al. 2011), and the auditory system can distinguish between the two types (Versteven et al. 2017).
BEHAVIORAL RESPONSES TO SONG PATTERNS
As insect songs contain patterns on multiple timescales (Figure 1), determining which features auditory systems are tuned for is paramount to understanding the sensorimotor transformations that connect song detection with behavior. Quantitative behavioral experiments in crickets, grasshoppers, and flies have provided insights into song feature tuning.
Female crickets walk towards a conspecific calling song. The directionality of phonotaxis can be measured in tethered females on a trackball and used as a readout of song attractiveness. Measuring the joint tuning for pulse duration and pulse pause (Figure 1a) has revealed diverse preferences across species. Whereas some species are narrowly tuned for pulse duration, others are highly selective for pause duration, and still others are selective for interpulse interval (Blankers et al. 2015, Gray et al. 2016). The diverse response functions imply divergence in behavioral algorithms and the underlying computations (Clemens & Hennig 2013). Crickets are also selective for the duration and pause between chirps (Grobe et al. 2012, Meckenhäuser et al. 2013), suggesting that they evaluate song structure on multiple timescales. Indeed, trackball experiments have revealed two distinct timescales in song-evoked motor responses. Pattern recognition on a long timescale controls female forward velocity, whereas the spatial location of each individual pulse guides the directionality of rapid steering responses (Hedwig & Poulet 2004, Poulet & Hedwig 2005). These observations imply that pattern recognition and sound localization are performed by parallel circuits.
Chorthippus biguttulus grasshoppers engage in duets. Females produce a response call only to attractive conspecific calling songs, and males use the response calls to locate a female (von Helversen 1972, von Helversen & von Helversen 1997). Measuring signal attractiveness via female response probability to song playback has revealed that females prefer songs within a narrow range of syllable:pause ratios. Females are also sensitive to fine syllable structure such as gaps or other modulations (Schmidt et al. 2008, Stumpner & Ronacher 1994). The presence of negative cues, such as those associated with unfit or heterospecific males, far outweighs the presence of positive cues (Clemens et al. 2014), which saves females from duetting with undesirable males.
Behavioral responses to cricket and grasshopper short-range courtship songs are not well studied compared with long-range calling songs. Once animals are in close proximity, chemical and visual cues play important roles in courtship, making it difficult to decompose the unique contribution of song (Finck et al. 2016, Libersat et al. 1994, Ostrowski et al. 2009).
In flies, the sound receiver detects near-field sounds, and males do not produce long-range calling songs. Understanding female responses to fly courtship songs is therefore subject to the same limitations of studying short-range songs in crickets and grasshoppers. However, high-throughput behavioral assays coupled with automated fly tracking and song segmentation (Arthur et al. 2013) have enabled fine mapping of dynamic female behavioral responses, such as changes in locomotor speed, to song (Coen et al. 2014). These studies have revealed that female locomotion is sensitive to song features over timescales extending to roughly 1 min (Bussell et al. 2014, Clemens et al. 2015, Coen et al. 2014). However, correlations between female behavior and short-timescale parameters, such as interpulse intervals, have not emerged from natural courtship experiments, likely because the song produced by a conspecific male is within the females’ acceptable range (Clemens et al. 2015). Instead, the role of short-timescale song parameters has been tested with playback experiments, which typically involve pairing females with wingless (mute) males, delivering synthetic song, and then measuring the time to copulation or the percentage of copulating pairs. Such experiments found that conspecific interpulse intervals decrease time to copulation relative to shorter or longer intervals and relative to mute males (Bennet-Clark & Ewing 1969). However, subsequent experiments have yielded inconsistent results on the role of species-specific parameters such as interpulse interval and carrier frequency (Li et al. 2018, Rybak et al. 2002, Talyn & Dowse 2004), likely because dynamic interactions between communication partners prevent the isolation of the unique contributions of song features. In contrast to females, male flies respond to song playback by increasing their walking speed (Crossley et al. 1995, Kowalski et al. 2004, Vaughan et al. 2014) and courting one another in courtship chains (Eberl et al. 1997, Kamikouchi et al. 2009, von Schilcher 1976, Yoon et al. 2013, Zhou et al. 2015). These responses are interpreted to reflect increased drive to find and court a female after hearing a nearby singing male (Eberl et al. 1997) and have been shown to be strongest for conspecific interpulse intervals (Yoon et al. 2013, Zhou et al. 2015).
From previous studies, it was not clear if male and female auditory systems were similarly or differently tuned to song. Assaying fast locomotor responses to song playback in solitary flies revealed sex-specific behavioral responses for all tested pulse song features (Clemens et al. 2018b). That is, females slow down, whereas males accelerate and sing, and these responses are strongest for conspecific parameters. These findings led to the hypothesis that sexually monomorphic processes may detect song, while sexually dimorphic downstream circuits may guide behavioral responses to song. This hypothesis is supported by the finding that male and female behavioral responses (male chaining and copulation latency) to interpulse intervals are shaped similarly by auditory experience (Li et al. 2018), even though the motor outputs are sex-specific. In contrast to pulse song, sine song elicits sexually monomorphic and broadly tuned locomotor responses (both sexes slow, and neither sex sings) (Clemens et al. 2018b).
In sum, careful examination of behavioral responses to song has been critical for identifying the multiple temporal patterns within conspecific songs to which insects are sensitive. Leveraging these behavioral results, several recent studies have investigated the tuning of auditory neurons across these diverse insect species and have found matches between neural tuning for acoustic parameters and behavioral preferences.
AUDITORY PATHWAYS
A major advantage of studying auditory processing in insects is the numerical simplicity of their nervous systems, which makes it feasible to determine how sound stimuli are transformed across sensory layers to extract behaviorally relevant features. Although we know the most about the anatomy and physiology of the sound receiver and mechanosensory neurons, several more recent studies have explored how central neurons represent songs, and future work will decipher how these representations are decoded to change behavior.
Crickets
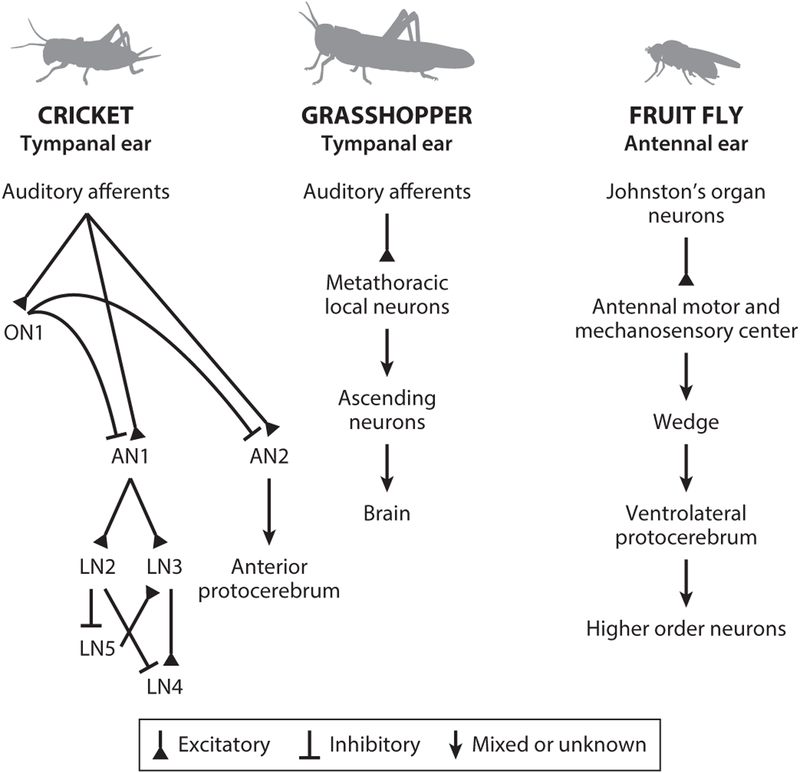
Crickets have a tympanic hearing organ on each front leg, which acts as a pressure gradient receiver since it receives sound directly on its external surface and indirectly on its internal surface through openings in the thorax and an auditory trachea. Auditory afferents can be broadly divided into low-frequency-preferring (most sensitive to the calling song) and high-frequency-preferring afferents (most sensitive to the courtship song or ultrasonic bat calls) (Imaizumi & Pollack 2005). Afferents project to three major auditory neurons in the prothoracic ganglion (Imaizumi & Pollack 2005, Wohlers & Huber 1982) (Figure 2): the local omega neuron ON1 and two ascending neurons (ANs), AN1 and AN2, that transmit auditory information to the brain. ON1 is broadly tuned for frequency and inhibits the contralateral AN1 and AN2 (Atkins et al. 1984, Hedwig 2016, Pollack & Hedwig 2017, Stumpner et al. 1995). AN1 and AN2 constitute labeled lines for two ecological sound categories: conspecific calling song and predatory bat ultrasound, respectively (Wyttenbach et al. 1996). AN1 encodes song patterns with high sensitivity and fidelity (Marsat & Pollack 2005) and projects to brain areas that are involved in temporal pattern analysis (Kostarakos & Hedwig 2012, Schöneich et al. 2015). AN2 activity is both necessary and sufficient to produce evasive maneuvers for predatory bats (Nolen & Hoy 1984).
Figure 2.
Insect auditory pathways. In crickets and grasshoppers, auditory afferents relay sound cues from tympanal ears to ganglia within the ventral nerve cord. ANs then project from the ventral nerve cord to the central brain. In flies, sensory neurons called Johnston’s organ neurons transduce sound vibrations from an antennal ear into the central brain. Abbreviations: AN, ascending neuron; LN, local neuron; ON, omega neuron.
Grasshoppers
Grasshoppers have a tympanic hearing organ on each side of the abdomen. Most auditory receiver cells are maximally sensitive to frequencies between 4 and 7 kHz, whereas a smaller number are maximally sensitive to frequencies higher than 15 kHz (Stumpner & Ronacher 1991). Afferents project to the metathoracic ganglion, which contains approximately 15 types of local neurons (LNs) with diverse morphologies and physiologies (Römer & Marquart 1984, Stumpner & Ronacher 1991) (Figure 2). These LNs then synapse onto approximately 20 ANs, which in turn project to the brain (Ronacher 2014). ANs are diverse in their selectivity for temporal features of sound and play important roles in song-feature extraction (Clemens et al. 2011). How this rich representation of song patterns is processed in the brain to generate behavioral responses is not known, but higher-order auditory neurons are beginning to be identified (Bhavsar et al. 2015).
Drosophila
Whereas crickets and grasshoppers have tympanal ears that are sensitive to sound pressure, flies detect sound using a feathery extension of the antenna called the arista, which is sensitive to particle displacements induced by the near-field component of sound or by wind. Particle velocity attenuates rapidly with distance (Windmill & Jackson 2016), which limits the effective signal space to nearby sounds. Antennal movements activate Johnston’s organ neurons (JONs) within the antenna. Three of five JON subsets respond to the range of antennal deflections representative of song carrier frequencies (Kamikouchi et al. 2009, Matsuo et al. 2014, Yorozu et al. 2009) and project to distinct areas of the antennal mechanosensory and motor area (AMMC) in the brain (Kamikouchi et al. 2006) (Figure 2).
From the AMMC, known auditory pathways travel to the wedge, then to the ventrolateral protocerebrum (VLP), and on to the lateral protocerebral complex (LPC) (Clemens et al. 2018b, Lai et al. 2012, Vaughan et al. 2014, Zhou et al. 2015). Neurons in several areas, including B1 (also known as aPN1) and aLN in the AMMC, vPN1 in the VLP, and neurons in the LPC, respond to pulse songs and are necessary for female receptivity and male locomotor responses to song (Clemens et al. 2018b, Vaughan et al. 2014, Zhou et al. 2014, 2015). Although numerous auditory neurons in the AMMC and beyond have been described (Azevedo & Wilson 2017; Clemens et al. 2015, 2018b; Lai et al. 2012; Matsuo et al. 2016; Tootoonian et al. 2012; Vaughan et al. 2014; Zhou et al. 2015), the Drosophila auditory system remains incompletely mapped. To more thoroughly understand Drosophila auditory processing, one would need to identify the full complement of sound-responsive neurons throughout the brain, precisely characterize each neuron’s tuning, and then map connectivity among auditory neurons and between auditory and motor pathways. This ambitious goal should be feasible given the available tools in Drosophila, including methods for whole-cell patch-clamp recordings from identified neurons (Azevedo & Wilson 2017, Clemens et al. 2015), imaging pan-neuronal activity and comparing across animals (Mann et al. 2017), mapping connections within electron microscopic data sets (Zheng et al. 2018), and precisely mapping behavioral outputs during the manipulation of neural activity (Cande et al. 2018). Calcium sensor dynamics are too slow for detailed studies of auditory coding mechanisms, but are still useful for assessing tuning and identifying neurons for further mechanistic studies (Clemens et al. 2018b, Lai et al. 2012, Patella & Wilson 2018, Zhou et al. 2015).
NEURAL COMPUTATIONS FOR EVALUATING SHORT-TIMESCALE SONG FEATURES
Insect songs contain patterns spanning multiple timescales, from pulse carrier frequencies and durations to chirp or bout duration (Figure 1). How do nervous systems detect and encode these patterns? Whereas frequency tuning first arises at the periphery, temporal features are not typically peripherally filtered and instead must be extracted centrally. Such processing usually involves adaptation, interactions between excitation and inhibition, and/or cell-intrinsic properties.
Frequency Tuning
Similar to mammalian cochleae, tympanic hearing organs decompose sounds into their constituent frequencies using receptor cells whose physical location within the organ determines the receptor’s most sensitive frequency. The correspondence of frequency sensitivity to physical location is called tonotopy and is generally maintained throughout ascending projections. Cross-frequency inhibition may further sharpen central tuning. For example, the convergence of inhibition evoked by low frequencies and broadband excitation in a grasshopper auditory neuron sharpens frequency tuning by suppressing responses to low frequencies (Römer et al. 1981). A similar principle is at work in the katydid AN1 neuron, where inhibition at high frequencies produces selectivity for the male song frequency (Stumpner 2002).
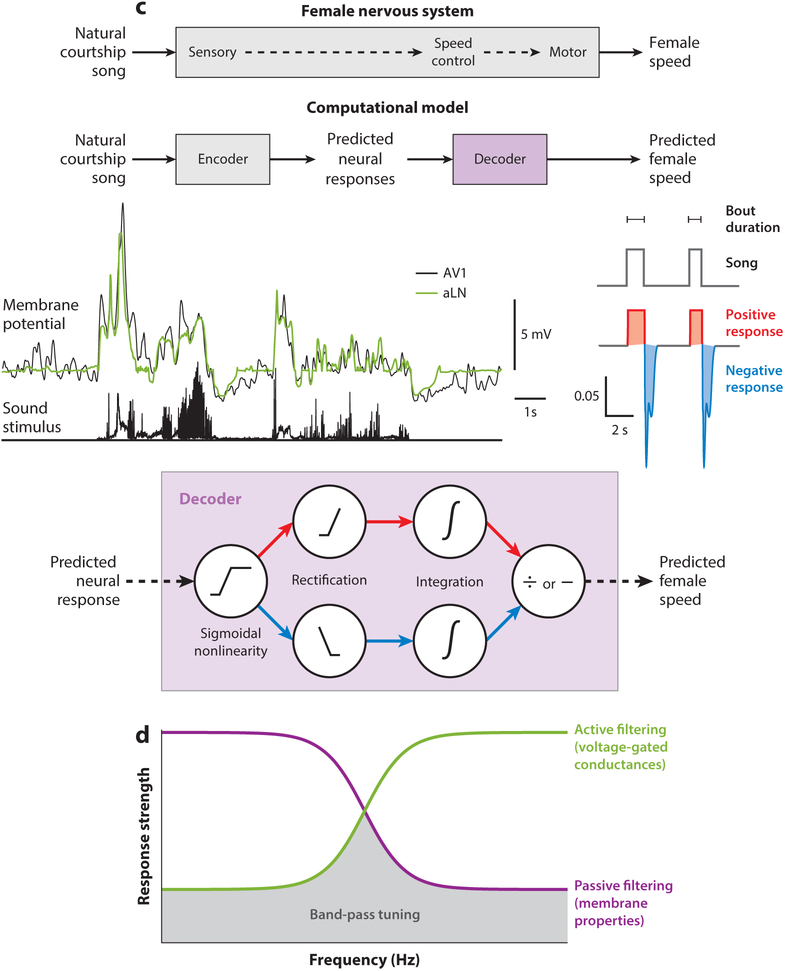
Whereas gradients in mechanical tympanic properties can spectrally decompose sound, this approach is not available to the fly antennal sound receiver. Instead, frequency selectivity in Drosophila auditory afferents likely arises intrinsically (Fettiplace & Fuchs 1999, Göpfert & Hennig 2016, Riabinina et al. 2011). Calcium imaging and local-field potential recordings of JON populations suggest broad frequency tuning within JON subsets: The A subset responds to frequencies higher than 100 Hz and the B subset responds to frequencies lower than 100 Hz (Kamikouchi et al. 2009, Matsuo et al. 2014, Yorozu et al. 2009). However, two recent studies found more selective frequency tuning within A and B JONs than indicated by population activity (Ishikawa et al. 2017, Patella & Wilson 2018). Further, calcium imaging recently revealed tonotopy in JON responses that was sharpened in higher auditory areas (Patella & Wilson 2018). Band-pass tuning for stimulus frequencies in B1 (aPN1) AMMC neurons arises through both active and passive cell-intrinsic mechanisms (Figure 3d) (Azevedo & Wilson 2017).
Figure 3.
Mechanisms for temporal pattern recognition in insects. (a) Cricket phonotaxis behavior is tuned to species-specific pulse periods, or the time intervals between successive pulses (left) (Schöneich et al. 2015). The responses of the neuron LN4 match behavioral tuning. LN4 achieves its interval tuning through a delay line and coincidence detection mechanism (right). AN1 provides sound-evoked excitation to LN2 and LN3. LN2 in turn inhibits LN5, which produces a postinhibitory rebound at the end of each song pulse. LN3 acts as a coincidence detector by combining postinhibitory rebound from LN5 with excitation from AN1. LN3 responds when the rebound co-occurs with AN1 excitation, which happens only for conspecific intervals. Finally, interval selectivity is enhanced by LN4, which spikes only when input from LN3 is maximal. Panel a adapted from (left) Schöneich et al. (2015) and (right) Hedwig (2016). (b) Grasshopper behavioral responses are selective for a narrow range of syllable and pause durations (top left) (Creutzig et al. 2010). An increase in temperature causes shorter syllables and pauses but leaves the syllable:pause ratio constant. The AN12 neuron in the metathoracic ganglion exhibits temperature-independent selectivity for conspecific syllable:pause ratios (top right) (Creutzig et al. 2009). AN12 generates bursts at the onset of each syllable, with the number of spikes per burst depending on the duration of the preceding pause but not syllable. Higher temperatures cause shorter pauses, evoking fewer spikes per burst, but more syllables per second, evoking more bursts (bottom). The overall result is the same total number of spikes per second. AN12’s responses are hypothesized to result from interactions between excitation and inhibition. AN12 could receive an excitatory copy of the adapted responses of auditory receptors and a delayed, inhibitory, low-pass version of the adapted responses. The upper subpanels of panel b are adapted with permission from Creutzig et al. (2009). (c) In flies, female locomotor speed is sensitive to song bout duration on timescales up to 1 s (Clemens et al. 2015). This bout duration sensitivity could arise from the integration of positive bout duration–dependent voltage changes during song and negative bout duration–independent voltage changes at song offset in early auditory neurons (middle). Summing the positive responses over a defined window provides a measure of the total amount of song, and counting the number of negative responses provides a measure of the total number of bouts. Dividing total song amount by total number of bouts provides an estimate of bout duration. Panel c adapted with permission from Clemens et al. (2015). (d) Band-pass frequency tuning (gray shading) in Drosophila B1 neurons results from a combination of passive and active filtering mechanisms (Azevedo & Wilson 2017). Active processes, such as voltage-gated conductances, suppress responses at low frequencies, and passive membrane properties, such as capacitive and leak currents, suppress responses at high frequencies. Variation in the relative strengths of passive and active filtering properties within B1 neurons gives rise to variation in frequency selectivity across the population. Abbreviations: AN, ascending neuron; LN, local neuron.
Song Gap Detection
Male C. biguttulus grasshoppers produce syllables with smooth and sustained plateaus. Millisecond-scale gaps in syllable structure are indicative of songs from injured males, conspecific females, and heterospecific males (Kriegbaum 1989, von Helversen & von Helversen 1997). Females are sensitive to song gaps as short as 2 ms (Ronacher & Stumpner 1988). The gap duration tuning of AN4 matches behavioral tuning and arises through the interplay of sustained excitation and fast transient inhibition (Ronacher & Stumpner 1988, Stumpner & Ronacher 1994). The fast inhibition suppresses spiking responses at pulse onset. Frequent interruptions of the syllable by gaps introduce many such onsets and thereby decrease AN4 spiking.
Drosophila females may also be sensitive to song interruptions. Inhibiting the motor neuron hg1 during singing prevents sine song production (Shirangi et al. 2013). The resulting bouts consist only of pulse song, leaving many gaps where sine would have been. These interrupted songs are less attractive to females, suggesting that the Drosophila auditory system is also able to detect song gaps, albeit on much longer timescales than grasshoppers.
Pulse Interval Selectivity
The clearest example of how an insect circuit establishes auditory feature selectivity is that of conspecific interpulse interval detection in crickets, which involves a delay line and coincidence detection mechanism (Figure 3a) (Schöneich et al. 2015). AN1 provides excitation onto LNs in the brain. LN2 inhibits LN5, which produces a postinhibitory rebound (excitatory potential) at the end of each sound pulse. A coincidence detector (LN3) then combines this postinhibitory rebound with direct excitation from AN1. LN3 will spike only when these two sources of excitation coincide, and synaptic delays in the network ensure that this happens only for the conspecific interval. Finally, the readout neuron LN4 further sharpens interval selectivity by responding only when excitatory input from LN3 is maximal (i.e., not to single pulses).
Fly auditory pathways also include interval-selective neurons (Clemens et al. 2015, 2018b; Tootoonian et al. 2012; Vaughan et al. 2014; Yamada et al. 2018; Zhou et al. 2015). Although many early auditory neurons constitute low-pass interval filters (Clemens et al. 2015), the match between neural and behavioral interval tuning gradually increases from the AMMC to the LPC (Clemens et al. 2018b, Yamada et al. 2018, Zhou et al. 2015). In B1 (aPN1) neurons, band-pass tuning arises through the inhibition of responses to short intervals by two GABAergic neurons, aLN and B2 (Yamada et al. 2018). In the LPC, the tuning of pC2 neurons is matched to conspecific pulse song parameters, including interpulse intervals (Clemens et al. 2018b). It is not yet clear (a) whether all band-pass interval tuning is inherited from B1 and (b) whether interval tuning in Drosophila also involves a coincidence detection mechanism, as in crickets (Schöneich et al. 2015).
Temperature-Independent Recognition of Conspecific Temporal Patterns
In poikilothermic animals, neural activity depends on temperature such that song pattern generators will produce slightly different songs at different temperatures. How do auditory systems deal with temperature-dependent songs? One solution has been identified in grasshoppers. Temperature increases induce a compression of the song’s temporal pattern, leading to shorter syllables and intersyllable pauses but leaving the ratio between syllable duration and pause constant. Grasshoppers exploit the syllable:pause ratio as a temperature-invariant cue for species recognition. The AN12 neuron fires a burst at each syllable onset in the calling song (Figure 3b), and the number of spikes per burst depends on the duration of the preceding pause but is independent of syllable duration (Creutzig et al. 2009). Higher temperatures cause an increase in the number of syllables per second and, accordingly, an increase in the number of bursts per second. At the same time, higher temperatures also shorten the pauses and thereby decrease the number of spikes per burst. The net result is the same absolute number of spikes per second, regardless of temperature.
The pause duration–dependent bursting responses of AN12 are hypothesized to arise from feedforward excitation and inhibition (Figure 3b) (Creutzig et al. 2010). Excitatory input to AN12 carries a copy of the adapted responses of auditory receptors, and inhibitory input provides a delayed low-pass version of these adapted responses. Integration of these inputs results in bursting only at syllable onset. The dependence on pause duration could result from adaptation in the auditory receptors, as longer pauses allow more recovery from adaptation and hence stronger excitatory inputs.
Female grasshoppers respond to a triangular range of pause and syllable durations. To more fully account for these behavioral preferences, the auditory system must measure two additional parameters: syllable duration and syllable number. AN6, which fires tonically and with little adaptation during a syllable, could represent syllable duration. A phasic, rapidly adapting neuron could count the number of syllables. Integration of the output of AN12, AN6, and a syllable-counting neuron comes very close to accurately modeling behavioral data (Creutzig et al. 2010).
NEURAL CODING OF LONG-TIMESCALE SONG FEATURES
Song features on the order of hundreds of milliseconds are too long to encode with mechanisms such as synaptic delays or postinhibitory rebounds. Instead, processes with longer time constants, such as adaptation or long-term plasticity, are likely to contribute. However, we are just beginning to understand how insect auditory circuits detect long-timescale parameters.
Male flies pattern their songs into bouts, which usually consist of both pulse and sine song modes and can last for about 50 ms to 3 s (Coen et al. 2014). Long-timescale song features such as bout duration are hypothesized to contribute to mate assessment (Coen & Murthy 2016). Indeed, female locomotor responses to conspecific songs are best predicted by song bout duration and not interpulse interval (Clemens et al. 2015), likely because males tend to always produce interpulse intervals within the acceptable range (Clemens et al. 2018b). Intracellular recordings and computational modeling revealed how bout duration sensitivity could arise (Clemens et al. 2015). First, early auditory neurons act as linear, biphasic filters of the adapted responses of auditory receiver neurons. This response property results in positive voltage changes throughout both pulse and sine song and transient negative voltage changes at song offset (Figure 3c). In contrast to positive phases, the negative phases appear to be independent of the amount of preceding song. Mathematically integrating positive responses over a defined time window yields the amount of song, and counting the number of negative responses reflects the number of bouts. A simple division of song amount by bout number yields bout duration, and a computational model of these operations was predictive of female speed during natural courtship (Clemens et al. 2015) (Figure 3c). This mechanism of combining sustained and transient responses to establish bout duration tuning is similar to that used by grasshoppers to recognize conspecific pulse patterns (Creutzig et al. 2010), with the processing in flies operating on longer timescales.
Female crickets are also sensitive to longer-timescale calling song features, including chirp structure (Grobe et al. 2012, Rothbart & Hennig 2012). This sensitivity could result from interactions between distinct short- and long-timescale temporal filters, or the simple integration over time of short-timescale filters (Clemens & Hennig 2013, Grobe et al. 2012). However, the underlying neural circuitry remains unknown.
PARALLELS ACROSS SENSORY SYSTEMS
All sensory systems are faced with the challenge of pattern recognition. Because different animals detect distinct patterns, understanding the underlying neural mechanisms in diverse species can reveal general coding principles. From insects, we are learning how nervous systems encode conspecific patterns using comparatively few neurons. Comparisons between insects and vertebrates suggest that as behaviorally relevant temporal patterns increase in complexity and/or diversity, so too do detection mechanisms (Baker & Carlson 2014, Creutzig et al. 2009, Goel & Buonomano 2014, Ronacher & Stumpner 1988, Rose 2014, Schöneich et al. 2015, Yamada et al. 2018). For instance, frog acoustic calls generally consist of sound pulses repeated at fixed interpulse intervals, with the intervals varying across social context and species (Fellers 1979, Rose & Brenowitz 2002). In contrast to crickets and grasshoppers, which primarily need to recognize the conspecific calling and courtship song patterns, frogs need to detect and respond to a number of distinct patterns that may vary in complexity, particularly during male-male competition (Rose 2017). In the frog auditory midbrain, some neurons respond selectively to long, short, or intermediate intervals and may even only respond after a specific number of preferred intervals (Alder & Rose 1998, Edwards et al. 2002, Rose & Capranica 1983). In long interval–preferring neurons, inhibition evoked by successive short intervals appears to coincide with excitation evoked by previous pulses, thereby preventing spikes to later pulses (Rose 2014). In short interval–preferring neurons, optimal intervals elicit rate-dependent excitation that eventually overcomes the inhibition to produce spikes (Rose 2014).
Another clear example of temporal pattern recognition occurs in mormyrid weakly electric fish, which vary the time intervals between successive electric pulses to communicate. In contrast to insect and frog songs, the intervals in electric signals are rapidly modulated, and the rate of interval change varies across signal classes (Carlson & Hopkins 2004). The electrosensory system establishes single-neuron interval sensitivity through both intrinsic and synaptic mechanisms (George et al. 2011, Kohashi & Carlson 2014), including short-term depression and temporal summation, with the combination of depression and summation resulting in a greater diversity of interval sensitivities than would either mechanism alone (Baker & Carlson 2014). Such diversity gives rise to a population of neurons with millisecond precision in temporal pattern encoding (Baker et al. 2016). Neurons preferring sounds with naturalistic spectrotemporal modulations have also been found in birds and mammals (Kanwal & Rauschecker 2007, Poremba et al. 2013, Schneider & Woolley 2013, Woolley & Portfors 2013), and the mechanisms by which specific tuning arises in more complex networks is currently being investigated.
FUTURE PROSPECTS
Studies of insect audition are revealing how small circuits can establish selectivity for behaviorally relevant patterns within sensory stimuli. A major outstanding question is how representations of distinct acoustic features are combined to influence mating decisions. At some stage, song feature information needs to be integrated to enable conspecific recognition, mate quality assessment, and finally motor output. Furthermore, many animals produce sounds in contexts that are independent of courtship, such that auditory information may need to be routed to different brain regions or may need to have differential effects on the same region depending on the signal (i.e., courtship versus agonistic versus predator sounds). Moreover, brains use cues from multiple sensory modalities to guide courtship behaviors, but how multisensory information is integrated during courtship is not well understood. Decades of work on cricket and grasshopper auditory systems have yielded deep insights into many aspects of song feature selectivity. Recent work on Drosophila audition, from behavior to neural coding, has now opened the door to solving the complete neural mechanisms underlying auditory encoding and decoding in this system. We posit that a comparative approach is essential for revealing general principles of neural coding and for informing hypotheses about similar processes across sensory systems.
ACKNOWLEDGMENTS
The authors thank David Deutsch and Frederic Roemschied for comments on this manuscript. C.A.B. was funded by a Jane Coffin Childs Memorial Fund Postdoctoral Fellowship, J.C. was funded by an Emmy Noether grant from the Deutsche Forschungsgemeinschaft (CL 596-1/1), and M.M. was funded by a New Innovator (DP2) award from the National Institute of Neurological Disorders and Stroke and a Faculty Scholar award from the Howard Hughes Medical Institute.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Alder TB, Rose GJ. 1998. Long-term temporal integration in the anuran auditory system. Nat. Neurosci 1(6):519–23 [DOI] [PubMed] [Google Scholar]
- Arthur BJ, Sunayama-Morita T, Coen P, Murthy M, Stern DL. 2013. Multi-channel acoustic recording and automated analysis of Drosophila courtship songs. BMC Biol. 11(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins G, Ligman S, Burghardt F, Stout JF. 1984. Changes in phonotaxis by the female cricket Acheta domesticusL. after killing identified acoustic interneurons. J. Comp. Physiol. A 154(6):795–804 [Google Scholar]
- Azevedo AW, Wilson RI. 2017. Active mechanisms of vibration encoding and frequency filtering in central mechanosensory neurons. Neuron 96:446–60.E9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker CA, Carlson BA. 2014. Short-term depression, temporal summation, and onset inhibition shape interval tuning in midbrain neurons. J. Neurosci 34(43):14272–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker CA, Ma L, Casareale CR, Carlson BA. 2016. Behavioral and single-neuron sensitivity to millisecond variations in temporally patterned communication signals. J. Neurosci 36(34):8985–9000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennet-Clark HC, Ewing AW. 1969. Pulse interval as a critical parameter in the courtship song of Drosophila melanogaster. Anim. Behav 17(4):755–59 [Google Scholar]
- Bhavsar MB, Heinrich R, Stumpner A. 2015. Multielectrode recordings from auditory neurons in the brain of a small grasshopper. J. Neurosci. Methods 256:63–73 [DOI] [PubMed] [Google Scholar]
- Blankers T, Hennig RM, Gray DA. 2015. Conservation of multivariate female preference functions and preference mechanisms in three species of trilling field crickets. J. Evol. Biol 28(3):630–41 [DOI] [PubMed] [Google Scholar]
- Bradbury JW, Vehrencamp SL. 2011. Principles of Animal Communication. Sunderland, MA: Sinauer Assoc. Inc.2nd ed. [Google Scholar]
- Bussell JJ, Yapici N, Zhang SX, Dickson BJ, Vosshall LB. 2014. Abdominal-B neurons control Drosophila virgin female receptivity. Curr. Biol 24(14):1584–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cande J, Namiki S, Qiu J, Korff W, Card GM, et al. 2018. Optogenetic dissection of descending behavioral control in Drosophila. eLife 7:e34275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson BA, Hopkins CD. 2004. Stereotyped temporal patterns in electrical communication. Anim. Behav 68(4):867–78 [Google Scholar]
- Chaverra-Rodriguez D, Macias VM, Hughes GL, Pujhari S, Suzuki Y, et al. 2018. Targeted delivery of CRISPR-Cas9 ribonucleoprotein into arthropod ovaries for heritable germline gene editing. Nat. Commun 9:3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T-W, Wardill TJ, Sun Y, Pulver SR, Renninger SL, et al. 2013. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499(7458):295–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens J, Coen P, Roemschied F, Pereira T, Mazumder D, et al. 2018a. Discovery of a new song mode in Drosophila reveals hidden structure in the sensory and neural drivers of behavior. Curr. Biol 28:2400–12.E6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens J, Deutsch D, Thiberge SY, Murthy M. 2018b. Shared song object detector neurons in Drosophila male and female brains drive divergent, sex-specific behaviors. bioRxiv 366765 10.1101/366765 [DOI] [Google Scholar]
- Clemens J, Girardin CC, Coen P, Guan X-J, Dickson BJ, Murthy M. 2015. Connecting neural codes with behavior in the auditory system of Drosophila. Neuron 87(6):1332–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens J, Hennig RM. 2013. Computational principles underlying the recognition of acoustic signals in insects. J. Comput. Neurosci 35(1):75–85 [DOI] [PubMed] [Google Scholar]
- Clemens J, Krämer S, Ronacher B. 2014. Asymmetrical integration of sensory information during mating decisions in grasshoppers. PNAS 111(46):16562–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens J, Kutzki O, Ronacher B, Schreiber S, Wohlgemuth S. 2011. Efficient transformation of an auditory population code in a small sensory system. PNAS 108(33):13812–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen P, Clemens J, Weinstein AJ, Pacheco DA, Deng Y, Murthy M. 2014. Dynamic sensory cues shape song structure in Drosophila. Nature 507(7491):233–37 [DOI] [PubMed] [Google Scholar]
- Coen P, Murthy M. 2016. Singing on the fly: sensorimotor integration and acoustic communication in Drosophila. Curr. Opin. Neurobiol 38:38–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen P, Xie M, Clemens J, Murthy M. 2016. Sensorimotor transformations underlying variability in song intensity during Drosophila courtship. Neuron 89(3):629–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowling DE, Burnet B. 1981. Courtship songs and genetic control of their acoustic characteristics in sibling species of the Drosophila melanogaster subgroup. Anim. Behav 29(3):924–35 [Google Scholar]
- Creutzig F, Benda J, Wohlgemuth S, Stumpner A, Ronacher B, Herz AVM. 2010. Timescale-invariant pattern recognition by feedforward inhibition and parallel signal processing. Neural Comput 22:1493–510 [DOI] [PubMed] [Google Scholar]
- Creutzig F, Wohlgemuth S, Stumpner A, Benda J, Ronacher B, Herz AVM. 2009. Timescale-invariant representation of acoustic communication signals by a bursting neuron. J. Neurosci 29(8):2575–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley SA, Bennet-Clark HC, Evert HT. 1995. Courtship song components affect male and female Drosophila differently. Anim. Behav 50(3):827–39 [Google Scholar]
- Eberl DF, Duyk GM, Perrimon N. 1997. A genetic screen for mutations that disrupt an auditory response inDrosophila melanogaster. PNAS 94:14837–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards CJ, Alder TB, Rose GJ. 2002. Auditory midbrain neurons that count. Nat. Neurosci 5(10):934–36 [DOI] [PubMed] [Google Scholar]
- Eisenberg JF, Kleiman DG. 1972. Olfactory communication in mammals. Annu. Rev. Ecol. Syst 3:1–32 [Google Scholar]
- Fellers GM. 1979. Aggression, territoriality, and mating behaviour in North American treefrogs. Anim. Behav 27:107–19 [Google Scholar]
- Fettiplace R, Fuchs PA. 1999. Mechanisms of hair cell tuning. Annu. Rev. Physiol 61:809–34 [DOI] [PubMed] [Google Scholar]
- Finck J, Kuntze J, Ronacher B. 2016. Chemical cues from females trigger male courtship behaviour in grasshoppers. J. Comp. Physiol. A 202(5):337–45 [DOI] [PubMed] [Google Scholar]
- Franconville R, Beron C, Jayaraman V. 2018. Building a functional connectome of the Drosophila central complex. eLife 7:e37017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George AA, Lyons-Warren AM, Ma X, Carlson BA. 2011. A diversity of synaptic filters are created by temporal summation of excitation and inhibition. J. Neurosci 31(41):14721–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel A, Buonomano DV. 2014. Timing as an intrinsic property of neural networks: evidence from in vivo and in vitro experiments. Philos. Trans. R. Soc. B Biol. Sci 369(1637):20120460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göpfert MC, Hennig RM. 2016. Hearing in insects. Annu. Rev. Entomol 61:257–76 [DOI] [PubMed] [Google Scholar]
- Gray DA, Gabel E, Blankers T, Hennig RM. 2016. Multivariate female preference tests reveal latent perceptual biases. Proc. Biol. Sci 283(1842):20161972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobe B, Rothbart MM, Hanschke A, Hennig RM. 2012. Auditory processing at two time scales by the cricket Gryllus bimaculatus. J. Exp. Biol 215(10):1681–90 [DOI] [PubMed] [Google Scholar]
- Halfwerk W, Jones PL, Taylor RC, Ryan MJ, Page RA. 2014. Risky ripples allow bats and frogs to eavesdrop on a multisensory sexual display. Science 343(6169):413–16 [DOI] [PubMed] [Google Scholar]
- Hartbauer M, Römer H. 2014. From microseconds to seconds and minutes—time computation in insect hearing. Front. Physiol 5:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedwig BG. 2016. Sequential filtering processes shape feature detection in crickets: a framework for song pattern recognition. Front. Physiol 7:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedwig BG, Poulet JFA. 2004. Complex auditory behaviour emerges from simple reactive steering. Nature 430(7001):781–85 [DOI] [PubMed] [Google Scholar]
- Hildebrandt KJ, Benda J, Hennig RM. 2015. Computational themes of peripheral processing in the auditory pathway of insects. J. Comp. Physiol. A 201(1):39–50 [DOI] [PubMed] [Google Scholar]
- Hill PSM. 2001. Vibration and animal communication: a review. Integr. Comp. Biol 41(5):1135–42 [Google Scholar]
- Hopkins CD. 1988. Neuroethology of electric communication. Annu. Rev. Neurosci 11:497–535 [DOI] [PubMed] [Google Scholar]
- Imaizumi K, Pollack GS. 2005. Central projections of auditory receptor neurons of crickets. J. Comp. Neurol 493(3):439–47 [DOI] [PubMed] [Google Scholar]
- Inagaki HK, Jung Y, Hoopfer ED, Wong AM, Mishra N, et al. 2014. Optogenetic control of Drosophila using a red-shifted channelrhodopsin reveals experience-dependent influences on courtship. Nat. Methods 11(3):325–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa Y, Okamoto N, Nakamura M, Kim H, Kamikouchi A. 2017. Anatomic and physiologic heterogeneity of subgroup-A auditory sensory neurons in fruit flies. Front. Neural Circuits 11:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson T, Kravitz EA, Heinrich R. 2011. Sound production during agonistic behavior of male Drosophila melanogaster. Fly 5(1):29–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamikouchi A, Inagaki HK, Effertz T, Hendrich O, Fiala A, et al. 2009. The neural basis of Drosophila gravity-sensing and hearing. Nature 458(7235):165–71 [DOI] [PubMed] [Google Scholar]
- Kamikouchi A, Shimada T, Ito K. 2006. Comprehensive classification of the auditory sensory projections in the brain of the fruit fly Drosophila melanogaster. J. Comp. Neurol 499(3):317–56 [DOI] [PubMed] [Google Scholar]
- Kanwal JS, Rauschecker JP. 2007. Auditory cortex of bats and primates: managing species-specific calls for social communication. Front. Biosci 12:4621–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klappert K, Reinhold K. 2003. Acoustic preference functions and sexual selection on the male calling song in the grasshopper Chorthippus biguttulus. Anim. Behav 65(1):225–33 [Google Scholar]
- Kohashi T, Carlson BA. 2014. A fast BK-type KCa current acts as a postsynaptic modulator of temporal selectivity for communication signals. Front. Cell. Neurosci 8:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostarakos K, Hedwig B. 2012. Calling song recognition in female crickets: Temporal tuning of identified brain neurons matches behavior. J. Neurosci 32(28):9601–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski S, Aubin T, Martin J-R. 2004. Courtship song in Drosophila melanogaster: a differential effect on male-female locomotor activity. Can. J. Zool 82(8):1258–66 [Google Scholar]
- Kriegbaum H 1989. Female choice in the grasshopper Chorthippus biguttulus. Naturwissenschaften 76(2):81–82 [Google Scholar]
- Lai JS-Y, Lo S-J, Dickson BJ, Chiang A-S. 2012. Auditory circuit in the Drosophila brain. PNAS 109(7):2607–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Ishimoto H, Kamikouchi A. 2018. Auditory experience controls the maturation of song discrimination and sexual response in Drosophila. eLife 7:e34348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libersat F, Murray JA, Hoy RR. 1994. Frequency as a releaser in the courtship song of two crickets, Gryllus bimaculatus (de Geer) and Teleogryllus oceanicus: a neuroethological analysis. J. Comp. Physiol. A 174(4):485–94 [DOI] [PubMed] [Google Scholar]
- Mann K, Gallen CL, Clandinin TR. 2017. Whole-brain calcium imaging reveals an intrinsic functional network in Drosophila. Curr. Biol 27(15):2389–96.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markow TA, O’Grady PM. 2005. Evolutionary genetics of reproductive behavior in Drosophila: connecting the dots. Annu. Rev. Genet 39:263–91 [DOI] [PubMed] [Google Scholar]
- Marsat G, Pollack GS. 2005. Effect of the temporal pattern of contralateral inhibition on sound localization cues. J. Neurosci 25(26):6137–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo E, Seki H, Asai T, Morimoto T, Miyakawa H, et al. 2016. Organization of projection neurons and local neurons of the primary auditory center in the fruit fly Drosophila melanogaster. J. Comp. Neurol 524(6):1099–164 [DOI] [PubMed] [Google Scholar]
- Matsuo E, Yamada D, Ishikawa Y, Asai T, Ishimoto H, Kamikouchi A. 2014. Identification of novel vibration-and deflection-sensitive neuronal subgroups in Johnston’s organ of the fruit fly. Front. Physiol 5:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meckenhäuser G, Hennig RM, Nawrot MP. 2013. Critical song features for auditory pattern recognition in crickets. PLOS ONE 8(2):e55349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J, Elsner N. 1996. How well are frequency sensitivities of grasshopper ears tuned to species-specific song spectra? J. Exp. Biol 199(7):1631–42 [DOI] [PubMed] [Google Scholar]
- Nolen TG, Hoy RR. 1984. Initiation of behavior by single neurons: the role of behavioral context. Science 226(4677):992–94 [DOI] [PubMed] [Google Scholar]
- Osorio D, Vorobyev M. 2008. A review of the evolution of animal colour vision and visual communication signals. Vision Res 48(20):2042–51 [DOI] [PubMed] [Google Scholar]
- Ostrowski TD, Sradnick J, Stumpner A, Elsner N. 2009. The elaborate courtship behavior of Stenobothrus clavatus (Acrididae: Gomphocerinae). J. Orthoptera Res 18(2):171–82 [Google Scholar]
- Otte D 1992. Evolution of cricket songs. J. Orthoptera Res 1:25–49 [Google Scholar]
- Patella P, Wilson RI. 2018. Functional maps of mechanosensory features in the Drosophila brain. Curr. Biol 28(8):1189–203.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack GS, Hedwig B. 2017. The cricket auditory pathway: neural processing of acoustic signals In The Cricket as a Model Organism, ed. Horch HW, Mito T, Popadić A, Ohuchi H, Noji S, pp. 155–67. Tokyo: Springer [Google Scholar]
- Pollack GS, Mason AC, Popper AN, Fay RR, eds. 2016. Insect Hearing. Cham, Switz.: Springer [Google Scholar]
- Poremba A, Bigelow J, Rossi B. 2013. Processing of communication sounds: contributions of learning, memory, and experience. Hear. Res 305:31–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulet JFA, Hedwig B. 2005. Auditory orientation in crickets: pattern recognition controls reactive steering. PNAS 102(43):15665–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichert MS, Gerhardt HC. 2014. Behavioral strategies and signaling in interspecific aggressive interactions in gray tree frogs. Behav. Ecol 25(3):520–30 [Google Scholar]
- Riabinina O, Dai M, Duke T, Albert JT. 2011. Active process mediates species-specific tuning of Drosophila ears. Curr. Biol 21(8):658–64 [DOI] [PubMed] [Google Scholar]
- Römer H, Marquart V. 1984. Morphology and physiology of auditory interneurons in the metathoracic ganglion of the locust. J. Comp. Physiol. A 155(2):249–62 [Google Scholar]
- Römer H, Rheinlaender J, Dronse R. 1981. Intracellular studies on auditory processing in the metathoracic ganglion of the locust. J. Comp. Physiol 144(3):305–12 [Google Scholar]
- Ronacher B 2014. Processing of species-specific signals in the auditory pathway of grasshoppers In Insect Hearing and Acoustic Communication: Animal Signals and Communication, Vol. 1, ed. Hedwig B, pp. 185–204. Berlin/Heidelberg: Springer [Google Scholar]
- Ronacher B, Hennig RM, Clemens J. 2015. Computational principles underlying recognition of acoustic signals in grasshoppers and crickets. J. Comp. Physiol. A 201(1):61–71 [DOI] [PubMed] [Google Scholar]
- Ronacher B, Stumpner A. 1988. Filtering of behaviourally relevant temporal parameters of a grasshopper’s song by an auditory interneuron. J. Comp. Physiol. A 163(4):517–23 [Google Scholar]
- Rose GJ. 2014. Time computations in anuran auditory systems. Front. Physiol 5:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose GJ. 2017. The numerical abilities of anurans and their neural correlates: insights from neuroethological studies of acoustic communication. Philos. Trans. R. Soc. Lond. B Biol. Sci 373(1740):20160512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose GJ, Brenowitz EA. 2002. Pacific treefrogs use temporal integration to differentiate advertisement from encounter calls. Anim. Behav 63(6):1183–90 [Google Scholar]
- Rose GJ, Capranica RR. 1983. Temporal selectivity in the central auditory system of the leopard frog. Science 219(4588):1087–89 [DOI] [PubMed] [Google Scholar]
- Rothbart MM, Hennig RM. 2012. The Steppengrille (Gryllus spec./assimilis): selective filters and signal mismatch on two time scales. PLOS ONE 7(9):e43975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybak F, Sureau G, Aubin T. 2002. Functional coupling of acoustic and chemical signals in the courtship behaviour of the male Drosophila melanogaster. Proc. R. Soc. B 269(1492):695–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Ronacher B, Hennig RM. 2008. The role of frequency, phase and time for processing of amplitude modulated signals by grasshoppers. J. Comp. Physiol. A 194(3):221–33 [DOI] [PubMed] [Google Scholar]
- Schneider DM, Woolley SMN. 2013. Sparse and background-invariant coding of vocalizations in auditory scenes. Neuron 79(1):141–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöneich S, Kostarakos K, Hedwig B. 2015. An auditory feature detection circuit for sound pattern recognition. Sci. Adv 1(8):e1500325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeholzer LF, Seppo M, Stern DL, Ruta V. 2018. Evolution of a central neural circuit underlies Drosophila mate preferences. Nature 559(7715):564–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirangi TR, Stern DL, Truman JW. 2013. Motor control of Drosophila courtship song. Cell Rep 5(3):678–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern DL, Crocker J, Ding Y, Frankel N, Kappes G, et al. 2017. Genetic and transgenic reagents for Drosophila simulans, D. mauritiana, D. yakuba, D. santomea, and D. virilis. G3 7(4):1339–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpner A 2002. A species-specific frequency filter through specific inhibition, not specific excitation.J. Comp. Physiol. A 188(3):239–48 [DOI] [PubMed] [Google Scholar]
- Stumpner A, Atkins G, Stout JF. 1995. Processing of unilateral and bilateral auditory inputs by the ON1 and L1 interneurons of the cricket Acheta domesticus and comparison to other cricket species. J. Comp. Physiol. A 177:379–88 [Google Scholar]
- Stumpner A, Ronacher B. 1991. Auditory interneurones in the metathoracic ganglion of the grasshopper Chorthippus biguttulus: I. Morphological and physiological characterization. J. Exp. Biol 158(3):391–410 [Google Scholar]
- Stumpner A, Ronacher B. 1994. Neurophysiological aspects of song pattern recognition and sound localization in grasshoppers. Integr. Comp. Biol 34(6):696–705 [Google Scholar]
- Sun D, Guo Z, Liu Y, Zhang Y. 2017. Progress and prospects of CRISPR/Cas systems in insects and other arthropods. Front. Physiol 8:608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talyn BC, Dowse HB. 2004. The role of courtship song in sexual selection and species recognition by female Drosophila melanogaster. Anim. Behav 68(5):1165–80 [Google Scholar]
- Tootoonian S, Coen P, Kawai R, Murthy M. 2012. Neural representations of courtship song in the Drosophila brain. J. Neurosci 32(3):787–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttle MD, Ryan MJ. 1981. Bat predation and the evolution of frog vocalizations in the neotropics. Science 214(4521):677–78 [DOI] [PubMed] [Google Scholar]
- Vaughan AG, Zhou C, Manoli DS, Baker BS. 2014. Neural pathways for the detection and discrimination of conspecific song in D. melanogaster. Curr. Biol 24(10):1039–49 [DOI] [PubMed] [Google Scholar]
- Versteven M, Vanden Broeck L, Geurten B, Zwarts L, Decraecker L, et al. 2017. Hearing regulates Drosophila aggression. PNAS 114(8):1958–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Helversen D 1972. Gesang des Männchens und Lautschema des Weibchens bei der Feldheuschrecke Chorthippus biguttulus (Orthoptera, Acrididae). J. Comp. Physiol 81(4):381–422 [Google Scholar]
- von Helversen D, von Helversen O. 1997. Recognition of sex in the acoustic communication of the grasshopper Chorthippus biguttulus (Orthoptera, Acrididae). J. Comp. Physiol. A 180(4):373–86 [Google Scholar]
- von Schilcher F 1976. The role of auditory stimuli in the courtship of Drosophila melanogaster. Anim. Behav 24(1):18–26 [Google Scholar]
- Windmill JFC, Jackson JC. 2016. Mechanical specializations of insect ears. See Pollack et al. 2016, pp. 125–57 [Google Scholar]
- Wohlers DW, Huber F. 1982. Processing of sound signals by six types of neurons in the prothoracic ganglion of the cricket, Gryllus campestris L. J. Comp. Physiol 146(2):161–73 [Google Scholar]
- Woolley SMN, Portfors CV. 2013. Conserved mechanisms of vocalization coding in mammalian and songbird auditory midbrain. Hear. Res 305:45–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyttenbach RA, May ML, Hoy RR. 1996. Categorical perception of sound frequency by crickets. Science 273(5281):1542–44 [DOI] [PubMed] [Google Scholar]
- Yamada D, Ishimoto H, Li X, Kohashi T, Ishikawa Y, Kamikouchi A. 2018. GABAergic local interneurons shape female fruit fly response to mating songs. J. Neurosci 38:4329–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HH, St-Pierre F. 2016. Genetically encoded voltage indicators: opportunities and challenges. J. Neurosci 36(3):9977–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J, Matsuo E, Yamada D, Mizuno H, Morimoto T, et al. 2013. Selectivity and plasticity in a sound-evoked male-male interaction in Drosophila. PLOS ONE 8(9):e74289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorozu S, Wong A, Fischer BJ, Dankert H, Kernan MJ, et al. 2009. Distinct sensory representations of wind and near-field sound in the Drosophila brain. Nature 458(7235):201–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Lauritzen JS, Perlman E, Robinson CG, Nichols M, et al. 2018. A complete electron microscopy volume of the brain of adult Drosophila melanogaster. Cell 174(3):730–43.E22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Franconville R, Vaughan AG, Robinett CC, Jayaraman V, Baker BS. 2015. Central neural circuitry mediating courtship song perception in male Drosophila. eLife 4:e08477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Pan Y, Robinett CC, Meissner GW, Baker BS. 2014. Central brain neurons expressing doublesex regulate female receptivity in Drosophila. Neuron 83(1):149–63 [DOI] [PubMed] [Google Scholar]
- Zuk M, Kolluru GR. 1998. Exploitation of sexual signals by predators and parasitoids. Q. Rev. Biol 73:415–38 [Google Scholar]
- Zuk M, Rebar D, Scott SP. 2008. Courtship song is more variable than calling song in the field cricket Teleogryllus oceanicus. Anim. Behav 76(3):1065–71 [Google Scholar]