Abstract
Motivational states modulate how animals value sensory stimuli and engage in goal-directed behaviors. The motivational states of thirst and hunger are represented in the brain by shared and unique neuromodulatory systems. However, it is unclear how such systems interact to coordinate expression of appropriate state-specific behavior. We show that the activity of two brain neurons expressing leucokinin (LK) neuropeptide is elevated in thirsty and hungry flies, and that LK release is necessary for state-dependent expression of water and sugar-seeking memories. LK inhibits two types of mushroom body (MB)-innervating dopaminergic neurons (DANs) to promote thirst-specific water-memory expression, whereas it activates other MB-innervating DANs to facilitate hunger-dependent sugar-memory expression. Selection of hunger- or thirst-appropriate memory emerges from competition between LK and other neuromodulatory hunger signals at the level of the DANs. Therefore, coordinated modulation of the dopaminergic system allows flies to prioritize the expression of the relevant state-dependent motivated behavior.
Introduction
Physiological needs elicit motivational states that promote goal-directed behaviors to potentially satisfy the animal’s requirements1. For example, hunger stimulates animals to seek and eat food, whereas water is sought to satiate thirst. Animals must therefore be able to select and execute the relevant behavior to procure the resource that satisfies their most pressing need. In mice, hunger induced by food deprivation and that evoked by synthetic neural activation, overrides the expression of other motivated behaviors, suggesting that internal states compete in the brain2. Although neuromodulatory systems are known to represent motivational states and to participate in behavioral control, it is unclear how these systems interact to prioritize the most appropriate behavior.
Drosophila behaviors are also regulated by internal motivational states and the relatively small brain and genetic tractability of the fruit fly make it possible to discover the underlying neural circuit mechanisms. Starvation in flies has been linked to release of a wide variety of neuromodulators, that in turn promote eating and food-seeking behavior by modulating peripheral and central neuronal circuits3. Interaction between hunger and thirst motivational systems has also been demonstrated. For example, the starvation-regulated adipokinetic hormone (AKH) activates four interoceptive neurons (ISNs) in the fly brain that promote food consumption, but inhibit water drinking4. Moreover, these ISNs are inhibited by high osmolality, like that resulting from dehydration. However, it is not known whether similar mechanisms also orchestrate more complex neural circuits and other hunger and thirst dependent behaviors.
In addition to modulating innate consummatory behaviors, motivational systems control whether flies express state-dependent learned behavioral responses. Flies can be trained to associate odors with water or sugar reward and they specifically express these memories when thirsty or hungry, respectively5,6. Prior work has shown that in hungry flies, neuropeptide F (dNPF) promotes sugar memory expression by inhibiting a specific subset of dopaminergic neurons (DANs), called MP1 or PPL1-γ1pedc, which project to a specific region of the mushroom body (MB)7. In contrast, thirst-dependent control of water memory expression is not understood. In this study, we investigated hunger- and thirst-dependent memory expression in flies as a means to elucidate a neural circuit mechanism that provides motivation state-specific behavioral control. We found that the neuropeptide Leucokinin (LK) released from a pair of neurons in the fly brain mediates both hunger- and thirst-dependent memory expression. However, LK controls expression of sugar and water memory by differentially regulating the activity of unique groups of MB-innervating DANs. Competition between LK and other modulatory hunger signals permits the relevant DANs to prioritize the expression of the appropriate hunger or thirst state-relevant memory.
Results
Expression of water-seeking memory is gated by thirst
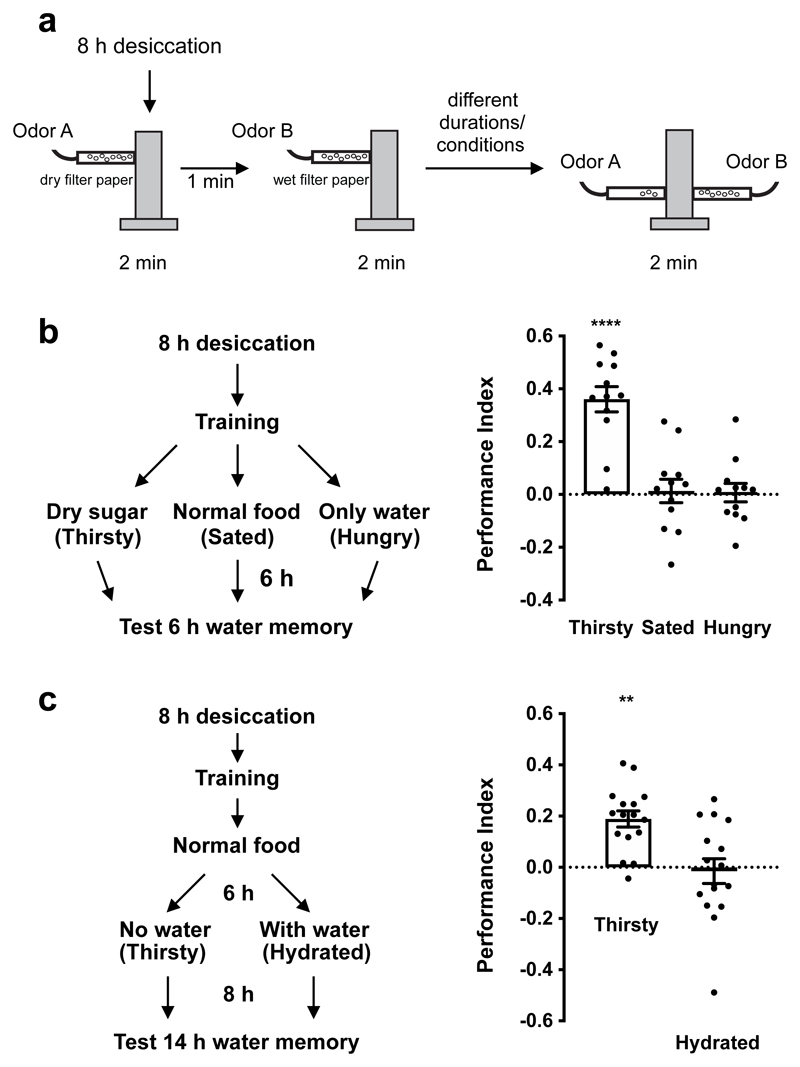
We previously reported that water-rewarded odor memories are more robustly expressed in thirsty than hungry flies5. We therefore first verified the state-specificity of water memory expression. Thirsty flies were trained with odors and water reward (Fig. 1a), then subjected to 3 different 6 h treatments after training (Fig. 1b). Flies were either transferred to empty vials with dry sugar paper to keep them thirsty, given normal fly food for 6 h to feed and drink to satiety, or housed in vials containing 1% agar to satiate thirst but keep them hungry. Only thirsty flies expressed robust 6 h water memory (Fig. 1b). Importantly, memory performance returned in water-sated flies that were made thirsty again. Robust 14 h water memory was expressed in sated flies that were water-deprived for 8 h, but not in flies kept hydrated (Fig. 1c). These data confirm that water memory expression is specifically gated by thirst.
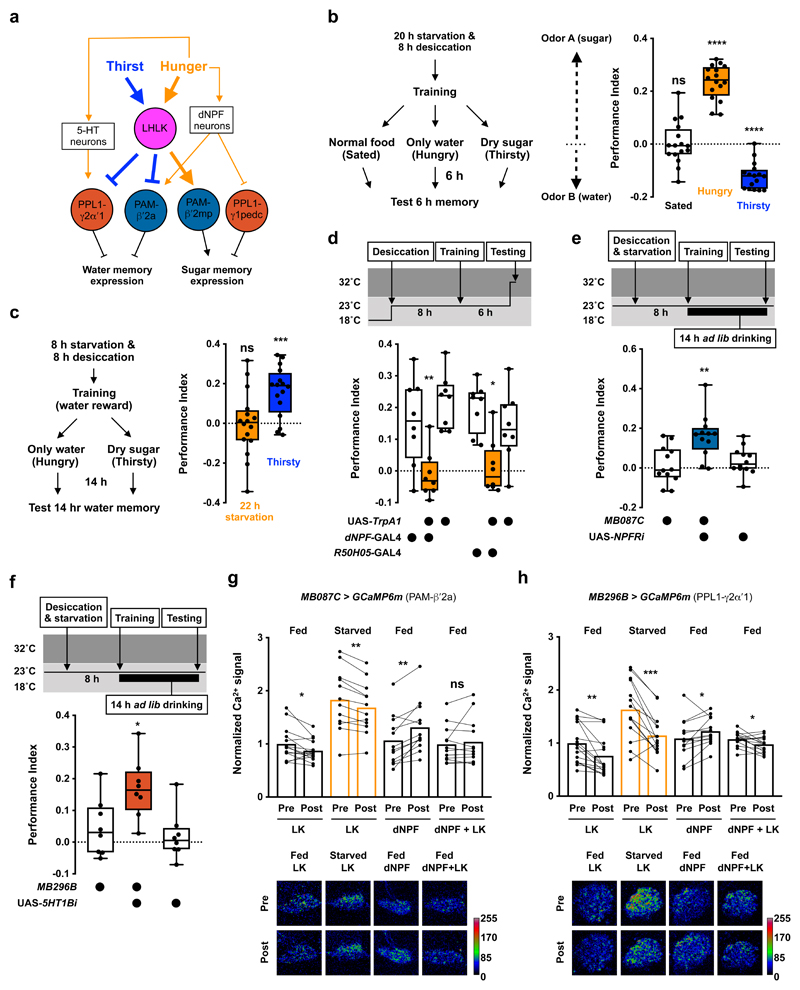
Figure 1. Thirst gates water memory expression.
a, Water-rewarded olfactory conditioning protocol. b, Left, experimental procedure. After training, flies were satiated, made hungry, or kept thirsty before testing 6 h memory. Thirsty flies show memory performance, which is significantly different from that of sated and hungry flies (p<0.0001, n = 12; one-way ANOVA with Tukey’s test). c, Re-dehydration protocol. Immediately after training, flies were satiated for 6 h, then made thirsty, or kept water sated before testing 14 h memory. Thirsty flies exhibited robust memory performance, that is significantly different to that of water-sated flies (p=0.0015, n = 16; two-tailed unpaired t-test with Welch’s correction). Data are mean ± standard error of mean (SEM). See Supplementary Table 3 for statistics details.
Leucokinin is a thirst signal that regulates water memory expression
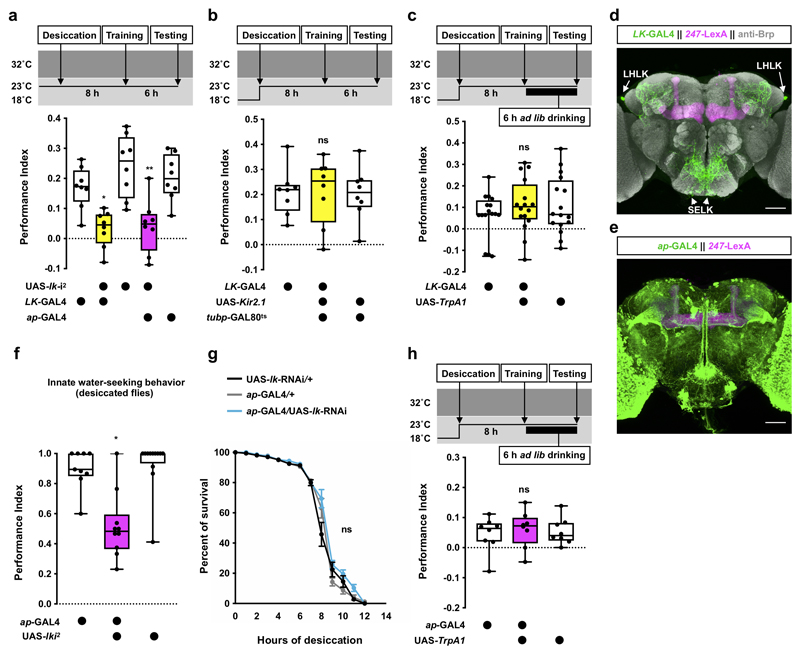
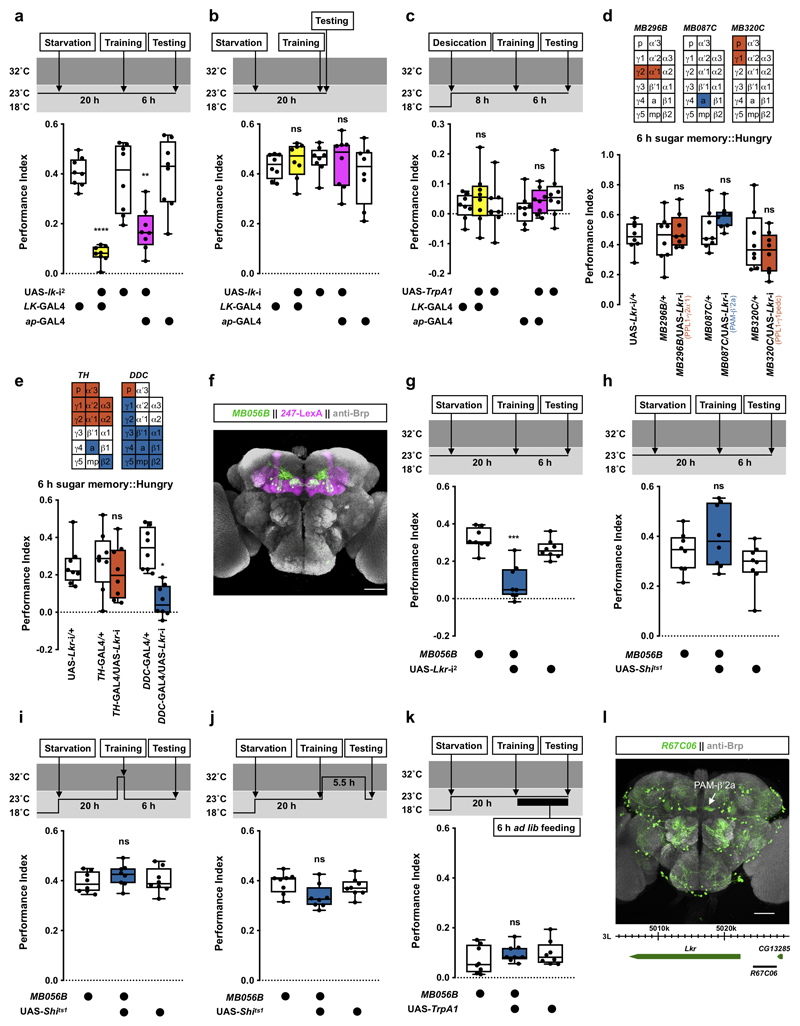
Several neuropeptides have been implicated as hunger or satiety signals in the fly8–17. However, less is known for thirst signals4,18. We found that electrically silencing neurons that release LK neuropeptide impaired thirst-dependent expression of 6 h water memory. We time-restricted expression of UAS-Kir2.1, encoding an inward-rectifier K+ channel19,20 in LK neurons by combining LK-GAL4 with ubiquitously expressed temperature-sensitive GAL80ts, an inhibitor of GAL421. Inducing Kir2.1 for 6 h after training, by shifting flies to 32°C to inactivate GAL80ts, abolished 6 h water memory performance (Fig. 2a). The 6 h water memory performance was similarly impaired in flies expressing either of two independent UAS-lk-RNAi transgenes in LK neurons (Fig. 2b, Extended Data Fig. 1a). No impairment was observed if Kir2.1 expression was suppressed by maintaining flies at permissive 23°C throughout the experiment (Extended Data Fig. 1b). Olfactory avoidance of UAS-Kir2.1 and UAS-lk-RNAi expressing flies was not significantly different from controls (Supplementary Table 1).
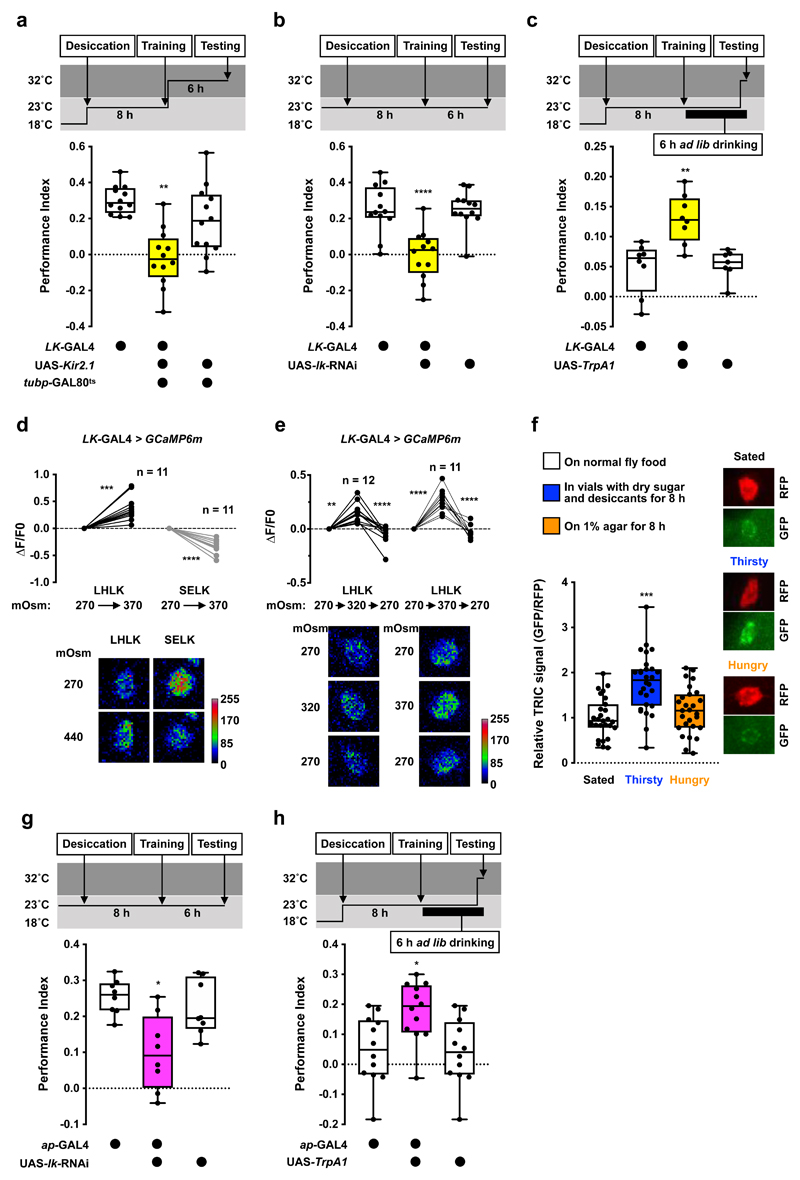
Figure 2. Leucokinin promotes thirst-dependent water memory expression.
a, Silencing LK neurons after training using UAS-Kir2.1 and tubp-GAL80ts impairs 6 h water memory performance. Memory performance is significantly lower than that of genetic controls (p<0.006, n = 8; one-way ANOVA with Tukey’s test). b, Knockdown of lk in LK neurons with LK-GAL4; UAS-lk-RNAi impairs 6 h water memory performance (p<0.0001, n = 8; one-way ANOVA with Tukey’s test). c, Activating LK neurons 10 min before and during testing enhances 6 h water memory performance in water-sated flies (p<0.0025, n = 8; one-way ANOVA with Tukey’s test). d, Increasing osmolality of the imaging buffer from 270 to 370 mOsm elevated GCaMP6m signal in LHLK neurons (p=0.0003, n = 11; two-tailed paired t-test) and decreased signal in SELK neurons (p<0.0001, n = 11; two-tailed paired t-test). e, Increasing osmolality from 270 to 320 or 370 mOsm elevated LHLK GCaMP6m signals (p<0.007, n = 12 or 320 mOsm; Friedman test with Dunn’s multiple comparisons test. p<0.0001, n = 11 for 370; repeated measures one-way ANOVA with Geisser-Greenhouse’s correction), which returned to baseline when osmolality was reverted to 270 mOsm. Representative images of GCaMP6m signal shown at bottom of each plot. f, TRIC signal in LHLK neurons is significantly higher in thirsty than sated or mildly hungry flies (p<0.00025, n = 27-28; one-way ANOVA with Tukey’s test). Representative TRIC images shown on the right of the plot. g, Knockdown of lk in LHLK neurons with ap-GAL4; UAS-lk-RNAi impairs 6 h water memory performance (p<0.015, n = 8; one-way ANOVA with Tukey’s test). h, Activating LHLK neurons 10 min before and during testing enhances 6 h water memory performance in water-sated flies (p<0.02, n = 8; one-way ANOVA with Tukey’s test). Temperature regimens shown above a-c and g-h. Box-plots: center line indicates median; box limits, upper and lower quartiles; whiskers, max to min range; dots, individual data points. Bar graphs: individual data points with mean ± SEM. See Supplementary Table 3 for statistics details.
We tested whether acutely activating LK neurons could provide a thirst signal and override water-replete suppression of water memory performance. We expressed the heat-sensitive TrpA1 Ca2+ channel22 in LK neurons which permits their depolarization by shifting flies to temperatures >25°C. Thirsty flies were trained at permissive 23°C and allowed to drink to satiety after training. Transient activation of LK neurons by raising flies to 32°C for 10 min before and during testing produced significant 6 h water memory performance in water-sated flies (Fig. 2c). However, water memory was not expressed in temperature-shifted control flies or when water sated LK-GAL4;UAS-TrpA1 flies were kept at 23°C during testing (Fig. 2c and Extended Data Fig. 1c). Therefore, LK neuron stimulation mimics water-deprivation in promoting water-memory expression.
Lateral horn LK neurons are osmosensitive and control water memory expression
There are two groups of LK-expressing neurons in the adult fly brain (LHLK and SELK; Extended Data Fig. 1d and Supplementary Video 1) and one in the abdominal ganglion of the ventral nerve cord (ABLK)23. Each LHLK cell body (one per hemisphere) in the lateral horn sends processes to the superior lateral protocerebrum, medial protocerebrum, and the neuropil around the MB calyx and peduncle23. SELK neuron processes are confined to the suboesophageal zone (SEZ). ABLK neurons regulate body water homeostasis, and their activities increase in water-satiated flies24,25. This is opposite to our behavioral data, which predict that LK should be released in thirsty flies to permit water memory expression. We therefore tested how LHLK and SELK neurons responded to osmolality change caused by desiccation. Fly hemolymph osmolality has been shown to increase from around 270 mOsm in the hydrated state to 320-370 mOsm when thirsty4. We bathed dissected brains with artificial hemolymph of differing osmolality and monitored the activity of LHLK and SELK neurons expressing the intracellular Ca2+ indicator GCaMP6m26. Surprisingly, elevating osmolality from 270 to 370 mOsm increased LHLK but decreased SELK neuron activity (Fig. 2d), suggesting that these LK neurons may have different roles in thirst-driven behavior, and that LHLK neurons could be those promoting water memory expression in dehydrated flies. We also tested whether LHLK neurons responded reversibly to osmolality changes within the physiological range. LHLK neuron activity increased when osmolality was elevated from 270 to 320 or 270 to 370 mOsm and decreased when osmolality was returned to 270 mOsm (Fig. 2e). We also assessed whether dehydration altered LHLK neuron activity using the transcriptional reporter of intracellular Ca2+, TRIC27. TRIC relies on calcium-dependent reconstitution of a functional transcription factor, which then drives GFP expression, allowing quantitative measurement of prolonged change in neural activity. Flies expressing TRIC in LHLK neurons were either dehydrated, made mildly hungry or kept fully satiated. The TRIC signal in LHLK neurons was significantly increased in thirsty compared to mildly hungry and fully satiated flies (Fig. 2f), suggesting that 8 h of water deprivation increases LHLK neuron activity. These data support a model where dehydration increases osmolality of fly hemolymph, which in turn activates LHLK neurons to release LK to regulate memory-related circuitry.
To test if LHLK neurons control water memory expression, we knocked down lk in LHLK neurons using apterous-GAL4 (ap-GAL4; Extended Data Fig. 1e and Supplementary Video 2). Although ap-GAL4 labels many cells including LHLK neurons, it does not label SELK or ABLK neurons23,28,29. Therefore, the intersection of lk RNAi and ap-GAL4 should specifically impair lk expression in LHLK neurons. The 6 h water memory performance of thirsty ap-GAL4;UAS-lk-RNAi flies was significantly impaired (Fig. 2g and Extended Data Fig. 1a). Knockdown of lk in LHLK neurons also impaired thirsty flies’ innate attraction to high humidity (Extended Data Fig. 1f) but not desiccation resistance (Extended Data Fig. 1g), water consumption (Supplementary Table 2), or olfactory avoidance (Supplementary Table 1). Lastly, heat-evoked activation of ap-GAL4 neurons during testing with ap-GAL4;UAS-TrpA1 promoted water memory expression in water-satiated flies (Fig. 2h and Extended Data Fig. 1h). Taken together, these data are consistent with LK released from LHLK neurons providing a signal that is critical to gate learned and innate water-seeking behaviors.
LK promotes water memory expression by inhibiting two types of MB-projecting DANs
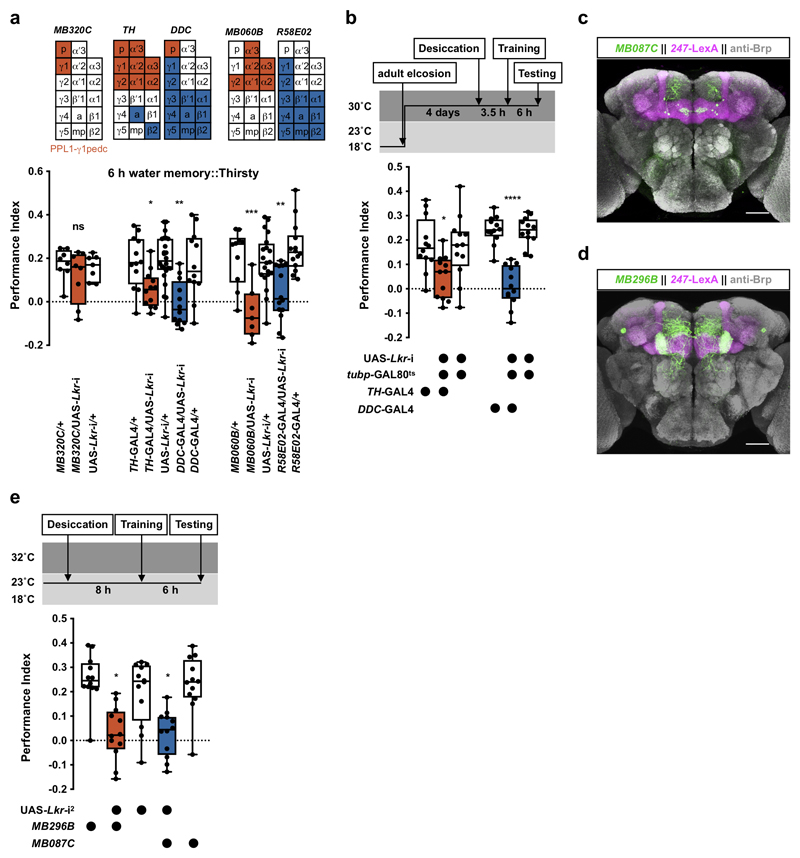
dNPF controls hunger-dependent expression of sugar-rewarded olfactory memory by releasing the inhibitory influence of PPL1-γ1pedc DANs innervating the MB7. We therefore tested whether LK might function similarly by RNAi knockdown of leucokinin receptor (Lkr) in MB-innervating DANs. Lkr-RNAi expression in PPL1-γ1pedc DANs (with MB320C-splitGAL4) did not affect 6 h water memory performance in thirsty flies (Extended Data Fig. 2a), suggesting hunger and thirst control differ. We next knocked down Lkr in neurons including the protocerebral anterior medial (PAM) and protocerebral posterior lateral (PPL1) clusters of DANs, that mostly innervate the vertical and horizontal MB lobes, respectively30,31. Knockdown of Lkr with either TH-GAL4 (labels all PPL1 DANs and a small number of PAM DANs) or DDC-GAL4 (labels all PAM DANs but also PPL1-γ1pedc DANs)31 impaired 6 h water memory performance in thirsty flies (Extended Data Fig. 2a). More specific expression of Lkr RNAi in PAM (R58E02-GAL4) or PPL1 (MB060B-splitGAL4) DANs confirmed these findings (Extended Data Fig. 2a). In addition, restricting Lkr RNAi knockdown to adult flies with tubp-GAL80ts and DDC-GAL4 or TH-GAL4 also impaired performance, suggesting that the defect does not have developmental origin (Extended Data Fig. 2b). We also knocked down Lkr in smaller subsets of MB-innervating DANs using Split-GAL4 lines31 (Fig. 3a). Although we could not test all the individual DAN classes, expressing Lkr-RNAi in PAM-β′2a or PPL1-γ2α′1 reduced 6 h water memory performance of thirsty flies (Fig. 3a, Extended Data Fig. 2c, d, and Supplementary Video 3 and 4). Water consumption and olfactory avoidance were unaffected (Supplementary Table 1 and 2). Similar memory impairments were apparent when another Lkr-RNAi was driven in PAM-β′2a or PPL1-γ2α′1 DANs (Extended Data Fig. 2e). These data indicate that LK signaling to PAM-β′2a and PPL1-γ2α′1 DANs gates water memory expression.
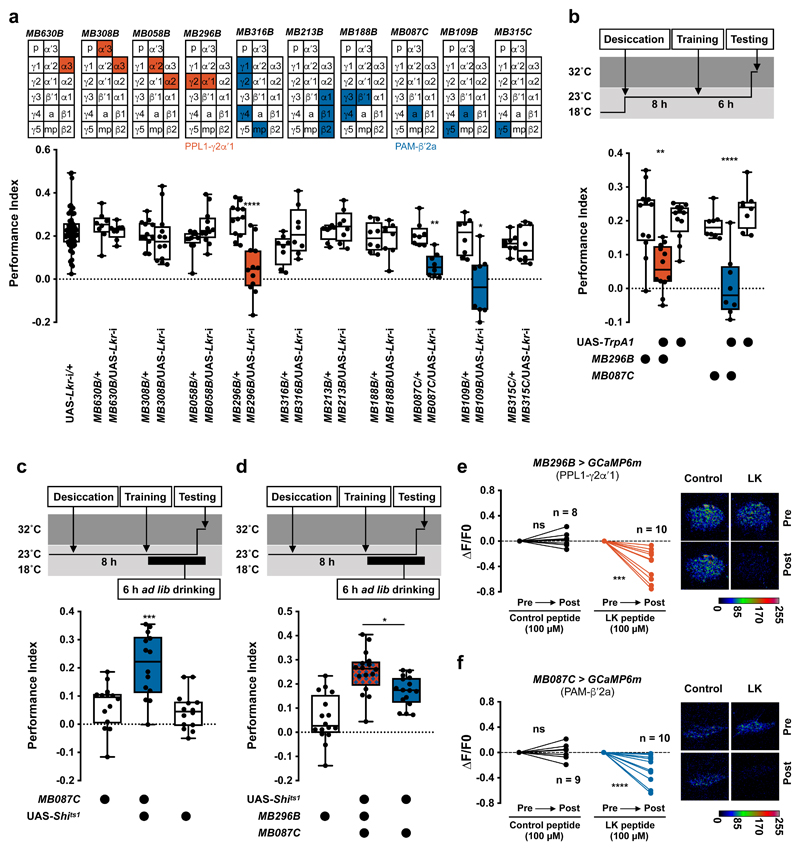
Figure 3. Leucokinin controls water memory expression by inhibiting two classes of dopaminergic neurons.
a, Lkr knockdown specifically in PPL1-γ2α′1 (MB296B-splitGAL4) or PAM-β′2a (MB087C-splitGAL4 and MB109B-splitGAL4) DANs impairs water memory expression (MB296B: p<0.0001, n = 12; MB087C: p<0.005, n = 8; MB109B: p<0.0015, n = 8; one-way ANOVA with Tukey’s test). Diagrams above the plot depict MB innervation of DANs labeled by each GAL4; p = peduncle, a = β′2a, and mp = β′2mp zones. b, Activating PAM-β′2a (MB087C-splitGAL4) or PPL1-γ2α′1 (MB296B-splitGAL4) DANs 10 min before and during testing impairs 6 h water memory expression in thirsty flies (PAM-β′2a: p<0.004, n = 12; Kruskal-Wallis test with Dunn’s multiple comparison test. PPL1-γ2α′1: p<0.0001, n = 8; one-way ANOVA with Tukey’s test). c, UAS-Shits1-mediated block of PAM-β′2a DANs (MB087C-splitGAL4) 20 min before and during testing reveals 6 h water memory expression in water-sated flies (p<0.0005, n = 14; one-way ANOVA with Tukey’s test). d, Blocking PAM-β′2a and PPL1-γ2α′1 DANs together (UAS-Shits1;MB087C/MB298B) 20 min before and during testing further enhances water memory expression in water-sated flies, compared to blocking PAM-β′2a alone (UAS-Shits1;MB087C) (p=0.0254, n = 16; one-way ANOVA with Tukey’s test). Temperature regimens shown above b-d. Box-plots: center line indicates median; box limits, upper and lower quartiles; whiskers, max to min range; dots, individual data points. e and f, Incubating explant brains with LK but not scrambled control peptides decreases GCaMP6m signal in PAM-β′2a (MB087C-splitGAL4) and PPL1-γ2α′1 (MB296B-splitGAL4) DANs (LK peptides for PAM-β′2a: p=0.001, n = 10; Scramble peptides PAM-β′2a: p=0.5399, n = 8; LK peptides for PPL1-γ2α′1: p=0.0054, n = 10; Scramble peptides PPL1-γ2α′1: p=0.5770, n = 9 ; two-tailed paired t-test). Representative images of GCaMP6m signal shown next to each plot. See Supplementary Table 3 for statistics details.
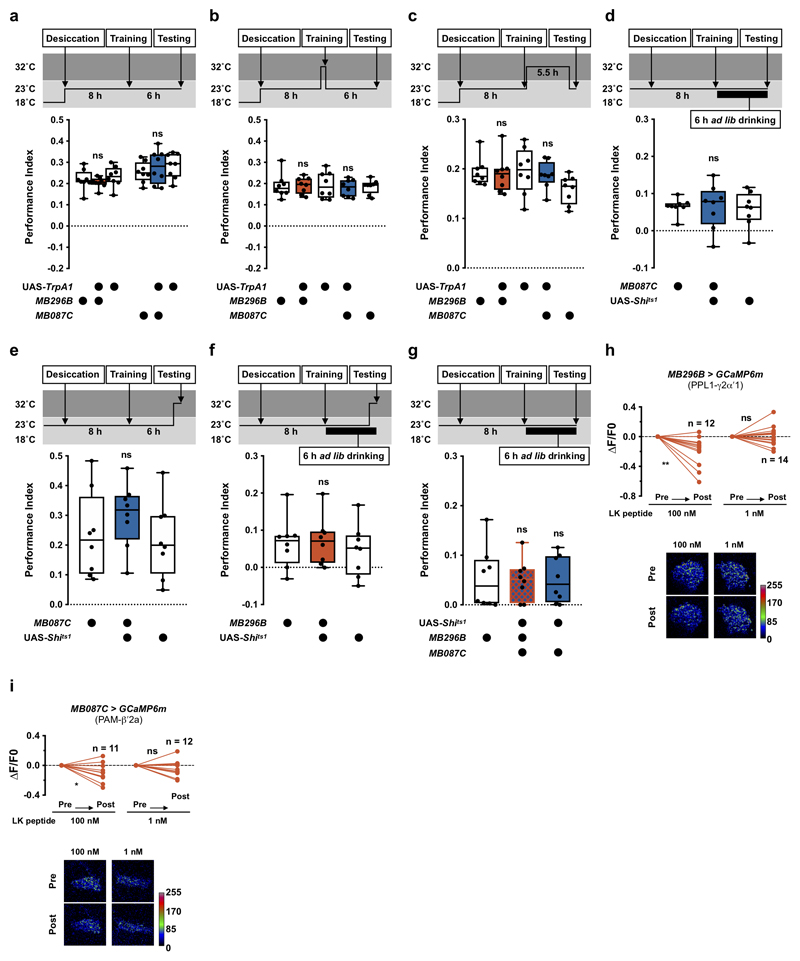
To directly assess a role for PAM-β′2a and PPL1-γ2α′1 DANs in water memory expression, we temporally manipulated their activity using UAS-TrpA1 or UAS-Shits1, the dominant temperature-sensitive dynamin that blocks neurotransmission at temperatures >29°C32. Activating either PAM-β′2a or PPL1-γ2α′1 DANs during testing in thirsty flies impaired 6 h memory performance but not odor avoidance (Fig. 3b, Extended Data Fig. 3a and Supplementary Table 1). In addition, activation during, or directly after, training had no effect (Extended Data Fig. 3b, c). We also tested whether silencing these DANs could mimic thirst and reveal water memory expression in sated flies. Thirsty flies with UAS-Shits1 driven in PAM-β′2a (MB087C-splitGAL4) or PPL1-γ2α′1 (MB296-splitGAL4) DANs were trained, then allowed to drink water to satiation before testing 6 h water memory. Flies were shifted to 32°C 20 min before and during testing to block DAN output. PAM-β′2a DAN block promoted water memory expression in water-sated flies (Fig. 3c). No enhancement was observed if the entire experiment was performed at permissive 23°C (Extended Data Fig. 3d). Moreover, blocking PAM-β′2a DANs did not further enhance memory performance of thirsty flies (Extended Data Fig. 3e). Blocking PPL1-γ2α′1 DANs during testing did not promote water memory expression in sated flies (Extended Data Fig. 3f). However, simultaneously blocking PPL1-γ2α′1 and PAM-β′2a DANs further enhanced memory expression in sated flies, as compared to blocking PAM-β′2a DANs alone (Fig. 3d and Extended Data Fig. 3g). PAM-β′2a and PPL1-γ2α′1 DANs may therefore work together to gate water memory expression. We also tested whether bath application of synthetic LK peptide to explant brains could regulate activity of PAM-β′2a and PPL1-γ2α′1 DANs expressing GCaMP6m. Applying 100 μM or 100 nM of synthetic LK significantly lowered Ca2+ signals in PAM-β′2a and PPL1-γ2α′1 DANs, whereas application of scrambled control peptides, or 1nM LK did not (Fig. 3e, f and Extended Data Fig. 3h, i). Although bath application experiments cannot accurately determine the effective concentration of LK peptide on PAM-β′2a and PPL1-γ2α′1 DANs, it is likely to be <100 nM. Together these results support a model where dehydration-induced LK release inhibits PAM-β′2a and PPL1-γ2α′1 DANs to promote water memory expression in thirsty flies.
LK is also a hunger signal that regulates sugar memory expression
Since LK has previously been implicated in feeding behavior33,34, we tested whether LK also contributed to hunger-dependent expression of food memory. Flies were starved for 20 h, trained to associate odors with sugar reward, then tested for 6 h sugar memory. Silencing LK neurons with UAS-Kir2.1 impaired memory performance of hungry flies (Fig. 4a). Knockdown of lk with two independent RNAi constructs in all LK neurons, or only LHLK neurons, also impaired 6 h sugar memory performance of hungry flies (Fig. 4b and Extended Data Fig. 4a). Interestingly, immediate memory performance was not disrupted in these flies, indicating that LK specifically regulates expression or formation of nutrient-dependent longer-term sugar memories35,36 (Extended Data Fig. 4b). Importantly, activation of LHLK neurons with LK-GAL4 or ap-GAL4 driven UAS-TrpA1 during testing promoted 6 h sugar memory expression in food-sated flies (Fig. 4c). No enhancement was observed if the entire experiment was performed at permissive 23°C (Extended Data Fig. 4c). Moreover, although 8 h starvation did not increase the TRIC signal in LHLK neurons (Fig. 2f), a significantly increased TRIC signal was evident after 20 h of starvation (Fig. 4d), consistent with a recent finding37. LK released from LHLK neurons therefore also contributes to hunger-dependent control of sugar memory expression.
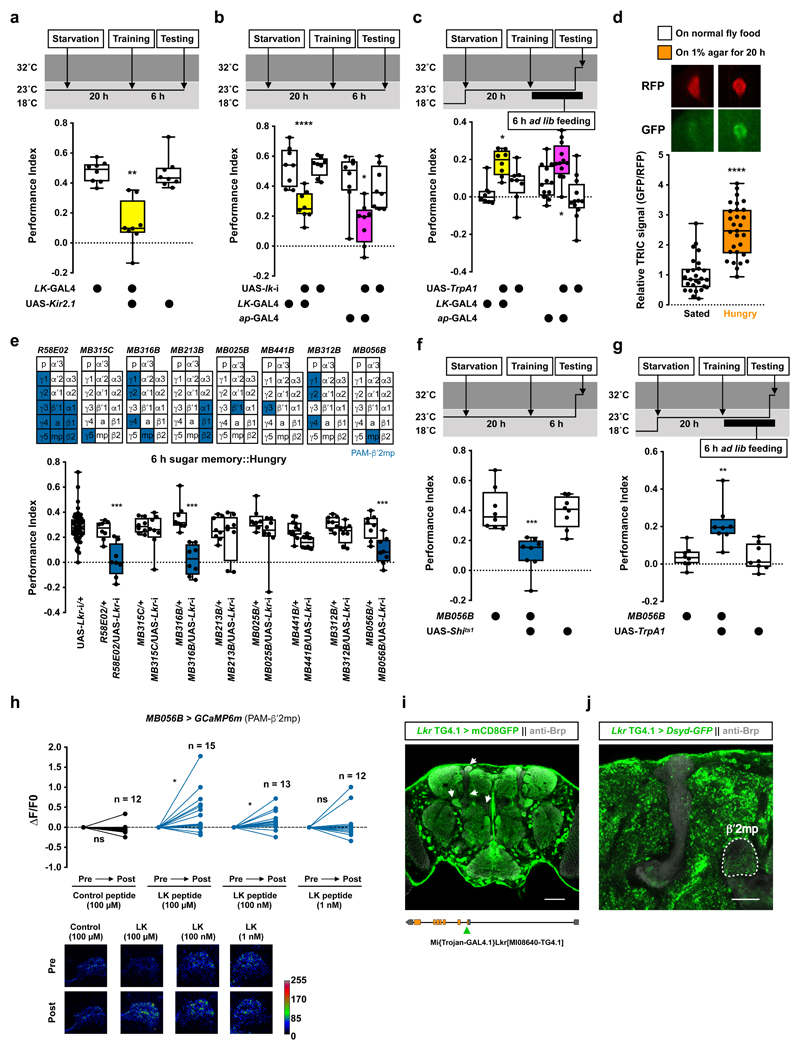
Figure 4. Leucokinin regulates hunger-dependent sugar memory expression via other dopaminergic neurons.
a, Silencing LK neurons with UAS-Kir2.1 impairs 6 h sugar memory performance in hungry flies (p<0.0045, n = 8; Kruskal-Wallis test with Dunn’s multiple comparisons test). b, RNAi knockdown of lk in all LK neurons (LK-GAL4) or LHLK neurons (ap-GAL4) impairs 6 h sugar memory performance in hungry flies (LK-GAL4: p<0.0001, n = 8; one-way ANOVA with Tukey’s test. ap-GAL4: p<0.045, n = 8; Kruskal-Wallis test with Dunn’s multiple comparisons test). c, Activating LHLK neurons with LK-GAL4 or ap-GAL4 driven UAS-TrpA1 10 min before and during testing enhances sugar memory performance in fed flies (LK-GAL4: p<0.025; ap-GAL4: p<0.035; n = 12; one-way ANOVA with Tukey’s test). d, LHLK neuron TRIC signal is increased following 20 h starvation (p<0.0001, n = 27 for Sated group and n = 29 for Hungry group; two-tailed unpaired t-test with Welch’s correction). Representative TRIC images shown above the plot. e, RNAi knockdown of Lkr in specific subsets of PAM DANs reveals PAM-β′2mp DANs (R58E02-GAL4, MB316B-splitGAL4, MB056B-splitGAL4) as critical for sugar memory expression (p<0.002, n = 8; one-way ANOVA with Tukey’s test). Diagrams above the plot depict MB innervation of DANs labeled by each GAL4. f, Blocking PAM-β′2mp DANs (MB056B-splitGAL4; UAS-Shits1) 20 min before and during testing impairs 6 h sugar memory in hungry flies (p<0.0006, n = 8; one-way ANOVA with Tukey’s test). g, Activation of PAM-β′2mp DANs (MB056B-splitGAL4; UAS-TrpA1) 10 min before and during testing enhances 6 h sugar memory in fed flies (p<0.0065, n = 8; Kruskal-Wallis test with Dunn’s multiple comparisons test). Temperature regimens shown above a-c and f-g. Box-plots: center line indicates median; box limits, upper and lower quartiles; whiskers, max to min range; dots, individual data points. h, Incubating explant brains with 100 μM or 100 nM, but not 1 nM LK or control peptide, increases GCaMP6m signal in PAM-β′2mp (MB056B-splitGAL4) DANs (Control peptide: p=0.2972, n = 12; 100 μM LK: p=0.0194, n = 15; 100 nM LK: p=0.0323, n = 13; 1 nM LK: p=0.4875, n = 12; two-tailed paired t-test). Representative images of GCaMP6m signal shown below the plot. See Supplementary Table 3 for statistics details. i, Adult brain expression of UAS-mCD8::GFP driven by Lkr Trojan-GAL4 (putative Lkr expressing neurons, green). Counterstained with anti-Brp antibody (gray). Single confocal plane is shown. Arrows indicate labeled neurites within the MB lobes. Four brains were examined and show the same expression pattern. Illustration below indicates position of Trojan-GAL4 insertion in the fly genome. Scale bar 50 μm. j, Adult brain expression of the presynaptic marker UAS-Dsyd-GFP (green) driven by Lkr Trojan-GAL4. Counterstained with anti-Brp antibody (gray). Single confocal plane is shown indicating innervation of the β′2mp zone (outlined in white). Four brains were examined and show the same expression pattern. Scale bar 20 μm.
LK promotes sugar memory expression by activating other MB-projecting DANs
Surprisingly, knocking down Lkr in PAM-β′2a or PPL1-γ2α′1 DANs did not impair 6 h sugar memory performance in hungry flies (Extended Data Fig. 4d), indicating LK regulates thirst and hunger behavior differently. Expressing Lkr-RNAi in PPL1-γ1pedc DANs, which dNPF modulates to control sugar memory expression7, or in all PPL1 DANs and a small number of PAM DANs (TH-GAL4) also had no effect (Extended Data Fig. 4d, e). In contrast, Lkr knockdown in all or subsets of PAM DANs (DDC-, R58E02-GAL4, or MB316B-splitGAL4) and specifically in PAM-β′2mp DANs (MB056B-splitGAL4) impaired 6 h sugar memory in hungry flies (Fig. 4e, Extended Data Fig. 4e, f and Supplementary Video 5). Flies expressing another Lkr-RNAi driven specifically in PAM-β′2mp DANs showed a similar memory defect (Extended Data Fig. 4g), confirming PAM-β′2mp DANs as the necessary site of Lkr expression for sugar memory. Knockdown of Lkr in PAM-β′2mp DANs did not affect sugar consumption or olfactory avoidance (Supplementary Table 1 and 2). Therefore, LK regulates water and sugar memory expression through different MB-directed DANs.
We further explored how PAM-β′2mp DANs regulate sugar memory expression. Blocking PAM-β′2mp DANs with Shits1 during testing significantly impaired 6 h memory performance in hungry flies (Fig. 4f and Extended Data Fig. 4h). No defect was observed when PAM-β′2mp DANs were blocked during or immediately after training (Extended Data Fig. 4i, j). In addition, TrpA1-mediated activation of PAM-β′2mp DANs during testing promoted sugar memory expression in fed flies (Fig. 4g and Extended Data Fig. 4k). Therefore, whereas output from PAM-β′2a and PPL1-γ2α′1 DANs inhibits water memory expression, PAM-β′2mp DAN output facilitates sugar memory expression. We also tested whether LK peptides could modulate PAM-β′2mp DANs by measuring their GCaMP6m Ca2+ signal in explant brains. PAM-β′2mp DAN Ca2+ signals were significantly increased in a dosage-dependent manner in brains incubated with LK, but not control peptides (Fig. 4h), indicating that LK regulates neuronal activity in a DAN-specific manner.
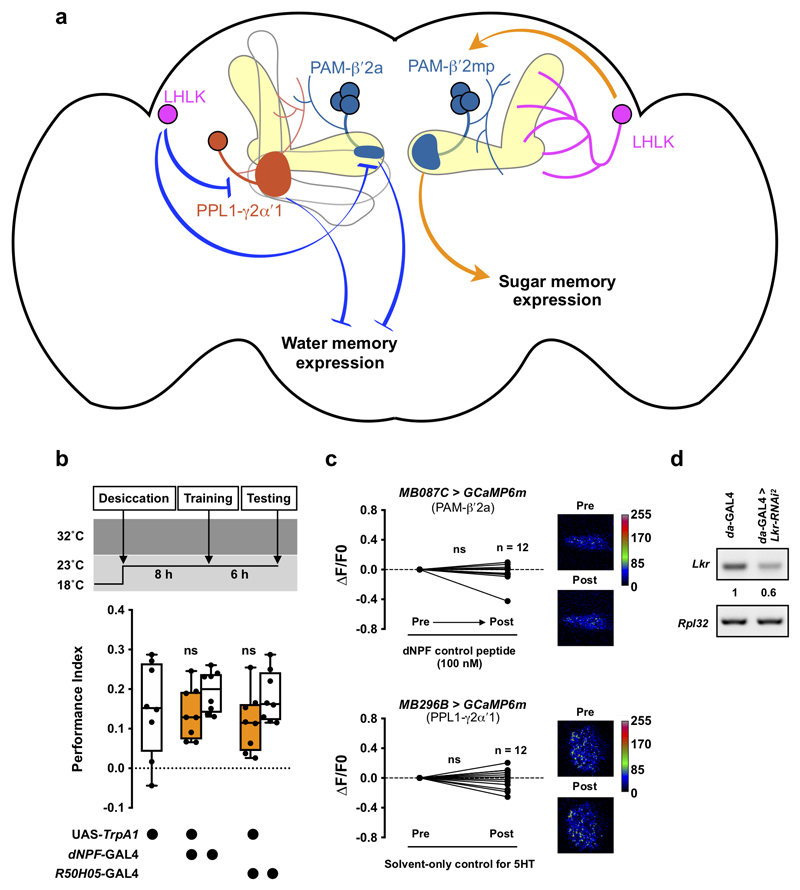
Our data suggest that LHLK neurons increase activity when flies are thirsty or very hungry. LK in turn modulates DANs to promote thirst- and hunger-dependent expression of water and sugar memories, respectively (Extended Data Fig. 5a). For thirst-dependent water memory, LK must lift the inhibitory influence of PAM-β′2a and PPL1-γ2α′1 DANs, reminiscent to how dNPF promotes sugar memory expression by inhibiting PPL1-γ1pedc DANs7. In contrast, LK promotes sugar memory performance in hungry flies by activating PAM-β′2mp DANs. Although LHLK neurons have neurites nearby the MB, they do not appear to be close to PAM-β′2a, PPL1-γ2α′1, and PAM-β′2mp DANs (Supplementary video 6-8), suggesting that LK might act through volume transmission. Other evidence supports Lkr expression in DANs. R67C06-GAL438, which is driven by an enhancer fragment from Lkr labels PAM-β′2a DANs, the only MB extrinsic neurons exclusively innervating the MB β′2a zones31 (Extended data fig. 4i). Furthermore, a Trojan-GAL4 (Lkr-TG4.1) inserted within the first intron of Lkr labels several MB extrinsic neurons, including PAM-β′2mp DANs as evident by the labeling of axonal termini in the MB β′2mp zones when the GAL4 is used to drive UAS-DSyd-GFP, a pre-synaptic marker39 (Fig. 4i, j and Supplementary Video 9). Although neither R67C06-GAL4 or Lkr-TG4.1 labels PPL1-γ2α′1 DANs, single-cell transcriptomics of the Drosophila midbrain reveals Lkr expression to be distributed across the brain, including in multiple DANs40,41. LK might therefore have a broader effect on the MB circuit than that reported here.
Flies express the memory that is relevant to their current deprivation state
It is unlikely that LK from the same LHLK source can distinguish between LKR on different DANs, and thereby generate state-specific memory expression5. The PAM-β′2a and PPL1-γ2α′1 DANs that are suppressed by LK also appear to be activated by dNPF and serotonin (5-HT) that represent hunger7,42,43 (Fig. 5a). We therefore hypothesized that dNPF and 5-HT might neutralize LK’s influence on PAM-β′2a and PPL1-γ2α′1 DANs to prevent water memory expression in hungry flies. We first confirmed that flies exhibit deprivation state-specific expression of water and sugar memories. Hungry and thirsty flies were trained to associate one odor with sugar and another odor with water, then subjected to 3 different 6 h treatments before testing odor preference (Fig. 5b). Flies were either given normal fly food to keep them fully satiated, transferred to 1% agar to make them hungry but not thirsty, or housed with dry sugar paper to satiate hunger but keep them thirsty. Fully satiated flies were indifferent to the two trained odors, whereas hungry flies approached the sugar-rewarded odor, and thirsty flies preferred the water-rewarded odor (Fig. 5b). These results demonstrate that flies can sequentially form water and sugar memories and express the relevant memory in the appropriate deprivation state. Furthermore, 22 h of starvation did not promote the expression of water memory, even though 20 h of starvation was sufficient to increase LHLK neuron activity (Fig. 4d and 5c).
Figure 5. Dopaminergic neurons control state-appropriate memory expression by integrating LK and other hunger signals.
a, Model for how leucokinin mediates thirst and hunger control of memory expression. Dehydration and prolonged starvation increase LK release from LHLK neurons. LK inhibits PPL1-γ2α′1 and PAM-β′2a DANs and activates PAM-β′2mp DANs. PPL1-γ2α′1 and PAM-β′2a DANs work together to limit water memory expression, whereas PAM-β′2mp DANs positively regulates sugar memory expression. Other hunger signals, such as dNPF and serotonin, are known to activate PPL1-γ2α′1 and PAM-β′2a and inhibit PPL1-γ1pedc DANs7,42,43. Hunger and thirst signals compete to allow the dopaminergic system to select expression of the appropriate sugar or water memory. b, Left, experimental procedure. Flies that were both hungry and thirsty were trained to associate odor A with sugar and another odor B with water. After training, flies were given normal fly food (fully-sated), 1% agar (hungry), or dry sugar-coated filter paper (thirsty) and tested for 6 h memory. Sated flies showed no odor preference (p=0.8932, n = 16, two-tailed one sample t-test), hungry flies approached the sugar-associated odor (p<0.0001, n = 16, two-tailed one sample t-test) and thirsty flies preferred the water-associated odor (p<0.0001, n = 16, two-tailed one sample t-test). c, Left, experimental protocol. Flies starved and desiccated for 8 h were trained with water reward. After training, flies were transferred to 1% agar (hungry) or vials with dry sugar-coated paper (thirsty), before testing 14 h memory. Thirsty, but not hungry, flies expressed 14 h memory (hungry: p=0.9714, n = 16; thirsty: p=0.0002, n = 16; two-tailed one sample t-test). d, Activating dNPF neurons (dNPF-GAL4) or hunger-promoting 5-HT neurons (R50H05-GAL4) 10 min before and during testing impairs 6 h water memory expression in thirsty flies (dNPF-GAL4: p<0.01; R50H05-GAL4: p<0.03; n = 8; one-way ANOVA with Tukey’s test). e, Knockdown of NPFR in PAM-β′2a DANs (MB087C-splitGAL4) promotes water memory expression in hungry flies (p<0.006, n = 12; one-way ANOVA with Tukey’s test). f, Knockdown of 5HT1B in PPL1-γ2α′1 DANs (MB296B-splitGAL4) promotes water memory expression in hungry flies (p<0.03, n = 8; one-way ANOVA with Tukey’s test). Temperature regimens shown above d-f. Box-plots: center line indicates median; box limits, upper and lower quartiles; whiskers, max to min range; dots, individual data points. g, Incubating explant brains from fed flies (fed brains) with 100 nM LK decreases Ca2+ signal in PAM-β′2a DANs (MB087C > GCaMP6m; p=0.0226, n = 14; two-tailed paired t-test). 20 h starvation increased Ca2+ signal in PAM-β′2a DANs (p<0.001, n = 12-14; one-way ANOVA with Tukey’s test). Incubating explant brains from flies starved for 20 h (starved brains) with 100 nM LK decreased Ca2+ signal in PAM-β′2a DANs (p=0.0016, n = 12; two-tailed paired t-test), but the Ca2+ signal remained higher than the baseline Ca2+signal of fed brains (p<0.015, n = 12-14; one-way ANOVA with Tukey’s test). Incubating fed brains with 100 nM dNPF peptide increased Ca2+ signal in PAM-β′2a DANs (p=0.0011, n = 12; two-tailed paired t-test) whereas applying 100 nM dNPF and 100 nM LK together did not (p=0.4744, n = 12; two-tailed paired t-test). All data points normalized to the mean of Fed LK data set. Representative images of GCaMP6m signal shown under the plot. h, Incubating fed brains with 100 nM LK decreases Ca2+ signal in PPL1-γ2α′1 DANs (MB296B > GCaMP6m; p=0.0002, n = 16; two-tailed Wilcoxon matched-pairs signed rank test). 20 h starvation increased Ca2+ signal in PPL1-γ2α′1 DANs (p<0.0045, n = 13-16; one-way ANOVA with Tukey’s test). 100 nM LK decreased Ca2+ signal in PPL1-γ2α′1 DANs in starved brains (p=0.0004, n = 14; two-tailed paired t-test), to a level that is indistinguishable from the baseline Ca2+ signal in fed brains (p>0.84, n = 13-16; one-way ANOVA with Tukey’s test). Incubating fed brains with 100 nM 5-HT increased PPL1-γ2α′1 DAN Ca2+ signals (p=0.0452, n = 13-16; two-tailed paired t-test). Incubating fed brains with both 100 nM 5-HT and 100 nM LK decreased PPL1-γ2α′1 DAN Ca2+ signals (p=0.0251, n = 14; two-tailed paired t-test), although the effect size was less than when incubated with 100 nM LK alone (-23.56 ± 4.89% vs. -8.48 ± 3.31%; p=0.0167, n = 15 for LK+5HT and 16 for LK alone; two-tailed unpaired t-test with Welch’s correction). Representative images of GCaMP6m signal shown under the plot. See Supplementary Table 3 for statistics details.
LK competes with other modulators to prevent inappropriate water memory expression
We next tested whether LK might interact with dNPF and 5-HT signals. Activation of dNPF neurons (dNPF-GAL47) or hunger-mediating 5-HT neurons (R50H05-GAL442) with TrpA1 during testing impaired 6h water memory in thirsty flies, without affecting olfactory avoidance (Fig. 5d, Extended Data Fig. 5b and Supplementary Table 1). Furthermore, knockdown of dNPF receptor (NPFR) in PAM-β′2a DANs or 5-HT receptor 1B (5-HT1B) in PPL1-γ2α′1 DANs promoted water memory expression in hungry flies (Fig. 5e, f). Enhanced water memory performance was not due to altered water consumption or odor avoidance (Supplementary Table 1 and 2). Expression of the relevant thirst- or hunger-dependent memory therefore involves interplay between LK, dNPF and 5-HT signals at the level of MB-innervating DANs.
We also tested whether starvation modulated PAM-β′2a and PPL1-γ2α′1 DAN activity using Ca2+ imaging in explant brains. 20 h starvation strongly increased Ca2+ signal in PAM-β′2a DANs (Fig. 5g). Incubating brains from both fed and starved flies with 100 nM LK decreased Ca2+ signals in PAM-β′2a DANs, but signals in starved brains incubated with LK remained higher than baseline Ca2+ signals in fed brains not exposed to LK (Fig. 5g). Applying 100 nM dNPF, but not scrambled control peptides also increased Ca2+ signal in PAM-β′2a DANs (Fig. 5g and Extended Data Fig. 5c). In addition, combining 100 nM dNPF with 100 nM LK, was sufficient to neutralize the LK-induced reduction in PAM-β′2a DAN Ca2+ signal (Fig. 5g). These data are consistent with hunger signals competing with LK to upregulate PAM-β′2a DAN activity. Similar competition between signals could also be observed in PPL1-γ2α′1 DANs. Starvation increased PPL1-γ2α′1 DAN Ca2+ signals (Fig. 5h) and application of 100 nM LK similarly decreased PPL1-γ2α′1 DAN Ca2+ signals in brains from fed and starved flies. However, PPL1-γ2α′1 DAN Ca2+ signals in starved brains with 100 nM LK were similar to those in fed brains without LK (Fig. 5h). 5-HT application also increased PPL1-γ2α′1 DAN Ca2+ signals (Fig. 5h and Extended Data Fig. 5c). In addition, co-delivery of 100 nM 5-HT with LK reduced the inhibitory influence of LK on the PPL1-γ2α′1 DAN Ca2+ signal (Fig. 5h). These imaging data indicate that LK and other internal state signals act together to differentially tune the dopaminergic system to allow the fly to prioritize thirst or hunger-state specific memory expression.
Discussion
DANs are proposed to relay behavioral and state information to the MB circuit, where the valence of olfactory cues is computed7,39,44. Multiple hunger signals have been found to modulate MB-projecting DANs7,43, but input signals representing other internal states remain elusive. Our data here suggest that LK released from LHLK neurons serves as a signal broadcasting the state of thirst to the MB. Interestingly, LK also plays a role in the representation of hunger to the MB. Hunger and thirst may share some signals controlling behavior because they both evoke appetitive behaviors. In addition, food and water often co-exist in nature, and eating and drinking behaviors are often tightly coupled45–47. However, hunger and thirst systems also need to be separable, so that animals can select whether to prioritize seeking food or water2,4.
LK promotes sugar memory expression by activating PAM-β′2mp DANs. Another hunger-mediating neuropeptide dNPF has been shown to control sugar memory expression by inhibiting PPL1-γ1pedc DANs7. Inactivity of these DANs is believed to increase the level of feedforward inhibition in the MB output neuron (MBON) network, which preferentially decreases activity of MBONs whose dendrites occupy the β′2 MB compartment48. LK modulation of PAM-β′2mp DANs thus represents a second and potentially more direct hunger-control pathway that converges with the dNPF pathway to regulate β′2 MBON activity. Interestingly, some of the critical thirst-dependent control also occurs in this MB area. Whereas LK activates PAM-β′2mp DANs in the context of hunger, LK inhibits PAM-β′2a (and PPL1-γ2α′1) DANs to permit thirst-dependent water memory expression. One possible mechanism that might explain the opposing effect of LK on different DANs would be having LKR coupled to excitatory G proteins, such as Gαs/q, in PAM-β′2mp and inhibitory Gαi in PAM-β′2a and PPL1-γ2α′1 DANs49. In addition, our findings demonstrate that state-specific selection of the appropriate memory emerges from the integrated action of multiple modulatory systems in the brain; hunger signals—dNPF and serotonin—inhibit water memory expression by counteracting LK in PAM-β′2a and PPL1-γ2α′1 DANs. Through these pathways the different motivational states are therefore able to regulate the relative valuation of learned sensory stimuli. Given that dopamine also plays an important role in motivated behavior in mammals50, it will be important to determine whether their control systems interact through a similar operating logic and neural circuit motifs to that of the fly.
Materials and Methods
Fly strains
Drosophila melanogaster were reared on standard cornmeal food and a 12 : 12 h light/dark cycle at 23°C and 60% humidity, unless stated otherwise. We used mixed sex 3-5 day-old flies in all behavioral experiments, except those involving UAS-TrpA1 for which flies were 8 days old. For imaging experiments, 3-5 day-old male flies were used. All strains used are previously described: white-eyed Canton-S51, LK-GAL4 (BDSC:51993)24, ap-GAL4 (BDSC:3041)29, dNPF-GAL47, R50H05-GAL442, G0239-GAL452, UAS-lk-RNAi (BDSC:25798)28,53,54, UAS-lk-RNAi2 (VDRC:14091)24,54,55, UAS-Lkr-RNAi (BDSC:25936)25,28,53, UAS-Lkr-RNAi2 (BDSC:65934)25,53, UAS-NPFRi (BDSC:25939)43,53, UAS-5HT1B-RNAi (BDSC:33418)43,53, UAS-TrpA122, UAS-Shi[ts1]32, UAS-Kir2.1 (DGRC:108846)19, tubp-GAL80ts (BDSC:7018)56, UAS-mCD8::GFP; MB247-LexA::P65, LexAop-rCD2::RFP57, TH-GAL458, DDC-GAL459, R58E02-GAL460, UAS-GCaMP6m (BDSC:42748)26, UAS-DenMark,UAS-Dsyd-1::GFP39, and 10XUAS-IVS-mCD8::RFP,13XLexAop2-mCD8::GFP;UAS-MKII::nlsLexADBDo,UAS-p65AD::CaM;UAS-p65AD::CaM (BDSC:62827)27. We also used the split-GAL4 strains31: MB060B, MB008B, MB131B, MB312B, MB630B, MB296B, MB308B, MB058B, MB316B, MB188B, MB194B, MB087C, MB320C, MB109B, MB315C, MB025B, MB441B, MB056B. Expression patterns of split-GAL4 lines can be viewed, http://splitgal4.janelia.org/cgi-bin/splitgal4.cgi. The efficacy of all RNAi lines used in this study have been verified previously24,28,43, except for UAS-Lkr-RNAi2, which we confirmed using RT-PCR to efficiently knock down Lkr transcript (Extended Data Fig. 5d).
RT-PCR
RT-PCR was performed using a S1000 Thermal Cycler (BioRad). Ubiquitously expressed da-GAL4 was used to drive UAS-Lkr-RNAi2. Total RNA from third-instar larvae was isolated using TRIzol RNA isolation reagent (Thermo Fisher Scientific: 15596026), followed by reverse transcription of cDNAs using SuperScript IV First-Strand Synthesis System for RT-PCR Kit (Thermo Fisher Scientific: 18091050). The Lkr transcripts were amplified with 30 PCR cycles using primers: 5′-GCAATGGACTTAATCGAGCAGG-3′ and 5′-CCGCGTATAGACGCTCAAATTC-3′. Intensity of PCR bands was quantified using Fiji/ImageJ61 and normalized to that of control ribosomal protein L32 (Rpl32) mRNA, which was amplified using primers: 5′-AGATCGTGAAGAAGCGCACCAAG-3′ and 5′-CACCAGGAACTTCTTGAATCCGG-3′.
Reward conditioning paradigms
To train flies with water reward, we first dehydrated them for 8 h in milk bottles (~100 flies/bottle) containing 20 mg desiccant beads (Dong-Syuan Co.) and a 2.5 cm × 8 cm dry sucrose-coated filter paper, in a sealed box maintained at 23°C and 10% humidity. When flies were required to be both hungry and thirsty, as for some experiments in Fig. 5, dry sucrose-coated filter paper was replaced with a clean filter paper without coating. Thirsty flies were conditioned in a T-maze (CelExplorer Labs: TM-101) under 630 nm red LED light. Flies were exposed to air for 2 min, followed by 2 min odor in a tube lined with dry filter paper. Following 60 s of air, flies were transferred to another tube lined with water-soaked filter paper and exposed to a second odor for 2 min, then 60 s of air. To test immediate memory, flies were transferred to a T-maze choice point and given 2 min to choose between the two odors used in training. To test 6 or 14 h memory, flies were transferred into vials containing a 1.5-cm × 6.5-cm dry sucrose-coated filter paper until testing. Performance Index (PI) was calculated as the number of flies choosing the conditioned odor minus the number of flies choosing the non-conditioned odor, divided by the total number of flies. A single PI value is the average score of two experiments in which different populations of flies of the same genotype were trained to associate water reward with the reciprocal odors. Odors used in conditioning were 3-octanol (OCT; Sigma: 218405) and 4-methyl-cyclohexanol (MCH; Sigma: 153095), diluted 20 μl OCT or 25 μl MCH in 10 ml mineral oil (Sigma: 330760). Airflow was maintained at 800 ml/min. To satiate flies with water or food, flies were transferred after training to vials containing 1% agar (BD: 214530) or standard fly food, respectively. All experiments were performed at 23°C and 50% humidity unless stated otherwise.
Sugar reward training was performed similarly to that with water reward, except that: 1) flies were starved in bottles containing 15 ml of 1% agar for 20 h at 23°C and 50% humidity before training; 2) filter paper coated with dried sucrose was used in the training tube; and 3) flies were kept in vials containing 1% agar between training and testing. For experiments using UAS-TrpA1, flies were grown at 18°C until starvation or desiccation began, which was performed at permissive 23°C. Flies were then shifted—the timing of which differed depending on the respective experiment—to restrictive 32°C for at least 10 min to activate neurons expressing TrpA1. Similarly, for experiments involving UAS-Shits1, flies were kept at permissive 23°C until shifting to restrictive 32°C for at least 20 min to block neurons expressing Shits1. In experiments in Fig. 2a with tubp-GAL80ts, flies were grown at 18°C, but desiccated and trained at permissive 23°C. Immediately after training, flies were shifted to restrictive 32°C until end of experiment. For experiments in Extended Data Fig. 2a, flies were grown at 18°C and shifted to 30°C after eclosion for 4 days, followed by 3.5 h desiccation, training, and testing at 30°C.
Innate water-seeking behavioral assay
The protocol was modified from previous studies62,63. Around 150 3-5 day old flies of both sexes were desiccated for 16 h in a milk bottle containing a 2.5 cm × 8 cm dry sucrose-coated filter paper, within a sealed box maintained at 23°C and 10% humidity. After desiccation, 30-40 flies were introduced into an 85 mm diameter arena to test humidity preference. The arena was separated into bottom and top levels by a sheet of mesh. The bottom was divided into two equal-sized chambers with a plastic divider and one chamber was filled with deionized water. Mesh was suspended 2mm above the water surface and flies were given 90 sec to choose between staying above the dry chamber (50% humidity) or the wet chamber (95% humidity). The number of flies in each area at the end of the 90 sec was recorded and a performance index was calculated as the number of flies above the wet area minus the number of flies above the dry area, divided by the total number of flies.
Ingestion assays
To measure water consumption, 20 flies (10 males, 10 females) were desiccated for 8 h and housed for 2 min in a tube lined with filter paper soaked with 0.4% FD&C blue # 1 dye (Spectrum Chemical: FD110) diluted in distilled water. Flies were then quickly frozen at −20°C to prevent excretion. Frozen flies were homogenized in 500 μl phosphate-buffered saline (PBS; Sigma: P4417) and centrifuged at 18,800 g for 3 min to clear debris. Supernatant was mixed with 100 μl PBS and centrifuged again at 18,800 g for 3 min. Dye in the supernatant was quantified by measuring absorbance at 625 nm using a Multiscan GO microplate spectrophotometer (Thermo Fisher Scientific). Sugar consumption was measured using the same protocol except that flies were starved for 20 h and placed in a tube lined with 1% agar mixed with 3M sucrose and 0.4% dye.
Olfactory avoidance test
Flies in the relevant motivational state (hungry or thirsty) were given a choice in a T-maze between air (10 ml mineral oil only) and OCT (20 μl in 10 ml mineral oil) or MCH (25 μl in 10 ml mineral oil). Avoidance index was calculated as the number of flies approaching air minus the number of flies in the odor, divided by the total number of flies in each experiment. Odor avoidance was tested at 23°C and for temperature-sensitive experiments, at 32°C.
Desiccation resistance assay
To measure desiccation resistance, around 70-80 flies were placed in an empty vial containing 2 g desiccant beads and a 1.5-cm × 6.5-cm filter paper coated with dry sucrose. Vials were covered with Parafilm. The number of dead flies in each vial was counted every hour after desiccation began.
Immunofluorescence staining
Fly brains were dissected in 1X PBS and fixed for 20 min at room temperature in 1X PBS containing 4% formaldehyde (Sigma: F8775). Fixed brains were washed 3 X 20 min in 1X PBS with 0.5% Triton X-100 (PBST; Sigma: T8787), followed by incubation in PBST with 5% normal goat serum (blocking solution; Jackson ImmunoResearch: 005-000-121) for 30 min. Brains were then incubated overnight with primary antibodies in blocking solution at 4°C, washed 3 X 20 min in PBST, before incubating overnight with secondary antibodies in PBST at 4°C. Brains were then washed 3 X 20 min in PBST and mounted on glass slides in Gold anti-fade reagent (Thermo Fisher Scientific: S36937). Antibodies used in this study are Mouse anti-Brp (1:50; DSHB: nc82), Rat anti-mCD8a (1:100; Thermo Fisher Scientific: MCD0800, lot# 1968949), chicken anti-GFP (1:5000; Abcam: 13970, lot# GR23665112), rabbit anti-Dsred (1:500; Clontech: 632496, lot# 1509043), donkey anti-chicken-488 (1:400; Jackson ImmunoResearch: 131753, lot# 131753), Goat anti-rat-488 (1:400; Thermo Fisher Scientific: A11006, lot# 1728142) and Goat anti-Rabbit-Cy3 (1:400; Jakson ImmumoResearch: 111-165-144, lot# 123834). Brains were imaged using an LSM 880 confocal microscope (Zeiss) and images were analyzed in Fiji/ImageJ61.
Brain registration
Adult brains with neurons labeled by different GAL4s and stained with anti-Brp antibody were registered to the IS2 template brain64. The registration was done using Fiji and the CMTK plugin (Developed in the lab of G. Jefferis, https://github.com/jefferis/fiji-cmtk-gui).
TRIC
Adult fly brains with the genotype 13XLexAop2-mCD8::GFP;LK-GAL4/UAS-MKII::nlsLexADBDo,UAS-p65AD::CaM;UAS-p65AD::CaM were dissected and stained following the procedure described in the Immunofluorescence staining section. The chicken anti-GFP and rabbit anti-Dsred primary antibodies and donkey anti-chicken-488 and Goat anti-Rabbit-Cy3 secondary antibodies were used to stain GFP and RFP. Mean intensities of GFP and RFP signals were measured in the cell bodies of LHLK neurons using Fiji/ImageJ, and the relative TRIC signal in each cell body was calculated as (GFP signal)/(RFP signal).
Explant brain two-photon calcium imaging
To image osmolality responses in LHLK neurons, dissected brains expressing GCaM6m were placed on a poly-lysine-coated glass coverslip bathed in 300 μl ALH buffer of 270 mOsm (NaCl 113 mM, KCl 5 mM, MgCl2 8.2 mM, CaCl2 2 mM, and HEPES-NaOH 5 mM; pH 7.3). Cell bodies of LHLK or SELK neurons were imaged at six frames per second using a Zeiss LSM 880 system with a two-photon laser (Spectra-Physics: Mai Tai HP 1040S) and a 20X water immersion objective. We selected the focal plane where the cell body covered the largest area of the visual field. After imaging for 300 frames, buffer was exchanged with ALH of 320 mOsm (NaCl 113 mM, KCl 5 mM, MgCl2 8.2 mM, CaCl2 2 mM, Sucrose 55 mM, and HEPES-NaOH 5 mM; pH 7.3) or 370 mOsm (NaCl 113 mM, KCl 5 mM, MgCl2 8.2 mM, CaCl2 2 mM, Sucrose 105 mM, and HEPES-NaOH 5 mM; pH 7.3) by repeatedly removing the original buffer and adding the new buffer (300 μl) to the brain for three times. To avoid movement caused by buffer exchange, we re-adjusted the focus to the plane where the cell body covered the largest area of the visual field. Brains were imaged for another 300 frames immediately after buffer exchange. The mean intensity of the GCaMP6m signal was measured using Fiji/ImageJ. For each brain, the average intensity of GCaMP6m signal of the 300 frames before buffer exchange was used as the baseline. ΔF/F0 was calculated as [(average intensity of GCaMP6m signal of the 300 frames post buffer exchange) – (average intensity of GCaMP6m signal of the 300 frames pre buffer exchange)]/(average intensity of GCaMP6m signal of the 300 frames pre buffer exchange). The same procedure was used to measure responses of DANs to LK, dNPF, and 5-HT (serotonin hydrochloride; Sigma-Aldrich: H9523), except that the high osmolality buffer was replaced with 270 mOsm ALH containing different concentrations of peptides or 5-HT and brains were incubated with peptides or 5-HT for 5 min before the second imaging. In addition, we imaged the DAN axon termini in the MB lobes, rather than their cell bodies. The LK peptide sequence is NSVVLGKKQRFHSWG-amide, and its scrambled control peptide GNWSSVHVFLRGQKK-amide. The dNPF peptide sequence is SNSRPPRKNDVNTMADAYKFLQDLDTYYGDRARVRFamide, and its scrambled control peptide FSRNVSRRAPRPDRGKYNYDTVDNLTDMQALDFAKYamide. Peptides were synthesized by GenScript.
Statistics and Reproducibility
Flies in an experimental group were selected randomly. Experimental and control groups were test in random orders. No blinding was used in data collection and analysis. All repeats in this study were biological replicates (i.e. different groups of animals in behavioral experiments and different fly brains in imaging experiments) and they were performed in at least two different days with similar results. Statistical analysis was performed in Prism 8. All behavioral data were tested for normality using the Shapiro-Wilk normality test, and no data point was excluded from the analyses. To compare more than two groups, normally distributed datasets were analyzed using one-way ANOVA and Tukey’s multiple comparisons. Data not conforming to a normal distribution were analyzed using Kruskal-Wallis and Dunn’s multiple comparisons test. When comparing two groups, we used a two-tailed unpaired Student’s t test with Welch’s correction for unpaired data or a two-tailed paired Student’s t test for paired data for normally distributed data. Paired data not conforming to a normal distribution were analyzed using two-tailed Wilcoxon matched-pairs signed rank test. Repeated measurements of paired data were analyzed using Friedman test with Dunn’s multiple comparisons test (non-normal distribution) or repeated measures one-way ANOVA with Geisser-Greenhouse’s correction (normal distribution). Data from the desiccation-resistance assay were analyzed using a two-way ANOVA with Tukey’s multiple comparisons test. No statistical methods were used to predetermine sample sizes but our sample sizes are similar to those reported in previous publications5,65,66.
Extended Data
Extended Data Fig. 1. Controls and additional experiments related to Figure 2.
Knockdown of lk in all LK neurons (LK-GAL4) or in LHLK neurons (ap-GAL4) using a second RNAi line impairs 6 h water memory performance in thirsty flies (LK-GAL4: p<0.015; ap-GAL4: p<0.025; n = 8; one-way ANOVA with Tukey’s test). b, Permissive 23°C control for experiments in Fig. 2a. No effect was observed (p>0.987, n = 8; one-way ANOVA with Tukey’s test). c, Permissive 23°C control for experiments in Fig. 2c. No effect was observed (p>0.552, n = 8; one-way ANOVA with Tukey’s test). d, Expression of UAS-mCD8::GFP driven by LK-GAL4 (LK neurons, green) and LexAop-rCD2::RFP driven by MB247-LexA::P65 (mushroom bodies, magenta). Brain also stained with anti-Brp antibody (gray). Four brains were examined and show the same expression pattern. Scale bar 50 μm. e, Expression of UAS-mCD8::GFP driven by ap-GAL4 (green) and LexAop-rCD2::RFP driven by MB247-LexA::P65 (magenta). Four brains were examined and show the same expression pattern. Scale bar 50 μm. f, Knockdown of lk in LHLK neurons using ap-GAL4 impairs innate water-seeking behavior (p<0.023, n = 9-12; Kruskal-Wallis test with Dunn’s multiple comparisons test). g, RNAi knockdown of lk in LHLK neurons using ap-GAL4 does not affect resistance to desiccation (p>0.098, n = 8; two-way ANOVA with Tukey’s test). Mean ± SEM are shown. h, Permissive 23°C control for experiments in Fig. 2h. No effect was observed (p>0.82, n = 8; one-way ANOVA with Tukey’s test). Temperature regimens shown above a-c and h. Box-plots: center line indicates median; box limits, upper and lower quartiles; whiskers, max to min range; dots, individual data points. See Supplementary Table 3 for statistics details.
Extended Data Fig. 2. Additional experiments related to Figure 3.
RNAi knockdown of Lkr in PPL1-γ1pedc DANs (MB320C-splitGAL4) does not affect 6 h water memory performance (p>0.48, n = 7-8; one-way ANOVA with Tukey’s test). RNAi knockdown of Lkr in PPL1 (TH-GAL4 and MB060B-splitGAL4) or PAM (DDC-GAL4 and R58E02-GAL4) DANs impairs 6 h performance (p<0.02, n=7-14; one-way ANOVA with Tukey’s test). b, Knockdown of Lkr after eclosion in TH-GAL4- and DDC-GAL4-labeled DANs with tubp-GAL80ts and UAS-Lkr-RNAi impairs 6 h water memory performance in thirsty flies (TH-GAL4: p<0.032; DDC-GAL4: p<0.0001; n = 12; one-way ANOVA with Tukey’s test). c, Adult brain expression of UAS-mCD8::GFP driven by MB087C-splitGAL4 (PAM-β′2a, green) and LexAop-rCD2::RFP driven by MB247-LexA::p65 (mushroom bodies, magenta). d, Adult brain expression of UAS-mCD8::GFP driven by MB296B-splitGAL4 (PPL1-γ2α′1, green) and LexAop-rCD2::RFP driven by MB247-LexA::p65 (magenta). Both brains counterstained with anti-Brp antibody (gray). Four brains were examined for each splitGAL4 and their expression patterns are consistent. Scale bars 50 μm. e, Knockdown of Lkr in PAM-β′2a (MB087C-splitGAL4) and PPL1-γ2α′1 (MB296B-splitGAL4) DANs with a second RNAi line impairs 6 h water memory performance (p<0.03, n = 12; Kruskal-Wallis test with Dunn’s multiple comparisons test). Temperature regimens shown above b and e. Box-plots: center line indicates median; box limits, upper and lower quartiles; whiskers, max to min range; dots, individual data points. See Supplementary Table 3 for statistics details.
Extended Data Fig. 3. Controls and additional experiments related to Figure 3.
Permissive 23°C controls for experiments in Fig. 3b. No defect was observed (MB296B: p>0.38; MB087C: p>0.72; n = 8; one-way ANOVA with Tukey’s test). b, Activating PAM-β′2a (MB087C) and PPL1-γ2α′1 (MB296B) DANs with UAS-TrpA1 at 32°C 10 min before and during training had no effect on 6 h water memory (MB087C: p>0.97; MB296B: p>0.9999; n = 8; one-way ANOVA with Tukey’s test). c, Activating PAM-β′2a (MB087C) and PPL1-γ2α′1 (MB296B) DANs with UAS-TrpA1 at 32°C immediately after training until 30 min before testing did not affect 6 h water memory (MB087C: p>0.46; MB296B: p>0.9999; n = 8; Kruskal-Willis test with Dunn’s multiple comparisons test). d, Permissive 23°C control for experiments in Fig. 3c. No enhancement was observed of 6 h water memory performance in water-satiated flies (p>0.9999, n = 8; Kruskal-Willis test with Dunn’s multiple comparisons test). e, Blocking PAM-β′2a (MB087C) 20 min before and during testing did not enhance 6 h water memory performance in thirsty flies (p>0.41, n = 8; one-way ANOVA with Tukey’s test). f, Blocking PPL1-γ2α′1 (MB296B) DANs 20 min before and during testing did not enhance 6 h water memory performance in water-satiated flies (p>0.73, n = 8; one-way ANOVA with Tukey’s test). g, Permissive 23°C control for experiments in Fig. 3d. No effect was observed (p>0.97, n = 8; one-way ANOVA with Tukey’s test). Temperature regimens shown above a-g. Box-plots: center line indicates median; box limits, upper and lower quartiles; whiskers, max to min range; dots, individual data points. h, Incubating explant brains with 100 nM, but not 1 nM, LK decreases GCaMP6m signal in PPL1-γ2α′1 (MB296B) DANs (100 nM: p=0.0057, n = 12; 1 nM: p=0.8659, n = 14; two-tailed paired t-test). i, Incubating explant brains with 100 nM, but not 1 nM, LK decreases GCaMP6m signal in PAM-β′2a (MB087C) DANs (100 nM: p=0.0225, n = 11; 1 nM: p=0.2423, n = 12; two-tailed paired t-test). Representative images of GCaMP6m signal shown under the plots in h and i. See Supplementary Table 3 for statistics details.
Extended Data Fig. 4. Controls and additional experiments related to Figure 4.
Knockdown of lk in all LK neurons (LK-GAL4) or only LHLK neurons (ap-GAL4) using a second RNAi line impairs 6 h sugar memory performance in hungry flies (LK-GAL4: p<0.0001; ap-GAL4: p<0.0025; n = 8; one-way ANOVA with Tukey’s test). b, RNAi knockdown of lk in all LK neurons (LK-GAL4) or only LHLK neurons (ap-GAL4) does not affect immediate sugar memory performance (LK-GAL4: p>0.98; ap-GAL4: p>0.81; n = 8; one-way ANOVA with Tukey’s test). c, Permissive 23°C control for experiments in Fig. 4c. No effect was observed (LK-GAL4: p>0.68; ap-GAL4: p>0.66; n = 8; one-way ANOVA with Tukey’s test). d, RNAi knockdown of Lkr in PAM-β′2a (MB087C-splitGAL4), PPL1-γ2α′1 (MB296B-splitGAL4), or PPL1-γ1pedc (MB320C-splitGAL4) DANs does not affect 6 h sugar memory performance (MB087C: p>0.49, n = 8; MB296B: p>0.98; MB320C: p>0.7; n = 8; one-way ANOVA with Tukey’s test). e, RNAi knockdown of Lkr in DDC-GAL4- but not TH-GAL4-labeled DANs impairs 6 h sugar memory performance in hungry flies (DDC-GAL4: p<0.036, n = 8; TH-GAL4: p>0.97, n = 8; one-way ANOVA with Tukey’s test). f, Adult brain expression of UAS-mCD8::GFP driven by MB056B-splitGAL4 (PAM-β′2mp, green) and LexAop-rCD2::RFP driven by MB247-LexA::p65 (mushroom bodies, magenta). Counterstained with anti-Brp antibody (gray). Four brains were examined and show the same expression pattern. Scale bar 50 μm. g, Knockdown of Lkr in PAM-β′2mp DANs with MB056B-splitGAL4 and a second RNAi line impairs 6 h sugar memory (p<0.0002, n = 8; one-way ANOVA with Tukey’s test). h, Permissive 23°C control for experiments in Fig. 4f. No effect was observed (p>0.079, n = 8; one-way ANOVA with Tukey’s test). i, Blocking PAM-β′2mp (MB056B) DANs with UAS-Shits1 at 32°C 20 min before and during training does not affect 6 h sugar memory performance (p>0.437, n = 8; one-way ANOVA with Tukey’s test). j, Blocking PAM-β′2mp (MB056B) DANs with UAS-Shits1 at 32°C immediately after training until 30 min before testing does not affect 6 h sugar memory performance (p>0.21, n = 8; one-way ANOVA with Tukey’s test). k, Permissive 23°C control for experiments in Fig. 4g. No effect was observed (p>0.55, n = 8; one-way ANOVA with Tukey’s test). l, Adult brain expression of JFRC2-10XUAS-IVS-mCDGFP driven by R67C06-GAL4 (putative Lkr expressing neurons, green). Counterstained with anti-Brp antibody (gray). Confocal stack downloaded from JFRC FlyLight database38. Illustration below indicates position of R67C06 enhancer in fly genome. Scale bar 50 μm. Temperature regimens shown above a-c and g-k. Box-plots: center line indicates median; box limits, upper and lower quartiles; whiskers, max to min range; dots, individual data points. See Supplementary Table 3 for statistics details.
Extended Data Fig. 5. Controls and additional experiments related to Figure 5.
Illustration of the anatomical and functional relationships between LHLK neurons and the three identified DANs that control water and sugar memory expression. b, Permissive 23°C control for experiments in Fig. 5d. No effect was observed (dNPF-GAL4: p>0.63; R50H05-GAL4: p>0.48; n = 8; one-way ANOVA with Tukey’s test). Temperature regimens shown above the plot. Box-plots: center line indicates median; box limits, upper and lower quartiles; whiskers, max to min range; dots, individual data points. c, Top: incubating explant brains with 100 nM scrambled control peptide for dNPF does not change GCaMP6m signal in PAM-β′2a (MB087C > GCaMP6m) DANs (p=0.5186, n = 12; two-tailed Wilcoxon matched-pairs signed rank test). Bottom: incubating explant brains with solvent-only (control for 5-HT experiments in Fig. 5h) does not change GCaMP6m signal in PPL1-γ2α′1 (MB296B > GCaMP6m) DANs (p=0.6645, n = 12; two-tailed paired t-Test). Representative images of GCaMP6m signal shown to right of each plot. See Supplementary Table 3 for statistics details. d, RT-PCR of Lkr and Rpl32 transcripts extracted from third-instar larvae. Pan-neuronal da-GAL4 was used to drive UAS-Lkr-RNAi2. Intensity of the PCR bands was normalized to the internal control Rpl32. Quantification of intensities relative to the da-GAL4-control is shown. The experiment was repeated three times with consistent results.
Supplementary Material
Acknowledgements
We thank P. Cognigni for experiments leading to those in Figure 5. We thank G. Wright, J. Felsenberg, E. Perisse, G. Das, and V. Croset for comments on the manuscript. We thank A-S. Chiang (National Tsing Hua University, Taiwan), G. Rubin (Janelia Farm Research Campus, USA), Y. Aso (Janelia Farm Research Campus, USA) and FlyLight (Janelia Farm Research Campus, USA), the Bloomington Drosophila Stock Center, Vienna Drosophila RNAi center, Kyoto Stock Center, Harvard TRiP RNAi stock center, and Taiwan Fly Core for fly stocks. C-L. W. is funded by Ministry of Science and Technology, Taiwan (106-2311-B-182-004-MY3) and Chang Gung Memorial Hospital, Taiwan (CMRPD1G0341-3 and BMRPC75). S. W. is funded by a Wellcome Principal Research Fellowship (200846/Z/16/Z), an ERC Advanced Grant (789274) and the Bettencourt–Schueller Foundation. S. L. is funded by Ministry of Science and Technology, Taiwan (105-2628-B-001-005-MY3 and 107-2311-B-001-042-MY3) and Academia Sinica, Taiwan.
Footnotes
Reporting summary
Further information on research design is available in the Life Sciences Reporting Summary linked to this article.
Data availability
Data supporting the findings of this study are available from the corresponding author upon request.
Author contributions
S.L., S.W., B.S., and C-H.T. designed the study and analyzed the data. B.S. performed behavioural experiments. C-H.T. performed imaging experiments. Y-A.J. performed naïve water seeking experiments. T-H.C. and C-L.W. assisted in the initial establishment of the water-reward olfactory conditioning paradigm. S.L. directed the research. S.L. and S.W. wrote the paper.
Competing interests
The authors declare no competing interests.
References
- 1.Toates F. Motivational systems. Cambridge University Press; 1986. [Google Scholar]
- 2.Burnett CJ, et al. Hunger-Driven Motivational State Competition. Neuron. 2016;92:187–201. doi: 10.1016/j.neuron.2016.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin S, Senapati B, Tsao C-H. Neural basis of hunger-driven behaviour in Drosophila. Open Biol. 2019;9:180259. doi: 10.1098/rsob.180259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jourjine N, Mullaney BC, Mann K, Scott K. Coupled sensing of hunger and thirst signals balances sugar and water consumption. Cell. 2016;166:855–866. doi: 10.1016/j.cell.2016.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin S, et al. Neural correlates of water reward in thirsty Drosophila. Nat Neurosci. 2014;17:1536–1542. doi: 10.1038/nn.3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shyu W, Chiu T, Chiang M, Cheng Y. Neural circuits for long-term water-reward memory processing in thirsty Drosophila. Nat Commun. 2017;8:1–13. doi: 10.1038/ncomms15230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krashes MJ, et al. A neural circuit mechanism integrating motivational state with memory expression in Drosophila. Cell. 2009;139:416–427. doi: 10.1016/j.cell.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dus M, et al. Nutrient Sensor in the Brain Directs the Action of the Brain-Gut Axis in Drosophila. Neuron. 2015;87:139–151. doi: 10.1016/j.neuron.2015.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inagaki HK, Panse KM, Anderson DJ. Independent, reciprocal neuromodulatory control of sweet and bitter taste sensitivity during starvation in Drosophila. Neuron. 2014;84:806–820. doi: 10.1016/j.neuron.2014.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loh K, et al. Insulin controls food intake and energy balance via NPY neurons. Mol Metab. 2017;6:574–584. doi: 10.1016/j.molmet.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee K-S, You K-H, Choo J-K, Han Y-M, Yu K. Drosophila short neuropeptide F regulates food intake and body size. J Biol Chem. 2004;279:50781–50789. doi: 10.1074/jbc.M407842200. [DOI] [PubMed] [Google Scholar]
- 12.Pool A-H, Scott K. Feeding regulation in Drosophila. Curr Opin Neurobiol. 2014;29:57–63. doi: 10.1016/j.conb.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rajan A, Perrimon N. Drosophila cytokine unpaired 2 regulates physiological homeostasis by remotely controlling insulin secretion. Cell. 2012;151:123–137. doi: 10.1016/j.cell.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Root CM, Ko KI, Jafari A, Wang JW. Presynaptic facilitation by neuropeptide signaling mediates odor-driven food search. Cell. 2011;145:133–144. doi: 10.1016/j.cell.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun J, et al. Drosophila FIT is a protein-specific satiety hormone essential for feeding control. Nat Commun. 2017;8:14161. doi: 10.1038/ncomms14161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu Q, Zhao Z, Shen P. Regulation of aversion to noxious food by Drosophila neuropeptide Y- and insulin-like systems. Nat Neurosci. 2005;8:1350–1355. doi: 10.1038/nn1540. [DOI] [PubMed] [Google Scholar]
- 17.Yu Y, et al. Regulation of starvation-induced hyperactivity by insulin and glucagon signaling in adult Drosophila. Elife. 2016;5:1–19. doi: 10.7554/eLife.15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gáliková M, Dircksen H, Nässel DR. The thirsty fly: Ion transport peptide (ITP) is a novel endocrine regulator of water homeostasis in Drosophila. PLoS Genet. 2018;14:e1007618. doi: 10.1371/journal.pgen.1007618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baines RA, Uhler JP, Thompson A, Sweeney ST, Bate M. Altered electrical properties in Drosophila neurons developing without synaptic transmission. J Neurosci. 2001;21:1523–1531. doi: 10.1523/JNEUROSCI.21-05-01523.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johns DC, Marx R, Mains RE, O’Rourke B, Marbán E. Inducible genetic suppression of neuronal excitability. J Neurosci. 1999;19:1691–1697. doi: 10.1523/JNEUROSCI.19-05-01691.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGuire SE, Mao Z, Davis RL. Spatiotemporal gene expression targeting with the TARGET and gene-switch systems in Drosophila. Sci STKE. 2004;2004:pl6. doi: 10.1126/stke.2202004pl6. [DOI] [PubMed] [Google Scholar]
- 22.Hamada FN, et al. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454:217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Haro M, et al. Detailed analysis of leucokinin-expressing neurons and their candidate functions in the Drosophila nervous system. Cell Tissue Res. 2010;339:321–336. doi: 10.1007/s00441-009-0890-y. [DOI] [PubMed] [Google Scholar]
- 24.Zandawala M, Marley R, Davies SA, Nässel DR. Characterization of a set of abdominal neuroendocrine cells that regulate stress physiology using colocalized diuretic peptides in Drosophila. Cell Mol Life Sci. 2018;75:1099–1115. doi: 10.1007/s00018-017-2682-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zandawala M, et al. Modulation of Drosophila post-feeding physiology and behavior by the neuropeptide leucokinin. PLoS Genet. 2018;14:e1007767. doi: 10.1371/journal.pgen.1007767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen T-W, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499:295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao XJ, et al. A transcriptional reporter of intracellular Ca(2+) in Drosophila. Nat Neurosci. 2015;18:917–925. doi: 10.1038/nn.4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cavey M, Collins B, Bertet C, Blau J. Circadian rhythms in neuronal activity propagate through output circuits. Nat Neurosci. 2016;19:587–595. doi: 10.1038/nn.4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herrero P, Magariños M, Torroja L, Canal I. Neurosecretory identity conferred by the apterous gene: lateral horn leucokinin neurons in Drosophila. J Comp Neurol. 2003;457:123–132. doi: 10.1002/cne.10555. [DOI] [PubMed] [Google Scholar]
- 30.Mao Z, Davis RL. Eight different types of dopaminergic neurons innervate the Drosophila mushroom body neuropil: anatomical and physiological heterogeneity. Front Neural Circuits. 2009;3:5. doi: 10.3389/neuro.04.005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aso Y, et al. The neuronal architecture of the mushroom body provides a logic for associative learning. Elife. 2014;3:e04577. doi: 10.7554/eLife.04577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kitamoto T. Conditional modification of behavior in Drosophila by targeted expression of a temperature-sensitive shibire allele in defined neurons. J Neurobiol. 2001;47:81–92. doi: 10.1002/neu.1018. [DOI] [PubMed] [Google Scholar]
- 33.Al-Anzi B, et al. The leucokinin pathway and its neurons regulate meal size in Drosophila. Curr Biol. 2010;20:969–78. doi: 10.1016/j.cub.2010.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Y, Luo J, Carlsson MA, Nässel DR. Serotonin and insulin-like peptides modulate leucokinin-producing neurons that affect feeding and water homeostasis in Drosophila. J Comp Neurol. 2015;523:1840–1863. doi: 10.1002/cne.23768. [DOI] [PubMed] [Google Scholar]
- 35.Huetteroth W, et al. Sweet taste and nutrient value subdivide rewarding dopaminergic neurons in Drosophila. Curr Biol. 2015;25:751–758. doi: 10.1016/j.cub.2015.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamagata N, et al. Distinct dopamine neurons mediate reward signals for short- and long-term memories. Proc Natl Acad Sci U S A. 2015;112:578–583. doi: 10.1073/pnas.1421930112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yurgel ME, et al. A single pair of leucokinin neurons are modulated by feeding state and regulate sleep-metabolism interactions. PLoS Biol. 2019;17:e2006409. doi: 10.1371/journal.pbio.2006409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jenett A, et al. A GAL4-driver line resource for Drosophila neurobiology. Cell Rep. 2012;2:991–1001. doi: 10.1016/j.celrep.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Owald D, et al. Activity of defined mushroom body output neurons underlies learned olfactory behavior in Drosophila. Neuron. 2015;86:417–427. doi: 10.1016/j.neuron.2015.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Croset V, Treiber CD, Waddell S. Cellular diversity in the Drosophila midbrain revealed by single-cell transcriptomics. Elife. 2018;7:1–31. doi: 10.7554/eLife.34550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davie K, et al. A Single-Cell Transcriptome Atlas of the Aging Drosophila Brain. Cell. 2018;174:982–998.e20. doi: 10.1016/j.cell.2018.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Albin SD, et al. A subset of serotonergic neurons evokes hunger in adult Drosophila. Curr Biol. 2015;25:2435–2440. doi: 10.1016/j.cub.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 43.Tsao C-H, Chen C-C, Lin C-H, Yang H-Y, Lin S. Drosophila mushroom bodies integrate hunger and satiety signals to control innate food-seeking behavior. Elife. 2018;7:e35264. doi: 10.7554/eLife.35264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cohn R, Morantte I, Ruta V. Coordinated and compartmentalized neuromodulation shapes sensory processing in Drosophila. Cell. 2015;163:1742–1755. doi: 10.1016/j.cell.2015.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Castro JM. A microregulatory analysis of spontaneous fluid intake by humans: evidence that the amount of liquid ingested and its timing is mainly governed by feeding. Physiol Behav. 1988;43:705–714. doi: 10.1016/0031-9384(88)90367-8. [DOI] [PubMed] [Google Scholar]
- 46.Kissileff HR. Food-associated drinking in the rat. J Comp Physiol Psychol. 1969;67:284–300. doi: 10.1037/h0026773. [DOI] [PubMed] [Google Scholar]
- 47.Fitzsimons TJ, Le Magnen J. Eating as a regulatory control of drinking in the rat. J Comp Physiol Psychol. 1969;67:273–283. doi: 10.1037/h0026772. [DOI] [PubMed] [Google Scholar]
- 48.Perisse E, et al. Aversive learning and appetitive motivation toggle feed-forward inhibition in the Drosophila mushroom body. Neuron. 2016;90:1086–1099. doi: 10.1016/j.neuron.2016.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hescheler J, Schultz G. G-proteins involved in the calcium channel signalling system. Curr Opin Neurobiol. 1993;3:360–367. doi: 10.1016/0959-4388(93)90129-m. [DOI] [PubMed] [Google Scholar]
- 50.Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 51.Tully T, Quinn WG. Classical conditioning and retention in normal and mutant Drosophila melanogaster. J Comp Physiol A. 1985;157:263–277. doi: 10.1007/BF01350033. [DOI] [PubMed] [Google Scholar]
- 52.Pai T-P, et al. Drosophila ORB protein in two mushroom body output neurons is necessary for long-term memory formation. Proc Natl Acad Sci U S A. 2013;110:7898–7903. doi: 10.1073/pnas.1216336110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ni J-Q, et al. A Drosophila resource of transgenic RNAi lines for neurogenetics. Genetics. 2009;182:1089–1100. doi: 10.1534/genetics.109.103630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murphy KR, et al. Postprandial sleep mechanics in Drosophila. Elife. 2016;5:1–19. doi: 10.7554/eLife.19334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dietzl G, et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- 56.McGuire SE, Le PT, Osborn AJ, Matsumoto K, Davis RL. Spatiotemporal rescue of memory dysfunction in Drosophila. Science. 2003;302:1765–1768. doi: 10.1126/science.1089035. [DOI] [PubMed] [Google Scholar]
- 57.Perisse E, et al. Different kenyon cell populations drive learned approach and avoidance in Drosophila. Neuron. 2013;79:945–956. doi: 10.1016/j.neuron.2013.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Friggi-Grelin F, et al. Targeted gene expression in Drosophila dopaminergic cells using regulatory sequences from tyrosine hydroxylase. J Neurobiol. 2003;54:618–627. doi: 10.1002/neu.10185. [DOI] [PubMed] [Google Scholar]
- 59.Li H, Chaney S, Roberts IJ, Forte M, Hirsh J. Ectopic G-protein expression in dopamine and serotonin neurons blocks cocaine sensitization in Drosophila melanogaster. Curr Biol. 2000;10:211–214. doi: 10.1016/s0960-9822(00)00340-7. [DOI] [PubMed] [Google Scholar]
- 60.Liu C, et al. A subset of dopamine neurons signals reward for odour memory in Drosophila. Nature. 2012;488:512–516. doi: 10.1038/nature11304. [DOI] [PubMed] [Google Scholar]
- 61.Schindelin J, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Knecht ZA, et al. Distinct combinations of variant ionotropic glutamate receptors mediate thermosensation and hygrosensation in Drosophila. Elife. 2016;5:1–35. doi: 10.7554/eLife.17879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Knecht ZA, et al. Ionotropic receptor-dependent moist and dry cells control hygrosensation in Drosophila. Elife. 2017;6:1–11. doi: 10.7554/eLife.26654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cachero S, Ostrovsky AD, Yu JY, Dickson BJ, Jefferis GSXE. Sexual dimorphism in the fly brain. Curr Biol. 2010;20:1589–1601. doi: 10.1016/j.cub.2010.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Felsenberg J, et al. Integration of Parallel Opposing Memories Underlies Memory Extinction. Cell. 2018;175:709–722.e15. doi: 10.1016/j.cell.2018.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boto T, Stahl A, Zhang X, Louis T, Tomchik SM. Independent contributions of discrete dopaminergic circuits to cellular plasticity, memory strength, and valence in Drosophila. Cell Rep. 2019;27:2014–2021.e2. doi: 10.1016/j.celrep.2019.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.