Abstract
Background
Atopic eczema (AE) is a non‐infective chronic inflammatory skin disease characterised by an itchy red rash.
Objectives
To assess the effects of dietary exclusions for the treatment of established atopic eczema.
Search methods
We searched The Cochrane Skin Group Specialised Register (to March 2006), The Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (Issue 1, 2006), MEDLINE (2003 to March 2006), EMBASE (2003 to March 2006), LILACS (to March 2006), PsycINFO (1806 to March 2006), AMED (1985 to March 2006), ISI Web of Science (March 2006), www.controlled‐trials.com, www.clinicaltrials.gov and www.nottingham.ac.uk/ongoingskintrials (March 2006). Pharmaceutical companies were contacted where appropriate for reviews or unpublished trials.
Selection criteria
People who have atopic eczema as diagnosed by a doctor.
Data collection and analysis
Two independent authors carried out study selection and assessment of methodological quality.
Main results
We found 9 RCTs involving a total of 421 participants of which 6 were studies of egg and milk exclusion (N=288), 1 was a study of few foods (N=85) and 2 were studies of an elemental diet (N=48).
There appears to be no benefit of an egg and milk free diet in unselected participants with atopic eczema. There is also no evidence of benefit in the use of an elemental or few‐foods diet in unselected cases of atopic eczema. There may be some benefit in using an egg‐free diet in infants with suspected egg allergy who have positive specific IgE to eggs ‐ one study found 51% of the children had a significant improvement in body surface area with the exclusion diet compared to normal diet (RR 1.51, 95% CI 1.07 to 2.11) and change in surface area and severity score was significantly improved in the exclusion diet compared to the normal diet at the end of 6 weeks (MD 5.50, 95% CI 0.19 to 10.81) and end of treatment (MD 6.10, 95% CI 0.06 to12.14).
Methodological difficulties have made it difficult to interpret these studies. Poor concealment of randomisation allocation, lack of blinding and high dropout rates without an intention‐to‐treat analysis indicates that these studies should be interpreted with great caution.
Authors' conclusions
There may be some benefit in using an egg‐free diet in infants with suspected egg allergy who have positive specific IgE to eggs. Little evidence supports the use of various exclusion diets in unselected people with atopic eczema, but that may be because they were not allergic to those substances in the first place. Lack of any benefit may also be because the studies were too small and poorly reported. Future studies should be appropriately powered focusing on participants with a proven food allergy. In addition a distinction should be made between young children whose food allergies improve with time and older children/adults.
Keywords: Humans; Dermatitis, Atopic; Dermatitis, Atopic/diet therapy; Egg Hypersensitivity; Egg Hypersensitivity/diet therapy; Food, Formulated; Milk Hypersensitivity; Milk Hypersensitivity/diet therapy; Randomized Controlled Trials as Topic
Dietary exclusions for improving established atopic eczema in adults and children
Atopic eczema is the most common inflammatory skin disease of childhood in developed countries. The cause of atopic eczema is probably due to a combination of genetic and environmental factors. Atopic eczema varies in severity, often from one hour to the next and the disease can be associated with complications such as bacterial and viral infections. There is a substantial economic cost not only to the family of the person with atopic eczema but also to health services. Although there is currently no cure for atopic eczema, a wide range of treatments are used to control the symptoms. One such approach is a dietary one, whereby certain foods such as cows' milk are excluded on the basis that they are thought to cause eczema to worsen. The reason for undertaking this review is because the effectiveness of removing various foods from the diet in the short term management of atopic eczema is unclear.
The general quality of the studies was poor. The main findings of the review suggest that there is some evidence from one study for the use of an egg‐free diet in infants with a suspected egg allergy who have positive specific IgE antibodies to eggs in their blood. Other studies that compared a dietary exclusion with ordinary diets did not test the people taking part to see if they were allergic to the foods concerned. There appears to be little benefit in eliminating cows milk from the diet or using an elemental (liquid diet containing only amino acids, carbohydrates, fat, minerals and vitamins) or 'few foods diet' for improving atopic eczema in people who have not undergone any form of testing.
Three of the studies used soya based substitute which itself can be allergenic to people with atopic eczema.
Adhering to elimination diets is difficult. The studies were performed in different populations with only one study describing the severity of the atopic eczema. The clinical relevance of changes in severity scores obtained in many studies is unknown.
Background
Description of the condition
Disease definition
Atopic eczema (AE) is a non‐infective chronic inflammatory skin disease characterised by an itchy red rash. The terms 'atopic eczema' and 'atopic dermatitis' are used synonymously. "Atopy" refers to a form of allergy in which there is a heritable tendency to develop "IgE" hypersensitivity reactions. However up to 40% of children with atopic eczema are not atopic, when defined according to allergy tests such as skin prick tests (Bohme 2001). Others have found that up to two thirds of people with atopic dermatitis are not atopic (Flohr 2004), implying that continued use of the term 'atopic dermatitis' is problematic.
A revised nomenclature for allergy (Johansson 2001) has been updated by the World Allergy Organisation (Johansson 2004). The new nomenclature is based on the mechanisms that initiate and mediate allergic reactions. The term 'eczema' is proposed to replace the provisional term 'atopic eczema/dermatitis syndrome' (AEDS). What is generally known as 'atopic eczema/dermatitis' is probably not one single disease but rather an aggregation of several diseases with certain characteristics in common. The term 'atopy' cannot be used until an IgE sensitisation has been documented by IgE antibodies in the blood of a person or by a positive skin prick test to common environmental allergens such as pollen, house dust mite, cow's milk or egg. If this is done then the term 'eczema' can be split into 'atopic eczema' and 'non‐atopic eczema'.
Whilst recognising the logic of the new nomenclature, most health care workers still think of atopic eczema as the clinical syndrome of an itchy inflammatory skin condition with a tendency to settle in the skin creases without doing any further investigations to find out if such individuals are atopic. For the purpose of this review therefore, we will use the term 'atopic eczema' throughout for ease of communication, knowing that in many cases, testing for atopy in such individuals has not been carried out. Since it is possible that individuals who are allergic to a particular substance are more likely to respond to a diet that excludes that substance than people who do not react to such tests, we will make it clear which studies have undertaken such allergy tests to further define the study populations in this review.
Epidemiology and causes
Atopic eczema is the most common inflammatory skin disease of childhood in developed countries, affecting 15 to 20% of children in the UK at any one time (Hoare 2000). Two‐thirds of people with the disease have a family history of atopic eczema, asthma or hay fever. The cumulative prevalence of atopic dermatitis varies from 20% in Northern Europe and the USA to 5% in the South‐Eastern Mediterranean (Thestrup 2002). Prevalence data for the symptoms of atopic eczema were collected in the global ISAAC study (International Study of Asthma and Allergies in Childhood). The results of this study suggest that atopic eczema is a worldwide problem affecting 15 to 20% of children (Williams 1999). Of those children with atopic eczema, only 2% under the age of 5 years have severe disease and 84% have mild disease (Emerson 1998). Two per cent of adults have atopic eczema and many of these have a more chronic and severe form (Charman 2002).
Atopic eczema is often associated with other atopic diseases e.g. asthma and rhinitis (Beck 2000). The incidence of common allergic disease has increased in the past 30 years (Fendrick 2001) and the increase in prevalence of atopic disease in the past 3 decades appears to be a real phenomenon and has been observed in countries as far apart as Japan, USA, Finland and Africa (Williams 1992). The cause of eczema is not well understood and probably is due to a combination of genetic and environmental factors (Cookson 2002). In recent years research has pointed to the possible role of environmental agents such as house dust mite (Van Bever 2002), pollution (Polosa 2001), and prenatal or early exposure to infections (Kalliomaki 2002). Food allergy may be common in atopic eczema especially if the eczema is severe (Guillet 1992; Zeiger 1995; Eigenmann 1998 ).
Clinical features
Atopic eczema may be acute (short and severe) with redness, scaling, oozing and vesicles, or it may be chronic (long‐term) with skin thickening, altered pigmentation and exaggerated surface markings. The condition affects mainly the creases of the elbows and knees, and the face and neck, although it can affect any part of the body. The severity of eczema is variable, ranging from localised mild scaling to generalised involvement of the whole body with redness, oozing and secondary infection. Itching is the predominant symptom which can induce a vicious cycle of scratching, leading to skin damage, which in turn leads to more itching ‐ the so called "itch scratch itch" cycle. There is a tendency to a dry sensitive skin even in those who have 'grown out' of the disease. This is thought to be due to a defect in the lipid barrier of the epidermis. In adulthood, the skin (especially of the hands) may be prone to inflammation in the presence of environmental irritants such as soaps (Archer 2000).
Natural history
Atopic eczema usually starts within the first 6 months of life, and by 1 year, 60% of those likely to develop it will have done so. Remission occurs by the age of 15 years, in 60 to 70% of cases, although some relapse later. In the more severely affected child, development and puberty may be delayed (Baum 2002). Many children with eczema go on to develop asthma and hay fever, which might also be triggered by food allergy (Ricci 2006)
Impact
Atopic eczema varies in severity, often from one hour to the next. Severity can be measured in a number of ways. A systematic review of named outcome scales for atopic eczema found that of the 13 named scales in current use, only one (Severity Scoring of Atopic Dermatitis, SCORAD) had been fully tested for validity, repeatability and responsiveness (Charman 2000). Itching and scratching can adversely affect quality of life through chronic sleep disturbance. This may have an impact on family life. The disease can be associated with complications such as bacterial and viral infections (McHenry 1995). The unsightly appearance of the skin and the need to apply greasy ointments can limit a child's inclination to participate in social and sporting activities and thus affect their confidence. Adults with atopic eczema often have low self‐esteem and relationships can be difficult to initiate and sustain. Everyday tasks such as housework, gardening, childcare and food preparation present problems when the skin on the hands is cracked. Promotion at work may be blocked for people who do not 'look good'.
There is a substantial economic cost not only to the family of the person with atopic eczema (Kemp 2003) but also to the health services of the country as a whole (Herd 1996; Verboom 2002). Direct costs to the family are encountered when purchasing treatments, special clothing and bedding; indirect costs are experienced from lost working days when parents are looking after a sick child. The wider economic implications lie in the costs of health professionals, the lost opportunities of parents of sick children who do not have the option of seeking employment and the child who, as a result of missing schooling, has limited employment prospects (Su 1997).
Description of the intervention
There is currently no cure for atopic eczema. However a wide range of treatments are employed which aim to control the symptoms (Fennessy 2000; Hoare 2000; Lamb 2002). Health professionals assist people in the management of their disease using a variety of treatment methods; these include emollients, topical steroids, topical tars, topical tacrolimus and pimecrolimus. Other treatments such as wet wrap dressings, phototherapy and complementary therapies are also tried (Ernst 2002). Many of the treatments are of unknown effectiveness (Hoare 2000). Emollients and topical corticosteroids are universally recommended (Smethurst 2002)
How the intervention might work
Diet and atopic eczema
Food hypersensitivity may be the first stage in the development of 'allergic diseases' such as atopic eczema (Chandra 2002). Food allergy may be an important factor in up to 20% of children with atopic eczema under 4 years (Oranje 2000). The incidence of food allergy is highest around the age of six to nine months. Many clinicians have found that elimination of specific foods found by food challenge to elicit symptoms can lead to significant improvement in eczematous symptoms (Sampson 2003). Challenges in people with food allergies can lead to eczematous lesions and infiltration of allergic inflammatory cells and animal studies have suggested that eczema may be caused by food allergies (Li 2001).
However, many food reactions in people with atopic eczema may not necessarily be mediated through immune reactions (David 2000). As sensitisation to food early in life may be a predisposing factor (Baena‐Cagnani 2001) it is important to investigate whether the elimination of dietary triggers could help to alleviate the symptoms of atopic eczema. The role of dietary factors in atopic eczema either as a cause or as a treatment, through the use of exclusion diets, remains unclear (Oranje 2000). Many trialists advocate double blind placebo controlled food challenges to establish whether a child has a true food allergy (Sampson 1992).
There is a vast amount of literature claiming that dietary elimination causes improvement of atopic eczema in some cases. However, much of the evidence fails to withstand close scrutiny (David 2000).
There are three main types of dietary exclusion (David 2000):
(1) the simple elimination of cow's milk protein and egg; (2) 'the few foods' diet (a diet in which all but a handful of foods is excluded) (David 1993). One problem with the 'few foods diet' is poor adherence because the diet is so restrictive (Hathaway 1983; David 1989); (3) the 'elemental' diet, more accurately described as a non‐macromolecular diet since ordinary foodstuffs are all avoided, and the participant receives a liquid diet which contains amino acids, carbohydrate, fat, minerals and vitamins. The palatability of the elemental formula is poor.
Many people, with or without their doctor's or dietician's help, experiment by excluding a particular food suspected of causing a reaction for a variable time. Most investigators would base elimination diets upon proven food allergies, either by challenge or serum food‐specific IgE antibodies exceeding specific diagnostic decision points (Sampson 2001).
The advantage of dietary interventions is that they may address one of the primary causes, as opposed to merely suppressing the symptoms, although there can be serious consequences to any dietary manipulation that leaves the individual deficient in calories, protein or minerals such as calcium. (David 1984; Devlin 1989). Avoidance of multiple foods is potentially hazardous and requires continued paediatric and dietary supervision (David 1984).
The role of food is much debated in atopic eczema, but the allergenic relevance of some proteins seems to be important only in a small number of people, especially in the first years of life (Svejgaard 1985).
Why it is important to do this review
The rationale for a review on dietary exclusions for established atopic eczema is:
currently there is no cure for atopic eczema;
diets excluding foods, such as cow's milk, are commonly tried (Graham‐Brown 2000);
the place for dietary elimination in the short‐term management of atopic eczema is unclear (Smethurst 2002);
some have claimed that there is no evidence of long‐term benefit from dietary elimination (David 2000).
Objectives
To assess the effects of general dietary avoidance practices for the treatment of established atopic eczema.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) of dietary exclusion for the treatment of established atopic eczema/dermatitis. We excluded double blind placebo controlled food challenges conducted in isolation, since these are not therapeutic trials but diagnostic or provocation tests.
Comparisons considered were active exclusion diet vs control or a comparison of two active diets.
Types of participants
We included participants who had atopic eczema diagnosed by a doctor. In the National Health Service Technology Assessment (HTA) systematic review of treatments for atopic eczema (Hoare 2000), specific terms were used to identify trial participants as listed in Table 8. The list classifies conditions into 'definite', 'possible' and 'not' atopic eczema and we have used this list as a guide. We excluded those studies using terms in the 'not atopic eczema' category such as 'allergic contact eczema' . We found some studies using terms in the 'possible atopic eczema' category, such as 'childhood eczema' . One or more authors scrutinised these and we included them if the description of the participants clearly indicated atopic eczema (i.e. itching and flexural involvement).
Table 1.
Terms used to categorise trial participants with atopic eczema (AE)
| Definite AE | Possible AE | Not AE |
| Atopic eczema | Periorbital eczema | Seborrheic eczema |
| Atopic dermatitis | Childhood eczema | Contact eczema |
| Besnier's prurigo | Infantile eczema | Allergic contact eczema |
| Neurodermatitis atopica (German) | 'Eczema' unspecified | Irritant contact eczema |
| Flexural eczema/ dermatitis | Constitutional eczema | Discoid/ nummular eczema |
| Endogenous eczema | Asteatotic eczema | |
| Chronic eczema | Varicose/ stasis eczema | |
| Neurodermatitis | Photo‐/ light‐sensitive eczema | |
| Neurodermatitis (German) | Chronic actinic dermatitis | |
| Dishydrotic eczema | ||
| Pompholyx eczema | ||
| Hand eczema | ||
| Frictional lichenoid dermatitis | ||
| Lichen simplex | ||
| Occupational dermatitis | ||
| Prurigo |
Types of interventions
We included studies with exclusions of any type of food, either singly or in combination with other foods.
Types of outcome measures
Primary outcomes
(a) Short‐term (within six weeks). Changes in parent‐rated or mother‐rated symptoms of atopic eczema such as itching (pruritus) or sleep loss. (b) Degree of long‐term (over six months) control, such as reduction in number of flares or reduced need for other treatments.
Secondary outcomes
(a) Global severity as rated by the participants or their physician.
Where the outcome was not available then the following was used: (b) Global changes in composite rating scales using a published named scale
Where this was not possible, we used: (c) The trial author's modification of existing scales or new scales
Also: (d) Quality of life (Finlay 1996; Chren 1997) (e) Palatability of the diet (f) Adverse events including long term consequences on growth.
Tertiary outcome measures
Changes in individual signs of atopic eczema as assessed by a physician e.g. erythema (redness), purulence (pus formation), excoriation (scratch marks), xerosis (skin dryness), lichenification (thickening of the skin), fissuring (cracks), exudation (weeping serum from the skin surface), pustules (pus spots), papules (spots that protrude from the skin surface), vesicles (clear fluid or 'water blisters' in the skin), crusts (dried serum on skin surface), infiltration/oedema (swelling of the skin), induration (a thickened feel to the skin).
Search methods for identification of studies
Electronic searches
We searched the Cochrane Skin Group Specialised Register (March 2006) using these terms: ((atopic AND eczema) OR (atopic AND dermatitis) OR (besnier* AND prurigo) OR (neurodermatitis) OR (infant* AND eczema) OR (childhood AND eczema) OR eczema) AND (diet* or (dietary and manipulation) or (diet* and therap*) or (milk and exclusion) or (egg and exclusion) or (food and allerg*))
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (Issue 1, 2006) using the strategy in Appendix 1
We searched MEDLINE (2003 to March 2006) using the strategy in Appendix 2
We searched EMBASE (2003 to March 2006) using the strategy in Appendix 3
We searched LILACS (Latin American and Caribbean Health Science Information database) (to March 2006) using the terms Eczema atópico [Palavras do título] or Dermatitis atópica [Palavras do título]
We searched PsycINFO (1806 to March 2006) using the strategy in Appendix 4
We searched AMED (Allied and Complementary Medicine) (1985 to March 2006) using the strategy in Appendix 5
We searched ISI Web of Science (March 2006) using the terms atopic eczema and diet
Searching other resources
References from published studies
References from published studies were checked for further trials.
Unpublished literature
Where possible unpublished, on‐going trials were obtained via correspondence with the trial authors. The metaRegister of Controlled Trials www.controlled‐trials.com, www.clinicaltrials.gov and www.nottingham.ac.uk/ongoingskintrials were searched in March 2006 for ongoing trials using the terms atopic eczema, atopic dermatitis.
Pharmaceutical companies
Pharmaceutical companies were contacted, where appropriate, for reviews or unpublished trials.
Language
No language restrictions were imposed and translations were obtained where possible.
Data collection and analysis
Selection of studies
One author (FD) ran the searches, then two authors (FB‐H and FD) checked the titles and abstracts for relevance. Three authors (FB‐H, HW and FD) then independently assessed the full text of the identified RCTs and decided whether they fitted our inclusion criteria. Where there were any disagreements they were resolved by discussion between the authors. Where there were missing data from the trials we contacted the trial authors.
Data extraction and management
Two authors (FB‐H and FD) independently extracted the data using a data extraction form for consistency. We resolved any discrepancies by discussion. One author (FB‐H) entered the data.
Assessment of risk of bias in included studies
Our quality assessment included an evaluation of the following components for each included study, since there is some evidence that these are associated with biased estimates of treatment effect (Juni 2001):
(a) the method of generation of the randomisation sequence; (b) the method of allocation concealment ‐ it was considered 'adequate' if the assignment could not be foreseen; (c) who was blinded/not blinded (participants, clinicians, outcome assessors); (d) how many participants were lost to follow up in each arm, and whether participants were analysed in the groups to which they were originally randomised (intention to treat).
In addition, the quality assessment also included: (e) degree of certainty that the participants had atopic eczema; (f) baseline comparability of the participants for age, sex and eczema severity; (g) assessment of compliance with treatment.
We recorded the information in a table of quality criteria Table 9 and a description of the quality of each study is given based on a summary of these components.
Table 2.
Quality components
| Study | Allocation generation | Allocation concealment | Masking | Loss to FU | Included in analysis | Certainty of AD | Baseline comparability | Compliance | Severity of AD |
| Atherton 1978 | unclear | unclear | participants and outcome assessors | 16 | n=20 (56%) | clinically typical AD | unclear | unclear | unclear |
| Cant 1986 | random number table | unclear | unclear | 2 | 17(87%) | criteria by Yates | unclear | unclear | unclear |
| Isolauri 1995 | unclear | unclear | unclear | ‐ | 100% | Hanifin | unclear | unclear | unclear |
| Leung 2004 | unclear | unclear | outcome assessor | 4 | 73.3% | Hanifin | unclear | clear | unclear |
| Lever 1998 | unclear | unclear | outcome assessor | 7 | 89% | unclear | no | unclear | unclear |
| Mabin 1995 | random number tables | unclear | outcome assessor | 39 | 54% | Hanifin | Yes | clear | unclear |
| Munkvad 1984 | unclear | unclear | participant, clinician, outcome assessor | 8 | 76% | Hanifin | unclear | clear | severe |
| Neild 1986 | unclear | unclear | participant and outcome assessor | 13 | 75% | unclear | unclear | clear | unclear |
| Niggemann 2001 | unclear | unclear | none | unclear | All | Hanifin | NS diff | unclear | unclear |
Measures of treatment effect
For studies with a similar type of intervention, we performed a meta‐analysis, to calculate a weighted treatment effect across trials, using a random effects model. We have expressed the results as risk ratio (RR) and 95% confidence intervals (CI) for dichotomous outcomes and mean differences (MD and 95% CI) for continuous outcomes. The results are also expressed as number needed to treat (NNT) where appropriate, for a range of plausible control event rates. Where it has not been possible to perform a meta‐analysis the data have been summarised for each trial.
Unit of analysis issues
Where paired data were available for cross‐over studies we calculated the conditional odds ratio with 95% CI using the methodology as described by Elbourne 2003. If paired data were not available then data, where available, were taken from the first phase of the cross‐over study and if appropriate then the first phase was treated as a parallel study. Cross‐over studies are not ideal for dietary exclusion studies since carry‐over effects may invalidate data in the second period. Non‐randomised controlled studies are listed but not discussed further. Studies relating to adverse effects are described qualitatively.
Assessment of heterogeneity
Heterogeneity was assessed using I2.
Data synthesis
Where participant‐rated symptoms were reported on categorical Likert scales (e.g. no improvement, mild improvement, good improvement, excellent), we dichotomised the data by defining a cut‐off at 'good to excellent improvement'. Where data were reported on continuous scales (e.g. number of days sleep loss), we regarded a 20% reduction/improvement compared to control as being clinically significant. Not enough studies used SCORAD for us to be able to split eczema severity into mild, moderate and severe where mild is 0 to 15, moderate is 15 to 40 and severe is >40.
Where data on existing medication usage were included, we have attempted to see whether this has increased differentially in one of the treatment arms as the main dietary intervention has proceeded.
Subgroup analysis and investigation of heterogeneity
Where substantial heterogeneity (I2>50%) existed between studies for the primary outcome, we have explored the reasons for heterogeneity, such as disease severity, whether food allergy was confirmed by a prior provocation/serum test, dosage etc. In future updates of this systematic review, we will perform further subgroup analysis, where there is sufficient information to do so. This will be done in those studies of infants (under one year old) and children (aged 1 to 16 years) versus adults and for those participants who had a positive food challenge prior to entry into the trial.
Sensitivity analysis
We may also conduct sensitivity analyses to examine the effects of excluding poor quality studies, defined as those studies that do not clearly report the randomisation process, blinding and no intention to treat.
Other
Where there was uncertainty, we contacted trial authors for clarification.
Results
Description of studies
Results of the search
We identified 12 RCTs of which 9 were included.
Included studies
Only two studies were considered sufficiently similar to pool (Isolauri 1995; Niggemann 2001).
The studies fell into three main categories ‐ see 'Characteristics of included studies'.
1. Egg and cow's milk exclusion diets 2. Few foods diet 3. Elemental diet
1. Egg and cows milk exclusion diets
Six RCTs, three of which were cross‐over studies (Atherton 1978; Cant 1986; Neild 1986) and three were parallel studies (Isolauri 1995; Lever 1998; Niggemann 2001)
Egg and cow's milk exclusion diet (with soya substitute) vs egg and cows' milk (Atherton 1978)
Egg and cow's milk exclusion diet (with soya substitute) vs egg and cows' milk in breastfeeding mothers (Cant 1986)
Whey hydrolysate vs amino acid derived formula (Isolauri 1995)
Egg and cow's milk exclusion diet (with soya substitute) vs normal diet (Neild 1986)
General advice on care of atopic eczema and specific advise about egg exclusion diet vs general advise from dietician only (Lever 1998)
Amino‐acid‐based (AA)formula vs extensively hydrolysed whey formula (Niggemann 2001)
2. Few foods diet
One RCT, a parallel study
Few foods diet plus whey hydrolysate vs few foods diet plus casein hydrolysate versus usual diet (Mabin 1995)
3. Elemental diet
Two RCTs, one was a cross‐over study (Leung 2004) and one was a parallel study (Munkvad 1984)
Elemental diet vs blended diluted diet of foodstuffs consumed by hospital inpatients (Munkvad 1984).
Hypoallergenic, milk free lactose and sucrose‐free, complete elemental formula with free amino‐acid (AA) vs participants pre existing formula (Leung 2004).
Excluded studies
We excluded three studies (see Characteristics of excluded studies) as they did not fit our inclusion criteria for 'types of intervention' (Majamaa 1997; Isolauri 2000; Kirjavainen 2003).
Risk of bias in included studies
Four studies were randomised, controlled, cross‐over designs (Atherton 1978; Cant 1986; Neild 1986; Leung 2004). Five studies were randomised controlled parallel designs (Munkvad 1984; Mabin 1995; Isolauri 1995; Lever 1998; Niggemann 2001).
Allocation
In seven of the studies in this review the method of randomisation was not described at all or was unclear. Two of the studies used a random number table (Cant 1986; Mabin 1995). In none of the studies did the trial authors clearly demonstrate adequate concealment of allocation.
Blinding
Only one study blinded participants, clinicians and outcome assessors (Munkvad 1984). Two studies blinded participants and outcome assessors (Atherton 1978; Neild 1986). Three studies blinded the outcome assessor only (Mabin 1995; Lever 1998; Leung 2004). In three studies blinding was unclear (Cant 1986; Isolauri 1995; Niggemann 2001)
Incomplete outcome data
Analysis should be performed according to the intention‐to‐treat principle, thus avoiding bias (Sackett 1979; May 1981; Altman 1991). However in many of the studies analysis of outcome was carried out only in those participants who completed the study. Only one study analysed by intention‐to‐treat (Isolauri 1995). For one study (Niggemann 2001) it was impossible to know if the analysis was intention‐to‐treat as no results tables were given or numbers mentioned in the text. All but two studies stated numbers and reasons for participants lost to follow up. One study (Isolauri 1995) had no loss to follow up, possibly due to the highly selected population.
Other potential sources of bias
Degree of certainty that participants had (atopic eczema) AE
The certainty of AE was clear for four studies (Munkvad 1984; Cant 1986; Mabin 1995; Niggemann 2001) that used criteria by Hanifin or Yates. One study stated that they included clinically typical AE (Atherton 1978) and all the other studies did not state how they diagnosed AE.
Baseline comparability of the participants for age, sex, and eczema severity
In one study the diet group had slightly more extensive and more severe involvement than the controls (Lever 1998). Two studies clearly stated that there were no differences in baseline comparability (Mabin 1995; Niggemann 2001). For all other studies it was not clear if there was baseline comparability of the participants.
Assessment of compliance
Compliance was clear in only three studies (Munkvad 1984; Neild 1986; Mabin 1995).
Severity of AE
Severity of AE was clear for only one study (Munkvad 1984).
Effects of interventions
The secondary outcome measures: (a) Global severity as rated by the participants or their physician, (b) Global changes in composite rating scales using a published named scale and (c) The trial author's modification of existing scales or new scales, have not been listed as we originally planned. This is because it was not possible to exactly match these outcomes with what we actually found. Instead we described the disease activity as we found it in each of the studies.
1. Egg and cows milk exclusion diets
Six RCTs of which three were cross‐over studies and three were parallel studies.
Cross‐over studies
All three studies (Atherton 1978;Cant 1986;Neild 1986) were conducted in different populations. One study was conducted in children of 2 to 8 yrs (Atherton 1978), one in breastfeeding mothers and babies (Cant 1986), and one in children and adults 1 to 23 yrs (Neild 1986). Three studies used soya milk as a control food which in itself can be allergenic in AE. All studies measured severity of AE in different ways. For these reasons the studies were not considered suitable to pool. Atherton 1978 The first cross‐over study (Atherton 1978) of 36 unselected children (2 to 8 yrs) compared a soya based preparation (egg and milk exclusion) vs dried egg and cows' milk over three four‐week periods. Main outcomes were eczema activity and area (using unpublished scale) pruritus, sleeplessness and antihistamine usage.
(1) Primary outcome measures
(a) Short‐term (within six weeks). Changes in participant‐rated or mother‐rated symptoms of atopic eczema such as itching (pruritus) or sleep loss.
Pruritus improved during the trial diet compared to the control diet. A small non significant order effect (i.e. improvements greater at the end of the first versus the second period whatever the diet content) with pruritus scores being lower during the first diet period than during the second diet period.
Sleeplessness was significantly lower during the trial period as compared to control period (p < 0.05), and the order effect was greater than the treatment effect. (b) Reduced need for other treatments. Significantly fewer antihistamine tablets were used in the trial diet period.
(2) Secondary outcome measures
Eczema area and activity (using unpublished composite score assessed by physician).
Eczema activity scores (major improvement +2; minor improvement +1; no change 0; minor deterioration ‐1; major deterioration ‐2) which recorded change of eczema activity during each diet period showed significantly more improvement after the trial diet than after control diet (p < 0.001). A significant order effect was apparent but this was smaller than the treatment effect.
Area scores (the body surface was divided into 20 separate zones, each of which was recorded as affected or unaffected; this gave an area score of up to 20) improved significantly during the trial period (p < 0.005). A significant order effect was apparent but this was smaller than the treatment effect.
From the paper we were able to calculate the difference between the proportions of children whose activity score improved and those whose score did not improve, based on paired observations from the same individual. Eczema activity scores improved significantly for children on the exclusion diet as compared to the control diet (Conditional OR 10.52, 95% CI 2.27 to 48.80; Analysis 1.1).
Analysis 1.1.
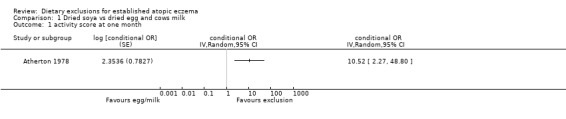
Comparison 1 Dried soya vs dried egg and cows milk, Outcome 1 activity score at one month.
(d) Quality of life No data given (e) Palatability of the diet. No data
(f) Adverse events Three participants (all on control diet) experienced a severe exacerbation of eczema within a few days of starting their diet.
Cant 1986 The second cross‐over study (Cant 1986) in 19 unselected breast feeding mothers and babies (6 weeks to 9 months of age) compared a soya based preparation (egg and milk exclusion) vs egg and milk diet over three four‐week periods. Due to insufficient data we were unable to calculate the conditional odds ratio for this study or analyse the first period only as from a parallel group trial.
1) Primary outcome measures
(a) Short‐term (within six weeks). Changes in parent‐rated or mother‐rated symptoms of atopic eczema such as itching (pruritus) or sleep loss. No data
(b) Reduced need for other treatments. There was no significant difference in the amount of steroid cream applied in each period.
(2) Secondary outcome measures
Two continuous scale eczema severity scores: area (number of involved body areas from 0 to 20) and activity (severity within each areas on 0 to 3 scale x number of involved areas)
Mean eczema severity scores decreased during the trial and at the end of the study when the mothers returned to a normal diet for four weeks. The scores were significantly lower than they had been at the beginning (p < 0.01).
There was a marked period effect in that children of mothers on the normal diet in the third period continued to improve.
(d) Quality of life No data given
(e) Palatability of the diet. No data given
(f) Adverse events Two mothers withdrew ‐ one mother vomited after soya milk and another mother's baby developed eczema and bloody diarrhoea within 24 hours of her taking the diet containing cows milk and egg. One infant developed watery stools with mucus when his mother took the substitute containing cow's milk and egg.
Neild 1986 A third cross‐over study (Neild 1986) in 53 unselected children and adults (1 to 23 yrs) compared a cow's milk exclusion diet (soya milk) vs milk containing egg and cows milk for 6 weeks. Due to insufficient data we were unable to calculate the conditional odds ratio for this study or analyse the first period only as from a parallel group trial.
1) Primary outcome measures
(a) Short‐term (within six weeks). Changes in participant‐rated or mother‐rated symptoms of atopic eczema such as itching (pruritus) or sleep loss. The trial authors reported no significant difference in total itch score at end of trial diet compared to end of normal diet
(b) Reduced need for other treatments. More topical steroids were used while on the trial diet .
(2) Secondary outcome measures
The body surface was divided into 20 zones using the method of Atherton 1978, and the presence of acute eczema (erythema and vesiculation) and chronic eczema (lichenification and prurigo papules) was noted, both on a scale from 0 to 3. The trial authors reported that no statistically significant difference was shown between the scores at the end of the trial diet compared with those at the end of the normal diet or between the scores at the end of the trial diet compared with those at the end of the control diet.
At the end of the study 25% (10/40) of adults and children appeared to have improved on the trial diet and had lower area scores and itch scores than on the normal diet or on the control. Of those who responded, five were in the youngest age group and two were non‐Caucasians. More responders had the trial diet first than the group as a whole.
(d) Quality of life
No data given
(e) Palatability of the diet.
No data given
(f) Adverse events
One participant developed gastrointestinal symptoms followed by itch and exacerbation of eczema
Parallel studies
(Isolauri 1995;Lever 1998;Niggemann 2001). Two of the these three studies were considered sufficiently similar to pool.
Isolauri 1995 and Niggemann 2001 The first study (Isolauri 1995) was in a population of 45 infants (6 to 7 months) with a positive reaction to cow's milk challenge that compared eHF (extensively hydrolysed whey formula) vs AA (amino acid formula) containing no peptides for 9 months. The second study (Niggemann 2001) in 73 infants (1 to 10 months) with a cows' milk allergy/intolerance (proved by a double‐blind, placebo controlled food challenge), compared an AA formula vs eHF for 6 months.
1) Primary outcome measures
(a) Short‐term (within six weeks). Changes in participant‐rated or mother‐rated symptoms of atopic eczema such as itching (pruritus) or sleep loss. No data for either study
(b) Degree of long‐term (over six months) control, such as reduction in number of flares or reduced need for other treatments. No data for either study
(2) Secondary outcome measures
There was no difference seen in eczema severity (using the SCORAD index) at 2 to 3 months (pooled analysis, 2 studies, MD 0.00, 95% CI ‐4.87 to 4.87; Analysis 2.1) or 6 to 8 months (pooled analysis, 2 studies, MD 1.06, 95% CI ‐1.67 to 3.80; Analysis 2.2). (d) Quality of life No data for either study
Analysis 2.1.
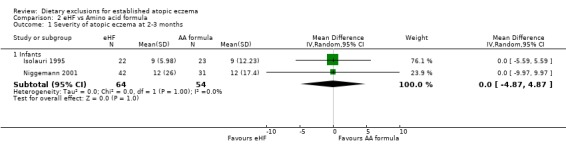
Comparison 2 eHF vs Amino acid formula, Outcome 1 Severity of atopic eczema at 2‐3 months.
Analysis 2.2.
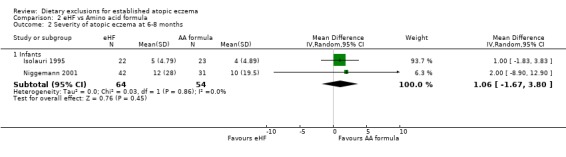
Comparison 2 eHF vs Amino acid formula, Outcome 2 Severity of atopic eczema at 6‐8 months.
(e) Palatability of the diet No data for either study
(f) Adverse events including long term consequences on growth No data for either study
Lever 1998 The third study (Lever 1998) in 62 infants (11 to 17 months ) with sensitivity to eggs compared an egg exclusion diet as advised by a dietician (in the diet group) vs general advice from a dietician (in the control group) for 4 weeks.
1) Primary outcome measures
(a) Short‐term (within six weeks). Changes in participant‐rated or mother‐rated symptoms of atopic eczema such as itching (pruritus) or sleep loss. No data available
(b) Degree of long‐term (over six months) control, such as reduction in number of flares or reduced need for other treatments. No data available
(2) Secondary outcome measures
Area affected (% of total skin area) using 'rule of nine'
Severity score in arbitary units which assesses six clinical features (extent, erythema, oedema/papulation, oozing/crusts, dryness, lichenification) on a scale of 0 to 3 units at 16 body sites.
At the end of the study 51% of the children had a significant improvement in body surface area with the exclusion diet compared to normal diet (RR 1.51, 95% CI 1.07 to 2.11; Analysis 3.1). Change in surface area and severity score (6 clinical features on a scale of 0 to 3 units at 16 body sites) was significantly improved in the egg exclusion diet group as compared to the normal diet at the end of 6 weeks and end of treatment (MD 5.50, 95% CI 0.19 to 10.81; Analysis 3.2 and MD 6.10, 95% CI 0.06 to 12.14; Analysis 3.3) respectively.
Analysis 3.1.
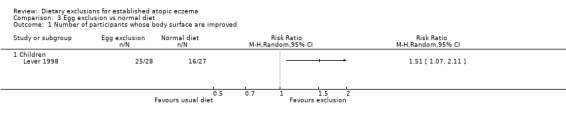
Comparison 3 Egg exclusion vs normal diet, Outcome 1 Number of participants whose body surface are improved.
Analysis 3.2.
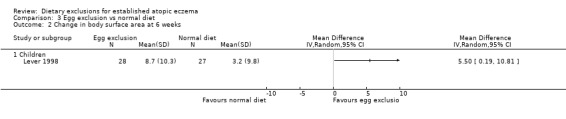
Comparison 3 Egg exclusion vs normal diet, Outcome 2 Change in body surface area at 6 weeks.
Analysis 3.3.
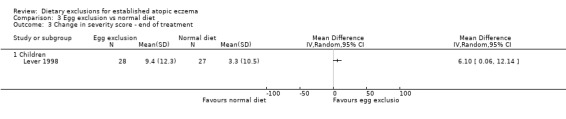
Comparison 3 Egg exclusion vs normal diet, Outcome 3 Change in severity score ‐ end of treatment.
(d) Quality of life No data available
(e) Palatability of the diet No data available
(f) Adverse events including long term consequences on growth No data available
2. Few foods diet
One RCT, which was a parallel study (Mabin 1995), randomised 85 children (0.3 to 13.3 yrs), with atopic eczema affecting more than 12% of the body, to one of three groups:
i) few foods diet (eliminating all but five to eight foods) plus whey;
ii) few foods diet plus casein hydrolysate; or
iii) usual diet.
1) Primary outcome measures
(a) Short‐term (within six weeks). Changes in participant‐rated or mother‐rated symptoms of atopic eczema such as itching (pruritus) or sleep loss. There was no significant difference in daytime itch when comparing: few foods diet with casein compared to normal diet (MD ‐0.6, 95% CI ‐ 1.46 to 0.26; Analysis 5.1); few foods diet with whey compared to few foods with casein (MD 0.5, 95% CI ‐ 1.69 to 2.69; Analysis 6.1); few foods with whey vs normal diet (MD ‐0.10, 95% CI ‐ 2.22 to 2.02; Analysis 4.1).
Analysis 5.1.
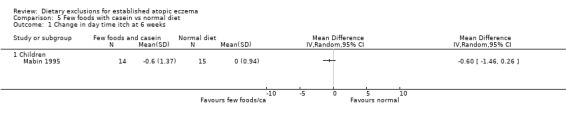
Comparison 5 Few foods with casein vs normal diet, Outcome 1 Change in day time itch at 6 weeks.
Analysis 6.1.
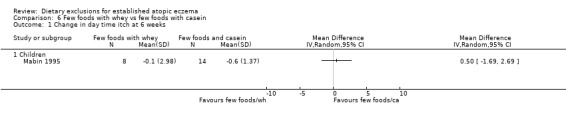
Comparison 6 Few foods with whey vs few foods with casein, Outcome 1 Change in day time itch at 6 weeks.
Analysis 4.1.
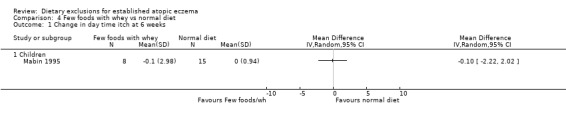
Comparison 4 Few foods with whey vs normal diet, Outcome 1 Change in day time itch at 6 weeks.
There were no significant differences in sleep disturbances at 6 weeks for: few foods with whey vs normal diet (MD ‐0.30, 95% CI ‐2.51 to 1.91; Analysis 4.2); few foods with casein vs normal diet (MD ‐0.10, 95% CI ‐0.90 to 0.70; Analysis 5.2); few foods with whey vs few foods with casein (MD ‐0.20, 95% CI ‐2.50 to 2.10; Analysis 6.2).
Analysis 4.2.
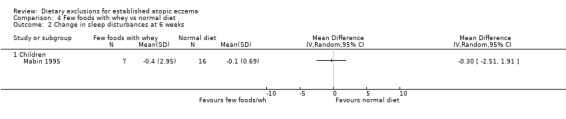
Comparison 4 Few foods with whey vs normal diet, Outcome 2 Change in sleep disturbances at 6 weeks.
Analysis 5.2.
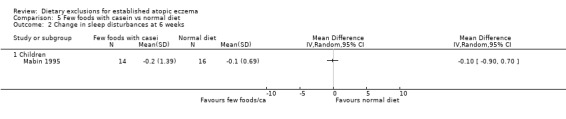
Comparison 5 Few foods with casein vs normal diet, Outcome 2 Change in sleep disturbances at 6 weeks.
Analysis 6.2.
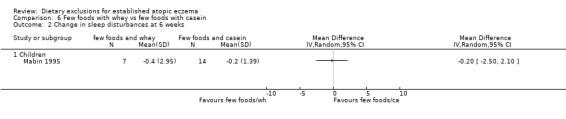
Comparison 6 Few foods with whey vs few foods with casein, Outcome 2 Change in sleep disturbances at 6 weeks.
(2) Secondary outcome measures
There were no significant differences in body surface area affected at 6 weeks when comparing: few foods and casein diet vs normal diet, (MD ‐0.10, 95% CI ‐18.91 to 18.71; Analysis 5.3); few foods and whey vs normal diet (MD ‐12.90, 95% CI ‐31.21 to 5.41; Analysis 4.3); few foods and whey vs few foods and casein (MD ‐12.80, 95% CI ‐36.75 to 11.15; Analysis 6.3).
Analysis 5.3.
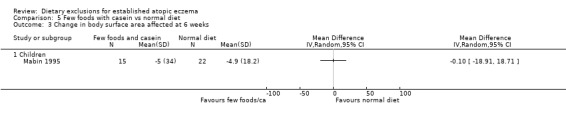
Comparison 5 Few foods with casein vs normal diet, Outcome 3 Change in body surface area affected at 6 weeks.
Analysis 4.3.
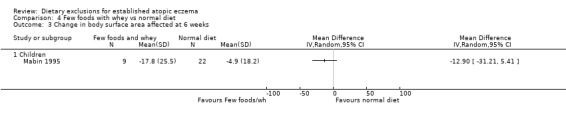
Comparison 4 Few foods with whey vs normal diet, Outcome 3 Change in body surface area affected at 6 weeks.
Analysis 6.3.
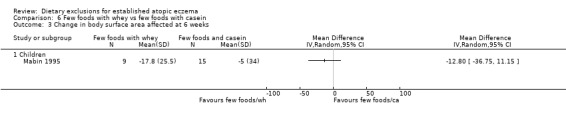
Comparison 6 Few foods with whey vs few foods with casein, Outcome 3 Change in body surface area affected at 6 weeks.
There were no significant differences in skin severity score at 6 weeks when comparing: few foods and whey diet vs normal diet (MD‐5.9, 95% CI ‐29.35 to 17.55; Analysis 4.4); few foods and casein diet vs normal diet (MD 2.40, 95% CI ‐22.64 to 27.44; Analysis 5.4); few foods and whey diet vs few foods and casein diet (MD ‐8.3, 95% CI ‐37.62 to 21.02; Analysis 6.4).
Analysis 4.4.
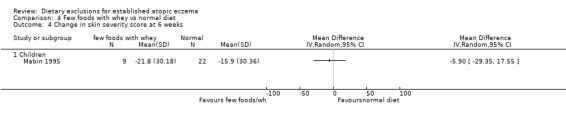
Comparison 4 Few foods with whey vs normal diet, Outcome 4 Change in skin severity score at 6 weeks.
Analysis 5.4.
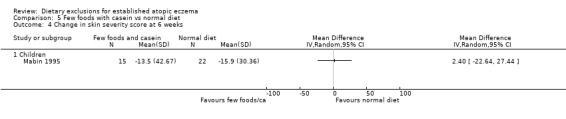
Comparison 5 Few foods with casein vs normal diet, Outcome 4 Change in skin severity score at 6 weeks.
Analysis 6.4.
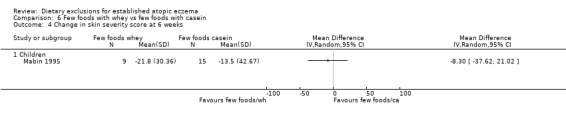
Comparison 6 Few foods with whey vs few foods with casein, Outcome 4 Change in skin severity score at 6 weeks.
d) Quality of life No data given
(e) Palatability of the diet No data given
(f) Adverse events including long term consequences on growth No data given
3. Elemental diet
Two RCTs, one was a cross‐over study (Leung 2004) and one was a parallel study ( Munkvad 1984).
Munkvad 1984 The first study (Munkvad 1984) randomised 33 adults (16 to 25 yrs) to either an elemental diet (amino acids, essential fatty acids, glucose, trace elements, sorbic acid and vitamins) or a blended diluted diet of foodstuffs consumed by hospital inpatients. Participants remained in hospital for the three week study.
1) Primary outcome measures
(a) Short‐term (within six weeks). Changes in participant‐rated or mother‐rated symptoms of atopic eczema such as itching (pruritus) or sleep loss. Short‐term (within six weeks).
There was no significant difference between the two groups for the combined outcome of pruritus, sleeplessness and antihistamine usage (RR 1.69, 95% CI 0.2 to 13.93; Analysis 7.2)
Analysis 7.2.
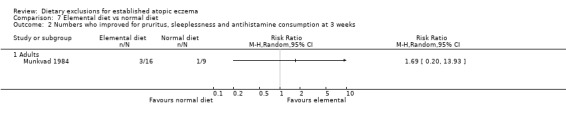
Comparison 7 Elemental diet vs normal diet, Outcome 2 Numbers who improved for pruritus, sleeplessness and antihistamine consumption at 3 weeks.
(2) Secondary outcome measures
There was no significant difference at three weeks between the two groups for improvement of intensity and extension of the eczema (RR 0.7, 95% CI, 0.25 to 1.97; Analysis 7.1) measured by a major activity score. A major activity score of > 100 was the criterion for a positive response to treatment. However there was a non significant trend in favour of the normal diet.
Analysis 7.1.
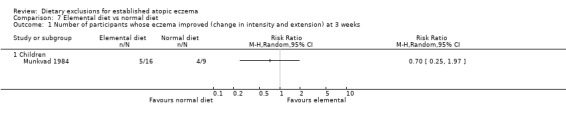
Comparison 7 Elemental diet vs normal diet, Outcome 1 Number of participants whose eczema improved (change in intensity and extension) at 3 weeks.
d) Quality of life No data given (e) Palatability of the diet Six withdrew due to a dislike of the diet (f) Adverse events including long term consequences on growth No data given
Leung 2004 A cross‐over study (Leung 2004) in 15 children (< 3 yrs) compared hypoallergenic, milk‐free, complete elemental formula with free AA (trial milk) vs participants pre existing formula (placebo) for 6 weeks.
1) Primary outcome measures
No data given
(2) Secondary outcome measures
Eczema activity score Median changes for SCORAD and its area, intensity pruritus and sleep loss components as well as global health score were reported not to be statistically significant during the active or placebo phase.
d) Quality of life No data given
(e) Palatability of the diet No data given
(f) Adverse events including long term consequences on growth No data given
Discussion
Summary of main results
We found some evidence to support the use of an egg‐free diet in infants with a suspected egg allergy who have a positive specific IgE to eggs in their blood. This perhaps highlights the importance of allergy testing beforehand. Only 2 of the other 11 included studies tested for food allergy (Isolauri 1995; Niggemann 2001), but those studies dealt with comparisons of 2 different forms of exclusion diets rather than a comparison of an exclusion diet versus normal diet, and have therefore not contributed to the question of whether any form of exclusion diet is helpful in such people. The other included studies of unselected people with atopic eczema did not find any evidence of benefit for exclusion diets. It is useful to know that exclusion diets given to unselected people with atopic eczema are not likely to be helpful, as benefit from dietary exclusions could be due to non‐allergic mechanisms. Not showing any benefit from such dietary exclusions in unselected people does not mean they are not helpful in people with proven allergy to that particular food. Three of the RCTs used potentially allergenic soya based milk substitute which itself can be allergenic in atopic eczema. Adverse events for people on exclusion diets included gastrointestinal symptoms followed by exacerbation of eczema or just exacerbation of eczema.
Overall completeness and applicability of evidence
This systematic review has only addressed dietary exclusion and has not addressed dietary supplements including probiotics which are the subject of another review. The clinical importance of changes in severity scores obtained in many studies is unknown. Drop‐out rates are particularly high for elimination diets and those containing hydrolysate milk substitutes and this will always remain a problem. Overall interpretation of the above studies was difficult due to the poor methodological quality of the studies.
Quality of the evidence
Nine poor quality studies were included in the review involving 421 participants: 6 egg and cow's milk exclusion diets; 1 was a few foods diet; 2 were elemental diets.
Of the egg and cow's milk exclusion diets, three studies used soya based milk substitute which itself can be allergenic in atopic eczema. Three small cross‐over studies studied various populations ranging from infants to adults. Only two studies followed participants for more than six months. Long‐term outcomes and consequences of an egg and milk free diet were not discussed by any of the studies. One study in unselected breast feeding mothers and babies found an improvement in their babies eczema during the exclusion period and when they went back to their normal diet ‐ however possible improvement may well have been spontaneous. One small study in unselected children found a significant improvement in eczema severity during the trials period when an egg and milk exclusion diet was compared to an egg and milk diet, however just under half of the participants were not included in the final analysis. One study in infants (11 to 17 months) with sensitivity to eggs found a significant improvement in body surface area with the exclusion diet compared to normal diet.
One study of the few foods diet found no significant change in body surface area, skin severity score, sleep disturbances in children when few foods diet plus whey compared to few foods diet plus casein hydrolysate or usual diet.
Two elemental diet studies were unable to find any significant difference in eczema severity when an elemental diet was compared to a normal hospital diet in adults or when an elemental diet was compared to a pre existing formula in children. Elemental diets are difficult since they are unpalatable for many and required hospitalisation and dietetic input.
Potential biases in the review process
Very few studies have been found and in seven of the included studies the method of randomisation was not described at all or was unclear.
Agreements and disagreements with other studies or reviews
The results of this review are in agreement with a previous HTA review (Hoare 2000).
Authors' conclusions
Elimination diets can be difficult to follow. The studies were performed in different populations with only one study giving results on the severity of atopic eczema. The clinical importance of small changes in severity scores obtained in many studies is unknown. Although diets excluding foods such as cows' milk are commonly tried there is little evidence for benefit in their use in unselected people with atopic eczema. That does not mean to say that they could not be beneficial in people with proven cows' milk allergy, but such studies have not been done yet. There may be some benefit in the use of an egg‐free diet in infants with a suspected egg allergy who have a positive specific IgE to eggs in their blood. There does not appear to be any benefit in the use of elemental or few foods diet in unselected people with atopic eczema.
Future studies should be big enough to answer the questions posed, and well reported according to CONSORT guidance (Moher 2001). Common sense suggests that studies of food allergy exclusions should be done on people with a history of suggested food allergy, confirmed by appropriate allergy testing or challenge tests. A distinction should be made between young children, older children and adults, because food allergy in children tends to improve in time. Disease severity should be measured using valid instruments and include quality of life assessments and patient‐centred outcomes that are easy to interpret clinically. Where possible, long‐term outcomes (greater than six months) should also be recorded in such studies.
Acknowledgements
The authors would like to thank Rosemary Humphries who contributed to the development of the protocol and the final readability of the review and to Weija Zhang who read and commented on the final protocol.
The editorial base would like to thank the following people who were the external referees for this review: Hugh Sampson and Peter Arkwright (content experts) and Shirley Manknell and Carol O'Brien (consumers).
Appendices
Appendix 1. CENTRAL (CLIB issue 1,2005) search strategy
| Search Strategy |
| #1 (atopic next dermatitis) or (atopic next eczema) or neurodermatitis in All Fields, from 1800 to 2005 in all products #2 (infantile next eczema) or (childhood next eczema) or (besniers next prurigo) in All Fields in all products #3 MeSH descriptor Dermatitis, Atopic explode all trees in MeSH products #4 MeSH descriptor Neurodermatitis explode all trees in MeSH products #5 (child near nutrition) or (adolescent near nutrition) or (infant near nutrition) or (bottle near feeding) or (breast near feeding) or weaning in All Fields, from 1800 to 2005 in all products #6 (dietary near protein*) or (egg near protein*) or (milk near protein*) or (vegetable near protein*) in All Fields, from 1800 to 2005 in all products #7 ((food or egg or milk or nut or peanut or wheat) near hypersensitivity) in All Fields, from 1800 to 2005 in all products #8 elimination or exclusion in All Fields in all products #9 elemental diet in All Fields in all products #10 MeSH descriptor Adolescent Nutrition explode all trees in MeSH products #11 MeSH descriptor Infant Nutrition explode all trees in MeSH products #12 MeSH descriptor Child Nutrition explode all trees in MeSH products #13 MeSH descriptor Bottle Feeding explode all trees in MeSH products #14 MeSH descriptor Breast Feeding explode all trees in MeSH products #15 MeSH descriptor Weaning explode all trees in MeSH products #16 MeSH descriptor Dietary Proteins explode all trees in MeSH products #17 MeSH descriptor Egg Proteins, Dietary explode all trees in MeSH products #18 MeSH descriptor Milk Proteins explode all trees in MeSH products #19 MeSH descriptor Vegetable Proteins explode all trees in MeSH products #20 MeSH descriptor Food Hypersensitivity explode all trees in MeSH products #21 MeSH descriptor Foods, Specialized explode all trees in MeSH products #22 (#1 OR #2 OR #3 OR #4) #23 (#5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21) #24 (#22 AND #23) |
Appendix 2. MEDLINE search strategy
| Search Strategy |
| 1. RANDOMIZED CONTROLLED TRIAL.pt.2. CONTROLLED CLINICAL TRIAL.pt. 3. RANDOMIZED CONTROLLED TRIALS.sh. 4. RANDOM ALLOCATION.sh. 5. DOUBLE BLIND METHOD.sh. 6. SINGLE‐BLIND METHOD.sh. 7. or/1‐6 8. animal/ not human/ 9. 7 not 8 10. CLINICAL TRIAL.pt. 11. exp CLINICAL TRIALS/ 12. (clin$ adj25 trial$).ti,ab. 13. ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).ti,ab. 14. PLACEBOS.sh. 15. placebo$.ti,ab. 16. random$.ti,ab. 17. RESEARCH DESIGN.sh. 18. or/10‐17 19. 18 not 8 20. 19 not 9 21. COMPARATIVE STUDY.sh. 22. exp EVALUATION STUDIES/ 23. FOLLOW UP STUDIES.sh. 24. PROSPECTIVE STUDIES.sh. 25. (control$ or prospectiv$ or volunteer$).ti,ab. 26. or/21‐25 27. 26 not 8 28. 27 not (9 or 20) 29. 9 or 20 or 28 30. exp Dermatitis, Atopic/ 31. dermatitis, atopic.mp. 32. eczema, atopic.mp. or exp Dermatitis, Atopic/ 33. atopic dermatitis.mp. 34. atopic eczema.mp. 35. infantile eczema.mp. 36. childhood eczema.mp. 37. exp CHILD/ 38. exp INFANT/ 39. neurodermatitis.mp. or exp NEURODERMATITIS/ 40. besniers prurigo.mp. 41. 30 or 31 or 32 or 33 or 34 42. 37 and 41 43. 38 and 41 44. 35 or 36 or 39 or 40 45. 41 or 42 or 43 or 44 46. child nutrition.mp. or exp Child Nutrition/ 47. adolescent nutrition.mp. or exp Adolescent Nutrition/ 48. infant nutrition.mp. or exp Infant Nutrition/ 49. bottle feeding.mp. or exp Bottle Feeding/ 50. breast feeding.mp. or exp Breast Feeding/ 51. exp WEANING/ or weaning.mp. 52. dietary proteins.mp. or exp Dietary Proteins/ 53. egg proteins.mp. or exp Egg Proteins/ 54. milk proteins.mp. or exp Milk Proteins/ 55. vegetable proteins.mp. or exp Vegetable Proteins/ 56. food hypersensitivity.mp. or exp Food Hypersensitivity/ 57. nut hypersensitivity.mp. or exp Nut Hypersensitivity/ 58. peanut hypersensitivity.mp. or exp Peanut Hypersensitivity/ 59. wheat hypersensitivity.mp. or exp Wheat Hypersensitivity/ 60. exp FOOD HYPERSENSITIVITY/ 61. foods, specialized.mp. or exp Foods, Specialized/ 62. (diet$ and (elimination or exclusion)).mp. [mp=title, original title, abstract, name of substance, mesh subject heading] 63. 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 or 62 64. 29 and 45 and 63 |
Appendix 3. EMBASE search strategey
| Search Strategy |
| 1. random$.mp.2. factorial$.mp. 3. crossover$.mp. 4. placebo$.mp. or PLACEBO/ 5. (doubl$ adj blind$).mp. [mp=title, abstract, subject headings, drug trade name, original title, device manufacturer, drug manufacturer name] 6. (singl$ adj blind$).mp. [mp=title, abstract, subject headings, drug trade name, original title, device manufacturer, drug manufacturer name] 7. assign$.mp. 8. volunteer$.mp. or VOLUNTEER/ 9. Crossover Procedure/ 10. Double Blind Procedure/ 11. Randomized Controlled Trial/ 12. Single Blind Procedure/ 13. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 14. atopic dermatitis.mp. or exp Atopic Dermatitis/ 15. atopic eczema.mp. 16. infantile eczema.mp. 17. childhood eczema.mp. 18. Child/ 19. Infant/ 20. neurodermatitis.mp. or exp NEURODERMATITIS/ 21. besniers prurigo.mp. 22. 14 or 15 23. 18 and 22 24. 19 and 22 25. 16 or 17 or 23 or 24 or 20 or 21 or 22 26. child nutrition.mp. or exp Child Nutrition/ 27. adolescent nutrition.mp. 28. infant nutrition.mp. or exp Infant Nutrition/ 29. bottle feeding.mp. or exp Bottle Feeding/ 30. breast feeding.mp. or exp Breast Feeding/ 31. weaning.mp. or exp WEANING/ 32. dietary proteins.mp. 33. milk proteins.mp. or exp Milk Protein/ 34. egg proteins.mp. or exp Egg Protein/ 35. vegetable proteins.mp. or exp Vegetable Protein/ 36. egg hypersensitivity.mp. 37. milk hypersensitivity.mp. 38. exp Food Allergy/ 39. nut hypersensitivity.mp. 40. peanut hypersensitivity.mp. 41. wheat hypersensitivity.mp. 42. elimination.mp. 43. exclusion.mp. 44. elemental diet.mp. or exp Elemental Diet/ 45. foods, specialized.mp. 46. 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 40 or 41 or 42 or 43 or 44 47. 13 and 25 and 46 |
Appendix 4. PsycINFO search strategy
| Search Strategy |
| 1. exp Dermatitis/ or atopic dermatitis.mp.2. exp Eczema/ or atopic eczema.mp. 3. exp NEURODERMATITIS/ or neurodermatitis.mp. 4. childhood eczema.mp. 5. 1 or 2 or 3 or 4 6. diet.mp. or exp DIETS/ 7. food allergy.mp. or exp Food Allergies/ 8. dietary exclusions.mp. 9. egg exclusion.mp. 10. diet$ manipulation$.mp. 11. 6 or 7 or 8 or 9 or 10 12. randomized controlled trial.pt. 13. clinical trial.mp. or exp Clinical Trials/ 14. 12 or 13 15. 5 and 11 and 14 |
Appendix 5. AMED search strategy
| Search Strategy |
| 1. randomized controlled trial$/ 2. random allocation/ 3. double blind method/ 4. single blind method.mp. 5. exp Clinical trials/ 6. (clin$ adj25 trial$).mp. [mp=abstract, heading words, title] 7. ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$ or dummy)).mp. [mp=abstract, heading words, title] 8. (placebo$ or random$).mp. [mp=abstract, heading words, title] 9. research design/ or clinical trials/ or comparative study/ or double blind method/ or random allocation/ 10. prospective studies.mp. 11. cross over studies.mp. 12. Follow up studies/ 13. control$.mp. 14. (multicent$ or multi‐cent$).mp. [mp=abstract, heading words, title] 15. ((stud or design$) adj25 (factorial or prospective or intervention or crossover or cross‐over or quasi‐experiment$)).mp. [mp=abstract, heading words, title] 16. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 17. exp Dermatitis, Atopic/ 18. dermatitis, atopic.mp. 19. eczema, atopic.mp. or exp Dermatitis, Atopic/ 20. atopic dermatitis.mp. 21. atopic eczema.mp. 22. infantile eczema.mp. 23. childhood eczema.mp. 24. exp CHILD/ 25. exp INFANT/ 26. neurodermatitis.mp. or exp NEURODERMATITIS/ 27. besniers prurigo.mp. 28. 17 or 18 or 19 or 20 or 21 29. 24 and 28 30. 25 and 28 31. 22 or 23 or 26 or 27 32. 28 or 29 or 30 or 31 33. child nutrition.mp. or exp Child Nutrition/ 34. adolescent nutrition.mp. or exp Adolescent Nutrition/ 35. infant nutrition.mp. or exp Infant Nutrition/ 36. bottle feeding.mp. or exp Bottle Feeding/ 37. breast feeding.mp. or exp Breast Feeding/ 38. exp WEANING/ or weaning.mp. 39. dietary proteins.mp. or exp Dietary Proteins/ 40. egg proteins.mp. or exp Egg Proteins/ 41. milk proteins.mp. or exp Milk Proteins/ 42. vegetable proteins.mp. or exp Vegetable Proteins/ 43. food hypersensitivity.mp. or exp Food Hypersensitivity/ 44. nut hypersensitivity.mp. or exp Nut Hypersensitivity/ 45. peanut hypersensitivity.mp. or exp Peanut Hypersensitivity/ 46. wheat hypersensitivity.mp. or exp Wheat Hypersensitivity/ 47. exp FOOD HYPERSENSITIVITY/ 48. foods, specialized.mp. or exp Foods, Specialized/ 49. (diet$ and (elimination or exclusion)).mp. [mp=abstract, heading words, title] 50. 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 51. 16 and 32 and 50 |
Data and analyses
Comparison 1.
Dried soya vs dried egg and cows milk
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 activity score at one month | 1 | conditional OR (Random, 95% CI) | Totals not selected |
Comparison 2.
eHF vs Amino acid formula
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severity of atopic eczema at 2‐3 months | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Infants | 2 | 118 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐4.87, 4.87] |
| 2 Severity of atopic eczema at 6‐8 months | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Infants | 2 | 118 | Mean Difference (IV, Random, 95% CI) | 1.06 [‐1.67, 3.80] |
Comparison 3.
Egg exclusion vs normal diet
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of participants whose body surface are improved | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Children | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Change in body surface area at 6 weeks | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Children | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Change in severity score ‐ end of treatment | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Children | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4.
Few foods with whey vs normal diet
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in day time itch at 6 weeks | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Children | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Change in sleep disturbances at 6 weeks | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Children | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Change in body surface area affected at 6 weeks | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Children | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Change in skin severity score at 6 weeks | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 Children | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 5.
Few foods with casein vs normal diet
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in day time itch at 6 weeks | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Children | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Change in sleep disturbances at 6 weeks | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Children | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Change in body surface area affected at 6 weeks | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Children | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Change in skin severity score at 6 weeks | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 Children | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 6.
Few foods with whey vs few foods with casein
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in day time itch at 6 weeks | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Children | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Change in sleep disturbances at 6 weeks | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Children | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Change in body surface area affected at 6 weeks | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Children | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Change in skin severity score at 6 weeks | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 Children | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 7.
Elemental diet vs normal diet
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of participants whose eczema improved (change in intensity and extension) at 3 weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Children | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Numbers who improved for pruritus, sleeplessness and antihistamine consumption at 3 weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Adults | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
What's new
| Date | Event | Description |
|---|---|---|
| 22 May 2008 | Amended | Converted to new review format. |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | UK, Single centre. RCT. D: cross over AC: unclear RS: unclear B: participant and outcome assessor. | |
| Participants | Setting: outpatients. Unselected children (n=36), Evaluable: (n=20). Age 2‐8 yrs SAE: unknown Dig: Typical AE C0‐T:hydrochortisone, emollients ,antihistamine | |
| Interventions | Three four week periods. During first and third period participants were placed on an egg and milk elimination diet and they were randomlly allocated to one of two milk substitutes. During 1 period the participants were given a dried soya‐based pepraration (trial period), during the other they were given a preparation containing a mixture of dried egg and cows' milk (control period). During the middle period participants resumed their normal diet, to minimise any carry‐over effect. | |
| Outcomes | Eczema area and activity (using unpublished composite score assessed by physician), degree of adherence to diet, skin prick tests, pruritus, sleeplessness and antihistamine usage. | |
| Notes | PP.16 loss to FU(44%). Nine excluded due to dietary lapses, seven withdrew voluntarily (four during or after control period, three during or after trial period), . Marked order effect i.e. improvements greater at end of first vs second period whatever the diet content. Soya milk (which itself can be allergenic in atopic eczema) used as 'control' food. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | UK, Single centre. RCT D: cross over AC: unclear RS: random number table B: unclear | |
| Participants | Setting: unclear. Unselected breast feeding mothers and babies (n=19), (8 infant girls and 9 infant boys) Evaluable: (n=17) Age 6 wks ‐6 mths SAE: unknown Dig: according to Yates et al. Co‐T: topical steroids and emollients | |
| Interventions | Twelve week study divided into three four week periods; during first two periods, mothers excluded cows' milk, egg and other foods (chocolate,wheat, nuts, fish, beef, chicken, citrus fruits, colourings and preservatives) from their diet. In the first period mothers were randomised to one of two milk substitutes containing either cows' milk and egg or just soya . Normal diet in third period. | |
| Outcomes | Two continuous‐scale eczema severity scores evaluated after four weeks on each diet: area (number of involved body areas from 0‐20) and 'activity' (severity within each area on 0‐3 scale x number of involved areas) | |
| Notes | ITT attempted. two mothers withdrew ‐ one vomited after soya milk , another mothers baby developed eczema and bloody diarrhoea. First study described as double‐blind, though almost half mothers correctly identified substitutes. Soya used as control diet. Marked period effect in that children of mothers on normal diet in third period continued to improve. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Finland, Single centre. RCT D:parallel AC:unclear RS: unclear B:unclear | |
| Participants | Setting: outpatients. Infants (n=45) who were not being breast‐fed, who had been fed substitute cows' milk for at least 6 months and who showed a positive reaction to a masked challenge with cows' milk Evaluable:45 Age 6‐7 months SAE: Dig: Hanifin C0‐T: | |
| Interventions | eHF (n=22) vs amino acid derived formula containing no peptides (n=23) | |
| Outcomes | Severity of atopic eczema measured by SCORAD. Infants growth also measured FU: 9 months. | |
| Notes | ITT. No loss to FU. Highly selected population Study mainly concerned in comparison of growth in AA vs eHF Main statistical comparison of change in eczema severity between the two groups not reported in results, though children in AA group higher baseline scores. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Hong Kong, single centre. RCT D: cross over AC:unclear RS: unclear B: outcome assessor blinded | |
| Participants | Setting: paediatric allergy and dermatology clinics. Unselected children (n=15) Evaluable: (n=11) Age <3 yrs SAE: unclear Dig:according to Hanifin criteria Co‐T: all drugs including topical corticosteroid mometasone furoate and oral sedating antihistmines. | |
| Interventions | Following a four week run in patients randomised to either Hypoallergenic, milk‐free, lactose and sucrose‐free, complete elemental formula with free AA (Neocate) or placebo (patients pre existing formulae) for six weeks. This was followed by a wash out period of six weeks and then crossed over to the other intervention for six weeks. | |
| Outcomes | AD severity was assessed using SCORAD before and after each phase. | |
| Notes | PP Four drop outs: two drank less than daily milk requirement, one refused to drink Neocate and one was discontinued due to too mild AD. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Scotland. Single centre. RCT D: parallel group AC:unclear RS: unclear B:Outcome assessor. randomised controlled trial. Method of randomisation and concealment of allocation unclear. | |
| Participants | Setting: outpatients. Young children(n=62) , with sensitivity to eggs. Evaluable: (n=55) Age: 11 to 17 months all with positive IgE blood antibodies to egg, only seven of which had a history suggestive of egg allergy. SAE:unclear Dig: as per dermatologist Co‐T: mild to moderate topical steroids) | |
| Interventions | a:General advice re AE and specific advice dietary advice vs b. general advice on AE and no dietary advice. Duration four weeks. | |
| Outcomes | Eczema severity assessed by extent in % terms and a composite severity score in 16 body sites. | |
| Notes | Randomisation done by same dietician who was giving the intervention PP. a: 4 withdrawals (2 defaulted and 2 incomplete records) b: 3 withdrawals (1 defaulted, 2 incomplete records) Co‐treatment use not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | UK, Single centre. RCT D:parallel AC:unclear RS: random no tables B: outcome assessors | |
| Participants | Setting: outpatients. Unselected children (n=85) Evaluable: (n=46 ) Age 0.3 to 13.3 yrs SAE:atopic eczema that persisted despite conventional treatment and >12% of body surface area. Dig: Hanifin and Rajka Co‐T:unclear | |
| Interventions | a:normal diet(n=26) b:few foods with whey hydrolysate formula (n=27)c: few foods diet with casein hydrolysate formula (n=32) for 6 weeks | |
| Outcomes | Clinical assessment consisted of the estimation of the body surface area affected by using charts that divided the body into 32 separate zones. Skin severity for each of the 32 zones was assessed by the extent of area affected and degree of erythema on an arbitrary scale of 0 to 3. Overall skin severity score with a possible range of 0 to 300 was achieved by summarising all 32 zones. | |
| Notes | PP.40 lost to FU, a:n=4 b:n=18 c:n=17 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Denmark,Single centre.RCT D:parallel AC:unclear RS:unclear B:participant, clinician,outcome assessor | |
| Participants | Setting: hospitalised. Unselected adults (n=33) Evaluable:(n=25) Age 16‐52 yrs SAE: severe, atopic eczema covering >10% of body Dig: Hanifin and Rajika Co‐T:emollients, topical steroid, antihistamines. | |
| Interventions | Elemental diet (amino acids, essential fatty acids, glucose, trace elements, sorbic acid and vitamins) vs blended diluted diet of foodstuffs consumed by hospital inpatients. Diet for three weeks. | |
| Outcomes | Various unpublished extent and intensity signs scored. A score between ‐3 and +3 was given to the degree of change for each of the clinical symptoms (erythema, infiltration and size of area involved). In addition photographs before and after, patient itch and sleep, and various serum markers of inflammation. In evaluation the mean value of these four figures was multiplied by 100 and named 'major activity score'. A 'major activity' score of >100 was defined as the criterion for a positive response to treatment. | |
| Notes | PP. Eight lost to FU‐ (24%) mostly due to dislike of diet. Small study of intervention that is unpalatable, impractical and requires hospitalisation and dietetic input. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | UK. Single centre. RCT. D: cross‐over AC: unclear RS: unclear B:participant and outcome assessor | |
| Participants | Setting: outpatients. Unselected children and adults (n=53), (29 female and 24 male) Evaluable:(n=40) Age 1‐23 yrs SAE: mild, moderate and severe. Dig: active atopic eczema Co‐T: usual treatment | |
| Interventions | Three six‐week periods; during first and third periods, patients placed on egg and cows' milk exclusion diet and randomised to either soya or a milk containing egg and cows' milk. | |
| Outcomes | Participants reported on itch and sleep loss, use of co‐treatments, composite score of area and intensity. | |
| Notes | PP. Thirteen lost to FU. Twelve patients excluded from analysis due to non compliance as diet too difficult to adhere to. One participant developed GI symptoms followed by itch and exacerbation of eczema. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Finland, Multicentre. RCT D: parallel AC: unclear RS: unclear B: none | |
| Participants | Setting: outpatients. Infants (n=73) Aged between 1 and 10 months SAE: Dig: Sampson and Seymour modified from Hanifin and Rajka with atopic and cows' milk intolerance, proven by DBPFC Co‐T:? | |
| Interventions | Amino acid based formulae (AA) group (n=31) vs eHF (n=42) for six months | |
| Outcomes | FU at three and six months. Clinical symptoms of AD according to SCORAD index to assess disease severity. Dietary intake measured using three‐day dietary record. | |
| Notes | No mention of ITT analysis or withdrawals. SCORAD results for two groups was not reported separately and no table of results. Data extracted from graph. Mean SCORAD was reduced significantly in all infants at three months and six months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
AA: amino acid formula AC: methods of allocation concealment AE: atopic eczema B: blinding (participant clinician outcome assessment) Co‐T: co treatments D: design DBPFC: double‐blind, placebo‐controlled food‐challenge Dig: diagnostic criteria eHF: extensively hydrolysed whey formula FU: follow‐up ITT: intention to treat PP: per protocol RAST: radio‐allergosorbent test RS: method of generating randomisation sequence SAE: severity of atopic eczema
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Isolauri 2000 | Breast fed infants weaned onto eHF vs eHF plus Bifidobacterium lactic Bb‐12 vs eHF plus Lactobacillus strain GG. This study was about probiotics rather than exclusion diets. |
| Kirjavainen 2003 | This study compared extensively hydrolysed whey formula vs the same formula supplemented with viable Lactobacillus GG or heat‐inactivated Lactobacillus GG. Although the groups excluded cows milk this study was about oral supplementation of viable and heat‐inactivated probiotic bacteria in the management of atopic disease. |
| Majamaa 1997 | This study aimed to evaluate effect of cow's milk elimination with and without the addition of Lactobacillus GG in eHF formula in infants. This study did not fit our inclusion criteria for types of studies. |
eHF ‐ extensively hydrolysed whey formula
Contributions of authors
Link with editorial base and co‐ordinate contributions from co‐authors (FB‐H) Draft protocol ( FB‐H, FD) Consumer ‐ input to draft protocol (RH) Run search (FD and FB‐H) Identify relevant titles and abstracts from searches (FB‐H, FD) Obtain copies of trials (FB‐H) Selection of trials (FB‐H, FD) Extract data from trials ( FB‐H, FD) Enter data into revman (FB‐H) Carry out analysis (FB‐H) Write up of full review (FB‐H) Interpret data (FB‐H, FD, HW)
Sources of support
Internal sources
University of Nottingham, School of Nursing, UK.
University of Nottingham, Cochrane Skin Group, CEBD., UK.
External sources
No sources of support supplied
Declarations of interest
None known
Edited (no change to conclusions)
References
References to studies included in this review
- Atherton DJ. Dietary antigen avoidance in the treatment of atopic eczema. Acta Dermato Venereologica 1980;Suppl 92:99‐102. [Google Scholar]; Atherton DJ, Sewell M, Soothill JF, Wells RS, Chilvers CE. A double‐blind controlled crossover trial of an antigen‐avoidance diet in atopic eczema. Lancet 1978;1(8061):401‐3. [DOI] [PubMed] [Google Scholar]
- Cant AJ, Bailes JA, Marsden RA, Hewitt D. Effect of maternal dietary exclusion on breast fed infants with eczema: two controlled studies. British Medical Journal Clinical Research 1986;293(6541):231‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isolauri E, Sutas Y, Makinen‐Kiljunen S, Oja SS, Isosomppi R, Turjanmaa K. Efficacy and safety of hydrolyzed cow milk and amino acid‐derived formulas in infants with cow milk allergy. Journal of Pediatrics 1995;127(4):550‐7. [DOI] [PubMed] [Google Scholar]
- Leung TF, Ma KC, Cheung LTF, Lam CWK, Wong E, Wang H, et al. A randomized, single‐blind and crossover study of an amino acid‐based milk formula in treating young children with atopic dermatitis. Pediatric Allergy & Immunology 1004;15:558‐61. [DOI] [PubMed] [Google Scholar]
- Lever R, MacDonald C, Waugh P, Aitchison T. Randomised controlled trial of advice on an egg exclusion diet in young children with atopic eczema and sensitivity to eggs. Pediatric Allergy & Immunology 1998;9(1):13‐9. [DOI] [PubMed] [Google Scholar]
- Mabin DC, Sykes AE, David TJ. Controlled trial of a few foods diet in severe atopic dermatitis. Archives of Disease in Childhood 1995;73(3):202‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munkvad M, Danielsen L, Hoj L, Povlsen CO, Secher L, Svejgaard E, et al. Antigen‐free diet in adult patients with atopic dermatitis. A double‐blind controlled study. Acta Dermato Venereologica 1984;64(6):524‐8. [PubMed] [Google Scholar]
- Neild VS, Marsden RA, Bailes JA, Bland JM. Egg and milk exclusion diets in atopic eczema. British Journal of Dermatology 1986;114(1):117‐23. [DOI] [PubMed] [Google Scholar]
- Niggemann B, Binder C, Dupont C, Hadji S, Arvola T, Isolauri E. Prospective, controlled, multi‐center study on the effect of an amino‐acid‐based formula in infants with cow's milk allergy/intolerance and atopic dermatitis. Pediatric Allergy & Immunology 2001;12(2):78‐82. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Isolauri E, Arvola T, Sutas Y, Moilanen E, Salminen S. Probiotics in the management of atopic eczema. Clinical and Experimental Allergy 2000;30:1604‐10. [DOI] [PubMed] [Google Scholar]
- Kirjavainen PV, Salminen SJ, Isolauri E. Probiotic bacteria in the management of atopic disease: underscoring the importance of viability. Journal of Pediatric Gastroenterology and Nutrition 2003;36:223‐7. [DOI] [PubMed] [Google Scholar]
- Majamaa H, Isolauri E. Probiotics: A novel approach in the management of food allergy. Journal of Allergy and Clinical Immunology 1997;99:179‐85. [DOI] [PubMed] [Google Scholar]
Additional references
- Altman DG. Practical statistics for medical research. London: Chapman and Hall, 1991. [Google Scholar]
- Archer CB. The pathophysiology and clinical features of atopic dermatitis. In: Hywel Williams editor(s). Atopic Dermatitis. The epidemiology, causes and prevention of atopic eczema. 1st Edition. Cambridge University Press, 2000:25‐40. [Google Scholar]
- Baena‐Cagnani CE, Teijeiro A. Role of food allergy in asthma in childhood. Current Opinion in Allergy & Clinical Immunology 2001;1(2):145‐9. [DOI] [PubMed] [Google Scholar]
- Baum WF, Schneyer U, Lantzsch AM, Kloditz E. Delay of growth and development in children with bronchial asthma, atopic dermatitis and allergic rhinitis. Experimental & Clinical Endocrinology & Diabetes 2002;110(2):53‐9. [DOI] [PubMed] [Google Scholar]
- Beck LA, Leung DY. Allergen sensitization through the skin induces systemic allergic responses. Journal of Allergy and Clinical Immunology 2000;106 Suppl 5:S258‐63. [DOI] [PubMed] [Google Scholar]
- Bohme M, Svensson A, Kull I, Nordvall SL, Wahlgren CF. Clinical features of atopic dermatitis at two years of age: a prospective, population‐based case‐control study. Acta Dermato Venereologica 2001;81(3):193‐7. [DOI] [PubMed] [Google Scholar]
- Chandra RK. Food hypersensitivity and allergic diseases. European Journal of Clinical Nutrition 2002;56 Suppl 3:S54‐6. [DOI] [PubMed] [Google Scholar]
- Charman C, Williams HC. Measures of disease severity in atopic eczema. Archives of Dermatology 2000;136:763‐9. [DOI] [PubMed] [Google Scholar]
- Charman C, Williams HC. Epidemiology of atopic dermatitis. Atopic Dermatitis. New York: Marcel Dekker Inc, 2002:21‐42. [Google Scholar]
- Chren MM, Lasek RT, Flocke SA, Zyzanski SJ. Improved discriminative and evaluative capability of a refined version of SKINDEX, a quality‐of‐life instrument for patients with skin diseases. Archives of Dermatology 1997;133:1433‐40. [PubMed] [Google Scholar]
- Cookson W. Genetics and genomics of asthma and allergic diseases. Immunological Reviews 2002;190:195‐206. [DOI] [PubMed] [Google Scholar]
- David TJ, Waddington E, Stanton RHJ. Nutritional hazards of elimination diets in children with atopic eczema. Archives of disease in childhood 1984;59:323‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David TJ. Dietary treatments of atopic eczema. Archives of Disease in Childhood 1989;64:1506‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David TJ. Food and Food Additive Intolerance in Childhood. Oxford: Blackwell Scientific, 1993. [Google Scholar]
- David TJ, Patel L, Ewing CI, Stanton RHJ. Dietary factors in established atopic dermatitis. In: Hywel Williams editor(s). Atopic Dermatitis. The epidemiology, causes and prevention of atopic eczema. Cambridge University Press, 2000:193‐201. [Google Scholar]
- Devlin J, Stanton RHJ, David TJ. Calcium intake and cows' milk free diets. Archives of Disease in Childhood 1989;64:1183‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigenmann PA, Sicherer SH, Borkowski TA, Cohen BD, Sampson HA. Prevalence of IgE‐mediated food allergy among children with atopic dermatitis. Pediatrics 1998;101:e8. [DOI] [PubMed] [Google Scholar]
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta‐analysis involving cross‐over trials: methodological issues. International Journal of Epidemiology 2003;31:140‐9. [DOI] [PubMed] [Google Scholar]
- Emerson RM, Williams HC, Allen BR. Severity distribution of atopic dermatitis in the community and its relationship to secondary referral. British Journal of Dermatology 1998;139(1):73‐6. [DOI] [PubMed] [Google Scholar]
- Ernst E, Pittler MH, Stevinson C. Complementary/alternative medicine in dermatology: evidence‐assessed efficacy of two diseases and two treatments. American Journal of Clinical Dermatology 2002;3(5):341‐8. [DOI] [PubMed] [Google Scholar]
- Fendrick AM, Baldwin JL. Allergen‐induced inflammation and the role of immunoglobulin E (IgE). American Journal of Therapeutics 2001;8(4):291‐7. [DOI] [PubMed] [Google Scholar]
- Fennessy M, Coupland S, Popay J, Naysmith K. The epidemiology and experience of atopic eczema during childhood: a discussion paper on the implications of current knowledge for health care, public health policy and research. Journal of Epidemiology & Community Health 2000;54(8):581‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay AY. Measurement of disease activity and outcome in atopic dermatitis. The British Journal of Dermatology 1996;135:509‐15. [PubMed] [Google Scholar]
- Flohr C, Johansson SGO, Wahlgren C‐F, Williams H. How atopic is atopic dermatitis?. Journal of Allergy and Clinical Immunology 2004;114:150‐8. [DOI] [PubMed] [Google Scholar]
- Graham‐Brown R. Atopic dermatitis: unapproved treatments or indications. Clinical Dermatology 2000;18(2):153‐8. [DOI] [PubMed] [Google Scholar]
- Guillet G, Guillet MH. Natural history of sensitisations in atopic dermatitis. A 3‐year follow‐up in 250 children: food allergy and high risk of respiratory symptoms. Archives of Dermatology 1992;128:187‐92. [DOI] [PubMed] [Google Scholar]
- Hathaway MJ, Warner JO. Problems in the dietary treatment of eczema. Archives of Disease in Childhood 1983;53:463‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herd RM, Tidman MJ, Prescott RJ, Hunter JA. The cost of atopic eczema. British Journal of Dermatology 1996;135(1):20‐3. [PubMed] [Google Scholar]
- Hoare C, Li Wan Po A, Williams H. Systematic review of treatments for atopic eczema. Health Technology Assessment 2000;4(37):1‐191. [PMC free article] [PubMed] [Google Scholar]
- Johansson SGO, O'B Hourihane J, Bousquet J, Bruijnzeel‐Koomen C, Dreborg S, Haahtela T. A revised nomenclature for allergy: an EAACI position statement from the EAACI nomenclature task force. Allergy 2001;56:813‐24. [DOI] [PubMed] [Google Scholar]
- Johansson SGO, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF. Revised nomenclature for allergy for global use: Report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. Journal of Allergy and Clinical Immunology 2004;113:832‐6. [DOI] [PubMed] [Google Scholar]
- Juni P, Altman DG, Egger M. Assessing the qualtiy of controlled clinical trials. BMJ 2001;323:42‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalliomaki M, Isolauri E. Pandemic of atopic diseases ‐a lack of microbial exposure in early infancy?. Current Drug Targets ‐ Infectious Disorders 2002;2(3):193‐9. [DOI] [PubMed] [Google Scholar]
- Kemp AS. Cost of illness of atopic dermatitis in children: a societal perspective. Pharmacoeconomics 2003;21(2):105‐13. [DOI] [PubMed] [Google Scholar]
- Lamb SR, Rademaker M. Pharmacoeconomics of drug therapy for atopic dermatitis. Expert Opinion on Pharmacotherapy 2002;3(3):249‐55. [DOI] [PubMed] [Google Scholar]
- Li XM, Kleiner GA, Huang CK, Lee SY, Schofield BH, Soter NA. Murine model of atopic dermatitis associated with food hypersensitivity. Journal of Allergy and Clinical Immunology 2001;107(4):693‐702. [DOI] [PubMed] [Google Scholar]
- May GS, Demets DL, Friedman LM, Furberg C, Passamani E. The randomised clinical trial: bias in analysis. Circulation 1981;64:669‐73. [DOI] [PubMed] [Google Scholar]
- McHenry PM, Williams HC, Bingham EA. Management of atopic eczema. Joint Workshop of the British Association of Dermatologists and the Research Unit of the Royal College of Physicians of London. BMJ 1995;310(6983):843‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Schulz KF, Altman DG, Lepage L. The CONSORT statement: revised recommendations for improving the quality of reports of parallel group randomised trials. Lancet 2001;357:1191‐4. [PubMed] [Google Scholar]
- Oranje AP, Waard‐van der Spek FB. Atopic dermatitis and diet. [comment]. Journal of the European Academy of Dermatology & Venereology 2000;14(6):437‐8. [DOI] [PubMed] [Google Scholar]
- Polosa R. The interaction between particulate air pollution and allergens in enhancing allergic and airway responses. Current Allergy & Asthma Reports 2001;1(2):102‐7. [DOI] [PubMed] [Google Scholar]
- Ricci G, Patrizi A, Baldi E, Menna G, Tabanelli M, Masi M. Long‐term follow‐up of atopic dermatitis: retrospective analysis of related risk factors and association with concomitant allergic diseases. Journal of the American Academy Dermatology 2006;55(5):765‐71. [DOI] [PubMed] [Google Scholar]
- Sackett DL, Gent M. Controversy in waiting and attributing events in clinical trials. New England Journal of Medicine 1979;301:1410‐2. [DOI] [PubMed] [Google Scholar]
- Sampson HA. The immunopathogenic role of food hypersensitivity in atopic dermatitis. Acta Dermato Venereologica Supplementum 1992;176:34‐7. [PubMed] [Google Scholar]
- Sampson HA. Utility of food‐specific IgE concentrations in predicting symptomatic food allergy. Journal of Allergy and Clinical Immunology 2001;107(5):891‐6. [DOI] [PubMed] [Google Scholar]
- Sampson HA. The evaluation and management of food allergy in atopic dermatitis. Clinics in Dermatology 2003;21:183‐92. [DOI] [PubMed] [Google Scholar]
- Smethurst D. Atopic eczema. Clinical Evidence 2002;7:1467‐82. [PubMed] [Google Scholar]
- Su JC, Kemp AS, Varigos GA, Nolan TM. Atopic eczema: its impact on the family and financial cost. Archives of Disease in Childhood 1997;76(2):159‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svejgaard E, Jakobsen B, Svejgaard A. Studies of HLA‐ABC and DR antigens in pure atopic dermatitis and atopic dermatitis combined with allergic respiratory disease. Acta Dermato Venereologica Supplementum 1985;114:72‐6. [DOI] [PubMed] [Google Scholar]
- Thestrup‐Pedersen K. Treatment principles of atopic dermatitis. Journal of the European Academy of Dermatology & Venereology 2002;16(1):1‐9. [DOI] [PubMed] [Google Scholar]
- Bever HP. Early events in atopy. European Journal of Pediatrics 2002;161(10):542‐6. [DOI] [PubMed] [Google Scholar]
- Verboom P, Hakkaart‐Van L, Sturkenboom M, Zeeuw R, Menke H, Rutten F. The cost of atopic dermatitis in the Netherlands: an international comparison. British Journal of Dermatology 2002;147(4):716‐24. [DOI] [PubMed] [Google Scholar]
- Williams HC. Is the prevalence of atopic dermatitis increasing?. Clinical & Experimental Dermatology 1992;17(6):385‐91. [DOI] [PubMed] [Google Scholar]
- Williams H, Robertson C, Stewart A, Ait‐Khaled N, Anabwani G, Anderson R, et al. Worldwide variations in the prevalence of symptoms of atopic eczema in the International Study of Asthma and Allergies in Childhood. Journal of Allergy & Clinical Immunology 1999;103(1 Pt 1):125‐38. [DOI] [PubMed] [Google Scholar]
- Zeiger R, Heller S. The development and prediction of atopy in high‐risk children: Follow up at seven years in a prospective randomised study of combined maternal and infant food allergen avoidance. Journal of Allergy and Clinical Immunology 1995;95:1179‐90. [DOI] [PubMed] [Google Scholar]


