Abstract
Background
Undernutrition contributes to five million deaths of children under five each year. Furthermore, throughout the life cycle, undernutrition contributes to increased risk of infection, poor cognitive functioning, chronic disease, and mortality. It is thus important for decision‐makers to have evidence about the effectiveness of nutrition interventions for young children.
Objectives
Primary objective
1. To assess the effectiveness of supplementary feeding interventions, alone or with co‐intervention, for improving the physical and psychosocial health of disadvantaged children aged three months to five years.
Secondary objectives
1. To assess the potential of such programmes to reduce socio‐economic inequalities in undernutrition. 2. To evaluate implementation and to understand how this may impact on outcomes. 3. To determine whether there are any adverse effects of supplementary feeding.
Search methods
We searched CENTRAL, Ovid MEDLINE, PsycINFO, and seven other databases for all available years up to January 2014. We also searched ClinicalTrials.gov and several sources of grey literature. In addition, we searched the reference lists of relevant articles and reviews, and asked experts in the area about ongoing and unpublished trials.
Selection criteria
Randomised controlled trials (RCTs), cluster‐RCTs, controlled clinical trials (CCTs), controlled before‐and‐after studies (CBAs), and interrupted time series (ITS) that provided supplementary food (with or without co‐intervention) to children aged three months to five years, from all countries. Adjunctive treatments, such as nutrition education, were allowed. Controls had to be untreated.
Data collection and analysis
Two or more review authors independently reviewed searches, selected studies for inclusion or exclusion, extracted data, and assessed risk of bias. We conducted meta‐analyses for continuous data using the mean difference (MD) or the standardised mean difference (SMD) with a 95% confidence interval (CI), correcting for clustering if necessary. We analysed studies from low‐ and middle‐income countries and from high‐income countries separately, and RCTs separately from CBAs. We conducted a process evaluation to understand which factors impact on effectiveness.
Main results
We included 32 studies (21 RCTs and 11 CBAs); 26 of these (16 RCTs and 10 CBAs) were in meta‐analyses. More than 50% of the RCTs were judged to have low risk of bias for random selection and incomplete outcome assessment. We judged most RCTS to be unclear for allocation concealment, blinding of outcome assessment, and selective outcome reporting. Because children and parents knew that they were given food, we judged blinding of participants and personnel to be at high risk for all studies.
Growth. Supplementary feeding had positive effects on growth in low‐ and middle‐income countries. Meta‐analysis of the RCTs showed that supplemented children gained an average of 0.12 kg more than controls over six months (95% confidence interval (CI) 0.05 to 0.18, 9 trials, 1057 participants, moderate quality evidence). In the CBAs, the effect was similar; 0.24 kg over a year (95% CI 0.09 to 0.39, 1784 participants, very low quality evidence). In high‐income countries, one RCT found no difference in weight, but in a CBA with 116 Aboriginal children in Australia, the effect on weight was 0.95 kg (95% CI 0.58 to 1.33). For height, meta‐analysis of nine RCTs revealed that supplemented children grew an average of 0.27 cm more over six months than those who were not supplemented (95% CI 0.07 to 0.48, 1463 participants, moderate quality evidence). Meta‐analysis of seven CBAs showed no evidence of an effect (mean difference (MD) 0.52 cm, 95% CI ‐0.07 to 1.10, 7 trials, 1782 participants, very low quality evidence). Meta‐analyses of the RCTs demonstrated benefits for weight‐for‐age z‐scores (WAZ) (MD 0.15, 95% CI 0.05 to 0.24, 8 trials, 1565 participants, moderate quality evidence), and height‐for‐age z‐scores (HAZ) (MD 0.15, 95% CI 0.06 to 0.24, 9 trials, 4638 participants, moderate quality evidence), but not for weight‐for‐height z‐scores MD 0.10 (95% CI ‐0.02 to 0.22, 7 trials, 4176 participants, moderate quality evidence). Meta‐analyses of the CBAs showed no effects on WAZ, HAZ, or WHZ (very low quality evidence). We found moderate positive effects for haemoglobin (SMD 0.49, 95% CI 0.07 to 0.91, 5 trials, 300 participants) in a meta‐analysis of the RCTs.
Psychosocial outcomes. Eight RCTs in low‐ and middle‐income countries assessed psychosocial outcomes. Our meta‐analysis of two studies showed moderate positive effects of feeding on psychomotor development (SMD 0.41, 95% CI 0.10 to 0.72, 178 participants). The evidence of effects on cognitive development was sparse and mixed.
We found evidence of substantial leakage. When feeding was given at home, children benefited from only 36% of the energy in the supplement. However, when the supplementary food was given in day cares or feeding centres, there was less leakage; children took in 85% of the energy provided in the supplement. Supplementary food was generally more effective for younger children (less than two years of age) and for those who were poorer/ less well‐nourished. Results for sex were equivocal. Our results also suggested that feeding programmes which were given in day‐care/feeding centres and those which provided a moderate‐to‐high proportion of the recommended daily intake (% RDI) for energy were more effective.
Authors' conclusions
Feeding programmes for young children in low‐ and middle‐income countries can work, but good implementation is key.
Keywords: Child, Preschool; Female; Humans; Infant; Male; Feeding Methods; Vulnerable Populations; Child Nutritional Physiological Phenomena; Controlled Before‐After Studies; Energy Intake; Malnutrition; Malnutrition/diet therapy; Randomized Controlled Trials as Topic; Sex Factors
Plain language summary
Supplementary feeding for children aged three months to five years: does it work to improve their health and well‐being?
Background
Undernutrition is a cause of child mortality; it contributed to the deaths of more than three million children in 2011. Furthermore, it can lead to higher risk of infection, poorer child development and school performance, and to chronic disease in adulthood. Evidence about the effectiveness of nutrition interventions for young children, therefore, is fundamentally important; not only for governments, funding agencies and nongovernmental organisations, but also for the children themselves.
Review question
How effective are supplementary food programmes for improving the health of disadvantaged children? What factors contribute to the effectiveness of such programmes?
Methods
We included studies that compared children who were given supplementary feeding (food, drink) to those who did not receive any feeding.
We followed careful systematic review methodology, including the use of broad searches. At least two people were involved in every stage of the review. Where possible, we performed analyses to combine results of several studies and get an average effect. We looked carefully for factors that may have impacted on the results (child age, sex and disadvantage, family sharing food, amount of energy given, etc.).
The evidence is current to January 2014.
Study characteristics
We included 32 studies; 21 randomised controlled trials (in which children were randomly assigned to receive either supplementary feeding (intervention group) or not (a control group), and 11 controlled before‐and‐after studies (in which outcomes were observed before and after treatment in a group of children who were not randomly assigned to an intervention and a control group). The number of children in them ranged from 30 to 3166. Most studies were from low‐ and middle‐income countries; three were from high‐income countries.
Key findings
We found that, in low‐ and middle‐income countries, providing additional food to children aged three months to five years led to small gains in weight (0.24 kg a year in both RCTs and CBAs) and height (0.54 cm a year in RCTs only; no evidence of an effect in other study designs),and moderate increases in haemoglobin. We also found positive impacts on psychomotor development (skills that involve mental and muscular activity). We found mixed evidence on effects of supplementary feeding on mental development.
In high‐income countries, two studies found no benefits for growth. The one effective study involved Aboriginal children.
We found that food was often redistributed ('leakage') within the family; when feeding was home‐delivered, children benefited from only 36% of the energy given in the supplement. However, when the supplementary food was given in day care centres or feeding centres, there was much less leakage; children took in 85% of the energy provided in the supplement. When we looked at different groups supplementary food was more effective for younger children (under two years old) and for those who were poorer or less well‐nourished. Results for sex were mixed. Feeding programmes that were well‐supervised and those that provided a greater proportion of required daily food for energy were generally more effective.
Quality of the evidence
We judged evidence from the RCTs to be of moderate quality and evidence from the CBAs to be of low quality.
Summary of findings
Summary of findings for the main comparison. Low‐ and middle‐income countries: Feeding compared to control ‐ growth RCTs for improving the physical and psychosocial health of disadvantaged children aged three months to five years.
| Low‐ and middle‐income countries: Feeding compared to control ‐ growth RCTs for improving the physical and psychosocial health of disadvantaged children aged three months to five years | ||||||
| Participants or population: Low‐ and middle‐income children aged 3 months to 5 years Settings: Low‐ and middle‐income countries Intervention: Feeding Comparison: Control ‐ growth RCTs | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants in meta‐analyses (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control ‐ growth RCT | Low‐ and middle‐income countries: Feeding | |||||
| Weight gain (kg) Follow‐up: 3 ‐ 12 months; average 6 months | Weight change of control group ranged from 0.32 to 2.42 kg | The mean weight gain in the intervention group was 0.12 higher (0.05 to 0.18 higher) | 1057 (9 studies) | ⊕⊕⊕⊝ moderate¹ | ||
| Height gain (cm) Follow‐up: 3 ‐ 12 months; average 6 months | Growth in height of control group ranged from 0.90 to 3.4 cm | The mean height gain in the intervention group was 0.27 cm higher (0.07 to 0.48 higher) | 1463 (9 studies) | ⊕⊕⊕⊝ moderate¹ | ||
| Weight‐for‐age: z‐scores (WAZ) Follow‐up: 3 ‐ 24 months; average 6.5 months | Change in WAZ in the control group ranged from ‐0.30 to 0.98 | The mean change in WAZ in the intervention group was 0.15 higher (0.05 to 0.24 higher) | 1565 (8 studies) | ⊕⊕⊕⊝ moderate¹ | ||
| Height‐for‐age: z‐scores (HAZ) Follow‐up: 3 ‐ 24 months; average 6.5 months | Change in HAZ in the control group ranged from ‐0.84 to 0.11 | The mean change in HAZ in the intervention group was 0.15 higher (0.06 to 0.24 higher) | 4544 (9 studies) | ⊕⊕⊕⊝ moderate¹ | ||
| Weight‐for‐height: z‐scores (WHZ) Follow‐up: 3 ‐ 12 months; average 6.5 months | Change in WHZ in the control group ranged from ‐0.70 to 0.10 | The mean change in WHZ in the intervention group was 0.10 higher (0.02 lower to 0.22 higher) | 4073 (7 studies) | ⊕⊕⊕⊝ moderate¹ | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Risk of bias rated as moderate because most studies lacked blinding and most studies report a completer analysis rather than intention‐to‐treat (ITT)
Summary of findings 2. Low‐ and middle‐income countries: Feeding compared to control. CBAs for improving the physical and psychosocial health of disadvantaged children aged three months to five years.
| Low‐ and middle‐income countries: Feeding compared to control. CBAs for improving the physical and psychosocial health of disadvantaged children aged three months to five years | ||||||
| Participant or population: Children aged 3 months to 5 years Settings: Low‐ and middle‐income countries Intervention: Feeding Comparison: Control ‐ CBAs | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants in meta‐analyses (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control ‐ CBA | Low‐ and middle‐income countries: Feeding | |||||
| Weight gain (kg) Follow‐up: 6 months ‐ 1.8 years; average 1 year | Weight change of control group ranged from 0.5 to 3.93 kg | The mean weight gain (kg) in the intervention group was 0.24 higher (0.09 to 0.39 higher) | 1784 (7 studies) | ⊕⊝⊝⊝ very low¹ | ||
| Height gain (cm) Follow‐up: 6 months ‐ 1.8 years; average 1 year | Growth in height of control group ranged from 1.88 to 20.1 cm | The mean height gain (cm) in the intervention group was 0.52 higher but non‐significant (0.07 lower to 1.10 higher) | 1782 (7 studies) | ⊕⊝⊝⊝ very low¹ | ||
| Weight‐for‐age: z‐scores (WAZ) Follow‐up: 9 ‐ 12 months | Change in WAZ in the control group ranged from ‐0.42 to 0.07 | The mean change in WAZ in the intervention group was 0.27 higher (0.13 lower to 0.68 higher) | 999 (4 studies) | ⊕⊝⊝⊝ very low¹ | ||
| Height‐for‐age: z‐scores (HAZ) Follow‐up: 9 ‐ 12 months | Change in HAZ in the control group ranged from ‐0.82 to 0.26 | There was little mean change in HAZ in the intervention group compared to the control group0.01 higher (0.10 lower to 0.12 higher) | 999 (4 studies) | ⊕⊝⊝⊝ very low¹ | ||
| Weight‐ for‐height: z‐scores (WHZ) Follow‐up: 9 ‐ 12 months | Change in WHZ in the control group ranged from ‐0.92 to ‐0.01 | The mean change in WHZ in the intervention group was 0.29 higher (0.11 lower to 0.69 higher) | 999 (4 studies) | ⊕⊝⊝⊝ very low¹ | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Studies are rated at high risk of bias due to lack of randomisation
Summary of findings 3. Low‐ and middle‐income countries: Feeding compared to control ‐ psychosocial development RCTs for improving the physical and psychosocial health of disadvantaged children aged three months to five years.
| Low‐ and middle‐income countries: Feeding compared to control ‐ psychosocial development RCTs for improving the physical and psychosocial health of disadvantaged children aged three months to five years | ||||||
| Participant or population: Children aged 3 months to 5 years Settings: Low‐ and middle‐income countries Intervention: Feeding Comparison: Control ‐ psychosocial development RCT | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants in meta‐analyses (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control ‐ psychosocial development RCT | Low‐ and middle‐income countries: Feeding | |||||
| Mental Development Index (total) Follow‐up: 3 ‐ 21 months | The mean change in mental development index score for the control group was 15.8 points | The standardised mean mental development index (total) in the intervention group was 0.40 lower (‐0.79 lower to ‐0.00) in one study | 113 (1 study) | ⊕⊕⊕⊝ moderate¹ | ||
| In another study, the standardized mean difference in change in cognitive ability was 0.58 over 21 months of supplementation (0.17 higher to 0.98 higher) | 99 (1 study) | |||||
| One study not included in the meta‐analysis, intervention group was significantlyhigher (F₁, ₁₀₇ = 4.44, P < 0.0) | 107 (1 study) | |||||
|
Psychomotor development Follow‐up: 3 months 6 ‐ 24 months for 4 other studies |
The mean change in psychomotor development index score for the control group was 2.7 points | The standardised mean psychomotor development in the intervention group was 0.41 higher (0.10 higher to 0.72 higher) | 178 (2 studies) | ⊕⊕⊕⊕ Moderate | ||
| Two‐year study: Mean gain in psychomotor development was 6.5 points higher in supplemented group and 13.4 points higher in the supplemented + stimulated group than controls. (Change in control compared to supplemented was ‐6.5 (‐11.1 to ‐1.9) points; change in control compared to supplemented + stimulated was ‐13.4 (‐17.9 to ‐8.8) points | 94 (1 study) | |||||
| One study: No main effect but change‐over‐time contrasts found that after 6 months of treatment, younger children in the experimental group showed significantly less decline on the Bayley Motor score than younger children in the placebo group (F₁,₄₈ = 6.01, P < 0.05). The differences in Bayley Motor Score disappeared at 12 months of intervention | 136; 48 younger children (1 study) | |||||
| One study: Boys who received 2½ years of supplementation beginning at 6 months had better overall scores on the Griffiths Mental Development Scales (GMDS) than those who had no supplementation; this was not true for girls. We could not test significance | 114 in analysis (1 study) | |||||
| One study: non‐significant | 30 (1 study) | |||||
| Follow‐up. 4 years after the end of supplementation | Supplemented and supplemented + stimulated performed better than controls on 14 out of 15 cognitive tests. Supplementation had a significant effect on the perceptual motor factor for children whose mothers had high baseline scores on the Peabody Picture Vocabulary Test (PPVT) | 122 (1 study) | ⊕⊕⊕⊝ moderate¹ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Risk of bias rated as moderate because of lack of blinding and lack of intention‐to‐treat (ITT) analysis
Background
Programmes that provide supplementary food for preschool‐aged children are intended to help address the biggest cause of the global burden of disease: undernutrition (Lopez 2006, p 297). Recent figures indicate that 842 million people globally were chronically undernourished between 2011 and 2013, with the vast majority of them (827 million) in low‐ and middle‐income countries (FAO 2013).
Many of those who are undernourished are children. Child and maternal undernutrition and suboptimal breastfeeding are responsible for about 35% of deaths of children under five years of age, and for 11% of the global burden of disease (Black 2008). Most of this burden falls onto low‐ and middle‐income countries, where 28% and 45% of children are underweight and stunted, respectively (WHO 2013). Most of the child deaths due to undernutrition are preventable (Horton 2008), and yet, distressingly, "Nutrition is a desperately neglected aspect of maternal, newborn, and child health" (Horton 2008, p 179).
Poverty and undernutrition are closely linked (Haddad 2000), with poverty as "the leading cause of hunger" (World Hunger Education Service 2012). In the 1990s, the percentage of underweight preschoolers declined sharply as gross domestic production rose (Haddad 2000). In high‐income countries, such as Canada (ONPP 2004) and the United States (Nord 2010), household food insecurity is strongly associated with low income.
Description of the condition
"Undernutrition is the outcome of insufficient food intake and repeated infectious diseases. It includes being underweight for one’s age, too short for one’s age (stunted), dangerously thin for one’s height (wasted) and deficient in vitamins and minerals (micronutrient malnutrition)” (UNICEF 2006). Throughout the life cycle, undernutrition contributes to increased risk of infection, lowered cognitive performance, chronic disease in adulthood, and mortality (United Nations ACC/SCN 2000). The consequences of undernutrition in early childhood are particularly severe; both physical and intellectual development may be affected (Ivanovic 2004; Petrou 2010). The main causes of child deaths are diarrhoea, pneumonia, malaria, measles, AIDS, and perinatal conditions; undernutrition is an underlying cause for most of these (Black 2003a; Black 2003b; Caulfield 2004). Zinc deficiency, for example, contributes to child morbidity and mortality through increased prevalence and severity of diarrhoea and pneumonia (Jones 2003). In turn, severe illness may lead to appetite loss, metabolic changes, and behavioural changes (Tomkins 1989), thus worsening nutritional status and may increase the risk of more prolonged or severe illness episodes (Fishman 2003). Early and persistent undernutrition may cause permanent changes in physiology, metabolism, and endocrine function (Barker 2001; Prentice 2005); it has been increasingly linked to chronic diseases, including obesity, stroke, and coronary heart disease (Barker 1992; Barker 2001; Caballero 2001; Gaskin 2000; Hoffman 2000; López‐Jaramillo 2008; Prentice 2005). Undernutrition also increases the risk of mortality from disease (Shankar 2000).
Although the brain continues to grow throughout childhood, the period between birth and three years of age is a time of particularly rapid growth. During these years, the brain is very sensitive to factors that can inhibit brain growth and cognitive development, such as protein‐energy malnutrition or micronutrient deficiency (Tanner 2002). Although it is sometimes difficult to disentangle the effects of undernutrition from other deprivations to which children living in poverty are exposed, early undernutrition is linked to lowered cognitive functioning and poorer school performance (Alderman 2004; Grantham‐McGregor 2007; Schrimshaw 1998; Tanner 2002; Worobey 1999). In the short term, skipping breakfast can lower performance on memory and verbal fluency tasks (Pollitt 1998). Animal studies show that malnutrition leads to changes in motivation, emotionality, and anxiety (Strupp 1995; Walker 2007). These effects may limit a child’s capacity to interact with his or her environment and to learn from these interactions (Beaton 1993; Pollitt 1994; Walker 2007). Chronic malnutrition in early childhood may result in partially irreversible structural and functional brain changes (Morgane 2002). Maternal, foetal, and early childhood undernutrition is also linked to lower educational attainment and lower economic productivity in later life (Grantham‐McGregor 2007; Victora 2008).
Description of the intervention
Supplementary feeding involves provision of energy (with nutrients or micronutrients or both) through food (meals/snacks) or beverage to children to ameliorate or prevent undernutrition. This may be given in preschool, day care, or community settings; take‐home or home‐delivered rations are also included. Programme goals generally include one or more of the following: improved survival, prevention or amelioration of growth failure, lowered morbidity, and promotion of normal cognitive and behavioural development (Beaton 1993). Figure 1 provides an overview of the interventions eligible for inclusion in this review.
1.
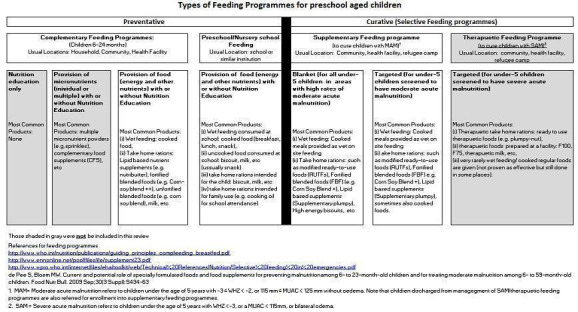
Types of feeding programs in the preschool review
How the intervention might work
It is important to intervene in early childhood to maximise developmental potential and lifelong health (Power 1997). Supplementary feeding for disadvantaged young children is designed to accomplish this. According to Beaton 1982, feeding programmes are usually designed to meet 40% to 70% of the estimated energy gap and should exist alongside usual home meals. The food or beverage may improve growth and micronutrient status by providing additional energy, macronutrients, and micronutrients; it may also boost immune status and reduce the risk of infection (Barker 2001; Prentice 2005; Schrimshaw 1998). The energy, nutrients, and micronutrients given may also improve motivation and psychosocial health, including cognitive functions such as intelligence, attention, psychomotor skills, language,and visuospatial skills. Nutrition can influence the development and function of a young child’s brain through several mechanisms: development of brain structure, including increased brain volume (Ivanovic 2004), myelination, and neurotransmitter operation (Tanner 2002; Wachs 2000). Feeding may also improve social behaviour, through increased interaction with the world, improved emotional state, and lowered anxiety (Barrett 1985). Increased social interaction may, in turn, enhance cognitive functioning and learning. Better nutrition in the first two years of life is associated with achieving a higher level of schooling (Martorell 2010; Victora 2008).
Several factors may affect intervention success. The amount of energy given and the macronutrient and micronutrient composition of the food are critical for achieving adequate growth and meeting physiological needs (Allen 1994; Beaton 1982; Rivera 1991; Rush 1998). The child's age may also be important; effects on growth, particularly linear growth, may be most pronounced for children aged two years and under (Dewey 2008; Schroeder 1995). Substitution and ration‐sharing can be a problem in both take‐home and on‐site feeding programmes (ACC/SCN 1993; Engle 1992b). In take‐home feeding programmes, only 40% to 60% of the food distributed appeared to reach targeted children, with the remainder either consumed by other family members or sold (Beaton 1982).
There is a dearth of research on effectiveness by socio‐economic status; however, some research has shown that feeding may be more effective for the most undernourished (typically very poor) young (Beaton 1982) and school‐aged children (Kristjansson 2007). Related to this, and based on their finding of different patterns of socio‐economic inequalities in stunting, Van de Poel 2008 suggested that, in countries with mass deprivation, a universal approach be used, while in situations of exclusion, targeted approaches should be used to improve the health of the poorest children.
However, despite the obvious benefits (reductions in underweight and wasting), supplementary feeding programmes in a few low‐ and middle‐income countries, particularly in Latin America, may be contributing to a slight rise in obesity prevalence (Kain 1998; Uauy 2001).
Our conceptual model of mechanisms through which supplementary feeding may or may not work is in Figure 2.
2.
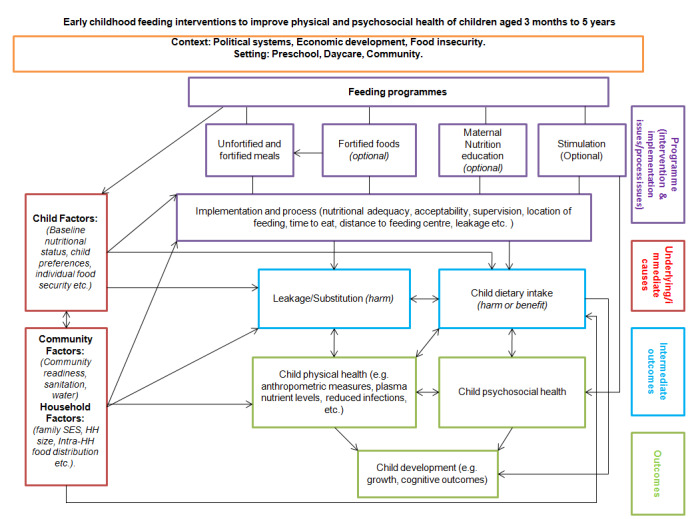
Conceptual model for feeding interventions to improve physical and psychosocial health of children aged two months to five years
Footnotes
SES ‐ socio‐economic status
HH ‐ household
Why it is important to do this review
Child undernutrition is a major global health issue that is responsible for lost potential, morbidity, and death. Thus, we need good evidence on which interventions work to reduce childhood undernutrition, and how and why they work. Systematic reviews on supplementary feeding for preschool‐aged children are especially timely in an era when governments and leading international organisations are placing increasing emphasis on evidence‐based strategies to improve the health of the poor. It is important for governments and non‐governmental organisations (NGOs) to have evidence about these programmes in order to make important decisions about the distribution of scarce resources (Irwin 2007).
Our review addresses important evidence gaps in the following ways: first, it is broad; it includes controlled before‐and‐after (CBA) studies, controlled clinical trials (CCTs), and interrupted time series (ITS). This is because it is increasingly recognised that reviews containing study designs other than randomised controlled trials (RCTs) are advantageous for capturing important population‐level (or population health) interventions (Ogilvie 2005; Tugwell 2010). Second, we used a rigorous process evaluation to elucidate pertinent information on factors that impact on effectiveness. Finally, we assessed the effect of the intervention on many outcomes, including physical and psychosocial development, physical activity, and infectious diseases. Thus our review may help to address one of the evidence gaps identified by Bhutta 2008; the lack of evidence about whether adverse effects of undernutrition on cognition and infectious disease may be ameliorated.
Objectives
Primary objective
To assess the effectiveness of supplementary feeding interventions, alone or with co‐intervention, for improving the physical and psychosocial health of disadvantaged children aged three months to five years.
Secondary objectives
To assess the potential of such programmes to reduce socio‐economic inequalities in undernutrition.
To evaluate implementation and to understand how this may impact on outcomes.
To determine whether there are any adverse effects of supplementary feeding.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs), clustered RCTs (c‐RCTs), controlled clinical trials (CCT), controlled before‐and‐after (CBA) studies, and interrupted time series (ITS; with three time points before and three after the intervention, with or without a control group) were eligible for inclusion in this review.
We also accepted RCTs with stepped‐wedge designs (treatments begun at different times for different groups of participants). In these cases, our baseline was the time at which the 'treated group' (longest treatment) began treatment and our endpoint was the point at which the 'control group' began treatment. We excluded all other study types.
Types of participants
Children aged three months to five years were eligible, from all countries of the world. We divided countries into low‐ and middle‐income and high‐income; classification was based on the 2011 World Bank List of Country Economies (World Bank 2011). Low‐ and middle‐income countries include those which the World Bank classified as low income, (USD 1035 Gross National Income (GNI) per capita or less) and lower middle‐income (USD 1036 to USD 4085 GNI per capita) countries. High‐income countries include both upper middle‐income (USD 4086 to USD 12,615 GNI per capita) and high‐income (USD 12,616 GNI per capita or more) countries. We analysed results separately for low‐ and middle‐income countries and high‐income countries.
Studies had to comprise children from:
Socio‐economically disadvantaged groups; OR
All socio‐economic groups if results are or can be stratified by some indicator of socio‐economic status (for example, high or low income, high or low education, rural or urban).
Studies also had to follow the same children.
Definition of socio‐economic disadvantage for low‐ and middle‐income countries and high‐income countries:
Low‐ and middle‐income countries: from rural areas, villages, provinces, or deprived urban areas OR parents have low average education (primary school or below) OR parents were manual workers (including small farmers) or unemployed OR families were materially disadvantaged or of low socio‐economic status (SES) OR children were described as low‐income, malnourished, undernourished, underweight or stunted.
High‐income countries: families or children described as low SES, low income, low education (high school or below), or from low‐income areas (ghettos).
We excluded severely malnourished children (those with a weight‐for‐height (WfH) z‐score of three standard deviations (SDs) or more below the mean). We also excluded studies that focused exclusively on children with diagnosed illnesses (e.g. HIV) or that fed children in emergency and refugee settings. Finally, we excluded interventions that provided supplementary food or drink to mothers in the prenatal period.
Types of interventions
Provision of energy and macronutrients through:
Hot or cold meals (breakfast or lunch);
Snacks (including both food and beverages such as milk or milk substitutes);
Meals or snacks in combination with take‐home rations;
Take‐home rations.
Studies had to compare children who received feeding (with or without co‐intervention such as maternal education) to a no‐feeding control. We accepted either no‐treatment controls (no feeding) or placebo controls (e.g. low‐energy foods (less than 5% of the energy provided by the intervention) or drinks (without fortification)). For example, a low‐energy, unfortified (e.g. 30 kcal) drink was acceptable as a control.
We excluded food stamps, food banks, and modifications to meals to lower the energy, fat or sodium content. We also excluded therapeutic feeding designed for children with severe acute malnutrition and illness. Feeding could not take place in a hospital setting.
Figure 1 shows the types of feeding programmes included in the review.
Types of outcome measures
The outcomes in this review cover both physical health and psychosocial health (including behaviour).
Primary outcomes
Physical health
Growth (weight, height, weight‐for‐age, height‐for‐age, weight‐for‐height).
Psychosocial health
Psychomotor development (the progressive attainment of skills that involve both mental and muscular activity; e.g. the ability to turn over, crawl, and walk).
Cognitive development or mental development (development of thought processes, including memory, reasoning, information processing, intelligence (the ability to learn or understand or deal with new or trying situations), and language).
Attention (the ability to apply one's mind to something or the condition of readiness for attention, including a selective narrowing of consciousness).
Language (the ability to comprehend receptive language and apply expressive language to communicate).
Memory (the ability to recover information about past events or knowledge).
Adverse effects
Substitution or leakage (the family cuts home rations for the child who has been fed in order to spread food to other family members, or shares the child's supplementary rations with other family members).
We used primary outcomes in physical health and psychosocial health to populate the 'Summary of findings' tables.
Secondary outcomes
Physical health
Biochemical markers of nutrition (Vitamin A, haemoglobin, hematocrit).
Physical activity (body movements that work muscles and require more energy than resting, for example, running, jumping, playing ball, walking around school yard).
Morbidity (physician diagnosis of acute illness such as pneumonia, diarrhoea, malaria).
Mortality (death).
Overweight or obesity (adverse outcome).
Psychosocial outcomes
Stigmitisation (adverse effect, involves being shamed).
Behaviour problems (aggression, disruptive behaviour).
Where possible, we extracted data on cost and resource use.
We excluded reduction of dental caries and increased nutritional knowledge (although the latter was included in the data extraction form to help elucidate findings). We also excluded intermediate health outcomes such as reduction of hunger and nutrient intake.
For cognitive and behavioural outcomes, we accepted reliable and valid psychometric measures (e.g. Wechsler Intelligence Scale for Children (WISC), Raven's Progressive Matrices (RPM)). For physical outcomes, we accepted clinical measures of growth (e.g. length or height boards, digital or balance beam weighing scales, skinfold thickness, mid‐upper‐arm circumference (MUAC)), biochemical nutritional status (e.g. blood tests), and morbidity (diagnosis by physician).
Equity outcomes
To assess equity, we conducted subgroup analyses, examining results that compared boys to girls and poor (or more undernourished) to less poor.
Search methods for identification of studies
Electronic searches
We ran the initial searches in July 2011, and updated them most recently on 28 January 2014 (Appendix 1), except where stated otherwise. We did not apply any date or language limits.
Cochrane Central Register of Controlled Studies (CENTRAL), 2014, Issue 1, part of The Cochrane Library.
Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE(R) 1946 to present.
Cochrane Database of Systematic Reviews (CDSR), 2014 Issue 1, part of The Cochrane Library.
Database of Abstracts of Reviews of Effects (DARE), 2014 Issue 1, part of The Cochrane Library.
Social Sciences Citation Index (SSCI) (Web of Science) 1970 to the present.
Conference Proceedings Citation Index‐Science (CPCI‐S) (Web of Science) 1990 to the present.
Conference Proceedings Citation Index‐Social Science & Humanities (CPCI‐SSH) (Web of Science) 1990 to the present.
ERIC – Education Resources Information Centre via Proquest, 1994 to the present.
Proquest Dissertations and Theses.
PsycINFO (Ovid) 1806 to January Week 3 2014.
Clinicaltrials.gov (clinicaltrials.gov/).
Searches last updated 3 May 2012 (Appendix 2)
EMBASE Classic and EMBASE (OVID) 1947 to 1 May 2012.
CINAHL (EBSCOhost) 1981 to 3 May 2012.
Healthstar (OVID) 1966 to 3 May 2012.
LILACS Last searched 10 May 2012.
Searches last updated 5 July 2011 (Appendix 3)
Social Services Abstracts (CSA).
Searching other resources
We searched the following grey literature sources:
OpenGrey (www.opengrey.eu/). Accessed: January 2014.
WHOLIS (dosei.who.int/uhtbin/cgisirsi/Wed+May+21+19:32:01+MEST+2014/0/49). Accessed: January 2014.
WHO nutrition databases (www.who.int/nutrition/databases/en/). Accessed: January 2014.
We sought information about ongoing and unpublished trials through members of our advisory panel of experts in nutrition and child development. We also scanned the references of included articles, relevant reviews, and annotated bibliographies for eligible studies, and searched the websites of selected development agencies or research firms (IDEAS: ideas.repec.org/, IFPRI www.ifpri.org/; JOLIS/World Bank: external.worldbankimflib.org/external.htm; NBER: www.nber.org/, USAID; www.usaid.gov/) in January 2014.
Data collection and analysis
Selection of studies
At least two review authors (SL, BK, DF), working independently, scanned all titles and abstracts of articles retrieved by the searches. One of the review authors retrieved copies of all those deemed eligible. Two review authors (SL and EK) reviewed the full text of all retrieved studies against the inclusion and exclusion criteria, with disagreements settled by a third author (DF).
The team comprised review authors fluent in Portuguese, Spanish, French, and English, and we were therefore able to assess articles written in these languages.
Data extraction and management
Four people (MBJ, SL, DF, and KM), working in pairs, extracted data. They compared their work and resolved discrepancies. We pilot‐tested the data extraction form on two studies by having these four review authors extract data and compare extractions.
Our data extraction forms were based on the data collection forms from the Cochrane Effective Practice and Organisation of Care (EPOC) review group (EPOC 2012) modified for this review. We extracted data on study design, description of the intervention (including process), details about participants (including number in each group, age, and socio‐economic status), length of intervention and follow‐up, definition of disadvantage, all primary and secondary outcomes, the process factors listed below, costs and resource use, risk of bias, and statistical analysis. Where possible, we recorded effects by socio‐economic status, geographic location, gender, race or ethnicity, and age.
Process evaluation
We assessed the following process elements (list modified from Arblaster 1996 and Kristjansson 2007, and based on our knowledge of the literature and our conceptual model).
Type of meal.
Energy provided, % of the dietary reference intake (DRI), and level of nutritional adequacy.
Multifaceted approaches (were other supports (nutrition education etc.) used in addition to providing food?).
Where the food was given: preschool, day care, community, home‐delivered, take‐home.
Agent administering the intervention (e.g. community, government).
Agent delivering intervention (e.g. mother, healthcare worker, day‐care worker).
Provision of material support (was food provided free of charge or for a reduced price according to income?).
Type of food given.
Control treatment.
Supervision: whether or not intake was monitored. Categorised into low, moderate, and strict (see below).
Total net energy intake of experimental and control participants. The comparison of this to energy given in the supplement allowed us to assess leakage.
Implementation fidelity.
Nutritional adequacy
A nutritionist (SL) assessed the nutritional adequacy of the meals provided to the children. Two other nutritionists (DF and MB) helped to develop the approach.
Methodology for calculating energy content, protein content, % DRI:
Energy: when the total kilocalories or % DRI of energy were provided in the text of the study, we used this figure. When this information was not provided but the descriptions of food were sufficient (quantity and type of food), we estimated energy content (kilocalories) of the meal or snack using the Food and Agriculture Organization (FAO) international food composition table.
Calculating % DRI for energy: we calculated the % DRI for energy by dividing the given or estimated average kilocalorie content of the meal or snack by the DRI for the age‐ or sex‐specific target group in each study. For children aged three years and older, we identified the estimated energy requirement assuming an active physical activity level. When the intervention group comprised different age and sex groups, and outcomes were given for the entire group only, we used a weighted average of the % DRI for each group to calculate the overall % DRI. When the number of boys and girls was not reported, we assumed that equal proportions took part in the study, and estimated an average DRI for both sexes.
Categorisation of the level of energy in the supplementary food: we categorised % DRI for energy into three levels: low (0 to 29%), moderate (30 to 59%), and high (60% and above). We used this categorisation in subgroup analyses. When different levels of energy were provided in one study, we used the highest level of energy to categorise the level of energy provided. When the same amount of food was provided to different age groups, we based calculations on the oldest age groups, as these had the highest energy requirements.
Protein: when the total protein or % DRI of protein was provided in the text of the study, we used this figure. When the amount of protein was not provided but the descriptions of food were sufficient (quantity and type of food), we estimated the protein content of the meal or snack using the FAO international food composition table.
Calculating % DRI for protein: we calculated the % DRI for protein by dividing the average protein content of the meal or snack by the DRI for the age‐ or sex‐specific target group in each study (DRI from Health Canada). DRI for protein is given in g/kg/d, and weight provided in the study was used to calculate DRI. When weight was not provided in the study, we considered World Health Organization (WHO) weight (average of boys and girls) to estimate the DRI.
Assessing leakage
Where possible, we used information on the energy content in the supplement as well as information on the reported energy intakes of the experimental and control children to calculate the net benefit that the children actually received from the supplement. We calculated this as follows: (Difference in energy intake between experimental and control at end of study) / total energy content of the supplement.
Level of supervision
We divided the studies into strict versus moderate versus low supervision (i.e. monitoring) of the supplementary feeding intake in the following manner.
Strict supervision. To be categorised as strictly supervised, the feeding had to be:
In day cares, preschools or feeding centres; OR
At home, with visits every two weeks (at least) AND collection of food packets or questions to parents, or both.
Moderate supervision. We characterised studies as moderately supervised if they:
Provided monthly home visits; OR
Delivered rations every week or every two weeks, but did not ask mothers about consumption.
Low supervision. We characterised studies as low supervision if they provided fewer than monthly home visits.
Organization of process findings
We created an EXCEL file that contained process elements for all studies. The studies were in rows, and the columns contained: type of study, cluster or not, whether it was corrected for clustering, setting, country, feeding duration, the final 'n' rate of attrition, whether the intervention was single or multiple, the type of food and energy provided, programme delivery site, level of supervision, and the outcome measures covered.
We performed subgroup analyses by factors that could impact on effectiveness, including child's age, sex and income level, nutritional adequacy of supplement, level of supervision, location of feeding, and single versus multiple interventions.
Assessment of risk of bias in included studies
Two review authors (EK and BS) independently assessed the risk of bias for most studies; EK and SL did this for a few of the later studies.
We used the Cochrane 'Risk of bias' tool (Higgins 2011b) to assess risk of bias in RCTs and c‐RCTs; there were no CCTs. Each component is covered by one or more items, and a dictionary gives thorough definitions for each item. Most items are scored as 'high risk', 'low risk' or 'unclear risk'. We gave component ratings, but did not give an overall rating. For CBAs, we used the 'Risk of bias' tool from the Cochrane EPOC group (EPOC 2009), in addition to the domains covered by the Cochrane 'Risk of bias' tool ‐ allocation, blinding, incomplete outcome data, selective reporting, and other risks of bias. See Table 4 ‐ it includes similarity of baseline outcome measurement, similarity of baseline characteristics, and protection against contamination.
1. Risk of bias domains and criteria for judgement*.
| Risk of bias domain | Criteria for judgement |
| 1. Was the allocation sequence adequately generated? | Score “Low risk” if a random component in the sequence generation process is described (e.g. Referring to a random number table). Score "High risk” when a nonrandom method is used (e.g. performed by date of admission). NRCTs and CBA studies should be scored “High risk”. Score “Unclear risk” if not specified in the paper |
| 2. Was allocation concealed | Score “Low risk” if the unit of allocation was by institution, team or professional and allocation was performed on all units at the start of the study; or if the unit of allocation was by patient or episode of care and there was some form of centralised randomisation scheme, an on‐site computer system or sealed opaque envelopes were used. CBA studies should be scored “High risk". Score “Unclear risk” if not specified in the paper |
| 3. Were baseline outcome measurements similar? | Score “Low risk” if performance or patient outcomes were measured prior to the intervention, and no important differences were present across study groups. In RCTs, score “Low risk” if imbalanced but appropriate adjusted analysis was performed (e.g. Analysis of covariance). Score “High risk” if important differences were present and not adjusted for in analysis. If RCTs have no baseline measure of outcome, score “Unclear risk” |
| 4. Were baseline characteristics similar? | Score “Low risk” if baseline characteristics of the study and control providers are reported and similar. Score “Unclear risk” if it is not clear in the paper (e.g. characteristics are mentioned in text but no data were presented). Score “High risk” if there is no report of characteristics in text or tables or if there are differences between control and intervention providers. Note that in some cases imbalance in patient characteristics may be due to recruitment bias whereby the provider was responsible for recruiting patients into the trial |
| 5. Were incomplete outcome data adequately addressed? | Score “Low risk” if missing outcome measures were unlikely to bias the results (e.g. the proportion of missing data was similar in the intervention and control groups or the proportion of missing data was less than the effect size i.e. unlikely to overturn the study result). Score “High risk” if missing outcome data was likely to bias the results. Score “Unclear risk” if not specified in the paper (Do not assume 100% follow up unless stated explicitly) |
| 6. Was knowledge of the allocated interventions adequately prevented during the study? | Score “Low risk” if the authors state explicitly that the primary outcome variables were assessed blindly, or the outcomes are objective, e.g. length of hospital stay. Primary outcomes are those variables that correspond to the primary hypothesis or question as defined by the authors. Score “High risk” if the outcomes were not assessed blindly. Score “Unclear risk” if not specified in the paper |
| 7. Was the study adequately protected against contamination? | Score “Low risk” if allocation was by community, institution or practice and it is unlikely that the control group received the intervention. Score “High risk” if it is likely that the control group received the intervention (e.g. if patients rather than professionals were randomised). Score “Unclear risk” if professionals were allocated within a clinic or practice and it is possible that communication between intervention and control professionals could have occurred (e.g. physicians within practices were allocated to intervention or control) |
| 8. Was the study free from selective outcome reporting? | Score “Low risk” if there is no evidence that outcomes were selectively reported (e.g. all relevant outcomes in the methods section are reported in the results section). Score “High risk” if some important outcomes are subsequently omitted from the results. Score “Unclear risk” if not specified in the paper |
| 9. Was the study free from other risks of bias? | Score “Low risk” if there is no evidence of other risk of biases |
| 10. Were participants unaware of allocation? | Score " Low risk" if control participants were given a placebo. Score "Unclear risk" if it is hard to tell. Score "High risk" if participants were aware of the allocation, even if this could not be prevented |
Domains one to nine taken directly from: EPOC risk of bias criteria. We added the tenth domain.
We included 'Risk of bias' assessments for the RCTs and CBAs in the 'Risk of bias' tables, beneath the 'Characteristics of included studies' tables.
Measures of treatment effect
We performed statistical analyses using Review Manager 5 (RevMan) (Review Manager 2012).
Continuous data
If continuous outcomes were measured identically across studies, we calculated an overall mean difference (MD) and 95% confidence interval (CI). If the same continuous outcome was measured differently across studies, we calculated an overall standardised mean difference (SMD) and 95% CI (Higgins 2011a).
We analysed continuous data from means and standard deviations wherever possible. When means and standard deviations were not reported, we used other available data (for example, confidence intervals, T values, P values) and appropriate methods as described in the Cochrane Handbook for Systematic Reviews of Interventions (Cochrane Handbook, Section 9.4.5, Higgins 2011b) to calculate the means and standard deviations, in consultation with our statistician. Where other available data were not sufficient to calculate standard deviations, we contacted the trial authors.
Change data
We used change data in all analyses. Data were either taken directly from the papers or calculated from other information presented. When we calculated change scores, we used means and standard deviation from baseline and end‐of‐study according to the methods described in section 16.1 of the Cochrane Handbook (Higgins 2011b). We used before‐and‐after correlations of 0.9 for height, weight, height‐for‐age z‐score (HAZ), weight‐for‐age z‐score (WAZ), and weight‐for‐height z‐score (WHZ). These correlations for growth are based on those provided by Zhang 2006 [pers comm]. For mental and psychomotor development, we used correlations of 0.71 and 0.69. We took these correlations from a publication on test‐retest reliability of the Bayley Scales of Infant Development (BSID, Cook 1989).
When studies provided insufficient data to calculate an effect estimate, we selected regression analyses, multilevel analyses, or analyses of variance (ANOVA) as providing the better estimate of effect because: (a) multilevel analyses account for clustering, and (b) other ANOVAs and regressions provided results that were corrected for important covariates.
We conducted separate meta‐analyses for RCTS and CBAs and, within those, for each outcome. We also separated low‐ and middle‐income countries and high‐income countries, as the two settings are very different in terms of the prevalence and severity of undernutrition; they also differ in many other ways, including political climate, traditions, and food delivery mechanisms.
Within each outcome, we assessed whether the tests used to assess that income were conceptually similar; in cases where the tasks covered by the test were too different, we did not combine them in a meta‐analysis.
Unit of analysis issues
Methods of analysis for cluster‐randomised trials
Studies allocated by village, neighbourhood, or day care could have unit of analysis errors if they did not adjust for between‐cluster correlations. Where trials used clustered allocation, we determined whether or not they had controlled appropriately for clustering (e.g. variance‐inflated standard errors, hierarchical linear models). If they had used appropriate methods, we used these data in our analyses. If they had not, we corrected for clustering where possible. Table 5 provides a summary of clustered studies.
2. Summary of studies with clustered design.
| RCTs | ||
| Study | Adjusted clustering appropriately? | Our adjustments |
| Fauveau 1992 | No | Not corrected because no standard deviations. Not in meta‐analysis. Reported narratively |
| Husaini 1991 | No | Cluster size: intervention = 7, control = 5. Used ICC of 0.025 for weight and length, used 0.15 for psychosocial outcomes |
| Isanaka 2009 | Yes | Not applicable |
| De Romana 2000 | No | Not corrected because there were no standard deviations. Not in meta‐analysis. Reported narratively |
| McKay 1978 | No | Cluster size: intervention = 16 and control = 16. Used ICC of 0.15 for psychological outcomes |
| Pollitt 2000a | No | Cluster size: intervention = 6 and control = 6. Used ICC of 0.025 for weight. For psychosocial outcomes, did not correct for clustering as did not have the appropriate data. Used ANOVAs from the papers as they controlled for covariates |
| Rivera 2004 | Yes | Not applicable |
| Roy 2005 | Yes | Not applicable |
| CBAs | ||
| Study | Adjusted clustering appropriately? | Our adjustments |
| Coyne 1980 | No | Cluster size: intervention = 15 and control = 9. Used ICC of 0.025 for weight and length |
| Devadas 1971 | No | Cluster size: intervention = 25 and control = 25. Used ICC of 0.025 for weight and length |
| Gershoff 1988 | No | Cluster size: 43 in intervention and control groups. Used ICC of 0.025 for weight and length |
| Joshi 1988 | No | Adjusted for clustering for % of children who improved nutritional status (reported narratively as outcome couldn't be combined with other). Cluster size 50 in intervention group and 42 in control group. Used ICC of 0.025 |
| Lutter 2008 | Yes, but the numbers we used were not adjusted | Cluster size: intervention = 17 and control = 25. Used ICC of 0.025 |
| Santos 2005 | Yes | Not applicable |
| Schroeder 2002 | No | Cluster size: 20 Used ICC of 0.025 for weight and length |
| Tomedi 2012 | Yes | Not applicable |
CBAs = controlled before‐and‐after trials ICC = intraclass correlation coefficient RCTs = randomised controlled trials
Methods used to correct for design effect in clustered trials or CBAs that were not adjusted for clustering
When we used a standardised mean difference (SMD) as the pooled estimate (because of varying metrics), we applied the methods outlined in Section 16.3 of the Cochrane Handbook (Higgins 2011b) to inflate the standard error. First, we calculated the unadjusted SMD and 95% confidence interval. We entered the unadjusted SMD as the effect estimate in the generic inverse variance method, and then we inflated the standard error of the effect estimate by multiplying by the square root of the variance inflation factor, calculated as: 1 + ((M ‐ 1) multiplied by ICC), where M is the average cluster size. We calculated the standard error as the confidence interval divided by 3.92.
When the pooled estimate was the mean difference (MD), we used the variance inflation factor (VIF) to adjust the standard deviations in the treatment and control groups separately. We then used these standard deviations in the meta‐analysis, and so incorporated them in the standard error of the mean difference and the weighting procedures. The result of this analysis is equivalent to the method outlined in the Cochrane Handbook when the variance inflation factors are the same in the treatment and control groups.
We used this approach because final cluster sizes often differed between the treatment and control groups and therefore the VIF, which depends on cluster size, would be different. As far as we know, the Cochrane Handbook does not provide for this eventuality.
Calculating the variance inflation factor
First, we calculated cluster size. When the number of participants in each analysis was provided, we divided this by the number of clusters to calculate cluster size. Otherwise, we used the number of participants provided in the Methods sections of the primary studies divided by the number of clusters.
-
Next, we found appropriate intra‐cluster correlation coefficients (ICCs).
For growth outcomes (weight, height, WAZ, HAZ, WHZ), we used ICCs of 0.025; these were based on those published in Du's 2005 letter to the editor of the British Journal of Nutrition (Du 2005). We conducted sensitivity analyses with ICCs of 0.10.
For the psychosocial outcomes, we used ICCs of 0.15, with sensitivity analyses at 0.20. These were based on the Schochet report (Schochet 2005) for maths and reading.
Then, for experimental and control groups separately, we calculated the VIF as follows:
1+ ((M ‐ 1) multiplied by ICC), where M is the average cluster size (Ukoumunne 1999). We then multiplied the original standard deviation by the square root of the VIF for experimental and control groups separately. We then entered these adjusted standard deviations into the Review Manager 5 data tables, combining them with estimates from individual level trials.
Dealing with missing data
Where possible (e.g. studies conducted after 1995), we contacted trial authors to supply any missing or unreported data such as group means, standard deviations, details of attrition or details of interventions received by the control groups. We describe missing data and attrition for each included study in the Characteristics of included studies tables.
Assessment of heterogeneity
We considered clinical (variation in participants, interventions, outcomes) and methodological (i.e. study design, risk of bias) heterogeneity as well as statistical heterogeneity. We assessed statistical heterogeneity using a standard Chi² test to assess whether observed differences in results were compatible with chance alone. We used the I² test to assess the impact of heterogeneity on the meta‐analysis. It shows the percentage of variability in effect estimates that are due to heterogeneity rather than to chance; values over 75% indicate a high level of heterogeneity (Higgins 2003).
If heterogeneity existed, we examined potential sources.
We obtained an estimate of the between‐studies variance component (Tau²) through a random‐effects meta‐analysis.
Assessment of reporting biases
We had planned to draw funnel plots to assess the presence of possible publication bias, as well as the relationship between effect size and study precision, but did not have the recommended minimum number of studies (10) for any analysis (see Differences between protocol and review).
Data synthesis
We conducted separate meta‐analyses for RCTS and CBAs. If we could not conduct meta‐analysis, we reported studies narratively.
In cases where studies provided insufficient data for meta‐analysis, we selected analyses of variance (ANOVA) as providing the better estimate of effect because they corrected for important covariates. We included one regression in a meta‐analysis using the generic inverse variance method. Grantham‐McGregor 1991 presented the regression coefficients of contrasts between groups over 24 months. We considered this contrast an effect size, and calculated the standard deviation from the 95% confidence limits, then calculated the standardized mean difference by dividing by the standard deviation, using the formulae provided in the Cochrane Handbook (Higgins 2011a). We entered this standardized mean difference into the generic inverse variance analysis to allow pooling with Husaini 1991, which also measured psychomotor development, with a different scale.
Randomised controlled trials (RCTs), cluster‐randomised controlled trials (c‐RCTs), controlled before‐and‐after studies (CBAs)
For continuous data, we incorporated data on means, standard deviations, and the number of participants for each outcome in the two groups. While we did not adjust these means and standard deviations for confounders, we adjusted them for clustering when needed.
In performing our meta‐analyses, we used the inverse‐variance random‐effects model. We calculated SMDs using Hedges g, taking the direction of effect into account. Following the Cochrane Handbook (Section 9.2.3.2), we interpreted results using clinical as well as statistical significance.
We compared the most intensive intervention (e.g. highest energy, co‐intervention) to a non‐intervention control. We also entered comparisons between baseline and the end of the feeding.
Interrupted time series (ITS)
We did not have any ITS studies in this review. However, should we find any suitable ITS studies in future updates, we will analyse them according to the methods in Appendix 4 (see also Kristjansson 2007).
'Summary of findings' tables
We constructed 'Summary of findings' tables and rated the quality of evidence using GRADE (Grades of Recommendation, Assessment, Development and Evaluation) (Guyatt 2011) for all primary outcomes in physical and psychological health. GRADE categorises the quality of the evidence as high, moderate, low, or very low. Randomised controlled trials start out at high or medium quality, and observational studies (including CBAs) are low or very low. Evidence from RCTs is downgraded if there is a high risk of bias across studies, if results are inconsistent or imprecise or in the presence of publication bias. Observational studies with no limitations can be upgraded if there is a large magnitude of effect, dose‐response or if plausible confounders would have reduced the effect.
Subgroup analysis and investigation of heterogeneity
We had planned to conduct subgroup analyses across six categories (Kristjansson 2012).
Age: three months to two years versus greater than two years to five years.
Sex: male versus female.
Socio‐economically disadvantaged: more versus less.
Undernourished (1 SD below mean) versus normal weight. We are using this definition as participants in the sample are limited in the range of underweight they will exhibit (none below ‐3). This will give us a reasonable proportion in each group.
Percentage of daily requirements for energy provided (less than 15%, 15% to 30%, 30% to 50%, above 50%).
Micronutrients added versus not added.
We hypothesised that feeding would be more effective for:
Younger children;
The most disadvantaged, poorest, lowest SES;
Those with the poorest nutritional status (underweight, stunted); and
Children who received a higher percentage of the daily energy requirements.
In the review, we conducted analyses one, two and five and combined analyses three and four, as undernourishment was seen as a proxy for low income. We did not perform analysis six. Furthermore, after learning more about other potential impacts on effectiveness, we added three more subgroup analyses; location of feeding, level of supervision, and single versus multiple interventions.
We hypothesised that feeding would be more effective if:
It was delivered in day cares or feeding centres;
It was strictly supervised (i.e. well‐monitored); and
If multiple interventions were given rather than single interventions.
In total, we performed subgroup analyses across seven categories.
Age: three to 12 months, one to two years, and two years and older for RCTs.
Sex: male versus female.
Socio‐economically disadvantaged: poor versus less poor; undernourished versus well‐nourished.
Nutritional adequacy: percentage of daily requirements (RDI) for energy provided by the supplement (low (0% to 29%), moderate (30% to 59%), and high (60% +)).
Location of feeding: take‐home rations versus feeding centre, or day care or preschool, or both.
Level of supervision (i.e. monitoring): low supervision versus moderate supervision versus strict supervision.
Single versus multiple interventions.
Assessing impact on socio‐economic inequities in health and psychosocial outcomes
We assessed this potential for primary outcomes. Our assessment of the potential for reductions in socio‐economic inequities in health was classified as: effective for reducing inequities in health; potentially effective for reducing inequities in health; ineffective for reducing inequities in health; or uncertain.
Effective: we rated an intervention as effective if the intervention worked, and if improvements in health were greater for children in lower socio‐economic groups than in higher groups.
Potentially effective: we classified an intervention as potentially effective if delivered only to children of lower socio‐economic groups, and if it showed statistically significant and meaningful effects.
Ineffective: we classified an intervention as ineffective for reducing socio‐economic inequities in health if it resulted in greater improvements for children in higher socio‐economic groups than for children in lower socio‐economic groups, or if it was not effective.
Uncertain: there is not enough evidence to judge.
Sensitivity analysis
We conducted sensitivity analyses to consider the impact of:
ICCs of 0.10 for height, weight, WAZ, HAZ, and WHZ; and
ICCs of 0.20 for psychosocial outcomes.
Results
Description of studies
Included studies are described below.
Results of the search
Three electronic searches yielded 52,015 records from all databases in all years, and we identified an additional 65 records from other sources; this resulted in 32,983 articles after duplicates were removed. After initial screening of titles and abstracts, we retrieved 301 articles. Review authors agreed that 48 studies were potentially relevant, and of the appropriate design, and read each in full. Of these, 32 studies met the inclusion criteria; we excluded 16.
Twenty‐one of the included studies were randomised controlled trials (RCTs) and 11 were controlled before‐and‐after studies (CBAs).
We were able to include 26 studies (16 RCTs and 10 CBAs) in one or more meta‐analyses. The study flow diagram is shown in Figure 3.
3.
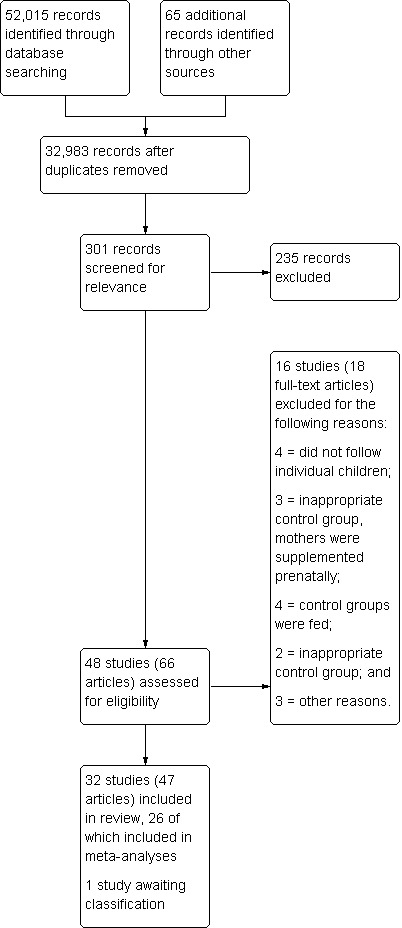
Study flow diagram
Included studies
Study setting
Twenty‐nine studies were from low‐ and middle income countries; three were from high‐income countries. Within low‐ and middle‐income countries, six were performed in India (Bhandari 2001; Devadas 1971; Gopalan 1973; Joshi 1988; Manjrekar 1986; Mittal 1980), two in Bangladesh (Fauveau 1992; Roy 2005), two in Jamaica (Grantham‐McGregor 1991; Heikens 1989), two in Indonesia (Husaini 1991; Pollitt 2000a), two in Columbia (McKay 1978; Waber 1981), three in Malawi (Kuusipalo 2006; Mangani 2014; Thakwalakwa 2010), and one each in Niger (Isanaka 2009), Nigeria (Obatolu 2003), Kenya (Tomedi 2012), Peru (De Romana 2000), South Africa (Oelofse 2003), Vietnam (Schroeder 2002), Thailand (Gershoff 1988), Brazil (Santos 2005), Ecuador (Lutter 2008), Haiti (Iannotti 2014), and Mexico (Rivera 2004). One study (Simondon 1996) was performed in four countries: Bolivia, Caledonia, Congo, and Senegal. All were conducted in poorer settings; these included urban and suburban slums and poor rural areas. Of the three studies from high‐income countries, one was implemented in Australia with Aboriginal children (Coyne 1980), one was performed in Canada (Yeung 2000), and one was performed in the United States (Ziegler 2009).
Participants
The participants comprised children aged three months to five years. In almost all studies in low‐ and middle‐income countries, a high proportion of children had low weight‐for‐age z‐scores (WAZ) or height‐for‐age z‐scores (HAZ). Eight studies allocated children on the basis of mild to moderate malnourishment or low WAZ. Very few children in these studies were severely malnourished (< 3 standard deviations (SDs) for WAZ or HAZ) or ill. Many children came from low income areas and had parents with low education or low income, or both. Many parents were employed as labourers, farmers, or fishermen; other parents were unemployed. The number of participants per study ranged from 30 (Obatolu 2003) to 3166 (Isanaka 2009).
In high‐income countries, two studies were aimed at low‐income children and one did not select on the basis of income.
Interventions
All interventions comprised supplementary food, with or without added micronutrients.
Single versus multiple interventions
In sixteen of the programmes in low‐ and middle‐income countries (De Romana 2000; Gopalan 1973; Heikens 1989; Husaini 1991; Iannotti 2014; Isanaka 2009; Joshi 1988; Kuusipalo 2006; Mangani 2014; Manjrekar 1986; Mittal 1980; Obatolu 2003; Oelofse 2003; Pollitt 2000a; Simondon 1996; Thakwalakwa 2010), and two programmes in high‐income countries (Yeung 2000; Ziegler 2009), supplementary feeding was the only difference between experimental and control groups.
Thirteen studies in low‐ and middle‐income countries provided adjunctive interventions. Seven programmes provided additional rations for the family (Bhandari 2001; Fauveau 1992; Grantham‐McGregor 1991; Rivera 2004; Santos 2005; Tomedi 2012; Waber 1981) to reduce redistribution of the child's supplement. The Progresa programme in Mexico (Rivera 2004) also provided cash transfers to families if they complied with healthcare requirements. Two studies (McKay 1978; Waber 1981) provided stimulation as well as supplementation. Four studies (Devadas 1971; Gershoff 1988; Lutter 2008; Schroeder 2002) provided health/nutrition education programmes for mothers as well as supplementation. Roy 2005 compared children who received supplementation + maternal education to children who received maternal education alone and to controls who received no treatment.
Some of these programmes (including Fauveau 1992; Heikens 1989; Roy 2005) provided health care, deworming or nutritional advice to both groups.
In Coyne 1980, a high‐income country, the children who received supplementation were in day care; the controls were not.
Location and supervision of supplementary feeding
Location. Nine studies in low‐ and middle‐income countries delivered the supplement at day‐care centres (Gershoff 1988; Husaini 1991; Pollitt 2000a) or feeding centres (Devadas 1971; Gopalan 1973; Joshi 1988; McKay 1978; Manjrekar 1986; Schroeder 2002). One study in high‐income countries (Coyne 1980) provided supplementation in day care. Take‐home rations were provided in the remaining 22 studies with different levels of supervision (i.e. monitoring).
Supervision (monitoring)
Strict supervision. Fourteen studies in low‐ and middle‐income countries (Bhandari 2001; Devadas 1971; Gershoff 1988; Gopalan 1973; Heikens 1989; Husaini 1991; Joshi 1988; Lutter 2008; Mangani 2014; Manjrekar 1986; McKay 1978; Pollitt 2000a; Schroeder 2002; Simondon 1996) and one study in a high‐income country (Coyne 1980) were judged to have strict supervision.
Moderate supervision. Ten studies (De Romana 2000; Fauveau 1992; Grantham‐McGregor 1991; Iannotti 2014; Isanaka 2009; Kuusipalo 2006; Oelofse 2003; Rivera 2004; Thakwalakwa 2010; Tomedi 2012) conducted in low‐ and middle‐income countries and two studies in high‐income countries (Yeung 2000; Ziegler 2009) provided moderate supervision.
Low supervision. Five studies in low‐ and middle‐income countries (Mittal 1980; Obatolu 2003; Roy 2005; Santos 2005; Waber 1981) were judged to have low supervision.
Intervention length
Intervention length ranged from three months (Heikens 1989; Husaini 1991; Isanaka 2009; Kuusipalo 2006; Roy 2005; Simondon 1996; Thakwalakwa 2010) to 32 months (Waber 1981). The average was 10 months and the median was nine months.
Food provided
Across all programmes in low‐ and middle‐income countries, a wide variety of food was provided. Eleven studies provided Ready‐to‐Use Theraputic Feeding (RUTF) with or without other foods. Six studies offered sweetened condensed milk, powdered milk or milk‐based formula (often high energy). One study provided bread with milk. Four studies gave cereal, flours or vegetable mixture, usually with milk. Seven others provided locally available foods such as fruit, vegetables, rice and lentils, or provided a fortified cookie.
Two of the studies in high‐income countries provided iron‐fortified cereal; one also provided meat. Food in a study in Australian day cares comprised "hot lunches, nutritious snacks, and vitamin supplementation" (Coyne 1980, p 369).
Sixteen studies in low‐ and middle‐income countries provided fortified foods.
Energy and RDI for energy of the Supplementary Food
The daily energy in the supplements offered was as follows:
For children under six months of age, energy in the supplementary food ranged from 103 kcal to 450 kcal;
For children aged 6 to 12 months, energy in the supplementary food ranged from 130 kcal to 899 kcal;
For children aged one to two years, energy in the supplementary food ranged from 130 kcal to 750 kcal;
For children aged two to three years, energy in the supplementary food ranged from 123 kcal to 500 kcal from 167 kcal;
For children aged three to four years, energy in the supplementary food ranged from 167 kcal to 960 kcal; and
For children aged four to five years, energy in the supplementary food ranged from 167 kcal to 1010 kcal.
The average amount of energy across the studies in low‐ and middle‐income countries was 398 kcal.
Table 6 shows the percentage (%) dietary reference intake (DRI) provided by the supplement for each age group. The % DRI for energy ranged from a low of 7.9 (Joshi 1988) in the oldest age group to 111.7 (Tomedi 2012) for the 6‐ to 12‐month‐old age group.
3. Adequacy of energy content of supplementation given.
| Study | Level of energy classified as low (L: 0 ‐ 29%), moderate (M: 30 ‐ 60%), and high (H: 60%+) of the dietary reference intake (% DRI) by children's age | |||||
| 4 ‐ 5 months | 6 ‐ 12 months | 12 ‐ 24 months | 24 ‐ 36 months | 36 ‐ 48 months | 48 ‐ 60 months | |
| Bhandari 2001 | H (89.9%) | H (94.7%) | ‐ | ‐ | ‐ | ‐ |
| Simondon 1996 | L (20.6%) | L (28.8%) | ‐ | ‐ | ‐ | ‐ |
| Rivera 2004 | M (38.7%) | L (27.4%) | ‐ | ‐ | ‐ | ‐ |
| Fauveau 1992 | ‐ | L (17.6%) | ‐ | ‐ | ‐ | ‐ |
| Oelofse 2003 | ‐ | M (42%) | ‐ | ‐ | ‐ | ‐ |
| Iannotti 2014 | ‐ | L (15%) | ‐ | ‐ | ‐ | ‐ |
| Mangani 2014 | ‐ | M (40%) | ‐ | ‐ | ‐ | ‐ |
| Grantham‐McGregor 1991 | ‐ | H (105.2%) | H (86.3%) | ‐ | ‐ | ‐ |
| ‐Husaini 1991 | ‐ | M (48.1%) | M (39.5%) | ‐ | ‐ | ‐ |
| Lutter 2008 | ‐ | M (38.6%) | M (31.6%) | ‐ | ‐ | ‐ |
| Mittal 1980 | ‐ | L (27.8%) | L (22.8%) | ‐ | ‐ | ‐ |
| Roy 2005 | ‐ | M (42.1%) | M (34.5%) | ‐ | ‐ | ‐ |
| Thakwalakwa 2010 | ‐ | M (30.9%) | L (25.4%) | ‐ | ‐ | ‐ |
| Kuusipalo 2006 | ‐ | M (55%) | M (44%) | ‐ | ‐ | ‐ |
| De Romana 2000 | ‐ | M (56.1) | M (46%) | ‐ | ‐ | ‐ |
| Santos 2005 | ‐ | H (60%) | H (60%) | ‐ | ‐ | ‐ |
| Tomedi 2012 | ‐ | H (136.2%) | H (111.7%) | ‐ | ‐ | ‐ |
| Pollitt 2000a | ‐ | ‐ | L (24.7%) | ‐ | ‐ | ‐ |
| Isanaka 2009 | ‐ | H (69.8%) | M (57.5%) | M (57.5%) | M (34.7%) | M (33%) |
| Manjrekar 1986 | ‐ | M (35.1%) | L (28.8%) | L (28.8%) | L (17.4%) | L (16.5%) |
| Gershoff 1988 | ‐ | M (42.1%) | M (34.5%) | M (34.5%) | L (20.8%) | L (19.8%) |
| Gopalan 1973 | ‐ | ‐ | M (30.6%) | M (30.6%) | L (18.5%) | L (17.5%) |
| Devadas 1971 | ‐ | ‐ | ‐ | L (14.2%) | ‐ | ‐ |
| McKay 1978 | ‐ | ‐ | ‐ | ‐ | M (53.6%) | ‐ |
| Joshi 1988 | ‐ | ‐ | ‐ | ‐ | L (8.3%) | L (7.9%) |
| Coyne 1980 | ‐ | ‐ | ‐ | ‐ | M (47.6%) | M (47.6%) |
This calculation was only done if the primary studies provided enough information. Therefore, six studies are missing as they did not provide enough information.
DRI ‐ dietary reference intake H ‐ high L ‐ low M ‐ moderate
Six studies (Heikens 1989; Obatolu 2003; Schroeder 2002; Waber 1981; Yeung 2000; Ziegler 2009) did not provide enough information to estimate energy or percent DRI.
Controls
In most of the studies, nothing was provided for children in control groups. Bhandari 2001 provided nutrition education, while three others (Heikens 1989; Isanaka 2009; Manjrekar 1986) provided health care, and Fauveau 1992 provided both health care and nutritional counselling. In all five of these latter studies, the experimental group also received the treatments given to the control children.
One study in a high‐income country (Yeung 2000) provided families of control children with vouchers for clothes and laundry so that they received the same economic benefit as families of the children who were given supplementation.
Outcomes
Nutritional outcomes
In low‐ and middle‐income countries, 28 out of 29 studies provided data on nutritional outcomes; 25 studies reported on weight and 23 studies reported on height. Twelve studies provided outcome data for WAZ, 13 for HAZ, and 12 for WHZ. Finally, eight studies reported on blood haemoglobin.
All three studies in high‐income countries provided data on nutritional outcomes. Coyne 1980 and Ziegler 2009 provided data on weight, height, and haemoglobin. Yeung 2000 provided data on WAZ, HAZ, WHZ, and haemoglobin.
Psychosocial outcomes
In low‐ and middle‐income countries, five studies provided outcome data on psychomotor development (Grantham‐McGregor 1991; Husaini 1991; Oelofse 2003; Pollitt 2000a; Waber 1981); three of these studies (Iannotti 2014; Mangani 2014: Pollitt 2000a) provided data on attainment of motor milestones. Three studies provided data on mental or cognitive development (Husaini 1991; McKay 1978; Pollitt 2000a).
Table 7, Table 8, Table 9, and Table 10 all provide an overview of outcomes reported in the included studies, split by study type and by low‐ and middle‐income country/high‐income country. Additional information on the included studies can be found in the Characteristics of included studies tables.
4. Summary of reported outcomes for RCTs in low‐ and middle‐income countries.
|
Outcome measure |
Systematic review | Meta‐analysis | ||
| No. of studies | No. of participants | No. of studies | No. of participants | |
| Weight gain | 11 | 1356 | 9 | 1057 |
| Height gain | 11 | 1814 | 9 | 1698 |
| WAZ | 9 | 2029 | 8 | 1747 |
| HAZ | 9 | 4837 | 9 | 4837 |
| WHZ | 6 | 4399 | 6 | 4399 |
| Psychomotor development | 5 | 430 | 1 | 113 |
| Cognitive development | 3 | 357 | 1 | 137 |
| Follow‐up of cognitive functioning | 3 | 505 | 1 | 142 |
| Language | 1 | 136 | 0 | 0 |
| Memory | 1 | 231 | 0 | 0 |
| Leakage and substitution | 5 | 1589 | 0 | 0 |
| Haemoglobin | 5 | 866 | 5 | 866 |
| Physical activity | 3 | 201 | 0 | 0 |
| Morbidity | 6 | 4099 | 0 | 0 |
| Mortality | 1 | 3103 | 0 | 0 |
CBAs ‐ controlled before‐and‐after trials HAZ ‐ height‐for‐age z‐score No. ‐ number RCT ‐ randomised controlled trial WAZ ‐ weight ‐for‐age z‐score WHZ ‐ weight‐for‐height z‐score
5. Summary of reported outcomes for RCTs in high‐income countries.
|
Outcome measure |
Systematic review | Meta‐analysis | ||
| No. of studies | No. of participants | No. of studies | No. of participants | |
| Weight gain | 1 | 45 | 1 | 45 |
| Height gain | 1 | 45 | 1 | 45 |
| WAZ | 1 | 97 | 1 | 97 |
| HAZ | 1 | 97 | 1 | 97 |
| WHZ | 1 | 97 | 1 | 97 |
HAZ ‐ height‐for‐age z‐score No. ‐ number RCT ‐ randomised controlled trial WAZ ‐ weight‐for‐age z‐score WHZ ‐ weight‐for‐height z‐score
6. Summary of reported outcomes for CBAs in low‐ and middle‐income countries.
|
Outcome measure |
Systematic review | Meta‐analysis | ||
| No. of studies | No. of participants | No. of studies | No. of participants | |
| Weight gain | 7 | 1574 | 7 | 1574 |
| Height gain | 7 | 1573 | 7 | 1573 |
| WAZ | 4 | 790 | 4 | 790 |
| HAZ | 5 | 873 | 4 | 790 |
| WHZ | 4 | 790 | 4 | 970 |
| Psychomotor development | 0 | 0 | 0 | 0 |
| Cognitive development | 0 | 0 | 0 | 0 |
| Follow‐up of cognitive functioning | 0 | 0 | 0 | 0 |
| Language | 0 | 0 | 0 | 0 |
| Memory | 0 | 0 | 0 | 0 |
| Leakage and substitution | 5 | 924 | 0 | 0 |
| Haemoglobin | 1 | 110 | 0 | 0 |
| Physical activity | 0 | 0 | 0 | 0 |
| Morbidity | 1 | 34 | 0 | 0 |
| Mortality | 0 | 0 | 0 | 0 |
CBAs ‐ controlled before‐and‐after trials HAZ ‐ height‐for‐age z‐score No. ‐ number WAZ ‐ weight‐for‐age z‐score WHZ ‐ weight‐for‐height z‐score
7. Summary of reported outcomes for CBAs in high‐income countries.
|
Outcome measure |
Systematic review | Meta‐analysis | ||
| No. of studies | No. of participants | No. of studies | No. of participants | |
| Weight gain | 1 | 116 | 1 | 116 |
| Height gain | 1 | 116 | 1 | 116 |
| WAZ | 0 | 0 | 0 | 0 |
| HAZ | 0 | 0 | 0 | 0 |
| WHZ | 0 | 0 | 0 | 0 |
CBAs ‐ controlled before‐and‐after trials HAZ ‐ height‐for‐age z‐score No. ‐ number WAZ ‐ weight‐for‐age z‐score WHZ ‐ weight‐for‐height z‐score
Sixteen studies were allocated by cluster (regions, neighbourhoods, or day cares). Of these 16, six (Isanaka 2009; Lutter 2008; Rivera 2004; Roy 2005; Santos 2005; Tomedi 2012) adjusted for clustering in some or all of their analyses. We performed this adjustment for eight studies: Coyne 1980; Devadas 1971; Gershoff 1988; Husaini 1991; Lutter 2008 (not all of their numbers were adjusted); McKay 1978; Schroeder 2002, and for the weight analyses in Pollitt 2000a (Beckett 2000). We did not adjust for clustering in the De Romana 2000, Fauveau 1992, Joshi 1988, and Pollitt 2000a studies, as appropriate data were not available. Table 5 provides a summary of the clustered studies.
Excluded studies
The Excluded studies table contains 16 studies. We excluded four studies because control groups received foods or drinks with energy; three studies because the sample included children whose mothers were supplemented prenatally; and another four because they did not follow specific children, but measured all children who resided in the area at the time of testing (and these may have been quite different children at follow‐up). We excluded two studies because the control groups were self‐selected, and three more because they included older children, had no outcomes of interest, or had too little information.
Risk of bias in included studies
For the 21 RCTs and 11 CBAs, we summarise judgements about the risk of bias in the 'Risk of bias' graph (Figure 4).
4.
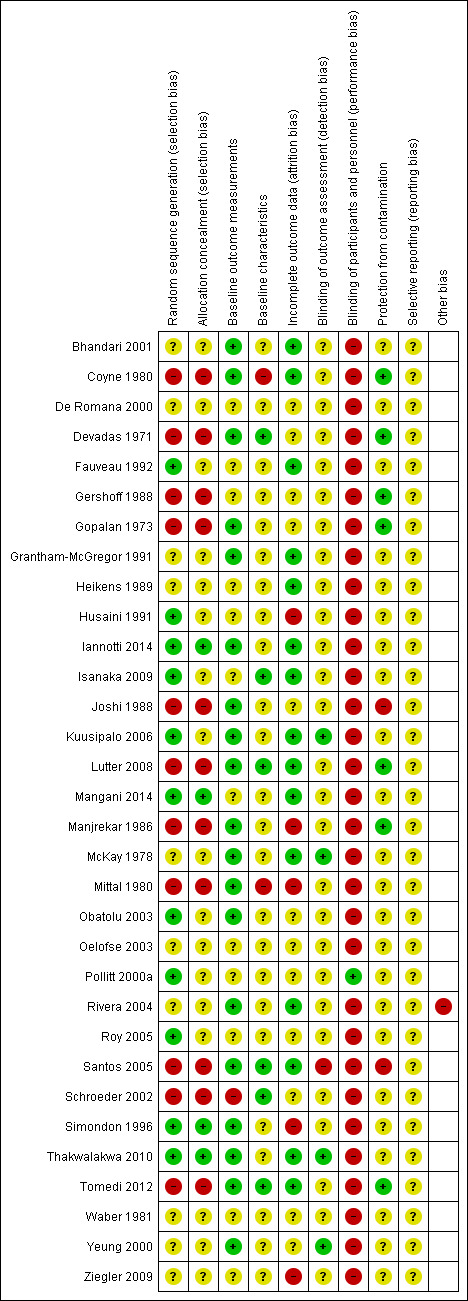
Risk of bias summary for RCTs: review authors' judgements about each risk of bias item for each included study
Allocation
We judged 11 RCTs (Fauveau 1992; Husaini 1991; Iannotti 2014; Isanaka 2009; Kuusipalo 2006: Mangani 2014; Obatolu 2003; Pollitt 2000a; Roy 2005; Simondon 1996; Thakwalakwa 2010) from low‐ and middle‐income countries to have a low risk of bias for random sequence generation, while the other eight from low‐ and middle‐income countries (Bhandari 2001; De Romana 2000; Grantham‐McGregor 1991; Heikens 1989; McKay 1978; Oelofse 2003; Rivera 2004; Waber 1981) we judged to have an unclear risk of bias for random sequence generation.
We judged both RCTs from high‐income countries (Yeung 2000; Ziegler 2009) to have an unclear risk of bias for random sequence generation.
We rated four RCTs from low‐ and middle‐income countries (Iannotti 2014; Mangani 2014; Simondon 1996; Thakwalakwa 2010) as having a low risk of bias for allocation concealment, while the other 15 were judged to have an unclear risk of bias.
We judged that both RCTs from high‐income countries (Yeung 2000; Ziegler 2009) had an unclear risk of bias for allocation concealment.
Due to the fact that these studies were not randomised, we rated all 11 CBAs at a high risk for random sequence generation and allocation concealment.
Blinding
In low‐ and middle‐income countries, we judged one RCT (Pollitt 2000a) to have a low risk of bias and the other 18 RCTs and 10 CBAs to have a high risk of bias because it is usually not possible to blind participants, parents, and personnel to supplementary feeding. This knowledge may lead to sharing of food within the family and changed behaviour, since providing food may lead to increased interaction with children.
In high‐income countries, we judged two RCTs (Yeung 2000) (Ziegler 2009 and one CBA (Coyne 1980) to have a high risk of bias for blinding of personnel and participants
For blinding of outcome assessment in low‐ and middle‐income countries, we judged three RCTs (Kuusipalo 2006; McKay 1978; Thakwalakwa 2010) to have a low risk of bias. We judged nine CBAs (Devadas 1971; Gershoff 1988; Gopalan 1973; Lutter 2008; Manjrekar 1986; Mittal 1980; Santos 2005; Schroeder 2002; Tomedi 2012) and the other 16 RCTs to have an unclear risk of bias. We judged the remaining CBA to have a high risk of bias (Joshi 1988).
In high‐income countries, we judged one RCT to have a low risk of bias (Yeung 2000) and one to have an unclear risk of bias (Ziegler 2009) for blinded outcome assessment. We also judged one CBA to have an unclear risk of bias (Coyne 1980).
Incomplete outcome data
We judged 11 RCTs in low‐ and middle‐income countries (Bhandari 2001; Fauveau 1992; Grantham‐McGregor 1991; Heikens 1989; Iannotti 2014; Isanaka 2009; Kuusipalo 2006; Mangani 2014; McKay 1978; Rivera 2004; Thakwalakwa 2010) and three CBAs from low‐ and middle‐income countries (Lutter 2008; Santos 2005; Tomedi 2012) to have a low risk of bias due to attrition. We rated six RCTs (De Romana 2000; Obatolu 2003; Oelofse 2003; Pollitt 2000a; Roy 2005; Waber 1981) and five CBAs (Devadas 1971; Gershoff 1988; Gopalan 1973; Joshi 1988; Schroeder 2002) at an unclear risk of bias. The remaining two RCTs (Husaini 1991; Simondon 1996) and two CBAs (Manjrekar 1986; Mittal 1980) we judged to have a high risk of bias due to attrition.
In high‐income countries, we judged one RCT (Yeung 2000) to have an unclear risk of bias and one RCT (Ziegler 2009) to have a high risk of bias due to attrition. We judged one CBA to have a low risk of bias (Coyne 1980).
Selective reporting
In low‐ and middle‐income countries, we judged 19 RCTs (Bhandari 2001; Fauveau 1992; De Romana 2000; Grantham‐McGregor 1991; Heikens 1989; Husaini 1991; Iannotti 2014; Isanaka 2009; Kuusipalo 2006; Mangani 2014; McKay 1978; Obatolu 2003; Oelofse 2003; Pollitt 2000a; Rivera 2004; Roy 2005; Simondon 1996; Thakwalakwa 2010; Waber 1981) and 10 CBAs to have an unclear risk of bias due to selective reporting.
In high‐income countries, we judged both RCTs (Yeung 2000 ; Ziegler 2009) and one CBA (Coyne 1980) to have an unclear risk of bias for selective reporting.
Other potential sources of bias
We judged Rivera 2004 to be at a high risk of bias due to the fact that 10% of the controls received treatment. We did not judge other sources of bias for the other RCTs or any of the CBAs.
Additional risk of bias domains assessed for CBAs
Baseline outcome measurement
This assesses whether the experimental groups were similar at baseline on the study outcomes. We scored nine CBAs from low‐ and middle‐income countries at a low risk of bias, as most or all of the baseline outcome measurements in each study were similar between the two groups. However, we rated Gershoff 1988 at an unclear risk of bias.
In high‐income countries, we judged Coyne 1980 to be at a low risk of bias.
Baseline characteristics
This assesses whether or not the baseline characteristics of study and control providers were similar. We judged five CBAs from low‐ and middle‐income countries (Devadas 1971; Lutter 2008; Mittal 1980; Schroeder 2002; Tomedi 2012) to have a low risk of bias. We rated three CBAs at an unclear risk of bias (Gershoff 1988; Gopalan 1973: Manjrekar 1986), and two studies at a high risk of bias (Joshi 1988; Santos 2005).
In high‐income countries, we judged Coyne 1980 to be at a low risk of bias.
Protection against contamination
This assesses the extent to which controls had access to treatments. We rated six CBAs in low‐ and middle‐income countries (Devadas 1971; Gershoff 1988; Gopalan 1973; Lutter 2008; Manjrekar 1986; Tomedi 2012) as being at a low risk of bias, three (Mittal 1980; Santos 2005; Schroeder 2002) as being at an unclear risk of bias, and one (Joshi 1988) as being at a high risk of bias.
In high‐income countries, we judged Coyne 1980 to be at a low risk of bias.
Effects of interventions
See: Table 1; Table 2; Table 3
Primary outcomes: Growth
Weight gain
Low‐ and middle‐income countries: RCTs
We included nine RCTs with 1057 children (Bhandari 2001; Grantham‐McGregor 1991 (see Walker 1991); Heikens 1989; Kuusipalo 2006; Mangani 2014; Oelofse 2003; Pollitt 2000a; Simondon 1996; Thakwalakwa 2010) in a meta‐analysis for weight. The average period of feeding in these studies was six months. Our meta‐analysis showed a small significant effect of feeding. Children who were given supplementation gained an average of 0.12 kg more than those who were not supplemented (95% confidence interval (CI) 0.05 to 0.18; Analysis 1.1). There was no heterogeneity (Chi² = 3.92, df = 8, P value = 0.86, I² = 0%). Sensitivity analyses using an intraclass correlation coefficient (ICC) of 0.10 made little difference (Analysis 2.1).
1.1. Analysis.
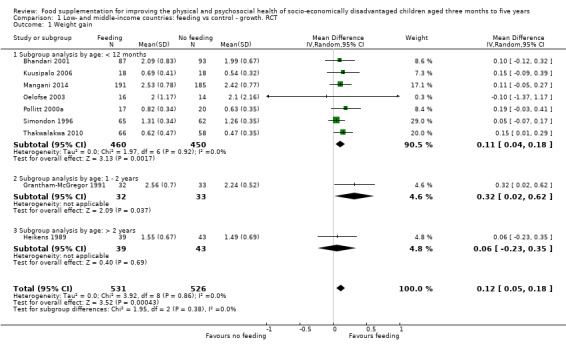
Comparison 1 Low‐ and middle‐income countries: feeding vs control ‐ growth. RCT, Outcome 1 Weight gain.
2.1. Analysis.
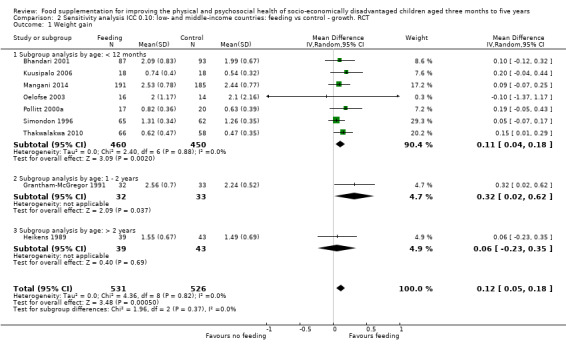
Comparison 2 Sensitivity analysis ICC 0.10: low‐ and middle‐income countries: feeding vs control ‐ growth. RCT, Outcome 1 Weight gain.
The 14‐month Obatolu 2003 RCT (60 children) found a large and significant effect of feeding on weight gain for boys (end‐of‐study difference of 3.91 kg statistically significant) and girls (end‐of‐study difference of 2.55 kg statistically significant).
The Fauveau 1992 study found that 48 children who received supplementary feeding gained an average of 39 grams more than the 43 controls (six‐month intervention: not significant).
Low‐ and middle‐income countries: CBAs
Seven CBAs in low‐ and middle‐income countries (Devadas 1971; Gopalan 1973; Gershoff 1988; Lutter 2008; Manjrekar 1986; Mittal 1980; Santos 2005) with 1784 children were included in this meta‐analysis. The average length of feeding was one year. There was a significant effect of feeding on weight. Children who were given supplementation gained an average of 0.24 kg more than those who were not supplemented (95% CI 0.09 to 0.39). There was moderate heterogeneity (Chi² = 30.07, df = 15, P value = 0.01, I² = 50%; Analysis 3.1). Sensitivity analyses with ICCs at 0.10 made little difference (Analysis 5.1).
3.1. Analysis.
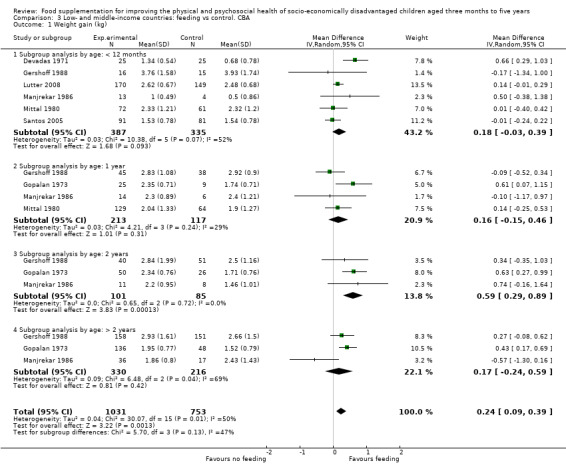
Comparison 3 Low‐ and middle‐income countries: feeding vs control. CBA, Outcome 1 Weight gain (kg).
5.1. Analysis.
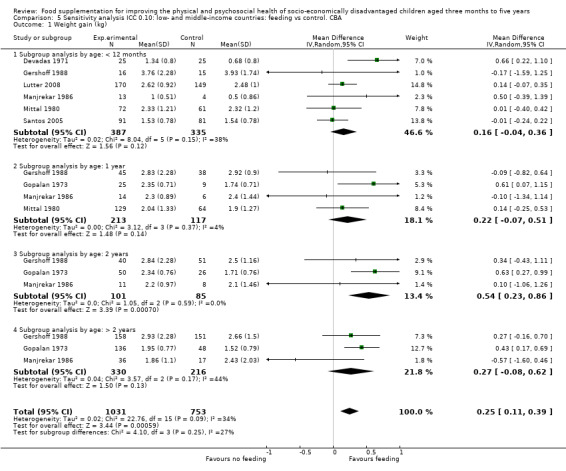
Comparison 5 Sensitivity analysis ICC 0.10: low‐ and middle‐income countries: feeding vs control. CBA, Outcome 1 Weight gain (kg).
High‐income countries: RCTs
Only one RCT in high‐income countries assessed weight gain (Ziegler 2009). Our analyses found that children who received supplementation in the form of an iron‐fortified cereal gained slightly less weight than children who received no supplementation (mean difference (MD) ‐0.10, 95% CI ‐0.52 to 0.32, n = 45; Analysis 4.1).
4.1. Analysis.
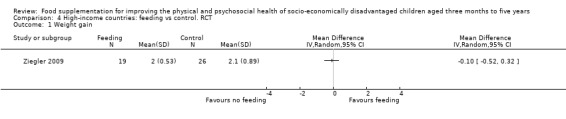
Comparison 4 High‐income countries: feeding vs control. RCT, Outcome 1 Weight gain.
High‐income countries: CBAs
We analysed results from Coyne 1980; 116 Aboriginal children were included. There were significant effects of four months of supplementation on weight (MD 0.95, 95% CI 0.58 to 1.33; Analysis 7.1).
7.1. Analysis.
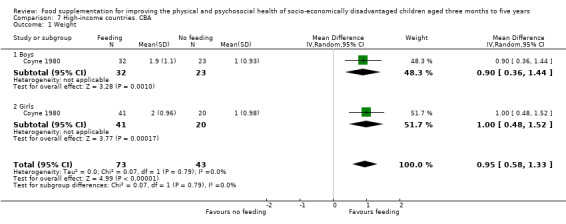
Comparison 7 High‐income countries. CBA, Outcome 1 Weight.
Height gain
Low‐ and middle‐income countries: RCTs
Nine RCTS (Bhandari 2001; Grantham‐McGregor 1991 (see Walker 1991); Heikens 1989; Kuusipalo 2006; Mangani 2014; Oelofse 2003; Rivera 2004; Simondon 1996; Thakwalakwa 2010), with 1463 children, contributed to this meta‐analysis. The average period of supplementation was six months. This analysis demonstrated that children who were given supplementation grew an average of 0.27 cm (95% CI 0.07 to 0.48) more than those who were not supplemented. There was little heterogeneity (Chi² = 11.33, df = 8, P value = 0.18, I² = 29%; Analysis 1.2). We did not perform a sensitivity analyses because no adjustments for clustering were needed.
1.2. Analysis.
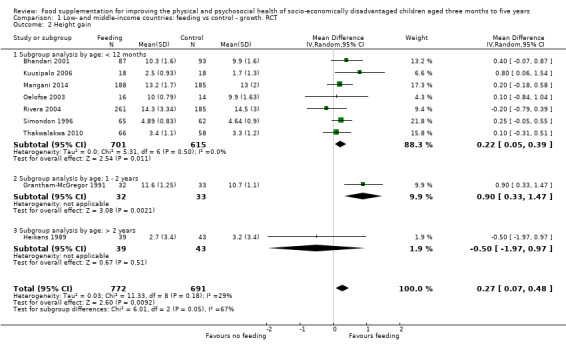
Comparison 1 Low‐ and middle‐income countries: feeding vs control ‐ growth. RCT, Outcome 2 Height gain.
Pollitt 2000a studied effectiveness for two age cohorts, 12 and 18 months old. They found that supplementary feeding had a significant effect on height for the younger (12‐month‐old) cohort only (see Beckett 2000). Obatolu 2003 (60 children) found a significant effect of feeding on length for boys (difference between experimental and controls: 5.12 cm; end‐of‐study difference of 5.02 statistically significant) and girls (difference: 6.95 cm; end‐of‐study difference of 5.92 cm statistically significant).
Low‐ and middle‐income countries: CBAs
We included seven CBAs in low‐ and middle‐income countries (Devadas 1971; Gopalan 1973; Gershoff 1988; Lutter 2008; Manjrekar 1986; Mittal 1980; Santos 2005) with 1782 children in this meta‐analysis. The average duration of feeding was one year. Overall, there was a non‐significant effect of feeding on height (MD 0.52, 95% CI ‐0.07 to 1.10). Heterogeneity was high (Chi² = 97.02 df = 15, P < 0.00001, I² = 85%; Analysis 3.2). A sensitivity analysis with the ICCs at 0.10 showed significant positive effects (MD 0.57, 95% CI 0.06 to 1.07) (Analysis 5.2) for height.
3.2. Analysis.
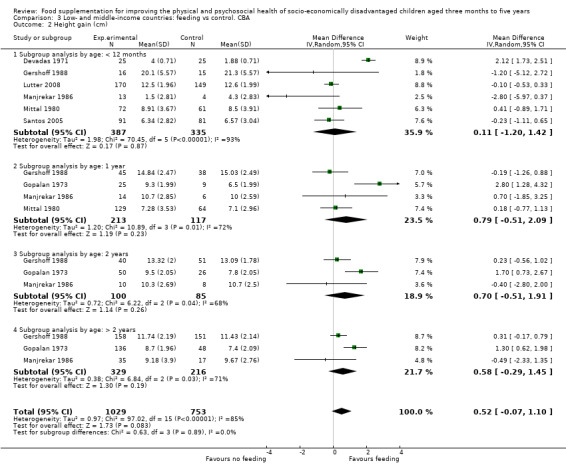
Comparison 3 Low‐ and middle‐income countries: feeding vs control. CBA, Outcome 2 Height gain (cm).
5.2. Analysis.
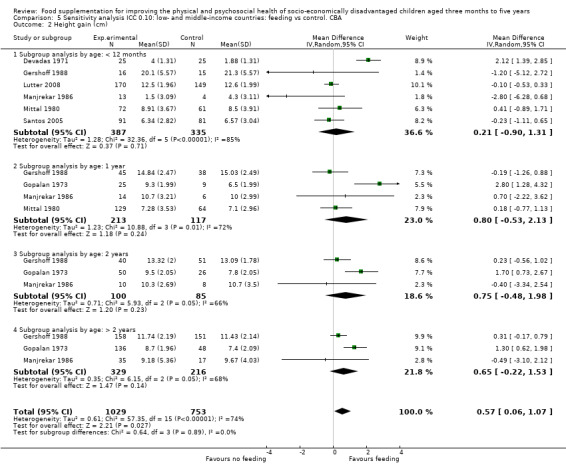
Comparison 5 Sensitivity analysis ICC 0.10: low‐ and middle‐income countries: feeding vs control. CBA, Outcome 2 Height gain (cm).
High‐income countries: RCTs
One RCT (Ziegler 2009) (45 children) studied height. Our analysis indicates that there was no significant difference between children who received iron‐fortified cereal and those who received no supplementation (MD ‐1.00, 95% CI ‐2.12 to 0.12; Analysis 4.2).
4.2. Analysis.
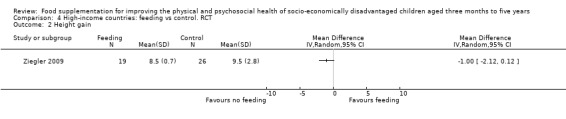
Comparison 4 High‐income countries: feeding vs control. RCT, Outcome 2 Height gain.
High‐income countries: CBAs
Our analysis of Coyne 1980 found no significant effects of supplementation on height (MD 0.61, 95% CI ‐0.31 to 1.54, n = 116; Analysis 7.2).
7.2. Analysis.
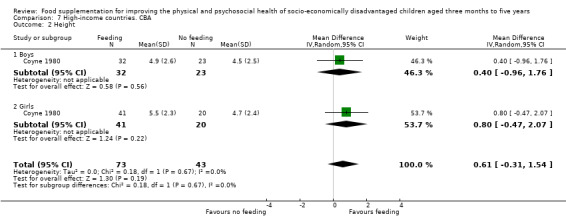
Comparison 7 High‐income countries. CBA, Outcome 2 Height.
Change in Weight for Age z‐score (WAZ)
Low‐ and middle‐income countries: RCTs
We included eight RCTs (Husaini 1991; Iannotti 2014; Kuusipalo 2006; Mangani 2014; McKay 1978; Oelofse 2003; Rivera 2004; Thakwalakwa 2010) and 1565 children in the meta‐analysis for WAZ. The average duration was six months. There were statistically significant differences between the supplemented and non‐supplemented groups (MD 0.15, 95% CI 0.05 to 0.24; Analysis 1.3). Heterogeneity was moderate (Chi² = 14.68, df = 7, P value = 0.04, I² = 52%). Sensitivity analyses with ICCs at 0.10 made little difference (Analysis 2.2).
1.3. Analysis.
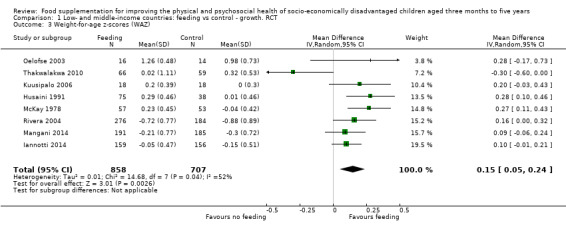
Comparison 1 Low‐ and middle‐income countries: feeding vs control ‐ growth. RCT, Outcome 3 Weight‐for‐age z‐scores (WAZ).
2.2. Analysis.
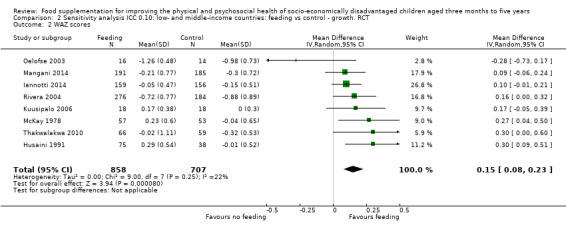
Comparison 2 Sensitivity analysis ICC 0.10: low‐ and middle‐income countries: feeding vs control ‐ growth. RCT, Outcome 2 WAZ scores.
In a cluster‐RCT with 282 children, Roy 2005 found significant effects of supplementation with maternal nutrition education. Those children in the intervention group gained 0.71 more in WAZ than the children who received no treatment (P < 0.001) and 0.26 more than the children who received only maternal nutrition education (not significant).
Low‐ and middle‐income countries: CBAs
Four CBAs (Lutter 2008; Santos 2005; Schroeder 2002; Tomedi 2012) with 999 children contributed to the meta‐analysis for WAZ. The average study period was eight months. There was no statistically significant difference between children who received supplementation and those who did not (MD 0.27; 95% CI ‐0.13 to 0.68). There was significant heterogeneity (Chi² = 87.47, df = 3, P < 0.00001, I² = 97%; Analysis 3.3). Sensitivity analyses with ICCs at 0.10 made little difference (Analysis 5.3).
3.3. Analysis.
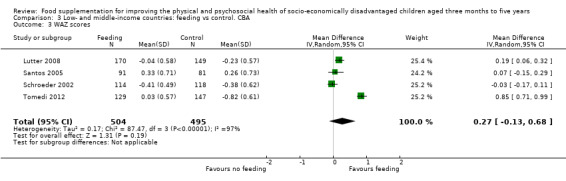
Comparison 3 Low‐ and middle‐income countries: feeding vs control. CBA, Outcome 3 WAZ scores.
5.3. Analysis.
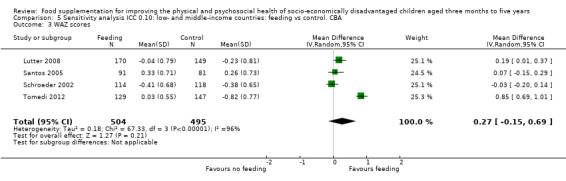
Comparison 5 Sensitivity analysis ICC 0.10: low‐ and middle‐income countries: feeding vs control. CBA, Outcome 3 WAZ scores.
High‐income countries: RCTs
One RCT (Yeung 2000) in 103 children in a high‐income country assessed WAZ; infants who received iron‐fortified cereals had a z‐score change of 0.02 (95% CI 0.01 to 0.03; Analysis 4.3).
4.3. Analysis.

Comparison 4 High‐income countries: feeding vs control. RCT, Outcome 3 WAZ scores.
High‐income countries: CBA
No CBAs assessed the change in WAZ in a high‐income country.
Change in height‐for‐age z‐scores (HAZ)
Low‐ and middle‐income countries: RCTs
We included nine RCTs (Husaini 1991; Iannotti 2014; Isanaka 2009; Kuusipalo 2006; Mangani 2014; McKay 1978; Oelofse 2003; Rivera 2004; Thakwalakwa 2010) with 4544 children in this analysis. The average study duration was six months. we found a significant effect of supplementation (MD 0.15, 95% CI 0.06 to 0.24). Heterogeneity was moderate (Chi² = 20.96, df = 8, P value = 0.007, I² = 62%; Analysis 1.4). Sensitivity analyses with ICCs at 0.10 made little difference (Analysis 2.3).
1.4. Analysis.
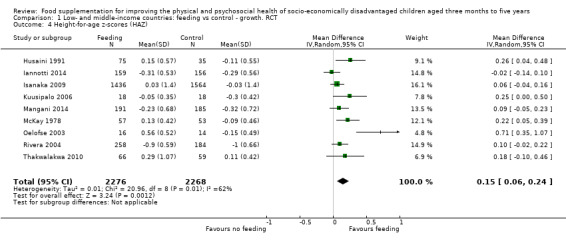
Comparison 1 Low‐ and middle‐income countries: feeding vs control ‐ growth. RCT, Outcome 4 Height‐for‐age z‐scores (HAZ).
2.3. Analysis.
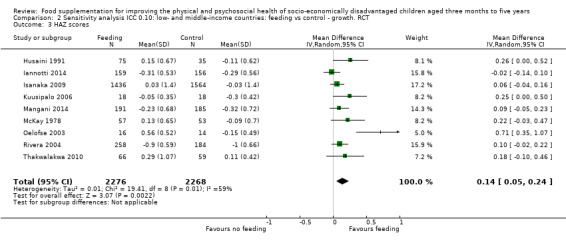
Comparison 2 Sensitivity analysis ICC 0.10: low‐ and middle‐income countries: feeding vs control ‐ growth. RCT, Outcome 3 HAZ scores.
Low‐ and middle‐income countries: CBAs
Four studies (Lutter 2008; Santos 2005; Schroeder 2002; Tomedi 2012) with 999 children contributed to this meta‐analysis. The average study period was eight months. There was no effect (MD 0.01; 95% CI ‐0.10 to 0.12) and little heterogeneity (Chi² = 3.95 df = 3, P value = 0.27, I² = 24%; Analysis 3.4). Sensitivity analyses with ICCs at 0.10 made little difference (Analysis 5.4).
3.4. Analysis.
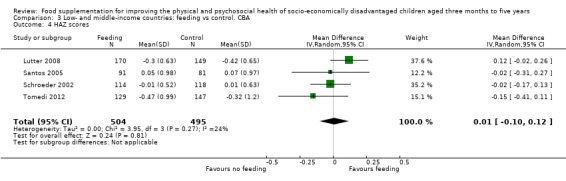
Comparison 3 Low‐ and middle‐income countries: feeding vs control. CBA, Outcome 4 HAZ scores.
5.4. Analysis.
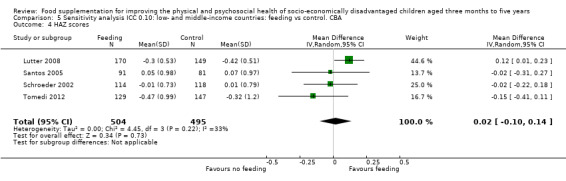
Comparison 5 Sensitivity analysis ICC 0.10: low‐ and middle‐income countries: feeding vs control. CBA, Outcome 4 HAZ scores.
De Romana 2000 (n = 250) found no significant difference between the experimental and the control groups in change in prevalence of stunting (i.e. height‐for‐age z scores (HAZ)).
High‐income countries: RCTS
One RCT (Yeung 2000) with 103 children assessed HAZ. Infants who received iron‐fortified cereals had a z‐score change of 0.04 (95% CI 0.04 to 0.05; Analysis 4.4).
4.4. Analysis.
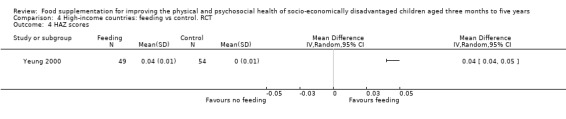
Comparison 4 High‐income countries: feeding vs control. RCT, Outcome 4 HAZ scores.
High‐income countries: CBAs
No CBAs assessed the change in HAZ scores in a high‐income country.
Change in weight‐for‐height z‐scores (WHZ)
Low‐ and middle‐Income countries: RCTs
Seven RCTs (Grantham‐McGregor 1991; Isanaka 2009; Kuusipalo 2006; Mangani 2014; Oelofse 2003; Rivera 2004; Thakwalakwa 2010) with 4073 children contributed to this meta‐analysis. The average study duration was six months. There was no effect of supplementation (MD 0.10, 95% CI ‐0.02 to 0.22; Analysis 1.5). Heterogeneity was high (Chi² = 18.39, df = 6, P < 0.005, I² = 67%). Sensitivity analyses with ICCs at 0.10 made little difference (Analysis 2.4).
1.5. Analysis.
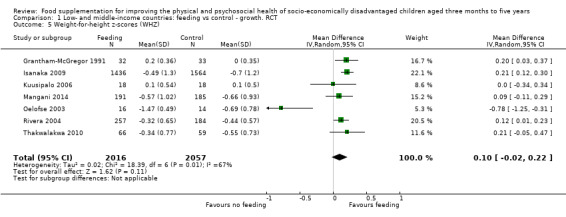
Comparison 1 Low‐ and middle‐income countries: feeding vs control ‐ growth. RCT, Outcome 5 Weight‐for‐height z‐scores (WHZ).
2.4. Analysis.
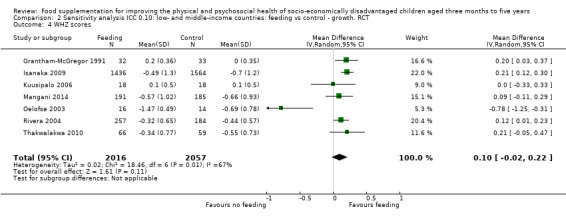
Comparison 2 Sensitivity analysis ICC 0.10: low‐ and middle‐income countries: feeding vs control ‐ growth. RCT, Outcome 4 WHZ scores.
Low‐ and middle‐income countries: CBAs
Four studies (Lutter 2008; Santos 2005; Schroeder 2002; Tomedi 2012) with 999 children contributed to this meta‐analysis for WHZ. The average study period was eight months. We found a non‐significant difference between children who received supplementation and those who did not (MD 0.29, 95% CI ‐0.11 to 0.69, Analysis 3.5). There was significant heterogeneity (Chi² = 67.31, df = 3, P < 0.00001, I² = 96%). Sensitivity analyses with ICCs at 0.10 made little difference (Analysis 5.5).
3.5. Analysis.
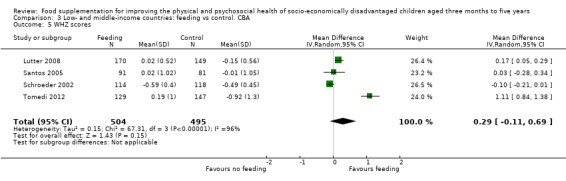
Comparison 3 Low‐ and middle‐income countries: feeding vs control. CBA, Outcome 5 WHZ scores.
5.5. Analysis.
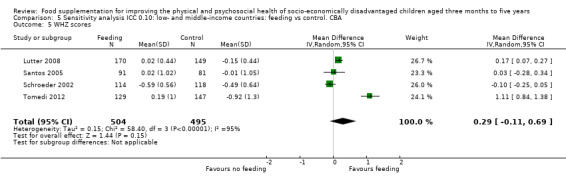
Comparison 5 Sensitivity analysis ICC 0.10: low‐ and middle‐income countries: feeding vs control. CBA, Outcome 5 WHZ scores.
High‐income countries: RCTS
One RCT (Yeung 2000) assessed WHZ. There was a very small, statistically significant effect: infants in the control group fared better than children who received supplementation (MD ‐0.06, 95% CI ‐0.07 to ‐0.05, n = 103; Analysis 4.5).
4.5. Analysis.

Comparison 4 High‐income countries: feeding vs control. RCT, Outcome 5 WHZ scores.
High‐income countries: CBAs
No CBAs assessed change in WHZ scores in a high‐income country.
Primary outcomes: Psychosocial
Psychomotor development
Low‐ and middle‐income countries: RCTs
Four RCTs in low‐ and middle‐income countries assessed the effect of supplementary feeding on psychomotor development.
Our meta‐analysis of two studies (Husaini 1991; Grantham‐McGregor 1991) found that children who received supplementary feeding had greater improvement on tests of psychomotor functioning than children who did not receive any supplementary food (SMD 0.41, 95% CI, 0.10 to 0.72; n = 178; Analysis 6.1). There was no heterogeneity (Chi² = 0.1, df = 1, P value = 0.75, I² = 0%).
6.1. Analysis.
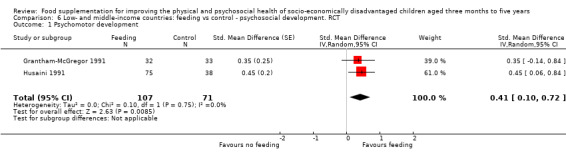
Comparison 6 Low‐ and middle‐income countries: feeding vs control ‐ psychosocial development. RCT, Outcome 1 Psychomotor development.
Waber 1981 reported that children who received 2.5 years of supplementation (Group B; n = 60) beginning at six months of age had better overall scores at the end of the study on the Griffiths Mental Development Scales (GMDS) than those who received no supplementation (n = 54), but significance was not given.
Pollitt 2000a reported no main effect of supplementary feeding on children's psychomotor performance in a Repeated Measures ANOVA (experimental group: n = 53 in 12‐month cohort; n = 83 in 18‐month cohort), but did find significant differences in change over time contrasts.
None of the CBAs in low‐ and middle‐income countries or RCTs and CBAs in high‐income countries assessed psychomotor development.
Motor milestones
Findings concerning the effect of supplementation on achievement of motor milestones are equivocal. Pollitt 2000a found that significantly more of the supplemented children walked by 18 months (100% compared to 50%: P² (Kruskal‐Wallace) = 11.4, df = 2, P < 0.01). Iannotti 2014 (n = 420) and Mangani 2014 (n = 840) found no significant effects.
None of the CBAs in low‐ and middle‐income countries or RCTs and CBAs in high‐income countries assessed motor milestones.
Cognitive development
Low‐ and middle‐Income countries: RCTs
Three RCTs in low‐ and middle‐income countries assessed change in cognitive development. The outcome measures in these studies were too different conceptually to be included in a meta‐analysis.
For McKay 1978, we compared results for T4 children (supplemented with stimulation from 42 to 84 months of age) to those of T2 children (supplemented from 63 to 84 months of age) at 63 months. Our analysis (n = 99) found that cognitive ability of the supplemented children improved more than the children who were not yet supplemented (SMD 0.58, 95% CI 0.17 to 0.98; Analysis 6.2).
6.2. Analysis.

Comparison 6 Low‐ and middle‐income countries: feeding vs control ‐ psychosocial development. RCT, Outcome 2 Cognitive development: test battery.
Our analysis of Husaini 1991 (n = 113) found a non‐significant difference in change on the Bailey Scales of Mental Development (BSMD) (SMD ‐0.40, 95% CI ‐0.79 to ‐0.00); Analysis 6.3).
6.3. Analysis.

Comparison 6 Low‐ and middle‐income countries: feeding vs control ‐ psychosocial development. RCT, Outcome 3 Cognitive development: Bayley's Mental Development Index (BMDI).
Pollitt 2000a found no main effects of supplementation on the BSMED (Bailey Scales of Mental Development). They reported positive effects in a contrast over time for the younger cohort but not for the older cohort (F₂, ₄₈= 4.58, P < 0.05; n = 53).
None of the CBAs in low‐ and middle‐income countries or RCTs and CBAs in high‐income countries assessed cognitive development.
Long‐term follow‐up of cognitive development
Low‐ and middle‐Income countries: RCTs
Grantham‐McGregor 1997 followed up 97% (n = 127) of the original cohort of stunted children (Grantham‐McGregor 1991; n = 129) after four years and tested them on a battery of cognitive and perceptual tests. A multiple regression found effects on perceptual motor tasks, but not on general cognition or memory. Interestingly, stimulation had a significant effect on later perceptual motor skills for all children (P < 0.05), but supplementation only had a significant effect for children whose mothers had higher scores on a test of verbal intelligence. (P < 0.05). Grantham‐McGregor 2007 also found that the supplemented children had higher average scores than the controls on 14 out of 15 cognitive tests (P value = 0.02).
Pollitt 1997 performed a seven‐year follow‐up of Husaini 1991. They found no differences between the experimental (n = 125) and control (n = 106) groups in the Peabody Picture Vocabulary Test (PPVT), emotionality, and maths. They did find small, (15‐second difference) positive effects of supplementation on working memory performance, although these are unlikely to be clinically significant.
None of the CBAs in low‐ and middle‐income countries or RCTs and CBAs in high‐income countries assessed long‐term follow‐up of cognitive development.
General development
Low‐ and middle‐Income countries: RCTs
Oelofse 2003 (n = 60) found no significant differences on the Denver Developmental Screening Test (DDST) between the group of South African infants (aged six months at baseline) given a micronutrient‐fortified supplement for six months and control infants.
None of the CBAs in low‐ and middle‐income countries or RCTs and CBAs in high‐income countries assessed general development.
Attention, Language and Memory
We found no reports of effects on attention or memory. For language, Pollitt 2000a reported that supplemented children in the younger cohort (n = 53) had greater increases in vocalisations over time than those who were not given supplementary feeding.
Primary outcomes: Adverse effects
Substitution or leakage
We were able to calculate the net benefit from supplementary feeding for seven studies that provided home‐delivered rations (RCTs: Bhandari 2001; De Romana 2000; Grantham‐McGregor 1991Rivera 2004; CBAs: Lutter 2008; Santos 2005; Tomedi 2012) and three of the day‐care/feeding centre studies (RCTs: Husaini 1991; Pollitt 2000a; CBA: Devadas 1971). We found important differences in the number of calories provided by the supplementary food and the number of extra calories that the children actually consumed in addition to their regular food. In the take‐home studies, we found that the net benefit to children was only 36% of the extra calories provide by the supplement. In the day‐care and feeding centres, the net benefit was 85% of the extra calories provided by the supplement.
Secondary outcomes: Physical health
Biochemical markers of nutrition
Low‐ and middle‐income countries: RCTs
Five RCTs (300 children) in low‐ and middle‐income countries (Husaini 1991; Kuusipalo 2006; Oelofse 2003; Rivera 2004; Thakwalakwa 2010) contributed to the meta‐analysis for haemoglobin. We found a significant effect of supplementation (SMD 0.49, 95% CI 0.07 to 0.91; Analysis 8.1); children who were supplemented showed positive change in haemoglobin status compared to controls. There was significant heterogeneity (Chi² = 10.78, df = 4, P value = 0.03; I² = 63%).
8.1. Analysis.
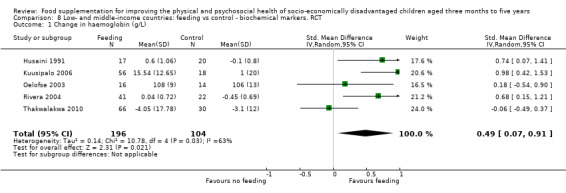
Comparison 8 Low‐ and middle‐income countries: feeding vs control ‐ biochemical markers. RCT, Outcome 1 Change in haemoglobin (g/L).
Low‐ and middle‐income countries: CBAs
Among the CBAs, Lutter 2008 reported a significant effect of supplementation on the risk of anaemia (P value = 0.003; n = 110 at final survey); those who were supplemented had lower risk of being anaemic (OR 0.58, 95% CI 0.24 to 0.75). Similarly, De Romana 2000 (n = 250) reported that while the prevalence of anaemia decreased by 27% in the intervention group, it decreased by only 13% in the control group.
High‐income countries: RCTs
Yeung 2000 (103 children) found no significant difference between the experimental and control group in change in haemoglobin.
High‐income countries: CBAs
Coyne 1980 (116 children) reported an increase in the number of children who had low haemoglobin levels in the experimental group and a decrease in the corresponding number in the control group.
Physical activity
Low‐ and middle‐income countries: RCTs
Pollitt 2000a (see Jahari 2000) reported a significant main effect of supplementation on motor activity in the youngest (12‐month‐old; n = 53) cohort (F₂,₄₈ = 4.8, P < 0.05). Over the 12‐month period of the supplementation, the supplemented group had significantly greater increases in high energy cost motor activity that began at 18 months of age and continued to the end of the study (24 months) (P < 0.05).
Grantham‐McGregor 1991 found no significant effect of supplementation alone (n = 26) or supplementation plus stimulation (n = 26) on changes in motor activity in stunted children (see Meeks Gardner 1999).
No CBAs in low‐ and middle‐income countries and no RCTs or CBAs in high‐income countries assessed physical activity.
Morbidity
Six studies (four RCTs; two CBAs) reported on morbidity. Three RCTs (Bhandari 2001; Iannotti 2014; Isanaka 2009) and two CBAS (Gopalan 1973; Tomedi 2012) found few differences between the supplemented group and the control group in the prevalence of morbidity. Roy 2005 (a CBA) reported mixed results; the prevalence of diarrhoea and fever was higher in the children who received supplementation (n = 99), while the prevalence of respiratory infection was higher in the control group (n = 90).
Mortality
Low‐ and middle‐income countries: RCTs
Isanaka 2009 reported that there was no significant difference in mortality between children supplemented with ready‐to‐use therapeutic feeding (RUTF; n = 1671) and those who were unsupplemented (adjusted hazard ratio (HR) 0.76, 95% CI 0.51 to 1.13; n = 1862).
No CBAs in low‐ and middle‐income countries and no RCTs or CBAs in high‐income countries assessed mortality.
Overweight or obesity
There were no reports of overweight or obesity in the included studies.
Secondary outcomes: Psychosocial outcomes
Stigmitisation and behaviour problems
There were no reports of stigmatisation or behaviour problems in the included studies.
Subgroup analyses
We conducted subgroup analyses across seven categories: age, sex, socio‐economic disadvantage (poor versus less poor or undernourished versus well‐nourished), nutritional adequacy, location of feeding, level of supervision (monitoring), and single intervention versus multiple interventions.
Age
We conducted subgroup analyses to explore the possible impact of age on weight and height. For the RCTs, we compared the following age groups: < 12 months, one to two years, and > 2 years. The age groups for the CBAs were: < 1 year, 1 year, 2 years, and > 2 years.
Weight
We found no significant differences in either the subgroup analyses of nine RCTs (Chi² = 1.95, df = 2, P value = 0.38, I² = 0%; n = 1057; Analysis 1.1) or that of seven CBAs (Chi² = 5.7, df = 3, P value = 0.13, I² = 47.4%; n = 1784; Analysis 3.1).
Height
This analysis showed significant subgroup differences (Chi² = 6.01, df = 2, P value = 0.05, I² = 66.7%). Supplementary feeding was effective for the youngest age groups (< 12 months: MD 0.22, 95% CI 0.05 to 0.39, 7 trials, n = 1316; and 1 to 2 years: MD 0.9, 95% CI 0.33 to 1.47, 1 trial, n = 65), while the height gains in the oldest age group (> 2 years old) were non‐significant (1 trial, n = 82; Analysis 1.2).
Seven CBAs (n = 1782) in low‐ and middle‐income countries contributed to this subgroup analysis. There were no significant differences among subgroups (Chi² = 0. 63, df = 3, P value = 0.89, I² = 0%) and no discernible pattern by age (Analysis 3.2).
Psychomotor performance
Pollitt 2000a reported that supplementation had greater impacts on psychomotor development for the younger (12‐month‐old) cohort (n = 53; see Jahari 2000).
Sex
Our subgroup analysis to explore effectiveness by sex comprised two CBAs from low‐ and middle‐income countries (Gershoff 1988; Mittal 1980) and 840 children. There were no significant subgroup differences in either the analysis for weight (Chi² = 0.06, df = 1, P value = 0.80, I² = 0%; Analysis 9.1) or height (Chi² = 0.54, df = 1, P value = 0.46, I² = 0%; Analysis 9.2).
9.1. Analysis.
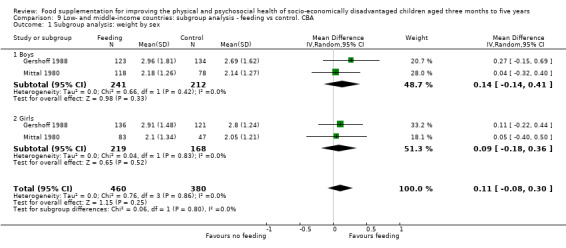
Comparison 9 Low‐ and middle‐income countries: subgroup analysis ‐ feeding vs control. CBA, Outcome 1 Subgroup analysis: weight by sex.
9.2. Analysis.
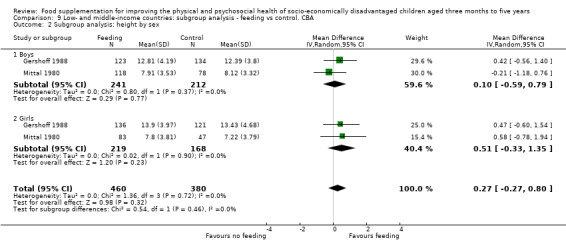
Comparison 9 Low‐ and middle‐income countries: subgroup analysis ‐ feeding vs control. CBA, Outcome 2 Subgroup analysis: height by sex.
Pollitt 2000a found stronger effects on weight and height for girls (n = 58) than for boys (n = 57); the interaction was significant only at the 0.10 level (see Beckett 2000). Coyne 1980 found that supplemented girls (n = 61) benefited from the intervention, but that supplemented boys (n = 55) did not.
Socio‐economic disadvantage: poor versus less poor; undernourished versus well‐nourished
Growth
Weight: we compared subgroups from Thakwalakwa 2010 and found significant differences in effectiveness between undernourished and well‐nourished children (Chi² = 4.76, df = 1, P value = 0.03 I² = 79%; 1 trial, n = 192; Analysis 10.1). Supplementary feeding of the undernourished children resulted in significant weight gain of 0.34 kg, (95% CI 0.18 to 0.50) relative to controls, while the intervention was ineffective for well‐nourished children at 0.08 kg. (95% CI ‐0.09 to 0.25). Gopalan 1973 found that children with low baseline WAZ gained more weight than controls while those whose WAZ was higher did not (n = 293) (see Rao 1977).
10.1. Analysis.
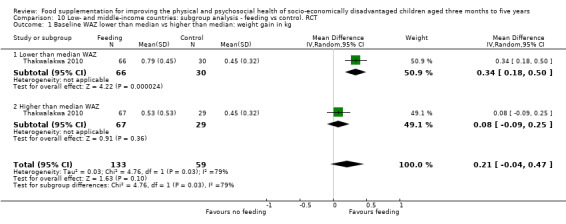
Comparison 10 Low‐ and middle‐income countries: subgroup analysis ‐ feeding vs control. RCT, Outcome 1 Baseline WAZ lower than median vs higher than median: weight gain in kg.
Height: we compared subgroups from Thakwalakwa 2010 and found non‐significant differences in effectiveness between undernourished and well‐nourished children (Chi² = 0.79, df = 1, P value = 0.38 I² = 0%; 1 trial, n = 192; Analysis 10.2). Rivera 2004 (n = 631) and Schroeder 2002 (n = 232 (but with no denominators reported for that particular analysis) both reported significant interactions between age, nutritional status and feeding; supplemented children who were poorer AND younger (< 6 months of age) at baseline grew more in height (Rivera 2004).
10.2. Analysis.
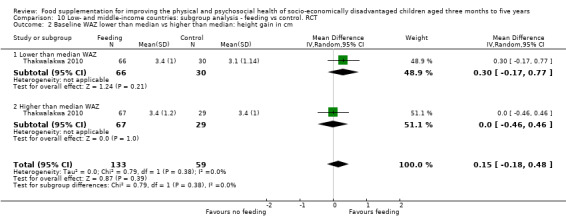
Comparison 10 Low‐ and middle‐income countries: subgroup analysis ‐ feeding vs control. RCT, Outcome 2 Baseline WAZ lower than median vs higher than median: height gain in cm.
Grantham‐McGregor 1991(n = 129) found that children who were more undernourished at baseline were more likely to gain more skinfold thickness than controls.
Two studies (one RCT: Husaini 1991; n = 113) and one CBA: Gershoff 1988) found no relationship between initial nutritional status, supplementation, and growth. Finally, Joshi 1988 (n = 247) reported that supplementary feeding was more effective for children living in areas of moderate socio‐economic status than for children living in slums. He suggested that poor environmental conditions may have reduced the effectiveness of the intervention.
Psychosocial outcomes
Husaini 1991 (n = 113) found no significant interaction between baseline nutritional status and treatment.
Nutritional adequacy
We explored the hypothesis that interventions which provided better nutritional adequacy (more calories) would be more effective.
Weight
The subgroup analysis for weight included eight RCTs with 975 children. There were no significant subgroup differences (Chi² = 0.63, df = 2, P value = 0.73, I² = 0%; Analysis 10.3).
10.3. Analysis.
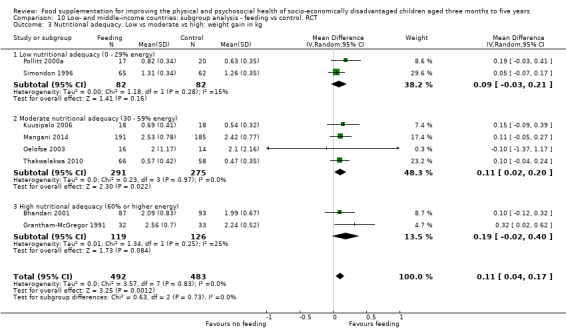
Comparison 10 Low‐ and middle‐income countries: subgroup analysis ‐ feeding vs control. RCT, Outcome 3 Nutritional adequacy. Low vs moderate vs high: weight gain in kg.
There were seven CBAs (1784 children) in the subgroup analysis for nutritional adequacy and weight. These included: Devadas 1971; Gershoff 1988; Gopalan 1973; Lutter 2008; Manjrekar 1986; Mittal 1980; and Santos 2005. There was no significant subgroup effect (Chi² = 3.35, df = 2, P value = 0.19, I2 = 40.3%; Analysis 9.3).
9.3. Analysis.
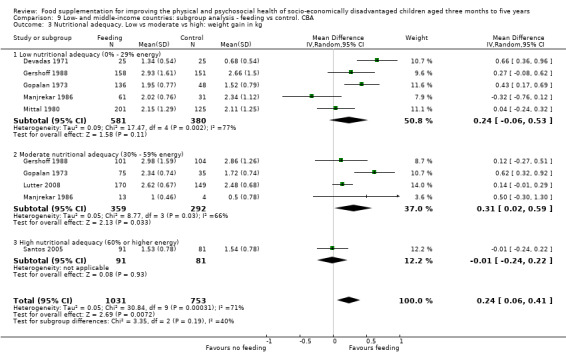
Comparison 9 Low‐ and middle‐income countries: subgroup analysis ‐ feeding vs control. CBA, Outcome 3 Nutritional adequacy. Low vs moderate vs high: weight gain in kg.
Height
The subgroup analysis for height contained eight RCTs (Bhandari 2001; Grantham‐McGregor 1991; Kuusipalo 2006; Mangani 2014; Oelofse 2003; Rivera 2004; Simondon 1996; Thakwalakwa 2010) with 1381 children. There were no significant subgroup differences (Chi² = 2.72, df = 2, P value = 0.26, I² = 26.4%; Analysis 10.4).
10.4. Analysis.
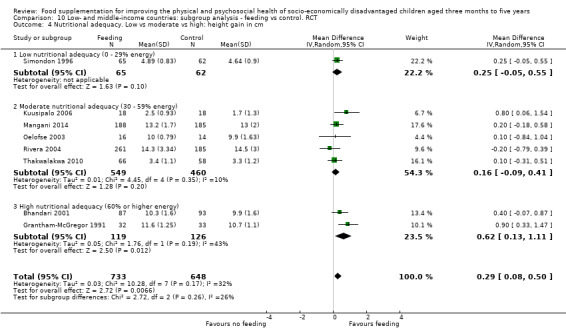
Comparison 10 Low‐ and middle‐income countries: subgroup analysis ‐ feeding vs control. RCT, Outcome 4 Nutritional adequacy. Low vs moderate vs high: height gain in cm.
Seven CBAS (1782 children) contributed to the subgroup analysis for nutritional adequacy and height. These included: Devadas 1971; Gershoff 1988; Gopalan 1973; Lutter 2008; Manjrekar 1986; Mittal 1980; Santos 2005. This analysis showed no subgroup effects (Chi² = 2.29, df = 2, P value = 0.32, I² = 12.5%; Analysis 9.4).
9.4. Analysis.
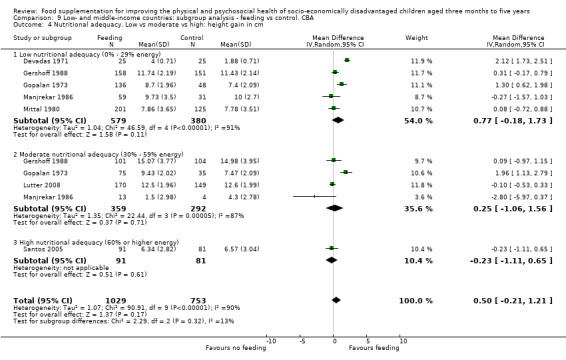
Comparison 9 Low‐ and middle‐income countries: subgroup analysis ‐ feeding vs control. CBA, Outcome 4 Nutritional adequacy. Low vs moderate vs high: height gain in cm.
Location of feeding (day care or preschool or feeding centre versus home)
Weight
The subgroup analysis for weight contained one RCT in day care (Pollitt 2000a) and eight RCTs that provided take‐home or home‐delivered rations. There was no significant subgroup effect (Chi² = 0.62, df = 1, P value = 0.43, I² = 0%; n = 1057; Analysis 10.5).
10.5. Analysis.
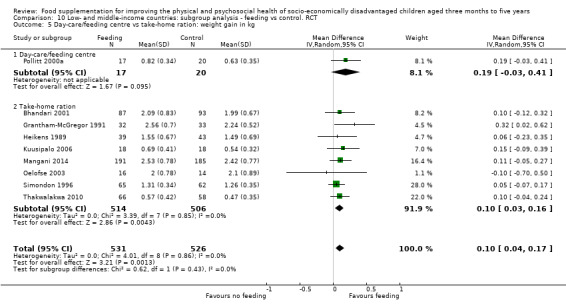
Comparison 10 Low‐ and middle‐income countries: subgroup analysis ‐ feeding vs control. RCT, Outcome 5 Day‐care/feeding centre vs take‐home ration: weight gain in kg.
The subgroup analysis for CBAs compared four studies in preschools or feeding centres (Devadas 1971; Gershoff 1988; Gopalan 1973; Manjrekar 1986; n = 967) with three studies that gave take‐home rations (Lutter 2008; Mittal 1980; Santos 2005; n = 817). We found no significant subgroup effects (Chi² = 1.84, df = 1, P value = 0.18, I² = 45.6%; Analysis 9.5). Sensitivity analyses with ICCs at 0.10 made no significant difference (Analysis 9.11).
9.5. Analysis.
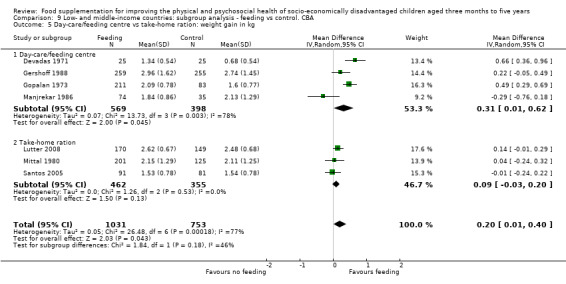
Comparison 9 Low‐ and middle‐income countries: subgroup analysis ‐ feeding vs control. CBA, Outcome 5 Day‐care/feeding centre vs take‐home ration: weight gain in kg.
9.11. Analysis.
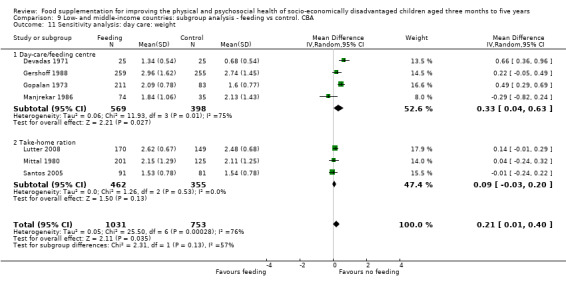
Comparison 9 Low‐ and middle‐income countries: subgroup analysis ‐ feeding vs control. CBA, Outcome 11 Sensitivity analysis: day care: weight.
Height and location
We were unable to perform this subgroup analysis for RCTs as there were no suitable data for meta‐analysis.
The subgroup analysis for height and location for the CBAs compared four studies in preschools or feeding centres (Devadas 1971; Gershoff 1988; Gopalan 1973; Manjrekar 1986) with three studies that provided take‐home rations (Lutter 2008; Mittal 1980; Santos 2005). There were no significant subgroup differences (Chi² = 2.52, df = 1, P value = 0.11 I² = 60.3%; n = 1782; Analysis 9.6). Sensitivity analyses with ICCs at 0.10 made little difference (Analysis 9.12).
9.6. Analysis.
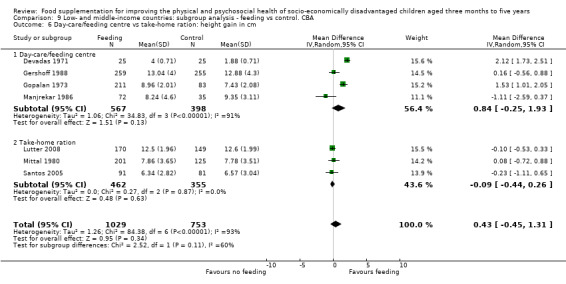
Comparison 9 Low‐ and middle‐income countries: subgroup analysis ‐ feeding vs control. CBA, Outcome 6 Day‐care/feeding centre vs take‐home ration: height gain in cm.
9.12. Analysis.
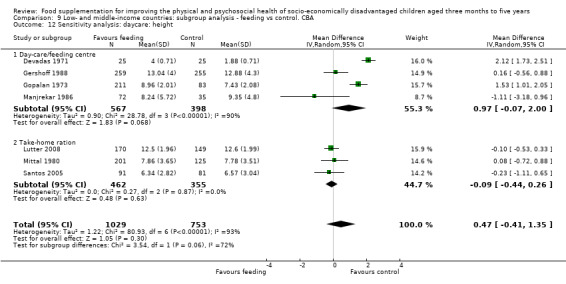
Comparison 9 Low‐ and middle‐income countries: subgroup analysis ‐ feeding vs control. CBA, Outcome 12 Sensitivity analysis: daycare: height.
Level of supervision
The studies were divided into strict, moderate, and no supervision (i.e. monitoring) of the supplementary feeding according to the principles outlined above in the Methods section.
Weight and supervision
Among the RCTs, five studies (Bhandari 2001; Heikens 1989; Mangani 2014; Pollitt 2000a; Simondon 1996) were classified as strictly supervised and four (Grantham‐McGregor 1991; Kuusipalo 2006; Oelofse 2003; Thakwalakwa 2010) were classified as moderately supervised. There were no significant subgroup differences (Chi² = 0.50, df = 1, P value = 0.48, I² = 0%; n = 1056; Analysis 10.6).
10.6. Analysis.
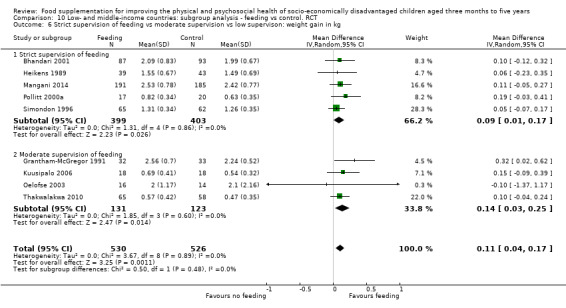
Comparison 10 Low‐ and middle‐income countries: subgroup analysis ‐ feeding vs control. RCT, Outcome 6 Strict supervision of feeding vs moderate supervision vs low supervison: weight gain in kg.
Among the CBAs, five studies were strictly supervised (Devadas 1971; Gershoff 1988; Gopalan 1973; Lutter 2008; Manjrekar 1986), one was moderately supervised (Mittal 1980), and one had little supervision (Santos 2005). This analysis showed non‐significant differences among the subgroups (Chi² = 3.04, df = 2, P value = 0.22, I² = 34.4%; n = 1784; Analysis 9.7).
9.7. Analysis.
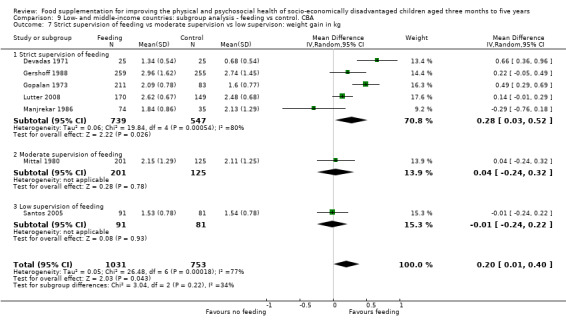
Comparison 9 Low‐ and middle‐income countries: subgroup analysis ‐ feeding vs control. CBA, Outcome 7 Strict supervision of feeding vs moderate supervision vs low supervison: weight gain in kg.
Height
Four studies (Bhandari 2001; Heikens 1989; Mangani 2014; Simondon 1996) were classified as strictly supervised and five (Grantham‐McGregor 1991; Kuusipalo 2006; Oelofse 2003; Rivera 2004; Thakwalakwa 2010) were classified as moderately supervised. There were no significant subgroup differences (Chi² = 0.11, df = 1, P value = 0.74; n = 1463 children; Analysis 10.7).
10.7. Analysis.
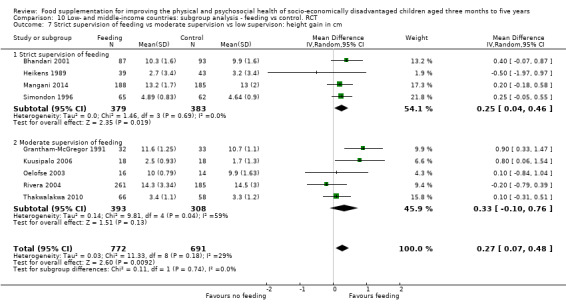
Comparison 10 Low‐ and middle‐income countries: subgroup analysis ‐ feeding vs control. RCT, Outcome 7 Strict supervision of feeding vs moderate supervision vs low supervison: height gain in cm.
Among the CBAs, five studies were strictly supervised (Devadas 1971; Gershoff 1988; Gopalan 1973; Lutter 2008; Manjrekar 1986), one was moderately supervised (Mittal 1980), and one provided little supervision (Santos 2005). This analysis showed no significant differences among subgroups (Chi² = 1.41, df = 2, P value = 0.49, I² = 0%; n = 1782; Analysis 9.8).
9.8. Analysis.
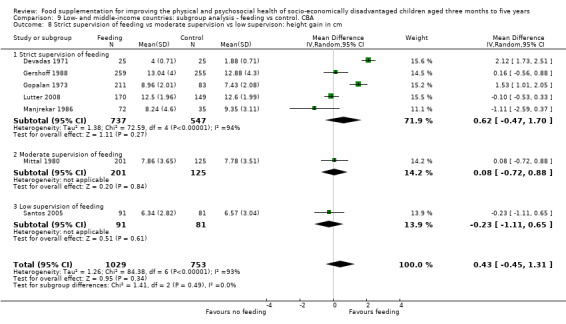
Comparison 9 Low‐ and middle‐income countries: subgroup analysis ‐ feeding vs control. CBA, Outcome 8 Strict supervision of feeding vs moderate supervision vs low supervison: height gain in cm.
Single intervention versus multiple interventions
Weight
Nine RCTs (Bhandari 2001; Grantham‐McGregor 1991 (feeding only); Heikens 1989; Kuusipalo 2006; Mangani 2014; Oelofse 2003; Pollitt 2000a; Simondon 1996; Thakwalakwa 2010; n = 1040) were classified as single interventions and one study (Grantham‐McGregor 1991: supplementation + stimulation; n = 49) was classified as a multiple intervention. There were no significant subgroup effects (Chi² = 2.59 df = 1, P value = 0.11, I² = 61.3%; Analysis 10.8).
10.8. Analysis.
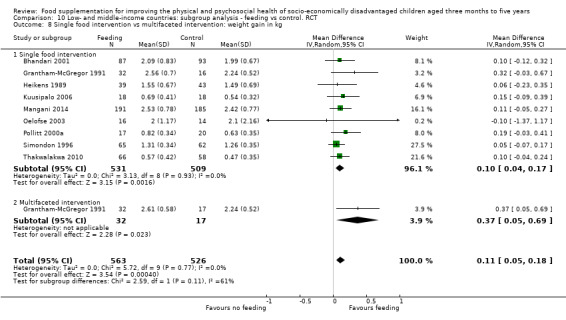
Comparison 10 Low‐ and middle‐income countries: subgroup analysis ‐ feeding vs control. RCT, Outcome 8 Single food intervention vs multifaceted intervention: weight gain in kg.
Four CBAs (Gopalan 1973; Manjrekar 1986; Mittal 1980; Santos 2005) were classified as single interventions while three (Devadas 1971; Gershoff 1988; Lutter 2008) provided multiple interventions. There were no significant subgroup differences (Chi² = 0.00, df = 1, P value = 0.99, n = 1784; Analysis 9.9).
9.9. Analysis.
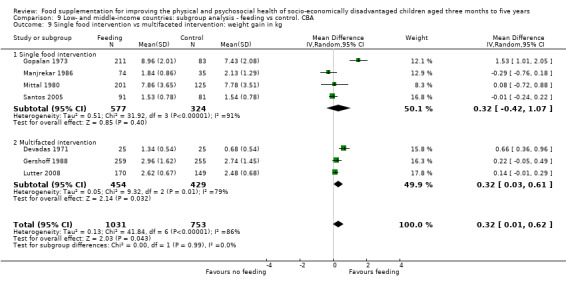
Comparison 9 Low‐ and middle‐income countries: subgroup analysis ‐ feeding vs control. CBA, Outcome 9 Single food intervention vs multifaceted intervention: weight gain in kg.
Height
Eight RCTs (Bhandari 2001; one arm of Grantham‐McGregor 1991; Heikens 1989; Kuusipalo 2006; Mangani 2014; Oelofse 2003; Simondon 1996; Thakwalakwa 2010) provided feeding only and two RCTs (Grantham‐McGregor 1991 (supplementation + stimulation); Rivera 2004) were classified as multiple interventions. There were no significant subgroup differences for height (Chi² = 0.04, df = 1, P value = 0.84, I² = 0%; n = 1512; Analysis 10.9; Figure 5).
10.9. Analysis.
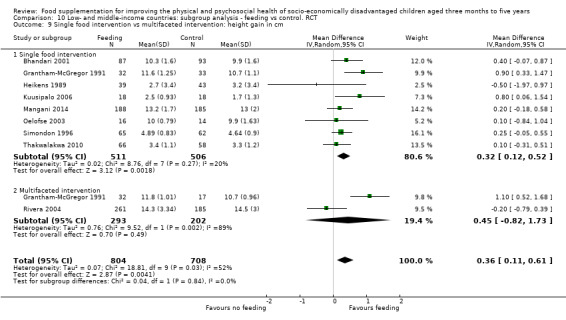
Comparison 10 Low‐ and middle‐income countries: subgroup analysis ‐ feeding vs control. RCT, Outcome 9 Single food intervention vs multifaceted intervention: height gain in cm.
5.
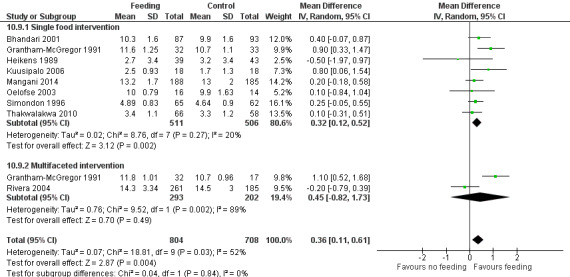
Forest plot of comparison: 11 Low‐ and middle‐income countries: subgroup analysis ‐ feeding vs control. RCT. Outcome: 11.9 Single food intervention vs multifaceted intervention: height gain in cm
Four CBAs (Gopalan 1973; Manjrekar 1986; Mittal 1980; Santos 2005) were classified as single interventions while three (Devadas 1971; Gershoff 1988; Lutter 2008) provided multiple interventions. There were no significant subgroup differences (Chi² = 0.32 df = 1, P value = 0.57, n = 1782; (Analysis 9.10).
9.10. Analysis.
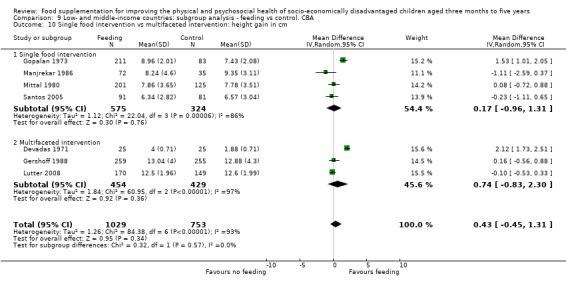
Comparison 9 Low‐ and middle‐income countries: subgroup analysis ‐ feeding vs control. CBA, Outcome 10 Single food intervention vs multifaceted intervention: height gain in cm.
Psychosocial outcomes
We compared two RCTs that provided feeding only (Grantham‐McGregor 1991, n = 32; Husaini 1991, n = 75) with the supplementation + stimulation group from Grantham‐McGregor 1991 (n = 32) and found non‐significant subgroup differences (Chi² = 2.34, df = 1, P value = 0.13, I² = 57.3%; Analysis 10.10).
10.10. Analysis.
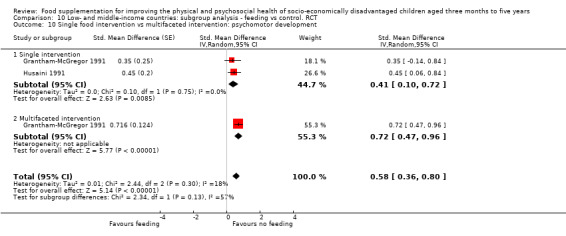
Comparison 10 Low‐ and middle‐income countries: subgroup analysis ‐ feeding vs control. RCT, Outcome 10 Single food intervention vs multifaceted intervention: psychomotor development.
Exploring heterogeneity
Analysis 3.3, Analysis 3.5, Analysis 5.3, and Analysis 9.10 were highly heterogeneous with I² values above 90%. We checked this in several ways. First, we examined any potential errors in data entry and found none. Second, we performed sensitivity analyses, taking out each study in these analyses one by one. We found that deleting Tomedi 2012 resulted in the largest drop in heterogeneity in analyses Analysis 3.3, Analysis 3.5, Analysis 5.3. We then compared Tomedi 2012 to the other studies and found that it had very good implementation procedures, including a provision of a high percentage of the recommended daily allowance, nutrition education, and take‐home rations for other children in the family. For Analysis 9.10 , we found the largest drop in heterogeneity when we deleted Gopalan 1973, but heterogeneity was still high at 70%.
Discussion
Summary of main results
After screening almost 33,000 references, we included 32 studies. These studies spanned the years from 1971 to 2014 and covered 22 countries.
Below, we summarise the major findings from the review.
Growth
Supplementary feeding young children has a small effect on gain in weight and weight‐for‐age z‐scores (WAZ) in low‐ and middle‐income countries
Of the randomised controlled trials (RCTs) in low‐ and middle‐income countries, meta‐analyses of weight gain (nine trials, 1057 children) and WAZ gain (eight trials, 1565 children) showed increases for children who were supplemented compared to those who were unsupplemented. However, these differences were small (0.12 kg for weight and 0.15 for WAZ over a period of six months).
Results from high‐income countries were mixed. An American study of infants from predominantly middle‐class families found no effects. However, large gains of 0.95 kg relative to controls over four months were realized in a study among 116 Aboriginal children in remote Australian communities; if a similar trajectory were maintained for a year, the children who were fed would have gained 2.85 kg. This may be because the Aboriginal children were less well nourished at baseline than those in the American study. In Australia, Aboriginal families are more likely to suffer food insecurity than non‐Aboriginal families (24% compared to 5%; Browne 2009).
Supplementary feeding for young children has a small effect on linear growth in low‐ and middle‐income countries
The meta‐analysis of the RCTs (nine trials, 1463 children) revealed that those who received supplementary food grew 0.27 cm more than controls over an average of six months. Results for height‐for‐age z‐scores (HAZ) in the RCTs also revealed a small impact: over five months children who received food supplementation (nine trials, 4638 children) gained 0.15 more than controls.
Psychosocial development
Supplementary feeding may have a moderate positive effect on psychomotor development in low‐ and middle‐income countries
While nearly all of the studies assessed growth, only eight assessed psychosocial outcomes in response to supplementary feeding.
Our meta‐analysis of two RCTs in low‐ and middle‐income countries (178 children) found greater gains in psychomotor development for children who were supplemented. Two other RCTs reported equivocal results.
The evidence on attainment of motor milestones is equivocal. Two studies (249 children) revealed that supplemented children reached motor milestones earlier, but the effects in one of them disappeared after maternal education was entered into the equation. Another study (747 children) found no differences.
The evidence of effects on cognitive development in low‐ and middle‐income countries is sparse and mixed
Our analyses of one study found effectiveness, our analysis of another study did not, and evidence from a third study was mixed.
There is sparse evidence that feeding may result in long‐term gains in intelligence or cognition in low‐ and middle‐income countries
One RCT (n = 129) found long‐term effects of supplementation and stimulation on perceptual motor skills. The effects of supplementation alone were limited to those children whose mothers had high scores on verbal intelligence at baseline while the effects of supplementation AND stimulation extended to all children. This suggests that supplementary feeding may be most effective if mothers have higher capacity to feed and stimulate their children. Another study (73 children) found that supplementation had very small long‐term positive impacts on working memory but not on reaction time or math performance.
Supplementary feeding results in increased haemoglobin and lowered anaemia in low‐ and middle‐income countries
Evidence from five RCTS (300 children) revealed a positive effect of supplementary feeding on haemoglobin that was equal to half of a standard deviation. Evidence from two controlled before‐and‐after studies (CBAs) (261 children) found that supplementary feeding reduced the risk of anaemia.
Overall completeness and applicability of evidence
We believe that our review provides very comprehensive coverage of the literature. We screened almost 33,000 studies from a well‐designed literature search and we carefully scanned reference lists of included studies and of reviews. Our included studies covered many countries and regions, including Latin America, Africa, Asia, North America, and Australia. Studies in low‐ and middle‐income countries predominated; this is not surprising, as 81% of the world's people who suffer from hunger live in them (World Hunger Education Service 2012). However, it does mean that results of the review are probably not generalisable to high‐income countries.
We found a range of feeding interventions, a variety of foods and a range of nutritional adequacy, different modes of delivery, and methods for implementation. Effects on outcomes are mixed and complex but subgroup analyses suggested some important hypotheses.
The effect sizes for weight and height were smaller than we expected. However, our finding of small effects on growth is consistent with Beaton 1982. In the past, the failure to show consistent effects on growth has been attributed to the use of inappropriate indicators of growth as well as to poor targeting of the intervention (Rivera 1991). In our review, we considered several indicators of growth and separately analysed interventions targeted at children under two years of age, a period in which linear growth velocity is highest (Baumgartner 1986). Many of the newer interventions were based on the latest scientific findings about composition of the supplements. However, for programmes to effect changes in growth and to be sustainable, there has to be a balance between nutritional science and feasibility of implementation (Griffiths 2000). For example, in three included studies (Bhandari 2001; Grantham‐McGregor 1991; Kuusipalo 2006), with particularly good implementation (moderate or strict supervision of feeding, provision of moderate to high nutritional adequacy), we found an average height gain of 0.76 cm (95% CI 0.30 to 1.22; n = 281) over nine months (Analysis 10.11). This finding supports the postulation that there is potential for a substantially larger effect on growth if feeding programmes are well implemented (Dewey 2008).
10.11. Analysis.
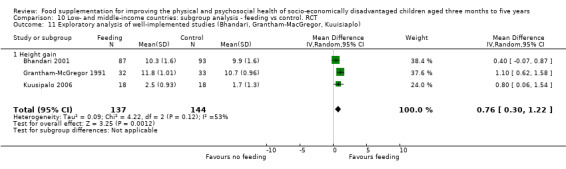
Comparison 10 Low‐ and middle‐income countries: subgroup analysis ‐ feeding vs control. RCT, Outcome 11 Exploratory analysis of well‐implemented studies (Bhandari, Grantham‐MacGregor, Kuuisiaplo).
The evidence base on psychosocial effects of supplementation is rather sparse; we found that only eight of 32 studies assessed psychosocial outcomes. We found some evidence for positive effects of feeding on psychomotor development and sparse mixed reports on cognition. Our findings on psychomotor development support Pollitt 1994. Interestingly, the effect sizes for psychomotor development were overall larger than those found for growth. There could be several reasons for this. First, most of the studies on motor development were among those that demonstrated better implementation, including higher nutritional adequacy. Second, they were relatively small studies and so were able to have tighter control over the intervention. Third, there were fewer studies on psychomotor development; it is possible that with more studies, effects might be diluted. Finally, the pathways between psychosocial development are probably different. It is possible that psychosocial outcomes are more sensitive to nutritional intervention (Dewey 2008). The concept of 'brain sparing' may be relevant here. This refers to the hypothesis that when nutritional resources are scarce early in life, they are preferentially directed to the developing brain at the expense of other parts of the body (Auestad 2000; Lumbers 2001). This is supported by animal studies (Seidler 1990). Brain sparing has been shown to occur during intra‐uterine growth and the neonatal period, resulting in slowed body growth (height and weight) with normal brain growth. Brain sparing has also been shown in the context of micronutrient deficiencies (Golub 1995). This suggests that supplementary energy, protein and micronutrients given to a child may be used for brain development first and then for growth and other aspects of health.
The possible link between increased nutrition and psychomotor and mental development is complex and involves a number of possible mechanisms. Such mechanisms include increased mylenation, increased alertness and curiosity (Meeks Gardner 1995), and increased motor activity resulting in enhanced motor development and consequently improved mental development (Pollitt 2000b). This latter mechanism is somewhat controversial; while support for this was found in the Tea Plantation study (Pollitt 2000b), the Jamaican study (Grantham‐McGregor 1991, reported in Meeks Gardner 1995), found that supplementation did not increase motor activity; they also found no effect of motor activity on later development. They suggested that effects of nutrition on increased motor activity might be dependent on context or age of the child, or both, and hypothesised that the quality of play and exploration might be more important for child development than the quantity of increased activity. Clearly, there is a need for more carefully developed studies on the mechanisms that link improved nutrition to psychosocial development.
Quality of the evidence
Feeding interventions for young children are complex interventions that are difficult and fairly costly to implement. Studying them therefore requires consideration of a number of factors pertaining to the context, the family, and the children.
Our judgements on the quality of the evidence ranged from very low (CBAs) to moderate (RCTs). However, it is important to note there are many old studies in the review, and that the quality of the studies, in terms of both design and implementation, has improved markedly in the last 10 to 15 years. In general, we placed more weight on the RCTs when drawing our conclusions.
One important problem was attrition rates. Among those that provided them, these rates ranged from 1% to 78%; 10 studies had attrition rates above 20%. Correspondingly, most of the analyses were conducted on completers rather than on an intention‐to‐treat (ITT) principle.
Another issue is that authors of several studies did not mention whether those who assessed study outcomes were blinded to the allocation status of the children. Blinding of outcome assessment is crucial in order to ensure that the outcome measurement is not influenced by assessors whose knowledge of the expected outcome may subtly influence their assessment (Viera 2007).
Finally, 10 study authors did not adequately control for clustering in their analyses. We adjusted for clustering for eight of them, but could not do so for the other two as we did not have access to the standard deviations.
Factors that may impact on effectiveness
As mentioned above, the findings of the review are mixed and complex. Furthermore, many of the effects are small, and we believe could be larger with improved implementation. Our process evaluation and related subgroup analyses shed some light on the reasons for this. In interpreting the subgroup analyses, we considered the evidence from both RCTs and CBAs to be important, but put slightly more emphasis on evidence from the RCTs as they provide stronger evidence for causation.
Age
Children who were younger at the start of the study may grow more in height/length than older children in response to supplementary feeding; results were mixed for weight
Our hypothesis that younger children would grow more in response to supplementary feeding was largely upheld. For example, our subgroup analysis for height (1463 children) was significant. Children in the two younger subgroups (< 12 months and ages one to two years; 1057 children; Analysis 1.2) gained significant amounts of weight, but those in the older age groups did not. There was no evidence of subgroup differences for weight, but the children in the two younger subgroups were the only ones who gained significant amounts of weight.
The meta‐analysis of the CBAs showed no subgroup differences for either weight (1784 children) or height (1782 children). For weight, children who were two years old gained more weight than controls, while the older and younger groups did not.
Our findings are consistent with those of Beaton 1992 and Dewey 2008, who concluded that feeding interventions can have maximum impact on linear growth if they are started in infancy, as the period between six and 24 months is a period of rapid growth (Dewey 2008). It is important to note that feeding can also have an impact on linear growth in older children (Beaton 1992). In fact, our review of school meals found that linear growth in school‐aged children increased by 0.25 to 1.47 cm per year (Galloway 2009; Kristjansson 2007). But it does mean that, for greatest impact on growth, and to help slow the rate of growth faltering, feeding should start when children are well below two years of age.
Only one of the studies (n = 53) reporting psychosocial outcomes assessed the impact of age; the authors report that feeding only benefited younger children (< 18 months).
Sex
The evidence is sparse but generally indicates few sex differences
Gender equity is an important consideration in low‐ and middle‐income countries. In some contexts, there is a family preference for favouring male adults and children in the distribution of food within the family. This was found in a qualitative study in Guatemala (Engle 1992b), in surveys in Bangladesh and the Philippines (Haanga 1987), and was reported in one of our included studies (Roy 2005). Thus, the question of whether boys and girls benefit equally from feeding interventions is an important one.
Our subgroup analyses of two CBAS (840 children) found no difference in effectiveness by sex. However, two CBAs (211 children) reported stronger effects on growth for girls than for boys. This latter finding is consistent with analyses from the Oriente Longitudinal Study, which found that girls benefited from supplementation more than boys in terms of growth and cognition (Engle 1992b). We cannot draw firm conclusions from this data as only two studies were included in the analysis. This should be explored further, both quantitatively and qualitatively, in future research.
Socioeconomic status or initial nutritional status
Children who are poorer or more undernourished at baseline may grow more in response to supplementary food
Our hypothesis that feeding would be more effective for children who were poorer or more undernourished was generally supported. For example, our analysis of one study (196 children) found greater effectiveness for weight gain if children were undernourished at baseline. Analyses from two primary studies also found greater effectiveness for undernourished children: one for weight and another for skinfold thickness. Two other studies (863 children) found that young undernourished children had greater height and WAZ gain in response to feeding, but that older undernourished children did not. Further evidence comes from the fact that the only study in a high‐income country that reported beneficial effects of feeding was performed among Aboriginal children, who are generally far more marginalised than non‐Aboriginals. For example, Australian Aboriginal families are much more likely to be food‐insecure (24% food‐insecure) than non‐Aboriginals (5% food‐insecure) (Browne 2009; Rosier 2011).
In contrast, as mentioned above, one primary study (n = 247) found that children living in very poor socio‐economic conditions did not respond as well to supplementary feeding as those living in better socio‐economic conditions. The author suggested that poor environmental conditions may have reduced effectiveness. Furthermore, in the follow‐up of the Jamaican study, supplemented children only experienced long‐term cognitive benefits if their mothers had higher verbal ability at baseline. Others have found that maternal education and intelligence are important contributors to infants’ dietary intake and nutritional status (Wachs 2005).
It makes biological sense that the children who are poorer or undernourished would benefit more from supplementary feeding. Our findings concur with those of Beaton 1982 and Kennedy 1987. We suggest that, in general, poorer children are more likely to benefit from feeding, but that feeding may not be all that is needed to overcome the effects of deprived environments.
It is important to point out that we were not able to assess whether or not the food actually reached those children who were most in need. Beaton 1982 and Rondo 1990 both noted that feeding programmes in developing countries often fail to do this.
Nutritional Adequacy
There is sparse evidence that programmes which provide more energy may more effective
Our hypothesis that higher nutritional adequacy would result in better outcomes was partially supported. Among the RCTs, there were few differences among subgroups for weight (eight trials, 975 children). For height, there was no evidence of subgroup differences in the RCTs, but the subgroup that provided high nutritional adequacy was the only group which found positive effects for feeding; the differences between high and low and moderate nutritional adequacy subgroups were 0.37 cm and 0.46 cm respectively. We believe that this subgroup analysis may have been non‐significant because of the low number of trials in the high (two trials, n = 254) and low (one trial, n = 127) nutritional adequacy groups.
Among the CBAs, there were no significant subgroup differences, but programmes which provided moderate nutritional adequacy (four trials, 651 children) had significant positive gains in weight after supplementary feeding, while the group who received low nutritional adequacy (five trials, 961 children) did not, but differences between the two groups were small. The mean difference for the moderate adequacy group was also 0.32 kg higher than that of the high‐energy group. It is important to note that the high‐energy intervention group for the CBAs contained only the Santos 2005 trial (n = 191), which had substantial issues with unreliable delivery and leakage within the family. In this study, 50% of the caregivers reported ‘gaps in delivery’; 36% of caregivers reported that these gaps occurred more than twice. Furthermore, only 32.5% of the participating children received the full ration. For the remainder of the children, the ration was shared with one to three other children and one to two adults. Despite the fact that the ration should have provided a high amount of energy, the supplemented group actually took in fewer calories than the control group.
The CBA results for height found no subgroup differences and the mean difference for the low‐energy group (five studies, n = 959) was higher than that for the moderate‐energy (four studies, n = 651) and high‐energy (one study, n = 172) groups.
Mode of delivery, amount of supervision of the supplementary feeding, leakage, and substitution
Location of feeding. There is some evidence that feeding given in day‐care may be more effective than that given at home
There were not enough data to fully test this in the RCTs, as only one study provided feeding on the spot. Among the CBAs, there was no evidence of subgroup differences but children who were fed in day‐care or feeding centres were the only ones who gained significant amounts of weight relative to controls (seven trials, 1784 children). For height, there was no evidence of an effect for any of the subgroups, but the subgroup who was fed 'on‐the spot' had a mean that was 0.93 cm higher than those who were fed in day‐cares. We believe that the lack of subgroup differences may have been due to other differences in implementation. An exploratory sensitivity analysis showed that when Manjrekar 1986 (whose results were markedly different from those of the other studies) was removed from the subgroup analyses for weight and height, heterogeneity was slightly lower, there was evidence of an effect for both subgroup analyses, and the effects in the day‐care group were stronger. It is notable that this study had a very high drop‐out rate.
Relatedly, our analysis in EXCEL found that when supplementary food was take‐home or home‐delivered, the children took in an average of only 36% of the energy provided by the supplement. In day‐cares and feeding centres, however, the children benefited from an average of 85% of this energy. This is consistent with findings reported in a synthesis by Kennedy 1987; 'on‐site' feeding resulted in higher intakes than did 'take‐home' feeding.
It is likely that this reduction in energy benefits from the home‐delivered food or poorly‐supervised programmes was at least partially due to 'leakage' within the family. In interviews with mothers, Santos 2005 found that the target child only received the full ration one‐third of the time; Tomedi 2012 reported that children in the experimental group received "at least" 50% of the supplement. This is an important issue for feeding programmes in developing countries (Patel 2005). With home‐based delivery, some of the food provided for one child often gets redistributed within poor families or sold to augment the family's income; this is one type of 'leakage'. When food is given at school or at day‐care, families may give that child less at home so that other family members can have more; this is known as 'substitution'.
Although, "this is understandable in the context of food‐insecure families" (Patel 2005, p 4), the result of such leakage is that the targeted child gets less food, and therefore less impact on growth and development can be expected. However, other researchers have pointed out that supplementary feeding may be seen as a net benefit to the whole family, and not just to one child.
Level of supervision. Our analyses suggest that stricter supervision of feeding may produce better child outcomes
Our hypothesis that programmes with stricter supervision would be more effective was partially supported. There was no evidence of subgroup differences for RCTs. For height, the supplemented children in the subgroup with the strictest supervision (four trials, 762 children) were the only ones who grew more than controls, although differences in means between subgroups were small. This analysis only compared moderate to strict supervision. There was no evidence of an effect in the CBAs, but we did find that children in the studies with the strictest supervision (five trials, 1286 children) gained more in weight from feeding than did children in the studies with moderate or little supervision (0.24 kg and 0.29 kg respectively). The same was true for height (0.54 cm and 0.85 cm difference between high and moderate and low supervision respectively). We believe that the lack of evidence for an effect in the CBA subgroup analyses may have been due to other differences in study implementation. An exploratory sensitivity analysis showed that when Manjrekar 1986 (whose results were markedly different from those of the other studies) was removed from the subgroup analyses for weight and height, heterogeneity was slightly lower and there was evidence of a subgroup effect for weight. It is notable that Manjrekar 1986 had a very high drop‐out rate.
Leakage in the supply chain
Two of our studies reported breakdowns in the supply chain; the supplements only reached the families part of the time. Such failures in delivery have been reported by others who have reviewed preschool feeding programmes (Kennedy 1987) and school feeding programmes (Galloway 2009).
Multiple interventions.There is little evidence to support our hypothesis that multiple interventions would be more effective for growth than single interventions
Our hypothesis that multiple interventions would be more effective for growth was unsupported. There were no subgroup differences. Among the RCTs, both single and multiple interventions were effective for weight gain but the effect size for multiple interventions was higher. For height, two RCTs that provided multiple interventions (495 children) did not show effects while the seven RCTs that provided single interventions (952 children) were effective for increasing height. Among the CBAs (1782 children), neither single nor multiple interventions were effective for increasing height.
For psychosocial outcomes, there was no evidence of subgroup differences, but the effect size for the supplementation + stimulation group in one study (n = 65) was twice as high as effects for feeding only in two studies (n =178 ). It is likely that stimulation combined with feeding is especially effective for psychosocial development.
Disruption of breastfeeding
When food is given to infants, it is important to ensure that it does not disrupt breastfeeding, as this could lead to a rise in morbidity (Dewey 2008). Only three studies in our review examined whether or not the feeding intervention interfered with breastfeeding, and they had contradictory results. Findings from a survey done in conjunction with the Ecuador study found that supplementary feeding did not interfere with breastfeeding practices (Lutter 2008; n = 110 at final survey). In Indonesia, supplementation did not interfere with breastfeeding boys (Pollitt 2000a; n = 47), but it did seem to decrease breast feeding of girls (n = 48). However, Bhandari 2001 found that the proportion of infants who were breastfed was lower in the food supplementation group (n = 96) compared with the visitation‐only group (n = 96).
Potential biases in the review process
We tried to reduce bias through careful attention to standard systematic review methodology. For example, we had at least two review authors involved in every aspect of identifying potential studies, deciding on inclusion and exclusion of studies, extracting data, and conducting analyses. However, a few potential sources of bias may remain.
Publication bias We have searched websites of relevant agencies and found a number of reports of evaluations of feeding programmes, but it is possible that we have missed some. However, this is probably not too serious as the reports found on the websites that we searched did not meet our inclusion criteria. Furthermore, we did not handsearch any relevant journals. Although this must be acknowledged as a potential limitation, we believe that our coverage of the literature was thorough; we used many key databases and searched websites of relevant organisations.
Bias in correcting for clustering As noted above, we corrected for clustering in a number of studies. This was vital in ensuring that confidence intervals were not inappropriately narrow. However, these corrections are highly dependent on the chosen intraclass correlation coefficients (ICCs). Having said that, we carried out a sensitivity analysis with different ICCs and were reassured that it made little difference.
Agreements and disagreements with other studies or reviews
We found one Cochrane review of RCTs of the effectiveness of supplementary feeding on growth (Sguassero 2012), two systematic reviews on complementary feeding (Dewey 2008: Lassi 2013), two earlier reviews of the effectiveness of supplementary feeding on growth (Beaton 1982) and other outcomes (Beaton 1993), and one short review and meta‐analysis of nutrition and cognition (Pollitt 1994).
Our review has a wider scope than the above reviews and is somewhat more recent. Nonetheless, our conclusions that feeding interventions for young children can be effective for growth are fairly consistent with those of the Beaton 1982, Dewey 2008 and Pollitt 1994 reviews, somewhat consistent with Lassi 2013, and inconsistent with Sguassero 2012. For example, like Beaton 1982 and Dewey 2008, we found small effects on growth and concluded that feeding interventions are currently underperforming. Our findings that feeding interventions were generally more effective for growth in younger children concur with those of Beaton 1982 and Beaton 1993. However, we feel that there has not been enough research on their effectiveness in older children. We also agree with Beaton 1993 that the pathways between feeding and growth and feeding and psychosocial development are quite different, and that feeding can have an important impact on psychosocial development beyond the age of two. Finally, we concur with Pollitt 1994 that feeding has positive impacts on psychomotor development.
Our findings on factors that can impact on success are very similar to some of those described by Kennedy 1987. For example, our findings concur with their paper on leakage within the family and substitution. Our results also support their findings that 'on site' feeding can markedly curtail leakages.
Authors' conclusions
Implications for practice.
Our review has found that child‐feeding interventions are underperforming. Although we provide evidence that feeding interventions can work, our results indicate that good implementation is key. This leads to several suggestions for programme development, implementation, and monitoring.
Target the poorest or most undernourished children or areas, if targeting is necessary. Our review provides some evidence that poorer and more undernourished children may be more responsive to supplementary feeding. Thus, when funding is limited, it is both an ethical imperative and necessary from a cost‐effectiveness point of view to target poorer areas, families, and children. However, careful attention needs to be paid to the other conditions in which the children are living. As previously noted, very poor environmental conditions may negate the positive effects of supplementary feeding.
Closely supervise the distribution and child’s intake of the supplement. Our work suggests that feeding may be more effective if delivered in a supervised feeding centre, day‐care centre, or preschool. We have also found that children in day‐cares or preschools benefit from more of the supplement. Another advantage to delivery in these settings is that feeding could easily be combined with hands‐on training for groups of mothers on topics such as child stimulation, nutrition, and breastfeeding (Kennedy 1987).
Providing extra rations for other family members may be helpful.Beaton 1982 suggests that instead of viewing 'leakage' as totally undesirable, it may be seen as a benefit to the whole family. He noted that, at the least, feeding interventions increase family purchasing power. We concur with the view that the net benefit to the entire family should be measured. However, we believe that emphasis should still be placed on providing adequate nutrition to the children most in need within the family. One way to facilitate this may be to provide some rations for the entire family in order to reduce redistribution of the target child's supplement. Seven studies in the current review gave the family extra rations to reduce sharing of the target child’s supplement. Similarly, the World Food Program's school feeding programmes are increasingly using take‐home rations to ensure that children, especially girls, are able to go to school regularly.
Build family capacity. Evidence from our review, and from other studies on household food distribution, suggests that education is essential for parents on the importance of feeding all children according to their needs.
Consider providing at least 30% of the RDI for energy. We found some suggestion that children may grow more in programmes that
provide moderate (30% to 59%) or high (60% or more) percentage of the dietary reference intake (DRI) for energy. This is consistent with findings from Kennedy 1987; programmes which gave only a few hundred calories were less effective than those that provided more energy. According to Kennedy 1987, it is important for programmes to account for leakage by providing more energy than needed to fill the 'existing calorie deficit' (the difference between the amount taken in and the amount needed).
Supplementation should begin early in the child's life. Our findings are somewhat supportive of other authors who have shown that younger children benefit more than older children in terms of growth. On this basis, we suggest that when it is to be given, supplementation should begin in infancy after a period of exclusive breastfeeding. As it may take time for supplementation to affect certain aspects of growth (Rivera 2013 [pers comm]) and cognitive development (see, for example, Grantham‐McGregor 1991), supplementation should continue for at least 18 months (Sguassero 2012) to two years (Rivera 2013 [pers comm]).
Monitor and evaluate on a continual basis. In addition to evaluating a range of appropriate outcomes, our review highlights the importance of evaluation that assesses all factors that can impact on the success of feeding. It is also important to monitor children's dietary intake, growth, and development on a regular basis.
Implications for research.
It seems inevitable that review authors will call for more research, and we follow this trend. However, we are not calling for more of the same research, but for research on relatively understudied areas. Furthermore, we believe that there should be guidelines for such research, and that process evaluation as well as outcome evaluation needs to be undertaken. We have identified the following research needs:
More research is needed on the impact of preschool feeding on psychosocial development. It is quite concerning that only eight out of 32 studies assessed effectiveness for psychosocial development. Yet, as Dewey 2008 noted, psychosocial outcomes may be particularly sensitive to nutrition intervention. Indeed, findings from our review indicate that feeding interventions can have positive effects on psychomotor and possibly cognitive development. Relatedly, we concur with Bhutta 2008 that it is important to learn to what extent the cognitive deficits caused by early undernutrition are reversible. We know that an individual's life chances are dependent on adequate motor, behavioural, and mental development in the first years of life. For example, early cognitive and social‐emotional development are major determinants of school progress in developed and developing countries, which in turn is related to adult employment status and income, and contributions to family, community, and society (Grantham‐McGregor 2007). We realise that psychomotor and mental testing can be time‐consuming and expensive to do on a large scale. However, more feasible and valid tests have been developed (Khan 2010). It is time that psychosocial development is given higher priority as an outcome of interventions.
More research is needed on the impact of feeding on older children. Our meta‐analyses on growth seem to show that feeding may not be effective at increasing the height or weight of children above two years of age. However, there is a dearth of research on feeding interventions for this age group; we only found four studies that assessed effectiveness for weight and height, and they were all conducted before 1990. Therefore, we believe that the jury is still out on the question of effectiveness of feeding interventions for growth after two years of age, and we concur with Bhutta 2008 that this is a major gap in our knowledge.
More research is needed on the impact of feeding on gender and income equity in growth and psychological development. Our review has provided some evidence that supplementary feeding might be more effective for poorer children and possibly for girls. Surprisingly few studies addressed this question. Relatedly, more research is needed on how to reduce inequities in the distribution of household food.
More high‐quality research is needed on the implementation of large‐scale programmes. Another area in which there is a dearth of high‐quality research is in the evaluation of large‐scale feeding programmes. Most of the evidence presented here is from small‐scale studies; only four evaluations of large‐scale studies met our inclusion criteria (Brazil's Milk Supplement Program (Santos 2005); PANN in Ecuador (Lutter 2008); Progresa in Mexico (Rivera 2004); and Vietnam's Integrated Health and Nutrition Program (Schroeder 2002)). While knowledge from these studies has contributed to the review and to our process analyses, there is a need for more high‐quality RCTs of such large‐scale programmes; we found a number of evaluations of such programmes in the literature but these evaluations were not rigorous enough to meet our inclusion criteria. In the future, we recommend cluster‐RCTS and process evaluations.
More research is needed on interventions of high quality. Many studies in this review were of relatively low quality in terms of implementation and design. It is encouraging that the more recent studies were generally of much better quality, although there are still issues concerning implementation. There is a need for careful attention to outcome measurement that is guided by theory and logic. Attention must also be paid to methods of randomisation, allocation concealment, blinding of outcome assessment, and to attrition. We need research that examines the causes of attrition and that determines how to reduce it.
History
Protocol first published: Issue 6, 2012 Review first published: Issue 3, 2015
| Date | Event | Description |
|---|---|---|
| 20 March 2014 | Amended | The comments from the statisticians have been addressed. Furthermore, an updated search has been conducted. |
Acknowledgements
We are very grateful for the advice and enormous amount of assistance received from members of the Cochrane Developmental, Psychosocial and Learning Problems Group: Margaret Anderson, Laura MacDonald, Joanne Wilson, Zulfiqar Bhutta, Geraldine Macdonald, and the copy‐editor. Your careful oversight resulted in a much stronger review.
Members of our advisory group: Rae Galloway, Chessa Lutter, Donald Bundy, Susan Walker, Aulo Gelli, and Dr. Martin Bloem have been very helpful and responsive to our requests for expert advice.
We also greatly appreciate the work of two research assistants: Katelyn Merritt and Micere Thuku.
Finally, we greatly appreciate the fact that several study authors were responsive to our requests for additional information. These include: Madalin Husaini, Chessa Lutter, Jean Rivera, and Susan Walker.
Appendices
Appendix 1. Strategies for searches last updated in January 2014
Cochrane Central Register of Controlled Studies (CENTRAL), Cochrane Database of Systematic Reviews (CDSR), and Database of Abstracts of Reviews of Effects (DARE)
CENTRAL 2014 Issue 1 of 12. Limited to 2012 to 2014. Searched 28 January 2014 [187 records]. CENTRAL May 2012. Limited to 2011 to 2012. Searched 3 May 2012 [140 records]. CENTRAL 2011 Issue 7. Searched 18 July 2011.
CDSR, 2014 Issue 1 of 12. Searched 28 January 2014 [111 records].
DARE, Issue 1 of 4. Searched 28 January 2014 [20 records]. DARE, May 2012. Limited to 2011 to 2012 Searched 3 May 2012 [12 records]. #1MeSH descriptor: [Dietary Supplements] this term only #2MeSH descriptor: [Diet Therapy] this term only#3MeSH descriptor: [Food, Fortified] this term only #4MeSH descriptor: [Functional Food] this term only #5MeSH descriptor: [Nutrition Therapy] explode all trees #6((extra or take‐home or take home) and (food* or feed* or ration*)):ti,ab #7MeSH descriptor: [Nutrition Policy] this term only #8((feed* or food*) and program*):ti,ab #9((fortif* or enrich*) and (food* or diet* or spread* or flour* or cereal*)):ti,ab #10(lunch* or dinner* or break‐fast* or breakfast* or break fast* or supper* or snack* or meal* or milk):ti,ab #11(plumpy* or nutri spread*):ti,ab #12((supplement* or complement*) and (food* or feed* or diet* or nutrition* or nutrient* or micronutrient* or micro‐nutrient*)):ti,ab #13(blended and food*):ti,ab #14(energy and supplement*):ti,ab #15(lipid based and supplement*):ti,ab #16(#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15) #17MeSH descriptor: [Infant] explode all trees #18MeSH descriptor: [Child, Preschool] explode all trees #19toddler*:ti,ab #20(baby or babies or infant* or preschool* or pre‐school* or child*):ti,ab #21(#17 or #18 or #19 or #20) #22(#16 and #21) #23MeSH descriptor: [Growth and Development] this term only #24*Growth #25MeSH descriptor: [Child Development] this term only #26milestone*:ti,ab #27MeSH descriptor Motor Skills explode all trees in MeSH products #28MeSH descriptor: [Psychomotor Performance] this term only #29MeSH descriptor: [Psychomotor Disorders] this term only #30(psychomotor and development):ti,ab #31psychosocial:ti,ab #32MeSH descriptor: [Stress, Psychological] this term only #33MeSH descriptor: [Adaptation, Psychological] this term only #34MeSH descriptor: [Social Support] this term only #35MeSH descriptor: [Cognition] this term only #36MeSH descriptor: [Cognition Disorders] this term only #37MeSH descriptor: [Learning Disorders] this term only #38(cognit* and ability):ti,ab #39cognit*:ti,ab #40MeSH descriptor: [Attention] this term only #41MeSH descriptor: [Attention Deficit Disorder with Hyperactivity] this term only #42MeSH descriptor: [Child Behavior Disorders] this term only #43(on task and behavio*r):ti,ab #44MeSH descriptor Vocabulary explode all trees in MeSH products #45MeSH descriptor Language Development explode all trees in MeSH products #46MeSH descriptor Intelligence explode all trees in MeSH products #47MeSH descriptor Intelligence Tests explode all trees in MeSH products #48MeSH descriptor Bone Density explode all trees in MeSH products #49(bone and mineral and test*):ti,ab #50MeSH descriptor Motor Activity explode all trees in MeSH products #51(physical and activit*):ti,ab #52*Exercise #53MeSH descriptor Morbidity explode all trees in MeSH products #54MeSH descriptor Stereotyping explode all trees in MeSH products #55stigma*:ti,ab #56MeSH descriptor: [Aggression] this term only #57(bully or bullying):ti,ab #58victimization:ti,ab #59disruptive behavio*r:ti,ab #60MeSH descriptor: [Obesity] this term only #61MeSH descriptor: [Weight Loss] this term only #62(excess* and weight and loss):ti,ab #63MeSH descriptor: [Memory] this term only #64MeSH descriptor: [Logic] this term only #65MeSH descriptor: [Problem Solving] this term only #66reasoning:ti,ab #67MeSH descriptor: [Psychometrics] this term only #68height:ti,ab #69weight:ti,ab #70length:ti,ab #71MeSH descriptor: [Anthropometry] this term only #72MeSH descriptor: [Body Weight] this term only #73MeSH descriptor: [Body Height] this term only #74MeSH descriptor: [Body Size] this term only #75MeSH descriptor: [Weight Gain] this term only #76MeSH descriptor: [Body Composition] this term only #77MeSH descriptor: [Physical Fitness] this term only #78fitness:ti,ab #79#23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 or #44 or #45 or #46 or #47 or #48 or #49 or #50 or #51 or #52 or #53 or #54 or #55 or #56 or #57 or #58 or #59 or #60 or #61 or #62 or #63 or #64 or #65 or #66 or #67 or #68 or #69 or #70 or #71 or #72 or #73 or #74 or #75 or #76 or #77 or #78 #80(#22 and #79)
Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE(R)
Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE(R) 1946 to Present. Limited to 2012 to 2014. Searched 28 January 2014 [1799 records]. Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE(R) 1948 to Present. Limited to 2011 to 2012. Searched 1 May 2012 [1050 records]. Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE(R) 1948 to Present. Searched July 2011 [10937 records]. 1 Dietary Supplements/ 2 Diet Therapy/ 3 Food, Fortified/ 4 Functional Food/ 5 Nutrition Therapy/ 6 ((extra or take‐home or takehome) adj3 (food$ or feed$ or ration$)).tw. 7 Nutrition Policy/ 5882) 8 ((feed$ or food$) adj3 program$).tw. 3245) 9 ((fortif$ or enrich$) adj3 (food$ or diet$ or spread$ or flour$ or cereal$)).tw. 10 (lunch$ or dinner$ or break‐fast$ or breakfast$ or break fast$ or supper$ or snack$ or meal$ or milk).tw. 11 (plumpy$ or nutri spread$).tw. 12 ((supplement$ or complement$) adj3 (food$ or feed$ or diet$ or nutrition$ or nutrient$ or micronutrient$ or micro‐nutrient$)).tw. 13 (blended adj3 food$).tw. 14 (energy adj3 supplement$).tw. 15 (lipid based adj3 supplement$).tw. 16 or/1‐15 17 Infant/ 18 Child, Preschool/ 19 toddler$.tw. 20 (baby or babies or infant$ or preschool$ or pre‐school$ or child$).tw. 21 or/17‐20 22 16 and 21 23 "Growth and Development"/ 24 *Growth/ 25 Child Development/ 26 milestone$.tw. 27 exp Motor Skills/ 28 Psychomotor Performance/ 29 Psychomotor Disorders/ 30 (psychomotor adj3 development).tw. 31 psychosocial.tw. 32 Stress, Psychological/ 33 Adaptation, Psychological/ 34 Social Support/ 35 Cognition/ 36 Cognition Disorders/ 37 Learning Disorders/ 38 (cognit$ adj4 ability).tw. 39 cognit$.tw. 40 Attention/ 41 Attention Deficit Disorder with Hyperactivity/ 42 Child Behavior Disorders/ 43 (on task adj4 behavio$r).tw. 44 exp Vocabulary/ 45 exp Language Development/ 46 exp Intelligence/ 47 exp Intelligence Tests/ 48 exp Bone Density/ 49 (bone adj3 mineral adj3 test$).tw. 50 exp Motor Activity/ 51 (physical adj3 activit$).tw. 52 *Exercise/ (43388) 53 exp Morbidity/ 54 exp Stereotyping/ 55 stigma$.tw. 56 Aggression/ 57 (bully or bullying).tw. 58 victimization.tw. 59 disruptive behavio$r.tw. 60 Obesity/ 61 Weight Loss/ 62 (excess$ adj3 weight adj3 loss).tw. 63 Memory/ 64 Logic/ 65 Problem Solving/ 66 reasoning.tw. 67 Psychometrics/ 68 height.tw. 69 weight.tw. 70 length.tw. 71 Anthropometry/ 72 Body Weight/ 73 Body Height/ 74 Body Size/ 75 Weight Gain/ 76 Body Composition/ 77 Physical Fitness/ 78 fitness.tw. 79 or/23‐78 80 22 and 79
Social Sciences Citation Index (SSCI) (Web of Science), Conference Proceedings Citation Index‐Science (CPCI‐S) (Web of Science), and Conference Proceedings Citation Index‐Social Science & Humanities (CPCI‐SSH) (Web of Science)
The following databases were searched via Web of Science on 28 January 2014 [1005 records].
SSCI 1970 to present. CPCI‐S 1990 to present. CPCI‐SSH 1990 to present. Title=(lunch* OR dinner* OR breakfast* OR snack* OR meal OR milk OR meat OR egg OR food OR feed) AND Title=(toddler* OR baby OR babies OR infant* OR preschool OR preschool OR child*)
Education Resources Information (ERIC) (Proquest)
ERIC 1994 to present. Searched 28 January 2014 [83 records].
TI(lunch* OR dinner* OR breakfast* OR snack* OR meal OR milk OR meat OR egg OR food OR feed) AND TI(toddler* OR baby OR babies OR infant* OR preschool OR preschool OR child*)
Proquest Dissertations and Theses
Proquest Dissertations and Theses. Searched 28 January 2014 [74 records]. Proquest Dissertations and Theses. Searched 18 July 2011 [6141 records]. TI(lunch* OR dinner* OR breakfast* OR snack* OR meal OR milk OR meat OR egg OR food OR feed) AND TI(toddler* OR baby OR babies OR infant* OR preschool OR preschool OR child*)
PsycINFO (Ovid)
PsycINFO 1806 to January Week 3 2014. Searched 28 January 2014.
1 Dietary Supplements/ 2 Diets/ 3 (Diet adj3 therapy).tw. 4 Food/ 5 Food Intake/ 6 Nutrition/ 7 fortifi$.tw. 8 (Functional adj3 Food).tw. 9 (fortified adj3 food).tw. 10 (Nutrition adj3 Therapy).tw. 11 ((extra or take‐home or takehome) adj3 (food$ or feed$ or ration$)).tw. 12 Nutrition Policy.tw. 13 ((feed$ or food$) adj3 program$).tw. 14 ((fortif$ or enrich$) adj3 (food$ or diet$ or spread$ or flour$ or cereal$)).tw. 15 (lunch$ or dinner$ or break‐fast$ or breakfast$ or break fast$ or supper$ or snack$ or meal$).tw. 16 plumpy$.tw. 17 (supplement$ adj3 (food$ or feed$ or diet$ or nutrition$ or nutrient$)).tw. 18 or/1‐17 19 Infant.tw. 20 Preschool Students/ 21 (baby or babies or infant$ or preschool$ or pre‐school$ or child$ or toddler$).tw. 22 19 or 20 or 21 23 18 and 22
Clinicaltrials.govvia National Institutes of Health (NIH)
Advanced Search
Intervention: (feed or food or meal)
Age Group: 0 to 17
Accessed: 28 January 2014
Appendix 2. Strategies for searches last updated in May 2012
EMBASE Classic and EMBASE (OVID)
Embase Classic and Embase 1947 to 1 May 2012. Searched 3 May 2012. Limited to 2011 to 2012 [257 records].
Embase Classic and Embase 1947 to 1 May 2012. Search 18 July 2011 [5611 records].
1 exp Dietary Supplements/
2 Diet Therapy/
3 Food, Fortified/
4 Food/
5 (Functional adj3 Food).tw.
6 Nutrition Therapy/
7 Diet Therapy/
8 ((extra or take‐home or takehome) adj3 (food$ or feed$ or ration$)).tw.
9 Nutrition Policy/
10 ((feed$ or food$) adj3 program$).tw.
11 ((fortif$ or enrich$) adj3 (food$ or diet$ or spread$ or flour$ or cereal$)).tw.
12 (lunch$ or dinner$ or break‐fast$ or breakfast$ or milk or break fast$ or supper$ or snack$ or meal$).tw.
13 (plumpy$ or nutri spread$).tw.
14 (blend$ food$ or lipid based supplement$).tw.
15 (energy adj3 supplement$).tw.
16 (supplement$ adj3 (food$ or feed$ or diet$ or nutrition$ or nutrient$)).tw.
17 or/1‐16
18 exp Infant/
19 Child, Preschool/
20 (baby or babies or infant$ or preschool$ or pre‐school$ or child$).tw.
21 or/18‐20
22 17 and 21
23 "Growth and Development"/
24 *Growth/
25 Child Development/
26 exp Motor Skills/
27 Psychomotor Performance/
28 Psychomotor Disorders/
29 (psychomotor adj3 development).tw.
30 Cognition/
31 Cognition Disorders/
32 Learning Disorders/
33 (cognit$ adj4 ability).tw.
34 Attention/
35 Attention Deficit Disorder with Hyperactivity/
36 Child Behavior Disorders/
37 (on task adj4 behavio$r).tw.
38 exp Vocabulary/
39 exp Language Development/
40 exp Intelligence/
41 exp Intelligence Tests/
42 exp Bone Density/
43 (bone adj3 mineral adj3 test$).tw.
44 exp Motor Activity/
45 (physical adj3 activit$).tw.
46 *Exercise/
47 exp Morbidity/
48 exp Stereotyping/
49 stigma$.tw.
50 Aggression/
51 (bully or bullying).tw.
52 victimization.tw.
53 disruptive behavio$r.tw.
54 Obesity/
55 Weight Loss/
56 (excess$ adj3 weight adj3 loss).tw.
57 or/23‐56
58 22 and 57
CINAHL (Ebscohost)
CINAHL 1981 to current. Searched 3 May 2012. Limited to 2011 to 2012 [27 records]. CINAHL 1981 to current. Searched 15 July 2011 [4582 records]. S15 (S1 or S2 or S3 or S4 or S7 or S9) and (S13 and S14) S14 S1 or S2 or S3 or S4 or S7 or S9 S13 S10 or S11 or S12 S12 "toddler" S11 (MH "Infant") S10 (MH "Child, Preschool") OR (MH "Schools, Nursery") OR (MH "Child") S9 (MH "Infant Feeding") OR (MH "Infant Food") OR (MH "Infant Nutrition") S8 ""food program"" S7 "feeding program" S6 "feed$ program or food$ program$" S5 "((feed$ or food$) adj3 program$)" S4 (MH "Diet Therapy") S3 (MH "Nutrition Policy") S2 (MH "Food") OR (MH "Snack Foods") OR (MH "Functional Food") OR (MH "Infant Food")
Healthstar (OVID)
Healthstar 1966 to 3 May 2012. Limited to 2011 to 2012 [348 records].
Healthstar 1966 to 18 July 2011 [3106 records].
1 exp Dietary Supplements/ 2 Diet Therapy/ 3 Food, Fortified/ 4 Food/ 5 (Functional adj3 Food).tw. 6 Nutrition Therapy/ 7 Diet Therapy/ 8 ((extra or take‐home or takehome) adj3 (food$ or feed$ or ration$)).tw. 9 Nutrition Policy/ 10 ((feed$ or food$) adj3 program$).tw. 11 ((fortif$ or enrich$) adj3 (food$ or diet$ or spread$ or flour$ or cereal$)).tw. 12 (lunch$ or dinner$ or break‐fast$ or breakfast$ or milk or break fast$ or supper$ or snack$ or meal$).tw. 13 (plumpy$ or nutri spread$).tw. 14 (blend$ food$ or lipid based supplement$).tw. 15 (energy adj3 supplement$).tw. 16 (supplement$ adj3 (food$ or feed$ or diet$ or nutrition$ or nutrient$)).tw. 17 or/1‐16 18 exp Infant/ 19 Child, Preschool/ 20 (baby or babies or infant$ or preschool$ or pre‐school$ or child$).tw. 21 or/18‐20 22 17 and 21 23 "Growth and Development"/ 24 *Growth/ 25 Child Development/ 26 exp Motor Skills/ 27 Psychomotor Performance/ 28 Psychomotor Disorders/ 29 (psychomotor adj3 development).tw. 30 Cognition/ 31 Cognition Disorders/ 32 Learning Disorders/ 33 (cognit$ adj4 ability).tw. 34 Attention/ 35 Attention Deficit Disorder with Hyperactivity/ 36 Child Behavior Disorders/ 37 (on task adj4 behavio$r).tw. 38 exp Vocabulary/ 39 exp Language Development/ 40 exp Intelligence/ 41 exp Intelligence Tests/ 42 exp Bone Density/ 43 (bone adj3 mineral adj3 test$).tw. 44 exp Motor Activity/ 45 (physical adj3 activit$).tw. 46 *Exercise/ 47 exp Morbidity/ 48 exp Stereotyping/ 49 stigma$.tw. 50 Aggression/ 51 (bully or bullying).tw. 52 victimization.tw. 53 disruptive behavio$r.tw. 54 Obesity/ 55 Weight Loss/ 56 (excess$ adj3 weight adj3 loss).tw. 57 or/23‐56 58 22 and 57
LILACS
LILACS. Searched 10 May 2012. Limited to 2011 to 2012 [42 records]. LILACS. Searched 15 July 2011.
(Dietary Supplements or Diet Therapy or Food, Fortified or Functional Food or Nutrition Therapy or Nutrition Policy or feed$ or food$ or fortif$ or enrich or food$ or diet$ or spread$ or flour$ or cereal$ or lunch$ or dinner$ or break‐fast$ or breakfast$ or break fast$ or supper$ or snack$ or meal$ or plumpy$ or supplement$ or diet$ or nutrition$ or nutrient$) AND (Infant or Child, Preschool or baby or babies or infant$ or preschool$ or pre‐school$ or child$)
Appendix 3. Strategies for searches last updated in 2011
Social Services Abstracts (CSA)
Social Services Abstracts. Last searched 15 July 2011 [423 records].
((DE=("food" or "food security" or "food stamps" or "diet")) or(KW=(meal$ or breakfast$ or (break fast$)) or KW=(lunch$ or snack$ or dinner$) or KW=(supper$ or ration$)) or(KW=(supplement$ or fortified or fortify) or KW=(enriched or milk or bread) or KW=plumpy)) and((DE=("preschool children" or "child care services" or "children" or "pediatrics" or "preschool education")) or(KW=((nursery school) or baby or babies) or KW=(infant or toddler)) or(DE="infants"))
Appendix 4. Methods for Interrupted time series (ITS) trials in future updates of this review
If our update of this review contains any interrupted time series (ITS) trials, we will analyse them in the following ways: we will calculate relative and absolute mean difference in before and after values. When possible, we will use time series regression to calculate mean change in level and mean change in slope.
For discrete outcomes (e.g. undernourished versus well‐nourished), we will present the relative risk (RR) of the outcome compared to the control group. We will also calculate the risk difference (RD), which is the absolute difference in the proportions in each treatment group. Finally, we will calculate the number needed‐to‐treat (NTT) to achieve one person with the desired outcome.
When possible, comparisons will be reported by socio‐economic group as well as by other relevant socio‐demographic variables, including baseline nutritional status, gender, race or ethnicity, and place of residence. Where results by socio‐economic variables are not available in the primary articles and reports, we will request these data from the authors and recalculate effect sizes and P values.
Data and analyses
Comparison 1. Low‐ and middle‐income countries: feeding vs control ‐ growth. RCT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight gain | 9 | 1057 | Mean Difference (IV, Random, 95% CI) | 0.12 [0.05, 0.18] |
| 1.1 Subgroup analysis by age: < 12 months | 7 | 910 | Mean Difference (IV, Random, 95% CI) | 0.11 [0.04, 0.18] |
| 1.2 Subgroup analysis by age: 1 ‐ 2 years | 1 | 65 | Mean Difference (IV, Random, 95% CI) | 0.32 [0.02, 0.62] |
| 1.3 Subgroup analysis by age: > 2 years | 1 | 82 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.23, 0.35] |
| 2 Height gain | 9 | 1463 | Mean Difference (IV, Random, 95% CI) | 0.27 [0.07, 0.48] |
| 2.1 Subgroup analysis by age: < 12 months | 7 | 1316 | Mean Difference (IV, Random, 95% CI) | 0.22 [0.05, 0.39] |
| 2.2 Subgroup analysis by age: 1 ‐ 2 years | 1 | 65 | Mean Difference (IV, Random, 95% CI) | 0.90 [0.33, 1.47] |
| 2.3 Subgroup analysis by age: > 2 years | 1 | 82 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐1.97, 0.97] |
| 3 Weight‐for‐age z‐scores (WAZ) | 8 | 1565 | Mean Difference (IV, Random, 95% CI) | 0.15 [0.05, 0.24] |
| 4 Height‐for‐age z‐scores (HAZ) | 9 | 4544 | Mean Difference (IV, Random, 95% CI) | 0.15 [0.06, 0.24] |
| 5 Weight‐for‐height z‐scores (WHZ) | 7 | 4073 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.02, 0.22] |
Comparison 2. Sensitivity analysis ICC 0.10: low‐ and middle‐income countries: feeding vs control ‐ growth. RCT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight gain | 9 | 1057 | Mean Difference (IV, Random, 95% CI) | 0.12 [0.05, 0.18] |
| 1.1 Subgroup analysis by age: < 12 months | 7 | 910 | Mean Difference (IV, Random, 95% CI) | 0.11 [0.04, 0.18] |
| 1.2 Subgroup analysis by age: 1 ‐ 2 years | 1 | 65 | Mean Difference (IV, Random, 95% CI) | 0.32 [0.02, 0.62] |
| 1.3 Subgroup analysis by age: > 2 years | 1 | 82 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.23, 0.35] |
| 2 WAZ scores | 8 | 1565 | Mean Difference (IV, Random, 95% CI) | 0.15 [0.08, 0.23] |
| 3 HAZ scores | 9 | 4544 | Mean Difference (IV, Random, 95% CI) | 0.14 [0.05, 0.24] |
| 4 WHZ scores | 7 | 4073 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.02, 0.22] |
Comparison 3. Low‐ and middle‐income countries: feeding vs control. CBA.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight gain (kg) | 7 | 1784 | Mean Difference (IV, Random, 95% CI) | 0.24 [0.09, 0.39] |
| 1.1 Subgroup analysis by age: < 12 months | 6 | 722 | Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.03, 0.39] |
| 1.2 Subgroup analysis by age: 1 year | 4 | 330 | Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.15, 0.46] |
| 1.3 Subgroup analysis by age: 2 years | 3 | 186 | Mean Difference (IV, Random, 95% CI) | 0.59 [0.29, 0.89] |
| 1.4 Subgroup analysis by age: > 2 years | 3 | 546 | Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.24, 0.59] |
| 2 Height gain (cm) | 7 | 1782 | Mean Difference (IV, Random, 95% CI) | 0.52 [‐0.07, 1.10] |
| 2.1 Subgroup analysis by age: < 12 months | 6 | 722 | Mean Difference (IV, Random, 95% CI) | 0.11 [‐1.20, 1.42] |
| 2.2 Subgroup analysis by age: 1 year | 4 | 330 | Mean Difference (IV, Random, 95% CI) | 0.79 [‐0.51, 2.09] |
| 2.3 Subgroup analysis by age: 2 years | 3 | 185 | Mean Difference (IV, Random, 95% CI) | 0.70 [‐0.51, 1.91] |
| 2.4 Subgroup analysis by age: > 2 years | 3 | 545 | Mean Difference (IV, Random, 95% CI) | 0.58 [‐0.29, 1.45] |
| 3 WAZ scores | 4 | 999 | Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.13, 0.68] |
| 4 HAZ scores | 4 | 999 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.10, 0.12] |
| 5 WHZ scores | 4 | 999 | Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.11, 0.69] |
Comparison 4. High‐income countries: feeding vs control. RCT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight gain | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Height gain | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 WAZ scores | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 HAZ scores | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 WHZ scores | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 5. Sensitivity analysis ICC 0.10: low‐ and middle‐income countries: feeding vs control. CBA.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight gain (kg) | 7 | 1784 | Mean Difference (IV, Random, 95% CI) | 0.25 [0.11, 0.39] |
| 1.1 Subgroup analysis by age: < 12 months | 6 | 722 | Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.04, 0.36] |
| 1.2 Subgroup analysis by age: 1 year | 4 | 330 | Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.07, 0.51] |
| 1.3 Subgroup analysis by age: 2 years | 3 | 186 | Mean Difference (IV, Random, 95% CI) | 0.54 [0.23, 0.86] |
| 1.4 Subgroup analysis by age: > 2 years | 3 | 546 | Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.08, 0.62] |
| 2 Height gain (cm) | 7 | 1782 | Mean Difference (IV, Random, 95% CI) | 0.57 [0.06, 1.07] |
| 2.1 Subgroup analysis by age: < 12 months | 6 | 722 | Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.90, 1.31] |
| 2.2 Subgroup analysis by age: 1 year | 4 | 330 | Mean Difference (IV, Random, 95% CI) | 0.80 [‐0.53, 2.13] |
| 2.3 Subgroup analysis by age: 2 years | 3 | 185 | Mean Difference (IV, Random, 95% CI) | 0.75 [‐0.48, 1.98] |
| 2.4 Subgroup analysis by age: > 2 years | 3 | 545 | Mean Difference (IV, Random, 95% CI) | 0.65 [‐0.22, 1.53] |
| 3 WAZ scores | 4 | 999 | Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.15, 0.69] |
| 4 HAZ scores | 4 | 999 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.10, 0.14] |
| 5 WHZ scores | 4 | 999 | Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.11, 0.69] |
Comparison 6. Low‐ and middle‐income countries: feeding vs control ‐ psychosocial development. RCT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Psychomotor development | 2 | 178 | Std. Mean Difference (Random, 95% CI) | 0.41 [0.10, 0.72] |
| 2 Cognitive development: test battery | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Cognitive development: Bayley's Mental Development Index (BMDI) | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 7. High‐income countries. CBA.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight | 1 | 116 | Mean Difference (IV, Random, 95% CI) | 0.95 [0.58, 1.33] |
| 1.1 Boys | 1 | 55 | Mean Difference (IV, Random, 95% CI) | 0.90 [0.36, 1.44] |
| 1.2 Girls | 1 | 61 | Mean Difference (IV, Random, 95% CI) | 1.0 [0.48, 1.52] |
| 2 Height | 1 | 116 | Mean Difference (IV, Random, 95% CI) | 0.61 [‐0.31, 1.54] |
| 2.1 Boys | 1 | 55 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.96, 1.76] |
| 2.2 Girls | 1 | 61 | Mean Difference (IV, Random, 95% CI) | 0.80 [‐0.47, 2.07] |
Comparison 8. Low‐ and middle‐income countries: feeding vs control ‐ biochemical markers. RCT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in haemoglobin (g/L) | 5 | 300 | Std. Mean Difference (IV, Random, 95% CI) | 0.49 [0.07, 0.91] |
Comparison 9. Low‐ and middle‐income countries: subgroup analysis ‐ feeding vs control. CBA.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Subgroup analysis: weight by sex | 2 | 840 | Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.08, 0.30] |
| 1.1 Boys | 2 | 453 | Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.14, 0.41] |
| 1.2 Girls | 2 | 387 | Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.18, 0.36] |
| 2 Subgroup analysis: height by sex | 2 | 840 | Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.27, 0.80] |
| 2.1 Boys | 2 | 453 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.59, 0.79] |
| 2.2 Girls | 2 | 387 | Mean Difference (IV, Random, 95% CI) | 0.51 [‐0.33, 1.35] |
| 3 Nutritional adequacy. Low vs moderate vs high: weight gain in kg | 7 | 1784 | Mean Difference (IV, Random, 95% CI) | 0.24 [0.06, 0.41] |
| 3.1 Low nutritional adequacy (0% ‐ 29% energy) | 5 | 961 | Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.06, 0.53] |
| 3.2 Moderate nutritional adequacy (30% ‐ 59% energy) | 4 | 651 | Mean Difference (IV, Random, 95% CI) | 0.31 [0.02, 0.59] |
| 3.3 High nutritional adequacy (60% or higher energy) | 1 | 172 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.24, 0.22] |
| 4 Nutritional adequacy. Low vs moderate vs high: height gain in cm | 7 | 1782 | Mean Difference (IV, Random, 95% CI) | 0.50 [‐0.21, 1.21] |
| 4.1 Low nutritional adequacy (0% ‐ 29% energy) | 5 | 959 | Mean Difference (IV, Random, 95% CI) | 0.77 [‐0.18, 1.73] |
| 4.2 Moderate nutritional adequacy (30% ‐ 59% energy) | 4 | 651 | Mean Difference (IV, Random, 95% CI) | 0.25 [‐1.06, 1.56] |
| 4.3 High nutritional adequacy (60% or higher energy) | 1 | 172 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐1.11, 0.65] |
| 5 Day‐care/feeding centre vs take‐home ration: weight gain in kg | 7 | 1784 | Mean Difference (IV, Random, 95% CI) | 0.20 [0.01, 0.40] |
| 5.1 Day‐care/feeding centre | 4 | 967 | Mean Difference (IV, Random, 95% CI) | 0.31 [0.01, 0.62] |
| 5.2 Take‐home ration | 3 | 817 | Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.03, 0.20] |
| 6 Day‐care/feeding centre vs take‐home ration: height gain in cm | 7 | 1782 | Mean Difference (IV, Random, 95% CI) | 0.43 [‐0.45, 1.31] |
| 6.1 Day‐care/feeding centre | 4 | 965 | Mean Difference (IV, Random, 95% CI) | 0.84 [‐0.25, 1.93] |
| 6.2 Take‐home ration | 3 | 817 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.44, 0.26] |
| 7 Strict supervision of feeding vs moderate supervision vs low supervison: weight gain in kg | 7 | 1784 | Mean Difference (IV, Random, 95% CI) | 0.20 [0.01, 0.40] |
| 7.1 Strict supervision of feeding | 5 | 1286 | Mean Difference (IV, Random, 95% CI) | 0.28 [0.03, 0.52] |
| 7.2 Moderate supervision of feeding | 1 | 326 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.24, 0.32] |
| 7.3 Low supervision of feeding | 1 | 172 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.24, 0.22] |
| 8 Strict supervision of feeding vs moderate supervision vs low supervison: height gain in cm | 7 | 1782 | Mean Difference (IV, Random, 95% CI) | 0.43 [‐0.45, 1.31] |
| 8.1 Strict supervision of feeding | 5 | 1284 | Mean Difference (IV, Random, 95% CI) | 0.62 [‐0.47, 1.70] |
| 8.2 Moderate supervision of feeding | 1 | 326 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.72, 0.88] |
| 8.3 Low supervision of feeding | 1 | 172 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐1.11, 0.65] |
| 9 Single food intervention vs multifaceted intervention: weight gain in kg | 7 | 1784 | Mean Difference (IV, Random, 95% CI) | 0.32 [0.01, 0.62] |
| 9.1 Single food intervention | 4 | 901 | Mean Difference (IV, Random, 95% CI) | 0.32 [‐0.42, 1.07] |
| 9.2 Multifacted intervention | 3 | 883 | Mean Difference (IV, Random, 95% CI) | 0.32 [0.03, 0.61] |
| 10 Single food intervention vs multifaceted intervention: height gain in cm | 7 | 1782 | Mean Difference (IV, Random, 95% CI) | 0.43 [‐0.45, 1.31] |
| 10.1 Single food intervention | 4 | 899 | Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.96, 1.31] |
| 10.2 Multifaceted intervention | 3 | 883 | Mean Difference (IV, Random, 95% CI) | 0.74 [‐0.83, 2.30] |
| 11 Sensitivity analysis: day care: weight | 7 | 1784 | Mean Difference (IV, Random, 95% CI) | 0.21 [0.01, 0.40] |
| 11.1 Day‐care/feeding centre | 4 | 967 | Mean Difference (IV, Random, 95% CI) | 0.33 [0.04, 0.63] |
| 11.2 Take‐home ration | 3 | 817 | Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.03, 0.20] |
| 12 Sensitivity analysis: daycare: height | 7 | 1782 | Mean Difference (IV, Random, 95% CI) | 0.47 [‐0.41, 1.35] |
| 12.1 Day‐care/feeding centre | 4 | 965 | Mean Difference (IV, Random, 95% CI) | 0.97 [‐0.07, 2.00] |
| 12.2 Take‐home ration | 3 | 817 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.44, 0.26] |
Comparison 10. Low‐ and middle‐income countries: subgroup analysis ‐ feeding vs control. RCT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Baseline WAZ lower than median vs higher than median: weight gain in kg | 1 | 192 | Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.04, 0.47] |
| 1.1 Lower than median WAZ | 1 | 96 | Mean Difference (IV, Random, 95% CI) | 0.34 [0.18, 0.50] |
| 1.2 Higher than median WAZ | 1 | 96 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.09, 0.25] |
| 2 Baseline WAZ lower than median vs higher than median: height gain in cm | 1 | 192 | Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.18, 0.48] |
| 2.1 Lower than median WAZ | 1 | 96 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.17, 0.77] |
| 2.2 Higher than median WAZ | 1 | 96 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.46, 0.46] |
| 3 Nutritional adequacy. Low vs moderate vs high: weight gain in kg | 8 | 975 | Mean Difference (IV, Random, 95% CI) | 0.11 [0.04, 0.17] |
| 3.1 Low nutritional adequacy (0 ‐ 29% energy) | 2 | 164 | Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.03, 0.21] |
| 3.2 Moderate nutritional adequacy (30 ‐ 59% energy) | 4 | 566 | Mean Difference (IV, Random, 95% CI) | 0.11 [0.02, 0.20] |
| 3.3 High nutritional adequacy (60% or higher energy) | 2 | 245 | Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.02, 0.40] |
| 4 Nutritional adequacy. Low vs moderate vs high: height gain in cm | 8 | 1381 | Mean Difference (IV, Random, 95% CI) | 0.29 [0.08, 0.50] |
| 4.1 Low nutritional adequacy (0 ‐ 29% energy) | 1 | 127 | Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.05, 0.55] |
| 4.2 Moderate nutritional adequacy (30 ‐ 59% energy) | 5 | 1009 | Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.09, 0.41] |
| 4.3 High nutritional adequacy (60% or higher energy) | 2 | 245 | Mean Difference (IV, Random, 95% CI) | 0.62 [0.13, 1.11] |
| 5 Day‐care/feeding centre vs take‐home ration: weight gain in kg | 9 | 1057 | Mean Difference (IV, Random, 95% CI) | 0.10 [0.04, 0.17] |
| 5.1 Day‐care/feeding centre | 1 | 37 | Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.03, 0.41] |
| 5.2 Take‐home ration | 8 | 1020 | Mean Difference (IV, Random, 95% CI) | 0.10 [0.03, 0.16] |
| 6 Strict supervision of feeding vs moderate supervision vs low supervison: weight gain in kg | 9 | 1056 | Mean Difference (IV, Random, 95% CI) | 0.11 [0.04, 0.17] |
| 6.1 Strict supervision of feeding | 5 | 802 | Mean Difference (IV, Random, 95% CI) | 0.09 [0.01, 0.17] |
| 6.2 Moderate supervision of feeding | 4 | 254 | Mean Difference (IV, Random, 95% CI) | 0.14 [0.03, 0.25] |
| 7 Strict supervision of feeding vs moderate supervision vs low supervison: height gain in cm | 9 | 1463 | Mean Difference (IV, Random, 95% CI) | 0.27 [0.07, 0.48] |
| 7.1 Strict supervision of feeding | 4 | 762 | Mean Difference (IV, Random, 95% CI) | 0.25 [0.04, 0.46] |
| 7.2 Moderate supervision of feeding | 5 | 701 | Mean Difference (IV, Random, 95% CI) | 0.33 [‐0.10, 0.76] |
| 8 Single food intervention vs multifaceted intervention: weight gain in kg | 9 | 1089 | Mean Difference (IV, Random, 95% CI) | 0.11 [0.05, 0.18] |
| 8.1 Single food intervention | 9 | 1040 | Mean Difference (IV, Random, 95% CI) | 0.10 [0.04, 0.17] |
| 8.2 Multifaceted intervention | 1 | 49 | Mean Difference (IV, Random, 95% CI) | 0.37 [0.05, 0.69] |
| 9 Single food intervention vs multifaceted intervention: height gain in cm | 9 | 1512 | Mean Difference (IV, Random, 95% CI) | 0.36 [0.11, 0.61] |
| 9.1 Single food intervention | 8 | 1017 | Mean Difference (IV, Random, 95% CI) | 0.32 [0.12, 0.52] |
| 9.2 Multifaceted intervention | 2 | 495 | Mean Difference (IV, Random, 95% CI) | 0.45 [‐0.82, 1.73] |
| 10 Single food intervention vs multifaceted intervention: psychomotor development | 2 | Std. Mean Difference (Random, 95% CI) | 0.58 [0.36, 0.80] | |
| 10.1 Single intervention | 2 | Std. Mean Difference (Random, 95% CI) | 0.41 [0.10, 0.72] | |
| 10.2 Multifaceted intervention | 1 | Std. Mean Difference (Random, 95% CI) | 0.72 [0.47, 0.96] | |
| 11 Exploratory analysis of well‐implemented studies (Bhandari, Grantham‐MacGregor, Kuuisiaplo) | 3 | 281 | Mean Difference (IV, Random, 95% CI) | 0.76 [0.30, 1.22] |
| 11.1 Height gain | 3 | 281 | Mean Difference (IV, Random, 95% CI) | 0.76 [0.30, 1.22] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bhandari 2001.
| Methods | Study date: 2001. Study design: RCT. Individual randomisation, stratified. Feeding of home‐delivered rations. Delivered twice‐weekly | |
| Participants | SES or context: Low‐ and middle‐income country: South Dehli, India. Urban slum of Nehru place. 80% of women and 40% of men have never been to school. Most families were migrants from rural areas. Median family income is 2000 Rupees (USD 50) per month. Live in dwellings made of mud, concrete or a mixture of both Nutritional status: 22% ‐ 25% had HAZ < 2 SD below mean Age: Children were enrolled at the age of 4 months Number: Supplemented = 87; nutritional counselling = 97; no intervention = 93; visitation = 91 Sex: Both. 42% ‐ 54% boys |
|
| Interventions | Intervention: Feeding alone: 50 g milk cereal supplement prepared with 50 ml water. Given to mothers to prepare and to give to infants twice daily. Twice‐weekly delivery and morbidity assessments Energy: 941 kj, 7 g fat, 8 g protein, 30 g carbohydrates, 2.5 g minerals Duration: 8 months % DRI for energy: 4 ‐ 5 months = 89.9%, 6 ‐ 11 months = 126% % DRI for protein: 4 ‐ 5 months = 191.84%, 6 ‐ 11 months = 354.63%. Protein energy ratio 14.21 Control: Home‐feeding as usual Provider: UNICEF Supervised: Twice‐weekly visits by staff. Asked mothers about consumption and collected packets Compliance: Empty containers collected to measure compliance |
|
| Outcomes | Physical: Weight and length | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not clear how randomisation was done |
| Allocation concealment (selection bias) | Unclear risk | Not clear |
| Baseline outcome measurements | Low risk | No difference in weight between group that was fed and controls |
| Baseline characteristics | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up rate was good, and not much different between experimental and control group |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Personnel who distributed the food were not blind, participant's mothers would have also known |
| Protection from contamination | Unclear risk | Not assessed |
| Selective reporting (reporting bias) | Unclear risk | No access to protocol |
Coyne 1980.
| Methods | Study date: 1980. Study Design: Cluster‐CBA. 5 communities with preschools were selected for the experimental group. 5 comparable communities were selected as controls | |
| Participants | SES or context: High‐income country. Aboriginal children in remote communities. Low SES, marginalised population. Weight and height consistently below average Nutritional status: Initial height, weight, nutrients below "acceptable levels" Age: Average of 4 years Number: 180 enrolled initially. 116 available at follow‐up, experimental = 73, control = 43 Sex: Both. More girls than boys in experimental group, slightly more boys in control group |
|
| Interventions | Intervention: Feeding with adjunctive intervention: Hot lunches in day cares. Provided 2/3 of the DRA for nutrients for the age group. Multivitamin supplements Energy: 941 kj, 7 g fat, 8 g protein, 30 g carbohydrates, 2.5 g minerals Duration: 8 months % DRI for energy: 4 ‐ 5 months = 89.9%, 6 ‐ 11 months = 126% % DRI for protein: Not enough information to calculate Control: Home‐feeding as usual. No day care Provider: Save The Children Supervised: Yes. In preschool |
|
| Outcomes | Physical: Height, weight, biochemical outcomes | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Non‐randomised study |
| Allocation concealment (selection bias) | High risk | Non‐randomised study |
| Baseline outcome measurements | Low risk | No statistically significant differences in age, weight, height at baseline |
| Baseline characteristics | High risk | Intervention is children attending preschool and control is children not in preschool, so the provider setting is different |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The mean height, weight, age, and haemoglobin did not differ from those included in the study vs those who did not return for a second visit |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are aware of intervention |
| Protection from contamination | Low risk | Preschool setting was unit of allocation, unlikely to contaminate non‐preschool control group |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
De Romana 2000.
| Methods | Study date: 2000. Study design: Cluster‐RCT, communities were chosen randomly as intervention or control communities (impact evaluation longitudinal with evaluations before and after the intervention) | |
| Participants | SES or context: Low‐ and middle‐income country: Peru. Area with high prevalence of infant malnutrition Nutritional status: 51% malnutrition in infants. High prevalence of diarrhoea, inadequate infant feeding practices, low prevalence of exclusive breastfeeding, and use of inadequate foods for complementary feeding Age: 6 ‐ 36 months Number: Experimental = 125, control = 125 Sex: Both |
|
| Interventions | Intervention: Feeding only. Precooked food with instant preparation and high nutritional value. 100% of the iron, zinc, iodine, vitamin A and vitamin C requirements, and 60% of the other micronutrient Feeding compared to controls. Nutrition education, but not clear whether both groups got it Energy: 33% of energy requirements for 6 ‐ 36‐month‐old children, 20% of animal protein Reconstituted to provide 1 kcal/g Intensity: Daily Duration: 12 months % DRI for energy: 6 ‐ 12 months = 56.1%, 12 ‐ 24 months = 21.4% % DRI for protein: 6 ‐ 12 months = 148.86%, 12 ‐ 24 months = 130.55% Control: None Provider: Government of Peru and private sector Supervised: Not mentioned Compliance: Not mentioned |
|
| Outcomes | Physical: Haemoglobin, height, and weight | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Says randomly chosen, but does not say how |
| Allocation concealment (selection bias) | Unclear risk | Not much information given in paper on how allocation was done |
| Baseline outcome measurements | Unclear risk | Some shown but not clear whether these are significantly different |
| Baseline characteristics | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data on initial numbers were reported, but outcome data were by percentage, very few numbers |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not really discussed, but probably difficult to blind as they gave food |
| Protection from contamination | Unclear risk | Not applicable |
| Selective reporting (reporting bias) | Unclear risk | No access to protocol |
Devadas 1971.
| Methods | Study date: 1971. Study design: Controlled Cohort Study. 25 children selected from community preschool for experimental group and similar number chosen from another village of comparable background as controls | |
| Participants | SES or context: Low‐ middle‐income country: India. Vulnerable groups in a community development block at Perianaickenpalayam, Coimbatore district, India Nutritional status: Not mentioned Age: Preschool (no age mentioned) Sex: Both Number: Experimental = 25, control = 25 |
|
| Interventions | Intervention: Feeding with adjunctive intervention (nutrition education). Supplement, including 28.4 g of skimmed milk given daily and 1 egg given 3 days a week. Not clear where it was given, but probably in day‐care or feeding centre Energy: 123 kcal and 11 g of protein % DRI for energy: 14.2 % DRI for protein: 89 Duration: 6 months Control: No intervention Provider: UNICEF, WHO, FAO Supervised: Supplement was provided at a feeding centre Compliance: Children's eating habits were evaluated |
|
| Outcomes | Physical: Height, weight, and haemoglobin | |
| Notes | Nutrition education to children and mothers through songs, skits, discussions, and demonstration programmes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Non‐randomised study |
| Allocation concealment (selection bias) | High risk | Non‐randomised study |
| Baseline outcome measurements | Low risk | Initial heights and weights seem comparable |
| Baseline characteristics | Low risk | An identical group of 25 preschool children in Thaliyur village was selected as controls. The nutrient intake of both the group was deficient in calories, iron, ascorbic acid and vitamin A, while the non ANP group did not consume adequate quantities of calcium |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not specified in the study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified in the study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were aware of intervention |
| Protection from contamination | Low risk | 2 different preschool settings were used for allocation 1.5 km apart |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
Fauveau 1992.
| Methods | Study date: 1992. Study design: Cluster‐RCT. Allocated by courtyard | |
| Participants | SES or context: Low‐ and middle‐income country: Urban slum in Bangladesh. 75% of slum dwellers were 'daily labourers'. Income per day less than USD 2. Among sample, only 22% of mothers employed; all with 'low wages'. Almost all of the sample had parents with wages less than USD 2 a day Nutritional status: Mid‐upper arm circumference between 110 and 129 mm, at risk of malnutrition Age: Average of almost 8 months in both groups Number: 127 entered. Experimental = 48, control = 43 (completed) Sex: Both. 60% ‐ 70% girls |
|
| Interventions | Intervention: Feeding + rations for family: Weekly ration of 450 g of pre‐mixed rice, wheat and lentil powder, and 90 g of cooking oil. Delivered to home. All local ingredients. Mothers were taught how to prepare the cereal Mothers of children in both groups received health education that focused on frequency of feedings and caloric content of food Duration: 6 months % DRI for energy: 17.6% % DRI for protein: Not enough information Control: Mothers taught how to prepare meals, but no feeding Provider: USDA Supervised: Visited every 2 weeks to assess. 6‐hour family food‐intake observation Compliance: Not mentioned Intervention: Home‐delivered rations to mothers |
|
| Outcomes | Physical: Weight gain | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used computerised random number generation |
| Allocation concealment (selection bias) | Unclear risk | Nothing mentioned about allocation concealment |
| Baseline outcome measurements | Unclear risk | Not given |
| Baseline characteristics | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | They lost 36 children out of 127 due to illness or movement out of area. Reasons seem to be unrelated to intervention or outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | None mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Children and parents knew that they were fed. Personnel delivering the interventions also knew |
| Protection from contamination | Unclear risk | Not applicable |
| Selective reporting (reporting bias) | Unclear risk | No access to protocol |
Gershoff 1988.
| Methods | Study date: 1988. Study design: CBA. Conducted in day‐care centres where children were enrolled for full day. Children brought lunches | |
| Participants | SES or context: Low‐ and middle‐income country: Thailand. 24 villages in Northern Thailand. Children delayed in growth compared to middle‐class children Nutritional status: Not provided Age: Children were enrolled between the ages of 6 months and 5 years Number and sex: 123 boys and 146 girls supplemented and full data; 144 boys and 121 girls day care no other intervention, full data Sex: Both |
|
| Interventions | Intervention: 5 groups: 1 = no intervention, 2 = health‐sanitation programme, 3 = day‐care centre only, 4 = day‐care centre + vitamin mineral supplement and sanitation, 5 = day‐care centre + everything and snack. We used 3 as the control group and 5 as the experimental Feeding: Locally‐baked fortified cookies given as mid‐morning snack in day care Energy: 300 kcal with 40% of fat and 8% of protein. Given once per day mid‐morning for 5 days per week Duration: 22 months % DRI for energy: 6 ‐ 12 months = 42.1%, 12 ‐ 36 months = 34.5%, 24 ‐ 48 months = 20.8%, 48 ‐ 60 months = 19.8% % DRI for protein: 6 ‐ 12 months = 68.8%, 12 ‐ 36 months = 60.4%, 24 ‐ 36 months = 48.6%, 36 ‐ 48 months = 41.4%, 48 ‐ 60 months = 36.4% Control: Home‐feeding as usual Provider: Thrasher Research Fund, Salt Lake City, Utah, and UNICEF Supervised: Yes. Feeding was done in day care Compliance: Records were kept for each child as to whether the cookies were eaten, partially eaten, or not eaten |
|
| Outcomes | Physical: Head, arm and chest circumference, triceps and subscapular skin folds, weight and length, WAZ, HAZ, WHZ | |
| Notes | Sanitary water provided to the family and health worker to family | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Non randomised study |
| Allocation concealment (selection bias) | High risk | Non randomised study |
| Baseline outcome measurements | Unclear risk | Not mentioned |
| Baseline characteristics | Unclear risk | We compared day care with feeding to day care without, but staff not specified |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not specified in the study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are aware of intervention |
| Protection from contamination | Low risk | Day‐care centres were used for allocation. Not likely to contaminate other groups |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
Gopalan 1973.
| Methods | Study date: 1973. Study design: CBA. Not cluster | |
| Participants | SES or context: Low‐ and middle‐income country: India. 9 villages near Hyderabad. Children from low‐income groups Nutritional status: Does not really say, but ingested 700 kcal/day in their regular diet Age: 1 ‐ 5 years Sex: Both Number: Experimental = 306 (211 reported), control = 108 (83 reported) |
|
| Interventions | Intervention; Feeding only, Sweet cakes supplement consisted of wheat flour (23 g), sugar (35 g), and edible oil (10 g). Given in a feeding centre once daily for 6 days a week Energy: 310 kcal, 3 g protein Duration: 14 months. Feeding was timed so that it would not interfere with home meals % DRI for energy: 12 ‐ 24 months = 35.7%, 24 ‐ 36 months = 35.7%, 36 ‐ 48 months = 21.5%, 48 ‐ 60 months = 20.5% % DRI for protein: 12 ‐ 24 months = 30.19%, 24 ‐ 36 months = 24.31%, 36 ‐ 48 months = 20.72%, 48 ‐ 60 months = 18.22%. Protein energy ratio 3.87 Control: Regular food at home. No supplement Provider: Not mentioned Supervised: Yes Compliance: It was ensured that children consumed all the supplement. 85% attendance rate |
|
| Outcomes | Physical: Weight and height | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Non randomised study |
| Allocation concealment (selection bias) | High risk | Non randomised study |
| Baseline outcome measurements | Low risk | The groups were matched initially with respect to sex, height and weight, and the prevalence of nutritional deficiency signs were therefore comparable. No significant difference in the intakes of home diets between the two periods were noticed |
| Baseline characteristics | Unclear risk | Not specified in the study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | During the course of the study, there was an outbreak of measles, with 114 of the 415 children being affected. Of these, 32 belonged to the control group and 82 to the experimental group.This provided an opportunity to examine the effect of food supplements on the response to the disease |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified in the study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants aware of intervention |
| Protection from contamination | Low risk | All children in the experimental group were assembled daily at a central place in the village and were fed the supplement 6 days a week. It was ensured that all children consumed the entire supplement |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
Grantham‐McGregor 1991.
| Methods | Study date: 1991. Study design: RCT | |
| Participants | SES or context: Low‐ and middle income country: Jamaica. Poor urban neighbourhoods in Kingston. Stunted children randomly assigned. A small group of non‐stunted children was used as a reference, but they are not included in the review Nutritional status: Below ‐2 SD. NCHS reference data for age and sex for height Age: 19 ‐ 24 months Number: 129 (control = 33, stimulated = 30, supplemented = 32, both = 32) Sex: Both |
|
| Interventions | Intervention: Three study arms. Feeding only, feeding + stimulation, stimulation only + control. We compared feeding only to control Feeding: 1 kg milk‐based formula per week. Supplement delivered to home. Supposed to be given once daily Energy: 750 kcal (3.15 MJ) per day, 20 g protein per day Duration: 2 years % DRI for energy: 9 ‐ 12 months = 105.2%, 12 ‐ 24 months = 86.3% % DRI for protein: 6 ‐ 12 months = 215.96%, 12 ‐ 20 months = 201.27%. Protein energy ratio 10.67 Control: Home food and breastfeeding Provider: Ford Foundation USA, Population Council Cow and Gate, Grace Kennedy Jamaica, and Seprod Jamaica Supervised: Weekly visits to encourage use Compliance: Community health workers made weekly visits to deliver supplement and encourage use |
|
| Outcomes | Physical: Weight, height, weight, mid‐upper arm circumference, WHZ Psychological: Developmental Quotient (locomotor, hearing and speech, hand and eye, and performance) |
|
| Notes | Additional 0.9 kg cornmeal and skimmed milk powder were given to the family to minimise sharing of the supplement, stimulation done by community health aides 1‐hour per week, mothers taught how to play with child to promote development and made homemade toys for children | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random assignment; nothing else mentioned |
| Allocation concealment (selection bias) | Unclear risk | None mentioned |
| Baseline outcome measurements | Low risk | Weight, WHZ almost identical |
| Baseline characteristics | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Almost all followed up |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear for height and weight. Low risk for cognitive as they were blinded |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of blinding and impossible to blind participants |
| Protection from contamination | Unclear risk | Not applicable |
| Selective reporting (reporting bias) | Unclear risk | No access to protocol |
Heikens 1989.
| Methods | Study date: 1989. Study design: RCT. Individually randomised | |
| Participants | SES or context: Low‐ and middle‐income country: Kingston, Jamaica Nutritional status: Malnourished children enrolled in community rehabilitation. < 80% of NCHS weight‐for‐age Age: 3 ‐ 36 months Number: Supplemented = 39, unsupplemented = 43 Sex: Both. 42% ‐ 54% boys |
|
| Interventions | Intervention: Feeding only. High‐energy supplement, delivered to home with instructions on how to prepare, and measuring cup Energy 526 kcal, 13.75 g protein. Delivered once a week Duration: 3 months of supplementation, 3 months of follow‐up % DRI for energy: Not enough information % DRI for protein: Not enough information Control: Home‐feeding as usual. Also received health care and micronutrient supplementation Provider: Ministry of Health, Jamaica Supervised: Some monitoring through food frequency questionnaires Compliance: Supplemented children took in more kcal |
|
| Outcomes | Physical: Weight, height, BMI | |
| Notes | Difference in weight gain was significant during supplementation, but disappeared once supplementation stopped. Difference in height still remained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Says children were allocated randomly but no information on how |
| Allocation concealment (selection bias) | Unclear risk | Little information |
| Baseline outcome measurements | Unclear risk | Not mentioned |
| Baseline characteristics | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Says 82 enrolled. 14 admitted to hospital. Equal numbers in each group |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants, caregivers, or personnel |
| Protection from contamination | Unclear risk | Not applicable |
| Selective reporting (reporting bias) | Unclear risk | No access to protocol |
Husaini 1991.
| Methods | Study date: 1991. Study design: Cluster‐RCT; stepwise by pairs. Randomised, except that they added 2 control day cares afterwards | |
| Participants | SES or context. Low‐ and middle‐income country: Indonesia. Tea plantations in Java, Indonesia. Tea plantation workers. Low education: fathers about 5 years, mothers about 3 years Nutritional status: Weight z‐scores average were ‐1.57 and ‐1.66 and height z‐scores were ‐2.34 and ‐2.42 Age: 6 ‐ 59 months (but up to 20 months are the only ones included in this paper) Number: 113. Experimental = 75, control = 38 Sex: Both. Boys experimental = 43, boys control = 19, girls experimental = 32, girls control = 19 |
|
| Interventions | Intervention: feeding only: Snacks, including rice, rice flour, wheat flour, bread, cassava, potatoes, sweet potatoes, coconut milk, refined sugar, brown sugar, and edible oil. Given in day care Energy: On average, the daily supplements provided 1660 kJ (400 kcal) and 5 g protein Duration: 6 days per week for 3 months. 6 months for haemoglobin % DRI for energy: 6 ‐ 12 months = 56.1%, 12 ‐ 20 months = 46.0% % DRI for protein: 6 ‐ 12 months = 57.37%, 12 ‐ 20 months = 50.32%. Protein energy ratio 5 Control: Usual Provider: Indonesian Government Supervised: Not mentioned Compliance: Mothers were encouraged to use supplements along with usual diet |
|
| Outcomes | Physical: WAZ, HAZ, skinfold thickness, arm circumference, head circumference, and chest circumference measured but not reported Psychological: Psychomotor Development Index and Mental Development Index |
|
| Notes | 32 tested recipes, 20 were used for intervention | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Cannot really tell how allocation was done |
| Baseline outcome measurements | Unclear risk | Low for WAZ, HAZ, Cognitive. For psychomotor, scores at baseline 8 points apart |
| Baseline characteristics | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only used the youngest cohort |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Low for PDI and MDI. Unclear for height or weight |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Children certainly did not know study goals, or whether they were in experimental or control. Day‐care centre personal certainly did |
| Protection from contamination | Unclear risk | Not applicable |
| Selective reporting (reporting bias) | Unclear risk | No access to protocol |
Iannotti 2014.
| Methods | Study date: 2014. Study Design: RCT | |
| Participants | SES or context: Urban slums of Haiti Nutritional status: The average WAZ ranged from ‐0.70 ‐0.85 Age: 6 ‐ 11 months at start of study Sex: Both. Slightly more girls than boys in all groups Number: 589 recruited to 3 groups (after 6 months follow‐up there were: control = 144, intervention = 150, other treatment = 126) |
|
| Interventions | Intervention: Feeding + 2 intervention groups: 3 month Lipid nutrient supplement, 6 month LIpid Nutritent supplement. Home‐delivered; 1 sachet per day. Parents asked to feed children Energy: On average, the daily supplements provided 108 kcal and 23% of protein Duration: 6 month% DRI for energy: 15% % DRI for protein: 23% Control: No supplement Provider: Researchers with funding from Bill & Melinda Gates Foundation, the Inter‐American Development Bank, the World Bank, and the United Nations World Food Program Supervised: Once monthly Compliance: 98% of mothers reported that the children ate all of the supplement |
|
| Outcomes | Physical: LAZ, WAZ, WHZ | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Says infants were randomly assigned. Drawn from container |
| Allocation concealment (selection bias) | Low risk | Sealed paper forms were drawn from a container |
| Baseline outcome measurements | Low risk | WAZ, HAZ, WHZ not significantly different |
| Baseline characteristics | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Approximately the same proportion missing in each group and reasons unlikely to be related to outcome (most moved to country) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned |
| Protection from contamination | Unclear risk | Not applicable |
| Selective reporting (reporting bias) | Unclear risk | No access to protocol |
Isanaka 2009.
| Methods | Study date: 2009. Study design: Cluster‐RCT | |
| Participants | SES or context: Low‐ and middle‐income country: Niger. 12 villages with a 15% or higher prevalence of wasting. Low income, diet dependent on annual crop harvest Nutritional status: Height for weight 80% or more of NCHS median Age: 6 ‐ 60 months. No longer fed once they reached 60 months Number: 3166; down to 3026 after 7 months Sex: Both |
|
| Interventions | Intervention: Feeding only. 92 g packet of RUTF. Monthly distribution enough for 1 sachet daily Energy: 500 kcal Duration: Intervention was 3 months long. Followed up for 32 weeks % DRI for energy: 6 ‐ 12 months = 69.8%, 12 ‐ 24 months = 57.5%, 24 ‐ 36 months = 57.5%, 36 ‐ 48 months = 34.7%, 48 ‐ 60 months = 33.0% % DRI for protein: Not enough information Control: Regular meal. No extra supplement Provider: MSF Supervised: Not mentioned Compliance: Not mentioned |
|
| Outcomes | Physical: Weight, length, HAZ, WAZ | |
| Notes | Adequate control for clustering | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Villages drawn from a hat; first 3 in each district went to experimental, second went to control |
| Allocation concealment (selection bias) | Unclear risk | Selection was made by a field worker not involved in identification of eligible villages |
| Baseline outcome measurements | Unclear risk | Not applicable |
| Baseline characteristics | Low risk | HAZ, WHZ not significantly different |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop out was similar in both groups; did an all‐available‐data analysis |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Does not say blinded |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel distributing the supplement had to know whether they were in the intervention or control group. But unlikely to affect anthropometrics |
| Protection from contamination | Unclear risk | Not applicable |
| Selective reporting (reporting bias) | Unclear risk | No access to protocol |
Joshi 1988.
| Methods | Study date: 1988. Study design: Cluster‐CBA. 2 Balwadies (preschools) selected (1 = poor, 1 = middle class). 2 kindergartens were selected per community (1 experimental and 1 control in each). 1 was implementing a feeding programme. They were chosen on the basis of implementing or not implementing a feeding programme | |
| Participants | SES or context: Low‐ and middle‐income country: India. 4 Balwadies in Pune City, India. 2 were in a poor living area consisting of families of low socioeconomic classes, slum dwellers and illiterate parents, without facilities for sanitation, sewage systems, and personal hygiene (LSE). 2 were in middle, socio‐economic classes with higher income and education level with enough space and clean surroundings (MSE) Participants were all children who had just enrolled in the schools. So there was a baseline Nutritional status: Ranged from normal to severe Age: 30 months ‐ 5 years Sex: Both Number: Experimental = 50 low SES and 74 middle SES, control = 42 low SES and 81 middle SES |
|
| Interventions | Intervention. Feeding only. Supplement included commonly consumed snacks with which the children were familiar such as milk, biscuits, curd, and seasonal fruits. Each child was served the same quantity of food on a clean plate. Given once daily in kindergarten Energy: 167 kcal and 5.1 g protein Duration: 7 months. 151 days of feeding in LSE area out of 210. 129 in MSE area % DRI for energy: 36 ‐ 48 months = 11.60%, 48 ‐ 60 months = 11.02% % DRI for protein: 36 ‐ 48 months = 35.2%, 48 ‐ 60 months = 31.0% Control: No feeding programme Provider: Not mentioned Supervised: Teachers monitored consumption as food was distributed in kindergarten Compliance: Not mentioned |
|
| Outcomes | Physical: Height and weight | |
| Notes | Gomez classification used for assessing impact of the intervention | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Non randomised study |
| Allocation concealment (selection bias) | High risk | Non randomised study |
| Baseline outcome measurements | Low risk | Initital weights and heights of the group that was fed and those that were unfed were very similar and the confidence intervals overlapped, meaning that differences were non‐significant |
| Baseline characteristics | Unclear risk | No information to judge |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not specified in the study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified in the study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants, parents, staff aware of intervention |
| Protection from contamination | High risk | Unit of allocation is the children; was consumed on the spot under the supervision of the teachers |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
Kuusipalo 2006.
| Methods | Study date: 2006. Study design: RCT, not cluster, with 7 intervention arms and 1 control. Intervention arms were varying intensity of spreads with 2 different formulations: soy and milk | |
| Participants | SES or context: Low‐ and middle‐income country: Rural Malawi. Most children were undernourished. Study conducted during rainy season when food security is the lowest and weight and height gain of the children is poorer than the rest of the year. Exclusive breastfeeding is almost non‐existent and diet is complemented with maize Nutritional status: Weight‐for‐age < ‐2, weight greater than 5.5 kg, and WHZ greater than ‐3 Age: 6 ‐ 17 months Number: Total: 128 started (18, 18, 18, and 9 children received 5, 25, 50, and 75 g of milk‐based fortified spread, respectively; 20, 18, and 9 children received 25, 50, and 75 g of soy‐based fortified spread, respectively). 125 finished. 18 ‐ 19 in each group, control = 18 Sex: Both |
|
| Interventions | Intervention: Feeding only with seven different intervention arms: Milk‐based fortified spread and soy‐based fortified spread of different quantities Energy: Supplementation provided 96, 544, 1105, and 1661 kcal and 1, 4, 8, and 11 g of protein in 5, 25, 50, and 75 g of milk‐based fortified spread, respectively. It provided 531, 1071, and 1615 kcal and 3, 7, and 10 g of protein in 25, 50, and 75 g of soy‐based fortified spread, respectively. Supplements delivered to homes prepackaged weekly for first 4 weeks and bi‐weekly thereafter Duration: 12 weeks % DRI for energy: Milk‐based formula 6 ‐ 12 months = 28.57% (avg.), soy‐based formula 6 ‐ 12 months = 35.98% (avg.), milk‐based formula 12 ‐ 24 months = 23.44% (avg.), soy‐based formula 12 ‐ 24 months = 29.52% (avg.) % DRI for protein: Milk‐based formula 6 ‐ 12 months = 68.84% (avg), soy‐based formula 6 ‐ 12 months = 76.50% (avg.), milk‐based formula 12 ‐ 24 months = 60.38% (avg.), soy‐based formula 12 ‐ 24 months = 67.10% (avg.) *Because it provided more of the DRI for energy, we used the children who received the 75 g soy‐based formula as our experimental group Control: No feeding programme Provider: Foundation for Paediatric, Research in Finland, and Medical Research Fund of Tampere Supervised: No, but empty sachets from the previous week were collected. Sometimes nurses visited homes during feeding time Compliance: No, but empty sachets from the previous week were collected |
|
| Outcomes | Physical: Haemoglobin, height, weight, WAZ, HAZ, WHZ | |
| Notes | At a daily dose of 25 and 50 g, spreads are somewhat more expensive than micronutrient‐fortified corn‐soy flour, tablets or sprinkles. USD 0.10 ‐ 0.20/day vs USD 0.02 ‐ 0.04/day | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by computer‐generated lists |
| Allocation concealment (selection bias) | Unclear risk | Nothing mentioned |
| Baseline outcome measurements | Low risk | Very little (and non‐significant) difference in weight, heights, WAZ, HAZ, WHZ |
| Baseline characteristics | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 3 dropped out |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Said that research assistant and lab assistant performing outcome assessment remained blinded until end of study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | For the comparison against the "untreated" group, there was no placebo, but within different energy‐densities, participants were blinded. "Thus, in total, 7 different supplementation groups and 1 unsupplemented group (that received no placebo spread) were included in the study. Soy‐containing formulas tasted slightly sweeter than the milk‐containing ones, but otherwise the look, taste and packing of different formulas were identical" |
| Protection from contamination | Unclear risk | Not applicable |
| Selective reporting (reporting bias) | Unclear risk | No access to protocol |
Lutter 2008.
| Methods | Study date: 2008. Study design: CBA | |
| Participants | SES or context: Low‐ and middle‐income country: Ecuador. Urban, peri‐urban and rural communities, low and insecure income, poor housing, and a general lack of 1 or more essential services (piped water, reliable electricity supply, sewage disposal) Nutritional status: Included all children in communities Age: 9 ‐ 14 months at enrolment Number: Experimental = 338 for anthropometry, 170 at end; 324 for morbidity, 324 at end. Control = 296 for anthropometry, 149 at end; 262 for morbidity, 262 at end |
|
| Interventions | Intervention: Feeding with nutrition education. Supplement was a 65 g dry milk‐based product. Given to mothers to prepare once daily Energy: Provided 275 kcal/day and 10 g of protein, 6 g lipid Duration: 44 weeks % DRI for energy: 9 ‐ 12 months = 38.6%, 12 ‐ 14 months = 31.6% % DRI for protein: 9 ‐ 12 months = 108.0%, 12 ‐ 14 months = 114.30% Control: Usual diet Provider: National Food Nutrition Program administered by Ministry of Public Health Supervised: Yes. Weekly home visits with dietary recall Compliance. The supplement was consumed 73% of the time. Based on dietary recall, consumption was ½ of the daily ration Difference between study and control groups at end of study was 180 kcal. But says that daily energy increased by 240 kcal and iron by 9 mg |
|
| Outcomes | Physical: Weight, length, anaemia, HAZ, WAZ, WHZ | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Non randomised study |
| Allocation concealment (selection bias) | High risk | Non randomised study |
| Baseline outcome measurements | Low risk | At baseline, program and control groups were similar with respect to many but not all variables (Table 3) |
| Baseline characteristics | Low risk | Field workers were trained and standardized using WHO guidelines |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The 80% follow‐up by the team responsible for weekly morbidity surveillance was due to the fact that it was done by community health workers who could easily revisit the home to collect complete data. In contrast, the other teams travelled from the capital to the evaluation area for baseline and final measurements in the health clinics and had less flexibility to follow up with children who did not come to the clinic. Loss to measurement did not appear to bias these results; this was determined using the method described in the ‘‘Methods’’ in which a dummy variable indicating loss to follow‐up status was regressed on the variables in the regression models |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified in the study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants aware of intervention |
| Protection from contamination | Low risk | Health centres in communities where the program began served as the program group and health centres in neighbouring, apparently similar communities, where the program was to be implemented 1 year later, served as the control group |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
Mangani 2014.
| Methods | Study date: 2014. Study design: RCT | |
| Participants | SES or context: Low‐ and middle‐income country: Rural Malawi Nutritional status: Not severely malnourished. Average WAZ of ‐0.70 to ‐0.80 Age: 6 months Number: 840 randomised into 4 groups. 183 ‐ 191 finished in each of the 4 groups Sex: 53% boys |
|
| Interventions | Intervention. Children randomised into 4 groups. Milk‐LNS, Soy‐LNS, Corn‐soy blend, and control Feeding: The Milk‐LNS group received a LNS with milk. There was also a Soy‐LNS, but we used milk Energy: provided 285 kcal/day for Milk‐LNS Duration: 12 months % DRI for energy: 40% % DRI for protein: 94.1% Control: Usual diet Provider: Academy of Finland, Foundation for Pediatric Research in Finland, Medical Research Fund of Tampere University Hospital, the American people, the Office of Health, Infectious Disease and Nutrition, Bureau for Global Health, United States Agency for International Development (USAID), Foundation and Singapore Ministry of Health’s National Medical Research Council Supervised: Yes, every 2 weeks visits were made and packets retrieved. Also asked mothers about compliance Compliance. All mothers reported that the infants consumed all of the packet. They also reported that the children were the only ones who received the supplement in almost all cases |
|
| Outcomes | Physical: Weight, length, HAZ, WAZ, WHZ | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Opaque envelopes shuffled and guardian was asked to choose 1 |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes shuffled and guardian was asked to choose 1 |
| Baseline outcome measurements | Unclear risk | Weight, height, WAZ, HAZ, WHZ similar and non‐significant differences |
| Baseline characteristics | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Number of drop outs not significantly different between groups |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not clear. Says that they did rotate outcome assessors so that they did not remember previous measurements |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants |
| Protection from contamination | Unclear risk | Not applicable |
| Selective reporting (reporting bias) | Unclear risk | No access to protocol |
Manjrekar 1986.
| Methods | Study date: 1986. Study design: CBA | |
| Participants | Context: Low‐ and middle‐income country: India. Mysore City in India for experimental food distribution centres SES: Control taken from a semi‐urban village in the vicinity of Mysore city where socio‐economic conditions were comparable to intervention group Nutritional status: Not clear Age: 0 ‐ 5 years Number: Experimental = 72 (13 = < 1 year, 14 = 1 ‐ 2 years, 10 = 2 ‐ 3 years, 19 = 3 ‐ 4 years, and 16 = 4 ‐ 5 years), control = 51 (8 = < 1 year, 9 = 1 ‐ 2 years, 10 = 2 ‐ 3 years, 6 = 3 ‐ 4 years, and 18 = 4 ‐ 5 years) Sex: Both |
|
| Interventions | Intervention: Feeding only. Bread and 'Miltone', a groundnut protein‐based milk substitute. Children received 2 slices of bread and 150 ml milk, infants received 1 slice of bread and 200 ml milk Energy: Child 250 kcal and infant 200 kcal. Given 6 days a week Duration: 18 months % DRI for energy: 6 ‐ 12 months = 35.1%, 12 ‐ 36 months = 28.8%, 36 ‐ 48 months = 17.4%, 48 ‐ 60 months = 16.5% % DRI for protein: Not enough information Control: Usual meals Provider: Government Supervised: In 2 centres the supplement was consumed under strict supervision at the centre. In the third centre, supplement was home‐delivered 'On the spot' consumption was strictly supervised |
|
| Outcomes | Physical: Height and weight | |
| Notes | Deworming, after stool examinations, was done at 6‐monthly interval | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Non randomised study |
| Allocation concealment (selection bias) | High risk | Non randomised study |
| Baseline outcome measurements | Low risk | No statistically significant difference was found between the initial and final measurements in the supplement and control children except between the weights of the children in the age group 4 ‐ 5 years and this difference was in favour of the control group |
| Baseline characteristics | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High drop‐out rate in a longitudinal study was felt a serious set‐back. With a turnover of 509 nutritionally assessed children, only 111 fulfilled the requisites till the final examination and still less for the follow‐up of height and weight. The average attendance of the centres throughout the feeding period was 207. For a regular follow‐up the control formed a still greater problem, since the children and their guardians were not motivated by regular food distribution and further prevented by superstitions, though medical care was given during the visit |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified in the study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were aware that they were being fed |
| Protection from contamination | Low risk | Not specified in the study |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
McKay 1978.
| Methods | Study date: 1978. Study design:Cluster‐RCT. 6 different groups with different treatment times. T1a = 1 treatment of supplementation + stimulation, T1b = 1 treatment of supplementation + stimulation, but prior nutritional supplementation + health care, T2 = 2 treatments; T3 = 3 treatments; T4 = 4 treatments; and T0 = 0 treatments, but only measured at end. Sectors of the community were randomly chosen to be in each group. We compared T4 to T2 at 63 months before T2 receive supplementation | |
| Participants | SES or context: Low‐ and middle‐income country: Cali, Colombia. Low‐income urban community Nutritional status: Subnormal (undernourished), except for T0 who were average Age: ˜ 3 years Sex: Both Number: T2 = 64, T4 = 62 |
|
| Interventions | Intervention: Feeding + simulation for different lengths of time (Groups given above). Given as part of the programme in centres Energy: Enough for 3 times a day Duration: 3.5 years divided into 4 treatment periods of 9 months each % DRI for energy: 75% of the recommended calories % DRI for protein: 75% of the recommended protein Control: Compared T4 to T2 at age 63 months before the T2 began treatment Provider: Human Ecology Research Foundation Supervised: Yes, provided at the treatment centre Compliance: Yes, provided at the treatment centre. Attendance above 95% for all groups |
|
| Outcomes | Physical: Weight and length reported in Perez‐Escamilla, WAZ, HAZ Psychological: Cognitive development |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Says randomised, but not clear how it was done |
| Allocation concealment (selection bias) | Unclear risk | Stratified according to initial height and weight and then randomised into groups |
| Baseline outcome measurements | Low risk | T4 and T2 the same on cognition |
| Baseline characteristics | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 53 lost; 2 died and 51 moved out of area. This is out of a total of 301. They report that there were no initial differences between those who dropped out and those who remained |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Say specifically that outcome assessors were randomly assigned and that they were blinded to allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and people delivering the programme could not be blinded as they were getting fed and delivering the intervention |
| Protection from contamination | Unclear risk | Not applicable |
| Selective reporting (reporting bias) | Unclear risk | No access to protocol |
Mittal 1980.
| Methods | Study date: 1980. Study design: CBA | |
| Participants | SES or context: Low‐ and middle‐income country: India. Single community block, low and insecure income, poor housing, and a general lack of 1 or more essential services (piped water, reliable electricity supply, sewage disposal) Age: 6 ‐ 24 months, pregnant in the last trimester and lactating women in the first 6 months Sex: Both Number: Experimental = 201, control = 125 |
|
| Interventions | Intervention: Feeding only. Take‐home feeding. 55 g nutritional supplement in packets collected by mother or older sibling at a distribution point. Collected once weekly. Measuring cup provided Energy: 100 g of the supplement provided 14 g of protein and 360 kcal. Given once a day Duration: 12 months % DRI for energy: 6 ‐ 11 months = 27.8%, 12 ‐ 23 months = 22.8% % DRI for protein: 6 ‐ 11 months = 88.35%, 12 ‐ 23 months = 77.49%. Protein energy ratio 15.66 Control: Usual diet Provider: Government of India in collaboration with World Bank and the Swedish International Development Agency Supervised: Not mentioned Compliance: Collection rate of 75% weekly at 4 distribution points. But do not know whether the targeted children consumed them |
|
| Outcomes | Physical: Weight and length Time‐points: Measured at baseline and end of study |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Non randomised study |
| Allocation concealment (selection bias) | High risk | Non randomised study |
| Baseline outcome measurements | Low risk | Both these groups were comparable in all respects, including nutritional status (as reflected by lack of significant difference between their heights and weights at the beginning of the study) |
| Baseline characteristics | High risk | Different staff for nutrition component |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High rate of dropout |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified in the study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were aware of intervention |
| Protection from contamination | Unclear risk | Unit of allocation was children within a block that was divided into experimental and control zone |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
Obatolu 2003.
| Methods | Study date: 2003. Study design: RCT. Individually randomised | |
| Participants | SES or context: Low‐ and middle‐income country: Nigeria. Low‐income group had low and insecure income. Most parents had no formal education or only primary education Age: 4 months at baseline Number: Experimental = 30 in low‐income feeding group; 15 boys and 15 girls. Control = 30 in low‐income non‐feeding group and 30 in high‐income non‐feeding group |
|
| Interventions | Intervention: Feeding only. Home‐delivered. Seems like once a week. Pre‐prepared gruel given to mothers to mix up. Instructions on how to prepare Energy: Not mentioned Duration: 14 months % DRI for energy: Not mentioned % DRI for protein: Not mentioned Control: No food provided Provider: International Development Research Centre, Canada Institute of Agricultural Research and Training, and International Institute of Tropical Agriculture Supervised: Not clear. Seems like once a week Compliance: Not clear. Nothing mentioned |
|
| Outcomes | Physical: Height and weight | |
| Notes | Little information on implementation, especially on compliance and attrition | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers used after children were stratified. 15 boys and 15 girls |
| Allocation concealment (selection bias) | Unclear risk | Nothing mentioned |
| Baseline outcome measurements | Low risk | Weight and length nearly identical |
| Baseline characteristics | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Nothing mentioned about attrition. Said that 30 were selected in each group. Had end‐of‐study data for 30 in each group. But too much information is lacking to make a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Nothing mentioned about blinding at all. Participants must be aware of food being provided so we judged this as high risk |
| Protection from contamination | Unclear risk | Not applicable |
| Selective reporting (reporting bias) | Unclear risk | No access to protocol |
Oelofse 2003.
| Methods | Study date: 2003. Study design: RCT. Feeding at home. Home‐delivered | |
| Participants | SES or context. Low‐ and middle‐income country: South Africa. Urban disadvantaged black community, low SES indicated by type of housing, possession of household appliances, and access to basic amenities. Most of the inhabitants work in industries in the city or as domestic workers in private homes Nutritional status: Birth weight ≧ 2.5 kg Age: 6 months Sex: Both Number: Experimental = 25, control = 21 at 6 months. Experimental = 16, control = 14 at 12 months |
|
| Interventions | Intervention: Feeding only. Supplement of 60 g dry cereal. Enough for 1½ weeks delivered to home Mothers instructed on how to prepare Energy: 1304 kj, 12 g protein, and 6 g fat Intensity: Once daily Duration: 6 months % DRI for energy: 6 ‐ 12 months 42% % DRI for protein: 6 ‐ 12 months 137.69%. Protein energy ratio 15.4 Control: Usual diet Provider: Researchers (Nutrition Intervention Unit, MRC South Africa) Supervised: Some supervision (research assistant visited once a week to check cereal consumption) Compliance: Not mentioned |
|
| Outcomes | Physical: Weight, length, WAZ, HAZ, WHZ | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors mentioned that children were randomly allocated but no explanation of how this was done |
| Allocation concealment (selection bias) | Unclear risk | Allocation method was not described |
| Baseline outcome measurements | Unclear risk | No significant difference on any outcome variable |
| Baseline characteristics | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Lost 35% of study infants to follow‐up. Reasons for 'default' were given and were plausible. Many moved out of the study area. It is unclear whether these reasons were the same for experimental and control groups |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Says research assistants conducted the test. Does not indicate if they were blinded |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study participants and their mothers could not be blinded as they received supplements. Unlcear if personnel were blinded |
| Protection from contamination | Unclear risk | Not applicable |
| Selective reporting (reporting bias) | Unclear risk | No access to protocol |
Pollitt 2000a.
| Methods | Study date: 2002. Study design: Cluster‐RCT. Randomised by day care. 3 Groups: Condensed milk + micronutrient. (2 of each age cohort). Skimmed milk + micronutrient (2 of each age cohort). Skimmed milk + placebo (3 groups for 6 months each. One 12‐month, one 18‐month, one 24‐month) | |
| Participants | SES or context: Low‐ and middle‐income country: Indonesia. Rural West Java. Children in government day care. Workers on tea plantation. Most were tea pickers; some were factory workers. A few had supplementary income. Income low; at time of study average was USD 68 ‐ USD 83 a month. Parental education averaged 3 years. Most families were Sudanese Nutritional status: Length for age ≦1 SD below mean. WFA between ‐1 and ‐2 SD of median Age: 2 cohorts. 12 and 18 months at enrolment Sex: Both Number: Experimental = 53 in 12‐month cohort, 83 in 18‐month cohort |
|
| Interventions | Intevention: Feeding only. 2 intervention groups (see above) Energy: E group: 1171 kj + 12 mg iron; M Group: 209 kj + 12 mg iron, or S group: 104 kj with placebo pill (no micro‐nutrients). We compared E group to S group Duration: 12 months % DRI for energy: 6 ‐ 12 months = 26.1%, 12 ‐ 36 months = 21.4%, 36 ‐ 48 months = 12.9%, 48 ‐ 60 months = 12.3% % DRI for protein: Not enough information Control: Placebo Provider: Nestlé Foundation Supervised: Day‐care workers Compliance: Given at day care |
|
| Outcomes | Physical: Weight Psychological: Standardised mental and cognitive assessment |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By day care. Success was tested through inter‐group comparisons |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Baseline outcome measurements | Unclear risk | Means look the same for height and weight |
| Baseline characteristics | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Difficult and complex to ascertain. No mention of attrition |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Tried to blind testers, but they noticed differences. However, they did switch testers around to avoid bias |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Controls received skimmed milk and experimental received condensed with micronutrient, and they were on different plantations, so they probably did not notice |
| Protection from contamination | Unclear risk | Not applicable |
| Selective reporting (reporting bias) | Unclear risk | No access to protocol |
Rivera 2004.
| Methods | Study date: 2004. Study design: Cross‐over RCT | |
| Participants | SES or context: Low‐ and middle‐income country: Mexico. Participants were from low‐income households in poor rural communities in 6 central Mexican states. Children and pregnant and lactating women in participating households received fortified nutrition supplements, and the families received nutrition education, health care, and cash transfers. Families enrolled in the programme (Progresa families) received 2 types of cash transfers every 2 months: A universal cash amount for all families and a specific cash transfer associated with school attendance Nutritional status: Included all children in communities Age: 12 months or younger at enrolment Number: 650 children (intervention group = 373, cross‐over intervention group = 277) |
|
| Interventions | Intervention. Feeding +take‐home rations + cash incentive for attending clinic. 240 g dry whole milk, sugar, maltodextrins, and micronutrient given in 3 flavours that required hydration before consumption. Packages were distributed at health centres. Mothers given instruction to add 4 spoons of boiled water to 1 ration. Families in program given incentives to attend health clinic Energy: 5 daily rations of 44 g provided 275 kcal/day and 10 g of protein, 6 g lipid Duration: 24 months % DRI for energy: 4 ‐ 5 months = 38.7%, 6 ‐ 12 months = 27.3% % DRI for protein: 4 ‐ 5 months = 69.54%, 6 ‐ 12 months = 66.55% Control: Cross‐over intervention group Provider: National Institute of Public Health, Ministry of Health Supervised: Not mentioned Compliance: Not mentioned |
|
| Outcomes | Physical: Weight, height, WAZ, HAZ, WHZ, haemoglobin levels (anaemia) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Does not say how randomisation was done |
| Allocation concealment (selection bias) | Unclear risk | No mention of how it was done or concealed |
| Baseline outcome measurements | Low risk | No significant differences between groups on any outcome variable |
| Baseline characteristics | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | They were very clear about attrition rates. At the first follow‐up 10% dropped out. Very little difference |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Low for anaemia; could not reasonably affect outcome. Unclear for growth |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Hard to blind. Mothers were given food packages at daycare, so judged as high risk of bias |
| Protection from contamination | Unclear risk | Not applicable |
| Selective reporting (reporting bias) | Unclear risk | No access to protocol |
| Other bias | High risk | There was some leakage. 10% of control communities got food |
Roy 2005.
| Methods | Study date: 2005. Study design: RCT | |
| Participants | SES or context. Low‐ and middle‐income country: Chandpur, Bangladesh. Most of the children came from families of low SES Nutritional status: WAZ between 61% and 75% of median of the NCHS standard Age: 6 ‐ 24 months Sex: Both Number: Supplementation + nutrition education = 94, nutrition education alone = 94, control = 94 |
|
| Interventions | Intervention: Feeding: Food made of roasted and powdered rice and pulse, molasses, and oil. One group feeding + education, one group nut. education only, control group. We compared to both groups Energy: 300 kcal (8 ‐ 9 g protein, 40 g rice, 20 g pulse, 10 g molasses, and 6 g oil) Intensity: Once a day for 6 days a week Duration: 3 months and followed up for 24 weeks % DRI for energy: 6 ‐ 12 months = 42.1%, 12 ‐ 24 months = 34.5% % DRI for protein: 6 ‐ 12 months = 103.27%, 12 ‐ 24 months = 90.57%. Protein energy ratio 12 Control: Regular diet and usual care Provider: Bangladesh Integrated Nutrition Project, Government of Bangladesh Supervised: Not mentioned Compliance: Not mentioned |
|
| Outcomes | Physical: Weight and length | |
| Notes | Mothers received intensive nutrition education on food security, caring practices, personal hygiene, and control for child nutrition. Intervention also included cooking demonstrations. Focus group discussions on mothers' perception of child feeding practices, food taboos, and health‐seeking behaviour during illnesses | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | No report of how this was done |
| Baseline outcome measurements | Unclear risk | No significant differences in outcome measures at baseline |
| Baseline characteristics | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reported numbers only at beginning of study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Hard to blind as participants know what they received and as personnel needed to know too |
| Protection from contamination | Unclear risk | Not applicable |
| Selective reporting (reporting bias) | Unclear risk | No access to protocol |
Santos 2005.
| Methods | Study date: 2005. Study Design: CBA | |
| Participants | SES or context: Low‐ and middle‐income country: Brazil. 20 municipalities in the State of Alagoas Nutritional status: Below 10th percentile of WFA Age: 6 ‐ 18 months Sex: Both Number: 191. Experimental = 99, control = 92 |
|
| Interventions | Intervention: Feeding + take‐home supplements. Milk powder and cooking oil to be added to prepared milk. MIlk to be distributed to other children < 5 to avoid redistribution. Supplement delivered to mothers at healthcare centres once a week. Take‐home rations. Mothers had to prepare them Energy: Supposed to be 60% of RDI Duration: 6 months % DRI for energy: 60% of the recommended calories % DRI for protein: 100% of the recommended protein Control: no feeding. Deworming given to both groups Provider: Brazilian government Supervised: Does not seem like there was much at all. A great deal of leakage Compliance: Reported that only 32.5% of children received the full supplement; for the others, it was shared between 1 and 3 other children and 1 and 2 other adults. Furthermore, 63.2% of the mothers did not add the oil to the supplement as directed, but rather used it for cooking family meals |
|
| Outcomes | Physical: Weight, length, WAZ, HAZ, WHZ | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Non randomised study |
| Allocation concealment (selection bias) | High risk | Non randomised study |
| Baseline outcome measurements | Low risk | No significant differences in outcome measures at baseline |
| Baseline characteristics | Low risk | No significant differences in outcome measures |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | To prevent the effect of misclassification error, 28 children were excluded for being above the 10th percentile of the weight‐for‐age index at enrolment (15 supplemented and 13 non‐supplemented). Analyses were restricted to 191 children who met the inclusion criterion satisfactorily. From the first to the second visit, 17 children were lost (6 supplemented and 11 controls), mainly due to change of address to a different city. 2 children died, both in the supplemented group. No migration from control to intervention group occurred during the study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants aware of intervention |
| Protection from contamination | High risk | Gaps in delivery were reported by nearly 50.0% of the mothers, thereby preventing their full access to the Program. Regarding utilization, it was clear at the second visit that the mean intake of calories (270kcal/d) and nutrients (14.7g protein, 524.4g calcium, 0.26mg iron, 1.87mg zinc, and 179mg retinol) from milk were considerably lower than the amount made available from the supplement, indicating major under‐utilization by beneficiary children |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
Schroeder 2002.
| Methods | Study date: 2002. Study design: Cluster‐CBA. Was RCT, but added 41 children | |
| Participants | SES or context: Low‐ and middle‐income country: Vietnam. 12 rural communes Nutritional status: Between ‐2 and ‐3 SD on WAZ; some nearer to normal Age: 5 months ‐ 30 months on entry Sex: Both Number: 238 at entry. Experimental = 119, control = 119. At month 6, experimental = 114, control = 118 |
|
| Interventions | Intervention: Feeding + nutrition education on positive deviant practices (behaviours used by families whose children grow well despite economic poverty). All children in both groups de‐wormed. Breastfeeding in addition to positive deviant local foods. Common local sources of protein, tofu, fish oil, etc. Caregivers prepared foods at health centres. Sounds like they prepared it in rotation Energy: 300 kcal Intensity: ONLY 12 days a month, but all day. 1 full meal Duration: 12 months. Data in meta‐analysis is from 6‐month follow‐up % DRI for energy: Not enough information % DRI for protein: Not enough information Control: No feeding. Dewormed Provider: Partnership between federal government, Save the Children and USAID linkages. But mothers asked to bring a handful of positive deviant foods Supervised: Mothers and children attended health centres all day. Sounds like pretty strict supervision, but not clear that intake was monitored |
|
| Outcomes | Physical: WAZ, HAZ, WHZ | |
| Notes | Seems like quite a good programme, but it was limited to every other day. The method was based on local behaviours that resulted in good child development. However, it is difficult to determine how randomisation and child selection were done | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Non randomised study |
| Allocation concealment (selection bias) | High risk | Non randomised study |
| Baseline outcome measurements | High risk | Despite matching of communes and random selection, the intervention families were somewhat better off on a number of characteristics, although this differential only reached statistical significance for child wasting |
| Baseline characteristics | Low risk | The field workers and supervisors, affiliated with the Research and Training Center for Community Development (RTCCD) in Hanoi, were bachelor’s level physicians and sociologists with previous health data collection experience in rural Vietnam. Every evening, the field workers reviewed forms for completeness and accuracy. Supervisors reviewed all forms and discussed any discrepancies. If necessary and logistically feasible, households were revisited to reconcile these discrepancies |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 241 children were enrolled in the study at baseline, including 2 children younger than 5 months and 2 children older than 25 months who were excluded from these analyses (table 1). At month 6, there were a total of 232 children with complete data |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Because participants, personnel, and parents couldn't be blinded as children received food |
| Protection from contamination | Unclear risk | Participants were randomised by commune and they were chosen to be non‐contiguous. But only half of participants attended and feeding was only 12 days a month |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
Simondon 1996.
| Methods | Study date: 1996. Study design: RCT. All babies born in selected hospital in 4 countries during certain time. Not cluster | |
| Participants | SES or context: Low‐ and middle‐income country. 4 areas in Central (peri‐urban) and West Africa (poor rural area), South America (peri‐urban), and the South Pacific (farming community) Nutritional status at baseline: HAZ ≧ ‐2.5 SD, WHZ ≧ ‐2 SD Age: 4 months Sex: Both Number: Congo: experimental = 74 (53 completed) and control = 74 (67 completed). Senegal: experimental = 66 (53 completed) and control = 68 (57 completed). Bolivia: experimental = 78 (65 completed) and control = 82 (62 completed). New Caledonia: experimental = 63 (43 completed) and control = 53 (47 completed) |
|
| Interventions | Intervention: Feeding only. Ready‐to‐use supplement (precooked wheat, maize, millet, soybean flour, milk powder, soybean oil, palm oil, and sugar, enriched with minerals and vitamins). Supplements taken home and feeding observed Energy: 4 ‐ 5 months 103 kcal/meal, and at 5 ‐ 7 months 205 kcal/ meal Intensity: twice daily for 7 days/week (1st meal at 0800 ‐ 1100; 2nd meal at 1500 ‐ 1900) Duration: 12 ‐ 13 weeks % DRI for energy: 4 ‐ 5 months = 20.6%, 5 ‐ 7 months = 28.8% % DRI for protein: 4 ‐ 5 months = 26.98%, 5 ‐ 7 months = 51.64%. Protein energy ratio 8.74 and 8.78 respectively Control: Usual diet Provider: Grant from French Ministry of Research Supervised: Female field workers assigned to 7 families each and visited daily for preparation and consumption of supplement Compliance: Female field workers assigned to 7 families each and visited daily for preparation and consumption of supplement |
|
| Outcomes | Physical: Weight and length | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Said that randomisation was accomplished by drawing lots |
| Allocation concealment (selection bias) | Low risk | With drawing lots, it is unlikely that researchers or participants could have foreseen who was going to be drawn |
| Baseline outcome measurements | Low risk | No significant differences at baseline on outcome measures |
| Baseline characteristics | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) All outcomes | High risk | In Congo, Senegal, and New Caledonia, far more families in the experimental group dropped out due to refusal. In Bolivia, it was the opposite. The authors say that the baseline statistics were no different for those who dropped out and for those who stayed |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | This is not stated anywhere in the article |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants' parents were aware of their status, as their children were given supplements. It is unlikely that this affected performance. Study personnel were probably also aware |
| Protection from contamination | Unclear risk | Not applicable |
| Selective reporting (reporting bias) | Unclear risk | No access to protocol |
Thakwalakwa 2010.
| Methods | Study date: 2010. Study design: RCT. Three groups: Lipid nutrient supplement (LNS), Corn‐Soy Blend (CSB). | |
| Participants | SES or context: Low‐ and middle‐income country: Malawi. Small African farming community. Underweight is very common, and study conducted during growing season when food levels are low Nutritional status: WAZ < ‐2 SD Age: 6 ‐ 15 months Sex: Both Number: Control = 59, LNS = 66, CBS = 67 |
|
| Interventions | Intervention: Feeding ONLY: 43 g LNS (peanut paste (26%), dried skimmed milk (25%), vegetable oil (20%), icing sugar (27.5%), and a pre‐made mineral and vitamin mix (1.5%) from Nutriset) or 71 g CSB Energy: 921 kj (10.4 g protein) or 1189 kj (6.0 g protein) Intensity: Twice daily. Food delivered to their homes Duration: 12 weeks % DRI for energy: 6 ‐ 12 months LNS = 39.9%, CBS = 30.9%, 12 ‐ 15 months LNS = 32.7%, CBS = 25.4% % DRI for protein: 6 ‐ 12 months LNS = 68.85%, CBS 68.58%, 12 ‐ 15 months LNS = 119.33%, CBS = 118.86%. Protein energy ratio LNS 8.44 and CBS 18.88 Control: Usual diet and breastfeeding Provider: Academy of Finland, stipends for researchers provided by Nestlé Supervised: Weekly home visits by trained research assistants Compliance: Not mentioned |
|
| Outcomes | Physical: Head circumference, mid‐upper arm circumference, weight, length, WAZ, HAZ, WHZ | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Had opaque envelopes in a cabinet. Then guardian picked an envelope |
| Allocation concealment (selection bias) | Low risk | Envelopes were opaque. Kept in a cabinet until they were selected by guardians |
| Baseline outcome measurements | Low risk | No significant differences in outcome measures at baseline |
| Baseline characteristics | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 188 out of 192 completed the trial (98%). No differences between groups |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Research assistants who assessed weight and height were blinded to allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants could not be blinded. However, investigator was blinded |
| Protection from contamination | Unclear risk | Not applicable |
| Selective reporting (reporting bias) | Unclear risk | No access to protocol |
Tomedi 2012.
| Methods | Study date: 2012. Study design: Quasi‐experimental design. Cluster controlled cohort. 20 villages intervention and 20 villages control | |
| Participants | SES or context: Low‐ and middle‐income country: Rural Kenya. Subsistence farmers who rely on rain‐fed agriculture (maize and beans as staple foods as well as cowpeas and pigeon peas). Small‐scale horticulture and animal husbandry are also practised. 23.9% unemployment in household. 98.1% and 96.6% of the caregivers attended school and had 7.8 years and 8.0 years of school in intervention and control areas, respectively Nutritional status: All children with WHZ ≧ ‐2 at baseline. Average WAZ was ‐0.51 and ‐0.37 Average HAZ was ‐1.23 and ‐1.21 Age: 6 ‐ 20 months Sex: Both Number: Experimental = 139, control = 147 |
|
| Interventions | Intervention: Feeding: Monthly rations given to family for child and the rest of family. Millet (150 g), pigeon peas (25 g), milk (125 g), eggs (50 g), vegetable oil (10 g), mango (100 g), and sugar (15 g) Energy: 4058 kj Intensity: Monthly but no information on time of day Duration: 7 months % DRI for energy: 6 ‐ 12 months = 136.2%, 12 ‐ 24 months = 111.7% % DRI for protein: Inestimable in all groups Control: Usual diet Provider: Global Health Partnership Supervised: Workers visited monthly Compliance: Caregiver reported that the index child was given at least 50% of the food. The index child was the only person in the household consuming the milk 79% of the time and the only person consuming eggs 78% of the time Intervention included education session on appropriate complementary feeding and hygiene |
|
| Outcomes | Physical: Weight, length, WAZ, HAZ, WHZ | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Non‐randomised |
| Allocation concealment (selection bias) | High risk | Non‐randomised |
| Baseline outcome measurements | Low risk | No significant differences in outcome measures at baseline |
| Baseline characteristics | Low risk | Both sub‐locations are governed by the same local chief and have community health workers (CHW) who participate in the screening of the households with children under 5 years of age for acute malnutrition |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | For the children who were lost to follow‐up, there were no significant differences in anthropometric measurements at baseline between those in the intervention group and those in the non‐intervention group |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Parents, children, and personnel not blinded to the fact that children were being fed |
| Protection from contamination | Low risk | Allocated by village. Food was given at home so unlikely that it was shared between villages |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
Waber 1981.
| Methods | Study date: 1981. RCT. Different arms. A = control, A1 = maternal education only, B = fed from 6 months, B1 = fed + maternal education from 6 months, D = mothers fed from third trimester | |
| Participants | SES or context: Low‐ and middle‐income country: Southern slums in Bogata, Colombia Nutritional status: Half of children in family below 85% percentile for weight Age: 6 months ‐ 3 years Sex: Approximately equal in both groups Number: 433 |
|
| Interventions | Intervention: Feeding: Enriched bread, dry skimmed milk, and cooking oil for entire family. Index child given dry skimmed milk, high protein vegetable mixture, and ferrous sulcate. Supplements delivered in store‐like atmosphere once a week Maternal education. Trained home visitors worked directly with the children and trained mothers to become more responsive Energy: 623 kcal per day. 30 g protein Duration: 32 months % DRI for energy: Not enough information % DRI for protein: Not enough information Control: Home‐feeding as usual, or education Provider: Not clear Supervised: Not clear. However, home visitors worked with children and educated mothers Compliance: Not mentioned |
|
| Outcomes | Psychological: Griffiths Mental Development Scales and Einsten IQ test | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Simply noted that study was randomised |
| Allocation concealment (selection bias) | Unclear risk | No detail |
| Baseline outcome measurements | Unclear risk | No significance given |
| Baseline characteristics | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 433 started trial; 318 reported. Does not say who or why |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Training mentioned, blinding not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not possible for participants |
| Protection from contamination | Unclear risk | Not applicable |
| Selective reporting (reporting bias) | Unclear risk | No access to protocol |
Yeung 2000.
| Methods | Study date: 2000. Study design: RCT | |
| Participants | SES or context: High‐income country: Toronto, Canada. Urban community, maternal education level: 51% primary or secondary school, 28.7% college, 20.2% university Nutritional status: Not stated Age: 6 months Sex: Both Number: Experimental = 49, control = 52 |
|
| Interventions | Intervention: Feeding: Puréed meat, iron‐fortified infant cereal, and whole cow's milk Energy: Not stated Intensity: Not stated Duration: 6 months % DRI for energy: Neither energy nor protein was provided % DRI for protein: Neither energy nor protein was provided Control: Usual diet Provider: Dairy farmers of Ontarario and the Ministry of Agriculture, Food and Rural Affairs Ontario Supervised: Monthly compliance questionnaire administered by trained nurses Compliance: Families of 6 infants were non‐compliant with intervention |
|
| Outcomes | Physical: Head circumference, weight, and length | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Does not say how randomisation was done |
| Allocation concealment (selection bias) | Unclear risk | Does not say how randomisation was done |
| Baseline outcome measurements | Low risk | No significant differences in outcome measures at baseline |
| Baseline characteristics | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reported well, and same reasons for dropping out, but significantly higher numbers in intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Low risk for blood tests |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Parents knew that they got coupons for the food |
| Protection from contamination | Unclear risk | Not applicable |
| Selective reporting (reporting bias) | Unclear risk | No access to protocol |
Ziegler 2009.
| Methods | Study date: 2009. Study design: Prospective, randomised, open‐label trial | |
| Participants | SES or context: High‐income country. Predominantly white population (< 10% African American, Asian, and Hispanic), middle‐income community Nutritional status: Birth weight > 2500 g Age: Enrolment 1 month but intervention at 4 months Sex: Both Number: Iron in medicine = 48, iron in cereal = 45, control = 59 |
|
| Interventions | Intervention: Feeding: 113 g wet ration fruit cereal, rice cereal with applesauce, mixed cereal with applesauce and bananas, and oatmeal with applesauce and bananas (Gerber Products Company) Energy: Not mentioned Intensity: Once daily Duration: 20 weeks % RDA for energy: 6 ‐ 12 months inestimable % DRI for protein: 6 ‐ 12 months inestimable Control: Usual diet and breastfeeding Provider: NIH, Gerber Products Company, and Mead Johnson Supervised: Monthly visits to lab Compliance: Empty containers collected at the time of visit |
|
| Outcomes | Physical: Weight and length | |
| Notes | No information on energy content of supplement provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Open‐label |
| Allocation concealment (selection bias) | Unclear risk | Open‐label |
| Baseline outcome measurements | Unclear risk | No significant differences in outcome measures at baseline |
| Baseline characteristics | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not much attrition, but it was related to side effects of the iron |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned. Seems that parents could not be blinded. Not sure about study personnel |
| Protection from contamination | Unclear risk | Not applicable |
| Selective reporting (reporting bias) | Unclear risk | No access to protocol |
ANP ‐ advanced nutrition programme AVG. ‐ average BMI ‐ body mass index CBA ‐ controlled before‐and‐after CBS ‐ corn‐soy blend CHW ‐ community health worker DRA ‐ daily recommended amounts DRI ‐ daily recommended intake FAO ‐ Food and Agriculture Organization HAZ ‐ height‐for‐age z‐scores LAZ ‐ length‐for‐age z‐scores LNS ‐ lipid‐based nutrient supplement LSE ‐ low socio‐economic status MDI ‐ mental development index MRC ‐ Medical Research Council MSE ‐ middle socio‐economic status MSF ‐ Médecins Sans Frontières NCHS ‐ National Center for Health Statistics NIH ‐ National Institutes of Health PDI ‐ psychomotor development index RCT ‐ randomised controlled trial RUTF ‐ ready‐to‐use therapeutic foods SD ‐ standard deviation SES ‐ socio‐economic status UNICEF ‐ United Nations Children's Fund USD ‐ United States dollars USDA ‐ United States Department of Agriculture VS. ‐ versus WAZ ‐ weight‐for‐age z‐scores WFA ‐ weight for age WHO ‐ World Health Organization WHZ ‐ weight‐for‐height z‐scores
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Baertl 1970 | Did not follow specific children. Survey of whole population before and after |
| Das Gupta 2005 | Did not follow specific children. Based on survey data |
| Gartner 2007 | Did not follow specific children. Survey of whole population before and after |
| Hanafy 1967 | All groups received feeding |
| Hicks 1982 | Intervention included supplementation for mothers prior to birth of child. Also, no appropriate control group |
| Hillis 1992 | No clear starting point of feeding and entry into day care. Children could have been in day care for a long time. No information on food supplement |
| Huybregts 2012 | Children were given RUTF in addition to a general food distribution programme |
| Khan 2011 | Supplemented mothers prenatally |
| Leroy 2008 | Control group not appropriate. They were the children of eligible families who opted not to take part in the Opportunades programme |
| Matilsky 2009 | All groups were fed |
| Meller 2012 | Inappropriate control group. Regression discontinuity design |
| Mora 1981 | Subset of McKay 1978, but only reported on the children whose mothers were supplemented before birth. In addition, children included were older than 5 years of age with no disaggregated data presented |
| Rivera 1991 | The INCAP study in Guatemala. Some were supplemented prenatally, some were supplemented from birth |
| Rosado 2010 | Control groups received more than 100 kcal |
| Van Hoan 2009 | No primary or secondary outcome of interest. Focused on energy intake and the effect on breastfeeding |
| Vermeersch 2004 | Did not follow same children. Examined test scores in schools |
INCAP ‐ Institute of Nutrition of Central America and Panama RUTF ‐ ready‐to‐use therapeutic food
Characteristics of studies awaiting assessment [ordered by study ID]
He 2005.
| Methods | Chose 7 schools, randomly divided all kindergarten children into yogurt supplementation and control group. One page of the methods is blank, as are some results tables |
| Participants | 402 preschool children |
| Interventions | Yogurt supplementation with 125 g of yogurt 5 days a week |
| Outcomes | Height, weight |
| Notes |
Differences between protocol and review
The original protocol can be read in Kristjansson 2012.
Outcomes
We changed the names of some psychosocial outcomes (mental and cognitive development to mental development) and reordered them (we put psychomotor development first). We also put intelligence under cognitive development.
Searches
In some cases we amended the choice of database or replaced it with an equivalent source from the source listed in the protocol due to availability of the resource. For example, we searched Social Sciences Citation Index (SSCI) instead of Sociofile, as the coverage was comparable and it was available in our institution. Similarly, Health Management Information Consortium (HMIC) and Dissertation Abstracts International were not available but have similar coverage to OVID Medline and Proquest Dissertations and Theses. We did not search SCOPUS as originally planned, neither did we run supplementary citation searches.
We added Clinicaltrials.gov for all years in January 2014.
We had planned to identify key researchers in the field and to write to them to ask about any unpublished or forthcoming works. However, we did not carry this out. We believe that the risk of missing key studies was low because of the extensive searching in many different databases (more than 30,000 references identified).
Subgroup Analyses
We added two subgroup analyses to those in the protocol: location of feeding (take‐home rations versus feeding centre or day‐care or preschool, or both) and level of supervision (i.e. monitoring). We added these analyses because it became evident from consultation with each other and from gaining a better understanding of the context that these were potentially important factors in success/failure.
We also used the EPOC 2009 risk of bias criteria to change the age groups in the analyses due to data constraints.
Risk of bias
We had planned to use the Effective Public Health Practice Project tool (EPHPP 2009) in addition to the Cochrane and EPOC tools to assess bias; however this proved to be too time‐consuming.
There were no ITS studies, so we could not assess their risks of bias. Our appraisal criteria for ITS studies were adapted from the 'Risk of bias' checklist developed by the EPOC Group (EPOC 2009). In assessing risk of bias in the ITS designs, we would have considered protection against secular changes, predefined shape of effect, effect on data collection, knowledge of allocated interventions, incomplete outcome data, selective outcome reporting, and other biases.
Analyses
We had planned to do an intention‐to‐treat (ITT) analysis, but nearly all studies reported only on completers. We wrote to some authors for other information but received very few replies. Our analyses, therefore, are completion analyses.
If scales had been measured in different directions (high on some representing greater disease severity while high on others represents less severity), we would have multiplied the mean values from one set of studies by –1 to ensure that all the scales measured in the same direction.
We would have analysed categorical and continuous data separately had there been any categorical data. We would have analysed categorical data using odds ratios (ORs) and risk ratios (RRs).
We had planned to draw funnel plots to assess the presence of possible publication bias as well as the relationship between effect size and study precision. However, we did not have the recommended minimum number of studies (10) for any analysis. Furthermore, while funnel plot asymmetry may indicate publication bias, this is not inevitably the case (Egger 1997).
We had planned to do sensitivity analyses by five factors: reliable primary outcome measure/not, placebo versus no treatment control, allocation concealment, attrition (< 10% versus > 10%), and imputed correlation coefficient. However, we did not do these and only did sensitivity analyses to check whether more conservative ICCs in the clustering adjustments would make a difference.
Finally, due to the high number of potential variables and insufficient number of studies, we were unable to conduct a meta‐regression as planned.
Contributions of authors
Elizabeth Kristjansson ‐ led the review. She led the funding application and development of the protocol, writing much of it. Dr. Kristjansson also screened studies, decided on inclusion and exclusion of studies, assessed risk of bias, oversaw data extraction and analyses (conducting many of them), and wrote the results and discussion sections. Damian K Francis ‐ was involved in proposal development and writing the protocol. He helped to assess the nutritional composition and quality of the meals (intervention) administered to the participants. He also extracted data, performed much of the data analysis, and helped with writing and knowledge translation. Selma Liberato ‐ contributed to the proposal and protocol writing. She screened studies, decided on inclusion and exclusion of retrieved studies, helped with data extraction and writing. She led the assessment of the nutritional composition and nutrition (intervention) administered to the participants; she also judged the amount of supervision in each programme. Maria Benkhalti Jandu ‐ was involved in writing the protocol and the development of the logic model. She also developed the data extraction sheet, performed data extraction, and edited the review. Vivian Welch ‐ contributed to the policy influence plan, proposal development, and development of the search strategy. She carried out the correction for clustering and advised on all analyses. She was also involved in the implementation analysis. Malek Batal ‐ was involved in the proposal and protocol writing and drafting the logic model. He also provided input into the assessment of nutritional quality of food or drink given and helped to edit the review. Trish Greenhalgh ‐ contributed to proposal writing and lead the process evaluation. She also contributed to writing and editing the final review. Tamara Rader ‐ developed and ran search strategies according to the Cochrane Handbook for Systematic Reviews of Interventions and in collaboration with subject experts. She also drafted the sections on searching. Eamonn Noonan ‐ assisted with development of the policy Influence plan, wrote the plain language summary, and helped with policy briefs and with knowledge translation. He also edited the review. Beverley Shea ‐ reviewed the protocol, assessed risk of bias, and edited the review. Laura Janzen ‐ contributed to the proposal and protocol writing, assessed the quality of the psychological measures, and contributed to the discussion of the cognitive and behavioural results. George A Wells ‐ provided statistical advice on analyses. Mark Petticrew ‐ reviewed the proposal and edited the final review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
3ie, UK.
The development and publication of the protocol and review was made possible thanks to a 86,000 US dollars grant from the International Initiative for Impact Evaluation (3ie) and Global Development Network. The grant funded contributions for the following authors: Damian Francis, Selma Liberato, Maria Benkhalti Jandu, Trish Greenhalgh, and Tamara Rader.
-
Canadian Institutes of Health Research, Canada.
Partial support for Tamara Rader's salary to work on several reviews, including this one.
-
Department of Health, UK.
Mark Petticrew's salary is partially funded from the Department of Health Research.
Declarations of interest
Elizabeth Kristjansson ‐ none known. Damian K Francis ‐ none known. Selma Liberato ‐ none known. Maria Benkhalti Jandu ‐ none known. Vivian Welch ‐ none known. Malek Batal ‐ none known. Trish Greenhalgh ‐ none known. Tamara Rader ‐ none known. Eamonn Noonan ‐ none known. Beverley Shea ‐ none known. Laura Janzen ‐ none known. George A Wells ‐ none known. Mark Petticrew ‐none known.
New
References
References to studies included in this review
Bhandari 2001 {published data only}
- Bhandari N, Bahl R, Nayyar B, Khokhar P, Rohde JE, Bhan MK. Food supplementation with encouragement to feed it to infants from 4 to 12 months of age has a small impact on weight gain. Journal of Nutrition 2001;131(7):1946‐51. [DOI] [PubMed] [Google Scholar]
Coyne 1980 {published data only}
- Coyne T, Dowling M, Condon‐Paoloni D. Evaluation of the preschool meals program on the nutritional health of Aboriginal children. Medical Journal of Australia 1980;2(7):369‐75. [DOI] [PubMed] [Google Scholar]
De Romana 2000 {published data only}
- Romano GL. Experience with complementary feeding in the FONCODES project. Food and Nutrition Bulletin 2000;21(1):43‐8. [Google Scholar]
Devadas 1971 {published data only}
- Devadas RP, Balambal M, Ushakumari N. Impact of an applied nutrition programme on the nutritional status of preschool children in a village. Indian Journal of Nutrition and Dietetics 1971;8(5):260‐3. [Google Scholar]
Fauveau 1992 {published data only}
- Fauveau C, Siddiqui M, Briend A, Silimperi D, Begum N, Fauveau V. Limited impact of a targeted food supplementation programme in Bangladeshi urban slum children. Annals of Tropical Paediatrics 1992;12(1):41‐6. [DOI] [PubMed] [Google Scholar]
Gershoff 1988 {published data only}
- Gershoff SN, McGandy RB, Nondasuta A, Tantiwongse P. Nutrition studies in Thailand: effects of calories, nutrient supplements, and health interventions on growth of preschool Thai village children. American Journal of Clinical Nutrition 1988;48(5):1214‐8. [DOI] [PubMed] [Google Scholar]
Gopalan 1973 {published data only}
- Gopalan C, Swaninathan MC, Kumari VK, Rao DH, Vijayaraghavan K. Effect of calorie supplementation on growth of undernourished children. American Journal of Clinical Nutrition 1973;26(5):563‐6. [DOI] [PubMed] [Google Scholar]
- Rao DH, Naidu N. Nutritional supplementation: whom does it benefit most?. American Journal of Clinical Nutrition 1977;30(10):1612‐6. [DOI] [PubMed] [Google Scholar]
Grantham‐McGregor 1991 {published data only}
- Gardner JM, Grantham‐McGregor SM, Himes J, Chang S. Behaviour and development of stunted and nonstunted Jamaican children. Journal of Child Psychology and Psychiatry 1999;40(5):819‐27. [DOI] [PubMed] [Google Scholar]
- Grantham‐McGregor SM, Powell CA, Walker SP, Himes JH. Nutritional supplementation, psychosocial stimulation, and mental development of stunted children: the Jamaican Study. Lancet 1991;338(8758):1‐5. [DOI] [PubMed] [Google Scholar]
- Grantham‐McGregor SM, Walker S, Chang SM, Powell CA. Effects of early childhood supplementation with and without stimulation on later development in stunted Jamaican children. American Journal of Clinical Nutrition 1997;66(2):247‐53. [DOI] [PubMed] [Google Scholar]
- Meeks Gardner JM, Grantham‐MacGregor SM, Himes J, Chang S. Behaviour and development of stunted and nonstunted Jamaican children. Journal of Child Psychology and Psychiatry 1999;40(5):819‐27. [DOI] [PubMed] [Google Scholar]
- Walker S, Powell C, Grantham‐McGregor S, Himes JH, Chang SM. Nutritional supplementation, psychosocial stimulation and growth of stunted children: the Jamaican study. American Journal of Clinical Nutrition 1991;54(4):642‐8. [DOI] [PubMed] [Google Scholar]
Heikens 1989 {published data only}
- Heikens GT, Schofiled WN, Dawson S, Grantham‐McGregor S. The Kingston Project I. Growth of malnourished children during rehabilitation in the community, given a high energy supplement. European Journal of Clinical Nutrition 1989;43(3):145‐60. [PubMed] [Google Scholar]
Husaini 1991 {published data only}
- Husaini M, Jahari B, Pollitt E. The effects of high energy and micronutrient supplementation on iron status in nutritionally at‐risk infants. Biomedical and Environmental Sciences 1996;9(2‐3):325‐40. [PubMed] [Google Scholar]
- Husaini MA, Karyadi L, Husaini YK, Sandjaja, Karyadi D, Pollitt E. Developmental effects of short‐term supplementary feeding in nutritionally‐at‐risk Indonesian infants. American Journal of Clinical Nutrition 1991;54(5):799‐804. [DOI] [PubMed] [Google Scholar]
- Pollitt E, Watkins W, Husaini M. Three‐month nutritional supplementation in Indonesian infants and toddlers benefits memory function 8 years later. American Journal of Clinical Nutrition 1997;66(6):1357‐63. [DOI] [PubMed] [Google Scholar]
Iannotti 2014 {published data only}
- Iannotti LL, Dulience SJ, Green J, Joseph S, Francois J, Antenor ML, et al. Linear growth increased in young children in an urban slum of Haiti: a randomized controlled trial of lipid‐based nutrient supplement. American Journal of Clinical Nutrition 2014;99(1):198‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Isanaka 2009 {published data only}
- Isanaka S, Nombela N, Djibo A, Poupard M, Beckhoven D, Gaboulaud V, et al. Effect of preventive supplementation with ready‐to‐use therapeutic food on the nutritional status, mortality, and morbidity of children aged 6 to 60 months in Niger: a cluster randomized trial. JAMA 2009;301(3):277‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Joshi 1988 {published data only}
- Joshi S, Rao S. Assessing supplementary feeding programmes in selected Balwadies. European Journal of Clinical Nutrition 1988;42(9):779‐85. [PubMed] [Google Scholar]
Kuusipalo 2006 {published data only}
- Kuusipalo H, Maleta K, Briend A, Manary M, Ashorn P. Growth and change in blood haemoglobin concentration among underweight Malawian infants receiving fortified spreads for 12 weeks: a preliminary trial. Journal of Pediatric Gastroentrentrology and Nutrition 2006;43(4):525‐32. [DOI] [PubMed] [Google Scholar]
Lutter 2008 {published data only}
- Lutter CK, Rodriguez A, Fuenmayor G, Avila L, Sempertegui F, Escobar J. Growth and micronutrient status in children receiving a fortified complementary food. Journal of Nutrition 2008;138(2):379‐88. [DOI] [PubMed] [Google Scholar]
Mangani 2014 {published data only}
- Mangani C, Cheung YB, Maleta K, Phuka J, Thakwalakwa C, Dewey, K, et al. Providing lipid‐based nutrient supplements does not affect developmental milestones among Malawian children. Acta Pædiatrica 2014;103(1):e17–26. [DOI] [PubMed] [Google Scholar]
- Mangani C, Maleta K, Phuka J, Cheung YB, Thakwalakwa C, Dewey K, et al. Effect of complementary feeding with lipid based nutrient supplements and corn‐soy blend on the incidence of stunting and linear growth among 6‐ to 18‐month‐old infants and children in rural Malawi. Maternal and Child Nutrition 2013 Jun 25 [Epub ahead of print]. [DOI: 10.1111/mcn.12068] [DOI] [PMC free article] [PubMed]
Manjrekar 1986 {published data only}
- Manjrekar C, Leelavathi K, Saraswathi A, Sujayalakshmi AN, Katyayani V. Evaluation of the special nutrition programme in Mysore City. Indian Journal of Medical Research 1986;83:404‐7. [PubMed] [Google Scholar]
McKay 1978 {published data only}
- McKay H, Sinisterra L, McKay A, Gomez H, Lloreda P. Improving cognitive ability in chronically deprived children. Science 1978;200(4339):270‐8. [DOI] [PubMed] [Google Scholar]
- Pérez‐Escamilla R, Pollitt E. Growth improvements in children above 3 years of age: the Cali Study. Journal of Nutrition 1995;125(4):885‐93. [DOI] [PubMed] [Google Scholar]
Mittal 1980 {published data only}
- Mittal S, Gupta MC. Evaluation of a supplementary feeding programme through take home system. Journal of Tropical Pediatrics 1980;26(2):50‐3. [DOI] [PubMed] [Google Scholar]
Obatolu 2003 {published data only}
- Obatolu V. Growth pattern of infants fed with a mixture of extruded malted maize and cowpea. Nutrition 2003;19(2):174‐8. [DOI] [PubMed] [Google Scholar]
Oelofse 2003 {published data only}
- Oelofse A, Raaij JM, Benade AJ, Dhansay MA, Tolboom JJ, Hautvast JG. The effect of a micronutrient‐fortified complementary food on micronutrient status, growth and development of 6‐ to 12‐month‐old disadvantaged urban South African infants. International Journal of Food Sciences and Nutrition 2003;54(5):399‐407. [DOI] [PubMed] [Google Scholar]
Pollitt 2000a {published data only}
- Aitchison T, Durnin J, Beckett C, Pollitt E. Effects of an energy and micronutrient supplement on growth and activity, correcting for non‐supplemental sources of energy input in undernourished children in Indonesia. European Journal of Clinical Nutrition 2000;54 Suppl 2:S69‐73. [DOI] [PubMed] [Google Scholar]
- Beckett C, Durnin JVGA, Aitchison T, Pollitt E. Effects of an energy and micronutrient supplement on anthropometry in undernourished children in Indonesia. European Journal of Clinical Nutrition 2000;54 Suppl 2:S52‐9. [DOI] [PubMed] [Google Scholar]
- Durnin JVGA, Aitchison TC, Beckett C, Husaini M, Pollitt E. Nutritional intake of an undernourished infant population receiving an energy and micronutrient supplement in Indonesia. European Journal of Clinical Nutrition 2000;54 Suppl 2:S43‐51. [DOI] [PubMed] [Google Scholar]
- Jahari AB, Saco‐Pollitt C, Husaini MA, Pollitt E. Effects of an energy and micronutrient supplement on motor development and motor activity in undernourished children in Indonesia. European Journal of Clinical Nutrition 2000;54 Suppl 2:S60‐8. [DOI] [PubMed] [Google Scholar]
- Pollitt E, Durnin JVGA, Husani M, Jahari A. Effect of an energy and micro‐nutrient supplement on growth and development in undernourished children in Indonesia: methods. European Journal of Clinical Nutrition 2000;54 Suppl 2:S16‐20. [DOI] [PubMed] [Google Scholar]
- Pollitt E, Jahari A, Walka H. A developmental view of the effects of an energy and micronutrient supplement in undernourished children in Indonesia. European Journal of Clinical Nutrition 2000;54 Suppl 2:S107‐13. [DOI] [PubMed] [Google Scholar]
- Pollitt E, Saco‐Pollitt C, Jahari A, Husaini M, Huang J. Effects of an energy and micronutrient supplement on mental development and behavior under natural conditions in undernourished children in Indonesia. European Journal of Clinical Nutrition 2000;54 Suppl 2:S80‐90. [DOI] [PubMed] [Google Scholar]
- Walka H, Triana N, Jahari A, Husaini M, Pollitt E. Effects of an energy and micronutrient supplement on play behavior in undernourished children in Indonesia. European Journal of Clinical Nutrition 2000;54 Suppl 2:S91‐106. [DOI] [PubMed] [Google Scholar]
Rivera 2004 {published data only}
- Rivera JA, Sotres‐Alvarez D, Habicht JP, Shamah T, Villalpando S. Impact of the Mexican program for education, health, and nutrition (Progresa) on rates of growth and anaemia in infants and young children: a randomized effectiveness study. JAMA 2004;291(21):2563‐70. [DOI] [PubMed] [Google Scholar]
Roy 2005 {published data only}
- Roy SK, Fuchs GJ, Mahmud Z, Ara G, Islam S, Shafique S, et al. Intensive nutrition education with or without supplementary feeding improves the nutritional status of moderately‐malnourished children in Bangladesh. Journal of Health, Population, and Nutrition 2005;23(4):320‐3. [PubMed] [Google Scholar]
Santos 2005 {published data only}
- Santos IS, Gigante DP, Coitinho DC, Haisma H, Valle NC, Valente G. Evaluation of the impact of a nutritional program for undernourished children in Brazil. Cadernos de Saúde Pública 2005;21(3):776‐85. [DOI] [PubMed] [Google Scholar]
Schroeder 2002 {published data only}
- Schroeder DG, Pachón H, Dearden KA, Kwon CB, Ha TT, Lang TT, et al. An integrated child nutrition intervention improved growth of younger, more malnourished children in northern Viet Nam. Food and Nutrition Bulletin 2002;23 Suppl 4:53‐61. [PubMed] [Google Scholar]
Simondon 1996 {published data only}
- Simondon KB, Gartner A, Berger J, Cornu A, Massamba JP, Miguel JL, et al. Effect of early, short‐term supplementation on weight and linear growth of 4‐7 month old infants in developing countries: a four‐country randomized trial. American Journal of Clinical Nutrition 1996;64(4):537‐45. [DOI] [PubMed] [Google Scholar]
Thakwalakwa 2010 {published data only}
- Thakwalakwa C, Ashorn P, Phuka J, Briend A, Puumalainen T, Maleta K. A lipid‐based nutrient supplement but not corn‐soy blend modestly increases weight gain among 6‐ to 18‐month‐old moderately underweight children in rural Malawi. Journal of Nutrition 2010;140(11):2008‐13. [DOI] [PubMed] [Google Scholar]
Tomedi 2012 {published data only}
- Tomedi A, Rohan‐Minjares F, McCalmont K, Ashton R, Opiyo R, Mwanthi M. Feasibility and effectiveness of supplementation with locally available foods in the prevention of child malnutrition in Kenya. Public Health Nutrition 2012;15(4):749‐56. [DOI] [PubMed] [Google Scholar]
Waber 1981 {published data only}
- Waber D, Vuori‐Christiansen L, Ortiz N, Clement JR, Christiansen NE, Mora JO, et al. Nutritional supplementation, maternal education, and cognitive development of infants at risk of malnutrition. American Journal Of Clinical Nutrition 1981;34(4):807‐13. [DOI] [PubMed] [Google Scholar]
Yeung 2000 {published data only}
- Yeung GS, Zlotkin SH. Efficacy of meat and iron‐fortified commercial cereal to prevent iron depletion in cow milk‐fed infants 6 to 12 months of age: a randomized controlled trial. Canadian Journal of Public Health 2000;91(4):263‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ziegler 2009 {published data only}
- Ziegler EE, Nelson SE, Jeter JM. Iron supplementation of breastfed infants from an early age. American Journal of Clinical Nutrition 2009;89(2):525‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Baertl 1970 {published data only}
- Baertl JM, Morales E, Verastegui G, Graham G. Diet supplementation for entire communities. Growth and mortality of infants and children. American Journal of Clinical Nutrition 1970;23(6):707‐15. [DOI] [PubMed] [Google Scholar]
Das Gupta 2005 {published data only}
- Das Gupta M, Loshkin M, Gragnotati M, Ivaschenko O. Improving child nutrition outcomes in India: can the integrated child development services be more effective?. http://bit.ly/1yRpqhi (accessed 30 January 2015).
Gartner 2007 {published data only}
- Gartner A, Kameli Y, Traissac P, Dhur A, Delpeuch F, Maire B. Has the first implementation phase of the Community Nutrition Project in urban Senegal had an impact?. Nutrition 2007;23(3):219‐28. [DOI] [PubMed] [Google Scholar]
Hanafy 1967 {published data only}
- Hanafy MM, Aref MK, Seddik Y, Zein MS, El‐Kashlan KM. Effect of supplementary feeding on the nutritional status of the pre‐school child. Journal of Tropical and Medical Hygiene 1967;70(10):238‐42. [PubMed] [Google Scholar]
Hicks 1982 {published data only}
- Hicks LE, Langham RA, Takenaka J. Cognitive and health measures following early nutritional supplementation: a sibling study. American Journal of Public Health 1982;72(10):1110‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hillis 1992 {published data only}
- Hillis SD, MIranda CM, McCann M, Bender D, Weigle K. Day care center attendance and diarrheal morbidity in Colombia. Pediatrics 1992;90(4):582‐8. [PubMed] [Google Scholar]
Huybregts 2012 {published data only}
- Huybregts L, Houngbe F, Salpeteur C, Brown R, Roberfroid D, Ait‐Aissa M, et al. The effect of adding ready‐to‐use supplementary food to a general food distribution on child nutritional status and morbidity: a cluster‐randomized controlled trial. PLoS Medicine 2012;9(9):e1001313. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khan 2011 {published data only}
- Khan A, Kabir I, Ekstrom E, Asling‐Monemi K, Alam D, Frongillo E, et al. Effects of prenatal food and micronutrient supplementation on child growth from birth to 54 months of age: a randomized trial in Bangladesh. Nutrition Journal 2011;10:134. [DOI: 10.1186/1475-2891-10-134] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leroy 2008 {published data only}
- Leroy JL, Garcia‐Guerra A, Guerra R, Dominguez C, Rivera J, Neufield LM. The Opportunitades program increases the linear growth of children enrolled at young ages in urban Mexico. Journal of Nutrition 2008;138(4):793‐8. [DOI] [PubMed] [Google Scholar]
Matilsky 2009 {published data only}
- Matilsky D, Maleta K, Castleman T, Manary M. Supplementary feeding with fortified spreads results in higher recovery rates than with a corn‐soy blend in moderately wasted children. Journal of Nutrition 2009;139(4):773‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meller 2012 {unpublished data only}
- Meller M, Lithschig S. Saving lives: evidence from a nutrition program in Ecuador. http://bit.ly/1zKWk3j (accessed 30 January 2015).
Mora 1981 {published data only}
- Mora JO, Herrera G, Suescun J, Navarro L, Wagner M. The effects of nutritional supplementation on physical growth of children at risk of malnutrition. American Journal of Clinical Nutrition 1981;34(9):1885‐92. [DOI] [PubMed] [Google Scholar]
Rivera 1991 {published data only}
- Martorell R, Habicht JP, Rivera JA. History and design of the INCAP longitudinal study (1969‐1977) and its follow‐up (1988‐89). Journal of Nutrition 1991;125(4 Suppl):1027S‐41S. [DOI] [PubMed] [Google Scholar]
- Rivera JA, Habicht JP, Robson DS. Effect of supplementary feeding on recovery from mild to moderate wasting in preschool children. American Journal of Clinical Nutrition 1991;54(1):62‐8. [DOI] [PubMed] [Google Scholar]
- Scroeder DG, Martorell R, Rivera JA, Ruela MT, Habicht JP. Age differences in the impact of nutritional supplementation on growth. Journal of Nutrition 1995;125 (4 Suppl):1051‐9S. [DOI] [PubMed] [Google Scholar]
Rosado 2010 {published data only}
- Rosado GL, Lopez P, Garcia OP, Alatorre J, Alvarado C. Effectiveness of the nutritional supplement used in the Mexican Oportunidades programme on growth, anaemia, morbidity and cognitive development in children aged 12‐24 months. Public Health Nutrition 2010;14(5):933‐7. [DOI] [PubMed] [Google Scholar]
Van Hoan 2009 {published data only}
- Hoan N, Phu P, Salvignol B, Berger J, Trèche S. Effect of the consumption of high energy dense and fortified gruels on energy and nutrient intakes of 6‐10‐month‐old Vietnamese infants. Appetite 2009;53(2):233‐40. [DOI] [PubMed] [Google Scholar]
Vermeersch 2004 {unpublished data only}
- Vermeersch C, Kremer M. School meals, educational achievement and school competition: evidence from a randomized evaluation. http://datatopics.worldbank.org/hnp/files/edstats/KENimp04.pdf (accessed 30 June 2014).
References to studies awaiting assessment
He 2005 {published data only}
- He M, Yang YX, Han H, Men JH, Bian LH, Wang GD. Effects of yogurt supplementation on growth of preschool children in Bejing suburbs. Biomedical and Environmental Sciences 2005;18(3):192‐7. [PubMed] [Google Scholar]
Additional references
ACC/SCN 1993
- ACC/SCN. SCN news, number 11 ‐ maternal and child nutrition. http://bit.ly/1zKWyr4 (accessed 30 June 2014).
Alderman 2004
- Alderman H, Berhman J, Hoddinott J. Improving child malnutrition for sustainable poverty reduction in Africa. http://bit.ly/1E1GEz5 (accessed 1 July 2011).
Allen 1994
- Allen L. Nutritional influences on linear growth: a general review. European Journal of Clinical Nutrition 1994;48 Suppl 1:S75‐89. [PubMed] [Google Scholar]
Arblaster 1996
- Arblaster L, Lambert M, Entwistle V, Forster M, Fullerton D, Sheldon T, et al. A systematic review of the effectiveness of health service interventions aimed at reducing inequalities in health. Journal of Health Services Research and Policy 1996;1(2):93‐103. [DOI] [PubMed] [Google Scholar]
Auestad 2000
- Auestad N. Infant nutrition – brain development ‐ disease in later life. An introduction. Developmental Neuroscience 2000;22(5‐6):472‐3. [DOI] [PubMed] [Google Scholar]
Barker 1992
- Barker DJP. The effect of nutrition of the fetus and neonate on cardiovascular disease in adult life. Proceedings of the Nutrition Society 1992;51(2):135‐44. [DOI] [PubMed] [Google Scholar]
Barker 2001
- Barker DJ. Fetal and infant origins of adult disease. Monatsschrift Kinderheilkunde 2001;149(1 Suppl):S2‐6. [Google Scholar]
Barrett 1985
- Barrett DE, Radke‐Yarrow M. Effects of nutritional supplementation on children's responses to novel, frustrating, and competitive situations. American Journal of Clinical Nutrition 1985;42(1):102‐20. [DOI] [PubMed] [Google Scholar]
Baumgartner 1986
- Baumgartner RN, Roche AF, Himes JH. Incremental growth tables: supplementary to previously published charts. American Journal of Clinical Nutrition 1986;43(5):711‐22. [DOI] [PubMed] [Google Scholar]
Beaton 1982
- Beaton GH, Ghassemi H. Supplementary feeding programs for young children in developing countries. American Journal of Clinical Nutrition 1982;35(4):863‐916. [DOI] [PubMed] [Google Scholar]
Beaton 1992
- Beaton G. Which age group should be targeted for supplementary feeding?. Proceedings of the ACC/SCN Symposium on Nutritional Issues in Food Aid; Rome. 1992.
Beaton 1993
- Beaton G. Nutritional issues in food aid − nutrition policy discussion paper no.12. Which age groups should be targeted for supplementary feeding?. http://bit.ly/1A2vQPJ (accessed 1 July 2011).
Beckett 2000
- Beckett C, Durnin JVGA, Aitchison T, Pollitt E. Effects of an energy and micronutrient supplement on anthropometry in undernourished children in Indonesia. European Journal of Clinical Nutrition 2000;54 Suppl 2:S52‐9. [DOI] [PubMed] [Google Scholar]
Bhutta 2008
- Bhutta ZA, Ahmed T, Black RE, Cousens S, Dewey K, Giugliani E, et al. What works? Interventions for maternal and child undernutrition and survival. Lancet 2008;371(9610):417‐40. [DOI] [PubMed] [Google Scholar]
Black 2003a
- Black R. Micronutrient deficiency ‐ an underlying cause of morbidity and mortality. Bulletin of the World Health Organization 2003;81(2):79‐156. [PMC free article] [PubMed] [Google Scholar]
Black 2003b
- Black RE, Morris SS, Bryce J. Where and why are 10 million children dying every year?. Lancet 2003;361(9376):2226‐34. [DOI] [PubMed] [Google Scholar]
Black 2008
- Black RE, Allen LH, Bhutta ZA, Caulfield LE, Onis M, Ezzati M, et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 2008;371(9608):243‐60. [DOI] [PubMed] [Google Scholar]
Browne 2009
- Browne J, Laurence S, Thorpe S. Acting on food insecurity in urban Aboriginal and Torres Strait Islander communities: policy and practice interventions to improve local access and supply of nutritious food. http://bit.ly/1ANxsxp (accessed 15 March 2014).
Caballero 2001
- Caballero B, Popkin BM (editors). The Nutrition Transition: Diet and Disease in the Developing World. London: Academic Press, 2001. [Google Scholar]
Caulfield 2004
- Caulfield LE, Onis M, Blossner M, Black RE. Undernutrition as an underlying cause of child deaths associated with diarrhea, pneumonia, malaria, and measles. American Journal of Clinical Nutrition 2004;80(1):193‐8. [DOI] [PubMed] [Google Scholar]
Cook 1989
- Cook MJ, Holder‐Brown L, Johnson LJ, Kilgo JL. An examination of the stability of the Bayley Scales of infant development with high‐risk infants. Journal of Early Intervention 1989;13(1):45‐9. [Google Scholar]
Dewey 2008
- Dewey KG, Adu‐Afarwauh S. Systematic review of the efficacy and effectiveness of complementary feeding interventions in developing countries. Maternal & Child Nutrition 2008;4 Suppl s1:24‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Du 2005
- Du X, Zhu K, Trube A, Fraser DR, Greenfield H, Zhang Q, et al. Effects of school‐milk intervention on growth and bone mineral accretion in Chinese girls aged 10‐12 year: accounting for cluster randomisation. British Journal of Nutrition 2005;94(6):1038‐9. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Engle 1992b
- Engle PL, Gorman K, Martorell R, Pollitt E. Infant and preschool psychological development. Food and Nutrition Bulletin 1992;14(3):201‐14. [Google Scholar]
EPHPP 2009
- Effective Public Health Practice Project. Quality assessment tool for quantitative studies 2009. www.ephpp.ca (accessed 1 July 2011).
EPOC 2009
- Effective Practice, Organization of Care. Risk of bias tool. http://goo.gl/6Ki2iy (accessed 1 July 2011).
EPOC 2012
- Effective Practice and Organization of Care Group. Data abstraction form. http://goo.gl/ojeDUj (accessed 1 May 2012).
FAO 2013
- Food, Agriculture Organization. The state of food insecurity in the world. http://bit.ly/1piFzNB (accessed 13 May 2014).
Fishman 2003
- Fishman L, Rappaport L, Schonwald A, Nurko S. Trends in referral to a single encopresis clinic over 20 years. Pediatrics 2003;111(5):e604‐7. [DOI] [PubMed] [Google Scholar]
Galloway 2009
- Galloway R, Kristjansson E, Gelli A, Meir U, Espejo F, Bundy D. School feeding: outcomes and costs. Food and Nutrition Bulletin 2009;30(2):171‐82. [DOI] [PubMed] [Google Scholar]
Gaskin 2000
- Gaskin PS, Walker SP, Forrester TE, Grantham‐McGregor S. Early linear growth retardation and later blood pressure. European Journal of Clinical Nutrition 2000;54(7):563‐7. [DOI] [PubMed] [Google Scholar]
Golub 1995
- Golub MS, Keen CL, Gershwin ME, Hendrickx AG. Developmental zinc deficiency and behavior. Journal of Nutrition 1995;125(8 Suppl):2263‐71S. [DOI] [PubMed] [Google Scholar]
Grantham‐McGregor 1997
- Grantham‐McGregor SM, Walker S, Chang SM, Powell CA. Effects of early childhood supplementation with and without stimulation on later development in stunted Jamaican children. American Journal of Clinical Nutrition 1997;66(2):247‐53. [DOI] [PubMed] [Google Scholar]
Grantham‐McGregor 2007
- Grantham‐McGregor S. Early child development in developing countries. Lancet 2007;369(9564):824. [DOI] [PubMed] [Google Scholar]
Griffiths 2000
- Griffiths M. Panel: what are the relative roles of processed complementary foods and behavioural change in improving nutritional status? The need for strategic planning, not a technological fix. Food and Nutrition Bulletin 2000;21(1):73‐5. [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knotterus A. GRADE guidelines: a new series of articles in the journal of clinical epidemiology. Journal of Clinical Epidemiology 2011;64(4):380‐2. [DOI] [PubMed] [Google Scholar]
Haanga 1987
- Haanga J, Mason J. Food distribution within the family: evidence and implications for research and programmes. Food Policy 1987;12(2):146‐60. [Google Scholar]
Haddad 2000
- Haddad L, Alderman H. Malnutrition: income growth or nutrition programs. IFPRI 1999‐2000 Annual Report 2000.
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altmam DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- HIggins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing the risk of bias in included studies. in: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hoffman 2000
- Hoffman DJ, Sawaya AL, Verreschi I, Tucker KL, Roberts SB. Why are nutritionally stunted children at increased risk of obesity? Studies of metabolic rate and fat oxidation in shantytown children from São Paulo, Brazil. American Journal of Clinical Nutrition 2000;72(3):702‐7. [DOI] [PubMed] [Google Scholar]
Horton 2008
- Horton R. Maternal and child undernutrition: an urgent opportunity. Lancet 2008;371(9608):179. [DOI] [PubMed] [Google Scholar]
Irwin 2007
- Irwin L, Siddiqi A, Hertzman C. The equalizing power of early child development: from the Commission on Social Determinants of Health to Action. Child Health and Education 2007;1(3):146‐61. [Google Scholar]
Ivanovic 2004
- Ivanovic DM, Leiva BP, Pérez HT, Olivares MG, Díaz NS, Urrutia MS, et al. Head size and intelligence, learning, nutritional status and brain development: head, IQ, learning, nutrition and brain. Neuropsychologia 2004;42(8):1118‐31. [DOI] [PubMed] [Google Scholar]
Jahari 2000
- Jahari AB, Saco‐Pollitt C, Husaini MA, Pollitt E. Effects of an energy and micronutrient supplement on motor development and motor activity in undernourished children in Indonesia. European Journal of Clinical Nutrition 2000;54 Suppl 2:S60‐8. [DOI] [PubMed] [Google Scholar]
Jones 2003
- Jones G, Steketee RW, Black RE, Bhutta ZA, Morris SS, Bellagio Child Survival Study Group. How many child deaths can we prevent this year?. Lancet 2003;362(9377):65‐71. [DOI] [PubMed] [Google Scholar]
Kain 1998
- Kain J, Vio F, Albala C. Childhood nutrition in Chile: from deficit to excess. Nutrition Research 1998;18(11):1825‐37. [Google Scholar]
Kennedy 1987
- Kennedy ET, Alderman HH. Comparative analyses of nutritional effectiveness of food subsidies and other food‐related interventions. http://bit.ly/1EJELoG (accessed 30 June 2014).
Khan 2010
- Khan NZ, Muslima H, Begum D, Shilpi AB, Akhter S, Bilkis K, et al. Validation of rapid neurodevelopmental assessment instrument for under‐two‐year‐old children in Bangladesh. Pediatrics 2010;125(4):e755‐62. [DOI] [PubMed] [Google Scholar]
Kristjansson 2007
- Kristjansson E, Petticrew M, MacDonald B, Krasevec J, Janzen L, Greenhalgh T, et al. School feeding for improving the physical and psychosocial health of disadvantaged students. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD004676.pub2] [DOI] [PubMed] [Google Scholar]
Lassi 2013
- Lassi ZS, Das JK, Zahid G, Imdad A, Bhutta ZA. Impact of education and provision of complementary feeding on growth and morbidity in children less than 2 years of age in developing countries: a systematic review. BMC Public Health 2013;13 Suppl 3:S13. [DOI: 10.1186/1471-2458-13-S3-S13] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lopez 2006
- Lopez A, Mathers C, Ezzati M, Jamison D, Murray C. Global burden of disease and risk factors. The World Bank 2006. www.dcp2.org/pubs/GBD (accessed 1 July 2011):1‐552.
Lumbers 2001
- Lumbers ER, Yu ZY, Gibson KJ. The selfish brain and the barker hypothesis. Clinical and Experimental Pharmacology & Physiology 2001;28(11):942‐7. [DOI] [PubMed] [Google Scholar]
López‐Jaramillo 2008
- López‐Jaramillo P, Silva SY, Rodriguez‐Salamanca N, Duran A, Mosquera W, Castillo V. Are nutrition‐induced epigenetic changes the link between socioeconomic pathology and cardiovascular diseases?. American Journal of Theraputics 2008;15(4):362‐72. [DOI] [PubMed] [Google Scholar]
Martorell 2010
- Martorell R, Horta B, Adair L, Stein A, Richter L, Fall CHD, et al. Weight gain in the first two years of life is an important predictor of school outcomes in pooled analyses from five birth cohorts in low‐ and middle‐income countries. Journal of Nutrition 2010;140(2):348‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meeks Gardner 1995
- Meeks Gardner J, Grantham‐McGregor SM, Chang SM, Himes JH, Powell CA. Activity and behavioral development in stunted and nonstunted children and response to nutritional supplementation. Child Development 1995;66(6):1785‐97. [PubMed] [Google Scholar]
Meeks Gardner 1999
- Meeks Gardner JM, Grantham‐McGregor S, Himes J, Chang S. Behaviour and development of stunted and nonstunted Jamaican children. Journal of Child Psychology and Psychiatry 1999;40(5):819‐27. [DOI] [PubMed] [Google Scholar]
Morgane 2002
- Morgane PJ, Mokler DJ, Galler JR. Effects of prenatal protein malnutrition on the hippocampal formation. Neuroscience and Biobehavioral Reviews 2002;26(4):471‐83. [DOI] [PubMed] [Google Scholar]
Nord 2010
- Nord M, Coleman‐Jensen A, Andrews M, Carlson S. Household food security in the United States, 2009. http://1.usa.gov/1CEDRay (accessed 30 June 2014).
Ogilvie 2005
- Ogilvie D, Egan M, Hamilton V, Petticrew M. Systematic reviews of health effects of social interventions: 2. Best available evidence: how low should you go?. Journal of Epidemiology and Community Health 2005;59(10):886‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
ONPP 2004
- Office of Nutrition Policy and Promotion, Health Policy and Food Branch. Income related household food insecurity in Canada. http://bit.ly/1azDJe9 (accessed 1 July 2011):1‐124.
Patel 2005
- Patel MP, Sandige HL, Ndekha MJ, Briend A, Ashom P, Manary MJ. Supplemental feeding with ready‐to‐use therapeutic food in Malawian children at risk of malnutrition. Journal of Health, Population and Nutrition 2005;23(4):351‐7. [PubMed] [Google Scholar]
Petrou 2010
- Petrou S, Kupek E. Poverty and childhood undernutrition in developing countries: a multi‐national cohort study. Social Science and Medicine 2010;71(7):1366‐73. [DOI] [PubMed] [Google Scholar]
Pollitt 1994
- Pollitt E, Oh SY. Early supplementary feeding, child development and health policy. Food and Nutrition Bulletin 1994;15(3):208‐14. [Google Scholar]
Pollitt 1997
- Pollitt E, Watkins WE, Husaini MA. Three‐month nutritional supplementation in Indonesian infants and toddlers benefits memory function 8 y later. American Journal of Clinical Nutrition 1997;66(6):1357‐63. [DOI] [PubMed] [Google Scholar]
Pollitt 1998
- Pollitt E, Mathews R. Breakfast and cognition: an integrative summary. American Journal of Clinical Nutrition 1998;67(4):804‐13S. [DOI] [PubMed] [Google Scholar]
Pollitt 2000b
- Pollitt E, Saco‐Pollitt C, Jahari A, Husaini MA, Huang J. Effects of an energy and micronutrient supplement on mental development and behavior under natural conditions in undernourished children in Indonesia. European Journal of Clinical Nutrition 2000;54 Suppl 2:S80‐90. [DOI] [PubMed] [Google Scholar]
Power 1997
- Power C, Hertzman C. Social and biological pathways linking early life and adult disease. British Medical Bulletin 1997;53(1):210‐21. [DOI] [PubMed] [Google Scholar]
Prentice 2005
- Prentice AM, Moore SE. Early programming of adult diseases in resource poor countries. Archives of Disease in Childhood 2005;90(4):429‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rao 1977
- Rao DH, Naidu N. Nutritional supplementation: whom does it benefit most?. American Journal of Clinical Nutrition 1977;30(10):1612‐6. [DOI] [PubMed] [Google Scholar]
Review Manager 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Rivera 2013 [pers comm]
- Rivera J. [personal communication]. E‐mail to: Elizabeth Kristjansson 2013.
Rondo 1990
- Rondo SP. Supplementary feeding programs: a critical analysis. Revista de Saúde Pública 1990;24(5):412‐9. [DOI] [PubMed] [Google Scholar]
Rosier 2011
- Rosier K. Food insecurity in Australia: what is it, who experiences it and how can child and family services support families experiencing it?. http://bit.ly/1E1VJRd (accessed 30 June 2014). [ISSN: 1838‐7330]
Rush 1998
- Rush D, Leighton J, Sloan NL, Alvir JM, Horvitz DG, Seaver WB, et al. The National WIC Evaluation: evaluation of the Special Supplemental Food Program for Women, Infants, and Children. VI. Study of infants and children. American Journal of Clinical Nutrition 1988;48(2 Suppl):484‐511. [DOI] [PubMed] [Google Scholar]
Schochet 2005
- Schochet PZ. Statistical power for random assignment evaluations for education programs. http://bit.ly/1EJJQgP (accessed 15 August 2006).
Schrimshaw 1998
- Schrimshaw NS. Malnutrition, brain development, learning, and behaviour. Nutrition Research 1998;18(2):351‐79. [Google Scholar]
Schroeder 1995
- Scroeder DG, Martorell R, Rivera J, Ruela MT, Habicht JP. Age differences in the impact of nutritional supplementation on growth. Journal of Nutrition 1995;125(4 Suppl):1051‐9S. [DOI] [PubMed] [Google Scholar]
Seidler 1990
- Seidler FJ, Bell JM, Slotkin TA. Undernutrition and overnutrition in the neonatal rat: long‐term effects on noradrenergic pathways in brain regions. Pediatric Research 1990;27(2):191‐7. [DOI] [PubMed] [Google Scholar]
Sguassero 2012
- Sguassero Y, Onis M, Bonotti AM, Carroli G. Community‐based supplementary feeding for promoting the growth of children under five years of age in low and middle income countries. Cochrane Database of Systematic Reviews 2012, Issue 6. [DOI: 10.1002/14651858.CD005039.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shankar 2000
- Shankar AH. Nutritional modulation of malaria morbidity and mortality. Journal of Infectious Diseases 2000;182 Suppl 1:S37‐53. [DOI] [PubMed] [Google Scholar]
Strupp 1995
- Strupp BJ, Levitsky DA. Enduring cognitive effects of child malnutrition: a theoretical reappraisal. Journal of Nutrition 1995;125 Suppl 8:2221‐32S. [DOI] [PubMed] [Google Scholar]
Tanner 2002
- Tanner EM, Finn‐Stevenson M. Nutrition and brain development: social policy implications. American Journal of Orthopsychiatry 2002;72(2):182‐93. [DOI] [PubMed] [Google Scholar]
Tomkins 1989
- Tomkins A, Watson F. Malnutrition and infection ‐ a review ‐ nutrition policy discussion paper No.5. http://bit.ly/1FX18rZ (accessed 30 June 2014).
Tugwell 2010
- Tugwell P, Petticrew M, Kristjansson E, Welch V, Euffing E, Waters E, et al. Assessing equity in systematic reviews: realising the recommendations of the Commission on Social Determinants of Health. BMJ 2010;341:c4739. [DOI] [PubMed] [Google Scholar]
Uauy 2001
- Uauy R, Albala C, Kain J. Obesity trends in Latin America: transiting from under‐ to overweight. Journal of Nutrition 2001;131(3):893‐9S. [DOI] [PubMed] [Google Scholar]
Ukoumunne 1999
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ. Methods for evaluating area‐wide and organisation based interventions in health and health care: a systematic review. Health Technology Assessment 1999;3(5):1‐108. [PubMed] [Google Scholar]
UNICEF 2006
- United Nations Children's Fund. Progress for children: a report card on nutrition. http://uni.cf/1L6M5Nn (accessed 1 July 2011).
United Nations ACC/SCN 2000
- United Nations ACC Sub‐Committee on Nutrition. 4th report on the world nutrition situation: nutrition throughout the life cycle. http://bit.ly/1DlFnDQ (accessed 20 June 2014).
Van de Poel 2008
- Poel E, Hosseinpoor AR, Speybroeck N, Ourti R, Vega J. Socio‐economic inequality in malnutrition in developing countries. Bulletin of the World Health Organization 2008;86(4):241‐320. [DOI] [PMC free article] [PubMed] [Google Scholar]
Victora 2008
- Victora CG, Adair L, Fall C, Hallal PC, Martorell R, Richter L, et al. Maternal and child undernutrition: consequences for adult health and human capital. Lancet 2008;371(9609):340‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Viera 2007
- Viera A, Bangdiwala S. Eliminating bias in randomized controlled trials: importance of allocation concealment and masking. Family Medicine 2007;39(2):132‐7. [PubMed] [Google Scholar]
Wachs 2000
- Wachs TD. Nutritional deficits and behavioural development. International Journal of Behavioral Development 2000;24(4):435‐41. [Google Scholar]
Wachs 2005
- Wachs TD, Creed‐Kanashiro H, Cueto S, Jacoby E. Maternal education and intelligence predict offspring diet and nutritional status. Journal of Nutrition 2005;135(9):2179‐86. [DOI] [PubMed] [Google Scholar]
Walker 1991
- Walker SP, Powell CA, Grantham‐McGregor SM, Himes JH, Chang SM. Nutritional supplementation, psychosocial stimulation, and growth of stunted children: the Jamaican study. American Journal of Clinical Nutrition 1991;54(4):642‐8. [DOI] [PubMed] [Google Scholar]
Walker 2007
- Walker SP, Wachs TD, Gardner JM, Lozoff B, Wasserman GA, Pollitt E, et al. Child development: risk factors for adverse outcomes in developing countries. Lancet 2007;369(9556):145‐57. [DOI] [PubMed] [Google Scholar]
WHO 2013
- World Health Organization. Global nutrition policy review: what does it take to scale up nutrition action?. http://bit.ly/1AA0Ybi (accessed 12 August 2013).
World Bank 2011
- World Bank. Country and lending groups. http://bit.ly/1baL18q (accessed 1 July 2011).
World Hunger Education Service 2012
- World Hunger Education Service. 2012 World hunger and poverty facts and statistics. http://bit.ly/1eGO0c7 (accessed 1 July 2011).
Worobey 1999
- Worobey J, Worobey HS. The impact of a two‐year school breakfast program for pre‐school children on their nutrient intake and pre‐academic performance. Child Study Journal 1999;29(2):113‐31. [Google Scholar]
Zhang 2006 [pers comm]
- Zhang Q. [personal communication]. Emails to: E Kristjansson and editorial base of the Cochrane Developmental, Psychosocial and Learning Problems Group June 2006.
References to other published versions of this review
Kristjansson 2012
- Kristjansson E, Francis DK, Liberato S, Benkhalti Jandu M, Welch V, Batal M, et al. Feeding interventions for improving the physical and psychosocial health of disadvantaged children aged three months to five years. Cochrane Database of Systematic Reviews 2012, Issue 6. [DOI: 10.1002/14651858.CD009924] [DOI] [PMC free article] [PubMed] [Google Scholar]


