Abstract
Background
Colorectal cancer is one of the most common cancer in the western world. Acute colonic obstruction is one of the common presentations of colon cancer. Emergency surgical decompression is the traditional treatment of choice but is associated with high morbidity and mortality. In recent years colonic stents have been used to relieve the obstruction.
Objectives
The aim was to compare the colonic stenting versus emergency surgical decompression with regards to benefits and risks.
Search methods
Searches were carried out May 2010 in the Cochrane Colorectal Cancer Specialised Register, the Cochrane Central Register of Controlled Trials, Ovid MEDLINE, Ovid EMBASE and Ovid CINAHL.
Selection criteria
Randomised clinical trials comparing colonic stenting versus surgical decompression for obstructing colorectal cancers were considered for inclusion.
Data collection and analysis
Data on the characteristics of the trial, methodological quality of the trials, mortality, morbidity, technical and clinical success rate, operating time, hospital stay and other measured secondary outcomes from each trial were collected. And the data were analysed with both the fixed‐effect and the random‐effects models using RevMan Analysis. For each outcome, odds ratio (OR) with 95% confidence intervals (CI) based on available data analysis was calculated.
Main results
Five randomised trials were identified with a total of 207 participants, 102 to colorectal stenting and 105 to emergency surgery. There was statistically significant higher clinical success rate in the emergency surgery group. The average time of clinical relief of obstruction was 0.66 day in the colonic stent group and was 3.55 days in the emergency surgery group. The stent insertion was successful in 86.02% of attempted stent placements. There was no statistically significant difference in the 30‐day mortality between two groups. The 30 day mortality rate was similar, 2.3% in both groups. The stent related perforation rate was 5.88%. The stent migration rate was 2.13%. The stent obstruction rate was 2.13%. There was no statistically significant difference in overall complication rate in both groups. The complication rate was 39.22% in the colonic stent group and was 45.71% in the emergency surgery group. The mean hospital stay was 11.53 days in the colonic stent group and was 17.15 days in the emergency surgery group. The mean procedure/operating time was 113.93 minutes in the colonic stent group compared to 143.85 minutes in the emergency surgery group. The median blood loss was 50 ml in the colonic stent group and 350 ml in the emergency surgery group.
Authors' conclusions
The use of colonic stent in malignant colorectal obstruction seems to have no advantage over emergency surgery. The clinical success rate was statistically higher in emergency surgery group. However, use of colorectal stents seems to be as safe in the malignant colorectal obstruction as the emergency surgery with no statistically significant difference in the mortality and morbidity. Colorectal stents are associated with acceptable stent perforation, migration and obstruction rates. The advantages of colorectal stent includes shorter hospital stay and procedure time and less blood loss. However, due to the variability in the sample size and trial designs in the included studies, further randomised trials with bigger sample size and well defined trial design are needed to achieve the robust evidence.
Keywords: Humans, Stents, Colorectal Neoplasms, Colorectal Neoplasms/complications, Emergencies, Intestinal Obstruction, Intestinal Obstruction/etiology, Intestinal Obstruction/mortality, Intestinal Obstruction/surgery, Randomized Controlled Trials as Topic, Survival Rate
Plain language summary
Colonic stenting has no decisive advantages to Emergency surgery.
Emergency surgical decompression has been the traditional treatment of choice for the malignant colorectal tumours presenting as an acute obstruction. This is associated with higher morbidity and mortality due to the emergency nature of the procedure along with other existing co morbidities. This systematic review of five randomised trial shows higher rates of clinical relief of obstruction in emergency surgery. Colonic stent has not been shown to be as effective as emergency surgery in malignant colorectal obstructions. However, use of colonic stent is associated with comparable mortality and morbidity with advantage of shorter hospital stay and procedure time and less blood loss. Further randomised controlled trials with larger sample size and robust trial design are required on this topic.
Summary of findings
Summary of findings for the main comparison. Summary of outcomes.
| Outcome | No. of Study | No. of Participants | Statistical Mehtod | Effect Size |
| Clinical Success | 3 | 168 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.01, 0.32] |
| 30 Day Mortality | 5 | 207 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.48, 4.14] |
| Complication | 5 | 207 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.47, 1.34] |
| Major Wound Complications | 2 | 146 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.05, 6.16] |
Background
Description of the condition
Colorectal cancer is one of the common cancers affecting the western population. It usually affects the older population. Acute colonic obstruction is one of the common clinical presentations of colorectal cancer. In such cases surgical decompression with colostomy with or without resection and eventual re‐anastomosis is the traditional treatment of choice; however, associated morbid conditions due to old age, along with emergency nature of surgical intervention, increases the chances of morbid complications intra‐ and post‐operatively.
Description of the intervention
The colonic stent placement to relieve the obstruction has been used since the last decade to avoid emergency surgery. The colonic stent insertion effectively decompresses the obstructed colon, allowing surgery to be performed electively at a later stage (Targownik 2004). 80% of the colonic obstruction is due to malignancy and 10% to 30% of patients with colonic cancer present with obstruction (Deans 1994; Rault 2005). Colonic stents are used increasingly as a palliation and as a bridge to surgery for obstructing colorectal cancer (Khot 2002). Colonic stents can be deployed either under fluorescence radiology using guidewire, through endoscopy or through the use of both techniques.
How the intervention might work
Technical and clinical success of the colonic stents varied from 70% to 95%. In a pooled analysis of 1198 colonic stent insertion the median technical and clinical success rates were 94% (i.q.r. 90‐100) and 91% (i.q.r. 84‐94), respectively. The clinical success, when used as a bridge to surgery, was 71.7% (Sebastian 2004). Among the complications perforation was 4%, stent migration and stent reabsorption were 10% each (Khot 2002). Colonic stenting can be used effectively, with acceptable morbidity, to manage patients presenting with large bowel obstruction (Watson 2005; Syn 2005). Self‐expanding metallic stent placement is a palliative alternative to colostomy for patients with inoperable malignant colonic strictures (Xinopoulos 2004). The colonic stent may provide an alternative option to emergency surgery with possible low morbidities and mortality.
Why it is important to do this review
Although systematic review on the efficacy and safety of colorectal stents has been presented (Khot 2002; Sebastian 2004), we were unable to identify any systematic review involving randomised controlled trials, questioning whether the colonic stents provides an alternative option to surgical intervention for the management of colonic malignant obstruction. Consequently, this review tried to reduce this uncertainty by summarising the available research evidence regarding the colonic stents in the malignant colorectal obstructions in comparison to emergency surgical interventions.
Objectives
The primary objective was to evaluate the clinical success rate of stent placement compared to emergency surgery. The secondary objectives were to evaluate the technical success rate of stent deployment, survival, mortality, morbidity, hospital stay, procedure time and patient comfort and quality of life in both groups. I also aimed to assess the cost effectiveness of colonic stenting in comparison with surgery.
Methods
Criteria for considering studies for this review
Types of studies
Only randomised controlled trials (RCTs) of parallel study design , where the allocation of concealment was adequate or not reported, were considered eligible for inclusion in this review. There were no quasi‐randomised trials found for this study.
Types of participants
RCTs involving adults (18 years and over) presented clinically with large bowel obstruction secondary to colorectal cancer, confirmed radiologically, were included irrespective of race, sex and associated medical conditions undergoing either colonic stent placement or surgical interventions.
Types of interventions
Studies reporting the following comparisons were considered eligible for inclusion in this review. Studies were included irrespective of the methods used for colonic stent deployment either through endoscopic or fluoroscopic radiology guided or through endoscopic and fluoroscopic radiology guidance together.
Colonic stent compared with stoma creation for malignant colonic obstruction as palliative procedure
Colonic stent compared with stoma creation for malignant colonic obstruction as a bridge to a definitive surgical procedure
One type of colonic stent compared with another type of colonic stent for malignant colonic obstruction
Types of outcome measures
Studies which reported the following outcomes were considered eligible for the inclusion.
Primary outcomes
Clinical success in the form of relief from colonic obstruction (defined by the author).
Secondary outcomes
Technical success (defined by the author)
Stent related perforation of bowel
Stent migration
Stent blockage or obstruction
Length of hospital stay
Survival
Patient's pain or discomfort (however measured)
Other outcomes
Search methods for identification of studies
Systematic search strategies was used to identify the relevant studies (Appendix 1) and performed May 2010.
Electronic searches
Searched databases are as follows:
Cochrane Colorectal Cancer Specialised Register (30/04/2010);
Cochrane Central Register of Controlled Trials (CENTRAL); 2010 issue 4
Ovid MEDLINE (1950 to May 2010);
Ovid EMBASE (1970 to May 2010);
Ovid CINAHL (1970 to May 2010).
A filter was used to identify RCTs for all databases except the Colorectal Cancer Group Specialised Register and CENTRAL, using the strategy described in the Cochrane Handbook (Higgins 2008).
The databases used for the literature search included MEDLINE, EMBASE and Cochrane Controlled Trials Register. These are considered the relevant bibliographic databases in this subject area.
The resulting search results were uploaded and managed in Reference Manager version 11.
Searching other resources
All relevant studies were considered, irrespective of language and publication status, for this review. The reference lists in the identified studies were also searched in order to identify further studies. We also searched to identify any ongoing or completed trials.
Data collection and analysis
The author independently assessed the titles and abstracts of the identified studies. We obtained the full articles for all studies that potentially meet the inclusion criteria and included all those that meet the inclusion criteria. Any differences were resolved by discussion with Cochrane colorectal group.
Selection of studies
Only randomised controlled trials were included in this review. All studies based on case series or Institutes' own experiences were studied to avoid any missed randomised trials. Doubts on inclusion were discussed and solved in collaboration with the Cochrane Cochrane Colorectal Group.
Data extraction and management
The author independently extracted data for the outcomes listed and independently assessed the methodological quality of each trial, without masking authors' names. In addition, the following data for each study using a custom designed extraction form were extracted:
Language of publication
Country where study conducted
Baseline characteristics of participants by group
Type of interventions ‐ colonic stent/surgery
Type of stents
Details of the comparison intervention
Co‐interventions (by group)
Duration of follow up
Inclusion and exclusion criteria.
Missing information was sought by contacting the study authors, as well as information on studies potentially sharing the same patients.
Assessment of risk of bias in included studies
Assessment of risk of bias in the trial was based on guidelines in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). If information was not available in the published study, authors were contacted to assess the trials correctly.
The elements of risk of bias assessment were generation of allocation sequence, allocation concealment, blinding, incomplete data outcomes, selective data reporting, and other bias (early stopping, baseline imbalance, source of funding): (1) Generation of the allocation sequence
Adequate, if investigators described a random component in the sequence generation process such as, use of a random number table, computer random number generator, coin tossing, shuffling cards or envelopes, throwing dice, drawing of lots or minimization.
Not adequate, if investigators described a non‐random component in the sequence generation process such as sequence generated based on odd or even date of birth, on date (or day) of admission or on hospital or clinic record number.
Unclear, if insufficient information was available to clarify the sequence generation as adequate or not.
(2) Allocation concealment
Adequate, if participants and investigators could not foresee assignment such as use of central allocation (including telephone, web‐based and pharmacy‐controlled randomisation), sequentially numbered drug containers of identical appearance or sequentially numbered, opaque, sealed envelopes.
Inadequate, if participants or investigators enrolling participants could possibly foresee assignments such as using an open random allocation schedule (e.g. a list of random numbers), assignment envelopes without appropriate safeguards (e.g. if envelopes were unsealed or nonopaque or not sequentially numbered), alternation or rotation, date of birth, case record number or any other explicitly unconcealed procedure.
Unclear, if insufficient information was provided to permit judgement as adequate or not.
(3) Blinding of participant, care‐provider and outcome assessor
Adequate, if any one of the following was followed.
No blinding, but the review authors judge that the outcome and the outcome measurement were not likely to be influenced by lack of blinding;
Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken;
Either participants or some key study personnel were not blinded, but outcome assessment was blinded and the non‐blinding of others unlikely to introduce bias.
Not adequate, if any one of the following was followed.
No blinding or incomplete blinding, and the outcome or outcome measurement was likely to be influenced by lack of blinding;
Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken;
Either participants or some key study personnel were not blinded, and the non‐blinding of others likely to introduce bias.
Unclear, if insufficient information was provided to permit judgement as adequate or not or the study did not address this outcome.
(4) Incomplete data outcomes
Adequate, if any one of the following was observed.
No missing outcome data;
Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias);
Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups;
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate;
For continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size;
Missing data have been imputed using appropriate methods.
Not adequate, if any one of the following was observed.
Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups;
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate;
For continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size;
‘As‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation;
Potentially inappropriate application of simple imputation.
Unclear, if insufficient reporting of attrition/exclusions to permit judgement as adequate or not (e.g. number randomised not stated, no reasons for missing data provided) or the study did not address this outcome.
(5) Selective outcome reporting
Adequate, if any of the following was observed.
The study protocol was available and all of the study’s pre‐specified (primary and secondary) outcomes that were of interest in the review had been reported in the pre‐specified way;
The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were pre‐specified (convincing text of this nature might be uncommon).
Inadequate, if any one of the following was observed.
Not all of the study’s pre‐specified primary outcomes had been reported;
One or more primary outcomes was reported using measurements, analysis methods or subsets of the data (e.g. sub scales) that were not pre‐specified;
One or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect);
One or more outcomes of interest in the review were reported incompletely so that they could not be entered in a meta‐analysis;
The study report failed to include results for a key outcome that would be expected to have been reported for such a study.
Unclear, if insufficient information to permit judgement as adequate or not.
(6) Other potential threats to validity
Adequate if the study appeared to be free of other sources of bias.
Not adequate, if there was at least one important risk of bias. For example, the study
Had a potential source of bias related to the specific study design used; or
Was stopped early due to some data‐dependent process (including a formal‐stopping rule); or
Had extreme baseline imbalance; or
Had been claimed to have been fraudulent; or
Had some other problem.
Unclear, if there might be a risk of bias, but there was either
Insufficient information to assess whether an important risk of bias exists; or
Insufficient rationale or evidence that an identified problem would introduce bias.
Measures of treatment effect
The software package, RevMan 5.0, provided by The Cochrane Collaboration was used for this review. For dichotomous variables, the odds ratio (OR) with 95% confidence interval was calculated. There were no continuous outcomes that were studied in this review.
Unit of analysis issues
The unit of allocation was individual participants. There were no cluster‐randomised trials.
Dealing with missing data
To deal with missing data, "available case analysis" was used i.e. whether participants were analysed in the groups to which they were originally randomised (Hollis 1999; Higgins 2008) without imputing any data for the patients for whom the outcomes were not reported. There were no continuous outcomes that were studied in this review. When medians were reported without the means, median was not used as a substitute for mean in the meta‐analysis . But median was considered as mean in other data results where meta‐analysis was not applicable. As there were no post‐randomisation drop‐outs or withdrawals from the included trials, we considered this as "intention‐to‐treat analysis".
Assessment of heterogeneity
The heterogeneity was explored using I2 and p‐value from the chi‐squared test whenever required.
Assessment of reporting biases
Duplicate publication bias
In case, there were any doubts whether a trial had been published twice or more (by identifying common authors, centres, and interventions), the authors were contacted to clarify whether the trial report had been duplicated.
Location bias
It was investigated whether effect estimate varies with the database from which the report was identified (MEDLINE indexed versus MEDLINE not indexed).
Language bias
There was no language bias as all the included trials were published in English.
Publication bias
It was investigated whether effect estimate varied with the publication of the trial report (as full text) or not. The bias was explored through a funnel plot of effect estimates and the standard error of the effect estimate using RevMan 5.0 (Egger 1997). The asymmetry in funnel plot of study size was used against treatment effect to identify this bias. The linear regression approach described by Egger (Egger 1997) was used to determine the funnel plot asymmetry.
Data synthesis
The meta‐analyses was performed according to the recommendations of The Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). The Mantel‐Haenzel's methods were used to perform the meta‐analysis. Both, fixed‐effects model (DerSimonian 1986) and a random‐effect model (Demets 1987) were used in analysis. In case of discrepancy between the two models, both results were reported; otherwise only the results from the fixed‐effect model were reported.
Subgroup analysis and investigation of heterogeneity
Although there were more than one trial, subgroup analysis was not performed. Due to limited number of trials with variability in their use of type of stent, subgroup analysis of different varieties of stents was not performed.
Sensitivity analysis
The sensitivity analysis was not performed as medians were not applicable in the data results where meta‐analysis was applicable. There was no 'zero‐event' trials (i.e., zero event in both groups) in statistically significant outcomes.
Results
Description of studies
A total of 85 references were identified through the electronic searches of Cochrane Colorectal Cancer Specialised Register; Cochrane Central Register of Controlled Trials (CENTRAL); Ovid MEDLINE; Ovid EMBASE and Ovid CINAHL. These 85 references were relevant and therefore their abstracts were retrieved for further assessment. No references were identified through scanning preference lists of identified randomised trials. Out of 85 references, six randomised trials were found eligible for inclusion. The rest of the studies were either non‐randomised studies, case series or Institutions' own experiences.
Results of the search
Out of six randomised studies, only five studies met the inclusion criteria and were considered for the review (Cheung 2009; Fiori 2004; Sankararajah 2005; van Hooft 2008; van Hooft 2011). One of these trials was published as interim results of the trial in the form of an abstract, and it was unclear whether the trial was completed or not (Sankararajah 2005). The data were very limited and attempts to contact the authors of this trial were unsuccessful. The characteristics of all the included trials are shown in the table "Characteristics of included studies". Two of the included trials were closed prematurely due to adverse events in the intervention group (van Hooft 2008; van Hooft 2011). The excluded study could not provide separate data for the malignant colorectal obstruction (Xinopoulos 2004) despite attempts to contact the authors.
Included studies
Five randomised controlled trials were identified for inclusion (Cheung 2009; Fiori 2004; Sankararajah 2005; van Hooft 2008; van Hooft 2011). A total of 208 patients, 112 males and 96 females, with large bowel obstruction were included in the five trials. In the study by Sankararajah 2005, one of the patients (gender not reported) in the emergency surgery group refused treatment after randomisation, and therefore not included in the analysis of the study. Consequently, 207 patients underwent intervention and were considered for the analysis. The median age of patients in two trials was 72.75 years (Sankararajah 2005; Cheung 2009), while mean age in three trial was 70.65 years (Fiori 2004; van Hooft 2008; van Hooft 2011).
Experimental intervention
Colorectal stenting was performed in 102 patients as either palliative or as a bridge to further surgical intervention. The details of endoluminal stenting was available in four trials (Cheung 2009; Fiori 2004; van Hooft 2008; van Hooft 2011) but was not provided in the trial by Sankararajah 2005. In one trial, authors used Precision stent system (Fiori 2004) while in two trials, authors used Wallstent enteral endoprosthesis (Cheung 2009; van Hooft 2008). In another trial, authors used enteral Wallstent and Wallflex colonic stents (van Hooft 2011). Control intervention
One hundred and five patients were randomised for surgical intervention. The details of surgical intervention were available in four trials (Cheung 2009;Fiori 2004; van Hooft 2008; van Hooft 2011) and was not provided in the trial by Sankararajah 2005. Concomitant intervention
Details of the concomitant intervention in the form of definitive surgery were available in three trials (Cheung 2009; Fiori 2004; van Hooft 2008; van Hooft 2011) and was not provided in the trial by Sankararajah 2005. In one trial authors used definitive laparoscopic surgery as concomitant intervention (Cheung 2009). Outcome measures
The primary outcome measures reported in these trials were different, one trial reported clinical success of stent as the primary outcome (Fiori 2004) while another trial reported successful one‐stage operation following stent placement (Cheung 2009). The third trial did not mention primary outcomes (Sankararajah 2005). The hospital free in good health and quality of life were the primary outcomes in the remaining two trials respectively (van Hooft 2011; van Hooft 2008). The other outcome measures reported were mortality rates, complication rates, operative time, blood loss, stoma rate, effectiveness of palliation, cost and hospital stay. One trial reported lymph node harvest, pain scoring and postoperative analgesia as secondary outcomes (Cheung 2009). Only one trial measured median survival and estimated one year survival rates (Sankararajah 2005).
Excluded studies
One randomised trial did not meet the inclusion criteria (Xinopoulos 2004). The reason for exclusion is described in the "characteristics of the excluded studies".
Risk of bias in included studies
Three trials had high methodological quality (Cheung 2009; van Hooft 2008; van Hooft 2011) as shown in Figure 1 and Figure 2.
1.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The generation of allocation sequence and allocation concealment was adequate in three trials (Cheung 2009; van Hooft 2008; van Hooft 2011), but was unclear in one trial (Sankararajah 2005). In another trial, allocation sequence generation was not clear but allocation concealment was adequate (Fiori 2004). It was not possible to get any responses to clarify these despite attempts to contact the authors of these studies.
Blinding
The blinding of participants, care providers and outcome assessors were adequate in all the trials.
Incomplete outcome data
Information about incomplete outcome data was adequate in all the trials.
Selective reporting
Information about selective outcome reporting was adequate in all the trials.
Other potential sources of bias
Information about other potential sources of bias was adequate in three trials (Cheung 2009; van Hooft 2008; van Hooft 2011), while it was unclear in two trials (Fiori 2004; Sankararajah 2005) as source of finding was not declared. We did not get any responses to clarify these despite attempts to contact the authors of these studies.
Effects of interventions
See: Table 1
Five trials including 207 patients were included for this review. One hundred and two patients were randomised to colonic stent group and 105 patients were randomised to emergency surgery group.
Clinical Success Rate: There was statistically significant difference between two groups for the clinical success rates (p = 0.001, OR 0.06, 95% CI 0.01 to 0.32) Figure 3. The emergency surgery group has high success rate of clinical relief (98.84%) compared to colonic stent group (78.05%). There was no change in the results by adopting the random‐effects model, available case analysis or by calculating the risk difference. The average time of clinical relief of obstruction was 0.66 day in colonic stent group and was 3.55 days in emergency surgery group.
3.

Forest plot of comparison: Clinical Relief of Obstruction
Technical Success Rate: The stent insertion was successful in 86.02% of attempted stent placements.
Other Outcomes:
30 Day Mortality: There was no statistically significant difference between two groups for the 30 day mortality rate using the fixed‐effect model (OR 1.41, 95% CI 0.48 to 4.14), random‐effects model, available case analysis or by calculating the risk difference Figure 4. The 30 day mortality rate was similar, 2.3% in both groups.
4.

Forest plot of comparison: 30 Day Mortality
Stent Related Perforation: The stent related perforation rate was 5.88%. However, there was no stent related perforation in three trials (Cheung 2009; Fiori 2004; Sankararajah 2005), but it was 54.55% and 12.77% in two trials respectively (van Hooft 2008; van Hooft 2011).
Complication Rate: There was no statistically significant difference between two groups regarding the total complication rate using the fixed‐effect model (OR 0.79, CI 0.47 to 1.34),random‐effects model, available case analysis, or by calculating the risk difference Figure 5. The complication rate was 39.22% in the colonic stent group and was 45.71% in the emergency surgery group.
5.
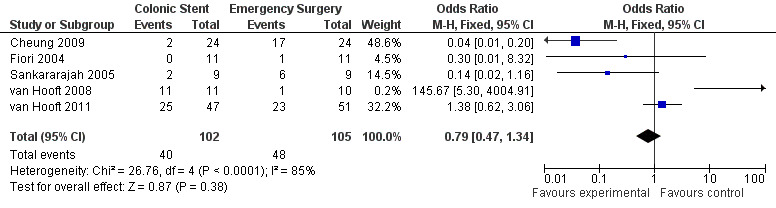
Forest plot of comparison: Complications
Major Wound Complication Rate: There was no statistically significant difference between two groups for the major wound complication rate using the fixed‐effect model (OR 0.54, 95% CI 0.05 to 6.16), random‐effects model, available case analysis, or by calculating the risk difference Figure 6. The major wound complication rate was 5.63% in the colonic stent group and was 12% in the emergency surgery group.
6.

Forest plot of comparison: Major Wound Complication
Stent Migration: The stent migration rate was 2.13%.
Hospital Stay: The mean hospital stay was 11.53 days in the colonic stent group and was 17.15 days in the emergency surgery group.
Procedure/Operating Time: The mean procedure/operating time was 113.93 minutes in the colonic stent group compared to 143.85 minutes in the emergency surgery group.
Blood Loss: The median blood loss was 50 ml in the colonic stent group and 350 ml in the emergency surgery group.
Stent Obstruction/Blockage: The stent obstruction rate was 2.13%.
Funnel plots did not reveal any bias for any of the outcomes measured. However, there were too few trials to perform the Egger's test for exploration of bias Figure 7; Figure 8; Figure 9; Figure 10.
7.

Funnel plot of comparison: Clinical Relief of Obstruction
8.

Funnel plot of comparison: 30 Day Mortality
9.

Funnel plot of comparison: Complications
10.

Funnel plot of comparison: Major Wound Complication
Subgroup analysis of the trials was not applicable.
Sensitivity analysis was not applicable.
Discussion
In this systematic review, there is statistically significant difference in the clinical relief of obstruction favouring emergency surgery for malignant colorectal obstruction. However, colonic stenting has the comparable mortality and morbidity to the emergency surgery. Colonic stenting also has the benefit of shorter hospital stay and procedure time and less blood loss.
Colonic obstruction is one of the common presentations of the large bowel pathology. About 80 % of colonic obstruction is due to malignant lesions (Deans 1994; Rault 2005). Before the advent of colorectal stents emergency surgical decompression, with or without primary resection of obstructing malignant tumour, was the traditional treatment of choice. It is still the preference in setup where facilities of colonic stenting are limited. In 1991, Dohmoto first described the use of a metal self expanding stent as the endoscopic palliative alternative for inoperable colon cancer (Dohmoto 1991). In the following three years, Tejero et al published their report of using stents as a bridge to surgery in two patients with colonic obstruction (Tejero 1994). However, patients presenting with acute large bowel obstruction suffer from high rates of complications due to associated co‐morbidities. About 10‐30% of patients with malignant colonic lesions present as bowel obstruction (Deans 1994; Rault 2005). Colonic stenting can be used effectively, with acceptable morbidity, to manage patients presenting with large bowel obstruction (Watson 2005; Syn 2005). Colorectal stenting becomes the treatment of choice in many centres with facilities available to relieve obstruction in malignant colonic obstruction (Khot 2002). Self‐expanding metallic stent placement is a palliative alternative to colostomy for patients with inoperable malignant colonic strictures (Xinopoulos 2004).
Although the use of colonic stents for the relief of obstruction seems exciting there is very limited data available to establish its use, compared to emergency surgical options, in terms of clinical and technical success rates. Technical and clinical success of colonic stents varied from 70% to 95%. In a pooled analysis of 1198 colonic stent insertion the median technical and clinical success rates were 94% and 91% respectively while the clinical success rate, when used as a bridge to surgery was 71.7% (Sebastian 2004). In this review, there was statistically significant difference between two groups for the clinical success rates Figure 3. The emergency surgery group has high success rate of clinical relief (98.84%) compared to colonic stent group (78.05%). The authors of different trials used their own criteria to define clinical success. In one trial, successful primary operation or successful stenting was considered as clinical success (Cheung 2009). In other trial "relief of colonic obstruction symptoms" was used as clinical success (van Hooft 2011). Oral intake was considered as clinical success in another trial (van Hooft 2008). Fiori et al. used the return of bowel function as clinical success in their trial (Fiori 2004). These differences of defining clinical success may impact the outcome of these trials. The average time of clinical relief of obstruction was 0.66 day in the colonic stent group and was 3.55 days in the emergency surgery group. These results were pooled from three of the five included trials (Cheung 2009; Fiori 2004; van Hooft 2008). The technical success rate was 86.02%. Those who failed in the colonic stent insertion were offered emergency surgical intervention. However the analysis was performed with the principle of "intention to treat". The technical success rate is associated with the site of obstruction. More proximal lesions are technically challenging, compared to the obstructions in the left colon or in the rectosigmoid colon (Sebastian 2004). In this study one trial has specified the site of obstructing lesion, 63.6% in the rectum and 36.3% in the rectosigmoid/sigmoid colon (Fiori 2004) and another trial reported the site of obstruction at rectosigmoid in 37%, sigmoid in 21%, splenic flexure in 16%, descending colon in 16%, rectum in 5% and ascending colon in 5% (Sankararajah 2005). Two trials reported the site of obstruction in the left sided colon in all cases but did not provide the details of their precise locations (Cheung 2009; van Hooft 2011). The last trial mentioned 76% obstruction in rectosigmoid and 24% obstruction in descending colon (van Hooft 2008). Another important factor affecting the technical success of colonic stent insertion is the length of obstruction. Only one trial mentioned the range of the stricture length (3‐7 cms) (Sankararajah 2005). The method of insertion of colonic stent might affect the technical success rates. Four of five trials have reported the method of colorectal stenting (Fiori 2004Cheung 2009; van Hooft 2008; van Hooft 2011) while one trial did not describe the method of colorectal stenting (Sankararajah 2005).
Although placement of a colonic stent is much less invasive and seems more appealing, there are a few possible inherent complications associated with this procedure. These include stent migration, stent stenosis, reocclusion or reabsorption and bowel perforation. The rate of perforation of the bowel is around 4% while stent migration and stent reabsorption rates are 10% each (Khot 2002). In the recent pooled analysis of 2287 patients, Dayte reported an overall perforation rate of 4.9% with no statistical difference between the use of stent as a bridge to surgery or palliation (Datye 2011). The majority of bowel perforations (>80%) occurred within 30 days of stent placement (Datye 2011). In another review, covering 54 studies, the bowel perforation rate was 3.76% while stent migration and re obstruction rates were 11.81% and 7.34% respectively (Sebastian 2004). In this review, the stent related perforation rate of 5.88%. However, there was no stent related perforation in three trials (Cheung 2009; Fiori 2004; Sankararajah 2005), but it was 54.55% and 12.77% in two trials respectively (van Hooft 2008; van Hooft 2011). The stent migration rate was 2.13%. The stent obstruction rate was 2.13%. Apart from endoscopists' or radiologists' experience, these complications do vary with different varieties of stents. In this review, four trials documented the type of stent used in their series: Precision stent system (Fiori 2004), Wallstent enteral endoprosthesis (Cheung 2009; van Hooft 2008) and enteral Wallstent and Wallflex colonic stents (van Hooft 2011). But there were no separate data available for comparison of these stents. In a retrospective study of self expanding metallic stents, stent dysfunction, stent related complications and the need for re intervention were reported to be higher in Enteral Wallstents compared to Precision colonic Ultraflex stents when used as a palliative option for the left sided colonic malignant obstruction (Small 2008). The newly designed double layered combination covered stent has not been shown to be superior to prevent stent migration in comparison to D weave uncovered stent in a prospective multicenter study (Moon 2010).
There are very limited data on the survival rates following stent placement and emergency surgery due to the nature of the disease (Tilney 2007). One would expect to raise doubt about the oncological pathogenesis and spread of the disease following colonic stent insertion. There is some data suggesting no adverse effects of colonic stents in the spread of cancer in the short term but seems to adversely affect the 5 year overall survival and 5 year disease free survival (Kim 2009). I found only one trial in this review that mentioned the median survival rate (Sankararajah 2005). The median survival was 23 months in the colonic stent group compared to 19 months in the emergency surgery group. In the same trial the reported estimated one year survival rate was 4.86 in the colonic stent group and was 5.7 in the emergency surgery group. Regarding mortality, one would expect higher mortality rate with emergency surgery, however in this review we found no statistically significant difference in the mortality rate between the two groups Figure 4. The 30 day mortality rate was similar, 2.3% in both groups. Although there are many non‐randomised studies favouring the colonic stents compared to emergency surgery, none of the randomised trials in this review showed any statistical difference in mortality. However, in a prospective non‐randomised trial, mortality associated with the emergency surgery group has been reported to be three times the mortality associated with the colonic stent group (Martinez‐Santos 2002). Similar findings have been reported in other non randomised studies and meta analysis (Ng 2006; Saida 2003; Targownik 2004; Tilney 2007).
In this review, there was no statistically significant difference in the overall complication rate among two groups. The complication rate was 39.22% in the colonic stent group and 45.71% in the emergency surgery group. This finding is contradictory to other non‐randomised studies that have shown much lower complication rate with colonic stents (Martinez‐Santos 2002; Ng 2006; Saida 2003; Targownik 2004; Tilney 2007). The major wound complication rate was 5.63% in the colonic stent group and was 12% in the emergency surgery group and this difference failed to reach statistical significance. Similar findings has been reported in different non‐randomised trials (Martinez‐Santos 2002; Ng 2006; Saida 2003; Targownik 2004; Tilney 2007). The higher complication rate with emergency surgery can be expected by the more invasive nature of the procedure along with existing co‐morbidities but this has not been shown in this review. Another important factor contributing to the morbidity and mortality is the duration of symptoms. Longer duration of symptoms is associated with lack of nutrition and thus contribute to higher mortality and morbidity. However, of five trials, only one trial has reported the mean symptom duration {5 days(1‐14 days)} (Sankararajah 2005).
The other secondary outcomes measured were hospital stay, procedure time and blood loss. In this review, the overall hospital stay, procedure time and blood loss were less in the colonic stent group compared to the emergency surgery group. These findings were similar to the findings reported in various non randomised studies (Martinez‐Santos 2002; Ng 2006; Saida 2003; Targownik 2004; Tilney 2007).The mean hospital stay was 11.53 days in the colonic stent group and 17.15 days in the emergency surgery group. The mean procedure/operating time was 113.93 minutes in the colonic stent group compared to 143.85 minutes in the emergency surgery group. The median blood loss was 50 ml in the colonic stent group and 350 ml in the emergency surgery group. Thus indirectly the cost effectiveness was favoured in the colonic stent group however the cost of colonic stents needs to be considered as well. Two decision analysis and one retrospective study reported the use of a colonic stent to be more cost effective (Targownik 2004; Singh 2006; Osman 2000).
Only one of the included trials studied the quality of life and patient comfort (van Hooft 2011) and they did not find any difference in both groups. Only one trial measured the immediate postoperative pain score and analgesia requirements (Cheung 2009). They reported lower pain score and less requirements of analgesia in the post operative period in the colonic stent followed by the laparoscopic surgery group. The other important issue is the stage of malignant disease on presentation. In only one trial the stage of the malignancy was reported for both groups separately (Cheung 2009). In another trial, authors reported the stage of disease cumulative for both groups (Sankararajah 2005). In two trials, the patients were selected after the diagnosis of advanced unresectable disease (Fiori 2004; van Hooft 2008).
Summary of main results
There was statistically significant difference in the clinical success rate between the colonic stent group and the emergency surgery group, favouring emergency surgery group. The technical success rate was comparable to other non randomised studies. The mortality, overall complications and major wound complication rates were comparable in both groups. The stent related complications are comparable to other non‐randomised studies and reviews. The hospital stay, procedure time and blood loss were less in the colonic stent group compared to the emergency surgery group.
Overall completeness and applicability of evidence
This review includes a meta‐analysis and summary from five randomised controlled trials. These studies had similar intervention groups although measured primary outcomes were different. The primary outcome was documented in four of the five trials (Cheung 2009; Fiori 2004; van Hooft 2008; van Hooft 2011), while all the secondary outcomes were measured in one or more trials. The sample size was variable, with a minimum number of participants in each group, being nine (Sankararajah 2005) and a maximum number of participants in each group, being 51 (van Hooft 2011). Considering the number of trials, along with the variable sample size and trial design, the applicability of evidence is limited. Further trials with bigger sample size and robust trial design are required to improve the level of evidence.
Quality of the evidence
In regards to the quality of trials, none of the trials was at high risk of bias. There were three trials with high quality with low risk of bias (Cheung 2009van Hooft 2008; van Hooft 2011) (Figure 1; Figure 2). Regarding adequate sequence generation and other bias factors, the other two trials were unclear (Fiori 2004; Sankararajah 2005) (Figure 1; Figure 2). One of these two trials was unclear in allocation concealment as well (Sankararajah 2005).
Potential biases in the review process
It is unlikely that I have missed any randomised controlled studies that address the role of colonic stent as a bridge or as palliation against emergency surgery. The characteristics of one excluded study has been documented in "Characteristics of the excluded studies" section. This trial was excluded due to lack of available separate data for malignant colorectal obstruction despite multiple attempts to contact the authors (Xinopoulos 2004).
Bias may have been introduced because single author has reviewed all the studies as a sole author. However, it was made sure to avoid any personal bias effect while writing this review.
Agreements and disagreements with other studies or reviews
Using the search strategy, We could not find any other systematic review which has reported meta‐analysis from the randomised controlled trials. However, a number of studies ( case series, case analysis, retrospective studies, non randomised prospective studies) comparing the colorectal stents against the emergency surgery were found. The majority of other studies agreed that the colorectal stents are as effective if not better compared to the emergency surgery considering the primary outcome of this review (Martinez‐Santos 2002; Ng 2006; Targownik 2004; Tilney 2007; Sebastian 2004), but in this review, we found statistically significant difference in the clinical relief of colonic obstruction favouring emergency surgery group. Regarding the secondary outcomes, most studies favoured the colonic stent compared to the emergency surgery (Martinez‐Santos 2002; Ng 2006; Sebastian 2004; Targownik 2004; Tilney 2007; Saida 2003). In this review, the only two outcomes which contradict other studies are the 30‐day mortality and overall complication rate. I could not find it to be statistically significant in this systematic review; however, in other studies, the 30‐day mortality has been documented up to three times more in the emergency surgery group compared to the colonic stent group (Martinez‐Santos 2002; Saida 2003; Targownik 2004; Tilney 2007). Similarly, overall complication rate has been reported to be high in the emergency surgery compared to colonic stenting. These discrepancies might be reflected due to the small and variable sample size of this review.
Authors' conclusions
Implications for practice.
Colorectal stenting has no advantages to Emergency surgery in malignant colorectal obstructions. Emergency surgery appears to have high clinical success rate compared to colorectal stenting. The stent related complications are acceptable. Colorectal stenting has the advantage of shorter hospital stay and procedure time and less blood loss with comparable mortality and morbidity to emergency surgery. However, further randomised trials with a large sample size and robust trial design are needed to improve the level of evidence on the use of colorectal stents.
Malignant colonic obstruction is a critical condition and relief by colorectal stents requires dedicated specialised units with endoscopic/radiological facilities.
Colorectal stent insertion should only be performed by experienced endoscopists or radiologists with adequate interventional experience.
Implications for research.
Further randomised clinical trials with larger sample size and well defined trial design are needed to compare the colonic stent versus emergency surgery.
Further randomised clinical trials are needed to compare the colonic stent as a palliative procedure versus emergency surgery.
Further randomised clinical trials are needed to compare the colonic stent as a bridge to definitive surgery versus emergency surgery or primary definitive surgical procedure.
Further randomised clinical trials are needed to compare the different varieties of colonic stents.
More trials on the management of malignant colonic obstruction need to adapt blinded assessments of outcome measures.
Trials need to be conducted and reported according to the CONSORT Statement (www.consort‐statement.org).
Acknowledgements
I would like to thank Dr. H K Andersen for his support in this review. I would also like to thank the Cochrane Colorectal Cancer Group for their support in search strategy and statistical analysis.
I would like to thank Prof. M Winslet for his encouragement to deal with the intricacies of this review and his initial contribution in this review.
I also would like to thank Mr. K Gurusamy, editor of the Cochrane hepatobiliary group for his valuable encouragement and advice.
At last but not least, I would like to thank Sue Stubbings for her valuable contribution to correct the language of this manuscript.
Appendices
Appendix 1. Search Strategies
The Cochrane Library :
#1 MeSH descriptor Colonic Pseudo‐Obstruction explode all trees 6
#2 (large bowel obstruction*) or (colonic obstruction*) or (colonic pseudo‐obstruction) or (ACPO) or (Ogilvie syndrome) 206
#3 (#1 OR #2) 206
#4 (colorectal stent*) or (colon stent*) 31
#5 MeSH descriptor Stents explode all trees 1856
#6 (#4 OR #5) 1871
#7 (#3 AND #6) 17
EMBASE (Webspirs 5.1, Silver Platter version 2.0)
#25 #7 and #24 43
#24 #19 not #23 1822036
#23 #21 not #22 2789434
#22 #20 and #21 521969
#21 (ANIMAL or NONHUMAN) in DER 3311403
#20 HUMAN in DER 6212409
#19 #16 or #17 or #18 2899291
#18 (SINGL* or DOUBL* or TREBL* or TRIPL*) near ((BLIND* or MASK*) in TI,AB) 90780
#17 (RANDOM* or CROSS?OVER* or FACTORIAL* or PLACEBO* or VOLUNTEER*) in TI,AB 506486
#16 #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 2693779
#15 "SINGLE‐BLIND‐PROCEDURE"/ all subheadings 7374
#14 "DOUBLE‐BLIND‐PROCEDURE"/ all subheadings 68332
#13 "PHASE‐4‐CLINICAL‐TRIAL"/ all subheadings 640
#12 "PHASE‐3‐CLINICAL‐TRIAL"/ all subheadings 7961
#11 "MULTICENTER‐STUDY"/ all subheadings 42285
#10 "CONTROLLED‐STUDY"/ all subheadings 2660891
#9 "RANDOMIZATION"/ all subheadings 25244
#8 "RANDOMIZED‐CONTROLLED‐TRIAL"/ all subheadings 155222
#7 #3 and #6 138
#6 #4 or #5 35019
#5 explode "stent‐" / all SUBHEADINGS in DEM,DER,DRM,DRR 35018
#4 (colorectal stent*) or (colon stent*) 47
#3 #1 or #2 1030
#2 explode "Ogilvie‐syndrome" / all SUBHEADINGS in DEM,DER,DRM,DRR 288
#1 (large bowel obstruction*) or (colonic obstruction*) or (colonic pseudo‐obstruction) or (ACPO) or (Ogilvie syndrome) 1030
MEDLINE (Webspirs 5.1, Silver Platter version 2.0)
#18 #7 and #17 6
#17 #15 and #16 527089
#16 tg=humans 10059983
#15 #8 or #9 or #10 or #11 or #12 or #13 or #14 606142
#14 trial in ti 74607
#13 randomly in AB 131362
#12 "Clinical‐Trials‐as‐Topic" / all SUBHEADINGS in MIME,MJME,PT 134901
#11 placebo in AB 108580
#10 randomized in AB 173544
#9 controlled‐clinical‐trial in pt 76495
#8 randomized‐controlled‐trial in pt 250518
#7 #3 and #6 134
#6 #4 or #5 29826
#5 explode "Stents‐" / all SUBHEADINGS in MIME,MJME,PT 29820
#4 (colorectal stent*) or (colon stent*) 43
#3 #1 or #2 1443
#2 explode "Colonic‐Pseudo‐Obstruction" / all SUBHEADINGS in MIME,MJME,PT 474
#1 (large bowel obstruction*) or (colonic obstruction*) or (colonic pseudo‐obstruction) or (ACPO) or (Ogilvie syndrome) 1443
Data and analyses
Comparison 1. Clinical Relief ‐ Colonic stent Vs Emergency surgery.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical Relief of Obstruction ‐ Numbers | 3 | 168 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.01, 0.32] |
| 2 Clinical Relief of Obstruction ‐ Time | Other data | No numeric data |
1.1. Analysis.

Comparison 1 Clinical Relief ‐ Colonic stent Vs Emergency surgery, Outcome 1 Clinical Relief of Obstruction ‐ Numbers.
1.2. Analysis.
Comparison 1 Clinical Relief ‐ Colonic stent Vs Emergency surgery, Outcome 2 Clinical Relief of Obstruction ‐ Time.
| Clinical Relief of Obstruction ‐ Time | ||
|---|---|---|
| Study | Colonic Stent | Emergency Surgery |
| Cheung 2009 | 1 day | Not mentioned |
| Fiori 2004 | 1 day | 3.1 days |
| van Hooft 2008 | 0 day | 4 days |
Comparison 2. Technical Success ‐ Colonic Stent.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Technical Success Rates ‐ Colonic Stent | Other data | No numeric data |
2.1. Analysis.
Comparison 2 Technical Success ‐ Colonic Stent, Outcome 1 Technical Success Rates ‐ Colonic Stent.
| Technical Success Rates ‐ Colonic Stent | ||
|---|---|---|
| Study | Successful Placement | Attempted Placement |
| Cheung 2009 | 20 | 24 |
| Fiori 2004 | 11 | 11 |
| Sankararajah 2005 | 7 | 9 |
| van Hooft 2008 | 9 | 11 |
| van Hooft 2011 | 33 | 47 |
Comparison 3. Other Outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 30 Day Mortality | 5 | 207 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.48, 4.14] |
| 2 Stent Related Perforation | Other data | No numeric data | ||
| 3 Complications | 5 | 207 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.47, 1.34] |
| 4 Major Wound Complication | 2 | 146 | Odds Ratio (M‐H, Random, 95% CI) | 0.54 [0.05, 6.16] |
| 5 Stent Migration | Other data | No numeric data | ||
| 6 Hospital Stay | Other data | No numeric data | ||
| 7 Procedure/Operating Time | Other data | No numeric data | ||
| 8 Blood Loss | Other data | No numeric data | ||
| 9 Stent Obstruction/Blockage | Other data | No numeric data |
3.1. Analysis.

Comparison 3 Other Outcomes, Outcome 1 30 Day Mortality.
3.2. Analysis.
Comparison 3 Other Outcomes, Outcome 2 Stent Related Perforation.
| Stent Related Perforation | ||
|---|---|---|
| Study | Stent Procedure Related Perforation (Clinical) | Total Attempted stents |
| Cheung 2009 | 0 | 24 |
| Fiori 2004 | 0 | 11 |
| Sankararajah 2005 | 0 | 9 |
| van Hooft 2008 | 6 | 11 |
| van Hooft 2011 | 6 | 47 |
3.3. Analysis.

Comparison 3 Other Outcomes, Outcome 3 Complications.
3.4. Analysis.

Comparison 3 Other Outcomes, Outcome 4 Major Wound Complication.
3.5. Analysis.
Comparison 3 Other Outcomes, Outcome 5 Stent Migration.
| Stent Migration | ||
|---|---|---|
| Study | Stent Migration | Total Successful Stents Inserted |
| Cheung 2009 | 0 | 20 |
| Fiori 2004 | 0 | 11 |
| Sankararajah 2005 | 1 | 7 |
| van Hooft 2008 | 1 | 9 |
| van Hooft 2011 | 0 | 47 |
3.6. Analysis.
Comparison 3 Other Outcomes, Outcome 6 Hospital Stay.
| Hospital Stay | ||
|---|---|---|
| Study | Colonic Stent | Emergency Surgery |
| Cheung 2009 | 13.5 Days (Median) | 14 Days (Median) |
| Fiori 2004 | 2.6 Days (Median) | 8.6 Days (Median) |
| Sankararajah 2005 | 18 Days (Mean) | 35 Days (Mean) |
| van Hooft 2008 | 12 Day (Median) | 11 Days (Median) |
3.7. Analysis.
Comparison 3 Other Outcomes, Outcome 7 Procedure/Operating Time.
| Procedure/Operating Time | ||
|---|---|---|
| Study | Colonic Stent | Emergency Surgery |
| Cheung 2009 | 130 Minutes (Median) | 170 Minutes (Median) |
| Fiori 2004 | 36.8 Minutes | 75.4 Minutes |
| Sankararajah 2005 | 175 Minutes | 208 Minutes |
| van Hooft 2008 | 122 Minutes | |
3.8. Analysis.
Comparison 3 Other Outcomes, Outcome 8 Blood Loss.
| Blood Loss | ||
|---|---|---|
| Study | Colonic Stent | Emergency Surgery |
| Cheung 2009 | 50 mls (Median) | 200 mls (Median) |
| van Hooft 2008 | <500 mls | |
3.9. Analysis.
Comparison 3 Other Outcomes, Outcome 9 Stent Obstruction/Blockage.
| Stent Obstruction/Blockage | ||
|---|---|---|
| Study | Blocked stents | Successful stents |
| Cheung 2009 | 0 | 20 |
| Fiori 2004 | 0 | 11 |
| Sankararajah 2005 | 0 | 7 |
| van Hooft 2008 | 2 | 9 |
| van Hooft 2011 | 0 | 47 |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cheung 2009.
| Methods | Randomised controlled trial ‐ unicentre | |
| Participants | 48 ( 24 randomised to stent followed by laparoscopic surgery, 24 randomised to emergency open surgery) (26 males and 22 females) | |
| Interventions | Intervention: stent placement followed by laparoscopic surgery, Control: Emergency open surgery | |
| Outcomes | Primary outcome ‐ successful one stage operation | |
| User defined 1 | ||
| Notes | Other outcomes measured include operative time, blood loss, conversion rate, postoperative pain score and analgesia requirements, hospital stay, operative mortality, complication rates, permanent stoma rate and number of harvested lymph nodes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Fiori 2004.
| Methods | Randomised controlled trial ‐ unicentre | |
| Participants | 22 (11 randomised to stent insertion and 11 randomised to elective surgery) (13 males and 9 females) ‐ diagnosed with malignant rectosigmoid obstruction as presentation of advanced unresectable stage | |
| Interventions | Intervention: Stent placement, Control: Right transverse loop colostomy | |
| Outcomes | Technical and clinical success rates, mortality and complications rates, operation time and hospital stay | |
| User defined 1 | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Author did not mention about allocation generation and we did not get any response when tried to contact the authors |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | Source of funding not reported |
Sankararajah 2005.
| Methods | Randomised controlled trial ‐ unicentre | |
| Participants | 19 (9 randomised to stent, 10 randomised for emergency surgery ‐ one refused treatment ‐ not included in analysis) (11 male and 8 females) | |
| Interventions | Intervention: Stent placement, Control: emergency surgery | |
| Outcomes | Techinical and clinical success rates, mortality and complication rates, operation time, hospital stay, overall survival and one year estimated survival | |
| User defined 1 | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Author did not mention about allocation generation and we did not get any response when tried to contact the authors |
| Allocation concealment (selection bias) | Unclear risk | Author did not mention about allocation concealment and we did not get any response when tried to contact the authors |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | Souce of funding not reported |
van Hooft 2008.
| Methods | Randomised controlled trial ‐ Multicentre | |
| Participants | 21 ( 11 randomised to stent, 10 randomised to emergency surgery) (11 male and 10 females) | |
| Interventions | Intervention: Stent placement Control: Emergency Surgery | |
| Outcomes | Hospital free in good health,effectiveness of palliation, quality of life, adverse events, costs, morbidity and mortality | |
| User defined 1 | ||
| Notes | Trial was closed early due to adverse events in intervention arm | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
van Hooft 2011.
| Methods | Randomised controlled trial ‐ Multicentre | |
| Participants | 98 (47 randomised to stent, 51 randomised to emergency surgery) (51 males and 47 females) | |
| Interventions | Intervention: Stent placement, Control: Emergency surgery | |
| Outcomes | Quality of life, morbidity and mortality | |
| User defined 1 | ||
| Notes | Trial closed early due to adverse events in intervention arm | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Xinopoulos 2004 | No separate data available for malignant colorectal obstruction from the obstruction secondary to ovarian cancer |
Contributions of authors
J Sagar wrote the protocol, and is the lead author of the protocol and the review. He extracted data including the methodological quality of the trials, performed the meta‐analyses, and drafted the review.
Declarations of interest
No potential conflict of interest
New
References
References to studies included in this review
Cheung 2009 {published data only}
- Cheung HY, Chung CC, Tsang WW, Wong JC, Yau KK, Li MK. Endolaparoscopic approach vs conventional open surgery in the treatment of obstructing left‐sided colon cancer: a randomized controlled trial.. Arch Surg 2009;144(12):1127‐1132. [DOI] [PubMed] [Google Scholar]
Fiori 2004 {published data only}
- Fiori E, Lamazza A, Volpino P, Burza A, Paparelli C, Cavallaro G, Schillaci A, Cangemi V. Palliative management of malignant antro‐pyloric strictures. Gastroenterostomy vs. endoscopic stenting. A randomized prospective trial.. Anticancer Research 2004;24:265‐268. [PubMed] [Google Scholar]
Sankararajah 2005 {published data only}
- D Sankararajah, MJ Forshaw, MC Parker. Multicentre prospective randomised controlledtrial of pre‐operative endoluminal stenting vs.surgery in large bowel obstruction – interimanalysis of short term outcomes. Colorectal Disease 2005;7(Supple 1):45 ‐ 143 (29). [Google Scholar]
van Hooft 2008 {published data only}
- Hooft JE, Fockens P, Marinelli AW, Timmer R, Berkel AM, Bossuyt PM, Bemelman WA, Dutch Colorectal Stent Group. Early closure of a multicenter randomized clinical trial of endoscopic stenting versus surgery for stage IV left‐sided colorectal cancer.. Endoscopy 2008;40:184‐191. [DOI] [PubMed] [Google Scholar]
van Hooft 2011 {published data only}
- Hooft JE, Bemelman WA, Oldenburg B, Marinelli AW, Holzik MF, Grubben MJ, Sprangers MA, Dijkgraaf MG, Fockens P, collaborative Dutch Stent‐In study group. Colonic stenting versus emergency surgery for acute left‐sided malignant colonic obstruction: a multicentre randomised trial.. Lancet Oncol 2011;12(4):344‐352. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Xinopoulos 2004 {published data only}
- Xinopoulos D, Dimitroulopoulos D, Theodosopoulos T, Tsamakidis K, Bitsakou G, Plataniotis G, Gontikakis M, Kontis M, Paraskevas I, Vassilobpoulos P, Paraskevas E. Stenting or stoma creation for patients with inoperable malignant colonic obstructions? Results of a study and cost‐effectiveness analysis.. Surg Endosc 2004;18:421‐426. [DOI] [PubMed] [Google Scholar]
Additional references
Datye 2011
- Datye A, Hersh J. Colonic perforation after stent placement for malignant colorectal obstruction : causes and contributing factors.. Minim Invasive Ther Allied Technol 2011;20(3):133‐140. [DOI] [PubMed] [Google Scholar]
Deans 1994
- Deans GT, Krukowski ZH, Irwin ST. Malignant obstruction of the left colon. Br J Surg 1994;81(9):1270‐7. [DOI] [PubMed] [Google Scholar]
Demets 1987
- Demets DL. Methods for combining randomized clinical trials: strengths and limitations. Stat Med 1987;6(3):341‐50. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Dohmoto 1991
- Dohmoto M. New Method: endoscopic implantation of rectal stent in palliative treatment of malignant stenosis. Endosc Dig 1991;3:1507‐12. [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008]. www.cochrane‐handbook.org, Feb 2008. [Google Scholar]
Hollis 1999
- Hollis S, Campbell F. What is meant by intention to treat analysis? Survey of published randomised controlled trials. BMJ 1999;319(7211):670‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khot 2002
- Khot UP, Lang AW, Murali K, Parker MC. Systematic review of the efficacy and safety of colorectal stents. Br J Surg 2002;89(9):1096‐102. [DOI] [PubMed] [Google Scholar]
Kim 2009
- Kim JS, Hur H, Min BS, Sohn SK, Cho CH, Kim NK. Oncologic outcomes of self‐expanding metallic stent insertion as a bridge to surgery in the management of left‐sided colon cancer obstruction: comparison with nonobstructing elective surgery.. World J Surg 2009 June;33(6):1281‐6. [DOI] [PubMed] [Google Scholar]
Martinez‐Santos 2002
- Martinez‐Santos C, Lobato RF, Fradejas JM, Pinto I, Ortega‐Deballón P, Moreno‐Azcoita M. Self‐expandable stent before elective surgery vs. emergency surgery for the treatment of malignant colorectal obstructions: comparison of primary anastomosis and morbidity rates.. Dis Colon Rectum 2002 March;45(3):401‐6. [DOI] [PubMed] [Google Scholar]
Moon 2010
- Moon CM, Kim TI, Lee MS, Ko BM, Kim HS, Lee KM, Byeon JS, Kim YS. Comparison of a newly designed double‐layered combination covered stent and D‐weave uncovered stent for decompression of obstructive colorectal cancer: a prospective multicenter study.. Dis Colon Rectum 2010 August;53(8):1190‐6. [DOI] [PubMed] [Google Scholar]
Ng 2006
- Ng KC, Law WL, Lee YM, Choi HK, Seto CL, Ho JW. Self‐expanding metallic stent as a bridge to surgery versus emergency resection for obstructing left‐sided colorectal cancer: a case‐matched study. J Gastrointest Surg 2006;10:798‐803. [DOI] [PubMed] [Google Scholar]
Osman 2000
- Osman HS, Rashid HI, Sathananthan N, Parker MC. The cost effectiveness of self‐expanding metal stents in the management of malignant left‐sided large bowel obstruction.. Colorectal Dis 2000;2:233‐7. [DOI] [PubMed] [Google Scholar]
Rault 2005
- Rault A, Collet D, Sa Cunha A, Larroude D, Ndobo'epoy F, Masson B. Surgical management of obstructed colonic cancer. Ann Chir. 2005 June;130(5):331‐5. [DOI] [PubMed] [Google Scholar]
Saida 2003
- Saida Y, Sumiyama Y, Nagao J, Uramatsu M. Long‐term prognosis of preoperative ?bridge to surgery? expandable metallic stent insertion for obstructive colorectal cancer: comparison with emergency operation. Dis Colon Rectum 2003;46:44‐9. [DOI] [PubMed] [Google Scholar]
Sebastian 2004
- Sebastian S, Johnston S, Geoghegan T, Torreggiani W, Buckley M. Pooled analysis of the efficacy and safety of self‐expanding metal stenting in malignant colorectal obstruction. Am J Gastroenterol. 2005 May;100(5):1203‐4. [DOI] [PubMed] [Google Scholar]
Singh 2006
- Singh H, Latosinsky S, Spiegel BM, Targownik LE. The cost‐effectiveness of colonic stenting as a bridge to curative surgery in patients with acute left‐sided malignant colonic obstruction: a Canadian perspective. Can J Gastroenterol 2006;20:779‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Small 2008
- Small AJ, Baroon TH. Comparison of Wallstent and Ultraflex stents for palliation of malignant left‐sided colon obstruction: a retrospective, case‐matched analysis.. Gastrointest Endosc. 2008 March;67(3):478‐88. [DOI] [PubMed] [Google Scholar]
Syn 2005
- Syn WK, Patel M, Ahmed MM. Metallic stents in large bowel obstruction: experience in a District General Hospital. Colorectal Dis. 2005 Jan;7(1):22‐6. [DOI] [PubMed] [Google Scholar]
Targownik 2004
Tejero 1994
- Tejero E, Mainar A, Fernandez L, Tobio R, Gregorio MA. New procedure for the treatment of colorectal neoplastic obstruction. Dis Colon Rectum 1994;37(11):1158‐59. [DOI] [PubMed] [Google Scholar]
Tilney 2007
- Tilney HS, Lovegrove RE, Purkayastha S, Sains PS, Weston‐Petrides GK, Darzi AW, Tekkis PP, Heriot AG. Comparison of colonic stenting and open surgery for malignant large bowel obstruction. Surg Endosc 2007;21:225‐33. [DOI] [PubMed] [Google Scholar]


