Abstract
Background
Cannulation techniques have been recognized to be important in causing post‐endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis (PEP). However, considerable controversy exists about the usefulness of the guidewire‐assisted cannulation technique for the prevention of PEP.
Objectives
To systematically review evidence from randomised controlled trials (RCTs) assessing the effectiveness and safety of the guidewire‐assisted cannulation technique compared to the conventional contrast‐assisted cannulation technique for the prevention of PEP.
Search methods
We searched CENTRAL (The Cochrane Library), MEDLINE, EMBASE, and CINAHL databases and major conference proceedings, up to February 2012, using the Cochrane Upper Gastrointestinal and Pancreatic Diseases model with no language restrictions.
Selection criteria
RCTs comparing the guidewire‐assisted cannulation technique versus the contrast‐assisted cannulation technique in patients undergoing ERCP.
Data collection and analysis
Two review authors conducted study selection, data extraction and methodological quality assessment independently. Using intention‐to‐treat analysis with random‐effects models, we combined dichotomous data to obtain risk ratios (RR) with 95% confidence intervals (CI). We assessed heterogeneity using the Chi² test (P < 0.15) and I² statistic (> 25%). To explore sources of heterogeneity, we conducted a priori subgroup analyses according to trial design, publication type, risk of bias, use of precut sphincterotomy, inadvertent guidewire insertion or contrast injection of the pancreatic duct (PD), use of a PD stent, cannulation device, and trainee involvement in cannulation. To assess the robustness of our results we carried out sensitivity analyses using different summary statistics (RR versus odds ratio (OR)) and meta‐analytic models (fixed‐effect versus random‐effects), and per protocol analysis.
Main results
Twelve RCTs comprising 3450 participants were included. There was statistical heterogeneity among trials for the outcome of PEP (P = 0.04, I² = 45%). The guidewire‐assisted cannulation technique significantly reduced PEP compared to the contrast‐assisted cannulation technique (RR 0.51, 95% CI 0.32 to 0.82). In addition, the guidewire‐assisted cannulation technique was associated with greater primary cannulation success (RR 1.07, 95% CI 1.00 to 1.15), less precut sphincterotomy (RR 0.75, 95% CI 0.60 to 0.95), and no increase in other ERCP‐related complications. Subgroup analyses indicated that this significant risk reduction in PEP with the guidewire‐assisted cannulation technique existed only in 'non‐crossover' trials (RR 0.22, 95% CI 0.12 to 0.42). The results were robust in sensitivity analyses.
Authors' conclusions
Compared with the contrast‐assisted cannulation technique, the guidewire‐assisted cannulation technique increases the primary cannulation rate and reduces the risk of PEP, and it appears to be the most appropriate first‐line cannulation technique.
Keywords: Humans; Common Bile Duct; Catheterization; Catheterization/adverse effects; Catheterization/instrumentation; Catheterization/methods; Cholangiopancreatography, Endoscopic Retrograde; Cholangiopancreatography, Endoscopic Retrograde/adverse effects; Cholangiopancreatography, Endoscopic Retrograde/instrumentation; Cholangiopancreatography, Endoscopic Retrograde/methods; Contrast Media; Pancreatitis; Pancreatitis/etiology; Pancreatitis/prevention & control; Randomized Controlled Trials as Topic; Safety
Techniques for gaining access to the bile duct for the prevention of post‐procedure pancreatitis
Endoscopic retrograde cholangiopancreatography (ERCP) combines endoscopy and x‐ray to diagnose and treat problems of the bile and pancreatic ducts. With the patient under sedation, an endoscope is passed down the oesophagus, through the stomach, and into the duodenum where the opening of the bile and pancreatic ducts (papilla) is located. A catheter is then inserted through the endoscope and through the papilla into the bile duct. Contrast dye is then injected into the bile duct and x‐rays are taken to look for gallstones or blockage. However, the major risk of ERCP is the development of pancreatitis due to irritation of the pancreatic duct by the contrast material or catheter, which can occur in 5% to 10% of all procedures. This may be self‐limited and mild, but it can also be severe and require hospitalisation. Rarely, it may be life threatening. There are additional small risks of bleeding or making a hole in the bowel wall.
In general, there are two techniques for gaining access to the bile duct during ERCP. The traditional technique involves inserting a catheter directly into the papilla and injecting contrast dye to confirm access to the bile duct. However, contrast dye may be unintentionally injected into the pancreatic duct. A second technique involves the use of a guidewire to probe the papilla to gain access to the bile duct. Once the guidewire is confirmed to be in the bile duct on x‐ray, contrast dye is injected into the bile duct. There has been much debate as to which technique is better for the prevention of post‐procedure pancreatitis.
This review compared the effect of the two techniques for gaining access to the bile duct in patients undergoing ERCP. Twelve studies, with a total of 3450 patients, were reviewed and provide the best available evidence. The use of a guidewire to gain access to the bile duct reduced the risk of post‐procedure pancreatitis and increased the success rate of gaining access to the bile duct compared to the traditional technique involving injection of contrast dye with a catheter.
Summary of findings
Summary of findings for the main comparison.
Guidewire‐assisted cannulation compared to contrast‐assisted cannulation, main analysis for the prevention of post‐endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis
| Guidewire‐assisted cannulation compared to contrast‐assisted cannulation, main analysis for the prevention of post‐endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis | ||||||
| Patient or population: patients undergoing diagnostic or therapeutic ERCP Settings: hospital Intervention: Guidewire‐assisted cannulation Comparison: Contrast‐assisted cannulation, Main analysis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Contrast‐assisted cannulation, Main analysis | Guidewire‐assisted cannulation | |||||
| Post‐ERCP pancreatitis (ITT) | 67 per 1000 | 34 per 1000 (22 to 55) | RR 0.51 (0.32 to 0.82) | 3450 (12 studies) | ⊕⊕⊕⊝ moderate1,2 | NNT was 31 (95% CI 19 to 78) |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Most information is obtained from studies with high risk of bias for blinding of participants and personnel (the endoscopists). Inability to blind the endoscopist may have an impact on cannulation success and the rates of PEP depending on the preference and the expertise of the endoscopist performing the procedure. The quality of evidence is downgraded because of risk of bias. 2 There is significant heterogeneity among studies mostly due to study design. However, the heterogeneity could be explained by trial design. Therefore, the quality of evidence is not downgraded for inconsistency / heterogeneity.
Background
Description of the condition
Endoscopic retrograde cholangiopancreatography (ERCP) is a commonly performed endoscopic procedure that has both diagnostic and therapeutic roles in various hepatobiliary and pancreatic disorders. Despite its potential benefits, ERCP is not without risks. Acute pancreatitis is one of the most common serious complications of ERCP (Cotton 1991). The incidence of post‐ERCP pancreatitis (PEP) varies between 5% and 10%, although it may exceed 25% in certain high‐risk patient populations (Freeman 2004a). While most PEP manifests as minor illness with two to three days of additional hospitalisation and expected full recovery, severe pancreatitis is a devastating illness with significant morbidity, such as pancreatic necrosis and multi‐organ failure, and mortality. Severe pancreatitis has been reported to occur in 0.1% to 0.5% of ERCPs in prospective series (Freeman 2004a).
The pathophysiologic mechanisms of PEP are likely to be multifactorial and are incompletely understood (Freeman 2004a; Pezzilli 2002). These may include:
mechanical injury to the papilla and pancreatic duct (PD) due to instrumental manipulation, resulting in obstruction or impairment of pancreatic flow;
chemical injury due to contrast injection into the PD;
hydrostatic injury due to contrast injection into the PD;
thermal injury due to the electrosurgical current used for biliary or pancreatic sphincterotomy;
enzymatic injury from introduction of activated proteolytic enzymes into the PD;
microbiological injury due to contamination or instillation of intestinal flora or bacteria into the PD.
Considerable efforts have been made to identify risk factors for PEP. Multivariate analyses of prospective studies have found a number of patient‐related risk factors for PEP, including young age, female gender, sphincter of Oddi dysfunction (SOD), recurrent pancreatitis and a history of PEP (Cheng 2006; Freeman 2001). Procedure‐related risk factors include difficult cannulation, multiple injections of the PD, precut sphincterotomy, pancreatic sphincterotomy and biliary sphincter balloon dilation (Cheng 2006; Freeman 2001). Operator‐related risk factors such as the endoscopist's expertise, case volume, and trainee involvement in the procedure have been considered to be potential factors that can influence the outcome of ERCP. Indeed, low case volumes have been found to be associated with higher ERCP failure and complication rates (Freeman 1996; Loperfido 1998). However, large prospective studies have provided conflicting evidence as to whether any of these operator‐related risk factors increases the risk of PEP (Cheng 2006; Colton 2009; Freeman 1996; Freeman 2001; Loperfido 1998; Testoni 2010; Vandervoort 2002; Wang 2009; Williams 2007b). This is likely to be due to the fact that any difference in the rates of PEP between low‐ and high‐volume centres or endoscopists is often blunted by a disparity in case mix. In contrast, trainee participation has been shown to be a significant risk factor for the development of PEP (Cheng 2006). This increased risk is possibly due to multiple cannulation attempts by trainees.
In clinical practice, as recommended by current guidelines (Banks 2006; Forsmark 2007; UK guidelines 2005), acute pancreatitis is diagnosed by the presence of two of the following three features:
abdominal pain typical of acute pancreatitis;
greater than or equal to three‐fold elevation in amylase or lipase;
computed tomography (CT) evidence of pancreatitis.
However, much controversy remains about the definition of PEP. In an attempt to establish reliable criteria for defining PEP, a consensus definition was developed in 1991 based on data collected from more than 15,000 procedures (Cotton 1991). PEP was defined as a rise in serum amylase levels to greater than or equal to three‐fold above the upper limit of normal, 24 hours after ERCP, accompanied by abdominal pain characteristic of pancreatitis and requiring an unplanned hospital stay or an extension of a planned hospital stay by at least two days (Cotton 1991). The severity of PEP (mild, moderate, severe) was graded according to the length of stay and local or systemic complications related to pancreatitis. However, this consensus definition (Cotton 1991) has not been adopted widely, and varying definitions of PEP have been used in clinical trials. This is likely to reflect the ongoing controversy in defining PEP in the context of post‐ERCP complications. The consensus definition (Cotton 1991) for PEP has not been updated since 1991 and is arguably distinct from that used in clinical practice for diagnosing acute pancreatitis. Furthermore, neither the consensus definition (Cotton 1991) nor the clinical definition has been shown to reliably diagnose PEP. This is due to the fact that asymptomatic transient elevations in amylase or lipase levels, or both, are often seen post‐ERCP (up to 70%) (Conn 1991; Skude 1976; Testoni 1999). Asymptomatic hyperamylasaemia with levels more than five times the upper limit of normal and lasting for 24 hours after ERCP has been reported in about 27% of cases (Testoni 1999). Moreover, serum lipase is now considered to be more sensitive and specific than serum amylase in the diagnosis of acute pancreatitis (Yadav 2002). In addition, abdominal pain post‐procedure could be due to a multitude of factors other than PEP (for example air insufflation). The duration of pain is, therefore, essential for defining PEP because pain that subsides within 24 hours is unlikely to indicate pancreatitis. Moreover, mild pain disappearing within 24 to 48 hours and not requiring analgesics or prolonged hospital stay still does not fulfil the criteria for clinical pancreatitis. Taken together, these two common findings post‐ERCP (pain and elevation in amylase) may lead to over‐diagnosis of PEP. Because of the lack of specificity of pain and hyperamylasaemia after ERCP, computed tomography (CT) has been proposed as the most appropriate method to confirm the diagnosis of PEP (Badalov 2009; Kiriyama 2010). To add to the controversy, the need for diagnostic criteria for PEP distinct from those used for pancreatitis has been challenged by a recent study suggesting that the consensus definition (Cotton 1991) may under‐diagnose PEP (Artifon 2010). On the other hand, the clinical definition may over‐diagnose PEP without having any significant impact on clinical management or patient outcomes.
Description of the intervention
ERCP involves passage of a side‐viewing endoscope into the duodenum and cannulation of the common bile duct (CBD) with a device (sphincterotome or catheter). Contrast can then be injected in a retrograde manner into the CBD. Selective deep cannulation of the CBD is a prerequisite to successful diagnostic and therapeutic ERCP.
Contrast‐assisted cannulation
Conventional contrast‐assisted cannulation of the CBD is the direct injection of contrast through a catheter or a sphincterotome into the papilla under fluoroscopy (Freeman 2005). With this technique, a catheter or a sphincterotome is first aligned with the CBD and advanced into the papilla. Contrast is then injected to determine if the CBD has been entered. Upon visualization of the CBD, more contrast can be injected for optimal opacification and the catheter or the sphincterotome is then advanced further into the CBD for deep cannulation. If contrast is noted to fill the PD, the catheter or sphincterotome is then withdrawn and reoriented to the direction of the CBD and the above steps repeated until the CBD is accessed. However, inadvertent contrast injection of the PD or the papilla itself (submucosal injection), as well as repeated cannulation attempts, may increase the risk of PEP (Cheng 2006; Freeman 2001).
Guidewire‐assisted cannulation
Guidewires were initially designed and utilized to maintain access to the CBD during therapeutic manoeuvers such as stent placement and stone extraction. Increasingly, guidewires are used to facilitate selective deep cannulation of the CBD. With the guidewire‐assisted cannulation technique, a guidewire is used to confirm selective cannulation of the CBD before contrast injection. If the guidewire inadvertently enters the PD, the guidewire is withdrawn into the catheter or the sphincterotome and attempts repeated to enter the CBD. Once the guidewire is noted to enter the CBD, the catheter or the sphincterotome can be advanced deeper into the CBD and contrast is injected for optimal opacification. It has been postulated that the guidewire‐assisted cannulation technique may improve biliary cannulation success and prevent PEP by avoiding papillary trauma and inadvertent contrast injection of the PD or the papilla itself. In general, there are two variations of the guidewire‐assisted cannulation technique (Freeman 2005):
a guidewire is extended slightly beyond the catheter or the sphincterotome and is advanced in small increments under fluoroscopy to probe and gain access to the CBD;
the tip of the catheter or the sphincterotome is first inserted into the papilla and oriented to the direction of the CBD followed by advancement of the guidewire to probe and gain access to the CBD.
Achieving deep cannulation of the CBD can be difficult. Success depends primarily on the skill and experience of the endoscopist but also on anatomical variations and underlying conditions. Even among experienced endoscopists, failure of biliary cannulation may occur in up to 10% to 20% of cases (Varadarajulu 2006; Williams 2007a). When access by conventional methods fails a precut sphincterotomy, by means of an incision into or just above the papilla, is often employed as a last resort to achieve CBD cannulation (Freeman 2005; Siegel 1989). Use of precut sphincterotomy has been reported to be associated with an increased risk of complications including PEP, bleeding and perforation (Cennamo 2010; Freeman 2001; Masci 2003). However, it remains controversial as to whether the increased risk is due to the precut itself or to the prolonged attempts at cannulation. In high risk patients, the placement of a prophylactic PD stent after ERCP has been shown to reduce the risk of PEP (Choudhary 2011; Mazaki 2010). However, PD stents can be technically difficult to place even for the most experienced endoscopists, with reported failure in up to 10% of cases (Freeman 2007). In high risk patients, PD manipulation followed by failure to place a PD stent may be associated with a higher risk of PEP than no attempt at all (Freeman 2004b). There is also a potential for inducing pancreatic ductal injury (Kozarek 1990).
How the intervention might work
Cannulation techniques have long been recognized to be important in causing PEP (Freeman 2001; Freeman 2004a). Mechanical injury to the papilla and PD from repeated cannulation attempts may lead to edema and obstruction of pancreatic ductal flow. In addition, inadvertent injection of contrast into the PD may lead to both chemical and hydrostatic injuries of the pancreas. These factors are thought to play an important role in the development of PEP with conventional contrast‐assisted cannulation of the CBD using a catheter or a sphincterotome. It has been postulated that the guidewire‐assisted cannulation technique may improve biliary cannulation success and prevent PEP by avoiding papillary trauma and inadvertent contrast injection of the PD or the papilla itself (submucosal injection). The rationale for more successful CBD cannulation with the guidewire‐assisted technique is that a small‐diameter guidewire with a hydrophilic tip can pass more easily through the small opening of the bile duct than a larger‐diameter catheter or sphincterotome. There are, however, potential concerns with the guidewire‐assisted cannulation technique including false passage, intramural dissection, perforation and PD injury (Freeman 2005).
Why it is important to do this review
Prevention of PEP has been the 'Holy Grail' of ERCP. Investigators have long searched for a pharmacologic agent that will prevent PEP, but nearly all agents evaluated (with the exception of rectal non‐steroidal anti‐inflammatory drugs) have failed to demonstrate efficacy in randomised controlled trials or logistic feasibility in real‐life settings (Elmunzer 2012; Testoni 2006). Similarly, numerous endoscopic interventions have been studied for the prevention of PEP (Freeman 2004a). The findings of these studies have often provided conflicting results due to different study designs, definitions of outcomes, patient populations and interventions used. In particular, considerable controversy remains about the usefulness of the guidewire‐assisted cannulation technique compared to the conventional contrast‐assisted cannulation technique for the prevention of PEP. A comprehensive meta‐analysis of the efficacy and safety of the guidewire‐assisted cannulation technique will allow us to make recommendations for clinical practice and research. This systematic review is part of a series of reviews examining endoscopic interventions for the prevention of PEP.
PEP is the most common serious complication of ERCP and carries significant morbidity and mortality. The cannulation technique is believed to be pivotal in the pathogenesis of PEP. We conducted this systematic review to evaluate the relative merits of the two different cannulation techniques for the prevention of PEP. The findings of this review are relevant to patients, physicians and to healthcare systems.
Objectives
We aimed to assess the clinical effectiveness of the guidewire‐assisted cannulation technique compared to the conventional contrast‐assisted cannulation technique for cannulation of the CBD in the prevention of PEP by systematic review and meta‐analysis of randomised controlled trials (RCTs).
The objectives of this review were two‐fold, to:
assess whether the guidewire‐assisted cannulation technique shows any overall benefit in reducing adverse clinical outcomes including PEP and other ERCP‐related complications (bleeding, cholangitis, perforation, mortality) compared to the contrast‐assisted cannulation technique;
assess whether the technical success of selective CBD cannulation (cannulation success) can be improved by the guidewire‐assisted cannulation technique compared to the contrast‐assisted cannulation technique.
Methods
Criteria for considering studies for this review
Types of studies
RCTs comparing the guidewire‐assisted cannulation technique versus the contrast‐assisted cannulation technique in patients undergoing diagnostic or therapeutic ERCP for biliary or pancreatic diseases. Trials that permitted other concomitant therapies were eligible as long as the therapies were administered to both the intervention and the control arms. We did not include trials that employed non‐random methods of allocation, such as judgment of the clinician or preference of the participant, results of a laboratory test or series of tests, or availability of the intervention, as the allocation was not truly random. We considered published and unpublished studies, full articles and abstracts for inclusion in this review.
Types of participants
Trials were eligible for inclusion in the review if they recruited men and women aged at least 18 years who were scheduled to undergo diagnostic or therapeutic ERCP for biliary or pancreatic diseases.
Types of interventions
Guidewire‐assisted cannulation technique compared with contrast‐assisted cannulation technique for cannulation of the CBD using a catheter or a sphincterotome.
Types of outcome measures
Primary outcomes
The primary outcome measure was post‐ERCP pancreatitis (PEP), as defined by the primary studies. If different definitions of PEP were provided by the same study, the consensus definition (Cotton 1991) was used for assessment of this outcome.
Secondary outcomes
The secondary outcome measures were as follows.
Severity of PEP, as defined by the primary studies. If different definitions of severity of PEP were provided by the same study, the consensus criteria (Cotton 1991) were used for assessment of this outcome.
Primary CBD cannulation success with the randomised technique.
Secondary CBD cannulation success after technique 'cross‐over', as defined by cannulation success with the 'cross‐over' technique (in trials that allowed technique 'cross‐over' after failed attempts with the randomised technique).
Overall CBD cannulation success.
Precut sphincterotomy.
Inadvertent guidewire cannulation or contrast injection of the pancreatic duct (PD) (inadvertent PD manipulation).
Post‐sphincterotomy bleeding.
Post‐ERCP cholangitis.
Perforation.
Mortality.
Search methods for identification of studies
The search strategies were constructed by using a combination of subject headings and text words relating to ERCP and acute pancreatitis. We applied the standard Cochrane search strategy filter for identifying RCTs to all searches. See also the Cochrane Upper Gastrointestinal and Pancreatic Diseases Group search strategy.
Electronic searches
We conducted a comprehensive literature search to identify all published and unpublished RCTs, with no language restriction. We searched the following electronic databases to identify potential studies:
the Cochrane Central Register of Controlled Trials (CENTRAL) (Appendix 1);
MEDLINE (1946 to February 2012) (Appendix 2);
EMBASE (1974 to February 2012) (Appendix 3); and
CINAHL (1982 to February 2012) (Appendix 4).
Searching other resources
Two review authors (YY, FT) handsearched the published abstracts from the conference proceedings in Digestive Disease Week (published in Gastroenterology and Gastrointestinal Endoscopy) and United European Gastroenterology Week (published in Gut) from 2004 to 2011. We handsearched references cites in studies found by the above search to identify further relevant trials.
Data collection and analysis
Selection of studies
Two review authors (YY, FT) independently screened titles and trial abstracts that were identified by the search strategy for potential inclusion in the review using predefined inclusion and exclusion criteria. We resolved differences by discussion and consensus. The same two review authors (YY, FT) retrieved and reviewed the complete reports of all selected articles. We contacted authors of trial reports if they were published only as abstracts or if additional data were required for analyses. In the case of duplicate publications, we retained only the most comprehensive report.
Data extraction and management
Two independent review authors (YY, FT) recorded the following study and patient characteristics:
setting (single or multi‐centre);
country of origin;
enrolment period;
year of publication, format (abstract or full publication);
study design;
inclusion and exclusion criteria used;
indications for ERCP;
types of ERCP performed (diagnostic or therapeutic ERCP);
diagnostic criteria for and severity of PEP;
endoscopists (number, trainee involvement);
number of patients assigned per intervention;
patient demographics and characteristics including gender, mean age, co‐morbidities, sphincter of Oddi (SOD), previous history of PEP or recurrent pancreatitis, difficult cannulation with definitions, or prior endoscopic sphincterotomy;
endoscopic interventions evaluated;
specific endoscopic interventions (types of guidewire, sphincterotome, catheter; electrosurgical generator and current used for sphincterotomy; use of PD stent; use of precut sphincterotomy; therapeutic interventions including stone extraction, stent placement, balloon dilatation of sphincter, SOD manometry);
pharmacological prophylaxis for PEP;
outcomes (PEP, severity of PEP, primary CBD cannulation success with the randomised technique, secondary CBD cannulation success after technique 'cross‐over', overall CBD cannulation success, precut, inadvertent guidewire cannulation or contrast injection of the PD, and other ERCP‐related complications including bleeding, cholangitis, perforation and mortality);
drop outs or loss to follow‐up; and
study quality (generation of allocation sequence, allocation concealment, blinding, incomplete outcome data, selective reporting, other bias).
Studies were summarized and, if appropriate, meta‐analysis was undertaken.
Assessment of risk of bias in included studies
Two review authors (YY, FT) independently assessed the methodological quality of the included studies based on the Cochrane Handbook for Systematic Reviews of Interventions. We assessed each included study regarding sequence generation, allocation sequence concealment, blinding, incomplete outcome data, selective outcome reporting and other potential sources of bias. We resolved disagreements by discussion and consensus.
Random sequence generation
Low risk, if the allocation sequence was generated by a computer or a random number table.
Unclear, if the trial was described as randomised, but the method used for generation of the allocation sequence was not described.
High risk, if a system involving dates, names or hospital record numbers was used for the allocation of patients.
Allocation concealment
Low risk, if the allocation of patients involved central allocation or sequentially numbered, opaque, sealed envelopes.
Unclear, if there is insufficient information to permit judgment of 'low risk' or 'high risk'.
High risk, if the allocation was based on using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes without appropriate safeguards, alternation or rotation; date of birth; case record number; or any other explicitly unconcealed procedure.
Blinding of participants and personnel (post‐ERCP pancreatitis)
Low risk, blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken.
Unclear risk, insufficient information to permit judgment of 'low risk' or 'high risk'.
High risk, no blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of study participants and personnel attempted but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding.
Blinding of outcome assessment (post‐ERCP pancreatitis)
Low risk, blinding of outcome assessment ensured, and unlikely that the blinding could have been broken.
Unclear risk, insufficient information to permit judgment of "low risk" or "high risk".
High risk, no blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding.
Incomplete outcome data
Low risk, if no missing outcome data; reasons for missing outcome data unlikely to be related to true outcome; missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; missing data have been imputed using appropriate methods.
Unclear, if insufficient reporting of attrition or exclusions to permit judgment of ‘low risk’ or ‘high risk’ (e.g. number randomised not stated, no reasons for missing data provided).
High risk, if reasons for missing outcome data are likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; the proportion of missing outcomes compared with observed event risk is enough to introduce clinically relevant bias in intervention effect estimate; per protocol analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation.
Selective reporting
Low risk, if the published reports include all expected outcomes, including those that were prespecified.
Unclear, if insufficient information to permit judgment of 'low risk' or 'high risk'.
High risk, if not all of the study’s prespecified primary outcomes have been reported; if one or more primary outcome is reported using measurements, analysis methods or subsets of the data that were not prespecified; one or more of the reported primary outcomes were not prespecified; one or more outcomes of interest were reported incompletely; or the study report failed to include results for a key outcome that would be expected to have been reported for such a study.
Measures of treatment effect
Primary outcome
The primary outcome was PEP. We expected dichotomous data for PEP and we expressed this as risk ratio (RR) with 95% confidence interval (CI). We defined RR as the risk of PEP in the guidewire‐assisted cannulation technique compared to the contrast‐assisted cannulation technique.
Secondary outcomes
We expressed dichotomous outcomes for severity of PEP, cannulation success (primary, secondary, overall), precut sphincterotomy, inadvertent guidewire cannulation or contrast injection of the PD, post‐ERCP complications (bleeding, cholangitis, perforation, mortality) as RR with 95% CI.
Unit of analysis issues
Trials that permitted technique 'cross‐over', in which patients were allowed to receive the alternative endoscopic technique if the randomised technique failed, were included in this review. However, these 'cross‐over' trials are at risk for contamination due to carry‐over effects in the subgroup of patients who received the alternative technique after failing the assigned technique. Therefore, we also performed subgroup analysis according to trial design (permission of technique 'cross‐over' versus non‐permission of technique 'cross‐over').
Dealing with missing data
We contacted authors for any data missing from the included studies. We performed analyses on an intention‐to‐treat (ITT) basis, with inclusion of data from all patients randomised whenever possible. Otherwise, we adopted the 'available‐case' analysis. We assumed there should not be any missing data with respect to cannulation success as this outcome is assessed during the procedure and is not dependent on follow‐up of patients. We assumed most patients with PEP would require admission to the hospital for treatment. Therefore, any missing data with respect to PEP is unlikely to be related to the actual outcome itself ('missing at random'). We did not assume a 'worse‐case scenario' (PEP) for the patients who were lost to follow‐up because the event rates for PEP were low and this assumption may be unrealistic.
Assessment of heterogeneity
We assessed heterogeneity using the Chi² test (P < 0.15, significant heterogeneity) and I² statistic (> 25%, heterogeneity) using a random‐effects model along with visual inspection of forest plots. When significant heterogeneity was found, possible explanations were investigated by subgroup and sensitivity analyses to test the robustness of the overall results. The potential sources of heterogeneity, hypothesized a priori, were the following.
Trial design (permission for technique 'cross‐over' versus non‐permission of technique 'cross‐over').
Precut sphincterotomy (yes versus no versus unclear).
Use of PD stent (yes versus no versus unclear).
Cannulation device (sphincterotome versus catheter).
Involvement of trainees in cannulation (yes versus no versus unclear).
Publication type (abstract versus full text).
Risk of bias (high versus low versus unclear).
Assessment of reporting biases
This review was designed to include published and unpublished studies, with no language restriction. We assessed publication bias visually by examining the relationship between the treatment effects and the standard error of the estimate using a funnel plot.
Data synthesis
We conducted a meta‐analysis for the comparison of the guidewire‐assisted cannulation technique and the contrast‐assisted cannulation technique for cannulation of the CBD. We performed meta‐analysis only if two or more trials with similar comparisons and outcome measures were found. Where appropriate, we combined data using a random‐effects model (the Mantel‐Haenszel method) to determine a summary estimate of the RR and 95% CI. We calculated the RR of the incidence of PEP as the primary outcome. We calculated the RRs of other dichotomous secondary outcomes including severity of PEP, primary CBD cannulation success, secondary CBD cannulation success, overall CBD cannulation success, precut sphincterotomy, inadvertent guidewire cannulation or contrast injection of the PD (inadvertent PD manipulation), post‐sphincterotomy bleeding, post‐ERCP cholangitis, perforation, and mortality. Number needed to treat (NNT) with CI were obtained from the risk difference (1/RD). We used the Cochrane Review Manager 5 software (RevMan 2011) to carry out the analysis based on the ITT principle. We presented results on forest plots, using a random‐effects model.
Subgroup analysis and investigation of heterogeneity
We decided to perform the following subgroup analyses for the incidence of PEP a priori.
Risk of bias (high or unclear versus low).
Publication type (abstract versus full text).
Trial design (permission for technique 'cross‐over' versus non‐permission of technique 'cross‐over'). In technique 'cross‐over' trials, patients were permitted to receive the alternative endoscopic technique if the randomised technique failed. These 'cross‐over' trials are at risk for contamination due to carry‐over effects in the subgroup of patients who received the alternative technique after failing the assigned technique.
Among all trials and within trials that did not permit technique 'cross‐over' ('non‐crossover' trials) but provided data for the following variables, further subgroup analyses for the incidence of PEP were performed:
precut sphincterotomy (yes versus no versus unclear);
inadvertent guidewire insertion or contrast injection into the PD (inadvertent PD manipulation) (yes versus no);
use of PD stent (yes versus no versus unclear);
cannulation device (sphincterotome versus catheter);
involvement of trainees in cannulation (yes versus no versus unclear).
Among all trials and within trials that did not permit technique 'cross‐over' ('non‐crossover' trials) but provided data for the following variables, further subgroup analyses for primary cannulation success were performed:
cannulation device (sphincterotome versus catheter);
involvement of trainees in cannulation (yes versus no versus unclear).
We performed tests for subgroup differences based on the fixed‐effect model inverse‐variance method (implemented in RevMan 5) for the above outcomes, with P < 0.05 considered statistically significant.
Sensitivity analysis
Sensitivity analyses were as follows:
ITT versus per protocol (PP) analysis;
Summary statistic (risk ratio versus odds ratio); and
meta‐analysis modelling (fixed‐effect versus random‐effects).
Results
Description of studies
See: Characteristics of included studies and Characteristics of excluded studies.
Results of the search
The search strategy used for CENTRAL, MEDLINE, EMBASE, and CINAHL identified 3413 articles (Figure 1). A recursive search of the reference lists of these articles and the handsearching of conference proceedings from Digestive Disease Week (published in Gastroenterology and Gastrointestinal Endoscopy) and United European Gastroenterology Week (published in Gut) (from 2004 to 2011) identified 26 further articles. After reviewing the abstracts of the above articles we excluded 3045 articles as they were clearly not relevant. We retrieved the full articles for the remaining 42 trials. Of these, 30 did not meet the eligibility criteria and were excluded for the following reasons: non‐randomised trial design (Bailey 2006b; Ito 2010; Kamata 2011; Lee 2004; Mariani 2012; Nakai 2011; Trifan 2011), inappropriate interventions (Angsuwatcharakon 2010; Angsuwatcharakon 2012; Balderas 2011; Cha 2011; Cote 2010; de Tejada 2007; de Tejada 2009; Ito 2008; Maeda 2003; Zheng 2010), meta‐analyses (Cennamo 2009; Cheung 2009; Choudhary 2009; Choudhary 2010a; Choudhary 2010b; Epstein 2009; Madhoun 2009; Shao 2009), and preliminary or duplicate data (Artifon 2005; Bailey 2006a; Bailey 2006c; Nambu 2009; Park 2008).
Figure 1.
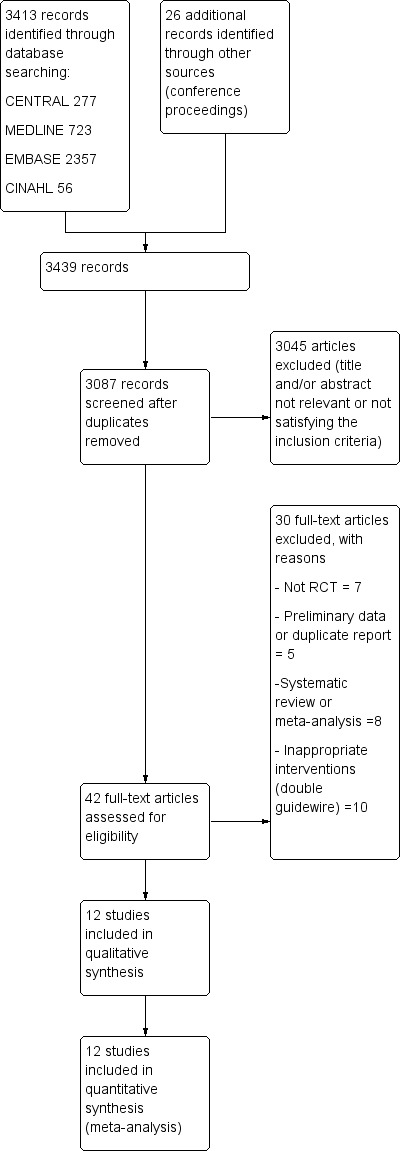
Study flow diagram.
Twelve RCTs (Apostolopoulos 2005; Artifon 2007; Bailey 2008; Gruchy 2007; Katsinelos 2008; Kawakami 2012; Kobayashi 2010; Lee 2009; Lella 2004; Mangiavillano 2007; Mangiavillano 2011; Nambu 2011) comprising 3450 participants were included. A detailed summary of all included and excluded studies can be found in Characteristics of included studies and Characteristics of excluded studies.
Included studies
Design
All 12 included studies were RCTs. Of these, five were 'non‐crossover' studies which did not report the use of the alternative technique when the randomised technique failed (Apostolopoulos 2005; Artifon 2007; Lee 2009; Lella 2004; Mangiavillano 2007), two of these were in abstract format (Apostolopoulos 2005; Mangiavillano 2007). Seven were 'cross‐over' studies which allowed patients to receive the alternative endoscopic technique when the randomised technique failed due to difficult cannulation (Bailey 2008; Gruchy 2007; Katsinelos 2008; Kawakami 2012; Kobayashi 2010; Mangiavillano 2011; Nambu 2011), three of which were in abstract format (Gruchy 2007; Kobayashi 2010; Mangiavillano 2011). One study did not report the permission of technique 'cross‐over' in the conference proceeding (Gruchy 2007). However, authors of the primary study (Gruchy 2007) were contacted and confirmed the use of technique 'cross‐over'. One 'cross‐over' study (Kawakami 2012) used a 2 x 2 factorial design and randomised patients to four intervention groups according to cannulation device (sphincterotome or catheter) and cannulation method (guidewire‐assisted or contrast‐assisted).
The criteria used to define difficult cannulation were highly variable among studies. Among the 'non‐crossover' studies, difficult cannulation was defined by a time limit of 20 minutes in one study (Apostolopoulos 2005) or greater than 10 unsuccessful cannulation attempts in another study (Artifon 2007) prior to the use of precut sphincterotomy as a rescue technique. One 'non‐crossover' study defined difficult cannulation as after a time limit of 10 minutes or five unintentional PD cannulation or two contrast injections into the PD (Lee 2009). Two 'non‐crossover' studies (Lella 2004; Mangiavillano 2007) did not define difficult cannulation. Most 'cross‐over' studies defined difficult cannulation by a time limit of 10 minutes (Bailey 2008; Katsinelos 2008; Kawakami 2012; Nambu 2011). One study allowed 'cross‐over' after a time limit of five minutes or five unsuccessful attempts, PD cannulation or three contrast injections into the PD (Mangiavillano 2011). One study allowed 'cross‐over' after three cannulation attempts (Gruchy 2007). In one 'cross‐over' study (Kawakami 2012) the subsequent cannulation techniques used to achieve selective biliary cannulation were left to the discretion of the endoscopists (including 'cross‐over' to the alternative technique and the use of precut sphincterotomy) after failure to achieve cannulation within 10 minutes. One 'cross‐over' study (Kobayashi 2010) did not define difficult cannulation.
Trainees were allowed to start cannulation in five studies (Bailey 2008; Gruchy 2007; Kawakami 2012; Kobayashi 2010; Nambu 2011). If cannulation was unsuccessful after a predefined cannulation time limit (five minutes in Bailey 2008, Kawakami 2012 and Nambu 2011; unclear in Kobayashi 2010 and Gruchy 2007), the experienced endoscopists took over the procedure. In other studies (Apostolopoulos 2005; Artifon 2007; Katsinelos 2008; Lee 2009; Lella 2004), experienced endoscopists performed all procedures. Two studies (Mangiavillano 2007; Mangiavillano 2011) did not provide information as to whether trainees were involved in cannulation. In one study (Apostolopoulos 2005) trainees manipulated the guidewire during cannulation.
Sample sizes
The number of participants per trial ranged from 88 (Mangiavillano 2011) to 430 (Bailey 2008). One study (Apostolopoulos 2005) excluded from the analysis any randomised participants who received precut sphincterotomy (N = 7). In one study (Bailey 2008), 17 participants were excluded after randomisation because of the presence of unsuspected prior sphincterotomy or surgically altered anatomy. In one study (Nambu 2011), two cases of bilio‐duodenal fistula were excluded from the analysis after randomisation. In one study (Gruchy 2007), participants who received precut sphincterotomy or a PD stent or were lost to follow‐up (N = 93) were excluded from the analysis after randomisation.
According to the ITT principle, we included all randomised participants for the main analyses (N = 3450). We used per protocol sample sizes (N = 3331) in sensitivity analysis.
Setting
Seven of the studies were conducted in a single centre (Apostolopoulos 2005; Bailey 2008; Gruchy 2007; Lee 2009; Lella 2004; Mangiavillano 2007; Nambu 2011). Five were multi‐centre studies (Artifon 2007; Katsinelos 2008; Kawakami 2012; Kobayashi 2010; Mangiavillano 2011). In six studies, the procedures were performed by one or two experienced endoscopists (Apostolopoulos 2005; Artifon 2007; Bailey 2008; Katsinelos 2008; Lee 2009; Lella 2004). In four studies, the procedures were performed by multiple endoscopists at single (Nambu 2011) or multiple centres (Kawakami 2012; Kobayashi 2010; Mangiavillano 2011). Two studies, in abstract format, did not report on who performed the procedures (Gruchy 2007; Mangiavillano 2007).
Participants
The 12 studies that were included in the main analyses comprised a total of 3450 participants (Apostolopoulos 2005; Artifon 2007; Bailey 2008; Gruchy 2007; Katsinelos 2008; Kawakami 2012; Kobayashi 2010; Lee 2009; Lella 2004; Mangiavillano 2007; Mangiavillano 2011; Nambu 2011). Of these, 1784 were randomised to the guidewire‐assisted cannulation technique and 1666 to the contrast‐assisted cannulation technique.
The included studies were heterogeneous in their patient selection criteria. The specific criteria for each study are outlined in the Characteristics of included studies section. In general, studies included participants with intact papilla who required ERCP for pancreaticobiliary diseases. Participants were excluded if they had previous sphincterotomy (Artifon 2007; Bailey 2008; Katsinelos 2008; Kawakami 2012; Lella 2004; Nambu 2011), surgically altered anatomy (Billroth II or Roux‐en‐Y anastomosis) (Artifon 2007; Bailey 2008; Katsinelos 2008; Kawakami 2012; Lee 2009; Lella 2004; Nambu 2011), ampullary neoplasm (Bailey 2008; Katsinelos 2008; Kawakami 2012; Lee 2009; Nambu 2011), pancreatic cancer (Bailey 2008), balloon dilatation of sphincter (Kawakami 2012; Nambu 2011), separate orifices of the CBD and PD (Katsinelos 2008; Kawakami 2012), acute pancreatitis (Artifon 2007; Kawakami 2012; Lee 2009), chronic pancreatitis (Kawakami 2012), impacted CBD stones (Kawakami 2012; Lee 2009), peri‐ampullary diverticulum (Katsinelos 2008) and pancreaticobiliary malunion (long common channel) (Kawakami 2012; Lee 2009; Nambu 2011). Indications for the procedure were provided by all (Apostolopoulos 2005; Artifon 2007, Bailey 2008; Katsinelos 2008; Kawakami 2012; Lee 2009; Lella 2004; Mangiavillano 2011; Nambu 2011) but three studies (Gruchy 2007; Kobayashi 2010; Mangiavillano 2007): CBD stones (64.8%), pancreaticobiliary malignancy (17.9%), SOD dysfunction (2.9%), idiopathic recurrent pancreatitis (1.2%) and other indications (13.2%). In addition, peri‐ampullary diverticulum was reported to be present in 11.4% of cases.
The age range of participants was 18 to 96 years. The mean age of participants was reported by seven studies: 53.4 years (Artifon 2007), 59.4 years (Bailey 2008), 69.0 years (Katsinelos 2008), 63.2 years (Lee 2009), 61.2 years (Lella 2004), 65.8 years (Mangiavillano 2011) and 70.5 years (Nambu 2011). One study (Kawakami 2012) reported a median age of 67.7 years. The gender of the participants was reported by eight studies (Artifon 2007; Bailey 2008; Katsinelos 2008; Kawakami 2012; Lee 2009; Lella 2004; Mangiavillano 2011; Nambu 2011). Overall, there were equal proportions of females and males: 100/200 (Artifon 2007), 251/162 (Bailey 2008), 193/139 (Katsinelos 2008), 147/253 (Kawakami 2012), 145/155 (Lee 2009), 218/182 (Lella 2004), 56/32 (Mangiavillano 2011) and 95/77 (Nambu 2011).
Interventions
See: intervention characteristics of Included studies (Table 11).
Table 1.
Intervention characteristics of included studies
| Study | Endoscopists | Trainees | Cannulation device | Guidewire | Guidewire Technique | Who advanced the guidewire | Cannulation limit | Precut (Yes/No) | PD stents (Yes/No) |
| 'Non‐crossover' trials | |||||||||
| Lella 2004 Single centre |
1 | None | Sphincterotome | 0.035 inch soft tipped Teflon Tracer guidewire (Wilson‐Cook) | Sphincterotome inserted into papilla then guidewire advanced | Endoscopist and radiologist | Unclear | No | No |
| Apostolopoulos 2005 Single centre |
2 | Handled guidewire | Sphincterotome | 0.035 inch Terumo guidewire (Terumo) | Guidewire directly advanced into CBD | Trainees | 20 minutes | Yes | No |
| Artifon 2007 Multi‐centre |
1 | None | Sphincterotome | 0.035 inch soft hydrophilic Teflon tipped guidewire (Boston Scientific) | Sphincterotome inserted into papilla then guidewire advanced | Unclear | 10 attempts | Yes | No |
| Mangiavillano 2007 Single centre |
Unclear | Unclear | Sphincterotome | Soft‐tipped Tracer guidewire | Sphincterotome inserted into papilla then guidewire advanced | Unclear | Unclear | Unclear | Unclear |
| Lee 2009 Single centre |
1 | None | Sphincterotome | 0.035 inch soft hydrophilic tipped Jagwire standard (Boston Scientific) | Sphincterotome inserted into papilla then guidewire advanced | Assistant | 10 minutes or 5 PD cannulations or 2 PD injections | Yes | No |
| 'Cross‐over' trials | |||||||||
| Gruchy 2007 Single centre |
Unclear | Started procedure | Sphincterotome | Hydrophilic guidewire | Unclear | Unclear | 3 attempts | Yes, but excluded from analysis | Yes, but excluded from analysis |
| Bailey 2008 Single centre |
2 | Started procedure | Sphincterotome | 0.035 inch soft hydrophilic tipped Jagwire standard (Boston Scientific) | Guidewire directly advanced into CBD | Assistant | 10 minutes (5 minutes trainee) | Yes | Yes |
| Katsinelos 2008 Multi‐centre |
2 | None | Catheter | 0.035 inch soft hydrophilic tipped Jagwire standard (Boston Scientific) | Guidewire directly advanced into CBD | Assistant or endoscopist | 10 minutes | Yes | Yes |
| Kobayashi 2010 Multi‐centre |
Multiple | Started procedure | Sphincterotome / Catheter | Unclear | Unclear | Unclear | Unclear | Yes | Yes |
| Mangiavillano 2011 Multi‐centre |
Multiple | Unclear | Unclear | Guidewire with a loop in the tip | Unclear | Unclear | 5 minutes or 5 PD cannulations or 3 PD injections | Yes | No |
| Nambu 2011 Single centre |
Multiple | Started procedure | Sphincterotome in the guidewire group and catheter in the contrast group | 0.035 inch soft hydrophilic angle‐ tipped Jagwire guidewire (Boston Scientific) | Guidewire directly advanced into CBD | Assisting endoscopist | 10 minutes (5 minutes trainee) | Yes | No |
| Kawakami 2012 Multi‐centre |
Multiple | Started procedure | Sphincterotome / Catheter | 0.035 inch soft hydrophilic tipped Jagwire standard (Boston Scientific) | Both techniques | Assisting endoscopist | 10 minutes (5 minutes trainee) | Yes | Yes |
PD: pancreatic duct
Guidewire‐assisted cannulation
In the guidewire‐assisted cannulation group, most studies used hydrophilic guidewires (Apostolopoulos 2005; Artifon 2007; Bailey 2008; Gruchy 2007; Katsinelos 2008; Kawakami 2012; Lee 2009; Nambu 2011) or Teflon‐coated guidewires (Lella 2004; Mangiavillano 2007). One study used guidewires with a loop in the tip (Mangiavillano 2011). One study did not report the type of guidewire used (Kobayashi 2010). Only sphincterotomes were used for cannulation in eight studies (Apostolopoulos 2005; Artifon 2007; Bailey 2008; Gruchy 2007; Lee 2009; Lella 2004; Mangiavillano 2007; Nambu 2011). One study used only catheters for cannulation (Katsinelos 2008). Two studies used either sphincterotomes or catheters (Kawakami 2012; Kobayashi 2010) and one study did not report the type of cannulation device used (Mangiavillano 2011). In terms of specific techniques used for guidewire‐assisted cannulation, a guidewire was directly advanced into the CBD in four studies (Apostolopoulos 2005; Bailey 2008; Katsinelos 2008; Nambu 2011). In four other studies, a sphincterotome was first inserted into the papilla followed by advancement of the guidewire into the CBD (Artifon 2007; Lee 2009; Lella 2004; Mangiavillano 2007). One study (Kawakami 2012) reported the use of both techniques. The specific technique used for guidewire‐assisted cannulation was not reported in three studies (Gruchy 2007; Kobayashi 2010; Mangiavillano 2011). It was unclear who advanced the guidewires in five studies (Artifon 2007; Gruchy 2007; Kobayashi 2010; Mangiavillano 2007; Mangiavillano 2011). In other studies, an assistant (Apostolopoulos 2005; Bailey 2008; Katsinelos 2008; Kawakami 2012; Lee 2009; Nambu 2011), a radiologist (Lella 2004), or the endoscopist (Katsinelos 2008; Lella 2004) advanced the guidewires.
Contrast‐assisted cannulation
Contrast‐assisted cannulation was performed with a sphincterotome in seven studies (Apostolopoulos 2005; Artifon 2007; Bailey 2008; Gruchy 2007; Lee 2009; Lella 2004; Mangiavillano 2007), a catheter in two studies (Katsinelos 2008; Nambu 2011), and either a sphincterotome or a catheter in two studies (Kawakami 2012; Kobayashi 2010). In one study, it was unclear what cannulation device was used (Mangiavillano 2011).
Precut sphincterotomy
Precut sphincterotomy was permitted as a rescue technique for difficult cannulation in 10 studies (Apostolopoulos 2005; Artifon 2007; Bailey 2008; Gruchy 2007; Katsinelos 2008; Kawakami 2012; Kobayashi 2010; Lee 2009; Mangiavillano 2011; Nambu 2011). One study (Lella 2004) did not permit the use of precut sphincterotomy. One study did not report the use of precut sphincterotomy (Mangiavillano 2007). The reported techniques for precut sphincterotomy included free‐hand needle knife papillotomy (an incision made starting at the papillary orifice and extending upward towards the direction of the CBD) (Bailey 2008; Katsinelos 2008; Kawakami 2012), fistulotomy (a puncture made above the papillary orifice and extending upward or downward towards the orifice) (Artifon 2007; Katsinelos 2008; Lee 2009) and transpancreatic precut sphincterotomy (inserting the tip of the sphincterotome in the PD and cutting through the septum in the direction of the CBD) (Katsinelos 2008; Kawakami 2012). The precut techniques were not described in five studies (Apostolopoulos 2005; Gruchy 2007; Kobayashi 2010; Mangiavillano 2011; Nambu 2011).
PD stents
Pancreatic duct (PD) stents were used for prophylaxis of PEP in five studies (Bailey 2008; Gruchy 2007; Katsinelos 2008; Kawakami 2012; Kobayashi 2010) in high risk patients including those with SOD (Katsinelos 2008), a history of acute pancreatitis (Katsinelos 2008), moderate to difficult cannulation (Katsinelos 2008), multiple cannulations or injections of the PD (Bailey 2008; Katsinelos 2008) and precut sphincterotomy (Bailey 2008; Katsinelos 2008).
Other aspects of trial design are discussed in Characteristics of included studies and Risk of bias in included studies.
Outcomes
Commonly reported outcomes included post‐ERCP pancreatitis (PEP), overall cannulation success rates and primary cannulation success rates with the randomised technique. Most studies (Apostolopoulos 2005; Bailey 2008; Gruchy 2007; Katsinelos 2008; Kawakami 2012; Kobayashi 2010; Lee 2009; Mangiavillano 2011; Nambu 2011) defined PEP as a rise in serum amylase level to greater than or equal to three‐fold above the upper limit of normal 24 hours after ERCP accompanied by abdominal pain characteristic of pancreatitis, according to the consensus definition (Cotton 1991). There was no mention of procedure‐related hospital stay as part of the criteria for defining the occurrence of PEP in all but one study (Gruchy 2007). However, one study defined PEP as pancreatic‐like pain for at least 24 hours after the procedure associated with serum amylase levels greater than five times the upper limit of normal (Lella 2004). One study (Artifon 2007) defined PEP as abdominal pain 24 hours following ERCP with CT evidence of pancreatitis, but also provided outcome data according to the consensus definition (Colton 2009) and the criteria used by Lella 2004. One study (Mangiavillano 2007), in abstract format, did not specify the criteria for the diagnosis of PEP. See Table 12.
Table 2.
Outcome definitions of included studies
| Study | Definitions of post‐ERCP pancreatitis | Severity Criteria | Incidence of post‐ERCP pancreatitis (%) | ||
| Guidewire‐assisted cannulation technique | Contrast‐assisted cannulation technique | Overall | |||
| 'Non‐crossover' trials | |||||
| Lella 2004 | abdominal pain > 24 h after ERCP and amylase > 5 times the upper limit of normal | Not reported | 0 | 4 | 2.0 |
| Apostolopoulos 2005 | abdominal pain > 24 h after ERCP and amylase > 3 times the upper limit of normal | Ranson"s criteria and Balthazar grading | 1.5 | 9.5 | 5.4 |
| Artifon 2007 | abdominal pain > 24 h after ERCP and CT evidence of pancreatitis | Ranson"s criteria and Balthazar grading | 8.6 | 16.6 | 8.3 |
| abdominal pain > 24 h after ERCP and amylase > 3 times the upper limit of normal | 3.3 | 12.0 | 7.7 | ||
| abdominal pain > 24 h after ERCP and amylase > 5 times the upper limit of normal | 3.3 | 6.7 | 5.0 | ||
| Mangiavillano 2007 | Not reported | Not reported | 2.0 | 6.0 | 4.0 |
| Lee 2009 | abdominal pain > 24 h after ERCP and amylase > 3 times the upper limit of normal | consensus criteria | 2.0 | 11.3 | 5.0 |
| 'Cross‐over' trials | |||||
| Gruchy 2007 | abdominal pain > 24 h after ERCP and amylase > / = 3 times the upper limit of normal requiring hospital admission | Not reported | 1.7 | 4.4 | 2.7 |
| Bailey 2008 | abdominal pain > 24 h after ERCP and amylase > 3 times the upper limit of normal | consensus criteria | 7.4 | 6.0 | 6.7 |
| Katsinelos 2008 | abdominal pain > 24 h after ERCP and amylase > 3 times the upper limit of normal | consensus criteria | 5.4 | 7.9 | 6.6 |
| Kobayashi 2010 | abdominal pain > 24 h after ERCP and amylase > 3 times the upper limit of normal | consensus criteria | 6.1 | 6.3 | 6.2 |
| Mangiavillano 2011 | abdominal pain > 24 h after ERCP and amylase > 3 times the upper limit of normal | Not reported | 4.3 | 9.5 | 6.8 |
| Nambu 2011 | abdominal pain > 24 h after ERCP and amylase > 3 times the upper limit of normal | consensus criteria | 2.3 | 5.8 | 4.1 |
| Kawakami 2012 | abdominal pain > 24 h after ERCP and amylase > 3 times the upper limit of normal | consensus criteria | 4.0 | 3.0 | 3.5 |
h: hours
Severity of PEP was graded using the consensus criteria in six studies (Bailey 2008; Katsinelos 2008; Kawakami 2012; Kobayashi 2010; Lee 2009; Nambu 2011).Two studies (Apostolopoulos 2005; Artifon 2007) graded severity using the Ranson's criteria (Ranson 1974) and the Balthazar grading system (Balthazar 1990). Two studies (Lella 2004; Mangiavillano 2007) graded the severity of pancreatitis as mild, moderate or severe, but did not specify the criteria for severity assessment. Two studies did not provide outcome data regarding the severity of PEP (Gruchy 2007; Mangiavillano 2011). See Table 12.
Overall cannulation success rates were reported by all but one study (Kobayashi 2010). Additional data regarding overall cannulation success rates were obtained from the authors of this primary study (Kobayashi 2010). All except two studies (Gruchy 2007; Mangiavillano 2011) provided outcome data regarding primary cannulation success rate with the randomised technique prior to technique 'cross‐over' or the use of precut sphincterotomy. Among the seven 'cross‐over' studies, secondary cannulation success rates as defined by success rates with the 'cross‐over' technique were reported only by one study (Katsinelos 2008). Additional data regarding secondary cannulation success rates were obtained from the authors of three primary studies (Bailey 2008; Kobayashi 2010; Nambu 2011).
Among the 10 studies that allowed the use of precut sphincterotomy in difficult cannulation, only one study (Artifon 2007) reported subgroup data regarding the rates of PEP between the two cannulation techniques. Additional subgroup data according to precut sphincterotomy were provided by the authors of two primary studies (Apostolopoulos 2005; Lee 2009).
Five studies (Artifon 2007; Kawakami 2012; Lee 2009; Lella 2004; Mangiavillano 2007) reported data regarding the rates of inadvertent guidewire cannulation or contrast injection of the PD (inadvertent PD manipulation) between the two cannulation techniques. One study (Apostolopoulos 2005) reported data regarding the rates of inadvertent contrast injection but not inadvertent guidewire cannulation of the PD. One study (Gruchy 2007) provided data regarding the rates of inadvertent contrast injection only in the guidewire‐assisted cannulation group. Three studies (Bailey 2008; Katsinelos 2008; Nambu 2011) only provided the mean or median number of inadvertent PD cannulations or injections. Additional outcome data regarding inadvertent guidewire cannulation or contrast injection of the PD were obtained from the authors of three primary studies (Bailey 2008; Kobayashi 2010; Nambu 2011).
Difficult and multiple cannulation attempts have been found to be a risk factor for PEP (Cheng 2006; Vandervoort 2002). Three studies reported the mean number of cannulation attempts (Katsinelos 2008; Kawakami 2012; Mangiavillano 2011). Due to the variable criteria used to define difficult cannulation and cannulation attempts (Udd 2010), we decided not to explore the differences in cannulation attempts between the two cannulation techniques.
Post‐ERCP complications including bleeding (Artifon 2007; Gruchy 2007; Katsinelos 2008; Lee 2009; Nambu 2011), perforation (Artifon 2007; Gruchy 2007; Katsinelos 2008; Kawakami 2012; Lee 2009; Nambu 2011) and cholangitis (Apostolopoulos 2005) were reported by seven studies. Mortality was reported by six studies (Apostolopoulos 2005; Artifon 2007; Katsinelos 2008; Lee 2009; Lella 2004; Nambu 2011).
Excluded studies
Thirty studies did not meet the eligibility criteria and were excluded. The main reasons for exclusion included: non‐randomised trial design, inappropriate interventions, meta‐analyses, and preliminary or duplicate data.
See: Characteristics of excluded studies and Results of the search.
Risk of bias in included studies
The methodological quality of the included studies is summarized in Characteristics of included studies and shown in Figure 2 and Figure 3.
Figure 2.
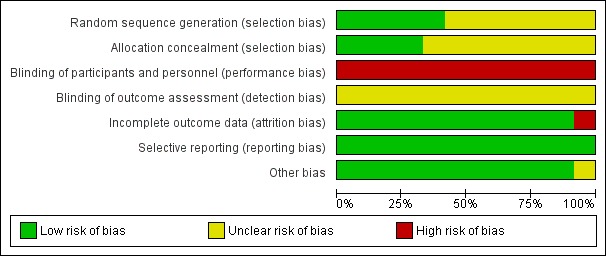
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Figure 3.
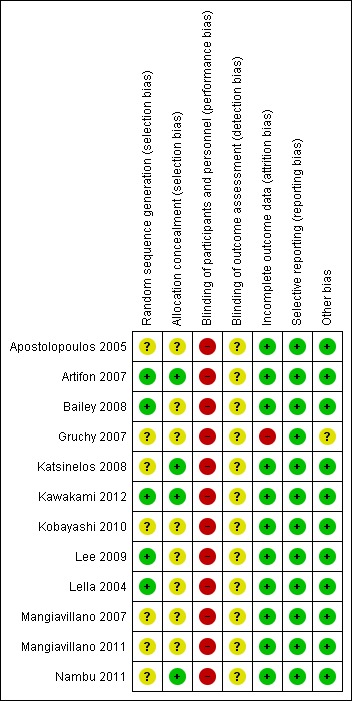
Risk of bias summary: review authors" judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
Five studies were considered to be at low risk of bias for random sequence generation: four studies (Bailey 2008; Kawakami 2012; Lee 2009; Lella 2004) generated the allocation sequence by a computer, and one study (Artifon 2007) provided some information regarding block randomisation. Five studies, in abstract format, were considered to be at unclear risk of bias for random sequence generation as no information was provided regarding the randomisation process (Apostolopoulos 2005; Gruchy 2007; Kobayashi 2010; Mangiavillano 2007; Mangiavillano 2011). The randomisation was done by a research centre (Gruchy 2007) in one study but the intervention groups appeared to be highly unbalanced in terms of numbers. This raised concerns as to whether the method used to generate random sequence was truly random. Two studies, in full text, were also considered to be at unclear risk of bias because they did not adequately describe the randomisation process: "randomisation was prepared by a biostatistician" in one study (Katsinelos 2008), and patients were "divided randomly into two groups" in another study (Nambu 2011).
Allocation concealment
Four studies were considered to be at low risk of bias for allocation concealment: three studies allocated patients by sealed (Artifon 2007; Nambu 2011) or opaque (Katsinelos 2008) envelopes, and one study (Kawakami 2012) involved central allocation. Eight studies (Apostolopoulos 2005; Bailey 2008; Gruchy 2007; Kobayashi 2010; Lee 2009; Lella 2004; Mangiavillano 2007; Mangiavillano 2011) had uncertain concealment.
Blinding
In all trials, the endoscopists performing the procedure could not be blinded. This may have had an impact on cannulation success and the rates of PEP depending on the preference and expertise of the endoscopists performing the procedure. Blinding of patients, health providers, data collectors and outcome assessors should be possible, but may be less important when an outcome can be objectively defined (for example death). In the case of PEP, there was some degree of subjectivity in the interpretation of pancreatic pain. Blinding of these groups was therefore essential for reducing performance and detection bias. Blinding of participants, personnel (other than the endoscopists) and outcome assessors was not reported by any of the included studies. One study (Kawakami 2012) explicitly stated that it was a "non‐double blinded" study, but it was unclear whether it was single‐blinded. One study (Artifon 2007), in full text, stated that it was a "single‐blinded" RCT, but it was unclear who was blinded. Therefore, all studies (Apostolopoulos 2005; Artifon 2007; Bailey 2008; Gruchy 2007; Katsinelos 2008; Kawakami 2012; Kobayashi 2010; Lee 2009; Lella 2004; Mangiavillano 2007; Mangiavillano 2011; Nambu 2011) were considered at high risk of bias for blinding of participants and personnel (the endoscopists), and unclear risk of bias for outcome assessment.
Incomplete outcome data
One study (Gruchy 2007) was considered at high risk of bias for incomplete outcome data as 93 patients (25%) were lost to follow‐up. This study (Gruchy 2007) also excluded from the analysis any randomised participants who received precut sphincterotomy or PD stents. As the treatment groups were highly unbalanced in numbers (Gruchy 2007), additional patients may have been excluded after randomisation. The other studies either had no losses to follow‐up (Apostolopoulos 2005; Artifon 2007; Katsinelos 2008; Kawakami 2012; Kobayashi 2010; Lee 2009; Lella 2004; Mangiavillano 2007; Mangiavillano 2011) or described withdrawals and drop outs in detail (Bailey 2008; Nambu 2011) and were considered low risk of bias for incomplete outcome data. One study (Apostolopoulos 2005) excluded from the analysis any randomised participants who received precut sphincterotomy due to difficult cannulation. Additional outcome data of these patients were provided by the authors of the primary study (Apostolopoulos 2005). Another study (Kobayashi 2010) reported inconsistent PEP rates between three conference abstracts published in the same year. Authors of the primary study (Kobayashi 2010) were contacted and provided the final PEP rates of the two intervention groups.
Selective reporting
All studies reported all important outcomes and were therefore considered at low risk of bias for selective reporting (Apostolopoulos 2005; Artifon 2007; Bailey 2008; Gruchy 2007; Katsinelos 2008; Kawakami 2012; Kobayashi 2010; Lee 2009; Lella 2004; Mangiavillano 2007; Mangiavillano 2011; Nambu 2011).
Other potential sources of bias
Unbalanced prognostic factors between groups
In the study by Artifon et al (Artifon 2007), there were more women in the guidewire‐assisted cannulation group than in the contrast‐assisted cannulation group (39.3% versus 27.3%). However, the difference between the two groups was likely to be due to chance since both the random sequence generation and allocation concealment were considered at low risk of bias for this study (Artifon 2007). Furthermore, despite this potential bias against the guidewire‐assisted cannulation group, the PEP rate was found to be lower in the guidewire‐assisted cannulation group than in the contrast‐assisted cannulation group.
Differential diagnostic activity
Increased diagnostic activity can potentially lead to biased outcome assessments. The results were particularly susceptible to detection bias when the patients and the outcome assessors were not blinded and the assessment of outcomes was based on rather subjective criteria (pancreatic pain). In the study by Artifon et al (Artifon 2007), all patients were admitted for overnight observation after ERCP. As a result, patients were more likely to undergo laboratory and radiological evaluation of abdominal pain as opposed to being discharged home following ERCP. Two studies explicitly stated that patients were discharged from the endoscopy unit (Bailey 2008) or within 24 hours after ERCP (Lella 2004). Other studies (Apostolopoulos 2005; Gruchy 2007; Katsinelos 2008; Kawakami 2012; Kobayashi 2010; Lee 2009; Mangiavillano 2007; Mangiavillano 2011; Nambu 2011) did not report on the disposition of patients after the procedure.
Interim analysis
One study (Gruchy 2007), in abstract format, stated that the results were based on an "interim analysis of an ongoing trial". However, there was no mention of a fixed time horizon for the final analysis, and it was unclear whether the interim analysis was preplanned and why such an analysis was carried out. Furthermore, although the conference proceeding (Gruchy 2007) stated that the "analyses were performed on an intention‐to‐treat basis", we were not able to convert the percentage of PEP in each group to round patient numbers based on ITT analysis. The full results have not been published, but the authors of the primary study provided us with data of the completed study (Gruchy 2007). Unfortunately, it appeared the authors of the primary study (Gruchy 2007) could only perform per protocol analyses because of high drop out rates. We decided to include the full data set in our analyses because interim report analysis may yield potentially biased estimates of treatment effect (Pocock 1989).
Effects of interventions
See: Table 1
The primary objective of the main analysis (Analysis 1) was to determine if the guidewire‐assisted cannulation technique compared to the contrast‐assisted cannulation technique had any benefit in reducing the risk of post‐ERCP pancreatitis (PEP). Twelve studies were included in the main analysis (Apostolopoulos 2005; Artifon 2007; Bailey 2008; Gruchy 2007; Katsinelos 2008; Kawakami 2012; Kobayashi 2010; Lee 2009; Lella 2004; Mangiavillano 2007; Mangiavillano 2011; Nambu 2011). The secondary objectives of this review were to determine if the guidewire‐assisted cannulation technique compared to the contrast‐assisted cannulation technique had any effect on the severity of PEP; primary, secondary, and overall CBD cannulation rates; the need for precut sphincterotomy; inadvertent guidewire insertion or contrast injection into the PD (inadvertent PD manipulation); and ERCP‐related complications including post‐sphincterotomy bleeding, post‐ERCP cholangitis, perforation and mortality.
To explore sources of heterogeneity, prespecified subgroup analyses were then performed according to trial design (permission of technique 'cross‐over' versus non‐permission of technique 'cross‐over') (Analysis 2), publication type (Analysis 3), risk of bias (Analysis 4), the use of precut sphincterotomy (Analysis 5), inadvertent guidewire insertion or contrast injection into the PD (inadvertent PD manipulation) (Analysis 6), and the use of a PD stent (Analysis 7) for the outcome of PEP. Prespecified subgroup analyses were also performed according to cannulation device (Analysis 8) and involvement of trainees in cannulation (Analysis 9) for both PEP and primary cannulation success.
As 'cross‐over' studies are at risk for contamination due to carry‐over effects in the subgroup of patients who received the alternative technique after failing the assigned technique, it was decided a priori that further subgroup analyses restricted to 'non‐crossover' studies would be performed. Among the 'non‐crossover' studies (Apostolopoulos 2005; Artifon 2007; Lee 2009; Lella 2004; Mangiavillano 2007), prespecified subgroup analyses were performed according to the use of precut sphincterotomy (Analysis 5), inadvertent guidewire insertion or contrast injection into the PD (inadvertent PD manipulation) (Analysis 6), the use of PD stent (Analysis 7), cannulation device (Analysis 8), and involvement of trainees in cannulation (Analysis 9).
Unweighted pooled rates and RRs with 95% CIs for each of the outcomes were calculated using a random‐effects model for the guidewire‐assisted cannulation technique compared to the contrast‐assisted cannulation technique. Data were analysed on an ITT basis.
To assess the robustness of our results, sensitivity analyses were carried out using different summary statistics (RR versus OR) and meta‐analytic models (fixed‐effect versus random‐effects). Per protocol analysis was also carried out for the primary outcome (PEP) in the main analysis (Analysis 1).
Analysis 1: guidewire‐assisted cannulation compared to contrast‐assisted cannulation
Post‐ERCP pancreatitis
All 12 studies included in the main analysis reported PEP rates and comprised a total of 1784 participants in the guidewire‐assisted cannulation technique and 1666 in the contrast‐assisted cannulation technique groups (Apostolopoulos 2005; Artifon 2007; Bailey 2008; Gruchy 2007; Katsinelos 2008; Kawakami 2012; Kobayashi 2010; Lee 2009; Lella 2004; Mangiavillano 2007; Mangiavillano 2011; Nambu 2011). There was significant heterogeneity among the studies (P = 0.04, I² = 45%). Unweighted pooled rates of PEP were 3.5% for the guidewire‐assisted cannulation technique and 6.7% for the contrast‐assisted cannulation technique. The guidewire‐assisted cannulation technique significantly reduced PEP compared to the contrast‐assisted cannulation technique based on ITT analysis (RR 0.51, 95% CI 0.32 to 0.82; P = 0.005; Analysis 1.1) or per protocol analysis (RR 0.51, 95% CI 0.32 to 0.83; P = 0.007; Analysis 1.2). The NNT was 31 (95% CI 19 to 78). In sensitivity analyses, the results remained robust with OR or a fixed‐effect model. The results also remained robust in a post hoc analysis with exclusion of the only high risk of bias study because of incomplete outcome data (Gruchy 2007).
Analysis 1.1.
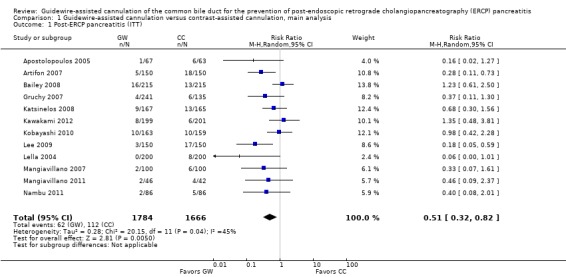
Comparison 1 Guidewire‐assisted cannulation versus contrast‐assisted cannulation, main analysis, Outcome 1 Post‐ERCP pancreatitis (ITT).
Analysis 1.2.
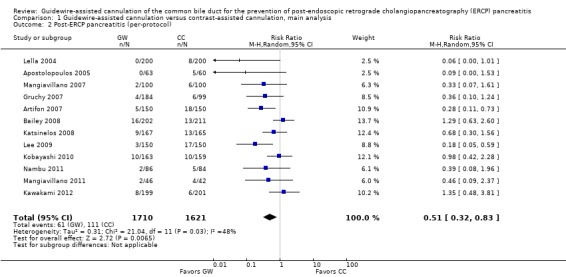
Comparison 1 Guidewire‐assisted cannulation versus contrast‐assisted cannulation, main analysis, Outcome 2 Post‐ERCP pancreatitis (per‐protocol).
Severity of post‐ERCP pancreatitis
Ten studies provided data regarding the severity of PEP for all randomised patients, and comprised a total of 1497 participants in the guidewire‐assisted cannulation technique and 1489 in the contrast‐assisted cannulation technique groups (Apostolopoulos 2005; Artifon 2007; Bailey 2008; Katsinelos 2008; Kawakami 2012; Kobayashi 2010; Lee 2009; Lella 2004; Mangiavillano 2007; Nambu 2011). There was significant heterogeneity among the studies for the outcome of mild PEP (P = 0.03, I² = 51%). However, there was no significant heterogeneity among the studies for moderate PEP (P = 0.72, I² = 0%) or severe PEP (P = 0.55, I² = 0%). Unweighted pooled rates of mild PEP were 2.6% for the guidewire‐assisted cannulation technique and 5.3% for the contrast‐assisted cannulation technique. The guidewire‐assisted cannulation technique significantly reduced the risk of mild PEP compared to the contrast‐assisted cannulation technique (RR 0.49, 95% CI 0.26 to 0.93; P = 0.03; Analysis 1.3). The NNT was 37 (95% CI 21 to 192). In sensitivity analyses, the results remained robust with OR or a fixed‐effect model. Unweighted pooled rates of moderate PEP were 0.7% for the guidewire‐assisted cannulation technique and 1.0% for the contrast‐assisted cannulation technique. There was no statistically significant difference in the rates of moderate PEP between the two cannulation techniques (RR 0.76, 95% CI 0.34 to 1.67; P = 0.49; Analysis 1.3). In sensitivity analyses, the results remained non‐significant with OR or a fixed‐effect model. Unweighted pooled rates of severe PEP were 0.4% for the guidewire‐assisted cannulation technique and 0.6% for the contrast‐assisted cannulation technique. There was no statistically significant difference in the rates of severe PEP between the two cannulation techniques (RR 0.84, 95% CI 0.28 to 2.48; P = 0.75; Analysis 1.3). In sensitivity analyses, the results remained non‐significant with OR or a fixed‐effect model.
Analysis 1.3.
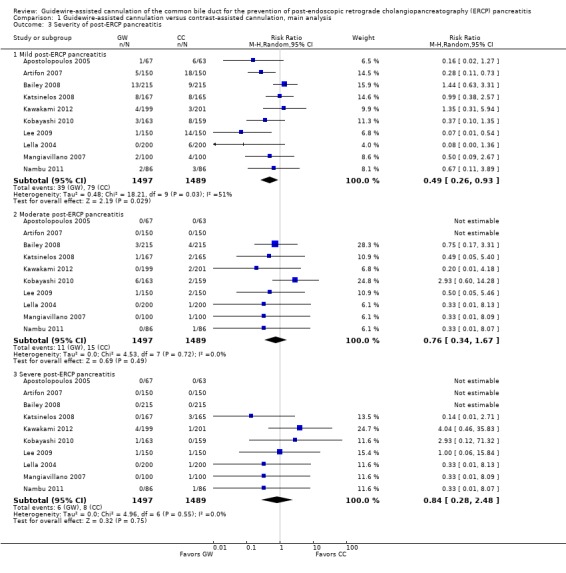
Comparison 1 Guidewire‐assisted cannulation versus contrast‐assisted cannulation, main analysis, Outcome 3 Severity of post‐ERCP pancreatitis.
Primary cannulation success
All except two studies (Gruchy 2007; Mangiavillano 2011) included in the main analysis provided primary cannulation success rates with the randomised technique, and comprised a total of 1497 participants in the guidewire‐assisted cannulation technique and 1489 in the contrast‐assisted cannulation technique groups. There was significant heterogeneity among the studies (P < 0.00001, I² = 83%). Unweighted pooled primary cannulation success rates were 83.6% for the guidewire‐assisted cannulation technique and 77.3% for the contrast‐assisted cannulation technique. There was significantly higher primary cannulation success with the guidewire‐assisted cannulation technique compared to the contrast‐assisted cannulation technique (RR 1.07, 95% CI 1.00 to 1.15; P = 0.05; Analysis 1.11). The NNT was 18 (95% CI 9 to 625). In sensitivity analyses, the results remained statistically significant with OR (OR 1.50, 95% CI 1.05 to 2.14; P = 0.03) and a fixed‐effect model (RR 1.08, 95% CI 1.04 to 1.12; P < 0.00001).
Analysis 1.11.
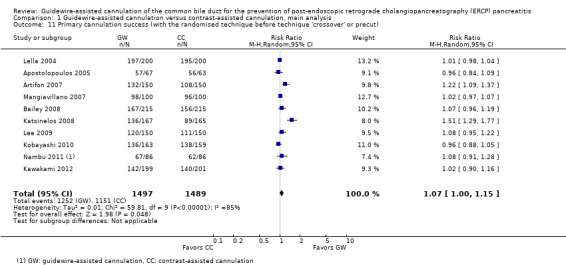
Comparison 1 Guidewire‐assisted cannulation versus contrast‐assisted cannulation, main analysis, Outcome 11 Primary cannulation success (with the randomised technique before technique 'crossover' or precut).
Secondary cannulation success after technique 'cross‐over' (in 'cross‐over' studies)
Among the seven 'cross‐over' studies, four (Bailey 2008; Katsinelos 2008; Kobayashi 2010; Nambu 2011) provided data regarding the number of patients requiring 'cross‐over' to the alternative technique when the randomised technique failed, comprising a total of 631 participants in the guidewire‐assisted cannulation technique and 625 in the contrast‐assisted cannulation technique groups. One hundred patients in the guidewire‐assisted cannulation group and 169 patients in the contrast‐assisted cannulation group required 'cross‐over' to the alternative technique. There was significant heterogeneity among the studies (P = 0.0008, I² = 82%). Unweighted pooled rates of 'cross‐over' to the alternative technique were 15.8% for the guidewire‐assisted cannulation technique and 27.0% for the contrast‐assisted cannulation technique. There was no statistically significant difference in the 'cross‐over' rates between the two cannulation techniques (RR 0.65, 95% CI 0.38 to 1.13; P = 0.13; Analysis 1.4). In sensitivity analyses, the results remained non‐significant with OR, but became statistically significant with a fixed‐effect model (RR 0.59, 95% CI 0.47 to 0.73; P < 0.0001) favouring the guidewire‐assisted cannulation technique for less 'cross‐over' to the alternative technique.
Analysis 1.4.
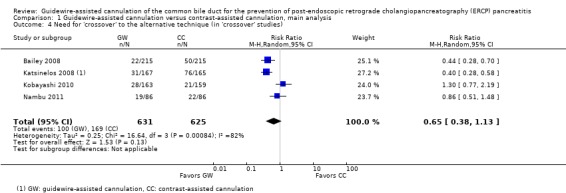
Comparison 1 Guidewire‐assisted cannulation versus contrast‐assisted cannulation, main analysis, Outcome 4 Need for 'crossover' to the alternative technique (in 'crossover' studies).
Among the seven 'cross‐over' studies, four (Bailey 2008; Katsinelos 2008; Kobayashi 2010; Nambu 2011) provided data regarding secondary cannulation success after technique 'cross‐over', and comprised a total of 100 participants in the guidewire‐assisted cannulation technique and 169 in the contrast‐assisted cannulation technique groups. There was significant heterogeneity among the studies (P = 0.04, I² = 64%). Unweighted pooled secondary cannulation rates were 34.0% after 'cross‐over' to the contrast‐assisted cannulation technique and 49.7% after 'cross‐over' to the guidewire‐assisted cannulation technique. There was no statistically significant difference in the cannulation success rates after 'cross‐over' to either technique (RR 0.74, 95% CI 0.41 to 1.31; P = 0.30; Analysis 1.5). In sensitivity analyses, the results remained non‐significant with OR. However, a significantly higher cannulation success after 'cross‐over' to the guidewire‐assisted cannulation technique was found with a fixed‐effect model (RR 0.66, 95% CI 0.47 to 0.93; P = 0.02).
Analysis 1.5.
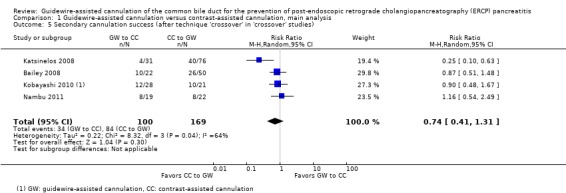
Comparison 1 Guidewire‐assisted cannulation versus contrast‐assisted cannulation, main analysis, Outcome 5 Secondary cannulation success (after technique 'crossover' in 'crossover' studies).
Overall cannulation success
All studies reported overall cannulation success rates, and comprised a total of 1784 participants in the guidewire‐assisted cannulation technique and 1666 in the contrast‐assisted cannulation technique groups. There was significant heterogeneity among the studies (P = 0.05, I² = 44%). Unweighted pooled overall cannulation success rates were 91.4% for the guidewire‐assisted cannulation technique and 90.1% for the contrast‐assisted cannulation technique. There was no statistically significant difference in the overall cannulation success rates between the two cannulation techniques (RR 1.01, 95% CI 0.99 to 1.04; P = 0.21; Analysis 1.6). In sensitivity analyses, the results became statistically significant with OR (OR 1.29, 95% CI 1.01 to 1.65; P = 0.04) and a fixed‐effect model (RR 1.02, 95% CI 1.00 to 1.04; P = 0.04) favouring the guidewire‐assisted cannulation technique for achieving overall cannulation success.
Analysis 1.6.
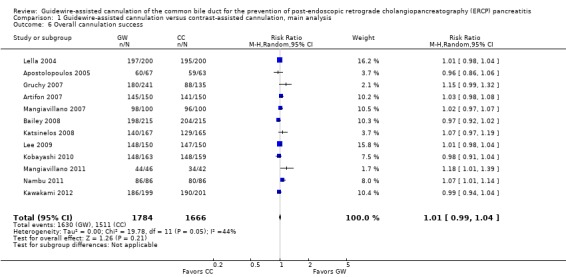
Comparison 1 Guidewire‐assisted cannulation versus contrast‐assisted cannulation, main analysis, Outcome 6 Overall cannulation success.
The need for precut sphincterotomy
Among the 10 studies that permitted precut sphincterotomy as a rescue technique for difficult cannulation, eight reported the precut sphincterotomy rate for each cannulation technique (Apostolopoulos 2005; Artifon 2007; Bailey 2008; Katsinelos 2008; Kawakami 2012; Kobayashi 2010; Lee 2009; Nambu 2011). There was no significant heterogeneity among the studies (P = 0.48, I² = 0%). Unweighted pooled precut sphincterotomy rates were 9.3% for the guidewire‐assisted cannulation technique and 12.4% for the contrast‐assisted cannulation technique. The guidewire‐assisted cannulation technique significantly reduced the need for precut sphincterotomy compared to the contrast‐assisted cannulation technique (RR 0.75, 95% CI 0.60 to 0.95; P = 0.02; Analysis 1.7). The NNT was 44 (95% CI 19 to 115). In sensitivity analyses, the results remained robust with OR or a fixed‐effect model.
Analysis 1.7.
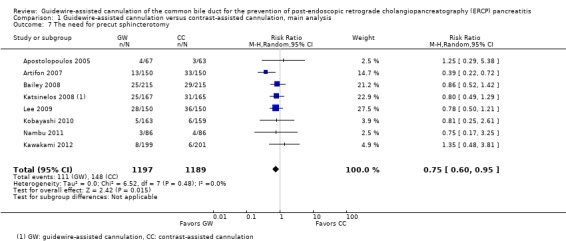
Comparison 1 Guidewire‐assisted cannulation versus contrast‐assisted cannulation, main analysis, Outcome 7 The need for precut sphincterotomy.
Inadvertent guidewire insertion or contrast injection into the pancreatic duct (PD) (inadvertent PD manipulation)
A total of eight studies provided data regarding the number of patients with inadvertent guidewire insertion or contrast injection into the PD (inadvertent PD manipulation): five studies (Artifon 2007; Kawakami 2012; Lee 2009; Lella 2004; Mangiavillano 2007) reported data in full text or abstract format, and additional outcome data were obtained from the authors of three primary studies (Bailey 2008; Kobayashi 2010; Nambu 2011). One study (Apostolopoulos 2005) was excluded from this analysis as it provided only data regarding the rates of inadvertent contrast injection but not inadvertent guidewire cannulation of the PD. Another study (Gruchy 2007) was excluded from this analysis as it provided only data regarding the rates of inadvertent contrast injection into the PD in the guidewire‐assisted cannulation group. There was significant heterogeneity among the studies (P = 0.04, I² = 53%). Unweighted pooled rates of inadvertent guidewire insertion or contrast injection into the PD (inadvertent PD manipulation) were 37.1% for the guidewire‐assisted cannulation technique and 43.1% for the contrast‐assisted cannulation technique. There was a non‐significant trend towards less inadvertent PD manipulation with the guidewire‐assisted cannulation technique compared to the contrast‐assisted cannulation technique (RR 0.88, 95% CI 0.76 to 1.01; P = 0.08; Analysis 1.8). In sensitivity analyses, the results remained non‐significant with OR but became statistically significant with a fixed‐effect model (RR 0.86, 95% CI 0.79 to 0.95; P = 0.002).
Analysis 1.8.
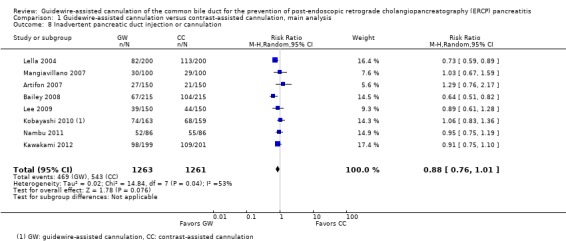
Comparison 1 Guidewire‐assisted cannulation versus contrast‐assisted cannulation, main analysis, Outcome 8 Inadvertent pancreatic duct injection or cannulation.
ERCP‐related complications
Post‐sphincterotomy bleeding was reported by five studies (Artifon 2007; Gruchy 2007; Katsinelos 2008; Lee 2009; Nambu 2011). The other seven studies did not report on this outcome (Apostolopoulos 2005; Bailey 2008; Kawakami 2012; Kobayashi 2010; Lella 2004; Mangiavillano 2007; Mangiavillano 2011). Most reported bleeding episodes either stopped spontaneously or with medical or endoscopic therapies. One patient required surgery (Katsinelos 2008). There was no significant heterogeneity among the studies (P = 0.79, I² = 0%). Unweighted pooled rates of post‐sphincterotomy bleeding were 2.6% for the guidewire‐assisted cannulation technique and 2.9% for the contrast‐assisted cannulation technique. There was no statistically significant difference in the rates of post‐sphincterotomy bleeding between the two cannulation techniques (RR 0.93, 95% CI 0.50 to 1.72; P = 0.82; Analysis 1.9).
Analysis 1.9.
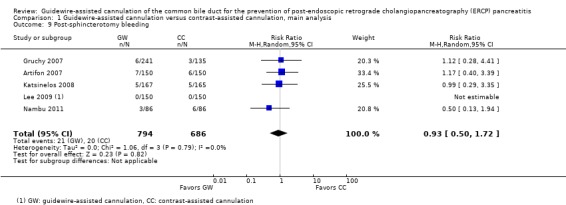
Comparison 1 Guidewire‐assisted cannulation versus contrast‐assisted cannulation, main analysis, Outcome 9 Post‐sphincterotomy bleeding.
Perforation was reported by six studies (Artifon 2007; Gruchy 2007; Katsinelos 2008; Kawakami 2012; Lee 2009; Nambu 2011). The other six studies did not report on this outcome (Apostolopoulos 2005; Bailey 2008; Kobayashi 2010; Lella 2004; Mangiavillano 2007; Mangiavillano 2011). There was significant heterogeneity among the studies (P = 0.07, I² = 69%). Unweighted pooled rates of perforation were 0.50% with the guidewire‐assisted cannulation technique and 0.34% with the contrast‐assisted cannulation technique. There was no statistically significant difference in the rates of perforation between the two cannulation techniques (RR 1.53, 95% CI 0.06 to 41.19; P = 0.80; Analysis 1.10).
Analysis 1.10.
Comparison 1 Guidewire‐assisted cannulation versus contrast‐assisted cannulation, main analysis, Outcome 10 Perforation.
Post‐ERCP cholangitis was specifically reported by one trial (Apostolopoulos 2005) and only one case was identified, with the contrast‐assisted cannulation technique.
Mortality was reported by six studies (Apostolopoulos 2005; Artifon 2007; Katsinelos 2008; Lee 2009; Lella 2004; Nambu 2011) and no procedure‐related death occurred out of 1634 patients.
Analysis 2: guidewire‐assisted cannulation compared to contrast‐assisted cannulation according to trial design
All five 'non‐crossover' studies reported PEP for all randomised patients, comprising a total of 667 participants in the guidewire‐assisted cannulation technique and 663 in the contrast‐assisted cannulation technique (Apostolopoulos 2005; Artifon 2007; Lee 2009; Lella 2004; Mangiavillano 2007). There was no significant heterogeneity among the studies (P = 0.81, I² = 0%). Unweighted pooled rates of PEP were 1.6% for the guidewire‐assisted cannulation technique and 8.3% for the contrast‐assisted cannulation technique. Among the 'non‐crossover' studies, the guidewire‐assisted cannulation technique significantly reduced PEP compared to the contrast‐assisted cannulation technique (RR 0.22, 95% CI 0.12 to 0.42; P < 0.0001; Analysis 2.1). The NNT was 17 (95% CI 11 to 33). In sensitivity analyses the results remained robust with OR or a fixed‐effect model.
Analysis 2.1.
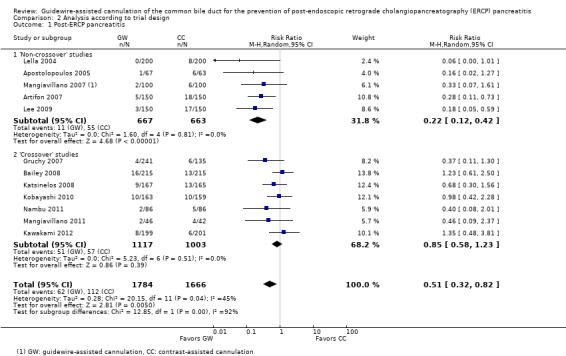
Comparison 2 Analysis according to trial design, Outcome 1 Post‐ERCP pancreatitis.
All seven 'cross‐over' studies reported PEP rates, and comprised a total of 1117 participants in the guidewire‐assisted cannulation technique and 1003 in the contrast‐assisted cannulation technique groups (Bailey 2008; Gruchy 2007; Katsinelos 2008; Kawakami 2012; Kobayashi 2010; Mangiavillano 2011; Nambu 2011). There was no significant heterogeneity among the studies (P = 0.51, I² = 0%). Unweighted pooled rates of PEP were 4.6% for the guidewire‐assisted cannulation technique and 5.7% for the contrast‐assisted cannulation technique. Among the 'cross‐over' studies, there was no statistically significant difference in the rates of PEP between the two cannulation techniques (RR 0.85, 95% CI 0.58 to 1.23; P = 0.39; Analysis 2.1). In sensitivity analyses, the results remained non‐significant with OR or a fixed‐effect model. The results also remained non‐significant in a post hoc analysis with the exclusion of the only high risk of bias study for incomplete outcome data (Gruchy 2007).
Most importantly, the test for subgroup differences indicated statistically significant differences between the two subgroups ('non‐crossover' versus 'cross‐over' studies) for the outcome of PEP, with the guidewire‐assisted cannulation technique favoured in 'non‐crossover' studies but not in 'cross‐over' studies (P = 0.0001).
Analysis 3: guidewire‐assisted cannulation compared to contrast‐assisted cannulation according to publication type
All seven studies published in full text reported PEP for all randomised patients, comprising a total of 1167 participants in the guidewire‐assisted cannulation technique and 1167 in the contrast‐assisted cannulation technique groups (Artifon 2007; Bailey 2008; Katsinelos 2008; Kawakami 2012; Lee 2009; Lella 2004; Nambu 2011). There was significant heterogeneity among the studies (P = 0.01, I² = 63%). Unweighted pooled rates of PEP were 3.7% for the guidewire‐assisted cannulation technique and 6.9% for the contrast‐assisted cannulation technique. Among fully published studies, there was a non‐significant trend towards less PEP with the guidewire‐assisted cannulation technique compared to the contrast‐assisted cannulation technique (RR 0.51, 95% CI 0.26 to 1.02; P = 0.06; Analysis 3.1). In sensitivity analyses, the results remained non‐significant with OR but became statistically significant in favour of the guidewire‐assisted cannulation technique with a fixed‐effect model (RR 0.54, 95% CI 0.38 to 0.77; P = 0.0008).
Analysis 3.1.
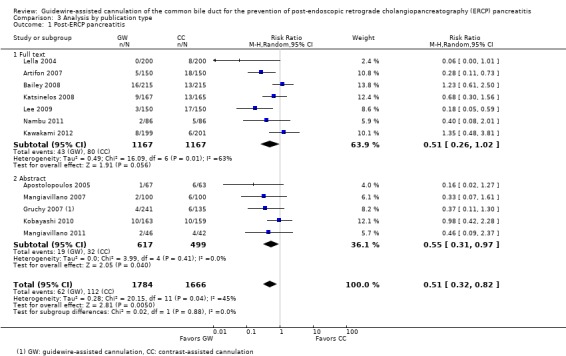
Comparison 3 Analysis by publication type, Outcome 1 Post‐ERCP pancreatitis.
All five studies published in abstract format reported PEP rates, and comprised a total of 617 participants in the guidewire‐assisted cannulation technique and 499 in the contrast‐assisted cannulation technique groups (Apostolopoulos 2005; Gruchy 2007; Kobayashi 2010; Mangiavillano 2007; Mangiavillano 2011). There was no significant heterogeneity among the studies (P = 0.41, I² = 0%). Unweighted pooled rates of PEP were 3.1% for the guidewire‐assisted cannulation technique and 6.4% for the contrast‐assisted cannulation technique. Among studies published in abstract format, the guidewire‐assisted cannulation technique significantly reduced PEP compared to the contrast‐assisted cannulation technique (RR 0.55, 95% CI 0.31 to 0.97; P = 0.04; Analysis 3.1). The NNT was 32 (95% CI 18 to 156). In sensitivity analyses, the results remained robust with OR or a fixed‐effect model. However, the results became statistically non‐significant in a post hoc analysis with the exclusion of the only high risk of bias study for incomplete outcome data (Gruchy 2007).
Most importantly, the test for subgroup differences indicated no statistically significant differences between the two subgroups (full text versus abstract) for the outcome of PEP (P = 0.89).
Analysis 4: guidewire‐assisted cannulation compared to contrast‐assisted cannulation according to risk of bias
All included studies were considered at low risk of bias for selective reporting, unclear risk of bias for blinding of outcome assessment, and high risk of bias for blinding of participants and personnel (the endoscopists). All except one study (Gruchy 2007) were considered at low risk of bias for incomplete outcome assessment. Therefore, subgroup analyses according to risk of bias for random sequence generation and allocation concealment were performed.
Random sequence generation
Five studies were considered as low risk (Artifon 2007; Bailey 2008; Kawakami 2012; Lee 2009; Lella 2004) and seven were considered as at unclear risk of bias (Apostolopoulos 2005; Gruchy 2007; Katsinelos 2008; Kobayashi 2010; Mangiavillano 2007; Mangiavillano 2011; Nambu 2011) for random sequence generation. There was significant heterogeneity among the studies considered as at low risk of bias for random sequence generation (P = 0.003, I² = 75%). Among studies considered as having an unclear risk of bias for random sequence generation, there was no significant heterogeneity (P = 0.63, I² = 0%). In studies considered as at low risk of bias for random sequence generation, there was no statistically significant difference in the rates of PEP between the two cannulation techniques (RR 0.46, 95% CI 0.17 to 1.25; P = 0.13; Analysis 4.1). In sensitivity analyses, the results remained non‐significant with OR but became statistically significant with a fixed‐effect model (RR 0.52, 95% CI 0.34 to 0.79; P = 0.002). In studies considered as at unclear risk of bias for random sequence generation, the guidewire‐assisted cannulation technique significantly reduced PEP compared to the contrast‐assisted cannulation technique (RR 0.57, 95% CI 0.36 to 0.90; P = 0.02; Analysis 4.1). In sensitivity analyses, the results remained robust with OR or a fixed‐effect model. Most importantly, the test for subgroup differences indicated no statistically significant differences between the two subgroups (according to risk of bias for random sequence generation) for the outcome of PEP (P = 0.88).
Analysis 4.1.
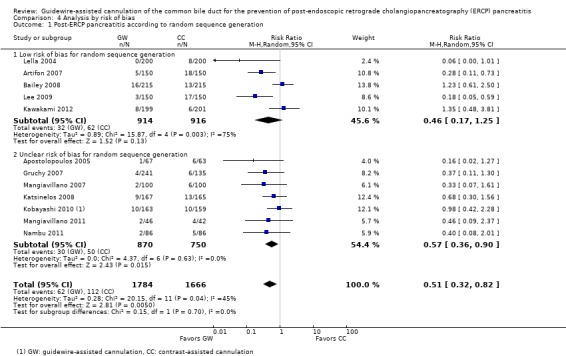
Comparison 4 Analysis by risk of bias, Outcome 1 Post‐ERCP pancreatitis according to random sequence generation.
Allocation concealment
Four studies were considered as low risk (Artifon 2007; Katsinelos 2008; Kawakami 2012; Nambu 2011) and eight were considered as at unclear risk of bias (Apostolopoulos 2005; Bailey 2008; Gruchy 2007; Kobayashi 2010; Lee 2009; Lella 2004; Mangiavillano 2007; Mangiavillano 2011) for allocation concealment. There was significant heterogeneity among studies considered as having low risk of bias for allocation concealment (P = 0.16, I² = 42%). Among studies considered as at unclear risk of bias for allocation concealment, there was also significant heterogeneity (P = 0.03, I² = 54%). In studies considered as at low risk of bias for allocation concealment, there was no statistically significant difference in the rates of PEP between the two cannulation techniques (RR 0.59, 95% CI 0.29 to 1.17; P = 0.13; Analysis 4.2). In sensitivity analyses, the results remained non‐significant with OR but became statistically significant with a fixed‐effect model (RR 0.57, 95% CI 0.35 to 0.93; P = 0.02). In studies considered as having unclear risk of bias for allocation concealment, the guidewire‐assisted cannulation technique significantly reduced PEP compared to the contrast‐assisted cannulation technique (RR 0.44, 95% CI 0.22 to 0.87; P = 0.02; Analysis 4.2). The NNT was 28 (95% CI 17 to 83). In sensitivity analyses, the results remained robust with OR or a fixed‐effect model. Most importantly, the test for subgroup differences indicated no statistically significant differences between the two subgroups (according to risk of bias for allocation concealment) for the outcome of PEP (P = 0.73).
Analysis 4.2.
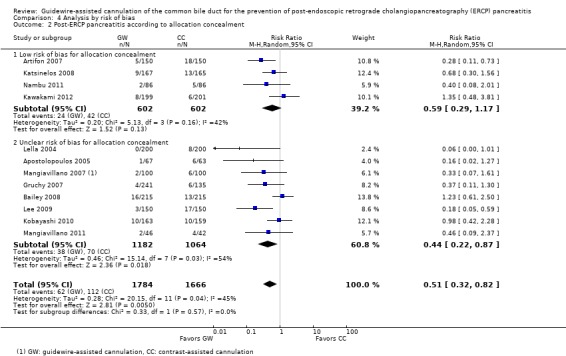
Comparison 4 Analysis by risk of bias, Outcome 2 Post‐ERCP pancreatitis according to allocation concealment.
Analysis 5: guidewire‐assisted cannulation compared to contrast‐assisted cannulation according to the use of precut sphincterotomy
'Cross‐over' and 'non‐crossover' studies
Precut sphincterotomy was permitted as a rescue technique for difficult cannulation in 10 studies (Apostolopoulos 2005; Artifon 2007; Bailey 2008; Gruchy 2007; Katsinelos 2008; Kawakami 2012; Kobayashi 2010; Lee 2009; Mangiavillano 2011; Nambu 2011). Two studies (Apostolopoulos 2005; Gruchy 2007) permitted precut sphincterotomy but excluded from the analysis any patients who received precut sphincterotomy (per protocol analysis). Additional ITT data were provided by one study (Apostolopoulos 2005). We included both studies (Apostolopoulos 2005; Gruchy 2007) under the subgroup of studies that permitted precut sphincterotomy based on the principle of ITT. One study (Lella 2004) did not permit the use of precut sphincterotomy. One study, in abstract format, did not report the use of precut sphincterotomy (Mangiavillano 2007).
Ten studies permitted the use of precut sphincterotomy in difficult cannulation and provided data regarding the rates of PEP (Apostolopoulos 2005; Artifon 2007; Bailey 2008; Gruchy 2007; Katsinelos 2008; Kawakami 2012; Kobayashi 2010; Lee 2009; Mangiavillano 2011; Nambu 2011), and comprised a total of 1484 participants in the guidewire‐assisted cannulation technique and 1366 in the contrast‐assisted cannulation technique groups. There was significant heterogeneity among the studies (P = 0.05, I² = 46%). Unweighted pooled rates of PEP for participants were 4.0% for the guidewire‐assisted cannulation technique and 7.2% for the contrast‐assisted cannulation technique. In studies that permitted the use of precut sphincterotomy, the guidewire‐assisted cannulation technique significantly reduced PEP compared to the contrast‐assisted cannulation technique (RR 0.56, 95% CI 0.35 to 0.90; P = 0.02; Analysis 5.1). The NNT was 31 (95% CI 18 to 147). In sensitivity analyses, the results remained robust with OR or a fixed‐effect model. The results also remained robust in a post hoc analysis with the exclusion of the only high risk of bias study for incomplete outcome data (Gruchy 2007).
Analysis 5.1.
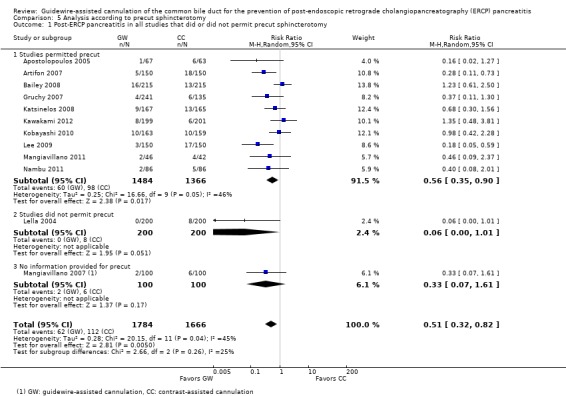
Comparison 5 Analysis according to precut sphincterotomy, Outcome 1 Post‐ERCP pancreatitis in all studies that did or did not permit precut sphincterotomy.
One study (Lella 2004) did not permit the use of precut sphincterotomy in difficult cannulation. The PEP rates for participants were 0% for the guidewire‐assisted cannulation technique and 4.0% for the contrast‐assisted cannulation technique. There was a non‐significant trend towards less PEP with the guidewire‐assisted cannulation technique compared to the contrast‐assisted cannulation technique (RR 0.06, 95% CI 0 to 1.01; P = 0.05; Analysis 5.1) (Lee 2009).
One study (Mangiavillano 2007) did not provide information as to whether precut sphincterotomy was used. The PEP rates for participants were 2.0% for the guidewire‐assisted cannulation technique and 6.0% for the contrast‐assisted cannulation technique. There was no statistically significant difference in the PEP rates between the two cannulation techniques (RR 0.33, 95% CI 0.07 to 1.61; P = 0.17; Analysis 5.1).
Most importantly, the test for subgroup differences indicated no statistically significant differences between the two subgroups (studies that permitted the use of precut versus studies that did not permit the use of precut) (P = 0.12) for the outcome of PEP.
'non‐crossover' studies
Three 'non‐crossover' studies (Apostolopoulos 2005; Artifon 2007; Lee 2009) provided subgroup data regarding the rates of PEP among patients who did or did not undergo precut sphincterotomy. Among participants who underwent precut sphincterotomy, the unweighted pooled rates of PEP were 4.4% in the guidewire‐assisted cannulation technique and 22.2% in the contrast‐assisted cannulation technique groups. There was no significant heterogeneity for this analysis (P = 0.46, I² = 0%). When precut was used, there was no statistically significant difference in the rates of PEP between the two cannulation techniques (RR 0.31, 95% CI 0.08 to 1.18; P = 0.09; Analysis 5.2). In sensitivity analyses, the results remained non‐significant with OR but became statistically significant with a fixed‐effect model (RR 0.23, 95% CI 0.06 to 0.86; P = 0.03). Among participants who did not undergo precut sphincterotomy, the unweighted pooled rates of PEP were 4.7% in the guidewire‐assisted cannulation technique and 11.0% in the contrast‐assisted cannulation technique. There was no significant heterogeneity for this analysis (P = 0.28, I² = 22%). When precut was not used, the guidewire‐assisted cannulation technique significantly reduced PEP compared to the contrast‐assisted cannulation technique (RR 0.42, 95% CI 0.19 to 0.92; P = 0.03; Analysis 5.2). The NNT was 14 (95% CI 9 to 33). In sensitivity analyses, the results remained robust with OR or a fixed‐effect model. Most importantly, the test for subgroup differences indicated no statistically significant differences between the two subgroups (participants who did or did not undergo precut sphincterotomy) for the outcome of PEP (P = 0.42).
Analysis 5.2.
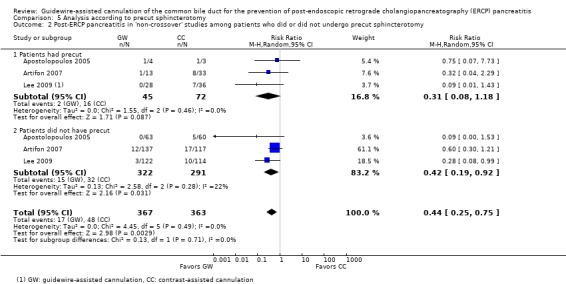
Comparison 5 Analysis according to precut sphincterotomy, Outcome 2 Post‐ERCP pancreatitis in 'non‐crossover' studies among patients who did or did not undergo precut sphincterotomy.
Analysis 6: guidewire‐assisted cannulation compared to contrast‐assisted cannulation according to inadvertent guidewire insertion or contrast injection into the PD (inadvertent PD manipulation)
'Cross‐over' and 'non‐crossover' studies
Four studies (Artifon 2007; Lee 2009; Lella 2004; Mangiavillano 2007) reported subgroup data regarding the rates of PEP among patients who did or did not have Inadvertent guidewire cannulation or contrast injection of the pancreatic duct (PD) (inadvertent PD manipulation) between the two cannulation techniques. Additional subgroup data regarding inadvertent PD manipulation were obtained from the author of one primary study (Nambu 2011). Among participants who had inadvertent PD manipulation, the unweighted pooled rates of PEP were 1.7% in the guidewire‐assisted cannulation technique and 8.7% in the contrast‐assisted cannulation technique. There was no significant heterogeneity for this analysis (P = 0.68, I² = 0%). Among participants with inadvertent PD manipulation, the guidewire‐assisted cannulation technique significantly reduced PEP compared to the contrast‐assisted cannulation technique (RR 0.28, 95% CI 0.11 to 0.71; P = 0.008; Analysis 6.1). The NNT was 15 (95% CI 8 to 82). In sensitivity analyses, the results remained robust with OR or a fixed‐effect model. Among participants who did not have inadvertent PD manipulation, the unweighted pooled rates of PEP were 2.2% in the guidewire‐assisted cannulation technique and 6.9% in the contrast‐assisted cannulation technique. There was no significant heterogeneity for this analysis (P = 0.69, I² = 0%). Among participants who did not have inadvertent PD manipulation, the guidewire‐assisted cannulation technique also significantly reduced PEP compared to the contrast‐assisted cannulation technique (RR 0.37, 95% CI 0.19 to 0.73; P = 0.004; Analysis 6.1). The NNT was 27 (95% CI 16 to 98). In sensitivity analyses, the results remained robust with OR or a fixed‐effect model. Most importantly, the test for subgroup differences indicated no statistically significant differences between the two subgroups (participants who did or did not have inadvertent PD manipulation) for the outcome of PEP (P = 0.52).
Analysis 6.1.
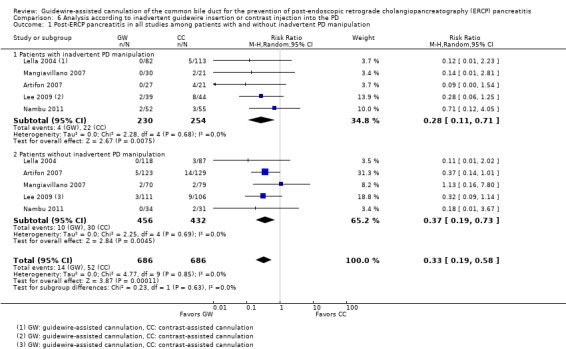
Comparison 6 Analysis according to inadvertent guidewire insertion or contrast injection into the PD, Outcome 1 Post‐ERCP pancreatitis in all studies among patients with and without inadvertent PD manipulation.
'Non‐crossover' studies
Four 'non‐crossover' studies (Artifon 2007; Lee 2009; Lella 2004; Mangiavillano 2007) provided subgroup data regarding the rates of PEP among patients who did or did not have inadvertent PD manipulation between the two cannulation techniques. Among participants who had inadvertent PD manipulation, the unweighted pooled rates of PEP were 1.1% in the guidewire‐assisted cannulation technique and 9.5% in the contrast‐assisted cannulation technique. There was no significant heterogeneity for this analysis (P = 0.88, I² = 0%). Among participants with inadvertent PD manipulation, the guidewire‐assisted cannulation technique significantly reduced PEP compared to the contrast‐assisted cannulation technique (RR 0.19, 95% CI 0.06 to 0.58; P = 0.003; Analysis 6.2). The NNT was 11 (95% CI 6 to 68). In sensitivity analyses, the results remained robust with OR or a fixed‐effect model. Among participants who did not have inadvertent PD manipulation, the unweighted pooled rates of PEP were 2.4% in the guidewire‐assisted cannulation technique and 7.0% in the contrast‐assisted cannulation technique. There was no significant heterogeneity for this analysis (P = 0.57, I² = 0%). Among participants who did not have inadvertent PD manipulation, the guidewire‐assisted cannulation technique also significantly reduced PEP compared to the contrast‐assisted cannulation technique (RR 0.38, 95% CI 0.19 to 0.78; P = 0.008; Analysis 6.2). The NNT was 28 (95% CI 15 to 222). In sensitivity analyses, the results remained robust with OR or a fixed‐effect model. Most importantly, the test for subgroup differences indicated no statistically significant differences between the two subgroups (participants who did or did not have inadvertent PD manipulation) for the outcome of PEP (P = 0.27).
Analysis 6.2.
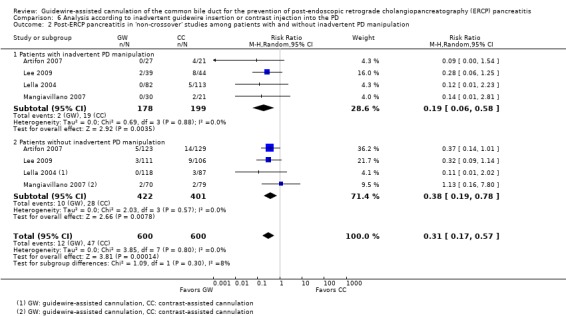
Comparison 6 Analysis according to inadvertent guidewire insertion or contrast injection into the PD, Outcome 2 Post‐ERCP pancreatitis in 'non‐crossover' studies among patients with and without inadvertent PD manipulation.
Analysis 7: guidewire‐assisted cannulation compared to contrast‐assisted cannulation according to the use of PD stent
'Cross‐over' and 'non‐crossover' studies
Pancreatic duct (PD) stents were used for prophylaxis of PEP in five studies (Bailey 2008; Gruchy 2007; Katsinelos 2008; Kawakami 2012; Kobayashi 2010). One study (Gruchy 2007) permitted the use of PD stents but excluded from the analysis any patients who received PD stents (per protocol analysis). We included this study under the subgroup of studies that permitted PD stents based on the principle of ITT. PD stents were not permitted in four studies (Apostolopoulos 2005; Artifon 2007; Lee 2009; Nambu 2011). Three studies did not report the use of PD stents (Lella 2004; Mangiavillano 2007; Mangiavillano 2011). Subgroup data of PEP rates among participants who did or did not receive PD stents were not reported by any of the included studies.
All five studies that permitted the use of PD stents provided data regarding the rates of PEP (Bailey 2008; Gruchy 2007; Katsinelos 2008; Kawakami 2012; Kobayashi 2010), and comprised a total of 985 participants in the guidewire‐assisted cannulation technique and 875 in the contrast‐assisted cannulation technique groups. There was no significant heterogeneity among the studies (P = 0.45, I² = 0%). Unweighted pooled rates of PEP for participants were 4.8% for the guidewire‐assisted cannulation technique and 5.5% for the contrast‐assisted cannulation technique. In studies that permitted the use of PD stents, there was no statistically significant difference in the rates of PEP between the two cannulation techniques (RR 0.92, 95% CI 0.62 to 1.36; P = 0.68; Analysis 7.1). In sensitivity analyses, the results remained non‐significant with OR or a fixed‐effect model. The results also remained non‐significant in a post hoc analysis with the exclusion of the only high risk of bias study for incomplete outcome data (Gruchy 2007).
Analysis 7.1.
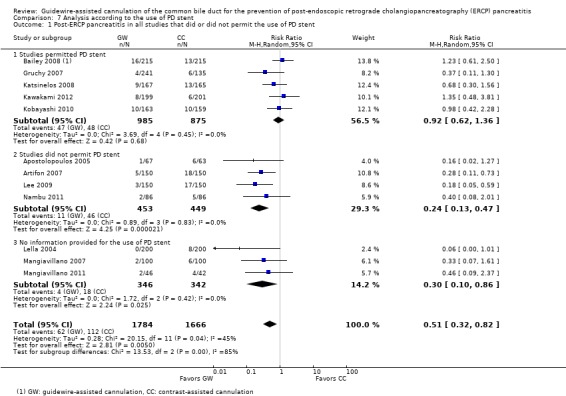
Comparison 7 Analysis according to the use of PD stent, Outcome 1 Post‐ERCP pancreatitis in all studies that did or did not permit the use of PD stent.
All four studies that did not permit the use of PD stents provided data regarding the rates of PEP among all randomised patients (Apostolopoulos 2005; Artifon 2007; Lee 2009; Nambu 2011), and comprised a total of 453 participants in the guidewire‐assisted cannulation technique and 449 in the contrast‐assisted cannulation technique groups. There was no significant heterogeneity among the studies (P = 0.83, I² = 0%). Unweighted pooled rates of PEP for participants were 2.4% for the guidewire‐assisted cannulation technique and 10.2% for the contrast‐assisted cannulation technique. In studies that did not permit the use of PD stent, the guidewire‐assisted cannulation technique significantly reduced PEP compared to the contrast‐assisted cannulation technique (RR 0.24, 95% CI 0.13 to 0.47; P < 0.0001; Analysis 7.1). The NNT was 14 (95% CI 10 to 23). In sensitivity analyses, the results remained robust with OR or a fixed‐effect model.
All three studies that did not report the use of PD stents provided data regarding the rates of PEP among all randomised patients (Lella 2004; Mangiavillano 2007; Mangiavillano 2011), and comprised a total of 346 participants in the guidewire‐assisted cannulation technique and 342 in the contrast‐assisted cannulation technique groups. There was no significant heterogeneity among the studies (P = 0.42, I² = 0%). Unweighted pooled rates of PEP for participants were 1.2% for the guidewire‐assisted cannulation technique and 5.3% for the contrast‐assisted cannulation technique. In studies that did not report the use of PD stents, the guidewire‐assisted cannulation technique significantly reduced PEP compared to the contrast‐assisted cannulation technique (RR 0.30, 95% CI 0.10 to 0.86; P = 0.03; Analysis 7.1). The NNT was 25 (95% CI 15 to 63). In sensitivity analyses, the results remained robust with OR or a fixed‐effect model.
Most importantly, the test for subgroup differences indicated statistically significant differences between the two subgroups (studies that permitted the use of PD stents versus studies that did not permit the use of PD stents) (P = 0.0004) and the three subgroups (P = 0.0004) for the outcome of PEP.
'Non‐crossover' studies
Among the five 'non‐crossover' studies, three did not permit the use of PD stents (Apostolopoulos 2005; Artifon 2007; Lee 2009) and two did not report the use of PD stents (Lella 2004; Mangiavillano 2007).
All three 'non‐crossover' studies that did not permit the use of PD stents provided data regarding the rates of PEP among all randomised patients (Apostolopoulos 2005; Artifon 2007; Lee 2009), and comprised a total of 367 participants in the guidewire‐assisted cannulation technique and 363 in the contrast‐assisted cannulation technique groups. There was no significant heterogeneity among the studies (P = 0.80, I² = 0%). Unweighted pooled rates of PEP for participants were 2.5% for the guidewire‐assisted cannulation technique and 11.3% for the contrast‐assisted cannulation technique. In 'non‐crossover' studies that did not permit the use of PD stents, the guidewire‐assisted cannulation technique significantly reduced PEP compared to the contrast‐assisted cannulation technique (RR 0.22, 95% CI 0.11 to 0.45; P < 0.0001; Analysis 7.2). The NNT was 11 (95% CI 8 to 20). In sensitivity analyses, the results remained robust with OR or a fixed‐effect model.
Analysis 7.2.
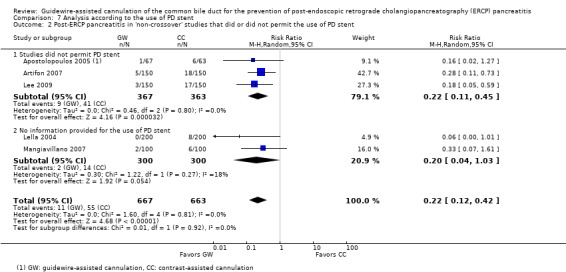
Comparison 7 Analysis according to the use of PD stent, Outcome 2 Post‐ERCP pancreatitis in 'non‐crossover' studies that did or did not permit the use of PD stent.
The two studies that did not report the use of PD stents provided data regarding the rates of PEP among all randomised patients (Lella 2004; Mangiavillano 2007), and comprised a total of 300 participants in the guidewire‐assisted cannulation technique and 300 in the contrast‐assisted cannulation technique. There was no significant heterogeneity among the studies (P = 0.27, I² = 18%). Unweighted pooled rates of PEP for participants were 0.7% for the guidewire‐assisted cannulation technique and 4.7% for the contrast‐assisted cannulation technique. In 'non‐crossover' studies that did not report the use of PD stents, there was a non‐significant trend towards reduced risk of PEP with the guidewire‐assisted cannulation technique compared to the contrast‐assisted cannulation technique (RR 0.20, 95% CI 0.04 to 1.03; P = 0.05; Analysis 7.2). In sensitivity analyses, the results became statistically significant with OR (OR 0.20, 95% CI 0.04 to 0.98; P = 0.05) or a fixed‐effect model (RR 0.17, 95% CI 0.05 to 0.65; P = 0.010).
Most importantly, the test for subgroup differences indicated no statistically significant differences between the two subgroups ('non‐crossover' studies that did not permit the use of PD stents versus 'non‐crossover' studies that did not report the use of PD stents) for the outcome of PEP (P = 0.76).
Analysis 8: guidewire‐assisted cannulation compared to contrast‐assisted cannulation according to cannulation device
'Cross‐over' and 'non‐crossover' studies
A sphincterotome was used with both the guidewire‐assisted cannulation technique and the contrast‐assisted cannulation technique in eight studies (Apostolopoulos 2005; Artifon 2007; Bailey 2008; Gruchy 2007; Kawakami 2012; Lee 2009; Lella 2004; Mangiavillano 2007), whereas a catheter was used with both techniques in two studies (Katsinelos 2008; Kawakami 2012). One study used a sphincterotome with the guidewire‐assisted cannulation technique and a catheter with the contrast‐assisted cannulation technique (Nambu 2011). One study, in abstract format, did not report what cannulation device was used with either cannulation technique (Mangiavillano 2011). One study, in abstract format, reported the use of a sphincterotome or a catheter with the guidewire‐assisted cannulation technique but did not report the cannulation device used with the contrast‐assisted cannulation technique (Kobayashi 2010).
Post‐ERCP pancreatitis (PEP)
Eight studies used a sphincterotome for both cannulation techniques (Apostolopoulos 2005; Artifon 2007; Bailey 2008; Gruchy 2007; Kawakami 2012; Lee 2009; Lella 2004; Mangiavillano 2007) and reported the rates of PEP, and comprised a total of 1220 participants in the guidewire‐assisted cannulation technique and 1113 in the contrast‐assisted cannulation technique groups. Unweighted pooled rates of PEP were 2.7% with the guidewire‐assisted cannulation technique and 6.8% with the contrast‐assisted cannulation technique. There was significant heterogeneity among the studies for this analysis (P = 0.03, I² = 54%). In studies that used a sphincterotome for both cannulation techniques, the guidewire‐assisted cannulation technique significantly reduced PEP compared to the contrast‐assisted cannulation technique (RR 0.37, 95% CI 0.18 to 0.76; P = 0.006; Analysis 8.1). The NNT was 26 (95% CI 16 to 74). In sensitivity analyses, the results remained robust with OR or a fixed‐effect model. The results also remained robust in a post hoc analysis with the exclusion of the only high risk of bias study for incomplete outcome data (Gruchy 2007).
Analysis 8.1.
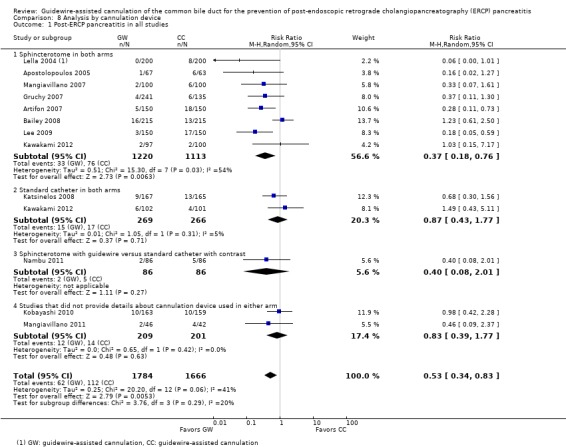
Comparison 8 Analysis by cannulation device, Outcome 1 Post‐ERCP pancreatitis in all studies.
Two studies used a catheter for both cannulation techniques (Katsinelos 2008; Kawakami 2012) and reported the rates of PEP for all randomised patients, comprising a total of 269 participants in the guidewire‐assisted cannulation technique and 266 in the contrast‐assisted cannulation technique groups. Unweighted pooled rates of PEP were 5.6% with the guidewire‐assisted cannulation technique and 6.4% with the contrast‐assisted cannulation technique. There was no significant heterogeneity among the studies for this analysis (P = 0.31, I² = 5%). In studies that used a catheter for both cannulation techniques, there was no statistically significant difference in the rates of PEP between the two cannulation techniques (RR 0.87, 95% CI 0.43 to 1.77; P = 0.71; Analysis 8.1).
One study used a sphincterotome with the guidewire‐assisted cannulation technique and a catheter with the contrast‐assisted cannulation technique (Nambu 2011). The rates of PEP were 2.3% with the guidewire‐assisted cannulation technique and 5.8% with the contrast‐assisted cannulation technique. There was no statistically significant difference in the rates of PEP between the two cannulation techniques (RR 0.40, 95% CI 0.08 to 2.01; P = 0.27; Analysis 8.1).
Two studies did not report the cannulation device used for either of the two cannulation techniques (Kobayashi 2010; Mangiavillano 2011) and reported the rates of PEP for all randomised patients, comprising a total of 209 participants in the guidewire‐assisted cannulation technique and 201 in the contrast‐assisted cannulation technique groups. Unweighted pooled rates of PEP were 5.7% for the guidewire‐assisted cannulation technique and 7.0% for the contrast‐assisted cannulation technique. There was no significant heterogeneity for this analysis (P = 0.42, I² = 0%). There was no statistically significant difference in the rates of PEP between the two cannulation techniques (RR 0.83, 95% CI 0.39 to 1.77; P = 0.63; Analysis 8.1).
Most importantly, the test for subgroup differences indicated no statistically significant differences between the two subgroups (sphincterotome with both techniques versus catheter with both techniques) (P = 0.06) or among the four subgroups (P = 0.17) for the outcome of PEP.
Primary cannulation success
Eight studies used a sphincterotome for both cannulation techniques (Apostolopoulos 2005; Artifon 2007; Bailey 2008; Gruchy 2007; Kawakami 2012; Lee 2009; Lella 2004; Mangiavillano 2007). All except one study (Gruchy 2007) reported the primary cannulation success rates for all randomised patients, comprising a total of 979 participants in the guidewire‐assisted cannulation technique and 978 in the contrast‐assisted cannulation technique groups. Unweighted pooled primary cannulation success rates were 85.6% for the guidewire‐assisted cannulation technique and 80.8% for the contrast‐assisted cannulation technique. There was significant heterogeneity among the studies for this analysis (P = 0.002, I² = 72%). In studies that used a sphincterotome for both cannulation techniques, there was no statistically significant difference in the primary cannulation success rates between the two cannulation techniques (RR 1.05, 95% CI 0.99 to 1.11; P = 0.12, Analysis 8.2). In sensitivity analyses, the results became statistically significant with OR (OR 1.44, 95% CI 1.05 to 1.96; P = 0.02) or a fixed‐effect model (RR 1.06, 95% CI 1.02 to 1.10; P = 0.004) favouring the guidewire‐assisted cannulation technique.
Analysis 8.2.
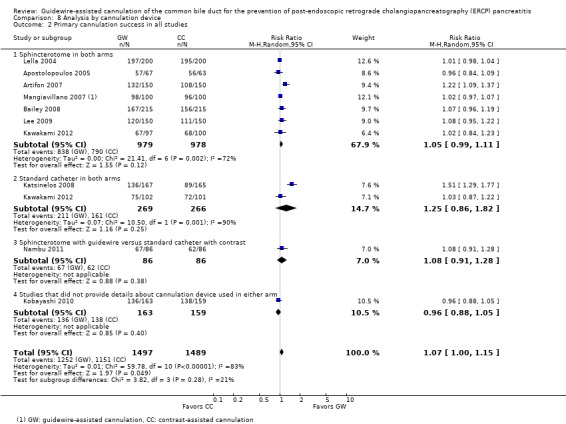
Comparison 8 Analysis by cannulation device, Outcome 2 Primary cannulation success in all studies.
Two studies used a catheter for both cannulation techniques (Katsinelos 2008; Kawakami 2012) and reported the primary cannulation success rates for all randomised patients, comprising a total of 269 participants in the guidewire‐assisted cannulation technique and 266 in the contrast‐assisted cannulation technique groups. Unweighted pooled primary cannulation success rates were 78.4% with the guidewire‐assisted cannulation technique and 60.5% for the contrast‐assisted cannulation technique. There was significant heterogeneity among the studies for this analysis (P = 0.001, I² = 90%). In studies that used a catheter for both cannulation techniques, there was no statistically significant difference in the primary cannulation success rates between the two cannulation techniques (RR 1.25, 95% CI 0.86 to 1.82; P = 0.25; Analysis 8.2). In sensitivity analyses, the results remained non‐significant with OR but became statistically significant with a fixed‐effect model (RR 1.30, 95% CI 1.15 to 1.46; P < 0.0001).
One study used a sphincterotome with the guidewire‐assisted cannulation technique and a catheter with the contrast‐assisted cannulation technique (Nambu 2011). The primary cannulation rates were 77.9% with the guidewire‐assisted cannulation technique and 72.1% with the contrast‐assisted cannulation technique. There was no statistically significant difference in the primary cannulation success rates between the two cannulation techniques (RR 1.08, 95% CI 0.91 to 1.28; P = 0.38; Analysis 8.2).
Two studies did not report the cannulation device used for either of the two cannulation techniques (Kobayashi 2010; Mangiavillano 2011), and only one study (Kobayashi 2010) provided primary cannulation success rates for all randomised patients. The primary cannulation rates were 83.4% with the guidewire‐assisted cannulation technique and 86.8% with the contrast‐assisted cannulation technique. There was no statistically significant difference in the primary cannulation rates between the two cannulation techniques (RR 0.96, 95% CI 0.88 to 1.05; P = 0.40; Analysis 8.2).
Most importantly, the test for subgroup differences indicated statistically significant differences between the two subgroups (sphincterotome with both techniques versus catheter with both techniques) (P = 0.001) and among the four subgroups (P = 0.001) for the outcome of primary cannulation success, significantly favouring the guidewire‐assisted technique when a catheter was used for cannulation.
'Non‐crossover' studies
Post‐ERCP pancreatitis (PEP)
All five 'non‐crossover' studies used a sphincterotome for both cannulation techniques and reported the rates of PEP for all randomised patients (Apostolopoulos 2005; Artifon 2007; Lee 2009; Lella 2004; Mangiavillano 2007), comprising a total of 667 participants in the guidewire‐assisted cannulation technique and 663 in the contrast‐assisted cannulation technique groups. There was no significant heterogeneity among the studies for this analysis (P = 0.81, I² = 0%). Unweighted pooled rates of PEP for participants were 1.6% for the guidewire‐assisted cannulation technique and 8.3% for the contrast‐assisted cannulation technique. In 'non‐crossover' studies that used a sphincterotome for both cannulation techniques, the guidewire‐assisted cannulation technique significantly reduced PEP compared to the contrast‐assisted cannulation technique (RR 0.22, 95% CI 0.12 to 0.42; P < 0.00001; Analysis 8.3). The NNT was 16 (95% CI 11 to 30). In sensitivity analyses, the results remained with robust with OR or a fixed‐effect model.
Analysis 8.3.
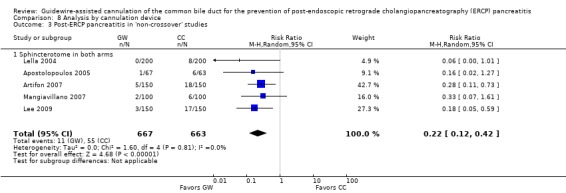
Comparison 8 Analysis by cannulation device, Outcome 3 Post‐ERCP pancreatitis in 'non‐crossover' studies.
Subgroup analysis according to cannulation device could not be performed as all 'non‐crossover' studies used a sphincterotome for both cannulation techniques.
Primary cannulation success
All five 'non‐crossover' studies used a sphincterotome for both cannulation techniques and reported the primary cannulation success rates for all randomised patients (Apostolopoulos 2005; Artifon 2007; Lee 2009; Lella 2004; Mangiavillano 2007), comprising a total of 667 participants in the guidewire‐assisted cannulation technique and 663 in the contrast‐assisted cannulation technique groups. There was significant heterogeneity among the studies for this analysis (P = 0.0002, I² = 82%). Unweighted pooled primary cannulation rates were 90.6% for the guidewire‐assisted cannulation technique and 85.4% for the contrast‐assisted cannulation technique. There was no statistically significant difference in the primary cannulation success rates between the two cannulation techniques (RR 1.05, 95% CI 0.97 to 1.13; P = 0.21; Analysis 8.4). In sensitivity analyses, the results became statistically significant with OR (OR 1.64, 95% CI 1.01 to 2.68; P = 0.05) and a fixed‐effect model (RR 1.06, 95% CI 1.02 to 1.10; P = 0.003).
Analysis 8.4.
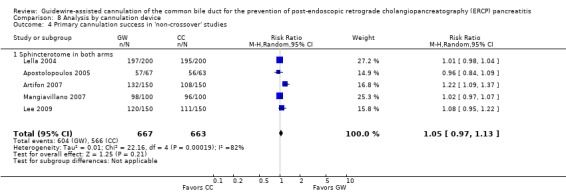
Comparison 8 Analysis by cannulation device, Outcome 4 Primary cannulation success in 'non‐crossover' studies.
Subgroup analysis according to cannulation device could not be performed as all 'non‐crossover' studies used a sphincterotome for both cannulation techniques.
Analysis 9: guidewire‐assisted cannulation compared to contrast‐assisted cannulation according to involvement of trainees in cannulation
'Cross‐over' and 'non‐crossover' studies
Trainees were allowed to start cannulation in five studies (Bailey 2008; Gruchy 2007; Kawakami 2012; Kobayashi 2010; Nambu 2011). If cannulation was unsuccessful after a predefined cannulation time limit, the experienced endoscopists would take over the procedure. In other studies (Apostolopoulos 2005; Artifon 2007; Katsinelos 2008; Lee 2009; Lella 2004), experienced endoscopists performed all procedures. Two studies (Mangiavillano 2007; Mangiavillano 2011) did not provide information as to whether trainees were involved in cannulation.
Post‐ERCP pancreatitis (PEP)
Five studies with involvement of only experienced endoscopists in cannulation reported the rates of PEP for all randomised patients (Apostolopoulos 2005; Artifon 2007; Katsinelos 2008; Lee 2009; Lella 2004), comprising a total of 734 participants in the guidewire‐assisted cannulation technique and 728 in the contrast‐assisted cannulation technique groups. There was significant heterogeneity among the studies (P = 0.18, I² = 37%). Unweighted pooled rates of PEP were 2.5% for the guidewire‐assisted cannulation technique and 8.5% for the contrast‐assisted cannulation technique. In studies with involvement of only experienced endoscopists, the guidewire‐assisted cannulation technique significantly reduced the rates of PEP compared to the contrast‐assisted cannulation technique (RR 0.29, 95% CI 0.14 to 0.60; P = 0.0009; Analysis 9.1). The NNT was 17 (95% CI 12 to 33). In sensitivity analyses, the results remained robust with OR or a fixed‐effect model.
Analysis 9.1.
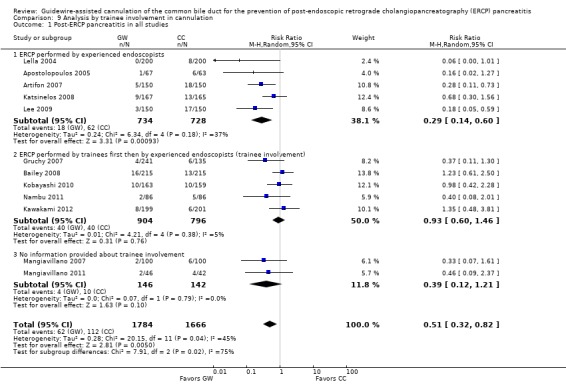
Comparison 9 Analysis by trainee involvement in cannulation, Outcome 1 Post‐ERCP pancreatitis in all studies.
Five studies had involvement of trainees in cannulation and reported the rates of PEP (Bailey 2008; Gruchy 2007; Kawakami 2012; Kobayashi 2010; Nambu 2011). Unweighted pooled rates of PEP were 4.4% for the guidewire‐assisted cannulation technique and 5.0% for the contrast‐assisted cannulation technique. There was no significant heterogeneity among the studies (P = 0.38, I² = 5%). In studies with involvement of trainees in cannulation, there was no statistically significant difference in the rates of PEP between the two cannulation techniques (RR 0.93, 95% CI 0.60 to 1.46; P = 0.76; Analysis 9.1). In sensitivity analysis, the results remained non‐significant with OR or a fixed‐effect model. The results also remained non‐significant in a post hoc analysis with the exclusion of the only high risk of bias study for incomplete outcome data (Gruchy 2007).
Two studies did not provide information as to whether trainees were involved in cannulation (Mangiavillano 2007; Mangiavillano 2011), and comprised a total of 146 participants in the guidewire‐assisted cannulation technique and 142 in the contrast‐assisted cannulation technique groups. There was no significant heterogeneity among the studies (P = 0.79, I² = 0%). Unweighted pooled rates of PEP were 2.7% for the guidewire‐assisted cannulation technique and 5.6% for the contrast‐assisted cannulation technique. In studies that did not provide information as to whether trainees were involved in cannulation, there was no statistically significant difference in the rates of PEP between the two cannulation techniques (RR 0.39, 95% CI 0.12 to 1.21; P = 0.10; Analysis 9.1).
Most importantly, the test for subgroup differences indicated statistically significant differences between the two subgroups (studies with versus without trainee involvement in cannulation) (P = 0.0006) or for the three subgroups (P = 0.002) for the outcome of PEP, favouring the guidewire‐assisted cannulation technique when only experienced endoscopists were involved in cannulation.
Primary cannulation success
Five studies had involvement of only experienced endoscopists in cannulation and reported the primary cannulation success rates for all randomised patients (Apostolopoulos 2005; Artifon 2007; Katsinelos 2008; Lee 2009; Lella 2004), comprising a total of 734 participants in the guidewire‐assisted cannulation technique and 728 in the contrast‐assisted cannulation technique groups. Unweighted pooled primary cannulation success rates were 87.5% for the guidewire‐assisted cannulation technique and 76.8% for the contrast‐assisted cannulation technique. There was significant heterogeneity among the studies (P < 0.00001, I² = 96%). In studies that had involvement of only experienced endoscopists in cannulation, there was no statistically significant difference in the primary cannulation success rates between the two cannulation techniques (RR 1.14, 95% CI 0.92 to 1.40; P = 0.23; Analysis 9.2). In sensitivity analyses, the results became statistically significant with OR (OR 1.96, 95% CI 1.11 to 3.45; P = 0.02) and a fixed‐effect model (RR 1.14, 95% CI 1.09 to 1.19; P < 0.00001).
Analysis 9.2.
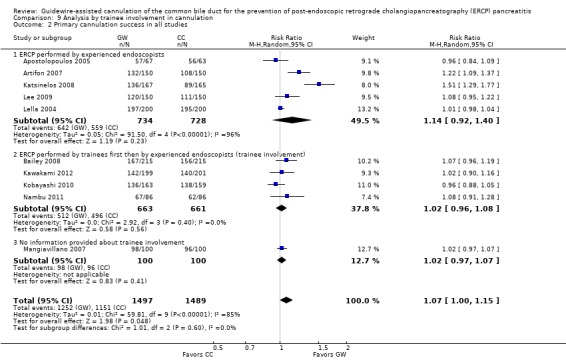
Comparison 9 Analysis by trainee involvement in cannulation, Outcome 2 Primary cannulation success in all studies.
Five studies had involvement of trainees in cannulation (Bailey 2008; Gruchy 2007; Kawakami 2012; Kobayashi 2010; Nambu 2011). All except one study (Gruchy 2007) reported primary cannulation success rates. Unweighted pooled primary cannulation rates were 77.2% for the guidewire‐assisted cannulation technique and 75.0% for the contrast‐assisted cannulation technique. There was no significant heterogeneity among the studies (P = 0.40, I² = 0%). In studies that had involvement of trainees in cannulation, there was no statistically significant difference in the primary cannulation rates between the two cannulation techniques (RR 1.02, 95% CI 0.96 to 1.08; P = 0.56; Analysis 9.2). In sensitivity analyses, the results remained non‐significant with OR or a fixed‐effect model.
Two studies did not provide information as to whether trainees were involved in cannulation (Mangiavillano 2007; Mangiavillano 2011). Only one study reported primary cannulation success rates (Mangiavillano 2007). The primary cannulation success rates were 98.0% for the guidewire‐assisted cannulation technique and 96.0% for the contrast‐assisted cannulation technique. There was no statistically significant difference in the primary cannulation rates between the two cannulation techniques (RR 1.02, 95% CI 0.97 to 1.07; P = 0.41; Analysis 9.2).
Most importantly, the test for subgroup differences indicated statistically significant differences between the two subgroups (studies with versus without trainee involvement in cannulation) (P = 0.008) or three subgroups (P = 0.002) for the outcome of primary cannulation success, favouring the guidewire‐assisted cannulation technique when only experienced endoscopists were involved in cannulation.
'Non‐crossover' studies
Post‐ERCP pancreatitis (PEP)
Among the five 'non‐crossover' studies, experienced endoscopists performed all procedures in four studies (Apostolopoulos 2005; Artifon 2007; Lee 2009; Lella 2004), and one study did not provide information as to whether trainees were involved in cannulation (Mangiavillano 2007). No 'non‐crossover' studies had involvement of trainees in cannulation.
All four 'non‐crossover' studies with involvement of only experienced endoscopists in cannulation reported the rates of PEP for all randomised patients (Apostolopoulos 2005; Artifon 2007; Lee 2009; Lella 2004), comprising a total of 567 participants in the guidewire‐assisted cannulation technique and 563 in the contrast‐assisted cannulation technique. There was no significant heterogeneity among the studies for this analysis (P = 0.73, I² = 0%). Unweighted pooled rates of PEP for participants were 1.6% for the guidewire‐assisted cannulation technique and 8.7% for the contrast‐assisted cannulation technique. In 'non‐crossover' studies with involvement of only experienced endoscopists in cannulation, the guidewire‐assisted cannulation technique significantly reduced PEP compared to the contrast‐assisted cannulation technique (RR 0.21, 95% CI 0.10 to 0.41; P < 0.00001; Analysis 9.3). The NNT was 15 (95% CI 10 to 30). In sensitivity analyses, the results remained robust with OR and a fixed‐effect model.
Analysis 9.3.
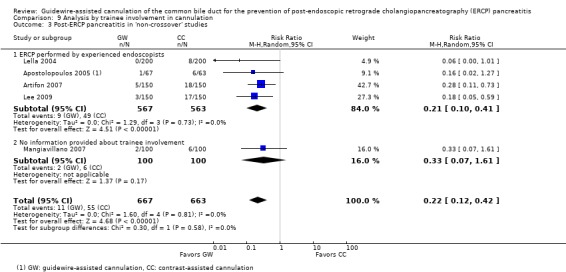
Comparison 9 Analysis by trainee involvement in cannulation, Outcome 3 Post‐ERCP pancreatitis in 'non‐crossover' studies.
One 'non‐crossover' study did not provide information as to whether trainees were involved in cannulation (Mangiavillano 2007), and comprised a total of 100 participants in the guidewire‐assisted cannulation technique and 100 in the contrast‐assisted cannulation technique groups. The rates of PEP for participants were 2.0% for the guidewire‐assisted cannulation technique and 6.0% for the contrast‐assisted cannulation technique. There was no statistically significant difference in the rates of PEP between the two cannulation techniques (RR 0.33, 95% CI 0.07 to 1.61; P = 0.17; Analysis 9.3).
Most importantly, the test for subgroup differences indicated no statistically significant differences between the two subgroups (studies with trainee involvement in cannulation versus studies with uncertain trainee involvement in cannulation) for the outcome of PEP (P = 0.52).
Primary cannulation success
All four 'non‐crossover' studies with involvement of only experienced endoscopists in cannulation reported primary cannulation success rates for all randomised patients (Apostolopoulos 2005; Artifon 2007; Lee 2009; Lella 2004), comprising a total of 567 participants in the guidewire‐assisted cannulation technique and 563 in the contrast‐assisted cannulation technique. Unweighted pooled primary cannulation success rates were 89.2% for the guidewire‐assisted cannulation technique and 83.5% for the contrast‐assisted cannulation technique. There was significant heterogeneity among the studies for this analysis (P < 0.0001, I² = 87%). There was no statistically significant difference in the primary cannulation success rates between the two cannulation techniques (RR 1.06, 95% CI 0.93 to 1.21; P = 0.37; Analysis 9.4). In sensitivity analyses, the results remained non‐significant with OR but became statistically significant with a fixed‐effect model (RR 1.07, 95% CI 1.02 to 1.12, P = 0.004).
Analysis 9.4.
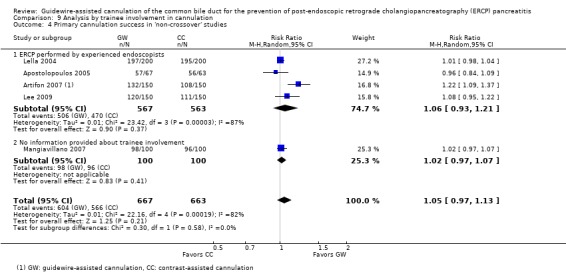
Comparison 9 Analysis by trainee involvement in cannulation, Outcome 4 Primary cannulation success in 'non‐crossover' studies.
One 'non‐crossover' study did not provide information as to whether trainees were involved in cannulation (Mangiavillano 2007), and comprised a total of 100 participants in the guidewire‐assisted cannulation technique and 100 in the contrast‐assisted cannulation technique groups. The primary cannulation success rates for participants were 98.0% for the guidewire‐assisted cannulation technique and 96.0% for the contrast‐assisted cannulation technique. There was no statistically significant difference in the rates of PEP between the two cannulation techniques (RR 1.02, 95% CI 0.97 to 1.07; P = 0.41; Analysis 9.4).
Most importantly, the test for subgroup differences indicated no statistically significant differences between the two subgroups (studies with trainee involvement in cannulation versus studies with uncertain trainee involvement in cannulation) for the outcome of primary cannulation success (P = 0.17).
Discussion
This systematic review and meta‐analysis assessed the clinical effectiveness and safety of the guidewire‐assisted cannulation technique compared to the contrast‐assisted cannulation technique in the common bile duct (CBD) for the prevention of post‐ERCP pancreatitis (PEP) and for achieving selective biliary cannulation. This has been a complex area to review systemically due to the variability in trial designs. These include permission versus non‐permission of technique 'cross‐over' when the randomised technique failed, the use of precut sphincterotomy as a rescue technique, the variable definitions used by the primary studies for difficult cannulation before technique 'cross‐over' or resorting to precut sphincterotomy, the use of prophylactic pancreatic duct (PD) stents, the different cannulation devices used between intervention arms, the variable number and experience of endoscopists, and the involvement of trainees in cannulation. Furthermore, studies have used different criteria to diagnose and grade the severity of PEP.
To standardize the definitions of PEP and to allow comparability between trials, we determined a priori that PEP, as defined by the consensus definition (Cotton 1991), to be the most important primary outcome. According to the consensus definition, PEP was defined by the presence of abdominal pain characteristic of pancreatitis associated with a serum amylase level of at least three times above the upper limit of normal at 24 hours after the procedure, together with an unplanned hospital stay or an extension of a planned hospital stay by at least two days (Cotton 1991). We also defined the severity of PEP based on the consensus criteria (Cotton 1991) depending on the number of days of hospitalisation and local complications secondary to pancreatitis as mild (hospital stay of up to three days), moderate (hospital stay for four to 10 days) and severe (hospital stay for more than 10 days with a significant complication). Although it appears the consensus definition (abdominal pain associated with a serum amylase level at least three times above the upper limit of normal at 24 hours after ERCP) was used by most studies to define PEP (Apostolopoulos 2005; Artifon 2007; Bailey 2008; Gruchy 2007; Katsinelos 2008; Kawakami 2012; Kobayashi 2010; Lee 2009; Mangiavillano 2011; Nambu 2011), procedure‐related hospital stay was specifically stated as part of the diagnostic criteria by only one study (Gruchy 2007). In one study (Lella 2004), a serum amylase level of at least five times the upper limit of normal was used, which may have reduced the apparent rate of PEP. In one study (Artifon 2007) all patients were admitted for overnight observation after ERCP. As a result, patients were more likely to undergo laboratory and radiological evaluation of abdominal pain as opposed to being discharged home following ERCP. This may have increased the apparent rate of PEP (Artifon 2007). One study (Mangiavillano 2007) did not specify the criteria for the diagnosis of PEP. Severity of PEP was graded using the consensus criteria in six studies (Bailey 2008; Katsinelos 2008; Kawakami 2012; Kobayashi 2010; Lee 2009; Nambu 2011). Two studies (Apostolopoulos 2005; Artifon 2007) graded severity using the Ranson's criteria (Ranson 1974) and the Balthazar grading system (Balthazar 1990). Two studies (Lella 2004; Mangiavillano 2007) did not specify the criteria used for severity assessment. Two other studies did not provide outcome data regarding the severity of PEP (Gruchy 2007; Mangiavillano 2011).
Because the definitions and grading of severity of PEP were variable between studies, we decided to accept the definitions used by the primary studies for this review. We acknowledge that the variable definitions used by the primary studies are likely to introduce heterogeneity in the analyses. However, the definition of PEP still remains a controversial issue. The consensus definition (Cotton 1991) has not been adopted widely, and varying definitions of PEP have been used both in research and in clinical practice. In our review, the incidence of PEP ranged from 2.0% (Lella 2004) to 8.3% (Artifon 2007) among the included studies (Table 12). This varying incidence of PEP may be attributable to a combination of factors, differences in patient populations (case mix), techniques performed during the procedure, endoscopic expertise, definitions of PEP used, methods of data collection, and completeness of follow‐up and assessment (Freeman 2004a; Testoni 2002).
Summary of main results
We have adopted the GRADE approach to evaluate and rate the quality of evidence for each clinical outcome reported in this systematic review (Guyatt 2008). RCTs without important limitations constitute high quality evidence. However, there are five factors that can lower the quality of evidence, 1. limitations in the design and implementation of available studies suggesting high likelihood of bias, 2. indirectness of evidence (indirect population, intervention, control, outcomes), 3. unexplained heterogeneity or inconsistency of results (including problems with subgroup analyses), 4. imprecision of results (wide confidence intervals), and 5. high probability of publication bias. In addition, there are three factors that can increase the quality of evidence, 1. large magnitude of treatment effect, 2. a dose‐response gradient, and 3. all plausible confounding would reduce a demonstrated effect or suggest a spurious effect when results show no effect.
Post‐ERCP pancreatitis (PEP)
Post‐ERCP pancreatitis (PEP) is the primary outcome of this analysis. We found that the guidewire‐assisted cannulation technique significantly reduced PEP compared to the conventional contrast‐assisted cannulation technique. Heterogeneity in this analysis could be explained by differences in trial design ('cross‐over' versus 'non‐crossover' to the alternative technique if the randomised technique failed), publication type and risk of bias (random sequence generation). Other factors such as the use of a prophylactic PD stent, cannulation devices (sphincterotome versus standard catheter), and involvement of trainees in cannulation also contribute to heterogeneity, but these factors could be confounded by trial design due to overlap in comparison groups between the trial design and these subgroups. Most information is obtained from studies with high risk of bias for blinding of participants and personnel (the endoscopists). This may have an impact on the rates of PEP depending on the preference and expertise of the endoscopists performing the procedure. Overall, the qualify of evidence for PEP is moderate. Hence, further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. See Table 1.
Severity of post‐ERCP pancreatitis (PEP)
The severity of PEP is an important clinical outcome as it correlates with mortality, complications and length of hospital stay. We found that the guidewire‐assisted cannulation technique significantly reduced the risk of mild PEP compared to the contrast‐assisted technique. However, there was no statistically significant difference in the rates of moderate or severe PEP between the two cannulation techniques. The event rates for moderate and severe PEP were low, resulting in wide confidence intervals around the estimates. The results are therefore consistent with either no effect or inadequate power to rule out clinically important differences between the two cannulation techniques. The quality of evidence for the severity of PEP is low due to significant heterogeneity and inclusion of high risk of bias studies for blinding of participants and personnel (the endoscopists).
Primary cannulation success
Primary cannulation success is an important benchmark of successful ERCP. A high primary cannulation success rate reduces the risk of difficult cannulation, repeated cannulation attempts and the need for precut sphincterotomy, all of which have been reported as independent procedure‐related risk factors for PEP (Freeman 2001). We found a significantly higher primary cannulation rate with the guidewire‐assisted cannulation technique than with the contrast‐assisted cannulation technique. Heterogeneity in this analysis could be explained by involvement of trainees in cannulation. However, this factor could also be confounded by trial design. The quality of evidence for primary cannulation success is low due to significant heterogeneity, which could be explained by trial design, and inclusion of high risk of bias studies for blinding of participants and personnel (the endoscopists). Lack of blinding of endoscopists may introduce bias due to the endoscopist's experience and preference of techniques.
Secondary cannulation success
Secondary cannulation success is defined as successful cannulation after 'cross‐over' to the alternative technique when the randomised technique failed. This is an important outcome as a high secondary cannulation success rate may reduce the need for precut sphincterotomy or the risk of a failed procedure. Among 'cross‐over' studies, we found no difference in the 'cross‐over' rates between the two cannulation techniques. We also found no difference in the cannulation success rate after 'cross‐over' to either technique. The quality of evidence for secondary cannulation success is low due to significant heterogeneity and the inclusion of high risk of bias studies for blinding of participants and personnel (the endoscopists). Lack of blinding of endoscopists may introduce bias due to the endoscopist's experience and preference of techniques.
Overall cannulation success
Overall cannulation success is an important outcome as failed procedures usually necessitate repeat ERCP or a radiological or a surgical procedure, which carry additional costs and risks (Perdue 2004). We found no statistically significant difference in the overall cannulation success rates between the two cannulation techniques. However, this outcome is difficult to interpret because the overall effect could be diluted by 'cross‐over' studies and the use of precut as a rescue technique. The quality of evidence for overall cannulation success is low due to significant heterogeneity and the inclusion of high risk of bias studies for blinding of participants and personnel (the endoscopists). Lack of blinding of endoscopists may introduce bias due to the endoscopist's experience and preference of techniques.
The need for precut sphincterotomy
The need for precut sphincterotomy is an important clinical outcome as it has been reported to be associated with an increased risk of complications including PEP, bleeding and perforation (Cennamo 2010; Freeman 2001; Masci 2003). We found that the need for precut sphincterotomy was significantly reduced by the use of the guidewire‐assisted cannulation technique compared to the contrast‐assisted cannulation technique. The quality of evidence for the use of precut sphincterotomy is low due to the inclusion of high risk of bias studies for blinding of participants and personnel (the endoscopists). Lack of blinding of endoscopists may introduce bias due to the endoscopist's experience and preference of techniques.
Inadvertent PD cannulation or injection
Inadvertent PD manipulation (cannulation or injection) has been reported to be associated with an increased risk of PEP (Masci 2003; Vandervoort 2002). We found a non‐significant trend towards less inadvertent PD manipulation with the guidewire‐assisted cannulation technique compared to the contrast‐assisted cannulation technique. The results are consistent with either no effect or inadequate power to rule out clinically important differences between the two cannulation techniques. The quality of the evidence for inadvertent PD cannulation or injection is low due to significant heterogeneity and inclusion of high risk of bias studies for blinding of participants and personnel (the endoscopists). Lack of blinding of endoscopists may introduce bias due to the endoscopist's experience and preference of techniques.
ERCP‐related complications
With regard to safety endpoints, there was no statistically significant difference in the risk of post‐sphincterotomy bleeding between the two cannulation techniques. However, the result is compatible with either no difference in bleeding risk between the two cannulation techniques or inadequate power to rule out clinically important differences. Nevertheless, the risk of bleeding appeared to be low and most bleeding episodes either stopped spontaneously or with medical or endoscopic therapies. Overall, the risks of perforation, cholangitis and mortality appeared to be very low.
Summary of findings on subgroup analyses
Meta‐analyses on subgroups of the studies were performed to explore sources of heterogeneity. Meta‐regression was not performed given the small number of included studies. We prespecified all subgroup analyses based on scientific rationale. Due to the observational nature of subgroup analyses, the following results should be considered hypothesis generating for further testing rather than evidence that should change practice. Furthermore, differences between subgroups, particularly those that correspond to differences between studies, need to be interpreted cautiously since chance variation between subgroups is inevitable. The rationale for and the limitations of the analyses are discussed followed by a summary of the findings on each subgroup analysis.
Trial design
Endoscopic trials usually involve comparison of an established technique with a new technique. 'Cross‐over' between interventions is not uncommon due to unforeseen technical challenges or endoscopic findings. This perceived need for 'cross‐over' may be motivated by the moral imperative to avoid the potential adverse consequences of failed ERCP, complications, and the need for repeat ERCP, percutaneous transhepatic cholangiography or surgery. This 'cross‐over' design, however, should not be confused with trials in which all participants are randomised to a sequence of treatments or interventions. In this review, technique 'cross‐over' was permitted in seven included studies (Bailey 2008; Gruchy 2007; Katsinelos 2008; Kawakami 2012; Kobayashi 2010; Mangiavillano 2011; Nambu 2011); patients were permitted to 'cross‐over' to the alternative technique if the randomised technique failed to achieve biliary cannulation within a predefined cannulation limit. Among 'cross‐over' studies, the percentage of patients requiring 'cross‐over' to the alternative technique ranged from 15.2% (Kobayashi 2010) to 32.2% (Katsinelos 2008). These 'cross‐over' studies are at risk for contamination due to the unavoidable carry‐over effects in the subgroup of patients who received the alternative technique after failing the assigned technique. Hence, an observed effect cannot be attributed to the randomised intervention alone. Furthermore, the 'cross‐over' effect can substantially reduce the power of a trial to find an overall treatment difference. There is also the concern of differential procedural 'cross‐over' bias. In some studies, there were disproportionately larger numbers of patients who required 'cross‐over' from the contrast‐assisted cannulation technique to the guidewire‐assisted cannulation technique (Bailey 2008; Katsinelos 2008) than vice versa. This may well be due to lower primary cannulation success with the contrast‐assisted cannulation technique than with the guidewire‐assisted cannulation technique. Alternatively, it is conceivable that this may also reflect the preference or expertise of the endoscopist, and the trial results may therefore be biased. Nevertheless, restricting analyses to patients who did not 'cross‐over' would certainly produce biased results because those who required 'cross‐over' were likely to be more difficult to cannulate and therefore carried a higher risk of PEP than those who did not 'cross‐over'. We therefore decided a priori to investigate trial design as a potential source of heterogeneity by performing subgroup analysis ('cross‐over' versus 'non‐crossover' studies).
In our prespecified subgroup analysis according to trial design, we found that the guidewire‐assisted cannulation technique significantly reduced PEP compared to the contrast‐assisted cannulation technique only in 'non‐crossover' studies, and not in 'cross‐over' studies. Most importantly, the test for subgroup differences indicated statistically significant differences between 'non‐crossover' and 'cross‐over' studies. Although it is unlikely that in clinical practice biliary cannulation is performed with either technique alone, our results may support the use of guidewire‐assisted cannulation as the most appropriate first‐line cannulation technique. Overall, trial design appears to be a significant source of heterogeneity for the outcome of PEP, and does have a significant impact on the effect estimates of PEP.
Publication type
The inclusion of unpublished data in meta‐analyses is controversial. There is empirical evidence to suggest that published studies are more likely to report statistically significant or clinically favourable results than unpublished studies (Dickersin 1987; Easterbrook 1991; Eyding 2010). This is likely to be due to studies with negative results not being submitted for publication rather than being rejected after submission. When unfavourable results of clinical trials are not published, meta‐analyses and systematic reviews that are based only on published data may overestimate the treatment effects. On the other hand, unpublished studies may be of lower methodological quality than published studies (Cook 1993), and their inclusion may therefore compromise the validity of a meta‐analysis. However, previous studies have found no significant methodological differences between published and unpublished studies (Dickersin 1987; Easterbrook 1991). To ameliorate the effects of publication bias, we included both published and unpublished studies in our meta‐analysis. We also decided a priori to investigate publication type as a potential source of heterogeneity by comparing the results of published and unpublished studies.
Among fully published studies, there was a non‐significant trend towards less PEP with the guidewire‐assisted cannulation technique compared to the contrast‐assisted cannulation technique. Among unpublished studies, the guidewire‐assisted cannulation technique significantly reduced PEP compared to the contrast‐assisted cannulation technique. However, the test for subgroup differences indicated no statistically significant differences between fully published versus unpublished studies for the outcome of PEP. Overall, publication type appears to be a source of heterogeneity for the outcome of PEP but does not have a significant impact on the effect estimates of PEP.
Risk of bias
The success of randomisation depends on two interrelated processes, random sequence generation and allocation concealment. Inadequate sequence generation has been shown to yield exaggerated estimates of intervention effects compared with trials with adequate sequence generation (Als‐Nielsen 2004; Kjaergard 2001; Schulz 2002). This is because selection bias can arise due to selective enrolment and non‐enrolment of participants into a study if the sequence generation is not truly random. Concealment of allocation is important in protecting the merit of randomisation. Without concealment of allocation, investigators may systematically influence group allocation. Empiric studies have shown that inadequate allocation concealment can also lead to exaggerated estimates of treatment effects (Kjaergard 2001; Schulz 1995) but with scope for bias in either direction. In addition, inadequate reporting has been associated with biased treatment estimates (Moher 1998; Schulz 1995). We therefore performed prespecified subgroup analyses according to random sequence generation (unclear versus low risk of bias) and allocation concealment (unclear versus low risk of bias).
Separating low and unclear risk of bias studies for random sequence generation (but not for allocation concealment) reduced statistical heterogeneity for the outcome of PEP. In unclear risk of bias studies for random sequence generation or allocation concealment, the guidewire‐assisted cannulation technique significantly reduced PEP compared to the contrast‐assisted cannulation technique. In contrast, there was no significant difference in the rates of PEP between the two cannulation techniques in low risk of bias studies for random sequence generation or for allocation concealment. However, the test for subgroup differences indicated no statistically significant differences between the two subgroups (according to risk of bias for random sequence generation or for allocation concealment) for the outcome of PEP. Overall, the results of the subgroup analyses would suggest that selection bias does not have a significant impact on the effect estimates for PEP.
Precut sphincterotomy
Precut sphincterotomy is often used after conventional methods of biliary cannulation have failed. Although the use of precut sphincterotomy may improve the cannulation success rate, prospective studies have suggested that it is an independent risk factor for post‐ERCP complications including PEP (Cennamo 2010; Freeman 2001; Masci 2003). However, it remains controversial as to whether precut alone or the repeated attempts at cannulation prior to precut is the culprit factor in the development of PEP (Testoni 2011). We were not able to perform meta‐analysis based on individual patient‐level data because none of the studies reported individual patient data. Subgroup data were provided by three 'non‐crossover' studies (Apostolopoulos 2005; Artifon 2007; Lee 2009). We therefore performed prespecified between‐study subgroup analysis according to the permission of precut sphincterotomy for all included studies and within‐study subgroup analysis for the studies that provided subgroup data (Apostolopoulos 2005; Artifon 2007; Lee 2009).
The permission of precut sphincterotomy as a rescue technique for difficult cannulation was not found to be a significant source of heterogeneity for the outcome of PEP because most of the included studies, with the exception of two (Lella 2004; Mangiavillano 2007), permitted the use of precut sphincterotomy. In our prespecified subgroup analyses according to the use of precut sphincterotomy (permission or non‐permission of precut at study level), the guidewire‐assisted cannulation technique significantly reduced PEP compared to the contrast‐assisted cannulation technique in studies that permitted the use of precut sphincterotomy. Only one study (Lella 2004) did not permit the use of precut sphincterotomy. Based on this one study (Lella 2004), there was a non‐significant trend towards less PEP with the guidewire‐assisted cannulation technique compared to the contrast‐assisted cannulation technique. One study (Mangiavillano 2007), in abstract format, did not report the use of precut sphincterotomy. Most importantly, the test for subgroup differences indicated no statistically significant differences between the two subgroups (studies that permitted the use of precut versus study that did not permit the use of precut). Interestingly, the within‐study subgroup analysis showed the opposite results, suggesting that the guidewire‐assisted cannulation technique significantly reduced the rates of PEP compared to the contrast‐assisted cannulation technique when precut was not used. When precut was used, there was no statistically significant difference in the rates of PEP between the two cannulation techniques. The results are therefore consistent with either no effect or inadequate power to rule out clinically important differences between the two cannulation techniques when precut was used. The test for subgroup differences indicated no statistically significant differences between participants who did or did not undergo precut sphincterotomy for the outcome of PEP. Overall, the results of the between‐study and within‐study subgroup analyses would suggest that the use of precut sphincterotomy does not have a significant impact on the effect estimates for PEP, and the guidewire‐assisted cannulation technique significantly reduced the rates of PEP regardless of whether or not precut was used.
Inadvertent guidewire insertion or contrast injection into the PD (inadvertent PD manipulation)
Several mechanisms have been postulated for the prevention of PEP with the guidewire‐assisted cannulation technique. They include facilitating selective biliary cannulation, limiting papillary trauma, and minimizing inadvertent contrast injection into the main PD or the papilla itself (submucosal injection) and, thereby, reducing the possibility of mechanical, chemical and hydrostatic injury to the pancreas when compared to the contrast‐assisted cannulation technique. However, inadvertent guidewire insertion into the PD (especially when performed repeatedly) may result in injury to the papilla or the PD, increasing the likelihood of PEP. Moreover, it remains unclear whether inadvertent guidewire insertion into the PD is safer than inadvertent contrast injection into the PD with regard to PEP. We were not able to perform meta‐analysis based on individual patient‐level data because none of the studies reported individual patient data. Subgroup data were provided by four 'non‐crossover' studies (Artifon 2007; Lee 2009; Lella 2004; Mangiavillano 2007) and one 'cross‐over' study (Nambu 2011). We therefore performed prespecified within‐study subgroup analysis for the studies that provided subgroup data according to inadvertent guidewire cannulation or contrast injection into the PD (inadvertent PD manipulation).
Among participants with inadvertent PD manipulation, the guidewire‐assisted cannulation technique significantly reduced PEP compared to the contrast‐assisted cannulation. This suggests that the guidewire‐assisted cannulation technique may reduce PEP by avoiding inadvertent contrast injection into the PD. Among participants who did not have inadvertent PD manipulation, the guidewire‐assisted cannulation technique also significantly reduced PEP compared to the contrast‐assisted cannulation technique. This suggests that the guidewire‐assisted cannulation technique may also reduce PEP by limiting repeated cannulation trauma to the papilla. The benefit of the guidewire‐assisted cannulation technique was, however, greater among participants with inadvertent PD manipulation. Most importantly, the test for subgroup differences indicated no statistically significant differences between the participants who did or did not have inadvertent PD manipulation for the outcome of PEP. Our results, therefore, support the hypothesis that the guidewire‐assisted cannulation technique reduces PEP not only by minimizing contrast injection into the PD but also by limiting papillary trauma.
PD stent
Outflow tract edema caused by cannulation trauma to the papilla, inadvertent PD manipulation, and contrast injection may cause obstruction to the flow of pancreatic secretions with subsequent acute pancreatic inflammation. It has been postulated that a stent placed across the injured outflow tract may help to maintain the flow of pancreatic secretions and reduce the intraductal pressure after ERCP. Indeed, PD stent placement in high risk patients has been found to significantly reduce the risk of PEP (Choudhary 2011). We were not able to perform meta‐analysis based on individual patient‐level data because none of the studies reported individual patient data. Separate subgroup data according to the use of a PD stent were also not reported by any of the included studies. We therefore performed prespecified between‐study subgroup analysis according to the permission of a PD stent for all included studies.
Separating studies for the use of PD stents (permission versus non‐permission at study level) reduced statistical heterogeneity for the outcome of PEP. However, there is significant overlap in trial design and the use of PD stents (permission versus non‐permission at study level). All studies that permitted the use of PD stents were 'cross‐over' studies, whereas all except one study (Nambu 2011) that did not permit the use of PD stents were 'non‐crossover' studies. Among the 'cross‐over' studies included for this analysis, the use of PD stents ranged from 4.7% (Bailey 2008) to 8.0% (Kawakami 2012). In contrast, the percentage of patients requiring 'cross‐over' to the alternative technique ranged from 15.2% (Kobayashi 2010) to 32.2% (Katsinelos 2008). Hence, trial design rather than the use of PD stents may be a more important source of heterogeneity for the outcome of PEP. In studies that did not permit the use of PD stents, the guidewire‐assisted cannulation technique significantly reduced PEP compared to the contrast‐assisted cannulation technique. In studies that permitted the use of PD stents, there was no statistically significant difference in the rates of PEP between the two cannulation techniques. The test for subgroup differences indicated statistically significant differences between the two subgroups (studies that permitted the use of PD stents versus studies that did not permit the use of PD stents), favouring the use of guidewire‐assisted cannulation technique in studies that did not permit the use of PD stents. However, the results could be confounded by trial design. Among the 'non‐crossover' studies that did not permit the use of PD stents, the guidewire‐assisted cannulation technique significantly reduced PEP compared to the contrast‐assisted cannulation techniques. As no 'non‐crossover' study permitted the use of PD stents, subgroup analyses restricted to 'non‐crossover' studies according to the use of PD stents (permission versus non‐permission at study level) could not be performed.
Cannulation device
Biliary cannulation is best performed from below with an upward view of the papilla and with the cannulation device in line with the axis of the CBD towards the 11 o'clock position (Freeman 2005). Standard catheters are limited in their ability to vary the angle of approach to the papilla independent of the endoscope to gain biliary access. The tip of the sphincterotome, however, can be adjusted to give preferential upward angulation for selective biliary cannulation. Indeed, RCTs have found the use of a sphincterotome to be superior to that of a standard catheter for achieving selective biliary cannulation, with a significant reduction in cannulation times and in the number of attempts required for selective biliary cannulation (Cortas 1999; Schwacha 2000). Whether the use of a sphincterotome for cannulation results in less PEP is not clear. We therefore performed prespecified between‐study subgroup analyses according to the use of cannulation device (sphincterotome versus standard catheter).
Separating studies according to cannulation device reduced statistical heterogeneity for the outcome of PEP. However, this is largely due to overlap in trial design and the use of a cannulation device, the two studies that used a catheter for both cannulation techniques were also 'cross‐over' studies. In studies that used a sphincterotome for both cannulation techniques, the guidewire‐assisted cannulation technique significantly reduced PEP compared to the contrast‐assisted cannulation technique. In the two studies that used a catheter for both cannulation techniques, there was no statistically significant difference in the rates of PEP between the two cannulation techniques. Most importantly, the test for subgroup differences indicated no statistically significant differences between the two subgroups (sphincterotome with both techniques versus catheter with both techniques) for the outcome of PEP. As all 'non‐crossover' studies used a sphincterotome for both cannulation techniques, subgroup analysis restricted to 'non‐crossover' studies according to cannulation device could not be performed. However, the results could be confounded by trial design.
In studies that used a sphincterotome for both cannulation techniques, there was no statistically significant difference in the primary cannulation success rates between the two cannulation techniques. Similarly, there was no statistically significant difference in the primary cannulation success rates between the two cannulation techniques in studies that used a catheter for both cannulation techniques. The test for subgroup differences, however, indicated statistically significant differences between the two subgroups (sphincterotome with both techniques versus catheter with both techniques) for the outcome of primary cannulation success, significantly favouring the guidewire‐assisted technique when a catheter was used for cannulation. As all 'non‐crossover' studies used a sphincterotome for both cannulation techniques, subgroup analysis restricted to 'non‐crossover' studies according to cannulation device could not be performed.
Involvement of trainees in cannulation
Trainee participation in the procedure has been shown to be a significant risk factor for the development of PEP (Cheng 2006). This increased risk is possibly due to multiple cannulation attempts by trainees. The findings of the UK National Confidential Enquiry into Patient Outcomes and Death relating to ERCP suggested that trainees with experience of > 200 ERCPs had an unsupervised cannulation rate of 66%; this fell to 40% for those with experience of < 200 ERCPs (Williams 2007a). This is in contrast to a cannulation success rate of over 90% in experienced endoscopists. We therefore performed prespecified between‐study subgroup analyses according to the involvement of trainees in cannulation.
Separating studies according to whether trainees were involved in cannulation reduced statistical heterogeneity for the outcome of PEP. However, there is significant overlap in trial design and the involvement of trainees in cannulation. All studies with involvement of trainees in cannulation were 'cross‐over' studies, whereas all but one study (Katsinelos 2008) with involvement of only experienced endoscopists were 'non‐crossover' studies. In studies with involvement of only experienced endoscopists, the guidewire‐assisted cannulation technique significantly reduced the rates of PEP compared to the contrast‐assisted cannulation technique. In studies with involvement of trainees in cannulation, there was no statistically significant difference in the rates of PEP between the two cannulation techniques. Most importantly, the test for subgroup differences indicated statistically significant differences between the two subgroups (studies with versus without trainee involvement in cannulation) for the outcome of PEP, favouring the guidewire‐assisted cannulation technique when only experienced endoscopists were involved in cannulation. However, the results could be confounded by trial design. Among the 'non‐crossover' studies without trainee involvement in cannulation, the guidewire‐assisted cannulation technique significantly reduced PEP compared to the contrast‐assisted cannulation techniques. As no 'non‐crossover' study involved trainees in cannulation, subgroup analyses restricted to 'non‐crossover' studies according to trainee involvement could not be performed.
In studies that involved only experienced endoscopists in cannulation, there was no statistically significant difference in the primary cannulation success rates between the two cannulation techniques. Similarly, in studies that involved trainees in cannulation, there was no statistically significant difference in the primary cannulation rates between the two cannulation techniques. The test for subgroup differences, however, indicated statistically significant differences between the two subgroups (studies with versus without trainee involvement in cannulation) for the outcome of primary cannulation success, favouring the guidewire‐assisted cannulation technique when only experienced endoscopists were involved in cannulation. However, the results could also be confounded by trial design. As no 'non‐crossover' study involved trainees in cannulation, subgroup analyses restricted to 'non‐crossover' studies according to trainee involvement could not be performed.
Overall completeness and applicability of evidence
This systematic review was designed to include trials from around the world regardless of publication status or language of publication. All studies identified by the search could be retrieved in full. Moreover, we were able to obtain unpublished data from authors of primary studies, including data of a completed trial that has been published in abstract format only as an interim analysis (Gruchy 2007). Hence, we believe this review is comprehensive and the results reflect the available evidence for the guidewire‐assisted cannulation technique compared to the conventional contrast‐assisted cannulation technique for the prevention of PEP. Most studies defined PEP as abdominal pain characteristic of pancreatitis associated with a rise in serum amylase level of at least three times the upper limit of normal at 24 hours after the procedure. The participants included in this meta‐analysis had intact papilla and required ERCP for a variety of pancreaticobiliary diseases including CBD stones, pancreaticobiliary malignancies, SOD and idiopathic recurrent pancreatitis. Participants included were of a wide age range (18 to 96 years). There were equal proportions of males and females. Hence, this review is applicable to most patients undergoing ERCP. Studies were conducted in high‐volume, tertiary care settings (seven single‐centre studies and five multi‐centre studies). Procedures were performed by either single or multiple experienced endoscopists, with or without the involvement of trainees. Therefore, the generalizability of findings to low‐volume centres with less expertise in ERCP, especially in the use of the guidewire technique, may be limited. Cannulation was carried out with either a sphincterotome (in most studies) or a catheter using either contrast or a 0.035 inch hydrophilic guidewire. Precut sphincterotomy or 'cross‐over' to the alternative cannulation technique was permitted if the randomised technique failed due to difficult cannulation in most studies. PD stents were used for prophylaxis of PEP in some studies. Overall, we found that the guidewire‐assisted cannulation technique significantly reduced PEP compared to the conventional contrast‐assisted cannulation technique. However, the benefit of the guidewire‐assisted cannulation technique was only apparent in 'non‐crossover' studies and not in 'cross‐over' studies. The results may therefore not be generalizable to endoscopists who employ technique 'cross‐over' in difficult cannulation cases. Additionally, the findings of the subgroup analyses according to the use of PD stents, cannulation device and involvement of trainees in cannulation should be interpreted keeping in mind the significant overlap with trial design.
Quality of the evidence
The quality of evidence for the outcome of PEP is moderate because most information is obtained from studies with high risk of bias for blinding of participants and personnel (the endoscopists). Lack of binding of the endoscopist may have an impact on cannulation success and PEP depending on the experience, expertise and preference of the endoscopist performing the procedure. One study (Gruchy 2007) was considered high risk of bias for incomplete outcome data as 25% of participants were lost to follow‐up. Nevertheless, the results remained robust with the exclusion of this only high risk of bias study for incomplete outcome data (Gruchy 2007). Furthermore, the robustness of the results during sensitivity analyses would support the overall quality and reliability of the evidence and conclusions reached by this review. There was significant heterogeneity for the outcome of PEP, which could be explained by differences in trial design, publication type, and risk of bias (random sequence generation). Therefore, the quality of evidence for the outcome of PEP was not downgraded because of inconsistency or heterogeneity. Other factors such as the use of prophylactic PD stents, cannulation devices, and involvement of trainees in cannulation also contribute to heterogeneity, but these factors could be confounded by trial design due to the overlap in comparison groups between trial design and these subgroups. The results of the subgroup analyses according to the use of PD stents, cannulation devices and involvement of trainees should therefore be considered to be less reliable due to overlap with trial design. Overall, the results of the main analyses (except perforation due to rare events) and subgroup analyses appeared to be precise with narrow confidence intervals. The quality of evidence for the outcomes of primary cannulation success, secondary cannulation success, overall cannulation success and the use of precut sphincterotomy was low because of inclusion of high risk of bias studies for blinding of participants and personnel (the endoscopists). Lack of blinding of the endoscopist may have a significant impact on these outcomes, which are highly dependent on the experience, expertise and preference of the endoscopist performing the procedure.
Potential biases in the review process
We explored small‐study effects (a trend for the smaller studies in a meta‐analysis to show larger treatment effects), of which publication bias is one potential cause, using funnel plots (Figure 4). Visual inspection of the funnel plot suggests asymmetry with a gap in the bottom right side of the graph. This impression is partially caused by one study (Lella 2004) at the bottom left of the most common effect. Although this study (Lella 2004) has the second largest sample size (N = 400) among the included studies, it has the highest standard error due to loss of statistical power resulting from low event rates (zero event in the guidewire‐assisted cannulation arm). The low event rates may be due to the use of a more stringent definition of PEP (amylase more than five times the upper limit of normal) (Lella 2004) compared to the other studies (amylase more than three times the upper limit of normal). In addition, the asymmetry may be due to a lack of negative studies with high standard error and low statistical power. Funnel plot asymmetry tests, however, showed discordant results with a positive Egger test (P = 0.01) and negative Harbord‐Egger test (P = 0.14).
Figure 4.
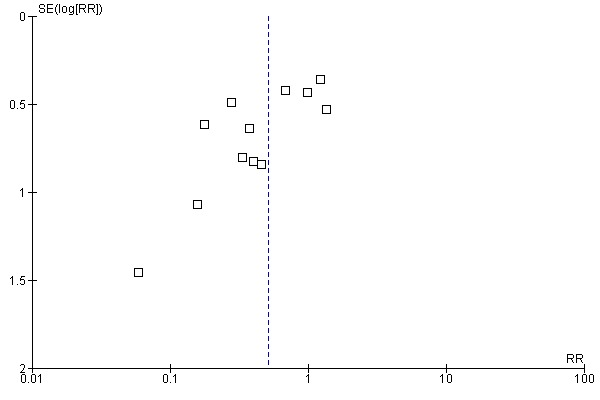
Funnel plot of comparison: 1 Guidewire‐assisted cannulation vs contrast‐assisted cannulation, Main analysis, outcome: 1.1 Post‐ERCP pancreatitis (ITT).
Funnel plot asymmetry cannot, however, be considered to be proof of publication bias in a meta‐analysis (Sterne 2000). True clinical or methodological heterogeneity between studies may also lead to funnel plot asymmetry (Sterne 2000). It has been recognized that the funnel plot itself is inappropriate in the presence of significant heterogeneity (Ioannidis 2007; Terrin 2003). This is because the funnel plot is based on the premise that studies come from a single underlying population and all studies estimate a single true effect (Light 1984). In the presence of significant heterogeneity, it is highly likely that studies are in fact estimating a range of effects rather than a single true effect (Terrin 2003). Furthermore, the application of funnel plot asymmetry tests such as the Egger (Egger 1997) and the Harbord‐Egger (Harbord 2006) tests to detect publication bias is inappropriate or not meaningful in the presence of significant heterogeneity, and may lead to false‐positive claims for publication bias (Harbord 2006; Ioannidis 2007), although the Harbord‐Egger test has better properties than the Egger test (Harbord 2006). Also, when the average event rate per trial is low, these tests may give false positive results (Sterne 2000). The trim and fill method for adjusting for publication bias has also been shown to spuriously adjust the estimate of the global treatment effect when the studies are heterogeneous (Terrin 2003). Indeed, there is significant heterogeneity for the outcome of PEP in our review that could be explained by differences in trial design ('cross‐over' versus 'non‐crossover'), publication type, and risk of bias (random sequence generation). In addition, other factors such as the use of a prophylactic PD stent, cannulation devices (sphincterotome versus standard catheter), and involvement of trainees in cannulation also contribute to heterogeneity, but these factors could be confounded by trial design due to overlap in comparison groups between trial design and these subgroups. For the primary outcome of PEP in our review, closer inspection of the funnel plot reveals that the 'non‐crossover' studies are scattered to the left of the most common effect, and most of the 'cross‐over' studies are scattered to the right, suggesting that trial design (a source of heterogeneity) may be an important source of funnel plot asymmetry (Figure 5). We were not able to pool the studies separately according to trial design to test for publication bias because each group would have less than 10 trials (Ioannidis 2007). Based on the reasons outlined above, the application of funnel plot and asymmetry tests should be considered inappropriate or not meaningful for assessing publication bias in this meta‐analysis.
Figure 5.
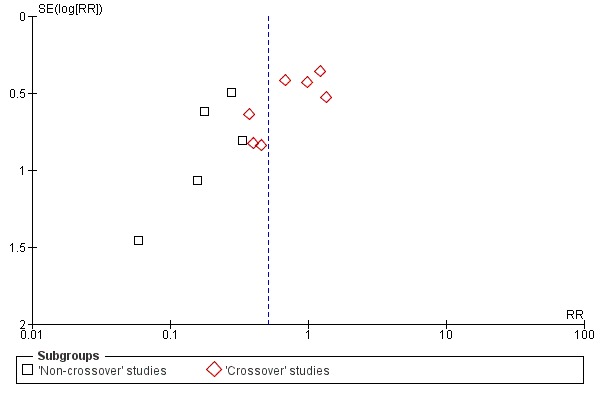
Funnel plot of comparison: 2 Analysis according to trial design, outcome: 2.1 Post‐ERCP pancreatitis.
A potential limitation of this review would be the variable definitions and grading of severity of PEP used by some trials (Apostolopoulos 2005; Artifon 2007; Lella 2004). The heterogeneity of criteria used to define PEP and classify its severity may make direct comparisons of these trials difficult. However, the definition of PEP still remains a controversial issue. It is well recognized that the rise in serum amylase may vary considerably without any clinical significance (Testoni 2000). However, patients with hyperamylasaemia post‐procedure (even with mild pain) are more likely to be carefully monitored with a prolonged hospital stay in both research and clinical settings. This adds to the confusion in the definition and evaluation of PEP, especially when procedure‐related hospital stay is part of the definition of PEP according to the consensus criteria (Cotton 1991). Nevertheless, the variable definitions used by the included studies likely reflect 'real world' practices with a highly variable incidence of PEP depending on the definition criteria adopted (Testoni 2000).
Another potential limitation of this review is the inclusion of 'cross‐over' studies in the main analysis, which may have diluted the treatment effect of the guidewire‐assisted cannulation technique for the prevention of PEP. Nevertheless, we still found a significant reduction of the risk of PEP with the guidewire‐assisted cannulation technique compared to the contrast‐assisted cannulation technique with the inclusion of 'cross‐over' studies. In addition, our subgroup analyses confirmed that trial design was a significant source of heterogeneity.
Finally, the inclusion of predominantly unclear risk of bias studies for random sequence generation, allocation concealment and blinding of outcome assessment, and high risk of bias studies for blinding of participants and personnel (the endoscopists) in the analyses may have biased our effect estimates. In particular, blinding of participants and outcome assessors is essential for reducing bias in the case of PEP when there is some degree of subjectivity in the interpretation of pancreatic pain. Lack of binding of the endoscopist may also have an impact on cannulation success and PEP depending on the experience, expertise and preference of the endoscopist performing the procedure.
Agreements and disagreements with other studies or reviews
There have been three fully published systematic reviews (Cennamo 2009; Cheung 2009; Shao 2009) on this topic that compared the guidewire‐assisted cannulation technique with the conventional contrast‐assisted cannulation technique for the prevention of PEP. There are some notable differences between these three reviews and our review. Due to inadequate information presented in abstract format, we were not able to contrast the methodologies and findings of four other meta‐analysis published only in conference proceedings (Choudhary 2009; Choudhary 2010b; Epstein 2009; Madhoun 2009) with our review.
The first systematic review by Shao et al (Shao 2009) included four fully published RCTs (Artifon 2007; Bailey 2008; Lella 2004; Lee 2009). It failed to show a significant association between the use of the guidewire‐assisted cannulation technique and reduction of PEP (RR 0.34, 95% CI 0.10 to 1.17; P = 0.09). Subgroup analysis, however, showed a benefit of the guidewire‐assisted cannulation technique in trials without 'cross‐over' design (RR 0.20, 95% CI 0.09 to 0.40; P < 0.00001). First, three potentially eligible trials were not included in this meta‐analysis, a fully published RCT (Katsinelos 2008) and two trials (Gruchy 2007; Mangiavillano 2007) published in abstract format. In particular, the latter two trials (Gruchy 2007; Mangiavillano 2007) should have been identified through their search of the 2006 to 2008 proceedings of the American Gastroenterological Digestive Disease Week (published in Gastroenterology and Gastrointestinal Endoscopy) and the United European Gastroenterology Week (published in Gut and Endoscopy). Because of the small number of studies included, this meta‐analysis (Shao 2009) may have been underpowered to detect a clinically important difference between the two cannulation techniques. All three trials (Gruchy 2007; Katsinelos 2008; Mangiavillano 2007) were included in our meta‐analysis. Second, it is important to highlight that there were some discrepancies in the rates of PEP for one trial (Bailey 2008) between this review (Shao 2009) and our review. For this 'cross‐over' trial (Bailey 2008), Shao et al restricted their analyses to patients who did not 'cross‐over' to the alternative cannulation technique. The PEP rates for Bailey 2008 were reported to be 10/202 in the guidewire‐assisted cannulation group and 7/211 in the contrast‐assisted cannulation group before 'cross‐over' of the cannulation technique (data requested from the primary authors by Shao 2009). Based on the publication by Bailey et al (Bailey 2008), and with confirmation from the authors of the primary study, PEP occurred in 29/413 patients, 16/202 in the guidewire arm and 13/211 in the contrast arm with 50 patients crossed over to guidewire and 22 patients crossed over to contrast. Therefore, it would appear the PEP rates for Bailey 2008 should be 10/180 in the guidewire‐assisted cannulation group and 7/161 in the contrast‐assisted cannulation group before 'cross‐over'. We included all patients with PEP in our main analysis and also in our subgroup analyses according to trial design based on ITT (16/215 in the guidewire‐assisted cannulation technique versus 13/215 in the contrast‐assisted cannulation technique) (Bailey 2008). In order to avoid bias, we decided a priori not to restrict our analyses to patients who did not 'cross‐over' because those who required 'cross‐over' were likely to be more difficult to cannulate and therefore carried a higher risk of PEP than those who did not 'cross‐over'.
The second systematic review by Cennamo et al (Cennamo 2009) included five fully published RCTs (Artifon 2007; Bailey 2008; Katsinelos 2008; Lee 2009; Lella 2004). Two potentially eligible trials published in abstract format (Gruchy 2007; Mangiavillano 2007) were not included in this meta‐analysis. It concluded that the guidewire‐assisted cannulation technique significantly increases the primary cannulation rate (OR 2.05, 95% CI 1.27 to 3.31) and reduces the risk of PEP (OR 0.23, 95% CI 0.13 to 0.41) compared with the contrast‐assisted cannulation technique. However, the conclusion was based on the exclusion of the two 'cross‐over' trials (Bailey 2008; Katsinelos 2008) from the analysis of the PEP outcome. The results may therefore be biased towards a reduction of PEP. Instead, we included all studies in the main analyses and explored subgroup differences according to trial design in our systematic review.
The third systematic review by Cheung et al (Cheung 2009) included seven RCTs (five 'non‐crossover' trials and two 'cross‐over' trials) (Artifon 2007; Bailey 2008; Gruchy 2007; Katsinelos 2008; Lee 2009; Lella 2004; Mangiavillano 2007). It was decided a priori that the analysis of PEP would be performed separately by trial design. The review concluded that there was a significant reduction in PEP when using the guidewire‐assisted cannulation technique compared with the contrast‐assisted cannulation technique (RR 0.38, 95% CI 0.19 to 0.76) among 'non‐crossover' trials only. One trial (Gruchy 2007), published in abstract format, was included under 'non‐crossover' trials. We included this trial (Gruchy 2007) under 'cross‐over' trials after confirming with the authors of the primary study that this was in fact a 'cross‐over' study by design. In addition, one potentially eligible trial (Apostolopoulos 2005), published in abstract format, was not included in this meta‐analysis. This trial (Apostolopoulos 2005) should have been identified through their search of the 2004 to 2008 conference abstracts (Digestive Disease Week, American College of Gastroenterology, British Society of Gastroenterology, and United European Gastroenterology Week). This meta‐analysis (Cheung 2009) also concluded that there was no significant reduction of precut with the guidewire‐assisted cannulation technique compared with the contrast‐assisted cannulation technique (RR 0.57, 95% CI 0.29 to 1.11). With a larger number of included studies, we found that the need for precut was significantly reduced by the use of the guidewire‐assisted cannulation technique compared to the contrast‐assisted cannulation technique (RR 0.75, 95% CI 0.60 to 0.95; P = 0.02). Among patients with precut, that review concluded that there was less PEP with the guidewire‐assisted cannulation technique compared with the contrast‐assisted cannulation technique (RR 0.21, 95% CI 0.04 to 1.04) (Cheung 2009). We also found no significant difference in the rates of PEP between the two cannulation techniques when precut was used (RR 0.31, 95% CI 0.08 to 1.18; P = 0.09), although there appeared to be a trend favouring the guidewire‐assisted cannulation technique among patients with precut. The results are therefore consistent with either no effect or inadequate power to rule out clinically important differences between the two cannulation techniques when precut was used. When precut was not used, the guidewire‐assisted cannulation technique significantly reduced PEP compared to the contrast‐assisted cannulation technique (RR 0.42, 95% CI 0.19 to 0.92; P = 0.03). The test for subgroup differences was non‐significant (precut versus no precut). Hence, our results would suggest that the use of precut does not have a significant impact on the effect estimates for PEP, and the guidewire‐assisted cannulation technique significantly reduced the rates of PEP regardless of whether or not precut was used.
In summary, this systematic review presents the current evidence regarding the clinical effectiveness and safety of the guidewire‐assisted cannulation technique compared to the conventional contrast‐assisted cannulation technique for the prevention of post‐ERCP pancreatitis. We explored sources of heterogeneity, including trial design, publication type, risk of bias, use of precut sphincterotomy or PD stents, cannulation device and trainee involvement, in subgroup analyses. We conclude that the guidewire‐assisted cannulation technique significantly reduced post‐ERCP pancreatitis compared to the contrast‐assisted cannulation technique. In addition, the guidewire‐assisted cannulation technique is associated with greater primary cannulation success, less precut sphincterotomy, and no increase in ERCP‐related complications.
Authors' conclusions
With the increasing availability of safer and less invasive diagnostic modalities including magnetic resonance cholangiopancreatography (MRCP) and endoscopic ultrasound (EUS), ERCP has become primarily a therapeutic procedure for a wide spectrum of biliary and pancreatic disorders. Contrast‐assisted cannulation is the conventional method commonly used to achieve selective deep biliary cannulation. When primary attempts with contrast‐assisted cannulation fail, guidewires are sometimes used as a secondary technique to facilitate biliary cannulation. Increasingly, guidewires are used as a primary cannulation technique despite conflicting evidence to support this practice (Löhr 2012). The present analysis found that the guidewire‐assisted cannulation technique significantly reduced PEP compared to the conventional contrast‐assisted cannulation technique. In addition, the guidewire‐assisted cannulation technique is associated with greater primary cannulation success, less precut sphincterotomy, and no increase in ERCP‐related complications. Our results suggest that the guidewire‐assisted cannulation technique reduces PEP by minimizing contrast injection into the PD and by limiting papillary trauma. Although it is unlikely that in clinical practice biliary cannulation is performed with either technique alone, our results support the use of the guidewire‐assisted cannulation technique as the most appropriate first‐line primary cannulation technique. However, the routine use of guidewires in biliary cannulation will be dependent on local expertise, availability and cost. Moreover, it remains to be proven whether the use of the guidewire‐assisted cannulation technique is cost effective in terms of prevention of PEP. Nevertheless, the cost of the guidewires may be partly offset by easier cannulation and less use of precut sphincterotomy, which necessitates the use of another device (for example a needle‐knife). In the era of therapeutic ERCP, the guidewire‐assisted cannulation technique using a sphincterotome may be considered as the first‐line primary cannulation technique, especially when guidewires have become essential in maintaining ductal access during therapeutic manoeuvers (for example stent placement, stone extraction).
This review has highlighted the need for further research on the optimal cannulation techniques for the prevention of PEP.
Standardized definitions are important for adequate communication in clinical practice and for research. The consensus definition (Cotton 1991) for PEP has not been adopted uniformly and studies have used different criteria to define and grade the severity of PEP. These variations in the definitions used in trials are likely to reflect the ongoing controversy in defining PEP in the context of post‐ERCP complications. The consensus definition for PEP has not been updated since 1991, and is arguably distinct from that used in clinical practice for diagnosing acute pancreatitis. Nonetheless, it is generally recognized that no consensus definition is perfectly accurate (sensitive and specific) (Cutlip 2007). The value of a consensus definition for clinical outcomes lies in its ability to provide consistency across studies that can facilitate the evaluation of safety and effectiveness of endoscopic procedures. Toward this end, the definition and reporting of PEP will need to be updated and standardized to reflect the current knowledge. To improve adoption of these standardized definitions by clinicians and researchers, there is a need to validate them in prospective clinical studies.
There are different variations of the guidewire‐assisted cannulation technique. Some endoscopists prefer the insertion of a sphincterotome into the ampulla during cannulation, while others use the non‐touch technique of probing the bile duct with the guidewire. While some endoscopists prefer an assistant to handle the guidewire, others prefer to handle the guidewire themselves. Recently, the short guidewire system has allowed endoscopists greater control of the wire during cannulation (ASGE Technology Committee 2007). Theoretically, the short guidewire systems may lead to faster device exchange, less use of fluoroscopy, reduced procedure time, decreased sedation requirements, improved wire stability and increased endoscopist control of the wire with less dependence on support staff (Reddy 2009). Furthermore, the physician‐controlled guidewire‐assisted cannulation technique has the potential to decrease papillary trauma and the risk of PEP. However, there are limited data on the ease of use and efficacy of the short guidewire systems (Draganov 2010). There is a need for further research on the efficacy and safety of these various guidewire‐assisted cannulation techniques.
Although our analysis suggested a benefit of the guidewire‐assisted cannulation technique in reducing the risk of PEP, our review also highlights the paucity of cost and cost effectiveness data in this area. Future RCTs should include data on cost and resource utilization. In addition, decision analyses and economic evaluations may help identify the most cost effective strategy for the prevention of PEP.
'Cross‐over' effect can substantially reduce the power of a trial to find an overall treatment difference. However, 'cross‐over' between techniques is not uncommon both in clinical trials and in clinical practice due to unforeseen technical challenges or endoscopic findings. The perceived need for 'cross‐over' is often motivated by the moral imperative to avoid the potential adverse consequences of failed ERCP and complications, and the need for repeat ERCP, percutaneous transhepatic cholangiography or surgery. The optimal solution to this problem is to avoid 'cross‐over' design or to keep 'cross‐over' or the use of the rescue technique to a reasonable minimum. However, 'cross‐over' of technique may be unavoidable due to ethical concerns (Holubkov 2009). Future trials should explicitly report trial design ('cross‐over' versus 'non‐crossover' of technique), criteria for technique 'cross‐over', and outcome pertaining to patients with and without 'cross‐over' to allow assessment of potential bias.
The endoscopist’s expertise, case volume and case mix have been considered to be potential factors that can influence the outcome of ERCP. Concerns have been raised about the potential impact of a new intervention over time (learning curve) on RCTs by distorting comparisons (Cook 2004). Failure to control for the learning curve effects may underestimate the treatment effect of any new intervention. In addition, variation in trainee involvement in one intervention arm compared to another may occur as technically challenging interventions are more likely to be performed by experienced endoscopists than by trainees. This differential involvement of trainees between arms implies a bias against the intervention with more trainee involvement. Furthermore, failure to maintain a consistently high quality of procedures may dilute any important treatment differences and may have an impact on patient outcomes. One solution is to avoid trainees altogether in RCTs. However, this would be impractical since most RCTs are done at academic centres. Another solution is to define competency thresholds and standards (based on procedural numbers and competency thresholds) for all endoscopists prior to their participation in RCTs. In addition, some degree of standardization in techniques may improve comparability between trials.
Acknowledgements
We acknowledge the following authors for kindly providing additional data on their trials: Dr Periklis Apostolopoulos (NIMTS Hospital, Athens, Greece), Dr Tomoko Nambu (Toho University Ohashi Medical Center, Toykyo, Japan), Dr Do Hyun Park (University of Ulsan College of Medicine, Asan Medical Center, Seoul, South Korea), Dr Go Kobayashi (Sendai City Medical Center, Sendai, Japan), Dr Michael J Bourke (Westmead Hospital, Sydney, Australia), and Dr Stephen Gruchy (Dalhousie University, Halifax, Canada).
We thank Karin Dearness, Managing Editor, Cochrane Upper Gastrointestinal and Pancreatic Diseases (UGPD) Group, for providing administrative and logistical support for the conduct of the current review.
Appendices
Appendix 1. CENTRAL search strategy
(Pancreatitis) and (acute)
MeSH descriptor Pancreatitis, explode all trees
(#1 OR #2)
MeSH descriptor Cholangiopancreatography, Endoscopic Retrograde explode all trees
MeSH descriptor Sphincterotomy, Endoscopic explode all trees
(endoscop* near sphincterotom*)
(endoscop* retrograde and (cholangio‐pancreatograph* or cholangiopancreatograph*)):ti,ab,kw
(ERCP):ti,ab,kw
(papillotomy):ti,ab,kw
(#4 OR #5 OR #6 OR #7 OR #8 OR #9)
(#3 AND #10)
(random* or trial*)
(#11 AND#12)
Appendix 2. MEDLINE search strategy
ERCP.mp. or exp endoscopic retrograde cholangiopancreatography/
(endoscop$ adj2 retrograd$ adj2 (cholangiopancreatograph$ or cholangio‐pancreatograph$)).tw.
exp Sphincterotomy, Endoscopic/
(endoscop$ adj3 sphincterotom$).tw.
papillotomy.tw.
or/1‐5
exp Pancreatitis, Acute Necrotizing/ or exp Pancreatitis/
Pancreatitis.mp.
or/7‐8
6 and 9
randomised controlled trial.pt.
controlled clinical trial.pt.
random$.ab.
trial.ab.
groups.ab.
or/11‐15
10 and 16
exp animals/ not humans.sh.
17 not 18
Appendix 3. EMBASE search strategy
ERCP.mp. or exp endoscopic retrograde cholangiopancreatography/
(endoscop$ adj2 retrograd$ adj2 (cholangiopancreatograph$ or cholangio‐pancreatograph$)).tw.
exp Sphincterotomy, Endoscopic/
(endoscop$ adj3 sphincterotom$).mp.
papillotomy.mp.
or/1‐5
hemorrhagic pancreatitis/ or acute hemorrhagic pancreatitis/
acute pancreatitis/
exp pancreatitis/ or pancreatitis.mp.
or/7‐9
6 and 10
exp clinical trial/ or clin$ trial$.mp.
exp Randomized controlled trial/
exp Randomization/
Single‐Blind Method/
Double‐Blind Method/
Cross‐Over Studies/ or (crossover$ or cross‐over$).tw.
exp Random Allocation/
RCT.tw.
random$.mp.
(Single blind$ or Double blind$ or ((treble or triple) adj2 blind$)).tw.
comparative study/
controlled study/
Prospective study/
or/12‐24
11 and 25
(animal not (humans and animal)).sh.
26 not 27
Appendix 4. CINAHL search strategy
(MH "Cholangiopancreatography, Endoscopic Retrograde") OR "endoscopic AND retrograde AND cholangiopancreatography"
TX endoscopic retrograde cholangiopancreatography OR ERCP
TX endoscopic AND sphincterotomy
TX papillotomy
1 or 2 or 3 or 4
(MH "Pancreatitis+") OR "pancreatitis"
5 and 6
random*
7 and 8
Data and analyses
Comparison 1.
Guidewire‐assisted cannulation versus contrast‐assisted cannulation, main analysis
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Post‐ERCP pancreatitis (ITT) | 12 | 3450 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.32, 0.82] |
| 2 Post‐ERCP pancreatitis (per‐protocol) | 12 | 3331 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.32, 0.83] |
| 3 Severity of post‐ERCP pancreatitis | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Mild post‐ERCP pancreatitis | 10 | 2986 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.26, 0.93] |
| 3.2 Moderate post‐ERCP pancreatitis | 10 | 2986 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.34, 1.67] |
| 3.3 Severe post‐ERCP pancreatitis | 10 | 2986 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.28, 2.48] |
| 4 Need for 'crossover' to the alternative technique (in 'crossover' studies) | 4 | 1256 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.38, 1.13] |
| 5 Secondary cannulation success (after technique 'crossover' in 'crossover' studies) | 4 | 269 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.41, 1.31] |
| 6 Overall cannulation success | 12 | 3450 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.99, 1.04] |
| 7 The need for precut sphincterotomy | 8 | 2386 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.60, 0.95] |
| 8 Inadvertent pancreatic duct injection or cannulation | 8 | 2524 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.76, 1.01] |
| 9 Post‐sphincterotomy bleeding | 5 | 1480 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.50, 1.72] |
| 10 Perforation | 6 | 1880 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [0.06, 41.19] |
| 11 Primary cannulation success (with the randomised technique before technique 'crossover' or precut) | 10 | 2986 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [1.00, 1.15] |
Comparison 2.
Analysis according to trial design
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Post‐ERCP pancreatitis | 12 | 3450 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.32, 0.82] |
| 1.1 'Non‐crossover' studies | 5 | 1330 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.12, 0.42] |
| 1.2 'Crossover' studies | 7 | 2120 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.58, 1.23] |
Comparison 3.
Analysis by publication type
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Post‐ERCP pancreatitis | 12 | 3450 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.32, 0.82] |
| 1.1 Full text | 7 | 2334 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.26, 1.02] |
| 1.2 Abstract | 5 | 1116 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.31, 0.97] |
Comparison 4.
Analysis by risk of bias
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Post‐ERCP pancreatitis according to random sequence generation | 12 | 3450 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.32, 0.82] |
| 1.1 Low risk of bias for random sequence generation | 5 | 1830 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.17, 1.25] |
| 1.2 Unclear risk of bias for random sequence generation | 7 | 1620 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.36, 0.90] |
| 2 Post‐ERCP pancreatitis according to allocation concealment | 12 | 3450 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.32, 0.82] |
| 2.1 Low risk of bias for allocation concealment | 4 | 1204 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.29, 1.17] |
| 2.2 Unclear risk of bias for allocation concealment | 8 | 2246 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.22, 0.87] |
Comparison 5.
Analysis according to precut sphincterotomy
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Post‐ERCP pancreatitis in all studies that did or did not permit precut sphincterotomy | 12 | 3450 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.32, 0.82] |
| 1.1 Studies permitted precut | 10 | 2850 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.35, 0.90] |
| 1.2 Studies did not permit precut | 1 | 400 | Risk Ratio (M‐H, Random, 95% CI) | 0.06 [0.00, 1.01] |
| 1.3 No information provided for precut | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.07, 1.61] |
| 2 Post‐ERCP pancreatitis in 'non‐crossover' studies among patients who did or did not undergo precut sphincterotomy | 3 | 730 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.25, 0.75] |
| 2.1 Patients had precut | 3 | 117 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.08, 1.18] |
| 2.2 Patients did not have precut | 3 | 613 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.19, 0.92] |
Comparison 6.
Analysis according to inadvertent guidewire insertion or contrast injection into the PD
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Post‐ERCP pancreatitis in all studies among patients with and without inadvertent PD manipulation | 5 | 1372 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.19, 0.58] |
| 1.1 Patients with inadvertent PD manipulation | 5 | 484 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.11, 0.71] |
| 1.2 Patients without inadvertent PD manipulation | 5 | 888 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.19, 0.73] |
| 2 Post‐ERCP pancreatitis in 'non‐crossover' studies among patients with and without inadvertent PD manipulation | 4 | 1200 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.17, 0.57] |
| 2.1 Patients with inadvertent PD manipulation | 4 | 377 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.06, 0.58] |
| 2.2 Patients without inadvertent PD manipulation | 4 | 823 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.19, 0.78] |
Comparison 7.
Analysis according to the use of PD stent
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Post‐ERCP pancreatitis in all studies that did or did not permit the use of PD stent | 12 | 3450 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.32, 0.82] |
| 1.1 Studies permitted PD stent | 5 | 1860 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.62, 1.36] |
| 1.2 Studies did not permit PD stent | 4 | 902 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.13, 0.47] |
| 1.3 No information provided for the use of PD stent | 3 | 688 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.10, 0.86] |
| 2 Post‐ERCP pancreatitis in 'non‐crossover' studies that did or did not permit the use of PD stent | 5 | 1330 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.12, 0.42] |
| 2.1 Studies did not permit PD stent | 3 | 730 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.11, 0.45] |
| 2.2 No information provided for the use of PD stent | 2 | 600 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.04, 1.03] |
Comparison 8.
Analysis by cannulation device
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Post‐ERCP pancreatitis in all studies | 12 | 3450 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.34, 0.83] |
| 1.1 Sphincterotome in both arms | 8 | 2333 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.18, 0.76] |
| 1.2 Standard catheter in both arms | 2 | 535 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.43, 1.77] |
| 1.3 Sphincterotome with guidewire versus standard catheter with contrast | 1 | 172 | Risk Ratio (M‐H, Random, 95% CI) | 0.4 [0.08, 2.01] |
| 1.4 Studies that did not provide details about cannulation device used in either arm | 2 | 410 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.39, 1.77] |
| 2 Primary cannulation success in all studies | 10 | 2986 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [1.00, 1.15] |
| 2.1 Sphincterotome in both arms | 7 | 1957 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.99, 1.11] |
| 2.2 Standard catheter in both arms | 2 | 535 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.86, 1.82] |
| 2.3 Sphincterotome with guidewire versus standard catheter with contrast | 1 | 172 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.91, 1.28] |
| 2.4 Studies that did not provide details about cannulation device used in either arm | 1 | 322 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.88, 1.05] |
| 3 Post‐ERCP pancreatitis in 'non‐crossover' studies | 5 | 1330 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.12, 0.42] |
| 3.1 Sphincterotome in both arms | 5 | 1330 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.12, 0.42] |
| 4 Primary cannulation success in 'non‐crossover' studies | 5 | 1330 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.97, 1.13] |
| 4.1 Sphincterotome in both arms | 5 | 1330 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.97, 1.13] |
Comparison 9.
Analysis by trainee involvement in cannulation
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Post‐ERCP pancreatitis in all studies | 12 | 3450 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.32, 0.82] |
| 1.1 ERCP performed by experienced endoscopists | 5 | 1462 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.14, 0.60] |
| 1.2 ERCP performed by trainees first then by experienced endoscopists (trainee involvement) | 5 | 1700 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.60, 1.46] |
| 1.3 No information provided about trainee involvement | 2 | 288 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.12, 1.21] |
| 2 Primary cannulation success in all studies | 10 | 2986 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [1.00, 1.15] |
| 2.1 ERCP performed by experienced endoscopists | 5 | 1462 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.92, 1.40] |
| 2.2 ERCP performed by trainees first then by experienced endoscopists (trainee involvement) | 4 | 1324 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.96, 1.08] |
| 2.3 No information provided about trainee involvement | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.97, 1.07] |
| 3 Post‐ERCP pancreatitis in 'non‐crossover' studies | 5 | 1330 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.12, 0.42] |
| 3.1 ERCP performed by experienced endoscopists | 4 | 1130 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.10, 0.41] |
| 3.2 No information provided about trainee involvement | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.07, 1.61] |
| 4 Primary cannulation success in 'non‐crossover' studies | 5 | 1330 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.97, 1.13] |
| 4.1 ERCP performed by experienced endoscopists | 4 | 1130 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.93, 1.21] |
| 4.2 No information provided about trainee involvement | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.97, 1.07] |
Differences between protocol and review
In the protocol, both the primary outcome of PEP and the secondary outcome of severity of PEP were defined by the consensus definition (Cotton 1991). Because the definitions of PEP and grading of severity of PEP were variable between studies, we decided to accept the definitions used by the primary studies for this review.
Among all trials and within trials that did not permit technique 'cross‐over' ('non‐crossover' trials), subgroup analysis for primary cannulation success was not performed for precut sphincterotomy as primary cannulation success was defined as successful cannulation with the randomised technique prior to 'cross‐over' or the use of rescue technique.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Single centre, RCT. Enrolment period: unclear. Endoscopist(s): all procedures were performed by 2 experienced endoscopists. Guidewire was handled by 2 GI interns with more than 2 years of training (information provided by authors). | |
| Participants | Country: Greece. 123 patients with suspected choledocholithiasis. | |
| Interventions | 1. Guidewire‐assisted cannulation: a regular 0.035 inch Terumo guidewire through a 5.5F sphincterotome. Guidewire was used to access the CBD, followed by cannulation and opacification. Unclear who advanced the guidewire. 2. Contrast‐assisted cannulation: standard method of cannulation through a 5.5 F sphincterotome. |
|
| Outcomes | PEP; successful cannulation of the CBD; inadvertent PD cannulation / injection; asymptomatic hyperamylasaemia; cholangitis; mortality. | |
| Notes | 1. PEP was defined according to the consensus definition (Cotton 1991) (information provided by authors). 2. Graded severity of PEP using the Ranson's criteria and Balthazar grading (information provided by authors). Per protocol data: all episodes of PEP in the contrast‐assisted group were mild according to the Ranson's criteria, whereas using Balthazar criteria, 3 patients developed Balthazar A and 2 patients developed Balthazar B pancreatitis. ITT data: all episodes of PEP were mild in both groups. 3. No technique cross‐ove" when cannulation failed (information provided by authors). 4. Precut was not permitted (information provided by authors). According to the study protocol, 20 minutes of biliary cannulation were allowed in both groups. When access to the CBD failed, precut fistulotomy was performed, but these patients were excluded from analysis (per‐protocol analysis). We included these patients in our analysis based on the ITT principle. Successful cannulation of the CBD after precut: 3/4 patients in the guidewire‐assisted group vs. 3/3 patients in the contrast‐assisted group. 1/4 patients (Ranson: mild, Balthazar A) in the guidewire‐assisted group vs. 1/3 (Ranson: mild, Balthazar B) patients in the contrast‐assisted group developed PEP. 1/4 patient in the guidewire‐assisted group vs. 1/3 patient in the contrast‐assisted group had inadvertent PD cannulation / injection. 5. PD stents were not used for the prevention of PEP (information provided by authors). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The patients were randomised into two groups." Conference proceeding, no further information was provided. |
| Allocation concealment (selection bias) | Unclear risk | Conference proceeding, no information was provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Conference proceeding, no information was provided. Endoscopists could not be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Conference proceeding, no information was provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | PEP data reported in per protocol sample. Patients who failed cannulation and underwent precut were excluded from the analysis of the primary study. Additional outcome data of these patients were provided by authors of the primary study. We performed our analysis based on ITT principle, and used PP data in sensitivity analysis. |
| Selective reporting (reporting bias) | Low risk | Reported all important outcomes. |
| Other bias | Low risk | No other risk of bias. |
| Methods | Multi‐centre (three tertiary care hospitals in Sao Paulo, Brazil), RCT. Enrolment period: July 2002 to October 2003. Endoscopist(s): a single experienced endoscopist performed all procedures. | |
| Participants | Country: Brazil. 300 patients undergoing ERCP for a biliary indication. | |
| Interventions | 1. Guidewire‐assisted cannulation: a soft hydrophilic tipped Teflon 0.035 inch guidewire (Boston Scientific or Wilson Cook) through a sphincterotome (Boston Scientific). The tip of the sphincterotome was inserted into the papilla, followed by advancement of the guidewire and opacification. Unclear who advanced the guidewire. 2. Contrast‐assisted cannulation: standard method of cannulation through a sphincterotome (Boston Scientific). |
|
| Outcomes | PEP; severity of PEP; ease of CBD cannulation (assessed by attempts required for CBD cannulation: easy [0 to 3 attempts], moderate [4 to 6 attempts], difficult [7 to 10 attempts]); rates of precut); inadvertent PD cannulation / injection; change in amylase / lipase / CRP levels over 24 hours; complications (bleeding, perforation). | |
| Notes | 1. Reported PEP rates based on 3 different definitions: 1) abdominal pain and CT scan evidence of pancreatitis; 2) consensus definitions (Cotton 1991) (abdominal pain 24 hours following ERCP + > 3‐fold hyperamylasaemia); 3) Lella et al definition (abdominal pain 24 hours following ERCP + > 5‐fold hyperamylasaemia) (Lella 2004). Our analysis was based on the consensus definition (Cotton 1991). 2. Graded severity of PEP using the Ranson's criteria and Balthazar grading. 3. Did not report on the use of technique 'cross‐over'. 4. Precut was permitted if there was difficulty accessing the CBD despite greater than 10 attempts on the major papilla with or without contrast injection. 5. PD stents were not used for the prevention of PEP. Authors (Dr Atul Kumar and Everson LA Artifon) contacted, but did not have additional data pertaining to severity of PEP based on the consensus criteria (Cotton 1991). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "by using block randomisation". |
| Allocation concealment (selection bias) | Low risk | "Following intubation of the duodenum and identification of the ampulla, a numbered envelope was drawn from a set of sealed envelopes containing the allocation on a card and the endoscopist was informed about the patient’s group assignment. The assignment was recorded by an independent staff member." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | In the abstract, the study was described as "a single‐centre, blinded, randomised trial". However, it is unclear as to who were blinded in the study. Endoscopist could not be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is unclear whether the outcome assessor was blinded. "A research assistant recorded patient and procedure‐related data prospectively at the time of ERCP" and "A research assistant carried out subsequent outcome assessments at follow‐up visit or by telephone interview and chart review." Following ERCP, all patients were admitted for overnight observation. As a result, patients were more likely to undergo laboratory and radiological evaluation of abdominal pain as opposed to being discharged home following ERCP. Results were therefore susceptible to detection bias if outcome assessors were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up. PEP data reported based on ITT sample. |
| Selective reporting (reporting bias) | Low risk | Reported all planned outcomes. |
| Other bias | Low risk | More women in the guidewire‐assisted cannulation group than in the contrast‐assisted cannulation group (39.3% vs. 27.3%, P = 0.03). This is likely a chance finding. |
| Methods | Single centre, RCT. Enrolment period: August 2003 to April 2006. Endoscopist(s): two experienced endoscopists supervised procedures performed by a dedicated ERCP training fellow. The fellow commenced the procedure in the majority of cases (77.5%). | |
| Participants | Country: Australia. 430 patients with an intact papilla who were referred for ERCP. | |
| Interventions | 1. Guidewire‐assisted cannulation: a hydrophilic tipped 0.035 inch guidewire (Jagwire, Boston Scientific) through a sphincterotome (Olympus). Guidewire was used to access the CBD, followed by cannulation and opacification. Guidewire was advanced by an assistant. 2. Contrast‐assisted cannulation: standard method of cannulation through a sphincterotome (Olympus). |
|
| Outcomes | PEP; cannulation success; time to successful cannulation; the number of cannulation attempts; the number of inadvertent PD cannulations or injections; independent predictors of PEP and adjusted odds ratios from multiple logistic regression. | |
| Notes | 1. Defined PEP according to the consensus definition (Cotton 1991). 2. Graded severity of PEP based on the consensus criteria (Cotton 1991). 3. 'Cross‐over' technique: The fellow attempted initially for five minutes. If unsuccessful, the consultant attempted for 5 minutes using the same technique, followed by 'cross‐over' to the other technique in the same sequence. Did not report PEP data for those with and without 'cross‐over' separately. Authors contacted: in the guidewire‐assisted group, total PEP = 16 (13 mild, 3 moderate) with 6 crossed over to contrast (5 mild, 1 moderate) and 10 did not crossover (8 mild, 2 moderate). In the contrast‐assisted group, total PEP = 13 (9 mild, 4 moderate) with 6 crossed over to guidewire (3 mild, 3 moderate) and 7 did not cross over (6 mild, 1 moderate). 4. Precut was permitted. If attempts at cannulation failed, a needle‐knife sphincterotomy (NKS) was performed by the consultant endoscopist where appropriate. The consultant could proceed directly to NKS without 'cross‐over' if it seemed that the alternate technique was likely to fail. 5. PD stents were used at the discretion of the endoscopists. 6. Did not report on the number of patients with inadvertent PD injection / cannulations. Authors contacted: in the guidewire‐assisted group, 67 had one or more inadvertent PD injection / cannulation and 119 had no PD injection / cannulation. In the contrast‐assisted group, 104 had one or more inadvertent PD injection / cannulation and 94 had no PD injection / cannulation. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomised using a computer‐generated randomisation program". |
| Allocation concealment (selection bias) | Unclear risk | No information was provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information was provided. Endoscopists could not be blinded. "The proceduralist was informed of which treatment had been assigned immediately after commencement of the procedure." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information was provided. "All patients were assessed clinically at the bedside before discharge from the endoscopy unit"; "All patients were asked to have serum collected for amylase and lipase levels the day after ERCP"; and "Telephone interviews were performed by the endoscopy fellow on day 1 and day 30 after ERCP". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up. Excluded 17 randomised patients (13 vs. 4) from the final analysis, primarily because of the presence of unsuspected prior sphincterotomy or surgically altered anatomy. ITT sample was used in our analysis. |
| Selective reporting (reporting bias) | Low risk | Reported all planned outcomes. |
| Other bias | Low risk | No other risk of bias. |
| Methods | Single centre, RCT. This study was an interim analysis of an ongoing trial presented in 2007 as a conference proceeding. We contacted the primary author and obtained data of the competed trial in April 2012. Enrolment period: unclear. Endoscopist(s): unclear. Authors contacted: There was one trainee with "minimal involvement in procedures". | |
| Participants | Country: Canada. In the conference proceeding, 216 patients underwent their first ERCP and had not previously been diagnosed with pancreatitis. Completed trial data were obtained from authors: a total of 376 patients were randomised. | |
| Interventions | 1. Guidewire‐assisted cannulation: a hydrophilic guidewire. No information provided regarding cannulation device. Authors contacted: Jagtome using a 0.035 inch guidewire (Boston Scientific). No information on technique. Unclear who advanced the guidewire. 2. Contrast‐assisted cannulation: standard method of cannulation. |
|
| Outcomes | PEP; successful cannulation of the CBD; bleeding, perforation, infection; cannulation success rate and incidence of 'cross‐over'. | |
| Notes | 1.Data set of the completed trial were obtained from authors of the primary study: 241 patients randomised to the guidewire‐assisted cannulation technique and 135 patients randomised to the contrast‐assisted cannulation technique. 57 versus 36 patients were lost to follow up because no blood test or unable to reach by follow up calls. Data was analysed based on PP sample (184 vs 99). PEP = 4 vs 6. Overall cannulation success: 180 versus 88, bleeding 6 versus 3. 2. Defined PEP by "standard criteria". Authors contacted: Pancreatitis was defined as new or increased abdominal pain requiring hospital admission associated with an elevated amylase level > = 3x upper limit of normal. Each patient received blood work pre‐ERCP and 24 hrs post ERCP. A research nurse obtained a follow up phone call at 24 hrs and 30 days. 3. Did not grade the severity of PEP or report outcome data regarding severity of PEP in abstract. Authors contacted: no data for the severity of PEP. 4. Did not report on the use of technique 'cross‐over'. Authors contacted: 9 in the guidewire‐assisted cannulation technique and 43 in the contrast‐assisted cannulation group 'cross‐over' to the other arm. Criteria for 'cross‐over' was 3 attempts at cannulation. 5. Did not report on the use of precut. Authors contacted: patients were excluded if a precut was performed. However, it is unclear how many patients were excluded after randomisation because of this reason. 6. Did not report on the use of PD stent. Authors contacted: patients were excluded if a PD stent was used. However, it is unclear how many patients were excluded after randomisation because of this reason. 7. Did not report on inadvertent guidewire cannulation or contrast injection of the PD. Authors contacted: 15 patients in the guidewire‐assisted cannulation group had inadvertent contrast injection of the PD, and 2 of these patients developed PEP. It is unclear how many patients had inadvertent guidewire cannulation of the PD in the guidewire‐assisted cannulation group or inadvertent contrast injection of the PD in the contrast‐assisted cannulation group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned", conference proceeding, no further information provided. Authors contacted: "randomisation performed by the research office". However, the groups appeared to be highly unbalanced in terms of numbers (241 in the guidewire‐assisted cannulation versus 135 patients in the contrast‐assisted cannulation). This raises concerns as to whether the method used to generate the random sequence was truly random. |
| Allocation concealment (selection bias) | Unclear risk | Conference proceeding, no information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Conference proceeding, no information provided. Endoscopists could not be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. Authors contacted: each patient received blood work pre‐ERCP and 24 hrs post ERCP. A research nurse obtained a follow up phone call at 24 hrs and 30 days. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Information obtained from authors: 23.7 % (57/241) patients in the guidewire‐assisted cannulation group and 26.6% (36/135) patients in the contrast‐assisted cannulation group were lost to follow up (dropouts). The reasons for "dropouts" included: unable to obtain blood work and unable to reach for follow‐up phone call. It is unclear how many of these "dropouts" had PEP because patients with PEP could be admitted to other hospitals. Authors of the primary study therefore performed per protocol analyses on the data. However, we performed all our analyses based on ITT principle. As it is unclear how many of the "dropouts" had successful cannulation or PEP, analysing the data of this study based on ITT may have underestimated both the cannulation success rates and the PEP rates. |
| Selective reporting (reporting bias) | Low risk | Reported all planned outcomes. |
| Other bias | Unclear risk | Authors stated that "analysis was based on ITT sample" in the conference abstract. However, the percentage provided for PEP incidence for each group cannot be translated into patient numbers based on ITT analysis. We contacted the authors and obtained data for the completed trial. However, data were analysed and reported only in PP sample. |
| Methods | Multi‐centre (two tertiary referral centres in Thessaloniki and Larissa, Greece), RCT. Enrolment period: June 2006 to December 2006. Endoscopist(s): all procedures were performed by two experienced endoscopists. | |
| Participants | Country: Greece. 332 patients referred for therapeutic ERCP. | |
| Interventions | 1. Guidewire‐assisted cannulation: a hydrophilic tipped 0.035 inch guidewire (Jagwire, Boston Scientific) through a 5.5F standard catheter (Wilson Cook, Winston Salem, NC). Guidewire was used to access the CBD, followed by cannulation and opacification. Guidewire was advanced by an assistant or the endoscopist. 2. Contrast‐assisted cannulation: standard method of cannulation through a 5.5F standard catheter (Wilson Cook). |
|
| Outcomes | PEP; successful cannulation of the CBD (within a period of 20 minutes); cannulation time; number of attempts at CBD cannulation; inadvertent PD cannulation / injection; complication rates (bleeding, perforation). | |
| Notes | 1. Defined PEP according to the consensus definition (Cotton 1991). 2. Graded severity of PEP based on the consensus criteria (Cotton 1991). 3. 'Cross‐over' technique: A period of up to 10 minutes was allowed for deep cannulation with the standard catheter or the guidewire. If access was not obtained within this time, a change was made to the other instrument (guidewire or catheter) and the cannulation attempt was continued for a further 10 minutes. If cannulation failed with both devices, the study procedure was terminated and alternative strategies were used, depending on the individual situation. 4. Precut was permitted. 5. PD stents were used for prevention of PEP. Attempts to contact authors for additional data were unsuccessful. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomization was prepared by a biostatistician." Unclear how the random sequence was generated. |
| Allocation concealment (selection bias) | Low risk | "A trainee in gastroenterology who was not participating in the study carried out the randomisation based on an opaque envelope system." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "It was impossible for the endoscopist to be blinded." It is unclear whether patients or personnel were blinded. No information was provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information was provided. "Patients were observed after the procedure for symptoms such as abdominal pain, nausea, and fever. Plain abdominal radiographs and CT scans were obtained in patients with post‐procedure symptoms." It is unclear whether patients were discharged home or admitted for observation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up. PEP reported based on ITT sample. |
| Selective reporting (reporting bias) | Low risk | Reported all planned outcomes. |
| Other bias | Low risk | No other risk of bias. |
| Methods | Multi‐centre (Fifteen referral endoscopy units), RCT with a 2 x 2 factorial design. Enrolment period: September 2009 to March 2010. Endoscopist(s): multi‐endoscopists with mixed operator expertise (low, moderate/high) performing or directly supervising dedicated ERCP fellows. | |
| Participants | 400 consecutive patients with naive papillae who were candidates for ERCP. | |
| Interventions | Patients were assigned to four groups according to both cannulation device type (catheter or sphincterotome) and method (contrast or guidewire): 1. Guidewire‐assisted cannulation: catheter and guidewire or sphincterotome and guidewire. Guidewire was used to access the CBD, followed by cannulation and opacification or the tip of the sphincterotome is inserted into the papilla, followed by advancement of the guidewire and opacification. Guidewire was advanced by an assistant endoscopist. 2. Contrast assisted cannulation: standard method of cannulation through a catheter or a sphincterotome. All procedures were performed by using a 15‐degree backward oblique angle duodenoscope with an elevator function (Olympus). Guidewire: a hydrophilic hard‐type 0.035 inch guidewire (Jagwire, Boston Scientific). Catheter: a variety of catheters (Olympus; Boston Scientific; MTW, Endoskopie). Sphincterotome: a single type of sphincterotome (Olympus). |
|
| Outcomes | Success rate of selective bile duct cannulation within 10 minutes; selective bile duct cannulation time; fluoroscopy time for selective bile duct cannulation; number of attempts at bile duct cannulation; number of PD opacifications; number of inadvertent PD insertions; use of precut; final success rate of selective bile duct cannulation; and complications including PEP, hyperamylasaemia, and ampulla of Vater perforation; univariate and multivariate analyses to identify risk factors for failure of selective bile duct cannulation. | |
| Notes | 1. Defined PEP according to the consensus definition (Cotton 1991). 2. Graded severity of PEP based on the consensus criteria (Cotton 1991). 3. 'Cross‐over' technique: "the time limit for selective bile duct cannulation was set at 5 minutes for the low or moderate career‐length (less than 10 years) of ERCP experience. If selective bile duct cannulation was not possible within 10 minutes, then there were no subsequent restrictions on centres or endoscopists." Patients may 'cross‐over' to alternative cannulation technique and / or cannulation device, double guidewire technique, precut and others (2 devices in 1 channel method). 4. Precut was permitted. 5. PD stents and nasopancreatic drainage were used. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization of patients was performed according to a computer‐generated schedule."; Randomization was performed just before the ERCP procedure, with stratification by each endoscopy unit." |
| Allocation concealment (selection bias) | Low risk | "The patients were enrolled via a dedicated web site and the method of selective bile duct cannulation identified just after enrolment."; "The person generating the randomisation schedule was not involved in determining patient eligibility, administering treatment, or determining outcome." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Non–double‐blind study". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information was provided. "Serum amylase was measured 24 hours after ERCP." It is unclear whether patients were discharged home or admitted for observation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients lost to follow up. PEP reported based on ITT sample. |
| Selective reporting (reporting bias) | Low risk | Reported all planned outcomes. |
| Other bias | Low risk | No other risk of bias. |
| Methods | Multi‐centre (six tertiary referral centres and three university hospitals), RCT. Enrolment period: April 2008 to March 2009. Endoscopist(s): multiple experienced endoscopists supervised procedures performed by dedicated ERCP fellows (information provided by authors). | |
| Participants | Country: Japan. 322 patients with indications for ERCP requiring selective biliary cannulation. | |
| Interventions | 1. Guidewire‐assisted cannulation: no information provided regarding the guidewire. Both sphincterotome and standard catheter were used in the guidewire‐assisted group (UEGW abstract). No information on technique. Unclear who advanced the guidewire. 2. Contrast‐assisted cannulation: no information provided regarding the cannulation device or technique. |
|
| Outcomes | PEP; ease of cannulation of the CBD; successful cannulation of the CBD; time required for cannulation of the CBD; inadvertent PD cannulation / injection. | |
| Notes | 1. PEP was defined according to the consensus definition (Cotton 1991) (information provided by authors). 2. PEP was graded based on the consensus criteria (Cotton 1991) (information provided by authors). 3. "Cross‐over" technique (information provided by authors): when the primary cannulation failed to achieve biliary cannulation within the allotted time, 'cross‐over' technique was generally applied according to everyday ERCP practice. 28 patients in guidewire assisted group vs. 21 patients in contrast assisted group 'cross‐over' to alternative technique. 4. Precut was permitted (information provided by authors). 3% (5/163) in the guidewire‐assisted group versus 4% (6/159) in the contrast‐assisted group had precut. 5. PD stents were used for the prevention of PEP (Information provided by authors). 9% (15/163) in the guidewire‐assisted group versus 4% (6/159) in the contrast‐assisted group had PD stents. 6. Slightly inconsistent PEP rates between the three conference abstracts in the same year. Authors contacted: 10/163 (3 mild, 6 moderate, 1 severe) in the guidewire‐assisted group versus 10/159 (8 mild, 2 moderate) in the contrast‐assisted group had PEP. 7. Did not report on the rates of inadvertent PD cannulation / injection. Authors contacted: 45% (74/163) in the guidewire‐assisted group versus 43% (68/159) in the contrast‐assisted group. 8. Reported primary cannulation success rates in abstract 83.4% (136/163) in the guidewire‐assisted group versus 86.8% (138/159) in the contrast‐assisted group. Authors contacted: overall cannulation success rates were 90.8% (148/163) in the guidewire‐assisted group versus 93.1% (148/159) in the contrast‐assisted group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Multicenter randomised, controlled trial". Conference proceeding, no information was provided |
| Allocation concealment (selection bias) | Unclear risk | Conference proceeding, no information was provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Conference proceeding, no information was provided. Endoscopists could not be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Conference proceeding, no information was provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Inconsistent PEP rates between three conference abstracts in the same year. Authors were contacted and provided ITT data. |
| Selective reporting (reporting bias) | Low risk | Reported all important outcomes. |
| Other bias | Low risk | No other risk of bias. |
| Methods | Single centre, RCT. Enrolment period: June 2006 to May 2007. Endoscopist(s): all procedures were performed by a single experienced endoscopists. | |
| Participants | Country: Korea. 300 consecutive patients with native papilla and pancreaticobiliary disease who were candidates for therapeutic biliary ERCP. | |
| Interventions | 1. Guidewire‐assisted cannulation: a hydrophilic tipped 0.035 inch guidewire (Jagwire, Boston Scientific) through a sphincterotome (Olympus). The tip of the sphincterotome was inserted into the papilla, followed by advancement of the guidewire and opacification. Guidewire was advanced by an assistant. 2. Contrast‐assisted cannulation: standard method of cannulation through a sphincterotome (Olympus). |
|
| Outcomes | PEP; successful cannulation of the CBD; hyperamylasaemia; inadvertent PD cannulation / injection; use of needle‐knife sphincterotomy; risk factors for PEP; procedure‐related complications (bleeding, perforation); mortality. | |
| Notes | 1. Defined PEP according to the consensus definition (Cotton 1991). 2. Graded the severity of PEP based on the consensus criteria (Cotton 1991). 3. Did not report on the use of technique 'cross‐over'. 4. Precut was permitted. A fistulotomy with a needle‐knife as rescue management was performed when access to the CBD failed despite five attempts of pancreatic cannulation or 10 minutes of biliary cannulation in both groups. 5. PD stents were not used for prevention of PEP. 6. Did not report on the PEP rate in patients who had precut. Authors contacted: 0% (0/28) in the guidewire‐assisted group vs. 19.4% (7/36) in the contrast‐assisted group who underwent precut developed PEP. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization "by means of computer‐generated numbers". |
| Allocation concealment (selection bias) | Unclear risk | No information was provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information was provided. Endoscopists could not be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information was provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patient was lost to follow‐up. PEP data reported in ITT sample. "The serum amylase level was measured before ERCP and 24 hours thereafter". It is unclear whether patients were discharged home or admitted for observation. |
| Selective reporting (reporting bias) | Low risk | Reported all planned outcomes. |
| Other bias | Low risk | No other risk of bias |
| Methods | Single Centre, RCT. Enrolment period: September 2000 to December 2002. Endoscopist(s): a single experienced endoscopist performed all procedures. | |
| Participants | Country: Italy. 400 consecutive patients with pancreatic and biliary disease who were candidates for therapeutic ERCP. | |
| Interventions | 1. Guidewire‐assisted cannulation: a soft‐tipped Teflon tracer 0.035‐inch guidewire through a 6F sphincterotome (Wilson Cook). The tip of the sphincterotome was inserted into the papilla, followed by advancement of the guidewire and opacification. Guidewire was advanced by both the endoscopist and the radiologist. 2. Contrast‐assisted cannulation: standard method of cannulation through a 6F sphincterotome (Wilson Cook). |
|
| Outcomes | PEP; hyperamylasaemia; successful cannulation of the CBD; mortality; ease of cannulation; number of cannulations: duration of the procedure; number of inadvertent PD cannulation / injection. | |
| Notes | 1. Defined PEP as pancreatic like pain that persisted for at least 24 hours after the procedure associated with serum amylase levels greater than 5 times the upper normal limit, with or without leukocytosis; CT was used to confirm pancreatitis. 2. Graded the severity of PEP (mild, moderate, severe). Unclear what criteria was used. 3. Did not report on the use of technique 'cross‐over'. 4. Precut was not permitted. 5. Did not report on the use of PD stent. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "using random numbers generated by a computer program." |
| Allocation concealment (selection bias) | Unclear risk | No information was provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information was provided. Endoscopists could not be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information was provided. "The presence / absence of pancreatic‐like pain was recorded by an endoscopy staff member who was unaware of the serum amylase and white blood cell count values before the procedure and at 2, 4, 8, and 24 hours afterwards". However, it is unclear whether the staff member was blinded to the assigned intervention. "Patients with serum amylase more than 5 times the upper limit of normal remained under observation in hospital until 48 hours after ERCP, whereas, all others were discharged within 24 hours after ERCP." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patient was lost to follow up. |
| Selective reporting (reporting bias) | Low risk | Reported all planned outcomes. |
| Other bias | Low risk | No other risk of bias. |
| Methods | Single centre, RCT. Enrolment period: unclear. Endoscopist(s): unclear. | |
| Participants | Country: Italy. 200 patients with biliary disease submitted to ERCP. | |
| Interventions | 1. Guidewire‐assisted cannulation: a soft tipped tracer guidewire through a sphincterotome. The tip of the sphincterotome was inserted into the papilla, followed by advancement of the guidewire and opacification. Unclear who advanced the guidewire. 2. Contrast‐assisted cannulation: standard method of cannulation through a sphincterotome. |
|
| Outcomes | PEP (reported according to type of duct cannulated or failed cannulation). | |
| Notes | 1. Did not define PEP in abstract. 2. Did not grade the severity of PEP or report outcome data regarding severity of PEP in abstract. 3. Did not report on the use of technique 'cross‐over'. 4. Did not report on the use of precut. 5. Did not report on the use of PD stent. 6. PEP incidence according to the type of duct cannulated in the table is unclear. Successful cannulation based on number of failed cannulation: 98 vs 96. Unclear whether PD cannulation was intentional or inadvertent. Unsuccessful attempts to contact authors for additional data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Conference proceeding, no information was provided. |
| Allocation concealment (selection bias) | Unclear risk | Conference proceeding, no information was provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Conference proceeding, no information was provided. Endoscopists could not be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Conference proceeding, no information was provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | PEP reported in ITT sample. All patients were accounted for with no loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Reported all important outcomes. Information pertaining to successful cannulation and inadvertent PD cannulation presented in the table is unclear |
| Other bias | Low risk | No other risk of bias. |
| Methods | Multi‐centre, RCT. Enrolment period: unclear. Endoscopist(s): unclear, but likely multiple endoscopists in multiple centres. | |
| Participants | Country: Italy. 88 PEP high‐risk patients (no definition provided for high risk patients). | |
| Interventions | 1. Guidewire‐assisted cannulation: a new guidewire with a loop in the tip. No information provided regarding cannulation device. No information on technique. Unclear who advanced the guidewire. 2. Contrast‐assisted cannulation: no information provided regarding the cannulation device or technique. |
|
| Outcomes | PEP; post‐ERCP 24 h serum amylase; number of CBD cannulation attempts; technical success (successful cannulation of the CBD). | |
| Notes | 1. Defined PEP according to the consensus definition (Cotton 1991). 2. Did not grade the severity of PEP or report outcome data regarding severity of PEP in abstract. 3. "Cross‐over" technique: Cannulation attempt was composed of two phases: phase one consisted of 5 minutes attempts or a maximum of five attempts of main PD cannulation or three attempts of main PD opacification if group 2. If phase 1 failed, would proceed to phase 2 which consisted of 5 minutes or a maximum of five main PD cannulation attempts with the wire. If there was no CBD cannulation after phase 2, "technical cannulation failure" was declared. The endoscopist may either stop the ERCP or use precut to obtain CBD access or continue the CBD cannulation attempt with the wire. 4. Precut was permitted. 5. Did not report on the use of PD stent. Unsuccessful attempts to contact authors for additional data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Conference proceeding, no information was provided. |
| Allocation concealment (selection bias) | Unclear risk | Conference proceeding, no information was provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Conference proceeding, no information was provided. Endoscopists could not be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Conference proceeding, no information was provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | PEP reported in ITT sample, no patients were lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Reported all important outcomes. |
| Other bias | Low risk | No other risk of bias. |
| Methods | Single centre, RCT. Enrolement period: July 2007 to December 2009. Endoscopist(s): Multiple endoscopists. First a trainee endoscopist attempted the cannulation and if it was not successful, an expert endoscopist tried. | |
| Participants | Country: Japan. 172 ERCP patients with native papilla undergoing cholangiography, bile or tissue sampling from the gallbladder or the bile duct, or treatment of biliary diseases. | |
| Interventions | 1. Guidewire‐assisted cannulation: a hydrophilic tipped 0.035 inch guidewire (Jagwire, Boston Scientific) through a sphincterotome (Boston Scientific). Guidewire was used to access the CBD, followed by cannulation and opacification. Guidewire was advanced by an assisting endoscopist. 2. Contrast‐assisted cannulation: standard method of cannulation through a 4F standard catheter (Olympus). All procedures were performed by using a 15‐degree backward oblique angle duodenoscope with an elevator function (Olympus). |
|
| Outcomes | PEP; successful cannulation of the CBD; time to achieve successful deep cannulation; number of cannulation attempts; number of accidental PD insertions; amount of contrast medium used; post‐ERCP complications other than pancreatitis. | |
| Notes | 1. Defined PEP according to the consensus definition (Cotton 1991). 2. Graded severity of PEP based on the consensus criteria (Cotton 1991). 3. "Crossover" technique: A trainee would first attempt the cannulation, and if selective biliary cannulation was not obtained during the first 5 min, an expert endoscopist would apply the same technique for another 5 min. If these attempts during the first 10 min failed, the expert endoscopist would switch and apply the 'cross‐over' technique for another 10 min. If both methods failed during these 20 min, cannulation according to the preference of the expert was continued until successful. 4. Precut was permitted. 5. PD stents were not used for the prevention of PEP. 6. Did not provide data on inadvertent PD cannulation / injection. Authors contacted: inadvertent PD cannulation or injection occurred in 52 patients in the guidewire‐assisted group vs. 55 patients in the contrast‐assisted group. Among these cases, PEP occurred in 2/52 patients in the guidewire‐assisted group vs. 3/55 patients in the contrast‐assisted group. Prior to crossover, PEP occurred in 2/35 patients in the guidewire‐assisted group vs. 2/34 in the contrast‐assisted group. After crossover, PEP occurred in 0/17 patients in the guidewire‐assisted group vs. 1/21 patients in the contrast‐assisted group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "divided randomly just prior to ERCP into two groups: biliary cannulation by wire‐guided cannulation or standard cannulation with contrast injection using a sealed envelope method by a physician who was not involved in performing the endoscopic procedure or in the critical care of the patient". |
| Allocation concealment (selection bias) | Low risk | "divided randomly using a sealed envelope method by a physician who was not involved in performing the endoscopic procedure or in the critical care of the patient". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information was provided. Endoscopists could not be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information was provided. "Following each procedure, we monitored patients for subjective symptoms such as abdominal pain and nausea and conducted physical examinations of the abdomen. Blood samples collected 2 h after ERCP were used to measure complete blood count and serum amylase level, and those collected after 18 h were used to measure complete blood count, hepatobiliary enzymes, serum amylase, lipase, pancreatic amylase, and CRP." It is unclear whether patients were discharged home or admitted for observation post procedure. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patient was lost to follow up. 2 cases of bilio‐duodenal fistula were excluded from the contrast‐assisted group in the analysis. We used ITT sample in our analysis. |
| Selective reporting (reporting bias) | Low risk | Reported all planned outcomes. But for some outcomes (median time for deep cannulation, median number of endoscopic procedures, median number of accidental contrast injections or guidewire insertions into the PD), no raw data was provided ("no significant differences were reported"). |
| Other bias | Low risk | No other risk of bias. |
CBD: Common bile duct. PEP: post‐ERCP pancreatitis. PD: pancreatic duct. RCT: randomised controlled trial.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Angsuwatcharakon 2010 | Inappropriate intervention: double‐guidewire cannulation versus precut sphincterotomy. Preliminary report (conference presentation) of Angsuwatcharakon 2012. |
| Angsuwatcharakon 2012 | Inappropriate intervention: double‐guidewire cannulation versus precut sphincterotomy. |
| Artifon 2005 | Preliminary report (conference presentation) of Artifon 2007. |
| Bailey 2006a | Preliminary report (conference presentation) of Bailey 2008. |
| Bailey 2006b | Post hoc analysis of two RCTs aimed to assess the effect of needle knife sphincterotomy (NKS). |
| Bailey 2006c | Preliminary report (conference presentation) of Bailey 2008. |
| Balderas 2011 | Inappropriate intervention: double‐guidewire technique versus pancreatic duct stent, not a RCT. |
| Cennamo 2009 | A meta‐analysis of five RCTs that compared primary biliary cannulation and post‐ERCP pancreatitis rates with the wire‐guided method and the standard cannulation technique. |
| Cha 2011 | Inappropriate intervention: double‐guidewire versus transpancreatic precut sphincterotomy in difficult biliary cannulation. |
| Cheung 2009 | A meta‐analysis of seven RCTs that compared guidewire‐guided with conventional contrast‐guided bile duct cannulation for the prevention PEP. |
| Choudhary 2009 | A meta‐analysis of 6 RCTs that compared guidewire with conventional methods for cannulation rate and PEP. |
| Choudhary 2010a | A meta‐analysis of seven RCTs that compared pancreatic guidewire use for deep biliary cannulation with conventional guidewire use without pancreatic cannulation. |
| Choudhary 2010b | A meta‐analysis of seven RCTs that compared guidewires with conventional methods for cannulation rate and PEP. |
| Cote 2010 | Inappropriate intervention: pancreatic duct guidewire vs. pancreatic duct stent. |
| de Tejada 2007 | Inappropriate intervention: double‐guidewire technique vs. standard cannulation technique. Preliminary report and duplicate data of de Tejada 2009. |
| de Tejada 2009 | Inappropriate intervention: double‐guidewire technique vs. standard cannulation technique. |
| Epstein 2009 | A systematic review of three RCTs that compared wire‐guided cannulation with conventional contrast injection in ERCP. |
| Ito 2008 | Inappropriate intervention: double‐guidewire technique, not a RCT. |
| Ito 2010 | Not a RCT. |
| Kamata 2011 | Not a RCT. |
| Lee 2004 | A case series, not a RCT. |
| Madhoun 2009 | A meta‐analysis of five RCTs that compared wire‐guided cannulation technique with conventional cannulation as a strategy to reduce PEP. |
| Maeda 2003 | Inappropriate intervention: pancreatic duct guidewire versus persistence with a conventional catheter in difficult cases of selective bile duct cannulation. |
| Mariani 2012 | Not a RCT. |
| Nakai 2011 | Not a RCT |
| Nambu 2009 | Preliminary report (conference presentation) of Nambu 2011. |
| Park 2008 | Preliminary report (conference presentation) of Lee 2009. |
| Shao 2009 | A meta‐analysis of four RCTs that compared wire‐guided cannulation with conventional contrast‐assisted cannulation for the incidence of PEP. |
| Trifan 2011 | Not a RCT. |
| Zheng 2010 | Inappropriate intervention: double‐guidewire versus standard cannulation technique. |
Contributions of authors
Frances Tse, Yuhong Yuan, Paul Moayyedi, and Grigorios I Leontiadis were responsible for designing the review protocol. Yuhong Yuan developed the search strategy with collaboration the Cochrane UGPD Group. Yuhong Yuan conducted literature searches. Frances Tse and Yuhong Yuan were responsible for performing eligibility checks on the search results, data extraction, data analysis, quality assessment, and interpretation of data. Frances Tse contributed to the manuscript preparation. Grigorios I Leontiadis, Yuhong Yuan and Paul Moayyedi contributed to review of the manuscript. All review authors contributed to final editing of the review and gave final approval.
Sources of support
Internal sources
McMaster University, Department of Gastroenterology, Canada.
External sources
No sources of support supplied
Declarations of interest
None known.
New
References
References to studies included in this review
- Apostolopoulos P, Alexandrakis G, Liatsos C, Giannakoulopoulou E, Alogari A, Kalantzis C, et al. Guide wire‐assisted selective access to the common bile duct could prevent post‐ERCP pancreatitis. Endoscopy 2005;37 Suppl 1:A278. Abstract WED‐E‐297. [Google Scholar]
- Artifon EL, Sakai P, Cunha JE, Halwan B, Ishioka S, Kumar A. Guidewire cannulation reduces risk of post‐ERCP pancreatitis and facilitates bile duct cannulation. American Journal of Gastroenterology 2007;102(10):2147‐53. [DOI] [PubMed] [Google Scholar]
- Bailey AA, Bourke MJ, Williams SJ, Walsh PR, Murray MA, Lee EY, et al. A prospective randomized trial of cannulation technique in ERCP: effects on technical success and post‐ERCP pancreatitis. Endoscopy 2008;40(4):296‐301. [DOI] [PubMed] [Google Scholar]
- Gruchy S, Macintosh DG, Farina D, Molinari M, Khaliq‐kareemi M. Is the incidence of post‐endoscopic cholangiopancreatography (ERCP) pancreatitis reduced in patients undergoing hydrophilic guide wire Cannulation of the common bile duct (CBD) versus standard cannulation with dye injection: a randomized controlled trial. Gastroenterology 2007;132(4 Suppl 2):A 643. Abstract W1019. [Google Scholar]
- Katsinelos P, Paroutoglou G, Kountouras J, Chatzimavroudis G, Zavos C, Pilpilidis I, et al. A comparative study of standard ERCP catheter and hydrophilic guide wire in the selective cannulation of the common bile duct. Endoscopy 2008;40(4):302‐7. [DOI] [PubMed] [Google Scholar]
- Kawakami H, Maguchi H, Mukai T, Hayashi T, Sasaki T, Isayama H, et al. A multicenter, prospective, randomized study of selective bile duct cannulation performed by multiple endoscopists: the BIDMEN study. Gastrointestinal Endoscopy 2012;75(2):362‐72. [DOI] [PubMed] [Google Scholar]
- Kobayashi G, Fujita N, Imaizumi K, Irisawa A, Suzuki M, Murakami A, et al. Can a guidewire cannulation technique reduce the risk of post‐ERCP pancreatitis? A multicenter randomized controlled trial. Endoscopy 2010;42 Suppl 1:A89. Abstract OP409. [DOI] [PubMed] [Google Scholar]; Kobayashi G, Fujita N, Imaizumi K, Irisawa A, Suzuki M, Murakami A, et al. Can a wire‐guided cannulation technique reduce risk of post‐ERCP pancreatitis and facilitate bile duct deep cannulation?. Gastrointestinal Endoscopy 2010;71(5):AB109‐10. Abstract 347a. [Google Scholar]; Kobayashi G, Fujita N, Imaizumi K, Irisawa A, Suzuki M, Murakami A, et al. Risk factors for Post‐ERCP pancreatitis: Evaluation in a prospective randomized trial comparing the guidewire cannulation technique with the conventional cannulation technique. Pancreatology 2010;10:94‐5. [Google Scholar]
- Lee TH, Park do H, Park JY, Kim EO, Lee YS, Park JH, et al. Can wire‐guided cannulation prevent post‐ERCP pancreatitis? A prospective randomized trial. Gastrointestinal Endoscopy 2009;69(3 Pt 1):444‐9. [DOI] [PubMed] [Google Scholar]
- Lella F, Bagnolo F, Colombo E, Bonassi U. A simple way of avoiding post‐ERCP pancreatitis. Gastrointestinal Endoscopy 2004;59(7):830‐4. [DOI] [PubMed] [Google Scholar]
- Mangiavillano B, Mariani A, Giussani A, Masci E, Testoni PA. Guidewire assisted cannulation of the papilla of Vater to reduce the risk of post‐ERCP pancreatitis: a prospective randomised study. Endoscopy 2007;39 Suppl I:A373. Abstract WED‐E‐426. [Google Scholar]
- Mangiavillano B, Mangano M, Limido E, Bernasconi G, Spinzi G, Teruzzi V, et al. Preliminary results on the comparison of loop‐tip COOK MEDICAL® Wire vs traditional endoscopic technique cannulation in the prevention of post‐ERCP pancreatitis and biliary tree access in high‐risk patients. Endoscopy 2011;43 Suppl 1:A86. Abstract OP379. [Google Scholar]
- Nambu T, Ukita T, Shigoka H, Omuta S, Maetani I. Wire‐guided selective cannulation of the bile duct with a sphincterotome: a prospective randomized comparative study with the standard method. Scandinavian Journal of Gastroenterology 2011;46(1):109‐15. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Angsuwatcharakon P, Ridtitid W, Rerknimitr R, Kongkam P, Treeprasertsuk S, Kullavanijaya P. Pancreatic guide‐wire placement assisting biliary cannulation (double guide‐wire) versus precut sphincterotomy in difficult Biliary cannulation: a prospective randomized study. Gastrointestinal Endoscopy 2010;71(5):AB164. [Google Scholar]
- Angsuwatcharakon P, Rerknimitr R, Ridtitid W, Ponauthai Y, Kullavanijaya P. Success rate and cannulation time between precut sphincterotomy and double‐guidewire technique in truly difficult biliary cannulation. Journal of Gastroenterology and Hepatology 2012;27(2):356‐61. [DOI] [PubMed] [Google Scholar]
- Artifon EL, Sakai P, Hondo FY, Maluf‐Filho F, Monteiro‐da‐Cunha JE, Ishioka S. Comparison of deep bile duct cannulation with cannulatome versus cannulatome with guide wire: a prospective randomized trial. Gastrointestinal Endoscopy 2005;61(5):AB198. Abstract T1228. [Google Scholar]
- Bailey AA, Bourke MJ, Williams SJ, Kwan V, Lynch PM, Lee EY, et al. Guidewire cannulation improves primary success rates but does not protect against post ERCP pancreatitis: final results of a prospective randomised trial. Endoscopy 2006;38(Suppl II):A 24. Abstract OP‐E‐99. [Google Scholar]
- Bailey AA, Bourke MJ, Williams SJ, Kaffes AJ, Lee EY. Post ERCP pancreatitis and precut needle knife sphincterotomy in difficult biliary cannulation: a prospective evaluation of the relationship. Endoscopy 2006;38(Suppl II):A 24. Abstract OP‐E‐103. [Google Scholar]
- Bailey AA, Lynch PM, Lee EY, Walsh PR, Michael M, Kwan V, et al. Results of a randomised trial of cannulation technique in ERCP: effects on technical success and post ERCP pancreatitis. Gastrointestinal Endoscopy 2006;63(5):AB284. Abstract W1385. [DOI] [PubMed] [Google Scholar]
- Balderas V, Linsteadt J, Surapaneni SN, Echavarria J, Patel S, Rosenkranz L. Does implementation of the double wire technique and prophylactic pancreatic duct stenting reduce post (ERCP) pancreatitis in patients undergoing needle Knife Sphincterotomy. Gastrointestinal Endoscopy 2011;73(4 Suppl 1):AB268. [Google Scholar]
- Cennamo V, Fuccio L, Zagari RM, Eusebi LH, Ceroni L, Laterza L, et al. Can a wire‐guided cannulation technique increase bile duct cannulation rate and prevent post‐ERCP pancreatitis?: A meta‐analysis of randomized controlled trials. American Journal of Gastroenterology 2009;104(9):2343‐50. [DOI] [PubMed] [Google Scholar]
- Cha SW, Yoo YW, Kim A, Kim SH, Lee HL, Lee YJ, et al. Double guidewire technique versus transpancreatic precut sphincterotomy in difficult biliary cannulation; randomized prospective study. Gastrointestinal Endoscopy 2001;73(4 Suppl 1):AB348. Abstract Mo1445. [Google Scholar]
- Cheung J, Tsoi KK, Quan WL, Lau JY, Sung JJ. Guidewire versus conventional contrast cannulation of the common bile duct for the prevention of post‐ERCP pancreatitis: a systematic review and meta‐analysis. Gastrointestinal Endoscopy 2009;70(6):1211‐9. [DOI] [PubMed] [Google Scholar]
- Choudhary A, Puli SR, Ibdah JA, Bechtold ML. Guidewire use for prevention of post ERCP pancreatitis: A meta‐analysis of randomized controlled trials. Gastrointestinal Endoscopy 2009;69 (5):AB309. Abstract T1488. [Google Scholar]
- Choudhary A, Szary NM, Arif M, Hammad HT, Thapar M, Hammoud GM, et al. Does guidewire use in the pancreatic duct during deep biliary cannulation prevent post‐ERCP pancreatitis? A meta‐analysis. Gastrointestinal Endoscopy 2010;71(5):AB302. Abstract T1533. [Google Scholar]
- Choudhary A, Szary NM, Arif M, Hammad HT, Thapar M, Hammoud GM, et al. Does guidewire use prevent post‐ERCP pancreatitis? A meta‐analysis of randomized controlled trials. Gastrointestinal Endoscopy 2010;71(5):AB163. Abstract S1443. [Google Scholar]
- Cote GA, Hovis CE, Keswani RN, Ansstas M, Edmundowicz SA, Jonnalagadda SS, et al. A randomized clinical trial comparing the use of a pancreatic duct stent or a pancreatic duct guidewire to facilitate bile duct cannulation. Gastrointestinal Endoscopy 2010;71(5):AB163‐4. Abstract S1445. [Google Scholar]
- Tejada AH, Calleja JL, Díaz G, Pertejo V, Espinel J, Pertejo V, et al. Double guide wire technique compared to conventional method in selected cases of difficult common bile duct cannulation in ERCP. Preliminary data from a controlled randomized study. Gastrointestinal Endoscopy 2007;65(5):AB231. Abstract M1334. [Google Scholar]
- Tejada AH, Calleja JL, Díaz G, Pertejo V, Espinel J, Cacho G, et al. Double‐guidewire technique for difficult bile duct cannulation: a multicenter randomized, controlled trial. Gastrointestinal Endoscopy 2009;70(4):700‐9. [DOI] [PubMed] [Google Scholar]
- Epstein I, Gruchy S, MacIntosh D. Wire guided cannulation versus conventional contrast injection in ERCP: a systematic review. Canadian Journal of Gastroenterology 2009;23:Abstracts TOC 64. [Google Scholar]
- Ito K, Fujita N, Noda Y, Kobayashi G, Obana T, Horaguchi J, et al. Pancreatic guidewire placement for achieving selective biliary cannulation during endoscopic retrograde cholangio‐pancreatography. World Journal of Gastroenterology 2008;14(36):5595‐5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Mimura T, Hara S, Kamata I, Kishimoto Y, Okano N, et al. Device and handle of difficult bile duct cannulation. Journal of Gastroenterology and Hepatology 2010;25 :A30. [Google Scholar]
- Kamata K, Kitano M, Kudov M, Kadosaka K, Miyata T, Imai H, et al. Does wire guided cannulation reduce the risk of pancreatic disorder?. Journal of Gastroenterology and Hepatology 2011;26:38. [Google Scholar]
- Lee YT, Chan FK, Sung JJ. Selective biliary cannulation aided by pancreatic duct guided technique (PDGT). Endoscopy 2004;36 Suppl I:A160. Abstract TUE‐E‐27. [Google Scholar]
- Madhoun M, Te CC, Stoner J, Maple JT. Does wire‐guided cannulation prevent post‐ERCP pancreatitis? A meta‐analysis. Gastrointestinal Endoscopy 2009;69 (5):AB132. Abstract 934. [Google Scholar]
- Maeda S, Hayashi H, Hosokawa O, Dohden K, Hattori M, Morita M, et al. Prospective randomized pilot trial of selective biliary cannulation using pancreatic guide‐wire placement. Endoscopy 2003;35(9):721‐4. [DOI] [PubMed] [Google Scholar]
- Mariani A, Giussani A, Leo M, Testoni S, Testoni PA. Guidewire biliary cannulation does not reduce post‐ERCP pancreatitis compared with the contrast injection technique in low‐risk and high‐risk patients. Gastrointestinal Endoscopy 2012 75;75(2):339‐46. [DOI] [PubMed] [Google Scholar]
- Nakai Y, Isayama H, Tsujino T, Sasahira N, Hirano K, Kogure H, et al. Impact of introduction of wire‐guided cannulation in therapeutic biliary endoscopic retrograde cholangiopancreatography. Journal of Gastroenterology and Hepatology 2011;26(10):1552‐8. [DOI] [PubMed] [Google Scholar]
- Nambu T, Ukita T, Shigoka H, Omuta S, Endo T, Maetani I. Wire‐guided selective cannulation of the bile duct with a sphincterotome: a prospective randomized comparative study with the standard method: an interim report. Endoscopy. 2009; Vol. 41 Suppl 1:A 24. Abstract OP114. [DOI] [PubMed] [Google Scholar]
- Park DH, Lee TH, Park JY, Park JH, Lee SH, Chung IK, et al. Can wire‐guided cannulation (WGC) prevent post‐ERCP pancreatitis? A prospective randomized trial. Gastrointestinal Endoscopy 2008;67 (5):AB328. Abstract W1617. [DOI] [PubMed] [Google Scholar]
- Shao LM, Chen QY, Chen MY, Cai JT. Can wire‐guided cannulation reduce the risk of post‐endoscopic retrograde cholangiopancreatography pancreatitis? A meta‐analysis of randomised controlled trials. Journal of Gastroenterology and Hepatology 2009;24(11):1710‐5. [DOI] [PubMed] [Google Scholar]
- Trifan A, Sfarti C, Cretu M, Cojocariu C, Hutanasu C, Singeap AM, et al. Guide‐wire versus conventional contrast cannulation of the common bile duct for the prevention of post‐ERCP pancreatitis in patients with choledocholithiasis. Journal of Gastrointestinal and Liver Diseases 2011;20(2):149‐52. [PubMed] [Google Scholar]
- Zheng FP, Guo YW, Tao LI, Lin XY. Double‐guidewire technique for difficult bile duct cannulation in patients with biliary. Journal of Gastroenterology and Hepatology 2010;25 Suppl 2:A51. Abstract 329. [Google Scholar]
Additional references
- Als‐Nielsen B, Chen W, Gluud LL, Siersma V, Hilden J, Gluud C. Are trial size and reported methodological quality associated with treatment effects? Observational study of 523 randomised trials. 12th International Cochrane Colloquium. Ottawa, Canada, 2004. [Google Scholar]
- Artifon EL, Chu A, Freeman M, Sakai P, Usmani A, Kumar A. A comparison of the consensus and clinical definitions of pancreatitis with a proposal to redefine post‐endoscopic retrograde cholangiopancreatography pancreatitis. Pancreas 2010;39(4):530‐5. [DOI] [PubMed] [Google Scholar]
- ASGE Technology Committee, Shah RJ, Somogyi L, Petersen BT, Tierney WM, Adler DG, et al. Short‐wire ERCP systems. Gastrointestinal Endoscopy 2007;66(4):650‐7. [DOI] [PubMed] [Google Scholar]
- Badalov N, Tenner S, Baillie J. The prevention, recognition and treatment of post‐ERCP pancreatitis. JOP 2009;10(2):88‐97. [PubMed] [Google Scholar]
- Balthazar EJ, Robinson DL, Megibow AJ, Ranson JH. Acute pancreatitis: value of CT in establishing prognosis. Radiology 1990;174:331‐6. [DOI] [PubMed] [Google Scholar]
- Banks PA, Freeman ML, Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis. American Journal of Gastroenterology 2006;101(10):2379‐400. [DOI] [PubMed] [Google Scholar]
- Cennamo V, Fuccio L, Zagari RM, Eusebi LH, Ceroni L, Laterza L, et al. Can early precut implementation reduce endoscopic retrograde cholangiopancreatography‐related complication risk? Meta‐analysis of randomized controlled trials. Endoscopy 2010;42(5):381‐8. [DOI] [PubMed] [Google Scholar]
- Cheng CL, Sherman S, Watkins JL, Barnett J, Freeman M, Geenen J, et al. Risk factors for post‐ERCP pancreatitis: a prospective multicenter study. American Journal of Gastroenterology 2006;101(1):139‐47. [DOI] [PubMed] [Google Scholar]
- Choudhary A, Bechtold ML, Arif M, Szary NM, Puli SR, Othman MO, et al. Pancreatic stents for prophylaxis against post‐ERCP pancreatitis: a meta‐analysis and systematic review. Gastrointestinal Endoscopy 2011;73(2):275‐82. [DOI] [PubMed] [Google Scholar]
- Colton JB, Curran CC. Quality indicators, including complications, of ERCP in a community setting: a prospective study. Gastrointestinal Endoscopy 2009;70(3):457‐67. [DOI] [PubMed] [Google Scholar]
- Conn M, Goldenberg A, Concepcion L, Mandeli J. The effect of ERCP on circulating pancreatic enzymes and pancreatic protease inhibitors. American Journal of Gastroenterology 1991;86(8):1011‐4. [PubMed] [Google Scholar]
- Cook D, Guyatt GH, Ryan G. Should unpublished data be included in meta‐analysis? Current convictions and controversies. JAMA 1993;269(21):2749‐53. [PubMed] [Google Scholar]
- Cook JA, Ramsay CR, Fayers P. Statistical evaluation of learning curve effects in surgical trials. Clinical Trials Journal 2004;1(5):421‐7. [DOI] [PubMed] [Google Scholar]
- Cortas GA, Mehta SN, Abraham NS, Barkun AN. Selective cannulation of the common bile duct: a prospective randomized trial comparing standard catheters with sphincterotomes. Gastrointestinal Endoscopy 1999;50(6):775‐9. [DOI] [PubMed] [Google Scholar]
- Cotton PB, Lehman G, Vennes J, Geenen JE, Russell RC, Meyers WC, et al. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointestinal Endoscopy 1991;37(3):383‐93. [DOI] [PubMed] [Google Scholar]
- Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, Es GA, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation 2007;114(17):2344‐51. [DOI] [PubMed] [Google Scholar]
- Dickersin K, Chan S, Chalmers TC, Sacks HS, Smith H Jr. Publication bias and clinical trials. Controlled Clinical Trials 1987;8(4):343‐53. [DOI] [PubMed] [Google Scholar]
- Draganov PV, Kowalczyk L, Fazel A, Moezardalan K, Pan JJ, Forsmark CE. Prospective randomized blinded comparison of a short‐wire endoscopic retrograde cholangiopancreatography system with traditional long‐wire devices. Digestive Diseases and Sciences 2010;55(2):510‐5. [DOI] [PubMed] [Google Scholar]
- Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet 1991;337(8746):867‐72. [DOI] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmunzer BJ, Scheiman JM, Lehman GA, Chak A, Mosler P, Higgins PD, et al. A randomized trial of rectal indomethacin to prevent post‐ERCP pancreatitis. New England Journal of Medicine 2012;366(15):1414‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyding D, Lelgemann M, Grouven U, Härter M, Kromp M, Kaiser T, et al. Reboxetine for acute treatment of major depression: systematic review and meta‐analysis of published and unpublished placebo and selective serotonin reuptake inhibitor controlled trials. BMJ 2010;341:c4737. [DOI: 10.1136/bmj.c4737] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsmark CE, Baillie J, AGA Institute Clinical Practice and Economics Committee, AGA Institute Governing Board. AGA Institute technical review on acute pancreatitis. Gastroenterology 2007;132(5):2022‐44. [DOI] [PubMed] [Google Scholar]
- Freeman ML, Nelson DB, Sherman S, Haber GB, Herman ME, Dorsher PJ, et al. Complications of endoscopic biliary sphincterotomy. New England Journal of Medicine 1996;335(13):909‐18. [DOI] [PubMed] [Google Scholar]
- Freeman ML, DiSario JA, Nelson DB, Fennerty MB, Lee JG, Bjorkman DJ, et al. Risk factors for post‐ERCP pancreatitis: a prospective, multicenter study. Gastrointestinal Endoscopy 2001;54(4):425‐34. [DOI] [PubMed] [Google Scholar]
- Freeman ML, Guda NM. Prevention of post‐ERCP pancreatitis: a comprehensive review. Gastrointestinal Endoscopy 2004;59(7):845‐64. [DOI] [PubMed] [Google Scholar]
- Freeman ML, Overby C, Qi D. Pancreatic stent insertion: consequences of failure and results of a modified technique to maximize success. Gastrointestinal Endoscopy 2004;59(1):8‐14. [DOI] [PubMed] [Google Scholar]
- Freeman ML, Guda NM. ERCP cannulation: a review of reported techniques. Gastrointestinal Endoscopy 2005;61(1):112‐25. [PUBMED: 15672074] [DOI] [PubMed] [Google Scholar]
- Freeman ML. Pancreatic stents for prevention of post‐endoscopic retrograde cholangiopancreatography pancreatitis. Clinical Gastroenterology and Hepatology 2007;5(11):1354‐65. [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schunemann HJ, and the GRADE working Group. What is 'quality of evidence' and why is it important to clinicians. British Medical Journal 2008;336:995‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbord RM, Egger M, Sterne JA. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443‐57. [DOI] [PubMed] [Google Scholar]
- Holubkov R, Dean JM, Berger J, Anand KJ, Carcillo J, Meert K, et al. Is "rescue" therapy ethical in randomized controlled trials?. Pediatric Critical Care Medicine 2009;10(4):431‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis JP, Trikalinos TA. The appropriateness of asymmetry tests for publication bias in meta‐analyses: a large survey. Canadian Medical Association Journal 2007;176(8):1091‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiriyama S, Gabata T, Takada T, Hirata K, Yoshida M, Mayumi T, et al. New diagnostic criteria of acute pancreatitis. Journal of Hepato‐Biliary‐Pancreatic Sciences 2010;17(1):24‐36. [DOI] [PubMed] [Google Scholar]
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
- Kozarek RA. Pancreatic stents can induce ductal changes consistent with chronic pancreatitis. Gastrointestinal Endoscopy 1990;36(2):93‐5. [DOI] [PubMed] [Google Scholar]
- Light RJ, Pillemer DB. Summing up. The science of reviewing research. Cambridge, MA: Harvard University Press, 1984. [Google Scholar]
- Loperfido S, Angelini G, Benedetti G, Chilovi F, Costan F, Berardinis F, et al. Major early complications from diagnostic and therapeutic ERCP: a prospective multicenter study. Gastrointestinal Endoscopy 1998;48(1):1‐10. [DOI] [PubMed] [Google Scholar]
- Löhr JM, Aabaken L, Arnelo U, Grönroos J, Halttunen J, Hauge T, et al. How to cannulate? A survey of the Scandinavian Association for Digestive Endoscopy (SADE) in 141 endoscopists. Scandinavian Journal of Gastroenterology 2012;Early Online:1‐9. [DOI: 10.3109/00365521.2012.672588] [DOI] [PubMed] [Google Scholar]
- Masci E, Mariani A, Curioni S, Testoni PA. Risk factors for pancreatitis following endoscopic retrograde cholangiopancreatography: a meta‐analysis. Endoscopy 2003;35(10):830‐4. [DOI] [PubMed] [Google Scholar]
- Mazaki T, Masuda H, Takayama T. Prophylactic pancreatic stent placement and post‐ERCP pancreatitis: a systematic review and meta‐analysis. Endoscopy 2010;42(10):842‐53. [DOI] [PubMed] [Google Scholar]
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [DOI] [PubMed] [Google Scholar]
- Perdue DG, Freeman ML, ERCOST study group. Failed biliary ERCP: A prospective multicenter study of risk factors, complications and resource utilization. Gastrointestinal Endoscopy 2004;59:T1479. [Google Scholar]
- Pezzilli R, Romboli E, Campana D, Corinaldesi R. Mechanisms involved in the onset of post‐ERCP pancreatitis. Journal of the Pancreas 2002;3(6):162‐8. [PubMed] [Google Scholar]
- Pocock SJ, Hughes MD. Practical problems in interim analyses with particular regard to estimation. Controlled Clinical Trials 1989;10:2095‐215. [DOI] [PubMed] [Google Scholar]
- Ranson JH, Rifkind KM, Roses DF, Fink SD, Eng K, Spencer FC. Prognostic signs and the role of operative management in acute pancreatitis. Surgery, Gynecology and Obstetrics 1974;139:69‐81. [PubMed] [Google Scholar]
- Reddy SC, Draganov PV. ERCP wire systems: the long and the short of it. World Journal of Gastroenterology 2009;15(1):55‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
- Schulz KF, Grimes DA. Generation of allocation sequences in randomised trials: chance, not choice. Lancet 2002;359(9305):515‐9. [DOI] [PubMed] [Google Scholar]
- Schwacha H, Allgaier HP, Deibert P, Olschewski M, Allgaier U, Blum HE. A sphincterotome‐based technique for selective transpapillary common bile duct cannulation. Gastrointestinal Endoscopy 2000;52(3):387‐91. [DOI] [PubMed] [Google Scholar]
- Siegel JH, Ben‐Zvi JS, Pullano W. The needle knife: a valuable tool in diagnostic and therapeutic ERCP. Gastrointestinal Endoscopy 1989;35(6):499‐503. [DOI] [PubMed] [Google Scholar]
- Skude G, Wehlin L, Maruyama T, Ariyama J. Hyperamylasaemia after duodenoscopy and retrograde cholangiopancreatography. Gut 1976;17(2):127‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta‐analysis: power of statistical tests and prevalence in the literature. Journal of Clinical Epidemiology 2000;53(11):1119‐29. [DOI] [PubMed] [Google Scholar]
- Terrin N, Schmid CH, Lau J, Olkin I. Adjusting for publication bias in the presence of heterogeneity. Statistics in Medicine 2003;22(13):2113‐26. [DOI] [PubMed] [Google Scholar]
- Testoni PA, Bagnolo F, Caporuscio S, Lella F. Serum amylase measured four hours after endoscopic sphincterotomy is a reliable predictor of postprocedure pancreatitis. American Journal of Gastroenterology 1999;94(5):1235‐41. [DOI] [PubMed] [Google Scholar]
- Testoni PA, Bagnolo F, Natale C, Primignani M. Incidence of post‐endoscopic retrograde‐cholangiopancreatography/sphincterotomy pancreatitis depends upon definition criteria. Digestive and Liver Disease 2000;32(5):412‐8. [DOI] [PubMed] [Google Scholar]
- Testoni PA. Why the incidence of post‐ERCP pancreatitis varies considerably? Factors affecting the diagnosis and the incidence of this complication. Journal of the Pancreas 2002;3(6):195‐201. [PubMed] [Google Scholar]
- Testoni PA. Facts and fiction in the pharmacologic prevention of post‐ERCP pancreatitis: a never‐ending story. Gastrointestinal Endoscopy 2006;64(5):732‐4. [DOI] [PubMed] [Google Scholar]
- Testoni PA, Mariani A, Giussani A, Vailati C, Masci E, Macarri G, et al. Risk factors for post‐ERCP pancreatitis in high‐ and low‐volume centers and among expert and non‐expert operators: a prospective multicenter study. American Journal of Gastroenterology 2010;105(8):1753‐61. [DOI] [PubMed] [Google Scholar]
- Testoni PA, Giussani A, Vailati C, Testoni S, Leo M, Mariani A. Precut sphincterotomy, repeated cannulation and post‐ERCP pancreatitis in patients with bile duct stone disease. Digestive and Liver Disease 2011;43(10):792‐6. [DOI] [PubMed] [Google Scholar]
- Udd M, Kylanpaa L, Halttunen J. Management of difficult bile duct cannulation in ERCP. World Journal of Gastrointestinal Endoscopy 2010;2(3):97‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Working Party of the British Society of Gastroenterology, Association of Surgeons of Great Britain and Ireland, Pancreatic Society of Great Britain and Ireland, Association of Upper GI Surgeons of Great Britain and Ireland. UK guidelines for the management of acute pancreatitis. Gut 2005;54(Suppl 3:iii):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandervoort J, Soetikno RM, Tham TC, Wong RC, Ferrari AP Jr, Montes H, et al. Risk factors for complications after performance of ERCP. Gastrointestinal Endoscopy 2002;56(5):652‐6. [DOI] [PubMed] [Google Scholar]
- Varadarajulu S, Kilgore ML, Wilcox CM, Eloubeidi MA. Relationship among hospital ERCP volume, length of stay, and technical outcomes. Gastrointestinal Endoscopy 2006;64(3):338‐47. [DOI] [PubMed] [Google Scholar]
- Wang P, Li ZS, Liu F, Ren X, Lu NH, Fan ZN, et al. Risk factors for ERCP‐related complications: a prospective multicenter study. American Journal of Gastroenterology 2009;104(1):31‐40. [DOI] [PubMed] [Google Scholar]
- Williams EJ, Taylor S, Fairclough P, Hamlyn A, Logan RF, Martin D, et al. Are we meeting the standards set for endoscopy? Results of a large‐scale prospective survey of endoscopic retrograde cholangio‐pancreatography practice. Gut 2007;56(6):821‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EJ, Taylor S, Fairclough P, Hamlyn A, Logan RF, Martin D, et al. Risk factors for complication following ERCP; results of a large‐scale, prospective multicenter study. Endoscopy 2007;39(9):793‐801. [DOI] [PubMed] [Google Scholar]
- Yadav D, Agarwal N, Pitchumoni CS. A critical evaluation of laboratory tests in acute pancreatitis. American Journal of Gastroenterology 2002;97(6):1309‐18. [DOI] [PubMed] [Google Scholar]


