Abstract
Background
Several erythropoiesis‐stimulating agents (ESAs) are available for treating anaemia in people with chronic kidney disease (CKD). Their relative efficacy (preventing blood transfusions and reducing fatigue and breathlessness) and safety (mortality and cardiovascular events) are unclear due to the limited power of head‐to‐head studies.
Objectives
To compare the efficacy and safety of ESAs (epoetin alfa, epoetin beta, darbepoetin alfa, or methoxy polyethylene glycol‐epoetin beta, and biosimilar ESAs, against each other, placebo, or no treatment) to treat anaemia in adults with CKD.
Search methods
We searched the Cochrane Renal Group's Specialised Register to 11 February 2014 through contact with the Trials' Search Co‐ordinator using search terms relevant to this review.
Selection criteria
Randomised controlled trials (RCTs) that included a comparison of an ESA (epoetin alfa, epoetin beta, darbepoetin alfa, methoxy polyethylene glycol‐epoetin beta, or biosimilar ESA) with another ESA, placebo or no treatment in adults with CKD and that reported prespecified patient‐relevant outcomes were considered for inclusion.
Data collection and analysis
Two independent authors screened the search results and extracted data. Data synthesis was performed by random‐effects pairwise meta‐analysis and network meta‐analysis. We assessed for heterogeneity and inconsistency within meta‐analyses using standard techniques and planned subgroup and meta‐regression to explore for sources of heterogeneity or inconsistency. We assessed our confidence in treatment estimates for the primary outcomes within network meta‐analysis (preventing blood transfusions and all‐cause mortality) according to adapted GRADE methodology as very low, low, moderate, or high.
Main results
We identified 56 eligible studies involving 15,596 adults with CKD. Risks of bias in the included studies was generally high or unclear for more than half of studies in all of the risk of bias domains we assessed; no study was low risk for allocation concealment, blinding of outcome assessment and attrition from follow‐up. In network analyses, there was moderate to low confidence that epoetin alfa (OR 0.18, 95% CI 0.05 to 0.59), epoetin beta (OR 0.09, 95% CI 0.02 to 0.38), darbepoetin alfa (OR 0.17, 95% CI 0.05 to 0.57), and methoxy polyethylene glycol‐epoetin beta (OR 0.15, 95% CI 0.03 to 0.70) prevented blood transfusions compared to placebo. In very low quality evidence, biosimilar ESA therapy was possibly no better than placebo for preventing blood transfusions (OR 0.27, 95% CI 0.05 to 1.47) with considerable imprecision in estimated effects. We could not discern whether all ESAs were similar or different in their effects on preventing blood transfusions and our confidence in the comparative effectiveness of different ESAs was generally very low. Similarly, the comparative effects of ESAs compared with another ESA, placebo or no treatment on all‐cause mortality were imprecise.
All proprietary ESAs increased the odds of hypertension compared to placebo (epoetin alfa OR 2.31, 95% CI 1.27 to 4.23; epoetin beta OR 2.57, 95% CI 1.23 to 5.39; darbepoetin alfa OR 1.83, 95% CI 1.05 to 3.21; methoxy polyethylene glycol‐epoetin beta OR 1.96, 95% CI 0.98 to 3.92), while the effect of biosimilar ESAs on developing hypertension was less certain (OR 1.18, 95% CI 0.47 to 2.99). Our confidence in the comparative effects of ESAs on hypertension was low due to considerable imprecision in treatment estimates. The comparative effects of all ESAs on cardiovascular mortality, myocardial infarction (MI), stroke, and vascular access thrombosis were uncertain and network analyses for major cardiovascular events, end‐stage kidney disease (ESKD), fatigue and breathlessness were not possible. Effects of ESAs on fatigue were described heterogeneously in the available studies in ways that were not useable for analyses.
Authors' conclusions
In the CKD setting, there is currently insufficient evidence to suggest the superiority of any ESA formulation based on available safety and efficacy data. Directly comparative data for the effectiveness of different ESA formulations based on patient‐centred outcomes (such as quality of life, fatigue, and functional status) are sparse and poorly reported and current research studies are unable to inform care. All proprietary ESAs (epoetin alfa, epoetin beta, darbepoetin alfa, and methoxy polyethylene glycol‐epoetin beta) prevent blood transfusions but information for biosimilar ESAs is less conclusive. Comparative treatment effects of different ESA formulations on other patient‐important outcomes such as survival, MI, stroke, breathlessness and fatigue are very uncertain.
For consumers, clinicians and funders, considerations such as drug cost and availability and preferences for dosing frequency might be considered as the basis for individualising anaemia care due to lack of data for comparative differences in clinical benefits and harms.
Keywords: Adult; Humans; Anemia; Anemia/drug therapy; Biosimilar Pharmaceuticals; Biosimilar Pharmaceuticals/adverse effects; Darbepoetin alfa; Epoetin Alfa; Erythropoietin; Erythropoietin/adverse effects; Erythropoietin/analogs & derivatives; Erythropoietin/therapeutic use; Hematinics; Hematinics/adverse effects; Hematinics/therapeutic use; Hypertension; Hypertension/chemically induced; Polyethylene Glycols; Polyethylene Glycols/adverse effects; Polyethylene Glycols/therapeutic use; Recombinant Proteins; Recombinant Proteins/adverse effects; Recombinant Proteins/therapeutic use; Renal Insufficiency, Chronic; Renal Insufficiency, Chronic/complications
The relative safety and effectiveness of different epoetin drugs for treating anaemia in people with chronic kidney disease
Several drugs are available to treat anaemia for people who have kidney disease but whether these drugs are similar or different in their ability to improve symptoms of anaemia, such as tiredness and breathlessness, and whether they are equally safe based on their risks of causing a stroke or a heart attack, is not clear. This is because research studies that compare the effects of one drug directly with another are not common. We have found 56 studies that measure the safety and how these drugs help to improve how patients who have kidney disease feel, function and survive that have involved 15,596 people. Our last search of the literature was in February 2014.
We are somewhat confident that four of the drugs (epoetin alfa, epoetin beta, darbepoetin beta and methoxy polyethylene glycol‐epoetin beta) are better than a placebo injection to prevent patients needing to have a blood transfusion. We are less certain that biosimilar drugs are better than placebo to help patients avoid a blood transfusion.
All erythropoiesis‐stimulating agents cause high blood pressure, but we cannot be very sure if biosimilar products have effects on blood pressure. We cannot be confident in the other important effects of these drugs ‐ we are not sure whether the drugs are similar or different in their effects on the chances of death, a heart attack or stroke; the risk of having a clot in a fistula or vascular catheter needed for dialysis; or the chances of needing dialysis for people who have milder kidney disease. We are unsure whether the different drugs are better at improving symptoms such as tiredness or breathlessness than others as the available research studies generally do not measure these aspects of treatment very well.
Overall, whether different drugs are safer or better at treating symptoms of anaemia for people with kidney disease is poorly known. It is likely that most if not all the drugs prevent the need for a patient to require a blood transfusion. The choice of which drug to use to treat anaemia when a patient has kidney disease can be decided between patients and health professionals based on shared preferences for how frequently the drug is given and considering drug costs and availability.
Summary of findings
Summary of findings for the main comparison.
Erythropoiesis‐stimulating agents (ESAs) for anaemia in adults with chronic kidney disease (CKD)
| ESAs for anaemia in adults with CKD | |||||
| Intervention | Comparison/intervention | Nature of the evidence | Confidence in the evidence | Reasons for downgrading our confidence in the evidence* | Network treatment estimate OR (95% CI) |
| Preventing blood transfusion | |||||
| Epoetin alfa | Placebo | Mixed | Low | Study limitations (‐1) Inconsistency (‐1) |
0.18 (0.05 to 0.59) |
| Epoetin beta | Placebo | Mixed | Low | Study limitations (‐1) Inconsistency (‐1) |
0.09 (0.02 to 0.38) |
| Darbepoetin alfa | Placebo | Mixed | Moderate | Inconsistency (‐1) | 0.17 (0.05 to 0.57) |
| Methoxy polyethylene glycol‐epoetin beta |
Placebo | Indirect | Low | Study limitations (‐1) Inconsistency (‐1) |
0.15 (0.03 to 0.70) |
| Biosimilar ESA | Placebo | Indirect | Very low | Study limitations (‐1) Imprecision (‐1) Inconsistency (‐1) |
0.27 (0.05 to 1.47) |
| Epoetin alfa | Epoetin beta | Indirect | Very low | Study limitations (‐1) Imprecision (‐1) Imprecision (‐1) |
2.04 (0.38 to 11.0) |
| Epoetin alfa | Darbepoetin alfa | Mixed | Very low | Study limitations (‐2) Inconsistency (‐1) Imprecision (‐1) |
1.06 (0.35 to 3.29) |
| Epoetin alfa | Methoxy polyethylene glycol‐epoetin beta |
Indirect | Very low | Study limitations (‐2) Inconsistency (‐1) Imprecision (‐1) |
1.14 (0.27 to 4.97) |
| Epoetin alfa | Biosimilar ESA | Mixed | Very low | Study limitations (‐1) Imprecision (‐1) Imprecision (‐1) |
0.66 (0.19 to 2.28) |
| Epoetin beta | Darbepoetin alfa | Indirect | Very low | Study limitations (‐1) Inconsistency (‐1) Imprecision (‐1) |
0.52 (0.10 to 2.67) |
| Epoetin beta | Methoxy polyethylene glycol‐epoetin beta |
Mixed | Very low | Study limitations (‐1) Inconsistency (‐1) Imprecision (‐1) |
0.56 (0.11 to 3.00) |
| Epoetin beta | Biosimilar ESA | Indirect | Very low | Study limitations (‐1) Inconsistency (‐1) Imprecision (‐1) |
0.33 (0.04 to 2.60) |
| Darbepoetin alfa | Methoxy polyethylene glycol‐epoetin beta |
Mixed | Very low | Study limitations (‐2) Inconsistency (‐1) Imprecision (‐1) |
1.08 (0.38 to 3.04) |
| Darbepoetin alfa | Biosimilar ESA | Indirect | Very low | Study limitations (‐1) Inconsistency (‐1) Imprecision (‐1) |
0.62 (0.12 to 3.30) |
| Methoxy polyethylene glycol‐epoetin beta |
Biosimilar ESA | Indirect | Very low | Study limitations (‐1) Inconsistency (‐1) Imprecision (‐1) |
0.58 (0.09 to 3.92) |
| All‐cause mortality | |||||
| Epoetin alfa | Placebo | Mixed | Low | Study limitations (‐1) Imprecision (‐1) |
1.25 (0.71 to 2.21) |
| Epoetin beta | Placebo | Mixed | Low | Study limitations (‐1) Imprecision (‐1) |
0.82 (0.45 to 1.48) |
| Darbepoetin alfa | Placebo | Mixed | Moderate | Imprecision (‐1) | 1.06 (0.91 to 1.24) |
| Methoxy polyethylene glycol‐epoetin beta |
Placebo | Indirect | Low | Study limitations (‐1) Imprecision (‐1) |
1.16 (0.74 to 1.82) |
| Biosimilar ESA | Placebo | Indirect | Low | Study limitations (‐1) Imprecision (‐1) |
1.31 (0.65 to 2.62) |
| Epoetin alfa | Epoetin beta | Indirect | Low | Study limitations (‐1) Imprecision (‐1) |
1.53 (077 to 3.03) |
| Epoetin alfa | Darbepoetin alfa | Mixed | Low | Study limitations (‐1) Imprecision (‐1) |
1.17 (0.68 to 2.05) |
| Epoetin alfa | Methoxy polyethylene glycol‐epoetin beta |
Indirect | Very low | Study limitations (‐1) Inconsistency (‐1) Imprecision (‐1) |
1.08 (0.54 to 2.15) |
| Epoetin alfa | Biosimilar ESA | Mixed | Very low | Study limitations (‐2) Inconsistency (‐1) Imprecision (‐1) |
0.95 (0.62 to 1.44) |
| Epoetin beta | Darbepoetin alfa | Mixed | Low | Study limitations (‐1) Imprecision (‐1) |
0.77 (0.43 to 1.38) |
| Epoetin beta | Methoxy polyethylene glycol‐epoetin beta |
Mixed | Low | Study limitations (‐1) Imprecision (‐1) |
0.71 (0.35 to 1.42) |
| Epoetin beta | Biosimilar ESA | Mixed | Low | Study limitations (‐1) Imprecision (‐1) |
0.62 (0.29 to 1.37) |
| Darbepoetin alfa | Methoxy polyethylene glycol‐epoetin beta |
Mixed | Very low | Study limitations (‐2) Imprecision (‐1) |
0.91 (0.60 to 1.40) |
| Darbepoetin alfa | Biosimilar ESA | Indirect | Low | Study limitations (‐1) Imprecision (‐1) |
0.81 (0.41 to 1.61) |
| Methoxy polyethylene glycol‐epoetin beta |
Biosimilar ESA | Indirect | Very low | Study limitations (‐2) Inconsistency (‐1) Imprecision (‐1) |
0.88 (0.40 to 1.97) |
|
CI: Confidence interval; OR: Odds Ratio *There was moderate heterogeneity in the network for preventing blood transfusion (τ = 0.89 which was between the 50th and 75th quartile of empirical distributions of heterogeneity variances specific to the type of outcome and types of treatments being compared) (Turner 2012) We did not downgrade for reasons of indirectness or publication bias as insufficient studies contributed to network treatment estimates to draw meaningful conclusions. We downgraded for inconsistency when the network did not include a closed loop of evidence for the comparison and accordingly the presence of inconsistency could not be excluded. | |||||
| GRADE Working Group grades of evidence (GRADE: Rating the quality of evidence 2011) High quality: We are very confidence that the true effect lies close to that of the estimate of effect Moderate quality: We are moderately confident in the estimate of effect: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of effect Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | |||||
Background
Description of the condition
Anaemia, literally meaning lack of blood, is defined as "a condition in which the number of red blood cells or their oxygen‐carrying capacity is insufficient to meet physiological needs" (http://www.who.int/topics/anaemia/en/). Circulating red blood cells transport oxygen to tissues bound to iron ions within the metalloprotein, haemoglobin. In anaemia, insufficient numbers of circulating red blood cells or inadequate quantities of iron or functional haemoglobin are available to transport and release oxygen to tissues, which is essential for aerobic (oxygen‐dependent) metabolism. Anaemia, defined by the World Health Organization as a haemoglobin level below 130 g/L in men and below 120 g/L in women, affects approximately a quarter of the world's population, particularly children and pregnant women (WHO 2008). Anaemia is common in the expanding global populations of chronic disease including people affected by solid malignancies (50%), blood cancers (60% to 70%) (Ludwig 2004), human immunodeficiency virus (HIV) causing acquired immunodeficiency syndrome (AIDS; 40%) (Shah 2007), chronic heart failure (20%) (Ezekowitz 2003) and nearly all individuals who have advanced chronic kidney disease (CKD). Symptoms caused by insufficient oxygen delivery to tissues in anaemia include weakness and fatigue, breathlessness, light‐headedness, and palpitations. Observational cohort data show that anaemia in people who have chronic disease is also consistently associated with negative effects on quality of life (Lefebvre 2006), role function (Ludwig 2004; Semba 2005), and survival (Caro 2001; Groenveld 2008; Locatelli 2004; Melekhin 2012).
Description of the intervention
Recombinant erythropoietin and its synthetic derivatives (epoetin alfa, epoetin beta, darbepoetin alfa, methoxy polyethylene glycol‐epoetin beta; collectively known as erythropoiesis‐stimulating agents (ESAs)), are widely used to treat anaemia. Erythropoietin is a glycoprotein made by peritubular cells in the kidney (with an additional smaller contribution from liver cells (15% total)) and is released in response to low tissue oxygen levels (hypoxia) through the actions of hypoxia‐inducible factor to stimulate the formation and viability of red blood cells in the bone marrow (erythropoiesis). The average red blood cell survives in the circulation for 120 days although red cell survival is reduced by chronic disease. Causes of anaemia are numerous and include: reduced production of erythropoietin in response to hypoxia (CKD; chronic inflammatory conditions); abnormal bone marrow responses to the actions of erythropoietin (chronic inflammatory conditions, bone marrow failure due to infiltration or drug‐related therapy); insufficient iron stores; abnormal production or function of haemoglobin (thalassaemia or haemoglobinopathies); excessive red blood cell losses (destruction within the circulation or haemorrhage); or reduced red blood cell survival (Figure 1).
Figure 1.
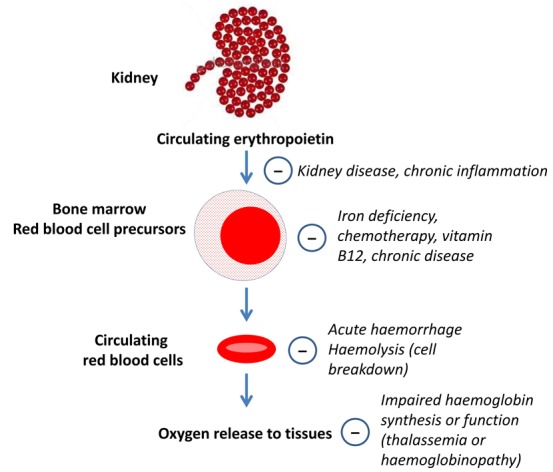
Overview of anaemia in chronic disease
Before the development of recombinant human erythropoietin (rHuEPO) in the late 1980s (Eschbach 1987), blood transfusions and iron supplementation (both oral and intravenous (IV)) were the mainstays of treatment for anaemia in populations with severe CKD, in which haemoglobin levels were commonly in the range of 70 to 80 g/L. Androgen treatment for anaemia was also used in CKD but provided small and unsustained responses in haemoglobin levels and was poorly tolerated (Neff 1981). In the pre‐recombinant erythropoietin era, blood transfusions effectively increased haemoglobin levels to provide acute symptom relief but were associated with hospitalisation, iron overload, antibody formation against blood cell antigens, sensitisation to transplant antigens, and transfusion‐related infections, particularly viral hepatitis. Technological advances and successful cloning of the erythropoietin gene enabled large‐scale production of rHuEPO which effectively and rapidly increases haemoglobin levels when administered IV or subcutaneously (SC). The United States Food and Drug Administration (FDA) approved rHuEPO for the treatment of anaemia in people with CKD on dialysis in 1989 and broadened approval to include people with CKD without dialysis, and in patients with HIV and anaemia on zidovudine (AZT) in 1990.
Clinical guidelines published soon after initial drug approval suggested that patients with CKD and haemoglobin concentrations below 80 g/L who were symptomatic should receive ESA treatment in conjunction with sufficient iron supplementation once other causes of anaemia were excluded (Macdougall 1990). However, rapid widespread uptake of ESAs occurred in numerous clinical settings, and by 2007, clinical practice guidelines recommended the use of ESAs to achieve target haemoglobin levels of 110 to 120 g/L in people with CKD (KDOQI 2007). ESA prescription also subsequently expanded to treat anaemia in cancer and heart failure populations, as well as for people undergoing surgery likely to require blood transfusion who could not undergo pre‐operative blood collection. Presently, epoetin alfa is approved by the FDA for treatment of anaemia due to CKD, zidovudine in HIV‐infected patients, effects of concomitant myelosuppressive chemotherapy and to reduce red blood cell transfusions in patients undergoing elective, noncardiac, nonvascular surgery. Darbepoetin alfa is currently approved by the FDA for the treatment of anaemia resulting from CKD or myelosuppressive chemotherapy (FDA website).
How the intervention might work
Despite an association between low haemoglobin levels and higher mortality in uncontrolled studies, prompting speculation that correcting anaemia with ESA therapy might lower cardiovascular events and mortality, the opposite was observed in subsequent meta‐analyses of RCTs (Bohlius 2009; Palmer 2010; Phrommintikul 2007; Strippoli 2006). Correction of anaemia and maintenance of haemoglobin levels to near normal levels with ESAs reduced the need for red blood cell transfusions, but increased mortality, cardiovascular events and cancer progression, without consistently improving quality of life. The precise mechanisms for treatment‐related harm are not understood, but observational studies suggest that impaired haemoglobin responses to erythropoietin treatment, together with higher erythropoietin doses are associated with increased treatment‐related toxicity (Kilpatrick 2008; Szczech 2008).
Treatment guidelines for ESAs to treat anaemia have become more conservative over the last decade and FDA labelling now suggests that ESA treatment should be considered in people with CKD when the haemoglobin level is less than 100 g/L, and treatment objectives are to increase haemoglobin levels sufficient to reduce the need for red cell transfusions (FDA website). Clinical practice guidelines have also responded to increasing evidence of harm when higher haemoglobin levels are targeted by ESA treatment (Bohlius 2009; Palmer 2010; Phrommintikul 2007). Recent clinical practice guidelines for the use of ESAs to treat anaemia in CKD suggest the potential benefits of reducing blood transfusions and anaemia‐related symptoms should be balanced against the risks of harm (e.g. stroke, vascular access thrombosis and hypertension) for individual patients. Currently, guidelines do not suggest specific haemoglobin targets for patients not treated with dialysis, while for dialysis patients, the recommended approach is to use ESA therapy to avoid a haemoglobin level below 9.0 g/dL (KDIGO 2010).
Why it is important to do this review
Darbepoetin alfa and methoxy polyethylene glycol‐epoetin beta (a continuous erythropoietin‐receptor activator (CERA)) are newer synthetic forms of naturally‐occurring erythropoietin that have a longer duration of action (Macdougall 2008). These agents have similar effects on haemoglobin concentrations as epoetin alfa and beta and require less frequent administration (Levin 2007; Macdougall 2001). Darbepoetin alfa treatment in people with earlier stages of CKD and diabetes mellitus has been shown to nearly halve the risk of blood transfusion but has no beneficial effects on survival and increases the risk of stroke and death related to cancer recurrence (TREAT Study 2005).
The apparent narrow therapeutic balance between potential treatment benefits (avoidance of red blood cell transfusions and improving symptoms of anaemia) and hazards (cardiovascular events and mortality) together with the availability of several agents in this drug class (epoetin alfa, epoetin beta, darbepoetin alfa, methoxy polyethylene glycol‐epoetin beta and biosimilar epoetins) to treat anaemia builds the case for a comprehensive and systematic head‐to‐head comparison of the available ESAs to treat anaemia. However, large‐scale studies directly comparing different epoetins have been relatively uncommon, and the comparative efficacy and safety of each agent relative to each other is poorly understood.
In addition, the expiration of several epoetin patents has prompted companies to produce similar biological medicinal products that are second versions of biological medicines that depend on the same mechanism of action and are intended to be used for the same therapeutic indication as the earlier product, known as "biosimilars" or "follow‐on biologicals". Global clinical guidelines assume that available epoetins are all equally safe and effective, including true biosimilar products (KDIGO 2010), although the drug formulations differ widely in molecular structure, cost, availability and duration of action.
While patient and policy decisions about anaemia management of CKD are highly dependent on the comparative effectiveness of ESAs, existing studies have focused mainly on the evaluation of targeting differing haemoglobin levels with treatment. Head‐to‐head studies of ESAs in CKD are lacking. To overcome the known limitations of single randomised studies, we have conducted a systematic review of the literature and a network meta‐analysis to estimate the comparative efficacy and safety of ESAs for treating anaemia in people with CKD.
Objectives
To compare the efficacy and safety of ESAs (epoetin alfa, epoetin beta, darbepoetin alfa, or methoxy polyethylene glycol‐epoetin beta, and biosimilar ESAs, against each other, placebo, or no treatment) to treat anaemia in adults with CKD.
Methods
Criteria for considering studies for this review
Types of studies
We included all RCTs comparing ESA versus ESA, placebo or no treatment to treat anaemia in people with CKD. We did not restrict inclusion based on language of publication. We did not include quasi‐RCTs (studies in which treatment allocation was by date of birth, alternation, or similar predictable method). We included studies in which allocation to treatment was not adequately concealed but considered study methodological quality in our analyses and discussion.
Types of participants
Inclusion criteria
Studies in adults aged 18 years or older with anaemia due to CKD were included. CKD was characterised by clinically relevant proteinuria, haematuria, and/or structural kidney disease with or without estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m², recipients of a kidney transplant, and people with Stage 5 CKD treated with dialysis (KDIGO 2013).
Exclusion criteria
As network meta‐analysis requires reasonable homogeneity in study design and populations, we excluded data in children and from studies in which follow‐up was less than three months.
Types of interventions
We included studies of ESAs (epoetin alfa, epoetin beta, darbepoetin beta, methoxy polyethylene glycol‐epoetin beta, biosimilar) to treat or prevent anaemia in CKD administered via any route (IV or SC), compared with each other, placebo or no treatment. Dose adaptation of ESAs and non‐randomised iron supplementation depending on haematological response were allowed. We included studies in which iron was administered as a randomised intervention in all arms of the study.
We coded the comparisons within a study where iron was a randomised co‐intervention in all study arms as follows.
ESA1 plus iron (any route) versus ESA2 plus iron (any route) = ESA1 versus ESA2
ESA plus oral iron versus oral iron = ESA versus no treatment
ESA plus oral iron versus oral iron plus placebo injection = ESA versus placebo
ESA plus intravenous iron versus intravenous iron plus placebo injection = ESA versus placebo
ESA plus intravenous iron versus intravenous iron = ESA versus no treatment.
We excluded studies in which iron therapy was a randomised co‐intervention combined with an ESA in a single arm of the study (e.g. ESA plus iron versus ESA alone, ESA plus iron versus placebo). Studies of hypoxia‐inducible factor stabilisers and peginesatide were excluded.
Types of outcome measures
We evaluated the following outcomes occurring at any time during study follow‐up.
Primary outcomes
We estimated the comparative effects of the competing interventions according to the following outcomes:
Response to treatment
Preventing blood transfusion
Safety
All‐cause mortality.
Secondary outcomes
Response to treatment
Fatigue (as defined by study authors)
Dyspnoea (as defined by study authors)
Safety
Cardiovascular mortality
Fatal or nonfatal MI
Fatal or nonfatal stroke
Vascular access thrombosis
Major adverse cardiovascular event (as adjudicated by investigators)
End‐stage kidney disease (ESKD).
Search methods for identification of studies
Electronic searches
We searched the Cochrane Renal Group's Specialised Register to 11 February 2014 through contact with the Trials' Search Co‐ordinator using search terms relevant to this review. The Cochrane Renal Group’s Specialised Register contains studies identified from several sources:
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
Weekly searches of MEDLINE OVID SP
Handsearching of renal‐related journals and the proceedings of major renal conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected renal journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Specialised Register are identified through search strategies for CENTRAL, MEDLINE, and EMBASE based on the scope of the Cochrane Renal Group. Details of these strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available in the Specialised Register section of information about the Cochrane Renal Group.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
Database of Abstracts of Reviews of Effects (DARE) (The Cochrane Library)
Health Technology Assessment (HTA) database (The Cochrane Library)
NHS Economic Evaluation Database (The Cochrane Library)
Reference lists of review articles, relevant studies and clinical practice guidelines
Letters seeking information about unpublished or incomplete studies to investigators known to be involved in previous studies.
Data collection and analysis
Selection of studies
The search strategy described was used to obtain titles and abstracts of studies that were relevant to the review. The titles and abstracts were screened independently by two authors, who discarded studies that were not applicable; however studies and reviews that might have included relevant data or information on studies were retained initially. Two authors independently assessed retrieved abstracts, and if necessary the full text, of these studies to determine which studies satisfied the inclusion criteria. Systematic reviews were screened to identify any studies not retrieved by the electronic database search.
Data extraction and management
Data extraction was carried out independently by two authors using standard data extraction forms. Data were cross checked between authors and discussed. Studies reported in non‐English language journals were translated electronically before assessment. Where more than one publication of one study existed, reports were grouped together and the publication with the most complete data was used in the analyses. Where relevant outcomes were only published in earlier versions, these data were used. Any disagreements in data extraction were discussed with a third author.
Any further information required from the original authors or sponsors of studies included in the review was requested by written correspondence (e.g. emailing or writing to corresponding author/s) and any relevant information obtained in this manner was included in the review. Data requested included numbers of events and numbers of participants at risk for important dichotomous clinical outcomes (blood transfusions, all‐cause mortality, cardiovascular mortality, fatal or nonfatal stroke, fatal or nonfatal MI, vascular access thrombosis, ESKD, major adverse cardiovascular events, fatigue, breathlessness). We also requested additional information on the use of iron supplementation in treatment arms where this was not clear from reading the study report.
Assessment of risk of bias in included studies
The following items were independently assessed by two authors using the risk of bias assessment tool (Higgins 2011) (see Appendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study (detection bias)?
Participants and personnel
Outcome assessors
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias (imbalance in interventions, publication only as abstract or letter, premature termination of study and industry sponsor involvement in authorship or data management and analysis)?
Measures of treatment effect
Relative treatment effects
We calculated comparative effect sizes for pairwise and network meta‐analysis as odds ratios (ORs) with their 95% confidence intervals (CIs).
Relative treatment rankings
To rank the treatments available according to safety or efficacy, we planned to use the surface under the cumulative ranking (SUCRA) probabilities which express as percentages each intervention to an imaginary intervention that is always the best without uncertainty (Salanti 2011). For example, a SUCRA of 80% means that the drug achieved 80% of the effectiveness of this imaginary drug, and accordingly, larger SUCRAs denote greater efficacy. However, the large uncertainty in the resulting estimates rendered ranking of the competing treatments imprecise.
Assessment of clinical and methodological heterogeneity within treatment comparisons
To evaluate the presence of clinical heterogeneity, we generated descriptive statistics for the population characteristics across all eligible studies that compared each pair of interventions. We assessed the presence of clinical heterogeneity within pairwise comparisons by comparing these characteristics.
Assessment of transitivity across treatment comparisons
The assumption of transitivity ‐ that one can learn about treatment A versus treatment B via treatment C (e.g. learning about epoetin alfa versus darbepoetin alfa via placebo) ‐ underlies network meta‐analysis (Salanti 2012). Evaluation of the assumption is important and its plausibility determines the validity of the network meta‐analysis results. We inferred about the assumption of transitivity:
We assessed whether the included interventions were similar when they were evaluated in studies with different designs, for example, whether ESAs are administered the same way in studies comparing ESAs to placebo and in those comparing ESAs to other ESAs
We compared the distribution of the potential effect modifiers (age, stage of CKD, duration of treatment) across the different pairwise comparisons.
Data synthesis
Methods for direct treatment comparisons
First, we conducted pairwise meta‐analyses by synthesising studies that compared the same interventions using a random‐effects model (DerSimonian 1986) that contained two or more studies. We compared treatments that used the same haemoglobin target (e.g. epoetin high target versus darbepoetin high target). For dichotomous outcomes (avoiding red blood cell transfusions; all‐cause and cardiovascular mortality; major cardiovascular event; fatal or nonfatal myocardial infarction; fatal or nonfatal stroke; vascular access thrombosis; ESKD; fatigue; breathlessness) results were expressed as an OR with 95% CI.
Methods for indirect and mixed comparisons
To determine comparative efficacy and safety, we then conducted network meta‐analysis. Network meta‐analysis is a method of synthesising information from a network of studies addressing the same questions but involving different interventions. Joint analysis of data within a network framework allows novel inferences on treatment comparisons that have not been previously addressed directly in any studies, and it may increase precision for comparisons with few data (Caldwell 2010; Lu 2004; Salanti 2008). For a given comparison, say A versus B, direct evidence is provided by studies that compare these two treatments directly (epoetin alfa versus darbepoetin alfa) as in standard direct comparisons meta‐analysis. In addition, indirect evidence for A versus B can be provided if studies that compare A versus C and B versus C are analysed jointly (e.g. epoetin alfa versus placebo studies and darbepoetin alfa versus placebo studies can allow indirect comparison of epoetin alfa versus darbepoetin alfa via the use of placebo). Network meta‐analysis aims to combine the direct and indirect evidence into a single effect size and thus may help to increase the precision of the comparison, while randomisation is respected. The combination of direct and indirect evidence for any given treatment comparison can be extended when ranking more than three types of treatments according to their effectiveness or safety; every study contributes evidence in the network about a subset of the competing treatments. We performed network meta‐analysis in STATA (www.stata.com) using the 'mvmeta' command (White 2012) and self‐programmed STATA routines described in Chaimani 2013 and available at http://www.mtm.uoi.gr/index.php/stata‐routines‐for‐network‐meta‐analysis.
Assessment of statistical heterogeneity
Assumptions when estimating heterogeneity
In standard pairwise meta‐analyses we estimated different heterogeneity variances for each pairwise comparison. In network meta‐analysis we assumed a common estimate for the heterogeneity variance across the different comparisons.
Measures and tests for heterogeneity
We evaluated for the presence of heterogeneity within meta‐analyses using the Cochran Q test and I² statistic (Higgins 2003) that measures the percentage of variability that cannot be attributed to random error. We considered the I² thresholds to represent heterogeneity that might not be important (0% to 40%), might be moderate heterogeneity (30% to 60%), might be substantial heterogeneity (50% to 90%), and was considerable heterogeneity (75% to 100%) considering also the magnitude and direction of treatment effects and strength of evidence for heterogeneity (P value from the Chi² test) (Higgins 2011). The assessment of statistical heterogeneity in the entire network was based on the magnitude of the heterogeneity variance parameter (τ²) estimated from the network meta‐analysis models. We compared the magnitude of a common heterogeneity variance for the specific network of interest with an empirical distribution of heterogeneity variances specific to the type of outcome and the types of treatments being compared (Turner 2012).
Assessment of statistical inconsistency
Local approaches for evaluating inconsistency
To evaluate the presence of inconsistency locally, we used the loop‐specific approach. A loop of evidence is formed by at least three treatment pairs which have been compared in studies forming a closed path. Indirect evidence can be contrasted to direct evidence and their difference defines their disagreement (inconsistency factor). To infer whether the inconsistency factor is incompatible with zero, we looked at the magnitude of the inconsistency factors and their 95% confidence intervals (Bucher 1997). We extended analysis to all closed triangular and quadratic loops assuming a single loop‐specific heterogeneity and examine the estimates of inconsistency together with 95% confidence intervals for each loop using a graphical representation (Salanti 2009), This approach can be easily applied and indicates loops with large inconsistency, but cannot infer consistency of the entire network or identify the particular comparison that is problematic. It should be noted that in a network of evidence there may be many loops and estimates of inconsistency factors and with multiple testing there is an increased likelihood that we might find an inconsistent loop by chance. Therefore, we were cautious deriving conclusions from this approach.
Global approaches for evaluating inconsistency
To check the assumption of consistency in the entire network, we used the design‐by‐treatment interaction model as fully explained in Higgins 2012 (pp. 102 to 103). This method accounts for different sources of inconsistency that can occur when studies with different designs (two‐arm studies versus three‐arm studies) give different results as well as disagreement between direct and indirect evidence. Using this approach, we inferred about the presence of inconsistency from any source in the entire network based on a Chi² test. The design‐by‐treatment model was performed in STATA using the 'mvmeta' command. Inconsistency and heterogeneity are interwoven: to distinguish between these two sources of variability we employed the I² for inconsistency that measures the percentage of variability that cannot be attributed to random error or heterogeneity (within comparison variability).
It should be noted in general that the power of statistical tests for inconsistency are low, which implies that the absence of statistically significant inconsistency is not evidence of consistency.
Investigation of heterogeneity and inconsistency
We planned to perform meta‐regression or subgroup analyses to explore important heterogeneity and/or inconsistency. When we identified potential evidence of inconsistency and heterogeneity, we first checked for any mistakes and inconsistencies in data extraction and entry. We then evaluated for evidence based on the following effect modifiers as possible sources of inconsistency and/or heterogeneity. However, insufficient data precluded these analyses.
Population: iron status at baseline (iron replete versus iron deficient); stage of CKD (CKD stages 1 to 3, CKD stage 4 to 5, CKD stage 5D, transplantation); baseline haemoglobin (< 10 g/dL, 10 to 12 g/dL, > 12 g/dL); mean age; gender; proportion with diabetes or cardiovascular disease
Intervention: dose, frequency or route; iron supplementation (fixed iron treatment, iron treatment as necessary, or not clear)
Risk of bias: allocation concealment; blinding of outcome assessment; attrition; premature termination of study; publication (full text publication, abstract publication, unpublished data); funding source
Study design: duration of ESA treatment (12 to 16 weeks; 16 to 24 weeks; 24 to 48 weeks; > 48 weeks); duration of follow‐up (≥ 12 months, versus < 12 months); number of participants; date of publication.
Sensitivity analysis
Insufficient data and wide confidence intervals for most treatment estimates precluded additional such analyses.
Summary of findings table
The main results of the review for the primary outcomes (preventing blood transfusion and all‐cause mortality) are presented in a summary of findings table (Table 1). The summary of findings table was provided for the network estimates only and included an overall grading of the evidence for these outcomes.
We used an adapted Grading of Recommendations Assessment, Development approach to grading evidence quality in pairwise meta‐analysis that was developed specifically for network meta‐analysis (Salanti 2014). We considered five components to evidence quality: study limitations, indirectness, inconsistency, imprecision, and publication bias. The interpretations of each of the grades are provided in GRADE: Rating the quality of evidence 2011 and described in the footnote of the Table 1.
For publication bias, due to small numbers of contributing studies (< 10), we considered that funnel plots would have insufficient power and specificity to evaluate for evidence of publication bias and therefore we did not downgrade our confidence in the evidence for reasons of publication bias in this review because of the comprehensive search strategy we followed.
In networks in which there were no closed loops (where three or four treatments were not connected by direct comparisons in individual studies, we couldn't evaluate for consistency between direct information (two drugs compared in a study) and indirect information (two drugs compared via a third treatment strategy using network meta‐analysis). In this situation, we downgraded evidence quality because we could not show absence of inconsistency between these two sources of information.
The adjudication of each component of evidence quality then resulted in maintaining or downgrading evidence quality from a high‐quality rating to moderate, low or very low.
Results
Description of studies
Results of the search
Figure 2 shows the results of the electronic search.
Figure 2.
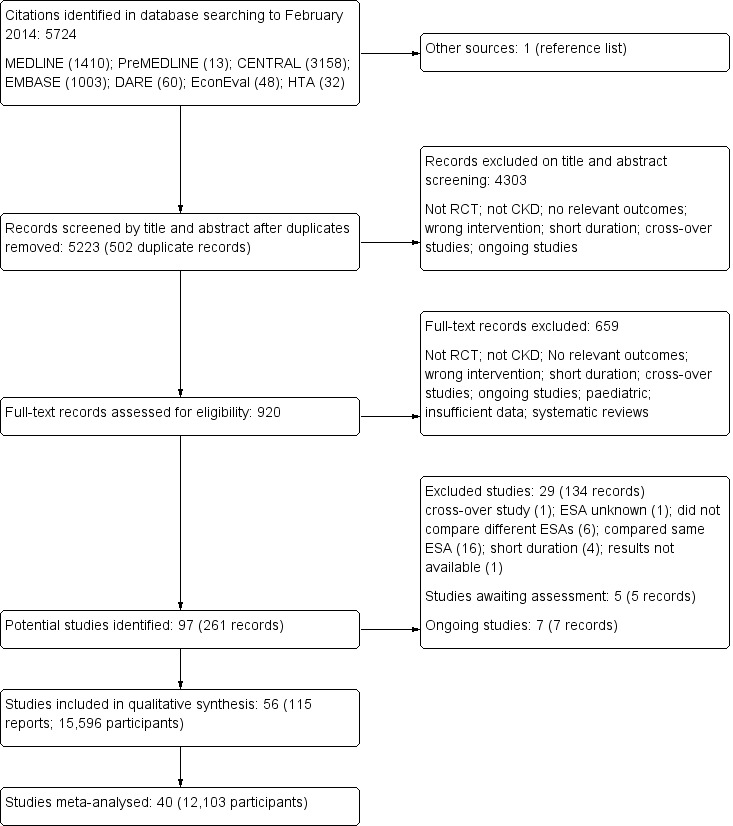
Study flow diagram.
Included studies
The search strategy identified 5223 unique citations. After exclusions during title and abstract screening (4303 citations excluded) and full text analysis (659 citations excluded), 56 studies involving 15,596 participants published between 1989 and 2013 were included in the systematic review and 40 studies involving 12,103 participants could be included within pairwise and network meta‐analyses (Characteristics of included studies). We received unpublished data from investigators of seven studies (Akizawa 2011; CORDATUS Study 2011; EPOCARES Study 2010; Hirakata 2010; Nissenson 2002; Patel 2012; TIVOLI Study 2013).
Median follow‐up was six months (range 3 to 29), with 77% of studies reporting outcomes before 12 months. The average age of participants was highly variable (range 43 to 84 years). Of the 40 studies contributing outcome data, 22 studies included 5583 dialysis patients, two studies provided data for 111 kidney transplant recipients, and 16 studies included in 6409 participants with an estimated GFR between 15 to 90 mL/min/1.73 m². Among studies included in meta‐analyses, seven were placebo controlled (4638 participants) and eight compared ESAs with standard care (787 participants). The remainder were head‐to‐head studies of epoetin alfa versus darbepoetin alfa (8 studies, 1783 participants), epoetin beta versus darbepoetin alfa (1 study, 219 participants), epoetin beta versus methoxy polyethylene glycol‐epoetin beta (2 studies, 462 participants), darbepoetin alfa versus methoxy polyethylene glycol‐epoetin beta (5 studies, 1505 participants), epoetin alfa versus biosimilar ESA (8 studies, 2419 participants) and epoetin beta versus biosimilar ESA (1 study, 290 participants).
Other studies
We identified seven ongoing studies (Besarab 2006; NCT00442702; NCT00559273; NCT00717821; NCT00773513; PRIMAVERA Study 2011; STIMULATE Study 2011) and there are five studies which appear to have been completed but as yet there are no results available (Barany 1998; Carrera 2003; Nissenson 2007; Ostrvica 2010; Palazzuoli 2011). These studies will be included in a future update of this review.
Excluded studies
After full‐text review we excluded 29 studies (134 records). Sixteen studies compared the same ESA derivative is the different treatment arms (ACORD Study 2004; Besarab 1998; CHOIR Study 2006; Cianciaruso 2008; CREATE Study 2001; ECAP Study 2006; Eschbach 1989; Foley 2000; Gouva 2004; Johnson 1999; Levin 2005; Linde 2001; Locatelli 2008; Parfrey 2005; Salek 2001; SLIMHEART Study 2004); six studies didn't compare different ESAs (BA16260 Study 2006; BA16285 Study 2007; BA16286 Study 2005; Brier 2010; CAPRIT Study 2012; Macdougall 2007); and one study in which the type of ESA was unknown (Acchiardo 1991a). We also excluded a cross‐over study (Wizemann 2008); four studies of insufficient duration (Kawanishi 2005; Neo‐PDGF Study 2010; Perez‐Oliva 2005; Sja'bani 1997), and one study in which there were insufficient data to determine eligibility (N0055116759).
Studies excluded from the meta‐analyses
The primary reasons for exclusion from meta‐analyses (16 studies involving 3493 participants) were that disaggregated data for different ESA types were not available separately (for example, both epoetin alfa and beta were administered within a single study arm) or that outcome data were not reported in extractable format (Akiba 2010; Arabul 2009; Chen 2008; Coyne 2000; Vanrenterghem 2002; MAXIMA Study 2007; Smith 2007; PROTOS Study 2007; RUBRA Study 2008; Shaheen 1993; Shand 1993; Sikole 1993; Alexander 2007; Teehan 1989; Watson 1990; Van Loo 1996).
Risk of bias in included studies
The risks of bias are summarised in Figure 3 and Figure 4.
Figure 3.
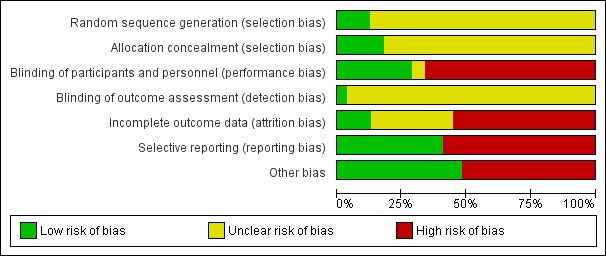
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies. The coloured bars correspond to each risk of bias adjudication summarised across all studies. The numbers shown in the bars indicate the raw number of studies which were adjudicated the corresponding risks of bias (green = low risk; yellow = unclear risk; red = high risk). The size of each coloured box within the bars indicates the proportion of studies with the adjudicated risk. For example, there were 7 studies (13%) which were adjudicated as low risk of bias from reported sequence generation methods. The description of the risk domains considered is given in the vertical axis. *Other threats to validity include one or more of: sponsor involved in study design, analysis, or authorship; imbalance between treatment comparisons and/or premature termination of trial
Figure 4.
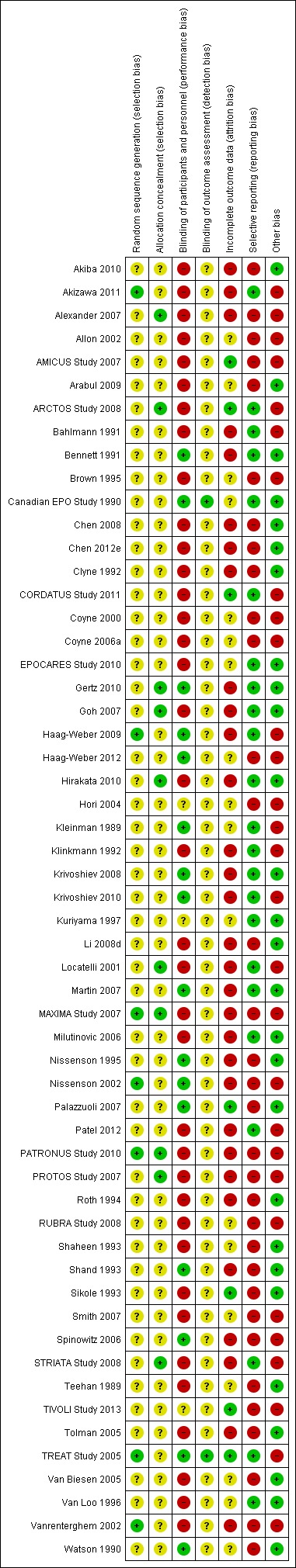
Risk of bias summary: review authors' judgements about each risk of bias item for each included study. Each study is shown in the vertical axis and the corresponding risk of bias for each domain adjudicated by two authors is shown by coloured circles within the grid. Green (+) = low risk, yellow (?) = unclear risk, red (‐) = high risk. Other threats to validity include one or more of: sponsor involved in study design, analysis, or authorship; imbalance between treatment comparisons and/or premature termination of trial
Allocation
Sequence generation
Of 56 included studies, seven (12.5%) reported low risk methods for sequence generation (Akizawa 2011; Vanrenterghem 2002; Haag‐Weber 2009; MAXIMA Study 2007; Nissenson 2002; PATRONUS Study 2010; TREAT Study 2005).
Allocation concealment
Of 56 included studies, 10 (18%) reported adequate methods for allocation concealment (low risk of bias) (ARCTOS Study 2008; Gertz 2010; Goh 2007; Hirakata 2010; Locatelli 2001; MAXIMA Study 2007; PATRONUS Study 2010; PROTOS Study 2007; STRIATA Study 2008; Alexander 2007). The remaining 46 studies (82%) did not provide sufficient information to enable adjudication risk of bias in allocation concealment (unclear risk).
Blinding
There were 16 studies (29%) which reported that participants and investigators were blinded (Bennett 1991; Canadian EPO Study 1990; Gertz 2010; Haag‐Weber 2009; Haag‐Weber 2012; Kleinman 1989; Krivoshiev 2008; Krivoshiev 2010; Martin 2007; Nissenson 1995; Nissenson 2002; Palazzuoli 2007; Shand 1993; Spinowitz 2006; TREAT Study 2005; Watson 1990). There were 35 studies (63%) that were open‐label (high risk of bias) and the remaining three studies (5%) did not provide sufficient information to enable assessment (unclear) (Hori 2004; Kuriyama 1997; TIVOLI Study 2013). Two studies (4%) reported adequate methods of blinding outcome assessment (Canadian EPO Study 1990; TREAT Study 2005) and the remainder did not provide sufficient information to assess risk (unclear risk of bias).
Incomplete outcome data
Seven studies (13%) were judged to meet criteria for low risk of bias (fewer than 10% missing from follow‐up analyses and balanced numbers across intervention groups with similar reasons for loss to follow‐up) for low risk of incomplete outcome data bias (AMICUS Study 2007; ARCTOS Study 2008; CORDATUS Study 2011; Palazzuoli 2007; Sikole 1993; TIVOLI Study 2013; TREAT Study 2005), 31 studies (55%) were at high risk of bias, and the remaining 18 studies (32%) did not provide sufficient information to assess risk of bias (unclear risk).
Selective reporting
Outcomes of interest (mortality, cardiovascular event (fatal or nonfatal) and hypertension) were reported in 22 studies (39%) (Akizawa 2011; ARCTOS Study 2008; Bahlmann 1991; Bennett 1991; Canadian EPO Study 1990; CORDATUS Study 2011; EPOCARES Study 2010; Gertz 2010; Goh 2007; Haag‐Weber 2009; Hirakata 2010; Kleinman 1989; Klinkmann 1992; Krivoshiev 2008; Krivoshiev 2010; Locatelli 2001; Martin 2007; Milutinovic 2006; Patel 2012; STRIATA Study 2008; TREAT Study 2005; Van Loo 1996).
Other potential sources of bias
Industrial sponsor on authorship or involved in data management or analysis
There were 29 studies (51%) that reported the sponsor was involved in authorship of the study report or in data management or analysis (Allon 2002; AMICUS Study 2007; ARCTOS Study 2008; Bahlmann 1991; CORDATUS Study 2011; Coyne 2000; Coyne 2006a; Vanrenterghem 2002; Gertz 2010; Haag‐Weber 2009; Haag‐Weber 2012; Kleinman 1989; Klinkmann 1992; Krivoshiev 2010; Locatelli 2001; Martin 2007; MAXIMA Study 2007; Nissenson 2002; Patel 2012; PATRONUS Study 2010; Smith 2007; PROTOS Study 2007; RUBRA Study 2008; Spinowitz 2006; STRIATA Study 2008; Alexander 2007; TIVOLI Study 2013; TREAT Study 2005; Watson 1990).
Abstract or letter only
Seven studies (18%) were published either as an abstract or letter (Brown 1995; Coyne 2000; Coyne 2006a; Hori 2004; Smith 2007; Alexander 2007; TIVOLI Study 2013).
Imbalance in interventions
In one study, two differing ESAs were prescribed according to differing haemoglobin targets, resulting in an imbalance in treatment doses (Akizawa 2011).
Premature termination of study
Two studies were terminated early (Haag‐Weber 2012; Alexander 2007) due to development of erythropoietin antibodies (Haag‐Weber 2012) and for uncertain reasons (Alexander 2007).
Effects of interventions
See: Table 1
The Table 1 provides overall estimates of treatment effects and the quality of the available evidence for the primary efficacy (preventing blood transfusion) and safety (all‐cause mortality) outcomes and Table 3 shows the treatment estimates from pairwise and network meta‐analyses. Treatment estimates from pairwise comparisons are shown in italics in the lower left portion of each table section and treatment estimates from network analyses are shown in the upper right portion of each table section.
Table 1.
Comparative effects of erythropoiesis‐stimulating agents on clinical outcomes in chronic kidney disease
| Outcomes / interventions | Comparators (treatment estimate (OR (95% CI)) | |||||
| Epoetin alfa | Epoetin beta | Darbepoetin alfa | Methoxy polyethylene‐glycol epoetin beta | Biosimilar ESA | Placebo | |
| Blood transfusion | ||||||
| Epoetin alfa | ‐‐ | 2.04 (0.38‐11.0) | 1.06 (0.35‐3.29) | 1.14 (0.27‐4.97) | 0.66 (0.19‐2.28) | 0.18 (0.05‐0.59) |
| Epoetin beta | Not estimable | ‐‐ | 0.52 (0.10‐2.67) | 0.56 (0.11‐3.00) | 0.33 (0.04‐2.60) | 0.09 (0.02‐0.38) |
| Darbepoetin alfa | 2.31 (1.34‐3.97) | Not estimable | ‐‐ | 1.08 (0.38‐3.04) | 0.62 (0.12‐3.30) | 0.17 (0.05‐0.57) |
| Methoxy polyethylene‐glycol epoetin beta |
Not estimable | 0.83 (0.17‐4.15) | 0.94 (0.45‐1.95) | ‐‐ | 0.58 (0.09‐3.92) | 0.15 (0.03‐0.70) |
| Biosimilar ESA | 0.72 (0.42‐1.22) | Not estimable | Not estimable | Not estimable | ‐‐ | 0.27 (0.05‐1.47) |
| Placebo | 0.07 (0.01‐0.84) | 0.07 (0.03‐0.21) | 0.53 (0.46‐0.63) | Not estimable | Not estimable | ‐‐ |
| All‐cause mortality | ||||||
| Epoetin alfa | ‐‐ | 1.53 (0.77‐3.03) | 1.17 (0.68‐2.05) | 1.08 (0.54‐2.15) | 0.95 (0.62‐1.44) | 1.25 (0.71‐2.21) |
| Epoetin beta | Not estimable | – | 0.77 (0.43‐1.38) | 0.71 (0.35‐1.42) | 0.62 (0.29‐1.37) | 0.82 (0.45‐1.48) |
| Darbepoetin alfa | 1.12 (0.59‐2.14) | 0.89 (0.38‐2.09) | ‐‐ | 0.91 (0.60‐1.40) | 0.81 (0.41‐1.61) | 1.06 (0.91‐1.24) |
| Methoxy polyethylene‐glycol epoetin beta | Not estimable | 0.81 (0.12‐5.35) | 0.90 (0.59‐1.40) | ‐‐ | 0.88 (0.40‐1.97) | 1.16 (0.74‐1.82) |
| Biosimilar ESA | 1.04 (0.53‐2.01) | 0.34 (0.04‐2.82) | Not estimable | Not estimable | ‐‐ | 1.31 (0.65‐2.62) |
| Placebo | 0.99 (0.14‐6.86) | 0.61 (0.17‐2.15) | 1.06 (0.91‐1.24) | Not estimable | Not estimable | ‐‐ |
| Fatigue | ||||||
| Epoetin alfa | ‐‐ | Not estimable | Not estimable | Not estimable | Not estimable | Not estimable |
| Epoetin beta | Not estimable | ‐‐ | Not estimable | Not estimable | Not estimable | Not estimable |
| Darbepoetin alfa | 0.94 (0.57‐1.55) | Not estimable | ‐‐ | Not estimable | Not estimable | Not estimable |
| Methoxy polyethylene‐glycol epoetin beta | Not estimable | Not estimable | Not estimable | ‐‐– | Not estimable | Not estimable |
| Biosimilar ESA | 0.18 (0.01‐3.91) | Not estimable | Not estimable | Not estimable | ‐‐ | Not estimable |
| Placebo | Not estimable | Not estimable | Not estimable | Not estimable | Not estimable | ‐‐ |
| Breathlessness | ||||||
| Epoetin alfa | ‐‐ | Not estimable | Not estimable | Not estimable | Not estimable | Not estimable |
| Epoetin beta | Not estimable | ‐‐ | Not estimable | Not estimable | Not estimable | Not estimable |
| Darbepoetin alfa | 0.71 (0.46‐1.10) | Not estimable | ‐‐ | Not estimable | Not estimable | Not estimable |
| Methoxy polyethylene‐glycol epoetin beta | Not estimable | Not estimable | Not estimable | ‐‐ | Not estimable | Not estimable |
| Biosimilar ESA | 0.68 (0.37‐1.25) | Not estimable | Not estimable | Not estimable | ‐‐ | Not estimable |
| Placebo | Not estimable | Not estimable | Not estimable | Not estimable | Not estimable | ‐‐ |
| Cardiovascular mortality | ||||||
| Epoetin alfa | ‐‐ | 2.12 (0.34‐13.1) | 1.48 (0.28‐7.96) | 1.02 (0.16‐6.48) | 0.55 (0.22‐1.38) | 1.56 (0.29‐8.37) |
| Epoetin beta | Not estimable | ‐‐ | 0.70 (0.12‐4.10) | 0.48 (0.07‐3.31) | 0.26 (0.04‐1.51) | 0.74 (0.13‐4.28) |
| Darbepoetin alfa | 2.15 (0.31‐14.9) | Not estimable | ‐‐ | 0.69 (0.32‐1.48) | 0.37 (0.06‐2.20) | 1.05 (0.87‐1.26) |
| Methoxy polyethylene‐glycol epoetin beta | Not estimable | Not estimable | 0.69 (0.32‐1.48) | ‐‐ | 0.54 (0.08‐3.74) | 1.52 (0.69‐3.34) |
| Biosimilar ESA | 0.53 (0.20‐1.35) | 0.34 (0.04‐2.82) | Not estimable | Not estimable | ‐‐ | 2.81 (0.47‐16.7) |
| Placebo | Not estimable | 0.45 (0.06‐3.75) | 1.05 (0.87‐1.26) | Not estimable | Not estimable | ‐‐ |
| Major adverse cardiovascular events | ||||||
| Epoetin alfa | ‐‐ | Not estimable | Not estimable | Not estimable | Not estimable | Not estimable |
| Epoetin beta | Not estimable | ‐‐ | Not estimable | Not estimable | Not estimable | Not estimable |
| Darbepoetin alfa | 0.20 (0.01‐4.17) | Not estimable | ‐‐ | Not estimable | Not estimable | Not estimable |
| Methoxy polyethylene‐glycol epoetin beta | Not estimable | Not estimable | Not estimable | ‐‐ | Not estimable | Not estimable |
| Biosimilar ESA | 0.49 (0.17‐1.47) | Not estimable | Not estimable | Not estimable | ‐‐ | Not estimable |
| Placebo | Not estimable | Not estimable | 1.08 (0.95‐1.24) | Not estimable | Not estimable | ‐‐ |
| Myocardial infarction | ||||||
| Epoetin alfa | ‐‐ | Not estimable | 1.04 (0.35‐3.11) | 0.55 (0.05‐5.69) | 1.18 (0.47‐3.02) | 1.00 (0.32‐3.09) |
| Epoetin beta | Not estimable | ‐‐ | Not estimable | Not estimable | Not estimable | Not estimable |
| Darbepoetin alfa | 0.87 (0.20‐3.81) | Not estimable | ‐‐ | 0.53 (0.07‐4.18) | 1.14 (0.27‐4.83) | 0.97 (0.75‐1.25) |
| Methoxy polyethylene‐glycol epoetin beta | Not estimable | Not estimable | 0.47 (0.06‐3.65) | ‐‐ | 2.17 (0.17‐27.1) | 1.83 (0.18‐19.1) |
| Biosimilar ESA | 1.23 (0.49‐3.12) | Not estimable | Not estimable | Not estimable | ‐‐ | 0.84 (0.20‐3.65) |
| Placebo | 3.46 (0.12‐100.51) | Not estimable | 0.97 (0.75‐1.25) | Not estimable | Not estimable | ‐‐ |
| Stroke | ||||||
| Epoetin alfa | ‐‐ | 4.56 (0.29‐71.8) | 1.39 (0.38‐5.16) | 2.36 (0.24‐23.6) | 0.92 (0.39‐2.16) | 2.74 (0.71‐10.5) |
| Epoetin beta | Not estimable | ‐‐ | 0.31 (0.02‐4.55) | 0.52 (0.02‐14.0) | 0.20 (0.01‐3.61) | 0.60 (0.04‐8.88) |
| Darbepoetin alfa | 1.44 (0.37‐5.54) | Not estimable | ‐‐ | 1.70 (0.26‐11.2) | 0.66 (0.14‐3.14) | 1.96 (1.40‐2.75) |
| Methoxy polyethylene‐glycol epoetin beta | Not estimable | Not estimable | 1.33 (0.17‐10.49) | ‐‐ | 0.38 (0.03‐4.50) | 1.16 (0.17‐7.90) |
| Biosimilar ESA | 0.92 (0.39‐2.15) | Not estimable | Not estimable | Not estimable | ‐‐ | 2.99 (0.61‐14.8) |
| Placebo | Not estimable | 0.33 (0.01‐8.21) | 1.97 (1.40‐2.76) | Not estimable | Not estimable | ‐‐ |
| Hypertension | ||||||
| Epoetin alfa | ‐‐ | 0.90 (0.41‐1.95) | 1.26 (0.81‐1.96) | 1.18 (0.64‐2.18) | 1.95 (0.97‐3.94) | 2.31 (1.27‐4.23) |
| Epoetin beta | Not estimable | ‐‐ | 1.41 (0.70‐2.82) | 1.31 (0.63‐2.72) | 2.18 (0.76‐6.22) | 2.57 (1.23‐5.39) |
| Darbepoetin alfa | 0.94 (0.62‐1.43) | 1.18 (0.38‐3.69) | ‐‐ | 0.93 (0.60‐1.45) | 1.55 (0.68‐3.55) | 1.83 (1.05‐3.21) |
| Methoxy polyethylene‐glycol epoetin beta | Not estimable | 1.38 (0.62‐3.09) | Not estimable | ‐‐ | 1.66 (0.65‐4.21) | 1.96 (0.98‐3.92) |
| Biosimilar ESA | 1.77 (1.02‐3.09) | Not estimable | Not estimable | Not estimable | ‐‐ | 1.18 (0.47‐2.99) |
| Placebo | 4.10 (2.16‐7.76) | 2.95 (1.19‐7.26) | 1.14 (0.99‐1.32) | Not estimable | Not estimable | ‐‐ |
| End‐stage kidney disease | ||||||
| Epoetin alfa | ‐‐ | Not estimable | Not estimable | Not estimable | Not estimable | Not estimable |
| Epoetin beta | Not estimable | ‐‐ | Not estimable | Not estimable | Not estimable | Not estimable |
| Darbepoetin alfa | 2.17 (0.37‐12.74) | Not estimable | ‐ | Not estimable | Not estimable | Not estimable |
| Methoxy polyethylene‐glycol epoetin beta | Not estimable | Not estimable | 1.83 (0.66‐5.09) | ‐‐ | Not estimable | Not estimable |
| Biosimilar ESA | Not estimable | Not estimable | Not estimable | Not estimable | ‐‐ | Not estimable |
| Placebo | Not estimable | Not estimable | 1.04 (0.88‐1.23) | Not estimable | Not estimable | ‐‐ |
| Vascular access thrombosis | ||||||
| Epoetin alfa | ‐‐ | 0.93 (0.28‐3.10) | 1.22 (0.78‐1.91) | 1.04 (0.48‐2.25) | 1.26 (0.45‐3.36) | 1.72 (0.58‐5.16) |
| Epoetin beta | Not estimable | ‐‐ | 1.30 (0.42‐4.04) | 1.11 (0.38‐3.24) | 1.35 (0.29‐6.34) | 1.85 (0.61‐5.63) |
| Darbepoetin alfa | 1.15 (0.73‐1.82) | Not estimable | ‐‐ | 0.86 (0.45‐1.61) | 1.04 (0.35‐3.05) | 1.42 (0.50‐4.03) |
| Methoxy polyethylene‐glycol epoetin beta | Not estimable | 1.74 (0.49‐6.24) | 0.76 (0.39‐1.47) | ‐‐ | 1.21 (0.35‐4.22) | 1.66 (0.54‐5.08) |
| Biosimilar ESA | 1.71 (0.30‐10.00) | Not estimable | Not estimable | Not estimable | ‐‐ | 1.37 (0.32‐5.93) |
| Placebo | 6.40 (0.80‐51.50) | 1.09 (0.28‐4.34) | 1.34 (0.30‐6.01) | Not estimable | Not estimable | ‐‐ |
Treatment estimates for pairwise meta‐analyses are shown in italics
Figure 5 shows the networks of evidence for the safety and efficacy of ESA drugs included in the review. Each line links the treatments which have been directly compared in studies. The thickness of the line is proportional to the number of studies included in the comparison and the width of each circle is proportional to the number of participants included in the comparison. Figure 6 shows the summary treatment effects for ESAs when compared against placebo within networks.
Figure 5.
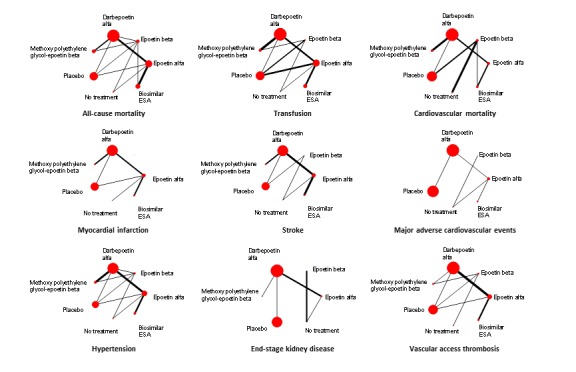
Networks of the treatment efficacy and safety of ESA drugs in the treatment of anaemia in chronic kidney disease. Values lower than 1 favour the active treatment in the comparison
Figure 6.
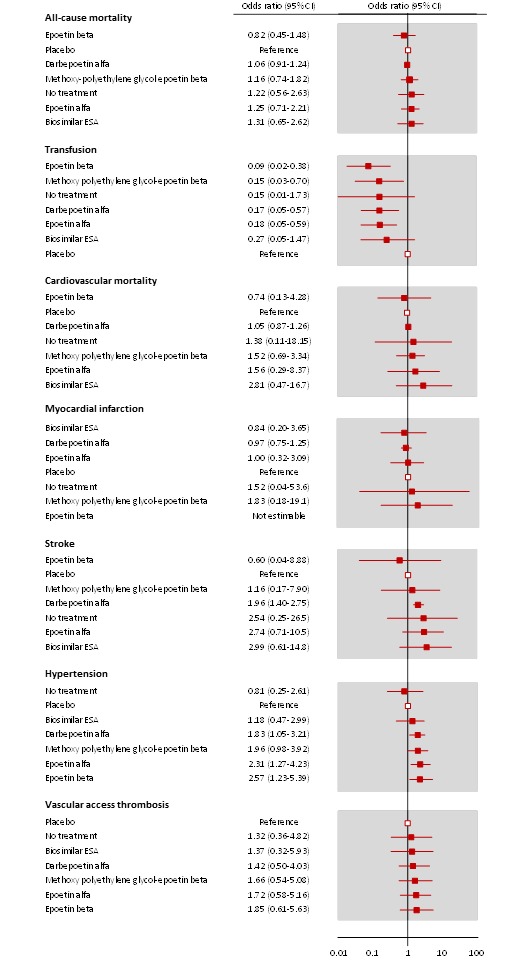
Forest plots for results from network meta‐analyses comparing ESAs versus placebo
1. Response to treatment (efficacy)
1.1 Pairwise meta‐analysis (direct comparisons)
Treatment estimates for pairwise meta‐analyses are shown in italics in Table 3.
Preventing blood transfusions
ESAs compared to placebo
Data for effects of ESA treatment compared to placebo or no treatment was provided in eight studies with 4661 participants (Bahlmann 1991; Bennett 1991; Canadian EPO Study 1990; Kleinman 1989; Patel 2012; Roth 1994; TREAT Study 2005; Van Biesen 2005). Three agents (epoetin alfa, epoetin beta and darbepoetin alfa) were assessed against placebo or no treatment. No study evaluated either methoxy polyethylene glycol‐epoetin beta or a biosimilar ESA with placebo or standard care.
The following results for blood transfusions were found:
Epoetin alfa reduced the odds of blood transfusion compared to placebo (Analysis 1.1.1 (3 studies, 196 participants): OR 0.07, 95% CI 0.01 to 0.84; I² = 81%) (Canadian EPO Study 1990; Kleinman 1989; Roth 1994) with evidence of heterogeneity that might be substantial
Epoetin beta reduced the odds of blood transfusion compared to placebo (Analysis 1.1. (2 studies, 230 participants): OR 0.07, 95% CI 0.03 to 0.21; I² = 0%) (Bahlmann 1991; Bennett 1991)
Darbepoetin alfa reduced the odds of blood transfusion compared to placebo (Analysis 1.1.3 (1 study, 4038 participants): OR 0.53, 95% CI 0.46 to 0.63) (TREAT Study 2005)
Epoetin alfa had uncertain effects on the odds of blood transfusion compared with no treatment (Analysis 1.1.4 (1 study, 157 participants): OR 3.10, 95% CI 0.16 to 58.97) (Patel 2012)
Epoetin beta had uncertain effects on the odds of blood transfusion compared with no treatment (Analysis 1.1.5 (1 study, 40 participants): OR 0.35, 95% CI 0.06 to 2.18) (Van Biesen 2005).
Analysis 1.1.
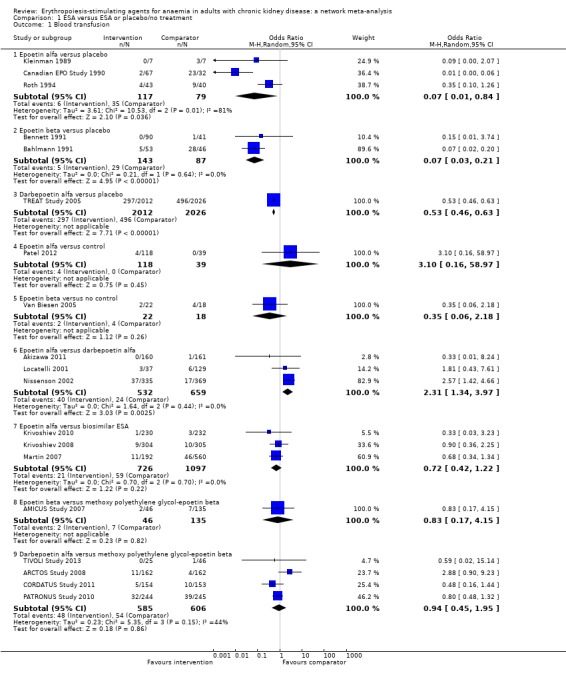
Comparison 1 ESA versus ESA or placebo/no treatment, Outcome 1 Blood transfusion.
ESAs compared to each other
Three studies (1191 participants) compared epoetin alfa with darbepoetin alfa (Akizawa 2011; Locatelli 2001; Nissenson 2002), three studies (1823 participants) compared epoetin alfa with a biosimilar ESA (Krivoshiev 2008; Krivoshiev 2010; Martin 2007), one study (181 participants) compared epoetin beta versus methoxy polyethylene glycol‐epoetin beta (AMICUS Study 2007), and four studies (1191 participants) compared darbepoetin alfa versus methoxy polyethylene glycol‐epoetin beta (ARCTOS Study 2008; CORDATUS Study 2011; PATRONUS Study 2010; TIVOLI Study 2013).
Epoetin alfa increased the odds of blood transfusion compared to darbepoetin alfa (Analysis 1.1.6 (3 studies, 1191 participants): OR 2.31, 95% CI 1.34 to 3.97; I² = 0%) (Akizawa 2011; Locatelli 2001; Nissenson 2002)
Epoetin alfa had uncertain effects on the odds of blood transfusion compared to a biosimilar ESA (Analysis 1.1.7 (3 studies, 1823 participants): OR 0.72, 95% CI 0.42 to 1.22; I² = 0%) (Krivoshiev 2008; Krivoshiev 2010; Martin 2007)
Epoetin beta had uncertain effects on the odds of blood transfusion compared to methoxy polyethylene glycol‐epoetin beta (Analysis 1.1.8 (1 study, 181 participants): OR 0.83, 95% CI 0.17 to 4.15) (AMICUS Study 2007)
Darbepoetin alfa had uncertain effects on the odds of blood transfusion compared to methoxy polyethylene glycol‐epoetin beta (Analysis 1.1.9 (4 studies, 1191 participants): OR 0.94, 95% CI 0.45 to 1.95; I² = 44%) (ARCTOS Study 2008; CORDATUS Study 2011; PATRONUS Study 2010; TIVOLI Study 2013) with evidence of moderate heterogeneity.
Fatigue
ESAs compared to placebo or no treatment
There were no placebo or standard care‐controlled studies providing extractable data for the effects of treatment on fatigue.
ESAs compared to each other
Data for effects of ESA treatment versus another ESA on fatigue were available in three studies with 730 participants (Allon 2002; Goh 2007; Nissenson 2002).
Epoetin alfa had uncertain effects on fatigue compared to darbepoetin alfa (Analysis 1.2.1 (2 studies, 551 participants): OR 0.94, 95% CI 0.57 to 1.55; I² = 0%) (Allon 2002; Nissenson 2002)
Epoetin alfa had uncertain effects on fatigue compared to a biosimilar ESA (Analysis 1.2.2 (1 study, 179 participants): OR 0.18, 95% CI 0.01 to 3.91) (Goh 2007).
Analysis 1.2.
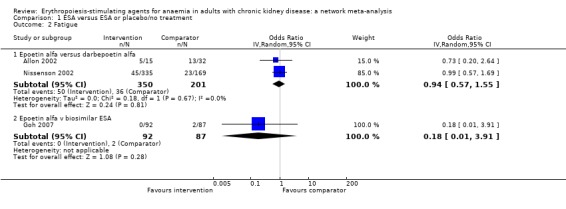
Comparison 1 ESA versus ESA or placebo/no treatment, Outcome 2 Fatigue.
Breathlessness
ESAs compared to placebo or no treatment
There were no placebo or standard care‐controlled studies providing extractable data for the effects of treatment on breathlessness.
ESAs compared to each other
Data for effects of ESA treatment versus another ESA on breathlessness were available in three studies involving 1298 participants (Goh 2007; Haag‐Weber 2009; Nissenson 2002).
Epoetin alfa had uncertain effects on breathlessness when compared to darbepoetin alfa (Analysis 1.3.1 (1 study, 504 participants): OR 0.71, 95% CI 0.46 to 1.10) (Nissenson 2002)
Epoetin alfa had uncertain effects on breathlessness when compared to a biosimilar ESA (Analysis 1.3.2 (2 studies, 794 participants): OR 0.68, 95% CI 0.37 to 1.25; I² = 0%) (Goh 2007; Haag‐Weber 2009).
Analysis 1.3.
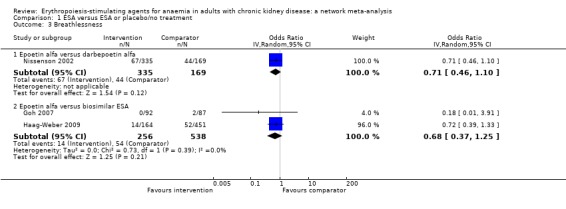
Comparison 1 ESA versus ESA or placebo/no treatment, Outcome 3 Breathlessness.
1.2 Network meta‐analysis (combination of direct and indirect comparisons)
Treatment estimates for network meta‐analyses are shown in Table 3 and network meta‐analyses for all ESAs against placebo are summarised in Figure 6. Studies grouped by comparison were deemed comparable for the effect modifiers of stage of CKD, haemoglobin target with ESA treatment, age of participants and duration of follow‐up, such that the assumption of transitivity might hold and that a network meta‐analytical approach was reasonable. However, we could not assess the comparability of treatment comparisons across different studies using statistical methods due to insufficient data. Overall, SUCRA rankings of the differing ESAs were imprecise due to sparse data rendering the analyses clinically irrelevant. Therefore, treatment rankings are not provided in the results.
Preventing blood transfusions
Blood transfusion data were provided in 19 studies (Akizawa 2011; AMICUS Study 2007; ARCTOS Study 2008; Bahlmann 1991; Bennett 1991; Canadian EPO Study 1990; CORDATUS Study 2011; Kleinman 1989; Krivoshiev 2008; Krivoshiev 2010; Locatelli 2001; Martin 2007; Nissenson 2002; Patel 2012; PATRONUS Study 2010; Roth 1994; TIVOLI Study 2013; TREAT Study 2005; Van Biesen 2005) involving 9047 participants with CKD (58.0% of the participants in this review). Most participants within the network were randomised to darbepoetin alfa or placebo due to the contribution of the large TREAT study (TREAT Study 2005).
In moderate to low quality evidence, epoetin alfa, epoetin beta, darbepoetin alfa and methoxy polyethylene glycol‐epoetin beta were all superior to placebo for preventing blood transfusion (epoetin alfa OR 0.18, 95% CI 0.05‐0.59, epoetin beta OR 0.09, 95% CI 0.02 to 0.38; darbepoetin alfa OR 0.17, 95% CI 0.05 to 0.57; methoxy polyethylene glycol‐epoetin beta OR 0.15, 95% CI 0.03 to 0.70). In very low quality evidence, biosimilar ESAs were possibly no better than placebo (OR 0.27, 95% CI 0.05 to 1.47). There were no statistical differences between all ESAs for their effects on blood transfusion in treatment estimates showing considerable uncertainty. The heterogeneity tau for this network overall was 0.89, which is consistent with moderate heterogeneity.
Fatigue
Network meta‐analysis was not possible for this outcome due to insufficient data.
Breathlessness
Network meta‐analysis was not possible for this outcome due to insufficient data.
2. Safety
2.1 Pairwise meta‐analysis (direct comparisons)
All‐cause mortality
ESAs compared to placebo
Data for effects of ESA treatment compared to placebo or no treatment on all‐cause mortality was provided in 10 studies involving 5209 participants (Bahlmann 1991; Bennett 1991; EPOCARES Study 2010; Klinkmann 1992; Kuriyama 1997; Nissenson 1995; Palazzuoli 2007; Patel 2012; Roth 1994; TREAT Study 2005). Three agents (epoetin alfa, epoetin beta and darbepoetin alfa) were assessed against placebo or no treatment. No study evaluated either methoxy polyethylene glycol‐epoetin beta or a biosimilar ESA with placebo or standard care.
The odds of all‐cause mortality were uncertain for epoetin alfa (Analysis 1.4.1 (2 studies, 235 participants): OR 0.99, 95% CI 0.14 to 6.86; I² = 0%), epoetin beta Analysis 1.4.2 (3 studies, 311 participants): OR 0.61, 95% CI 0.17 to 2.15; I² = 0%) and darbepoetin alfa (Analysis 1.4.3 (Analysis 1.4.3 (1 study, 4038 participants): OR 1.06, 95% 0.91 to 1.24) when compared with placebo (Bahlmann 1991; Bennett 1991; Nissenson 1995; Palazzuoli 2007; Roth 1994; TREAT Study 2005)
The odds of all‐cause mortality were uncertain for epoetin alfa (Analysis 1.4.4 (1 study, 157 participants): OR 1.06, 95% CI 0.39 to 2.87) and epoetin beta (Analysis 1.4.5 (3 studies, 468 participants): OR 0.69, 95% CI 0.36 to 1.33; I² = 0%) when compared with standard care (EPOCARES Study 2010; Klinkmann 1992; Kuriyama 1997).
Analysis 1.4.
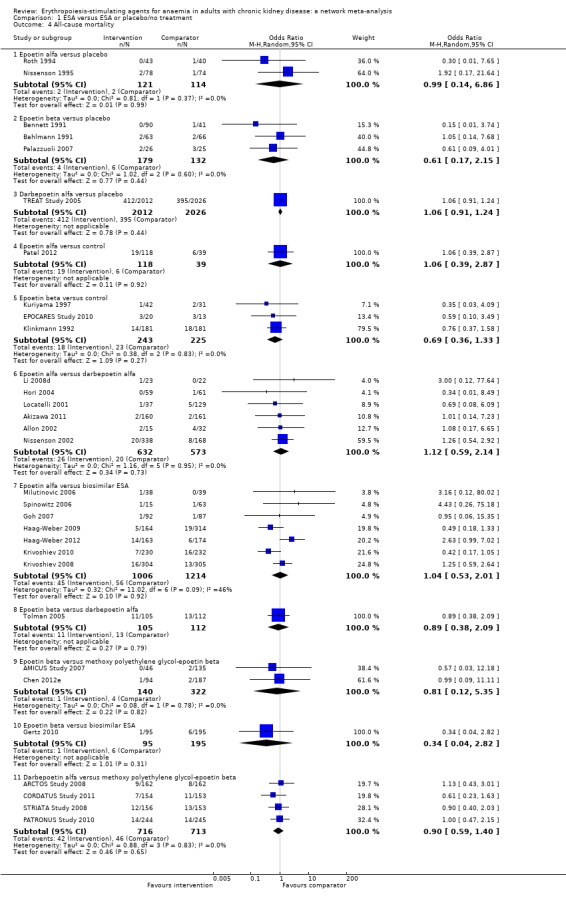
Comparison 1 ESA versus ESA or placebo/no treatment, Outcome 4 All‐cause mortality.
ESAs compared to each other
Epoetin alfa was compared to darbepoetin alfa in six studies involving 1205 participants (Akizawa 2011; Allon 2002; Hori 2004; Li 2008d; Locatelli 2001; Nissenson 2002), epoetin alfa was compared to a biosimilar ESA in seven studies involving 2220 participants (Goh 2007; Haag‐Weber 2009; Haag‐Weber 2012; Krivoshiev 2008; Krivoshiev 2010; Milutinovic 2006; Spinowitz 2006), epoetin beta was compared versus darbepoetin alfa in one study and 217 participants (Tolman 2005), epoetin beta was compared to methoxy polyethylene glycol‐epoetin beta in two studies involving 462 participants (AMICUS Study 2007; Chen 2012e), epoetin beta was compared to a biosimilar ESA in one study involving 290 participants (Gertz 2010) and darbepoetin alfa was compared to methoxy polyethylene glycol‐epoetin beta in four studies involving 1429 participants (ARCTOS Study 2008; CORDATUS Study 2011; PATRONUS Study 2010; STRIATA Study 2008).
The odds of all‐cause mortality with epoetin alfa were uncertain when compared to darbepoetin alfa (Analysis 1.4.6 (6 studies, 1205 participants): OR 1.12, 95% CI 0.59 to 2.14; I² = 0%) or biosimilar ESAs (Analysis 1.4.7 (7 studies, 2220 participants): OR 1.04, 95% CI 0.53 to 2.01; I² = 46%)
The odds of all‐cause mortality with epoetin beta were uncertain when compared to darbepoetin alfa (Analysis 1.4.8 (1 study, 217 participants): OR 0.89, 95% CI 0.38 to 2.09), methoxy polyethylene glycol‐epoetin beta (Analysis 1.4.9 (2 studies, 462 participants): OR 0.57, 95% CI 0.03 to 12.18; I² = 0%) or a biosimilar ESA (Analysis 1.4.10 (1 study, 290 participants): OR 0.34, 95% CI 0.04 to 2.82)
The odds of all‐cause mortality with darbepoetin alfa were uncertain when compared to methoxy polyethylene glycol‐epoetin beta (Analysis 1.4.11 (4 studies, 1429 participants): OR 0.90, 95% CI 0.59 to 1.40; I² = 0%).
Cardiovascular mortality
ESAs compared to placebo
Data for effects of ESA treatment compared to placebo or no treatment on cardiovascular mortality were provided in six studies with 4766 participants (Bahlmann 1991; Bennett 1991; EPOCARES Study 2010; Klinkmann 1992; Kuriyama 1997; TREAT Study 2005). Two agents (epoetin beta and darbepoetin alfa) were assessed against placebo or no treatment. No study evaluated either epoetin alfa, methoxy polyethylene glycol‐epoetin beta or a biosimilar ESA with placebo or standard care.
The odds of cardiovascular mortality were uncertain for epoetin beta (Analysis 1.5.1 (2 studies, 260 participants): OR 0.45, 95% CI 0.06 to 3.75, I² = 0%) and darbepoetin alfa (Analysis 1.5.2 (1 study, 4038 participants): OR 1.05, 95% 0.87 to 1.26) when compared to placebo (Bahlmann 1991; Bennett 1991; TREAT Study 2005)
The odds of cardiovascular mortality were uncertain for epoetin beta (Analysis 1.5.3 (3 studies, 430 participants): OR 0.28, 95% CI 0.08 to 1.03; I² = 0%) when compared with no treatment (EPOCARES Study 2010; Klinkmann 1992; Kuriyama 1997).
Analysis 1.5.
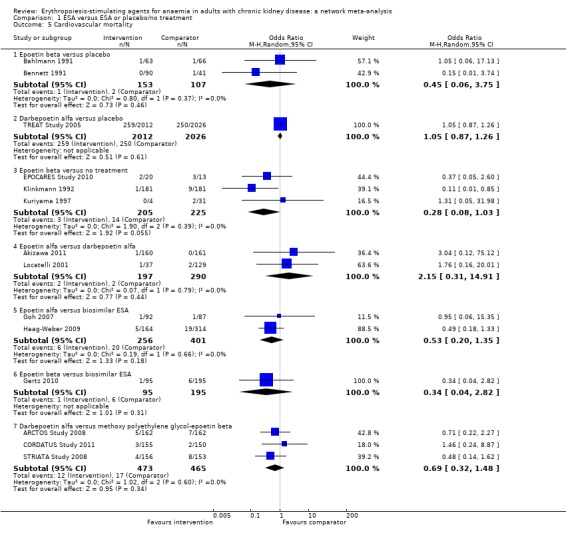
Comparison 1 ESA versus ESA or placebo/no treatment, Outcome 5 Cardiovascular mortality.
ESAs compared to each other
Epoetin alfa was compared to darbepoetin alfa in two studies and 487 participants (Akizawa 2011; Locatelli 2001) and a biosimilar ESA in one study and 478 participants (Haag‐Weber 2009). Epoetin beta was compared to a biosimilar ESA in 1 study and 290 participants (Gertz 2010). Darbepoetin alfa was compared to methoxy polyethylene glycol‐epoetin beta in two studies and 629 participants (ARCTOS Study 2008; CORDATUS Study 2011).
The odds of cardiovascular mortality were uncertain for epoetin alfa when compared to darbepoetin alfa (Analysis 1.5.4 (2 studies, 487 participants): OR 2.15, 95% CI 0.31 to 14.91; I² = 0%) or a biosimilar ESA (Analysis 1.5.5 (2 studies, 657 participants): OR 0.53, 95% CI 0.20 to 1.35; I² = 0%)
The odds of cardiovascular mortality were uncertain for epoetin beta when compared to a biosimilar ESA (Analysis 1.5.6 (1 study, 290 participants): OR 0.34, 95% CI 0.04 to 2.82)
The odds of cardiovascular mortality were uncertain for darbepoetin alfa when compared to methoxy polyethylene glycol‐epoetin beta (Analysis 1.5.7 (3 studies, 938 participants): OR 0.69, 95% CI 0.32 to 1.48; I² = 0%).
Myocardial infarction (MI)
ESAs compared to placebo
Data for effects of ESA treatment compared to placebo or no treatment on MI were provided in three studies involving 4209 participants (Kleinman 1989; Patel 2012; TREAT Study 2005). Two agents (epoetin alfa and darbepoetin alfa) were assessed against placebo or no treatment. No study evaluated either epoetin beta, methoxy polyethylene glycol‐epoetin beta or a biosimilar ESA with placebo or standard care.
The odds of MI were uncertain for epoetin alfa (Analysis 1.6.1 (1 study, 14 participants): OR 3.46, 95% CI 0.12 to 100.51) and darbepoetin alfa (Analysis 1.6.2 (1 study, 4038 participants): OR 0.97, 95% CI 0.75 to 1.25) when compared to placebo (Kleinman 1989; TREAT Study 2005)
The odds of MI were uncertain for epoetin alfa (Analysis 1.6.3 (1 study, 157 participants): OR 1.01, 95% CI 0.04 to 25.26) when compared to no treatment (Patel 2012).
Analysis 1.6.
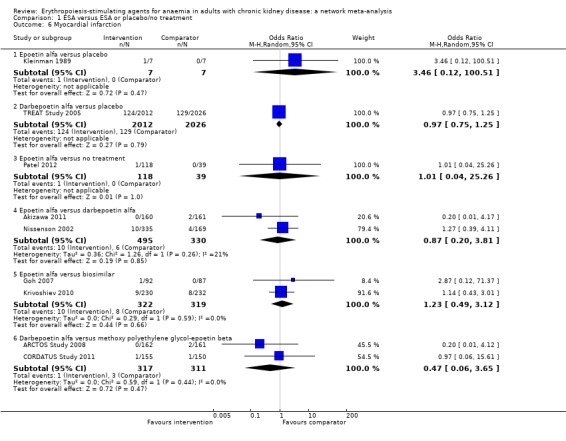
Comparison 1 ESA versus ESA or placebo/no treatment, Outcome 6 Myocardial infarction.
ESAs compared to each other
Epoetin alfa was compared to darbepoetin alfa in two studies involving 825 participants (Akizawa 2011; Nissenson 2002), and a biosimilar ESA in two studies involving 641 participants (Goh 2007; Krivoshiev 2010). Darbepoetin alfa was compared to methoxy polyethylene glycol‐epoetin beta in two studies involving 629 participants (ARCTOS Study 2008; CORDATUS Study 2011).
The odds of MI were uncertain for epoetin alfa when compared to darbepoetin alfa (Analysis 1.6.4 (2 studies, 825 participants): OR 0.87, 95% CI 0.20 to 3.81; I² = 21%) and a biosimilar ESA (Analysis 1.6.5 (2 studies, 641 participants): OR 1.23, 95% CI 0.49 to 3.12; I² = 0%)
The odds of MI were uncertain for darbepoetin alfa when compared to methoxy polyethylene glycol‐epoetin beta (Analysis 1.6.6 (2 studies, 628 participants): OR 0.47, 95% CI 0.06 to 3.65; I² = 0%).
Stroke
ESAs compared to placebo
Data for effects of ESA treatment compared to placebo or no treatment on stroke were provided in four studies and 4334 participants (Bahlmann 1991; EPOCARES Study 2010; Patel 2012; TREAT Study 2005). Three agents (epoetin alfa, epoetin beta and darbepoetin alfa) were assessed against placebo or no treatment. No study evaluated either methoxy polyethylene glycol‐epoetin beta or a biosimilar ESA with placebo or standard care.
The odds of stroke were uncertain for epoetin beta when compared to placebo (Analysis 1.7.1 (1 study, 106 participants): OR 0.33, 95% CI 0.01 to 8.21) but were increased with darbepoetin alfa compared to placebo (Analysis 1.7.2 (1 study, 4038 participants): OR 1.97, 95% CI 1.40 to 2.76)
The odds of stroke were uncertain for epoetin alfa (Analysis 1.7.3 (1 study, 157 participants): OR 0.99, 95% CI 0.10 to 9.82) and epoetin beta (Analysis 1.7.4 (1 study, 33 participants): OR 0.20, 95% CI 0.01 to 5.39) compared to control.
Analysis 1.7.
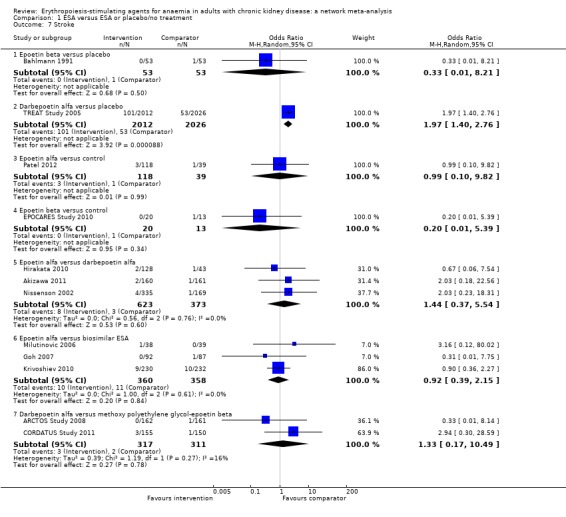
Comparison 1 ESA versus ESA or placebo/no treatment, Outcome 7 Stroke.
ESAs compared to each other
Epoetin alfa was compared to darbepoetin alfa in three studies involving 996 participants (Akizawa 2011; Hirakata 2010; Nissenson 2002) and a biosimilar ESA in three studies involving 539 participants (Goh 2007; Krivoshiev 2010; Milutinovic 2006). Darbepoetin alfa was compared to methoxy polyethylene glycol‐epoetin beta in two studies and 629 participants (ARCTOS Study 2008; CORDATUS Study 2011).
The odds of stroke were uncertain for epoetin alfa versus darbepoetin alfa (Analysis 1.7.5 (3 studies, 996 participants): OR 1.44, 95% CI 0.37 to 5.54; I² = 0%) and a biosimilar ESA (Analysis 1.7.6 (3 studies, 718 participants): OR 0.92, 95% CI 0.39 to 2.15; I² = 0%)
The odds of stroke were uncertain for darbepoetin alfa when compared to methoxy polyethylene glycol‐epoetin beta (Analysis 1.7.7 (2 studies, 628 participants): OR 1.33, 95% CI 0.17 to 10.49; I² = 16%).
Hypertension
ESAs compared to placebo
Data for the effects of ESA treatment compared to placebo or no treatment on hypertension were provided in eight studies and 5058 participants (Bahlmann 1991; Bennett 1991; Canadian EPO Study 1990; Clyne 1992; Klinkmann 1992; Nissenson 1995; Patel 2012; TREAT Study 2005). Three agents (epoetin alfa, epoetin beta and darbepoetin alfa) were assessed against placebo or no treatment. No study evaluated either methoxy polyethylene glycol‐epoetin beta or a biosimilar ESA with placebo or standard care.
The odds of hypertension were increased with epoetin alfa (Analysis 1.8.1 (2 studies; 251 participants): OR 4.10, 95% CI 2.16 to 7.76; I² = 0%); epoetin beta (Analysis 1.8.2 (2 studies, 230 participants): OR 2.95, 95% CI 1.19 to 7.26; I² = 0%) and darbepoetin alfa (Analysis 1.8.3 (1 study, 4038 participants): OR 1.14, 95% CI 0.99 to 1.32) when compared to placebo (Bahlmann 1991; Bennett 1991; Canadian EPO Study 1990; Nissenson 1995; TREAT Study 2005)
The odds of hypertension were uncertain for epoetin alfa compared to no treatment (Analysis 1.8.4 (1 study, 157 participants): OR 5.31, 95% CI 0.30 to 95.20) but were increased with epoetin beta compared to no treatment (Analysis 1.8.5 (2 studies, 382 participants): OR 2.99, 95% CI 1.34 to 6.69; I² = 0%) (Clyne 1992; Klinkmann 1992; Patel 2012).
Analysis 1.8.
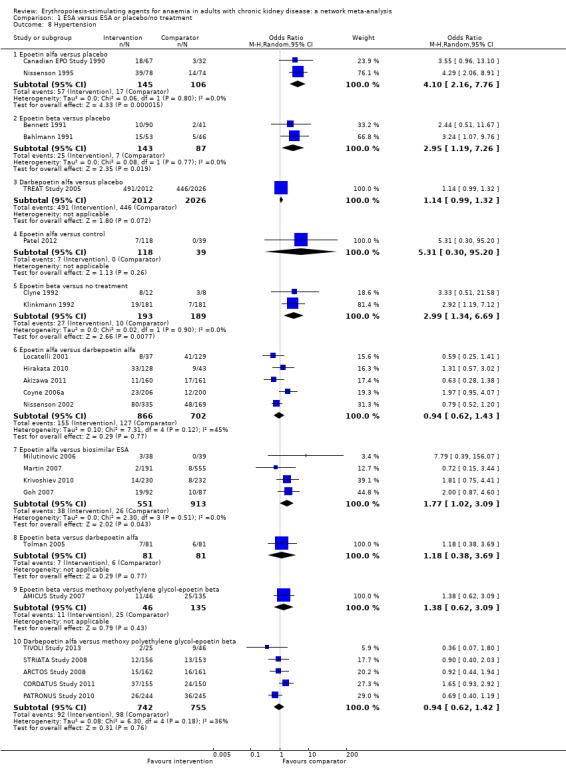
Comparison 1 ESA versus ESA or placebo/no treatment, Outcome 8 Hypertension.
ESAs compared to each other
Epoetin alfa was compared to darbepoetin alfa in five studies and 1568 participants (Akizawa 2011; Coyne 2006a; Hirakata 2010; Locatelli 2001; Nissenson 2002) and a biosimilar ESA in four studies involving 1464 participants (Goh 2007; Krivoshiev 2010; Martin 2007; Milutinovic 2006). Epoetin beta was compared to darbepoetin alfa in one study and 162 participants (Tolman 2005) and methoxy polyethylene glycol‐epoetin beta in one study and 181 participants (AMICUS Study 2007). Darbepoetin alfa was compared to methoxy polyethylene glycol‐epoetin beta in five studies and 1497 participants (ARCTOS Study 2008; CORDATUS Study 2011; PATRONUS Study 2010; STRIATA Study 2008; TIVOLI Study 2013).
The odds of hypertension were uncertain for epoetin alfa compared to darbepoetin alfa (Analysis 1.8.6 (5 studies, 1568 participants): OR 0.94, 95% CI 0.62 to 1.43; I² = 45%) with evidence of moderate heterogeneity or a biosimilar ESA (Analysis 1.8.7 (4 studies, 1464 participants): OR 1.77, 95% CI 1.02 to 3.09; I² = 0%)
The odds of hypertension were uncertain for epoetin beta compared to darbepoetin alfa (Analysis 1.8.8 (1 study, 162 participants): OR 1.18, 95% CI 0.38 to 3.69)
The odds of hypertension were uncertain for epoetin beta compared to methoxy polyethylene glycol‐epoetin beta (Analysis 1.8.9 (1 study, 181 participants): OR 1.38, 95% CI 0.62 to 3.09)
The odds of hypertension were uncertain for darbepoetin alfa compared to methoxy polyethylene glycol‐epoetin beta (Analysis 1.8.10 (5 studies, 1497 participants): OR 0.94, 95% CI 0.62 to 1.42; I² = 36%) with evidence of moderate heterogeneity.
Vascular access thrombosis
ESAs compared to placebo
Data for effects of ESA treatment compared to placebo or no treatment on thrombosis of vascular access were provided in four studies and 4617 participants (Bahlmann 1991; Canadian EPO Study 1990; Klinkmann 1992; TREAT Study 2005). Three agents (epoetin alfa, epoetin beta and darbepoetin alfa) were assessed against placebo or no treatment. No study evaluated either methoxy polyethylene glycol‐epoetin beta or a biosimilar ESA with placebo or standard care.
The odds of vascular access thrombosis were uncertain for epoetin alfa (Analysis 1.9.1 (1 study, 118 participants): OR 6.40, 95% CI 0.80 to 51.50), epoetin beta (Analysis 1.9.2 (1 study, 99 participants): OR 1.09, 95% CI 0.28 to 4.34) and darbepoetin alfa (Analysis 1.9.3 (1 study, 4038 participants): OR 1.34, 95% CI 0.30 to 6.01) compared to placebo (Bahlmann 1991; Canadian EPO Study 1990; TREAT Study 2005)
The odds of vascular access thrombosis were uncertain for epoetin beta (Analysis 1.9.4 (1 study, 363 participants): OR 1.40, 95% CI 0.72 to 2.73) when compared to no treatment (Klinkmann 1992).
Analysis 1.9.
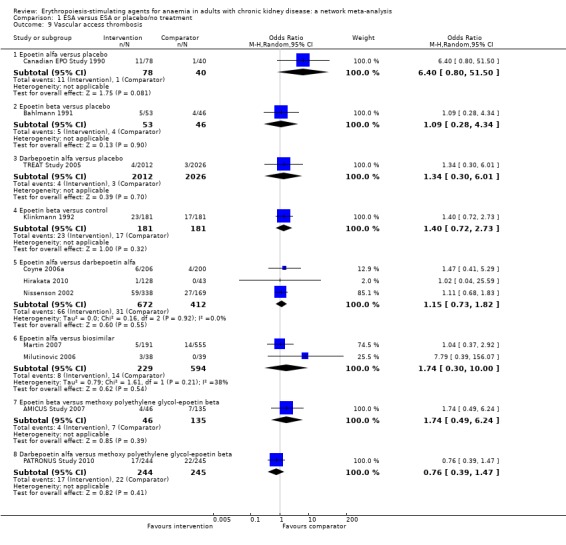
Comparison 1 ESA versus ESA or placebo/no treatment, Outcome 9 Vascular access thrombosis.
ESAs compared to each other
Epoetin alfa was compared to darbepoetin alfa in three studies and 1084 participants (Coyne 2006a; Hirakata 2010; Nissenson 2002) and a biosimilar ESA in two studies and 823 participants (Martin 2007; Milutinovic 2006). Epoetin beta was compared to methoxy polyethylene glycol‐epoetin beta in one study and 181 participants (AMICUS Study 2007). Darbepoetin alfa was compared to methoxy polyethylene glycol‐epoetin beta in one study and 489 participants (PATRONUS Study 2010).
The odds of vascular access thrombosis were uncertain for epoetin alfa when compared to darbepoetin alfa (Analysis 1.9.5 (3 studies, 1084 participants): OR 1.15, 95% CI 0.73 to 1.82; I² = 0%) or a biosimilar ESA (Analysis 1.9.6 (2 studies, 823 participants): OR 1.74, 95% CI 0.30 to 10.00; I² = 38%) with evidence of moderate heterogeneity
The odds of vascular access thrombosis were uncertain for epoetin beta when compared to methoxy polyethylene glycol‐epoetin beta (Analysis 1.9.7 (1 study, 181 participants): OR 1.74, 95% CI 0.49 to 6.24)
The odds of vascular access thrombosis were uncertain for darbepoetin alfa when compared to methoxy polyethylene glycol‐epoetin beta ((Analysis 1.9.8 (1 study, 489 participants): OR 0.76, 95% CI 0.39 to 1.47).
End‐stage kidney disease
ESAs compared to placebo
Data for effects of ESA treatment compared to placebo or no treatment on ESKD were provided in four studies and 4161 participants (Brown 1995; EPOCARES Study 2010; Kuriyama 1997; TREAT Study 2005). Three agents (epoetin alfa, epoetin beta and darbepoetin alfa) were assessed against placebo or no treatment. No study evaluated either methoxy polyethylene glycol‐epoetin beta or a biosimilar ESA with placebo or standard care.
The odds of ESKD were uncertain for darbepoetin alfa compared to placebo (Analysis 1.10.1 (1 study, 4038 participants): OR 1.04, 95% CI 0.88 to 1.23) (TREAT Study 2005)
The odds of ESKD were uncertain for epoetin alfa (Analysis 1.10.2 (1 study, 17 participants): OR 0.27, 95% CI 0.03 to 2.12) and epoetin beta (Analysis 1.10.3 (2 studies, 106 participants): OR 0.40, 95% CI 0.08 to 1.93; I² = 26%) (Brown 1995; EPOCARES Study 2010; Kuriyama 1997) when compared to no treatment with evidence of moderate heterogeneity.
Analysis 1.10.
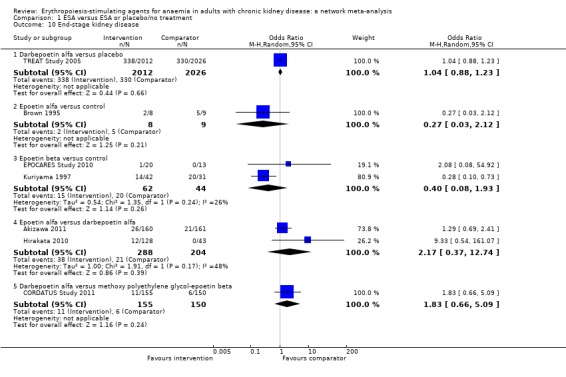
Comparison 1 ESA versus ESA or placebo/no treatment, Outcome 10 End‐stage kidney disease.
ESAs compared to each other
Epoetin alfa was compared to darbepoetin alfa in two studies and 492 participants (Akizawa 2011; Hirakata 2010). Darbepoetin alfa was compared to methoxy polyethylene glycol‐epoetin beta in one study and 305 participants (CORDATUS Study 2011).
The odds of ESKD were uncertain for epoetin alfa when compared to darbepoetin alfa (Analysis 1.10.4 (2 studies, 492 participants): OR 2.17, 95% CI 0.37 to 12.74; I² = 48%) with evidence of moderate heterogeneity
The odds of ESKD were uncertain for darbepoetin alfa when compared to methoxy polyethylene glycol‐epoetin beta (Analysis 1.10.5 (1 study, 305 participants): OR 1.83, 95% CI 0.66 to 5.09).
Major cardiovascular events
ESAs compared to placebo
Data for effects of ESA treatment compared to placebo or no treatment on major cardiovascular events were provided in three studies and 4228 participants (EPOCARES Study 2010; Patel 2012; TREAT Study 2005). Three agents (epoetin alfa, epoetin beta and darbepoetin alfa) were assessed against placebo or no treatment. No study evaluated either methoxy polyethylene glycol‐epoetin beta or a biosimilar ESA with placebo or standard care.
Darbepoetin alfa may increase odds of major cardiovascular events compared to placebo (Analysis 1.11.1 (1 study, 4038 participants): OR 1.08, 95% CI 0.95 to 1.24) (TREAT Study 2005)
Epoetin alfa (Analysis 1.11.2 (1 study, 157 participants): OR 2.40, 95% CI 0.29 to 20.11) and epoetin beta (Analysis 1.11.3 (1 study, 33 participants): OR 0.61, 95% CI 0.07 to 4.98) have uncertain effects on major cardiovascular events when compared to no treatment (EPOCARES Study 2010; Patel 2012).
Analysis 1.11.
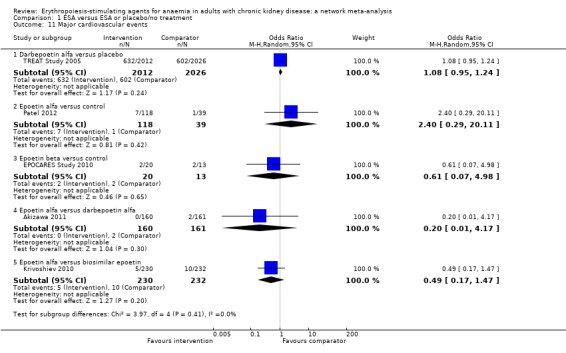
Comparison 1 ESA versus ESA or placebo/no treatment, Outcome 11 Major cardiovascular events.
ESAs compared to each other
Epoetin alfa was compared to darbepoetin alfa in one study and 321 participants (Akizawa 2011) and a biosimilar ESA in one study and 462 participants (Krivoshiev 2010).
The odds of a major cardiovascular event was uncertain for epoetin alfa when compared to darbepoetin alfa (Analysis 1.11.4 (1 study, 321 participants): OR 0.20, 95% CI 0.01 to 4.17) and a biosimilar ESA (Analysis 1.11.5 (1 study, 462 participants): OR 0.49, 95% CI 0.17 to 1.47).
2.2 Network meta‐analysis (combination of direct and indirect comparisons)
Treatment estimates for network meta‐analyses are shown in Table 3 and network meta‐analyses for all ESAs against placebo are summarised in Figure 6. Studies grouped by comparison were deemed comparable for the effect modifiers of stage of CKD, haemoglobin target with ESA treatment, age of participants and duration and follow‐up, such that the assumption of transitivity might hold and that a network meta‐analytical approach was reasonable. Overall, SUCRA rankings of the differing ESAs were imprecise due to sparse data rendering the analyses clinically irrelevant. Therefore, treatment rankings are not provided in the results.
All‐cause mortality
All‐cause mortality data were provided in 31 studies (Akizawa 2011; Allon 2002; AMICUS Study 2007; ARCTOS Study 2008; Bahlmann 1991; Bennett 1991; Chen 2012e; CORDATUS Study 2011; EPOCARES Study 2010; Gertz 2010; Goh 2007; Haag‐Weber 2009; Haag‐Weber 2012; Hori 2004; Klinkmann 1992; Krivoshiev 2008; Krivoshiev 2010; Kuriyama 1997; Li 2008d; Locatelli 2001; Milutinovic 2006; Nissenson 1995; Nissenson 2002; Palazzuoli 2007; Patel 2012; PATRONUS Study 2010; Roth 1994; Spinowitz 2006; STRIATA Study 2008; Tolman 2005; TREAT Study 2005) involving 11,024 participants with CKD (70.7% of the participants in this review). Most participants within the network were randomised to darbepoetin alfa or placebo due to the contribution of the large TREAT study (TREAT Study 2005). Effects of all ESA formulations on the odds of death from any cause were imprecise when compared with placebo or other ESA drug and were not statistically significant but there was considerable uncertainty in the comparative treatment effects. The heterogeneity tau for the network was 0.0, indicating no statistical evidence of heterogeneity.
Cardiovascular mortality
Cardiovascular mortality were provided in 14 studies (Akizawa 2011; ARCTOS Study 2008; Bahlmann 1991; Bennett 1991; CORDATUS Study 2011; EPOCARES Study 2010; Gertz 2010; Goh 2007; Haag‐Weber 2009; Klinkmann 1992; Kuriyama 1997; Locatelli 2001; STRIATA Study 2008; TREAT Study 2005) in 7138 participants (45.8% of the participants in this review). Effects of all ESA formulations on the odds of death caused by a cardiovascular event were imprecise when compared with placebo or other ESA drug and were not statistically significant but there was considerable uncertainty in the comparative treatment effects. The heterogeneity tau for the network was 0%, indicating no statistical evidence of heterogeneity.
Myocardial infarction
Nine studies provided data for one or more MI outcomes (Akizawa 2011; ARCTOS Study 2008; CORDATUS Study 2011; Kleinman 1989; Krivoshiev 2010; Nissenson 2002; Patel 2012; TREAT Study 2005) in 6303 participants (40.4% of the participants in this review). Effects of all ESA formulations on the odds of MI were imprecise when compared with placebo or other ESA drug and were not statistically significant but there was considerable uncertainty in the comparative treatment effects. The heterogeneity tau for the network was 0.0, indicating no statistical evidence of heterogeneity.
Stroke
There were 12 studies that provided data for one or more stroke events (Akizawa 2011; ARCTOS Study 2008; Bahlmann 1991; CORDATUS Study 2011; EPOCARES Study 2010; Goh 2007; Hirakata 2010; Krivoshiev 2010; Milutinovic 2006; Nissenson 2002; Patel 2012; TREAT Study 2005) in 6676 participants (42.8% of the participants in this review). Effects of all ESA formulations on the odds of stroke were imprecise when compared with placebo or other ESA drug and were not statistically significant except for the comparison between darbepoetin alfa and placebo (OR 1.96, 95% CI 1.40 to 2.75), but there was considerable uncertainty in the comparative treatment effects. The heterogeneity tau for the network was 0.0, indicating no statistical evidence of heterogeneity.
Hypertension
Hypertension data were provided in 24 studies (Akizawa 2011; AMICUS Study 2007; ARCTOS Study 2008; Bahlmann 1991; Bennett 1991; Canadian EPO Study 1990; Clyne 1992; CORDATUS Study 2011; Coyne 2006a; Goh 2007; Hirakata 2010; Klinkmann 1992; Krivoshiev 2010; Martin 2007; Milutinovic 2006; Nissenson 1995; Nissenson 2002; Patel 2012; PATRONUS Study 2010; STRIATA Study 2008; TIVOLI Study 2013; Tolman 2005; TREAT Study 2005) in 9930 participants (63.7% of participants in this review). All proprietary ESA drugs were significantly worse than placebo for the odds of inducing hypertension (epoetin alfa OR 2.31, 95% CI 1.27 to 4.23; epoetin beta OR 2.57, 95% CI 1.23 to 5.39; darbepoetin alfa OR 1.83, 95% CI 1.05 to 3.21) except for methoxy polyethylene glycol‐epoetin beta for which the treatment estimate was less precise and marginally included the possibility of no effect (OR 1.96, 95% CI 0.98 to 43.92). The effects on biosimilar ESAs on the odds of hypertension were less certain (OR 1.18, 95% CI 0.47 to 2.99). The heterogeneity tau for the network was 0.37, consistent with low heterogeneity.
End‐stage kidney disease
The network for this outcome provided no closed loops of evidence (Figure 5) and conventional pairwise meta‐analysis was the primary source of evidence for this outcome, showing generally imprecise estimates of comparative treatment effects.
Vascular access thrombosis
Eleven studies provided data for one or more episodes of vascular access thrombus (AMICUS Study 2007; Bahlmann 1991; Canadian EPO Study 1990; Coyne 2006a; Hirakata 2010; Klinkmann 1992; Martin 2007; Milutinovic 2006; Nissenson 2002; PATRONUS Study 2010; TREAT Study 2005) in 7194 participants (46.1% of the participants in this review). Effects of all ESA formulations on the odds of vascular access thrombosis were imprecise when compared with placebo or other ESA drug and were not statistically significant but there was considerable uncertainty in the comparative treatment effects. The heterogeneity tau for the network was 0.0, indicating no statistical evidence of heterogeneity.
Major cardiovascular events
The network for this outcome provided no closed loops of evidence (Figure 5) and conventional pairwise meta‐analysis was the primary source of evidence for this outcome, showing generally imprecise estimates of comparative treatment effects.
3. Assessment of heterogeneity and inconsistency within network analyses
There was important clinical diversity in studies based on the age of the participants, stage of CKD and duration of treatment. Treatment estimates from direct and indirect evidence in networks with closed loops did not show evidence of statistical inconsistency except for three of the five loops for hypertension (Table 4). However, the results for inconsistency were very imprecise as individual direct and indirect estimates were themselves imprecise and so the possibility of inconsistency in network analyses for other outcomes could not be excluded. When comparing a common heterogeneity variance in networks and with empirical distributions of heterogeneity variances specific to the outcome and types of treatment being compared, networks for blood transfusion (τ = 0.89) and hypertension (τ = 0.37) possessed heterogeneity variances that indicated the presence of low to moderate heterogeneity. Similarly, when evaluating the inconsistency in the networks as a whole, there was an indication that global inconsistency was present within the networks for blood transfusion (Chi² = 6.38; P = 0.01) and hypertension (Chi² = 6.40; P = 0.04). We therefore downgraded the credibility of the evidence provided by these two networks as the risk of important inconsistency was high. Meta‐regression to explore potential sources of inconsistency was not possible due to sparse data for direct treatment comparisons.
Table 2.
Evaluation of consistency using loop specific approach
| Treatments included in the loop of evidence | Inconsistency factor* | 95% CI |
| All‐cause mortality | ||
| Epoetin alfa – epoetin beta – darbepoetin alfa – biosimilar ESA | 0.87 | 0.00‐3.32 |
| Epoetin beta – darbepoetin alfa – placebo | 0.40 | 0.00‐1.82 |
| Epoetin alfa – epoetin beta – biosimilar ESA – no treatment | 0.66 | 0.00‐3.36 |
| Epoetin beta – darbepoetin alfa – methoxy polyethylene glycol‐epoetin beta | 0.02 | 0.00‐2.08 |
| Epoetin alfa – epoetin beta – biosimilar ESA – placebo | 0.64 | 0.00‐3.99 |
| Epoetin alfa – darbepoetin alfa – placebo | 0.17 | 0.00‐2.17 |
| Epoetin alfa – epoetin beta – darbepoetin alfa – no treatment | 0.16 | 0.00‐1.58 |
| Epoetin alfa – epoetin beta – placebo – no treatment | 0.07 | 0.00‐2.54 |
| Transfusion | ||
| Epoetin alfa – epoetin beta – placebo – no treatment | 2.09 | 0.00‐6.91 |
| Epoetin alfa – darbepoetin alfa – placebo | 1.97 | 0.00‐4.20 |
| Epoetin beta – darbepoetin alfa ‐ methoxy polyethylene glycol‐epoetin beta ‐ placebo | 1.26 | 0.00‐3.39 |
| Myocardial infarction | ||
| Epoetin alfa – darbepoetin alfa – placebo | 1.13 | 0.00‐4.37 |
| Hypertension | ||
| Epoetin alfa – darbepoetin alfa – placebo | 1.55 | 0.26‐2.84 |
| Epoetin alfa – epoetin beta – darbepoetin alfa – no treatment | 2.03 | 0.00‐4.66 |
| Epoetin beta – darbepoetin alfa – placebo | 1.56 | 0.73‐2.38 |
| Epoetin alfa – epoetin beta – placebo – no treatment | 2.15 | 0.00‐4.91 |
| Epoetin beta – darbepoetin alfa – methoxy polyethylene glycol‐epoetin beta | 2.49 | 0.76‐4.22 |
| Vascular access thrombosis | ||
| Epoetin beta – darbepoetin alfa – placebo | 1.32 | 0.00‐3.86 |
| Epoetin beta – darbepoetin alfa – methoxy polyethylene glycol‐epoetin beta – placebo | 0.98 | 0.00‐3.35 |
*The inconsistency factor is the absolute difference in the log odds ratio estimated from indirect and direct treatment comparisons and is reported together with the 95% confidence interval. A 95% confidence interval that includes zero indicates that the result is compatible with zero inconsistency between effect estimates using indirect (network meta‐analysis) and direct (conventional pairwise meta‐analysis) treatment comparisons. We used the'ifplot' command in STATA to estimate inconsistency (Chaimani 2013) allowing for all comparisons within a loop to share a common heterogeneity variance
4. Subgroup and sensitivity analyses
Subgroup and meta‐regression analyses to explore potential sources of heterogeneity and inconsistency in networks were precluded by sparse data for direct treatment comparisons (four studies or fewer for all comparisons). Differences in treatment estimates between studies and between direct and indirect evidence in network analyses may have been due to differing prescribing approaches, changing use of ESA across time, differing policies for blood transfusions and outcome adjudication and differing stages of CKD in contributing studies.
5. Grading of the evidence
When grading our confidence in the evidence using the methods of Del Giovane 2012, we first generated contribution matrices for the networks providing evidence for the primary outcomes (preventing blood transfusions (Figure 7) and all‐cause mortality (Figure 8)). In these matrices, the size of each square is proportional to the weight attached to each direct summary effect (horizontal axis) for the estimation of each network summary effect (vertical axis) with the numbers re‐expressing weights as percentages. We then evaluated risk of bias assessments for treatment comparisons obtained in network meta‐analyses proportional to study contributions (Figure 9 for the outcome of preventing blood transfusions and Figure 10 for the outcome of all‐cause mortality). We then considered the overall study limitations obtained from risk of bias assessments, imprecision in estimated treatment effects and inconsistency within networks.
Figure 7.
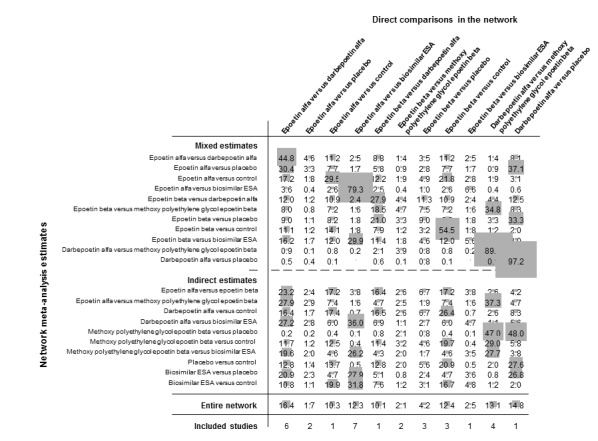
Contributions matrix: percentage contribution of each direct estimate to the network meta‐analysis estimates for all‐cause mortality. Rows correspond to network meta‐analysis odds ratios (separated for mixed and indirect evidence) and the columns correspond to direct meta‐analysis odds ratios. The size of the shaded boxes are proportional to the percentage contribution of each direct estimate to the network meta‐analysis and to the entire network (lowest row). The last row shows the number of included direct comparisons. The names of the treatment comparisons are shown in the first column. For example, information for the network estimate of epoetin alfa versus darbepoetin alfa is derived from both direct and indirect evidence (generating a mixed estimate. Of this mixed network estimate, trials directly comparing epoetin alfa versus darbepoetin alfa contribute 44.8 % of the information to the network estimate of effect and trials directly comparing epoetin alfa versus placebo contribute 4.6% of the network estimated effect, etc. We used the 'netweight' command in STATA to generate the plot. The contribution matrix shows how much each direct comparison in the network contributes to each network (mixed or indirect) estimate
Figure 8.
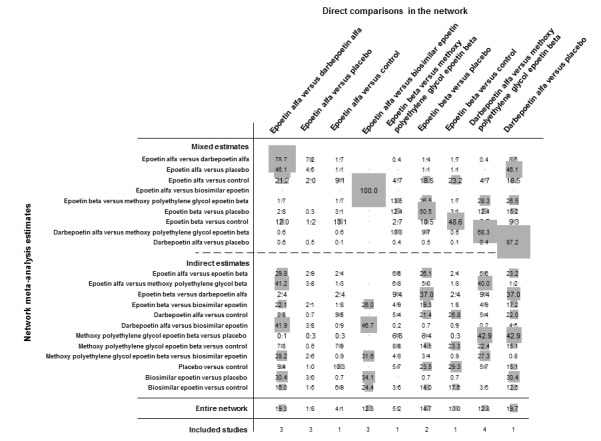
Contributions matrix: percentage contribution of each direct estimate to the network meta‐analysis estimates for preventing blood transfusions. Rows correspond to network meta‐analysis odds ratios (separated for mixed and indirect evidence) and the columns correspond to direct meta‐analysis odds ratios. The size of the shaded boxes are proportional to the percentage contribution of each direct estimate to the network meta‐analysis and to the entire network (lowest row). The last row shows the number of included direct comparisons. The names of the treatment comparisons are shown in the first column. For example, information for the network estimate of epoetin alfa versus darbepoetin alfa is derived from both direct and indirect evidence (generating a mixed estimate. Of this mixed network estimate, trials directly comparing epoetin alfa versus darbepoetin alfa contribute 78.7% of the information to the network estimate of effect and trials directly comparing epoetin alfa versus placebo contribute 7.2% of the network estimated effect, etc. We used the 'netweight' command in STATA to generate the plot. The contribution matrix shows how much each direct comparison in the network contributes to each network (mixed or indirect) estimate
Figure 9.
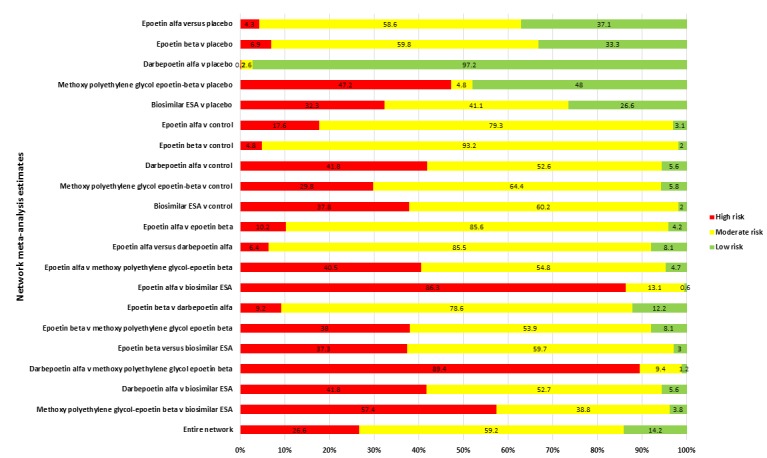
Study limitations distribution for each network estimate for pairwise comparisons of erythropoiesis‐stimulating agents on the primary safety outcome (all‐cause mortality). Calculations are based on the contributions of direct evidence to the network estimates and the overall risks of bias from all bias domains (sequence generation, allocation concealment, blinding of participants and investigators, blinding of outcome assessment, attrition from follow‐up, selective outcome reporting and other sources of bias) within studies contributing to the direct evidence. The colours represent risk (green, low; yellow unclear; red, high). The direct comparisons are described in the vertical axis
Figure 10.
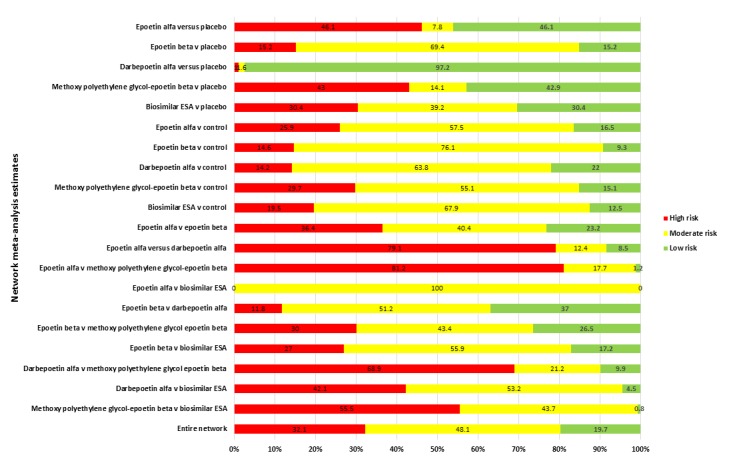
Study limitations distribution for each network estimate for pairwise comparisons of erythropoiesis‐stimulating agents on the primary efficacy outcome (preventing blood transfusions). Calculations are based on the contributions of direct evidence to the network estimates and the overall risks of bias from all bias domains (sequence generation, allocation concealment, blinding of participants and investigators, blinding of outcome assessment, attrition from follow‐up, selective outcome reporting and other sources of bias) within studies contributing to the direct evidence. The colours represent risk (green, low; yellow unclear; red, high). The direct comparisons are described in the vertical axis
For each comparison, the quality of the evidence for preventing blood transfusions and all‐cause mortality was frequently downgraded from high‐quality due to important study limitations (Table 1). Our confidence in the treatment estimates was generally moderate or low for comparisons of ESAs against placebo and was low or very low quality particularly for direct comparisons between two ESAs.
Discussion
Summary of main results
Clinical guidelines recommend ESA treatment to avoid blood transfusions and anaemia‐related symptoms for patients with CKD (NICE 2011; KDIGO 2013). However, whether all the available ESAs are equally effective and safe has not been adequately evaluated by individual RCTs and is central to informed patient choices and rational pharmaceutical policy. To date, no studies have compared methoxy polyethylene glycol‐epoetin beta or biosimilar ESAs directly against placebo or have provided head‐to‐head comparisons for darbepoetin alfa and methoxy‐polyethylene glycol‐epoetin beta. This review of the effects of ESA treatment for anaemia in CKD included 56 studies involving 15,596 randomised adult participants and provides the first evidence for the comparative efficacy and safety of all ESAs in the setting of CKD.
Network meta‐analysis showed that all proprietary ESAs (epoetin alfa, epoetin beta, darbepoetin alfa, and methoxy polyethylene glycol‐epoetin beta) prevented blood transfusions compared to placebo with ORs ranging between 0.09 and 0.18. Our confidence in these beneficial effects was considered generally moderate or low using the GRADE approach. The efficacy of biosimilar ESAs for preventing blood transfusions was less certain and included the possibility of no effect; our confidence in this evidence was very low. The estimated treatment effects of the differing ESAs in head‐to‐head comparisons on preventing blood transfusions were imprecise and we could not be sure whether any of the formulations were similar or different for their effects on this outcome. The comparative effectiveness of different ESA formulations against each other or placebo on other potentially beneficial effects of therapy (such as reductions in fatigue and breathlessness) was inconclusive due to sparse data and the inconsistent methods used to report these outcomes in existing studies.
Although ESAs as a drug class are known to increase the odds of vascular and mortality outcomes when used to target higher haemoglobin levels (Palmer 2010; Phrommintikul 2007), the comparative safety of the available agents against each other and placebo is uncertain. All proprietary ESAs (epoetin alfa, epoetin beta, darbepoetin alfa, and methoxy polyethylene glycol‐epoetin beta) increased the odds of hypertension to a similar extent when compared with placebo, but estimated effects for biosimilar ESAs were much less precise. Greater precision in estimates for darbepoetin alfa (due to the availability of data from the large TREAT Study 2005 which provided 26% of all participants included in this review) suggested that darbepoetin alfa increased the odds of stroke compared to placebo but there was no clear evidence that this or other potential harms of darbepoetin alfa differed from those of other ESA derivatives. We could not discern any differences in the differing ESAs compared with placebo or each other for their effects on all‐cause mortality, cardiovascular mortality, myocardial infarction or vascular access thrombosis either in direct evidence or direct and indirect treatment comparisons in network analyses. Networks of evidence for the outcomes of end‐stage kidney disease and major cardiovascular events did not provide any closed loops of evidence; information from direct comparisons in available studies were uninformative and we could not identify whether ESAs differed from each other with regards to these outcomes.
The risks of bias in the contributing studies and imprecise treatment estimates limited our overall confidence in the results and inconsistency between direct and indirect evidence in analyses for preventing blood transfusion and hypertension reduced the credibility of treatment estimates derived by network analysis. Evidence from network meta‐analyses comparing ESA formulations against placebo were generally moderate or low quality while treatment estimates for head‐to‐head comparisons between ESAs were down‐graded to generally very low quality. Thus, presently there is no evidence for a preferred ESA to treat anaemia in CKD based on considerations of efficacy or safety including for biosimilar ESA preparations
Overall completeness and applicability of evidence
We included all eligible studies to February 2014 of ESA formulations that are currently available for the treatment of anaemia in CKD, but we did not include other potential interventions for anaemia in this setting such as iron supplementation. The information presented in this review is derived primarily from studies of epoetin alfa and darbepoetin alfa which contributed to outcome data for 5290 participants (33.9%) in the network of evidence for all‐cause mortality and 4749 participants (30.5%) in the network of evidence for preventing blood transfusions, due to a large contribution from the placebo‐controlled RCT of darbepoetin alfa which contributed 4038 participants (TREAT Study 2005). Data for biosimilar ESAs were limited to nine studies providing 2709 (17.4%) of participants in the meta‐analyses. Due to the few studies and data for biosimilar ESAs, we elected to combine information for all the different non‐proprietary ESAs studied (alfa, delta, omega, theta, zeta), but recognise that these agents may have important clinical and biological differences in their effects. In addition, selective outcome reporting reduced our confidence in the estimated treatment effects.
Participants involved in the meta‐analyses were nearly equally distributed between those treated with dialysis or those with milder forms of CKD, however participants who were recipients of a kidney transplant for treatment of end‐stage kidney disease were rarely involved in contributing studies and the findings of this review may not be directly applicable to this clinical setting. In addition, it was unclear how many participants in studies in dialysis were conducted in the setting of peritoneal dialysis.
While we aimed to incorporate many patient‐important outcomes including symptoms of anaemia such as breathlessness and fatigue, other relevant outcomes for participants were not included (such as health‐related quality of life) as these are infrequently reported in anaemia studies in CKD and are frequently at risk of selective reporting (Clement 2009). In addition, the core outcomes of greatest importance to clinicians and patients in the management of CKD remain poorly explored, in comparison to other clinical specialities (such as rheumatology (the OMERACT (Outcome Measures in Rheumatology) initiative; www.omeract.org/)), and as such we could not align our review with the outcomes considered most important to consumers, health professionals and other stakeholders. We deliberately did not include analysis of treatment effects on haemoglobin levels as this is a surrogate outcome that adds little if anything to our understanding of clinical outcomes.
Quality of the evidence
Risks of bias
Risks of bias in the included studies was generally high or unclear for more than half of studies in all of the risk of bias domains we assessed, limiting our confidence in the estimated treatment effects from these data. No study was low risk for allocation concealment, blinding of outcome assessment and attrition from follow‐up. Allocation concealment, in which investigators are unaware of the treatment allocation for individual participants, was reported using low risk methods in only 10 (18%) studies. Blinding of outcome assessment was clearly documented as low risk in two studies (4%), and differences in haemoglobin levels between groups made it likely that investigators were aware of the treatment allocation in the remaining placebo‐ or no treatment‐controlled studies. Follow‐up data was incomplete (for more than 10% of randomised participants and/or markedly discrepant between treatment arms) in 31 (55%) studies and unclearly documented in a further 18 (32%) studies.
Heterogeneity
Evidence for moderate to substantial heterogeneity was present for many pairwise meta‐analysis beyond that expected from random variation; however, analyses lacked power for subgroup or meta‐regression analyses due to the small number of studies (≤ four studies) in these meta‐analyses. There were substantial differences in the treatment effects in studies comparing epoetin alfa with placebo on blood transfusion that may have related to the characteristics of the patient populations or transfusion policies within the studies. Evidence of moderate heterogeneity was common in treatment effects estimated from studies comparing darbepoetin with methoxy polyethylene glycol‐epoetin beta, which limited our confidence in estimated treatment effects for myocardial infarction and hypertension. In addition, moderate to substantial heterogeneity in treatment estimates for epoetin alfa compared with darbepoetin alfa were present in analyses for all‐cause mortality, hypertension and end‐stage kidney disease.
Inconsistency
Notably, there was important clinical diversity in the included studies and evidence of inconsistency between treatment effects estimated from direct evidence (within head‐to‐head studies) and mixed evidence (from both direct and indirect evidence) generated using network analyses for the outcomes of blood transfusion and hypertension. Our confidence in the results obtained from network meta‐analysis was reduced by these differences. Importantly, as data were generally sparse, our ability to ascertain evidence of inconsistency was relatively low, and important inconsistency within analyses could not be excluded.
Potential biases in the review process
While this review was prepared using a sensitive electronic search strategy to identify eligible studies, was conducted according to a prespecified protocol and is reported using Cochrane Collaboration methods, the review has limitations which should be considered when interpreting the results. First, relatively few data were available for most comparisons resulting in inconclusive evidence for many outcomes including cardiovascular events, anaemia‐related symptoms, and end‐stage kidney disease. Second, while the included studies appeared similar in their treatment approaches to anaemia, there was evidence of heterogeneity in treatment effects estimated by individual studies and inconsistency between direct and indirect evidence that reduced the credibility of estimated treatment safety and efficacy which could not be explored meaningfully using subgroup or meta‐regression analyses. Third, data for a single study (the TREAT Study 2005 study) dominated many of the analyses and most studies had high or unclear risks of bias for key domains. Finally, outcome data for patient‐important outcomes were not available in most studies or were reported ad hoc and therefore reduced our confidence in the reliability of these treatment effects. This was particularly the case for reporting of quality of life domains such as fatigue; the reporting of this outcome did not allow many data to be included in analyses.
In addition, we originally included network analyses for the effects of higher and lower haemoglobin targets with ESAs in the peer‐reviewed protocol of this review. As these comparisons are adequately addressed by existing meta‐analyses, and network analyses are unlikely to provide more information for these comparisons than conventional pairwise meta‐analyses and hinder the readability and usefulness of this review, we have not included the results of these networks in the final version of this review.
Agreements and disagreements with other studies or reviews
To our knowledge, this is the first systematic review directly comparing different ESA preparations against each other or placebo in the setting of kidney disease and is the first to evaluate the evidence for biosimilar ESA formulations against proprietary ESAs and placebo. Previous systematic reviews of ESA drugs to treat anaemia in CKD have largely focused on treatment strategies that compare higher with lower haemoglobin targets using the same ESA or ESAs against placebo or no treatment (Palmer 2010; Phrommintikul 2007; Strippoli 2006) which have generally shown increased cardiovascular events and mortality in patients who have been prescribed ESA treatment to achieve a higher haemoglobin target. The findings in this review are similar to an earlier Cochrane meta‐analysis comparing darbepoetin alfa against placebo or other ESAs which found that darbepoetin alfa reduced transfusion without showing beneficial effects on mortality or quality of life, while the treatment effects of darbepoetin alfa compared to other ESAs were uncertain (Palmer 2014).
Our findings agree with a review of RCTs comparing the efficacy and safety of epoetin (alfa or beta) with darbepoetin alfa in patients undergoing cancer treatment (AHRQ 2006). In analyses of head‐to‐head studies comparing epoetin with darbepoetin (7 studies, 1415 participants), epoetin versus placebo or no treatment (48 studies, 4518 participants) and darbepoetin versus placebo or no treatment (4 studies, 598 participants), those authors found that there was no statistical difference between epoetin alfa or beta and darbepoetin alfa on need for blood transfusion or risk of thromboembolic events and that both epoetin alfa or beta and darbepoetin alfa were better than placebo or no treatment at preventing blood transfusions, although many analyses showed evidence of important heterogeneity. Similar to our analyses, the authors reported that many studies were not designed to evaluate survival and that reporting of quality of life outcomes was frequently unusable for analyses. In an updated report dated 2013, treatment effects for darbepoetin alfa compared to epoetin alfa or beta on preventing blood transfusions, on‐study mortality, and thromboembolic events remained inconclusive and information for quality of life was assessed as low quality (AHRQ 2013).
While a study of the comparative effectiveness of IV iron preparations on patient outcomes for adults who have ESKD is ongoing, as this is a non‐randomised analysis, it is unlikely that treatment effects will be sufficiently free of confounding by treatment indication and other patient and health services related characteristics to inform clinical practice and policy (Boulware 2012) and does not address the use of ESAs.
Authors' conclusions
Our review includes direct and indirect comparisons of ESAs for anaemia in CKD and is currently the best available evidence for consumers and health professionals on the relative safety and efficacy of differing ESA prescribing patterns. On the basis of moderate to low quality evidence, epoetin alfa, epoetin beta, darbepoetin alfa and methoxy polyethylene glycol‐epoetin beta are all superior to placebo for preventing blood transfusions. It is unclear whether ESA formulations have similar or different efficacy for patient‐centred benefits including blood transfusions, fatigue and breathlessness in very low quality data.
There are presently insufficient high quality data for a definitive statement on whether differing ESAs differ from placebo or each other for their effects on mortality and cardiovascular outcomes including stroke, myocardial infarction or death due to a cardiovascular cause.
In general, data for biosimilar ESA formulations are sparse and very low quality, and are not suitable to inform patients and health providers about the balance of their benefits and risks.
As seen in earlier reviews, reporting of treatment effects of ESAs on potentially patient‐important outcomes is infrequent and heterogeneous, precluding a robust understanding of the effects of ESA therapy on the way patients feel and function. Given the inconclusive effects of the differing ESAs on quality of life and survival, decisions about different agents in clinical practice and policy might be based on drug cost and availability and patient preferences for treatment frequency until additional data become available.
We believe there is a key need that the research agenda should address. Large RCTs of ESAs on patients‐centred outcomes that are considered most relevant to patients and health services should be undertaken using consistent methods for reporting of outcomes and consideration of clinically important benefits for these drugs. Currently, given the lack of evidence for treatment benefits from ESA therapy (Clement 2009; Phrommintikul 2007) studies of treatment effects on health‐related quality of life and key symptoms of anaemia and advanced kidney disease are required before widespread ongoing use of these agents can be justified. Additional work to identify core research outcomes that are priorities for consumers and health providers would inform the design of future studies for treatment of anaemia of CKD to increase their research relevance to health and clinical practice.
Acknowledgements
We wish to acknowledge the support of the editorial office at the Cochrane Renal Group. In particular we wish to thank Narelle Willis and Ann Jones. We are also very grateful to our specialist Trials Search Coordinator, Ruth Mitchell. We wish to thank the referees for their comments and feedback during the preparation of this review.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimisation (minimisation may be implemented without a random element, and this is considered to be equivalent to being random) |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention | |
| Unclear: Insufficient information about the sequence generation process to permit judgement | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes) |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure | |
| Unclear: Randomisation stated but no information on method used is available | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon) |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias |
Data and analyses
Comparison 1.
ESA versus ESA or placebo/no treatment
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Blood transfusion | 19 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Epoetin alfa versus placebo | 3 | 196 | Odds Ratio (M‐H, Random, 95% CI) | 0.07 [0.01, 0.84] |
| 1.2 Epoetin beta versus placebo | 2 | 230 | Odds Ratio (M‐H, Random, 95% CI) | 0.07 [0.03, 0.21] |
| 1.3 Darbepoetin alfa versus placebo | 1 | 4038 | Odds Ratio (M‐H, Random, 95% CI) | 0.53 [0.46, 0.63] |
| 1.4 Epoetin alfa versus control | 1 | 157 | Odds Ratio (M‐H, Random, 95% CI) | 3.10 [0.16, 58.97] |
| 1.5 Epoetin beta versus no control | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.35 [0.06, 2.18] |
| 1.6 Epoetin alfa versus darbepoetin alfa | 3 | 1191 | Odds Ratio (M‐H, Random, 95% CI) | 2.31 [1.34, 3.97] |
| 1.7 Epoetin alfa versus biosimilar ESA | 3 | 1823 | Odds Ratio (M‐H, Random, 95% CI) | 0.72 [0.42, 1.22] |
| 1.8 Epoetin beta versus methoxy polyethylene glycol‐epoetin beta | 1 | 181 | Odds Ratio (M‐H, Random, 95% CI) | 0.83 [0.17, 4.15] |
| 1.9 Darbepoetin alfa versus methoxy polyethylene glycol‐epoetin beta | 4 | 1191 | Odds Ratio (M‐H, Random, 95% CI) | 0.94 [0.45, 1.95] |
| 2 Fatigue | 3 | Odds Ratio (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Epoetin alfa versus darbepoetin alfa | 2 | 551 | Odds Ratio (IV, Random, 95% CI) | 0.94 [0.57, 1.55] |
| 2.2 Epoetin alfa v biosimilar ESA | 1 | 179 | Odds Ratio (IV, Random, 95% CI) | 0.18 [0.01, 3.91] |
| 3 Breathlessness | 3 | Odds Ratio (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Epoetin alfa versus darbepoetin alfa | 1 | 504 | Odds Ratio (IV, Random, 95% CI) | 0.71 [0.46, 1.10] |
| 3.2 Epoetin alfa versus biosimilar ESA | 2 | 794 | Odds Ratio (IV, Random, 95% CI) | 0.68 [0.37, 1.25] |
| 4 All‐cause mortality | 31 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Epoetin alfa versus placebo | 2 | 235 | Odds Ratio (M‐H, Random, 95% CI) | 0.99 [0.14, 6.86] |
| 4.2 Epoetin beta versus placebo | 3 | 311 | Odds Ratio (M‐H, Random, 95% CI) | 0.61 [0.17, 2.15] |
| 4.3 Darbepoetin alfa versus placebo | 1 | 4038 | Odds Ratio (M‐H, Random, 95% CI) | 1.06 [0.91, 1.24] |
| 4.4 Epoetin alfa versus control | 1 | 157 | Odds Ratio (M‐H, Random, 95% CI) | 1.06 [0.39, 2.87] |
| 4.5 Epoetin beta versus control | 3 | 468 | Odds Ratio (M‐H, Random, 95% CI) | 0.69 [0.36, 1.33] |
| 4.6 Epoetin alfa versus darbepoetin alfa | 6 | 1205 | Odds Ratio (M‐H, Random, 95% CI) | 1.12 [0.59, 2.14] |
| 4.7 Epoetin alfa versus biosimilar ESA | 7 | 2220 | Odds Ratio (M‐H, Random, 95% CI) | 1.04 [0.53, 2.01] |
| 4.8 Epoetin beta versus darbepoetin alfa | 1 | 217 | Odds Ratio (M‐H, Random, 95% CI) | 0.89 [0.38, 2.09] |
| 4.9 Epoetin beta versus methoxy polyethylene glycol‐epoetin beta | 2 | 462 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.12, 5.35] |
| 4.10 Epoetin beta versus biosimilar ESA | 1 | 290 | Odds Ratio (M‐H, Random, 95% CI) | 0.34 [0.04, 2.82] |
| 4.11 Darbepoetin alfa versus methoxy polyethylene glycol‐epoetin beta | 4 | 1429 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.59, 1.40] |
| 5 Cardiovascular mortality | 14 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Epoetin beta versus placebo | 2 | 260 | Odds Ratio (M‐H, Random, 95% CI) | 0.45 [0.06, 3.75] |
| 5.2 Darbepoetin alfa versus placebo | 1 | 4038 | Odds Ratio (M‐H, Random, 95% CI) | 1.05 [0.87, 1.26] |
| 5.3 Epoetin beta versus no treatment | 3 | 430 | Odds Ratio (M‐H, Random, 95% CI) | 0.28 [0.08, 1.03] |
| 5.4 Epoetin alfa versus darbepoetin alfa | 2 | 487 | Odds Ratio (M‐H, Random, 95% CI) | 2.15 [0.31, 14.91] |
| 5.5 Epoetin alfa versus biosimilar ESA | 2 | 657 | Odds Ratio (M‐H, Random, 95% CI) | 0.53 [0.20, 1.35] |
| 5.6 Epoetin beta versus biosimilar ESA | 1 | 290 | Odds Ratio (M‐H, Random, 95% CI) | 0.34 [0.04, 2.82] |
| 5.7 Darbepoetin alfa versus methoxy polyethylene glycol‐epoetin beta | 3 | 938 | Odds Ratio (M‐H, Random, 95% CI) | 0.69 [0.32, 1.48] |
| 6 Myocardial infarction | 9 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Epoetin alfa versus placebo | 1 | 14 | Odds Ratio (M‐H, Random, 95% CI) | 3.46 [0.12, 100.51] |
| 6.2 Darbepoetin alfa versus placebo | 1 | 4038 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.75, 1.25] |
| 6.3 Epoetin alfa versus no treatment | 1 | 157 | Odds Ratio (M‐H, Random, 95% CI) | 1.01 [0.04, 25.26] |
| 6.4 Epoetin alfa versus darbepoetin alfa | 2 | 825 | Odds Ratio (M‐H, Random, 95% CI) | 0.87 [0.20, 3.81] |
| 6.5 Epoetin alfa versus biosimilar | 2 | 641 | Odds Ratio (M‐H, Random, 95% CI) | 1.23 [0.49, 3.12] |
| 6.6 Darbepoetin alfa versus methoxy polyethylene glycol‐epoetin beta | 2 | 628 | Odds Ratio (M‐H, Random, 95% CI) | 0.47 [0.06, 3.65] |
| 7 Stroke | 12 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Epoetin beta versus placebo | 1 | 106 | Odds Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.21] |
| 7.2 Darbepoetin alfa versus placebo | 1 | 4038 | Odds Ratio (M‐H, Random, 95% CI) | 1.97 [1.40, 2.76] |
| 7.3 Epoetin alfa versus control | 1 | 157 | Odds Ratio (M‐H, Random, 95% CI) | 0.99 [0.10, 9.82] |
| 7.4 Epoetin beta versus control | 1 | 33 | Odds Ratio (M‐H, Random, 95% CI) | 0.20 [0.01, 5.39] |
| 7.5 Epoetin alfa versus darbepoetin alfa | 3 | 996 | Odds Ratio (M‐H, Random, 95% CI) | 1.44 [0.37, 5.54] |
| 7.6 Epoetin alfa versus biosimilar ESA | 3 | 718 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.39, 2.15] |
| 7.7 Darbepoetin alfa versus methoxy polyethylene glycol‐epoetin beta | 2 | 628 | Odds Ratio (M‐H, Random, 95% CI) | 1.33 [0.17, 10.49] |
| 8 Hypertension | 24 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Epoetin alfa versus placebo | 2 | 251 | Odds Ratio (M‐H, Random, 95% CI) | 4.10 [2.16, 7.76] |
| 8.2 Epoetin beta versus placebo | 2 | 230 | Odds Ratio (M‐H, Random, 95% CI) | 2.95 [1.19, 7.26] |
| 8.3 Darbepoetin alfa versus placebo | 1 | 4038 | Odds Ratio (M‐H, Random, 95% CI) | 1.14 [0.99, 1.32] |
| 8.4 Epoetin alfa versus control | 1 | 157 | Odds Ratio (M‐H, Random, 95% CI) | 5.31 [0.30, 95.20] |
| 8.5 Epoetin beta versus no treatment | 2 | 382 | Odds Ratio (M‐H, Random, 95% CI) | 2.99 [1.34, 6.69] |
| 8.6 Epoetin alfa versus darbepoetin alfa | 5 | 1568 | Odds Ratio (M‐H, Random, 95% CI) | 0.94 [0.62, 1.43] |
| 8.7 Epoetin alfa versus biosimilar ESA | 4 | 1464 | Odds Ratio (M‐H, Random, 95% CI) | 1.77 [1.02, 3.09] |
| 8.8 Epoetin beta versus darbepoetin alfa | 1 | 162 | Odds Ratio (M‐H, Random, 95% CI) | 1.18 [0.38, 3.69] |
| 8.9 Epoetin beta versus methoxy polyethylene glycol‐epoetin beta | 1 | 181 | Odds Ratio (M‐H, Random, 95% CI) | 1.38 [0.62, 3.09] |
| 8.10 Darbepoetin alfa versus methoxy polyethylene glycol‐epoetin beta | 5 | 1497 | Odds Ratio (M‐H, Random, 95% CI) | 0.94 [0.62, 1.42] |
| 9 Vascular access thrombosis | 11 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Epoetin alfa versus placebo | 1 | 118 | Odds Ratio (M‐H, Random, 95% CI) | 6.40 [0.80, 51.50] |
| 9.2 Epoetin beta versus placebo | 1 | 99 | Odds Ratio (M‐H, Random, 95% CI) | 1.09 [0.28, 4.34] |
| 9.3 Darbepoetin alfa versus placebo | 1 | 4038 | Odds Ratio (M‐H, Random, 95% CI) | 1.34 [0.30, 6.01] |
| 9.4 Epoetin beta versus control | 1 | 362 | Odds Ratio (M‐H, Random, 95% CI) | 1.40 [0.72, 2.73] |
| 9.5 Epoetin alfa versus darbepoetin alfa | 3 | 1084 | Odds Ratio (M‐H, Random, 95% CI) | 1.15 [0.73, 1.82] |
| 9.6 Epoetin alfa versus biosimilar | 2 | 823 | Odds Ratio (M‐H, Random, 95% CI) | 1.74 [0.30, 10.00] |
| 9.7 Epoetin beta versus methoxy polyethylene glycol‐epoetin beta | 1 | 181 | Odds Ratio (M‐H, Random, 95% CI) | 1.74 [0.49, 6.24] |
| 9.8 Darbepoetin alfa versus methoxy polyethylene glycol‐epoetin beta | 1 | 489 | Odds Ratio (M‐H, Random, 95% CI) | 0.76 [0.39, 1.47] |
| 10 End‐stage kidney disease | 7 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 Darbepoetin alfa versus placebo | 1 | 4038 | Odds Ratio (M‐H, Random, 95% CI) | 1.04 [0.88, 1.23] |
| 10.2 Epoetin alfa versus control | 1 | 17 | Odds Ratio (M‐H, Random, 95% CI) | 0.27 [0.03, 2.12] |
| 10.3 Epoetin beta versus control | 2 | 106 | Odds Ratio (M‐H, Random, 95% CI) | 0.40 [0.08, 1.93] |
| 10.4 Epoetin alfa versus darbepoetin alfa | 2 | 492 | Odds Ratio (M‐H, Random, 95% CI) | 2.17 [0.37, 12.74] |
| 10.5 Darbepoetin alfa versus methoxy polyethylene glycol‐epoetin beta | 1 | 305 | Odds Ratio (M‐H, Random, 95% CI) | 1.83 [0.66, 5.09] |
| 11 Major cardiovascular events | 5 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 Darbepoetin alfa versus placebo | 1 | 4038 | Odds Ratio (M‐H, Random, 95% CI) | 1.08 [0.95, 1.24] |
| 11.2 Epoetin alfa versus control | 1 | 157 | Odds Ratio (M‐H, Random, 95% CI) | 2.40 [0.29, 20.11] |
| 11.3 Epoetin beta versus control | 1 | 33 | Odds Ratio (M‐H, Random, 95% CI) | 0.61 [0.07, 4.98] |
| 11.4 Epoetin alfa versus darbepoetin alfa | 1 | 321 | Odds Ratio (M‐H, Random, 95% CI) | 0.20 [0.01, 4.17] |
| 11.5 Epoetin alfa versus biosimilar epoetin | 1 | 462 | Odds Ratio (M‐H, Random, 95% CI) | 0.49 [0.17, 1.47] |
Differences between protocol and review
We did not include the outcomes of end of treatment haemoglobin level as this is a surrogate outcome and not indicative of efficacy or safety. We did not include continuous measures (number of blood transfusions or number of hospital admissions for blood transfusions) in the final review as these were largely not reported in the included studies. We have not included cancer as an outcome as this was relevant to an earlier version of the protocol (which included all populations receiving ESA therapy, not just CKD but which we subsequently excluded from the published protocol as assumptions of transitivity were likely to have been breached using this approach). We added biosimilar ESAs as a single node of interest in the review (which was not mentioned in the protocol) as these are of interest to patients, clinicians and policy‐makers and the network analysis approach is the ideal vehicle to consider the comparative safety and efficacy of these ESA formulations.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Iron supplementation
|
|
| Outcomes | Primary study outcome
Outcomes included in meta‐analysis
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Lost to follow‐up 14% in epoetin alfa arm and 17% in biosimilar epoetin kappa arm. As this was > 10% this was judged high risk |
| Selective reporting (reporting bias) | High risk | No data for cardiovascular outcomes |
| Other bias | Low risk | None apparent |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Iron supplementation
|
|
| Outcomes | Primary study outcome
Outcomes included in the meta‐analyses
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Eligible patients were assigned to either of two groups by a computer according to a minimisation method" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "We conducted this randomised, multicentre, open‐label, parallel group study..." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported for secondary outcomes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition was 49/160 (30.6%) in the intervention group and 43/161 (26.7%) in the control group. As this was > 10% of all randomised participants, we adjudicated this as high risk |
| Selective reporting (reporting bias) | Low risk | Low risk (extractable data for major cardiovascular events were available) |
| Other bias | High risk | There was an imbalance in the doses used when comparing two different epoetin drugs |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Iron supplementation
|
|
| Outcomes | Primary trial outcome
Outcomes extracted for meta‐analysis
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Randomly assigned by a central randomisation centre. Randomisation numbers were allocated sequentially to patients in the order in which they were enrolled |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 14/62 lost to follow‐up in darbepoetin alfa arm (23%) and 16/19 lost to follow‐up in control arm (84%). As this was imbalanced between groups and > 10% this judged to be high risk |
| Selective reporting (reporting bias) | High risk | Data for major cardiovascular events not available |
| Other bias | High risk | Industrial sponsor on authorship; abstract only publication |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Iron supplementation
|
|
| Outcomes | Primary study outcomes
Endpoint included in meta‐analysis
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "This was a multicenter, randomized, open‐label study." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not clearly described |
| Selective reporting (reporting bias) | High risk | Data for major cardiovascular events were not available |
| Other bias | High risk | Employees of the sponsor were authors |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Iron supplementation
|
|
| Outcomes | Primary study outcome
Outcomes included in the meta‐analysis
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "AMICUS was an open‐label, randomized, multicenter, epoetin‐controlled, parallel‐group, phase 3 study" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition 3/46 in intervention arm (6.5%) and 3/135 in control arm (2.2%). As these were similar and the overall attrition was <10% this was adjudicated as low risk. |
| Selective reporting (reporting bias) | High risk | High risk as major adverse cardiovascular events were not extractable for meta‐analysis |
| Other bias | High risk | Employees of the sponsor were authors |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Iron supplementation
|
|
| Outcomes | Primary study outcomes
Outcomes included in meta‐analyses
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described sufficiently to adjudicate |
| Selective reporting (reporting bias) | High risk | No extractable data for meta‐analysis |
| Other bias | Low risk | None apparent |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Iron supplementation
|
|
| Outcomes | Primary outcome
Outcomes included in meta‐analysis
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | "Patients were assigned to study treatment via a central randomization center with stratification by geographic region." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "ARCTOS was an open‐label, randomized, multicenter, darbepoetin alfa– controlled, parallel‐group Phase III study" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition 11/162 (6.8%) in intervention arm and 17/162 (10.55 in control arm. As this was similar between groups and below 10% overall, we adjudicated this as low risk |
| Selective reporting (reporting bias) | Low risk | All major expected outcomes reported |
| Other bias | High risk | Sponsor employees were listed on the authorship. |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Iron supplementation
|
|
| Outcomes | Primary study outcomes
Outcomes included in meta‐analyses
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Double blinded for the first 4 weeks then open label |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition 10/63 (15.9%) in intervention arm and 20/66 (30.3%) in the control arm. As this was higher than 10% overall, this was judged high risk |
| Selective reporting (reporting bias) | Low risk | Major cardiovascular events were extractable for meta‐analysis |
| Other bias | High risk | Sponsor employees were listed on the authorship |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Iron supplementation
|
|
| Outcomes | Primary study outcome
Outcomes included in meta‐analyses
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition 8/90 (8.9%) in intervention arm and 1/40 (0.25%) in control arm. As this was markedly different between groups we judged this as high‐risk |
| Selective reporting (reporting bias) | Low risk | Major cardiovascular events reported |
| Other bias | Low risk | None apparent |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes | Primary study outcome
Outcomes included in the meta‐analysis
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not clearly reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition was unclearly reported |
| Selective reporting (reporting bias) | High risk | No extractable data for key outcomes |
| Other bias | High risk | Abstract only publication; funded by Ortho Biotech |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
Iron supplementation
|
|
| Outcomes | Primary study outcome
Outcomes extracted for meta‐analysis
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded for adverse reactions, other clinical events, and quality of life assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition 5/38 (13%) in high dose epoetin alfa arm, 6/40 (15%) in low dose epoetin alfa arm and 8/40 (20%) in placebo arm. As this was cumulatively > 10% then this was judged high risk |
| Selective reporting (reporting bias) | Low risk | Data for all‐cause mortality and major cardiovascular events were available |
| Other bias | Low risk | None apparent |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes | Primary study outcome
Outcomes extracted for meta‐analysis
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Prospective open‐label single centre study..." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition was 4/20 (20% in the epoetin alfa arm and 6/22 (27%) in the darbepoetin alfa arm. As this was > 10% overall, this was judged as high risk |
| Selective reporting (reporting bias) | High risk | No data for cardiovascular events were available |
| Other bias | Low risk | None apparent |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Iron supplementation
|
|
| Outcomes | Primary outcome
Outcomes included in meta‐analysis
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open trial |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 28/281 lost to follow‐up (10% of randomised participants) |
| Selective reporting (reporting bias) | High risk | Major cardiovascular events not reported |
| Other bias | Low risk | None apparent |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Iron supplementation
|
|
| Outcomes | Primary study outcome
Outcome extracted for meta‐analysis
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Open randomised parallel‐group study" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition was 1/12 (8%) in the epoetin beta arm and 2/10 (20%) in the control arm. As this was > 10% overall this was judged to be high risk |
| Selective reporting (reporting bias) | High risk | Major cardiovascular outcomes were not available |
| Other bias | Low risk | None apparent |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Iron supplementation
|
|
| Outcomes | Primary study outcomes
Outcomes extracted for meta‐analysis
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "The CORDATUS study was an open‐label, randomized, controlled, multicentre, parallel‐group study in patients with CKD not on dialysis." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition 17/154 in the darbepoetin alfa group (11%) and 12/153 (7.8%) in the methoxy polyethylene glycol‐epoetin beta group. As this was below 105 overall, we judged attrition to be low risk |
| Selective reporting (reporting bias) | Low risk | Data for major cardiovascular outcomes were available |
| Other bias | High risk | Industry sponsor employees on authorship |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Iron supplementation
|
|
| Outcomes | Primary study outcome
Outcomes extracted for meta‐analysis
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmatched interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | High risk | No data extractable for meta‐analysis |
| Other bias | High risk | Industry sponsor on authorship |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Iron supplementation
|
|
| Outcomes | Primary study outcome
Outcomes extracted for meta‐analysis
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmatched interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | High risk | Cardiovascular events not available for analysis |
| Other bias | High risk | Industrial sponsor on authorship |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment
Control
Iron supplementation
|
|
| Outcomes | Primary study outcome
Outcomes extracted for meta‐analysis
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Open‐label randomised trial" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | Low risk | Major cardiovascular outcomes available |
| Other bias | Low risk | None apparent; |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Iron supplementation
|
|
| Outcomes | Primary study outcome
Outcomes for meta‐analysis
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Low risk | Interactive voice‐response system |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Drug administered by third party who was aware of treatment assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 20/95 lost to follow‐up in epoetin beta arm (21%) and 34/193 lost to follow‐up in biosimilar epoetin theta arm (18%). As this was > 10%, this was judged to be high risk |
| Selective reporting (reporting bias) | Low risk | Data for major cardiovascular events available |
| Other bias | Low risk | Sponsor on authorship and involved in statistical analysis |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Iron supplementation
|
|
| Outcomes | Primary trial outcome
Outcomes extracted for meta‐analysis
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Randomised centrally |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not double‐dummy controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Discrepancy in proportion of patients lost in biosimilar ESA arm |
| Selective reporting (reporting bias) | Low risk | All patient‐relevant outcomes reported |
| Other bias | Low risk | None apparent |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Iron supplementation
|
|
| Outcomes | Primary trial outcome
Outcomes extracted for meta‐analysis
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised sequence |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The principal investigator adjudicated deaths with an independent expert but unclear whether they were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition 22/164 in epoetin alfa arm (13.4%) and 53/314 in biosimilar arm (16.9%). As this was above 10% this was judged as high risk |
| Selective reporting (reporting bias) | Low risk | Data for cardiovascular events provided |
| Other bias | High risk | Sponsor on authorship |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Iron supplementation
|
|
| Outcomes | Primary outcome
Outcome extracted for meta‐analysis
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition was 44/163 in epoetin alfa arm (27%) and 30/174 (17.2%) in the biosimilar ESA arm. As this was > 10%, then adjudicated as high risk |
| Selective reporting (reporting bias) | High risk | Data for major cardiovascular events not provided |
| Other bias | High risk | Sponsor on authorship: premature termination |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group 1
Control group 2
Control group 3
Iron supplementation
|
|
| Outcomes | Primary study outcome
Outcomes extracted for meta‐analysis
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Centrally allocated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition 8/43 (19%) in 30 µg darbepoetin alfa arm; 8/42 (19%) in 60 µg darbepoetin alfa arm; 7 /43 (16%) in 90 µg darbepoetin alfa arm; and 5/43 (12%) in epoetin alfa arm. As this was > 10% this was judged as high risk |
| Selective reporting (reporting bias) | Low risk | Major cardiovascular event data available |
| Other bias | Low risk | None apparent |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Iron supplementation
|
|
| Outcomes | Primary trial outcome
Outcomes extracted for meta‐analysis
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | High risk | No major cardiovascular event data provided |
| Other bias | High risk | Abstract only publication |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Iron supplementation
|
|
| Outcomes | Primary study outcome
Outcomes extracted for meta‐analysis
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | Low risk | Major cardiovascular event data available |
| Other bias | High risk | Sponsor on authorship |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Iron supplementation
|
|
| Outcomes | Primary trial outcome
Outcomes extracted for meta‐analysis
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 34/181 (19%) lost in epoetin alfa group and 39/181 (21.5%) lost in control group. As this is > 10% this was judged to be high risk |
| Selective reporting (reporting bias) | Low risk | Major cardiovascular event data available |
| Other bias | High risk | Industrial sponsor on authorship |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group:
Iron supplementation
|
|
| Outcomes | Primary study outcome
Outcomes extracted for meta‐analysis
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation list provided by an independent clinical research organisation |
| Allocation concealment (selection bias) | Unclear risk | Patients enrolled at each centre were allocated consecutive numbers |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 46/304 lost to follow‐up in epoetin alfa arm (15%) and 32/305 lost to follow‐up in biosimilar epoetin arm (10%). As this was > 10%, this was adjudicated as high risk |
| Selective reporting (reporting bias) | Low risk | Data for cardiovascular events available |
| Other bias | Low risk | None apparent |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Iron supplementation
|
|
| Outcomes | Primary trial outcome
Outcomes extracted for meta‐analysis
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 65/230 lost to follow‐up in epoetin alfa arm (28%) and 78/232 lost to follow‐up in biosimilar epoetin arm (34%). As this was > 10%, this was adjudicated as high risk |
| Selective reporting (reporting bias) | Low risk | Data for cardiovascular events available |
| Other bias | High risk | Sponsor on authorship |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Iron supplementation
|
|
| Outcomes | Primary trial outcome
Outcomes extracted for meta‐analysis
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | Low risk | Data for cardiovascular outcomes available |
| Other bias | Low risk | None apparent |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Iron supplementation
|
|
| Outcomes | Primary trial outcome
Outcomes extracted for meta‐analysis
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 4/23 lost from epoetin alfa arm (17%) and 4/22 lost from darbepoetin alfa arm (17%). As this was > 10% this was judged as high risk |
| Selective reporting (reporting bias) | High risk | Data for cardiovascular outcomes not available |
| Other bias | Low risk | None apparent |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Iron supplementation
|
|
| Outcomes | Primary trial outcome
Outcomes extracted for meta‐analysis
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 32/166 lost to follow‐up (19.3%). As this was > 10%, this was judged to be high risk |
| Selective reporting (reporting bias) | Low risk | Data for major cardiovascular events available |
| Other bias | High risk | Industrial sponsor on authorship |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Iron supplementation
|
|
| Outcomes | Primary trial outcome
Outcomes extracted for meta‐analysis
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 39/192 lost to follow‐up in epoetin alfa arm (20%) and 130/560 lost to follow‐up in epoetin delta arm (23%). As this was >10% this was adjudicated as high risk. |
| Selective reporting (reporting bias) | Low risk | Data for cardiovascular events available. |
| Other bias | Low risk | None apparent |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Iron supplementation
|
|
| Outcomes | Primary trial outcome
Outcomes extracted for meta‐analysis
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation numbers were generated by computer at a co‐ordinating centre |
| Allocation concealment (selection bias) | Low risk | Allocated sequentially by centre, in the order in which patients were enrolled. Investigators received numbers by telephone and recorded them on patients’ case‐report forms |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 46/226 lost from epoetin arm (20.4%) and 110/337 lost from methoxy polyethylene glycol epoetin beta arm (32.6%). As this was > 10%, this was judged as high risk |
| Selective reporting (reporting bias) | High risk | Major cardiovascular events were not available |
| Other bias | High risk | Industrial sponsor on authorship |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Iron supplementation
|
|
| Outcomes | Primary study outcome
Outcomes extracted for meta‐analysis
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Recruiting physician blinded to randomisation list |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 1/38 lost to follow‐up in epoetin arm (2.6%) and 5/39 lost to follow‐up in biosimilar epoetin omega arm (12.8%). As there was a marked difference between arms, this was judged as high risk |
| Selective reporting (reporting bias) | Low risk | Major cardiovascular events available |
| Other bias | Low risk | None apparent |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Iron supplementation
|
|
| Outcomes | Primary trial outcome
Outcomes extracted for meta‐analysis
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The randomisation sequence was designed to ensure that approximately equal numbers of patients were randomised. At each centre, treatment unit numbers were assigned consecutively by date of randomisation |
| Allocation concealment (selection bias) | Unclear risk | The randomisation sequence was designed to ensure that approximately equal numbers of patients were randomised. At each centre, treatment unit numbers were assigned consecutively by date of randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 9/78 in epoetin alfa arm lost to follow‐up (11.5%) and 7/74 in placebo arm lost to follow‐up (9.5%). As the loss to follow‐up in the trial overall was > 10% this was judged as high risk |
| Selective reporting (reporting bias) | High risk | No data for major cardiovascular events |
| Other bias | Low risk | None apparent |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Iron supplementation
|
|
| Outcomes | Primary trial outcome
Outcomes extracted for meta‐analysis
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central computerised system |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 54/338 loss from epoetin alfa arm (16%) and 27/169 lost from darbepoetin alfa arm (16%). As this is higher than 10%, we judged this to be high risk. |
| Selective reporting (reporting bias) | High risk | Data for major cardiovascular events not available |
| Other bias | High risk | Industrial sponsor on authorship |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Iron supplementation
|
|
| Outcomes | Primary trial outcome
Outcome extracted for meta‐analysis
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind placebo controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described for all‐cause mortality |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5/56 lost to follow‐up and reasons given (< 10%) |
| Selective reporting (reporting bias) | High risk | No data for cardiovascular events extractable for analysis |
| Other bias | Low risk | None apparent |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Iron supplementation
|
|
| Outcomes | Primary trial outcome
Outcomes extracted for meta‐analysis
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 27/118 lost to follow‐up in epoetin alfa arm (22.9%) and 10/39 lost to follow‐up in standard treatment arm (25.6%) |
| Selective reporting (reporting bias) | Low risk | Data for major cardiovascular events available |
| Other bias | High risk | Sponsor on authorship; change in protocol; medical writing assistance by sponsor |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group:
Iron supplementation
|
|
| Outcomes | Primary trial outcome
Outcomes extracted for meta‐analysis
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation numbers were generated by computer at a coordinating centre |
| Allocation concealment (selection bias) | Low risk | Allocated to the two treatment groups in a 1:1 ratio using a permuted block randomisation with a block size of four. Investigators received numbers by telephone and recorded them on electronic case‐report forms. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 97/245 lost to follow‐up in methoxy polyethylene glycol‐epoetin beta arm (40%) and 58/245 lost to follow‐up in darbepoetin alfa arm (24%). As this is > 10% and there is a marked difference between groups, this is judged as high risk |
| Selective reporting (reporting bias) | High risk | Data for cardiovascular events not available |
| Other bias | High risk | Industrial sponsor on authorship |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Iron supplementation
|
|
| Outcomes | Primary trial outcome
Outcomes extracted for meta‐analysis
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Randomly assigned to treatment via a central randomisation centre |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 74/381 lost to follow‐up in methoxy polyethylene glycol epoetin beta arm (19%) and 24/191 lost to follow‐up in epoetin alfa or epoetin beta arm (13%). As this is > 10% and there was a difference between trial arms, this was judged as high risk |
| Selective reporting (reporting bias) | High risk | Data for major cardiovascular events not available |
| Other bias | High risk | Industrial sponsor on authorship |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Iron supplementation
|
|
| Outcomes | Primary trial outcome
Outcomes extracted for meta‐analysis:
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 23/43 lost to follow‐up in epoetin alfa arm (53%) and 25/40 lost to follow‐up in control arm (53%). As this was >10%, this was considered high risk |
| Selective reporting (reporting bias) | High risk | Data for major cardiovascular events/mortality not available |
| Other bias | Low risk | None apparent |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Iron supplementation
|
|
| Outcomes | Primary trial outcome
Outcomes extracted for meta‐analysis
|
|
| Notes | Funding: F. Hoffman‐La Roche Trials registration: NCT00081484 Contact with authors: not contacted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 45/168 lost to follow‐up in methoxy polyethylene glycol‐epoetin beta arm (27%) and 35/168 lost to follow‐up in epoetin alfa or beta arm (21%). As this was > 10%, this was judged high risk |
| Selective reporting (reporting bias) | High risk | Data for cardiovascular death or events not available |
| Other bias | High risk | Industrial sponsor on authorship |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Iron supplementation
|
|
| Outcomes | Primary trial outcome
Outcomes extracted for meta‐analysis
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | High risk | Data for cardiovascular events not extractable for analysis |
| Other bias | Low risk | None apparent |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Iron supplementation
|
|
| Outcomes | Primary outcome
Outcomes extracted for meta‐analysis
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | > 10% of randomised participants not included in analyses |
| Selective reporting (reporting bias) | High risk | Data not available for analysis |
| Other bias | Low risk | None apparent |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Iron supplementation
|
|
| Outcomes | Primary trial outcome
Outcomes extracted for meta‐analysis
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/40 lost to follow‐up (5%). As this was < 10% this was judged low risk |
| Selective reporting (reporting bias) | High risk | Data for cardiovascular events not available |
| Other bias | Low risk | None apparent |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Iron supplementation
|
|
| Outcomes | Primary trial outcome
Outcomes extracted for meta‐analysis
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | High risk | No data for cardiovascular events provided |
| Other bias | High risk | Sponsor on authorship; abstract only |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Iron supplementation
|
|
| Outcomes | Primary study outcome
Outcomes extracted for meta‐analysis
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 9/78 (11.5%) lost to follow‐up after randomisation which was > 10% |
| Selective reporting (reporting bias) | High risk | Major cardiovascular events not provided |
| Other bias | High risk | Sponsor on authorship |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Iron supplementation
|
|
| Outcomes | Primary trial outcome
Outcomes extracted for meta‐analysis
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Randomly assigned by a central randomisation centre. Randomisation numbers were allocated sequentially to patients in the order in which they were enrolled |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 30/156 lost to follow‐up in darbepoetin arm (19%) and 34/157 (22%) lost to follow‐up in methoxy polyethylene glycol‐epoetin beta arm. As this was >10% in both arms, this was considered high risk |
| Selective reporting (reporting bias) | Low risk | Data for cardiovascular events available |
| Other bias | High risk | Industrial sponsor on authorship |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Iron supplementation
|
|
| Outcomes | Primary trial outcome
Outcomes extracted for meta‐analysis
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | High risk | Data for cardiovascular outcomes not available |
| Other bias | Low risk | None apparent |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Iron supplementation
|
|
| Outcomes | Primary study outcome
Outcomes extracted for meta‐analysis
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/24 lost to follow‐up in darbepoetin arm (13%) and 1/46 lost to follow‐up in methoxy polyethylene glycol epoetin beta arm (2%). As this was <10% overall and differences between arms were not reliable due to small numbers of events, this was adjudicated as low risk |
| Selective reporting (reporting bias) | High risk | Data for cardiovascular events not available |
| Other bias | High risk | Published as letter only; writing supported and funded by sponsor |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Iron supplementation
|
|
| Outcomes | Primary trial outcome
Outcomes extracted for meta‐analysis
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 26/107 in epoetin beta arm (24%) lost to follow‐up and 29/112 in darbepoetin alfa arm (26%) lost to follow‐up. As this was > 10%, this was judged as high risk |
| Selective reporting (reporting bias) | High risk | Data for cardiovascular events not available |
| Other bias | Low risk | None apparent |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Iron supplementation
|
|
| Outcomes | Primary study outcome
Outcomes extracted for meta‐analysis
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Adjudicated by an endpoint committee whose members were unaware of the treatment assignments and the HCT and Hb values were redacted from the documents under review |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 153/2102 (7.5%) lost to follow‐up in darbepoetin alfa arm and 164/2026 (8.1%) lost to follow‐up in placebo arm. As this was <10%, this was judged low risk. |
| Selective reporting (reporting bias) | Low risk | Data for cardiovascular events available |
| Other bias | High risk | Imbalance of percentage with CV disease: interim analyses; sponsor provided independent statistical support |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Iron supplementation
|
|
| Outcomes | Primary study outcome
Outcomes extracted for meta‐analysis
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | High risk | Data for cardiovascular events not available |
| Other bias | Low risk | None apparent |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Iron supplementation
|
|
| Outcomes | Primary trial outcome:
Outcomes extracted for meta‐analysis:
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | Low risk | Data for major cardiovascular events available |
| Other bias | Low risk | None apparent |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Iron supplementation
|
|
| Outcomes | Primary study outcome
Outcome extracted for meta‐analysis
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central computerised system |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Open‐label comparative study" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition 63/175 (36%) in epoetin alfa arm and 123/347 (35%) in darbepoetin alfa arm |
| Selective reporting (reporting bias) | High risk | No data for major cardiovascular events available |
| Other bias | High risk | Industrial sponsor on authorship |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Iron supplementation
|
|
| Outcomes | Primary trial outcome
Outcomes extracted for meta‐analysis
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | High risk | No cardiovascular events reported |
| Other bias | Low risk | Sponsor responsible for randomisation |
AUC ‐ area under the curve; CKD ‐ chronic kidney disease; CrCl ‐ creatinine clearance; CRP ‐ C‐reactive protein; DBP ‐ diastolic blood pressure; eGFR ‐ estimated glomerular filtration rate; ESA ‐ erythropoiesis‐stimulating agents; ESKD ‐ end‐stage kidney disease; HCT ‐ haematocrit; HD ‐ haemodialysis; iPTH ‐ intact parathyroid hormone; IQR ‐ interquartile range; IV ‐ intravenous; LVEF ‐ left ventricular ejection fraction; NS ‐ not stated; NSAID ‐ nonsteroidal anti‐inflammatory drug; PD ‐ peritoneal dialysis; RBC ‐ red blood cell; rHuEPO ‐ recombinant human erythropoietin; RRT ‐ renal replacement therapy; TSAT ‐ transferrin saturation; SBP ‐ systolic blood pressure; SCr ‐ serum creatinine
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Acchiardo 1991a | ESA type not defined |
| ACORD Study 2004 | Comparing the same ESA derivative in different treatment arms |
| BA16260 Study 2006 | Not comparing different ESAs |
| BA16285 Study 2007 | Not comparing different ESAs |
| BA16286 Study 2005 | Not comparing different ESAs |
| Besarab 1998 | Comparing the same ESA derivative in different treatment arms |
| Brier 2010 | Not comparing different ESAs |
| CAPRIT Study 2012 | Not comparing different ESAs |
| CHOIR Study 2006 | Comparing the same ESA derivative in different treatment arms |
| Cianciaruso 2008 | Comparing the same ESA derivative in different treatment arms |
| CREATE Study 2001 | Comparing the same ESA derivative in different treatment arms |
| ECAP Study 2006 | Comparing the same ESA derivative in different treatment arms |
| Eschbach 1989 | Comparing the same ESA derivative in different treatment arms |
| Foley 2000 | Comparing the same ESA derivative in different treatment arms |
| Gouva 2004 | Comparing the ESA epoetin derivative in different treatment arms |
| Johnson 1999 | Comparing the ESA epoetin derivative in different treatment arms |
| Kawanishi 2005 | Short duration |
| Levin 2005 | Comparing the same ESA derivative in different treatment arms |
| Linde 2001 | Comparing the same ESA derivative in different treatment arms |
| Locatelli 2008 | Comparing the same ESA derivative in different treatment arms |
| Macdougall 2007 | Not comparing different ESAs |
| N0055116759 | No results available despite attempted contact with authors |
| Neo‐PDGF Study 2010 | Short duration |
| Parfrey 2005 | Comparing the same ESA derivative in different treatment arms |
| Perez‐Oliva 2005 | Short duration |
| Salek 2001 | Comparing the same ESA derivative in different treatment arms |
| Sja'bani 1997 | Short duration |
| SLIMHEART Study 2004 | Comparing the same ESA derivative in different treatment arms |
| Wizemann 2008 | Cross‐over study |
ESA ‐ erythropoiesis‐stimulating agents
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
Iron supplementation
|
| Outcomes |
|
| Notes |
|
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
Iron supplementation
|
| Outcomes | Primary study outcome
Outcomes included in meta‐analysis
|
| Notes |
|
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
Iron supplementation
|
| Outcomes |
|
| Notes |
|
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
Control group
Iron supplementation
|
| Outcomes |
|
| Notes |
|
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
Control group
Iron supplementation
|
| Outcomes |
|
| Notes |
|
CKD ‐ chronic kidney disease; CrCl ‐ creatinine clearance; Hb ‐ haemoglobin; HD ‐ haemodialysis; IV ‐ intravenous; NS ‐ not stated; RBC ‐ red blood cell; RCT ‐ randomised controlled trial; SC ‐ subcutaneous; TSAT ‐ transferrin saturation
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Randomized comparison of IV C.E.R.A. (Continuous Erythropoietin Receptor Activator) and darbepoetin alfa (DA) at extended administration intervals for the maintenance of Hb levels in patients with CKD on dialysis |
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
|
| Outcomes |
|
| Starting date | April 2006 |
| Contact information | Not available |
| Notes | Abstract only publication |
| Trial name or title | A study of subcutaneous Mircera in patients with chronic kidney disease, not on dialysis |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | September 2007 |
| Contact information | Hoffman‐La Roche |
| Notes | No publications provided by sponsor |
| Trial name or title | A study of subcutaneous Mircera once monthly in the treatment of anemia in patients with chronic kidney disease not on dialysis |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | December 2007 |
| Contact information | Hoffmann‐La Roche |
| Notes | No publications provided by sponsor to clinicaltrials.gov |
| Trial name or title | A study of once monthly intravenous or subcutaneous Mircera in patients with chronic kidney disease on hemodialysis |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | October 2008 |
| Contact information | Hoffman‐La Roche |
| Notes | No publications provided by sponsor to clinicaltrials.gov |
| Trial name or title | A study of all‐cause mortality and cardiovascular morbidity in CKD patients on dialysis and those not on renal replacement therapy receiving Mircera or reference ESAs |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | December 2008 (estimated completion date November 2019) |
| Contact information | Hoffman‐La Roche |
| Notes |
| Trial name or title | The PRIMAVERA study protocol design: evaluating the effect of continuous erythropoiesis receptor activator (C.E.R.A.) on renal function in non‐anemic patients with chronic kidney disease |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Starting date | The results of PRIMAVERA are expected in 2013 |
| Contact information | D Fliser |
| Notes |
| Trial name or title | STIMULATE Study: anemia correction and HRQoL outcomes in elderly CKD patients |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | August 2006 |
| Contact information | Amgen |
| Notes | Study now terminated due to poor recruitment and lack of timely enrolment |
CKD ‐ chronic kidney disease; eGFR ‐ estimated glomerular filtration rate; Hb ‐ haemoglobin; HD ‐ haemodialysis; IV ‐ intravenous; MI ‐ myocardial infarction; PD ‐ peritoneal dialysis; RBC ‐ red blood cell; SC ‐ subcutaneous; TSAT ‐ transferrin saturation
Contributions of authors
Draft the protocol: SP, GS, JC, GFMS
Study selection: SP, VS
Extract data from studies: SP, VS
Enter data into RevMan: SP
Carry out the analysis: SP, GS, DM
Interpret the analysis: SP, VS, DM, GS, JC, MT, NW, GFMS
Revising the review drafts for important intellectual content: SP, VS, DM, GS, JC, MT, NW, GFMS
Draft the final review: SP
Disagreement resolution: GS
Update the review: SP
Sources of support
Internal sources
Cochrane Renal Group, Australia.
External sources
Georgia Salanti and Dimitris Mavridis receive research funding from the European Research Council Starting Grant (Grant Nr. IMMA 260559), Other.
Declarations of interest
Suetonia C Palmer: none known
Valeria Saglimbene: none known
Dimitris Mavridis: none known
Georgia Salanti: none known
Jonathan C Craig: none known
Marcello Tonelli: Dr Tonelli has received an investigator‐initiated grant and honoraria from Amgen Australia for an academic lecture series ‐‐ neither were related to ESA or anaemia. All honoraria were donated to charity
Natasha Wiebe: none known
Giovanni FM Strippoli: none known
New
References
References to studies included in this review
- Akiba T, Akizawa T, Kakuma T. Randomized double‐blind comparative study of recombinant human erythropoietin (epoetin kappa, produced by serum‐free culture) in renal anemia patients on hemodialysis. Japanese Pharmacology & Therapeutics 2010;38(2):181‐98. [EMBASE: 2010186120] [Google Scholar]
- Akaishi M, Hiroe M, Hada Y, Suzuki M, Tsubakihara Y, Akizawa T, et al. Effect of anemia correction on left ventricular hypertrophy in patients with modestly high hemoglobin level and chronic kidney disease. Journal of Cardiology 2013;62(4):249‐56. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Akizawa T, Gejyo F, Nishi S, Iino Y, Watanabe Y, Saito A, et al. Positive outcomes of high hemoglobin target in patients with chronic kidney disease not on dialysis: a randomized controlled study. Therapeutic Apheresis & Dialysis 2011;15(5):431‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Akizawa T, Tsubakihara Y. Target level for hemoglobin correction by darbepoetin alfa (KRN321) for patients with chronic kidney disease (CKD) not on dialysis in randomized controlled study; from the viewpoint of the efficacy [abstract no: SU‐PO804]. Journal of the American Society of Nephrology 2007;18(Abstracts Issue):762A. [CENTRAL: CN‐00740554] [Google Scholar]; Tsubakihara Y, Akizawa T. High target hemoglobin with erythropoiesis stimulating agent (ESA) in chronic kidney disease (CKD) slows the occurrence rate of events related to decline of renal function [abstract no: SA‐FC341]. Journal of the American Society of Nephrology 2009;20:79A. [CENTRAL: CN‐00793981] [Google Scholar]; Tsubakihara Y, Akizawa T. Target level for hemoglobin correction by darbepoetin alfa (KRN321) for patients with chronic kidney disease (CKD) not on dialysis in randomized controlled study; from the viewpoint of the safety [abstract no: SU‐PO818]. Journal of the American Society of Nephrology 2007;18(Abstracts Issue):765A. [CENTRAL: CN‐00740542] [Google Scholar]
- Alexander M, Kewalramani R, Agodoa I, Globe D. Association of anemia correction with health related quality of life in patients not on dialysis. Current Medical Research & Opinion 2007;23(12):2997‐3008. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Thadhani R, Cheriyan R, Brenner R, Ford J, Powers K, Rahman SN. Treatment of anemia with ARANESP (darbepoetin alfa) improves health related quality of life (HRQOL) in patients with chronic kidney disease (CKD) [abstract]. Journal of the American Society of Nephrology 2002;13(September, Program & Abstracts):637a. [CENTRAL: CN‐00447983] [Google Scholar]
- Allon M, Kleinman AK, Walczyk M, Kaupke C, Maroni BJ, Heatherington A, et al. The pharmacokinetics of novel erythropoiesis stimulating protein (NESP) following chronic intravenous administration is time‐and dose‐linear [abstract no: A1308]. Journal of the American Society of Nephrology 2000;11(Sept):248A. [CENTRAL: CN‐00626053] [Google Scholar]; Allon M, Kleinman K, Walczyk M, Kaupke C, Messer‐Mann L, Olson K, et al. Pharmacokinetics and pharmacodynamics of darbepoetin alfa and epoetin in patients undergoing dialysis. Clinical Pharmacology & Therapeutics 2002;72(5):546‐55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Chanu P, Gieschke R, Reigner B, Dougherty FC. Pharmacokinetic parameters of C.E.R.A. and are not affected by age in patients with chronic kidney disease on dialysis [abstract no: SaP351]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi351. [CENTRAL: CN‐00757500] [Google Scholar]; Chanu P, Gieschke R, Reigner B, Dougherty FC. Pharmacokinetics of C.E.R.A. and stable maintenance of haemoglobin (Hb) levels with once‐monthly dosing in patients with chronic kidney disease (CKD) [abstract no: SaP325]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi342. [CENTRAL: CN‐00757502] [Google Scholar]; Fishbane S, Dalton C, Beswick R, Dutka P, Schmidt R. Efficacy of C.E.R.A., a continuous erythropoietin receptor activator, in the treatment of renal anemia: overview of 6 global phase 3 trials [abstract no: 59]. American Journal of Kidney Diseases 2007;49(4):A39. [CENTRAL: CN‐00756571] [Google Scholar]; Klinger M, Arias M, Vargemezis V, Besarab A, Sulowicz W, Gerntholtz T, et al. C.E.R.A. (Continuous Erythropoietin Receptor Activator) administered at extended intervals corrects Hb levels in patients with CKD on dialysis [abstract no: SA‐PO212]. Journal of the American Society of Nephrology 2006;17(Abstracts):620A. [CENTRAL: CN‐00644218] [Google Scholar]; Klinger M, Arias M, Vargemezis V, Besarab A, Sulowicz W, Gerntholtz T, et al. Efficacy of intravenous methoxy polyethylene glycol‐epoetin beta administered every 2 weeks compared with epoetin administered 3 times weekly in patients treated by hemodialysis or peritoneal dialysis: a randomized trial. American Journal of Kidney Diseases 2007;50(6):989‐1000. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Provenzano R, Macdougall IC, Law A, Ouyang Y, Bexon M. Anemia correction with C.E.R.A. in patients (pts) with chronic kidney disease (CKD) is unaffected by baseline hemoglobin (Hb) level [abstract no: SU‐PO796]. Journal of the American Society of Nephrology 2007;18(Abstracts Issue):760A. [CENTRAL: CN‐00747311] 17267745 [Google Scholar]
- Arabul M, Gullulu M, Yilmaz Y, Eren MA, Baran B, Gul CB, et al. Influence of erythropoietin therapy on serum prohepcidin levels in dialysis patients. Medical Science Monitor 2009;15(11):CR583‐7. [MEDLINE: ] [PubMed] [Google Scholar]
- Fishbane S, Dalton C, Beswick R, Dutka P, Schmidt R. Efficacy of C.E.R.A., a continuous erythropoietin receptor activator, in the treatment of renal anemia: overview of 6 global phase 3 trials [abstract no: 59]. American Journal of Kidney Diseases 2007;49(4):A39. [CN‐00756571] [Google Scholar]; Kessler M, Martinez‐Castelao A, Siamopoulos KC, Villa G, Spinowitz B, Dougherty FC, et al. C.E.R.A. once every 4 weeks in patients with chronic kidney disease not on dialysis: The ARCTOS extension study. Hemodialysis International 2010;14(2):233‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Macdougall IC, Walker R, Provenzano R, Alvaro F, Locay HR, Nader PC, et al. C.E.R.A. (Continuous Erythropoietin Receptor Activator) administered at extended intervals corrects anemia and maintains stable Hb levels in patients with CKD not on dialysis [abstract no: SA‐PO208]. Journal of the American Society of Nephrology 2006;17(Abstracts):619A. [Google Scholar]; Macdougall IC, Walker R, Provenzano R, Alvaro F, Locay HR, Nader PC, et al. C.E.R.A. corrects anemia in patients with chronic kidney disease not on dialysis: results of a randomized clinical trial. Clinical Journal of The American Society of Nephrology: CJASN 2008;3(2):337‐47. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]; Provenzano R, Macdougall IC, Law A, Ouyang Y, Bexon M. Anemia correction with C.E.R.A. in patients (pts) with chronic kidney disease (CKD) is unaffected by baseline hemoglobin (Hb) level [abstract no: SU‐PO796]. Journal of the American Society of Nephrology 2007;18(Abstracts Issue):760A. [CENTRAL: CN‐00747311] 17267745 [Google Scholar]; Walker R, Macdougall IC, Levin A, Kessler M, Noble S, Burgos‐Calderon R, et al. C.E.R.A. corrects anaemia and maintains stable haemoglobin (Hb) levels at extended administration intervals in a 52‐week study of patients with chronic kidney disease (CKD) not on dialysis [abstract no: SuO003]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi412. [CENTRAL: CN‐00644215] [Google Scholar]
- Bahlmann J, Schöter KH, Scigalla P, Gurland HJ, Hilfenhaus M, Koch KM, et al. Morbidity and mortality in hemodialysis patients with and without erythropoietin treatment: a controlled study. Contributions to Nephrology 1991;88:90‐106. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bennett WM. A multicenter clinical trial of epoetin beta for anemia of end‐stage renal disease. Journal of the American Society of Nephrology 1991;1(7):990‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Brown CD, Zhao ZH, Thomas LL, Friedman EA. Erythropoietin delays the onset of uremia in anemic azotemic diabetic predialysis patients [abstract]. Journal of the American Society of Nephrology 1995;6(3):447. [CENTRAL: CN‐00483340] [Google Scholar]
- Anonymous. Association between recombinant human erythropoietin and quality of life and exercise capacity of patients receiving haemodialysis. Canadian Erythropoietin Study Group. BMJ 1990;300(6724):573‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]; Anonymous. Effect of recombinant human erythropoietin therapy on blood pressure in hemodialysis patients. Canadian Erythropoietin Study Group. American Journal of Nephrology 1991;11(1):23‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Canadian Erythropoietin Study Group. The clinical effects and side‐effects of recombinant human erythropoietin (EPO) in anaemic patients on chronic hemodialysis [abstract]. Kidney International 1990;37:278. [CENTRAL: CN‐00583134] [Google Scholar]; Canadian Erythropoietin Study Group. The effect of recombinant human erythropoietin (EPO) upon quality of life and exercise capacity of anemic patients on chronic hemodialysis [abstract]. Kidney International 1990;37:278. [CENTRAL: CN‐00583135] [Google Scholar]; Keown PA. Quality of life in end‐stage renal disease patients during recombinant human erythropoietin therapy. The Canadian Erythropoietin Study Group. Contributions to Nephrology 1991;88:81‐9. [MEDLINE: ] [PubMed] [Google Scholar]; Keown PA, Canadian Erythropoietin Study Group. The effect of recombinant human erythropoietin (EPO) upon quality of life (QL) and functional capacity (FC) of anemic patients on chronic hemodialysis [abstract]. Kidney International 1989;35(1):195. [CENTRAL: CN‐00583136] [Google Scholar]; Keown PA, Churchill DN, Poulin‐Costello M, Lei L, Gantotti S, Agodoa I, et al. Dialysis patients treated with Epoetin alfa show improved anemia symptoms: A new analysis of the Canadian Erythropoietin Study Group trial. Hemodialysis International 2010;14(2):168‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Laupacis A. A randomized double‐blind study of recombinant human erythropoietin in anaemic hemodialysis patients. Canadian Erythropoietin Study Group. Transplantation Proceedings 1991;23(2):1825‐6. [MEDLINE: ] [PubMed] [Google Scholar]; Laupacis A. Changes in quality of life and functional capacity in hemodialysis patients treated with recombinant human erythropoietin. The Canadian Erythropoietin Study Group. Seminars in Nephrology 1990;10(2 Suppl 1):11‐9. [MEDLINE: ] [PubMed] [Google Scholar]; Laupacis A, Wong C, Churchill D. The use of generic and specific quality‐of‐life measures in hemodialysis patients treated with erythropoietin. The Canadian Erythropoietin Study Group. Controlled Clinical Trials 1991;12(4 Suppl):168S‐79S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Muirhead N, Keown P, Churchill DN, Lei L, Gitlin M, Mayne TJ. An intent‐to‐treat (ITT) analysis of anemia symptoms in the Canadian erythropoietin study group (CESG) [abstract no: PUB537]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):932A. [CENTRAL: CN‐00790629] [Google Scholar]; Muirhead N, Keown P, Gitlin M, Mayne TJ, Churchill DN. A reanalysis of the Canadian erythropoietin study group (CESG) patient‐reported outcomes (PRO) trial [abstract no: 177]. American Journal of Kidney Diseases 2008;51(4):A72. [CENTRAL: CN‐00790972] [Google Scholar]; Muirhead N, Keown P, Lei L, Gitlin M, Mayne TJ, Churchill D. The relationship between achieved hemoglobin (HB) & exercise tolerance [abstract no: 161]. American Journal of Kidney Diseases 2008;51(4):A68. [CENTRAL: CN‐00796608] [Google Scholar]; Muirhead N, Keown PA, Churchill DN, Poulin‐Costello M, Gantotti S, Lei L, et al. Dialysis patients treated with Epoetin alpha show improved exercise tolerance and physical function: A new analysis of the Canadian Erythropoietin Study Group trial. Hemodialysis International 2011;15(1):87‐94. [EMBASE: 2011063916] [DOI] [PubMed] [Google Scholar]; Muirhead N, Laupacis A, Wong C. Erythropoietin for anaemia in haemodialysis patients: results of a maintenance study (the Canadian Erythropoietin Study Group). Nephrology Dialysis Transplantation 1992;7(8):811‐6. [MEDLINE: ] [PubMed] [Google Scholar]
- Chen HH, Tarng DC, Lee KF, Wu CY, Chen YC. Epoetin alfa and darbepoetin alfa: effects on ventricular hypertrophy in patients with chronic kidney disease. Journal of Nephrology 2008;21(4):543‐9. [MEDLINE: ] [PubMed] [Google Scholar]
- Chen N, Qian JQ, Mei CL, Zhang AH, Xing CY, Wang L, et al. The efficacy and safety of continuous erythropoietin receptor activator in dialytic patients with chronic renal anemia: an open, randomized, controlled, multi‐center trial. Chung‐Hua Nei Ko Tsa Chih [Chinese Journal of Internal Medicine] 2012;51(7):502‐7. [MEDLINE: ] [PubMed] [Google Scholar]
- Clyne N, Jogestrand T. Effect of erythropoietin treatment on physical exercise capacity and on renal function in predialytic uremic patients. Nephron 1992;60(4):390‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Martinez‐Castelao A. C.E.R.A. once every 4 weeks (Q4W) corrects anaemia in chronic kidney disease (CKD) patients with low incidence of HB values outside the target range [abstract no: OSu057]. NDT Plus 2010;3(Suppl 3):iii298. [EMBASE: 70484207] [Google Scholar]; Roger S. C.E.R.A. once every 4 weeks (Q4W) corrects anaemia in patients with chronic kidney disease (CKD) not on dialysis and demonstrates comparable safety to darbepoetin alfa [abstract no: 202]. Nephrology 2010;15(Suppl 4):79‐80. [EMBASE: 70467206] [Google Scholar]; Roger SD. C.E.R.A. once every 4 weeks (Q4W) corrects anaemia in patients with chronic kidney disease (CKD) not on dialysis and demonstrates comparable safety to darbepoetin alfa weekly (QW) or once every 2 weeks (Q2W) [abstract no: Sa535]. NDT Plus 2010;3(Suppl 3):iii219‐20. [EMBASE: 70484001] [Google Scholar]; Roger SD, Locatelli F, Woitas RP, Laville M, Tobe SW, Provenzano R, et al. C.E.R.A. once every 4 weeks corrects anaemia and maintains haemoglobin in patients with chronic kidney disease not on dialysis. Nephrology Dialysis Transplantation 2011;26(12):3980‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne DW, Ling BN, Toto R, McDermott‐Vitak AD, Trotman ML, Jackson L. Novel erythropoiesis stimulating protein (NESP) corrects anemia in dialysis patients when administered at reduced dose frequency compared with recombinant‐human erythropoietin (r‐huEPO) [abstract no: 1380]. Journal of the American Society of Nephrology 2000;11(Sept):263A. [CENTRAL: CN‐00583382] [Google Scholar]
- Coyne D, Zeig R, Benz R, Berns J, Varma N, Nakanishi A, et al. A randomized, double‐blind study comparing darbepoetin alfa and recombinant human erythropoietin (rHuEPO) in the treatment of anemia in African‐American (AA) subjects with chronic kidney disease (CKD) receiving hemodialysis (HD) [abstract no: TH‐PO365]. Journal of the American Society of Nephrology 2006;17(Abstracts):184A. [CENTRAL: CN‐00740574] [Google Scholar]
- Emans ME, Braam B, Diepenbroek A, Putten K, Cramer MJ, Wielders JP, et al. Neutrophil gelatinase‐associated lipocalin (NGAL) in chronic cardiorenal failure is correlated with endogenous erythropoietin levels and decreases in response to low‐dose erythropoietin treatment. Kidney & Blood Pressure Research 2013;36(1):344‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Emans ME, Putten K, Velthuis BK, Vries JJ, Cramer MJ, America YG, et al. Atherosclerotic renal artery stenosis is prevalent in cardiorenal patients but not associated with left ventricular function and myocardial fibrosis as assessed by cardiac magnetic resonance imaging. BMC Cardiovascular Disorders 2012;12:76. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]; Emans ME, Putten K, Rooijen KL, Kraaijenhagen RJ, Swinkels D, Solinge WW, et al. Determinants of red cell distribution width (RDW) in cardiorenal patients: RDW is not related to erythropoietin resistance. Journal of Cardiac Failure 2011;17(8):626‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Jie KE, Putten K, Bergevoet MW, Doevendans PA, Gaillard CA, Braam B, et al. Short‐ and long‐term effects of erythropoietin treatment on endothelial progenitor cell levels in patients with cardiorenal syndrome. Heart 2011;97(1):60‐5. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]; Jie KE, Putten K, Wesseling S, Joles JA, Bergevoet MW, Pepers‐de Kort F, et al. Short‐term erythropoietin treatment does not substantially modulate monocyte transcriptomes of patients with combined heart and renal failure. PloS ONE 2012;7(9):e41339. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]; Putten K, Jie KE, Emans ME, Verhaar MC, Joles JA, Cramer MJ, et al. Erythropoietin treatment in patients with combined heart and renal failure: objectives and design of the EPOCARES study. Journal of Nephrology 2010;23(4):363‐8. [MEDLINE: ] [PubMed] [Google Scholar]; Putten K, Jie KE, Broek D, Kraaijenhagen RJ, Laarakkers C, Swinkels DW, et al. Hepcidin‐25 is a marker of the response rather than resistance to exogenous erythropoietin in chronic kidney disease/chronic heart failure patients. European Journal of Heart Failure 2010;12(9):943‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Putten K, Broek D, Rooijen KL, Kraaijenhagen RJ, Swinkels DW, Braam B, et al. Erythropoetin (EPO) induced decrease in hepcidin determines bone marrow response in patients with combined heart and renal failure [abstract no: SA‐PO2668]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):712A. [CENTRAL: CN‐00765053] [Google Scholar]
- Gertz B, Kes P, Essaian A, Bias P, Buchner A, Zellner D. Epoetin theta: efficacy and safety of subcutaneous administration in anemic pre‐dialysis patients in the maintenance phase in comparison to epoetin beta. Current Medical Research & Opinion 2012;28(7):1101‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Gertz B, Kohler E, Kes P, Essaian A, Bias P, Buchner A, et al. Epoetin theta: efficacy and safety of IV administration in anaemic haemodialysis patients in the maintenance phase in comparison to epoetin beta. Current Medical Research & Opinion 2010;26(10):2393‐402. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Goh BL, Ong LM, Sivanandam S, Lim TO, Morad Z, Biogeneric EPO Study Group. Randomized trial on the therapeutic equivalence between Eprex and GerEPO in patients on haemodialysis. Nephrology 2007;12(5):431‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Haag‐Weber M, Vetter A, Thyroff‐Friesinger U, INJ‐Study Group. Therapeutic equivalence, long‐term efficacy and safety of HX575 in the treatment of anemia in chronic renal failure patients receiving hemodialysis. Clinical Nephrology 2009;72(5):380‐90. [MEDLINE: ] [PubMed] [Google Scholar]; Vetter A, Haag‐Weber M, Thyroff‐Friesinger U. Efficacy and safety of intravenous (IV) HX575 (Binocrita) in the treatment of anaemia in hemodialysis patients [abstract no: SA‐PO2660]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):710A. [CENTRAL: CN‐00747269] [Google Scholar]
- Haag‐Weber M, Eckardt KU, Horl WH, Roger SD, Vetter A, Roth K. Safety, immunogenicity and efficacy of subcutaneous biosimilar epoetin‐alpha (HX575) in non‐dialysis patients with renal anemia: a multi‐center, randomized, double‐blind study. Clinical Nephrology 2012;77(1):8‐17. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hirakata H, Gejyo F, Suzuki M, Saito A, Lino Y, Watanabe Y, et al. Effect of darbepoetin alfa (KRN321) subcutaneous treatment on hemoglobin levels, health‐related QOL (HRQOL) and left ventricular mass index (LVMI) in patients with chronic kidney disease (CKD) not on dialysis [abstract no: SA‐PO204]. Journal of the American Society of Nephrology 2006;17(Abstracts):618A. [CENTRAL: CN‐00740524] [Google Scholar]; Hirakata H, Tsubakihara Y, Gejyo F, Nishi S, Iino Y, Watanabe Y, et al. Maintaining high hemoglobin levels improved the left ventricular mass index and quality of life scores in pre‐dialysis Japanese chronic kidney disease patients. Clinical & Experimental Nephrology 2010;14(1):28‐35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Inaguma D, Tsubakihara Y, Hirakata H, Hiroe M, Hada Y, Akizawa T, et al. Monthly subcutaneous treatment of darbepoetin alfa (KRN321) could maintain higher Hb safely and have beneficial effects on cardiac function of Japanese CKD patients not on dialysis [abstract no: SaP353]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi352. [CENTRAL: CN‐00740573] [Google Scholar]; Suzuki M, Hada Y, Akaishi M, Hiroe M, Aonuma K, Tsubakihara Y, et al. Effects of anemia correction by erythropoiesis‐stimulating agents on cardiovascular function in non‐dialysis patients with chronic kidney disease. International Heart Journal 2012;53(4):238‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hori K, Tsujimoto Y, Ohmori H, Nakamura H, Suga A, Iwasaki M, et al. Randomized, double‐blind, comparative study of intravenous KRN321 (darbepoetin alfa) compared to intravenous recombinant human erythropoietin (rHuEPO) for treatment of anemia in subjects with chronic renal failure (CRF) receiving hemodialysis in Japan [abstract no: F‐PO502]. Journal of the American Society of Nephrology 2004;15(Oct):177A. [CENTRAL: CN‐00740529] [Google Scholar]
- Kleinman KS, Schweitzer SU. Human recombinant erythropoietin (rHuEPO) treatment of severe anemia associated with progressive renal failure may delay the need to initiate regular dialytic therapy [abstract]. Kidney International 1990;37(1):240. [CENTRAL: CN‐00626057] [Google Scholar]; Kleinman KS, Schweitzer SU, Perdue ST, Abels RI. The use of recombinant human erythropoietin in the correction of anemia in pre‐dialysis patients and its effects on renal function: a double blind placebo controlled trial [abstract]. Kidney International 1989;35(1):229. [CENTRAL: CN‐00636148] [DOI] [PubMed] [Google Scholar]; Kleinman KS, Schweitzer SU, Perdue ST, Bleifer KH, Abels RI. The use of recombinant human erythropoietin in the correction of anemia in predialysis patients and its effect on renal function: a double‐blind, placebo‐controlled trial. American Journal of Kidney Diseases 1989;14(6):486‐95. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Klinkmann H, Schmidt R, Wieczorek L, Scigalla P. Adverse events of subcutaneous recombinant human erythropoietin therapy. Contributions to Nephrology 1992;100:127‐38. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Klinkmann H, Wieczorek L, Scigalla P. Adverse events of subcutaneous recombinant human erythropoietin therapy: results of a controlled multicenter European study. Artificial Organs 1993;17(4):219‐25. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Baldamus C, Krivoshiev S, Wolf‐Pflugmann M, Siebert‐Weigel M, Koytchev R, Bronn A. Long‐term safety and tolerability of epoetin zeta, administered intravenously, for maintenance treatment of renal anemia. Advances in Therapy 2008;25(11):1215‐28. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Krivoshiev S, Todorov VV, Manitius J, Czekalski S, Scigalla P, Koytchev R, et al. Comparison of the therapeutic effects of epoetin zeta and epoetin alpha in the correction of renal anaemia. Current Medical Research & Opinion 2008;24(5):1407‐15. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Krivoshiev S, Wizemann V, Czekalski S, Schiller A, Pljesa S, Wolf‐Pflugmann M, et al. Therapeutic equivalence of epoetin zeta and alfa, administered subcutaneously, for maintenance treatment of renal anemia. Advances in Therapy 2010;27(2):105‐17. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kuriyama S, Tomonari H, Hashimoto T, Kawaguchi Y, Sakai O. Reversal of anemia by EPO therapy retards the progression of chronic renal failure in non‐diabetic pre‐dialysis patients [abstract]. Nephrology 1997;3(Suppl 1):S506. [CENTRAL: CN‐00461123] [DOI] [PubMed] [Google Scholar]; Kuriyama S, Tomonari H, Yoshida H, Hashimoto T, Kawaguchi Y, Sakai O. Reversal of anemia by erythropoietin therapy retards the progression of chronic renal failure, especially in nondiabetic patients. Nephron 1997;77(2):176‐85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Li WY, Chu TS, Huang JW, Wu MS, Wu KD. Randomized study of darbepoetin alfa and recombinant human erythropoietin for treatment of renal anemia in chronic renal failure patients receiving peritoneal dialysis. Journal of the Formosan Medical Association 2008;107(11):843‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Johnson DW, European/Australian NESP 980202 Study Group. Novel erythropoiesis stimulating protein (darbepoietin alpha) corrects anaemia of early chronic kidney disease (CKD) at a reduced dose frequency compared with recombinant human erythropoietin (rHuEPO) [abstract no: P156]. Nephrology 2002;7(Suppl 3):A40. [CENTRAL: CN‐00794721] [Google Scholar]; Locatelli F, European/Australian NESP 980202 Study Group. Novel erythoropoiesis stimulating protein (NESP) corrects anemia of chronic renal insufficiency (CRI) at a reduced dose frequency compared with rHuEPO [abstract]. Nephrology Dialysis Transplantation 2001;16(6):A92. [CENTRAL: CN‐00671771] [Google Scholar]; Locatelli F, Olivares J, Walker R, Wilkie M, European/Australian NESP 980202 Study Group. Novel erythropoiesis stimulating protein (NESP) administered subcutaneously corrects anemia in subjects with chronic renal insufficiency (CRI) when administered at a reduced dose frequency compared with recombinant‐human erythropoietin (r‐huEPO) [abstract]. Journal of the American Society of Nephrology 2000;11(Sept):283A. [CENTRAL: CN‐00626023] 10665935 [Google Scholar]; Locatelli F, Olivares J, Walker R, Wilkie M, Jenkins B, Dewey C, et al. Novel erythropoiesis stimulating protein for treatment of anemia in chronic renal insufficiency. Kidney International 2001;60(2):741‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Martin KJ. Epoetin delta for the management of renal anemia: a one year study [abstract no: TH‐PO374]. Journal of the American Society of Nephrology 2006;17(Abstracts):186A. [CN‐00765049] [Google Scholar]; Martin KJ. The first human cell line‐derived erythropoietin, epoetin‐delta (Dynepo), in the management of anemia in patients with chronic kidney disease. Clinical Nephrology 2007;68(1):26‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Martin KJ, Epoetin Delta 3001 Study Group. Epoetin delta in the management of renal anaemia: results of a 6‐month study. Nephrology Dialysis Transplantation 2007;22(10):3052‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Smyth M, Pratt RD. Epoetin delta, erythropoietin produced in a human cell line, is as effective as epoetin alfa in the treatment of anemia [abstract no: 1296]. Blood 2006;108(11):380a. [CENTRAL: CN‐00740519] [Google Scholar]
- Barany P, Besarab A, Macdougall IC, Law A, Ouyang Y, Heifets M. Median hemoglobin (Hb) decline following C.E.R.A. dose interruption is similar to that with other erythropoiesis stimulating agents (ESAs) [abstract no: SU‐PO795]. Journal of the American Society of Nephrology 2007;18(Abstracts Issue):760A. [CENTRAL: CN‐00794522] 17267745 [Google Scholar]; Chanu P, Gieschke R, Reigner B, Dougherty FC. Pharmacokinetic parameters of C.E.R.A. and are not affected by age in patients with chronic kidney disease on dialysis [abstract no: SaP351]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi351. [CENTRAL: CN‐00757500] [Google Scholar]; Chanu P, Gieschke R, Reigner B, Dougherty FC. Pharmacokinetics of C.E.R.A. and stable maintenance of haemoglobin (Hb) levels with once‐monthly dosing in patients with chronic kidney disease (CKD) [abstract no: SaP325]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi342. [CENTRAL: CN‐00757502] [Google Scholar]; Fishbane S, Bernardo M, Locatelli F, Aguila M, Edwardes M, Bexon M. Once‐monthly intravenous (IV) C.E.R.A. maintains stable hemoglobin (Hb) in dialysis patients (pts), irrespective of age or gender [abstract no: SU‐PO779]. Journal of the American Society of Nephrology 2007;18(Abstracts Issue):756A. [CENTRAL: CN‐00757405] [Google Scholar]; Fishbane S, Dalton C, Beswick R, Dutka P, Schmidt R. Efficacy of C.E.R.A., a continuous erythropoietin receptor activator, in the treatment of renal anemia: overview of 6 global phase 3 trials [abstract no: 59]. American Journal of Kidney Diseases 2007;49(4):A39. [CENTRAL: CN‐00756571] [Google Scholar]; Fishbane S, Levin NW, Mann JFE, Lewis JL, Bernardo M, Lunde NM, et al. IV C.E.R.A. (Continuous Erythropoietin Receptor Activator) once every 2 weeks or once monthly maintains stable Hb levels after converting directly from IV epoetin 1‐3 times per week in patients with CKD on dialysis [abstract no: SA‐PO205]. Journal of the American Society of Nephrology 2006;17(Abstracts):618A. [CENTRAL: CN‐00757097] [Google Scholar]; Imbasciati E, Bernardo M, Caraman P, David‐Neto E, Harris K, Law A, et al. Stable haemoglobin (Hb) levels are maintained with once‐monthly C.E.R.A. in dialysis patients with varying C‐reactive protein (CRP), albumin or dialysis adequacy [abstract no: SuO005]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi412. [CENTRAL: CN‐00758076] [Google Scholar]; Levin NW, Fishbane S, Canedo FV, Zeig S, Nassar GM, Moran JE, et al. Intravenous methoxy polyethylene glycol‐epoetin beta for haemoglobin control in patients with chronic kidney disease who are on dialysis: a randomised non‐inferiority trial (MAXIMA). Lancet 2007;370(9596):1415‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Levin NW, Imbasciati E, Combe C, Rocco MV, Lok CE, Donnelly SM, et al. Adequate Hb levels are maintained with IV C.E.R.A. (Continuous Erythropoietin Receptor Activator) administered up to once monthly in dialysis patients irrespective of age, gender or diabetic status [abstract no: SA‐PO206]. Journal of the American Society of Nephrology 2006;17(Abstracts):619A. [CENTRAL: CN‐00755210] [Google Scholar]; Mann J, Locatelli F, Sulowicz W, Nissenson A, Portoles J, Levin N, et al. C.E.R.A. provides stable haemoglobin (Hb) levels in CKD patients on dialysis with and without coronary artery disease (CAD) or diabetes mellitus (DM) when administered once monthly [abstract no: SuO002]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi411. [CENTRAL: CN‐00791257] [Google Scholar]; Ryckelynck JP, Valdes Canedo F, Riella M, Lempert K, Donnelly S, Adrogue H, et al. Once‐monthly C.E.R.A. maintains stable haemoglobin concentrations in dialysis patients regardless of gender or age [abstract no: SUO001]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi411. [CENTRAL: CN‐00644372] [Google Scholar]
- Milutinovic S, Krpan D, Drenovac M. Erythropoietin (EPO) omega improves cognitive functioning and quality of life in dialysis patients in comparison to ALFA [abstract no: PUB233]. Journal of the American Society of Nephrology 2002;13(Program & Abstracts):718a. [CENTRAL: CN‐00583281] [Google Scholar]; Milutinovic S, Milutinovic E, Plavljanic D, Kusec V. Differences in glycosylation structures have an important impact on potency and pharmacokinetics of erythropoietin (EPO) in dialyzed uremics [abstract]. Nephrology Dialysis Transplantation 2000;15(9):A156. [CENTRAL: CN‐00461327] [Google Scholar]; Milutinovic S, Milutinovic E, Plavljanic D, Kusec V. Erythropoietin‐induced hypertension in dialyzed uremics is influenced by glycosylation patterns of the molecule [abstract]. Nephrology Dialysis Transplantation 2001;16(6):A91. [CENTRAL: CN‐00583279] [Google Scholar]; Milutinovic S, Milutinovic E, Plavljanic D, Kusec V. Molecular glycosylation patterns have an impact on erythropoietin‐induced hypertension in dialyzed uremics [abstract]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):399A. [CENTRAL: CN‐00626113] [Google Scholar]; Milutinovic S, Plavljani E, Trkulja V. Comparison of two epoetin brands in anemic hemodialysis patients: results of two efficacy trials and a single‐dose pharmacokinetic study. Fundamental & Clinical Pharmacology 2006;20(5):493‐502. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Milutinovic S, Trkulja V. Reduced responsiveness to epoetin at re‐exposure after prolonged epoetin‐free period in anemic hemodialysis patients with end‐stage renal disease. Croatian Medical Journal 2006;47(3):424‐32. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
- Nissenson AR, Korbet S, Faber M, Burkart J, Gentile D, Hamburger R, et al. Multicenter trial of erythropoietin in patients on peritoneal dialysis. Journal of the American Society of Nephrology 1995;5(7):1517‐29. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Nissenson A, Krishnan M, Liu W, McCary L, Stehman‐Breen C, Mix C. Hemoglobin (Hb) variability does not differ between hemodialysis (HD) patients treated with epoetin alfa and darbepoetin alfa [abstract no: TH‐PO360]. Journal of the American Society of Nephrology 2006;17(Abstracts):183A. [CENTRAL: CN‐00740539] [Google Scholar]; Nissenson AR. Dosing darbepoetin alfa. American Journal of Kidney Diseases 2002;40(4):872. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Nissenson AR, Swan SK, Lindberg JS, Soroka SD, Beatey R, Wang C, et al. Randomized, controlled trial of darbepoetin alfa for the treatment of anemia in hemodialysis patients. American Journal of Kidney Diseases 2002;40(1):110‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Nissenson AR, Swan SK, Lindberg JS, Soroka SD, McDermott‐Vitak AD, Wang C, et al. Novel Erythropoiesis stimulating protein (NESP) safely maintains hemoglobin concentration levels in hemodialysis patients as effectively as r‐huEPO when administered once weekly [abstract no: A1326]. Journal of the American Society of Nephrology 2000;11(Sept):252A. [CENTRAL: CN‐00794722] [Google Scholar]; Nissenson AR, US/Canadian NESP 980117 Study Group. Once weekly IV novel erythoropoiesis stimulating protein (NESP) effectively maintains Hb in hemodialysis patients [abstract]. Nephrology Dialysis Transplantation 2001;16(6):A92. [CENTRAL: CN‐00446969] [Google Scholar]
- Palazzuoli A, Silverberg DS, Iovine F, Calabro A, Campagna MS, Gallotta M, et al. Effects of beta‐erythropoietin treatment on left ventricular remodeling, systolic function, and B‐type natriuretic peptide levels in patients with the cardiorenal anemia syndrome. American Heart Journal 2007;154(4):645.e9‐15. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Patel M, Thimons DG, Winston JL, Langholff W, McGowan T. An open‐label, randomized, multicenter, controlled study of epoetin alfa for the treatment of anemia of chronic kidney disease in the long term care setting. Journal of the American Medical Directors Association 2012;13(3):244‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Carrera F. C.E.R.A. vs darbepoetin alfa as maintenance therapy for anaemia in patients with chronic kidney disease (CKD): The PATRONUS Study [abstract no: M558]. World Congress of Nephrology; 2009 May 22‐26; Milan, Italy. 2009. [Google Scholar]; Carrera F, Lok CE, Francisco A, Locatelli F, Mann JF, Canaud B, et al. Maintenance treatment of renal anaemia in haemodialysis patients with methoxy polyethylene glycol‐epoetin beta versus darbepoetin alfa administered monthly: a randomized comparative trial. Nephrology Dialysis Transplantation 2010;25(12):4009‐17. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]; Locatelli F, PATRONUS Study Group. Once‐monthly C.E.R.A. is superior to darbepoetin alfa for maintaining hemoglobin levels in hemodialysis patients regardless of age, gender, diabetic status or presence of hyperlipidemia [abstract no: SA‐PO2412]. Journal of the American Society of Nephrology 2009;20:663A. [CENTRAL: CN‐00747328] [Google Scholar]; Mann JF, PATRONUS Study Group. Risk factors for vascular events in hemodialysis patients receiving once‐monthly C.E.R.A. or once‐monthly darbepoetin alfa: post hoc analysis of the PATRONUS Study [abstract no: PUB414]. Journal of the American Society of Nephrology 2009;20:921A. [CENTRAL: CN‐00747329] [Google Scholar]; Francisco AL, PATRONUS Study Group. Significantly higher hemoglobin response rates are achieved in hemodialysis patients with once‐monthly C.E.R.A. compared with darbepoetin alfa in hemodialysis, regardless of etiology of chronic kidney disease [abstract no: SA‐PO2411]. Journal of the American Society of Nephrology 2009;20:663A. [CENTRAL: CN‐00747327] [Google Scholar]
- Barany P, Besarab A, Macdougall IC, Law A, Ouyang Y, Heifets M. Median hemoglobin (Hb) decline following C.E.R.A. dose interruption is similar to that with other erythropoiesis stimulating agents (ESAs) [abstract no: SU‐PO795]. Journal of the American Society of Nephrology 2007;18(Abstracts Issue):760A. [CENTRAL: CN‐00794522] 17267745 [Google Scholar]; Chanu P, Gieschke R, Reigner B, Dougherty FC. Pharmacokinetic parameters of C.E.R.A. and are not affected by age in patients with chronic kidney disease on dialysis [abstract no: SaP351]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi351. [CENTRAL: CN‐00757500] [Google Scholar]; Chanu P, Gieschke R, Reigner B, Dougherty FC. Pharmacokinetics of C.E.R.A. and stable maintenance of haemoglobin (Hb) levels with once‐monthly dosing in patients with chronic kidney disease (CKD) [abstract no: SaP325]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi342. [CENTRAL: CN‐00757502] [Google Scholar]; Fishbane S, Dalton C, Beswick R, Dutka P, Schmidt R. Efficacy of C.E.R.A., a continuous erythropoietin receptor activator, in the treatment of renal anemia: overview of 6 global phase 3 trials [abstract no: 59]. American Journal of Kidney Diseases 2007;49(4):A39. [CENTRAL: CN‐00756571] [Google Scholar]; Imbasciati E, Bernardo M, Caraman P, David‐Neto E, Harris K, Law A, et al. Stable haemoglobin (Hb) levels are maintained with once‐monthly C.E.R.A. in dialysis patients with varying C‐reactive protein (CRP), albumin or dialysis adequacy [abstract no: SuO005]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi412. [CENTRAL: CN‐00758076] [Google Scholar]; Locatelli F, Sulowicz W, Harris K, Selgas R, Kaufman J, Klinger M, et al. SC C.E.R.A. (Continuous Erythropoietin Receptor Activator) once every 2 weeks or once monthly maintains stable Hb levels after converting directly from SC epoetin 1‐3 times per week in patients with CKD on dialysis [abstract no: SA‐PO207]. Journal of the American Society of Nephrology 2006;17(Abstracts):619A. [CENTRAL: CN‐00626009] [Google Scholar]; Mann J, Locatelli F, Sulowicz W, Nissenson A, Portoles J, Levin N, et al. C.E.R.A. provides stable haemoglobin (Hb) levels in CKD patients on dialysis with and without coronary artery disease (CAD) or diabetes mellitus (DM) when administered once monthly [abstract no: SuO002]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi411. [CENTRAL: CN‐00791257] [Google Scholar]; Ryckelynck JP, Harris K, Selgas R, Stompor T, Ladanyi E, Opatrna S, et al. SC C.E.R.A. (Continuous Erythropoietin Receptor Activator) administered up to once monthly in patients with CKD on dialysis maintains adequate Hb levels regardless of age, gender or diabetic status [abstract no: SA‐PO210]. Journal of the American Society of Nephrology 2006;17(Abstracts):620A. [CENTRAL: CN‐00644371] [Google Scholar]; Ryckelynck JP, Valdes Canedo F, Riella M, Lempert K, Donnelly S, Adrogue H, et al. Once‐monthly C.E.R.A. maintains stable haemoglobin concentrations in dialysis patients regardless of gender or age [abstract no: SUO001]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi411. [CENTRAL: CN‐00644372] [Google Scholar]; Sulowicz W, Locatelli F, Balla J, Csiky B, Rikker C, Aldigier J, et al. Subcutaneous (SC) C.E.R.A. (Continuous Erythropoietin Receptor Activator) administered once every 2 weeks or once monthly maintains haemoglobin (Hb) levels in patients with chronic kidney disease (CKD) on dialysis [abstract no: SP424]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv156‐7. [CENTRAL: CN‐00601914] [Google Scholar]; Sulowicz W, Locatelli F, Ryckelynck JP, Balla J, Csiky B, Harris K, et al. Once‐monthly subcutaneous C.E.R.A. maintains stable hemoglobin control in patients with chronic kidney disease on dialysis and converted directly from epoetin one to three times weekly. Clinical Journal of the American Society of Nephrology 2007;2(4):637‐46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Benz R, Teehan B, Roth D, Buckalew V, Freedman B, Hatch F, et al. Renal function and quality of life (QOL) studies in anemic, pre‐dialysis chronic renal failure (CRF) patients receiving recombinant human erythropoietin (r‐HuEPO): results of a multi‐center trial [abstract]. 12th International Congress of Nephrology; 1993 Jun 13‐18; Jerusalem, Israel. 1993:316. [CENTRAL: CN‐00602029] [Google Scholar]; Revicki DA, Brown RE, Feeny DH, Henry D, Teehan BP, Rudnick MR, et al. Health‐related quality of life associated with recombinant human erythropoietin therapy for predialysis chronic renal disease patients. American Journal of Kidney Diseases 1995;25(4):548‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Roth D, Smith RD, Schulman G, Steinman TI, Hatch FE, Rudnick MR, et al. Effects of recombinant human erythropoietin on renal function in chronic renal failure predialysis patients. American Journal of Kidney Diseases 1994;24(5):777‐84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Barany P, Besarab A, Macdougall IC, Law A, Ouyang Y, Heifets M. Median hemoglobin (Hb) decline following C.E.R.A. dose interruption is similar to that with other erythropoiesis stimulating agents (ESAs) [abstract no: SU‐PO795]. Journal of the American Society of Nephrology 2007;18(Abstracts Issue):760A. [CENTRAL: CN‐00794522] 17267745 [Google Scholar]; Fishbane S, Dalton C, Beswick R, Dutka P, Schmidt R. Efficacy of C.E.R.A., a continuous erythropoietin receptor activator, in the treatment of renal anemia: overview of 6 global phase 3 trials [abstract no: 59]. American Journal of Kidney Diseases 2007;49(4):A39. [CENTRAL: CN‐00756571] [Google Scholar]; Spinowitz B, Coyne DW, Fraticelli M, Azer M, Dalal S, Villa G, et al. C.E.R.A. (continuous erythropoietin receptor activator) administered once every 2 weeks via pre‐filled syringe (PFS) maintains stable Hb levels in patients with CKD on dialysis [abstract no: PUB376]. Journal of the American Society of Nephrology 2006;17(Abstracts):895A. [CENTRAL: CN‐00653773] [Google Scholar]; Spinowitz B, Coyne DW, Lok CE, Fraticelli M, Azer M, Dalal S, et al. C.E.R.A. maintains stable control of hemoglobin in patients with chronic kidney disease on dialysis when administered once every two weeks. American Journal of Nephrology 2008;28(2):280‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Shaheen FAM, Al‐Aqeil N A, Badawi L, Sheikh IA, Shalabi NM, Adiku W, et al. Correction of anemia by erythropoietin in pre‐dialysis patients. Saudi Kidney Diseases & Transplantation Bulletin 1993;4(3):215‐9. [CENTRAL: CN‐00525736] [Google Scholar]
- Lynn KL, Buttimore AL, Inkster JA, Divakar D, Mylius AL, Ikram H, et al. Echocardiographic assessment of cardiac effects of erythropoietin in haemodialysis patients [abstract]. 9th Asian Colloquium in Nephrology; 1992 May 17‐21; Seoul, Korea. 1992:183. [CENTRAL: CN‐00461224] [Google Scholar]; Lynn KL, Richards AM, Buttimore AL, Inkster JA, Bailey RR, Robson RA, et al. Placebo‐controlled study of blood pressure and vasoactive hormones in haemodialysis patients on erythropoietin [abstract]. 9th Asian Colloquium in Nephrology; 1992 May 17‐21; Seoul, Korea. 1992:183. [CN‐00461225] [Google Scholar]; Shand B I, Buttimore AL, Hurrell MA, Wells JE, Inkster JA, Bailey RR, et al. Hemorheology and fistula function in home hemodialysis patients following erythropoietin treatment: a prospective placebo‐controlled study. Nephron 1993;64(1):53‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sikole A, Polenakovic M, Spirovska V, Polenakovic B, Masin G. Analysis of heart morphology and function following erythropoietin treatment of anemic dialysis patients. Artificial Organs 1993;17(12):977‐84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Sikole A, Polenakovic M, Spirovska V, Polenakovic B, Masin G. Echocardiographic analysis in patients on haemodialysis treated with erythropoietin [abstract]. Nephrology Dialysis Transplantation 1993;8(9):964. [CENTRAL: CN‐00260828] [Google Scholar]
- Pratt R. Pharmacokinetics of erythropoietin by a human cell line (Epoetin I): subcutaneous vs intravenous dosing in patients with chronic kidney disease [abstract no: 1137]. Haematologica 2006;91(Suppl 1):414. [CENTRAL: CN‐00716122] [Google Scholar]; Pratt RD. Epoetin delta for the treatment of anemia in patients with CKD not requiring hemodialysis [abstract no: TH‐PO377]. Journal of the American Society of Nephrology 2006;17(Abstracts):187A. [CENTRAL: CN‐00765050] [Google Scholar]; Pratt RD, Dowell J. Pharmacokinetics of epoetin delta: a new erythropoietin produced by gene‐activation in a human cell line [abstract no: TH‐PO378]. Journal of the American Society of Nephrology 2006;17(Abstracts):187A. [CENTRAL: CN‐00644158] [Google Scholar]; Smith WB, Dowell JA, Pratt RD. Pharmacokinetics and pharmacodynamics of epoetin delta in two studies in healthy volunteers and two studies in patients with chronic kidney disease. Clinical Therapeutics 2007;29(7):1368‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pratt R. Epoetin delta, erythropeitin produced by a human cell line, is effective in the treatment of renal anemia [abstract]. Haematologica 2006;91(Suppl):213. [CENTRAL: CN‐00716123] [Google Scholar]; Spinowitz BS, Pratt RD, Epoetin Delta 2002 Study Group. Epoetin delta is effective for the management of anaemia associated with chronic kidney disease. Current Medical Research & Opinion 2006;22(12):2507‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Barany P, Besarab A, Macdougall IC, Law A, Ouyang Y, Heifets M. Median hemoglobin (Hb) decline following C.E.R.A. dose interruption is similar to that with other erythropoiesis stimulating agents (ESAs) [abstract no: SU‐PO795]. Journal of the American Society of Nephrology 2007;18(Abstracts Issue):760A. [CENTRAL: CN‐00794522] 17267745 [Google Scholar]; Canaud B, Braun J, Locatelli F, Villa G, Vlem B, Sanz Guajardo D, et al. Intravenous (IV) C.E.R.A. (continuous erythropoietin receptor activator) administered once every 2 weeks maintains stable haemoglobin (Hb) levels in patients with chronic kidney disease (CKD) on dialysis [abstract no: SP425]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv157. [CENTRAL: CN‐00690645] [Google Scholar]; Canaud B, Mingardi G, Braun J, Aljama P, Kerr PG, Locatelli F, et al. Intravenous C.E.R.A. maintains stable haemoglobin levels in patients on dialysis previously treated with darbepoetin alfa: results from STRIATA, a randomized phase III study. Nephrology Dialysis Transplantation 2008;23(11):3654‐61. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]; Fishbane S, Dalton C, Beswick R, Dutka P, Schmidt R. Efficacy of C.E.R.A., a continuous erythropoietin receptor activator, in the treatment of renal anemia: overview of 6 global phase 3 trials [abstract no: 59]. American Journal of Kidney Diseases 2007;49(4):A39. [CENTRAL: CN‐00756571] [Google Scholar]
- Teehan BP, Sigler MH, Brown JM, Benz RL, Gilgore GS, Schleifer CR, et al. Hematologic and physiologic studies during correction of anemia with recombinant human erythropoietin in predialysis patients. Transplantation Proceedings 1989;21(6 Suppl 2):63‐6. [EMBASE: 1990284570] 2545019 [Google Scholar]
- Campistol JM, Carreno A, Morales JM, Pallardo L, Franco A, Navarro D, et al. Once‐monthly pegylated epoetin beta versus darbepoetin alfa every two weeks in renal transplant recipients: a randomized trial. Transplantation 2013;95(2):e6‐e10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Carreno A, Campistol JM, Arias M, Morales JM, Pallardo L, Franco A. A randomised, multicenter, phase IIIb clinical trial to evaluate efficacy and safety of C.E.R.A. once a month versus darbepoetin alfa in renal transplant recipients with chronic renal anaemia (TIVOLI Study Group) [abstract no: F164]. NDT Plus 2011;4(Suppl 2):4.s2.32. [Google Scholar]; Carreno A, Campistol JM, Arias M, Morales JM, Pallardo L, Franco A. A randomised, multicenter, phase IIIb clinical trial to evaluate efficacy and safety of C.E.R.A. once a month versus darbepoetin alfa in renal transplant recipients with chronic renal anaemia (TIVOLI study group) [abstract no: 27]. American Journal of Transplantation 2011;11(Suppl 2):36. [EMBASE: 70405062] [Google Scholar]
- Tolman C, Richardson D, Bartlett C, Will E. A randomised study of weekly subcutaneous aranesp and neorecormon in a large unselected haemodialysis cohort, managed with computer assisted anaemia alogrithms [abstract]. 41st Congress. European Renal Association. European Dialysis and Transplantation Association; 2004 May 15‐18; Lisbon, Portugal. 2004:229‐30. [CENTRAL: CN‐00509514] [Google Scholar]; Tolman C, Richardson D, Bartlett C, Will E. Application of computer assisted anaemia management algorithms in haemodialysis patients produces predictable haemoglobin outcomes regardless of the erythropoietic agent or frequency of administration: results of a randomised study [abstract]. 41st Congress. European Renal Association. European Dialysis and Transplantation Association; 2004 May 15‐18; Lisbon, Portugal. 2004:110. [CENTRAL: CN‐00509513] [Google Scholar]; Tolman C, Richardson D, Bartlett C, Will E. Structured conversion from thrice weekly to weekly erythropoietic regimens using a computerized decision‐support system: a randomized clinical study. Journal of the American Society of Nephrology 2005;16(5):1463‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Tolman C, Richardson D, Bartlett C, Will EJ. Dose conversion ratio (DCR) from subcutaneous epoetin‐b (EPO) to weekly darbepoetin‐a (DA) is dependent on baseline sensitivity: results from a randomised study [abstract no: SA‐PO461]. Journal of the American Society of Nephrology 2004;15(Oct):404A. [CENTRAL: CN‐00583282] [Google Scholar]; West RM, Harris K, Gilthorpe MS, Tolman C, Will EJ. Functional data analysis applied to a randomized controlled clinical trial in hemodialysis patients describes the variability of patient responses in the control of renal anemia. Journal of the American Society of Nephrology 2007;18(8):2371‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Desai AS, Toto R, Jarolim P, Uno H, Eckardt KU, Kewalramani R, et al. Association between cardiac biomarkers and the development of ESRD in patients with type 2 diabetes mellitus, anemia, and CKD. American Journal of Kidney Diseases 2011;58(5):717‐28. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Lewis EF, Pfeffer MA, Feng A, Uno H, McMurray JJ, Toto R, et al. Darbepoetin alfa impact on health status in diabetes patients with kidney disease: a randomized trial. Clinical Journal of The American Society of Nephrology: CJASN 2011;6(4):845‐55. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]; McMurray JJ, Uno H, Jarolim P, Desai AS, Zeeuw D, Eckardt KU, et al. Predictors of fatal and nonfatal cardiovascular events in patients with type 2 diabetes mellitus, chronic kidney disease, and anemia: an analysis of the Trial to Reduce cardiovascular Events with Aranesp (darbepoetin‐alfa) Therapy (TREAT). American Heart Journal 2011;162(4):748‐55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Mix TC, Brenner RM, Cooper ME, Zeeuw D, Ivanovich P, Levey AS, et al. Rationale‐‐Trial to Reduce Cardiovascular Events with Aranesp Therapy (TREAT): evolving the management of cardiovascular risk in patients with chronic kidney disease. American Heart Journal 2005;149(3):408‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Pfeffer MA, Burdmann EA, Chen C, Cooper ME, Zeeuw D, Eckardt K, et al. Trial to reduce cardiovascular events with Aranesp therapy [abstract]. Circulation 2010;120(21):2154‐5. [EMBASE: 70089295] [Google Scholar]; Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, Zeeuw D, Eckardt KU, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. New England Journal of Medicine 2009;361(21):2019‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, Zeeuw D, Eckardt KU, et al. Baseline characteristics in the Trial to Reduce Cardiovascular Events With Aranesp Therapy (TREAT). American Journal of Kidney Diseases 2009;54(1):59‐69. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Pfeffer MA, TREAT Executive Committee. An ongoing study of anemia correction in chronic kidney disease. New England Journal of Medicine 2007;356(9):959‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Skali H, Lin J, Pfeffer MA, Chen CY, Cooper ME, McMurray JJ, et al. Hemoglobin stability in patients with anemia, CKD, and type 2 diabetes: an analysis of the TREAT (Trial to Reduce Cardiovascular Events With Aranesp Therapy) placebo arm. American Journal of Kidney Diseases 2013;61(2):238‐46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Skali H, Parving HH, Parfrey PS, Burdmann EA, Lewis EF, Ivanovich P, et al. Stroke in patients with type 2 diabetes mellitus, chronic kidney disease, and anemia treated with darbepoetin alfa: the trial to reduce cardiovascular events with Aranesp therapy (TREAT) experience. Circulation 2011;124(25):2903‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Solomon SD, Uno H, Lewis EF, Eckardt KU, Lin J, Burdmann EA, et al. Erythropoietic response and outcomes in kidney disease and type 2 diabetes. New England Journal of Medicine 2010;363(12):1146‐55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Thomas MC, Cooper ME, Rossing K, Parving HH. Anaemia in diabetes: Is there a rationale to TREAT?. Diabetologia 2006;49(6):1151‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Toto R, Ivanovich P, Levey A, Parfrey PS, Pereira BJ, Remuzzi G, et al. Trial to Reduce cardiovascular Events with Aranesp® (darbepoetin alfa) Therapy (TREAT) [abstract no: SU‐PO239]. Journal of the American Society of Nephrology 2004;15(Oct):584A. [CENTRAL: CN‐00550766] [Google Scholar]
- Biesen W, Vanholder R, Veys N, Verbeke F, Lameire N. Efficacy of erythropoietin administration in the treatment of anemia immediately after renal transplantation. Transplantation 2005;79(3):367‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Loo A, Vanholder R. Recombinant human erythropoietin (Rhu‐EPO) corrects anaemia during the first weeks after renal transplantation (RTP) [abstract]. Nephrology Dialysis Transplantation 1996;11(6):A265. [CENTRAL: CN‐00261322] [DOI] [PubMed] [Google Scholar]; Loo A, Vanholder R, Bernaert P, Roose J, Lameire N. Recombinant human erythropoietin corrects anaemia during the first weeks after renal transplantation: a randomized prospective study. Nephrology Dialysis Transplantation 1996;11(9):1815‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Loo A, Vanholder R, Bernaert P, Lamiere N. Recombinant human erythropoietin (Rhu‐EPO) corrects anaemia during the first weeks after renal transplantation (RTP) [abstract]. Nephrology Dialysis Transplantation 1997;12(3):631. [CN‐00261344] [DOI] [PubMed] [Google Scholar]; Vanholder R, Bernaert P, Loo A, Lameire N, Ringoir S. Recombinant human erythropoietin corrects early post‐transplantation anemia: a randomized prospective study [abstract]. Journal of the American Society of Nephrology 1995;6(3):1120. [CENTRAL: CN‐00486286] [Google Scholar]
- Canaud B. Darbepoetin alfa dose requirements for IV and SC administration are equivalent in anaemic dialysis patients [abstract no: M319]. Nephrology Dialysis Transplantation 2002;17(Suppl 12):137. [CENTRAL: CN‐00550478] [Google Scholar]; Kerr PG, Harris D, Hawley C, Walker R, European/Australian NESP 970290 Study Group. Novel erythropoiesis stimulating protein (NESP) maintains haemoglobin in ESRD patients with administered once weekly or once every other week [abstract no: 181]. Nephrology 2000;5(3):A112. [CENTRAL: CN‐00509271] [Google Scholar]; Vanrenterghem Y, Barany P, Mann J, European/Australian NESP 970290 Study Group. Novel erythropoiesis stimulating protein (NESP) maintains hemoglobin (hgb) in ESRD patients when administered once weekly or once every other week [abstract]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):270A. [CENTRAL: CN‐00583820] [Google Scholar]; Vanrenterghem Y, Barany P, Mann JF, Kerr PG, Wilson J, Baker NF, et al. Randomized trial of darbepoetin alfa for treatment of renal anemia at a reduced dose frequency compared with rHuEPO in dialysis patients. Kidney International 2002;62(6):2167‐75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Watson A, Gimenez L, Walser M, Cotton S, Spivak J. A prospective double‐blind study of subcutaneous recombinant human erythropoietin in predialysis renal failure [abstract]. Journal of Clinical Pharmacology 1989;29(929):856. [Google Scholar]; Watson AJ, Gimenez LF, Cotton S, Walser M, Spivak JL. Treatment of the anemia of chronic renal failure with subcutaneous recombinant human erythropoietin. American Journal of Medicine 1990;89(4):432‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Acchiardo SR, Quinn BP, Moore LW, Burk LB, Miles DE. Evaluation of hemodialysis patients treated with erythropoietin. American Journal of Kidney Diseases 1991;17(3):290‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Laville M, Anaemia CORrection in Diabetes trial. New strategies in anaemia management: ACORD (Anaemia CORrection in Diabetes) trial. Acta Diabetologica 2004;41 Suppl 1:S18‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Ritz E, Bilous R, O'Donoghue D, Laville M, Alvaro F. Prescription patterns of cardio‐ and reno‐protective agents in early diabetic nephropathy: baseline data from the ACORD trial [abstract no: PUB100]. Journal of the American Society of Nephrology 2004;15(Oct):783A. [CENTRAL: CN‐00550505] [Google Scholar]; Ritz E, Bilous RW, Alvaro F, Laville M, O'Donoghue D, Archerhag A. Anemia correction with epoetin beta in patients with diabetes and chronic kidney disease ‐ primary results of the anaemia correction in diabetes (ACORD) study [abstract no: SO022]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv11. [CENTRAL: CN‐00763625] [Google Scholar]; Ritz E, Laville M, Bilous R W, O'Donoghue D, Scherhag A, Burger U, et al. Target level for hemoglobin correction in patients with diabetes and CKD: primary results of the Anemia Correction in Diabetes (ACORD) Study. American Journal of Kidney Diseases 2007;49(2):194‐207. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Francisco AL, Sulowicz W, Dougherty FC. Subcutaneous CERA (continuous erythropoiesis receptor activator) has potent erythropoietic activity in dialysis patients with chronic renal anemia: an exploratory multiple‐dose study [abstract]. Journal of the American Society of Nephrology 2003;14(Nov):27A‐8A. [CENTRAL: CN‐00550366] [Google Scholar]; Francisco AL, Sulowicz W, Klinger M, Niemczyk S, Vargemezis V, Metivier F, et al. Continuous Erythropoietin Receptor Activator (C.E.R.A.) administered at extended administration intervals corrects anaemia in patients with chronic kidney disease on dialysis: a randomised, multicentre, multiple‐dose, phase II study. International Journal of Clinical Practice 2006;60(12):1687‐96. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Besarab A, Beyer U, Dougherty FC. Long‐term intravenous CERA (Continuous Erythropoietin Receptor Activator) maintains hemoglobin concentrations in hemodialysis patients [abstract no: W‐PO40127]. Nephrology 2005;10(Suppl 1):A312‐3. [CENTRAL: CN‐00747326] [Google Scholar]; Besarab A, Salifu MO, Lunde NM, Bansal V, Fishbane S, Dougherty FC, et al. Efficacy and tolerability of intravenous continuous erythropoietin receptor activator: a 19‐week, phase II, multicenter, randomized, open‐label, dose‐finding study with a 12‐month extension phase in patients with chronic renal disease. Clinical Therapeutics 2007;29(4):626‐39. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Dougherty FC, Beyer U. No changes in blood pressure in dialysis patients after 12 months of treatment with IV/SC CERA (Continuous Erythropoietin Receptor Activator) [abstract no: W‐PO40131]. Nephrology 2005;10(Suppl 1):A313‐4. [CENTRAL: CN‐00602138] [Google Scholar]; Dougherty FC, Beyer U. Safety and tolerability profile of continuous erythropoietin receptor activator (CERA) with extended dosing intervals in patients with chronic kidney disease on dialysis [abstract no: W‐PO40130]. Nephrology 2005;10(Suppl 1):A313. [CENTRAL: CN‐00602137] [Google Scholar]; Dougherty FC, Loghman‐Adham M, Schultze N, Beyer U. Adequate hemoglobin levels are maintained with continuous erythropoietin receptor activator (CERA) in dialysis patients regardless of gender, age, race and diabetic status [abstract no: MP206]. Nephrology Dialysis Transplantation 2005;20(Suppl 5):v269. [CENTRAL: CN‐00602143] [Google Scholar]; Dutka P, Tilocca P, BA16285 and BA16286 Study Groups. CERA (Continuous Erythropoietin Receptor Activator) maintains stable hemoglobin concentrations in dialysis patients irrespective of gender, age, race or diabetic status [abstract]. Nephrology Nursing Journal 2006;33(2):138. [CENTRAL: CN‐00602144] [Google Scholar]
- Dougherty FC, Beyer U. No changes in blood pressure in dialysis patients after 12 months of treatment with IV/SC CERA (Continuous Erythropoietin Receptor Activator) [abstract no: W‐PO40131]. Nephrology 2005;10(Suppl 1):A313‐4. [CENTRAL: CN‐00602138] [Google Scholar]; Dougherty FC, Beyer U. Safety and tolerability profile of continuous erythropoietin receptor activator (CERA) with extended dosing intervals in patients with chronic kidney disease on dialysis [abstract no: W‐PO40130]. Nephrology 2005;10(Suppl 1):A313. [CENTRAL: CN‐00602137] [Google Scholar]; Dougherty FC, Loghman‐Adham M, Schultze N, Beyer U. Adequate hemoglobin levels are maintained with continuous erythropoietin receptor activator (CERA) in dialysis patients regardless of gender, age, race and diabetic status [abstract no: MP206]. Nephrology Dialysis Transplantation 2005;20(Suppl 5):v269. [CENTRAL: CN‐00602143] [Google Scholar]; Dutka P, Tilocca P, BA16285 and BA16286 Study Groups. CERA (Continuous Erythropoietin Receptor Activator) maintains stable hemoglobin concentrations in dialysis patients irrespective of gender, age, race or diabetic status [abstract]. Nephrology Nursing Journal 2006;33(2):138. [CENTRAL: CN‐00602144] [Google Scholar]; Locatelli F, Villa G, Arias M, Marchesi D, Dougherty FC, Beyer U, et al. CERA (Continuous Erythropoietin receptor activator) maintains hemoglobin levels in dialysis patients when administered subcutaneously up to once every 4 weeks. [abstract no: SU‐PO051]. Journal of the American Society of Nephrology 2004;15(Oct):543A. [CENTRAL: CN‐00626008] [Google Scholar]; Locatelli F, Villa G, Beyer U, Dougherty FC. Subcutaneous CERA (Continuous Erythropoietin Receptor Activator) maintains hemoglobin concentrations with dosing intervals up to 4 weeks in dialysis patients [abstract no: MP183]. Nephrology Dialysis Transplantation 2005;20(Suppl 5):v261. [CN‐00602142] [Google Scholar]; Locatelli F, Villa G, Francisco AL, Albertazzi A, Adrogue HJ, Dougherty FC, et al. Effect of a continuous erythropoietin receptor activator (C.E.R.A.) on stable haemoglobin in patients with CKD on dialysis: once monthly administration. Current Medical Research & Opinion 2007;23(5):969‐79. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Salifu M, Villa G, Dougherty FC. Adequate hemoglobin levels are maintained with continuous erythropoietin receptor activator (CERA) in dialysis patients with different ranges of iron status and pre‐existing conditions. [abstract no: 138]. American Journal of Kidney Diseases 2006;47(4):A53. [CENTRAL: CN‐00602141] [Google Scholar]
- Berns JS, Rudnick MR, Cohen RM, Bower JD, Wood BC. Effects of normal hematocrit on ambulatory blood pressure in epoetin‐treated hemodialysis patients with cardiac disease. Kidney International 1999;56(1):253‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Berns JS, Rudnick MR, Cohen RM, Maloney A. Effect of normal v. anemic hematocrit on ambulatory blood pressure (ABP) in erythropoietin‐treated hemodialysis (HD) patients [abstract]. Journal of the American Society of Nephrology 1995;6(3):520. [CENTRAL: CN‐00483215] [Google Scholar]; Besarab A, Bolton WK, Browne JK, Egrie JC, Nissenson AR, Okamoto DM, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. New England Journal of Medicine 1998;339(9):584‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Besarab A, Goodkin DA, Nissenson AR, Normal Hematocrit Cardiac Trial Authors. The normal hematocrit study‐‐follow‐up. New England Journal of Medicine 2008;358(4):433‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Conlon P, Kovalik E, Minda SN, Schumm D, Gutman R, Schwab SJ. Normalizing hematocrit in hemodialysis patients does not increase blood pressure [abstract]. Journal of the American Society of Nephrology 1995;6(3):526. [CENTRAL: CN‐00483579] [Google Scholar]; Conlon PJ, Kovalik E, Schumm D, Minda S, Schwab SJ. Normalization of hematocrit in hemodialysis patients does not affect silent ischemia. Renal Failure 2000;22(2):205‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Conlon PJ, Kovalik E, Schumm D, Minda S, Schwab SJ. Normalization of hematocrit in hemodialysis patients with cardiac disease does not increase blood pressure. Renal Failure 2000;22(4):435‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Coyne DW. The health‐related quality of life was not improved by targeting higher hemoglobin in the Normal Hematocrit Trial. Kidney International 2012;82(2):235‐41. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]; Goodkin DA. The Normal Hematocrit Cardiac Trial revisited. Seminars in Dialysis 2009;22(5):495‐502. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Kilpatrick R, Critchlow C, Besarab A, Fishbane S, Stehman‐Breen C, Krishnan M, et al. Epoetin alfa (EPO) responsiveness predicts survival in the normal hematocrit study (NHS) [abstract no: TH‐PO382]. Journal of the American Society of Nephrology 2006;17(Abstracts):188A. [CENTRAL: CN‐00615892] 16338966 [Google Scholar]; Kilpatrick RD, Critchlow CW, Fishbane S, Besarab A, Stehman‐Breen C, Krishnan M, et al. Greater epoetin alfa responsiveness is associated with improved survival in hemodialysis patients. Clinical Journal of The American Society of Nephrology: CJASN 2008;3(4):1077‐83. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brier ME, Gaweda AE, Dailey A, Aronoff GR, Jacobs AA. Randomized trial of model predictive control for improved anemia management. Clinical Journal of The American Society of Nephrology: CJASN 2010;5(5):814‐20. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]; Dailey A, Gaweda A, Jacobs A, Aronoff G, Brier M. Computational intelligence based anemia management system for the dosing of erythropoietin [abstract no: F‐PO833]. Journal of the American Society of Nephrology 2007;18(Abstracts):285A. [CENTRAL: CN‐00774216] [Google Scholar]
- Choukroun G, Kamar N, Dussol B, Etienne I, Cassuto‐Viguier E, Toupance O, et al. Correction of postkidney transplant anemia reduces progression of allograft nephropathy. Journal of the American Society of Nephrology 2012;23(2):360‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]; Choukroun G, Kamar N, Dussol B, Etienne I, Cassuto‐Viguier E, Toupance O, et al. The complete correction of post‐transplant anemia reduces the rate of progression of chronic allograft nephropathy [abstract no: 340]. American Journal of Transplantation 2010;10(Suppl 4):141. [EMBASE: 70463701] [Google Scholar]; Choukroun G, Kamar N, Dussol B, Etienne I, Cassuto‐Viguier E, Toupance O, et al. The complete correction of post‐transplant anemia reduces the rate of progression of chronic allograft nephropathy [abstract no: Sa667]. NDT Plus 2010;3(Suppl 3):iii267‐8. [EMBASE: 70484133] [Google Scholar]; Choukroun G, Rostaing L, Dussol B, Etienne I, Cassuto E, Toupance O, et al. Anemia correction improves quality of life of renal transplant recipients: results of the CAPRIT study [abstract no: SA‐FC440]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):99A‐100A. [CENTRAL: CN‐00724883] [Google Scholar]
- Cassels C. CHOIR silenced as findings show increased risk of CVD outcomes/death. Medscape Medical News, 2006. http://www.medscape.com/viewarticle/539039 (accessed 2 October 2014). ; Inrig JK, Barnhart HX, Reddan D, Patel UD, Sapp S, Califf RM, et al. Effect of hemoglobin target on progression of kidney disease: a secondary analysis of the CHOIR (Correction of Hemoglobin and Outcomes in Renal Insufficiency) trial. American Journal of Kidney Diseases 2012;60(3):390‐401. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]; Inrig JK, Sapp S, Barnhart H, Patel UD, Reddan D, Singh A, et al. Impact of higher hemoglobin targets on blood pressure and clinical outcomes: a secondary analysis of CHOIR. Nephrology Dialysis Transplantation 2012;27(9):3606‐14. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]; McCullough PA, Barnhart HX, Inrig JK, Reddan D, Sapp S, Patel UD, et al. Cardiovascular toxicity of epoetin‐alfa in patients with chronic kidney disease. American Journal of Nephrology 2013;37(6):549‐58. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Reddan D, Szczech L, Sapp S, Bhaduri S, Klausner M, Singh AK, et al. ECG abnormalities among CKD patients with Anemia: baseline data from the CHOIR study [abstract no: SU‐PO241]. Journal of the American Society of Nephrology 2004;15(Oct):585A. [CENTRAL: CN‐00550515] 14978160 [Google Scholar]; Reddan D, Tran L, Singh A. Correction of hemoglobin and outcomes in renal insufficiency (CHOIR): study design [abstract]. American Journal of Kidney Diseases 2002;39(4):A27. [CENTRAL: CN‐00402331] [Google Scholar]; Reddan D, Tran LL, Jollis J, Singh A. Anemia correction and left ventricular hypertrophy: an echocardiographic substudy of the correction of hemoglobin and outcomes in renal insufficiency (CHOIR) study [abstract no: PUB028]. Journal of the American Society of Nephrology 2002;13(Program & Abstracts):676A. [CENTRAL: CN‐00447363] [Google Scholar]; Singh AK, Bhaduri S, Tang KL, Klausner M, Corwin M, Reddan D. Factors associated with hemoglobin (HB) response in patients (pts) with anemia of chronic kidney disease (CKD) [abstract no: SA‐PO726]. Journal of the American Society of Nephrology 2003;14(Nov):457A. [CENTRAL: CN‐00550451] [Google Scholar]; Singh AK, Day B, Szczech L, Bhaduri S, Klausner M, Obeng A, et al. Iron deficiency in CKD patients: baseline data from the CHOIR study [abstract no: SU‐PO240]. Journal of the American Society of Nephrology 2004;15(Oct):585A. [CENTRAL: CN‐00550452] 14978160 [Google Scholar]; Singh AK, Day B, Szczech L, Bhaduri S, Klausner M, Reddan D, et al. Medication use among CKD patients with cardiac disease: baseline data from the CHOIR study [abstract no: PUB173]. Journal of the American Society of Nephrology 2004;15(Oct):799A. [CENTRAL: CN‐00550455] [Google Scholar]; Singh AK, Day B, Szczech L, Bhaduri S, Klausner M, Reddan D, et al. Medication use among diabetic and non‐diabetic CKD patients: baseline data from the CHOIR study [abstract no: SU‐PO200]. Journal of the American Society of Nephrology 2004;15(Oct):575A‐6A. [CENTRAL: CN‐00550456] 14978159 [Google Scholar]; Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, et al. Correction of anemia with epoetin alfa in chronic kidney disease. New England Journal of Medicine 2006;355(20):2085‐98. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Singh AK, Szczech LA, Tang L, Sapp S, Wolfson M, Reddan DN. The effect of correcting anemia using epoietin‐alfa in patients with chronic kidney disease: results of the correction of hemoglobin and outcomes in renal insufficiency (CHOIR) study [abstract no: F‐FC091]. Journal of the American Society of Nephrology 2006;17(Abstracts):56A. [CENTRAL: CN‐00601974] [Google Scholar]; Szczech L. Post hoc analysis of the associations between hemoglobin and outcomes in CHOIR [abstract]. Renal Week; 2007 Oct 31‐Nov 5; San Francisco, CA. 2007. [CENTRAL: CN‐00757554] [Google Scholar]; Szczech LA, Barnhart HX, Inrig JK, Reddan DN, Sapp S, Califf RM, et al. Secondary analysis of the CHOIR trial epoetin‐alpha dose and achieved hemoglobin outcomes. Kidney International 2008;74(6):791‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]; Szczech LA, Barnhart HX, Sapp S, Felker GM, Hernandez A, Reddan D, et al. A secondary analysis of the CHOIR trial shows that comorbid conditions differentially affect outcomes during anemia treatment. Kidney International 2010;77(3):239‐46. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianciaruso B, Ravani P, Barrett BJ, Levin A, ITA‐EPO‐7 investigators. Italian randomized trial of hemoglobin maintenance to prevent or delay left ventricular hypertrophy in chronic kidney disease. Journal of Nephrology 2008;21(6):861‐70. [MEDLINE: ] [PubMed] [Google Scholar]; Cianciaruso B, Ravani P, Torraca S, Andreucci VE, ITA‐7 Study Group. Effect of anemia correction with epoietin a (EPO) on left ventricular mass (LVM) in patients with chronic kidney disease (CKD): a multicenter randomized controlled trial [abstract no: SU‐PO066]. Journal of the American Society of Nephrology 2004;15(Oct):547A. [CENTRAL: CN‐00602132] [Google Scholar]
- Clyne N, Drueke T, Eckardt K, Locatelli F, Macdougall I, Tsakiris D. Quality of life assessment in the 'cardiovascular risk reduction by early anaemia treatment with epoetin beta' (CREATE) study [abstract]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):155‐6. [CENTRAL: CN‐00444859] [Google Scholar]; Clyne N, Drueke TB, Eckardt K, Locatelli F, Macdougall IC, Tsakiris D, et al. Diagnostic value of NT‐proBNP in CKD patients: baseline and 6‐month data from the CREATE Study [abstract no: F‐PO320]. Journal of the American Society of Nephrology 2004;15(Oct):136A. [CENTRAL: CN‐00550685] [Google Scholar]; Clyne N, Macdougall I, Bilous R, Ritz E, CREATE Study Group, ACORD Study Group. Haemoglobin control with epoetin beta: results from the Cardiovascular risk Reduction by Early Anaemia Treatment with Epoetin beta (CREATE) and Anaemia Correction in Diabetes (ACORD) studies [abstract no: SP457]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv169. [CENTRAL: CN‐00583337] [Google Scholar]; Drueke T, Clyne N, Eckardt K, Locatelli F, Macdougall I, Tsakiris D, et al. Diagnostic value of NT‐proBNP and cardiac troponin T in chronic kidney disease patients: correlation with baseline characteristics in the CREATE study [abstract no: P210]. 41st Congress. European Renal Association. European Dialysis and Transplantation Association; 2004 May 15‐18; Lisbon, Portugal. 2004:85. [CENTRAL: CN‐00509163] [Google Scholar]; Drueke T, Clyne N, Eckardt K, Locatelli F, Macdougall I, Tsakiris D, et al. Homocysteine as a cardiovascular risk marker in patients with chronic kidney disease: baseline data and risk profiles from the CREATE study [abstract no: SP207]. 41st Congress. European Renal Association. European Dialysis and Transplantation Association; 2004 May 15‐18; Lisbon, Portugal. 2004:84. [CENTRAL: CN‐00509164] [Google Scholar]; Drueke T, Clyne N, Eckardt K, Locatelli F, Macdougall IC, Tsakiris D, et al. Homocysteine as a cardiovascular risk marker in patients with CKD: baseline and 6‐month data from the CREATE Study [abstract no: F‐PO335]. Journal of the American Society of Nephrology 2004;15(Oct):139A‐40A. [CENTRAL: CN‐00550688] [Google Scholar]; Drueke T, Clyne N, Eckardt KU, Locatelli F, Macdougell I, Tsakiris D, et al. Baseline characteristics of chronic renal failure patients not yet receiving renal replacement therapy enrolled in the CREATE study [abstract no: T135]. Nephrology Dialysis Transplantation 2002;17(Suppl 12):227. [CENTRAL: CN‐00509165] [Google Scholar]; Drueke T, Locatelli F, Clyne N, Eckardt KU, Macdougall I, Tsakiris D. Cardiovascular disease (CVD) characteristics of chronic kidney disease (CKD) patients enrolled in the 'cardiovascular risk reduction by early anaemia treatment with epoetin beta' (CREATE) study [abstract]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):156. [CENTRAL: CN‐00520329] [Google Scholar]; Drueke TB, Clyne N, Eckardt KU, Locatelli F, Macdougall IC, Tsakiris D. Characteristics of chronic kidney disease (CKD) patients enrolled in the 'cardiovascular risk reduction by early anemia treatment with epoetin beta' (CREATE) study [abstract no: SU‐P025]. Journal of the American Society of Nephrology 2002;13(September, Program & Abstracts):520A. [CENTRAL: CN‐00445149] [Google Scholar]; Drueke TB, Locatelli F, Clyne N, Eckardt KU, Macdougall IC, Tsakiris D, et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. New England Journal of Medicine 2006;355(20):2071‐84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Eckardt K, Clyne N, Drueke T, Locatelli F, Macdougall I, Tsakiris D, et al. Variables of left ventricular geometry and function in patients enrolled in the CREATE trial [abstract no: T136]. Nephrology Dialysis Transplantation 2002;17(Suppl 1):227. [CENTRAL: CN‐00583726] [Google Scholar]; Eckardt K, Macdougall I, Locatelli F, Tsakiris D, Clyne N, Drueke T. Effects of epoetin beta on left ventricular mass in patients with chronic kidney disease: echocardiographic results from the CREATE study [abstract no: TH‐FC172]. Journal of the American Society of Nephrology 2005;16:37A. [CENTRAL: CN‐00583370] [Google Scholar]; Eckardt KU, Clyne N, Drueke T, Locatelli F, Macdougall I, Tsakiris D. Left ventricular hypertrophy and associated variables in the 'cardiovascular risk reduction by early anaemia treatment with epoetin beta (CREATE) trial [abstract]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):155. [CENTRAL: CN‐00445194] [Google Scholar]; Eckardt KU, Scherhag A, Macdougall IC, Tsakiris D, Clyne N, Locatelli F, et al. Left ventricular geometry predicts cardiovascular outcomes associated with anemia correction in CKD. Journal of the American Society of Nephrology 2009;20(12):2651‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]; Eckardt KU, The Cardiovascular risk Reduction by Early Anemia Treatment with Epoetin Beta (CREATE) Trial. The CREATE trial‐‐building the evidence. Nephrology Dialysis Transplantation 2001;16 Suppl 2:16‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Locatelli F, Clyne N, Drueke T, Eckardt KU, Macdougall I, Tsakiris D, et al. Distribution of cardiovascular disease (CVD) across three geographical regions in patients with chronic renal failure (CRF) not yet receiving renal replacement therapy (RRT) enrolled in the CREATE study [abstract]. Nephrology Dialysis Transplantation 2002;17(Suppl 1):227‐8. [CENTRAL: CN‐00550716] [Google Scholar]; Locatelli F, Vecchio L, Pozzoni P. Anemia and cardiovascular risk: the lesson of the CREATE Trial. Journal of the American Society of Nephrology 2006;17(12 Suppl 3):S262‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Macdougall IC, Clyne N, Drueke TB, Eckardt K, Tsakiris D, Locatelli F, et al. Left ventricular hypertrophy and related variables in chronic kidney disease patients not receiving RRT enrolled in the cardiovascular risk reduction by early anemia treatment with epoetin beta (CREATE) study [abstract no: SU‐PO629]. Journal of the American Society of Nephrology 2003;14(Nov):672A. [CENTRAL: CN‐00550747] [Google Scholar]; Macdougall IC, Steering Committee of the CREATE trial, CREATE Study Group. CREATE: new strategies for early anaemia management in renal insufficiency. Nephrology Dialysis Transplantation 2003;18 Suppl 2:ii13‐6. [MEDLINE: ] [PubMed] [Google Scholar]; Tsakiris D, Clyne N, Drueke T, Eckardt K, Macdougall I, Locatelli F, et al. Impaired quality of life in chronic kidney disease patients enrolled in the cardiovascular risk reduction by early anemia treatment with epoetin beta (CREATE) study [abstract no: SA‐PO723]. Journal of the American Society of Nephrology 2003;14(Nov):456A. [CENTRAL: CN‐00550393] [Google Scholar]
- Jurkovitz C, Garelnabi M, Rossert J, Frei D, McClellan W. Is there an association between kidney function and oxidative stress? Results from the early correction of anemia on the progression of chronic kidney disease study [abstract no: SU‐PO236]. Journal of the American Society of Nephrology 2004;15(Oct):584A. [CENTRAL: CN‐00550521] [Google Scholar]; McClellan W, Gassmann‐Mayer C, Frei D, Rossert J. Body weight and c‐reactive protein interact to accelerate the progression of chronic kidney disease: analysis of the early correction of anemia on the progression of chronic renal insufficiency (ECAP) study [abstract no: SA‐PO113]. Journal of the American Society of Nephrology 2004;15(Oct):325A. [CENTRAL: CN‐00550698] [Google Scholar]; Rossert J, Gassmann‐Mayer C, Frei D, McClellan W. Prevalence and predictors of epoetin hyporesponsiveness in chronic kidney disease patients. Nephrology Dialysis Transplantation 2007;22(3):794‐800. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Rossert J, Gassmann‐Mayer C, Frei D, McClellan W. Prevalence and risk factors for erythropoetin hyporesponsiveness in chronic kidney disease: analysis of the ECAP study [abstract no: F‐PO343]. Journal of the American Society of Nephrology 2004;15(Oct):141A. [CENTRAL: CN‐00550504] [Google Scholar]; Rossert J, Levin A, Roger S, Horl W, Gassman‐Mayer C, Frei D, et al. Effect of early correction of anemia on the progression of chronic kidney disease: final results ECAP study [abstract no: SU‐PO063]. Journal of the American Society of Nephrology 2004;15(Oct):546A. [CENTRAL: CN‐00550517] [Google Scholar]; Rossert J, Levin A, Roger SD, Horl WH, Fouqueray B, Gassmann‐Mayer C, et al. Effect of early correction of anemia on the progression of CKD. American Journal of Kidney Diseases 2006;47(5):738‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Rossert J, Roger S, Levin A, Horl W, McClellan W. Effect on early correction of anemia on the progression of chronic kidney disease (ECAP) [abstract no: PUB180]. Journal of the American Society of Nephrology 2003;14(Nov):811A. [CENTRAL: CN‐00550519] [Google Scholar]
- Eschbach JW, Kelly MR, Haley NR, Abels RI, Adamson JW. Treatment of the anemia of progressive renal failure with recombinant human erythropoietin. New England Journal of Medicine 1989;321(3):158‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Foley RN, Parfrey PS, Morgan J, Barre P, Campbell P, Cartier P, et al. A randomized controlled trial of complete vs partial correction of anemia in hemodialysis patients with asymptomatic concentric lV hypertrophy or lV dilation [abstract no: A1064]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):208A. [CENTRAL: CN‐00445361] [Google Scholar]; Foley RN, Parfrey PS, Morgan J, Barre P, Campbell P, Cartier P, et al. Diastolic dysfunction in hemodialysis patients: the Canadian Normalization of Hemoglobin Study Group [abstract]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):261A. [CENTRAL: CN‐00550674] [Google Scholar]; Foley RN, Parfrey PS, Morgan J, Barre PE, Campbell P, Cartier P, et al. Effect of hemoglobin levels in hemodialysis patients with asymptomatic cardiomyopathy. Kidney International 2000;58(3):1325‐35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Foley RN, Parfrey PS, Morgan J, Barre PE, Campbell P, Cartier P, et al. Hemoglobin levels and hospitalization in hemodialysis patients without symptomatic cardiac disease [abstract no: SA‐PO818]. Journal of the American Society of Nephrology 2002;13(September, Program & Abstracts):432A. [CENTRAL: CN‐00445362] [Google Scholar]; Wells GA, Coyne D, Lee KM, Foley RN, Parfrey PS, et al. Quality of life effects of normalization of hemoglobin in asymptomatic hemodialysis patients [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):230A. [CENTRAL: CN‐00448336] [Google Scholar]
- Gouva C, Katapodis K, Siamopoulos K, Investigators of the Study Group. Effect of erythropoietin administration on lipid parameters in chronic renal failure patients. a randomized control trial [abstract no: SP294]. 41st Congress. European Renal Association. European Dialysis and Transplantation Association; 2004 May 15‐18; Lisbon, Portugal. 2004:114. [CENTRAL: CN‐00509216] [Google Scholar]; Gouva C, Nikolopoulos P, Ioannidis JP, Siamopoulos KC. Treating anemia early in renal failure patients slows the decline of renal function: a randomized controlled trial. Kidney International 2004;66(2):753‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Gouva CD, Pappas KD, Katopodis KP, Nikolopoulos PM, Michalis LK, Goudevenos IA, et al. The beneficial effects of erythropoietin on cardiac function and geometry in patients with chronic kidney disease (stage 3 or 4). A randomized control study [abstract no: MP180]. Nephrology Dialysis Transplantation 2005;20(Suppl 5):v260. [CENTRAL: CN‐00691682] [Google Scholar]; Papavasiliou EC, Gouva C, Siamopoulos KC, Tselepis AD. Erythrocyte PAF‐acetylhydrolase activity in various stages of chronic kidney disease: Effect of long‐term therapy with erythropoietin. Kidney International 2005;68(1):246‐55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Papavasiliou EC, Gouva C, Siamopoulos KC, Tselepis AD. PAF‐acetylhydrolase activity in plasma of patients with chronic kidney disease. Effect of long‐term therapy with erythropoietin. Nephrology Dialysis Transplantation 2006;21(5):1270‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Pappas KD, Gouva CD, Katopodis KP, Nikolopoulos PM, Korantzopoulos PG, Michalis LK, et al. Correction of anemia with erythropoietin in chronic kidney disease (stage 3 or 4): effects on cardiac performance. Cardiovascular Drugs & Therapy 2008;22(1):37‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Siamopoulos KC, Gouva C, Katopodis KP, Tzallas C, Nikolopoulos P, Papavasiliou EC, et al. Long‐term treatment with EPO increases serum levels of high‐density lipoprotein in patients with CKD. American Journal of Kidney Diseases 2006;48(2):242‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Johnson CA, Wakeen M, Taylor CA 3rd, Zimmerman SW, Burkart J, Bhattacharya A, et al. Comparison of intraperitoneal and subcutaneous epoetin alfa in peritoneal dialysis patients. Peritoneal Dialysis International 1999;19(6):578‐82. [MEDLINE: ] [PubMed] [Google Scholar]
- Kawanishi H, Iwasaki M, Akizawa T, Koshikawa S, KRN321 Study Group. Dose‐finding and long‐term studies of intravenous KRN321 (darbepoetin alfa) in chronic renal failure patients (CRF) on hemodialysis (HD) in Japan [abstract no: SA‐PO943]. Journal of the American Society of Nephrology 2005;16:763A. [CENTRAL: CN‐00740571] 15659562 [Google Scholar]
- Levin A, Djurdjev O, Thompson C, Barrett B, Ethier J, Carlisle E, et al. Canadian randomized trial of hemoglobin maintenance to prevent or delay left ventricular mass growth in patients with CKD. American Journal of Kidney Diseases 2005;46(5):799‐811. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Levin A, Djurdjev O, Thompson CR, Barrett BS. Change in GFR and change in hemoglobin predict change in LVMI [abstract no: SA‐PO088]. Journal of the American Society of Nephrology 2004;15(Oct):318A. [CENTRAL: CN‐00583328] [Google Scholar]; Levin A, Djurdjev O, Thompson CR, Barrett BS, Euan CJ. Results of the Canadian multicentre randomized control trial (RCT) of erythropoietin therapy for progression anemia in chronic kidney disease [abstract no: SU‐PO073]. Journal of the American Society of Nephrology 2004;15(Oct):548A. [CENTRAL: CN‐00583330] [Google Scholar]
- Danielson BG, Furuland H, Ahlmen J, Christensson A, Linde T, Strombom U. Scandinavian study of normalizing hemoglobin with rHu‐EPO in end stage renal failure [abstract no: A0822]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):160A. [CENTRAL: CN‐00550642] 9890322 [Google Scholar]; Furuland H, Linde T, Ahlmen J, Christensson A, Strombom U, Danielson BG. A randomized controlled trial of haemoglobin normalization with epoetin alfa in pre‐dialysis and dialysis patients. Nephrology Dialysis Transplantation 2003;18(2):353‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Furuland H, Linde T, Danielson BG. Cardiac function in patients with end‐stage renal disease after normalization of hemoglobin with erythropoietin (EPO) [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):337A. [CENTRAL: CN‐00445402] [Google Scholar]; Furuland H, Linde T, Danielson BG. Dialysis adequacy after normalization of hemoglobin with erythropoietin (EPO) [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):296A. [CENTRAL: CN‐00445403] [Google Scholar]; Furuland H, Linde T, Danielson BG. Physical exercise capacity in patients with end‐stage renal disease after normalizaton of hemoglobin with erythropoietin (EPO) [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):337A. [CENTRAL: CN‐00445404] [Google Scholar]; Furuland H, Linde T, Sandhagen B, Andren B, Wikstrom B, Danielson BG. Hemorheological and hemodynamic changes in predialysis patients after normalization of hemoglobin with epoetin‐alpha. Scandinavian Journal of Urology & Nephrology 2005;39(5):399‐404. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Furuland H, Linde T, Wikstrom B, Danielson BG. Reduced hemodialysis adequacy after hemoglobin normalization with epoetin. Journal of Nephrology 2005;18(1):80‐5. [MEDLINE: ] [PubMed] [Google Scholar]; Linde T, Ekberg H, Forslund T, Furuland H, Holdaas H, Nyberg G, et al. The use of pretransplant erythropoietin to normalize hemoglobin levels has no deleterious effects on renal transplantation outcome. Transplantation 2001;71(1):79‐82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Linde T, Wahlberg J, Furuland H, Danielson BG. Results of renal transplantation in patients randomized to EPO treatment aimed to reach a subnormal or normal Hb [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):684A. [CENTRAL: CN‐00446408] 9580370 [Google Scholar]
- Locatelli F, Villa G, Messa P, Filippini A, Cannella G, Ferrari G, et al. Efficacy and safety of once‐weekly intravenous epoetin alfa in maintaining hemoglobin levels in hemodialysis patients. Journal of Nephrology 2008;21(3):412‐20. [MEDLINE: ] [PubMed] [Google Scholar]
- Kwan JT, Temple M, Macdougall I. Is early treatment of anemia with epoetin‐alfa beneficial to predialysis renal patients? An UK multi‐centre study [abstract no: SU‐PO057]. Journal of the American Society of Nephrology 2004;15(Oct):545A. [CENTRAL: CN‐00765503] [Google Scholar]; Macdougall IC, Kwan J, Temple RM, EPO‐GBR‐2 Investigator Study Group. UK multicentre randomised controlled study of epoetin alfa in early renal insufficiency (ERI) ‐ a 12‐month interim analysis [abstract]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):395A. [CENTRAL: CN‐00766947] [Google Scholar]; Macdougall IC, Temple RM, Kwan JT. Is early treatment of anaemia with epoetin‐alpha beneficial to pre‐dialysis chronic kidney disease patients? Results of a multicentre, open‐label, prospective, randomized, comparative group trial. Nephrology Dialysis Transplantation 2007;22(3):784‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bennett‐Jones D. Use of epoetin alpha in the treatment in anaemia in predialysis patients. www.nihr.ac.uk/Profile/Pages/NRRResults.aspx?publication_id=N0055116759 (last accessed 3 July 2014). [National Research Register (NRR) Archive, UK]
- Choukroun G, Kamar N, Lang P, Durrbach A, Lebranchu Y, Adem A, et al. High dose epoetin beta in the first weeks following renal Tx had no influence on renal function in patients at risk for DGF [abstract no: 437]. American Journal of Transplantation 2010;10(Suppl 4):168. [EMBASE: 70463798] [DOI] [PubMed] [Google Scholar]; Martinez F, Kamar N, Pallet N, Lang P, Durrbach A, Lebranchu Y, et al. High dose epoetin beta in the first weeks following renal transplantation and delayed graft function: Results of the Neo‐PDGF Study. American Journal of Transplantation 2010;10(7):1695‐700. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Foley RN, Curtis BM, Parfrey PS. Erythropoietin therapy, hemoglobin targets, and quality of life in healthy hemodialysis patients: a randomized trial. Clinical Journal of The American Society of Nephrology: CJASN 2009;4(4):726‐33. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]; Foley RN, Curtis BM, Parfrey PS. Hemoglobin targets and blood transfusions in hemodialysis patients without symptomatic cardiac disease receiving erythropoietin therapy. Clinical Journal of The American Society of Nephrology: CJASN 2008;3(6):1669‐75. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]; Foley RN, Curtis BM, Parfrey PS. Hemoglobin targets, blood transfusions and quality of life in hemodialysis patients without symptomatic cardiac disease [abstract no: SA‐PO2745]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):730A. [CENTRAL: CN‐00756852] [DOI] [PMC free article] [PubMed] [Google Scholar]; Foley RN, Curtis BM, Randell EW, Parfrey PS. Left ventricular hypertrophy in new hemodialysis patients without symptomatic cardiac disease. Clinical Journal of The American Society of Nephrology: CJASN 2010;5(5):805‐13. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]; Foley RN, Parfrey PS, Wittreich BH, Sullivan DJ, Zagari MJ, Frei D, et al. The effect of higher haemoglobin levels on left ventricular cavity volume in patients starting haemodialysis: a blinded, randomised, controlled trial in 596 patients without symptomatic cardiac disease [abstract]. 41st Congress. European Renal Association. European Dialysis and Transplantation Association; 2004 May 15‐18; Lisbon, Portugal. 2004:217. [CENTRAL: CN‐00509197] [Google Scholar]; Parfrey PS, Foley RN, Wittreich BH, Sullivan DJ, Zagari MJ, Frei D. Double‐blind comparison of full and partial anemia correction in incident hemodialysis patients without symptomatic heart disease. Journal of the American Society of Nephrology 2005;16(7):2180‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Parfrey PS, Foley RN, Wittreich BH, Sullivan DJ, Zagari MJ, Frei D, et al. Double‐blind comparison of full and partial anemia correction with erythropoietin in incident hemodialysis patients without symptomatic heart disease [abstract no: PUB002]. Journal of the American Society of Nephrology 2004;15(Oct):762A. [CENTRAL: CN‐00583759] [DOI] [PubMed] [Google Scholar]; Parfrey PS, Foley RN, Wittreich BH, Sullivan DJ, Zagari MJ, Frei D, et al. The effect of higher haemoglobin levels on quality of life in patients starting haemodialysis: a blinded, randomised, controlled trial in 596 patients without symptomatic cardiac disease [abstract]. 41st Congress. European Renal Association. European Dialysis and Transplantation Association; 2004 May 15‐18; Lisbon, Portugal. 2004:229. [CENTRAL: CN‐00509402] [Google Scholar]
- Perez‐Oliva JF, Casanova‐Gonzalez M, Garcia‐Garcia I, Porrero‐Martin PJ, Valenzuela‐Silva CM, Hernandez‐Montero T, et al. Comparison of two recombinant erythropoietin formulations in patients with anemia due to end‐stage renal disease on hemodialysis: a parallel, randomized, double blind study. BMC Nephrology 2005;6:5. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salek M S, Pratheepawanit N. Use of erythropoietin in early anaemia improves health‐related quality of life in predialysis chronic renal failure patients [abstract]. Quality of Life Research 2001;10(3):198. [CENTRAL: CN‐00495402] [Google Scholar]
- Sja'bani M, Asdie AH. Effect of erythropoietin on pruritus and quality of life in chronic hemodialyzed end stage renal disease patients [abstract]. Journal of Clinical Epidemiology 1997;50(Suppl 1):10S. [CENTRAL: CN‐00550491] [Google Scholar]
- McMahon LP, Roger SD, Levin A, Slimheart Investigators Group. Development, prevention, and potential reversal of left ventricular hypertrophy in chronic kidney disease. Journal of the American Society of Nephrology 2004;15(6):1640‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; McMahon LP, Roger SD, Schou M. Does early intervention and treatment with epoetin prevent left ventricular hypertrophy (LVH) in chronic kidney disease (CKD)? (SLIMHEART study) [abstract no: SA‐P0852]. Journal of the American Society of Nephrology 2002;13(September, Program & Abstracts):440a. [CENTRAL: CN‐00446699] [Google Scholar]; Roger SD, McMahon LP, Clarkson A, Disney A, Harris D, Hawley C, et al. Effects of early and late intervention with epoetin alpha on left ventricular mass among patients with chronic kidney disease (stage 3 or 4): results of a randomized clinical trial. Journal of the American Society of Nephrology 2004;15(1):148‐56. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Roger SD, McMahon LP, Schou IM, Aus‐14 Investigators Group. Impact of epoetin alfa (EPO) treatment on cardiac and renal function in chronic kidney disease (CKD) [abstract]. Nephrology 2002;7(Suppl 3):70‐1. [CENTRAL: CN‐00447444] [Google Scholar]
- Baldamus C, Krivoshiev S, Wolf‐Pflugmann M, Siebert‐Weigel M, Koytchev R, Bronn A. Long‐term safety and tolerability of epoetin zeta, administered intravenously, for maintenance treatment of renal anemia. Advances in Therapy 2008;25(11):1215‐28. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Wizemann V, Rutkowski B, Baldamus C, Scigalla P, Koytchev R, Epoetin Zeta Study Group. Comparison of the therapeutic effects of epoetin zeta to epoetin alfa in the maintenance phase of renal anaemia treatment. Current Medical Research & Opinion 2008;24(3):625‐37. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
- Barany P. Treatment of anemia in hemodialysis (HD) patients (PTS) to a normal hemoglobin concentration (HB) ‐ results of an open randomized clinical trial of epoetin beta [abstract no: M387]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):243A. [CENTRAL: CN‐00444332] 9527400 [Google Scholar]
- Carrera F, Anunciada AI, Nogueira C, Silva JG. Comparison of HB levels in dialysis patients receiving three‐times weekly rHuepo switched to once‐weekly darbepoetin alfa: results of a randomized study [abstract]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):164. [CENTRAL: CN‐00444694] [Google Scholar]
- Nissenson A, Nassar G, Edwardes M, Beswick R, Berns J. C.E.R.A. maintains hemoglobin in dialysis patients directly switched from epoetin (EPO) without increasing iron therapy requirements [abstract no: F‐PO855]. Journal of the American Society of Nephrology 2007;18(Abstracts):290A‐1A. [Google Scholar]
- Ostrvica E, Mesic E, Ostrvica D, Delic J, Delic‐Custendil S, Hukic F. Effectiveness of treating the renal anemia in chronic hemodialyzed patients by epoietin alpha and beta. Medicinski Arhiv 2010;64(1):4‐6. [MEDLINE: ] [PubMed] [Google Scholar]
- Palazzuoli A, Quatrini I, Calabro A, Antonelli G, Caputo M, Campagna MS, et al. Anemia correction by erythropoietin reduces BNP levels, hospitalization rate, and NYHA class in patients with cardio‐renal anemia syndrome. Clinical & Experimental Medicine 2011;11(1):43‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
- Besarab A, Canaud B, Francisco AL, Kerr P, Locatelli F, Lok CE, et al. Randomized comparison of IV C.E.R.A. (Continuous Erythropoietin Receptor Activator) and Darbepoetin Alfa (DA) at extended administration intervals for the maintenance of Hb levels in patients with CKD on dialysis: rationale and design [abstract no: PUB377]. Journal of the American Society of Nephrology 2006;17(Abstracts):896A. [CENTRAL: CN‐00740564] [Google Scholar]
- NCT00442702. A randomized, open label study to compare the effect of monthly subcutaneous mircera with that of darbepoetin alfa, given according to local label, on the management of anemia in patients with chronic kidney disease not on dialysis. www.clinicaltrials.gov/show/NCT00442702 (accessed 8 October 2014).
- NCT00559273. An open‐label, randomized, multicenter, parallel‐group study to demonstrate correction of anemia using once every 4 weeks subcutaneous injections of mircera in patients with chronic kidney disease who are not on dialysis. www.clinicaltrials.gov/ct2/show/NCT00559273 (accessed 8 October 2014).
- NCT00717821. A randomized, controlled, open label, French multicenter parallel group study to compare the hemoglobin maintenance with once monthly administration of mircera versus epoetin beta or darbepoetin alfa in patients with chronic kidney disease on hemodialysis. www.clinicaltrials.gov/ct2/show/NCT00717821 (accessed 8 October 2014).
- NCT00773513. A randomized, open label study to assess all‐cause mortality and cardiovascular morbidity in patients with chronic kidney disease on dialysis and those not on renal replacement therapy under treatment with mircera or reference ESAs. www.clinicaltrials.gov/ct2/show/NCT00773513 (accessed 8 October 2014).
- Fliser D, Dellanna F, Koch M, Seufert J, Witzke O, Hauser IA. The Primavera study protocol design: evaluating the effect of continuous erythropoiesis receptor activator (C.E.R.A.) on renal function in non‐anemic patients with chronic kidney disease. Contemporary Clinical Trials 2011;32(6):786‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- NCT00364845. A randomised single‐blind study to improve health‐related quality of life as measured by the sf‐36 vitality score by correcting anemia with aranesp (darbepoetin alfa) in the elderly. www.clinicaltrials.gov/ct2/show/NCT00364845 (accessed 8 October 2014).
Additional references
- Seidenfeld J, Piper M, Bohlius J, Weingart O, Trelle S, Engert A, et al. Comparative effectiveness of epoetin and darbepoetin for managing anemia in patients undergoing cancer treatment. Comparative effectiveness review No. 3. (Prepared by Blue Cross and Blue Shield Association Technology Evaluation Center Evidence‐based Practice Center under Contract No. 290‐02‐0026). Rockville, MD: Agency for Healthcare Research and Quality; May 2006. www.effectivehealthcare.ahrq.gov/reports/final.cfm (accessed 8 October 2014).
- Grant MD, Piper M, Bohlius J, Tonia T, Robert N, Vats V, et al. Epoetin and darbepoetin for managing anemia in patients undergoing cancer treatment: comparative effectiveness update. Comparative Effectiveness Review No. 113. (Prepared by the Blue Cross and Blue Shield Association Technology Evaluation Center Evidence‐based Practice Center under Contract No. 290‐2007‐10058‐I). AHRQ Publication No. 13‐EHC077‐EF. Rockville, MD: Agency for Healthcare Research and Quality. 2013. http://effectivehealthcare.ahrq.gov/ehc/products/170/1481/cancer‐anemia‐treatment‐executive‐130425.pdf (accessed 8 October 2014). [PubMed]
- Bohlius J, Schmidlin K, Brillant C, Schwarzer G, Trelle S, Seidenfeld J, et al. Erythropoietin or Darbepoetin for patients with cancer ‐ meta‐analysis based on individual patient data. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD007303.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware LE, Tangri N, Ephraim PL, Scialla JJ, Sozio SM, Crews DC, et al. Comparative effectiveness studies to improve clinical outcomes in end stage renal disease: the DEcIDE patient outcomes in end stage renal disease study. BMC Nephrology 2012;13:167. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta‐analysis of randomized controlled trials. Journal of Clinical Epidemiology 1997;50(6):683‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Caldwell DM, Welton NJ, Ades AE. Mixed treatment comparison analysis provides internally coherent treatment effect estimates based on overviews of reviews and can reveal inconsistency. Journal of Clinical Epidemiology 2010;63(8):875‐82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Caro JJ, Salas M, Ward A, Goss G. Anemia as an independent prognostic factor for survival in patients with cancer: a systemic, quantitative review. Cancer 2001;91(12):2214‐21. [MEDLINE: ] [PubMed] [Google Scholar]
- Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta‐analysis in STATA. PLoS ONE 2013;8:e76654. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement FM, Klarenbach S, Tonelli M, Johnson JA, Manns BJ. The impact of selecting a high hemoglobin target level on health‐related quality of life for patients with chronic kidney disease: a systematic review and meta‐analysis. Archives of Internal Medicine 2009;169(12):1104‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Giovane C, Chaimani A, Caldwell D, Salanti G. Exploring the applicability and adaptation of the GRADE system to results from network analysis: a pilot study. 20th Cochrane Colloquium, Auckland, New Zealand. October 2012. [Google Scholar]
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Eschbach JW, Egrie JC, Downing MR, Browne JK, Adamson JW. Correction of the anemia of end‐stage renal disease with recombinant human erythropoietin. Results of a combined phase I and II clinical trial. New England Journal of Medicine 1987;316(2):73‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ezekowitz JA, McAlister FA, Armstrong PW. Anemia is common in heart failure and is associated with poor outcomes: insights from a cohort of 12 065 patients with new‐onset heart failure. Circulation 2003;107(2):223‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Groenveld HF, Januzzi JL, Damman K, Wijngaarden J, Hillege HL, Veldhuisen DJ, et al. Anemia and mortality in heart failure patients a systematic review and meta‐analysis. Journal of the American College of Cardiology 2008;52(10):818‐27. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
- Higgins JP, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta‐analysis: concepts and models for multi‐arm studies. Research Synthesis Methods 2012;3(2):98‐110. [DOI: 10.1002/jrsm.1044] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group. KDIGO Clinical practice guideline for anemia in chronic kidney disease. Kidney International Supplements 2010;2(4):279‐335. [DOI: 10.1038/kisup.2012.37] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney International Supplements 2013;3(1):1‐150. [DOI: 10.1038/kisup.2012.73] [DOI] [PMC free article] [PubMed] [Google Scholar]
- KDOQI. KDOQI Clinical Practice Guideline and Clinical Practice Recommendations for anemia in chronic kidney disease: 2007 update of hemoglobin target. American Journal of Kidney Diseases 2007;50(3):471‐530. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kilpatrick RD, Critchlow CW, Fishbane S, Besarab A, Stehman‐Breen C, Krishnan M, et al. Greater epoetin alfa responsiveness is associated with improved survival in hemodialysis patients. Clinical Journal of The American Society of Nephrology: CJASN 2008;3(4):1077‐83. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre P, Vekeman F, Sarokhan B, Enny C, Provenzano R, Cremieux PY. Relationship between hemoglobin level and quality of life in anemic patients with chronic kidney disease receiving epoetin alfa. Current Medical Research & Opinion 2006;22(10):1929‐37. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Levin NW, Fishbane S, Canedo FV, Zeig S, Nassar GM, Moran JE, et al. Intravenous methoxy polyethylene glycol‐epoetin beta for haemoglobin control in patients with chronic kidney disease who are on dialysis: a randomised non‐inferiority trial (MAXIMA). Lancet 2007;370(9596):1415‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Locatelli F, Pisoni RL, Combe C, Bommer J, Andreucci VE, Piera L, et al. Anaemia in haemodialysis patients of five European countries: association with morbidity and mortality in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrology Dialysis Transplantation 2004;19(1):121‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Statistics in Medicine 2004;23(20):3105‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ludwig H, Belle S, Barrett‐Lee P, Birgegard G, Bokemeyer C, Gascon P, et al. The European Cancer Anaemia Survey (ECAS): a large, multinational, prospective survey defining the prevalence, incidence, and treatment of anaemia in cancer patients. European Journal of Cancer 2004;40(15):2293‐306. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Macdougall IC, Hutton RD, Cavill I, Coles GA, Williams JD. Treating renal anaemia with recombinant human erythropoietin: practical guidelines and a clinical algorithm. BMJ 1990;300(6725):655‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdougall IC. An overview of the efficacy and safety of novel erythropoiesis stimulating protein (NESP). Nephrology Dialysis Transplantation 2001;16 Suppl 3:14‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Macdougall IC. Novel erythropoiesis‐stimulating agents: a new era in anemia management. Clinical Journal of The American Society of Nephrology: CJASN 2008;3(1):200‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Melekhin VV, Shepherd BE, Stinnette SE, Rebeiro PF, Turner MM, Sterling TR. Hemoglobin may contribute to sex differences in mortality among HIV‐infected persons in care. PLoS ONE 2012;7(9):e44999. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff MS, Goldberg J, Slifkin RF, Eiser AR, Calamia V, Kaplan M, et al. A comparison of androgens for anemia in patients on hemodialysis. New England Journal of Medicine 1981;304(15):871‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence. Anaemia management in people with chronic kidney disease: full guidance. 2011. http://www.nice.org.uk/guidance/CG114/Guidance (accessed 8 October 2014).
- Palmer SC, Navaneethan SD, Craig JC, Johnson DW, Tonelli M, Garg AX, et al. Meta‐analysis: erythropoiesis‐stimulating agents in patients with chronic kidney disease. Annals of Internal Medicine 2010;153(1):23‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Palmer SC, Saglimbene V, Craig JC, Navaneethan SD, Strippoli GF. Darbepoetin for the anaemia of chronic kidney disease. Cochrane Database of Systematic Reviews 2014, Issue 3. [DOI: 10.1002/14651858.CD009297.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phrommintikul A, Haas SJ, Elsik M, Krum H. Mortality and target haemoglobin concentrations in anaemic patients with chronic kidney disease treated with erythropoietin: a meta‐analysis. Lancet 2007;369(9559):381‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Salanti G, Higgins JP, Ades AE, Ioannidis JP. Evaluation of networks of randomized trials. Statistical Methods in Medical Research 2008;17(3):279‐301. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Salanti G, Marinho V, Higgins JP. A case study of multiple‐treatments meta‐analysis demonstrates that covariates should be considered. Journal of Clinical Epidemiology 2009;62(8):857‐64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple‐treatment meta‐analysis: an overview and tutorial. Journal of Clinical Epidemiology 2011;64(2):163‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Salanti G. Indirect and mixed‐treatment comparison, network, or multiple‐treatments meta‐analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Research Synthesis Methods 2012;3(2):80‐97. [DOI: 10.1002/jrsm.1037] [DOI] [PubMed] [Google Scholar]
- Salanti G, Giovane C, Chaimani A, Caldwell DM, Higgins JP. Evaluating the quality of evidence from a network meta‐analysis. PLoS One 2014;9(7):e99682. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semba RD, Martin BK, Kempen JH, Thorne JE, Wu AW. The impact of anemia on energy and physical functioning in individuals with AIDS. Archives of Internal Medicine 2005;165(19):2229‐36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Shah S, Smith CJ, Lampe F, Youle M, Johnson MA, Phillips AN, et al. Haemoglobin and albumin as markers of HIV disease progression in the highly active antiretroviral therapy era: relationships with gender. HIV Medicine 2007;8(1):38‐45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Strippoli GF, Navaneethan SD, Craig JC, Palmer SC. Haemoglobin and haematocrit targets for the anaemia of chronic kidney disease. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD003967.pub2] [DOI] [PubMed] [Google Scholar]
- Szczech LA, Barnhart HX, Inrig JK, Reddan DN, Sapp S, Califf RM, et al. Secondary analysis of the CHOIR trial epoetin‐alpha dose and achieved hemoglobin outcomes. Kidney international 2008;74(6):791‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RM, Davey J, Clarke MJ, Thompson SG, Higgins JP. Predicting the extent of heterogeneity in meta‐analysis, using empirical data from the Cochrane Database of Systematic Reviews. International Journal of Epidemiology 2012;41(3):818‐27. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- White IR, Barrett JK, Jackson D, Higgins JP. Consistency and inconsistency in network meta‐analysis: model estimation using multivariate meta‐regression. Research Synthesis Methods 2012;3(2):111‐25. [DOI: 10.1002/jrsm.1045] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoist B, McLean E, Egli I, Cogswell M (editors). Worldwide prevalence of anaemia 1993–2005 : WHO global database on anaemia. Geneva: World Health Organization, 2008. [ISBN: 978 92 4 159665 7] [Google Scholar]
References to other published versions of this review
- Palmer SC, Salanti G, Craig JC, Mavridis D, Strippoli GF. Erythropoiesis stimulating agents for anaemia in adults with chronic kidney disease: a network meta‐analysis. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD010590] [DOI] [PMC free article] [PubMed] [Google Scholar]


