Abstract
Background
Given the relatively high prevalence of age‐related macular degeneration (AMD) and the increased incidence of AMD as populations age, the results of trials of novel treatments are awaited with much anticipation. The complement cascade describes a series of proteolytic reactions occurring throughout the body that generate proteins with a variety of roles including the initiation and promotion of immune reactions against foreign materials or micro‐organisms. The complement cascade is normally tightly regulated, but much evidence implicates complement overactivity in AMD and so it is a logical therapeutic target in the treatment of AMD.
Objectives
To assess the effects and safety of complement inhibitors in the prevention or treatment of advanced AMD.
Search methods
We searched CENTRAL (which contains the Cochrane Eyes and Vision Group Trials Register) (The Cochrane Library 2013, Issue 11), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to November 2013), EMBASE (January 1980 to November 2013), Allied and Complementary Medicine Database (AMED) (January 1985 to November 2013), Latin American and Caribbean Literature on Health Sciences (LILACS) (January 1982 to November 2013), OpenGrey (System for Information on Grey Literature in Europe) (www.opengrey.eu/), Web of Science Conference Proceedings Citation Index ‐ Science (CPCI‐S) (January 1990 to November 2013), the metaRegister of Controlled Trials (mRCT) (www.controlled‐trials.com), ClinicalTrials.gov (www.clinicaltrials.gov) and the WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 21 November 2013. We also performed handsearching of proceedings, from 2012 onwards, of meetings and conferences of specific professional organisations.
Selection criteria
We planned to include randomised controlled trials (RCTs) with parallel treatment groups which investigated either the prevention or treatment of advanced AMD by inhibition of the complement cascade.
Data collection and analysis
Two authors (MW and GMcK) independently evaluated all the titles and abstracts resulting from the searches. We contacted companies running clinical trials which had not yet reported results to request information. Since no trials met our inclusion criteria, we undertook no assessment of quality or meta‐analysis.
Main results
We identified and screened 317 references but there were no published RCTs that met the inclusion criteria. We identified two ongoing studies: one phase I study and one phase II study.
Authors' conclusions
There is insufficient information at present to generate evidence‐based recommendations on the potential safety and efficacy of complement inhibitors for prevention or treatment of AMD. However we anticipate the results of ongoing trials.
Keywords: Humans, Complement Inactivating Agents, Complement Inactivating Agents/therapeutic use, Macular Degeneration, Macular Degeneration/drug therapy, Macular Degeneration/immunology, Macular Degeneration/prevention & control
Complement inhibitors for age‐related macular degeneration
Advanced age‐related macular degeneration (AMD) is an eye condition which principally affects the over 65s. In this age group it is the most common cause of loss of vision in the developed world, and the third most common cause globally. AMD causes loss of central vision. Despite advances in the understanding of AMD and the recent introduction of new treatments for some forms of AMD, the visual loss it causes is irreversible in most cases. When it affects both eyes, the impact on day to day functioning is huge. As the proportion of older people increases, larger numbers of people are likely to be affected by AMD in the future.
The complement cascade is the name for a series of proteins which form part of the body's immune system, helping to fight infection. The complement system is constantly active at a low level, and is tightly regulated. However evidence suggests that complement cascade overactivity may play a role in AMD, and so it is logical that drugs which inhibit the cascade may have a role in the treatment of AMD. This review sought to identify trials investigating the potential benefits and safety of complement inhibitors for AMD. No relevant trials have been completed but we anticipate that updates of this review will in future have results to analyse. There is insufficient information at present to generate evidence‐based recommendations on the potential safety and efficacy of complement inhibitors for prevention or treatment of AMD.
Background
Advanced age‐related macular degeneration (AMD) is the most common cause of blindness in those aged over 65 years in the developed world and the third most common cause globally (Augood 2006). In Great Britain (England, Wales and Scotland) it is estimated that AMD is the cause of vision impairment in 3.7% (95% confidence interval 3.2% to 4.2%) of the population over 75 years of age (Evans 2004). Given the relatively high prevalence of AMD and the increased incidence of AMD as populations age, the results of trials of novel treatments such as complement inhibitors are awaited with much anticipation. Patients, physicians and funders of health care require access to a critical evaluation of the evidence base on which to base treatment decisions.
Description of the condition
Age‐related changes that occur in the macula as a matter of age include the appearance of drusen. These are focal extracellular deposits between the retina and the retinal pigment epithelium (RPE), visible ophthalmoscopically as yellow dots. Drusen, pigment abnormalities and patchy atrophy of the RPE are a cluster of signs which constitute early AMD. While early AMD does not have an impact on central visual function, these signs may herald progression to advanced AMD (Hogg 2007). This involves geographic atrophy (or atrophic AMD), which is confluent atrophy with loss of the RPE and the choriocapillaris or neovascular AMD, which is an acute exudative pathology that affects the central macular tissues. This latter form of AMD is due to the onset of neovascularisation arising in the choroid or de novo in the retina. It can be classified on the basis of indocyanine green angiography and fluorescein angiography into polypoidal choroidopathy, choroidal neovascularisation and retinal angiomatous proliferation. These abnormal vascular complexes leak serous fluid, are unstable and rupture, which leads to frank haemorrhage. Eventually they form fibrous scars that destroy the architecture of the choroid, RPE and retina tissue. Population‐based studies show that, in cases of late AMD, neovascular forms are more common than geographic atrophy (Owen 2003).
Patients with neovascular AMD typically present with acute onset of visual symptoms such as difficulty reading, particularly small print, distortion, waviness of straight lines or in some cases a total loss of central vision. Sometimes patients are not aware of unilateral AMD, particularly when the fellow eye has normal vision. However, once a patient has lost central vision in one eye, the onset of symptoms in the fellow eye is invariably noticed immediately. Clinical examination by an ophthalmologist consists of functional and morphological assessments. The latter typically include colour fundus photography, fluorescein angiography, indocyanine green angiography and ocular coherence tomography (OCT). The improvements in quality and resolution of OCT scanning combined with its non‐invasive nature have led to its widespread use as a primary method for diagnosis and monitoring of responsiveness to treatments.
The effect of advanced AMD on central vision is often devastating. There are no effective treatments to restore vision following the confluent cell loss of the atrophic variety. The introduction of biologicals which inhibit vascular endothelial cell growth factor (VEGF) in the management of neovascular AMD has led to improved outcomes in recent times. Despite this, most patients do not recover lost vision. Not only is the advanced form of the disease common, but when one eye is affected by advanced AMD, the risk of progression to late‐stage disease in the fellow eye rises to 45% over a five‐year period (AREDS 2001). Bilateral late AMD impairs central vision and has a huge impact on visual functioning, and thus the handicap is great.
The risk of having both early and late AMD is strongly related to age. Pooled population‐based studies of predominately white populations from different continents have revealed estimates of a prevalence of advanced AMD of 3.32% of those over 65 years and approximately 11% of over 90s (Owen 2003). Given that current trends in the developed world predict an ageing population, the burden of AMD on populations and public interest in its causes and potential cures will increase. A recent systematic review (Chakravarthy 2010) has confirmed the importance of previously identified risk factors which include family history (Seddon 1997), smoking, cardiovascular disease, poor diet and nutrition, hypertension and obesity. There appears to be a dose‐response relationship between smoking and risk of AMD (Thornton 2005). Most of the risk that a family history poses has a genetic basis. Several genes have been associated with AMD (Patel 2008). In 2005 the results of four studies were published which each independently identified an association between a specific genetic change and the disease (Edwards 2005; Hageman 2005; Haines 2005; Klein 2005). The change was a single nucleotide polymorphism in amino acid 402 (Y402H) of the gene for a key regulator of the complement cascade, complement factor H (CFH). Since then another study has replicated the findings (Despriet 2006), and there has been great interest in polymorphisms in other genes which code for proteins involved in the complement cascade and which may have a role in the pathogenesis of AMD (Haines 2007).
The complement cascade describes a pathway which, when activated, results in the sequential proteolytic cleavage of a series of proteins, termed C1 to C9. Components of complement are distributed throughout the body, ready to engage in a variety of roles. These include initiating and promoting immune reactions against foreign materials or micro‐organisms, clearing apoptotic debris in utero or during the development of neuroplasticity and facilitating the clearance of necrotic debris.
Much evidence, other than the genetic associations, also implicates excessive complement activity in causing AMD (Sivaprasad 2006). For example, components such as C5 (Hageman 2001), inhibitors such as complement factor H (Hageman 2005), regulators such as vitronectin (Crabb 2002) and potential activators such as beta‐amyloid (Johnson 2002) of the complement cascade have been identified in drusen and in the retina‐choroid complex of eyes with AMD. Messenger ribonucleic acid (mRNA) for a complement component has been detected in RPE cells, suggesting that this component is produced locally in the retina (Johnson 2000). Animal models suggest that the complement cascade has a major contributory role in laser‐induced choroidal neovascularisation, possibly through the stimulation of increased levels of angiogenic growth factors (Bora 2005). Such insights into possible aetiologies have led to attempts to develop and apply therapeutic interventions that inhibit complement.
Description of the intervention
The complement cascade consists of a series of steps, from initial activation to production of terminal components. Any stage therefore is, in theory, a potential target for inhibition. Complement cascade inhibition as a therapeutic intervention for AMD is a novel concept, and there are challenges to overcome in the development of any novel therapeutic agent. It must be understood, for example, that preventing one step of the cascade could in fact stop the formation of all downstream products. Agents designed to inhibit the complement cascade must effectively dampen hyperactivity without compromising the ability to perform its normal functions. To understand how a complement inhibitor might work, an overview of complement is necessary.
Three pathways of complement activation exist (Markiewski 2007; Ricklin 2007; Rodriguez 2004). They converge on the activation by cleavage of C3, reaching that step in different ways. The classic pathway is initiated by the component C1q interacting either with immune complexes or non‐immune complexes such as C‐reactive protein or fibrillar beta‐amyloid (Heneka 2007). The lectin pathway is activated by recognition and binding of densely arranged mannose on bacterial surfaces by mannose‐binding lectin proteins. The result of either classic or lectin pathway activation is cleavage of C4 by a serine protease to C4a and C4b. C2 then binds to C4b. The result of further protease activity is the generation of C4bC2a which acts as C3 convertase. The alternative pathway describes the hydrolysis of C3 to C3w: the resultant conformational change allows binding of factor B to C3w, and the complex acts as an alternative C3 convertase. The hydrolysis can be activated by foreign pathogens, cellular debris such as apoptotic bodies or by macromolecular complexes such as lipofuscin or amyloid‐beta aggregates. However, as C3 also hydrolyses spontaneously, the alternative pathway allows a continuous low level of activity of complement. This pathway results in the deposition of C3b on foreign or abnormal surfaces. Once complement is activated, a series of proteolytic reactions ensues, producing a succession of complement proteins. C3 is cleaved to form C3a and C3b. C3b plays a central role in several ways. It covalently binds to pathogen surfaces: this is opsonisation and facilitates phagocytosis. Surface‐bound C3b combines with factor B, and cleavage by factor D leads to C3bBb: a C3 convertase. Thus amplification of complement activation occurs. C5 convertase results either from combination of two surface‐bound C3b molecules and factor B or from an assembly of C4b, C2a and C3b. C3a, C4a and C5a which are potent chemo‐attractants for phagocytes. As the cascade continues, conversion occurs of C5 to products (C6, C7, C8 and C9) which eventually assemble as a membrane attack complex (MAC). MACs insert into cellular plasma membranes and if enough MACs attack a cell, it can lyse. A full understanding of the complement cascade, however, is lacking. Some evidence is emerging that it can be activated in other ways, perhaps, for example, by interaction with the coagulation cascade.
A balance exists between permitting and dampening complement activity. Given the constitutive nature of the alternative pathway, tight regulation of the cascade is essential. Control is partly achieved by C3 convertase and C3b having short half‐lives. C3b that is deposited on foreign cells is stabilised by the protein properdin. However, C3b attached to host cell surfaces is degraded by a number of regulators, both surface‐bound and soluble. These include complement factor H (CFH), decay‐accelerating factor (CD55), membrane cofactor protein (CD46) and complement receptor 1 (CD35). The formation of the MAC is prevented by another regulator, namely CD59.
In animal models, the lack of CFH results in uncontrolled alternative pathway complement activation (Rodriguez 2004). CFH reins in complement in two ways. Firstly, it accelerates the decay of C3 and C3b convertases and, secondly, it is a cofactor for complement factor I (CFI). CFI degrades soluble and surface‐bound C3b to an inactive form. The interaction of CFH with bound C3b depends on the simultaneous presence of negatively charged molecules, such as sialic acid and heparin, also bound to cell surfaces or extracellular matrix. It is the degree of affinity between C3b and CFH which determines CFH's ability to inactivate C3b, and thus binding with these cell surface polyanions is crucial. As amino acid 402 of CFH, the position of the single nucleotide polymorphism most strongly associated with AMD, is located in a region of CFH which binds heparin, the Y402H change may impair the binding efficiency or some other aspect of the function of the molecule and hence reduce its inhibitory abilities.
Whatever stage of the complement cascade is targeted, the active agent could be delivered to the eye by several means: topically, intravitreally or systemically. Similarly, the potential vehicles for delivery of the active agent to its intended site of action in the retina are in theory legion. As this avenue of treatment is nascent, it is difficult to discuss complement inhibition for AMD in anything other than general terms.
How the intervention might work
Several potential classes of complement inhibitors acting as therapeutic agents exist: protease inhibitors, natural complement regulators, antibodies against specific complement components, functional complement component inhibitors and anaphylatoxin receptor antagonists (Ricklin 2007).
Eculizumab, for example, a monoclonal antibody against C5, prevents C5's cleavage to C5a and C5b. It is licensed for the treatment of paroxysmal nocturnal haemoglobinuria. The pathogenesis of this disorder is increased MAC formation on erythrocyte and platelet surfaces. Eculizumab may have potential for the treatment of atrophic AMD, and a phase II clinical trial on intravenous eculizumab is recruiting (ClinicalTrials.gov identifier: NCT00935883) (Alexion Pharmaceuticals: www.alexionpharm.com/). TT30, a CFH‐recombinant fusion protein which inhibits the alternative complement pathway and TA106, an antibody directed against factor B, are in the pipeline as potential treatment for AMD (Alexion Pharmaceuticals: www.alexionpharm.com/). Functional complement component inhibitors include compstatin, a peptide which blocks the cleavage of C3 to its active products. Although the exact mechanism of its action is unclear, it is said to be a promising drug candidate given its small size and high efficacy. An analogue of compstatin, POT‐4, has been investigated in phase I clinical trials (ClinicalTrials.gov identifier: NCT00473928) as a potential intravitreal agent for AMD (Potentia Pharmaceuticals: www.potentiapharma.com/). Through the use of Macugen, ophthalmologists are familiar with the concept of aptamers. ARC1905 is an aptamer which prevents the cleavage of C5 and may form the basis of future treatments for neovascular or atrophic AMD. Phase I clinical trials are ongoing under Archimex (ClinicalTrials.gov identifier: NCT00709527) (www.archemix.com/website/index.php) and Ophthotech (ClinicalTrials.gov identifiers: NCT00950638 and NCT00709527) (www.ophthotech.com/). Much of the pro‐inflammatory effects of complement are mediated through the anaphylatoxins C3a and C5a. C5a receptors are found on a variety of cells, including inflammatory cells such as neutrophils and macrophages. JPE1375 is a C5a receptor antagonist that has undergone preclinical trials and may have efficacy in the prevention of atrophic AMD (Jerini AG: www.jerini.com/cms/en/home.php). Botany may also offer treatments: a transgenic moss has been used to produce human CFH, with a signal peptide resulting in localisation of the recombinant CFH to culture supernatant (Büttner‐Mainik 2011).
Why it is important to do this review
As far as the authors are aware, no phase III trials on complement inhibitors for either the treatment of advanced AMD or its prevention have reported results at the time of writing this review. However, several complement inhibitors are in development and appear to have promise in the prevention or treatment of AMD. We developed the protocol for this review in anticipation of the progress of phase III controlled clinical trials. We have no expectations about the potential efficacy or safety of complement inhibitors, but given the wide interest in AMD, we consider it worthwhile to have the means in place to summarise evidence as it appears on this new class of potentially therapeutic agents. The review is necessarily widely inclusive of different classes of complement inhibitors, different clinical endpoints and different types of AMD. As the usefulness or otherwise of complement inhibitors becomes clear, we may refine the review to focus on individual drug classes, dosage variations, clinical endpoints and AMD types which are of most interest.
Objectives
The aim of this review was to assess the effects and safety of complement inhibitors in the prevention or treatment of advanced age‐related macular degeneration (AMD). Specifically, we sought to clarify the direction of any effect, the size of any effect, the consistency of any effects across studies and assess the strength of available evidence.
Methods
Criteria for considering studies for this review
Types of studies
We planned to include randomised controlled trials (RCTs) with parallel treatment groups. We planned not to include other trial designs such as cross‐over or cluster designs.
Types of participants
We planned to include two types of trials. Firstly, we planned to include trials with participants with advanced AMD which examined the treatment of advanced AMD in the treatment and control arms. We anticipate most RCTs on the use of complement inhibitors in AMD will be in this category. Secondly, we planned to include trials with participants who have non‐advanced AMD which looked at the prevention of advanced AMD in the treatment and control arms. The nomenclature may vary between studies, but for this review advanced AMD was defined as geographic atrophy involving the fovea or neovascular AMD that could be extrafoveal, juxtafoveal or subfoveal, as identified by clinical examination, angiography, OCT or other validated criteria. We excluded causes of neovascularisation other than AMD. We defined non‐advanced AMD as early age‐related maculopathy or drusen abnormalities, pigmentary abnormalities or both without neovascularisation or central geographic atrophy. We excluded studies on the normal healthy population. There were no age, sex or ethnic restrictions on study participants.
Types of interventions
We planned to include studies which compared therapeutic agents designed to treat or prevent advanced AMD by inhibition of the complement cascade to an active treatment, sham treatment or no treatment.
The agents under investigation may be locally or systemically applied and may inhibit any stage of the complement cascade by any means. We refer to these agents as complement inhibitors. There were no restrictions regarding delivery, dose, duration or co‐interventions.
Types of outcome measures
Primary outcomes
The primary outcomes for this review were as follows:
Loss of 15 letters or more of best corrected visual acuity (BCVA) at one year or more follow‐up, measured using charts adhering to Bailie‐Lovie principles. We converted BCVA data derived from other types of charts to equivalent log Mean Angle of Resolution (logMAR) units.
Change in BCVA after one year or more follow‐up, measured using charts adhering to Bailie‐Lovie principles, with BCVA considered as a continuous variable, either as number of letters or logMAR units.
The rationale for one year as a time point for outcomes is that recent clinical trials investigating interventions for AMD have consistently presented results at one year. One year is considered to be clinically meaningful.
Secondary outcomes
We considered the following secondary outcomes, each measured at one year or more follow‐up:
For those trials examining prevention of progression of non‐advanced to advanced AMD: development of advanced AMD.
Maintenance of BCVA; gain in 15 or more Early Treatment Diabetic Retinopathy Study (ETDRS) letters; loss of 30 or more ETDRS letters and blindness, defined as visual acuity worse than 20/200.
Contrast sensitivity, reading speed or any other validated measure of visual function.
Any quantitative measure of retinal morphology, such as thickness or lesion size, measured by ocular coherence tomography (OCT), fluorescein angiography or indocyanine green angiography.
Adverse outcomes, specifically hypersensitive reactions, complications of intravitreal injection (uveitis, infectious endophthalmitis, retinal detachment) or other adverse events as they emerged.
Quality of life, assessed using any validated measures.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (which contains the Cochrane Eyes and Vision Group Trials Register) 2013, Issue 11, part of The Cochrane Library. www.thecochranelibrary.com (accessed 21 November 2013), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to November 2013), EMBASE (January 1980 to November 2013), Allied and Complementary Medicine Database (AMED) (January 1985 to November 2013), Latin American and Caribbean Literature on Health Sciences (LILACS) (January 1982 to November 2013), OpenGrey (System for Information on Grey Literature in Europe) (www.opengrey.eu/), Web of Science Conference Proceedings Citation Index‐ Science (CPCI‐S) (January 1990 to November 2013), the metaRegister of Controlled Trials (mRCT) (www.controlled‐trials.com), ClinicalTrials.gov (www.clinicaltrials.gov) and the WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 21 November 2013.
See: Appendices for details of search strategies for CENTRAL (Appendix 1), MEDLINE (Appendix 2), EMBASE (Appendix 3), AMED (Appendix 4), LILACS (Appendix 5), OpenGrey (Appendix 6), CPCI‐S (Appendix 7), mRCT (Appendix 8), ClinicalTrials.gov (Appendix 9) and the ICTRP (Appendix 10).
Searching other resources
We searched the Science Citation Index. We contacted companies known to be conducting any stage of trials of complement inhibitors for AMD or having complement inhibitors for AMD in their pipeline and requested information on any ongoing or completed trials we may not have identified in the electronic search.
We handsearched abstracts from the following organisations' meetings and conferences from 2012 onwards:
the Association for Research in Vision and Ophthalmology;
the American Academy of Ophthalmology;
the UK Royal College of Ophthalmologists Annual Congress;
the Macula Society and the Retina Society.
Data collection and analysis
Selection of studies
Two review authors (MW and GMcK) independently evaluated all the titles and abstracts resulting from the searches. We obtained full copies of all the reports that definitely or potentially met the criteria for consideration in this review according to each review author's independent assessment. We discussed these reports and thus compiled a definitive list of selected studies.
As we did not find any completed RCTs that could be included in the review, we plan to follow the methods below in future updates.
Data extraction and management
Two review authors will independently extract data on all selected studies using specially developed paper forms available from the editorial base. We will compare results and resolve discrepancies by discussion between all three authors. When data are not available in the published report on the primary or secondary outcomes of interest to this review, we will contact the study authors and ask for relevant data in an effort to overcome any selective reporting biases. When necessary, we will extract data from figures in the reports and contact the authors to confirm or refute the accuracy of data so obtained.
Assessment of risk of bias in included studies
We will use Chapter 8 of theCochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) to guide the assessment of the methodological quality of each trial included in the review. Consequently each of two review authors (MW and GMcK) will consider the following for each trial.
selection bias (as addressed by sequence generation and allocation concealment);
performance bias (as addressed by masking of participants and personnel);
detection bias (as addressed by masking of outcome assessors);
attrition bias (as addressed by completeness of outcome data and documentation of exclusions);
reporting bias; and
other bias.
We will assess outcomes for each trial as 'low risk of bias', 'high risk of bias' or 'unclear'. We will make such assessments using the interpretations set out in tables 8.5a, 8.5c and 8.7a in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We will contact the authors of trials in which outcomes are categorised as unclear for additional information.
Bias may vary between different outcomes within the same study, for example for some outcomes, assessors and participants may be more easily masked (e.g. grade of AMD on retinal photographs) than for others (e.g. visual acuity). Therefore we will comment on bias at the level of outcomes rather than the study. If other types of bias are detected, they will be presented.
We will not include in the meta‐analysis data on a specific outcome from an individual trial if the outcome has an unclear or high risk of bias in that trial. Having made these assessments independently, the authors will discuss outcomes for each trial to agree on its bias level and whether to include the data. We will perform a sensitivity analysis for each outcome classed for any trial as 'high risk of bias' or 'unclear risk of bias' to determine whether the inclusion of all trials' data for that outcome would affect the conclusions. We will present all judgements and steps relating to bias in the text.
Measures of treatment effect
Primary outcomes for this review will be i) loss of 15 or more letters of BCVA in the treated eye and ii) change in BCVA as a continuous variable. We will therefore calculate the following for each trial for one year or more of follow‐up: i) risk ratio (RR) of loss of 15 or more ETDRS letters of BCVA, ii) the mean difference (MD) of BCVA (expressed as number of letters or as logMAR) between baseline and follow‐up, as described in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011).
Thus, we will consider BCVA as both a dichotomous and continuous outcome. For data pertaining to dichotomous outcomes, we will calculate RRs as this meets best the criteria set out in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011).
We will summarise secondary outcomes as continuous data and calculate MDs. We will allow for two exceptions which we considered as dichotomous, and therefore calculate an RR: i) the risk of adverse events and ii) secondary outcomes relating to visual acuity, i.e. the risk of maintenance of BCVA; the risk of gain in 15 or more ETDRS letters; the risk of loss of 30 or more ETDRS letters and the risk of blindness (visual acuity worse than 20/200). If the scale used to measure secondary outcomes varies between studies for any continuous outcome, such as retinal thickness, then we will calculate standardised mean difference.
We will perform statistical analyses using The Cochrane Collaboration's Review Manager (RevMan) software (Review Manager 2012). We will seek the advice of a statistician on how to deal with multiplicity issues, as well as other issues as necessary, for example if it is suspected that continuous data may have been skewed.
Unit of analysis issues
It is likely that randomisation will occur at the level of individuals as treatment given to one eye, or given systemically, potentially affects both eyes. The individual will therefore be our unit of analysis. If both eyes are treated, then we will seek advice as to whether to analyse the better visual acuity, the worse visual acuity or an average.
Trials may compare outcomes between each individual's treated eye and their fellow eye. If such paired study designs are encountered, we will seek statistical advice. It may be possible, for example, to combine paired and unpaired trial results using the generic inverse variance method.
Dealing with missing data
We will conduct a sensitivity analysis to examine any systematic bias caused by exclusion of participants after randomisation, including those lost to follow‐up. We will do this by analysing the following outcomes looking at two scenarios: i) the worst case scenario, i.e. assuming either that participants lost to follow‐up lost 15 or more letters visual acuity or that they all developed advanced AMD and ii) the best case scenario, i.e. either that none of those lost to follow‐up lost 15 or more letters visual acuity at one year follow‐up or that none developed advanced AMD.
Assessment of heterogeneity
We will calculate an I2 statistic. We will also assess heterogeneity using a Chi2 test. Given the low numbers of studies anticipated for the initial reviews, we will use a P value of 0.1 to address the null hypothesis of no significant heterogeneity. We will also assess methodological variability by careful review of manuscripts.
Assessment of reporting biases
We will present a funnel plot for each outcome of five or more study results included in the meta‐analysis. We chose the number five arbitrarily. We will plot effect size on the horizontal axis and standard error of each trial on the vertical axis. We will judge funnel plot asymmetry by visual inspection. We will try to judge whether asymmetry is due to publication bias or due to the tendency of smaller studies to produce different effect sizes for various reasons as outlined in Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Sterne 2011). We will present a full description of funnel plot interpretation with the cautionary note that such interpretation will be subjective and probably speculative. For any funnel plot which either author thinks is asymmetrical and for which 10 or more trials are included, we will seek statistical advice as to whether and how to formally test for funnel plot asymmetry.
Searching as comprehensively as possible will be the main means of avoiding reporting biases for studies.
It is possible that trials may report only their most statistically significant measure of visual improvement. We will overcome such selective outcome reporting by stating the precise outcome measures a priori as above. We will present a review outcome matrix as described in the Outcome Reporting Bias In Trials (ORBIT) study (Kirkham 2010). We will use this review outcome matrix to summarise, for each outcome and each trial, whether the outcome was reported, partially reported (selectively or incompletely) or not reported. If we believe that the outcome may have been recorded or analysed (or both) but not reported, we will contact the authors to ask for the relevant outcome data.
Data synthesis
We will perform meta‐analysis if possible. Meta‐analysis as described in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011) may not be appropriate, however in such circumstances we will give a structured summary. We will calculate weighted averages as follows: i) RR of loss of 15 or more ETDRS letters of BCVA, ii) the MD of BCVA and iii) when appropriate (for preventative trials), the RR of developing advanced AMD. From the weighted averages we will derive confidence intervals, a P value and number needed to treat.
If there is no evidence of significant heterogeneity between study estimates, we will use two approaches. For both the dichotomous and continuous data, we will use a fixed‐effect model: for dichotomous data we will apply the Mantel‐Haenszel method, and for continuous data we will use the inverse variance as the default approaches of RevMan. Where there are more than three studies, we will use a random‐effects model. In the absence of heterogeneity, these should give the same results. We will compare the results obtained using the two models. We will take care to interpret the results of the fixed and random‐effects models appropriately (Riley 2011).
If heterogeneity is found, we will use a random‐effects model (the inverse variance method as the default approach of RevMan). If we consider the heterogeneity to be substantial, we will give a narrative summary. We will record the Chi2 test results and the cut‐off I2 statistic (Higgins 2003) for defining heterogeneity as substantial. We will present a forest plot to allow visual assessment of overlap between confidence intervals of studies.
Subgroup analysis and investigation of heterogeneity
We anticipated that several characteristics may emerge as potential causes of heterogeneity, representing potential modifiers of the effectiveness of complement inhibitors. If other characteristics emerge, they may be the basis for post hoc subgroup analysis if authors judge them to be of major importance and if this judgement is supported by external evidence.
We will perform subgroup analysis for each of the primary outcomes of different classes of complement inhibitors. We will adopt the definition of classes of complement inhibitors as defined by Ricklin et al, as described above (Ricklin 2007): i) protease inhibitors; ii) natural complement regulators; iii) antibiotics against specific complement components; iv) functional complement component inhibitors and v) anaphylatoxin receptor antagonists. Future reviews may focus entirely on one class of complement inhibitor and dosage variations within that group if one emerges as a leader in the field.
We will perform subgroup analysis for each of the primary outcomes according to the type of advanced AMD: neovascular or atrophic.
For the data on treatment of neovascular AMD, we will define and analyse subgroups according to the sub‐type of neovascularisation, guided by the definitions adopted in the included trials.
We will also perform subgroup analysis based on reported ethnicity.
We will use fixed‐effect analyses based on the inverse variance method if there are fewer than 10 studies available for the characteristic. Otherwise we will use meta‐regression. We will be mindful, however, of the dangers of spurious associations derived from subgroup analyses due to small numbers of studies or too many comparisons.
Sensitivity analysis
We will perform sensitivity analyses: i) for each outcome to determine whether the inclusion of all outcomes classed as 'high risk of bias' or 'unclear risk of bias' would affect the conclusions, and to assess the effect of excluding all of the following trials: ii) unpublished data and iii) industry‐funded studies. If the only data available are unpublished or industry‐funded, we will not perform sensitivity analyses.
Results
Description of studies
Results of the search
The electronic searches yielded a total of 560 references (Figure 1). After removing duplicates we screened 402 references to identify potentially relevant studies but found no studies that met the inclusion criteria. We found two ongoing trials which may be eligible for inclusion in the review (NCT00935883: NCT00950638) and we will assess these studies when data become available. One trial was a phase II study on eculizumab for the treatment of non‐exudative macular degeneration (NCT00935883) for which the final data collection date will be June 2012: the results of this study are not yet available. The other is a phase I study, NCT00950638, investigating ARC1905 with a final data collection date of September 2012.
Figure 1.
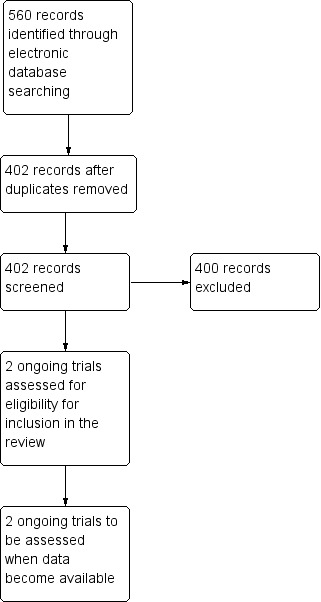
Results from searching for studies for inclusion in the review.
Included studies
No studies were eligible for potential inclusion in this review.
Excluded studies
No studies were eligible for potential inclusion in this review.
Risk of bias in included studies
We did not assess for risk of bias as no studies were included.
Effects of interventions
There were no data on which to perform meta‐analysis.
Discussion
Examples of relevant phase I and II trials include the following two. 'Safety of intravitreal POT‐4 therapy for patients with neovascular AMD (ASap)' (NCT00473928) was a phase I, single group assignment study primarily investigating safety, with the secondary aims of further characterising the efficacy of POT‐4 as assessed by changes in visual acuity, retinal thickness and choroidal neovascularisation lesion size. Data were presented at the American Academy of Ophthalmology (AAO) 2008 Annual Meeting (RET03 Section II: AMD Part II, Friday Nov 7, 9:01AM). The drug showed good tolerability and no drug‐related side effects (Anonymous 2009). The Potentia website states: "Interim results of this trial revealed no drug‐related toxicity based on clinical signs, ophthalmic examinations, or laboratory results at any time point monitored in patients treated with up to 150 ug/dose of POT‐4. Additionally, no serious adverse events and no identifiable intraocular inflammation were reported" (www.potentiapharma.com/). Potentia Pharmaceuticals entered into licensing and purchase option agreements regarding the drug with Alcon in 2009. The final data collection date for ASap was February 2010 and the study is now complete. New analogues of compstatin are under development (Tamamis 2012) and Anosos Biotherapeutics, a company linked with the University of Pennsylvania 'UPstart' programme (www.ctt.upenn.edu/upstart.html) is developing related compounds with greater potency for AMD.
'ARC1905 (ANTI‐C5 APTAMER) given either in combination therapy with Lucentis® 0.5 mg/eye in subjects with neovascular AMD' (NCT00709527) was a non‐randomised, open‐label, phase I, dose‐escalating, prospective study investigating intravitreal ARC1905 in combination with ranibizumab for neovascular AMD. The final data collection date was March 2011. Data were reported at the Association for Research in Vision and Ophthalmology (ARVO) 2010 meeting. Fifty‐eight participants were initiated in to the study and 48 had received at least two doses of the experimental regimen. There was no evidence of dose‐limiting toxicity and 35% of participants experienced a gain in 3 or more ETDRS lines of BCVA.
Such phase I and II trials may provide an impetus for phase III trials and eventually for the use of complement inhibitors in practice. We hope that in updates of this review we will have trials on which to perform meta‐analysis. Having the protocol for this review published in advance of such data will allow a systematic review and meta‐analysis to proceed without delay at the update stage, and also highlights an area of growing interest.
The two ongoing studies mentioned above are described in Characteristics of ongoing studies.
Authors' conclusions
There is insufficient information at present to generate evidence‐based recommendations on the potential safety and efficacy of complement inhibitors for prevention or treatment of AMD. However we anticipate the results of two trials: one phase I and one phase II trial, presently ongoing.
The treatment of AMD has been revolutionised by the advent of VEGF‐antagonists. There are a multitude of other potential treatments currently under investigation. In time these, along with knowledge of a patient's relevant genetic make‐up (Schwartz 2011), may allow individualised treatment. Preclinical evidence implicating complement overactivity in the pathogenesis of AMD suggests that inhibition of pathways leading to complement activation may in the future have a prominent role in treatment of AMD, although there are challenges to overcome first (Issa 2011; Khandhadia 2012; Troutbeck 2012). Phase III studies investigating the safety and efficacy of complement inhibitors for AMD in comparison to existing treatments are anticpated with interest.
Acknowledgements
To the Cochrane Eyes and Vision Group (CEVG) for their patience and help with the editorial process (Anupa Shah) and creating/executing the electronic searches (Iris Gordon). We thank Jennifer Evans for her assistance, Xue Wang, Sandy Forman and Sobha Sivaprasad for their comments on the review and Catey Bunce for her comments on the protocol.
Richard Wormald (Co‐ordinating Editor for CEVG) acknowledges financial support for his CEVG research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology. The views expressed in this publication are those of the authors and not necessarily those of the Department of Health.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor Macular Degeneration #2 MeSH descriptor Retinal Degeneration e #3 MeSH descriptor Retinal Neovascularization #4 MeSH descriptor Choroidal Neovascularization #5 MeSH descriptor Macula Lutea #6 maculopath* #7 (macul* or retina* or choroid*) near/3 (degener*) #8 (macul* or retina* or choroid*) near/3 (neovasc*) #9 macula* near/2 lutea #10 AMD or ARMD or CNV #11 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10) #12 MeSH descriptor Complement System Proteins #13 (cascad* or inhibit*) near/3 (complement) #14 C3 or C5 #15 eculizumab #16 compstatin #17 POT 4 #18 (#12 OR #13 OR #14 OR #15 OR #16 OR #17) #19 (#11 AND #18)
Appendix 2. MEDLINE (OvidSP) search strategy
1. randomized controlled trial.pt. 2. (randomized or randomised).ab,ti. 3. placebo.ab,ti. 4. dt.fs. 5. randomly.ab,ti. 6. trial.ab,ti. 7. groups.ab,ti. 8. or/1‐7 9. exp animals/ 10. exp humans/ 11. 9 not (9 and 10) 12. 8 not 11 13. exp macular degeneration/ 14. exp retinal degeneration/ 15. exp retinal neovascularization/ 16. exp choroidal neovascularization/ 17. exp macula lutea/ 18. maculopath$.tw. 19. ((macul$ or retina$ or choroid$) adj3 degener$).tw. 20. ((macul$ or retina$ or choroid$) adj3 neovasc$).tw. 21. (macula$ adj2 lutea).tw. 22. (AMD or ARMD or CNV).tw. 23. or/13‐22 24. exp complement system proteins/ 25. (complement adj3 (cascad$ or inhibit$)).tw. 26. (C3 or C5).tw. 27. eculizumab.tw. 28. compstatin.tw. 29. POT 4.tw. 30. or/24‐29 31. 23 and 30 32. 12 and 31
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville et al (Glanville 2006).
Appendix 3. EMBASE (OvidSP) search strategy
1. exp randomized controlled trial/ 2. exp randomization/ 3. exp double blind procedure/ 4. exp single blind procedure/ 5. random$.tw. 6. or/1‐5 7. (animal or animal experiment).sh. 8. human.sh. 9. 7 and 8 10. 7 not 9 11. 6 not 10 12. exp clinical trial/ 13. (clin$ adj3 trial$).tw. 14. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 15. exp placebo/ 16. placebo$.tw. 17. random$.tw. 18. exp experimental design/ 19. exp crossover procedure/ 20. exp control group/ 21. exp latin square design/ 22. or/12‐21 23. 22 not 10 24. 23 not 11 25. exp comparative study/ 26. exp evaluation/ 27. exp prospective study/ 28. (control$ or prospectiv$ or volunteer$).tw. 29. or/25‐28 30. 29 not 10 31. 30 not (11 or 23) 32. 11 or 24 or 31 33. exp retina macula degeneration/ 34. exp retina degeneration/ 35. exp retina neovascularization/ 36. exp subretinal neovascularization/ 37. maculopath$.tw. 38. ((macul$ or retina$ or choroid$) adj3 degener$).tw. 39. ((macul$ or retina$ or choroid$) adj3 neovasc$).tw. 40. exp retina macula lutea/ 41. (macula$ adj2 lutea$).tw. 42. (AMD or ARMD or CNV).ti,ab. 43. or/33‐42 44. exp complement/ 45. (complement adj3 (cascad$ or inhibit$)).tw. 46. (C3 or C5).tw. 47. eculizumab.tw. 48. compstatin.tw. 49. POT 4.tw. 50. or/44‐49 51. 43 and 50 52. 32 and 51
Appendix 4. AMED (OvidSP) search strategy
1. exp eye disease/ 2. exp vision disorders/ 3. exp retinal disease/ 4. maculopath$.tw. 5. ((macul$ or retina$ or choroid$) adj3 degenerat$).tw. 6. ((macul$ or retina$ or choroid$) adj3 neovasc$).tw. 7. or/1‐6 8. (complement adj3 (cascad$ or inhibit$)).tw. 9. (C3 or C5).tw. 10. eculizumab.tw. 11. compstatin.tw. 12. POT 4.tw. 13. or/8‐12 14. 7 and 13
Appendix 5. LILACS search strategy
macul$ or retina$ or choroid$ and degenerat$ or neovascula$ and complement or C3 or C5 or eculizumab or compstatin or POT 4
Appendix 6. OpenGrey search strategy
macul$ or retina$ or choroid$ AND degenerat$ or neovascula$ AND complement or C3 or C5 or eculizumab or compstatin or POT 4
Appendix 7. Web of Science CPCI‐S search strategy
#4 #2 AND #3 #3 TS= (complement or C3 or C5 or eculizumab or compstatin or POT 4) #2 TS= (degenerat* or neovasc*) AND #1 #1 TS= (macula* or retina* or choroid*)
Appendix 8. metaRegister of Controlled Trials search strategy
macula AND (complement or C3 or C5 or eculizumab or compstatin or POT 4)
Appendix 9. ClinicalTrials.gov search strategy
macula AND complement
Appendix 10. ICTRP search strategy
macula = Condition AND complement = Intervention
Characteristics of studies
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | 'Complement Inhibition With Eculizumab for the Treatment of Non‐Exudative Macular Degeneration' (COMPLETE) |
| Methods | Randomised, double‐arm, double‐masked, phase II trial |
| Participants | Over 50 years with nonexudative AMD and no history of CNV in the study eye, BCVA of 20/63 (or 59 letters) or better and no confounding ocular conditions |
| Interventions | Intravenous eculizumab or intravenous saline |
| Outcomes | Primary outcome: growth in GA/change in drusen volume |
| Starting date | July 2009 |
| Contact information | Principal investigator: Philip J Rosenfeld, MD, PhD |
| Notes | NCT00935883 is active but not recruiting, having had a final data collection date of June 2012 |
| Trial name or title | 'A Study of ARC1905 (Anti‐C5 Aptamer) in Subjects With Dry AMD' |
| Methods | Randomised, open‐label, dose comparison, parallel assignment, phase I study |
| Participants | Over 50 years of age with dry AMD in both eyes |
| Interventions | ARC1905 intravitreal injection (dose comparison) |
| Outcomes | Presence of any dose‐limiting toxicity; safety endpoints include adverse events, vital signs, ophthalmic variables and outcomes |
| Starting date | July 2009 |
| Contact information | Ophthotech Corporation, New York, USA |
| Notes | NCT00950638 is examining the safety and tolerability of intravitreal ARC‐1905 for GA secondary to AMD. It is active but not recruiting, with a final data collection date of September 2012 |
AMD: age‐related macular degeneration BCVA: best corrected visual acuity CNV: choroidal neovascularisation GA: geographic atrophy
Contributions of authors
MA Williams (MAW) initiated and wrote the protocol; GJ McKay (GMcK) and U Chakravarthy (UC) advised on and edited the protocol. MAW and GMcK independently evaluated all the titles and abstracts resulting from the searches, and then discussed findings. UC subsequently advised.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Dunhill Medical Trust/Royal College of Physicians Clinical Research Fellowship, UK.
Supported MA Williams
-
Alzheimer's Research Trust Emergency Grant, UK.
Supported MA Williams
Declarations of interest
The authors have no conflicts of interest.
New
References
References to ongoing studies
- NCT00935883. Eculizumab for the treatment of non‐exudative age‐related macular degeneration: an exploratory study to evaluate the effects of C5 inhibition on drusen and geographic atrophy. clinicaltrials.gov/show/NCT00935883 (accessed 28 May 2013).
- NCT00950638. A phase I study of ARC1905 (anti‐C5 aptamer) in subjects with dry age‐related macular degeneration. clinicaltrials.gov/show/Nct00950638 (accessed 28 May 2013).
Additional references
- Anonymous. Deal watch: Alcon licenses complement pathway inhibitor for macular degeneration. Nature Reviews Drug Discovery 2009;8:922. [DOI] [PubMed] [Google Scholar]
- Age‐Related Eye Disease Study Research Group. A randomized, placebo‐controlled, clinical trial of high‐dose supplementation with vitamins C and E, beta carotene, and zinc for age‐related macular degeneration and vision loss: AREDS report no. 8. Archives of Ophthalmology 2001;119(10):1417‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augood CA, Vingerling JR, Jong PT, Chakravarthy U, Seland J, Soubrane G, et al. Prevalence of age‐related maculopathy in older Europeans: the European Eye Study (EUREYE). Archives of Ophthalmology 2006;124(4):529‐35. [DOI] [PubMed] [Google Scholar]
- Bora PS, Sohn JH, Cruz JM, Jha P, Nishihori H, Wang Y, et al. Role of complement and complement membrane attack complex in laser‐induced choroidal neovascularization. Journal of Immunology 2005;174(1):491‐7. [DOI] [PubMed] [Google Scholar]
- Büttner‐Mainik A, Parsons J, Jérôme H, Hartmann A, Lamer S, Schaaf A, et al. Production of biologically active recombinant human factor H in Physcomitrella. Plant Biotechnology Journal 2011;9(3):373‐83. [DOI] [PubMed] [Google Scholar]
- Chakravarthy U, Wong TY, Fletcher A, Piault E, Evans C, Zlateva G, et al. Clinical risk factors for age‐related macular degeneration: a systematic review and meta‐analysis. BMC Ophthalmology 2010;10:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabb JW, Miyagi M, Gu X, Shadrach K, West KA, Sakaguchi H, et al. Drusen proteome analysis: an approach to the etiology of age‐related macular degeneration. Proceedings of the National Academy of Sciences of the United States of America 2002;99(23):14682‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011 Available from www.cochrane‐handbook.org.
- Despriet DD, Klaver CC, Witteman JC, Bergen AA, Kardys I, Maat MP, et al. Complement factor H polymorphism, complement activators, and risk of age‐related macular degeneration. JAMA 2006;296(3):301‐9. [DOI] [PubMed] [Google Scholar]
- Edwards AO, Ritter R 3rd, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age‐related macular degeneration. Science 2005;308(5720):421‐4. [DOI] [PubMed] [Google Scholar]
- Evans JR, Fletcher AE, Wormald RP. Age‐related macular degeneration causing visual impairment in people 75 years or older in Britain: an add‐on study to the Medical Research Council Trial of Assessment and Management of Older People in the Community. Ophthalmology 2004;111(3):513‐7. [DOI] [PubMed] [Google Scholar]
- Glanville JM, Lefebvre C, Miles JN, Camosso‐Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130‐6. [PMC free article] [PubMed] [Google Scholar]
- Hageman GS, Luthert PJ, Chong NHV, Johnson LV, Anderson DH, Mullins RF. An integrated hypothesis that considers drusen as biomarkers of immune‐mediated processes at the RPE‐Bruch's membrane interface in aging and age‐related macular degeneration. Progress in Retinal and Eye Research 2001;20(6):705‐32. [DOI] [PubMed] [Google Scholar]
- Hageman GS, Anderson DH, Johnson LV, Hancox LS, Taiber AJ, Hardisty LI, et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age‐related macular degeneration. Proceedings of the National Academy of Sciences of the United States of America 2005;102(20):7227‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines JL, Hauser MA, Schmidt S, Scott WK, Olson LM, Gallins P, et al. Complement factor H variant increases the risk of age‐related macular degeneration. Science 2005;308(5720):419‐21. [DOI] [PubMed] [Google Scholar]
- Haines JL, Spencer KM, Pericak‐Vance MA. Bringing the genetics of macular degeneration into focus. Proceedings of the National Academy of Sciences of the United States of America 2007;104(43):16725‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, O'Banion MK. Inflammatory processes in Alzheimer's disease. Journal of Neuroimmunology 2007;184(1‐2):69‐91. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011 Available from www.cochrane‐handbook.org.
- Hogg RE, Stevenson MR, Chakravarthy U, Beirne RO, Anderson RS. Early features of AMD. Ophthalmology 2007;114(5):1028. [DOI] [PubMed] [Google Scholar]
- Issa CP, Chong NV, Scholl HP. The significance of the complement system for the pathogenesis of age‐related macular degeneration ‐ current evidence and translation into clinical application. Graefe's Archive for Clinical and Experimental Ophthalmology 2011;249(2):163‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LV, Ozaki S, Staples MK, Erickson PA, Anderson DH. A potential role for immune complex pathogenesis in drusen formation. Experimental Eye Research 2000;70(4):441‐9. [DOI] [PubMed] [Google Scholar]
- Johnson LV, Leitner WP, Rivest AJ, Staples MK, Radeke MJ, Anderson DH. The Alzheimer's A beta ‐peptide is deposited at sites of complement activation in pathologic deposits associated with aging and age‐related macular degeneration. Proceedings of the National Academy of Sciences of the United States of America 2002;99(18):11830‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandhadia S, Cipriani V, Yates JR, Lotery AJ. Age‐related macular degeneration and the complement system. Immunobiology 2012;217(2):127‐46. [DOI] [PubMed] [Google Scholar]
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340:365. [DOI] [PubMed] [Google Scholar]
- Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, et al. Complement factor H polymorphism in age‐related macular degeneration. Science 2005;308(5720):385‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markiewski MM, Lambris JD. The role of complement in inflammatory diseases from behind the scenes into the spotlight. American Journal of Pathology 2007;171(3):715‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safety of intravitreal POT‐4 therapy for patients with neovascular age‐related macular degeneration. ClinicalTrials.gov/show/NCT00473928 (accessed 28 May 2013).
- ARC1905 (ANTI‐C5 APTAMER) given either in combination therapy with Lucentis® 0.5 mg/eye in subjects with neovascular age‐related macular degeneration. ClinicalTrials.gov/show/NCT00709527 (accessed 28 May 2013).
- Owen CG, Fletcher AE, Donoghue M, Rudnicka AR. How big is the burden of visual loss caused by age related macular degeneration in the United Kingdom?. British Journal of Ophthalmology 2003;87(3):312‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel N, Adewoyin T, Chong NV. Age‐related macular degeneration: a perspective on genetic studies. Eye 2008;22(6):768‐76. [DOI] [PubMed] [Google Scholar]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
- Ricklin D, Lambris JD. Complement‐targeted therapeutics. Nature Biotechnology 2007;25(11):1265‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta‐analyses. BMJ 2011;342:594. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Cordoba S, Esparza‐Gordillo J, Goicoechea de Jorge E, Lopez‐Trascasa M, Sanchez‐Corral P. The human complement factor H: functional roles, genetic variations and disease associations. Molecular Immunology 2004;41(4):355‐67. [DOI] [PubMed] [Google Scholar]
- Schwartz SG, Brantley MA, Jr. Pharmacogenetics and age‐related macular degeneration. Journal of Ophthalmology2011:Article ID 252549. [DOI: 10.1155/2011/252549] [DOI] [PMC free article] [PubMed]
- Seddon JM, Ajani UA, Mitchell BD. Familial aggregation of age‐related maculopathy. American Journal of Ophthalmology 1997;124(2):199‐206. [DOI] [PubMed] [Google Scholar]
- Sivaprasad S, Chong NV. The complement system and age‐related macular degeneration. Eye 2006;20(8):867‐72. [DOI] [PubMed] [Google Scholar]
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011 Available from www.cochrane‐handbook.org.
- Tamamis P, López de Victoria A, Gorham RD Jr, Bellows‐Peterson ML, Pierou P, Floudas CA, et al. Molecular dynamics in drug design: new generations of compstatin analogs. Chemical Biology and Drug Design 2012;79(5):703‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton J, Edwards R, Mitchell P, Harrison RA, Buchan I, Kelly SP. Smoking and age‐related macular degeneration: a review of association. Eye 2005;19(9):935‐44. [DOI] [PubMed] [Google Scholar]
- Troutbeck R, Al‐Qureshi S, Guymer RH. Therapeutic targeting of the complement system in age‐related macular degeneration: a review. Clinical and Experimental Ophthalmology 2012;40(1):18‐26. [DOI] [PubMed] [Google Scholar]
- www.alexionpharm.com/.
- www.archemix.com/website/index.php.
- UPstart at the University of Pennsylvania. www.ctt.upenn.edu/upstart.html (accessed 24 May 2012).
- www.jerini.com/cms/en/home.php.
- www.ophthotech.com/.
- www.potentiapharma.com/about/news.htm (accessed 13 May 2012).
References to other published versions of this review
- Williams MA, McKay GJ, Chakravarthy U. Complement inhibitors for age‐related macular degeneration. Cochrane Database of Systematic Reviews 2011, Issue 9. [DOI: 10.1002/14651858.CD009300] [DOI] [PMC free article] [PubMed] [Google Scholar]


