Abstract
Adjustment of cerebral blood flow (CBF) to neuronal activity via neurovascular coupling (NVC) plays an important role in the maintenance of healthy cognitive function. Strong evidence demonstrates that age-related cerebromicrovascular endothelial dysfunction and consequential impairment of NVC responses contribute importantly to cognitive decline. Recent studies demonstrate that NAD+ availability decreases with age in the vasculature and that supplemental NAD+ precursors can ameliorate cerebrovascular dysfunction, rescuing NVC responses and improving cognitive performance in aged mice. The mechanisms underlying the age-related decline in [NAD+] in cells of the neurovascular unit are likely multifaceted and may include increased utilization of NAD+ by activated poly (ADP-ribose) polymerase (PARP-1). The present study was designed to test the hypothesis that inhibition of PARP-1 activity may confer protective effects on neurovascular function in aging, similar to the recently demonstrated protective effects of treatment with the NAD+ precursor nicotinamide mononucleotide (NMN). To test this hypothesis, 24-month-old C57BL/6 mice were treated with PJ-34, a potent PARP inhibitor, for 2 weeks. NVC was assessed by measuring CBF responses (laser speckle contrast imaging) in the somatosensory whisker barrel cortex evoked by contralateral whisker stimulation. We found that NVC responses were significantly impaired in aged mice. Treatment with PJ-34 improved NVC responses by increasing endothelial NO-mediated vasodilation, which was associated with significantly improved spatial working memory. PJ-34 treatment also improved endothelium-dependent acetylcholine-induced relaxation of aorta rings. Thus, PARP-1 activation, likely by decreasing NAD+ availability, contributes to age-related endothelial dysfunction and neurovascular uncoupling, exacerbating cognitive decline. The cerebromicrovascular protective effects of pharmacological inhibition of PARP-1 highlight the preventive and therapeutic potential of treatments that restore NAD+ homeostasis as effective interventions in patients at risk for vascular cognitive impairment (VCI).
Keywords: Cellular energetics, Oxidative stress, ROS, Endothelial dysfunction, Functional hyperemia, Microcirculation, Senescence
Introduction
There is growing evidence that micro-vascular contributions to cognitive impairment and dementia (VCID) play a critical role in elderly individuals (Tarantini et al. 2017a). Proper brain homeostasis requires an adequate, highly-controlled and uninterrupted supply of oxygen and nutrients to match neuronal energetic demand. Cerebral blood flow (CBF), which represents 15% of cardiac output, must continuously and dynamically be adjusted due to brain’s limited energy reserves and shifting energy requirements. During periods of intense neuronal activity there is a need for rapid adjustment of regional oxygen and glucose delivery to metabolic demand through spatially localized adaptive increases in CBF. This is ensured by an evolutionarily conserved physiological homeostatic mechanism known as neurovascular coupling (NVC) or functional hyperemia (Tarantini et al. 2017a, 2018, 2019; Ungvari et al. 2017). The cellular mechanisms of NVC include release of vasodilator NO from the microvascular endothelium, in response to increased neuronal and astrocytic activation (Toth et al. 2014, 2015a). Translational studies show that endothelial function and NVC responses are compromised both in elderly subjects (Zaletel et al. 2005; Topcuoglu et al. 2009; Stefanova et al. 2013; Fabiani et al. 2013; Lipecz et al. 2019) and aged laboratory animals, which importantly contribute to the age-related decline in higher cortical function (Tarantini et al. 2018, 2017a, 2019). Experimental studies provide further evidence that inhibition of NO-mediated NVC responses in mice is associated with impaired cognitive performance (Tarantini et al. 2015, 2017b). Recent studies demonstrate that interventions that improve endothelial function and NVC responses exert protective effects on cognition in mouse models of aging (Tarantini et al. 2018, 2019). Understanding the cellular and molecular mechanisms underlying microvascular aging will enable the development of novel, clinically translatable interventions for restoration of neurovascular function and improvement of cerebral blood supply to prevent development and delay progression of vascular cognitive impairment.
Pre-clinical studies show that cellular NAD+ levels decline in old age in multiple tissues and that treatment with NAD+ precursors can ameliorate many age-related cellular impairments (Tarantini et al. 2019; Kiss et al. 2019; Yoshino et al. 2018; Kim et al. 2019; Mills et al. 2016; Gomes et al. 2013). We have recently shown that a decrease in NAD+ availability with age also plays a critical role in impaired NVC responses in aged mice (Tarantini et al. 2019; Csiszar et al. 2019). NAD+ is a rate-limiting co-substrate for sirtuin enzymes (including SIRT-1 (Csiszar et al. 2008a, 2009)), which are key regulators of endothelial function, cellular energetics and mitochondrial production of reactive oxygen species (ROS) (Tarantini et al. 2019; Kiss et al. 2019; Gomes et al. 2013; Csiszar et al. 2019; Bonkowski and Sinclair 2016; Das et al. 2018; Schultz and Sinclair 2016). The mechanisms responsible for endothelial NAD+ deficiency in vascular aging are likely multifaceted and may include increased activation of NAD+ utilizing poly (ADP-ribose) polymerase (PARPs, including PARP-1) enzymes (Csiszar et al. 2019). PARP-1 is a constitutive factor of the DNA damage surveillance network, which is activated upon oxidative/nitrative stress-induced DNA damage in aged cells. PARP-1 cleaves NAD+ and transfers the resulting ADP-ribose moiety onto target nuclear proteins and onto subsequent polymers of ADP-ribose, depleting cellular NAD+ pools in the process. Importantly, genetic depletion (Bai et al. 2011) and/or pharmacological inhibition of PARP-1 were shown to increase tissue NAD+ levels in rodent models of accelerated aging. Pharmacological inhibition of PARP-1 also improves endothelial function in large arteries of aged rodents (Pacher et al. 2002a, b, 2004).
The present study was designed to test the hypothesis that chronic inhibition of PARP-1 can improve cerebromicrovascular endothelial function and thereby rescue neurovascular coupling responses in aged mice. To achieve this goal, aged mice were treated with PJ-34, a potent PARP-1 inhibitor, for 2 weeks. Mice were behaviorally evaluated and functional tests for endothelial NO-mediated NVC responses were performed. To substantiate the in vivo findings the effects of PJ-34 on endothelium-dependent vasorelaxation were obtained in vitro in isolated aortic ring preparations.
Methods
Animals, PJ-34 treatment
Young (3 months, n = 20) and aged (24 months, n = 40) male C57BL/6 mice were purchased from the aging colony maintained by the National Institute on Aging at Charles River Laboratories (Wilmington, MA). Animals were housed under specific pathogen-free barrier conditions in the Rodent Barrier Facility at University of Oklahoma Health Sciences Center under a controlled photoperiod (12 h light/12 h dark) with unlimited access to water and were fed a standard AIN-93G diet (ad libitum). Mice in the aged cohort were assigned to two groups (n = 20 each group). One group of the aged mice received a daily dose of the potent PARP inhibitor PJ-34 (10 mg/kg/day, i.p., for 14 days from Cayman Chemical Ann Arbor, MI, USA). All procedures were approved by the Institutional Animal Use and Care Committees of the University of Oklahoma Health Sciences Center.
Behavioral studies
Previous studies suggest that alterations of neurovascular coupling responses associate with changes in cognition [12]. Thus, after the treatment period spatial memory and long-term memory was tested using the radial arms water maze as reported (Tarantini et al. 2018, 2019; Ungvari et al. 2017). In brief, the maze consisted of eight arms 9 cm wide that radiated out from an open central area, with a submerged escape platform located at the end of one of the arms. Paint was added into the water to make it opaque. The maze was surrounded by privacy blinds with extramaze visual cues. Intramaze visual cues were placed at the end of the arms. The mice were monitored by a video tracking system directly above the maze as they waded and parameters were measured using Ethovision software Noldus Information Technology Inc., Leesburg, VA, USA). Experimenters were unaware of the experimental conditions of the mice at the time of testing. During the learning period each day, mice were given the opportunity to learn the location of the submerged platform during two sessions each consisting of four consecutive acquisition trials. On each trial, the mouse was started in one arm not containing the platform and allowed to wade for up to 1 mi to find the escape platform. All mice spent 30 s on the platform following each trial before beginning the next trial. The platform was located in the same arm on each trial. Over the 3 days of training, mice in the young control group gradually improved performance as they learned the procedural aspects of the task. Upon entering an incorrect arm (all four paws within the distal half of the arm) or failing to select an arm after 15 s, the mouse was charged an error. Learning capability was assessed by comparing performance on days 2 and 3 of the learning period. A fourth trial, the probe trial, was conducted 7 days after the last learning trial to asses long-term memory retention. The reversal trials were carried out the following day by replacing the hidden platform in another arm while the errors were continuously assessed as during the learning phase. The reversal trials are a shorter version of the learning task which is performed after the animals have become proficient with the task and aims to evaluate the ability of each mouse to extinguish and re-learn the correct escape platform location.
Measurement of neurovascular coupling responses
After behavioral testing, mice in each group were anesthetized with isoflurane (4% induction and 1% maintenance), endotracheally intubated and ventilated (MousVent G500; Kent Scientific Co, Torrington, CT, USA). A thermostatic heating pad (Kent Scientific Co, Torrington, CT, USA) was used to maintain rectal temperature at 37 °C [6]. End-tidal CO2 was controlled between 3.2% and 3.7% to keep blood gas values within the physiological range, as described (Tarantini et al. 2015; Toth et al. 2015b). The right femoral artery was cannulated for arterial blood pressure measurement (Living Systems Instrumentations, Burlington, VT, USA) (Toth et al. 2014). The blood pressure was within the physiological range throughout the experiments (90–110 mmHg). Mice were immobilized and placed on a stereotaxic frame (Leica Microsystems, Buffalo Grove, IL, USA), the scalp and periosteum were pulled aside and the skull was gently thinned using a dental drill while cooled with dripping buffer. A laser speckle contrast imager (Perimed, Järfälla, Sweden) was placed 10 cm above the thinned skull, and to achieve the highest CBF response the right whiskers were stimulated for 30 s at 10 Hz from side to side (Tarantini et al. 2018). Differential perfusion maps of the brain surface were captured. Changes in CBF were assessed above the left barrel cortex in six trials in each group, separated by 5–10 min intervals. To assess the role of NO mediation, CBF responses to whisker stimulation were repeated 15 min after intravenous administration of the nitric oxide synthase inhibitor Nω-Nitro-L-arginine methyl ester (L-NAME). Changes in CBF were averaged and expressed as percent (%) increase from the baseline value (Kazama et al. 2004). All reagents used in this study were purchased from Sigma-Aldrich (St Louis, MO) unless otherwise indicated.
Assessment of endothelial function in the aorta
To assess the specific effect of PJ-34 treatment on endothelial function, endothelium-dependent vasorelaxation was assessed in isolated aorta ring preparations as described previously (Csiszar et al. 2009; Bailey-Downs et al. 2012; Csiszar et al. 2008b; Pearson et al. 2008; Horvath et al. 2005). In brief, aortas were cut into ring segments 1.5 mm in length and mounted in myographs chambers (Danish Myo Technology A/S, Inc., Denmark) for measurement of isometric tension. The chambers were filled with Krebs buffer solution (118 mM NaCl, 4.7 mM KCl, 1.5 mM CaCl2, 25 mM NaHCO3, 1.1 mM MgSO4, 1.2 mM KH2PO4, and 5.6 mM glucose; at 37 °C; gassed with 95% air and 5% CO2). After an equilibration period of 1 h during which an optimal passive tension was applied to the rings (as determined from the vascular length-tension relationship), they were pre-contracted with 10−6 M phenylephrine and relaxation in response to acetylcholine was measured in the absence and presence of the NO synthase inhibitor L-NAME (3 × 10−4 mol/L).
Statistical analysis
Statistical analysis was carried out by one-way ANOVA followed by Tukey’s post hoc test, as appropriate. Dose-response curves for vascular relaxations were analyzed by two-way ANOVA for repeated measures followed by Bonferroni multiple comparison test. A p value less than 0.05 was considered statistically significant. Data are expressed as mean ± S.E.M.
Results
PJ-34 treatment rescues NVC responses in aged mice by restoring endothelial NO mediation
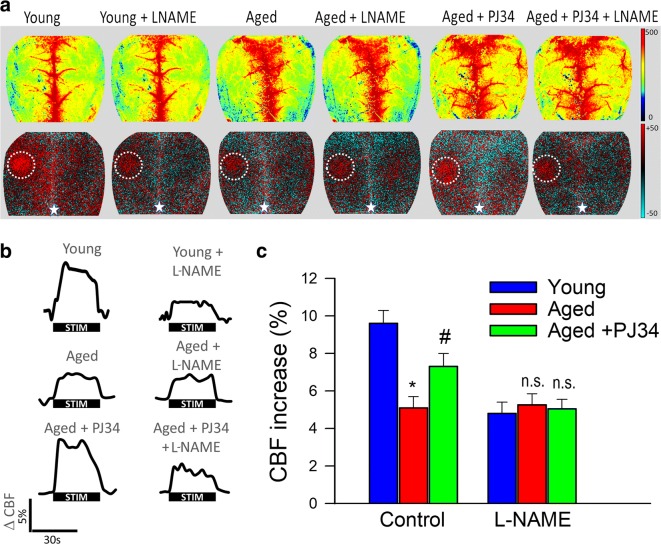
NVC responses measured in the somatosensory whisker barrel cortex elicited by contralateral whisker stimulation were significantly decreased in 24-month-old mice compared to young animals indicating impaired functional hyperemia in old age (Fig. 1) (Park et al. 2007). Two-week treatment with PJ-34 significantly increased CBF responses induced by contralateral whisker stimulation in aged mice, restoring NVC to levels observed in young mice (Fig. 1). There is strong experimental evidence, obtained using both pharmacological inhibitors and genetically modified animals, that NO production by the microvascular endothelium plays a critical role in NVC responses and that cerebromicrovascular endothelial dysfunction significantly contributes to age-related neurovascular dysfunction (Toth et al. 2014, 2015a). Accordingly, in untreated aged animals, administration of the NO synthase inhibitor L-NAME was without effect, whereas in young mice, it significantly decreased NVC responses, eliminating the differences between the age groups (Fig. 1c). In PJ-34-treated aged mice, L-NAME significantly decreased CBF responses elicited by whisker stimulation (Fig. 1c), suggesting that PARP-1 inhibition improves NO mediation of NVC responses in aged animals.
Fig. 1.
Treatment with the PARP-1 inhibitor PJ-34 improves NO mediation of neurovascular coupling responses in aged mice. a Representative pseudocolor laser speckle flowmetry maps of baseline CBF (upper row; shown for orientation purposes) and CBF changes in the whisker barrel field relative to baseline during contralateral whisker stimulation (bottom row, right oval, 30 s, 5 Hz) in young (3 months old), aged (24 months old), and PJ-34-treated aged mice. Color bar represents CBF as percent change from baseline. b The time-course of CBF changes after the start of contralateral whisker stimulation (horizontal bars). Summary data are shown in c. Data are mean ± S.E.M. (n = 6–8 in each group), *P < 0.05 vs. young; #P < 0.05 vs. aged. (one-way ANOVA with post hoc Tukey’s tests). n.s., not significant
Our results show that treatment with PJ-34 treatment also restores acetylcholine-induced, endothelium-dependent relaxation of aged mouse aortas (Fig. 2), extending previous findings obtained with a structurally different PARP inhibitor (Pacher et al. 2004). To assess the role of endothelium-derived NO, L-NAME was applied. L-NAME abolished acetylcholine-induced vasorelaxation, eliminating the differences between the three groups (data not shown). These finding provide additional evidence that PARP inhibition significantly improves endothelial function by restoring endothelial NO mediation in aged vessels.
Fig. 2.
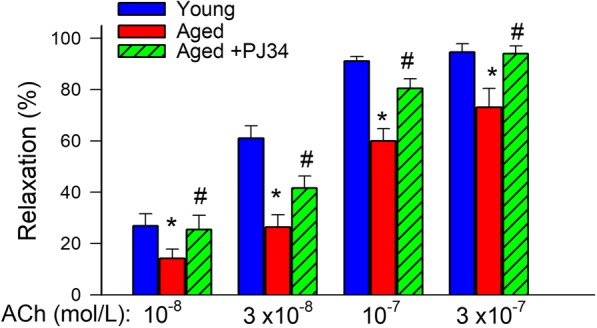
Treatment with PJ-34 improves NO-mediated, endothelium-dependent vasorelaxation in aged mice. Shown are acetylcholine (ACh)-induced relaxations in the absence and presence of the NO synthase inhibitor L-NAME (3 × 10−4 mol/L) in aortic ring preparations isolated from young (4 months old), aged (24 months old), and PJ-34-treated aged mice. Age-related declines in endothelial function were reversed by PJ-34 treatment. Data are mean ± S.E.M. (n = 5–8 for each data point).*P < 0.05 vs. young; #P < 0.05 vs. aged
Restoration of cerebromicrovascular function is associated with improved cognitive function in aged mice treated with PJ-34
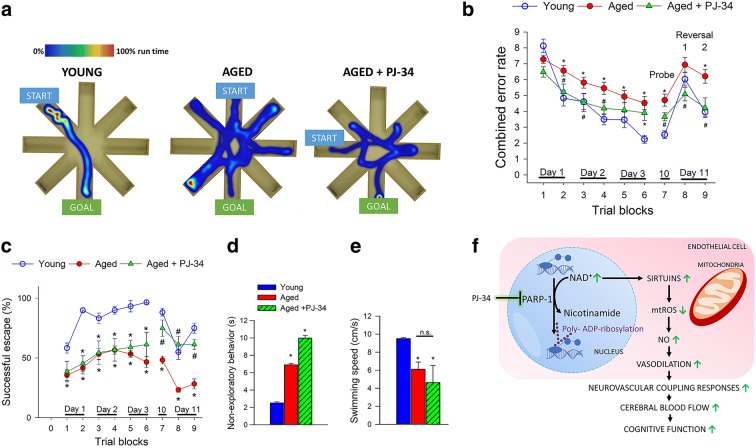
Experimental studies provide proof-of-concept that pharmacologically induced neurovascular uncoupling is associated with detectable cognitive impairment (Tarantini et al. 2015). To determine how rescue of cerebromicrovascular function by PJ-34 treatment impacts cognitive performance in aged mice, animals were tested in the radial arms water maze (Fig. 3). We compared the learning performance of mice in each experimental group by analyzing the day-to-day changes in the combined error rate and successful escape rate. During acquisition, or learning phase, mice from all groups showed a decrease in the combined error rate (Fig. 3b) across days, indicating improved proficiency at the task. After the first day of learning, young mice consistently had lower combined error rate than aged mice (Fig. 3b). PJ-34 treatment resulted in statistically significant improvement in cognitive performance during the learning phase as compared to aged animals. In the probe trial, error rates did not differ from that of the respective last trial of the learning phase in any of the groups. During the reversal trials, young and treated aged mice performed significantly better at re-learning the task compared to aged control animals.
Fig. 3.
Treatment of aged mice with the potent PARP-1 inhibitor PJ-34 associates with improved radial-arm water maze (RAWM) performance. a Heatmap representing the percentage of time spent in different locations in the maze for a randomly selected animal from each group during experimental day 3. b Older animals have higher combined error rates throughout the learning phase and probe day 10 (P < 0.0001). c Older animals are significantly less successful at solving the RAWM as compared to young mice. In contrast, aged mice treated with PJ-34 perform this task significantly better than untreated aged mice during the reversal phase (P < 0.01). d PJ-34 treatment did not affect the time spent engaged in non-exploratory behavior in old mice as compared to old control animals. (P < 0.0001). e There was no significant difference in swimming speed with PJ-34 treatment. n = 20 in each group. All data are shown as mean ± SEM. Statistical significance was calculated using one-way ANOVA with Tukey’s post hoc test to determine differences among groups. f Scheme depicting the proposed cellular mechanisms by which PARP-1 inhibition improves endothelial function in aged mice
Successful escape rate from the maze was assessed by measuring the percent of animals that could find the hidden platform within the 60 s allowed for each trial. During acquisition, mice from all groups showed an increase in successful escape rate consistent with the learning of the task. Young mice exhibited significantly better escape success than untreated aged mice (Fig. 3c). Performance of aged mice treated with PJ-34 was statistically identical to young mice in successful escape rate during the retrieval (day 10) and reversal phase (day 11).
The analyses of non-cognitive parameters revealed a slight age-related decline in swimming speed and an age-dependent increase in non-exploratory behavior (the cumulative time the mice spent not actively looking for the platform, e.g., floating), which were unaffected by PJ-34 treatment (Fig. 3d, e).
Discussion
The key finding of his study is that chronic treatment with the PARP inhibitor PJ-34 improves endothelial function, NVC responses and cognitive function in a mouse model of aging that recapitulates key aspects of cerebromicrovascular dysfunction and deficits of higher brain function manifested in elderly patients.
There is strong evidence that aging is associated with increased oxidative stress-mediated PARP-1 activation in multiple tissues, including the vasculature (Pacher et al. 2002a, 2004; Braidy et al. 2011; Jagtap and Szabo 2005; Massudi et al. 2012). Our findings that PJ-34 improves endothelium-mediated NVC responses and endothelium-dependent aorta relaxation in aged mice suggest that age-related PARP-1 overactivation is causally linked to generalized endothelial dysfunction, extending the results of previous investigations in aged rats (Pacher et al. 2004). Importantly, increased oxidative stress-mediated PARP-1 activation associated with accelerated vascular aging in diabetes mellitus (Soriano et al. 2001; Szabo et al. 2002) has also been causally linked to generalized endothelial dysfunction.
The mechanisms by which PARP-1 overactivation leads to endothelial dysfunction likely involve NAD+ depletion. PARP-1 is a critical NAD+ utilizing enzyme: it cleaves NAD+ and transfers the resulting ADP-ribose moiety onto target proteins and onto subsequent polymers of ADP-ribose. NAD+ depletion is known to impair the activity of sirtuins, which are known to confer multifaceted endothelial protective effects (e.g., regulation of eNOS activity, mitochondrial function and mitochondrial-free radical production, NADPH oxidase activity). Thus, we posit that PJ-34 treatment restores cellular NAD+ levels in the aged vasculature, promoting sirtuin-mediated endothelial protective effects. Indirect support for this hypothesis is provided by recent observations that restoration of cellular NAD+ levels by treatment with the NAD+ precursor nicotinamide mononucleotide (NMN) confers significant endothelial protective effects, including restoration of endothelium-mediated NVC responses and improvement of endothelium-dependent vasorelaxation in the cerebral microvessels and the aorta (Tarantini et al. 2019; Kiss et al. 2019). Additional evidence in support of this concept comes from the observations that chronic NMN treatment also improves NO release from aged cerebromicrovascular endothelial cells in vitro in a SIRT-1-dependent manner (Tarantini et al. 2019). Further studies are evidently needed to demonstrate the direct effects of chronic treatment with PJ-34 and/or other PARP inhibitors on vascular PARP-1 and SIRT-1 activity and endothelial NAD+ levels. Additional mechanisms contributing to age-related decline in NAD+ in endothelial cells may include downregulation of nicotinamide phosphoribosyltransferase (NAMPT; which catalyzes the first rate-limiting step in the biosynthesis of NAD+) (Csiszar et al. 2019). Thus, it is possible that combination treatments that simultaneously increase NAD+ production and inhibit its degradation may offer additional benefits for endothelial and neurovascular protection. It should be also noted that PARP-1 activation may also regulate vascular gene transcription through mechanisms independent of SIRT-1 activation, including epigenetic modifications and interactions with and modification of transcription factors (e.g., NF-κB). Thus, combination treatments inhibiting PARP-1 and boosting NAD+ levels may harness both the power of the anti-aging SIRT-1 pathway and the anti-inflammatory effects mediated by reduced NF-κB activation. These combination treatments can likely have clinical significance beyond restoration of NVC responses, potentially exerting multifaceted protective effects both on the cerebral microvasculature and physiological function of other cell types in the brain, including neurons, astrocytes, and microglia. As several classes of PARP inhibitors move towards clinical development, or have already entered clinical trials, we expect that in the upcoming few years, clinical proof of PARP inhibitors’ vasoprotective and neuroprotective effect in older individuals will be obtained.
There is a growing evidence from clinical (Sorond et al. 2013; Sorond et al. 2011) and experimental (Tarantini et al. 2015) studies that impairment of NVC responses contributes to the age-related decline in higher cortical functions. Thus, elucidating the mechanisms by which aging impairs cerebromicrovascular endothelial function and NVC responses is critical for the development of effective therapies for prevention of vascular cognitive impairment. Restoration of this key homeostatic mechanism matching energy supply with the needs of active neuronal tissue is expected to exert beneficial effects on brain function in aging. The present study is the first to demonstrate that improvement of NVC responses in PJ-34 treated aged mice associates with improvement of hippocampal encoded functions of learning and memory. Similar trends for improved cognitive function have been observed recently in aged mice treated with NMN2 and the mitochondria-targeted antioxidative peptide SS-31 (Tarantini et al. 2018). Future studies should determine whether the effects of combination treatment with PARP inhibitors, NAD+ boosters, and antioxidants are additive.
In conclusion, our findings show that PJ-34 treatment exerts significant cerebromicrovascular protective effects in aged mice by improving endothelial function and increasing NVC responses, which likely contribute to the observed significant cognitive benefit (Fig. 3F). Our model predicts that (1) aging is associated with PARP-1 overactivation that leads to increased NAD+ utilization and (2) that PJ-34 increases cellular NAD+ levels available for sirtuins by attenuating its utilization by PARP-1. We posit that the resulting increase in SIRT-1 activity attenuates endothelial oxidative stress, increase eNOS activity, and improves endothelial function, rescuing endothelium-dependent NVC responses in the aged cortex (Fig. 3f). The model predicts that increased DNA damage induced by age-related increases in reactive oxygen species is the underlying cause for PARP-1 overactivation. If this is indeed the case, it is predicted that anti-aging interventions that delay aging processes (e.g., rapamycin, endocrine factors) upregulate DNA repair mechanisms and/or attenuate oxidative stress will exert significant protective effects on this pathway (An et al. 2017; Ashpole et al. 2017; Deepa et al. 2017; Fang et al. 2017; Podlutsky et al. 2017; Unnikrishnan et al. 2017; Nacarelli et al. 2018; Reglodi et al. 2018). Importantly, NVC is compromised both in patients with Alzheimer’s disease (AD) and in mouse models of AD, which is believed to accelerate clinical deterioration (Tarantini et al. 2017a, c). Thus, our findings are likely relevant to the treatment of AD in elderly patients as well. Important in that regard is that PARP-1 inhibitors were also reported to restore cellular energetics in neurons and inhibit neuroinflammation in animal models of AD (Martire et al. 2016; Martire et al. 2015; Abeti and Duchen 2012).
Acknowledgements
This work was supported by grants from the American Heart Association (ST), the Oklahoma Center for the Advancement of Science and Technology (to AC, AY, ZU), the National Institute on Aging (R01-AG047879; R01-AG038747; R01-AG055395), the National Institute of Neurological Disorders and Stroke (NINDS; R01-NS056218 to AC, R01-NS100782 to ZU), the Oklahoma Shared Clinical and Translational Resources (OSCTR) program funded by the National Institute of General Medical Sciences (GM104938, to AY and JW), the Presbyterian Health Foundation (to ZU, AC, AY), the NIA-supported Geroscience Training Program in Oklahoma (T32AG052363), the Oklahoma Nathan Shock Center (P30AG050911), and the Cellular and Molecular GeroScience CoBRE (1P20GM125528, sub#5337). The funding sources had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Compliance with ethical standards
Conflict of interest
None.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Stefano Tarantini, Andriy Yabluchanskiy, Tamas Csipo and Gabor Fulop contributed equally to this work.
References
- Abeti R, Duchen MR. Activation of PARP by oxidative stress induced by beta-amyloid: implications for Alzheimer’s disease. Neurochem Res. 2012;37:2589–2596. doi: 10.1007/s11064-012-0895-x. [DOI] [PubMed] [Google Scholar]
- An JY, Quarles EK, Mekvanich S, Kang A, Liu A, Santos D, Miller RA, Rabinovitch PS, Cox TC, Kaeberlein M. Rapamycin treatment attenuates age-associated periodontitis in mice. Geroscience. 2017;39:457–463. doi: 10.1007/s11357-017-9994-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashpole NM, Logan S, Yabluchanskiy A, Mitschelen MC, Yan H, Farley JA, Hodges EL, Ungvari Z, Csiszar A, Chen S, Georgescu C, Hubbard GB, Ikeno Y, Sonntag WE. IGF-1 has sexually dimorphic, pleiotropic, and time-dependent effects on healthspan, pathology, and lifespan. Geroscience. 2017;39:129–145. doi: 10.1007/s11357-017-9971-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai P, Canto C, Oudart H, Brunyanszki A, Cen Y, Thomas C, Yamamoto H, Huber A, Kiss B, Houtkooper RH, Schoonjans K, Schreiber V, Sauve AA. Menissier-de Murcia J and Auwerx J. PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab. 2011;13:461–468. doi: 10.1016/j.cmet.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey-Downs LC, Sosnowska D, Toth P, Mitschelen M, Gautam T, Henthorn JC, Ballabh P, Koller A, Farley JA, Sonntag WE, Csiszar A, Ungvari Z. Growth hormone and IGF-1 deficiency exacerbate high-fat diet-induced endothelial impairment in obese Lewis dwarf rats: implications for vascular aging. J Gerontol A Biol Sci Med Sci. 2012;67:553–564. doi: 10.1093/gerona/glr197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonkowski MS, Sinclair DA. Slowing ageing by design: the rise of NAD+ and sirtuin-activating compounds. Nat Rev Mol Cell Biol. 2016;17:679–690. doi: 10.1038/nrm.2016.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braidy N, Guillemin GJ, Mansour H, Chan-Ling T, Poljak A, Grant R. Age related changes in NAD+ metabolism oxidative stress and Sirt1 activity in wistar rats. PLoS One. 2011;6:e19194. doi: 10.1371/journal.pone.0019194. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Csiszar A, Labinskyy N, Podlutsky A, Kaminski PM, Wolin MS, Zhang C, Mukhopadhyay P, Pacher P, Hu F, de Cabo R, Ballabh P, Ungvari Z. Vasoprotective effects of resveratrol and SIRT1: attenuation of cigarette smoke-induced oxidative stress and proinflammatory phenotypic alterations. Am J Physiol Heart Circ Physiol. 2008;294:H2721–H2735. doi: 10.1152/ajpheart.00235.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Labinskyy N, Perez V, Recchia FA, Podlutsky A, Mukhopadhyay P, Losonczy G, Pacher P, Austad SN, Bartke A, Ungvari Z. Endothelial function and vascular oxidative stress in long-lived GH/IGF-deficient Ames dwarf mice. Am J Physiol Heart Circ Physiol. 2008;295:H1882–H1894. doi: 10.1152/ajpheart.412.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Labinskyy N, Jimenez R, Pinto JT, Ballabh P, Losonczy G, Pearson KJ, de Cabo R, Ungvari Z. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: role of circulating factors and SIRT1. Mech Ageing Dev. 2009;130:518–527. doi: 10.1016/j.mad.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Tarantini S, Yabluchanskiy A, Balasubramanian P, Kiss T, Farkas E, Baur JA, Ungvari ZI (2019) Role of endothelial NAD+ deficiency in age-related vascular dysfunction. Am J Physiol Heart Circ Physiol in press [DOI] [PMC free article] [PubMed]
- Das A, Huang GX, Bonkowski MS, Longchamp A, Li C, Schultz MB, Kim LJ, Osborne B, Joshi S, Lu Y, Trevino-Villarreal JH, Kang MJ, Hung TT, Lee B, Williams EO, Igarashi M, Mitchell JR, Wu LE, Turner N, Arany Z, Guarente L, Sinclair DA. Impairment of an endothelial NAD(+)-H2S signaling network is a reversible cause of vascular aging. Cell. 2018;173:74–89 e20. doi: 10.1016/j.cell.2018.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deepa SS, Bhaskaran S, Espinoza S, Brooks SV, McArdle A, Jackson MJ, Van Remmen H, Richardson A. A new mouse model of frailty: the cu/Zn superoxide dismutase knockout mouse. Geroscience. 2017;39:187–198. doi: 10.1007/s11357-017-9975-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiani Monica, Gordon Brian A., Maclin Edward L., Pearson Melanie A., Brumback-Peltz Carrie R., Low Kathy A., McAuley Edward, Sutton Bradley P., Kramer Arthur F., Gratton Gabriele. Neurovascular coupling in normal aging: A combined optical, ERP and fMRI study. NeuroImage. 2014;85:592–607. doi: 10.1016/j.neuroimage.2013.04.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, McFadden S, Darcy J, Hill CM, Huber JA, Verhulst S, Kopchick JJ, Miller RA, Sun LY, Bartke A. Differential effects of early-life nutrient restriction in long-lived GHR-KO and normal mice. Geroscience. 2017;39:347–356. doi: 10.1007/s11357-017-9978-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes AP, Price NL, Ling AJ, Moslehi JJ, Montgomery MK, Rajman L, White JP, Teodoro JS, Wrann CD, Hubbard BP, Mercken EM, Palmeira CM, de Cabo R, Rolo AP, Turner N, Bell EL, Sinclair DA. Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell. 2013;155:1624–1638. doi: 10.1016/j.cell.2013.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath B, Orsy P, Benyo Z. Endothelial NOS-mediated relaxations of isolated thoracic aorta of the C57BL/6J mouse: a methodological study. J Cardiovasc Pharmacol. 2005;45:225–231. doi: 10.1097/01.fjc.0000154377.90069.b9. [DOI] [PubMed] [Google Scholar]
- Jagtap P, Szabo C. Poly(ADP-ribose) polymerase and the therapeutic effects of its inhibitors. Nat Rev Drug Discov. 2005;4:421–440. doi: 10.1038/nrd1718. [DOI] [PubMed] [Google Scholar]
- Kazama K, Anrather J, Zhou P, Girouard H, Frys K, Milner TA, Iadecola C. Angiotensin II impairs neurovascular coupling in neocortex through NADPH oxidase-derived radicals. Circ Res. 2004;95:1019–1026. doi: 10.1161/01.RES.0000148637.85595.c5. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Cao W, Oh GS, Lee S, Shen A, Khadka D, Lee SB, Sharma S, Kim SY, Choe SK, Kwak TH, Kim JM, Park R, So HS (2019) Augmentation of cellular NAD(+) by NQO1 enzymatic action improves age-related hearing impairment. Aging Cell. 2019 Oct; 18(5): e13016. [DOI] [PMC free article] [PubMed]
- Kiss T, Balasubramanian P, Valcarcel-Ares MN, Tarantini S, Yabluchanskiy A, Csipo T, Lipecz A, Reglodi D, Zhang XA, Bari F, Farkas E, Csiszar A, Ungvari Z (2019) Nicotinamide mononucleotide (NMN) treatment attenuates oxidative stress and rescues angiogenic capacity in aged cerebromicrovascular endothelial cells: a potential mechanism for prevention of vascular cognitive impairment. GeroScience:in press [DOI] [PMC free article] [PubMed]
- Lipecz A, Csipo T, Tarantini S, Hand RA, Ngo BN, Conley S, Nemeth G, Tsorbatzoglou A, Courtney DL, Yabluchanska V, Csiszar A, Ungvari ZI, Yabluchanskiy A. Age-related impairment of neurovascular coupling responses: a dynamic vessel analysis (DVA)-based approach to measure decreased flicker light stimulus-induced retinal arteriolar dilation in healthy older adults. Geroscience. 2019;41:341–349. doi: 10.1007/s11357-019-00078-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martire S, Mosca L, d'Erme M. PARP-1 involvement in neurodegeneration: a focus on Alzheimer’s and Parkinson’s diseases. Mech Ageing Dev. 2015;146-148:53–64. doi: 10.1016/j.mad.2015.04.001. [DOI] [PubMed] [Google Scholar]
- Martire S, Fuso A, Mosca L, Forte E, Correani V, Fontana M, Scarpa S, Maras B, d'Erme M. Bioenergetic impairment in animal and cellular models of Alzheimer’s disease: PARP-1 inhibition rescues metabolic dysfunctions. J Alzheimers Dis. 2016;54:307–324. doi: 10.3233/JAD-151040. [DOI] [PubMed] [Google Scholar]
- Massudi H, Grant R, Braidy N, Guest J, Farnsworth B, Guillemin GJ. Age-associated changes in oxidative stress and NAD+ metabolism in human tissue. PLoS One. 2012;7:e42357. doi: 10.1371/journal.pone.0042357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KF, Yoshida S, Stein LR, Grozio A, Kubota S, Sasaki Y, Redpath P, Migaud ME, Apte RS, Uchida K, Yoshino J, Imai SI. Long-term administration of nicotinamide mononucleotide mitigates age-Associated Physiological Decline in mice. Cell Metab. 2016;24:795–806. doi: 10.1016/j.cmet.2016.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacarelli T, Azar A, Altinok O, Orynbayeva Z, Sell C. Rapamycin increases oxidative metabolism and enhances metabolic flexibility in human cardiac fibroblasts. Geroscience. 2018;40:243–256. doi: 10.1007/s11357-018-0030-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Mabley JG, Soriano FG, Liaudet L, Szabo C. Activation of poly(ADP-ribose) polymerase contributes to the endothelial dysfunction associated with hypertension and aging. Int J Mol Med. 2002;9:659–664. [PubMed] [Google Scholar]
- Pacher P, Mabley JG, Soriano FG, Liaudet L, Komjati K, Szabo C. Endothelial dysfunction in aging animals: the role of poly(ADP-ribose) polymerase activation. Br J Pharmacol. 2002;135:1347–1350. doi: 10.1038/sj.bjp.0704627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Vaslin A, Benko R, Mabley JG, Liaudet L, Hasko G, Marton A, Batkai S, Kollai M, Szabo C. A new, potent poly(ADP-ribose) polymerase inhibitor improves cardiac and vascular dysfunction associated with advanced aging. J Pharmacol Exp Ther. 2004;311:485–491. doi: 10.1124/jpet.104.069658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park L, Anrather J, Girouard H, Zhou P, Iadecola C. Nox2-derived reactive oxygen species mediate neurovascular dysregulation in the aging mouse brain. J Cereb Blood Flow Metab. 2007;27:1908–1918. doi: 10.1038/sj.jcbfm.9600491. [DOI] [PubMed] [Google Scholar]
- Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podlutsky A, Valcarcel-Ares MN, Yancey K, Podlutskaya V, Nagykaldi E, Gautam T, Miller RA, Sonntag WE, Csiszar A, Ungvari Z. The GH/IGF-1 axis in a critical period early in life determines cellular DNA repair capacity by altering transcriptional regulation of DNA repair-related genes: implications for the developmental origins of cancer. Geroscience. 2017;39:147–160. doi: 10.1007/s11357-017-9966-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reglodi D, Atlasz T, Szabo E, Jungling A, Tamas A, Juhasz T, Fulop BD, Bardosi A. PACAP deficiency as a model of aging. Geroscience. 2018;40:437–452. doi: 10.1007/s11357-018-0045-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz MB, Sinclair DA. Why NAD(+) declines during aging: It's destroyed. Cell Metab. 2016;23:965–966. doi: 10.1016/j.cmet.2016.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano FG, Pacher P, Mabley J, Liaudet L, Szabo C. Rapid reversal of the diabetic endothelial dysfunction by pharmacological inhibition of poly(ADP-ribose) polymerase. Circ Res. 2001;89:684–691. doi: 10.1161/hh2001.097797. [DOI] [PubMed] [Google Scholar]
- Sorond FA, Kiely DK, Galica A, Moscufo N, Serrador JM, Iloputaife I, Egorova S, Dell'Oglio E, Meier DS, Newton E, Milberg WP, Guttmann CR, Lipsitz LA. Neurovascular coupling is impaired in slow walkers: the MOBILIZE Boston study. Ann Neurol. 2011;70:213–220. doi: 10.1002/ana.22433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorond FA, Hurwitz S, Salat DH, Greve DN, Fisher ND. Neurovascular coupling, cerebral white matter integrity, and response to cocoa in older people. Neurology. 2013;81:904–909. doi: 10.1212/WNL.0b013e3182a351aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanova I, Stephan T, Becker-Bense S, Dera T, Brandt T, Dieterich M. Age-related changes of blood-oxygen-level-dependent signal dynamics during optokinetic stimulation. Neurobiol Aging. 2013;34:2277–2286. doi: 10.1016/j.neurobiolaging.2013.03.031. [DOI] [PubMed] [Google Scholar]
- Szabo C, Zanchi A, Komjati K, Pacher P, Krolewski AS, Quist WC, LoGerfo FW, Horton ES, Veves A. Poly(ADP-ribose) polymerase is activated in subjects at risk of developing type 2 diabetes and is associated with impaired vascular reactivity. Circulation. 2002;106:2680–2686. doi: 10.1161/01.cir.0000038365.78031.9c. [DOI] [PubMed] [Google Scholar]
- Tarantini S, Hertelendy P, Tucsek Z, Valcarcel-Ares MN, Smith N, Menyhart A, Farkas E, Hodges E, Towner R, Deak F, Sonntag WE, Csiszar A, Ungvari Z, Toth P. Pharmacologically-induced neurovascular uncoupling is associated with cognitive impairment in mice. J Cereb Blood Flow Metab. 2015;35:1871–1881. doi: 10.1038/jcbfm.2015.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Tran CHT, Gordon GR, Ungvari Z, Csiszar A. Impaired neurovascular coupling in aging and Alzheimer’s disease: contribution of astrocyte dysfunction and endothelial impairment to cognitive decline. Exp Gerontol. 2017;94:52–58. doi: 10.1016/j.exger.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Yabluchanksiy A, Fulop GA, Hertelendy P, Valcarcel-Ares MN, Kiss T, Bagwell JM, O'Connor D, Farkas E, Sorond F, Csiszar A, Ungvari Z. Pharmacologically induced impairment of neurovascular coupling responses alters gait coordination in mice. Geroscience. 2017;39:601–614. doi: 10.1007/s11357-017-0003-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Fulop GA, Kiss T, Farkas E, Zolei-Szenasi D, Galvan V, Toth P, Csiszar A, Ungvari Z, Yabluchanskiy A. Demonstration of impaired neurovascular coupling responses in TG2576 mouse model of Alzheimer’s disease using functional laser speckle contrast imaging. Geroscience. 2017;39:465–473. doi: 10.1007/s11357-017-9980-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Valcarcel-Ares NM, Yabluchanskiy A, Fulop GA, Hertelendy P, Gautam T, Farkas E, Perz A, Rabinovitch PS, Sonntag WE, Csiszar A, Ungvari Z. Treatment with the mitochondrial-targeted antioxidant peptide SS-31 rescues neurovascular coupling responses and cerebrovascular endothelial function and improves cognition in aged mice. Aging Cell. 2018;17:e12731. doi: 10.1111/acel.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Valcarcel-Ares MN, Toth P, Yabluchanskiy A, Tucsek Z, Kiss T, Hertelendy P, Kinter M, Ballabh P, Sule Z, Farkas E, Baur JA, Sinclair DA, Csiszar A, Ungvari Z. Nicotinamide mononucleotide (NMN) supplementation rescues cerebromicrovascular endothelial function and neurovascular coupling responses and improves cognitive function in aged mice. Redox Biol. 2019;24:101192. doi: 10.1016/j.redox.2019.101192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topcuoglu MA, Aydin H, Saka E. Occipital cortex activation studied with simultaneous recordings of functional transcranial Doppler ultrasound (fTCD) and visual evoked potential (VEP) in cognitively normal human subjects: effect of healthy aging. Neurosci Lett. 2009;452:17–22. doi: 10.1016/j.neulet.2009.01.030. [DOI] [PubMed] [Google Scholar]
- Toth P, Tarantini S, Tucsek Z, Ashpole NM, Sosnowska D, Gautam T, Ballabh P, Koller A, Sonntag WE, Csiszar A, Ungvari ZI. Resveratrol treatment rescues neurovascular coupling in aged mice:role of improved cerebromicrovascular endothelial function and down-regulation of NADPH oxidas. Am J Physiol Heart Circ Physiol. 2014;306:H299–H308. doi: 10.1152/ajpheart.00744.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Tarantini S, Davila A, Valcarcel-Ares MN, Tucsek Z, Varamini B, Ballabh P, Sonntag WE, Baur JA, Csiszar A, Ungvari Z. Purinergic glio-endothelial coupling during neuronal activity: role of P2Y1 receptors and eNOS in functional hyperemia in the mouse somatosensory cortex. Am J Physiol Heart Circ Physiol. 2015;309:H1837–H1845. doi: 10.1152/ajpheart.00463.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Tarantini S, Ashpole NM, Tucsek Z, Milne GL, Valcarcel-Ares NM, Menyhart A, Farkas E, Sonntag WE, Csiszar A, Ungvari Z. IGF-1 deficiency impairs neurovascular coupling in mice: implications for cerebromicrovascular aging. Aging Cell. 2015;14:1034–1044. doi: 10.1111/acel.12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Tarantini S, Hertelendy P, Valcarcel-Ares MN, Fulop GA, Logan S, Kiss T, Farkas E, Csiszar A, Yabluchanskiy A. Cerebromicrovascular dysfunction predicts cognitive decline and gait abnormalities in a mouse model of whole brain irradiation-induced accelerated brain senescence. Geroscience. 2017;39:33–42. doi: 10.1007/s11357-017-9964-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unnikrishnan A, Jackson J, Matyi SA, Hadad N, Wronowski B, Georgescu C, Garrett KP, Wren JD, Freeman WM, Richardson A. Role of DNA methylation in the dietary restriction mediated cellular memory. Geroscience. 2017;39:331–345. doi: 10.1007/s11357-017-9976-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino J, Baur JA, Imai SI. NAD(+) intermediates: the biology and therapeutic potential of NMN and NR. Cell Metab. 2018;27:513–528. doi: 10.1016/j.cmet.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaletel M, Strucl M, Pretnar-Oblak J, Zvan B. Age-related changes in the relationship between visual evoked potentials and visually evoked cerebral blood flow velocity response. Funct Neurol. 2005;20:115–120. [PubMed] [Google Scholar]